Abstract
Purpose
Heparin is commonly used to anticoagulate the hemodialysis (HD) circuit. Despite the bleeding risk, no American standards exist for its administration. We identified correlates and quantified sources of variance in heparin dosing for HD.
Methods
We performed a cross-sectional study of patients aged ≥67 years who underwent HD with heparin on one of two randomly chosen days in 2008 at a national chain of dialysis facilities. Using a mixed effects model with random intercept for facility and fixed patient and facility characteristics, we examined heparin dosing at patient and facility levels.
Results
The median heparin dose among the 17,722 patients treated in 1366 facilities was 4000 (IQR: 2625–6000) units. In multivariable-adjusted analyses, higher weight; longer session duration; catheter use; and dialyzer reuse were significantly associated with higher heparin dose. Dose also varied considerably among census divisions. Of the overall variance in dose, 21% was due to between-facility differences, independent of facilities’ case-mix, geography, size, or rurality; 79% was due to differences at the patient level. The patient and facility characteristics in our model explained only 25% of the variance at the patient level.
Conclusions
Despite the lack of standards for heparin administration, we noted patterns of use, including weight-based and time-dependent dosing. Most of the variance was at the patient level; however, only a quarter of it could be explained. The high amount of unexplained variance suggests that factors other than clinical need are driving heparin dosing, and that there is likely room for more judicious dosing of heparin.
Keywords: anti-coagulation, hemodialysis, heparin, facility
Introduction
Heparin is the most commonly used anticoagulant for maintenance hemodialysis (HD).1 In a study of older patients who initiated HD with a U.S. dialysis provider from 2007–2008, 93% received the drug.2 Since heparin is an anticoagulant, it carries with it an increased risk of bleeding, which is of particular importance to patients on HD, who experience gastrointestinal bleeding rates almost 100 times that of the general population.3 Yet, despite its widespread use and potentially adverse side effects, there are no American standards for heparin administration for HD.4 Many facilities have developed protocols for heparin administration, but a wide variety of dosing schedules exist.4
Variation in clinical care is inevitable. Every patient is unique, so patient-centered care will naturally lead to variation stemming from differences in the medical conditions and preferences of patients.5 However, variation can also result from differences in provider practice patterns. When these patterns are driven by financial incentives, regional preferences, or protocols designed primarily for the providers’ convenience, they risk compromising patient care.5 Thus, identifying sources of variation may highlight areas for improvement in patient-centered care.
In order to better understand the patterns of heparin administration in the absence of universal guidelines, we aimed to identify correlates of heparin dosing as well as to determine the amount and sources of variance in heparin administration for maintenance HD in an older U.S. population.
Methods
Data Source
We used data merged from the United States Renal Data System (USRDS) and the electronic medical records (EMR) of a national dialysis provider using a crosswalk of anonymized patient identifiers that the USRDS Coordinating Center generated upon approval by the Centers for Medicare and Medicaid Services (CMS) and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). The USRDS contains demographic information for almost all Americans with end-stage renal disease; billing claims to Medicare (Part A and B); as well as comorbidities and dialysis facility information from forms submitted to the CMS. The EMR includes records on heparin and warfarin use, vital signs, laboratory measurements, and HD prescriptions.
Study Population
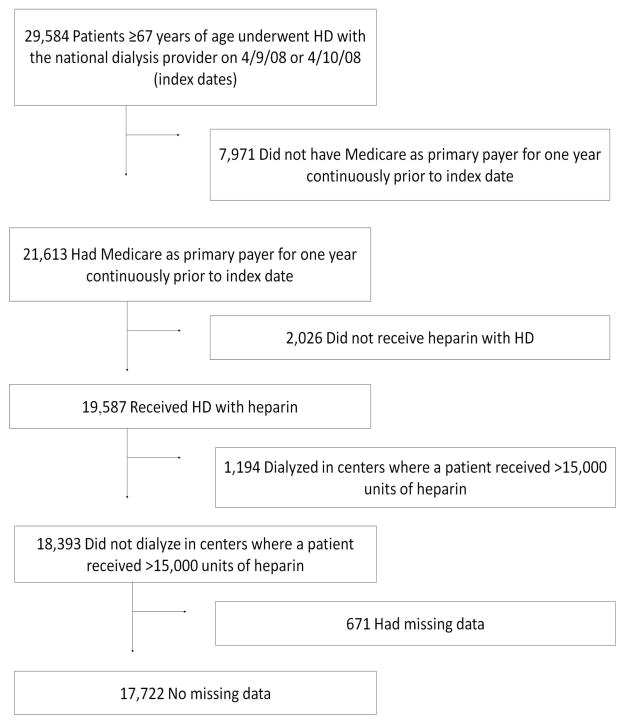
We selected a contemporary cohort of patients who underwent maintenance HD with heparin at a national chain of dialysis facilities on either a randomly chosen Wednesday or the adjacent Thursday in 2008 (April 9 or 10). We restricted our population to patients 67 years of age or older whose primary payer was Medicare for at least one year prior to the index date so that we could uniformly ascertain comorbidities from Medicare claims data (Figure 1). Of these 21,613 patients, 19,587 received heparin during their session (patients receiving heparin-free HD were excluded as previous studies have found that this population is quite different from those who do receive heparin).2 Eighty-six patients in 60 facilities received >15,000 units of heparin in one session, which we felt was implausible, not representative of the general dialysis population, and possibly the result of errors in data entry. Thus, we excluded all 1194 patients who dialyzed at these 60 facilities. After excluding an additional 671 patients with missing data, our final cohort included 17,722 patients from 1366 facilities.
Figure 1.
Study population selection from the United States Renal Data System. We selected a cohort of patients 67 years of age or older whose primary payer was Medicare who underwent hemodialysis with heparin at a participating facility on April 9 or 10, 2008. HD=hemodialysis.
Heparin Dose
We analyzed the total dose of heparin delivered during the index dialysis session, excluding hemodialysis catheter locks. We abstracted these data from the EMR.
Other Variables
Demographics, comorbidities, vital signs, laboratory measurements, dialysis characteristics, and facility factors were considered potential correlates of heparin dose. All variables are listed in Table 2.
Table 2.
Associations of variables with heparin dose for maintenance hemodialysis in older U.S. patients expressed as changes in heparin dose from a multivariable mixed effects model with random intercept for facility.1 N=17,722
| Variable | Coefficient estimate (units of heparin) | 95% Confidence interval (units of heparin) |
|---|---|---|
| Patient characteristics | ||
| Demographics | ||
| Age (per year) | −20 | (−25, −14) |
| Male (vs. female) | 145 | (80, 209) |
| Race | ||
| Black | 103 | (20, 185) |
| White | Reference | Reference |
| Other | −76 | (−224, 72) |
| Hispanic ethnicity | 79 | (−75, 155) |
| Reported comorbidities | ||
| Arrhythmia | −109 | (−176, −42) |
| Cancer | 10 | (−76, 95) |
| Coronary artery disease | −94 | (−158, −30) |
| Deep vein thrombosis | 25 | (−81, 130) |
| Diabetes mellitus | 9 | (−54, 72) |
| Gastrointestinal bleeding | −244 | (−347, −140) |
| Heart failure | −84 | (−145, −22) |
| Hemorrhagic stroke | −214 | (−528, 101) |
| Ischemic stroke | −87 | (−165, −9) |
| Liver disease | −111 | (−256, 34) |
| Peripheral vascular disease | −4 | (−67, 58) |
| Pulmonary disease | 92 | (22, 163) |
| Pulmonary embolism | −131 | (−390, 127) |
| Warfarin use | −288 | (−457, −118) |
| Vital Signs and laboratory measurements | ||
| Weight (kg) by quartile | P for trend<0.001 | |
| <61 | Reference | Reference |
| 61–71.4 | 385 | (300, 470) |
| 71.5–83 | 678 | (588, 768) |
| >83 | 1430 | (1332, 1527) |
| Pre-dialysis systolic blood pressure (mmHg) | P for trend=0.13 | |
| <120 | 84 | (−11, 178) |
| 120–139 | Reference | Reference |
| 140–159 | −20 | (−99, 58) |
| ≥160 | −10 | (−89, 69) |
| Hemoglobin (g/dL) | P for trend=0.001 | |
| <9 | −375 | (−621, −129) |
| 9–9.9 | −142 | (−292, 8) |
| 10–10.9 | −122 | (−215, −28) |
| 11–11.9 | Reference | Reference |
| 12–12.9 | 37 | (−35, 110) |
| 13–13.9 | 77 | (−20, 174) |
| ≥14 | 285 | (128, 442) |
| Platelets (x103/μL) | P for trend<0.001 | |
| <150 | −513 | (−613, −413) |
| 150–229 | −225 | (−294, −157) |
| 230–310 | Reference | Reference |
| 311–400 | 137 | (39, 234) |
| >400 | 188 | (36, 341) |
| Albumin (g/dL) | P for trend=0.39 | |
| <2.5 | 1 | (−288, 291) |
| 2.5–2.9 | 51 | (−103, 204) |
| 3–3.4 | Reference | Reference |
| 3.5–3.9 | −8 | (−89, 73) |
| ≥4 | −80 | (−171, 12) |
| Dialysis characteristics | ||
| Vascular access used during index session | ||
| Fistula or graft | Reference | Reference |
| Central venous catheter | 1391 | (1321, 1461) |
| Length of session (minutes) | P for trend<0.001 | |
| <180 | −712 | (−876, −548) |
| 180–194 | −278 | (−389, −168) |
| 195–209 | Reference | Reference |
| 210–224 | 301 | (196, 406) |
| 225–239 | 615 | (482, 747) |
| ≥240 | 1068 | (951, 1186) |
| Reuse of dialysis filter | 502 | (419, 585) |
| Years on dialysis (per year) | 1 | (−11, 9) |
| Facility characteristics | ||
| Number of outpatient hemodialysis patients (by quartile)2 | P for trend=0.04 | |
| <54 | Reference | Reference |
| 54–74 | −182 | (−351, −14) |
| 75–105 | −164 | (−348, 21) |
| ≥106 | −141 | (−350, 67) |
| Urban (vs. rural)3 | −276 | (−442, −111) |
| Census division4 | Pwald<0.001 | |
| East North Central | 848 | (426, 1270) |
| East South Central | 1440 | (973, 1908) |
| Middle Atlantic | 86 | (−348, 520) |
| Mountain | 840 | (374, 1307) |
| New England | Reference | Reference |
| Pacific | 780 | (360, 1200) |
| South Atlantic | 792 | (391, 1194) |
| West North Central | 1357 | (902, 1812) |
| West South Central | 923 | (499, 1346) |
All of the variables listed were included in the model. P for trend was calculated treating the categorical variable as an ordinal variable.
Reflects the number of outpatient hemodialysis patients treated at a facility at the end of 2008, per the ESRD Facility Survey (form CMS-2744).
Facilities were considered urban if they were classified as a metropolitan area in the Rural-Urban Commuting Area (RUCA) Codes version 2.0, which are based on 2000 Census commuting data and 2004 zip codes; all other areas were considered to be rural.
Facilities were categorized into one of nine U.S. Census Bureau Divisions based on their state.31
Demographic factors were obtained from the USRDS. Comorbidity status was derived from both the Medical Evidence Report (CMS form 2728) and Medicare claims data predating the index date by up to one year. The comorbidity definitions, which were derived from both previously validated algorithms and a comprehensive search of ICD-9 diagnosis codes, are provided in Supplementary Table 1. Comorbidities were assigned if coded in at least one inpatient claim or two outpatient claims more than 30 days apart in the year prior to the index date.6 Vital signs, laboratory measurements, and dialysis characteristics were abstracted from the EMR. Facility factors were taken from USRDS (see Table 2 footnotes for more detail). Variables were categorized into clinically relevant ranges, except for weight and facility size, which were categorized by quartile.
Statistical Analysis
We described baseline characteristics of the cohort using means and standard deviations for normally distributed continuous data, medians and 25th and 75th percentile values for non-normally distributed data, and counts and proportions for categorical data.
We performed unadjusted and adjusted analyses of potential correlates of heparin dose. For the multivariable model, we included all of the 59 variables previously described and listed in Table 2. All statistical tests were two-sided and conducted at the 0.05 level of significance.
We used a two-level mixed effects linear regression model with random intercept for facility where patients (level 1) were nested within facilities (level 2, see appendix for more detail). We fitted two models: 1) adjusting for patient characteristics only; and 2) adjusting for patient and facility characteristics (full model). Using the mixed effects model described above, we conducted three variance decomposition analyses. First, using the full model, we separated the total variance in the population into variance between facilities and variance between patients. We used the intraclass correlation coefficient (ICC) to characterize the proportion of total variance due to between facilities differences. Next, using both fitted models, we estimated the amount of patient-level variance explained by fixed patient and facility characteristics (patient-level R2). Finally, we estimated the amount of facility-level variance explained by the fitted models (facility-level R2, see appendix for more detail).7 We computed 95% percentile bootstrap confidence intervals (CI) for all three statistics: ICC, patient-level R2, and facility-level R2 based on 1999 bootstrap samples.8 We used non-parametric sampling to sample with replacement from the facilities and then included all patients for each facility selected (multiple times if a facility was selected more than once).9
We also performed a sensitivity analysis in a population of patients aged 67 years and older who initiated maintenance HD from 2007–2008. We analyzed the dose of heparin received during the HD session on day 90 (±10) after the initiation of dialysis to see if correlates of heparin dose differed in incident (sensitivity analysis) vs. prevalent (main analysis) patients. Day 90 was selected as any personalization (fine tuning) of the heparin dose would likely be completed by then.
Additional sensitivity analyses included: 1) an analysis based on the square root of the heparin dose, as this normalized the distribution of doses, 2) an analysis restricted to non-warfarin users as use of anticoagulants may substantially change results, and 3) an analysis where the dose was adjusted for the weight of the patient (i.e. Units/kg vs. units) as dosing for weight is a common practice.
Model selection was not performed for any of the analyses; variables were chosen a priori as potentially clinically relevant factors, rather than based on statistical significance. All analyses were performed using SAS Enterprise Guide 4.3 (SAS Institute Inc., Cary, NC). This study was approved by the Institutional Review Board of Stanford University.
Results
Patient Characteristics
Characteristics of the cohort are shown in Table 1. The mean age of this older cohort was 76 years, and almost a third was of black race. They had a high prevalence of comorbidities, with over half diagnosed with diabetes or heart failure, and over a third with coronary artery disease or peripheral vascular disease. Few patients had a history of previous bleeding (e.g., 9% had a history of gastrointestinal bleeding, 1% had suffered a hemorrhagic stroke), or a disorder associated with bleeding risk (e.g., 4% had liver disease). By contrast, disorders linked to thromboembolisms were more common: 27% had a history of arrhythmia, 13% had cancer, and 18% had a history of ischemic stroke, though only 9% had a history of a deep vein thrombosis and 1% had experienced a pulmonary embolism. The average vital signs and laboratory measurements were within normal limits, and the median time since first initiation of dialysis was 2.3 years. Less than a third used a central venous catheter for vascular access, but nearly two-thirds reused their dialyzers. The vast majority dialyzed in urban facilities, and every census division was represented.
Table 1.
Characteristics of older U.S. patients receiving maintenance hemodialysis with heparin on April 9 or 10, 2008.1
| Variable | |
|---|---|
| Patient Characteristics | N = 17,722 |
| Demographics | |
| Age (yr), mean ± SD | 76±6 |
| Male sex, N (%) | 9087 (51) |
| Race, N (%) | |
| Black | 5323 (30) |
| White | 11,354 (64) |
| Other | 1045 (6) |
| Hispanic ethnicity, N (%) | 2004 (11) |
| Reported comorbidities, N (%) | |
| Arrhythmia | 4803 (27) |
| Cancer | 2392 (13) |
| Coronary artery disease | 6576 (37) |
| Deep vein thrombosis | 1513 (9) |
| Diabetes mellitus | 10,593 (60) |
| Gastrointestinal bleeding | 1564 (9) |
| Heart failure | 9368 (53) |
| Hemorrhagic stroke | 152 (1) |
| Ischemic stroke | 3111 (18) |
| Liver disease | 765 (4) |
| Peripheral vascular disease | 6680 (38) |
| Pulmonary disease | 4178 (24) |
| Pulmonary embolism | 226 (1) |
| Warfarin use | 539 (3) |
| Vital signs and laboratory measurements | |
| Weight (kg), mean ± SD | 74 ± 17 |
| Pre-dialysis systolic blood pressure (mmHg), mean ± SD | 147 ± 26 |
| Hemoglobin (g/dL), mean ± SD | 11.9 ± 1.2 |
| Platelet count (x103/μL), median (25th–75th percentile) | 226 (180–281) |
| Albumin (g/dL), mean ± SD | 3.7 ± 0.4 |
| Kt/V2 | 1.7 ± 0.3 |
| Dialysis characteristics | |
| Vascular access, N (%) | |
| Central venous catheter | 4760 (27) |
| Fistula or graft | 12,963 (73) |
| Length of session (minutes), mean ± SD | 209 ± 26 |
| Reused dialyzer, N (%) | 11,365 (64) |
| Years on dialysis, median (25th–75th percentile) | 2.3 (1.1–4.3) |
| Facility characteristics | |
| Number of outpatient hemodialysis patients3, median (25th–75th percentile) | 76 (54–107) |
| Rural4, N (%) | 3343 (19) |
| Census division5, N (%) | |
| East North Central | 2505 (14) |
| East South Central | 920 (5) |
| Middle Atlantic | 1969 (11) |
| Mountain | 1018 (6) |
| New England | 700 (4) |
| Pacific | 2599 (15) |
| South Atlantic | 4848 (27) |
| West North Central | 1044 (6) |
| West South Central | 2119 (12) |
SD=Standard deviation
Variables are described using means and standard deviations for normally distributed continuous data, medians and 25th and 75th percentile values for non-normally distributed data, and counts and proportions for categorical data.
Kt/V is a measure of the adequacy of dialysis treatment. The National Kidney Foundation recommends that the Kt/V target for hemodialysis be ≥1.2.
Reflects the number of outpatient hemodialysis patients treated at a facility at the end of 2008, per the ESRD Facility Survey (form CMS-2744).
Facilities were considered urban if they were classified as a metropolitan area in the Rural-Urban Commuting Area (RUCA) Codes version 2.0, which are based on 2000 Census commuting data and 2004 zip codes; all other areas were considered to be rural.
Facilities were categorized into one of nine U.S. Census Bureau Divisions based on their state.31
Correlates of Heparin Dose
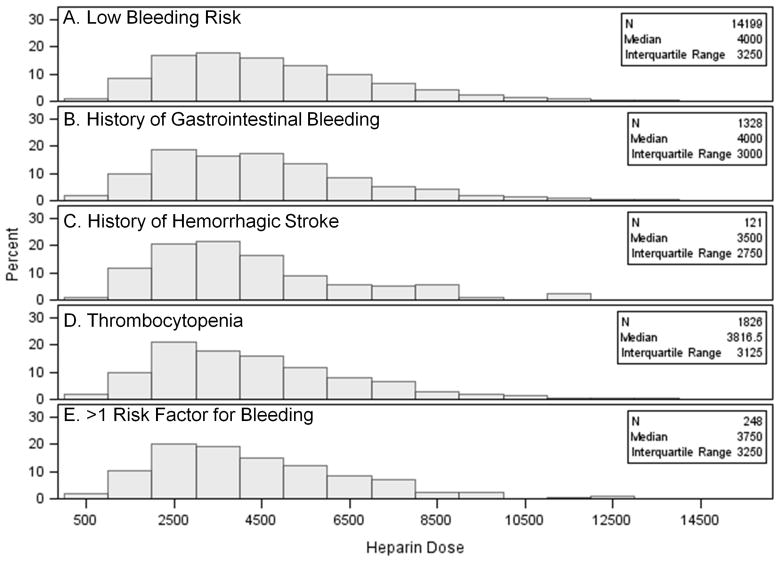
The median heparin dose in the full cohort was 4000 units, and the 25th and 75th percentile doses were 2625 and 6000 units, respectively. The distribution was similar in subgroups with a low risk of bleeding and those with a high risk of bleeding (Figure 2).
Figure 2.
Distribution of heparin dose in subgroups with different risk factors for bleeding: Panel A) patients with low bleeding risk (no history of gastrointestinal bleeding, hemorrhagic stroke, or thrombocytopenia); Panel B) patients with only a history of gastrointestinal bleeding; Panel C) patients with only a history of hemorrhagic stroke; Panel D) patients with only thrombocytopenia (platelet count <150,000/μL within 90 days of heparin administration); Panel E) Patients with more than one risk factor for bleeding. Subgroups are mutually exclusive.
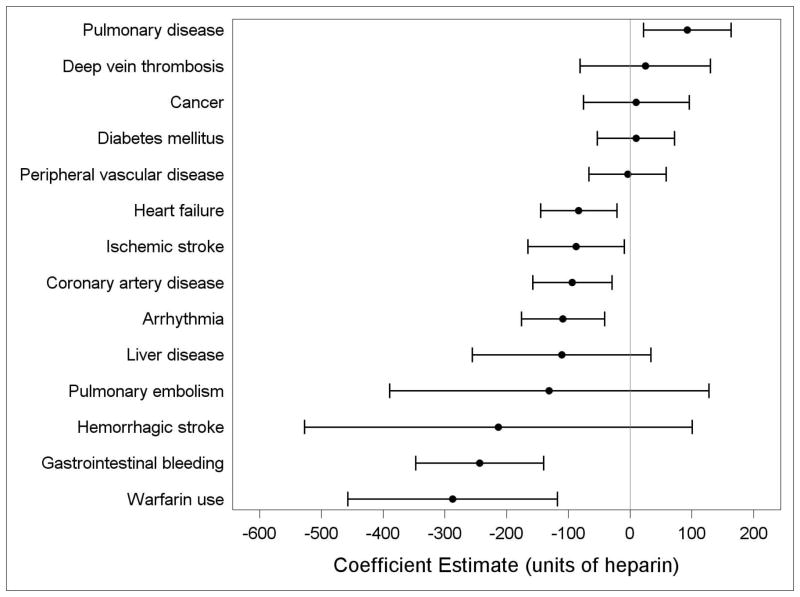
The results of the multivariable analysis of potential correlates of heparin dose are shown in Table 2. Younger, male, and black patients tended to receive higher doses of heparin. In general, patients with comorbid conditions received lower doses of heparin (Figure 3).
Figure 3.
Association of comorbidities with heparin dose in maintenance hemodialysis in older U.S. patients (coefficient and 95% confidence intervals) estimated from a multivariable mixed effects model for heparin dose with a random intercept for facility.
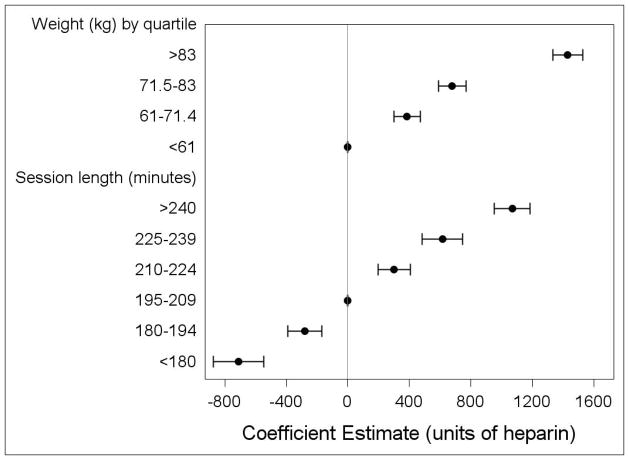
There was a clear pattern of weight-based dosing, with heavier patients receiving up to 1430 more units of heparin than lighter patients, depending on their quartile of weight (Figure 4). Similarly, those with higher hemoglobin and platelet levels received higher doses of heparin than those with lower values.
Figure 4.
Association of weight and time with heparin dose in maintenance hemodialysis in older U.S. patients (coefficient and 95% confidence intervals) estimated from a multivariable mixed effects model for heparin dose with a random intercept for facility. P for trend<0.001 for both variables.
With the exception of time since first dialysis, each dialysis characteristic was significantly linked to heparin dose. One of the strongest correlates was treatment duration: heparin dose varied by up to 1780 units based on the length of the session, with longer sessions associated with higher doses (Figure 4). Vascular access was a determinant of dose as well, with patients using central venous catheters receiving significantly more (1391 units) heparin than those with arteriovenous fistulas or grafts. Similarly, patients who reused their dialysis filters used higher doses of heparin.
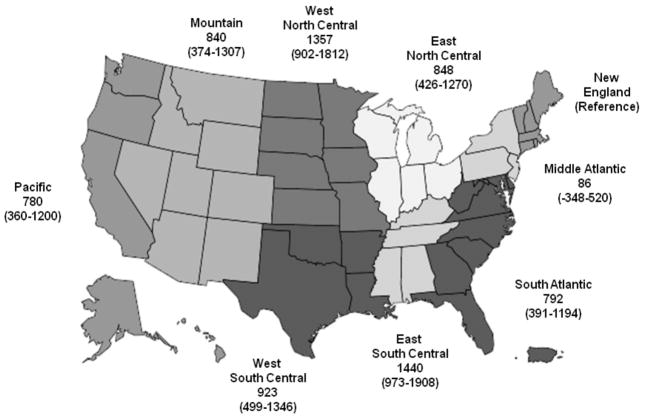
Heparin dose also correlated with facility characteristics. Urban facilities tended to use less than rural units. There was a considerable range in heparin dose by census division, up to 1440 units, with the lowest doses associated with the East Coast divisions of New England and Middle Atlantic, and the highest doses concentrated in the middle of the country, in the West North Central and East South Central divisions (Figure 5).
Figure 5.
Association of Census Bureau Divisions with heparin dose (in units) in maintenance hemodialysis in older U.S. patients estimated (coefficient estimate and 95% confidence intervals) from a multivariable mixed effects model for heparin dose with a random intercept for facility.
Variance in Heparin Dose
We found that 21% (95% CI: 19%–23%) of the total variance in heparin dose (the ICC) was due to between-facility differences, after adjusting for the case-mix, geography, size and rurality of the facility. Most of the variance, 79% (95% CI: 77%–81%), was at the patient level.
At the patient level, all patient characteristics, including demographics, comorbidities, vital signs, lab values, and dialysis characteristics, jointly explained 23% (95% CI: 22%–25%) of the variance in heparin dose between patients (as represented by the patient-level R2 for the model adjusted for patient characteristics, Table 3). Facility characteristics, such as the geography, size, and rurality of the unit where the patient dialyzes, only explained an additional 2% of this variance, for a total of 25% (95% CI: 24%–27%) variance explained by patient and facility factors.
Table 3.
Sources of variance in heparin dose for maintenance hemodialysis in older U.S. patients.
| Model |
Patient level R-squared: % variance at patient level explained by the model (95% confidence interval) |
Facility level R-squared: % variance at facility level explained by the model (95% confidence interval) |
|---|---|---|
| Adjusted for patient characteristics only1 | 23% (22%–25%) | 21% (18%–24%) |
| Adjusted for patient + facility characteristics2 | 25% (24%–27%) | 27% (24%–31%) |
Patient characteristics included age, sex, race, ethnicity, comorbidities, weight, blood pressure, hemoglobin, platelet count, albumin, vascular access, length of session, reuse status of the dialyzer, and years on dialysis.
Facility characteristics included number of outpatient hemodialysis patients, by quartile, urban/rural status, and census division.
Similarly, patient characteristics (i.e. the case mix of a facility), only explained 21% (95% CI: 18%–24%) of the variance in heparin dose between facilities (as represented by the facility-level R2 for the model adjusted for patient characteristics only, Table 3). Adding facility characteristics to the model explained an additional 6%.
In other words, in models of variance at both patient and facility levels, all of the patient and facility characteristics could only account for about a quarter of the variance in heparin dose; the vast majority (73–75%) of the variance was unexplained.
Sensitivity Analyses
We performed the same analysis on a cohort of 5,502 older patients who initiated maintenance hemodialysis at one of the participating facilities from 2007–2008, using the heparin dose given on day 90 (or closest dialysis session) after they started dialysis.
The median heparin dose was 4400 units (interquartile range: 3000–6000 units). The correlates of heparin dose were largely consistent with the primary analysis (Supplementary Table 2). In contrast to the primary analysis, where many of the comorbidities were significantly linked to heparin dose, only a history of ischemic stroke and warfarin use were associated with lower doses of heparin in the incident cohort. Despite a much higher prevalence of catheter use in this incident population (70% vs. 27%), catheter use remained associated with much higher doses of heparin (1006 units). We continued to observe a pattern of weight- and session length-dependent dosing and geographic variability as well.
The sources of variance in heparin dose were virtually identical to those in the primary analysis (Supplementary Table 3). Seventy-nine percent of the variance in the incident cohort was due to between-patient (vs. between-facility) differences. As in the primary analysis, all of the patient and facility characteristics could still only explain roughly a quarter of the variance in heparin dose between patients and between facilities.
The results for the sensitivity analyses where we analyzed the variance of the square root of the heparin dose and where we restricted the cohort to non-warfarin users were the same as the main analysis (Supplementary Tables 4 and 5). Furthermore, all of the associations that were significant in the main analysis were also significant in the subgroup of non-warfarin users, with the exception of a history of ischemic stroke (results not shown).
The variance decomposition of heparin dose adjusted for weight is shown in Supplementary Table 6. As expected, the amount of variance explained by the model was lower than that in the main analysis, because the sensitivity analysis did not include weight as a variable in the model. The associations between variables and heparin dose did not materially change between the main analysis and this sensitivity analysis (results not shown).
Discussion
Despite the lack of national standards for heparin administration in maintenance HD, we still observed clear patterns in heparin dosing. Most were related to patients’ clinical characteristics. For instance, two of the strongest correlates of dose were a patient’s weight and the duration of her or his dialysis session. Although American dialysis units commonly adjust heparin doses empirically based on clinical signs of over- and under-anticoagulation, a variety of dosing schedules exist to guide baseline prescriptions.4 While some protocols propose fixed doses for every patient, sliding scales based on weight and time are common because the drug’s effectiveness theoretically differs based on these two parameters.4,10–15 While neither approach has been proven to be superior, judging from our results, most clinicians are using sliding scales.12–15
We discovered a sizeable increase in heparin dose, 1391 units, for patients using a central venous catheter as their vascular access (vs. fistula or graft). This was analogous to a study on heparin-free HD that found that patients using catheters were more likely to receive HD with heparin compared to patients using peripheral accesses.2 Clotting of the dialysis circuit should prompt physicians to increase the heparin dose, since the goal of heparin administration is to prevent thrombosis. Reduced blood flow and recirculation can both lead to increased clotting.16 These complications are more common with catheter use than with peripheral accesses, which might account for the clinically justifiable difference in dose by access type.17
We also found that conditions associated with an increased risk of bleeding, such as a history of gastrointestinal bleeding, warfarin use, and low hemoglobin concentration and platelet count, correlated with lower heparin doses. Warfarin use in particular has been associated with a three-fold increase in the risk of hemorrhage in patients on HD and a doubling of the risk for hemorrhagic stroke in older HD patients with atrial fibrillation, compared to non-users.18–20 Our results mirror those from a study of heparin-free HD which showed that patients who shared these same pro-hemorrhagic conditions were more likely to dialyze heparin-free compared to patients without these comorbidities.2 Both studies suggest that physicians are taking their patients’ histories into account when prescribing heparin, lowering (or in the heparin-free HD study, eliminating) the dose of heparin in high-risk patients to mitigate the risk of hemorrhage.
Why, then, in the sensitivity analysis, did we not observe lower doses in incident patients with a predisposition to bleeding? It is possible that for incident patients, comorbidities only influence a physician’s decision to withhold heparin, but not to adjust the initial dose. If patients later bleed on that dose, the physician may, in response, lower the dose of these prevalent patients. Again, this suggests a patient-centered decision-making process.
Not all correlates of heparin dose were unequivocally aligned with patient safety. The positive association between heparin dose and reuse of the dialysis filter is one example. According to the 2006 National Kidney Foundation Dialysis Outcomes Quality Initiative (K/DOQI) guidelines, dialyzers should not be reused once the blood compartment volume has dropped to less than 80% of the original volume.21,22 Thus, there is more motivation to minimize clotting, which reduces dialyzer volume, in patients who reuse their dialyzers, presumably by increasing the dose of heparin. Other studies have come to the same conclusion.23–25 However, although reuse may be cost effective and environmentally responsible, it is debatable whether it represents the best care for patients as it exposes them to more heparin than they would have received using single use dialyzers, without a clear clinical benefit.26
Similarly, our study confirmed that there is a great deal of geographic variation in heparin dose. This is consistent with multiple other studies that have shown regional variation in the care of dialysis patients independent of patient characteristics, including vascular access placement, modality selection, anemia management, and end-of-life care.27–30 In particular, our results reflect similar geographic patterns previously observed with the use of heparin-free HD.2 The Northeast and Mid-Atlantic census divisions were the most conservative; patients in these regions were the least likely to dialyze with heparin, and those that did received lower doses than otherwise similar patients in other divisions. On the other end of the spectrum, patients in the West North Central, West South Central, and East South Central divisions were more likely to receive heparin with HD and at higher doses. Notably, our results were independent of (measured) potential confounders such as patient demographics, insurance type, and size and rurality of the dialysis units, and strongly suggest that regional differences in physician preferences, and not just the clinical needs of patients, are at play.
Many dialysis units use protocols for heparin administration, but these protocols vary by facility. Thus, we had expected that heparin dose would vary more by facility than by patient. While there was a substantial facility effect on heparin dose (between-facility differences accounted for 21% of the variance in heparin dose), the majority of variance was due to patient level differences. In other words, when it comes to heparin dose, who you are (patient level differences) matters more than where you receive your dialysis treatment (facility level differences). At first glance, this seems encouraging: practitioners are not solely relying on facility-specific protocols to dose heparin.
However, differences in heparin dose were only partially explained by case-mix and not well explained by the geographic location, size, or rurality of the dialysis units. So, while a patient’s heparin dose might not be primarily determined by facility-specific practices, neither is it being principally driven by a patient’s clinical characteristics. We could not adjust for a number of potentially influential factors, including the use of anti-platelet medications, a history of clotting of the dialysis circuit, or a history of bleeding at the access site. Still, 75% is a high percentage of variance in dose to remain unexplained, suggesting a high degree of randomness in heparin dosing practices.
The limited role that clinical judgment plays in determining dose is further reflected in the wide variation in heparin dose that exists even in subgroups with a high risk of bleeding. For instance, most practitioners would agree that patients with a history of a serious bleeding event such as a hemorrhagic stroke should receive a much lower dose of heparin than those with a low risk of bleeding. Yet, the distribution of heparin dose was markedly similar in these two groups. Even if a patient with a high bleeding risk were clotting his circuit on a moderate dose of heparin, practitioners have the option of using intermittent saline flushes instead of increasing the heparin dose to potentially unsafe levels. Patient-centered care would dictate that this alternative, though more labor and cost intensive, should be chosen because it represents the best interest of the patient. There is clearly room for more judicious use of heparin in these high-risk patients.
Our study has some limitations. We were unable to adjust for several potential correlates of heparin use, including the use of anti-platelet medications, previous clotting of the dialysis circuit, and the existence of facility-specific protocol for heparin administration and rationale for deviation from such a protocol. We ascertained comorbidities from administrative data so were unable to determine the severity of the conditions. Our population was also restricted to an older Medicare population dialyzing with a specific provider, which potentially limits the generalizability of the results and may underestimate the total variance in prescribing practices occurring nationwide.
However, the study has several strengths. We used a large contemporary national database with an unusual amount of detail, incorporating comorbidities, recent vital signs, laboratory measurements, dialysis characteristics, as well as facility-level factors. We are also not aware of any other study on heparin dose performed on a national scale.
Our study showed that there is a fair amount of variance in heparin dose. While some can be explained by a patient’s clinical characteristics, most of it remains unexplained, suggesting there is room for improvement in dosing heparin for the patient’s benefit.
Supplementary Material
Key points.
Considerable variance exists in the dosing of heparin for maintenance hemodialysis.
Some of the variance can be explained by clinically relevant patient characteristics such as a history of bleeding disorders.
A large percentage (75%) of the variance is unexplained by either patient or facility characteristics.
Factors other than clinical need are driving heparin dosing, suggesting that there is likely room for more judicious dosing of heparin.
Acknowledgments
This study was conducted under a data use agreement between the NIDDK and Dr. Winkelmayer. The manuscript was reviewed by an NIDDK officer and approved for publication. The data reported here have been supplied by the United States Renal Data System and by DaVita, Inc. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation.
Footnotes
Conflict of Interest Statement: The authors have no pertinent conflicts of interest to disclose. The study sponsors had no involvement in the study design, in the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the report for publication.
Prior Presentation: Portions of this manuscript were presented at a poster session at the National Kidney Foundation Spring Clinical Meetings in April 2013 in Orlando, FL.
Disclosure of Funding: This work was supported by a 2010 American Kidney Fund-Amgen Clinical Scientist in Nephrology Fellowship and grant F32DK096765 from the National Institute for Diabetes and Digestive and kidney Diseases to Dr. Shen.
References
- 1.Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: still the best option. Semin Dial. 2010 Sep-Oct;23(5):510–515. doi: 10.1111/j.1525-139X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen JI, Mitani AA, Chang TI, Winkelmayer WC. Use and safety of heparin-free maintenance hemodialysis in the USA. Nephrol Dial Transplant. 2013 Apr 5; doi: 10.1093/ndt/gft067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol. 2012 Mar;23(3):495–506. doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen JI, Winkelmayer WC. Use and safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis. 2012 Sep;60(3):473–486. doi: 10.1053/j.ajkd.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz HM. Variations in health care, patient preferences, and high-quality decision making. JAMA. 2013 Jul 10;310(2):151–152. doi: 10.1001/jama.2013.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in Acute Nonvariceal Upper Gastrointestinal Bleeding in Dialysis Patients. J Am Soc Nephrol. 2012 Jan 19; doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snijders TAB, Bosker RJ. Modeled Variance in 2-Level Models. Sociol Method Res. 1994 Feb;22(3):342–363. [Google Scholar]
- 8.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Statistics in medicine. 2000 May 15;19(9):1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge; New York, NY, USA: Cambridge University Press; 1997. [Google Scholar]
- 10.Brunet P, Simon N, Opris A, et al. Pharmacodynamics of unfractionated heparin during and after a hemodialysis session. Am J Kidney Dis. 2008 May;51(5):789–795. doi: 10.1053/j.ajkd.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Mingardi G, Perico N, Pusineri F, et al. Heparin for hemodialysis: practical guidelines for administration and monitoring. Int J Artif Organs. 1984 Sep;7(5):269–274. [PubMed] [Google Scholar]
- 12.Wilhelmsson S, Lins LE. Heparin elimination and hemostasis in hemodialysis. Clin Nephrol. 1984 Dec;22(6):303–306. [PubMed] [Google Scholar]
- 13.Ward RA, Farrell PC. Precise anticoagulation for routine hemodialysis using nomograms. Trans Am Soc Artif Intern Organs. 1978;24:439–442. [PubMed] [Google Scholar]
- 14.Low CL, Bailie G, Morgan S, Eisele G. Effect of a sliding scale protocol for heparin on the ability to maintain whole blood activated partial thromboplastin times within a desired range in hemodialysis patients. Clin Nephrol. 1996 Feb;45(2):120–124. [PubMed] [Google Scholar]
- 15.Kandrotas RJ, Gal P, Douglas JB, Deterding J. Pharmacokinetics and pharmacodynamics of heparin during hemodialysis: interpatient and intrapatient variability. Pharmacotherapy. 1990;10(5):349–355. [PubMed] [Google Scholar]
- 16.Peter Gerard Blake JTD, Ing Todd S. Handdbook of Dialysis. 4. Philadelphia: Lippincott Williams & Wilkins; 2007. Lippincott Williams & Wilkins handbook 2007. [Google Scholar]
- 17.Besarab A, Pandey R. Catheter management in hemodialysis patients: delivering adequate flow. Clin J Am Soc Nephrol. 2011 Jan;6(1):227–234. doi: 10.2215/CJN.04840610. [DOI] [PubMed] [Google Scholar]
- 18.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009 Oct;20(10):2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011 Nov;6(11):2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010 Jun;77(12):1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 21.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006 Jul;48(Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Gotch FA. Mass transport in reused dialyzers. Proc Clin Dial Transplant Forum. 1980;10:81–85. [PubMed] [Google Scholar]
- 23.Sievers SG, Stack JL, Piering WF, Cohen EP. Determinants of dialyzer reuseability. ASAIO Trans. 1991 Jul-Sep;37(3):M185–186. [PubMed] [Google Scholar]
- 24.Ouseph R, Brier ME, Ward RA. Improved dialyzer reuse after use of a population pharmacodynamic model to determine heparin doses. Am J Kidney Dis. 2000 Jan;35(1):89–94. doi: 10.1016/S0272-6386(00)70306-4. [DOI] [PubMed] [Google Scholar]
- 25.Mitwalli AH, Abed J, Tarif N, et al. Dialyzer reuse impact on dialyzer efficiency, patient morbidity and mortality and cost effectiveness. Saudi J Kidney Dis Transpl. 2001 Jul-Sep;12(3):305–311. [PubMed] [Google Scholar]
- 26.Twardowski ZJ. Dialyzer reuse--part II: advantages and disadvantages. Semin Dial. 2006 May-Jun;19(3):217–226. doi: 10.1111/j.1525-139X.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirth RA, Turenne MN, Woods JD, et al. Predictors of type of vascular access in hemodialysis patients. JAMA. 1996 Oct 23–30;276(16):1303–1308. [PubMed] [Google Scholar]
- 28.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002 Aug;13(8):2117–2124. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 29.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol. 2002 May;13(5):1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 30.Reddan DN, Frankenfield DL, Klassen PS, et al. Regional variability in anaemia management and haemoglobin in the US. Nephrol Dial Transplant. 2003 Jan;18(1):147–152. doi: 10.1093/ndt/18.1.147. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau GD. [Accessed January 5, 2012, 2012.];Census Bureau Regions and Divisions with State FIPS Codes. http://www.census.gov/geo/www/reg_div.txt.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.