Abstract
Individual differences in coping response lie at the core of vulnerability to conditions like post traumatic stress disorder (PTSD). Like humans, not all animals exposed to severe stress show lasting change in affect. Predator stress is a traumatic experience inducing long-lasting fear, but not in all rodents. Thus, individual variation may be a cross species factor driving responsiveness to stressful events. The present study investigated neurobiological bases of variation in coping with severe stress. The amygdala was studied because it modulates fear and its function is affected by stress. Moreover, stress-induced plasticity of the amygdala has been related to induction of anxiety, a comorbid symptom of psychiatric conditions like PTSD. We exposed rodents to predator stress and grouped them according to their adaptability based on a standard anxiety test (the elevated plus maze). Subsequently we investigated if well-adapted (less anxious) and mal-adapted (extremely anxious) stressed animals differed in the structure of dendritic trees of their output neurons of the right basolateral amygdala (BLA). Two weeks after exposure to stress, well-adapted animals showed low anxiety levels comparable to unstressed controls, whereas mal-adapted animals were highly anxious. In these same animals, Golgi analysis revealed that BLA neurons of well-adapted rats exhibited more densely packed and shorter dendrites than neurons of mal-adapted or unstressed control animals, which did not differ. These data suggest that dendritic hypotrophy in the BLA may be a resilience marker against lasting anxiogenic effects of predator stress.
Keywords: stress, anxiety, amygdala, neuron, morphology, plasticity, resilience
Introduction
Stress and trauma affect individuals differently. While traumatic experience leads to post traumatic stress disorder (PTSD) in some, others exposed to severe stressors are less affected (29;30;47). Relatively little is known about molecular and neural substrates of such individual differences in coping (47). However correlational behavioral research implicates a variety of possible factors, including personality traits (28;36), interaction of genetic factors and experiential factors, such as reduced functioning polymorphisms in the serotonin transporter (5-HTTLPR), and life stress or social support at the time of stress (4;27;31). Moreover, reactivity of amygdala to environmental threat is modulated by 5-HTTLPR (27) which has also been implicated in PTSD (31;34). So factors affecting functional amygdala reactivity may be important contributors to vulnerability to stress. In PTSD patients right amygdala activity is enhanced in response to both trauma reminder and general negative stimuli (43;45).
These studies are suggestive, but being correlative, do not reveal causal factors (47;48). One way to identify putative causal substrates, however, is to study impact of stress on brain and behavior of more and less stress vulnerable animals. A useful paradigm in this regard is exposure of rodents to brief predator stress, a putative model of hyperarousal and generalized anxiety characteristics of PTSD (3).
Domesticated strains of laboratory rats retain the fear of predators like a cat, even if they have never been exposed to predators (5;13). On exposure to a cat (predator stress) or cat stimuli (predator scent stress-PSS), laboratory rats and mice develop long-lasting (3 weeks or longer) anxiety (7;9;10;17-19). However, not all stressed animals respond similarly. Some remain unaffected, showing little fear sensitization (19;20).
Reasons for these individual differences remain largely unknown, though there has been recent progress. In hippocampus (area CA1), up regulation of ARC gene expression (mRNA) was found in well-adapted rats unaffected by PSS (33), whereas down regulation of BDNF and up regulation of TrkB receptors was observed in mal-adapted rats made extremely fearful by PSS (32). In addition serotonin transporter gene knockout mice are more vulnerable to predator stress (4;8), providing an interesting parallel to the human clinical literature, and in that context, implicating modulation of amygdala function in vulnerability to stress. Interestingly, predator stress induces a lasting enhancement of excitability of right rodent amygdala, detected as a potentiation of afferent and efferent transmission in basolateral (BLA) and central amygdala (2;12). Furthermore, degree of anxiogenic effect of predator stress is tightly predicted by degree of potentiation in amygdala circuitry (11). Moreover, electrophysiological studies suggest that one mechanism mediating predator stress potentiation of amygdala circuitry could be changes in dendritic morphology (12). Structural variation which alters neural transmission in BLA could alter fearful response which highly correlates with BLA transmission (11).
Indeed, variation in dendritic arbors of BLA neurons is related to the ability of restraint stress to generate anxiety (49;50). Anxiety generated by stress and stress hormone is accompanied by BLA hypertrophy (39;49;50), and experimental reduction of dendritic length results in reduction of anxiety (37). Moreover, once generated, BLA hypertrophy is as long lasting as stress induced anxiety (50).
Given the above considerations, it is timely to ask if neurons of the BLA and their plasticity are involved in individual differences in coping with predator stress. Two hypotheses can be proposed to explain individual differences. First, in mal-adapted animals, stress causes neural expansion in BLA related to the enhanced anxiety they experience. Second, stress causes neural retraction in well-adapted animals, and this plasticity prevents maladaptive effects of trauma. In this report, we attempted to test these two hypotheses.
Methods and Materials
Subjects and Groups
A total of 81 adult male Long-Evans hooded rats were used. At arrival from Charles River Canada, rats were approximately 4 weeks of age and weighed between 76g and 100g. Rats were housed individually in standard clear polycarbonate cages. The animals were fed and watered ad lib, and were maintained on a 12 hour light-dark schedule (lights on at 07:00). Rats were first habituated to their home cage for one day, after which they were handled once per day for one minute over the following five days. Finally, rats were randomly assigned to either the predator stressed group (to be exposed to a cat, n = 71) or handled control (n = 10). Observations reported in this manuscript refer to young animals (5 weeks at start of experiment); an important variable because stress sensitivity can vary across lifespan of rodents.
Predator Stress and Handling
One week after arrival, predator stress group animals were exposed to one of two cats. Exposures were unprotected and occurred between the hours of 09:00 and 12:00. All exposures took place in a large enclosed room with a floor area of approximately 35 square feet as described elsewhere (7). Exposures lasted for ten minutes and were videotaped to capture the activities of both the rat and the cat. Cat response consisted of watching the rat from a distance, followed by several approaches, pawing, and the occasional mild attack. No rats were injured. Handled animals were handled for one minute on the day of cat exposure of predator stressed groups. Handled and predator stressed rats were housed in separate rooms and did not come into contact with each other. Time of treatment was counterbalanced among all groups. Following treatment, all rats were returned to their home cages and left unhandled until testing for lasting effects on rodent anxiety.
Behavioral Measures taken from Cat Exposures
Behavior of both the rat and cat was analyzed from videotape. Cat behaviors consisted of latency to approach the rat, the number of approaches, and time spent near the rat; latency to sniff the rat and the time spent sniffing; latency to bite the rat, number of bites, and frequency of pawing. The cat was considered near the rat when it moved to within one foot of the rat determined from one foot square floor markings.
Rat behavior in response to the cat was also analyzed. Defensive behavior was categorized as frequency of active, passive, or escape as described elsewhere (7).
Post Treatment Behavioral Testing
Two weeks after handling or predator stress, anxiety-like behavior was examined using the hole board and elevated plus maze (EPM) tests. Such tests are commonly used to assess rodent exploration, activity, and anxiety (23-25). Behavior in all tests was video taped remotely for later blind analysis. All tests were 5 minutes in duration and conducted between 09:00 and 11:00 under normal room lighting. Rats were tested first in the hole board immediately followed by testing in the EPM.
Hole Board Testing
The hole board test provided independent measures of activity and exploratory tendency (25). The hole board apparatus was an open top square wooden box measuring 60 × 60 × 35 cm (length × width × height). In addition four evenly spaced holes were drilled 14 cm from the walls in a floor that was raised 12 cm above the ground. Both floor and walls were painted with grey enamel. Tape marked a square inside the box, separating it into center (containing the 4 holes) and perimeter (near the wall) segments.
Rats were placed in the center of the hole board apparatus and allowed to explore freely for 5 minutes. Rats were then immediately transferred to the EPM located in the same room for a further 5 minutes of testing. After each test the box was cleaned with a 5 % alcohol solution.
Measures of activity and exploratory behavior were taken from video taped records. Activity was recorded as time spent in motion of any kind. Exploratory tendency was scored as the number of head dips (placing the head or snout into one of the four holes drilled in the floor). In addition the amount of time spent near the walls of the box was measured. Rats were considered to be near the wall of the box when all four feet were outside the center square marked by tape.
Elevated Plus Maze (EPM)
Immediately following the hole board test, rats were placed in the EPM. The EPM was a wooden four armed platform with arms arranged in the shape of a plus. The platform was painted with gray enamel, and was raised 50 cm above the floor. All arms were 10 cm wide and 50 cm long and joined in the center to a 10 cm square platform. Two arms facing each other were closed arms, the other two were open. Closed arms were surrounded by 40 cm high wooden walls which were open at the top, while open arms were bounded by a 3 cm high edge only.
At the start of each test, rats were placed in the center square facing the same open arm, and were allowed to move freely for 5 minutes. At the conclusion of each test, rats were returned to their home cage and the maze was cleaned and wiped dry using a 5% alcohol solution.
A number of behavioral measures were taken from videotape. These included standard measures of rodent anxiety: ratio time and ratio entry. Ratio time refers to the total time spent in the open arms of the maze divided by the total time spent in any arm of the maze. Ratio entry refers to the total entries into the open arms of the maze divided by the total entries into any arm of the maze. Smaller ratios indicate less open arm exploration, or more “anxiety”. A rat was considered within an arm of the maze when all four feet were within the arm.
In addition, entries into the closed arms of the maze were taken as a measure of activity/exploratory tendency. Finally, risk assessment was measured. Risk assessment was scored when a rat poked its head into the open arm of the maze with its hindquarters in one of the closed arms. Frequency of risk assessment was recorded. The frequency of risk assessment was divided by the total time spent in the closed arms of the maze to produce a relative frequency risk assessment measure.
Selection of Mal-Adapted and Well-Adapted Predator Stressed Rats and Handled Controls
On the day of EPM testing, ratio time measures were calculated for each rat. Inclusion into the mal-adapted group of predator stressed rats required a ratio time score of 0. Well-adapted predator stressed rats were identified as those with ratio time scores falling between .25 and .50. This was based on an extensive data base of handled hooded rat data in the Adamec laboratory and was considered characteristic of the range of handled control EPM response (95% confidence intervals around a mean of .375). From the 71 predator stressed rats it was possible to select four well-adapted rats; thus, four mal-adapted rats were also selected. Four handled rats were randomly selected from the 10 handled controls.
Morphological studies and analysis
Animals were sacrificed under deep (chloral hydrate, 1 ml, 1 g/ml) anesthesia one day after EPM testing and 15 days after predator exposure or handling. Fresh brain tissue was removed and cut into a block containing right posterior amygdala from approximately 2.80 mm posterior to bregma back. This was done to capture that part of the right posterior BLA in which potentiation of ventral hippocampal afferent transmission is produced by predator stress (2;12). Dissected brain tissues containing posterior amygdala were processed for staining individual neurons using rapid Golgi method. Golgi-stained BLA tissue was sectioned (120 μm thick), mounted with cover slips and used for morphological analysis. Camera Lucida tracings (500 ×) were obtained (Nikon, USA) from up to ten selected neurons per rat (a mix of stellate and pyramidal principle output cells) and were then scanned (8-bit grayscale TIFF images with 600 dpi resolution; HP Scan Jet 6200C) along with a calibrated scale for subsequent computerized image-analysis. Custom-designed macros embedded in ‘Scion Image’ software (http://www.scioncorp.com/) were used for morphometric analysis of digitized images. Using the center of the soma as the reference point, dendritic length and branch points were measured as a function of radial distance from the soma by adding up all values in each successive concentric segment (Sholl's analysis). Golgi analysis was done blind to the group assignment of the brain under study. A total of four brains from each group were processed except for the predator stressed well-adapted group, which for technical reasons had three brains with well enough stained sections to process.
Statistical analysis
Values are reported as mean ± SEM. One way analysis of variance (ANOVA) tested differences between handled controls, mal- and well-adapted animals. Post-hoc Fisher's Least Significant Difference (LSD) test was used for mean contrasts. For the purpose of morphological studies each neuron was considered as a data point (49), a widely accepted method of analysis in golgi studies of dendritic morphology similar to the present study (14;22;42;44;46;52;53).
Ethical Approval
All procedures involving animals in this study adhered to the guidelines of the Canadian Council on Animal care, and were approved by the Institutional Animal Care committee of Memorial University. All efforts were made to minimize pain, stress, and the number of animals used.
Results
Mal-adapted animals are more anxious in the EPM than well-adapted and handled controls two weeks after treatment
While rats in the mal- and well-adapted groups were selected based on ratio time criteria, handled controls were randomly selected from a larger group. It was therefore important to confirm the expected pattern of differences in open arm exploration (ratio time and ratio entry) among the groups. Moreover, it was necessary to compare groups on measures of activity and exploration in order to ensure that differences in ratio time could be interpreted as differences in anxiety (open arm avoidance due to fear) and not differences in activity/exploration.
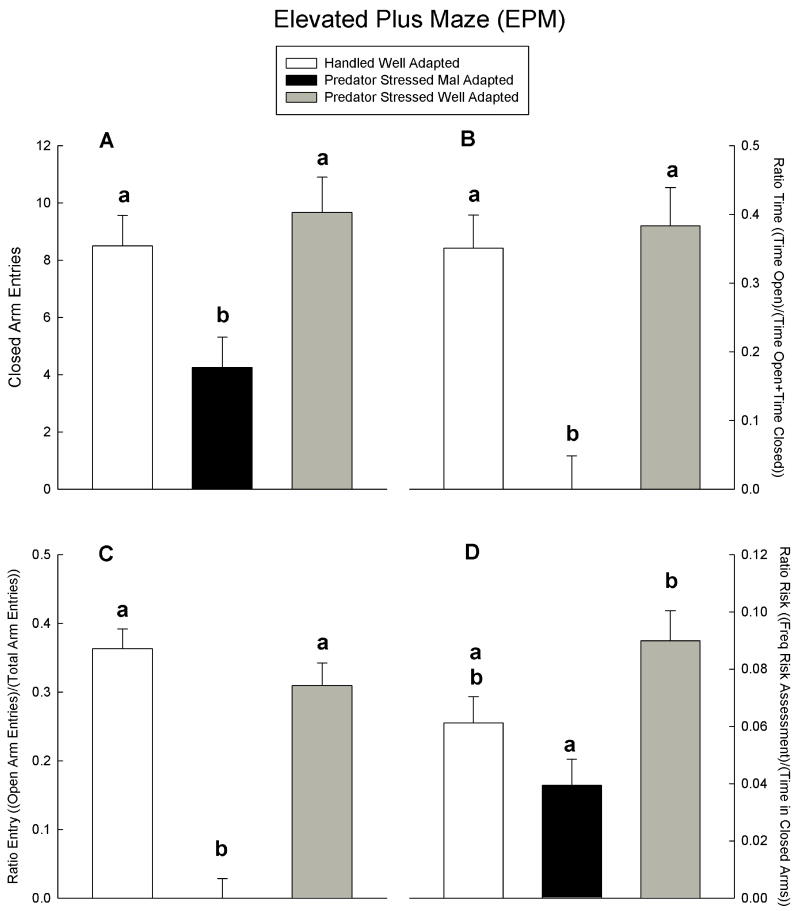
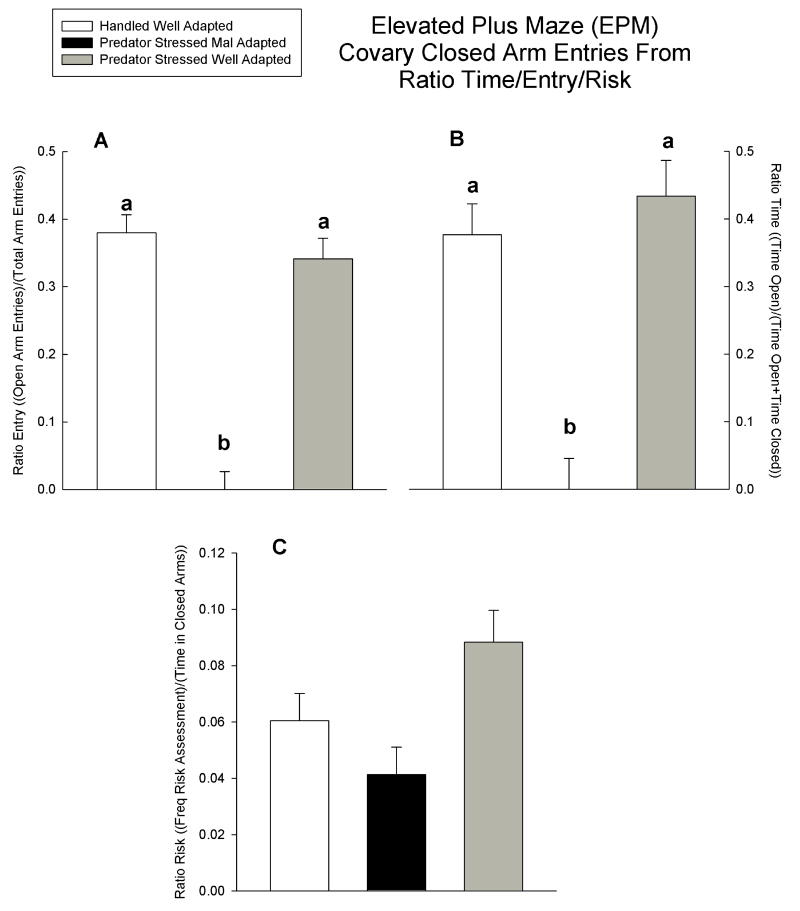
As expected, one way ANOVA confirmed that mal-adapted animals exhibited reduced open arm exploration (reduced ratio time and entries) relative to the other groups (Figure 1 B,C, all F(2,8) ≥ 18.23 all p<.001, mean contrasts p < .05 LSD), which did not differ (p > .05 LSD). Similar group differences were observed in closed arm entries (Figure 1 A, F(2,8) = 6.56, p<.021, mean contrasts p < .05 LSD), suggesting reduced locomotor activity in mal-adapted rats. To assess if locomotor activity contributed to group differences in anxiety (i.e., reduced open arm exploration), closed arm entries were used as a covariate in a reanalysis of ratio time and ratio entry. Reduced locomotor activity in the EPM did not contribute to reduced open-arm exploration, as the original pattern of group differences was preserved in the analysis of covariance (Figure 2 A,B, all F(2,7) ≥ 12.81 all p<.01, mean contrasts p < .05 LSD). Consistent with this analysis, there were no group differences in the hole board measures of activity/ exploration (time active or head dips), nor did the groups differ in time near the wall (all F(2,8) ≤ 2.72, all p>.12). These data support the conclusion that mal-adapted rats are selectively more anxious in the EPM than well-adapted rats and handled controls, which do not differ in anxiety.
Figure 1.
Plotted across handled, and stressed mal- and well-adapted groups in Figures A - D are mean + SEM of behaviors measured in the EPM. Within a given behavioral plot, means marked with the same letter do not differ, means marked differently differ, and means marked with two letters fall between and do not differ from means marked with either letter.
Figure 2.
Plotted across handled, and stressed mal- and well-adapted groups in Figures A - C are mean + SEM of measures of open arm exploration (ratio time/entry) and risk in the EPM after covarying closed arm entries from them. Within a given behavioral plot, means marked with the same letter do not differ, means marked differently differ. Unmarked means in C do not differ.
Mal-adapted rats displayed less risk assessment in the EPM than well-adapted rats, and handled controls fell between these groups, differing from neither (Figure 1 D, F(2,8) = 6.54, p<.03; mean contrasts, p<.05, LSD). Though reduced risk assessment in mal-adapted rats is consistent with previous reports of effects of predator stress on this measure (7;12), in the present study the group differences appear to reflect differences between groups in EPM activity (closed arm entries). Reanalysis of risk assessment data with closed arm entries as a covariate eliminated the group differences (Figure 2 C, F(2,7) = 2.45, p<.16).
The predator stress experience
Well- and mal-adapted rats were compared with respect to cat response to them and their responses to the cat. There were no group differences (all F(1,5) ≤ .75, p>.43). Therefore the predator stress experience, as measured, did not differ between well and mal-adapted rats.
Predator stressed animals per se are more anxious in the EPM than handled controls two weeks after treatment
It is important to confirm that there was an overall anxiogenic effect of predator stress in the group from which mal-adapted and well-adapted rats were selected. Therefore, behavioral responses in the EPM and hole board of all handled (n=10) and all stressed (n=71) rats were compared. Stressed animals exhibited significantly reduced open arm exploration (reduced ratio times and entries) relative to handled animals (Table 1). Of interest, groups did not differ in closed arm entries (F(1,79) = 0.50, p>.48, means ± SEM of handled, stressed respectively: 9.4 ± .88, 8.7 ± .33). These data suggest that locomotor activity did not contribute to group differences in anxiety, a conclusion drawn in the selected group analysis above. Consistent with this conclusion, there were no group differences in time active or head dips in the hole board (all F(1,79) ≤ 2.36, all p>.12). Interestingly, stressed rats spent more time near the wall than handled controls (Table 1), consistent with increased EPM anxiety. Taken together the data support the conclusion that overall predator stressed rats were more anxious in the EPM than handled controls. Finally, consistent with the smaller group analyses above, groups did not differ in risk assessment (F(1,79) = 2.20, p<.15)
Table 1. Comparision between handled and stressed animals.
| Parameter | df | ANOVA values | Mean ± SeM | ||
|---|---|---|---|---|---|
| F | P | Handled | Stressed | ||
| Ratio open-arm time in elevated plus-maze | 1, 79 | ≥ 6.26 | < 0.02 | 0.31 ± 0.04 | 0.19 ± 0.02 |
| Ratio open arm entries in elevated plus-maze | 1, 79 | ≥ 6.26 | < 0.02 | 0.36 ± 0.03 | 0.27 ± 0.01 |
| Time spent near wall in hole-board | 1, 79 | 6.03 | < 0.02 | 154.3 ± 9.36 sec | 178.9 ± 3.51 sec |
Predator stress well-adapted animals show differences from other groups in dendritic morphology of BLA neurons
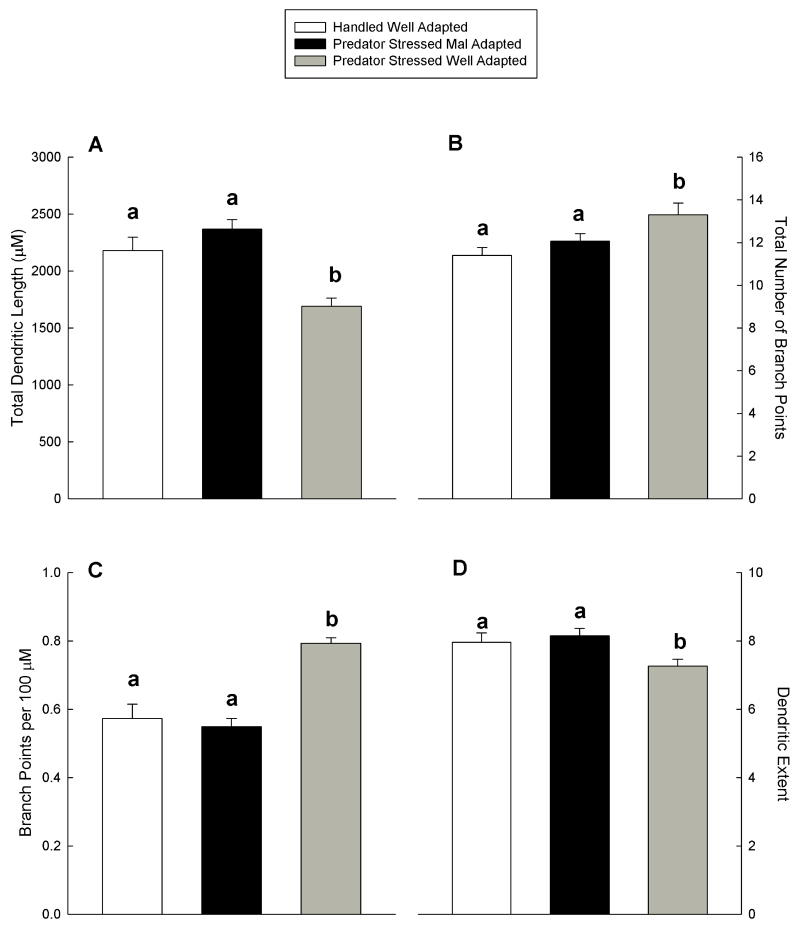
BLA stellate and pyramidal principle output neurons of the groups differed in dendritic length, number of branch points, branch packing (number of branches per 100 μm of dendrite length) and extent of dendritic tree (farthest distance from soma that any dendrite could be detected) (all F(2,91) ≥ 4.20, p<.02). Mal-adapted animals did not differ significantly from handled controls in any dendritic parameter (p > 0.25). Total dendritic length was lowest in well-adapted animals compared to mal-adapted (26% reduction, p < 0.001 LSD, Figure 3 A) and to handled control animals (22% reduction, p = 0.001 LSD). In contrast, total number of branch points was highest in well-adapted animals compared to handled controls (p < 0.01 LSD, Figure 3 B) and to mal-adapted animals (p < 0.05 LSD). Thus, the number of branches per 100 μm of dendritic length (branch packing) was highest in animals well-adapted to predator stress (> 38 % increase over handled controls, p < 0.001 LSD, Figure 3 C). Additionally well-adapted animals exhibited the shortest dendritic extent, significantly differing from the other two groups (p < 0.01 LSD, Figure 3 D).
Figure 3.
Plotted across handled, and stressed mal- and well-adapted groups in Figures A - D are mean + SEM of dendritic morphological measures taken from BLA neurons (n = 30 neurons for well-adapted [10 cells from each of 3 rats], n = 25 for handled [6 cells from each of 3 rats and 7 cells from the fourth] and n = 39 [10 cells from each of 3 rats and 9 cells from the fourth] for mal-adapted groups of animals). Within a given plot, means marked with the same letter do not differ, means marked differently differ.
In summary, well-adapted animals showed retracted dendritic arbors with higher branch packing, compared to both handled controls and mal-adapted animals. Dendritic trees of mal-adapted animals, on the other hand, were not different from control animals. Representative neurons from the three groups and a typical golgi-stained BLA neuron are depicted in Figures 4 A and B respectively.
Figure 4.
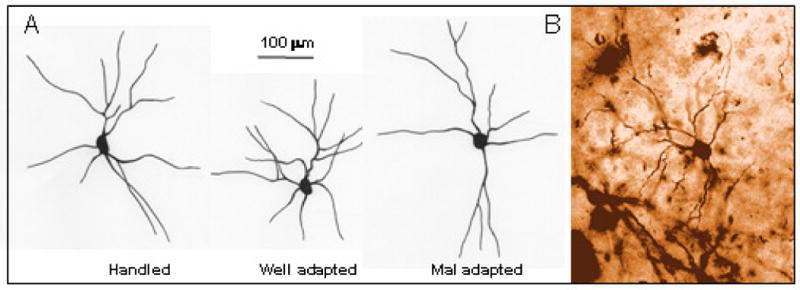
Qualitative representation of neurons from each group of animals. A. Schematic diagram of neuronal tracing from handled (left), well-adapted (middle) and mal-adapted (right) animals. Scale bar = 100 mm. B. A typical BLA field stained with Golgi, showing a stellate neuron at 500×.
Well-adapted animals show maximum branch packing at radial distance 75-125 μm from the soma
We conducted a segmental analysis of branch packing to qualitatively determine if dendritic changes were localized to certain parts of the arbor. Using the soma as the center, dendritic trees of individual neurons were sub-divided into successive concentric circles (25 μm successive increase in radius; Figure 5 B). Group means of dendritic length in each segment were divided by number of branch points of cells in a given group at that segment to generate a coefficient of packing for that group (Figure 5 A, no SEM plotted, none calculated since this is a ratio of a group mean to total group branch points at a given segment). Mal-adapted animals and handled controls exhibited sparser packing (more dendritic length per branch point) compared to well-adapted animals. These differences were evident between 75 μm to 125 μm distance from the cell soma. So well-adapted animals exhibited greater branch packing at a radial distance of 75 μm to 125 μm from the soma.
Figure 5.
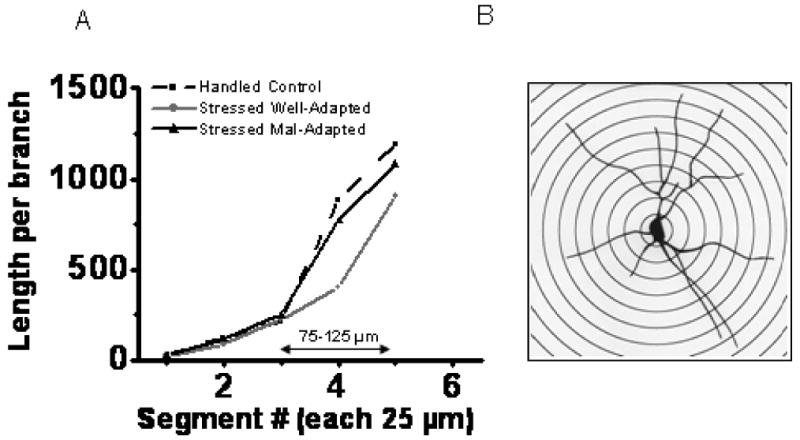
Segmental analysis showing packing density (length per branch). A. Well-adapted animals show highest packing density compared to other two groups at a radial distance 75-125 mm from cell soma. B. Sholl's analysis (with a typical tracing of a golgi-stained neuron) used for determination of segmental branch-packing in all groups; each concentric circle with 25 mm radial distance away from inner one.
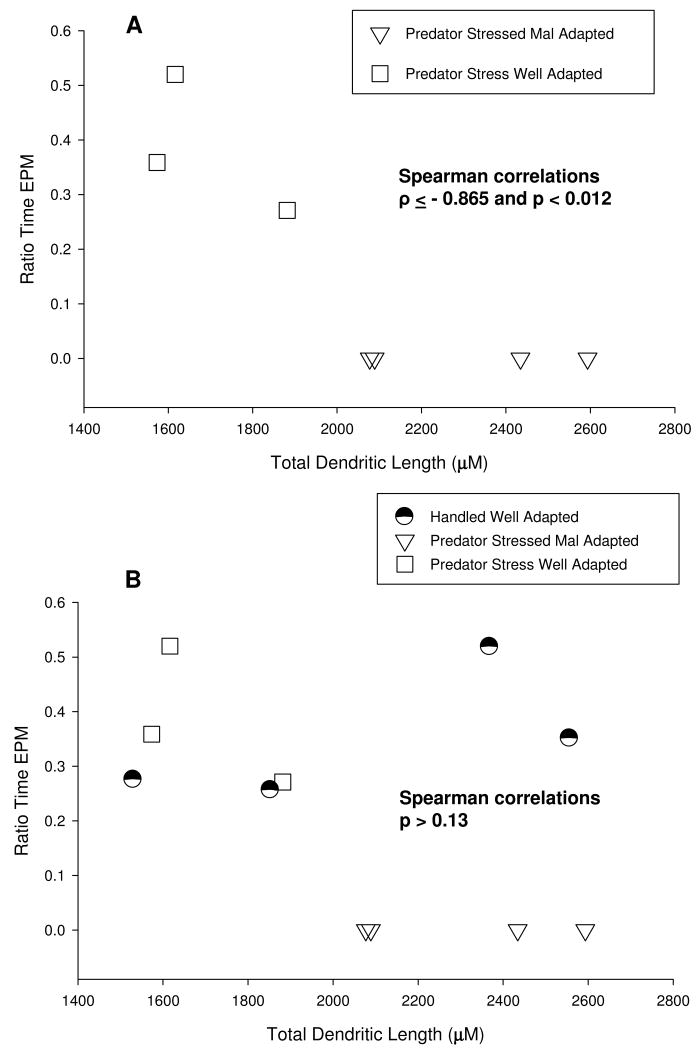
Total dendritic length correlates selectively with EPM anxiety
Total dendritic length, dendritic extent, total branch points and branch packing (branch points per 100. μm) were correlated with behavior in the EPM. Data consisted of dendritic parameter values averaged over cells for each rat in the different groups, as well as EPM measures that differed between groups: ratio time and entry and closed arm entries for each rat. Spearman non-parametric correlations were used since some variables were not normally distributed (Kolmogorov-Smirnov test > .304, p<.05). Correlations were calculated on stressed mal- and well-adapted rats as well as all three groups together (including handled controls).
Correlating BLA dendritic morphology and behavior in stressed rats revealed significant negative correlations between total dendritic length and ratio time and ratio entry only (all Spearman correlations or Spearman's rho - ρ ≤ - 0.865 all p < .012; see scatter plot example for ratio time vs length; Figure 6 A). The negative correlations indicate that the shorter the length and extent of dendrites, the larger the ratio time and entry, and the less the anxiety. In addition, dendritic extent correlated negatively with ratio time and entry (all ρ ≤ -0.866 all p < .012). However this correlation was apparently mediated by total dendritic length as the correlation was not significant when the influence of total dendritic length was removed by partial correlation (p >.20). Total branch points and branch packing did not correlate with ratio time or ratio entry (all p > .05). In contrast, branch packing was positively related to closed arm entries (ρ = 0.873 p < .011), while closed arm entries did not correlate with total dendritic length, dendritic extent or total branch points (all p > .05).
Figure 6.
Scatter plots illustrating the variation of EPM ratio time with total dendritic length - A. scatter plot of predator stressed rats (well- and mal- adapted) only; B. scatter plot including all groups (handled and well- and mal- adapted).
When handled controls were included, dendritic morphology and behavior correlations broke down (all p > .13; see Figure 6 B), with one exception. Branch packing remained positively correlated with closed arm entries (ρ = 0.626, p < .040). Therefore total dendritic length is selectively predictive of EPM anxiety in stressed rats only. Moreover, a different aspect of dendritic morphology (branch packing) is selectively predictive of activity (closed arm entries) in stressed and unstressed rats.
Of importance to the interpretation is the apparent overlap of average total dendritic length of pairs of handled controls with that of well- and mal-adapted stressed rats (Figure 6 B). Handled rats segregated into pairs with cells of comparable total dendritic length (all p >.20), and the cells of the segregated pairs differed from each other in total dendritic length (t(28)=3.26, p<.003). In addition similar comparisons of averages of the other dendritic parameters for each rat within each group showed no differences between rats within a given group. Thus, the segregation is particular to total dendritic length. Moreover the analysis also suggests that within groups, there were no outlier rats dominating a group's average, an important point, given the small group sizes.
Discussion
Exposure to a cat induces long-lasting increases in anxiety in rats (7). Yet, not all animals show increased anxiety. A subset that we referred to here as well-adapted remains unaffected by the cat exposure. Thus, the same stress experience evokes different degrees of behavioral response. Here we report that animals showing these disparate behavioral outcomes differ in dendritic architecture of basolateral amygdala (BLA) neurons, which form part of the neural circuitry mediating stress-induced anxiety (11;12;39;49). Well-adapted animals exhibit retracted dendrites and increased packing density of dendrites compared to mal-adapted animals with high anxiety, and surprisingly, compared to unstressed handled controls. These findings point to a putative neurobiological substrate for resilience to the anxiogenic effects of severe (predator) stress. Interestingly, these resilient individuals do not differ from their less resilient counterparts in their interaction with the cat. Rather their differences are limited to the fact that these individuals do not generalize fear experienced during the trauma. Animal models have been successfully used to study key aspects of stress effects on anxiety and fear. We believe that this naturally occurring variation gives us an important animal model to study resilience.
There is significant interest in understanding resilience, that is, the process of adapting well in face of adversity or trauma (16;21;26;35;41). Understanding mechanisms behind such resilience is important from the perspective of designing effective therapies. Our findings implicate variation in dendritic arbor of amygdala neurons as a candidate mechanism for variation in stress resilience. Correlation analysis suggests that dendritic length is particularly relevant to anxiety levels. Moreover, a reduced dendritic arbor as a mediator of resilience to stress makes functional sense. As reviewed in the introduction, the BLA is important in mediating anxiogenic effects of stress. The BLA can undergo structural reorganization in response to stressors as diverse as immobilization, maternal stress and external application of the stress hormone, corticosterone (38;39;49;51). A prominent feature of such structural reorganization is dendritic expansion (hypertrophy) of excitatory neurons of the BLA. Once evoked, BLA hypertrophy is as lasting as long-lasting anxiety (50). Conversely, dendritic retraction, achieved by viral-mediated over expression of inhibitory SK2 potassium channels in BLA, results in reduction of anxiety (37).
Based on a positive relationship between dendritic structure and anxiety, three hypothesis can be proposed to explain individual differences. First, mal-adapted animals showing greater anxiety undergo dendritic expansion, while well-adapted animals are resistant to such dendritic expansion. Second, well-adapted animals undergo dendritic retraction, thus countering anxiogenic effects of predator exposure. Third, preexisting differences in dendritic morphology in BLA cells predispose towards differences in the neuroplastic excitatory effect of stress on BLA response to afferent input, and towards differences in anxiety. Data presented here support the second and third possibilities. BLA dendritic trees of well-adapted animals either undergo dendritic retraction relative to mal-adapted animals and handled controls in response to stress, or well-adapted animals have a preexisting less extensive dendritic tree which works against the enhancement of anxiety by stress.
Variation in dendritic arbors can directly influence electrical properties of the neuron. Effects of a reduced arbor range from altered passive electrotonic properties to shorter surface area for receiving synaptic inputs. With regard to the latter possibility, reduced BLA response to excitatory input from the ventral hippocampus is associated with less EPM anxiety in predator stressed rats (11;12). A working hypothesis requiring further testing is that reduced dendritic arbors in well-adapted rats reduces excitatory transmission in BLA circuitry and thereby counters the normal predator stress-induced potentiation in BLA afferent transmission. Interestingly, reduced dendritic length in hippocampal CA1 neurons is associated with impaired LTP (15). Moreover, the concentration of dendritic branches near the soma might have inhibitory influences, which would be in agreement with a recent report (40).
In this study only a small number of animals (4 out of 71) showed resilience in the face of predator stress. From our present data, it is difficult to determine if morphometric differences between well- and mal-adapted animals were stress-induced, or were pre-existing differences. The segregation of handled controls into greater and lesser dendritic arbor lengths is suggestive of the latter, however. It is not possible to achieve paired measurements before and after stress, because of the post-mortem nature of Golgi staining. Nor is it yet possible to reliably predict mal- or well-adapted responses to predator stress, so a test of whether arbor retraction is induced by stress or is preexisting is difficult at this time. The fact that the correlations between dendritic length and behavior hold only for stressed rats, together with the finding that well-adapted stressed rats show smaller dendritic arbors than do handled controls with similar EPM anxiety, are consistent with a stress-induced retraction of dendritic arbor in well-adapted stressed rats.
Perhaps more compelling is the following: the pattern of findings point to total dendritic length as a critical neurostructural parameter underlying vulnerability to anxiogenic response to predator stress. It is likely that handled controls contain a mixture of dendritic length profiles akin to stress mal-adapted and stress well-adapted animals; thereby suggesting that dendritic length profiles are individual phenotypic differences. It is possible that smaller dendritic length prior to stress presents less of a substrate for the neuroplastic (LTP-like) changes in afferent inputs to the BLA shown to be induced by predator stress and to predict the degree of EPM anxiety (11). The opposite would apply to greater dendritic length profiles prior to stress. Animals with smaller total dendritic length profiles would be expected to show well-adapted responses to stress, whereas animals with greater total dendritic length would show mal-adapted stress responses. This logic is also consistent with the data showing anxiety-total dendritic length correlations only in stressed rats, since stress must act on the preexisting dendritic substrate to change neural response and behavior.
Finally, the present data also suggest that different aspects of dendritic morphology may contribute to different behaviors. A variety of studies, including the present study, point to differences in neural substrates mediating stress effects on EPM anxiety (open arm avoidance, ratio time/entry) and risk assessment and activity (closed arm entries) in EPM (1;6). Intriguingly, the present data suggest no relationship between BLA output, dendritic morphology and risk assessment, but a relationship between branch packing of BLA dendrites and EPM activity, while variation in dendritic length of the same cells may contribute to stress effects on EPM anxiety.
In conclusion, we show that well-adapted and mal-adapted animals systematically differ in terms of BLA dendritic arbors. Moreover, dendritic differences relate to disparate changes in anxiety exhibited by these animals. Thus different patterns of plasticity in BLA-neurons in response to stress could form the basis of widely reported individual differences observed in coping response to stress and trauma.
Acknowledgments
This work was supported by the CIHR (grant ROP 91548) to Dr. R. Adamec, and NIH RO1 (AGO20633) to Prof R M Sapolsky. Sincere gratitude is extended to Kim Pearcey and Chris Muir for their technical assistance.
Footnotes
Financial Disclosures
Dr. Mitra has no biomedical financial interests or potential conflicts of interest.
Dr. Adamec has no biomedical financial interests or potential conflicts of interest.
Dr. Sapolsky has no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rupshi Mitra, Stanford University, Stanford, California, USA.
Robert Adamec, Memorial University, St. John's, Newfoundland, Canada.
Robert Sapolsky, Stanford University, Stanford, California, USA.
Reference List
- 1.Adamec R. Does Long Term Potentiation in Periacqueductal Gray (PAG) Mediate Lasting Changes in Rodent ALB Produced by Predator Stress? -Effects of Low Frequency Stimulation (LFS) of PAG on Place Preference and Changes in ALB Produced by Predator Stress. Behavioural Brain Research. 2001;120:111–135. doi: 10.1016/s0166-4328(00)00366-1. [DOI] [PubMed] [Google Scholar]
- 2.Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiology & Behavior. 2005;86(1-2):75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Adamec R, Blundell J, Strasser K, Burton P. Mechanisms of lasting change in anxiety induced by severe stress. In: Sato N, Pitman R, editors. PTSD: Brain Mechanisms and Clinical Implications. Tokyo: Springer-Verlag; 2006. pp. 61–81. [DOI] [PubMed] [Google Scholar]
- 4.Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: Sex, serotonin and other factors-Relevance to PTSD. Neuroscience & Biobehavioral Reviews. 2008;32:1287–1292. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals -- implications for understanding and treating affective disorder following traumatic stress in humans. Neuroscience & Biobehavioral Reviews. 1998;23(2):301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 6.Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle - Effect on behavior depends on the hemisphere. Physiology & Behavior. 1999;65(4-5):739–751. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- 7.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiology & Behavior. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 8.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioural Brain Research. 2006;170(1):126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiology & Behavior. 2006;88(1-2):12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Adamec R, Walling S, Burton P. Long-lasting, selective, anxiogenic effects of feline predator stress in mice. Physiology & Behavior. 2004;80(3):401–410. doi: 10.1016/j.physbeh.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neuroscience & Biobehavioral Reviews. 2005;29(8):1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Adamec RE, Blundell J, Collins A. Neural plasticity and stress induced changes in defense in the rat. Neuroscience & Biobehavioral Reviews. 2001;25(7-8):721–744. doi: 10.1016/s0149-7634(01)00053-7. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarty S, Saiepour MH, Bence M, et al. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proceedings of the National Academy of Science, USA. 2006;103(4):1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champagne DL, Bagot RC, vanHasselt F, et al. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charney DS. Psychobiological and vulnerability : Implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 17.Cohen H, Friedberg S, Michael M, Kotler M, Zeev K. Interaction of CCK-4 induced anxiety and post-cat exposure anxiety in rats. Depress Anxiety. 1996;4(3):144–145. doi: 10.1002/(SICI)1520-6394(1996)4:3<144::AID-DA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Cohen H, Geva B, Matar MA, Zohar J, Kaplan Z. Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? International Journal of Neuropsychopharmacology. 2007:1–12. doi: 10.1017/S1461145707007912. Published On Line. [DOI] [PubMed] [Google Scholar]
- 19.Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biological Psychiatry. 2003;53(6):463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- 20.Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: The use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29(11):1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 21.DeRijk RH, DeKloet ER. Corticosteroid receptor polymorphisms: Determinants of vulnerability and resilience. European Journal of Pharmacology. 2008;583(2-3):303–311. doi: 10.1016/j.ejphar.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 22.Dolen G, Osterweil E, Rao BS, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.File SE. The contribution of behavioural studies to the neuropharmacology of anxiety. Neuropharmacology. 1987;26:877–886. doi: 10.1016/0028-3908(87)90065-7. [DOI] [PubMed] [Google Scholar]
- 24.File SE, Wardill AG. The reliability of the hole-board apparatus. Psychopharmacologia. 1975;44(1):47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- 25.File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44(1):53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- 26.Freeman T, Kimbrell T, Booe L, et al. Evidence of resilience: Neuroimaging in former prisoners of war. Psychiatric Research: Neuroimaging. 2006;146(1):59–64. doi: 10.1016/j.pscychresns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 28.Heinrichs M, Wagner D, Schoch W, Soravia LM, Hellhammer DH, Ehlert U. Predicting posttraumatic stress symptoms from pretraumatic risk factors: A 2-year prospective follow-up study in firefighters. American Journal of Psychiatry. 2005;162(12):2276–2286. doi: 10.1176/appi.ajp.162.12.2276. [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 32.Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. International Journal of Neuropsychopharmacology. 2007;10(6):741–758. doi: 10.1017/S1461145707007560. [DOI] [PubMed] [Google Scholar]
- 33.Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H. The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. European Neuropsychopharmacology. 2008;18(2):107–116. doi: 10.1016/j.euroneuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depression and Anxiety. 2005;21(3):135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 36.McNally RJ. Psychological mechanisms in acute response to trauma. Biological Psychiatry. 2003;53(9):779–788. doi: 10.1016/s0006-3223(02)01663-3. [DOI] [PubMed] [Google Scholar]
- 37.Mitra R, Ferguson D, Sapolsky R. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Molecular Psychiatry. 2009 Oct 2;:1–9. doi: 10.1038/mp.2009.9. 2-10-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Science USA. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Science USA. 2008;105(14):5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comparative Neurology. 2006;494(4):635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. Journal of Psychiatric Research. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Quach TT, Massicotte G, Berlin MF, Honnorat J, Glasper ER, DeVries AC. CRMP3 is required for hippocampal CA1 dendritic organization and plasticity. FASEB J. 2008;22(2):401–409. doi: 10.1096/fj.07-9012com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research - Past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Sherren N, Pappas BA. Selective acetylcholine and dopamine lesions in neonatal rats produce distinct patterns of cortical dendritic atrophy in adulthood. Neuroscience. 2005;132(2):445–456. doi: 10.1016/j.neuroscience.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 45.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 46.Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. European Journal of Neuroscience. 2005;22(9):2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- 47.Stam R. PTSD and stress sensitisation: A tale of brain and body Part 1: Human studies. Neuroscience & Biobehavioral Reviews. 2007;31(4):530–557. doi: 10.1016/j.neubiorev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Stam R. PTSD and stress sensitisation: A tale of brain and body Part 2: Animal models. Neuroscience & Biobehavioral Reviews. 2007;31(4):558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience & Biobehavioral Reviews. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62(8):566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of Neurobiology. 2006;66(6):578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]