Abstract
Objective
To find the effect of apolactoferrin administration on the middle and inner ears after experimentally induced pneumococcal otitis media.
Design
Histopathologic and morphometric analysis of the middle and inner ears.
Setting
University of Minnesota, Minneapolis.
Subjects
Ten chinchillas.
Interventions
The middle ear cavities of chinchillas were inoculated bilaterally with type 2 wild-type Streptococcus pneumoniae. Twenty-four hours later, the ears of 5 of the animals were injected with phosphate-buffered saline (PBS) and the other 5 with human apolactoferrin. The animals were killed 24 hours after the last injection. Bacterial counts were made of the middle ear effusions, and the cochleae were processed for histologic analysis. The thickness of the round window membranes and bacterial and inflammatory cell infiltration of the round window membranes, and scala tympani and damage of the hair cells and stria vascularis were compared for these 2 groups of animals.
Main Outcome Measures
Comparison of inflammatory and bacterial cells in the middle and inner ears, and damage to inner ear structures.
Results
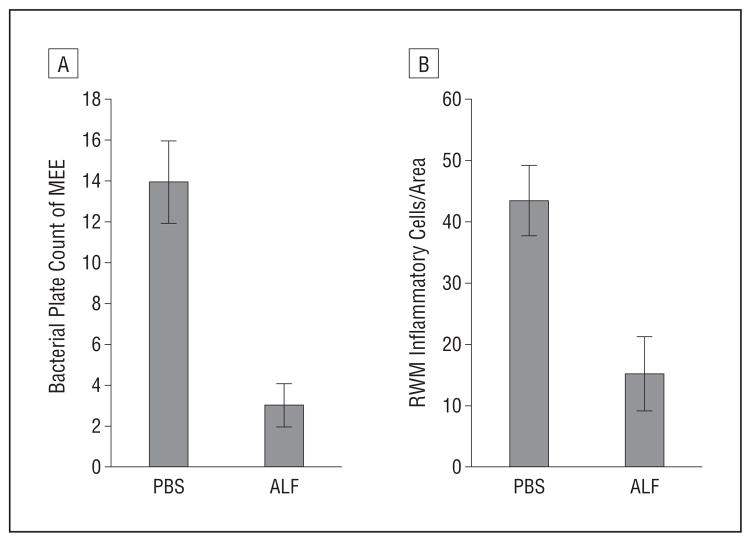
Bacterial plate counts of middle ear effusions (P = .005) and the number of inflammatory cells in the round window membrane (P = .047) were significantly lower in the apolactoferrin group compared with the group treated with PBS.
Conclusion
Further investigation of apolactoferrin as a nonantibiotic approach for the treatment of otitis media and its complications is needed to confirm its safety and efficacy.
Otitis media is one of the most common pediatric infectious diseases. Approximately 80% of children have had at least 1 episode of acute otitis media by 3 years of age.1 Acute otitis media is the most common indication for the prescription of antibiotics, resulting in an increase in antibiotic-resistant bacteria.2,3 The growing problem of bacterial resistance to antibiotics has prompted a search for new approaches, including host defense molecules as potential candidates for the treatment of otitis media.
Molecules of the innate immune system in the eustachian tube and middle ear include the β defensins, lysozyme, lactoferrin, and surfactant proteins.4 Previously, we studied mutant strains of Streptococcus pneumoniae that were deficient in specific proteins in an effort to find those with potential for vaccine development. We found that mutations deficient in pneumococcal surface protein A (PspA) caused less pathological changes in the middle5 and inner ears6 than the wild-type strain. One of the functions of PspA is its ability to bind apolactoferrin (ALF), the iron-free form of lactoferrin, to the surface of S pneumoniae to prevent the killing of S pneumoniae by ALF. This binding has been shown to be entirely dependent on PspA.7
Secreted antimicrobial peptides and other components of innate immunity, such as lactoferrin, are important components of the mucosal defense system and constitute the first line of defense protecting host mucosal surfaces.8 Resistance to pneumococcal carriage has been shown to be dependent on mucosal rather than systemic immunity.9 As potential therapeutic agents, these innate defenses have antimicrobial activities against major otitis media pathogens (S pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis) in bacterial assays,8 while producing little or no development of bacterial resistance. Lactoferrin-secreting cells have been found in tubotympanum of chinchillas.10,11 Notably higher concentrations of lactoferrin were also found in culture-positive middle ear fluids compared with sterile middle ear fluids from children with chronic otitis media12 and in patients with otitis media with effusion13; however, the effects of middle ear administration of lactoferrin in otitis media have not been determined. We studied the effect of ALF injected directly into the middle ear cavity for the potential therapeutic targeting of S pneumoniae and the prevention of ear damage.
METHODS
Wild-type S pneumoniae D39, serotype 2 strain, was used in this study. The growth of bacteria and measurement of their concentration were performed as previously described.10 Briefly, bacteria were grown in Todd-Hewitt broth (Bacto Todd-Hewitt Broth, BD Diagnostics, Sparks, Maryland) containing 0.5% yeast extract (Bacto Yeast Extract, BD Diagnostics) and plated on sheep blood agar plates. The bacterial strains were stored in 10% glycerin at −80°C. After growing the colonies overnight, they were transferred into broth, incubated at 37°C until early log phase, and centrifuged at 2000 rpm for 15 minutes. Bacteria were suspended in 0.15M of phosphate-buffered saline (PBS), and optical densities were measured at 660 nm on a spectrophotometer. Estimated concentration was determined, based on optical densities, and the solution was diluted to the desired concentration in PBS. Actual bacterial concentrations were confirmed by plating multiple 10-fold dilutions onto blood agar plates, incubating overnight at 37°C in 5% to 10% carbon dioxide, and counting viable cells.
Animals were housed and fed under standard conditions at our institutional animal care facility. Experiments were performed on young chinchillas weighing 250 to 350 g, with normal external auditory canals and tympanic membranes. The care and use of animals were approved by the Institutional Animal Care and Use Committee of the University of Minnesota, Minneapolis. All animals were anesthetized prior to intrabullar inoculations with a combination of ketamine, 100 mg/kg, and acepromazine, 10 mg/kg. A total of 10 chinchillas were given bilateral intrabullar inoculations with 0.5 mL of 500 colony-forming units/mL of wild-type S pneumoniae per ear. Twenty-four hours later, bullae of 5 of these animals were injected bilaterally with 0.5 mL of 1 mg/mL ALF (human ALF, L0520; Sigma, St Louis, Missouri) in 0.1M PBS, and bullae from the other 5 animals were bilaterally injected with 0.5 mL of 0.1M PBS.
Two days after bacterial inoculation, the animals were killed by overdose of sodium pentobarbital, and their bullas were removed. Bacterial counts in the middle ear effusions were estimated using BD BBL Stacker plates (BD Diagnostics). The cochleae were perfused via the apex and oval window with 2% glutaraldehyde in 0.2M phosphate buffer (pH 7.4). Samples were then immersed in fixative for 2 hours, decalcified for 3 days in 10% EDTA, washed in phosphate buffer, and postfixed in 1% osmium tetroxide in phosphate buffer (pH 7.4) for 1 hour. They were washed again in buffer, dehydrated in a graded series of ethanol followed by propylene oxide, and embedded in epoxy resin. Samples were cut at a thickness of 1 μm and stained with toluidine blue for light microscopic assessment.
Measurements of the thickness of the round window membrane were made at the midpoint of the sample and midway between the midpoint and the edge of the sample on each slide, using a 10 × 10-U eyepiece grid calibrated in units of 0.16 μm. Measurements were averaged. The areas of greatest inflammatory cell infiltration in the round window membrane and scala tympani were counted within the 10×10-U grid for each sample. Measurements were not taken near the annulus, as this was not present in every sample. Thickness and inflammatory cell infiltration were assessed at a magnification of ×600. Multiple slides from each ear were averaged and the data presented per ear. To compare the types of inflammatory cells infiltrating the round window membrane, the areas of greatest infiltration were photographed at a magnification of ×600 and the images enlarged for counting. The average number of polymorpho-nuclear leukocytes (PMNs), lymphocytes, and macrophages were compared for the 2 groups. Bacterial infiltration of the scala tympani and neurons and damage of the organs of Corti and stria vascularis were noted.
All results were expressed as mean ± SE. Differences of bacterial plate counts, thickness of the round window membrane, and inflammatory cell counts in the round window membrane and scala tympani between PBS- and ALF-treated animal groups were analyzed with paired samples t test using SPSS software (SPSS Inc, Chicago, Illinois). Differences were considered to be significant if P≤.05.
RESULTS
Two of the animals in the PBS-treated group were found dead on the final day of the experiment, presumably from the infection. One animal from the ALF-treated group died immediately after anesthesia administration, presumably from the anesthesia. At 48 hours after bacterial inoculation (24 hours after PBS or ALF injections) animals from both groups had middle ear effusions. The Table compares the findings of bacterial counts, round window membrane thickness, inflammation of the round window membrane and scala tympani, bacterial presence in the scala tympani and neurofilaments, and damage to the hair cells and stria vascularis. Bacterial plate counts of middle ear effusions and the number of inflammatory cells in the round window membrane were significantly lower in the ALF group compared with the group treated with PBS. Although histopathologic findings were not analyzed statistically, because of the limited number of animals, pathological changes did show a trend to be greater in the PBS-treated group. Figure 1A demonstrates the most severe findings of the round window membrane in the ALF-treated group, showing the round window membrane with only a slightly elevated thickness and a small number of inflammatory cells. Figure 1B demonstrates the most severe findings in the PBS-treated group, showing a large number of bacterial and inflammatory cells in the round window membrane and a substantially increased thickness of the round window membrane. Bacteria can be seen both in the round window membrane and the adjacent scala tympani. Figure 2A shows findings from this PBS-treated animal that are characteristic of the inner ear changes seen in both groups, including bacterial penetration of the scala tympani and neurofilaments, strial edema, and hair cell damage. The number of ears showing this type of pathological change, however, were greater in the PBS-treated group. Figure 2B is a higher magnification of the organ of Corti with bacteria in the area of the neurofilaments. The percentage of inflammatory cell types per total inflammatory cells were similar in each group. In the PBS-treated group, PMNs were 20%, lymphocytes were 9%, and macrophages were 71%; and in the ALF-treated group, PMNs were 20%, lymphocytes were 13%, and macrophages were 67%. Although the percentage of the total of each type of cell was similar in both groups, the average number of cells was higher in the PBS-treated group.
Table 1.
Table Comparison of Disease in the ALF- and PBS-Treated Animal Groups
| Middle/Inner Ear Parameters | Type of Treatment
|
P Value | |
|---|---|---|---|
| PBS | ALF | ||
| Bacterial plate count of MEEa | 14 (3) [n = 7] | 2 (1) [n = 7] | .005 |
| RWM thickness, μma | 29.6 (1.7) [n = 6] | 22.4 (1.9) [n = 6] | .11 |
| RWM inflammatory cells, all types/areaa | 43 (6) [n = 6) | 15 (6) [n = 6] | .047 |
| ST inflammatory cells, all types/area adjacent to RWMa | 62 (38) [n = 6] | 12 (6) [n = 6] | .27 |
| Bacteria in RWMb | 5 of 5 | 1 of 6 | NA |
| ST bacteria adjacent to RWMb | 3 of 5 | 1 of 6 | NA |
| Hair cell damageb | 4 of 6 | 3 of 8 | NA |
| Stria vascularis damageb | 3 of 6 | 2 of 8 | NA |
| Bacteria in area of neuronsb | 4 of 6 | 2 of 8 | NA |
Abbreviations: ALF, apolactoferrin; MEE, middle ear effusion; n, number of ears used in statistical analysis; NA, not applicable; PBS, phosphate-buffered saline; RWM, round window membrane; ST, scala tympani.
Values are given as mean (SE).
Data are given as the number of ears involved. Nonparametric test was not performed because of the small number of animals; however; the percentage of ears with these pathologic changes have a trend to be higher in PBS-treated animals compared with those treated with ALF.
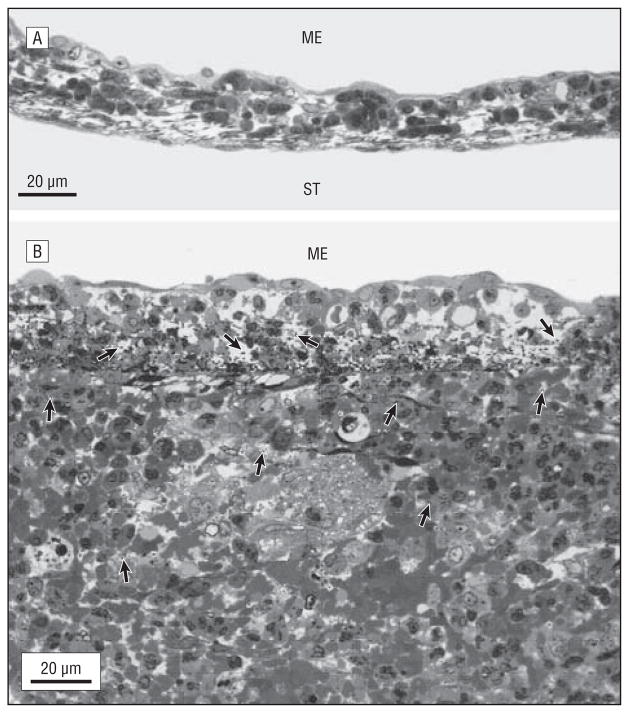
Figure 1.
Inflammation of the round window membrane (RWM) (toluidine blue, original magnification ×600). A, The most severe pathological changes observed in an RWM from the apolactoferrin-treated group, showing a slightly elevated thickness and a small number of inflammatory cells. B, The most severe pathological changes seen in the RWM of the phosphate-buffered saline–treated group, showing a large number of bacteria and inflammatory cells in the RWM and a substantial increasing of its thickness. Bacteria (arrows) are seen within the RWM and adjacent scala tympani (ST). ME indicates middle ear; arrows indicate bacteria.
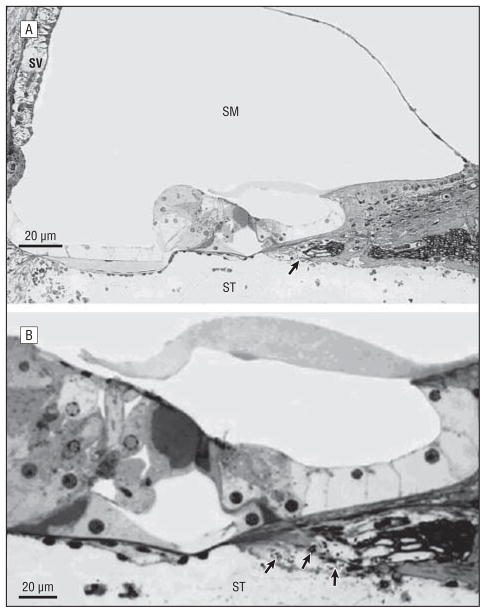
Figure 2.
Inner ear inflammation (toluidine blue; original magnification ×600). A, Inner ear pathological changes were seen in both the apolactoferrin- and phosphate-buffered saline (PBS)-treated groups; however, it was seen more often in the PBS-treated group. There is inflammatory cell infiltration in the scala tympani (ST), severe strial edema, hair cell damage, and bacterial cells (arrow) in this PBS-treated ear. B, Higher magnification shows bacteria (arrows) within the area of the neurofilaments. SM indicates scala media; SV, stria vascularis.
Paired t tests between PBS- and ALF-treated groups showed statistically significant differences for bacterial plate counts and for the number of inflammatory cells in the round window membrane (Table and Figure 3).
Figure 3.
Histogram shows a significant difference between the phosphate-buffered saline (PBS)- and apolactoferrin (ALF)-treated groups of animals. A, Bacterial plate counts of middle ear effusion (P = .005). B, Inflammatory cell infiltration of the round window membrane (RWM) (P = .047). Error bars indicate standard errors.
COMMENT
Antibiotic-resistant bacteria have become an increasing problem. Lynch and Zhanel14 recently reported that 15% to 30% of S pneumoniae strains worldwide are multi-drug resistant. Although the 7-valent polysaccharide-based pneumococcal conjugate vaccine (PCV7) has been successful in decreasing pneumococcal disease due to PCV7 serotypes, pneumococcal disease due to other non-PCV7 serotypes is increasing.15 These concerns suggest the need for the development of other methods of treatment, including the use of molecules of the innate immune system, of antibiotic-resistant ear infection.
The innate immune system of the tubotympanum contains a number of naturally produced antimicrobial agents that kill various microorganisms. One component of this system is the iron-binding glycoprotein, lactoferrin, which is secreted into mucosal fluids.16 Pneumococcal carriage has been shown to be dependent on mucosal rather than systemic immunity,9 and lactoferrin has been shown to play an important role as a first line of host defense against infection and inflammation at the mucosal surface.8 The functions and mechanisms of lactoferrin are broad and include among others that it (1) has antimicrobial properties, (2) is a key component of the innate immune response at the mucosal barrier, (3) has an anti-inflammatory role, (4) has pleiotropic immunodulatory activities affecting many cell types, and (5) has the ability to modulate cellular signaling pathways.16
Lactoferrin protection is less effective against S pneumoniae than against other bacterial types because of the protective effect of one of its virulence factors, PspA, which is common to all serotypes of pneumococcus. PspA was shown to prevent activation of complement by reducing host complement-mediated clearance and phagocytosis.17,18 Binding ALF, the iron-free form of lactoferrin, was demonstrated to protect pneumococci against its bactericidal effects.19,20 The mechanism by which ALF kills pneumococci was suggested to be membrane destabilization.19 In vitro experiments have demonstrated that ALF can kill actively growing pneumococci at a concentration of 1 mg/mL.19 We used a much higher concentration of ALF in our experiments, in an effort to overwhelm the protective binding capacity of the PspA, thus making the pneumococci vulnerable to the ALF.
We found that middle ear administration of ALF (the commercially available iron-free form of lactoferrin), after experimentally induced pneumococcal otitis media, reduced the number of bacteria in the middle and inner ears and damage of the round window membrane and inner ear compared with the PBS-treated controls. The safety of human lactoferrin pepide 1–11 was tested in a sequential, randomized, double-blind, placebo-controlled study using ascending single and multiple intravenous doses in healthy volunteers, and open-label, single intravenous doses in autologous human stem cell transplant recipients. It was shown to be well tolerated in both populations, with few possibly related side effects.21 Further studies, perhaps using topical application of exogenous ALF (or its peptides) alone or in combination with other antimicrobial and/or anti-inflammatory agents via tympanostomy tubes for the treatment of acute otitis media, are warranted.
Acknowledgments
Funding/Support: This work was supported by National Institute on Deafness and Other Communication Disorders grant R01 DC006452, the National Institute of Allergy and Infectious Disease grant RDI A1021458, The International Hearing Foundation, and The Starkey Foundation.
Footnotes
Financial Disclosure: None reported.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Schachern, Tsuprun, Cureoglu, Ferrieri, Briles, Paparella, and Juhn. Acquisition of data: Schachern, Tsuprun, Cureoglu, and Ferrieri. Analysis and interpretation of data: Schachern, Tsuprun, Cureoglu, Ferrieri, Briles, and Juhn. Drafting of the manuscript: Schachern, Tsuprun, Cureoglu, and Briles. Critical revision of the manuscript for important intellectual content: Schachern, Tsuprun, Cureoglu, Ferrieri, Briles, Paparella, and Juhn. Statistical analysis: Tsuprun, Cureoglu, and Briles. Administrative, technical, and material support: Schachern, Tsuprun, Cureoglu, Ferrieri, and Juhn. Study supervision: Ferrieri, Briles, Paparella, and Juhn.
References
- 1.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Bergus GR, Levy SM, Kirchner HL, Warren JJ, Levy BT. A prospective study of antibiotic use and associated infections in young children. Paediatr Perinat Epidemiol. 2001;15(1):61–67. doi: 10.1046/j.1365-3016.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- 3.Leibovitz E, Dagan R. Antibiotic treatment for acute otitis media. Int J Antimicrob Agents. 2000;15(3):169–177. doi: 10.1016/s0924-8579(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 4.Lim DJ, Chun YM, Lee HY, et al. Cell biology of tubotympanum in relation to pathogenesis of otitis media: a review. Vaccine. 2000;19(1 suppl 1):S17–S25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 5.Schachern PA, Tsuprun V, Cureoglu S, et al. The round window membrane in otitis media: effect of pneumococcal proteins. Arch Otolaryngol Head Neck Surg. 2008;134(6):658–662. doi: 10.1001/archotol.134.6.658. [DOI] [PubMed] [Google Scholar]
- 6.Schachern PA, Tsuprun V, Cureoglu S, et al. Virulence of pneumococcal proteins on the inner ear. Arch Otolaryngol Head Neck Surg. 2009;135(7):657–661. doi: 10.1001/archoto.2009.72. [DOI] [PubMed] [Google Scholar]
- 7.Håkansson A, Roche H, Mirza S, McDaniel LS, Brooks-Walter A, Briles DE. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect Immun. 2001;69(5):3372–3381. doi: 10.1128/IAI.69.5.3372-3381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175(4):839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 10.Hanamure Y, Lim DJ. Normal distribution of lysozyme- and lactoferrin-secreting cells in the chinchilla tubotympanum. Am J Otolaryngol. 1986;7(6):410–425. doi: 10.1016/s0196-0709(86)80017-5. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Lim DJ. Development of secretory elements in murine tubotympanum: lysozyme and lactoferrin immunohistochemistry. Ann Otol Rhinol Laryngol. 1993;102(5):385–395. doi: 10.1177/000348949310200512. [DOI] [PubMed] [Google Scholar]
- 12.Giebink GS, Carlson BA, Hetherington SV, Hostetter MK, Le CT, Juhn SK. Bacterial and polymorphonuclear leukocyte contribution to middle ear inflammation in chronic otitis media with effusion. Ann Otol Rhinol Laryngol. 1985;94 (4 pt 1):398–402. [PubMed] [Google Scholar]
- 13.Suzuki T, Somekawa Y, Yamanaka N, Niida Y, Kataura A. Lactoferrin in middle ear effusion. Auris Nasus Larynx. 1985;12(1 suppl 1):S154–S155. doi: 10.1016/s0385-8146(85)80132-2. [DOI] [PubMed] [Google Scholar]
- 14.Lynch JP, III, Zhanel GG. Streptococcus pneumoniae: does antimicrobial resistance matter? Semin Respir Crit Care Med. 2009;30(2):210–238. doi: 10.1055/s-0029-1202939. [DOI] [PubMed] [Google Scholar]
- 15.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 16.Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62(22):2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67(9):4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren B, Szalai AJ, Hollingshead SK, Briles DE. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun. 2004;72(1):114–122. doi: 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaper MS, Hollingshead SK, Benjamin WH, Jr, Briles DE. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [published correction appears in Infect Immun. 2004;72(12):7379] Infect Immun. 2004;72(9):5031–5040. doi: 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedrzejas MJ. Unveiling molecular mechanisms of pneumococcal surface protein A interactions with antibodies and lactoferrin. Clin Chim Acta. 2006;367 (1–2):1–10. doi: 10.1016/j.cca.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Velden WJ, van Iersel TM, Blijlevens NM, Donnelly JP. Safety and tolerability of the antimicrobial peptide human lactoferrin 1–11 (hLF1-11) BMC Med. 2009;7:44–48. doi: 10.1186/1741-7015-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]