Abstract
Background
Detoxification with psychosocial counseling remains a standard opioid-use disorder treatment practice but is associated with poor outcomes. This study tested the efficacy of a newly-developed psychosocial intervention, Community Reinforcement Approach and Family Training for Treatment Retention (CRAFT-T), relative to psychosocial treatment as usual (TAU), for improving treatment outcomes.
Methods
A randomized, 14-week trial with follow-up visits at 6 and 9 months post-randomization conducted at two substance use disorder (SUD) treatment programs. Opioid-dependent adults (i.e., identified patient - IP) enrolled in a residential buprenorphine-detoxification program and their identified concerned significant other (CSO) were randomized to CRAFT-T (n=28 dyads) or TAU (n=24 dyads). CRAFT-T consisted of 2 sessions with the IP and CSO together and 10 with the CSO alone, over 14 weeks. TAU for the CSOs was primarily educational and referral to self-help. All IPs received treatment as usually provided by the SUD program in which they were enrolled. The primary outcome was time to first IP drop from treatment lasting 30 days or more. Opioid and other drug use were key secondary outcomes.
Results
CRAFT-T resulted in a moderate but non-significant effect on treatment retention (p = 0.058, hazard ratio = 0.57). When the CSO was parental family, CRAFT-T had a large and significant effect on treatment retention (p < 0.01, hazard ratio = .040). CRAFT-T had a significant positive effect on IP opioid and other drug use (p<0.0001).
Conclusion
CRAFT-T is a promising treatment for opioid use disorder but replication is needed to confirm these results.
Keywords: addiction, dependence, family, treatment
1. Introduction
In 2009, there were an estimated 2.3 million Americans with an opioid-dependence disorder (Substance Abuse and Mental Health Services Administration, 2010). Over four decades of research indicates that agonist maintenance therapy (AMT) utilizing methadone (a full opioid agonist) and, more recently, buprenorphine (a partial opioid agonist) is the most effective treatment for opioid dependence (Kleber, 2008; Kreek et al., 2010; Mattick et al., 2008). Social, economic and regulatory barriers limit access to AMT and consequently detoxification followed by psychosocial counseling, with accompanying high relapse rates, is the most common approach to opioid dependence treatment (Mattick et al., 2009; Mayet et al., 2005). Availability of buprenorphine has improved the effectiveness of opioid detoxification (Brigham et al., 2007; Ling et al., 2005) however, without AMT, treatment drop-out and relapse rates are high and potentially lethal (Strang et al., 2003). Interventions are needed to increase retention in treatment and prevent relapse.
Community Reinforcement and Family Training (CRAFT), developed by Robert Meyers, works with concerned significant others (CSOs) to motivate treatment-refusing persons with a substance use disorder to volunteer for treatment. CRAFT has demonstrated a robust effect in several randomized clinical trials (Kirby et al., 1999; Meyers et al., 1998, 2003; Miller et al., 1999).
Between April of 2009 and November of 2010 fifteen dyads, each consisting of an opioid dependent adult identified patient (IP) and their respective CSO, were enrolled in stages 1a and 1b of a therapy development study (Rounsaville, 2001) to modify CRAFT. The new manualized treatment, Community Reinforcement and Family Training for Treatment Retention (CRAFT-T), works with the CSOs of IPs already in treatment, to increase the IP’s retention in treatment and recovery support. This report presents the results of a randomized clinical pilot evaluating CRAFT-T.
2. METHODS
2.1 Participants
We enrolled 104 participants into an intent to treat (ITT), 2-group randomized clinical trial at two Ohio locations: Site 1, in a metropolitan county, with 1.2 million residents, and Site 2, in a smaller county, with 178,000 residents. The study was IRB approved and sponsored by the National Institute on Drug Abuse. A detailed study protocol is available (Brigham et al., 2009).
Participants enrolled as dyads consisting of an IP and a CSO. IPs were approached during a detoxification program and, if interested, provided contact information for a CSO. CSOs and IPs were consented and screened separately. IPs were adults who: met DSM-IV-TR criteria for opioid dependence; planned to transfer from detoxification to outpatient; and had a CSO willing to participate. CSOs were relatives, spouses, or intimate partners, or planned to live with the IP following randomization. IPs and CSOs were ineligible if they had: a history of violence with each other; current suicide or homicide intent; a medical or psychiatric condition that would make participation difficult; or were court ordered to complete treatment.
2.2 Procedures
Participants were randomized to CRAFT-T or TAU using urn randomization balanced on site (1 or 2), race (Black or other), and CSO type (parent or other). The study treatment phase was 14 weeks during which there were 2 weekly research assessment visits for IPs and 12 for CSOs. The follow-up phase extended to 38 weeks with research visits for IPs and CSOs at weeks 14, 26, and 38. Randomization began in January of 2011 and follow-up was completed in June of 2012. Participants were compensated for research visits by gift cards ($20 for baseline and screening, $10 for each weekly treatment assessment, and $20 each for the end of treatment assessment and two follow-ups).
2.2.1 Treatments
2.2.1.1 Treatment as usual (TAU) for IPs
All IPs received the usual services offered at the treatment program which began with a 13-day BUP taper detoxification (Brigham et al., 2007). At Site 1 the taper was initiated in a residential sub-acute medical detoxification setting followed by step-down to ambulatory detoxification. At Site 2 the entire taper was completed in an ambulatory setting. At both sites IPs transferred to outpatient treatment following detoxification.
2.2.1.2 Treatment as usual (TAU) for CSOs
The TAU for CSOs was minimal consisting of an invitation to attend a volunteer-facilitated support group and an informal referral to self-help (Al-Anon or Nar-Anon).
2.2.1.3 Community Reinforcement and Family Training for Treatment Retention (CRAFT-T)
This unilateral family intervention worked primarily with the CSO with the goal of influencing the IP's behavior. CRAFT-T used a cognitive behavioral approach to assist the CSO in using behavioral principles to increase the IP’s treatment retention and reduce their drug use. CRAFT-T departed from the CRAFT model in five important ways: it worked with the CSO’s of IP’s who were in treatment; CSOs were identified by the IP; it targeted retention in treatment; the IP participated in two initial sessions; and it targeted reduction of HIV risk behavior.
CRAFT-T consisted of twelve weekly one-hour sessions. The IP and CSO attended the first two sessions together and the remaining ten sessions were attended by the CSO alone. Two optional sessions were also available. The CRAFT-T manual was designed to supplement the book “Motivating Substance Abusers to Enter Treatment” (Smith and Meyers, 2004).
2.2.2 CRAFT-T Therapist, Training, and Fidelity
Four therapists were recruited. Two had master’s degrees with less than one year of post-graduate experience and two were non-degreed licensed drug abuse counselors with over ten years of experience. Therapists attended a two-day training followed by training cases. Prior to the start of the trial eleven participant dyads were enrolled to serve as training cases. All therapist training case sessions were audio-recorded and rated for fidelity. Therapists were certified to see trial participants after ratings of two training cases reached a criterion threshold. During the treatment phase of the study all CRAFT-T sessions were audio-recorded and 25% were rated by the study PI [G.B.]. All therapists maintained acceptable fidelity with an overall compliance rating of 87%.
2.3 Measures
The primary outcome was days to the IP’s first drop of 30 days or more from all treatment as recorded in the clinic’s electronic health record. Secondary outcomes included days of opioid use and any drug use. A Timeline Follow-back (TLFB) procedure (Robinson et al., 2012; Sobell et al., 1988), was used to record the IP’s day-to-day use of alcohol, opioids, cocaine, marijuana, benzodiazepines, methamphetamine, and other illicit drugs. Urine samples were collected at each of the IPs research visits (weeks 1, 2, 14, 26, and 38) and were analyzed for opioids, cocaine, marijuana, benzodiazepines, methamphetamine using the Redi Test rapid screen system from Redwood Toxicology Laboratory. The Structured Clinical Interview for DSM-IV (First et al., 1996) was used with the IP to obtain the opioid-dependence diagnosis.
2.4 Data Analysis
Baseline measures are summarized in Table 1. Each measure was tested for between-treatment-arm differences using the Pearson Chi Square, Fisher Exact, Wilcoxon Rank Sum or Student’s t.
Table 1.
Participant Comparison at Baseline by Treatment Group
| TAU (N=24) |
CRAFT-T (N=28) |
Total (N=52) |
|
|---|---|---|---|
| Site (n, %): | |||
| Site 1 | 16, 66.7% | 20, 71.4% | 36, 69.2% |
| Site 2 | 8, 33.3% | 8, 28.6% | 16, 30.8% |
| IP: | |||
| Age in yrs. (mean, std.dev.) | 28.7, 6.7 | 29.5, 9.2 | 29.2, 8.1 |
| Males (n, %) | 18, 75.0% | 23, 82.1% | 41, 78.8% |
| Race (n,%): | |||
| Black | 1, 4.2% | 1, 3.6% | 2, 3.8% |
| White | 22, 91.7% | 27, 96.4% | 49, 94.2% |
| Other | 1, 4.2% | 0, 0.0% | 1, 1.9% |
| CSO: | |||
| Age in yrs. (mean, std.dev.) | 40.3, 14.8 | 28 44.3, 12.1 | 42.5, 13.4 |
| Males (n, %) | 5, 20.8% | 4, 14.3% | 9, 17.3% |
| Race (n,%): | |||
| Black | 2, 8.3% | 1, 3.6% | 3, 5.8% |
| White | 21, 87.5% | 26, 92.9% | 47, 90.4% |
| Other | 1, 4.2% | 1, 3.6% | 2, 3.8% |
| CSO Relation (n, %): | |||
| Parent/Aunt/Grandparent | 11, 45.8% | 15, 53.6% | 26, 50.0% |
| Spouse/Common Law | 2, 8.3% | 5, 17.9% | 7, 13.5% |
| Girlfriend/Boyfriend/Fiancee | 8, 33.3% | 5, 17.9% | 13, 25.0% |
| Sibling | 2, 8.3% | 1, 3.6% | 3, 5.8% |
| Friend | 1, 4.2% | 2, 7.1% | 3, 5.8% |
| CSO in Parental Family* (n, %) | 13, 54.2% | 16, 57.1% | 29, 55.8% |
| IP Secondary SUD Diagnosis (n, %): | |||
| None | 19, 79.2% | 23, 82.1% | 42, 80.8% |
| Sedative-Hyp-Anx Abuse | 1, 4.2% | 0, 0.0% | 1, 1.9% |
| Cannabis Abuse | 0, 0.0% | 2, 7.1% | 2, 3.8% |
| Cannabis Dependence | 1, 4.2% | 1, 3.6% | 2, 3.8% |
| Stimulant Dependence | 0, 0.0% | 1, 3.6% | 1, 1.9% |
| Cocaine Abuse | 1, 4.2% | 0, 0.0% | 1, 1.9% |
| Cocaine Dependence | 1, 4.2% | 1, 3.6% | 2, 3.8% |
| Poly Drug Dependence | 1, 4.2% | 0, 0.0% | 1, 1.9% |
None of these variables showed significant between-treatment differences.
CSO is parent, aunt, grandparent, or sibling
Each outcome analysis was performed twice: grouping participants by treatment arm, and then by CRAFT-T participants with parental family CSO (parent, aunt, grandparent or sibling) vs. all others. This second grouping resulted from previous indication of CSO relationship as a potential moderator (Meyers et al., 1998; Miller et al., 1999), and from the small, pilot study sample size which precluded all but the simplest regression models.
The primary outcome variable was treated as survival data and tested for group differences using Cox Proportional Hazard regressions. There were no missing data on the primary outcome. Daily TLFB IP opioid and drug use indicators, were assessed using random intercept mixed-model logistic regressions testing for both group effects and group-by-time interaction effects over weeks 1–2 (IP study treatment), over weeks 3–14 (CSO treatment after IP study treatment), and finally over the remaining follow-up weeks. No attempt was made to account for multiple analyses or missing data (on the drug use outcomes).
Urine drug screens (UDS) were too sparse for meaningful between-group comparisons. Instead, the Cohen Kappa was used to compare the UDS opioid indicators to TLFB results compiled over three-day periods ending with respective UDS days.
3. RESULTS
3.1 Participants
A total of 136 potential participants were pre-screened, 108 consented and screened, and 104 (52 dyads) randomized (Supplementary Figure S11). For weeks 14, 28 and 38 respectively, follow-up rates were 52%, 56% and 62% for IP, and 62%, 54%, and 79% for CSO. No reported baseline characteristics indicated significant between-treatment differences. IP participants averaged 29 years old and were 79% male, 94% white, and 73% unemployed (Table 1.). Based on the Risk Assessment Battery (RAB) self-reports, the CSO’s substance use appeared minimal (data not shown).
3.2 Study Treatment Exposure
IPs attended an average of 1.78 (median of 2) of their 2 scheduled CRAFT-T sessions. CSOs attended an average of 7.62 (median of 9.5) of their 12 scheduled study treatment sessions. Three CRAFT-T dyads dropped out before their first session. Of the two optional CSO sessions, 8 CSOs attended at least one session, and 2 attended both.
3.3 Primary Outcome
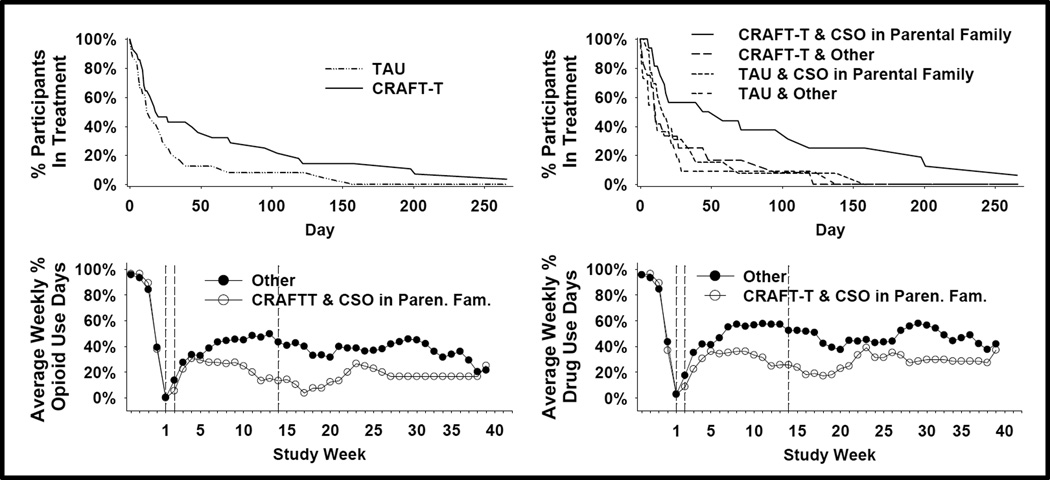
The primary outcome measure had 3 censored participants (2 for early withdrawal and one for outlasting the 38-day assessment period) and no missing data. CRAFT-T participants showed a longer time-to-dropout which approached significance with p = 0.058 and a hazard ratio of 0.57 indicating they were 57% as likely as TAU participants to dropout at any given point in time. CRAFT-T participants with parental-family CSOs showed a longer time-to-dropout with p < 0.01 and hazard ratio = 0.40 (Figure 1).
Figure 1.
(a,b) Survival curves for IP retention in drug abuse treatment are shown. Time point 0 is baseline and 1 – 250 are days in drug abuse treatment following randomization. (c,d) Comparison of treatments on TLFB reports of weekly percentage of opioid use and any drug use days are shown. Weeks 1 – 2 are treatment weeks in which both the CSO and IP attend CRAFTT sessions, 3 –14 are the weeks in which only the CSO attends CRAFT-T sessions, and weeks 15–40 are follow-up.
3.4 Daily Drug Use Outcomes
For both of the participant groupings, week 3–14 regressions and follow-up regressions demonstrated significant time-by-treatment interaction effects for both opioid and drug use TLFB indicators (p < 0.0001). The corresponding graphs in Figure 1 suggest divergence during weeks 3–14 favoring CRAFT-T and CRAFT-T-with-parental-family-CSO respectively, with differences diminishing during follow-up. A Cohen Kappa of 0.54 resulted from testing the TLFB opioid results for agreement with UDS results: disagreement balanced between positive and negative urines.
4. DISCUSSION
The goal was to determine if adding CRAFT-T to opioid detoxification followed by outpatient, would improve treatment retention and drug use outcomes. The primary outcome was days to the IP’s first drop of 30 days or more from treatment. Compared to TAU, CRAFT-T resulted in a moderate-sized effect that approached significance. We also evaluated the effect of type of CSO and found that when dyads with both CRAFT-T and CSOs from parental family were compared to all others, the effect on retention was large (Hazard Ratio = 0.4, Cohen’s d = 0.95) and significant. This is consistent with previous CRAFT research by Meyers (1998) who found parents were significantly more effective than spouses. Retention in treatment is important as it is consistently associated with improved drug use outcomes (Mertens et al., 2012; Simpson et al., 1997).
We also examined effects on drug use and found that assignment to CRAFT-T resulted in significant reductions in both opioid and drug use days reported on the TLFB. While this observed effect on drug use is encouraging, the overall rates of relapse and drug use were high. These outcomes should be interpreted with caution due to the relatively low follow-up rates.
CSOs attended an average of 8 of the 12 planned sessions, which is low compared to previous CRAFT research. In CRAFT research the CSO initiates involvement. In CRAFT-T, IPs invite a specific CSO and the relationships often appeared strained with a sense that the CSO was being engaged with reluctance.
This study had numerous strengths: the ITT randomized trial design, a manual guided treatment, and no missing data on the primary outcome measure. Some limitations resulted from the small sample size: lack of generalizability, lack of power to evaluate therapist effects and to fully evaluate the effects of CSO relationship type, and possibly distorted estimates of effect sizes (Kraemer et al., 2006). CSO’s utilization of CRAFT-T skills was not measured and therefore we cannot conclude that use of these skills caused the observed effects.
In conclusion, these preliminary results suggest that CRAFT-T is a promising intervention for improving treatment retention and drug use outcomes in adults with opioid use disorder.
Supplementary Material
Acknowledgements
Role of Funding Source:
This study was supported by the following grants from the National Institute on Drug Abuse (NIDA): 5K23DA021512-02 to University of Cincinnati (Dr. Brigham), U10-DA013732 to University of Cincinnati (Dr. Winhusen). NIDA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors:
Dr. Brigham lead the design and implementation of the study in collaboration with Drs. Somoza and Slesnick. Mr. Lewis and Dr. Guo conducted the analyses and provided critical review of the manuscript. Dr. Brigham drafted the manuscript and Drs. Winhusen, Slesnick, and Somoza contributed to the interpretation of the findings and critically revised the manuscript for intellectual content. All authors have approved the final manuscript.
Conflict of Interest
The authors have no conflicts to declare.
REFERENCES
- Brigham GS, Amass L, Winhusen T, Harrer JM, Pelt A. Using buprenorphine short-term taper to facilitate early treatment engagement. J. Subst. Abuse. Treat. 2007;32:349–356. doi: 10.1016/j.jsat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Brigham GS, Slesnick N, Somoza E, Horn P, Rich J. Community reinforcement and family training for treatment retention and HIV behavioral risk reduction: a study protocol. J. Behav. Anal. Health Sports Fitn. Med. 2009;2:91–108. [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Garvey KA, La Monaca V. Community reinforcement training for family and significant others of drug abusers: a unilateral intervention to increase treatment entry of drug users. Drug Alcohol Depend. 1999;56:85–96. doi: 10.1016/s0376-8716(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303–2305. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J. Addict. Dis. 2010;29:200–216. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Brigham G, Harrer J, Reid M, Muir J, Buchan B, Orr D, Woody G, Krejci J, Ziedonis D. A multicenter randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst. Rev. 2005:CD004330. doi: 10.1002/14651858.CD004330.pub2. [DOI] [PubMed] [Google Scholar]
- Meyers RJ, Miller WR, Hill DE, Tonigan JS. Community reinforcement and family training (CRAFT): engaging unmotivated drug users in treatment. J. Subst. Abuse. 1998;10:291–308. doi: 10.1016/s0899-3289(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Meyers RJ, Smith JE, Lash DN. The community reinforcement approach. Recent Dev. Alcohol. 2003;16:183–195. [PubMed] [Google Scholar]
- Miller WR, Meyers RJ, Tonigan JS. Engaging the unmotivated in treatment for alcohol problems: a comparison of three strategies for intervention through family members. J. Consult. Clin. Psychol. 1999;67:688–697. doi: 10.1037//0022-006x.67.5.688. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. 2012;104:734–741. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll Kathleen M, Onken Lisa S. A stage model of behavioral therapies research: getting started and moving on from stage 1. Am. Psychol. 2001;8:9. [Google Scholar]
- Smith JE, Meyers RJ. Motivating Substance Abusers to Enter Treatment: Working with Family Members. New York: Guilford Press; 2004. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Strang J, McCambridge J, Best D, Beswick T, Bearn J, Rees S, Gossop M. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326:959–960. doi: 10.1136/bmj.326.7396.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.