Abstract
Cholesterol's interfacial interaction with different sphingomyelins and phosphatidylcholines has been investigated using a Langmuir film balance. The average molecular area of cholesterol/sphingomyelin (SM) or cholesterol/phosphatidylcholine (PC) mixed monolayers was determined as a function of film composition from the force–area isotherms measured at 24 °C. In contrast to previous results, little difference was observed in equimolar cholesterol's “condensing effect” of SMs compared to PCs when their phase state was similar and when their hydrocarbon structural differences were minimized. For PCs, this meant that one acyl chain had to be long and capable of assuming an extended conformation and thus configurationally similar to the long-chain base of SM. This condition facilitated strong van der Waals attractive interactions with cholesterol's planar steroid ring and was satisfied when the sn-1 acyl chain of PC was either myristate or palmitate. Under these conditions, the structural requirements of the sn-2 chain of PC were mitigated. For instance, at equimolar cholesterol, almost no difference was observed in the apparent molecular area condensations of 1-palmitoyl-2-oleoyl-PC and 1-palmitoyl-2-arachidonoyl-PC at surface pressures between 10 and 40 mN/m. In contrast, the apparent molecular area condensations of dioleoyl-PC and diarachidonoyl-PC were substantially reduced under identical experimental conditions. The results are discussed in terms of the relative importance of phospholipid/sphingolipid hydrocarbon and headgroup structure in determining the extent of interaction with cholesterol. Conclusions were based on investigation of egg SM, bovine brain SM, N-oleoyl-SM, dipalmitoyl-PC, dimyristoyl-PC, 1-myristoyl-2-palmitoyl-PC, 1-palmitoyl-2-oleoyl-PC, 1-palmitoyl-2-arachidonoyl-PC, dioleoyl-PC, dinervonoyl-PC, diarachidonoyl-PC, and diphytanoyl-PC as well as comparison with cholesterol's condensation of various galactosylceramide molecular species.
Cholesterol, glycerol-based phospholipids, and sphingomyelin make up the bulk of lipid components in mammalian plasma membranes, and as a result, a great deal of effort has been devoted to understanding their interactions in model membrane assemblies [e.g., see Finegold (1993)]. Renewed interest in cholesterol–lipid interactions has been fueled by recent findings in the viral fusion and lipid signal transduction fields. For instance, human immunodeficiency virus envelopes reportedly contain high cholesterol-to-phospholipid ratios and are selectively enriched in sphingomyelin relative to host cell membranes [e.g., Aloia et al. (1993)]. Also, the low-pH-induced fusion activity of Semliki Forest virus requires cholesterol in the target membrane for viral binding, whereas the fusion event is dependent on simple sphingolipids such as sphingomyelin, ceramide, or galactosylceramide being present in the target membrane (Nieva et al., 1994). With respect to lipid signal transduction events, certain phospholipids, sphingolipids, and their metabolic derivatives are known to act as second messengers. Sphingomyelin, in particular, has been the focus of much recent study [for reviews, see Koval and Pagano (1991), Kolesnick (1991), and Hannun and Linardic (1993)]. Because biological evidence suggests coordinate regulation of sphingomyelin and cholesterol content in cellular membranes and certain biophysical evidence indicates that cholesterol has a greater “affinity” for sphingomyelin compared to phosphatidylcholine, the possibility exists that cholesterol–lipid interactions may be involved in regulating viral fusion and/or lipid signal transduction events. Hence, understanding cholesterol's interactions with simple sphingolipids is important from the biological as well as physical perspective.
Recently, we characterized the interfacial behavior of several molecular species of another simple sphingolipid, galactosylceramide (GalCer)1 (Ali et al., 1993, 1994b) and investigated cholesterol's ability to condense monolayers of these GalCer species (Ali et al., 1994a). Our results showed that acyl structure plays an important role in determining whether condensation occurs and that a large condensing effect is observed only with liquid-expanded molecular species of GalCer. In addition, we determined the relative magnitude of cholesterol's condensing effect on GalCer compared to that on sphingomyelin. Our condensation values at equimolar concentrations of cholesterol and either egg or bovine brain sphingomyelin were very similar to those of Lund-Katz et al. (1988). The results clearly showed the area condensation in cholesterol/sphingomyelin mixed films to be significantly greater than that in cholesterol/GalCer mixed films and pointed to headgroup structure being a major regulator of cholesterol interaction when differences in acyl structure and monolayer phase state were minimized (Ali et al., 1994a). In fact, in another recent study of cholesterol's interactions with mono- and dihexosylceramides, Slotte et al. (1993) concluded that the polar headgroup of these simple glycosphingolipids is the most important determinant of how the molecules associate with cholesterol in mixed monolayers. This conclusion is difficult to reconcile in light of an earlier study by Lund-Katz et al. (1988) in which cholesterol's interfacial condensation of N-palmitoylsphingomyelin was reported to be significantly greater than that of dipalmitoylphosphatidylcholine at liquid-expanded surface pressures despite the fact that both of these lipids contain identical phosphocholine headgroups. Indeed, if headgroup structure is “the most important determinant” of cholesterol interaction with sphingolipids and phospholipids, then small, if any, differences in area condensation would be expected for cholesterol/sphingomyelin and cholesterol/phosphatidylcholine mixed films. To resolve the issue, we reexamined cholesterol's interfacial interaction with phosphatidylcholine and sphingomyelin in monomolecular films and analyzed the data by the classic means of average molecular area versus composition. The results indicate that cholesterol induces very similar apparent molecular area reductions in sphingomyelin and phosphatidylcholine when these two lipids are in similar phase states and when the phosphatidylcholine contains one long, saturated acyl chain. The results provide new insight into the essential structural elements required for optimal interaction in cholesterol/phosphatidylcholine or cholesterol/sphingomyelin model membrane systems.
MATERIALS AND METHODS
Lipid, Buffer, and Solvent Preparation
Cholesterol was obtained from Nu-Chek Prep (Elysian, MN). All phospholipids including the sphingomyelins were obtained from Avanti Polar Lipids (Alabaster, AL). Purity was greater than 99% based on thin-layer chromatographic (TLC) analysis using petroleum ether/diethyl ether/acetic acid (8.5:1.5:0.1) for cholesterol analysis and CHCl3/CH3OH/H2O (65:35:4) for phospholipid analysis. Acyl compositions of egg SM were 78% palmitate (16:0), 7% stearate (18:0), 2% arachidate (20:0), 4% behenate (22:0), 2% lignocerate (24:0), and 3% nervonate (24:1Δ15) and of bovine brain SM were 58% stearate (18:0), 2% palmitate (16:0), 6% arachidate (20:0), 9% behenate (22:0), 7% lignocerate (24:0), and 15% nervonate (24:1Δ15). Stock lipid solutions for film balance studies were prepared by dissolving in petroleum ether/ethanol(95:5) that had been purified as described by Smaby and Brockman (1985). When not in use, these stocks were stored under argon at –70 °C. Quantitation of cholesterol was determined by dry weight. Phospholipid and sphingomyelin concentrations were assayed on the basis of phosphate content (Bartlett, 1959). Water for the subphase buffer was purified by reverse osmosis, mixed-bed deionization, adsorption to charcoal, and filtration through a 0.2-μm polycarbonate membrane. Subphase buffer consisted of 10 mM potassium phosphate (pH 6.6) containing 100 mM NaCl and 0.02% sodium azide (analytical grade reagents). After preparation, the buffer was filtered through a Diaflo hollow fiber sieve with a molecular weight cutoff of 10 000 (Amicon Corp., Danvers, MA) and stored under argon. Solvent purity was assessed by a π–A method (Ali et al., 1991) and by monitoring the surface potential versus molecular area (ΔV–A) behavior (Smaby & Brockman, 1991).
Monolayer Studies
A computer-controlled, Langmuir-type film balance, calibrated according to the equilibrium spreading pressures of known lipid standards (Smaby & Brockman, 1990) and housed in a laboratory equipped with a filtered air supply, was used to measure π–A isotherms (Brockman et al., 1980). Glassware was acid cleaned and was rinsed thoroughly with deionized water and then with hexane/ethanol (95:5) prior to use. Lipids were spread in 51.7-μL solvent aliquots onto a trough filled with ~800 mL of phosphate–saline buffer (pH 6.6). Film compression was initialized after a 4-min delay period and proceeded at a rate of ≤4 Å2/(molecule·min). Subphase temperature was maintained at 24 ± 1 °C by a temperature-controlled water bath. Surface potential was measured using a 210Po ionizing electrode.
Analysis of Isotherms
Mixing behavior in two-component lipid monolayers was analyzed by mean molecular area–composition diagrams (Goodrich, 1957; Ali et al., 1994a). The mean molecular area (Aπ) of two-component mixed monolayers at a given surface pressure (π) was calculated using the equation:
where X1 is the mole fraction of component 1 and (A1)π and (A2)π are the mean molecular areas of pure components 1 and 2 at identical surface pressures. Deviations between the experimentally observed areas of the mixtures and the areas calculated by adding the molecular areas of the pure components (apportioned by mole fraction in the mix) reflected the nature of lipid mixing. Negative deviations from additivity indicated condensation and implied intermolecular accommodation and/or dehydration interactions between lipids in the mixed films [e.g., Cadenhead and Müller-Landau (1980)].
The cholesterol-induced condensation or interfacial area reduction of the phospholipid (PL) molecules was calculated at constant surface pressure as follows:
where PL condensation units are Å2/PL molecule, APL is the area/molecule of the pure phospholipid, Amix is the average area/molecule of the PL/Chol mixed monolayer, Achol is the area/molecule of the pure cholesterol, Xchol is the mole fraction of cholesterol in the mixed monolayer, and XPL is the mole fraction of phospholipid in the mixed film. Calculating the area reduction in terms of PL condensation is valid because the surface area of cholesterol changes very little with increasing surface pressure (Pethica, 1955; Phillips, 1972). We determined the cross-sectional areas of pure cholesterol to be 37.6,37.2, and 36.8 Å2 at surface pressures of 5, 15, and 30 mN/m (Ali et al., 1994a).
RESULTS
To minimize differences in headgroup structure and to compare the contributions that the ceramide and diacylglycerol regions play in modulating interactions with cholesterol, we investigated cholesterol's interfacial interactions with selected sphingomyelins (SMs) and phosphatidylcholinw (PCs). Using X-ray diffraction techniques, Pascher (1976) showed that the first three carbons in the 18-carbon sphingosine base of simple sphingolipids are configurationally equivalent to the glycerol backbone of PC. The entire sphingosine base chain of SM and the combined glycerol backbone and sn-1 acyl chain of PC are oriented similarly with respect to the lamellar interface. Thus, the structural configuration in which SM and PC should be most similar is one in which the sn-1 acyl chain of PC is saturated and roughly 15–16 carbons long. The sole acyl chain of SM and the sn-2 acyl chain of PC also are configurationally similar in being oriented parallel to the long-chain base of SM and to the sn-1 acyl chain of PC, respectively [see Barenholz and Thompson (1980) and Kolesnick (1991)]. This parallel orientation of the sphingoid base and acyl chain in simple sphingolipids and of the sn-1 and sn-2 acyl chains in PCs occurs because the fatty acyl chain (sn-2 in the case of PC) bends sharply at carbon 2 such that only the portion beyond this point is parallel to the molecular long axis [see Hamilton et al. (1993) and references within]. Hence, N-oleoylsphingosylphosphocholine (18:1Δ9-SM) and 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) should be configurationally and structurally quite similar, aside from the specific chemical groups characteristic of ceramide and diacylglycerol. Any differences measured in cholesterol's interactions with these molecules would be the result of chemical differences rather than any large configurational differences.
We first compared the interfacial behavior of cholesterol/18:1Δ9-SM mixed monolayers with that of cholesterol/POPC mixed monolayers. Figure 1A shows the π–A isotherms of 18:1Δ9-SM in the absence and presence of various mole fractions of cholesterol, and Figure 1B shows similar data for POPC. In the absence of cholesterol, both of these structurally similar lipids displayed classic liquid-expanded behavior. However, the lateral packing density of 18:1Δ9-SM was significantly higher than that of POPC at equivalent surface pressures possibly due to the ability of SM to form interfacial hydrogen bonds [e.g., Barenholz and Thompson (1980), Boggs (1987), and Kolesnick (1991)]. A higher lateral packing density for SM was also noted by Lund-Katz et al. (1988), who compared the π–A isotherms of 18:1Δ9-SM and egg PC.
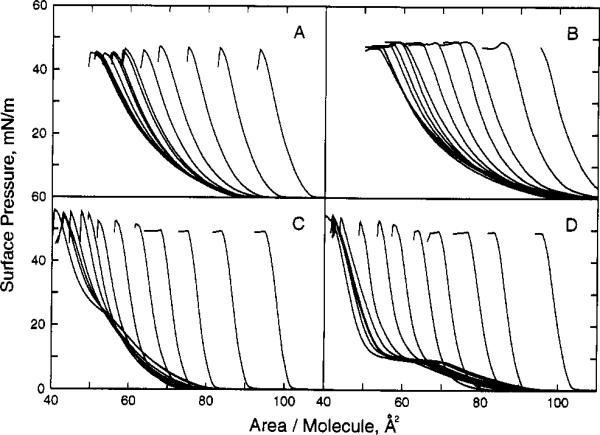
Figure 1.
Surface pressure vs apparent molecular area of sphingolipid/phospholipid. Isotherms were recorded at 24 °C on a subphase of 10 mM potassium phosphate (pH 6.6) containing 100 mM NaCl and 0.02% NaN3. Panel A shows N-oleoyl-SM with increasing mole fractions of cholesterol (from left to right at π = 30 mN/m; Xchol = 0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6). Panel B shows 1-palmitoyl-2-oleoyl-PC with increasing mole fractions of cholesterol (same Xchol as panel A). Panel C shows egg SM with increasing mole fractions of cholesterol (same Xchol as panel A). Panel D shows dipalmitoyl-PC with increasing mole fractions of cholesterol (same Xchol as panel A).
To facilitate viewing of the isotherms containing cholesterol, data in Figures 1 and 2 were plotted as the apparent molecular area of phospholipid (either SM or PC) versus surface pressure. The apparent molecular area of phospholipid was calculated by dividing the total, measured surface area of the cholesterol/phospholipid mixed monolayers by the number of phospholipid molecules in the surface. The π–A isotherms show that, at low cholesterol mole fractions (e.g., Xchol < 0.3), very little expansion occurred in the apparent molecular area of phospholipid derivatives. In fact, this behavior indicated that cholesterol has a large condensing effect on the phospholipid derivatives.
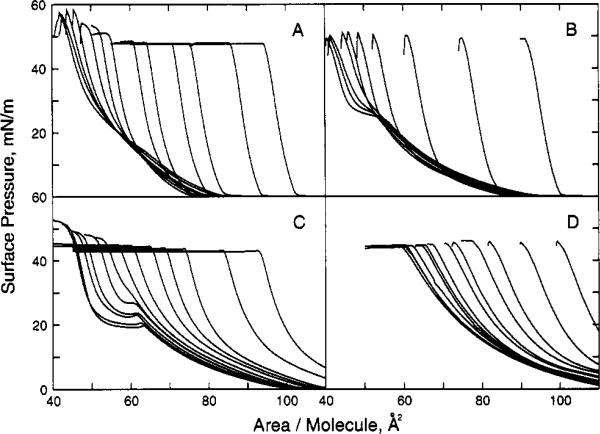
Figure 2.
Surface pressure vs apparent molecular area of sphingolipid/phospholipid. Experimental conditions same as in Figure 1. Panel A shows bovine brain SM with increasing mole fractions of cholesterol (same Xchol as Figure 1A). Panel B shows 1-myristoyl-2-palmitoyl-PC with increasing mole fractions of cholesterol (from left to right at π = 30 mN/m; Xchol = 0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.4, 0.5, 0.6). Panel C shows dinervonoyl (24:1Δ15)-PC with increasing mole fractions of cholesterol (same Xchol as Figure 1A). Panel D shows 1-palmitoyl-2-arachidonoyl-PC with increasing mole fractions of cholesterol (same Xchol as Figure 1A).
To compare quantitatively the magnitude of cholesterol's condensing effect on N-18:1Δ9-SM and POPC, we determined the additivity of the mean molecular areas as a function of composition (Goodrich, 1957). Figure 3A,B shows the results at three different surface pressures (5, 15, and 30 mN/m). Solid lines represent calculated ideal area additivity (see Materials and Methods). Substantial negative deviations from area additivity occurred for both the POPC and 18:1Δ9-SM. To compare our data with those of previous reports, we also calculated the condensing effect as the change in phospholipid/sphingolipid mean molecular area that occurred as cholesterol content increased from zero to 0.5 mole fraction [e.g., Lund-Katz et al. (1988) and Ali et al. (1994a)]. Because cholesterol's cross-sectional surface area is very insensitive to changes in surface pressure, the experimentally observed changes in the areas of mixed films can be attributed almost exclusively to configurational changes, i.e., ordering that cholesterol imparts to the phospholipid/sphingolipid (Pethica, 1955; Phillips, 1972). We found that including equimolar cholesterol in the SM monolayers decreased the mean molecular area of N-18: 1Δ9-SM by 28 Å2 (79 to 51 Å2/SM molecule), by 18 Å2 (63 to 45 Å2/SM molecule), and by 15 Å2 (58 to 43 Å2/SM molecule) at 5, 20, and 30 mN/m, respectively (Figure 6B). In comparison, including equimolar cholesterol in the PC monolayers decreased the mean molecular area of POPC by 30 Å2 (88 to 58 Å2/PC molecule), by 18 Å2 (68 to 50 Å2/PC molecule), and by 14 Å2 (61 to 47 Å2/PC molecule) at 5, 20, and 30 mN/m, respectively (Figure 6A). We also obtained very similar mean molecular areas for SOPC in the presence and absence of equimolar cholesterol (data not shown). Thus, although SM packed at higher average molecular density than POPC (or SOPC) at equal surface pressures in either the presence or absence of cholesterol, nearly identical cholesterol-induced condensations or interfacial area reductions were observed for N-18:1Δ9-SM and POPC.
Figure 3.
Mean molecular area vs composition. In each panel: (●) 5 mN/m; (■) 15 mN/m; (▲) 30 mN/m. Ideal additivity of mean molecular area is represented by the solid lines. Panel A: N-Oleoyl-SM; panel B: 1-palmitoyl-2-oleoyl-PC; panel C: egg SM; panel D: dipalmitoyl-PC.
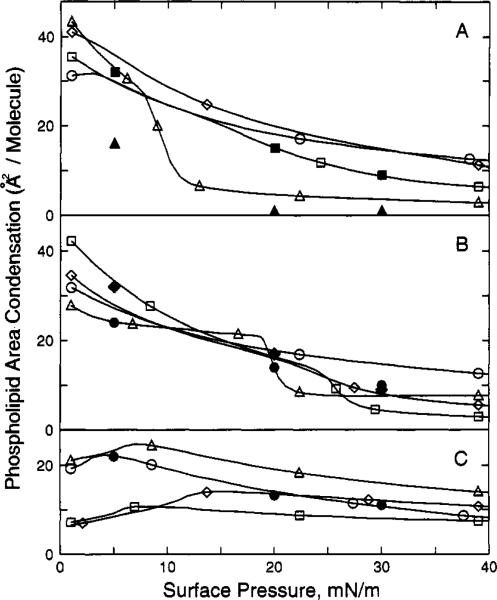
Figure 6.
Phospholipid/sphingolipid area condensation vs surface pressure. Area condensation was calculated as described in the Materials and Methods. All values are for equimolar mixtures with cholesterol. Each line represents data calculated at 1 mN/m intervals over the surface pressure range from 1 to 40 mN/m. Panel A: (△) dipalmitoyl-PC; (◇) dimyristoyl-PC; (○) 1-palmitoyl-2-oleoyl-PC; (□) bovine brain SM; panel B: (□) 1-myristoyl-2-palmitoyl-PC; (◇) egg SM; (○) N-oleoyl-SM; (△) dinervonoyl-PC; panel C: (△) 1-palmitoyl-2-arachidonoyl-PC; (○) dioleoyl-PC; (□) diphytanoyl-PC; (◇) diarachidonoyl-PC. Solid symbols are values from Lund-Katz et al. (1988). Panel A: (■) Bovine brain SM; (▲) dipalmitoyl-PC; panel B: (◆) egg SM; (●) N-oleoyl-SM; panel C: (●) dioleoyl-PC.
We found this result to be quite intriguing because earlier reports had indicated that cholesterol has a significantly larger condensing effect on SM compared to PC (Lund-Katz et al., 1988). To verify our results, we investigated cholesterol's condensing effect on other SMs and PCs. We compared cholesterol's condensing effect of egg SM with that of DPPC. Egg SM was used because its major acyl residue (78%) is palmitate (see Materials and Methods) and because its mixing behavior with cholesterol is almost identical to that of N-palmitoyl-SM (Lund-Katzet al., 1988). Our π–A isotherm of egg SM showed a phase transition from a liquid-expanded to liquid-condensed phase at 18 mN/m (Figure 1C). The similarity of the π–A isotherms of egg SM and N-palmitoyl-SM (Yedgar et al., 1982; Lund-Katz et al., 1988) was not surprising given the high content of palmitoyl acyl residues in egg SM. Likewise, our π–A isotherm of pure DPPC was consistent with previous reports in that a distinct phase transition from a liquid-expanded to liquid-condensed state was observed at 8.5 mN/m (Figure 1D) [e.g., Phillips and Chapman (1968), Demel and de Kruijff (1976), Müller-Landau and Cadenhead (1979), Albrecht et al. (1981), and Lund-Katz et al. (1988)].
If increasing amounts of cholesterol were included in either egg SM or DPPC monolayers, a marked condensation was observed in mean molecular area (Figure 3C,D). At 24 °C and in the presence of equimolar cholesterol, the mean molecular area of egg SM decreased by 28 Å2 (71 to 43 Å2/SM molecule), by 16 Å2 (57 to 41 Å2/SM molecule), and by 8 Å2 (48 to 40 Å2/SM molecule) at 5, 20, and 30 mN/m, respectively (Figure 6B). In comparison, the mean molecular area of DPPC decreased by 33 Å2 (80 to 47 Å2/PC molecule), by 6 Å2 (50 to 44 Å2/PC molecule), and by 5 Å2 (47 to 42 Å2/PC molecule) at 5, 20, and 30 mN/m, respectively (Figure 6A). Thus, at surface pressures where egg SM and DPPC were in comparable phase states (e.g., 5 and 30 mN/m), the surface density of egg SM was higher than that of DPPC both in the presence and in the absence of cholesterol. Nevertheless, the “condensing effect” or interfacial area reduction induced by cholesterol was quite similar. The larger cholesterol-induced condensation of egg SM compared to DPPC at 20 mN/m can be explained by the differences in the phase state of the two phospholipids in the absence of cholesterol. Egg SM was quite near its two-dimensional phase transition pressure; whereas, DPPC was clearly in a liquid-condensed state.
Toverify that other SMs and PCs (containing one saturated acyl chain) which display two-dimensional phase transitions show similar area condensations when mixed with cholesterol, we compared bovine brain SM/cholesterol and MPPC/cholesterol mixed monolayers (Figure 2, panels A and B, respectively). Increasing amounts of cholesterol in combination with either bovine SM or MPPC elicited negative deviations from ideal additivity in monolayer mean molecular areas (Figure 4A,B). At 24 °C and in the presence of equimolar cholesterol, the mean molecular area of bovine brain SM decreased by 29 Å2 (74 to 45 Å2/SM molecule), by 15 Å2 (57 to 42 Å2/SM molecule), and by 8 Å2 (49 to 41 Å2/SM molecule) at 5, 20, and 30 mN/m, respectively (Figure 6A). In comparison, the mean molecular area of MPPC decreased by 34 Å2 (79 to 45 Å2/PC molecule), by 17 Å2 (59 to 42 Å2/PC molecule), and by 5 Å2 (46 to 41 Å2/PC molecule), respectively, at 5, 20, and 30 mN/m (Figure 6B). Hence, as was observed with egg SM and DPPC, cholesterol's “condensing effect” on bovine brain SM and MPPC was quite similar. Large area changes were observed at pressures where bovine brain SM and MPPC were liquid-expanded, and diminished area reductions were observed at surface pressures where bovine brain SM and MPPC were already condensed prior to inclusion of cholesterol.
Figure 4.
Mean molecular area vs composition. In each panel: (●) 5 mN/m; (■) 15 mN/m; (▲) 30 mN/m. Ideal additivity of mean molecular area is represented by the solid lines. Panel A: Bovine brain SM; panel B: 1-myristoyl-2-palmitoyl-PC; panel C: dinervonoyl (24:1Δ15)-PC; panel D: dimyristoyl-PC.
Figure 6 (panels A and B) illustrates quite dramatically how surface pressure (and phase state) affect the change in mean molecular area of phospholipid/sphingolipid in the presence of equimolar cholesterol. At surface pressures where both DPPC and egg SM were liquid-expanded (e.g., π < 8 mN/m), cholesterol had a large, but similar condensing effect on either lipid. A dramatic drop in condensation occurred in DPPC between 8.5 and 12 mN/m, whereas, a clearly discernible but less dramatic drop in condensation occurred between 22 and 30 mN/m in egg SM. At surface pressures between 30 and 40 mN/m, which approximate the conditions of biological membranes (Demel et al., 1975; Blume, 1979), the cholesterol-induced condensation of DPPC was slightly less than that of egg SM. From Figure 6 (panels A and B), it is clear that other PC and SM derivatives which displayed two-dimensional phase transitions also behaved quite similarly (e.g., MPPC, DNPC, and bovine brain SM).
Taken together, these results clearly illustrate that monolayer phase state has a major impact on the cholesterol-induced condensation of SM and PC. In fact, we always observed quite similar levels of cholesterol-induced condensation of phase-state equivalent SM and PC (at π > 10 mN/m) as long as the PC derivative contained at least one acyl chain in which the first 10 carbons contained no structural constraints (e.g., cis double bonds; branched methyl groups) capable of disrupting close apposition of the planar steroid ring via van der Waals interactions. This was evident not only from the data presented above but also from our cholesterol-induced condensation data of DOPC, DNPC, PAPC, DAPC, diphytanoyl-PC, and DMPC (Figures 4–6). With the exception of DNPC, which displayed a liquid-expanded to liquid-condensed phase transition at 20 mN/m and 64 Å2/molecule (Figure 2C), all of these PC derivatives formed liquid-expanded films at π between 1 and 40 mN/m. However, only PAPC, DMPC, and liquid-expanded DNPC showed condensation levels in the presence of equimolar cholesterol similar to those of liquid-expanded SMs (e.g., compare π at 15 mN/m in Figure 6). Even though the other PCs (DOPC, DAPC, and diphytanoyl-PC) also were liquid-expanded, the packing constraints produced by having either cis double bonds or branched methyl groups in the first 10 carbons of both acyl chains presumably interfered with cholesterol being able to produce maximal condensation. As a result, at 30 mN/m, the observed phospholipid area condensations (Figure 6) are quite similar to those of condensed-phase egg SM and bovine SM. By having methyl groups at positions 3, 7, 11, and 15 (e.g., diphytanoyl-PC) or cis unsaturation and associated Δtg kinks at positions 9 or 5, 8, 11, and 14 (e.g., DOPC and DAPC, respectively), maximal condensation with cholesterol's planar steroid ring cannot be achieved. In the case of DNPC, the cis double bond and associated Δtg kinks were located at position 15 and, thus, probably did not interfere with cholesterol's steroid ring adjacently aligning with the first 10 or so carbons in the acyl chain(s) of PC. Indeed, X-ray studies have shown that cholesterol's steroid ring aligns with the hydrocarbon region proximal to the headgroup of PC (McIntosh, 1978).
Interestingly, we observed that as π increased from 1 to 8 mN/m, the cholesterol-induced condensation of PAPC increases (Figure 6C). Although the surface pressure dependence varied somewhat, we observed this same phenomena for all the PCs that had a cis double bond or branched methyl group in the first 10 carbons of an acyl chain (e.g., DAPC, DOPC, PAPC, POPC, and DPhytPC). This behavior has also been reported by Tinoco and colleagues (Evans & Tinoco, 1978; Evans et al., 1987).
DISCUSSION
Cholesterol's “Condensing Effect” on SMs versus PCs
The results of this study demonstrate clearly that, under conditions where the ceramide structure of SM and the diacylglycerol structure of PC are similar, the interfacial area condensation of SM induced by cholesterol is nearly identical to that of PC. Cholesterol induces a larger condensation of SM compared to PC only under certain limiting structural conditions, i.e., when the ceramide structure of SM and the diacylglycerol structure of PC differ sufficiently so as to put the two phospholipids in different physical states in the absence of cholesterol. When this situation occurs, whichever phospholipid is liquid-expanded always shows a 5- to 10-fold larger cholesterol-induced interfacial area reduction compared to that of the liquid-condensed phospholipid.
The importance of the monolayer phase state in determining the extent to which cholesterol reduces the average molecular packing density of either glycerol-based phospholipids (Chapman et al., 1969) or simple sphingolipids such as galactosylceramide (GalCer) (Ali et al., 1994a) has been noted previously. In fact, calculation of cholesterol-induced area reductions at surface pressures too close to the phase transition of other pure lipid species appears to influence the condensation value. We found this to be true for egg SM at 20 mN/m as well as for certain GalCer species (Ali et al., 1994a). This finding probably explains why Grönberg et al. (1991) observed a difference in cholesterol's condensation of N-18:0-SM, which has a phase transition just below 15 mN/m (Yedgar et al., 1982), compared to distearoyl-PC, which shows only liquid-condensed behavior.
When both SM and PC are in the liquid-expanded state (and thus potentially strongly condensable by cholesterol), subtle structural differences in the ceramide and diacylglycerol structures can still mitigate cholesterol's interaction with either SM or PC. The structural differences include the presence or absence of cis double bonds (and their associated Δtg kinks) as well as cis double bond position(s) along the hydrocarbon chains. The importance of the acyl structure of glycerol-based phospholipids in mitigating interfacial interactions with cholesterol also has been noted previously (Demel et al., 1967, 1972; Chapman et al., 1969; Ghosh et al., 1973; Evans & Tinoco, 1978; Evans et al., 1987). Similar findings have been reported in bilayer assemblies (Ayanoglu et al., 1986, 1988, 1990; Davis & Keough, 1984a,b; Keough et al., 1989; Davies et al., 1990; Kariel et al., 1991).
Our finding, that the cholesterol-induced interfacial area reductions of “phase-state equivalent” PC and SM are quite similar, is based on investigation of cholesterol's interfacial interactions with nine different PCs and three different SMs. The need to investigate so many PCs and SMs arose because of inconsistencies between some of our results and earlier studies in which cholesterol's interfacial interactions with SM and PC were compared (Lund-Katz et al., 1988). Interestingly, the reductions in average area per SM molecule that we observe when cholesterol is mixed with equimolar amounts of either bovine brain SM or egg yolk SM are quite similar to values reported by Lund-Katzet al. (1988). Our cholesterol-induced condensation values for DOPC are also nearly identical to the data reported by Lund-Katz et al. (1988). Only the cholesterol-induced condensation values for DPPC differ significantly. Rather than values of 16, 0, and 0 Å2/PC molecule at 5, 20, and 30 mN/m (Lund-Katz et al., 1988), we observed values of 33, 6, and 5 Å2/PC molecule at 5, 20, and 30 mN/m at equimolar cholesterol concentrations (Figure 6). We found our values to be highly reproducible using different lots of both cholesterol and DPPC. Careful examination of the data sets revealed that our π–A isotherms of DPPC/cholesterol mixed monolayers occupied smaller surface areas at equivalent cholesterol mole fractions than the isotherms of Lund-Katz et al. (1988). The reason for this difference is not clear. The behavior could be caused by an unknown surface-active impurity in their DPPC or cholesterol, by partial oxidation of their cholesterol (Kamel et al., 1971), or if their cholesterol mole fraction in the mixed monolayers were less than was stated. Interestingly, our DPPC/cholesterol π–A isotherms agree quite well with earlier results [e.g., Müller-Landau and Cadenhead (1979) and Albrecht et al. (1981)]. In any case, the differences in the DPPC/cholesterol π–A behavior and the resulting condensation values explain why Lund-Katz et al. (1988) reported that cholesterol has a larger condensing effect on SM compared to PC. Unfortunately, DPPC was the only homogeneous PC species containing at least one saturated acyl chain and possessing liquid-expanded character (at 5 mN/m) that Lund-Katz et al. (1988) investigated in mixed films with cholesterol.
It is important to note that, although cholesterol's condensation of phase-state equivalent SM and PC is quite similar, the interfacial lipid packing densities of phase-state equivalent SM and PC do differ, and the way that cholesterol influences the lipid packing density appears to depend on acyl structure. For instance, even though their cholesterol-induced interfacial area changes are quite similar, N-18:1-SM packs more densely than POPC at equivalent surface pressures both in the absence and in the presence of equimolar cholesterol. However, this is not the case for egg SM/cholesterol and DPPC/cholesterol. Although egg SM is more densely packed than DPPC at 5 mN/m, in the presence of equimolar cholesterol, DPPC ends up packed just as densely packed as egg SM above 30 mN/m.
The structural configuration promoting maximal and nearly equivalent cholesterol-induced condensation of SM and PC is one in which at least one hydrocarbon chain is long and capable of assuming an extended conformation. In PC, this condition is satisfied when the sn-1 acyl chain is myristate or palmitate. By the same token, the 18-carbon sphingosine base of SM satisfies this condition because its 4,5 trans double bond is quite close to the interfacial region (carbons 1–3 serve the equivalent role as glycerol in PC molecules) and does nothing to disrupt the potential linear extension of the sphingosine base of SM. This condition is essential for obtaining a large condensation effect because of cholesterol's known ability to reduce the number of trans-gauche isomerizations in the saturated acyl chains of liquid-crystalline phospholipids, thereby achieving optimal van der Waals attractive interactions with the planar-like steroid ring [e.g., Davies et al. (1990)].
The configurational requirements of the sn-2 acyl chain of PC or the sole acyl chain of SM appear to be less stringent but clearly mitigate interfacial interaction with cholesterol. Aside from influencing lipid phase state, the presence and location of cis unsaturation in the acyl chain also affect in-plane interactions with cholesterol. Maximal condensation is observed over a wide range of liquid-expanded surface pressures only when the acyl chain is saturated. This is evident fromour data for DPPC, DMPC, MPPC, egg SM, and bovine brain SM. Interestingly, if the sn-2 acyl chain of PC contains multiple unsaturation with some of the cis double bonds occurring in thefirst 10 carbons but the sn-1 chain is saturated, then maximal condensation by cholesterol is only observed at surface pressures above 8 mN/m. Indeed, our cholesterol-induced condensation data of PAPC are no different from that of DMPC, POPC, or N-oleoyl-SM at π between 8 and 40 mN/m. In general, our findings are supported by other studies of cholesterol's “condensing effect” on PCs containing palmitate or stearate as their sn-1 acyl chain and different polyunsaturated sn-2 acyl chains (e.g., 20:4, 225, or 22:6). One exception is for the PCs containing 22:6 in which an unusually compact conformation appears possible, thus yielding a diminished area reduction in the presence of cholesterol (Demel et al., 1972; Ghosh et al., 1973; Parks & Thuren, 1993). Recent computer-based molecular modeling also predicts three different energy-minimized conformations for diglycerides depending on the number and location of cis double bonds in the sn-2 chain (Applegate & Glomset, 1991).
If neither acyl chain in PC is saturated, then cis double bond position plays a more important role in determining the degree of cholesterol-induced interfacial area reduction. Only when the cis double bond is beyond the first 10 carbons (e.g., DNPC at π between 10 and 18 mN/m) is cholesterol-induced condensation similar to SM or to PC containing a saturated acyl chain.
Hydrocarbon structure is not the sole determinant of cholesterol's “condensing effect” on phospholipids and sphingolipids. Headgroup structure also plays a role. This is evident from recent studies of dihexosyl- and monohexosylceramides (Slotte et al., 1993; Ali et al., 1994a). For instance, it is clear that cholesterol's condensation of galactosylceramide (GalCer) is less than that of sphingomyelin even under conditions where GalCer's acyl composition has been altered so as to maximize cholesterol's “condensing effect” (Ali et al., 1994a). Similar headgroup effects have been shown in comparisons of cholesterol's condensing effect of chain-equivalent PCs and PES [e.g., Chapman et al. (1969)].
Other Insights into Cholesterol's Interaction with SMs versus PCs
Other classical indicators of mixing behavior in two-component monolayers, such as collapse pressure versus composition (Crisp, 1949), have quite limited value for many of the phospholipids studied here. A discussion of the difficulties associated with this approach was included in our recent report of cholesterol's interactions with GalCer (Ali et al., 1994a). However, the presence of a liquid-expanded-to-condensed phase transition in some of the SMs and PCs does provide an avenue for gaining limited insight into their interfacial mixing with cholesterol. Using the transition onset pressure as an indicator [e.g., Ali et al. (1991); Smaby et al. (1994a)], there are indications of differences in cholesterol's mixing with egg and bovine SM compared to DPPC and MPPC. For instance, less cholesterol is required to eliminate the transition onset pressure in egg SM (0.05 < Xchol < 0.07) and in bovine SM (0.04 < Xchol < 0.05) than in either DPPC (0.2 < Xchol < 0.25) or MPPC (0.1 < Xchol < 0.15). A similar disappearance of the 2-D transition of DPPC (0.2 < Xchol < 0.3) has been reported previously (Müller-Landau & Cadenhead, 1979; Albrecht et al., 1981). More recently, McConnell and others have utilized fluorescence microscopy to gain direct insight into the mixing behavior of cholesterol in DPPC or DMPC [for reviews, see McConnell (1991) and Weis (1991)]. However, to our knowledge, no fluorescence studies of cholesterol's interfacial mixing behavior with SM have been reported.
Implications
Our monolayer results clarify the lipid structural parameters controlling cholesterol's in-plane interactions with SMs and PCs. Earlier studies involving bilayer assemblies of cholesterol/PC and cholesterol/SM are consistent with our results. Calhoun and Shipley (1979) reported no difference in cholesterol's interaction with phase-state equivalent N-16:0-SM or DMPC on the basis of thermotropic behavior of multilamellar dispersions. Also, McIntosh et al. (1992) proposed that, in terms of strong interaction with cholesterol, the critical features of SM were its high degree of hydrocarbon chain saturation and double bond location in the acyl chain.
Interestingly, in the majority of tissues, it appears that less than 10% of the PC molecular species contain unsaturated acyl chains in both the sn-1 and sn-2 positions. These tissues include liver, skeletal muscle, brain, lung, egg yolk, and red cells [e.g., Kuksis (1985)]. Thus, some 90–95% of the PCs contain a saturated sn-1 acyl chain and, based on our results, would show similar cholesterol-induced condensation as sphingomyelin. On the basis of the known transbilayer distributions of these three lipid types in erythrocytes, it appears that cells probably regulate the amount of cholesterol present in surfaces enriched in SM and PC so as to prevent too much cholesterol from accumulating in these surfaces. As much as 75% of cholesterol reportedly resides in the cytofacial lamellar leaflet compared to the exofacial lamellar leaflet in red cells (Schroeder et al., 1991a,b). In contrast, 90% of the SM and 80% of the PC reside in the erythrocyte exofacial lamellar leaflet (Verleij et al., 1973).
Figure 5.
Mean molecular area vs composition. In each panel: (●) 5 mN/m; (■) 15 mN/m; (▲) 30 mN/m. Ideal additivity of mean molecular area is represented by the solid lines. Panel A: Diphytanoyl-PC; panel B: diarachidonoyl-PC; panel C: 1 -palmitoyl-2-arachidonoyl-PC; panel D: dioleoyl-PC.
ACKNOWLEDGMENT
We thank C. Perleberg for typing and spellchecking this report.
Footnotes
This investigation was supported by USPHS Grants GM45928 (R.E.B.) and HL49180 (H.L.B.) and the Hormel Foundation. Portions of this investigation were presented at the Biophysical Society meeting (Smaby et al., 1994b).
Abbreviations: GalCer, galactosylccramidc; POPC, 1-palmitoyl-2-olcoyl-sn-glycero-3-phosphochotine; SOPC, 1-stearoyl-2-olcoyl-sn-glycero-3-phosphocholinc; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholiie; DPPC, dipalmitoyl-sn-glycero-3-phosphocholine; DMPC, dimyristoyl-sn-glycero-3-phosphochotine; MPPC, 1-myristoyl-2-palmitoyl-sn-glycero-3-phosphocholine; DOPC, diolmyl-sn-glyccro-3-phosphocholine; DAPC, diarachidonoyl-sn-glycero-3-phosphocholine; DNPC, dinervonoyl-sn-glyccro-3-phosphocholine; DPhytPC, diphytanoyl-sn-glycero-3-phosphocholine; BSM, bovine brain sphingomyelin; ESM, egg yolk sphingomyelin.
REFERENCES
- Albrecht O, Gruler H, Sackmann E. J. Colloid Interface Sci. 1981;79:319–338. [Google Scholar]
- Ali S, Brockman HL, Brown RE. Biochemistry. 1991;30:11198–11205. doi: 10.1021/bi00111a002. [DOI] [PubMed] [Google Scholar]
- Ali S, Smaby JM, Brown RE. Biochemistry. 1993;32:11696–11703. doi: 10.1021/bi00094a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Smaby JM, Brockman HL, Brown RE. Biochemistry. 1994a;33:2900–2906. doi: 10.1021/bi00176a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Smaby JM, Brown RE. Thin Solid Films. 1994b;244:860–864. [Google Scholar]
- Aloia RC, Tian H, Jensen FC. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate KR, Glomset JA. J. Lipid Res. 1991;32:1645–1655. [PubMed] [Google Scholar]
- Ayanoglu E, Düzgünes N, Wijekoon WMD, Djerassi C. Biochim. Biophys. Acta. 1986;863:110–114. doi: 10.1016/0005-2736(86)90392-5. [DOI] [PubMed] [Google Scholar]
- Ayanoglu E, Li H, Djerassi C, Düzgünes N. Chem. Phys. Lipids. 1988;47:165–175. doi: 10.1016/0009-3084(88)90138-7. [DOI] [PubMed] [Google Scholar]
- Ayanoglu E, Chiche BH, Beatty M, Djerassi C, Düzgünes N. Biochemistry. 1990;29:3466–3471. doi: 10.1021/bi00466a007. [DOI] [PubMed] [Google Scholar]
- Barenholz Y, Thompson TE. Biochim. Biophys. Acta. 1980;604:129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Bartlett GR. J. Biol. Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- Blume A. Biochim. Biophys. Acta. 1979;557:32–44. doi: 10.1016/0005-2736(79)90087-7. [DOI] [PubMed] [Google Scholar]
- Boggs JM. Biochim. Biophys. Acta. 1987;906:353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Brockman HL, Jones CM, Schwebke CJ, Smaby JM, Jarvis DE. J. Colloid Interface Sci. 1980;78:502–512. [Google Scholar]
- Cadenhead DA, Müller-Landau F. J. Colloid Interface Sci. 1980;78:269–270. [Google Scholar]
- Calhoun WI, Shipley GG. Biochim. Biophys. Acta. 1979;555:436–441. doi: 10.1016/0005-2736(79)90397-3. [DOI] [PubMed] [Google Scholar]
- Chapman D, Owens NF, Phillips MC, Walker DA. Biochim. Biophys. Acta. 1969;183:458–465. doi: 10.1016/0005-2736(69)90160-6. [DOI] [PubMed] [Google Scholar]
- Crisp DJ. Surface Chemistry. Butterworths; London: 1949. pp. 17–25. [Google Scholar]
- Davies MA, Schuster HF, Brauner JW, Mendelsohn R. Biochemistry. 1990;29:4368–4373. doi: 10.1021/bi00470a016. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Keough KMW. Biochemistry. 1984a;22:6334–6340. [Google Scholar]
- Davis PJ, Keough KMW. Biochim. Biophys. Acta. 1984b;778:305–310. [Google Scholar]
- Demel RA, de Kruijff B. Biochim. Biophys. Acta. 1976;457:109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Demel RA, van Deenen LLM, Pethica BA. Biochim. Biophys. Acta. 1967;135:11–19. doi: 10.1016/0005-2736(67)90054-5. [DOI] [PubMed] [Google Scholar]
- Demel RA, Geurts van Kessel WSM, van Deenen LLM. Biochim. Biophys. Acta. 1972;266:26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Demel RA, Geurts van Kessel WSM, Zwaal RFA, Roelofson B, van Deenen LLM. Biochim. Biophys. Acta. 1975;406:97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Evans RW, Tinoco J. Chem. Phys. Lipids. 1978;22:207220. doi: 10.1016/0009-3084(78)90027-0. [DOI] [PubMed] [Google Scholar]
- Evans RW, Williams MA, Tinoco J. Biochem. J. 1987;245:455–462. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold L, editor. Cholesterol in Membrane Models. CRC Press; Boca Raton, FL.: 1993. [Google Scholar]
- Ghosh D, Williams MA, Tinoco J. Biochim. Biophys. Acta. 1973;291:351–362. doi: 10.1016/0005-2736(73)90488-4. [DOI] [PubMed] [Google Scholar]
- Goodrich FC. Proc. 2nd International Congress Surface Activity. I. Butterworths; London: 1957. pp. 85–91. [Google Scholar]
- Grönberg L, Ruan Z, Bittman R, Slotte JP. Biochemistry. 1991;30:10746–10754. doi: 10.1021/bi00108a020. [DOI] [PubMed] [Google Scholar]
- Hamilton KS, Jarrell HC, Briere KM, Grant CWM. Biochemistry. 1993;32:4022–4028. doi: 10.1021/bi00066a024. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Linardic CM. Biochim. Biophys. Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Kamel AM, Weiner ND, Felmeister A. J. Colloid Interface Sci. 1971;35:163–166. doi: 10.1016/0021-9797(71)90200-1. [DOI] [PubMed] [Google Scholar]
- Kariel N, Davidson E, Keough KMW. Biochim. Biophys. Acta. 1991;1062:70–76. doi: 10.1016/0005-2736(91)90336-7. [DOI] [PubMed] [Google Scholar]
- Keough KMW, Giffin B, Matthews PLJ. Biochim. Biophys. Acta. 1989;983:51–55. doi: 10.1016/0005-2736(89)90379-9. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN. Prog. Lipid Res. 1991;30:1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- Koval M, Pagano RE. Biochim. Biophys. Acta. 1991;1082:113–125. doi: 10.1016/0005-2760(91)90184-j. [DOI] [PubMed] [Google Scholar]
- Kuksis A. In: Lecithins. Szuhaj BF, List GR, editors. American Oil Chemists' Society; Champaign, IL.: 1985. pp. 122–139. [Google Scholar]
- Lund-Katz S, Laboda HM, McLean LR, Phillips MC. Biochemistry. 1988;27:3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- McConnell HM. Annu. Rev. Phys. Chem. 1991;42:171–195. [Google Scholar]
- McIntosh TJ. Biochim. Biophys. Acta. 1978;513:43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- McIntosh TJ, Simon SA, Needham D, Huang C. Biochemistry. 1992;31:2012–2020. doi: 10.1021/bi00122a017. [DOI] [PubMed] [Google Scholar]
- Müller-Landau F, Cadenhead DA. Chem. Phys. Lipids. 1979;25:315–328. [Google Scholar]
- Nieva JL, Bron R, Corver J, Wilschut J. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks JS, Thuren TY. J. Lipid Res. 1993;34:779–788. [PubMed] [Google Scholar]
- Pascher I. Biochim. Biophys. Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- Pethica BA. Faraday Trans. Soc. 1955;51:1402–1411. [Google Scholar]
- Phillips MC. In: Progress in Surface and Membrane Science. Danielli JF, Rosenburg MD, Cadenhead DA, editors. Vol. 5. Academic Press; New York: 1972. pp. 139–221. [Google Scholar]
- Phillips MC, Chapman D. Biochim. Biophys. Acta. 1968;163:301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Jefferson JR, Kier AB, Knittel J, Scallen TJ, Wood WG, Hapala I. Proc. Soc. Exp. Biol. Med. 1991a;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Nemecz G, Wood WG, Joiner C, Morrot G, Ayraut-Jarrier M, Devaux PF. Biochim. Biophys. Acta. 1991b;1066:183–192. doi: 10.1016/0005-2736(91)90185-b. [DOI] [PubMed] [Google Scholar]
- Slotte JP, Ostman A-L, Kumar ER, Bittman R. Biochemistry. 1993;32:7886–7892. doi: 10.1021/bi00082a008. [DOI] [PubMed] [Google Scholar]
- Smaby JM, Brockman HL. Biophys. J. 1985;48:701–708. doi: 10.1016/S0006-3495(85)83828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby JM, Brockman HL. Biophys. J. 1990;58:195–204. doi: 10.1016/S0006-3495(90)82365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby JM, Brockman HL. Chem. Phys. Lipids. 1991;58:249–252. doi: 10.1016/0009-3084(91)90099-w. [DOI] [PubMed] [Google Scholar]
- Smaby JM, Muderhwa JM, Brockman HL. Biochemistry. 1994a;33:1915–1922. doi: 10.1021/bi00173a039. [DOI] [PubMed] [Google Scholar]
- Smaby JM, Brockman HL, Ali S, Brown RE. Biophys. J. 1994b;65:A286. doi: 10.1016/S0006-3495(98)77791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij AJ, Zwaal RFA, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LLM. Biochim. Biophys. Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- Weiss RM. Chem. Phys. Lipids. 1991;57:227–239. doi: 10.1016/0009-3084(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Yedgar S, Cohen R, Gatt S, Barenholz Y. Biochem. J. 1982;201:597–603. doi: 10.1042/bj2010597. [DOI] [PMC free article] [PubMed] [Google Scholar]