Abstract
The development of robust neuropsychological measures of social and affective function—which link critical dimensions of mental health to their underlying neural circuitry—could be a key step in achieving a more pathophysiologically-based approach to psychiatric medicine. In this article, we summarize research indicating that self-reflection (the inward attention to personal thoughts, memories, feelings, and actions) may be a useful model for developing such a paradigm, as there is evidence that self-reflection is (1) measurable with self-report scales and performance-based tests, (2) linked to the activity of a specific neural circuit, and (3) dimensionally related to mental health and various forms of psychopathology.
Keywords: self-reflection, psychiatric illness, depression, anxiety, psychopathy, autism, neuropsychology, rest-state functional neuroimaging, medial prefrontal cortex, default mode network
Introduction
A major goal in psychiatric medicine is to develop a system of diagnosis and treatment that is pathophysiologically-based (Insel et al., 2010). At present, psychiatric patient assessments are primarily based on clinical observation of overt behavior and patient self-report, without corresponding evaluation of underlying biological mechanisms of dysfunction. By contrast, other medical specialties routinely use biological and physiological assays to inform diagnosis and treatment. For example, in the field of cardiology, objective measurements of physiological parameters (e.g., pulse, blood pressure, cholesterol levels, EKG) are standard, and the prescribed interventions (e.g., dietary changes, exercise programs, drug regiments, surgical procedures) are tailored to address specific aspects of the underlying pathophysiology.
In psychiatric disorders, the pathophysiology is rooted in the brain. Thus, a key step toward developing a new system of diagnosis and treatment in psychiatry is to identify specific and objectively measurable domains of psychological or behavioral dysfunction that are related to particular aspects of brain structure and/or function. Neuropsychology offers a promising approach in this regard. For certain cognitive functions, extensive neuropsychological batteries of performance-based tests have long been established. For example, in the domain of memory, there are standardized performance-based tests that probe the integrity of specific competencies (e.g., verbal vs. non-verbal, short-term vs. long-term memory). Similar batteries of performance-based psychometric tests have been developed to probe aspects of language, perception and executive function. Performance on these measures has been associated with the integrity of specific neural systems (e.g., the link between declarative memory and the medial temporal lobe), and these brain-behavior links are guiding translational research in memory disorders, such as Alzheimer’s disease.
Although significant advances have been made in our understanding of the neural correlates of affective and social functions, this has not yet translated into an analogous battery of well-validated performance-based clinical measures. Self-reflection is an example of a particular domain of psychological dysfunction that (1) is routinely disrupted in psychiatric illness and cuts across traditional diagnostic categories, (2) is measurable with self-report scales and performance-based tests and (3) can be dimensionally linked to activity within a particular neural circuit. In this article, we review relevant literature, identify outstanding questions, and propose an agenda of research that would support the use of neuropsychological measures of self-reflection combined with neuroimaging to serve as model for establishing a neuro-psycho-pathophysiological basis for mental healthcare.
Self-reflection and psychiatric illness
The ability to self-reflect—to turn our attention inward to consider our own thoughts, memories, feelings, and actions—is a fundamental aspect of human cognition. Interpersonally, self-reflection can help us to perceive social cues (Eisenberger & Lieberman, 2004; Lombardo et al., 2010) and generate social emotions (e.g., guilt) (Beer, Heerey, Keltner, Scabini, & Knight, 2003; Tracy & Robins, 2004), which can promote prosocial behavior and enhance relationships (Baumeister, Stillwell, & Heatherton, 1994; Keltner, 1995). Self-reflection can also contribute to emotion regulation (Leary, 2003), self-awareness (Duval & Wicklund, 1972), and self-insight (Beck, Baruch, Balter, Steer, & Warman, 2004)—processes that are essential for successful psychotherapy outcomes (Mansell, 2011). However, maladaptive levels of self-reflection, such as ruminative, self-critical thought in depression, can have detrimental consequences for health and well-being. In fact, a prominent psychological theory proposes that heightened self-focused attention is a common feature across several mental illnesses (Ingram, 1990). While there is substantial evidence to support heightened self-focus in certain types of mental illness, in this article we suggest that both heightened and diminished self-reflection may contribute to mental illness. Thus, the premise of the present article is that self-reflection may be a critical transdiagnostic dimension of social and affective function in mental illness; whereas moderate levels of self-reflection support normal social and affective functioning, significant deviations in the engagement of self-reflection (either elevated or diminished) may be associated with distinct forms of psychopathology.
Excessive self-reflection in mood and anxiety disorders
Heightened self-reflection is a central feature of mood and anxiety disorders (American Psychiatric Association, 2000; Clark & Wells, 1995; Nolen-Hoeksema, 2000; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Depression and anxiety are associated with pathological levels of rumination and worry. Although rumination and worry are not necessarily or exclusively self-focused in nature, they often consist of perseverative self-focused thoughts including feelings of guilt, self-blame, and negative self-appraisal (Clark et al., 1995; Nolen-Hoeksema et al., 2008). Consistent with these clinical descriptions, numerous empirical studies have found elevated levels of self-focused thought in depression, social anxiety, and social phobia (Ingram, Lumry, Cruet, & Sieber, 1987; Woodruff-Borden, Brothers, & Lister, 2001). For instance, self-report measures of self-reflection have been used to associate depression, social anxiety, and social phobia with heightened self-reflection and self-consciousness (Hope & Heimberg, 1988; Jostes, Pook, & Florin, 1999; Smith, Ingram, & Roth, 1985). Additionally, a sentence completion task has also been used to demonstrate increased self-focused thought in depression and anxiety disorders (Ingram et al., 1987; Woodruff-Borden et al., 2001). Heightened self-reflection is also evident in social situations, where depressed patients have been shown to refer to themselves more frequently during conversation, even when unsolicited (Jacobson & Anderson, 1982). Such self-focused cognition tends to prolong negative affect, predict and maintain psychopathology, and engender social isolation (Just & Alloy, 1997; Mor & Winquist, 2002; Nolen-Hoeksema et al., 2008). For example, in depression, chronic self-focused rumination can lead to diminished social support and perceived social conflict with others (Nolen-Hoeksema et al., 2008). Similarly, self-focused cognition in social anxiety and social phobia has been linked to deficits in social perception (e.g., perceiving neutral facial expressions as critical) (Clark et al., 1995; Smith & Sarason, 1975). In sum, elevated levels of self-reflection in depression and anxiety are evident clinically and experimentally, and appear to be associated with impairments in social and affective functioning and overall morbidity.
Diminished self-reflection in autism and psychopathy
In contrast to mood and anxiety disorders, autism and psychopathy are examples of disorders where levels of self-reflection may be pathologically low. Although autism has been associated with alterations in several different aspects of self-related cognition (i.e., autobiographical memory, emotional awareness) (e.g., Klein, Chan, & Loftus, 1999; Lombardo, Barnes, Wheelwright, & Baron-Cohen, 2007), we focus here on self-reflection. It is also important to note that although autism and psychopathy may share impoverished self-reflection as one dimension of dysfunction, these disorders are vastly different across many other behavioral and psychological domains. In autism, diminished self-reflection is thought to contribute to impairments in empathy, theory of mind, and social communication (Frith, 2003; Lombardo et al., 2007). Consistent with this hypothesis, significantly reduced levels of self-reflection (on both performance-based and self-report measures) have been observed in individuals with autism and Asperger syndrome (Lee & Hobson, 1998; Lee, Hobson, & Chiat, 1994; Lombardo et al., 2007). For example, reduced self-reflection was demonstrated in children and adolescents with autism in the diminished use of first-person pronouns (“I”, “me”, “my”) in an experimental setting, and the use of fewer self-referents in a social context (Lee et al., 1998; Lee et al., 1994). Moreover, diminished self-reflection is predictive of lower empathy and higher autism spectrum quotient scores (Lombardo et al., 2007). Given the clinical heterogeneity across autism spectrum disorders (Georgiades et al., 2013; Pelphrey, Shultz, Hudac, & Vander Wyk, 2011), future research is warranted to investigate self-reflection in ASD in individuals with different levels of functioning. In psychopathy, deficient self-reflection may similarly explain the hallmark affective characteristics of the disorder (callous lack of empathy and guilt), as well as laboratory findings indicating a reduced tendency to pause and consider the negative consequences of their actions (Koenigs, Kruepke, & Newman, 2010; Newman & Lorenz, 2003). Further work is necessary in psychopathy, as few studies have directly examined the relationship between self-reflection and social or affective features of the disorder.
Altered self-reflection in other psychiatric disorders
In addition to the psychiatric illnesses mentioned above, there is evidence for alterations in self-reflection in other psychiatric disorders. Two notable examples are schizophrenia and anorexia nervosa. In schizophrenia, empirical studies have found evidence for both diminished (e.g., Bedford, Surguladze, Giampietro, Brammer, & David, 2012; Holt et al., 2011) and heightened (e.g., Exner, 1973; Morrison & Haddock, 1997; Puentf & Morrisey, 1981) self-reflection. Some researchers have suggested that diminished self-reflection in schizophrenia and other psychotic disorders may contribute to well-known impairments in self-insight, self-monitoring, and self-agency (Beck et al., 2004; Dimaggio, Vanheule, Lysaker, Carcione, & Nicolo, 2009; van der Meer et al., 2012). However, self-reflection cannot fully explain these impairments and symptoms in schizophrenia. For example, other cognitive processes, including executive functions, have been associated with poor self-insight in schizophrenia (Cooke et al., 2010). In anorexia nervosa, similar to depression and anxiety, elevated self-reflection is a common symptom, characterized by increased negative evaluation of one’s own body weight or appearance (American Psychiatric Association, 2000).
Spectrum-based model of self-reflection and well-being
Taken together, these clinical and laboratory observations suggest that different types of psychiatric illness can be associated with either excessive or deficient levels of self-reflection. We thus propose that self-reflection is a psychological process existing on a spectrum that cuts across traditional psychiatric diagnoses. We further suggest that the relationship between health and degree of self-reflection may follow an inverted u-shape, with moderate levels associated with optimal well-being (Fig. 1). Similar relationships are observed across a wide array of biological parameters, including pain, stress, blood pressure, and caloric intake (Calabrese & Mattson, 2011). In this spectrum-based model, we conceive of optimal well-being as a state of overall psychological health and life satisfaction, with the absence of any disruptions of thinking, feeling, mood, ability to relate to others, or daily functioning. At a more basic level, well-being can be operationalized as the absence of any diagnosable psychopathology or mental illness.
Figure 1.
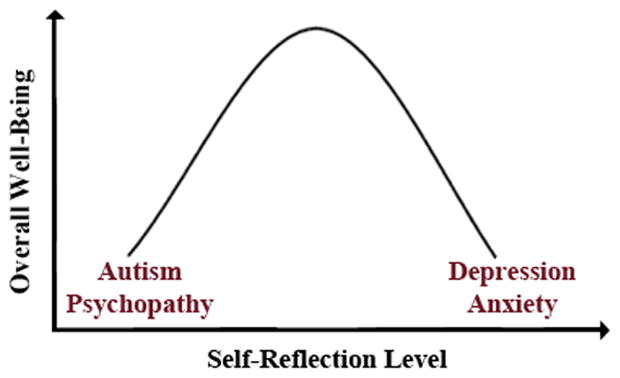
An inverted u-shaped curve illustrates the proposed relationship between self-reflection (x-axis) and overall well-being (y-axis). Both high (depression and anxiety) and low (autism and psychopathy) levels of self-reflection are associated with lower overall well-being.
With this spectrum-based model of self-reflection in psychopathology in mind, we next consider several self-report scales and experimental paradigms that could be useful for developing clinically-relevant self-report and performance-based measures of self-reflection.
Self-report scales of self-reflection
Several self-report scales have been developed to evaluate self-reflection in healthy and clinical populations. Rumination scales have also been designed to capture both adaptive (curiosity about one’s self) and maladaptive (repetitive focus on one’s negative traits) aspects of self-reflection (i.e., Rumination-Reflection Questionnaire and Ruminative Responses Scale) (Trapnell & Campbell, 1999; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). Here, we will focus on two scales that have been used most frequently to measure self-reflection and rumination in clinical populations: the Self-Consciousness Scale Revised (SCSR) and the Ruminative Responses Scale (RRS) (Hope et al., 1988; Joormann, Dkane, & Gotlib, 2006; Jostes et al., 1999; Lombardo et al., 2007; Nolen-Hoeksema et al., 2008; Saboonchi, Lundh, & Öst, 1999; Siegle, Moore, & Thase, 2004). The SCSR is a 22-item questionnaire that includes questions regarding both public (e.g., related to one’s appearance) and private self-consciousness (e.g., related to one’s inner thoughts) (Scheier & Carver, 1985). Some example items include “I’m usually aware of my appearance” and “I think about myself a lot”. The RRS is 22-item questionnaire with items assessing the frequency of self-reflection, brooding, and ruminative thought (Treynor et al., 2003). Example items from the RRS include “Go someplace alone to think about your feelings”, “Think, what am I doing to deserve this”, and “Think about how alone you feel”. The SCSR and RRS have been used to indicate heightened levels of self-reflection and rumination in depression, social anxiety, and social phobia (Hope et al., 1988; Joormann et al., 2006; Jostes et al., 1999; Nolen-Hoeksema et al., 2008; Saboonchi et al., 1999; Siegle et al., 2004). Diminished levels of self-reflection, by contrast, have been found in autism using the SCSR (Lombardo et al., 2007).
Although these self-report questionnaires have provided important insights into the relationship between self-reflection and psychiatric illness, there are some potential limitations to these findings. Compared to performance-based tasks, self-report scales can be less sensitive to changes over time, and potentially inaccurate (Offer, Kaiz, Howard, & Bennett, 2000; Reuben, Siu, & Kimpau, 1992), in particular when applied in clinical populations with reduced insight (Orfei, Robinson, Bria, Caltagirone, & Spalletta, 2008). These limitations in self-report scales highlight the need for complementary, clinically valid performance-based measures of self-reflection.
Performance-based measures of self-reflection
Judgment paradigms requiring explicit self-reflection
Although a variety of paradigms have been developed to explicitly prompt self-reflection, the most commonly used approach involves personality trait judgment (Rogers, Kuiper, & Kirker, 1977). In this task, personality traits are evaluated on the basis of: (i) self-relevance (e.g., “Am I self-disciplined?”), (ii) other-relevance to a familiar person (e.g., “Is my mom generous?”), and (iii) semantic or shallow processing (e.g., “Is this trait desirable/capitalized?”). After a delay, a recognition test is administered where participants are asked to make old/new judgments of presented traits. This task can be used to measure the interaction between self-reflection and memory, termed the “self-reference effect” (SRE), in which traits processed for self-relevance are better remembered than traits processed for other-relevance (Rogers et al., 1977). This task has already been used to demonstrate diminished SRE in autism (Lombardo et al., 2007), but increased memory for negative self-relevant traits in depression (Bradley & Mathews, 1983).
Personality trait judgment tasks have also been used to examine the relationship between self-reflection and emotion. For example, in a task developed by Moran and colleagues (2006), personality traits are judged for self-relevance, while trait valence is manipulated by including both positive and negative traits (i.e., “kind” or “mean”). The interaction between self-relevance and emotion can then be measured by calculating the proportion of traits rated for self-relevance as a function of valence. In this task, psychologically healthy participants tend to show a self-related positivity bias (endorsing more positive than negative traits as self-relevant), whereas depressed patients have exhibited a bias toward negative self-appraisal (Poulsen, Luu, Crane, Quiring, & Tucker, 2009).
Open-ended self-reflection tasks
A distinct, but related, approach is to assess aspects of self-reflection in an open-ended format. One such example is the self-focus sentence completion task (Exner, 1973), in which participants are presented with a number of open-ended sentence stems (e.g., “I think…”, or “My father…”) to complete as they choose. Sentence responses can then be scored for degree of self-focus by independent raters, or by more objective measures such as counting the total number of first-person pronouns, and for valence (positive/negative/neutral). Studies using this task have demonstrated increased self-focus in depression and social phobia (Ingram et al., 1987; Woodruff-Borden et al., 2001), and reduced self-focus in individuals with autism (Lombardo et al., 2007). A second open-ended approach is thought sampling, which has been used to assess the content of thoughts following rumination or worry inductions (McLaughlin, Borkovec, & Sibrava, 2007). In this approach, thought probes are given at unexpected times during an ongoing task and participants are asked to write down whatever is on their mind at that time. These thought samples can then be scored for self-relevance (i.e., first-person pronoun use) and valence (positive/negative/neutral).
In addition to the development of behavioral tests, a neuropsychological approach to self-reflection implores a link between behavior and brain. To this end, we will briefly review research findings that have begun to elucidate the neural circuitry underlying self-reflection.
Neural circuitry associated with self-reflection
Research in cognitive neuroscience has highlighted the role of two key brain areas in self-reflection: the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC) (Fig. 2). Evidence for the importance of these two regions in self-reflection has been derived from behavioral studies of neurological lesion patients and patients with neurodegenerative diseases as well as functional neuroimaging studies of healthy and psychiatric populations.
Figure 2.
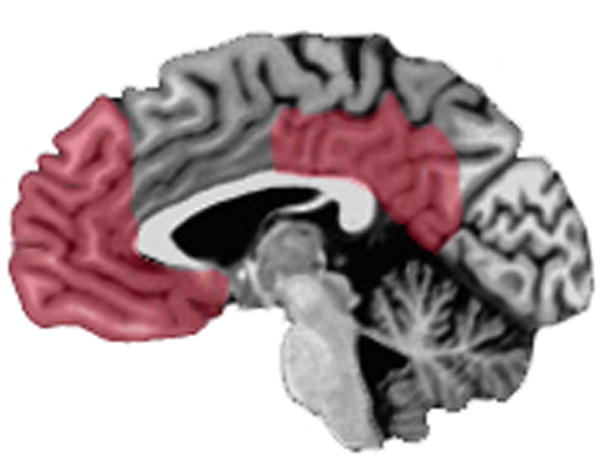
Midsagittal view of the brain depicting components of the default mode network most consistently implicated in self-reflection: the medial prefrontal cortex (mPFC) and posterior cingulate (PCC) (red).
Impaired self-reflection following prefrontal brain injury
Clinical observations from neurological lesion and neurodegenerative cases have documented the critical role of mPFC in self-reflection and self-insight. Deficits in self-reflection in patients with mPFC brain injury are most apparent in social interactions, which often require thinking about how one’s own behavior might affect others. For example, patients with mPFC lesions show diminished empathy, guilt, shame, embarrassment, and social disinhibition (Barrash, Tranel, & Anderson, 2000; Beer et al., 2003; Eslinger & Damasio, 1985). Experimentally, mPFC lesion patients do not exhibit the normal memory advantage for self-related information (SRE) (Philippi, Duff, Denburg, Tranel, & Rudrauf, 2012). Furthermore, damage to ventral mPFC has been shown to lower the risk for developing cognitive and affective symptoms of depression, such as self-dislike, self-criticalness, and feelings of worthlessness (Koenigs et al., 2008). Similarly, frontotemporal dementia (FTD) is associated with degeneration of the mPFC (in addition to temporal and insular regions) (Seeley et al., 2008) and a diminution of self-reflection. In FTD, symptoms of the behavioral variant are partially overlapping with psychopathic traits/behaviors (i.e., lack of empathy, disinhibition and impulsivity), which may be mediated in part by deficits in self-reflection (Rascovsky et al., 2011). Several studies have also associated FTD with a loss of self-processes requiring self-reflection, such as self-conscious emotions (i.e., embarrassment), self-monitoring, self-awareness and self-knowledge of one’s cognitive and behavioral changes (Eslinger et al., 2005; Sturm, Ascher, Miller, & Levenson, 2008).
Together, the neurological lesion and neurodegenerative disorder patient studies suggest that mPFC is a critical neural substrate for self-reflection. These findings are consistent with complementary results from functional brain imaging studies.
The “default mode network” and self-reflection
Functional neuroimaging research has identified a “default mode network” (DMN)—consisting of mPFC, posterior cingulate cortex (PCC), and lateral parietal regions—that is more active at ‘rest’ (during periods of unconstrained thought) than during externally directed cognitive tasks (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001). Functional magnetic resonance imaging (fMRI) studies also show a high degree of correlated activity (“functional connectivity”) within the DMN during rest conditions (Greicius, Krasnow, Reiss, & Menon, 2003). While the precise functional significance of the DMN is not fully understood, there is increasing evidence that the DMN plays an important role in self-reflection. The association between the DMN and self-reflection is based primarily on two lines of evidence. First, during rest conditions subjects frequently report that they are engaged in self-reflection—thinking about themselves, such as past memories and future goals—and self-reports of self-reflection are associated with DMN activity (Andreasen, O’Leary, Cizadlo, Arndt, & et al., 1995; D’Argembeau et al., 2005; Mason et al., 2007; McKiernan, D’Angelo, Kaufman, & Binder, 2006). Second, mPFC and PCC regions of the DMN are consistently activated during a variety of self-referential processing tasks, including the personality trait judgment tasks mentioned earlier (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Qin & Northoff, 2011 for meta-analysis; Whitfield-Gabrieli et al., 2011). Moreover, recent neuroimaging studies have provided further support for the specific role of mPFC and PCC in self-reflection. For example, Whitfield-Gabrieli and colleagues (2011) found that the same ventral mPFC and PCC regions were activated both during rest and self-referential processing task conditions. Similarly, Andrews-Hanna and colleagues (2010) showed that ventral mPFC and PCC regions were commonly recruited when subjects were making self-relevant decisions, regardless of the time period (present or future). Additionally, task-based fMRI studies have shown that activity in the mPFC (Macrae, Moran, Heatherton, Banfield, & Kelley, 2004; Moran et al., 2006) and PCC (Moran et al., 2006) are modulated by the degree of self-relevance (e.g., How relevant is this trait to me?) during personality trait judgment tasks. Similarly, a positron emission tomography (PET) study found that the amount of self-reflection correlated with cerebral metabolism in the mPFC during a personality trait judgment task (D’Argembeau et al., 2005). Besides self-reflection, DMN activity, including lateral temporal and parietal regions, has also been associated with other cognitive processes including prospective memory, episodic memory retrieval, theory of mind, and moral decision making (Andrews-Hanna et al., 2010; Buckner et al., 2008; Greene, Sommerville, Nystrom, Darley, & Cohen, 2001; Spreng & Grady, 2010; Spreng, Mar, & Kim, 2009). Nonetheless, the mPFC and PCC components of the DMN appear to be most reliably linked to self-reflection (e.g., Andrews-Hanna et al., 2010; Whitfield-Gabrieli et al., 2011).
Given these empirical links between self-reflection and the DMN, in particular the mPFC and PCC, we next consider the evidence for DMN dysfunction in psychiatric populations with ostensibly maladaptive levels of self-reflection.
DMN dysfunction in psychopathology and the spectrum-based model of self-reflection
Although aberrant DMN activity and connectivity at rest have been reported across a variety of psychopathological conditions (Broyd et al., 2009; Whitfield-Gabrieli & Ford, 2012), here we will focus on DMN dysfunction in the aforementioned psychiatric illnesses associated with excessive or deficient self-reflection, respectively.
Neuroimaging studies in patients with major depression and anxiety have demonstrated heightened DMN activity and connectivity in both rest-state and task-based neuroimaging. In depression, increased resting metabolism in the ventral mPFC (subgenual ACC) is well-documented (Drevets, Savitz, & Trimble, 2008; Mayberg, 1997), and is a site of deep brain stimulation for the treatment of depression (Mayberg et al., 2005). More recently, evidence of DMN hyperactivity has been found in rest-state fMRI, where increased functional connectivity within the DMN, in particular the ventral mPFC, has been documented in depressed patients (Berman et al., 2011; Greicius et al., 2007; Zhu et al., 2012). Importantly, heightened DMN activity and functional connectivity in depression also correlates with rumination and depression severity (Berman et al., 2011; e.g., Greicius et al., 2007; Hamilton et al., 2011). Task-based fMRI research has also shown reduced DMN task-related suppression in depressed patients during various paradigms involving affective processing (Whitfield-Gabrieli et al., 2012 for review). Relatedly, a task-based fMRI study in patients with anxiety disorders has found diminished task-related suppression of the mPFC component of the DMN in an emotional listening task during the neutral word condition (Zhao et al., 2007). Another fMRI study has shown reduced task-related suppression of the PCC component of the DMN in patients with social phobia during a facial perception task with emotional faces (Gentili et al., 2009). Additionally, increased rest-state functional connectivity of mPFC within the DMN was found in patients with social anxiety disorder (Liao et al., 2010). Given the putative role of the DMN in self-reflection, such elevated DMN activity/connectivity in patients with depression and anxiety, relative to mentally healthy individuals, may be linked to the excessive self-reflection (rumination and worry) characteristic of these disorders.
At the other end of the spectrum, autism and psychopathy have been associated with diminished DMN activity and connectivity. Individuals with autism and autism spectrum conditions have been found to exhibit diminished mPFC activity during a self-reflection task (Lombardo et al., 2010), as well as reduced functional connectivity within the DMN at rest (e.g., Kennedy & Courchesne, 2008; Monk et al., 2009). Similarly, psychopathic prison inmates have demonstrated significantly lower resting-state functional connectivity within the DMN (particularly between the PCC and mPFC) (Motzkin, Newman, Kiehl, & Koenigs, 2011; Pujol et al., 2012). Together, these fMRI findings in autism and psychopathy are largely consistent with the decrements in self-reflection associated with the disorders. Coupled with the aforementioned studies of depression and anxiety, these findings suggest that DMN dysfunction, in particular mPFC and PCC, in psychopathology could serve as a functional neuroimaging biomarker of either excessive or decreased self-reflection.
While we concentrated our review of neuroimaging literature on the psychiatric illnesses mentioned above, emerging fMRI research in patients with schizophrenia has found evidence for both hypoactivity (Bedford et al., 2012; Holt et al., 2011; Liemburg et al., 2012) and hyperactivity (Whitfield-Gabrieli et al., 2012 for review) within the DMN. There seem to be at least two potential explanations for these discrepancies in DMN activity in schizophrenia. First, different methods (e.g., resting-state versus task-based fMRI), tasks, and analytic approaches have been used to examine activity and connectivity within the DMN in schizophrenia. For example, two task-based fMRI studies found hypoactivity within the mPFC during a personality trait judgment task (Bedford et al., 2012; Holt et al., 2011). By contrast, in a different task-based fMRI study decreased deactivation or “hyperactivity” in the mPFC was found during a working memory task (Whitfield-Gabrieli et al., 2009). Thus, due to basic task differences, these results are difficult to compare. However, it appears that there is some consistency in decreased mPFC activity during tasks presumably engaging self-reflection in schizophrenia (e.g., Bedford et al., 2012; Holt et al., 2011). Second, symptom heterogeneity within schizophrenia (Albus, 2012; Sawa & Snyder, 2002) may also contribute to the disparities observed in DMN activity. For instance, differences between severity and presence of positive and negative symptoms could relate to differences in DMN activity. In line with this hypothesis, a resting-state fMRI study showed a correlation between positive symptom severity and DMN connectivity in patients with schizophrenia (Whitfield-Gabrieli et al., 2009). Thus, given the variability in these imaging findings and symptom heterogeneity within schizophrenia (Albus, 2012; Sawa et al., 2002), future work is necessary to examine the precise relationship between different aspects of self-referential processing and the DMN in schizophrenia. For example, studies could examine the relationship between particular symptoms and/or symptom severity and activity within the DMN using both task-based and resting-state fMRI.
From a developmental perspective, it will also be important to consider the potential influence of age on DMN connectivity. For example, rest-state fMRI findings have demonstrated reduced functional connectivity between mPFC and PCC regions of the DMN in children (Fair et al., 2008) and older adults (Andrews-Hanna et al., 2007). However, these studies did not associate DMN activity or connectivity with corresponding measures of self-reflection. Thus, further research will be crucial to determine whether the development/decline of self-reflection is linked with corresponding changes in DMN function in development, aging, and psychopathology.
DMN changes associated with treatment
If DMN dysfunction does play a role in psychopathology, then we would expect successful treatments to be associated with changes in DMN activity. Indeed, recent fMRI work suggests that therapeutic interventions may restore aberrant DMN connectivity in clinical populations (Li et al., 2012; Liston et al., 2014; Posner et al., 2013; Yoshimura et al., 2013). For example, an fMRI study in patients with dysthymia found that pharmacological treatment with antidepressants normalized DMN activity at rest (Posner et al., 2013). Another fMRI study showed that patients with major depression had decreased rest-state functional connectivity within posterior regions of the DMN, including the PCC, after antidepressant treatment (Li et al., 2012). Similarly, a recent fMRI study found that treatment with transcranial magnetic stimulation normalized rest-state DMN connectivity, in particular within the mPFC, in patients with depression (Liston et al., 2014). Additionally, a 12-week cognitive behavioral therapy program for patients with major depression was associated with reduced sgACC activity during processing of negative self-relevant stimuli and increased sgACC activity during processing of positive self-relevant stimuli (Yoshimura et al., 2013). While this early work is promising, more clinical studies are necessary to determine the impact of different types of treatment on DMN functioning and self-reflection (excessive or diminished) across psychiatric illnesses.
Caveats to the spectrum-based model of self-reflection
In reviewing evidence for a spectrum-based model of self-reflection, we focused on quantitative differences in self-reflection (excessive or diminished) across different mental illnesses. However, there may also be qualitative differences in self-reflection, such as the content of self-reflective thought. For example, in depression ruminative thought is focused on negative aspects about oneself (e.g., “I am worthless”), whereas in generalized anxiety disorder worry is concentrated on potential threats, such as health problems (American Psychiatric Association, 2000). Thus, future studies may also investigate qualitative differences in self-reflection to further understand distinct and overlapping self-reflective processes in psychiatric illness. Another aspect of self-reflection that should be considered is the accuracy of self-appraisals. For instance, psychological and neuropsychological research has examined the accuracy in the knowledge of one’s own personality traits (Klein & Gangi, 2010 for review) and of one’s own behavioral/cognitive impairments (Prigatano & Fordyce, 1986). Similar methods, comparing self-ratings with collateral-ratings (e.g., spouse, parent, sibling) of one’s own self-reflective thoughts, could be used to investigate self-reflective accuracy in various psychiatric conditions.
While the functional neuroimaging literature has reliably implicated the mPFC and PCC in self-reflection, fewer studies have examined whether this circuit is modulated by the amount of self-reflection (D’Argembeau et al., 2005; Macrae et al., 2004; Moran et al., 2006). It is also unclear whether activity in this circuit is critical for self-reflection or other potentially related processes, such as mentalizing or autobiographical memory retrieval. It is possible that subcomponents of the default mode network (DMN) are associated with qualitatively different types of self-reflection. For example, recent work has shown that thinking about one’s future preferentially engages the medial temporal subsystem (including parahippocampal gyrus), while self-relevant and emotion-related cognition in general engages the dorsomedial subsystem, including the mPFC and PCC (Andrews-Hanna et al., 2010). Altogether, these unanswered questions highlight the need for the development of better tests of self-reflection, which will be essential for identifying the neural circuitry necessary for different types and levels of self-reflection.
To date, previous neuropsychological research supports the causal role of the mPFC and PCC in self-reflection. However, in humans, naturally occurring lesions (e.g., after a stroke) may commonly encompass multiple functional subregions and white matter pathways. Due to these limitations in the human lesion method, it can be difficult to identify patients with focal lesions resulting in a single cognitive or behavioral deficit. That being said, we typically do not see widespread cognitive deficits following mPFC and PCC lesions. For example, mPFC lesion patients showing deficits in the self-reference effect for memory had intact scores on assessments of IQ, language, memory, and visuospatial ability (Philippi et al., 2012). Nonetheless, future studies using more anatomically focused interventions (e.g., deep brain stimulation, transcranial magnetic stimulation) combined with neuropsychological tests will be necessary to establish the causal role of the mPFC and PCC in self-reflection.
As a coarse starting point, we have adopted the phrase “amount of self-reflection” for the purposes of this review. However, we are hopeful that future studies in this area will help to refine this phrase and test the spectrum-based model with new findings from a variety of healthy, neuropsychological, and psychiatric patient populations using different self-reflection tasks. Through this additional research we may also find that particular disorders do not have self-reflection as a core feature or symptom, and/or that self-reflection could vary independently of other core features of particular psychiatric disorders. Although there is not yet evidence that the amount of self-reflection reliably distinguishes between different psychiatric disorders, we suggest that the ultimate benefit of this line of research may not be to discriminate between members of different psychiatric groups. Rather, consistent with dimensional approach outlined by the National Institute of Mental Health (NIMH) (Insel et al., 2010), self-reflection may serve as a psychological factor that cuts across traditional psychiatric diagnoses. As such, self-reflection could be a dimension of psychological health that is diagnosed and treated in its own right. Nevertheless, future research will be crucial to determine the applicability and scope of this theoretical perspective across different psychiatric disorders.
Although we concentrate on self-reflection in this review, other types of self-related processing, including bodily self-processes (e.g., self-recognition, self-agency, interoceptive awareness), are also relevant to mental illness and may rely on different neural circuitry. Several studies have associated schizophrenia with impairments in bodily self-recognition and self-agency (Blakemore, Smith, Steel, Johnstone, & Frith, 2000; Daprati et al., 1997; Haggard, Martin, Taylor-Clarke, Jeannerod, & Franck, 2003; Metcalfe, Van Snellenberg, DeRosse, Balsam, & Malhotra, 2012; Synofzik, Thier, Leube, Schlotterbeck, & Lindner, 2010). For example, in a study of bodily self-recognition, patients with schizophrenia and hallucinations/delusions of control had greater difficulty distinguishing between their own action and an action that was not their own (movement of an alien hand produced by experimenter) (Daprati et al., 1997). Further, neuroimaging studies suggest that other self-related processes may rely on distinct neural circuitry also relevant to psychiatric illness, e.g, the mirror neuron system (Zhao, Luo, Li, & Kendrick, 2013). For instance, the judgment of self-agency has been associated with insular activity in healthy populations (e.g., Farrer & Frith, 2002), but increased inferior parietal activity in schizophrenic patients (Spence et al., 1997).
In addition, other self-related processing constructs have also been studied extensively in relation to psychiatric illness, including self-monitoring, alexithymia (diminished ability to identify/describe emotions), self-insight, and self-awareness (David, Bedford, Wiffen, & Gilleen, 2012; Frith, Blakemore, & Wolpert, 2000; Stephan, Friston, & Frith, 2009; Taylor, Michael Bagby, & Parker, 1991). For instance, Frith proposed that delusions of control in schizophrenia could be explained by fundamental impairments in self-monitoring and awareness of self-generated action (Frith et al., 2000; Frith & Done, 1989). Alexithymia, characterized by an inability to identify and describe feelings, is a significant predictor of psychopathology, is associated with autism, and with poor psychotherapy outcomes (Conrad, Wegener, Imbierowicz, Liedtke, & Geiser, 2009; Lombardo et al., 2007; Ogrodniczuk, Piper, & Joyce, 2011). In the context of this review, it is interesting to consider the relationship between self-reflection and these other constructs. One possibility is that self-reflection is a process that contributes to or is required for self-monitoring, emotional awareness, self-insight, and self-awareness. Consistent with this hypothesis, Beck and colleagues suggest that self-insight is composed of two component processes: self-reflectiveness and self-certainty (Beck et al., 2004). Moreover, recent work has examined the relationship between impaired self-reflection and insight in patients with schizophrenia, again suggesting that self-reflection may contribute to self-insight (Lysaker et al., 2011; van der Meer et al., 2012). Similarly, other theories propose that self-reflection is crucial for self-monitoring of thoughts for agency (e.g., “Is this thought my own?”), increasing emotional awareness in alexithymia, and introspective aspects of self-awareness (Duval et al., 1972; Synofzik, Vosgerau, & Newen, 2008; Taylor et al., 1991).
Although the precise relationships among these various facets of self-related processing remain to be fully elucidated, it is clear that the degree and nature of self-reflection is a critically important psychological feature of mental illness.
Future Directions
In this article we have outlined support for the development of a neuropsychological approach to evaluating self-reflection in psychiatric illness. However, in order to more conclusively demonstrate the clinical benefit of this approach, we suggest several questions to guide future research:
Can neuropsychological assessment of self-reflection (behavioral test performance and/or functional imaging of mPFC-based neural circuits) provide information about the risk for developing a particular disorder?
Developmentally, could self-reflection assessment (in parents and children) be useful in determining risk for childhood psychopathology?
Can specifically targeting the psychological and neurobiological processes underlying self-reflection lead to improved patient outcomes? For instance, clinicians may select therapies or develop treatments aimed at decreasing excessive self-reflection (e.g., mindfulness-based therapy) or increasing diminished self-reflection (e.g., dialectical behavioral therapy).
Could pharmacological or brain stimulation techniques be employed to modulate activity in the mPFC circuit, and would these interventions affect self-reflection?
Could the diagnostic neuropsychological assessment information be used to predict the patient’s response to the various treatment options, and thus to tailor treatments accordingly?
Could the behavioral and/or neuroimaging assessment information be used to monitor the efficacy of the treatment?
Pending answers to these questions, the translation of cognitive neuroscience research into clinical practice may be close at hand.
Conclusion
In conclusion, we believe that neuropsychology offers a promising perspective for advancing psychiatric medicine toward a more pathophysiologically-based system of diagnosis and treatment. This perspective is consistent with the NIMH’s goal of using a dimensional approach to classify mental disorders based on objective behavioral and neurobiological measures. We are optimistic that continued progress in elucidating the brain-behavior relationships critical for social and affective function, such as the work described here on self-reflection, will provide the foundational knowledge necessary for achieving this goal.
Acknowledgments
Role of the Funding Source
This work was supported by NIH grants T32MH018931-23 and MH086787.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
C.L.P. wrote the first draft of the manuscript, and M.K. revised and edited subsequent versions of the manuscript. All authors have contributed to and approved the final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albus M. Clinical Courses of Schizophrenia. Pharmacopsychiatry. 2012;45:S31–S35. doi: 10.1055/s-0032-1308968. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, et al. Remembering the past: Two facets of episodic memory explored with positron emission tomography. The American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle Marcus E, Buckner RL. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Stillwell AM, Heatherton TF. Guilt: an interpersonal approach. Psychol Bull. 1994;115:243. doi: 10.1037/0033-2909.115.2.243. [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC psychiatry. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Smith J, Steel R, Johnstone E, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Bradley B, Mathews A. Negative self-schemata in clinical depression. Br J Clin Psychol. 1983;22 (Pt 3):173–181. doi: 10.1111/j.2044-8260.1983.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner, Andrews-Hanna J, Schacter D. The Brain’s Default Network. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment, and treatment. New York, NY, US: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Conrad R, Wegener I, Imbierowicz K, Liedtke R, Geiser F. Alexithymia, temperament and character as predictors of psychopathology in patients with major depression. Psychiatry Research. 2009;165:137–144. doi: 10.1016/j.psychres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Cooke MA, Peters ER, Fannon D, Aasen I, Kuipers E, Kumari V. Cognitive insight in psychosis: The relationship between self-certainty and self-reflection dimensions and neuropsychological measures. Psychiatry Research. 2010;178:284–289. doi: 10.1016/j.psychres.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Daprati E, Franck N, Georgieff N, Proust J, Pacherie E, Dalery J, Jeannerod M. Looking for the agent: an investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition. 1997;65:71–86. doi: 10.1016/s0010-0277(97)00039-5. [DOI] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, Gilleen J. Failures of metacognition and lack of insight in neuropsychiatric disorders. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:1379–1390. doi: 10.1098/rstb.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaggio G, Vanheule S, Lysaker PH, Carcione A, Nicolo G. Impaired self-reflection in psychiatric disorders among adults: a proposal for the existence of a network of semi independent functions. Conscious Cogn. 2009;18:653–664. doi: 10.1016/j.concog.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Wicklund R. A theory of objective self-awareness. New York: Academic Press; 1972. [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eslinger P, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Exner JE. The Self Focus Sentence Completion: A Study of Egocentricity. Journal of Personality Assessment. 1973;37:437–455. doi: 10.1080/00223891.1973.10119902. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore S-J, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Research Reviews. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19:359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. Malden, MA: Blackwell; 2003. [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Haxby JV, Pietrini P, Guazzelli M. Beyond Amygdala: Default mode network activity differs between patients with Social Phobia and healthy controls. NeuroImage. 2009;47(Supplement 1):S49. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Boyle M, Hanna S, Duku E, Zwaigenbaum L, Bryson S, Fombonne E, Volden J, Mirenda P. Investigating phenotypic heterogeneity in children with autism spectrum disorder: a factor mixture modeling approach. Journal of Child Psychology and Psychiatry. 2013;54:206–215. doi: 10.1111/j.1469-7610.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI Investigation of Emotional Engagement in Moral Judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14:1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. 1010.1097/1001.wnr.0000073684.0000000308.c0000073680. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-Mode and Task-Positive Network Activity in Major Depressive Disorder: Implications for Adaptive and Maladaptive Rumination. Biological Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biological Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG. Public and private self-consciousness and social phobia. Journal of Personality Assessment. 1988;52:626–639. doi: 10.1207/s15327752jpa5204_3. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Self-focused attention in clinical disorders: Review and a conceptual model. Psychol Bull. 1990;107:156–176. doi: 10.1037/0033-2909.107.2.156. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Lumry AE, Cruet D, Sieber W. Attentional processes in depressive disorders. Cognitive Therapy and Research. 1987;11:351–360. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Anderson EA. Interpersonal skill and depression in college students: An analysis of the timing of self-disclosures. Behav Ther. 1982;13:271–282. [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behav Ther. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Jostes A, Pook M, Florin I. Public and private self-consciousness as specific psychopathological features. Personality and Individual Differences. 1999;27:1285–1295. [Google Scholar]
- Just N, Alloy LB. The response styles theory of depression: tests and an extension of the theory. Journal of Abnormal Psychology. 1997;106:221. doi: 10.1037//0021-843x.106.2.221. [DOI] [PubMed] [Google Scholar]
- Keltner D. Signs of appeasement: Evidence for the distinct displays of embarrassment, amusement, and shame. J Pers Soc Psychol. 1995;68:441–441. [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Klein SB, Chan RL, Loftus J. Independence of episodic and semantic self-knowledge: The case from autism. Social Cognition. 1999;17:413–436. [Google Scholar]
- Klein SB, Gangi CE. The multiplicity of self: neuropsychological evidence and its implications for the self as a construct in psychological research. Ann N Y Acad Sci. 2010;1191:1–15. doi: 10.1111/j.1749-6632.2010.05441.x. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Kruepke M, Newman JP. Economic decision-making in psychopathy: A comparison with ventromedial prefrontal lesion patients. Neuropsychologia. 2010;48:2198–2204. doi: 10.1016/j.neuropsychologia.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR. The self and emotion: The role of self-reflection in the generation and regulation of affective experience. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. The Handbook of Affective Sciences. New York: Oxford University Press; 2003. pp. 773–786. [Google Scholar]
- Lee A, Hobson RP. On Developing Self-concepts: A Controlled Study of Children and Adolescents with Autism. Journal of Child Psychology and Psychiatry. 1998;39:1131–1144. [PubMed] [Google Scholar]
- Lee A, Hobson RP, Chiat S. I, you, me, and autism: An experimental study. Journal of autism and developmental disorders. 1994;24:155–176. doi: 10.1007/BF02172094. [DOI] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng L-L, Hu D. A treatment-resistant default mode subnetwork in major depression. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS ONE. 2012;7:e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS ONE. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S. Atypical neural self-representation in autism. Brain. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Dimaggio G, Buck KD, Callaway SS, Salvatore G, Carcione A, Nicolò G, Stanghellini G. Poor insight in schizophrenia: links between different forms of metacognition with awareness of symptoms, treatment need, and consequences of illness. Comprehensive psychiatry. 2011;52:253–260. doi: 10.1016/j.comppsych.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mansell W. Core processes of psychopathology and recovery: “does the Dodo bird effect have wings?”. Clin Psychol Rev. 2011;31:189–192. doi: 10.1016/j.cpr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. The Journal of neuropsychiatry and clinical neurosciences. 1997 doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Borkovec TD, Sibrava NJ. The effects of worry and rumination on affect states and cognitive activity. Behav Ther. 2007;38:23–38. doi: 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Van Snellenberg JX, DeRosse P, Balsam P, Malhotra AK. Judgements of agency in schizophrenia: an impairment in autonoetic metacognition. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:1391–1400. doi: 10.1098/rstb.2012.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychol Bull. 2002;128:638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical Evidence for Distinct Cognitive and Affective Components of Self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Haddock G. Self-focused attention in schizophrenic patients with and without auditory hallucinations and normal subjects: A comparative study. Personality and Individual Differences. 1997;23:937–941. [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. J Neurosci. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Lorenz AR. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York, NY, US: Oxford University Press; 2003. pp. 904–929. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Offer D, Kaiz M, Howard KI, Bennett ES. The altering of reported experiences. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:735–742. doi: 10.1097/00004583-200006000-00012. [DOI] [PubMed] [Google Scholar]
- Ogrodniczuk JS, Piper WE, Joyce AS. Effect of alexithymia on the process and outcome of psychotherapy: A programmatic review. Psychiatry Research. 2011;190:43–48. doi: 10.1016/j.psychres.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. The Neuroscientist. 2008;14:203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Duff MC, Denburg NL, Tranel D, Rudrauf D. Medial PFC damage abolishes the self-reference effect. J Cogn Neurosci. 2012;24:475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants Normalize the Default Mode Network in Patients With Dysthymia. JAMA Psychiatry. 2013:1–10. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Crane SM, Quiring J, Tucker DM. Frontolimbic activity and cognitive bias in major depression. J Abnorm Psychol. 2009;118:494–506. doi: 10.1037/a0015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigatano G, Fordyce D. Cognitive dysfunction and psychosocial adjustment after brain injury. In: Prigatano G, Fordyce D, Zeiner H, Roueche J, Pepping M, Wood B, editors. Neuropsychological rehabilitation after brain injury. Baltimore, MD: Johns Hopkins University Press; 1986. pp. 96–118. [Google Scholar]
- Puentf AE, Morrisey S. Self-consciousness in schizophrenics. Psychological Reports. 1981;49:631–634. doi: 10.2466/pr0.1981.49.2.631. [DOI] [PubMed] [Google Scholar]
- Pujol J, Batalla I, Contreras-Rodríguez O, Harrison BJ, Pera V, Hernández-Ribas R, Real E, Bosa L, Soriano-Mas C, Deus J. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social Cognitive and Affective Neuroscience. 2012;7:917–923. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini M-L, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. Journal of Gerontology. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Saboonchi F, Lundh L-G, Öst L-G. Perfectionism and self-consciousness in social phobia and panic disorder with agoraphobia. Behav Res Ther. 1999;37:799–808. doi: 10.1016/s0005-7967(98)00183-1. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. The Self-Consciousness Scale: A Revised Version for Use with General Populations. Journal of Applied Social Psychology. 1985;15:687–699. [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Moore PM, Thase ME. Rumination: One construct, many features in healthy individuals, depressed individuals, and individuals with lupus. Cognitive Therapy and Research. 2004;28:645–668. [Google Scholar]
- Smith RE, Sarason IG. Social anxiety and the evaluation of negative interpersonal feedback. J Consult Clin Psychol. 1975;43:429. doi: 10.1037/h0076855. [DOI] [PubMed] [Google Scholar]
- Smith TW, Ingram RE, Roth DL. Self-focused attention and depression: Self-evaluation, affect, and life stress. Motivation and emotion. 1985;9:381–389. [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120 doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8:861. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Thier P, Leube DT, Schlotterbeck P, Lindner A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain. 2010;133:262–271. doi: 10.1093/brain/awp291. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Vosgerau G, Newen A. Beyond the comparator model: A multifactorial two-step account of agency. Conscious Cogn. 2008;17:219–239. doi: 10.1016/j.concog.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Michael Bagby R, Parker JD. The alexithymia construct: a potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32:153–164. doi: 10.1016/s0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Robins RW. Putting the Self Into Self-Conscious Emotions: A Theoretical Model. Psychological Inquiry. 2004;15:103–125. [Google Scholar]
- Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. J Pers Soc Psychol. 1999;76:284. doi: 10.1037//0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol M-J, Nolen WA, David AS, Aleman A. Insight in Schizophrenia: Involvement of Self-Reflection Networks? Schizophr Bull. 2012 doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Woodruff-Borden J, Brothers AJ, Lister SC. Self-focused attention: Commonalities across psychopathologies and predictors. Behavioural and Cognitive Psychotherapy. 2001;29:169–178. [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yoshino A, Ueda K, Suzuki S-i, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Luo L, Li Q, Kendrick KM. What can psychiatric disorders tell us about neural processing of the self? Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, Tang XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63:373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]


