Abstract
Background
Tobacco smoking in pregnancy remains one of the few preventable factors associated with complications in pregnancy, stillbirth, low birthweight and preterm birth and has serious long-term implications for women and babies. Smoking in pregnancy is decreasing in high-income countries, but is strongly associated with poverty and increasing in low- to middle-income countries.
Objectives
To assess the effects of smoking cessation interventions during pregnancy on smoking behaviour and perinatal health outcomes.
Search methods
In this fifth update, we searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 March 2013), checked reference lists of retrieved studies and contacted trial authors to locate additional unpublished data.
Selection criteria
Randomised controlled trials, cluster-randomised trials, randomised cross-over trials, and quasi-randomised controlled trials (with allocation by maternal birth date or hospital record number) of psychosocial smoking cessation interventions during pregnancy.
Data collection and analysis
Two review authors independently assessed trials for inclusion and trial quality, and extracted data. Direct comparisons were conducted in RevMan, and subgroup analyses and sensitivity analysis were conducted in SPSS.
Main results
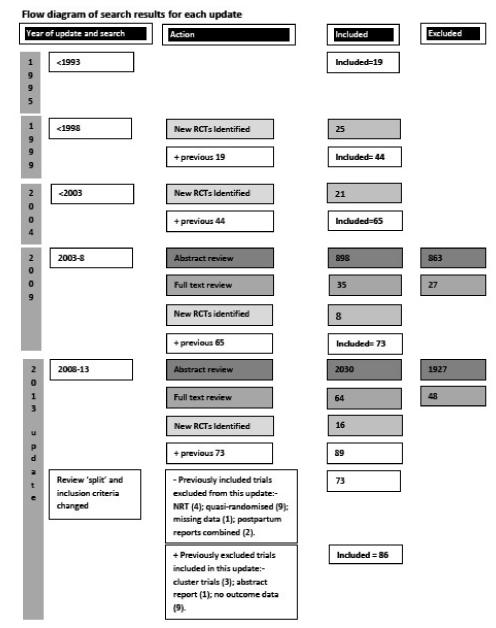
Eighty-six trials were included in this updated review, with 77 trials (involving over 29,000 women) providing data on smoking abstinence in late pregnancy.
In separate comparisons, counselling interventions demonstrated a significant effect compared with usual care (27 studies; average risk ratio (RR) 1.44, 95% confidence interval (CI) 1.19 to 1.75), and a borderline effect compared with less intensive interventions (16 studies; average RR 1.35, 95% CI 1.00 to 1.82). However, a significant effect was only seen in subsets where counselling was provided in conjunction with other strategies. It was unclear whether any type of counselling strategy is more effective than others (one study; RR 1.15, 95% CI 0.86 to 1.53). In studies comparing counselling and usual care (the largest comparison), it was unclear whether interventions prevented smoking relapse among women who had stopped smoking spontaneously in early pregnancy (eight studies; average RR 1.06, 95% CI 0.93 to 1.21). However, a clear effect was seen in smoking abstinence at zero to five months postpartum (10 studies; average RR 1.76, 95% CI 1.05 to 2.95), a borderline effect at six to 11 months (six studies; average RR 1.33, 95% CI 1.00 to 1.77), and a significant effect at 12 to 17 months (two studies, average RR 2.20, 95% CI 1.23 to 3.96), but not in the longer term. In other comparisons, the effect was not significantly different from the null effect for most secondary outcomes, but sample sizes were small.
Incentive-based interventions had the largest effect size compared with a less intensive intervention (one study; RR 3.64, 95% CI 1.84 to 7.23) and an alternative intervention (one study; RR 4.05, 95% CI 1.48 to 11.11).
Feedback interventions demonstrated a significant effect only when compared with usual care and provided in conjunction with other strategies, such as counselling (two studies; average RR 4.39, 95% CI 1.89 to 10.21), but the effect was unclear when compared with a less intensive intervention (two studies; average RR 1.19, 95% CI 0.45 to 3.12).
The effect of health education was unclear when compared with usual care (three studies; average RR 1.51, 95% CI 0.64 to 3.59) or less intensive interventions (two studies; average RR 1.50, 95% CI 0.97 to 2.31).
Social support interventions appeared effective when provided by peers (five studies; average RR 1.49, 95% CI 1.01 to 2.19), but the effect was unclear in a single trial of support provided by partners.
The effects were mixed where the smoking interventions were provided as part of broader interventions to improve maternal health, rather than targeted smoking cessation interventions.
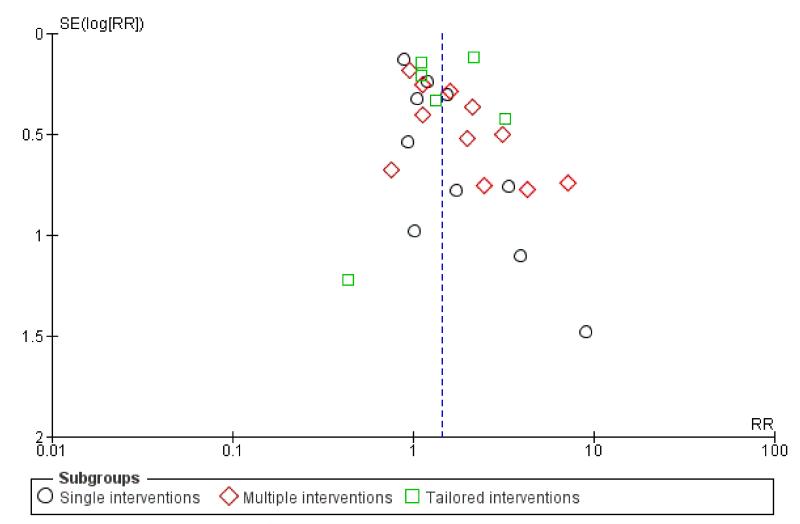
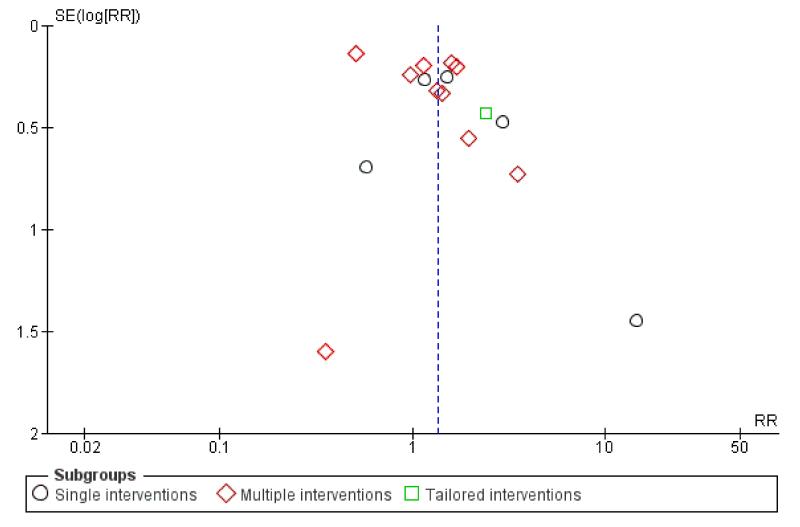
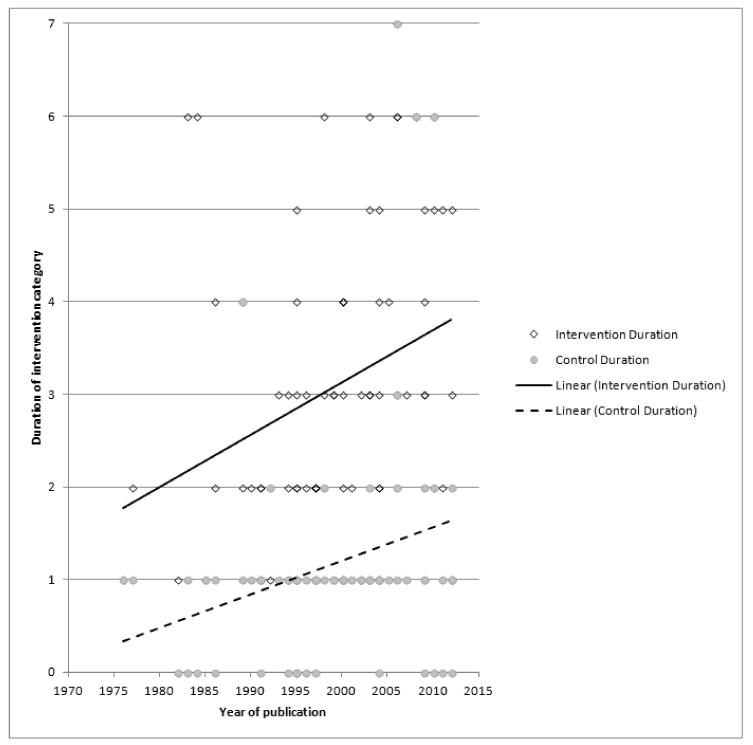
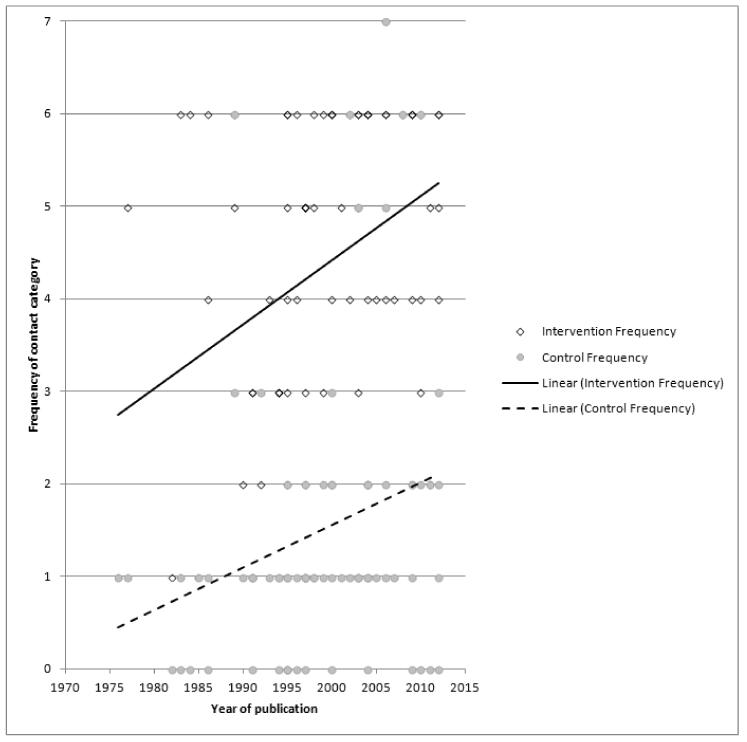
Subgroup analyses on primary outcome for all studies showed the intensity of interventions and comparisons has increased over time, with higher intensity interventions more likely to have higher intensity comparisons. While there was no significant difference, trials where the comparison group received usual care had the largest pooled effect size (37 studies; average RR 1.34, 95% CI 1.25 to 1.44), with lower effect sizes when the comparison group received less intensive interventions (30 studies; average RR 1.20, 95% CI 1.08 to 1.31), or alternative interventions (two studies; average RR 1.26, 95% CI 0.98 to 1.53). More recent studies included in this update had a lower effect size (20 studies; average RR 1.26, 95% CI 1.00 to 1.59), I2= 3%, compared to those in the previous version of the review (50 studies; average RR 1.50, 95% CI 1.30 to 1.73). There were similar effect sizes in trials with biochemically validated smoking abstinence (49 studies; average RR 1.43, 95% CI 1.22 to 1.67) and those with self-reported abstinence (20 studies; average RR 1.48, 95% CI 1.17 to 1.87). There was no significant difference between trials implemented by researchers (efficacy studies), and those implemented by routine pregnancy staff (effectiveness studies), however the effect was unclear in three dissemination trials of counselling interventions where the focus on the intervention was at an organisational level (average RR 0.96, 95% CI 0.37 to 2.50). The pooled effects were similar in interventions provided for women with predominantly low socio-economic status (44 studies; average RR 1.41, 95% CI 1.19 to 1.66), compared to other women (26 studies; average RR 1.47, 95% CI 1.21 to 1.79); though the effect was unclear in interventions among women from ethnic minority groups (five studies; average RR 1.08, 95% CI 0.83 to 1.40) and aboriginal women (two studies; average RR 0.40, 95% CI 0.06 to 2.67). Importantly, pooled results demonstrated that women who received psychosocial interventions had an 18% reduction in preterm births (14 studies; average RR 0.82, 95% CI 0.70 to 0.96), and infants born with low birthweight (14 studies; average RR 0.82, 95% CI 0.71 to 0.94). There did not appear to be any adverse effects from the psychosocial interventions, and three studies measured an improvement in women’s psychological wellbeing.
Authors’ conclusions
Psychosocial interventions to support women to stop smoking in pregnancy can increase the proportion of women who stop smoking in late pregnancy, and reduce low birthweight and preterm births.
BACKGROUND
Description of the condition
Risks associated with smoking in pregnancy
Tobacco smoking in pregnancy remains one of the few preventable factors associated with complications in pregnancy, such as placental abruption, miscarriage, low birthweight (Kramer 1987), preterm birth (US DHHS 2004; Hammoud 2005; Salihu 2007; Rogers 2009; Vardavas 2010; Baba 2012), stillbirth and neonatal death (Kallen 2001). Tobacco smoking also has serious long-term health implications for women and infants; 5.4 million people per year currently die from tobacco use, and this is expected to rise to eight million per year in the next 30 years (WHO 2008a).
Nicotine and other harmful compounds in cigarettes are developmental toxicants (Rogers 2009), which impact on the brain at critical developmental periods (Dwyer 2008) restricting the supply of oxygen and other essential nutrients, fetal growth (Crawford 2008), development of organs (Morales-Suarez-Varela 2006), including the lungs (Maritz 2008) and neurological development (Herrmann 2008; Blood-Siegfried 2010). Growing evidence suggests these ‘developmental origins of disease’ have life-long implications (Gluckman 2008).
Young women start smoking for many reasons including: belief it is a rite of passage into adult life, a gesture against authority, trying to appear modern and affluent, or to fit in with social networks (Todd 2001). Tobacco addiction is then caused by nicotine in tobacco which produces a cascade of actions, including release of “pleasure enhancing” dopamine, which strengthens associations of positive feelings with smoking behaviour and appears to be involved in all addictive behaviours (Schmidt 2004). Some suggest the negative feelings of “nicotine hunger” and unpleasant symptoms associated with nicotine withdrawal (Balfour 2004; Hughes 2007) may be stronger for pregnant women due to the physiological adaptations in pregnancy which accelerate nicotine metabolism (Ebert 2009; Ussher 2012a), however a recent study reported less severe withdrawal symptoms among pregnant women in the first 24 hours of abstinence, compared to non-pregnant women (Ussher 2012b).
Epidemiology of smoking in pregnancy
In high-income countries, such as Australia, Canada, Denmark, New Zealand, Sweden, the United Kingdom (UK) and the United States (US), the prevalence of smoking in pregnancy has declined from between 20% to 35% in the 1980s to between 10% and 20% in the early 2000s (Cnattingius 2004; US DHHS 2004; Giovino 2007; Dixon 2009b; Tong 2009; Al-Sahab 2010; Tappin 2010), with significant declines in the last decade bringing the prevalence of smoking in pregnancy well below 10% by 2010 (Lanting 2012). However, the decline has not been consistent across all sectors of society, with lower rates of decline among women with lower socio-economic status (US DHHS 2004; Pickett 2009; Graham 2010; Johnston 2011b; Lanting 2012). Tobacco smoking in high-income countries is a marker of social disadvantage and has been cited as one of the principal causes of health inequality between rich and poor (Wanless 2004), and understanding these disparities are central to understanding the tobacco epidemic (Graham 2010). In Scotland, 30% of women living in the most deprived areas continued to smoke during pregnancy in 2008, compared to 7% in the least deprived areas (Tappin 2010). Women who continue to smoke in pregnancy are more likely to: have a low income, higher parity, no partner, low levels of social support, limited education; access publicly funded maternity care; and feel criticised by society (Graham 1977; Frost 1994; Graham 1996; Tappin 1996; Wakschlag 2003; US DHHS 2004; Ebert 2007; Schneider 2008; Pickett 2009). The World Health Organization (WHO) report into the Social Determinants of Health recognises a paradigm whereby disadvantaged people are more likely to use substances in response to their circumstances (WHO 2008b). There is also a significantly higher prevalence of smoking in pregnancy in several ethnic and aboriginal minority groups (Wiemann 1994; Kaplan 1997; Chan 2001; US DHHS 2004; Wood 2008; Dixon 2009b; Johnston 2011b). In Australia, smoking during pregnancy is three times more prevalent among Aboriginal and Torres Strait Islander women (53%) than among non-Aboriginal women (16%) (Johnston 2011b), and similar disparities are reported between Maori and non-Maori women in New Zealand (Dixon 2009b). These disparities are largely in accord with social and material deprivation. However, in some migrant groups, cultural differences may cut across this social gradient (Troe 2008), which suggests that there are aspects of smoking socialisation not entirely explained by material deprivation. In the United States, the highest rates of pre-pregnancy smoking were reported among Alaskan Native women (55.6%), American Indian women (46.9%), and White women (46.4%), with significantly lower rates (less than 20%) reported among African American, Hispanic and Asian-Pacific women (Tong 2011; Watt 2012). Women who are migrants or refugees to Australia, Canada, New Zealand, Northern Europe, the UK, or the US or who originate from South East Asia also retain a lower prevalence of smoking, despite major social disadvantage (Potter 1996; Small 2000; Bush 2003; Dixon 2009b). However, second-generation migrant women are more likely to smoke during pregnancy than first-generation women (Troe 2008), reflecting movement between stages of ‘the tobacco epidemic’ (Lopez 1994).
In low- and middle-income countries there is marked variation in prevalence of smoking in pregnancy, which reflects the dynamic nature of the tobacco epidemic in these regions (Richmond 2003; Polanska 2004; Bloch 2008). Smoking rates among pregnant women have been comparatively low (9%) compared to men (50%), due to historical cultural constraints on women’s smoking in many low- to middle-income countries (Bloch 2008). However, the prevalence of tobacco smoking among women is increasing and is expected to rise to 20% by 2025, shifting the global tobacco smoking epidemic from high-income countries to low- and middle-income countries (Samet 2001; Richmond 2003). The highest rates of smoking during pregnancy were reported in Latin America (18.3% in Uruguay 2004 to 2005) (Bloch 2008) and Eastern Europe (15% in Romania 2005 to 2006) (Meghea 2010). Low rates were reported in Pakistan (3%) (Bloch 2008), South East Asia (1.3%) (Barraclough 1999; Ostrea 2008), and China (2% in 1999), though increasing rates among female school children are causing concern (Kong 2008). In India and Africa, rates of cigarette smoking were low (1.7% and 6.1% pregnant women reporting smoking cigarettes, respectively), (Steyn 2006; Bloch 2008; Palipudi 2009), while use of smokeless tobacco products was high among Indian (4.9% to 33.5%) (Palipudi 2009; Bloch 2008) and African women (6% to 7.5%) (Steyn 2006; Bloch 2008). The WHO has identified this rise of tobacco use in young females in low-income, high population countries as one of the most ominous developments of the tobacco epidemic (WHO 2008a), jeopardizing efforts to improve maternal and child health (Cnattingius 2004; Bloch 2008). This increase is being driven by aggressive marketing from tobacco companies, who are predicting high profits from sales in low- and middle-income countries (Kaufman 2001), along with increased tobacco production in these regions (FAO 2003), which further entrenches the countries’ tobacco dependence. Marketing strategies are specifically targeted at women and weak regulation of tobacco company marketing has been linked to a rapid increase in smoking among women, particularly those who are vulnerable (Kaufman 2001; Gilmore 2004; Graham 2009). A survey of women’s knowledge in two African countries suggests women’s knowledge of the risks of tobacco products was extremely limited (Chomba 2010), making women more vulnerable to tobacco marketing.
Issues around smoking in pregnancy are complicated by the intersection of gender (Healton 2009), where a woman’s role is seen primarily as a ‘reproducer’, and emphasis is placed on the rights of the unborn fetus (pxii; World Health Organization 2001). There is a risk these arguments may be used to impose authority over women’s behaviour, ‘blaming’ women for their own plight and that of their children, and using guilt or other means to undermine self-confidence; further reducing the control women have in their lives (Greaves 2007a).
In addition to the socio-economic factors associated with continued smoking, there are strong psychological associations, especially with depression and stress (Blalock 2005; Aveyard 2007; Crittenden 2007; Orr 2012), including race-related stress (Heath 2006; Fernander 2010; Nguyen 2012a). Depressed women are up to four times more likely to smoke during pregnancy than non-depressed women (Blalock 2005). Despite these strong associations, there is limited information available about the effects of smoking and interventions in pregnant women with psychological symptoms, as they are often excluded from trials (Blalock 2005). Furthermore, while tobacco control initiatives in high-income countries have been effective in reducing smoking, the stigmatisations of smokers has been an unintended consequence (Burgess 2009; Wigginton 2012), which is being increasingly recognised by the tobacco control community (Farrimond 2006; Thompson 2007a; Burgess 2009). Anti-smoking campaigns strive to inform, shock or shame people into quitting smoking and rarely take into account low self-esteem, low self-efficacy, poverty, stress and increased caring responsibilities that are common among women who continue to smoke during pregnancy (Gilbert 2005). A systematic review of qualitative experiences of women describes how smoking in pregnancy triggered “intense feelings of personal responsibility and inadequacy” and that women’s responses to social disapproval varied (Flemming 2013). For some, it provided an incentive to attempt to quit, while among others it resulted in increased smoking, either in response to the stress of social pressure or as an act of rebellion against it (Flemming 2013). Some argue that health risk narratives and the associated social stigma produced through anti-smoking campaigns contribute to oppression among marginalised people, and a consequence is that these strategies may inspire resistance and resentment rather than compliance (Bond 2012; Wigginton 2012; Flemming 2013).
Although commercial cigarettes are the most prevalent form of tobacco use worldwide, the use of other forms of tobacco (e.g. smokeless tobacco, cigars and pipes, and waterpipes) are becoming more popular in many parts of the world, especially low- and middle-income countries (England 2010). Of particular concern are increasing efforts by the tobacco industry to commercialise and market smokeless tobacco products to young adults (Lambe 2007). In high-income countries, the use of smokeless tobacco appears to be highly localised among some indigenous groups in Canada and the US, including Lumbee Indian, Navajo, and Alaskan Native communities (Strauss 1997; Spangler 2001; Patten 2009; Kim 2009a; Kim 2010). In India, one-third (33.5%) of all pregnant women reported using smokeless tobacco (Bloch 2008). In the Democratic Republic of Congo, 6% to 41.8% of pregnant women surveyed reported using other forms of tobacco, primarily snuff (Bloch 2008; Chomba 2010). In South Africa 7.5% of pregnant women surveyed reported using snuff (Steyn 2006). In Iran there has been concern over the 8% prevalence of local waterpipe tobacco smoking among pregnant women (Mirahmadizadeh 2008). These tobacco products may be cheaper and viewed as less harmful than cigarettes (England 2010). In some cases use may be a traditional cultural norm or a medicinal aid to reduce nausea in early pregnancy. However, these products can be high in nicotine content and cause nicotine addiction. Use of these products has been associated with increased oral and pancreatic cancer, and cardiovascular disease (England 2010). There is a paucity of research into the effect of these products on pregnancy outcomes and studies into the effects of these products can be challenging as the chemical content of various toxic compounds is variable and often poorly regulated. However, limited evidence suggests smokeless tobacco use is associated with decreased birthweight and preterm birth (Verma 1983; Gupta 2004; Pratinidhi 2010), stillbirth (Gupta 2006; Gupta 2012), maternal anaemia (Subramoney 2008), degenerative placental changes (Ashfaq 2008), and adverse infant neurobehavioural outcomes (Hurt 2005). Smoking more than one waterpipe per day (Tamim 2008) or starting to smoke waterpipes during the first trimester (Mirahmadizadeh 2008) was also associated with an increased risk of having a low birthweight baby.
Exposure to environmental tobacco smoke (ETS) also poses risks to pregnant women and their infants (Yang 2010). Studies suggest the risk may be exacerbated in low-income countries where exposure to indoor cooking smoke is also common (Kadir 2010). In China, 75.1% of pregnant non-smoking women were regularly exposed to environmental tobacco smoke from their husbands’ smoking (Yang 2010). Studies in high-income countries demonstrate that eliminating smoking in the workplace and other public spaces significantly reduces environmental tobacco smoke exposure and improves health outcomes, including preterm births (Cox 2013). One study in Indonesia reported increased collective efficacy when environmental tobacco smoke exposure was addressed through a well-publicised community household smoking ban (Nichter 2010). However, as these measures do not extend to homes (Oncken 2009), some argue domestic environmental tobacco smoke exposure may be increasing as public health policies restrict smoking of partners in public places, and the social position of women may limit their ability to enforce smoke-free policies within their homes (Tong 2009).
A positive theme emerging from this literature is that a higher proportion of women stop smoking during pregnancy than at other times in their lives. Up to 49% of women who smoked before pregnancy ‘spontaneously quit’ before their first antenatal visit (Quinn 1991; Woodby 1999; Hotham 2008), a quit rate substantially higher than reported in the general population (Ershoff 1999; McBride 2003; Tong 2008). However, these spontaneous quitting rates may be lower among women with lower socio-economic status (Mullen 1999). There are significant psychosocial differences between women who ‘spontaneously quit’ and women who continue to smoke in late pregnancy. Women who spontaneously quit usually smoke less, are more likely to have stopped smoking before, have a non-smoking partner, have more support and encouragement at home for quitting, are less seriously addicted, and have stronger beliefs about the dangers of smoking (Baric 1976; Ryan 1980; Cinciripini 2000; Passey 2012). Pregnant women are also more likely to use coping strategies to avoid relapse than non-pregnant women (Ortendahl 2007c; Ortendahl 2008a; Ortendahl 2009a), however less than a third of these women remain abstinent after one year postpartum (CDCP 2002; Fang 2004), supporting qualitative evidence that many women see pregnancy as a temporary period of abstinence for the sake of the baby (Stotts 1996; Lawrence 2005a; Flemming 2013). Despite high relapse rates, some studies suggest that the long-term effects of spontaneous quitting in pregnancy are significant (Rattan 2013), and others argue this success is important to recognise to avoid ‘pathologising’ smoking cessation and eroding confidence in human agency to overcome problems (Chapman 2010).
Given the complexity of the health and social dimensions of smoking in pregnancy there are conflicting perspectives regarding the most appropriate approaches. A dominant theme is that smoking in pregnancy is a lifestyle choice, however, there is concern this can lead to ‘victim blaming’ (Bond 2005), that individualised, behaviourist approaches are unlikely to adequately address health inequalities alone (Baum 2009), and that drug dependence and addiction is best dealt with in the domain of social policy and public health (Ebert 2009). Nevertheless, some suggest there is a role for individual support which is positive, not punitive (Bond 2012), and others express a concern that framing smoking in pregnancy solely as a social problem may make health professionals reluctant to intervene and offer support (McLellan 2000).
Description of the intervention
This review evaluates the effectiveness of individual psychosocial interventions that aim to motivate and support women to stop smoking in pregnancy, or prevent smoking relapse among women who have spontaneously quit. Psychosocial interventions are defined as non-pharmacological strategies that use cognitive-behavioural, motivational and supportive therapies to help women to quit, including counselling, health education, feedback, financial incentives, and social support from peers and/or partners (see Types of interventions), as well as dissemination trials.
Other smoking cessation intervention reviews
At the time of this update there were 73 other Cochrane reviews assessing the effectiveness of tobacco smoking cessation interventions for all populations (see Appendix 1). These include reviews on the following.
Population wide measures such as: legislative smoking bans, mass media campaigns, organisational interventions (workplace and school-based interventions), healthcare financing systems for increasing use of tobacco dependence treatment, advertising and promotion to reduce tobacco use, preventing tobacco smoking in public places, and impact of advertising on adolescent smoking.
Community interventions including family-based programmes, group behaviour interventions, family and carer interventions for reducing environmental tobacco smoke, school-based programmes, and school policies.
Individual psychosocial interventions, including aversive smoking, acupuncture, hypnotherapy, self-help, exercise, individual behavioural counselling, motivational interviewing, stage-based interventions, competitions and incentives, telephone counselling, mobile phone-based interventions, Internet-based interventions, nursing and physician advice, enhancing partner support, feedback, community pharmacy interventions, training health professionals in smoking cessation, use of electronic records, prevention of weight gain after smoking cessation, improving recruitment into cessation programs, harm reduction, reduction versus abrupt cessation, biomedical risk assessments, electronic cigarettes, incentives to prevent smoking in young people, relapse prevention, and interventions to reduce non-cigarette tobacco use, including waterpipe smoking cessation.
Individual pharmacological interventions, including antidepressants, anxiolytics, nicotine replacement therapy (NRT), clonidine, mecamylamine, nicobrevin, nicotine agonists, opioid agonists, cannabinoid type 1 receptor agonists, silver acetate, lobeline, and nicotine vaccines, increasing adherence to medications for tobacco dependence, behavioural interventions as adjuncts to pharmacotherapies, combined pharmacotherapy and behavioural interventions;and an ‘overview of pharmacological reviews’.
Interventions in specific population groups, including people with: schizophrenia and serious mental illness, depression, substance abuse, cardiovascular and pulmonary disease; pre-operative and hospitalised patients; Indigenous populations and Indigenous youth; and people in dental settings.
Other reviews, assessing effectiveness of interventions to recruit patients into smoking cessation programs, and reduce harm from continued tobacco use.
How the intervention might work
Pregnancy has been described as a ‘window of opportunity’ for smoking cessation (McBride 2003). Pregnancy increases a woman’s perception of risk and personal outcomes, therefore strong affective or emotional responses are more likely to be prompted (Slade 2006; Ortendahl 2008b). It also redefines a woman’s self-concept or social role (Ortendahl 2007b), especially when failure to comply with a social role results in social stigmatisation (Ortendahl 2007a; Ortendahl 2008c). Psychosocial interventions involve a range of social and psychological components which aim to increase motivation or affective or emotional responses to support pregnant women to stop smoking and support women to develop coping strategies to avoid relapse (Ortendahl 2007c; Pilling 2010). For example, counselling, feedback and financial incentives are all designed to enhance motivation to quit and move women closer towards the ‘action’ stage of change. Thirty-seven individual ‘behaviour change techniques’ or observable components used in interventions in the previous version of this review have been identified (Lorencatto 2012).
Psychosocial interventions to support women to stop smoking in pregnancy increasingly incorporate theoretical frameworks to inform, develop and evaluate strategies designed to influence behaviour (Green 2005b; Glanz 2008; Michie 2008; Bartholomew 2011). Using behaviour change theories in the context of addiction has been identified as a useful way to identify modifiable determinants and/or behaviour change techniques (Webb 2010). There are many theories of behaviour, which provide a summary of constructs, procedures and methods for understanding behaviour, and present hypothesised relationships or causal pathways that influence behaviour (Michie 2012). While some argue there is little apparent consensus about which theories are best to use in designing interventions (Noar 2005), most theories of behaviour change postulate a role for six broad classes of variables (Glanz 2008):
attitudes and beliefs about the behaviours or the outcomes of change (used in health education and counselling strategies);
beliefs about self-efficacy or perceived ability to enact and/or maintain the target behaviour change (used in counselling strategies such as motivational interviewing or cognitive behaviour therapy);
the role of contextual factors, particularly social factors, either directly and/or mediated through people’s beliefs (used in social support strategies);
previous experience with the behaviour either directly or indirectly through the processes of modelling (modelling can be seen as an element of social influence) (used in social support strategies);
priority for action, a person can only pursue a limited number of goals of any one time; and
the notion of a stage-based or systematic step-like progression towards behaviour change, which is incorporated into the assessment stage of many smoking cessation interventions (Prochaska 1992).
Why it is important to do this review
There are many psychosocial interventions that have been evaluated to support women to stop smoking during pregnancy. This review synthesises the evidence from these trials to generate evidence, which is of direct relevance for practitioners, policy-makers, and researchers. Synthesis enables comparison of whether interventions have been shown to be effective in individual studies and whether this effect has been replicated in other settings. Importantly, individual studies are unlikely to have sufficient power to evaluate the effect of interventions on perinatal outcomes or to conduct subgroup analyses to assess if there are differential effects among vulnerable subpopulations with high rates of smoking during pregnancy. Finally, collation of the body of evidence helps to identify any gaps for future research.
This is the fifth update of this Cochrane review, previously entitled ‘Interventions to promote smoking cessation during pregnancy’. The first version was published in 1995 on CD Rom and previously updated in The Cochrane Library in 1999, 2004 and 2009. Previous versions of this review have demonstrated the potential for individual interventions during pregnancy to have a modest but significant effect on reducing smoking, preterm births and infants born with low birthweight (Lumley 2009). This evidence has been instrumental in individual psychosocial interventions becoming a part of routine pregnancy care in many high-income countries in the past decade (Flenady 2005; Ministry of Health 2007; Fiore 2008; NICE 2010; Wong 2011). These guidelines generally incorporate a number of interventions, including identifying women who smoke during pregnancy, providing advice about risks, and supporting women to stop smoking.
In this review update, we have ‘split’ the previous version into two reviews: (1) this review focusing on psychosocial interventions to support women to stop smoking in pregnancy; and (2) a second review specifically focusing on pharmacological interventions to promote smoking cessation in pregnancy (Coleman 2012b). This split was necessary as there are different issues of concern for psychosocial and pharmacological interventions. Psychosocial interventions are now part of routine care in many high-income countries and contemporary issues focus on strategies to increase efficacy, and adaptation of psychosocial interventions to different contexts and settings, sometimes requiring different study designs (e.g. cluster trials of implementation). As many interventions involve multiple strategies or use of components which are tailored to individual women, it is very difficult to assess the independent effect of individual components of psychosocial interventions. As the efficacy and safety of pharmacological treatment (e.g. Nicotine Replacemernt Therapy, Bupropion) during pregnancy (Slotkin 2008) remains uncertain, more rigid study designs (i.e. randomised double-blind placebo-controlled trials) are required to assess the risks and efficacy.
To complement what is known from research literature about smoking in pregnancy, direct contributions to this review were sought from women who smoked before or during pregnancy in 1999. Women were identified through community networks, and their views emphasised the need to focus attention on potential adverse effects of smoking cessation programmes; in particular, the consequent guilt, anxiety and additional stress experienced by those who continue to smoke, especially through ‘high-risk’ pregnancies, and the detrimental effect on their relationships with their family and maternity care providers (Oliver 2001).
In this update, we indirectly considered women’s views reported in a systematic review of qualitative studies (Flemming 2013), which reinforce the previous contributions, identifying four main themes which have implications for interventions to support women to stop smoking in pregnancy.
Smoking is an embedded part of the lives of many women living in disadvantaged circumstances.
Women see smoking in pregnancy in terms of the risks it presents to their unborn baby, which can trigger guilt.
Quitting was not seen in unambiguously positive terms and was seen to have downsides, disrupting relationships and removing a habit perceived as helping women cope.
Partners play an important role in influencing women’s smoking behaviour in pregnancy, either as barriers or facilitators to quitting.
We also indirectly considered the views of pregnancy care providers reported in consultation for a Clinical Practice Guideline on Smoking Cessation in pregnancy (Williams 2010) in the UK; and the views of guideline developers requesting evidence for an international guideline on ‘Management of Tobacco Use in Pregnancy’ (CDCP 2013). Some of the major issues and gaps included:
whether psychological interventions are effective;
whether interventions are effective for pregnant teens and other hard-to-reach and vulnerable groups, including ethnic and minority populations;
whether interventions are effective for women who are mentally unwell or experiencing substance misuse;
whether interventions are effective in low- and middle-income countries.
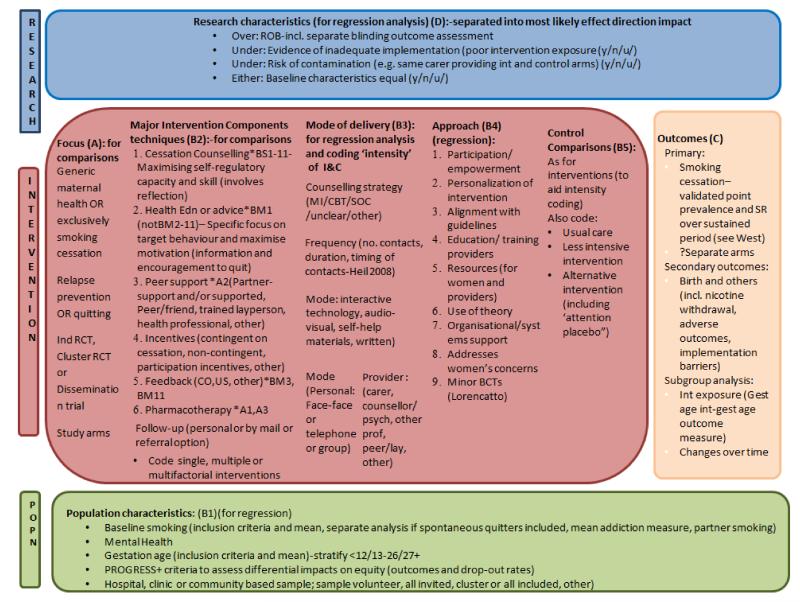
In addition to consideration of women’s views and feedback from guideline developers, we also considered thesis critiques of the previous version of this review (Gilligan 2008; Vilches 2009), health programme planning models (Green 2005b; Bartholomew 2011), various publications on factors affecting intervention efficacy (Greenhalgh 2004; Hoddinott 2010), descriptions of intervention components (Lorencatto 2012), and the ‘critical factors’ identified by authors of included studies reported in the results or discussion. As smoking in pregnancy has important impacts on health inequalities, we have introduced a focus on equity in this review, as recommended in the ‘PRISM-Equity’ guidelines for reporting interventions with a potential impact on equity (Welch 2012). We have synthesised this information into a logic model to identify key variables that may impact on intervention effectiveness (see Figure 1), to guide analysis and subgroup analyses planning ‘a priori’ (Petticrew 2012).
Figure 1. Logic model for systematic review analysis of potential factors impacting on efficacy of interventions for supporting women to stop smoking in pregnancy.
OBJECTIVES
This review evaluated the effect of psychosocial interventions designed to support women to stop smoking in pregnancy and aimed to address the following questions.
Primary objectives
To identify whether psychosocial interventions can support women to stop smoking in pregnancy
To compare the effectiveness of the main psychosocial intervention strategies in supporting women to stop smoking in pregnancy (i.e. counselling, health education, feedback, social support, incentives)
Secondary objectives
To identify if the intensity of the intervention corresponds to an effect size
To identify any specific intervention components associated with an effect (e.g. telephone counselling, self-help manuals)
To identify if psychosocial interventions in pregnancy have an impact on health outcomes for the mother (i.e. caesarean section, breastfeeding) and infant (i.e. mean birthweight, low birthweight, preterm births, very preterm births, perinatal mortality)
To identify if there are any positive or negative psychological effects reported among women receiving psychosocial interventions in pregnancy
To identify participants (women and pregnancy care providers) views of the psychosocial interventions in this review
To identify if psychosocial interventions have an effect on family functioning or other relationships for the mother, including non-accidental injury
To identify if psychosocial interventions during pregnancy can reduce the proportion of women who start smoking postpartum
To identify whether any methods for training and implementing psychosocial interventions have an effect on the knowledge, attitudes and behaviour of pregnancy care providers
To identify whether psychosocial interventions provided for women who have spontaneously quit smoking in early pregnancy, can reduce the proportion of women who start smoking by late pregnancy (relapse)
To identify whether psychosocial interventions are effective for women in vulnerable subpopulation groups (including women categorised as having low socio-economic status, young women (less than 20 years), ethnic minority and aboriginal women, and women in low- and middle-income countries
To identify whether psychosocial interventions, which are shown to be effective when implemented under trial conditions by a dedicated research team (efficacy studies), are still effective when implemented in a routine pregnancy care setting by existing staff (effectiveness studies)
To identify if psychosocial interventions to support women to stop smoking in pregnancy are cost-effective
To identify if there are any adverse effects reported as a result of women receiving psychosocial interventions to support them to stop smoking in pregnancy
To identify whether recently included studies are as effective as studies included in previous versions of this review
To identify if any of the risk of bias assessments have a significant impact on the effect size of the intervention
METHODS
Criteria for considering studies for this review
Types of studies
All randomised controlled trials, cluster-randomised controlled trials, and randomised cross-over trials of psychosocial interventions where a primary aim of the study was smoking cessation in pregnancy. Quasi-randomised studies were only considered for inclusion if there was a very low risk of interference with the sequence generation (e.g. allocation by odd or even maternal birth date or hospital record number).
Types of participants
Women who are currently smoking or have recently quit smoking and are pregnant, in any care setting.
Women who are currently smoking or have recently quit smoking and are seeking a pre-pregnancy consultation.
Health professionals in trials of implementation strategies of psychosocial interventions to support pregnant women to stop smoking.
Where possible, we have separated outcomes for women who spontaneously quit smoking when they become pregnant, and women who continue to smoke during pregnancy, as significant differences have been reported previously (Baric 1976; Ryan 1980; Cinciripini 2000; Passey 2012).
Types of interventions
Counselling interventions are those which provide motivation to quit, support to increase problem solving and coping skills (Ortendahl 2007c; Ortendahl 2008a; Ortendahl 2009b), and may incorporate ‘transtheoretical’ models of change (Prochaska 1992; Prochaska 2007). This includes interventions such as motivational interviewing, cognitive behaviour therapy, psychotherapy, relaxation, problem solving facilitation, and other strategies. Counselling interventions may be provided face-to-face, by telephone, via interactive computer programs, or using audiovisual equipment. The duration of counselling may range from brief interventions (less than five minutes) to more intensive interventions, which can last for up to an hour and be repeated over multiple sessions. Counselling may be provided by a range of personnel, including pregnancy care providers, trained counsellors, or others, on-site or by referral to specialist stop smoking services. Interventions that involved provision of videos with personal stories were included as counselling in this review.
Health education interventions are defined as those where women are provided with information about the risks of smoking and advice to quit, but are not given further support or advice about how to make this change. Interventions where the woman was provided with automated support such as self-help manuals or automated text messaging, but there was no personal interaction at all, were coded as health education in this review.
Feedback interventions are those where the mother is provided with feedback with information about the fetal health status or measurement of by-products of tobacco smoking to the mother. This includes interventions such as ultrasound monitoring and carbon monoxide or urine cotinine measurements, with results fed back to the mother (does not include where measurements are used for confirming smoking abstinence in the study).
Incentive-based interventions include those interventions where women receive a financial incentive, contingent on their smoking cessation; these incentives may be gift vouchers. Interventions that provided a ‘chance’ of incentive (e.g. lottery tickets) were not included as ‘incentives’ in this update, but were included in counselling and subgroup analysis of trials incorporating use of lottery tickets will be reported. Gifts and other incentives to promote participation in the study (but were not contingent on smoking cessation), were not coded as incentive-based interventions in this review.
Social support (peer and/or partner) includes those interventions where the intervention explicitly included provision of support from a peer (including self-nominated peers, ‘lay’ peers trained by project staff, or support from healthcare professionals), or partners, as a strategy to promote smoking cessation.
Other strategies, which could not be included in the categories listed above, including exercise, and dissemination interventions (where both intervention and control group received the same intervention, but the dissemination strategy differed).
In this review we have categorised interventions according to the ‘main’ strategy used, however many interventions incorporate several components. Therefore, interventions are coded according to whether the strategy was a:
single intervention - with only one main strategy used;
multiple intervention - which included several strategies being offered to all women;
tailored intervention - where additional optional strategies were available for women.
Trials that combined strategies for smoking cessation with other interventions to promote maternal health in pregnancy were considered for the review for smoking cessation and reduction outcomes but not for infant outcome measures such as birthweight, preterm birth, breastfeeding and perinatal mortality, which might be attributable to other components of an intervention package. We have included interventions that offered pharmacological therapies as part of a tailored intervention where there were higher levels of psychosocial support provided to participants in the intervention arm, compared with the control arm. Trials were excluded where the sole aim was to reduce: smokeless tobacco use; environmental tobacco smoke exposure; where the primary population was not pregnant women (e.g. partners, non-pregnant women); or the intervention was not primarily aimed at cessation during pregnancy (e.g. postpartum interventions). Studies were included where smokeless tobacco use, environmental tobacco smoke exposure or partner smoking were targeted in conjunction with interventions addressing the primary aim of supporting pregnant women to stop smoking in pregnancy. We have included dissemination studies, where the primary intervention includes strategies to disseminate smoking cessation interventions in pregnancy care settings (e.g. training, audit and feedback).
Types of comparisons
Any type of comparison group was included and was coded according to the following.
‘ Usual care’ or no additional intervention reported.
Less intensive interventions where the control group received some of the intervention or an approximation of ‘usual care’ consistently provided by the research team.
Alternative interventions, where the control group received different intervention components than the intervention group, of the same intensity.
Types of settings
Any setting, including residential and community settings, family planning clinics, pre-pregnancy planning clinics or general practitioner clinics, prenatal care clinics and hospitals.
The ‘PROGRESS-Plus’ criteria (Oliver 2008b; Ueffing 2009) were used to categorise interventions which were provided for vulnerable populations, including: social capital; place of residence; occupation; education; socio-economic status; ethnicity; age; or other factors which might impact on vulnerability. These categories are described in more detail in the methods.
Types of outcome measures
Primary outcomes
- Smoking abstinence in late pregnancy (point prevalence abstinence):
- self-reported or biochemically validated;
- biochemically validated only.
Secondary outcomes
Continued abstinence in late pregnancy after spontaneous quitting (relapse prevention) in early pregnancy (self-reported or biochemically validated).
- Smoking abstinence in the postpartum period (self-reported or biochemically validated):
- zero to five months;
- six to 11 months;
- 12 to 17 months;
- 18 months or longer.
- Smoking reduction from the first antenatal visit to late pregnancy:
- numbers of women reducing smoking (any definition, > 50% self-reported, or biochemically validated);
- biochemical measures (mean cotinine and thiocynate);
- mean cigarettes per day (self-reported).
- Perinatal outcomes:
- mean birthweight;
- low birthweight (proportion less than 2500 g);
- very low birthweight (less than 1500 g);
- preterm births (proportion less than 37 weeks);
- stillbirths;
- neonatal deaths;
- all perinatal deaths.
Mode of birth (caesarean section).
Breastfeeding initiation and breastfeeding at three and six months after birth.
Psychological effects: measures of anxiety, depression and maternal health status in late pregnancy and after birth.
Impact on family functioning and other relationships in late pregnancy and postpartum.
Participants’ views of the interventions, both women’s and pregnancy care providers’ views.
Measures of knowledge, attitudes and behaviour of health professionals (obstetricians, midwives and family physicians) with respect to facilitating smoking cessation in pregnancy.
Cost-effectiveness.
Adverse effects of smoking cessation programmes.
Search methods for identification of studies
This is the fifth update of this review and the details of previous searches are described in other published versions of this review (Lumley 1995a; Lumley 1995b; Lumley 1995c; Lumley 1995d; Lumley 1999; Lumley 2004; Lumley 2009).
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (1 March 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We also checked cited studies while reviewing the trial reports and key reviews. Where necessary, we contacted trial authors to locate additional unpublished data.
We did not apply any language restrictions.
[In addition, authors conducted a supplementary search for non-randomised studies, for the background and discussion, in MEDLINE, Embase, PsycLIT, and CINAHL (June 2008 to 1 March 2013) using the search strategy detailed in Appendix 2.]
Data collection and analysis
Selection of studies
Two review authors independently reviewed the full text of search results from the Cochrane Pregnancy and Childbirth Group and potential trials identified through other sources (CC/SP) to determine if they met the inclusion criteria for this review. Where there was disagreement, advice from co-authors was sought (SO/JC/AO/JT) and consensus reached by discussion.
Data extraction and management
Two review authors independently extracted data from the published reports without blinding as to journal, author, or research group. For each trial the following aspects were reported and coded into EPPI-Reviewer software (Thomas 2010). Independent data extraction was checked and areas of conflicting judgement were resolved by consensus, and where necessary discussion with co-authors. A summary of data collected is outlined in Appendix 3 and a summary reported for individual studies in the Characteristics of included studies table.
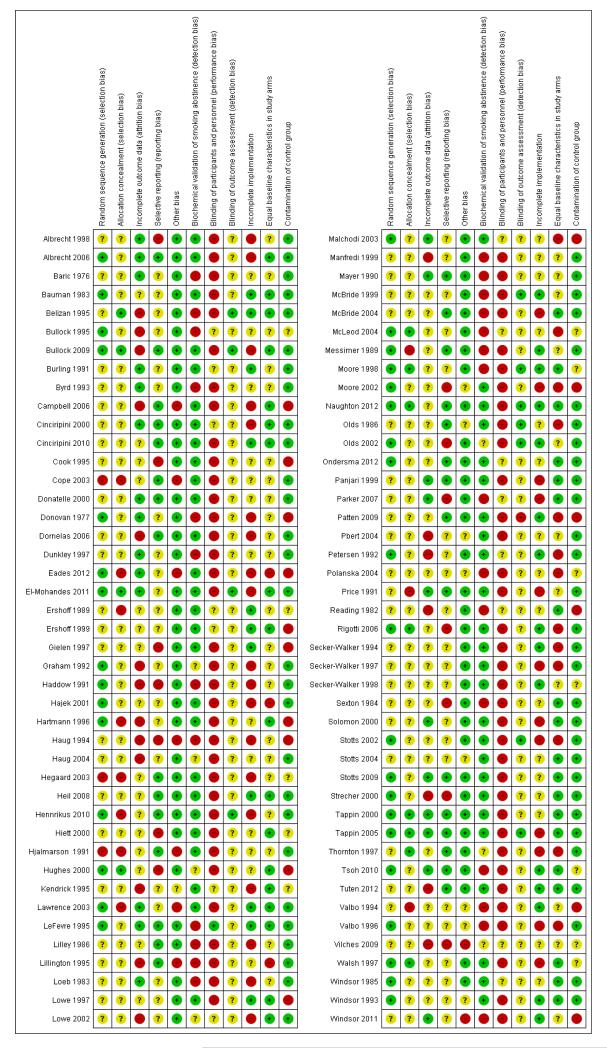
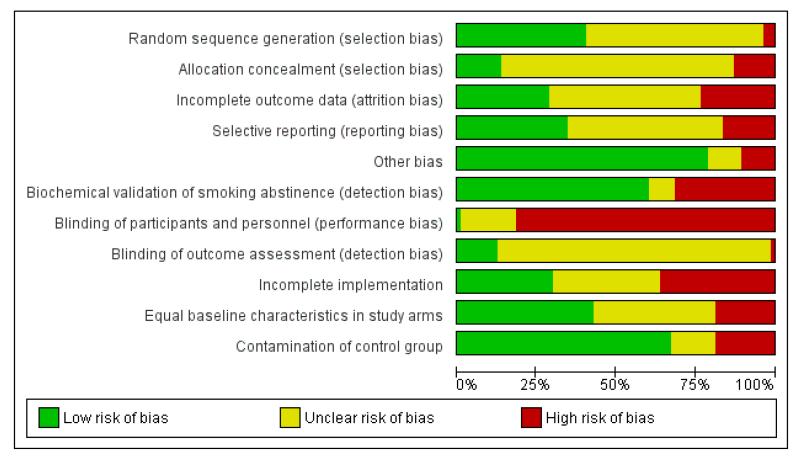
Assessment of risk of bias in included studies
We assessed the methodological quality of the included studies as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). The ‘quality assessment’ from previous reviews has been replaced with the ‘Risk of bias’ assessment.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the methods used to generate the allocation sequence, and have assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non random process, e.g. alternate clinic date; odd or even date of birth; hospital or clinic record number);
or unclear risk of bias.
Studies where sequence generation was assessed as inadequate and there is a reasonable opportunity to interfere with random allocation (e.g. alternate clinic date) have been excluded in this update of the review. Studies randomised by odd or even date of birth or medical record number have continued to be included in this review as there is limited reasonable opportunity to manipulate the allocation.
(2) Equal baseline characteristics (checking for possible selection bias)
To further assess the risk of selection bias, we assessed whether the baseline characteristics were equal in each included study, and have assessed them as:
low risk of bias (baseline characteristics were assessed and equal in both study arms);
high risk of bias (where there were significant differences in baseline characteristics, suggesting possible bias in the selection of participants);
or unclear risk of bias.
(3) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We have assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (e.g. open random allocation; unsealed or non-opaque envelopes; medical record number; date of birth);
or unclear risk of bias.
(4) Blinding (checking for possible performance bias) of study participants and intervention providers
We have described for each included study the methods used, if any, to blind study participants and intervention providers from knowledge of which intervention a participant received. However, it is rarely feasible in psychosocial interventions to blind women or the intervention providers to group allocation. We have assessed the methods as:
low risk of bias;
high risk of bias;
or unclear risk of bias.
(5) Blinding (checking for possible performance bias) of outcome assessor
We have described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received as recommended (West 2005). We have assessed the methods as:
low risk of bias;
high risk of bias;
or unclear risk of bias.
(6) Dealing with incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations, and intention-to-treat analysis)
We have described for each included study and for each outcome or class of outcomes the completeness of data including attrition and exclusions from the analysis. We have noted whether attritions and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups. We considered it was reasonable to exclude women from the final analysis who had experienced miscarriage or fetal demise, developed serious medical conditions, moved out of the area, or changed to another provider of care. However, as there are also clear associations between these outcomes and smoking, we have categorised the risk of attrition bias as ‘unclear’. Where possible, we included all other randomised women in the meta-analysis. Where data were not provided in such a way to enable inclusion of all other randomised participants, we have categorised these studies as high risk of attrition bias. We have assessed the methods as:
low risk of bias (outcomes for all randomised participants included in analysis);
high risk of bias (outcomes for all participants not reported, particularly if unequal attrition in both study arms);
or unclear risk of bias, which includes exclusions for medical conditions or moving.
(7) Reporting all outcomes (checking for possible selective reporting bias)
We have described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the studies’ prespecified primary outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the studies’ pre-specified outcomes have been reported); one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
or unclear risk of bias.
(8) Reliability of outcome measures used (checking for possible detection bias)
The unreliability of self-report as a measure of smoking status in healthcare settings, especially in maternity care (Pettiti 1981), was noted even in the first pregnancy trial (Donovan 1977). While this finding has not always been consistent (Fox 1989; Pickett 2009; Windsor 1985), the majority of other trials show substantial misclassification by self-report, with up to a quarter or a third of women who describe themselves as non-smokers having levels of salivary or urine cotinine (a metabolite of nicotine) incompatible with self-description (Mullen 1991; Petersen 1992; Kendrick 1995; Lillington 1995; Walsh 1997; Moore 2002; Tappin 2005; Parker 2007). A degree of misclassification is not surprising given the social stigma associated with smoking in pregnancy, and there appears to be less misclassification in non-pregnant populations (Patrick 1994). Some studies suggest that measurement of abstinence is reasonably accurate, but that there is greater inconsistency with reporting the amount of cigarettes smoked (Klebanoff 1998; Venditti 2012). Given this potential for bias, biochemical validation of smoking abstinence is now the standard for smoking cessation studies (West 2005; Shipton 2009). Use of cotinine concentration (saliva, urine or plasma) is the most sensitive and specific (saliva less than 15 ng/mL and urine less than 50 ng/mL). However, cotinine does not distinguish between smoking and use of nicotine replacement products, so expired air carbon monoxide is the preferred method for detecting recent smoking (less than 9 ppm) in many studies. Trials measuring cotinine need to ask participants about NRT use (available over the counter), ignore high levels in NRT users, and verify smoking abstinence with carbon monoxide levels (West 2005). However, several studies including use of NRT did use cotinine cut-offs to distinguish between smokers and non-smokers (Hegaard 2007). There may also be differential misclassification between intervention and control groups, though no investigations have published this effect. We have described for each included study whether the smoking outcome was biochemically validated (including measures used) or assessed by self-report only, and have included data on misclassification by self-report where they have been reported:
low risk of bias (biochemical validation);
high risk of bias (no biochemical validation);
or unclear risk of bias (including partial biochemical validation of a sample of the study population).
(9) Implementation of intervention
There are three main types of potential implementation problems trials (Walsh 2000):
not all participants in the intervention groups receiving the intervention;
intervention group participants not receiving all components of the intervention;
control groups receiving the intervention.
Failure to implement the intervention as planned limits the exposure of women to the intervention, and may negatively impact on the effectiveness of the intervention. Where possible, we included a description of any process evaluation reported. We have assessed the implementation of the intervention as:
low risk of bias (where process evaluation suggests the majority of participants received the intervention as planned);
high risk of bias (where process evaluation suggests a significant proportion of women did not receive the intervention as planned);
or unclear risk of bias (where process evaluation is not reported).
(10) Risk of control group contamination
Exposure of the control group to aspects of the intervention is a common challenge for intervention trials, particularly studies where healthcare providers are required to offer an intervention to some women, and not to others. Some trials use cluster-randomisation in order to reduce the risk of contamination, particularly when healthcare providers are involved in the intervention. The most likely impact is to increase the effect in the control arm, reducing the potential effect size between the intervention and control arms of the study. We have assessed the methods as:
low risk of bias, where the intervention providers are separate from the control group or strategies are employed to minimise the risk (such as cluster-randomisation);
high risk of bias, where the same provider is required to administer the intervention to both study arms, or there is specific reporting of suspected contamination in the trial report;
or unclear risk of bias.
(11) Other bias
We have considered any other potential sources of bias in the study, including whether recruitment was equal in both arms of cluster-randomised trials, and assessed these as:
low risk of bias;
high risk of bias;
or unclear risk of bias.
Measures of treatment effect
Dichotomous data
All data were entered into RevMan 5.2.5 and SPSS 20 for analysis. For dichotomous data, we have presented risk ratios (RR) with 95% confidence intervals. Analysis was conducted on the logged risk ratio, and then converted back to risk ratios for presentation purposes. In this update, smoking cessation outcomes have been converted from an ‘odds ratio’ for continued smoking, to a ‘RR’ for quitting, in line with other Cochrane Tobacco Group reviews. Therefore, an average RR > 1 in smoking cessation outcomes are positive in this review. Where less outcome events are desirable (e.g. preterm births, low birthweight infants, mean cigarettes per day), an average RR < 1 is a positive outcome. Analysis tables are labelled accordingly.
For two of the binary outcomes, abstinence in late pregnancy and perinatal deaths, zero cell counts for events in both the treatment and control groups were evident for one study each. The affected studies were Olds 1986 (abstinence in late pregnancy) and Valbo 1996 (perinatal deaths). This is problematic because the formula for calculating relative risk effect sizes requires non-zero cells (i.e., the numerator cannot be zero). Whilst RevMan 5.2.5 automatically corrects for zero events in one group, a manual ‘fix’ is required when both groups have zero events. The solution as recommended by the Cochrane statistician peer reviewer was to enter the values as zero in the analysis, which means the effect sizes are not estimable and those studies are effectively excluded from those analyses. The affected analyses are Analysis 9.1 for Olds 1986 and Analysis 1.16 and Analysis 11.15 for Valbo 1996. For all three of these affected analyses, the initial set of relevant studies was two; the result is that no pooled effect could be calculated because instead of two effect sizes we only have one effect size for each of these analyses. These instances are clearly marked in the results section.
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials (e.g. birthweight). We used the standardised mean difference (SMD) to combine trials that measured the same outcome, using different methods (e.g. biochemically-validated smoking reduction).
Where standard errors (SE) were reported instead of standard deviations (SD), we used the RevMan calculator to calculate the effect size estimate. In one study, the SD was calculated from the SE. Where no SDs or SEs were reported, we estimated the mean SD from available studies, as recommended in the Cochrane Handbook 16.1.3.1 (Higgins 2008). The mean birthweight SD was calculated from 13 studies with available SDs (mean SD 578), and imputed for six studies. The mean cigarettes per day SD was calculated from 14 studies with available SDs (mean SD 6.5), and imputed for five studies.
Unit of analysis issues
There are good reasons for considering random allocation of midwives, clinics, health educators, hospitals, general practitioners, or antenatal classes to intervention or comparison group, rather than random allocation of pregnant women. It may be difficult for pregnancy care providers to treat women differentially according to the intervention or usual care protocol, and not to introduce co-interventions in one or other groups (contamination). As women within a cluster are more likely to be similar to one another, and less like the women in another cluster, outcomes from cluster-randomised trials were adjusted for the intra-cluster correlation for the data to be included in this review. Adjusting for the clustering of studies means that cluster trials could be analysed in the same models as individual randomised trials.
Adjustment for cluster randomisation was conducted using a reported intra-cluster correlation (ICC) if available, and if not, a range of ICCs (from 0.003 to 0.20) was assumed and a sensitivity analysis conducted as recommended by (Merlo 2005). The results of the sensitivity analyses showed no substantial difference between the different ICCs (RRs were the same to at least three decimal places across ICC calculations). As such, for studies in which an ICC was not reported, an ICC value of 0.10 was used for the primary analysis and the cluster trials were included by adjusting the SEs (reported ICCs were used where available). The methods used for individual studies are reported in the Characteristics of included studies and Table 2. The adjustment involved reducing the size of each trial to its ‘effective sample size’ by dividing the sample size by the ‘design effect’, where the design effect is equal to 1 + (m - 1) × ICC, and m is the average cluster size (see Section 16.3.4 of the Cochrane Handbook, Higgins 2008).
Table 2. Cluster-randomised trial adjustment details.
| ICC | Trial ID |
Timing | Timing code |
Outcome description |
Outcome code |
Mean cluster size |
No. of clusters |
Sample size |
Ceased smoking % |
Continued smoking % |
Ceased smoking n |
Continued smoking n |
ICC | Between cluster var |
IF(int) | IF(comp) | Effective sample size, denominator |
Effective sample size, continue |
Effective sample size, ceased |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mi | mc | ci | cc | ni | nc | i% | c% | i% | c% | i | c | i | c | ri | rc | s^2c | ni | nc | i | c | OR | i | c | OR | RR | ||||||||
| 0.003 | |||||||||||||||||||||||||||||||||
| Campbell 2006 | 2nd or subsequent visit | 0 | 1 | 71.0 | 62.5 | 11 | 11 | 781 | 688 | 10.5 | 6.4 | 89.5 | 93.6 | 82 | 44 | 699 | 644 | 0.003 | 0.003 | 1.21 | 1.18 | 645 | 581 | 578 | 544 | 0.583 | 68 | 37 | 1.72 | 1.641 | |||
| Hajek 2001 | Birth | 0 | 1 | 5.9 | 6.7 | 92 | 86 | 545 | 575 | 22.0 | 20.0 | 78.0 | 80.0 | 120 | 115 | 425.1 | 460 | 0.003 | 0.003 | 1.01 | 1.017 | 537.1 | 565.4 | 419 | 452.3 | 0.886 | 118 | 113 | 1.13 | 1.1 | |||
| Haug 1994 | 0 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.003 | 0.003 | 1.00 | 1.00 | 251 | 98 | 205 | 90 | 0.398 | 46 | 8 | 2.51 | 2.237 | ||||
| Kendrick 1995 | 36/40 gest | 0 | 1 | 27.8 | 36.8 | 32 | 32 | 888 | 1177 | 5.9 | 6.1 | 94.1 | 93.9 | 52 | 72 | 835.6 | 1105 | 0.003 | 0.003 | 1.08 | 1.11 | 822 | 1063 | 774 | 998 | 1.036 | 48 | 65 | 0.97 | 0.967 | |||
| Lawrence 2003 | 30 wk gest | 0 | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 5.6 | 1.73 | 94.44 | 98.27 | 18 | 5 | 306 | 284 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 292 | 278 | 0.299 | 17 | 5 | 3.34 | 3.211 | |||
| Lillington 1995 | late preg | 0 | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 43.04 | 24.66 | 56.96 | 75.34 | 34 | 36 | 45 | 110 | 0.003 | 0.003 | 1.12 | 1.22 | 71 | 120 | 40 | 90 | 0.433 | 30 | 30 | 2.31 | 1.745 | |||
| McLeod 2004 | 36/40 gest | 0 | 1 | 6.273 | 4.615 | 11 | 13 | 69 | 60 | 20.3 | 13.3 | 79.71 | 86.67 | 14 | 8 | 55 | 52 | 1.096 | 1.100 | 63 | 55 | 50 | 47 | 0.604 | 13 | 7 | 1.65 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 9 | 7.5 | 12 | 8 | 108 | 60 | 17.6 | 10.0 | 82.41 | 90 | 19 | 6 | 89 | 54 | 1.075 | 1.100 | 100 | 55 | 83 | 49 | 0.52 | 18 | 5 | 1.92 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 7.696 | 5.714 | 23 | 21 | 177 | 120 | 18.6 | 11.7 | 81.36 | 88.33 | 33 | 14 | 144 | 106 | * | 1.10 | 163 | 109 | 133 | 96 | 0.577 | 30 | 13 | 1.73 | ||||||
| Messimer 1989 | 32-36 weeks' gest | 0 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 25.0 | 14.0 | 75 | 85.96 | 15 | 8 | 45 | 49 | 0.003 | 0.003 | 1.03 | 1.03 | 58 | 55 | 44 | 48 | 0.49 | 15 | 8 | 2.04 | 1.781 | |||
| Moore 2002 | 24-28/40 gest | 0 | 1 | 10.59 | 11.83 | 64 | 64 | 678 | 757 | 16.7 | 19.0 | 83.33 | 80.98 | 113 | 144 | 565 | 613 | 0.031 | 0.031 | 1.30 | 1.34 | 523 | 567 | 435 | 459 | 1.175 | 87 | 108 | 0.85 | 0.876 | |||
| Pbert 2004 | 36/40 gest | 0 | 1 | 63.67 | 100.5 | 3 | 2 | 191 | 201 | 20.0 | 11.0 | 80.0 | 89.0 | 38 | 22 | 152.8 | 178.9 | 0.003 | 0.003 | 1.19 | 1.30 | 161 | 155 | 129 | 138 | 0.493 | 32 | 17 | 2.03 | 1.822 | |||
| 0.05 | |||||||||||||||||||||||||||||||||
| Campbell 2006 | 2nd or subsequent visit | 0 | 1 | 71.0 | 62.5 | 11 | 11 | 781 | 688 | 10.5 | 6.4 | 89.5 | 93.6 | 82 | 44 | 699 | 644 | 0.050 | 0.050 | 4.50 | 4.08 | 174 | 169 | 155 | 158 | 0.583 | 18 | 11 | 1.72 | 1.641 | |||
| Hajek 2001 | Birth | 0 | 1 | 5.9 | 6.7 | 92 | 86 | 545 | 575 | 22.0 | 20.0 | 78.0 | 80.0 | 120 | 115 | 425.1 | 460 | 0.050 | 0.050 | 1.25 | 1.284 | 437.3 | 447.7 | 341 | 358.2 | 0.886 | 96 | 90 | 1.13 | 1.1 | |||
| Haug 1994 | 0 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.050 | 0.050 | 1.05 | 1.03 | 240 | 95 | 196 | 87 | 0.398 | 44 | 8 | 2.51 | 2.237 | ||||
| Kendrick 1995 | 36/40 gest | 0 | 1 | 27.8 | 36.8 | 32 | 32 | 888 | 1177 | 5.9 | 6.1 | 94.1 | 93.9 | 52 | 72 | 835.6 | 1105 | 0.003 | 0.003 | 1.08 | 1.11 | 822 | 1063 | 774 | 998 | 1.036 | 48 | 65 | 0.97 | 0.967 | |||
| Lawrence 2003 | 30 wk gest | 0 | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 5.6 | 1.73 | 94.44 | 98.27 | 18 | 5 | 306 | 284 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 292 | 278 | 0.299 | 17 | 5 | 3.34 | 3.211 | |||
| Lillington 1995 | late preg | 0 | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 43.04 | 24.66 | 56.96 | 75.34 | 34 | 36 | 45 | 110 | 0.050 | 0.050 | 2.93 | 4.60 | 27 | 32 | 15 | 24 | 0.433 | 12 | 8 | 2.31 | 1.745 | |||
| McLeod 2004 | 36/40 gest | 0 | 1 | 6.273 | 4.615 | 11 | 13 | 69 | 60 | 20.3 | 13.3 | 79.71 | 86.67 | 14 | 8 | 55 | 52 | 1.096 | 1.100 | 63 | 55 | 50 | 47 | 0.604 | 13 | 7 | 1.65 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 9 | 7.5 | 12 | 8 | 108 | 60 | 17.6 | 10.0 | 82.41 | 90 | 19 | 6 | 89 | 54 | 1.075 | 1.100 | 100 | 55 | 83 | 49 | 0.52 | 18 | 5 | 1.92 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 7.696 | 5.714 | 23 | 21 | 177 | 120 | 18.6 | 11.7 | 81.36 | 88.33 | 33 | 14 | 144 | 106 | * | 1.10 | 163 | 109 | 133 | 96 | 0.577 | 30 | 13 | 1.73 | ||||||
| Messimer 1989 | 32-36 weeks' gest | 0 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 25.0 | 14.0 | 75 | 85.96 | 15 | 8 | 45 | 49 | 0.050 | 0.050 | 1.50 | 1.47 | 40 | 39 | 30 | 33 | 0.49 | 10 | 5 | 2.04 | 1.781 | |||
| Moore 2002 | 24-28/40 gest | 0 | 1 | 11 | 12 | 64 | 64 | 678 | 757 | 16.7 | 19.0 | 83.33 | 80.98 | 113 | 144 | 565 | 613 | 0.031 | 0.031 | 1.30 | 1.34 | 523 | 567 | 435 | 459 | 1.175 | 87 | 108 | 0.85 | 0.876 | |||
| 36/40 gest | 0 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 20.0 | 11.0 | 80.0 | 89.0 | 38 | 22 | 152.8 | 178.9 | 0.050 | 0.050 | 4.13 | 5.98 | 46 | 34 | 37 | 30 | 0.493 | 9 | 4 | 2.03 | 1.822 | ||||
| 0.1 | |||||||||||||||||||||||||||||||||
| Campbell 2006 | 2nd or subsequent visit | 0 | 1 | 71 | 63 | 11 | 11 | 781 | 688 | 10.5 | 6.4 | 89.5 | 93.6 | 82 | 44 | 699 | 644 | 0.100 | 0.100 | 8.00 | 7.15 | 98 | 96 | 87 | 90 | 0.583 | 10 | 6 | 1.72 | 1.641 | |||
| Hajek 2001 | Birth | 0 | 1 | 6 | 7 | 92 | 86 | 545 | 575 | 22.0 | 20.0 | 78.0 | 80.0 | 120 | 115 | 425.1 | 460 | 0.100 | 0.100 | 1.49 | 1.569 | 365.2 | 366.6 | 285 | 293.3 | 0.886 | 80 | 73 | 1.13 | 1.1 | |||
| Haug 1994 | 0 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.100 | 0.100 | 1.10 | 1.06 | 229 | 93 | 187 | 85 | 0.398 | 42 | 8 | 2.51 | 2.237 | ||||
| Kendrick 1995 | 36/40 gest | 0 | 1 | 28 | 37 | 32 | 32 | 888 | 1177 | 5.9 | 6.1 | 94.1 | 93.9 | 52 | 72 | 835.6 | 1105 | 0.003 | 0.003 | 1.08 | 1.11 | 822 | 1063 | 774 | 998 | 1.036 | 48 | 65 | 0.97 | 0.967 | |||
| Lawrence 2003 | 30 wk gest | 0 | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 5.6 | 1.73 | 94.44 | 98.27 | 18 | 5 | 306 | 284 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 292 | 278 | 0.299 | 17 | 5 | 3.34 | 3.211 | |||
| Lillington 1995 | late preg | 0 | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 43.04 | 24.66 | 56.96 | 75.34 | 34 | 36 | 45 | 110 | 0.100 | 0.100 | 4.85 | 8.20 | 16 | 18 | 9 | 13 | 0.433 | 7 | 4 | 2.31 | 1.745 | |||
| McLeod 2004 | 36/40 gest | 0 | 1 | 6.273 | 4.615 | 11 | 13 | 69 | 60 | 20.3 | 13.3 | 79.71 | 86.67 | 14 | 8 | 55 | 52 | 1.096 | 1.100 | 63 | 55 | 50 | 47 | 0.604 | 13 | 7 | 1.65 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 9 | 7.5 | 12 | 8 | 108 | 60 | 17.6 | 10.0 | 82.41 | 90 | 19 | 6 | 89 | 54 | 1.075 | 1.100 | 100 | 55 | 83 | 49 | 0.52 | 18 | 5 | 1.92 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 7.696 | 5.714 | 23 | 21 | 177 | 120 | 18.6 | 11.7 | 81.36 | 88.33 | 33 | 14 | 144 | 106 | * | 1.10 | 163 | 109 | 133 | 96 | 0.577 | 30 | 13 | 1.73 | ||||||
| Messimer 1989 | 32-36 weeks' gest | 0 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 25.0 | 14.0 | 75 | 85.96 | 15 | 8 | 45 | 49 | 0.100 | 0.100 | 1.99 | 1.94 | 30 | 29 | 23 | 25 | 0.49 | 8 | 4 | 2.04 | 1.781 | |||
| Moore 2002 | 24-28/40 gest | 0 | 1 | 11 | 12 | 64 | 64 | 678 | 757 | 16.7 | 19.0 | 83.33 | 80.98 | 113 | 144 | 565 | 613 | 0.031 | 0.031 | 1.30 | 1.34 | 523 | 567 | 435 | 459 | 1.175 | 87 | 108 | 0.85 | 0.876 | |||
| Pbert 2004 | 36/40 gest | 0 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 20.0 | 11.0 | 80.0 | 89.0 | 38 | 22 | 152.8 | 178.9 | 0.100 | 0.100 | 7.27 | 10.95 | 26 | 18 | 21 | 16 | 0.493 | 5 | 2 | 2.03 | 1.822 | |||
| 0.2 | |||||||||||||||||||||||||||||||||
| Campbell 2006 | 2nd or subsequent visit | 0 | 1 | 71 | 63 | 11 | 11 | 781 | 688 | 10.5 | 6.4 | 89.5 | 93.6 | 82 | 44 | 699 | 644 | 0.200 | 0.200 | 15.00 | 13.31 | 52 | 52 | 47 | 48 | 0.583 | 5 | 3 | 1.72 | 1.641 | |||
| Hajek 2001 | Birth | 0 | 1 | 6 | 7 | 92 | 86 | 545 | 575 | 22.0 | 20.0 | 78.0 | 80.0 | 120 | 115 | 425.1 | 460 | 0.200 | 0.200 | 1.98 | 2.137 | 275 | 269 | 214 | 215 | 0.886 | 60 | 54 | 1.13 | 1.1 | |||
| Haug 1994 | 0 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.200 | 0.200 | 1.20 | 1.12 | 210 | 88 | 172 | 81 | 0.398 | 38 | 7 | 2.51 | 2.237 | ||||
| Kendrick 1995 | 36/40 gest | 0 | 1 | 28 | 37 | 32 | 32 | 888 | 1177 | 5.9 | 6.1 | 94.1 | 93.9 | 52 | 72 | 835.6 | 1105 | 0.003 | 0.003 | 1.08 | 1.11 | 822 | 1063 | 774 | 998 | 1.036 | 48 | 65 | 0.97 | 0.967 | |||
| Lawrence 2003 | 30 wk gest | 0 | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 5.6 | 1.73 | 94.44 | 98.27 | 18 | 5 | 306 | 284 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 292 | 278 | 0.299 | 17 | 5 | 3.34 | 3.211 | |||
| Lillington 1995 | late preg | 0 | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 43.04 | 24.66 | 56.96 | 75.34 | 34 | 36 | 45 | 110 | 0.200 | 0.200 | 8.70 | 15.40 | 9 | 9 | 5 | 7 | 0.433 | 4 | 2 | 2.31 | 1.745 | |||
| McLeod 2004 | 36/40 gest | 0 | 1 | 6.273 | 4.615 | 11 | 13 | 69 | 60 | 20.3 | 13.3 | 79.71 | 86.67 | 14 | 8 | 55 | 52 | 1.096 | 1.100 | 63 | 55 | 50 | 47 | 0.604 | 13 | 7 | 1.65 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 9 | 7.5 | 12 | 8 | 108 | 60 | 17.6 | 10.0 | 82.41 | 90 | 19 | 6 | 89 | 54 | 1.075 | 1.100 | 100 | 55 | 83 | 49 | 0.52 | 18 | 5 | 1.92 | ||||||
| McLeod 2004 | 36/40 gest | 0 | 1 | 7.696 | 5.714 | 23 | 21 | 177 | 120 | 18.6 | 11.7 | 81.36 | 88.33 | 33 | 14 | 144 | 106 | * | 1.10 | 163 | 109 | 133 | 96 | 0.577 | 30 | 13 | 1.73 | ||||||
| Messimer 1989 | 32-36 weeks' gest | 0 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 25.0 | 14.0 | 75 | 85.96 | 15 | 8 | 45 | 49 | 0.200 | 0.200 | 2.98 | 2.87 | 20 | 20 | 15 | 17 | 0.49 | 5 | 3 | 2.04 | 1.781 | |||
| Moore 2002 | 24-28/40 gest | 0 | 1 | 11 | 12 | 64 | 64 | 678 | 757 | 16.7 | 19.0 | 83.33 | 80.98 | 113 | 144 | 565 | 613 | 0.031 | 0.031 | 1.30 | 1.34 | 523 | 567 | 435 | 459 | 1.175 | 87 | 108 | 0.85 | 0.876 | |||
| Pbert 2004 | 36/40 gest | 0 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 20.0 | 11.0 | 80.0 | 89.0 | 38 | 22 | 152.8 | 178.9 | 0.200 | 0.200 | 13.53 | 20.90 | 14 | 10 | 11 | 9 | 0.493 | 3 | 1 | 2.03 | 1.822 | |||
| Key: | Outcome | Data given | Sensitivity analysis |
From formula in Merlo | * wt'd ave of IF in 2 intv arms | ||||||||||||||||||||||||||||
| ADDITIONAL OUTCOMES found 21/11/08 | |||||||||||||||||||||||||||||||||
| Eades 2012 | late preg | 0 | continued smoking for spontaneous quitters in late pregnancy | 2 | 24 | 8 | 14 | 6 | |||||||||||||||||||||||||
| Hajek 2001 | late preg | 0 | 2 | 1.2 | 1.6 | 92 | 86 | 114 | 135 | 22.0 | 20.0 | 64.9 | 53.3 | 40 | 63 | 74 | 72 | 0.003 | 0.003 | 1.00 | 1.002 | 113.9 | 134.8 | 74 | 71.88 | 1.619 | 40 | 63 | 0.62 | 0.752 | |||
| Lillington 1995 | late preg | 0 | 2 | 38 | 127.0 | 2 | 2 | 76 | 254 | 5.263 | 10.63 | 94.74 | 89.37 | 4 | 27 | 72 | 227 | 0.003 | 0.003 | 1.11 | 1.38 | 68 | 184 | 65 | 165 | 2.141 | 4 | 20 | 0.47 | 0.495 | |||
| Pbert 2004 | late preg | 0 | 2 | 27 | 39 | 3 | 2 | 81 | 77 | 70.4 | 77.9 | 29.6 | 22.1 | 57 | 60 | 24 | 17 | 0.003 | 0.003 | 1.08 | 1.11 | 75 | 69 | 22 | 15 | 1.486 | 53 | 54 | 0.67 | 0.903 | |||
| Polanska 2004 | late preg | 0 | 2 | 5.6 | 7.4 | 10 | 5 | 56 | 37 | 100 | 100 | 0 | 0 | 56 | 37 | 0 | 0 | 0.003 | 0.003 | 1.01 | 1.02 | 55 | 36 | 0 | 0 | ##### | 55 | 36 | #DIV/0! | 1 | |||
| Haug 1994 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.003 | 0.003 | 1.00 | 1.00 | 251 | 98 | 205 | 90 | 0.398 | 46 | 8 | 2.51 | 2.237 | ||
| Lawrence 2003 | 10 days pp | 1 | 10 days pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 8.0 | 3.5 | 92.0 | 96.5 | 26 | 10 | 298 | 279 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 284 | 273 | 0.411 | 25 | 10 | 2.43 | |||
| Lillington 1995 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 25.32 | 11.64 | 74.68 | 88.36 | 20 | 17 | 59 | 129 | 0.003 | 0.003 | 1.12 | 1.22 | 71 | 120 | 53 | 106 | 0.389 | 18 | 14 | 2.57 | 2.174 | ||
| McLeod 2004 | 4/12pp | 1 | 1 | 8 | 6 | 23 | 21 | 177 | 120 | 15.8 | 10.8 | 84.2 | 89.2 | 28 | 13 | 149 | 107 | 0.003 | 0.003 | 1.02 | 1.01 | 174 | 118 | 146 | 106 | 0.647 | 27 | 13 | 1.55 | ||||
| Messimer 1989 | 0-5 mo pp | 1 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 8.3 | 10.5 | 91.67 | 89.47 | 5 | 6 | 55 | 51 | 0.003 | 0.003 | 1.03 | 1.03 | 58 | 55 | 53 | 50 | 1.294 | 5 | 6 | 0.77 | 0.792 | |||
| Pbert 2004 | 0-5 mo pp | 1 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.2 | 3.0 | 95.8 | 97.0 | 8 | 6 | 183 | 195 | 0.003 | 0.003 | 1.19 | 1.30 | 161 | 155 | 154 | 150 | 0.704 | 7 | 5 | 1.42 | 1.403 | |||
| Polanska 2004 | 0-5 mo pp | 1 | 1 | 14.9 | 28.8 | 10 | 5 | 149 | 144 | 44.3 | 16.7 | 55.7 | 83.3 | 66 | 24 | 82.99 | 120 | 0.003 | 0.003 | 1.04 | 1.08 | 143 | 133 | 80 | 111 | 0.252 | 63 | 22 | 3.97 | 2.653 | |||
| Hajek 2001 | 6 mo pp | 2 | maintained cessation at 6-11 mo pp | 1 | 4.7 | 5.1 | 92 | 86 | 431 | 440 | 22.0 | 20.0 | 97.0 | 97.0 | 13 | 13 | 418 | 427 | 0.003 | 0.003 | 1.01 | 1.012 | 426.3 | 434.6 | 413 | 421.8 | 0.979 | 13 | 13 | 1.02 | 1.021 | ||
| Haug 1994 | 6-11 mo pp | 2 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 84.52 | 88.78 | 39 | 11 | 213 | 87 | 0.003 | 0.003 | 1.00 | 1.00 | 251 | 98 | 212 | 87 | 0.691 | 39 | 11 | 1.45 | 1.379 | |||
| Pbert 2004 | 6-11 mo pp | 2 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.7 | 2.5 | 95.3 | 97.5 | 9 | 5 | 182 | 196 | 0.003 | 0.003 | 1.19 | 1.30 | 161 | 155 | 153 | 151 | 0.516 | 8 | 4 | 1.94 | 1.894 | |||
| Haug 1994 | 12-17 mo pp | 3 | maintained cessation at 12-17 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 85.32 | 92.86 | 37 | 7 | 215 | 91 | 0.003 | 0.003 | 1.00 | 1.00 | 251 | 98 | 214 | 91 | 0.447 | 37 | 7 | 2.24 | 2.056 | ||
| Polanska 2004 | 12-17 mo pp | 3 | 1 | 20.5 | 36.2 | 10 | 5 | 205 | 181 | 31.7 | 12.7 | 68.29 | 87.29 | 65 | 23 | 140 | 158 | 0.003 | 0.003 | 1.06 | 1.11 | 194 | 164 | 132 | 143 | 0.314 | 61 | 21 | 3.19 | 2.495 | |||
| Lawrence 2003 | 18 mo pp | 4 | 18 mo pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 4.6 | 2.4 | 95.4 | 97.6 | 15 | 7 | 309 | 282 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 295 | 276 | 0.511 | 14 | 7 | 1.96 | |||
| 0.05 | |||||||||||||||||||||||||||||||||
| Eades 2012 | late preg | 0 | continued smoking for spontaneous quitters in late pregnancy | 2 | 24 | 8 | 14 | 6 | |||||||||||||||||||||||||
| Hajek 2001 | late preg | 0 | 2 | 1.2 | 1.6 | 92 | 86 | 114 | 135 | 22.0 | 20.0 | 64.9 | 53.3 | 40 | 63 | 74 | 72 | 0.050 | 0.050 | 1.01 | 1.028 | 112.7 | 131.3 | 73 | 70.01 | 1.619 | 40 | 61 | 0.62 | 0.752 | |||
| Lillington 1995 | late preg | 0 | 2 | 38 | 127.0 | 2 | 2 | 76 | 254 | 5.263 | 10.63 | 94.74 | 89.37 | 4 | 27 | 72 | 227 | 0.050 | 0.050 | 2.85 | 7.30 | 27 | 35 | 25 | 31 | 2.141 | 1 | 4 | 0.47 | 0.495 | |||
| Pbert 2004 | late preg | 0 | 2 | 27 | 39 | 3 | 2 | 81 | 77 | 70.4 | 77.9 | 29.6 | 22.1 | 57 | 60 | 24 | 17 | 0.050 | 0.050 | 2.30 | 2.88 | 35 | 27 | 10 | 6 | 1.486 | 25 | 21 | 0.67 | 0.903 | |||
| Polanska 2004 | late preg | 0 | 2 | 5.6 | 7.4 | 10 | 5 | 56 | 37 | 100 | 100 | 0 | 0 | 56 | 37 | 0 | 0 | 0.050 | 0.050 | 1.23 | 1.32 | 46 | 28 | 0 | 0 | ##### | 46 | 28 | #DIV/0! | 1 | |||
| Haug 1994 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.050 | 0.050 | 1.05 | 1.03 | 240 | 95 | 196 | 87 | 0.398 | 44 | 8 | 2.51 | 2.237 | ||
| Lawrence 2003 | 10 days pp | 1 | 10 days pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 8.0 | 3.5 | 92.0 | 96.5 | 26 | 10 | 298 | 279 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 284 | 273 | 0.411 | 25 | 10 | 2.43 | |||
| Lillington 1995 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 25.32 | 11.64 | 74.68 | 88.36 | 20 | 17 | 59 | 129 | 0.050 | 0.050 | 2.93 | 4.60 | 27 | 32 | 20 | 28 | 0.389 | 7 | 4 | 2.57 | 2.174 | ||
| McLeod 2004 | 4/12pp | 1 | 1 | 8 | 6 | 23 | 21 | 177 | 120 | 15.8 | 10.8 | 84.2 | 89.2 | 28 | 13 | 149 | 107 | 0.050 | 0.050 | 1.33 | 1.24 | 133 | 97 | 112 | 87 | 0.647 | 21 | 11 | 1.55 | ||||
| Messimer 1989 | 0-5 mo pp | 1 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 8.3 | 10.5 | 91.67 | 89.47 | 5 | 6 | 55 | 51 | 0.050 | 0.050 | 1.50 | 1.47 | 40 | 39 | 37 | 35 | 1.294 | 3 | 4 | 0.77 | 0.792 | |||
| Pbert 2004 | 0-5 mo pp | 1 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.2 | 3.0 | 95.8 | 97.0 | 8 | 6 | 183 | 195 | 0.050 | 0.050 | 4.13 | 5.98 | 46 | 34 | 44 | 33 | 0.704 | 2 | 1 | 1.42 | 1.403 | |||
| Polanska 2004 | 0-5 mo pp | 1 | 1 | 15 | 29 | 10 | 5 | 149 | 144 | 44.3 | 16.7 | 55.7 | 83.3 | 66 | 24 | 82.99 | 120 | 0.050 | 0.050 | 1.70 | 2.39 | 88 | 60 | 49 | 50 | 0.252 | 39 | 10 | 3.97 | 2.653 | |||
| Hajek 2001 | 6 mo pp | 2 | maintained cessation at 6-11 mo pp | 1 | 4.7 | 5.1 | 92 | 86 | 431 | 440 | 22.0 | 20.0 | 97.0 | 97.0 | 13 | 13 | 418 | 427 | 0.050 | 0.050 | 1.18 | 1.206 | 363.9 | 364.9 | 353 | 354.1 | 0.979 | 11 | 11 | 1.02 | 1.021 | ||
| Haug 1994 | 6-11 mo pp | 2 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 84.52 | 88.78 | 39 | 11 | 213 | 87 | 0.050 | 0.050 | 1.05 | 1.03 | 240 | 95 | 203 | 85 | 0.691 | 37 | 11 | 1.45 | 1.379 | |||
| Pbert 2004 | 6-11 mo pp | 2 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.7 | 2.5 | 95.3 | 97.5 | 9 | 5 | 182 | 196 | 0.050 | 0.050 | 4.13 | 5.98 | 46 | 34 | 44 | 33 | 0.516 | 2 | 1 | 1.94 | 1.894 | |||
| Haug 1994 | 12-17 mo pp | 3 | maintained cessation at 12-17 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 85.32 | 92.86 | 37 | 7 | 215 | 91 | 0.050 | 0.050 | 1.05 | 1.03 | 240 | 95 | 205 | 88 | 0.447 | 35 | 7 | 2.24 | 2.056 | ||
| Polanska 2004 | 12-17 mo pp | 3 | 1 | 20.5 | 36.2 | 10 | 5 | 205 | 181 | 31.7 | 12.7 | 68.29 | 87.29 | 65 | 23 | 140 | 158 | 0.050 | 0.050 | 1.98 | 2.76 | 104 | 66 | 71 | 57 | 0.314 | 33 | 8 | 3.19 | 2.495 | |||
| Lawrence 2003 | 18 mo pp | 4 | 18 mo pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 4.6 | 2.4 | 95.4 | 97.6 | 15 | 7 | 309 | 282 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 295 | 276 | 0.511 | 14 | 7 | 1.96 | |||
| 0.1 | |||||||||||||||||||||||||||||||||
| Eades 2012 | late preg | 0 | continued smoking for spontaneous quitters in late pregnancy | 2 | 24 | 8 | 14 | 6 | |||||||||||||||||||||||||
| Hajek 2001 | late preg | 0 | 2 | 1.2 | 1.6 | 92 | 86 | 114 | 135 | 22.0 | 20.0 | 64.9 | 53.3 | 40 | 63 | 74 | 72 | 0.100 | 0.100 | 1.02 | 1.057 | 111.3 | 127.7 | 72 | 68.12 | 1.619 | 39 | 60 | 0.62 | 0.752 | |||
| Lillington 1995 | late preg | 0 | 2 | 38 | 127.0 | 2 | 2 | 76 | 254 | 5.263 | 10.63 | 94.74 | 89.37 | 4 | 27 | 72 | 227 | 0.100 | 0.100 | 4.70 | 13.60 | 16 | 19 | 15 | 17 | 2.141 | 1 | 2 | 0.47 | 0.495 | |||
| Pbert 2004 | late preg | 0 | 2 | 27 | 39 | 3 | 2 | 81 | 77 | 70.4 | 77.9 | 29.6 | 22.1 | 57 | 60 | 24 | 17 | 0.100 | 0.100 | 3.60 | 4.75 | 23 | 16 | 7 | 4 | 1.486 | 16 | 13 | 0.67 | 0.903 | |||
| Polanska 2004 | late preg | 0 | 2 | 5.6 | 7.4 | 10 | 5 | 56 | 37 | 100 | 100 | 0 | 0 | 56 | 37 | 0 | 0 | 0.100 | 0.100 | 1.46 | 1.64 | 38 | 23 | 0 | 0 | ##### | 38 | 23 | #DIV/0! | 1 | |||
| Haug 1994 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.100 | 0.100 | 1.10 | 1.06 | 229 | 93 | 187 | 85 | 0.398 | 42 | 8 | 2.51 | 2.237 | ||
| Lawrence 2003 | 10 days pp | 1 | 10 days pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 8.0 | 3.5 | 92.0 | 96.5 | 26 | 10 | 298 | 279 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 284 | 273 | 0.411 | 25 | 10 | 2.43 | |||
| Lillington 1995 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 25.32 | 11.64 | 74.68 | 88.36 | 20 | 17 | 59 | 129 | 0.100 | 0.100 | 4.85 | 8.20 | 16 | 18 | 12 | 16 | 0.389 | 4 | 2 | 2.57 | 2.174 | ||
| McLeod 2004 | 4/12pp | 1 | 1 | 8 | 6 | 23 | 21 | 177 | 120 | 15.8 | 10.8 | 84.2 | 89.2 | 28 | 13 | 149 | 107 | 0.100 | 0.100 | 1.67 | 1.47 | 106 | 82 | 89 | 73 | 0.647 | 17 | 9 | 1.55 | ||||
| Messimer 1989 | 0-5 mo pp | 1 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 8.3 | 10.5 | 91.67 | 89.47 | 5 | 6 | 55 | 51 | 0.100 | 0.100 | 1.99 | 1.94 | 30 | 29 | 28 | 26 | 1.294 | 3 | 3 | 0.77 | 0.792 | |||
| Pbert 2004 | 0-5 mo pp | 1 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.2 | 3.0 | 95.8 | 97.0 | 8 | 6 | 183 | 195 | 0.100 | 0.100 | 7.27 | 10.95 | 26 | 18 | 25 | 18 | 0.704 | 1 | 1 | 1.42 | 1.403 | |||
| Polanska 2004 | 0-5 mo pp | 1 | 1 | 15 | 29 | 10 | 5 | 149 | 144 | 44.3 | 16.7 | 55.7 | 83.3 | 66 | 24 | 82.99 | 120 | 0.100 | 0.100 | 2.39 | 3.78 | 62 | 38 | 35 | 32 | 0.252 | 28 | 6 | 3.97 | 2.653 | |||
| Hajek 2001 | 6 mo pp | 2 | maintained cessation at 6-11 mo pp | 1 | 4.7 | 5.1 | 92 | 86 | 431 | 440 | 22.0 | 20.0 | 97.0 | 97.0 | 13 | 13 | 418 | 427 | 0.100 | 0.100 | 1.37 | 1.412 | 314.9 | 311.7 | 305 | 302.5 | 0.979 | 9 | 9 | 1.02 | 1.021 | ||
| Haug 1994 | 6-11 mo pp | 2 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 84.52 | 88.78 | 39 | 11 | 213 | 87 | 0.100 | 0.100 | 1.10 | 1.06 | 229 | 93 | 194 | 82 | 0.691 | 35 | 10 | 1.45 | 1.379 | |||
| Pbert 2004 | 6-11 mo pp | 2 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.7 | 2.5 | 95.3 | 97.5 | 9 | 5 | 182 | 196 | 0.100 | 0.100 | 7.27 | 10.95 | 26 | 18 | 25 | 18 | 0.516 | 1 | 0 | 1.94 | 1.894 | |||
| Haug 1994 | 12-17 mo pp | 3 | maintained cessation at 12-17 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 85.32 | 92.86 | 37 | 7 | 215 | 91 | 0.100 | 0.100 | 1.10 | 1.06 | 229 | 93 | 195 | 86 | 0.447 | 34 | 7 | 2.24 | 2.056 | ||
| Polanska 2004 | 12-17 mo pp | 3 | 1 | 20.5 | 36.2 | 10 | 5 | 205 | 181 | 31.7 | 12.7 | 68.29 | 87.29 | 65 | 23 | 140 | 158 | 0.100 | 0.100 | 2.95 | 4.52 | 69 | 40 | 47 | 35 | 0.314 | 22 | 5 | 3.19 | 2.495 | |||
| Lawrence 2003 | 18 mo pp | 4 | 18 mo pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 4.6 | 2.4 | 95.4 | 97.6 | 15 | 7 | 309 | 282 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 295 | 276 | 0.511 | 14 | 7 | 1.96 | |||
| 0.2 | |||||||||||||||||||||||||||||||||
| Eades 2012 | late preg | 0 | continued smoking for spontaneous quitters in late pregnancy | 2 | 24 | 8 | 14 | 6 | |||||||||||||||||||||||||
| Hajek 2001 | late preg | 0 | 2 | 1.2 | 1.6 | 92 | 86 | 114 | 135 | 22.0 | 20.0 | 64.9 | 53.3 | 40 | 63 | 74 | 72 | 0.200 | 0.200 | 1.05 | 1.114 | 108.8 | 121.2 | 71 | 64.63 | 1.619 | 38 | 57 | 0.62 | 0.752 | |||
| Lillington 1995 | late preg | 0 | 2 | 38 | 127.0 | 2 | 2 | 76 | 254 | 5.263 | 10.63 | 94.74 | 89.37 | 4 | 27 | 72 | 227 | 0.200 | 0.200 | 8.40 | 26.20 | 9 | 10 | 9 | 9 | 2.141 | 0 | 1 | 0.47 | 0.495 | |||
| Pbert 2004 | late preg | 0 | 2 | 27 | 39 | 3 | 2 | 81 | 77 | 70.4 | 77.9 | 29.6 | 22.1 | 57 | 60 | 24 | 17 | 0.200 | 0.200 | 6.20 | 8.50 | 13 | 9 | 4 | 2 | 1.486 | 9 | 7 | 0.67 | 0.903 | |||
| Polanska 2004 | late preg | 0 | 2 | 5.6 | 7.4 | 10 | 5 | 56 | 37 | 100 | 100 | 0 | 0 | 56 | 37 | 0 | 0 | 0.200 | 0.200 | 1.92 | 2.28 | 29 | 16 | 0 | 0 | ##### | 29 | 16 | #DIV/0! | 1 | |||
| Haug 1994 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 81.75 | 91.84 | 46 | 8 | 206 | 90 | 0.200 | 0.200 | 1.20 | 1.12 | 210 | 88 | 172 | 81 | 0.398 | 38 | 7 | 2.51 | 2.237 | ||
| Lawrence 2003 | 10 days pp | 1 | 10 days pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 8.0 | 3.5 | 92.0 | 96.5 | 26 | 10 | 298 | 279 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 284 | 273 | 0.411 | 25 | 10 | 2.43 | |||
| Lillington 1995 | 0-5 mo pp | 1 | maintained cessation at 0-5 mo pp | 1 | 39.5 | 73.0 | 2 | 2 | 79 | 146 | 25.32 | 11.64 | 74.68 | 88.36 | 20 | 17 | 59 | 129 | 0.200 | 0.200 | 8.70 | 15.40 | 9 | 9 | 7 | 8 | 0.389 | 2 | 1 | 2.57 | 2.174 | ||
| McLeod 2004 | 4/12pp | 1 | 1 | 8 | 6 | 23 | 21 | 177 | 120 | 15.8 | 10.8 | 84.2 | 89.2 | 28 | 13 | 149 | 107 | 0.200 | 0.200 | 2.34 | 1.94 | 76 | 62 | 64 | 55 | 0.647 | 12 | 7 | 1.55 | ||||
| Messimer 1989 | 0-5 mo pp | 1 | 1 | 10.91 | 10.36 | 5.5 | 5.5 | 60 | 57 | 8.3 | 10.5 | 91.67 | 89.47 | 5 | 6 | 55 | 51 | 0.200 | 0.200 | 2.98 | 2.87 | 20 | 20 | 18 | 18 | 1.294 | 2 | 2 | 0.77 | 0.792 | |||
| Pbert 2004 | 0-5 mo pp | 1 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.2 | 3.0 | 95.8 | 97.0 | 8 | 6 | 183 | 195 | 0.200 | 0.200 | 13.53 | 20.90 | 14 | 10 | 14 | 9 | 0.704 | 1 | 0 | 1.42 | 1.403 | |||
| Polanska 2004 | 0-5 mo pp | 1 | 1 | 15 | 29 | 10 | 5 | 149 | 144 | 44.3 | 16.7 | 55.7 | 83.3 | 66 | 24 | 82.99 | 120 | 0.200 | 0.200 | 3.78 | 6.56 | 39 | 22 | 22 | 18 | 0.252 | 17 | 4 | 3.97 | 2.653 | |||
| Hajek 2001 | 6 mo pp | 2 | maintained cessation at 6-11 mo pp | 1 | 4.7 | 5.1 | 92 | 86 | 431 | 440 | 22.0 | 20.0 | 97.0 | 97.0 | 13 | 13 | 418 | 427 | 0.200 | 0.200 | 1.74 | 1.823 | 248.1 | 241.3 | 241 | 234.2 | 0.979 | 7 | 7 | 1.02 | 1.021 | ||
| Haug 1994 | 6-11 mo pp | 2 | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 84.52 | 88.78 | 39 | 11 | 213 | 87 | 0.200 | 0.200 | 1.20 | 1.12 | 210 | 88 | 178 | 78 | 0.691 | 33 | 10 | 1.45 | 1.379 | |||
| Pbert 2004 | 6-11 mo pp | 2 | 1 | 64 | 101 | 3 | 2 | 191 | 201 | 4.7 | 2.5 | 95.3 | 97.5 | 9 | 5 | 182 | 196 | 0.200 | 0.200 | 13.53 | 20.90 | 14 | 10 | 13 | 9 | 0.516 | 1 | 0 | 1.94 | 1.894 | |||
| Haug 1994 | 12-17 mo pp | 3 | maintained cessation at 12-17 mo pp | 1 | 2 | 2 | 125 | 62 | 252 | 98 | 18.25 | 8.16 | 85.32 | 92.86 | 37 | 7 | 215 | 91 | 0.200 | 0.200 | 1.20 | 1.12 | 210 | 88 | 179 | 82 | 0.447 | 31 | 6 | 2.24 | 2.056 | ||
| Polanska 2004 | 12-17 mo pp | 3 | 1 | 20.5 | 36.2 | 10 | 5 | 205 | 181 | 31.7 | 12.7 | 68.29 | 87.29 | 65 | 23 | 140 | 158 | 0.200 | 0.200 | 4.90 | 8.04 | 42 | 23 | 29 | 20 | 0.314 | 13 | 3 | 3.19 | 2.495 | |||
| Lawrence 2003 | 18 mo pp | 4 | 18 mo pp | 1 | 17 | 8 | 23 | 41 | 324 | 289 | 4.6 | 2.4 | 95.4 | 97.6 | 15 | 7 | 309 | 282 | 0.003 | 0.003 | 1.05 | 1.02 | 309 | 283 | 295 | 276 | 0.511 | 14 | 7 | 1.96 | |||
| Key: | Outcome | Data given | Sensitivity analysis |
From formula in Merlo | |||||||||||||||||||||||||||||
Timing codes:
0. Late pregnancy
1. 0-5 mo pp
2. 6-11 mo pp
3. 12-17 mo pp
4. 18 mo pp
Outcome codes:
1. abstinence
2. relapse prevention for spontaneous quitters
Dealing with missing data
Due to the nature of the intervention, there is a high likelihood that women withdrawing from the study or not providing a biochemical sample for analysis, without a ‘plausible explanation’ (e.g. miscarriage/fetal demise, moving out of the area or changed to another provider of care) are likely to be continuing smokers. Where sufficient information has been reported or has been supplied by the trial authors, we have re-included missing data from each treatment group in the analyses to comply with recommended outcome criteria assessment for smoking cessation trials (West 2005). Only data which were excluded for medical reasons (e.g. miscarriage or preterm birth) or moving from study site were not re-included in this review. We have indicated where an intention-to-treat (ITT) (or available case) analysis was carried out for the smoking cessation outcome in the published report, or adjusted for this review. These assessments and any adjustments are reported in the ‘Risk of bias’ tables (see incomplete outcome data). Where data could not be re-included, we conducted sensitivity analysis to determine the effect of inclusion of trials assessed as ‘high risk’ of attrition bias.
Assessment of heterogeneity
We examined levels of heterogeneity in all pooled analyses (Cochran 1954). We used the I2 statistic to quantify heterogeneity (i.e., inconsistency) among the trials in each analysis (Higgins 2008) and Chi2 tests to assess the presence of significant variation amongst effect sizes (i.e., whether the observed effects are significantly different from chance) (Lipsey 2001; Higgins 2008). Forthe Chi2 tests, in addition to the P value, we report the Q-statistic calculated by the test and the degrees of freedom of the test.
We expected to find a substantial degree of heterogeneity given the breadth of types of interventions, which are broadly categorised as ‘psychosocial’ and the differences in comparisons. Therefore, we attempted to minimise heterogeneity in this update by reporting separate comparisons for each main intervention strategy (counselling, health education, feedback, incentives, and social support; and whether the intervention was provided as a specific smoking intervention or as part of a broader intervention to improve maternal health) and comparison type (usual care, less intensive intervention, or alternative intervention). Further, we grouped studies within each comparison according to whether the intervention was provided as a single, multiple or tailored intervention.
To indicate considerable statistical heterogeneity, we set a threshold of inconsistency of I2 > 75% and a Chi2 significance level of P < 0.05. Where considerable heterogeneity was evident, we did not present pooled results. We further explored heterogeneity by prespecified secondary analysis identified during development of a logic model (see Figure 1 and section on Subgroup analysis and investigation of heterogeneity for a description).
Assessment of reporting biases
Concerns about publication bias have been raised after observations that research evaluations showing beneficial and/or statistically significant findings are more likely to be published than those that have undesirable outcomes or non-significant findings (Higgins 2008). If this phenomenon does occur, then reviews of a biased evidence base will draw biased conclusions. Unfortunately, it is difficult to assess publication bias because there is no way of knowing the extent of what has not been published.
As a result of these concerns, researchers have developed ways of estimating the extent to which there may be some publication bias in the evidence base. Funnel plots (scatter plots in which the effect size from individual studies are plotted against a measure of study precision) are a common method for assessing the possibility of publication bias. Ideally, the spread of effect sizes should be such that there is more scattering of effect sizes at the bottom of the plot, where there is less precision, with a narrowing of the scattering towards the top, where there is greater precision.
Following guidance (Sterne 2001; Higgins 2008), we produced a funnel plot of the RR for the primary outcome on the x-axis, and the SE of the log RR on the y-axis, for each of the main comparisons (Analyses 1 through 10). Only the funnel plots for ‘counselling versus usual care’ (Analysis 1.1, Figure 2) and ‘counselling versus less intensive intervention’ (Analysis 2.1, Figure 3) are shown, because the remaining comparisons had too few effect sizes to reliably detect asymmetry in the funnel plot. In the figures, the vertical line indicates the random-effects pooled effect size estimate. In the absence of publication bias, we would expect a roughly symmetrical distribution of effect sizes in the inverted funnel shape. Two review authors examined the plot for publication bias; under the assumption that publication bias is detectable in these funnel plots, we conclude that it is unlikely that publication bias has biased the findings of this review.
Figure 2. Funnel plot of comparison: 1 Smoking cessation interventions: counselling vs usual care, outcome: 1.1 Abstinence in late pregnancy.
Figure 3. Funnel plot of comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention, outcome: 2.1 Abstinence in late pregnancy.
Data synthesis
We used the statistical methods described in the Cochrane Handbook (Higgins 2008). We adopted a random-effects approach using method of moments estimators. The comparison analyses and forest plots were generated in RevMan 5.2.5, and meta-regressions and other subgroup analyses (using an analog to the ANOVA) were conducted in SPSS 20.0 using macros developed by Wilson 2005. When examining statistical significance, P values greater than 0.05 were considered non-significant. Where only one study was included in the comparison, the outcomes are not displayed in a separate comparison table and are reported in text only in the results, and data used is displayed in Comparison 11 of ‘all outcomes by main intervention strategy’ (see Analysis 11.1 for primary outcome and subsequent analyses for secondary outcomes). Effect sizes that were included in the subgroup analyses for the primary outcome (reported in Section 1.2 of the results) were checked for outliers. First, skewness and SE of the skewness were calculated for the primary outcome in SPSS. Skewness was considered to be statistically significant at the 0.05 level when the skewness value divided by its SE was greater than 1.96. Second, given that skewness was detected, we checked for univariate outliers, which were defined as effect sizes greater than two SDs above or below the unweighted mean.
A sensitivity analysis was conducted to test whether Winsorising the outliers (i.e. changing the value of the effect size estimate to the mean ± 2 SDs), which is recommended in Lipsey 2001, affected the pooled effect size estimates. The analyses on the Winsorised datasets were conducted in SPSS, while the unchanged datasets were analysed in RevMan.
There was no substantial difference between pooled effect size estimate for the primary outcome when outliers unchanged (risk ratio (RR) 1.45, 95% confidence interval (CI) 1.27 to 1.64) and pooled effect size estimate with outliers Winsorised (RR 1.44, 95% CI 1.27 to 1.63).
Multivariate outliers of the primary outcome (i.e. abstinence in late pregnancy) were also explored using the predictor variables main intervention strategy (counselling, feedback, incentives, and social support, with health education and the one study with ‘other’ intervention type as the reference category). As recommended by Tabachnick 2001, the Mahalanobis distance of each study was compared to the Chi2 critical value of 18.47 (based on P < .001 and df =4). The Mahalanobis distance of none of the studies exceeded this value. Therefore, no multivariate outliers were identified for the primary outcome in terms of intervention strategy.
For the comparison analyses (conducted in RevMan and reported in Section 1.1 of the Results), we used the raw (i.e. not Winsorised) effect sizes in the analyses. This is because the subsets of studies are typically too small to reliably detect outliers.
The number needed to treat for benefit (NNTB) (Altman 1998) was calculated to give an approximation of how many women would need to receive the intervention for one of them to avoid an adverse outcome. We used the Visual Rx programme (Cates 2008) and based the computation on the random-effects pooled odds ratio effect size calculated in RevMan 5.2.5. We used the odds ratio rather than the risk ratio as this is invariant to whether the outcome is presented as a beneficial or adverse outcome (Cates 2002).
Subgroup analysis and investigation of heterogeneity
Investigation of heterogeneity is critical in such a large review that includes many different types of interventions and comparisons. It is possible that there are significant differences between subgroups of studies based on characteristics of the interventions, participants, comparisons, study bias etc, as outlined in Figure 1. In the section on Assessment of heterogeneity above, we described how we identified the presence or absence of heterogeneity; in the current section, we describe how we attempted to identify the main sources of variability in the effect size estimates, that is, to attempt to explain inconsistency across studies. We therefore explored how the observed effectiveness differs under different conditions.
Subgroup analyses
Where subgroup analyses were possible for the primary outcome, they were conducted on the whole dataset in SPSS 20 using an adapted ANOVA test. Ideally, the results of the subgroup analyses should produce a non-significant within-group heterogeneity statistic (i.e. the P value for QW should be > 0.05) to indicate that the effect sizes within a group are statistically similar to each other. If the subgroups are significantly different from each other, then the between-group heterogeneity statistic will be significant (i.e. the P value for QB will be < 0.05). If the between-group heterogeneity statistic QB is not statistically significant, then the proposed subgroup variable does not significantly explain differences between the effect sizes.
Two investigations of heterogeneity required meta-regression analyses. These were (1) a model that included two indicators of the difference in intensity of the intervention and control conditions and (2) a model that included both self-help manuals and telephone support as predictors. Meta-regressions were conducted in SPSS 20 using an adapted regression analysis. The overall fit of the regression model is indicated by two statistics: QM and QR QM is the variability associated with the regression model, while QR is the random error variability (that which is not accounted for by the model). A significant QM suggests that significant variation in the effect size distribution has been explained by the model, and is therefore desired. A significant QR, on the other hand, suggests that variability beyond that explained by the model remains, and is thus not ideal (Lipsey 2001).
Subgroup analyses for the primary outcome
We considered both clinical and statistical heterogeneity in the dataset. For the primary outcome, we did not calculate an overall pooled effect size for all intervention types versus all comparison types because clinical heterogeneity makes the overall effect size difficult to interpret. Instead, we focused our analysis of the primary outcome on subgroup analyses, which statistically test the significance of differences between groups, and trends in the pooled effects for different subgroups. The following variables were included in subgroup analyses conducted in SPSS 20 for the primary outcome of smoking abstinence in late pregnancy.
Main intervention strategy (counselling, health education, incentives, feedback, social support, or other).
Comparison type (usual care, less intensive interventions, or alternative interventions).
Biochemically validated versus self-report outcomes.
Intensity of the intervention (duration and frequency).
Features of the intervention (self-help manuals and telephone support).
Socio-economic status of the participants.
Newly included studies in this review update.
It is important to note that the subgroup analyses described below do not take into account interactions in the data. For example, the models do not include both intervention type and comparison type in the same model, so we did not test how these factors might interact. Whilst this is a limitation of the analyses presented, we feel that there is still value in determining overall trends across the dataset. Firstly, this allows better comparison with previous versions of the review, for which the review had not separated the studies by comparison. Secondly, it allows us to consider whether what the corpus of studies looks like and whether there are trends across all of the studies. Throughout, we have distinguished between statistical heterogeneity and conceptual (or clinical) heterogeneity, and we hope that these subgroup analyses help to explore these different types of variation more thoroughly. We also note that in future updates of the review, we hope to be able to incorporate the increasingly popular methods of network meta-analysis to better address all of these issues.
Heterogeneity in the secondary outcomes
For most secondary outcomes, we did not calculate an overall pooled effect but instead focused on comparisons within clinically homogeneous subsets. However, for infant outcomes, we calculated overall pooled effect sizes for all intervention types versus all comparison types, for two reasons. Firstly, there was less extreme clinical heterogeneity in terms of intervention strategy in the infant outcomes. Secondly, as a primary objective of this review is to determine whether psychosocial interventions to support women to abstain from smoking in pregnancy have an impact on infant and maternal health outcomes, and large numbers are needed to detect relatively rare events, the pooled infant outcomes are informative. The overall pooled effect size estimates demonstrate the relationship between being randomised to a smoking cessation intervention and birth outcomes only, rather than the effectiveness of any particular intervention strategy.
Due to the small number of studies reporting the secondary outcomes, we were limited in the range of subgroup analyses (i.e. tests for statistical heterogeneity) that we could conduct. As such, comparisons for the secondary outcomes were limited to description of pooled effect sizes for the subgroups, rather than statistical tests of between-group differences.
Descriptions of trends across studies
To gain a greater understanding of key issues that we were not able to synthesise statistically, we present narrative summaries of the intervention effectiveness for dissemination trials; intervention effectiveness by ethnicity of the participants; and other participant characteristic analyses reported by study authors.
Sensitivity analysis
Concerns have been raised about whether clinical trial efficacy will translate to clinical effectiveness when implemented in healthcare practice (Walsh 2000). To determine whether effectiveness studies (defined as those assessing the implementation of an intervention that uses existing service providers) demonstrate a beneficial outcome in the absence of efficacy trials (those provided by dedicated research staff), we conducted a sensitivity analysis with efficacy trials excluded. The pooled effect size estimate, 95% confidence interval, and I2 value of the effectiveness-only studies was then compared with the overall pooled effect size estimate and its precision and I2 value.
A number of potentially significant factors were identified during data extraction and coding of the trials (e.g. where ‘counselling’ was provided by a video-tape rather than in person; where ‘counselling’ included optional provision of nicotine replacement therapy or incentives etc.). The studies with these characteristics were highlighted and sensitivity analyses conducted for these studies, and the effect that removing them had on the remaining studies in the comparison.
Assessment of risk of bias across studies
Assessment of the risk of bias across studies was conducted through subgroup analyses in SPSS 20 using an adapted ANOVA test. We used subgroup analyses rather than an elimination approach to sensitivity analysis for two reasons. Firstly, the subgroup analysis allows us to test whether high or low risk of bias studies have statistically different pooled effect sizes. Secondly, we included the ‘unclear risk of bias’ studies as a subgroup in the analyses, which allows us to check for missing data problems. For some of the risk of bias types, many of the studies did not report sufficient information to be able to assess the potential risk of bias. Through the subgroup analysis, we could test whether there was a systematic difference between poorly reported studies and those with assessable risk of bias.
We conducted risk of bias analyses for the following bias types on the primary outcome.
Random sequence generation selection bias.
Allocation concealment selection bias.
Incomplete outcome data attrition bias.
Selective reporting bias.
Detection bias (biochemical validation of abstinence).
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete implementation.
Equal baseline characteristics in study arms.
Contamination of control group.
Other bias.
Due to the small numbers of effect size estimates for the 16 secondary outcomes for which we calculated effect size estimates, very few subgroup analyses by risk of bias type were possible. Only four of the outcomes had sufficient data to be analysed in terms of only one or two of the 12 possible risk of bias types. Given this, we did not conduct risk of bias analyses for the secondary outcomes. However, where possible we reported the average RR for studies assessed as having a high and low risk bias.
RESULTS
Description of studies
Results of the search
The original version of this review included a total of 19 studies identified up until 1993 included as separate reports in the Pregnancy and Childbirth CD Rom: behavioural strategies for reducing smoking (n = 9) (Lumley 1995a); counselling for reducing smoking in pregnancy (n = 1) (Lumley 1995b); advice as a strategy for reducing smoking (n = 6) (Lumley 1995c); and feedback as a strategy for reducing smoking (n = 3) (Lumley 1995d).
Following publication of a protocol in 1998, a search was conducted by the Pregnancy and Childbirth Group for the second update of the review published in The Cochrane Library in 1999. This update included a total of 44 trials: 37 trials including 16,916 women providing data on smoking cessation and over 800 women in five trials of relapse prevention (Lumley 1999).
The third update in 2004 was based on a search until July 2003 conducted by the Pregnancy and Childbirth Group, the Tobacco Addiction Group Trials Register and a search of MEDLINE, Embase, PsycLIT and AustHealth. A total of 65 trials were included involving over 20,000 women: 48 trials provided data on smoking cessation, six additional cluster trials involving over 7500 women were not included in the meta-analysis (Lumley 2004).
In the fourth update, published in 2009; a search from January 2003 to June 2008 identified 898 reports which were screened, the full text of 35 reports were reviewed and a total of 73 studies, involving over 20,000 women, were included (72 provided outcome data): 56 randomised and quasi-randomised trials and nine cluster-randomised trials provided primary outcome data for this update (Lumley 2009).
In this fifth update of the review, we screened 2030 abstracts (in addition to the search of the Pregnancy and Childbirth Group’s Trials Register) and reviewed the full text of 64 reports. We identified 16 new studies meeting the inclusion criteria. As a result of a change in the inclusion criteria we excluded 13 studies from the previous version of the review, including nine quasi-randomised trials, as well as four randomised controlled trials of pharmacological interventions which are now included in a separate review (Coleman 2012b). These are listed in Characteristics of excluded studies. We also included four studies that had been previously excluded (three cluster trials and one abstract report of a trial), as well as nine studies that did not report any outcomes which could be used in meta-analyses, and which are reported in a separate table. We combined two reports of relapse prevention (Ershoff 1995; Secker-Walker 1995) as ‘Associated References’ to the primary papers reporting smoking cessation (Ershoff 1989; Secker-Walker 1994), and another paper which did not report any usable outcomes (Solomon 1996) as an ‘Associated reference’ to the primary report (Secker-Walker 1998). A total of 77 randomised controlled trials, involving over 29,000 women with relevant outcome data, were included in the meta-analysis for this report (primary outcome data for 21,948 women participating in 70 trials and secondary outcome data only for a further 7404 women participating in seven trials). A further nine without outcomes are included but results summarised in Table 1, making a total of 86 studies included in this update. See Figure 4 for summary of search results.
Table 1. Primary outcomes from studies which met inclusion criteria, however outcomes were not able to be included in metaanalysis.
| Study ID | Main findings | Rationale for not including outcomes in meta-analysis |
|---|---|---|
| Byrd 1993 | There was no statistically significant difference in smoking status among those who received either type ofmedia or nurse counselling | Results could not be included as smoking cessation rates were not reported by intervention group |
| Graham 1992 | There was no decrease in the rate of low birthweight for women who received the intervention | Smoking outcomes were not reported. Birthweight outcomes were not included in this review, as aspects other than the smoking component of the intervention may have had an effect on birthweight, and it is unclear how many smokers were in each group, or what proportion quit |
| Haug 2004 | There was no significant difference in smoking between the intervention (motivational enhancement therapy) and control groups on self-reported cigarettes per day, mean carbon monoxide or mean cotinine | Study reports actual outcome data for movement in stages of change only. Outcome data for smoking cessation, cigarettes per day, carbon monoxide and cotinine levels are not reported |
| Hiett 2000 | Significantly more women were able to quit smoking when enrolled in the intervention | Actual cessation rates not reported (poster abstract only available) |
| Hughes 2000 | There was no difference between intervention and control groups in mean delta stage of change or 12-month rate of maintained cessation in pregnant women (-0.62 vs -0.65) | Data from intervention and control Outcomes were combined for intervention and control groups in pregnant women. Unable to extract numbers |
| Lowe 2002 | At 1 month, 65% of behaviourally-based intervention hospitals agreed to provide materials about smoking cessation, compared to 3% control hospitals. After 1 year, 43%intervention hospitals still providedmaterials, compared to 9% of control hospitals. McNemar’s Chi2 indicates a statistically meaningful difference between the proportion of intervention hospitals implementing the program and the proportion of control hospitals implementing the program (2 1 = 12, P = 0.0005) | Implementation data only included. No smoking cessation data provided |
| Manfredi 1999 | Compared to controls, smokers attending family planning, prenatal and well-child clinics, exposed to the intervention were more likely to have quit (14.5% vs 7. 7%) | It was not possible to separate out which data was related to pregnantwomen, as opposed towomen recruited from family planning and well child clinics. Further, it was not clear at what stage in pregnancy women were recruited and what the post-partum time points were |
| Moore 1998 | There was no significant difference in LBWwere 10.9% in the intervention group and 14.0% in controls (RR = 0.75, 95% CI 0.55 to 1.03). Preterm births rates were 9.7 in the intervention group and 11.0 in the controls (RR = 0.87, 95% CI 0.62 to 1.22) | Smoking outcomes were not reported. Birthweight and pretermbirth outcomes were not included in this review, as aspects other than the smoking component of the intervention may have had an effect on birthweight and preterm births |
| Olds 2002 | Significant reduction in mean cotinine among women who smoked at baseline. Mean reduction of 12.32 ng/mL in the control group, compared to asmean reduction of 259.00 ng/mL in nurse-home visiting group | Study reports the mean cotinine reduction only, not mean cotinine levels or smoking cessation rates. It is also unclear how many randomised women were included in this analysis |
CI: confidence interval
LBW: low birthweight
RR: risk ratio
Figure 4. Search flow chart.
Included studies
Participants
Over 29,000 pregnant women participating in 77 trials with outcomes included in the meta-analysis were assessed as current or recent ‘smokers’ at recruitment. The criteria used to assess a woman as a ‘smoker’ varied substantially between trials, and are detailed for each study in the Characteristics of included studies table. There were 1740 women who reported they had ‘spontaneously quit’ smoking when they became pregnant, and had outcomes reported separately from women who continued to smoke. In one study only one third of the study population smoked commercial cigarettes, while two thirds chewed traditional or commercial smokeless tobacco (Patten 2009).
Participants were generally healthy pregnant adult women over 16 years of age, with 19 trials explicitly excluding women with medical or psychological complications. The majority of trials (n = 47) included women categorised as having low socio-economic status; 43 of these measured the primary outcome. Most trials included women over 16 years of age, with only two trials explicitly targeting young women under 20 years (Albrecht 1998; Albrecht 2006) and one study including women over 15 years of age (Donatelle 2000). Four trials were specifically targeted towards women with ‘psychosocial risk factors’ (Graham 1992; Belizan 1995; Albrecht 1998; El-Mohandes 2011) and two trials were conducted among women requiring methadone treatment for opioid addiction (Haug 2004; Tuten 2012). Most trials recruited women at the first antenatal clinic visit and during the second trimester of pregnancy, excluding women in the last trimester due to limited time remaining to receive the intervention. However, four trials were explicitly targeted towards women who continued to smoke in late pregnancy (‘heavy smokers’) (Valbo 1994; Valbo 1996; Stotts 2002; Stotts 2009). Seven studies included mainly women belonging to an ethnic minority population (Graham 1992; Lillington 1995; Gielen 1997; Manfredi 1999; Malchodi 2003; El-Mohandes 2011; Ondersma 2012). Two trials were conducted in aboriginal communities (Creative Spirits 2013) among Aboriginal women in Australia (Eades 2012) and Alaskan Native women the US (Patten 2009), and one trial included more than 40% Maori women in New Zealand (McLeod 2004). Twenty-eight studies explicitly excluded women who were not able to speak English (n = 26), Danish (Hegaard 2003) or Swedish (Hjalmarson 1991). In eight studies access to a telephone or video recorder was required for participation in the study. In two studies, women using nicotine replacement therapy were excluded (Malchodi 2003; Tuten 2012).
Interventions
Of the studies which had outcomes included in the meta-analysis (n = 77/86), the main intervention strategies were categorised as counselling (n = 48), health education (n = 7), feedback (n = 7), incentives (n = 4), and social support (n = 10). In one study the intervention was classified as ‘intensive dissemination’ as both arms received the same counselling intervention, with only the dissemination differing (Campbell 2006), and is therefore reported as a separate comparison. In seven studies, the primary aim of the study was to improve maternal health, which included a smoking cessation component of counselling (El-Mohandes 2011); feedback (Reading 1982; LeFevre 1995) and social support (Olds 1986; Belizan 1995; Bullock 1995; Bullock 2009). These studies are reported as separate comparisons and only smoking outcomes are included, as there is potential for other aspects of these interventions to impact on birth outcomes.
One trial was designed exclusively for women who had spontaneously quit smoking (Lowe 1997), and 11 trials included a relapse prevention component for women who had spontaneously quit. Interventions which were provided only during the postpartum period were excluded from this review, though many interventions during pregnancy continued support into the postpartum period and measured postpartum outcomes.
Smoking cessation interventions implemented during pregnancy differ substantially in their intensity, their duration, and the people involved in their implementation. In 31/77 studies the intervention was coded as a single intervention, therefore the ‘main intervention strategy’ most accurately reflects the type of intervention. However in 33 studies the intervention was coded as ‘multiple’, where other components of the intervention were offered to all women. In 12 studies the intervention was coded as ‘tailored’ whereby different intervention components were offered and tailored to women’s needs. For example, two trials offered optional nicotine replacement therapy as part of a counselling intervention (Hegaard 2003; Eades 2012), and one trial offered nicotine replacement therapy to both intervention and control participants (Patten 2009). Most counselling studies involved face-to-face contact, using a variety of strategies either alone or in combination (such as motivational interviewing, cognitive behavioural therapy, stages of change). Three trials with the main intervention strategy coded as counselling included a lottery chance for women who reported quitting (Sexton 1984; Walsh 1997; Parker 2007); five included support for peers (Donatelle 2000; Solomon 2000; Hajek 2001; Vilches 2009; Eades 2012) and three included support for partners to quit (Thornton 1997; Vilches 2009; Eades 2012). The duration and frequency of the intervention also varied considerably, as illustrated in Figure 5 and Figure 6.
Figure 5. Duration of contact for each condition by publication year.
Figure 6. Frequency of contact for each condition by publication year.
Thirteen of the counselling interventions involved telephone counselling and in five of these studies all counselling was provided via telephone (Ershoff 1989; Bullock 1995; Solomon 2000; Stotts 2002; Rigotti 2006), and one had only brief additional face-to-face contact (Bullock 2009). Twenty-six studies included self-help manuals as part of the intervention, and in five studies there was a brief introduction to the manuals (less than five minutes) and the intervention was therefore coded as counselling (Ershoff 1989; Messimer 1989; Price 1991; Valbo 1994; Moore 2002), with sensitivity analysis conducted to assess the independent effect of these five studies. In six studies the intervention was provision of a video alone (Secker-Walker 1997; Cinciripini 2000), with a brief intervention (Price 1991) or as part of a counselling intervention (Walsh 1997; Manfredi 1999; Windsor 2011), and these were also coded as counselling as the videos included stories from women. Five studies included use of computers in the intervention, three of which were part of another main strategy (Lawrence 2003; Vilches 2009; Ondersma 2012); one which included interaction with a pregnancy care provider and was therefore coded as counselling (Tsoh 2010) and another in which the computer-generated messages were the only intervention and was therefore coded as health education (Strecher 2000). In one study the provision of the self-help manual was the only intervention (Hjalmarson 1991), and was therefore coded as health education only as there was no explicit personal component to the interaction. One study provided a mailed audiotape and self-help manual only (Petersen 1992) and one study provided only automated text-messaging (Naughton 2012); these were coded as health education, as there was no clear personal component. Three other studies that reported the intervention consisted of advice to quit only, either in person (Donovan 1977; Lilley 1986) or by post (Burling 1991) were coded as health education.
Five dissemination trials were identified, carried out in Australia (Lowe 2002; Campbell 2006) and the US (Manfredi 1999; Pbert 2004; Windsor 2011), two of which reported only dissemination outcomes (Manfredi 1999; Lowe 2002) and not the primary outcomes of abstinence in late pregnancy, therefore outcomes not able to be included in the meta-analysis are reported in Table 1. In 26 studies the intervention was provided by staff involved in routine pregnancy care (coded as effectiveness studies), and in 43 studies the intervention was provided by dedicated research project staff (coded as efficacy studies), or via automated technology (n = 8), (coded as unclear).
Comparisons
Women in the control arms in 44 of the 77 trials received information about the risks of smoking in pregnancy and were advised to quit as part of ‘usual care’. In 16 of these 44 trials the comparison/control group was described as receiving ‘usual care’ without specifying further what constituted usual practice (at a particular time and in a particular setting) with respect to advice and assistance. In 31 trials the comparison group received some kind of ‘less intensive’ intervention, which included studies where a dedicated research team consistently provided what they considered to be ‘usual care’ for women in the comparison group. In two studies the comparison group received an ‘alternative intervention’, which was categorised as having the same intensity as the intervention group. One was a counselling intervention using cognitive behavioural therapy compared with traditional health education (Cinciripini 2010) and another compared provision of incentives, contingent or not contingent on smoking status (Heil 2008). As expected, the intensity of interventions and controls has increased over time, as indicated by the change in duration (Figure 5) and frequency of contact during the interventions (Figure 6).
Setting
Included trials were conducted between 1976 and 2012 and almost all trials were conducted in high-income countries. This includes the USA (57), Canada (1), the UK (13), Norway (3), Sweden (1), Holland (1), Spain (1), Australia (5), and New Zealand (2). Only two trials have been conducted in middle-income countries: one trial was conducted in four Latin American countries (Argentina, Brazil, Cuba and Mexico) (Belizan 1995), and the other in Poland (Polanska 2004). Neither trial had biochemically validated smoking outcomes. Most trials of interventions to support pregnant women were conducted in public hospitals or community antenatal clinics.
Outcomes reported
Primary outcomes
Sixty randomised controlled trials and 10 cluster-randomised trials reported the primary outcome measure of smoking abstinence in late pregnancy, up to and including the period of hospitalisation for birth (21,948 women), and in 49 trials (including seven cluster-randomised trials), the abstinence was biochemically validated. Nineteen studies reported whether there was a differential effect among women from different ethnic groups, socio-economic status, or other factors such as depression or partner smoking. Nine studies did not report any outcomes which could be included in meta-analysis and a summary table of outcomes for these studies is reported in Table 1.
Secondary outcomes included in meta-analysis
Fourteen trials reported continued abstinence in late pregnancy among women who had quit spontaneously before the intervention, one of which was a trial exclusively for women who had spontaneously quit, so did not also report the primary outcome (Lowe 1997).
Thirty-two trials reported continued abstinence in the postpartum period at zero to five months (n = 26), six to 11 months (n = 13), 12 to 17 months (n = 5) and 18 months and over (n = 2). Two of these trials did not have outcomes in late pregnancy as the assessment was undertaken at home after birth (Strecher 2000; Polanska 2004). Continued abstinence for baseline smokers and spontaneous quitters are combined in this outcome measure for some studies, with abstinence among baseline smokers only reported where available. The details of the outcomes for each study are reported in the Characteristics of included studies table. Thirty-four trials reported various measures of smoking reduction in late pregnancy, including self-reported ‘any reduction’ (n = 7), self-reported reduction greater than 50% (n = 5), and biochemically validated reduction (n = 6). Two trials recorded both self-reported and biochemically validated reduction (Windsor 1985; Tappin 2005); in these cases we have included only the validated data in the analysis. Other reduction measures of reduced smoking included mean biochemical cotinine (n = 6) thiocyanate (n = 1), or mean cigarettes per day (n = 20). Three studies that reported smoking reduction did not include the primary outcomes of smoking abstinence (Donovan 1977; LeFevre 1995; Vilches 2009).
Nineteen trials reported mean birthweight, one of which had not reported any smoking cessation outcomes (Haddow 1991). Fourteen trials reported rates of low birthweight babies (less than 2500 g) and three reported rates of very low birthweight babies (less than 1500 g). Fourteen studies reported rates of preterm births less than 37 weeks’ gestation (n = 14). Other trials reporting perinatal outcomes included: perinatal deaths (n = 4), stillbirths (n = 7), neonatal deaths (n = 4), and neonatal intensive care unit (NICU) admissions (4).
Other perinatal outcome measures reported included fetal growth (Cope 2003; Heil 2008), mean Apgar scores (Tuten 2012), and head circumference (Cope 2003).
Secondary outcomes included in narrative synthesis
Three trials measured mode of birth (Thornton 1997; Cope 2003; Tappin 2005).
Three trials measured breastfeeding initiation and/or duration (Panjari 1999; McLeod 2004 and an associated reference to Heil 2008) (Higgins 2010a).
Nineteen studies reported baseline psychological measures of interventions, three studies reported associations between smoking outcomes and psychological measures, and nine studies reported psychological outcomes.
No studies reported measures of family functioning. However three studies reported perceptions of partner (McBride 2004)) and peer support (Bullock 2009; Hennrikus 2010), and one study provided analysis of social networks (Stotts 2009).
Twenty-six trials addressed issues identified as important to women in a consultation for this review; with two associated references (Berg 2008; Washio 2011) to included studies (Rigotti 2006; Heil 2008), reporting effects of smoking cessation on maternal weight gain.
Seven studies explicitly included the views of women or community in development of the intervention; and 32 trials reported women’s views about the content or delivery of the intervention. Three studies reported measures of knowledge, attitudes or practice among pregnancy care providers (Haug 1994; Secker-Walker 1994; Lawrence 2003).
Five studies reported cost-effectiveness measures (Windsor 1985; Ershoff 1989; Dornelas 2006; Parker 2007; Heil 2008).
Two studies reported rates of women who reported an increase in smoking (adverse events) (Haug 1994; Tappin 2005).
Excluded studies
Seventy-five studies did not meet the eligibility criteria and were excluded from the review, for the following reasons:
design not adequately randomised (e.g. cohort studies, pre-post design, quasi-experimental designs);
primary population was not pregnant women or intervention was not primarily aimed at cessation during pregnancy (e.g. postpartum interventions, intervention for partners, non-pregnant women);
trial evaluated efficacy of pharmacological treatment with equal psychosocial support in both arms;
cluster-randomised trials with insufficient information (e.g. number of clusters) provided to enable adjustment for clustering.
See Characteristics of excluded studies for details.
Risk of bias in included studies
Allocation
Sequence generation was described and adequate in 35 trials. In 48 trials the sequence generation was not described or simply described as ‘randomised’ so it was unclear whether this was adequate or not. Three trials were included which had non-random sequence generation, such as allocation by medical record numbers and birthdate, as it was considered the risk of interference with this sequence is low. There are also many studies where the method of sequence generation was not reported. Quasi-randomised trials where there was a potential for interference, such as clinic attendance day or other quasi-randomised methods were excluded from this update of the review and the reasons are listed in the Characteristics of excluded studies table.
The method of randomisation was not described in sufficient detail to permit assessment of whether the allocation was concealed at the time of trial entry in 63 studies. In only 12 studies was the allocation adequately concealed and in 11 studies there was clearly no concealment of group allocation.
Equal baseline characteristics
As the sequence generation was not reported in the majority of trials, we assessed whether the baseline characteristics were equal and these were assessed as adequate in 37 studies, unclear (minor differences or not reported) in 33 studies, and inadequate or significant differences in 16 studies. Of the 48 trials with unclear sequence generation, 18 had equal baseline characteristics, seven had unequal baseline characteristics and in 23 there were some minor differences or the baseline characteristics were not reported.
Blinding
Very few trials had any blinding of participants or providers, as this is not practicable in delivering most psychosocial interventions. In 60 studies the participants and providers were clearly aware of group allocation, it was unclear in 15 studies, and in one study they were able to blind participants and/or providers to group allocation.
Blinding of the outcome assessment was rarely reported and was assessed as adequate in 11 studies, unclear in 74 studies, and inadequate in one study.
Incomplete outcome data
Withdrawals from the trials were common. When women were recruited at their first antenatal visit some participants had a miscarriage or a termination of pregnancy before the time when smoking behaviour was reassessed. These women were often excluded from outcome measurement, which means that important outcomes linked in observational studies to smoking exposure were not ascertained. Assessing smoking at 20 to 28 weeks instead of at 36 to 38 weeks would reduce the need to exclude women withparticularly adverse outcomes, since their smoking status in mid-pregnancy would have been ascertained before preterm birth or a perinatal death had occurred. Others moved out of the area or changed to another provider of care. The latter was a common cause of attrition in those trials carried out among populations characterised by severe poverty and the receipt of special needs benefits such as Medicaid, or WIC (food program for women, infants and children) clinics.
In studies where there was longer-term follow-up, attrition was sometimes high; approximately half of the included studies had high levels of missing data (greater than 20%) for some outcomes. All randomised women were included in analysis for the primary outcome (abstinence in late pregnancy) in 25 trials. In 41 trials, some women were excluded from the analysis due to miscarriage or pregnancy loss, or moving, and these were assessed as unclear risk of attrition bias as there are some associations with smoking. In 20 trials, primary outcome data were missing and were unable to be included in this review, and they were assessed as inadequate due to risk of attrition bias. Levels of attrition for each study and information about any intention-to-treat analysis have been reported in the ‘Risk of bias’ tables .
Selective reporting
It was not clear in many trials the extent of outcome data that were collected and therefore, unclear whether the outcomes were selectively reported in 42 studies. All primary outcomes were adequately reported in 30 studies, and 14 studies were assessed as inadequately reporting primary outcomes.
Other potential sources of bias
Detection bias from misclassification by self-report
Fifty-two trials reported biochemical validation of the primary outcome measure, smoking abstinence. In seven trials there was unclear or partial validation of smoking status. Twenty-seven trials measured smoking status by self-report and are included in this review as ‘high risk’ of bias. Later trials more often relied on a definition of smoking abstinence requiring biochemical validation.
Implementation of intervention
Some studies reported process evaluation demonstrating challenges implementing the intervention and delivering it to all women (Walsh 2000). In 26 studies, process evaluation suggested that the majority of women received the intervention as planned, however 31 studies reported that many women had not received the intervention as planned and in 29 studies it was unclear or not reported.
Smoking cessation interventions implemented during pregnancy differ substantially in their intensity, their duration, and the people involved in their implementation. The timing of the final antenatal assessment of smoking status varied considerably between trials between the second and third trimester. This may have affected the amount of time the participants were exposed to the intervention (if it involved ongoing support), as well as the number of those lost to follow-up and measurement of perinatal outcomes.
Exposure of the control group to the intervention
Another problem with trials in this area can be ‘contamination’ or exposure of the control group to intervention components, particularly if the study is being implemented in a routine care setting. Fifty-eight trials were implemented by dedicated research staff or technology and were assessed as having a low risk of exposing the control group to the intervention. In 12 studies it was unclear, and in 16 studies the authors reported problems with exposure of the control group, or the intervention was provided by routine care providers and the study design was assessed as having a ‘high risk’ of control group exposure.
Other bias
No other risk of bias was suspected in 68 studies. However, in nine studies there were some other risks, such as unequal recruitment to study arms in cluster-randomised trials or financial conflicts of interest, and in nine studies it was unclear if there may be other risks of bias.
Change in ‘usual care’
In many cases the comparison/control group was described as receiving ‘usual care’ without specifying further what constituted usual practice (at a particular time and in a particular setting) with respect to advice and assistance. It can be seen from Figure 5 and Figure 6 that current ‘usual care’ may be a more substantial intervention than the defined intervention in some of the earliest trials (for example, Baric 1976).
A summary of Risk of bias’ assessments in the included trials is set out in Figure 7 and Figure 8.
Figure 7. ‘Risk of bias’ summary: review authors’ judgments about each risk of bias item for each included study.
Figure 8. ‘Risk of bias’ graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Effects of interventions
A total of 88 meta-analyses are reported in this review. Meta-analyses were conducted and are presented in data tables for a total of 11 comparisons involving 59 outcomes. Data for comparisons with only one study reporting an outcome are reported in text, but not displayed. In addition, eight non-prespecified meta-analyses conducted in Revman 5.2.5 were reported in text, to assess the effect of factors identified during data extraction and coding (e.g. where ‘counselling’ involved provision of a videotape only). The results of 21 meta-analyses conducted in SPSS 20 to assess risk of bias and sensitivity analyses are also reported in text and not reported in tables.
1. Primary outcome: Smoking abstinence in late pregnancy
1.1 Comparisons: Main intervention strategy compared with usual care, less intensive intervention, or an alternative intervention, and subgrouped by single, multiple or tailored components
Table 3 presents a cross-tabulation of the main intervention strategies and comparison type, for studies that report the primary outcome. The large number of cells that have very few (i.e., n ≤ 2) or zero studies means that it is not appropriate to run an interaction analysis with these two variables. Therefore, the synthesis in this section was not achieved through meta-analytic subgroup analyses; rather, the synthesis is a description of trends in the weighted pooled effect size estimate for subsets of studies based on the intervention strategy, the comparison type, and the number of components in the intervention (single component, multiple components, and tailored components). As such, we cannot draw any conclusions about statistical differences between subsets of studies in this section.
1.1.1 Counselling versus usual care
In trials where the main intervention strategy was counselling and the control group received ‘usual care’, the difference between intervention and control groups was significantly different from zero (27 studies; average risk ratio (average RR) 1.44, 95% confidence interval (CI) 1.19 to 1.75), I2 = 55%, see Analysis 1.1.
In subsets of studies, the effect size estimate was significantly different from zero where counselling was combined with other strategies (11 studies; average RR 1.59, 95% CI 1.15 to 2.21), I2 = 45% or tailored to the needs of individual women (six studies; average RR 1.49, 95% CI 1.01 to 2.20), I2 = 75%, but the effect was unclear when counselling was provided as a single intervention (10 studies; average RR 1.12, 95% CI 0.89 to 1.42), I2 = 11%. There was no significant difference in biochemically validated abstinence in late pregnancy in a single study where smoking cessation counselling was provided as part of a broader intervention to improve maternal health (El-Mohandes 2011) and the control group received usual care (RR 1.00, 95% CI 0.72 to 1.40). The analysis for this comparison is not displayed in a table as only one study met the criteria.
1.1.2 Counselling versus less intensive interventions
In trials where the main intervention strategy was counselling and the control group received a less intensive intervention, the effect size had borderline significance (16 studies; average RR 1.35, 95% CI 1.00 to 1.82), I2 = 74%, see Analysis 2.1. In subsets of studies, the effect size was significantly different from zero for the single trial (Walsh 1997) where counselling was tailored to individual needs (RR 2.39, 95% CI 1.03 to 5.56), and included lottery tickets for women who were abstinent from smoking, but there was no clear difference where counselling was provided alone (n = 5), or in combination with other strategies (n = 10).
1.1.3 Counselling versus alternative intervention
There was no significant effect in the single study (Cinciripini 2010) that compared one counselling strategy (CBT) to an alternative counselling intervention (traditional health education or motivational interviewing) (RR 1.15, 95% CI 0.86 to 1.53). The analysis for this comparison is not displayed in a table as only one study met the criteria.
Other counselling subset analyses (not displayed)
In two studies where counselling was provided as part of a tailored intervention that included optional nicotine replacement therapy and was compared with usual care (Eades 2012; Hegaard 2003), the effect was not significantly different from zero (average RR 1.63, 95% CI 0.25 to 10.50), I2 = 59%.
In two studies where ‘counselling’ involved only provision of a video tape (Secker-Walker 1997; Cinciripini 2000) compared with a less intensive intervention, the effect was unclear as it was not significantly different from zero and there was considerable heterogeneity (average RR 2.31, 95% CI 0.08 to 65.02), I2 = 78%, and the effect on the subgroup of ‘single’ counselling interventions compared with usual care continued to be borderline non-significant when these two studies were removed from the pooled results (average RR 1.52, 95% CI 0.99 to 2.34). The effect was not significantly different from zero in a single study (Price 1991), which provided brief advice (less than five minutes) in conjunction with provision of a video, compared with usual care (RR 3.94, 95% CI 0.45 to 34.41).
Five studies coded as counselling provided brief advice (less than five minutes) and a self-help manual (Ershoff 1989; Messimer 1989; Price 1991; Valbo 1994; Moore 2002). Four of these studies reported abstinence in late pregnancy and the combined effect was not significantly different from zero (average RR 1.28, 95% CI 0.79 to 2.07), I2 = 54%.
Four studies coded as counselling included peer and/or partner support as part of a tailored intervention (Solomon 2000 Hajek 2001; Vilches 2009; Eades 2012) compared with usual care, and the combined effect of two studies that reported abstinence in late pregnancy (Hajek 2001; Eades 2012) was not significantly different from zero (average RR 1.09, 95% CI 0.82 to 1.44), I2 = 0%.
Three studies coded as counselling (tailored) included support for partners to quit smoking (Thornton 1997; Vilches 2009; Eades 2012) compared with usual care, and two studies that reported abstinence in late pregnancy (Thornton 1997; Eades 2012) did not show a combined effect that was significantly different from zero (average RR 1.23, 95% CI 0.66 to 2.31), I2 = 0%.
Three studies coded as multiple or tailored counselling that included a lottery chance for women who reported abstinence (Sexton 1984; Walsh 1997; Parker 2007) had a combined effect that was significantly different from zero (average RR 1.98, 95% CI 1.61 to 2.42), I2 = 6%. Two studies that measured self-reported abstinence compared with usual care (Sexton 1984) and a less intensive intervention (Parker 2007) showed a significant effect (average RR 1.69, 95% CI 1.21 to 2.36), and the effect of the single study that reported biochemically validated abstinence (Walsh 1997) was also significantly different from zero (RR 2.39, 95% CI 1.03 to 5.56).
1.1.5 Health education versus usual care
For studies in which the main intervention strategy was health education and the control group received usual care, the pooled effect size estimate was not significantly different from zero (three studies; average RR 1.51, 95% CI 0.64 to 3.59), I 2= 28%, see Analysis 3.1. The effect size estimate was not significant in subsets of trials where health education was provided alone (n = 2) or in combination with other strategies (n = 1); or when the analysis was restricted to studies with biochemical validation of abstinence, see Analysis 3.2.
1.1.6 Health education versus less intensive interventions
The effect was not significantly different from zero in trials where health education was compared with a less intensive intervention (two studies; average RR 1.50, 95% CI 0.97 to 2.31), I2 = 0%, and there was little difference whether health education was provided alone (n = 1), or in combination with other strategies (n = 1), see Analysis 4.1.
Other health education subset analyses (not displayed)
Two studies coded as health education involved provision of self-help manuals with no additional advice (Hjalmarson 1991) or an audiotape (Petersen 1992) and the combined effect was not significantly different from zero (average RR 1.28, 95% CI 0.79 to 2.07), I2 = 7%. When these studies were removed from the health education subgroup, the combined effect of the remaining three studies (Lilley 1986; Burling 1991; Naughton 2012) was statistically significantly different from zero (average RR 1.93, 95% CI 1.01 to 3.69), I2 = 0%.
A single study coded as health education that provided advice via a computer (Strecher 2000), compared with a less intensive intervention reported an effect that was not significantly different from zero in abstinence at six weeks postpartum (RR 1.00, 95% CI 0.91 to 1.09).
The effect of a single study coded as health education that provided advice and motivational statements via text compared with a less intensive intervention (Naughton 2012), was not significantly different from zero (RR 1.59, 95% CI 0.68 to 3.73).
1.1.7 Feedback versus usual care
For the two trials where the main intervention was feedback, provided in combination with other strategies, and the control group received usual care (Valbo 1994; Cope 2003), the combined effect size estimate was significantly different from zero (average RR 4.39, 95% CI 1.89 to 10.21), I = 0%, see Analysis 5.1.
The effect of self-reported smoking abstinence in late pregnancy was not significantly different from zero in a single study that provided ultrasound feedback alone (with no smoking cessation advice) as part of a broader intervention to improve maternal health and usual care for the control group (Reading 1982) (RR 2.11, 95% CI 0.98 to 4.52). The analysis for this comparison is not displayed in a table as only one study met the criteria.
1.1.8 Feedback versus less intensive interventions
Two studies assessed the effectiveness of feedback compared with less intensive interventions. The effect size estimates of both studies - one in which feedback was provided alone (Bauman 1983) and one in which feedback was provided in combination with other strategies, for women still smoking in late pregnancy (Stotts 2009), were not significantly different from zero; (average RR 1.19, 95% CI 0.45 to 3.12), I = 49%, see Analysis 6.1.
1.1.9 Incentives versus usual care
There was no significant difference in rates of biochemically validated abstinence in the pooled results of two studies where the main intervention strategy was financial incentives and the control group received usual care (average RR 3.59, 95% CI 0.10 to 130.49). However, there was significant heterogeneity ( I2 = 82%) and interaction between the subgroups (Chi2 4.03, P = 0.04), so caution is needed considering the combined effect of these trials. The analysis included a trial of incentives (single intervention) (Tuten 2012) (RR 20.72, 95% CI 1.28 to 336.01) and a trial of ‘low intensity’ incentives (multiple intervention) provided with assistance of a computer program and counselling via a computerised program (Ondersma 2012) (RR 0.90, 95% CI 0.25 to 3.23), see Analysis 7.1.
1.1.10 Incentives versus less intensive or alternative interventions
The effect was significantly different from zero in the single trial where incentives were provided in combination with peer support and the control group received a less intensive intervention (Donatelle 2000) (RR 3.64, 95% CI 1.84 to 7.23). The analysis for this comparison is not displayed in a table as only one study met the criteria.
The effect was also significantly different from zero in the single study where the intervention group received incentives contingent on smoking status (single intervention), and the control group received an equally intensive alternative intervention of incentives which were not contingent on smoking status (Heil 2008) (RR 4.05, 95% CI 1.48 to 11.11). The analysis for this comparison is not displayed in a table as only one study met the criteria.
Another trial of incentives included a second comparison arm of non-contingent incentives (Tuten 2012), which demonstrated a significant effect (RR 18.21, 95% CI 1.33 to 294.43), although this effect size estimate was not included in the meta-analysis (only the comparison with the usual care condition was included in the meta-analyses in this review).
1.1.11 Social support versus less intensive interventions
The combined effect size estimate of six trials where the main intervention strategy included peer or partner (social) support and the control group received a less intensive intervention was not significantly different from zero (average RR 1.29, 95% CI 0.94 to 1.78), I = 18%, see Analysis 8.1. However, the effect was significantly different from zero in five trials which included peer support (average RR 1.49, 95% CI 1.01 to 2.19), I2 = 3%, see Analysis 8.2. In the single trial where the intervention involved partner support (McBride 2004), there was no significant effect in self-reported abstinence (RR 1.02, 95% CI 0.70 to 1.50).The analysis for this comparison is not displayed in a table as only one study met the criteria.
1.1.12 Social support as a component of a broader maternal health intervention versus usual care
The effect size was significantly different from zero in one study where tailored peer support was provided as part of a broader intervention to improve maternal health and compared with usual care (RR 1.83, 95% CI 1.22 to 2.73), see Analysis 9.1. A further study in which tailored peer support was provided as part of a broader intervention to improve maternal health and compared with usual care with biochemically validation smoking cessation (Olds 1986) had zero events in both study arms and the effect size estimate was therefore ‘not estimable’ in Revman 5.2.5. As such, we could not calculate a pooled effect for this comparison.
1.1.13 Social support as a component of a broader maternal health intervention versus less intensive intervention
There was no significant effect in two studies where telephone peer support was provided as part of a broader intervention to improve maternal health, and the control group received a less intensive intervention (average RR 0.80, 95% CI 0.46 to 1.39); see Analysis 10.1 and Analysis 10.2.
1.2 Subgroup analyses
The following subgroup analyses were conducted on the whole dataset using all studies for the primary outcome (smoking abstinence in late pregnancy) (see Analysis 11.1 for list of studies). These analyses were conducted in SPSS using Winsorised data.
1.2.1 Subgroup analysis 1: Main intervention strategy
Three of the main intervention strategy subgroups had pooled effect size estimates that were significantly different from a null effect, indicating that abstinence in late pregnancy was significantly greater in the treatment than in the control group for these strategies: incentives (four studies; average RR 2.95, 95% CI 1.55 to 5.63, I2 = 15%), feedback (five studies; average RR 2.08, 95% CI 1.23 to 3.50, I2 = 26%), and counselling (45 studies; RR 1.36, 95% CI 1.17 to 1.57, I2 = 0%). However, there was no significant difference between treatment and control groups in subgroup analyses of trials where the main intervention strategy was social support (10 studies; average RR 1.29, 95% CI 0.92 to 1.80, I2 = 0%), or health education (five studies; RR 1.50, 95% CI 0.90 to 2.51, I2 = 0%). There was not a significant between-group difference (QB (4) = 7.70, P = 0.10) and there was within-group homogeneity (as indicated by low I2 in each subgroup and non-significant Q-statistics for each subgroup; overall QW (64) = 57.86, P = 0.69). One study, Campbell 2006, was treated as missing from this analysis as the intervention type category was unclear.
1.2.2 Subgroup analysis 2: Comparison type
We conducted a subgroup analysis to test for differences in the pooled effect size estimate of studies grouped by their comparison type. As there were only two studies with alternative intervention comparators that also reported the primary outcome, we used a pooled estimate of the between-study variance (τ 2) following the method described in Borenstein 2009. The results suggests that there is no statistically significant difference between effect size estimates grouped by comparison type (QB (2) = 1.53, P = 0.47). Studies with comparisons consisting of usual care comparisons had the highest pooled effect size estimate (37 studies; average RR 1.34, 95% CI 1.25 to 1.44), I2 = 53%, followed by less intensive interventions (30 studies, average RR 1.20, 95% CI 1.08 to 1.31), I2 = 64%, and the effect size estimate for studies with an alternative intervention comparisons was not statistically different from zero (two studies, average RR 1.26, 95% CI 0.98 to 1.53), I2 = 82%. Forest plot not shown. It should be noted that studies where the comparison group received only ‘usual care’ were also more likely to provide a low intensity intervention, as shown in Figure 5 and Figure 6, and discussed below.
1.2.3 Subgroup analysis 3: Biochemically validated versus self-report outcomes
Given concerns about the potential biases (e.g. social desirability bias) of self-report measures of smoking behaviours, we conducted a subgroup analysis comparing biochemically validated smoking abstinence and self-reported abstinence. The results suggest that there is no statistically significant difference between the two groups of effect sizes (QB (1) = 0.06, P = 0.80; QW (67) = 61.33, P = 0.67), and there was a similar pooled effect size estimate for biochemically validated outcomes (49 studies; average RR 1.43, 95% CI 1.22 to 1.67, I2 = 0%), compared to self-reported outcomes (20 studies; average RR 1.48, 95% CI 1.17 to 1.87, I2 = 11%). Although this does not help us to explain the significant heterogeneity in the dataset, it gives us greater confidence in combining self-report with biochemically validated outcomes in further analyses. One study, Thornton 1997, was treated as missing from this analysis as the use of biochemical validation was unclear.
1.2.4 Subgroup analysis 4: Intensity of the intervention
There was no significant difference between effect sizes estimates subgrouped according to the frequency of contact in the intervention (QB (5) = 8.88, P = 0.11); see Table 4 for the pooled effect size estimates by group. Moreover, there was no significant difference between effect sizes estimates subgrouped according to the duration of contact in the intervention (QB (5) = 5.43, P = 0.37); see Table 5 for the pooled effect size estimates by group.
To explore whether the difference in intensity between conditions was a significant predictor of the outcome, a meta-regression was conducted. The model included two predictor variables: the difference between the intervention and control group frequency of contact categorisations, and the difference between the intervention and control group duration of contact categorisations. The analyses indicated that neither the magnitude of the difference in duration nor frequency of contact significantly predicted the primary outcome (QM (2) = 0.17, P = 0.92; QR (65) = 63.14, P = 0.54;R 2 = 0.00).
1.2.5 Subgroup analysis 5: Features of the intervention (self-help manuals and telephone support)
A meta-regression with two dichotomous predictor variables - the use of self-help manuals and the availability of telephone support - was conducted. Of the studies that reported the primary outcome, 24 studies offered self-help materials to participants and 13 provided telephone support (three of these offered both). The analyses indicated that neither self-help materials (B = −0.14, SE = 0.13) nor telephone support (B = −0.14, SE = 0.15) significantly predicted the primary outcome (QM (2) = 1.83, P = 0.40; QR (67) = 63.54, P = 0.60;R 2 = 0.03).
1.2.6 Subgroup analysis 6: Socio-economic status (SES) of the participants
For the primary outcome of abstinence in late pregnancy, there was no significant difference between the two groups of studies with women categorised as ‘low’ or ‘not low’ SES (QB (1) = 0.11, P = 0.74). The pooled effect size estimate for interventions provided for women categorised as ‘low’ SES interventions was similar (44 studies; average RR 1.41, 95% CI 1.19 to 1.66, I2 = 1%), to those provided for women categorised as ‘not low’ SES (26 studies; average RR 1.47, 95% CI 1.21 to 1.79, I2 = 0%).
1.2.7 Subgroup analysis 7: Newly included studies in this review update
Of the 70 studies reporting smoking abstinence in late pregnancy outcomes, 50 came from studies in the previous review (Lumley 2009), while 20 were from new studies identified in the updated search. We conducted this subgroup analysis to address concerns that newer trials may have a reduced effect due to the increased information about the risks of smoking in pregnancy in the general population. Although effect sizes from the newly-included studies tended to be lower (20 studies; average RR 1.26, 95% CI 1.00 to 1.59, I2= 3%), than those from the previous version of the review (50 studies; average RR 1.50, 95% CI 1.30 to 1.73, I2= 0%), this difference was not statistically significant (QB (1) = 1.51, P = 0.22).
1.3 Description of trends in intervention effectiveness: dissemination trials (not displayed)
There were five dissemination trials, defined as trials where the intervention was provided at an organisational level and strategies were employed to influence the practice of pregnancy care providers (Manfredi 1999; Lowe 2002; Pbert 2004; Campbell 2006; Windsor 2011). The combined effect of three trials that reported abstinence in late pregnancy (Pbert 2004; Campbell 2006; Windsor 2011) was not significantly different from zero (average RR 0.96, 95% CI 0.37 to 2.50), I2 = 72%.
1.4 Description of trends in intervention effectiveness: ethnic and aboriginal participants (not displayed)
The synthesis in this section was not achieved through meta-analytic subgroup analyses; rather, the synthesis is a description of trends in the weighted pooled effect size estimate for subsets of studies based on ethnicity of the participants. As such, we cannot draw any conclusions about statistical differences between subsets of studies in this section.
The combined effect of five studies (four counselling trials, one incentives trial) among women predominantly from a minority ethnic group (African-American and/or Hispanic) that reported abstinence in late pregnancy was not significantly different from zero (average RR 1.08, 95% CI 0.83 to 1.40), I2 = 0%. Of those five trials, three were conducted with African-American women (Gielen 1997; El-Mohandes 2011; Ondersma 2012) (average RR 1.01, 95% CI 0.75 to 1.37), I2 = 0%. The effect size estimate in a single trial among African-American and Hispanic women (Lillington 1995) was not significantly different from zero (RR 1.97, 95% CI 0.70 to 5.50). A single trial of social support developed specifically for Hispanic women in this review (Malchodi 2003) did not demonstrate a significant effect size estimate (RR 1.12, 95% CI 0.61 to 2.06).
The combined effect for the two tailored counselling interventions provided for aboriginal women in Australia (Eades 2012) and Canada (Patten 2009) did not show a significant difference between treatment and control groups in rates of abstinence in late pregnancy (average RR 0.40, 95% CI 0.06 to 2.67), I2 = 0%.
1.5 Description of participant characteristic analyses reported by study authors
The following is a narrative synthesis of the findings of subgroup analyses reported by primary study authors.
Low socio-economic status (SES)
Of seven studies which reported sensitivity analysis by a measure of SES, four reported lower abstinence rates or a negative association with quitting among women with lower SES (Baric 1976; McLeod 2004; Pbert 2004; Rigotti 2006), two reported no significant difference (Ershoff 1989; Tappin 2005), and one study reported 4/5 successful quitters had not graduated from high school (Secker-Walker 1997).
Ethnicity or race
Of nine studies which reported outcomes or sensitivity analysis by ethnic status, one study reported the intervention was less effective among Hispanic and African-American women (Kendrick 1995), one study reported the intervention was less effective among Hispanic compared to African American women (Lillington 1995), three studies reported no difference in outcomes by race or ethnicity (Burling 1991; Strecher 2000; Dornelas 2006), and four studies reported higher quit rates among African-American and/or Hispanic women compared to other women (Petersen 1992; Windsor 1993; Pbert 2004; Parker 2007).
Depression
Two studies that reported outcomes by rates of depression reported a negative association between smoking abstinence and depression (Cinciripini 2000; Rigotti 2006).
Low social support
Three studies that reported measures of social support reported a negative association with low social support (e.g. single mothers) and quitting (Loeb 1983; Thornton 1997; Rigotti 2006).
Partner smoking
Of four studies reporting associations with partner smoking and abstinence in late pregnancy, two reported no significant difference (Rigotti 2006; Stotts 2009) and two reported a negative association (i.e. lower rates of quitting among women whose partners’ smoked) (McLeod 2004; Polanska 2004).
1.6 Sensitivity analysis
1.6.1 Efficacy versus effectiveness trials
Given concerns about whether clinical trial efficacy will translate to clinical effectiveness when implemented in healthcare practice (Walsh 2000), we conducted a sensitivity analysis to determine whether effectiveness studies (defined as those assessing the implementation of an intervention that uses existing service providers) demonstrate a beneficial outcome. That is, efficacy trials (those provided by dedicated research staff, n = 43) were excluded from the analysis. The frequencies of key variables for the 26 effectiveness studies (three of which did not report the primary outcome and so were not included in the aforementioned analysis) are presented in Table 6. For the 23 effectiveness trials with primary outcome data, the pooled effect size estimate significantly favoured the intervention group (average RR 1.42, 95% CI 1.11 to 1.82). This group of studies, however, was substantially heterogeneous (I2 = 67%; Q (22) = 66.37, P < .001). The pooled effect size estimate for effectiveness studies is very similar to the overall pooled effect size estimate (average RR 1.44, 95% CI 1.27 to 1.63) of the full sample (n = 70), although the effectiveness studies have a wider confidence interval and slightly greater heterogeneity. We can therefore conclude that our overall pooled effect size estimate (n = 70 studies) is not likely to be an over-estimate, although the addition of the efficacy trials introduced greater precision to the estimate.
1.6.2 Assessment of risk of bias across studies
Random sequence generation selection bias
Not calculable due to insufficient numbers of studies with high risk of bias. Twenty-seven studies were classified as low risk of bias, three were high risk of bias, and the remainder were unclear.
Allocation concealment selection bias
Ten studies were classified as low risk of bias, 11 were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity (QB (2) = 5.22, P = 0.07), although high risk studies had a larger pooled effect size estimate (average RR 2.11, 95% CI 1.48 to 3.00, I2= 0%) compared to low-risk studies (average RR 1.33, 95% CI 0.99 to 1.79, I2= 0%), or unclear bias studies (average RR 1.36, 95% CI 1.17 to 1.58, I2= 1%).
Incomplete outcome data attrition bias
Twenty-two studies were classified as low risk of bias, 13 were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity (QB (2) = 0.13, P = 0.94). The mean effect size was largest for studies rated as high on this type of bias (average RR 1.47, 95% CI 1.09 to 1.99, I2= 0%), followed by unclear risk of bias (average RR 1.45, 95% CI 1.22 to 1.73, I2= 0%), and low risk of bias (average RR 1.39, 95% CI 1.10 to 1.75, I2= 13%).
Selective reporting bias
Twenty-nine studies were classified as low risk of bias, eight were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity (QB (2) = 3.56, P = 0.17). The mean effect size was largest for studies rated as low on this type of bias (average RR 1.67, 95% CI 1.34 to 2.06, I2= 0%), followed by high risk of bias (average RR 1.50, 95% CI 1.09 to 2.08, I2= 0%), and unclear risk of bias (average RR 1.28, 95% CI 1.08 to 1.52, I2= 0%).
Detection bias (biochemical validation of smoking abstinence)
Forty-nine studies were classified as low risk of bias, 20 were high risk of bias, and one was unclear. There was no significant between-group heterogeneity (QB (1) = 0.06, P = 0.80). The mean effect size was similar, but largest, for studies rated as high on this type of bias (average RR 1.48, 95% CI 1.17 to 1.87, I2= 11%), followed by low risk of bias (average RR 1.43, 95% CI 1.22 to 1.67, I2= 0%); the one unclear study was treated as missing in this analysis.
Blinding of participants and personnel performance bias
Not calculable due to insufficient numbers of studies with low risk of bias.
Blinding of outcome assessment detection bias
Not calculable due to insufficient numbers of studies with high or low risk of bias.
Other bias (such as unequal recruitment to study arms in cluster trials; potential conflict of interest)
Fifty-four studies were classified as low risk of bias, eight were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity (QB (2) = 1.28, P = 0.53). The mean effect size was largest for studies rated as low on this type of bias (average RR 1.47, 95% CI 1.28 to 1.69, I2= 0%), followed by high risk of bias (average RR 1.38, 95% CI 0.96 to 1.99, I2= 0%), and unclear risk of bias (average RR 1.18, 95% CI 0.82 to 1.70, I2= 0%).
Incomplete implementation
Twenty-two studies were classified as low risk of bias, 27 were high risk of bias, and the remainder were unclear. There was a significant between-group difference for this type of bias (QB (2) = 7.07, P = 0.03), though this is due to the difference in studies coded as ‘unclear’ (average RR 1.87, 95% CI 1.47 to 2.38, I2= 0%). Low risk of bias studies, assessed as having good implementation, had a similar effect size (average RR 1.33, 95% CI 1.10 to 1.62, I2= 17%) to high risk of bias studies (average RR 1.27, 95% CI 1.06 to 1.51, I2= 0%).
Equal baseline characteristics in study arms
Thirty studies were classified as low risk of bias, 15 were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity for this type of bias (QB (2) = 4.79, P = 0.09). The mean effect size was largest for studies with unclear risk of this type of bias (average RR 1.67, 95% CI 1.33 to 2.10, I2= 20%), followed by low risk of bias (average RR 1.45, 95% CI 1.21 to 1.74, I2= 0%), and high risk of bias (average RR 1.13, 95% CI 0.86 to 1.47, I2= 0%).
Contamination of control group
Forty-nine studies were classified as low risk of bias, 13 were high risk of bias, and the remainder were unclear. There was no significant between-group heterogeneity (QB (2) = 2.12, P = 0.35). The mean effect size was largest for studies with unclear risk of this type of bias (average RR 1.50, 95% CI 1.07 to 2.11, I2= 0%), followed by low risk of bias (average RR 1.48, 95% CI 1.28 to 1.71, I2= 0%), and high risk of bias (average RR 1.19, 95% CI 0.90 to 1.56, I2= 29%), which were not significantly different from the null effect.
2. Secondary outcomes
2.1 Relapse prevention
In examining trends in separate comparisons of studies, the effect was not statistically different from zero in eight trials where the intervention was counselling and the control group received usual care (average RR 1.06, 95% CI 0.93 to 1.21; see Analysis 1.3) or four trials comparing counselling with a less intensive intervention (average RR 1.05, 95% CI 0.98 to 1.13; see Analysis 2.3). Single studies comparing health education with usual care (Petersen 1992) and social support with a less intensive intervention (McBride 2004) also did not show a significant difference between intervention and control groups (RR 0.97, 95% CI 0.71 to 1.31 and RR 1.02, 95% CI 0.89 to 1.16, respectively), figures not displayed as comparisons as only single studies.
2.2 Continued abstinence in the postnatal period
2.2.1 Zero to five months
In examining trends in separate comparisons of studies, a significant difference in abstinence at zero to five months was seen between intervention and control groups only in trials where counselling was compared with usual care (10 studies; average RR 1.76, 95% CI 1.05 to 2.95, see Analysis 1.4). However there was considerable heterogeneity between trials (I2 = 83%) and subgroups (Chi2 25.05 P < 0.0001), so these results should be considered with caution. Within this comparison, there was a significant effect in single interventions (average RR 1.52, 95% CI 1.13 to 2.05) and multiple interventions (average RR 2.32, 95% CI 1.44 to 3.72), but not in the single tailored intervention (average RR 0.88, 95% CI 0.80 to 0.97). There was also a significant difference in abstinence in a single trial where incentives were compared with an alternative intervention (Heil 2008) (RR 9.73, 95% CI 1.29 to 73.13, analysis not displayed in a table as only one study met the criteria).
However, the difference between intervention and control groups was not statistically significant in trials where: counselling was compared with a less intensive intervention (six studies; average RR 1.17, 95% CI 0.82 to 1.66; see Analysis 2.4); or where social support was compared with a less intensive intervention (two studies; average RR 1.36, 95% CI 0.46 to 4.07; see Analysis 8.3); There was also no clear effect where health education was compared with a less intensive intervention (two studies; average RR 1.29, 95% CI 0.52 to 3.22, see Analysis 4.2), but there is considerable heterogeneity in this comparison (I2 = 93%, Chi2 = 25.03, P < 0.0001), so these pooled results should be considered with caution. No significant difference between intervention and control groups was noted in single studies (analyses not displayed in a table as only one study met the criteria) comparing two alternative counselling interventions (Cinciripini 2010) (RR 1.05, 95% CI 0.63 to 1.76); health education versus usual care (Petersen 1992) (RR 1.02, 95% CI 0.75 to 1.38); or counselling as part of a broader intervention to improve maternal health (El-Mohandes 2011) (RR 1.46, 95% CI 0.97 to 2.19); or where social support was provided as part of a broader strategy to improve maternal health (Bullock 2009) (RR 0.96, 95% CI 0.51 to 1.81).
2.2.2 Six to 11 months
In examining trends in separate comparisons of studies, the effect bordered on a significant difference from zero between intervention and control groups in a separate comparison of counselling and usual care (six studies; average RR 1.33, 95% CI 1.00 to 1.77; Analysis 1.5), but not when counselling was compared with a less intensive intervention (three studies; average RR 1.08, 95% CI 0.83 to 1.40, see Analysis 2.5 . Additionally, there was not a significant difference between intervention and control groups when social support was compared with a less intensive intervention (two studies; average RR 1.09, 95% CI 0.83 to 1.42; see Analysis 8.4), or in single studies comparing two alternative counselling interventions (Cinciripini 2010) (RR 0.76, 95% CI 0.33 to 1.73) or contingent and non-contingent incentives (Heil 2008) (RR 3.24, 95% CI 3.24, 95% CI 0.35 to 29.82) (results not displayed as there was only one study in these comparisons).
2.2.3 12 to 17 months
In examining trends in separate comparisons of studies, there was a significant difference between the treatment and control in the two trials comparing counselling versus usual care (average RR 2.20, 95% CI 1.23 to 3.96, see Analysis 1.6), but not in two trials where counselling was compared with a less intensive intervention (RR 1.25, 95% CI 0.71 to 2.20, see Analysis 2.6); or a single trial (McBride 2004) where a multiple social support intervention was compared with a less intensive intervention (RR 1.22, 95% CI 0.92 to 1.64, analysis not displayed in a table as only one study met the criteria).
2.2.4 18+ months
Two trials of counselling combined with other strategies, and compared with usual care, measured self-reported continued abstinence beyond 17 months postpartum (Secker-Walker 1994; Lawrence 2003). However, no significant difference was reported between intervention and control groups (average RR 1.25, 95% CI 0.57 to 2.73, see Analysis 11.7).
2.3 Smoking reduction
No significant biochemically validated reductions were reported in any comparisons, including a comparison of counselling with usual care (three studies; RR 1.11, 95% CI 0.54 to 2.26, see Analysis 1.8) or counselling with less intensive interventions (two studies; RR 1.35, 95% CI 0.98 to 1.87, see Analysis 2.8). No significant difference in biochemically validated reduction was seen in single study by Tuten 2012 (analyses not displayed in a table as only one study met the criteria) comparing incentives with usual care (RR 7.62, 95% CI 1.92 to 30.25), which also demonstrated a significant difference between intervention and control groups in mean cotinine (standardised mean difference (SMD) −0.87, 95% CI −1.36 to −0.39). El-Mohandes 2011, comparing counselling as part of a broader maternal health strategy similarly did not report a significant difference between intervention and control groups in mean cotinine (SMD 0.11, 95% CI −0.17 to 0.39). The difference was also statistically different from zero for one study (Sexton 1984) measuring mean thiocynate (SMD −0.29, 95% CI −0.44 to −0.15), but not for mean cotinine (SMD −0.05, 95% CI −0.14 to 0.05), see Analysis 1.10.
There was also no statistically significant difference in self-reported reduction in smoking (mean cigarettes per day) seen in comparisons of: counselling and less intensive interventions (two studies; SMD −0.11, 95% CI −0.30 to 0.09, see Analysis 2.9); or health education compared with usual care (two studies, pooled effect not calculated due to considerable heterogeneity I2 = 76.8%, see Analysis 3.3). No difference in self-reported smoking (mean cigarettes per day) was also seen in several single studies (results not displayed as only one study met criteria), including: Hjalmarson 1991, which compared health education with a less intensive intervention (SMD 0.02, 95% CI −0.15 to 0.18); Tuten 2012 which compared incentives with usual care (SMD −0.23, 95% CI −0.69 to 0.23); LeFevre 1995 which compared feedback as part of a broader maternal health intervention with usual care (SMD 0.23, 95% CI 0.16 to 0.30); or Bullock 1995 which compared social support as part of a broader maternal health intervention with a less intensive intervention (SMD 0.15, 95% CI −0.34 to 0.64). The difference was not significantly different from zero in self-reported reduction (over 50%) in a single study (Hartmann 1996) which compared counselling and usual care (RR 1.59, 95% CI 0.98 to 2.57); or (Solomon 2000) which compared social support with a less intensive intervention (RR 0.96, 95% CI 0.64 to 1.44). Similarly, no difference in self-reported ‘any’ reduction in smoking was seen in a single study (Reading 1982) where feedback as part of a broader maternal intervention was compared with usual care (RR 0.95, 95% CI 0.42 to 2.18).
However, significant differences in self-reported reductions in smoking were seen in separate comparisons of: counselling and usual care for ‘any self-reported reduction’ (two studies; average RR 1.61, 95% CI 1.06 to 2.43, Analysis 1.9) and mean cigarettes per day (nine studies; SMD −0.25, 95% CI −0.46 to −0.03, Analysis 1.11); counselling and less intensive interventions (two studies; average RR 1.35, 95% CI 1.07 to 1.71, Analysis 2.7); feedback and usual care (two studies; average RR 1.69, 95% CI 1.24 to 2.31, see Analysis 5.2); and social support as part of a broader maternal health intervention with usual care in mean cigarettes per day (SMD −0.28, 95% CI −0.45 to −0.11, see Analysis 9.2). One single study comparing feedback and usual care (Valbo 1994) also reported a significant reduction in mean cigarettes per day (RR −0.63, 95% CI −1.03 to −0.24; results not displayed as only one study in comparison).
2.4 Infant outcomes
As a primary objective of this review is to determine if psychosocial interventions to support women to stop smoking in pregnancy have an impact on infant and maternal health outcomes, and large numbers are needed to detect relatively rare events, the pooled infant outcomes are included in this section of the review. These outcomes demonstrate the relationship between being randomised to a smoking cessation intervention and birth outcomes only, rather than the effectiveness of any particular intervention strategy.
2.4.1 Low birthweight
The pooled results of 14 trials which reported low birthweight (less than 2500 g) demonstrated a significant reduction (average RR 0.82, 95% CI 0.71 to 0.94; see Analysis 11.11). This pooled effect represents the following intervention strategies: eight counselling, two health education, one feedback, two incentives, and one social support. The number needed to treat for benefit (NNTB) in terms of low birthweight is 61, with a 95% CI of 38 to 204. Presented in a different way, nine out of every 100 participants in the control group experienced low birthweight births, compared to seven (95% CI six to eight) out of 100 for the intervention group. In contrast, there was no significant difference in three trials (two counselling and one feedback intervention) which reported infants born very low birthweight (less than 1500 g) (average RR 1.11, 95% CI 0.62 to 2.01, see Analysis 11.12).
In separate comparisons of studies, the effect was no longer significantly different from zero in smaller comparisons of counselling and usual care (six studies; average RR 0.87, 95% CI 0.70 to 1.08, see Analysis 1.12) or less intensive interventions (two studies; average RR 0.58, 95% CI 0.32 to 1.04, see Analysis 2.10), as large sample sizes are required to detect a significant difference in this outcome. There was no significant effect on the proportion of infants born low birthweight (less than 2500 g) in any of the single studies (results not displayed in tables) comparing: health education and usual care (Donovan 1977) (RR 1.10, 95% CI 0.66 to 1.84) or a less intensive intervention (Hjalmarson 1991) (RR 0.60, 95% CI 0.28 to 1.29); feedback and usual care (Haddow 1991) (RR 0.82, 95% CI 0.63 to 1.06); incentives and usual care (Tuten 2012) (RR 0.47, 95% CI 0.20 to 1.11) or an alternative intervention (Heil 2008) (RR 0.43, 95% CI 0.12 to 1.49); or social support and a less intensive intervention (Malchodi 2003) (RR 1.00, 95% CI 0.33 to 2.99). The effect remained non-significant in the three trials reporting very low birthweight infants (less than 1500 g) when separated into comparison of counselling and usual care (Analysis 1.13) and in a single study (Haddow 1991) comparing feedback and usual care (RR 0.90, 95% CI 0.35 to 2,32).
2.4.2 Preterm births
Pooled data from 14 studies reporting preterm births (less than 37 weeks’ gestation) showed a statistically significant reduction in preterm births among women receiving psychosocial interventions (average RR 0.82, 95% CI 0.70 to 0.96; see Analysis 11.13), compared to women in the control groups. This pooled effect represents eight counselling, two health education, two feedback, and two incentives intervention strategies. The number needed to treat for benefit in terms of preterm births is 71, with a 95% CI of 42 to 341. Presented in a different way, eight out of every 100 participants in the control group experienced preterm births, compared to seven (95% CI six to eight) out of 100 for the intervention group.
In separate comparisons of studies, the effect was no longer significantly different from zero in comparisons of counselling and usual care (five studies; average RR 0.90, 95% CI 0.64 to 1.27, Analysis 1.14), counselling and less intensive interventions (three studies; average RR 0.82, 95% CI 0.47 to 1.42, Analysis 2.11), or feedback and usual care (two studies; average RR 0.60, 95% CI 0.28 to 1.29, Analysis 5.3), as large sample sizes are required to detect these relatively rare outcomes. Nor was a significant effect seen in comparisons which had only a single study (results not displayed in tables), including: health education and usual care (Donovan 1977) (RR 1.05, 95% CI 0.53 to 2.00) or a less intensive intervention (Hjalmarson 1991) (RR 0.76, 95% CI 0.32 to 1.80); or incentives compared with usual care (Tuten 2012) (RR 0.58, 95% CI 0.20 to 1.66) or an alternative intervention of non-contingent incentives (Heil 2008) (RR 0.38, 95% CI 0.11 to 1.30).
2.4.3 Mean birthweight
Pooled data from 19 studies reporting mean birthweight showed there was a statistically significant increase in mean birthweight of 40.78 g among women receiving the intervention (95% CI 18.45 to 63.10g, see Analysis 11.14), compared to women in the control group. The difference in mean birthweight was statistically significantly different from zero in subgroups of trials using counselling (n = 12) and incentives (n = 2) as the main intervention strategy, but was not significant in subgroups of trials using health education (n = 2), feedback (n = 2), or social support (n = 1) as a main intervention strategy.
In examining trends in separate comparisons of studies, the effect was borderline significant in comparisons of counselling and usual care (nine studies; MD 36.72, 95% CI 0.70 to 72.74, z = 2.00, P = 0.05, see Analysis 1.15), but not for comparisons of counselling and less intensive interventions (three studies; MD 56.02, 95% CI −31.46 to 143.50, see Analysis 2.12), or feedback and usual care (two studies; MD 79.43, 95% CI −53.05 to 211.91, see Analysis 5.4). There was no significant difference in mean birthweight in single studies (results not displayed in separate comparisons, only in comparison 1) comparing: health education and usual care (Donovan 1977) (MD −12.00, 95% CI −102.29 to 78.29) or less intensive interventions (Hjalmarson 1991) (MD 71, 95% CI −26.58 to 168.58); incentives and usual care (Tuten 2012) (MD 162, 95% CI −132.93 to 456.93) or non-contingent (alternative) incentives (Heil 2008) (MD 253, 95% CI-3.67 to 509.67); or social support provided as part of a broader maternal health intervention and a less intensive intervention (Malchodi 2003) (MD 28, 95% CI −152.48 to 208.48).
2.4.4 Perinatal deaths
Pooled data did not show a significant difference between intervention and control groups in perinatal deaths (four studies; average RR 1.13, 95% CI 0.72 to 1.77, see Analysis 11.15; although note that Valbo 1996 had a non-estimable effect), stillbirths (seven studies; average RR 1.22, 95% CI 0.76 to 1.95, see Analysis 11.16), neonatal deaths (four studies; average RR 1.15, 95% CI 0.44 to 3.06, see Analysis 11.17) or neonatal intensive care unit (NICU) admissions (four studies; average RR 0.78, 95% CI 0.59 to 1.04, see Analysis 11.18). These pooled effect size estimates, however, were based on small numbers of studies and had low power to detect clinically important differences. A number of trials also excluded women who had a perinatal death or a preterm birth from the study population.
In separate comparisons of studies, there was no significant effect seen in comparisons of counselling and usual care for: stillbirths (four studies; average RR 1.08, 95% CI 0.51 to 2.30, Analysis 1.17), neonatal deaths (three studies; average RR 2.06, 95% CI 0.61 to 6.92, Analysis 1.18), or NICU admissions (two studies; average RR 0.82, 95% CI 0.52 to 1.29, Analysis 1.19). There was unclear evidence in relation to counselling and usual care for perinatal deaths because the effect size for one of the two studies (Valbo 1996) was not estimable due to zero events in both groups, therefore pooled effect size not calculable (see Analysis 1.16). There was no significant effect observed for feedback and usual care in stillbirths (two studies; average RR 1.28, 95% CI 0.69 to 2.39, Analysis 5.5). There was no difference in single studies (results not displayed in comparison tables, only in comparison 1) comparing: counselling and a less intensive intervention (Ershoff 1989) in stillbirths (RR 1.84, 95% CI 0.17 to 20.04); health education and usual care (Donovan 1977) in perinatal deaths (RR 4.40, 95% CI 0.49 to 39.08); feedback and usual care (Haddow 1991) in peri-natal deaths (RR 1.05, 95% CI 0.59 to 1.87) or neonatal deaths (RR 0.40, 95% CI 0.08 to 2.07); incentives and usual care (Tuten 2012) in NICU admissions (RR 0.75, 95% CI 0.45 to 1.25); or incentives and an alternative (non-contingent incentive) intervention (Heil 2008) in NICU admissions (RR 0.76, 95% CI 0.24 to 2.49).
NB. The following sections for outcomes 2.4.5 to 2.12 are narrative descriptions based on the findings reported in the studies, rather than on results of statistical synthesis
2.4.5 Other infant outcomes
Two trials (Cope 2003; Heil 2008) reported significant increases in fetal growth measures including fetal femur length and fetal abdominal circumference, and infant length, but no significant difference in head circumference between control and intervention groups. Two trials reported no significant difference in Apgar scores at one and five minutes post-birth (Cope 2003; Tuten 2012).
2.5 Mode of birth
None of the three trials measuring mode of birth by intervention group (Thornton 1997; Cope 2003; Tappin 2005) reported a significant difference in the rate of operative births by intervention group.
2.6 Breastfeeding
There were mixed results for the effect of interventions on breast-feeding. Two trials that measured breastfeeding initiation (Panjari 1999; McLeod 2004) showed no significant difference in initiation or duration of breastfeeding in control or intervention arms. One trial of contingency management measured a significant effect on breastfeeding duration (Heil 2008) at both eight weeks and 12 weeks postpartum.
2.7 Psychological effects
Nineteen studies reported baseline psychological measures of interventions, reinforcing the findings from observational studies that there are significant psychological symptoms among many pregnant women who smoke. Up to 75% of pregnant women who smoked had current or previous psychological symptoms (Belizan 1995; Ershoff 1999; Cinciripini 2010; Ondersma 2012) and approximately 20% to 25% of women reported major depression based on CES-D scale assessments (Blalock 2005; Dornelas 2006; Bullock 2009; Cinciripini 2010; El-Mohandes 2011). Four studies identified baseline depression or stress as a ‘mediator’ or ‘predictor’ of continued smoking at follow-up (Crittenden 2007; Linares 2009; Stotts 2009; El-Mohandes 2011), suggesting depressive symptoms may be an ‘independent contributor to the problem of continued smoking during pregnancy’ (Linares 2009). Nine trials reported post-intervention psychological outcome measures and none reported any negative psychological effects. Six trials showed that smoking cessation interventions in pregnancy do not increase stress and psychological symptoms for women (Manfredi 1999; Panjari 1999; Aveyard 2004; Rigotti 2006; Solomon 2006; El-Mohandes 2011). Furthermore, three studies demonstrated that smoking cessation interventions have the potential to improve women’s psychological wellbeing and self-esteem (Stotts 2004; Bullock 2009; Cinciripini 2010) and self-efficacy (Stotts 2004).
2.8 Impact on family functioning and other relationships
No studies reported measures of family functioning. Studies reporting analysis of social networks (Stotts 2009), suggest a significant interaction between smoking networks (household and other) or partner smoking (Bullock 2009) and continued smoking of participants in late pregnancy. Two studies reporting perceptions of partner (McBride 2004) and peer support (Hennrikus 2010) had mixed findings. Pregnant women reported less negative partner support through pregnancy, but this increased in the postpartum period (McBride 2004). Women in another study reported an increase in both positive and negative support from a peer including: comments about the woman’s lack of willpower, trying to make them feel guilty, expressing anger about smoking and trying to scare women about smoking (Hennrikus 2010).
2.9 Participants views
Twenty-six trials included women’s views of the interventions, 12 studies reported providers’ views of the interventions and two studies reported measures of knowledge, attitudes or practice among pregnancy care providers.
Women’s views
Twenty-nine studies reported that they addressed in the intervention issues identified as concerns by women when consulted for this review (Oliver 2001); including ‘coping with stress and emotions’, misconceptions about smoking risks, and feelings of guilt. Two studies described using interactive discussions to address issues of concern to individual women (Sexton 1984; Hennrikus 2010).
Three studies reported outcomes related to maternal weight gain. One study (Sexton 1984) reported a slightly higher mean weight gain in the intervention group (12.9 kg) compared to the control group (11.9 kg). Two other studies did not report weight gain by intervention exposure but reported that women with a ‘high concern’ about weight gain were less likely to quit smoking during pregnancy or remain abstinent postpartum (Berg 2008), and another reported an increased weight gain of 2.8 kg in women who were abstinent compared to women who continued to smoke (P = 0.04), with an estimated 0.34 kg increase in weight gain for every 10% increase in smoking abstinence (Washio 2011).
Two studies explicitly mentioned consideration of women’s views in developing the intervention (Albrecht 1998; Cinciripini 2010), and six studies described the involvement of women or community members in the development of the intervention (Windsor 1985; Belizan 1995; Gielen 1997; Albrecht 2006; Patten 2009; Eades 2012).
Thirty-two studies reported women’s views about the content and delivery of the interventions. When asked, most women gave favourable feedback on the intervention and intervention materials (Baric 1976; Ershoff 1989; Belizan 1995; Bullock 1995; Lillington 1995; Secker-Walker 1997; Walsh 1997; Cinciripini 2000; Strecher 2000; Tappin 2000; Hajek 2001; Cope 2003; Tappin 2005; El-Mohandes 2011; Ondersma 2012), particularly audiovisual materials (Windsor 1993; Patten 2009; Ondersma 2012) and telephone support (Bullock 1995; Solomon 2000; Rigotti 2006; Bullock 2009). Women offered personal contact and a manual considered the personal contact the most important element and women appreciated printed materials much less if they were also offered a video, although the video combined with printed materials was no more effective than the printed materials alone (Secker-Walker 1997; Cinciripini 2000). Similarly, women offered motivational interviewing for relapse prevention were more likely to be satisfied than those offered a booklet, although the motivational interviewing was no more effective (Ershoff 1999. Women participating in a study in Ireland (Thornton 1997) reported the importance of providing the intervention in privacy, and suggested that telephone follow-up between visits and a video would have been helpful components in that intervention. Two studies reported that even if they did not like it, women expected to be asked about smoking from their care provider (Walsh 1997; McLeod 2004). Two trials using computer-assisted technology were rated positively (Strecher 2000; Ondersma 2012), but in an earlier trial women expressed concern about entering personal information into a computer (Ershoff 1999).
Despite positive feedback about the content of the intervention, several trials reported difficulty recruiting and retaining women’s participation in the intervention (Loeb 1983; Secker-Walker 1994; Cinciripini 2000; Stotts 2004; Patten 2009), and many studies had low participation rates. In a multimodal intervention including counselling and nicotine replacement therapy (NRT), only 87/327 women in the intervention group participated in counselling and only 75 women used NRT (Hegaard 2003).
Offering additional group sessions for smoking cessation was generally a poorly accepted intervention even in otherwise successful trials (Loeb 1983; Windsor 1985), though one study reported groups were well accepted (Sexton 1984). Hypnosis was also a poorly accepted intervention in two studies (Sexton 1984; Valbo 1996). Five studies reported women’s negative views of intervention components, including: use of carbon monoxide monitoring and prompt cards (Thornton 1997); some peer support behaviours (Hennrikus 2010), limited perceived efficacy of booklets (Moore 2002), and phone messages (Ershoff 1999).
Providers’ views
Ten studies reported providers’ views of the intervention. While providers’ views about the interventions were generally positive, a recurrent theme was their concern about the time taken by the intervention (Kendrick 1995; Hajek 2001; Moore 2002; Campbell 2006) and the impact on their relationship with women (Hajek 2001; Valbo 1996). Sixty-five per cent of midwives asked to use a carbon monoxide monitor and provide ‘stage of change’-based advice considered that this could not be achieved in the time available. This led to less than full implementation and variable motivation to promote smoking cessation counselling among staff in some studies (Kendrick 1995; Moore 2002), but not all (Windsor 2011). One of the reasons given for tailoring messages to ‘stages of change’ was to address providers’ concerns that interventions may alienate women not ready to quit (Hajek 2001). A survey of general practitioners suggested the smoking status of the provider influenced participation in intervention delivery (Haug 1994). Despite these challenges, engagement and involvement of providers was identified as a critical element of implementation (Lowe 1997; McLeod 2004; Campbell 2006) and providers reported that they would like more involvement (Tappin 2000).
2.10 Measures of knowledge attitudes and behaviour of health professionals with respect to facilitating smoking cessation in pregnancy
Two trials reported positive effects of the interventions on midwives’ understanding, confidence in delivering the intervention, optimism that the intervention may influence women’s smoking behaviour (Lawrence 2003) and obstetric knowledge and practice (Secker-Walker 1992).
2.11 Cost-effectiveness
Four studies reported that the interventions were cost-effective using a variety of measures. Pregnancy-specific, self-help materials were more cost-effective than standard smoking cessation information or self-help materials (Windsor 1985). Specific estimates include: a benefit-cost ratio of 2.8:1 (Ershoff 1990); 1 (non-smoker): $84 (Parker 2007); and an average cost of $56 per person for each smoking cessation intervention, and $299 to produce a non-smoker at the end of pregnancy (Dornelas 2006).
2.12 Adverse effects
Three studies that measured whether women increased their smoking following exposure to the intervention showed mixed results. One trial reported a slightly lower level of cotinine in the intervention group, compared to the control group (Tappin 2005), another reported no difference in self-reported smoking (Hjalmarson 1991), and another reported an increase in smoking among women who did not quit (Haug 1994).
DISCUSSION
Summary of main results
Studies in this review demonstrate that psychosocial interventions can support women to stop smoking in pregnancy. Importantly, the interventions do not appear to have any negative physical or psychological effects, are positively received by most women, and may improve psychological wellbeing. Incentives had the largest effect size, but only when provided intensively. Counselling was effective when provided in conjunction with other strategies or tailored to individual women, but it is unclear whether any types of counselling are more effective than others. Peer support appeared to be effective, but only when provided as a targeted intervention and not as part of a broader intervention to improve maternal health. It is unclear whether partner-assisted support helps women to quit. Feedback appeared to be effective when combined with other strategies, such as counselling, and compared with usual care, but not less intensive interventions. Health education was not effective in separate comparisons, but the pooled effect was significantly different from zero in subgroup analyses. Among women who received psychosocial interventions there was a significant reduction (18%) in preterm births (less than 37 weeks’ gestation), the proportion of babies born low birthweight (18%) (less than 2500 g), and a significant increase in mean birthweight of 41 g. Using data from this review, the NNTB to prevent one infant being born low birthweight is 61 (95% CI 38 to 204); and 71 interventions (95% CI 42 to 341) to prevent one infant being born preterm. These findings provide strong and clear evidence about the risks of smoking during pregnancy, supporting recommendations that it may be an integral part of strategies to reduce preterm births (Green 2005a). Given the benefits of stopping smoking in pregnancy for the woman and her infant, this would seem to be an important intervention, particularly when applied at a population level. However, it remains unclear from dissemination trials whether interventions are effective when implemented into routine pregnancy care.
Among the subgroups of ‘main intervention strategies’ categorised in this review, the four studies that included use of incentives had the strongest effect. Three trials that compared provision of intensive incentives with usual care (Tuten 2012), incentives and social support compared with a less intensive intervention (Donatelle 2000), and contingent incentives compared with non-contingent incentives (Heil 2008), were significantly different from zero. A three-armed trial, which included a non-contingent arm (Tuten 2012), also showed a significant effect. These non-contingent comparisons provide a ‘time-matched’ alternative comparison of similar intensity, which helps to identify if it is the ‘additional assistance’ or incentives which are effective (Mantzari 2012). The effect was also significantly different from zero in the pooled results of three counselling interventions that included lottery tickets (Sexton 1984; Walsh 1997; Parker 2007). These findings are consistent with other reviews of financial incentives in pregnancy (Higgins 2012) and the mechanisms for the effectiveness of incentives for reducing substance abuse more generally has been well documented (Higgins 2008b). However, the results of the incentives trials should be considered with caution as they are based on few trials with a very small number of women (less than 500), all of whom were in the US. Additionally, there was no effect from one trial of ‘low intensity’ incentives (‘CM Lite’) combined with an interactive computer-generated counselling program (Ondersma 2012), which relied on women initiating contact with the research team for urine cotinine testing, and provided a maximum of only five verification and ‘incentive’ interactions, with less than half the women in this arm submitting even one urine test. Interestingly, women in this four-armed trial who received the interactive computer-generated counselling program alone were more likely to quit than women who received the combined incentive and computer-counselling intervention (see Ondersma 2012).
Pooled results of interventions in which counselling was the main intervention strategy showed a significant effect in abstinence in late pregnancy. However, in separate comparisons, the effect of counselling was only significantly different from zero when combined with other strategies or tailored to individual needs. There was no significant difference seen when one type of counselling (cognitive behavioural therapy (CBT)) was compared with traditional health education (Cinciripini 2010), or when counselling was provided as part of a broader intervention to improve maternal health (El-Mohandes 2011). Group interventions were generally not well accepted in this population of pregnant women, despite being reported as a potentially well accepted intervention in the general population (Bauld 2010). Feedback was effective when combined with other strategies such as counselling, and only when compared with usual care. Findings from this review support recommendations that pregnant women may need more support than just brief advice or health education (Coleman 2004), as it was unclear whether health education alone helped women to quit. However, there was a significant pooled effect among the three trials of health education when two studies were removed providing only self-help materials or an audiotape with no additional personal advice, which is similar to findings in another review (Murthy 2010), and which concluded that apart from brief physician advice, there was limited clarity on the duration of interventions required by other professionals.
Social networks have been suggested as a major cause of relapse (Nguyen 2012b), and a systematic review of qualitative studies identified partners as one of the most important influences on women’s smoking and relapse (Flemming 2013). In this review, peer support appeared to be effective when provided as a targeted intervention, and when social support was provided as part of a broader intervention to improve maternal health, but not when [telephone] support was compared with a less intensive intervention. It is unclear from the single trial of partner-assisted support (McBride 2004) that this strategy can help women to stop smoking. Furthermore, counselling interventions that included support for partners to quit also did not show a significant effect, and there were mixed results in the four studies reporting associations between quitting and partner smoking. Mixed results have similarly been reported in a systematic review of five randomised controlled trials (Duckworth 2012), and another review of seven studies reported a non-significant effect (Hemsing 2012), concluding that, “Despite the importance of partner smoking, there are very few effective smoking cessation interventions for pregnant/postpartum women that include or target male partners”. This raises questions about arguments that a major reason for the modest effect of smoking interventions is the focus on individual behavioural change rather than acknowledging social factors and focusing on external motivation (Okoli 2010). Additionally, feedback from women demonstrates the support from both partners and peers can sometimes be negative, which raises concerns about the potential risks for vulnerable women in physically or emotionally violent relationships. Evidence from this review suggests that while partner and peer support may be important factors influencing smoking behaviour, eliciting peer and partner support that is positive and can actually support women to stop smoking in pregnancy is a challenge.
The lack of a clear difference in effect seen by increasing intervention intensity challenges the validity of the assumption that ever-increasing the intensity of support will increase quit rates, as has been reported by other commentators (Lando 2001), and supports views that there may be an upper limit of what women accept (Chapman 2012). Newly included studies in this review had lower effect sizes than older studies in the previous version, despite a general trend towards higher intensity interventions in more recent trials. It may be that women who continue to smoke are not getting ‘more hard core’ but that there are many options already available and additional strategies may not be offering a lot of extra benefit, as risks of smoking during pregnancy, due to health education campaigns, are well known in high-income countries (Campion 1994; Eriksson 1996; Eriksson 1998). One study found relapse within the first two weeks was predictive of continued abstinence, and suggested this indicates that intensive support during the earlier period of nicotine withdrawal may be an important component of interventions (Higgins 2006b).
Studies in this review suggest the effect during pregnancy continues into the postpartum period, up until approximately 18 months postpartum, though the smaller effect size shows many women who did quit during pregnancy relapse postpartum. Some suggest that many pregnant smokers simply suspend their smoking for the duration of pregnancy as opposed to quitting altogether or they commit to ‘temporary abstinence’ for pregnancy (Stotts 1996; Lawrence 2005a; Flemming 2013), but these relapse rates are similar for non-pregnant women (Bombard 2012). Rather than being disappointed by these limited effects, some authors suggest healthcare workers should focus on the positive aspects of these findings and reinforce the positive decisions many women are making when pregnant (Hotham 2008). High post-pregnancy relapse rates have led to some commentators calling for an extension of the period of support for women to stop smoking (Coleman-Cowger 2012). Hjalmarson 1991 reported a high proportion of women abstaining from smoking during their hospital stay for the birth, and suggests this may be an opportunity for intervention to reduce the risk of postpartum relapse. These findings suggest there may be a need for different approaches to promote continued abstinence postpartum, including focusing on the benefits for the mother, without excessive emphasis solely on the benefits for the baby.
While results are mixed, studies in this review suggest there is a reduction in self-reported smoking but not biochemically validated smoking. Continued nicotine and cigarette exposure may have effects on other outcomes not measured in this review. The level of reduction required to improve health outcomes remains unclear (Secker-Walker 2002a). One study analysing data from Kendrick 1995 suggested that reduction in smoking to fewer than eight cigarettes a day is necessary to avoid reduction in infant birthweight (England 2001), and estimated approximately a mean birthweight which was 200 g higher among women who quit smoking after enrolment, compared to women who continued to smoke during pregnancy. Therefore, extrapolating these data to this review, if all women in the intervention groups stopped smoking and none of those in the control group did, the expected mean birthweight difference would be about 200 g, rather than 41 g. With an absolute difference of six in every 100 women stopping smoking, the expected mean difference from the extent of smoking cessation alone would have been about 12 g. This suggests that smoking reduction is also happening to a greater extent in the intervention than comparison groups, in line with self-reported changes.
There was no evidence from studies in this review that smoking cessation increases the rate of caesarean section (Thornton 1997; Cope 2003; Tappin 2005), contrary to concerns raised by women about the effects of increased fetal size (Sexton 1984). One observational study modelled increases in birthweight (from 2450 g to 2550 g) in Guatemala and found an increased risk in caesarean section due to obstruction of eight in every 1000 cases, but this was outweighed by a reduction in caesarean section due to fetal distress of 34 per 1000 cases (Merchant 2001).
Women who smoke are less likely to initiate breastfeeding (Amir 2001a; Amir 2002a; Donath 2004; Einarson 2009; Disantis 2010b), and breastfeed for shorter duration (Sayers 1995; Horta 1997). Therefore, supporting women to initiate and maintain breastfeeding should be considered an important part of any intervention in this population group, and reported as an outcome in intervention studies. Studies in this review had mixed reports of the effect of smoking cessation interventions on breastfeeding (Panjari 1999; McLeod 2004; Higgins 2010b).
Studies in this review (Cinciripini 2000; Rigotti 2006) support a recent qualitative study that concluded “Pregnant women with mental disorders appear more motivated…yet find it more difficult, to stop smoking” (Howard 2013), and other studies that report higher rates of quitting among women with higher self-esteem and self-efficacy (Massey 2013). For these reasons, healthcare workers have reported difficulty addressing smoking with pregnant women (Valbo 1996). Qualitative studies have identified concerns about adverse effects of quitting, or increased guilt over continued smoking, on women’s psychological wellbeing and capacity to cope with adverse circumstances, with follow-on effects to the women’s families (Oliver 2001; Valbo 1996; Flemming 2013). In earlier versions of this review, it has been difficult to assess the effect of interventions on depression, as, despite the strong associations with poor mental health and smoking in pregnancy, women with mental illness were frequently excluded from trials. However, mental wellbeing has been addressed in more recent trials and, contrary to the above concerns, there is no evidence from studies in this review that there are any negative psychological consequences from delivery of individual smoking cessation interventions in pregnancy. Rather, feedback from women from studies in this review was positive with women feeling that “somebody cared” (Bullock 1995). Three studies have shown that provision of psychosocial support can in fact improve women’s psychological wellbeing, which has the potential to have enormous benefits for the mother, the infant, and the whole family (Bullock 1995; Stotts 2004; Cinciripini 2010).
In earlier versions of this review, there appeared to be little evidence of the involvement of pregnant women who smoked or caregivers being involved in the design and evaluation of interventions (Oliver 2001). However, there has been increasing discussion of women’s preferences for cessation support in recent years (Ussher 2004). Studies included in this review suggest women prefer individual personal contact, particularly by telephone, though studies inclusive of telephone support in this review did not appear to be significantly more effective. Rates of satisfaction with interventions delivered by computers or mobile phones were generally positive, but again there was no evidence in this review that the use of these technologies increased the rate of abstinence in late pregnancy. Nevertheless, acceptability of an intervention is an important aspect of population-based interventions.
Some evidence suggests that women in high-income countries are more likely to smoke to control their weight, and that female body image is extensively targeted by tobacco marketing campaigns (Pomerleau 2000; CDCP 2002; Levine 2006), although concerns about gaining weight through stopping smoking during pregnancy were not raised by any of the women consulted for this review (Oliver 2001). The systematic review of qualitative studies of women smoking in pregnancy (Flemming 2013) found two studies mentioning weight gain as a factor in considering smoking cessation. Hotham 2002 found that fear of weight gain was a barrier to smoking cessation for some women and Lawson 1994 found some women used smoking to cope with weight gain. Three studies in this update of the review (Sexton 1984; Berg 2008; Washio 2011) address weight gain. Only one study reported a small increase in weight gain among women in the intervention group (Sexton 1984). This concern should be considered in interventions, with interventions available to support women to avoid unwanted weight gain (Farley 2012). It should be noted that weight gain in pregnancy may not necessarily be a negative outcome for many women, particularly women in low- and middle-income countries. The association between smoking and glucose intolerance, a potential mechanism for these effects, remains unclear (Wendland 2008). A Cochrane systematic review of interventions for preventing weight gain after smoking cessation mentioned neither pregnancy nor breastfeeding (Parsons 2009) and therefore cannot be relied upon for evidence relevant to a population where weight may fluctuate for normal physiological reasons and where babies may be sensitive to drug treatments in utero or when breast-feeding.
Public health impact of the interventions
Importantly, psychosocial interventions to support women to stop smoking during pregnancy reduce the population-attributable risk of preterm birth (by 18%) and low birthweight (by 18%), with approximately 71 interventions required to prevent one preterm birth and 61 interventions to prevent one infant being born with low birthweight. As such, smoking cessation is recommended as a key recommendation for reducing the risk of recurrent preterm birth (Chang 2012; Cypher 2012). The number of interventions needed to treat for benefit is extraordinarily low, given the serious clinical consequences of these adverse outcomes. Based on the effectiveness published in the 2004 version of this Cochrane review, if 75% of pregnant women in the US disclosed their smoking status and all received the intervention, then it has been estimated that 31,573 (6%) ‘new quitters’ would be gained and the prevalence of smoking in pregnancy would potentially decrease from 16.4% to 15.6% (Kim 2009b). While these effect size estimates may appear modest, the response to interventions is similar to that of psychosocial interventions to reduce type 2 diabetes mellitus, hypertension and asthma, all of which are conditions that involve a combination of medical illness, personal choice and environmental factors (McLellan 2000). Importantly, the high prevalence of these conditions in the community means that interventions with a modest effect size estimate can have a substantial impact on population health if widely implemented.
Economic costs
Studies in this review report variable cost-effectiveness measures and costs of interventions. Based on a NNTB of one quitter for each 19 interventions, our cost estimates ($US1,064) based on $US56 per interventions is significantly higher than the $US299 reported in Dornelas 2006. However, even with higher estimates, other studies that evaluated the cost-effectiveness of these interventions clearly show that there is a ‘rapid return on investment’ (Lightwood 1999). Early studies estimated the smoking-attributable maternal costs during pregnancy alone ranged from $US150 million to $US995 million in the early 1990s (Adams 1998), with 2004 estimates of $US122 million or $US279 per smoker (Adams 2011). Estimated birth and first year costs for both mothers and infants attributed to smoking were $1142 to $1358 per smoking woman over a decade ago (Aligne 1997; Miller 2001; Adams 2002). Infant costs are approximately 10 times maternal costs, accounting for 90% of costs in the first year. Low birthweight produces the highest economic burden as it is the most common adverse outcome (Hueston 1994; Miller 2001). A 1% drop in smoking prevalence was estimated to prevent approximately 1300 low birthweight live births and save $US21 million in direct medical costs (Lightwood 1999). Inclusion of smoking attributable and environmental tobacco smoke exposure costs in birth and childhood conditions, pushes estimates into the billions (Aligne 1997), and long-term costs due to chronic disease up to $US57 billion in 1997, in the US alone (Bartlett 1994). An economic evaluation of data provided in the 2009 version of this review estimated the societal benefits from these interventions could be in excess of 500 million pounds sterling per annum in the United Kingdom (Taylor 2009). In contrast with that finding, the quality of diet in pregnancy (in high-income countries) has not been shown to affect the mean birthweight of infants over 32 weeks’ gestation (Rogers 1998). While there is variation in reported costs dependent on conditions included and changing healthcare costs (Ayadi 2006), it is clear that healthcare costs due to smoking in pregnancy are substantial.
Impact on health inequalities
In high-income countries, the reduction in rates of smoking has not been as substantial in women experiencing psychosocial disadvantage, as for the general population. Hence smoking has been identified as a major preventable cause of the health inequalities experienced by women who suffer psychosocial disadvantage, including psychological illness, low educational attainment, young early motherhood, lack of social support, and limited employment (Graham 2006). Some of the reasons may be that disadvantaged women are unable to change the environmental factors that increase the risk of smoking; population-based interventions may have the effect of being judgemental and alienate women; and women are unable to change generational patterns (Graham 2009). Several authors have suggested that women who continue to smoke in late pregnancy would be unlikely to benefit from the usual antenatal interventions, which rely on women’s capacity for self-initiation, self-control and social resources, which they suggest helps to explain why it remains such an intractable problem (Wakschlag 2003; Pickett 2009) and that individual interventions alone are unlikely to impact on inequalities (Baum 2009). However, subgroup analysis of studies included in this review refutes these arguments and suggests that individual interventions provided during pregnancy have similar effectiveness among women with low socio-economic status (SES), as women who are not classified as having low SES, despite several studies reporting a lower effect among participants with lower SES (Baric 1976; McLeod 2004; Pbert 2004; Rigotti 2006). This supports qualitative studies that suggest individual support, which is positive rather than punitive, has an important role (Bond 2012). Therefore, individual psychosocial support should form a part of the tobacco control ‘package’ to reduce smoking during pregnancy, in conjunction with population-based measures, which have also been shown to have a significant impact on birth outcomes (Adams 2012; Cox 2013) and reducing smoking in disadvantaged populations (Thomas 2008).
The pooled results were not significantly different from zero in eight studies, which were developed predominantly or specifically for ethnic and aboriginal minority women, including African-American women (Gielen 1997; Manfredi 1999; El-Mohandes 2011; Ondersma 2012), African American and Hispanic women (Lillington 1995), Hispanic women (Malchodi 2003), Alaskan Native Women (Patten 2009) and Australian Aboriginal and Torres Strait Islander women (Eades 2012). This is despite primary authors in several studies reporting subgroup analysis of higher quitting rates among African-American and Hispanic women than other women (Petersen 1992; Windsor 1993; Pbert 2004; Parker 2007). These studies tended to involve women more in the development of the intervention and all used several recommended strategies to tailor the intervention (American Legacy Foundation 2012) for initiatives that aim to address the disparities in tobacco use; including hiring culturally competent staff, conducting formative research to identify community needs, piloting and field-testing programs, ‘cultural tailoring’ of smoking cessation resources, and collaborating with key stakeholders and community organisations. Three studies adapted ‘SCRIPT’ materials in the US (see Windsor 2011), which include: ‘asking’ about smoking status; ‘advising’ women to quit; ‘assisting’ women to quit by providing advice on skills and materials such as video’s and self-help materials; and arranging for follow-up by referral at future appointments. Two studies developed audiovisual resources for African American (Ondersma 2012) and Alaskan Indian (Patten 2009) women, and these resources received positive feedback. Despite interventions being reported as feasible and acceptable to communities, there were challenges with implementation and few demonstrated an effect size estimate that was significantly different from zero. Further suggestions included trying to recruit from different settings and including elders to improve recruitment, and recognising the importance of broader social interventions for potentially reaching a larger proportion of pregnant women (Patten 2009). Other reviews of interventions in non-pregnant aboriginal peoples have demonstrated interventions can be effective (Carson 2012), and suggest mobile phone technology may be a feasible intervention strategy (Johnston 2013). Only one study included women using smokeless tobacco products, and identified conflicting beliefs about the effect of these products during pregnancy and the primary change recommended by participants in the study was to provide “more objective” information on the risks of Iqmik (smokeless tobacco) use for the infant (Patten 2009).
Most interventions have been developed in high-income countries and there is very limited information about the effectiveness of psychosocial interventions for individual women in low- to middle-income countries (Murthy 2010). The restrictions on tobacco marketing in high-income countries may result in an increase in tobacco marketing companies in low- and middle-income countries. Smoking has the potential to undermine health improvements in low- and middle-income countries and a range of interventions are needed to manage the emerging epidemic (Lopez 1994; Abdullah 2004). However, given the modest effect size estimate of individual interventions, population-based tobacco control strategies are an urgent priority, as there is now a brief ‘window of opportunity’ to prevent the increase of smoking among women in many low-income countries (Chomba 2010).
Translation of evidence into practice
The first trials of anti-smoking interventions during pregnancy were published more than 30 years ago (Baric 1976; Donovan 1977). The first trial to demonstrate the reversibility of the birthweight reduction associated with smoking by an intensive intervention during pregnancy was published in 1984 (Sexton 1984). Since then, attempts at widespread implementation of psychosocial interventions to support women to stop smoking in pregnancy have demonstrated many of the challenges of translating ‘evidence into practice’, particularly non-pharmacological evidence (Windsor 1998; Windsor 2000b; Lowe 2002; Moore 2002; NICS 2003; McLeod 2004; Herbert 2005; McDermott 2006; Abatemarco 2007; Manfredi 2011).
Studies in this review can be conveniently categorised within a framework for translation of research into practice (Nutbeam 2006), which suggests progression through several stages from; problem definition (descriptive studies) and formative research for intervention design; intervention efficacy research; to implementation in routine/normal settings (effectiveness research); dissemination across several settings; and institutionalisation (as interventions are provided as part of routine care). Many studies in this review clearly defined the problem and conducted formative research for intervention development (Katz 2008; Gilligan 2009), particularly interventions developed for vulnerable women, including young women (Albrecht 1998; Albrecht 2006). The modest but significant efficacy of psychosocial interventions provided by researchers has been well demonstrated by studies in this review, including counselling interventions.
The transfer of an intervention from one setting to another may reduce its effectiveness if elements are changed or aspects of the materials are culturally inappropriate. An example in these trials was the performance of the Windsor self-help manual. This was developed and shown to be effective in Birmingham, Alabama (Windsor 1985; Windsor 1993). However, when it was implemented into routine care (Windsor 2011), used in Baltimore with peer counsellors who received minimal training instead of trained health educators (Gielen 1997), adapted for Alaskan Native women (Patten 2009) and transferred to other countries (Lowe 1998a; Lowe 1998b), the effectiveness was much lower. An analysis of health promotion trials has concluded that where the providers are also the researchers (more likely in single centre studies than multicentre studies), they appear to be better providers for influencing behavioural outcomes and about the same as other providers for other outcome domains (Oliver 2008a). The larger, multicentre trials may therefore be a more accurate representation of implementing policy than smaller, single centre trials. In this review, interventions provided by usual care providers were as effective as interventions provided by researchers, including counselling interventions. However, there was substantial heterogeneity in sensitivity analyses of trials provided by usual care providers in this review, which supports the views that there are many variables to consider when implementing interventions in routine settings (Hoddinott 2010).
Despite evidence of efficacy and effectiveness, dissemination trials of counselling interventions into pregnancy care settings suggest challenges to translating this efficacy research into routine practice and policy. Data from the five dissemination trials that targeted the intervention at the organisational level, demonstrated significant effects in terms of increased implementation of interventions in routine practice, although challenges were reported and this did not translate into a significant reduction in rates of smoking among women in the intervention arms of these studies. One study that provided clinics with resources and referral options reported an increase in women’s recall of receiving interventions (Manfredi 1999). A significantly higher program implementation rate was reported when using an intervention based on Rogers’ ‘Diffusion of Innovation’ theory (43% compared with only 9% implementation in the control group after one year), but there were no data on the impact on smoking outcomes (Lowe 2002). An increased uptake of the intervention by staff was demonstrated using ‘active’ dissemination compared to a simple mail-out of information (Cooke 2001), but not at levels sufficient to have a significant impact on smoking outcomes in women (Campbell 2006), which was similar to other dissemination trials reporting smoking outcomes (Pbert 2004; Windsor 2011). Another nonrandomised study compared the use of the RE-AIM dissemination model to increase the reach, efficacy, adoption, implementation, maintenance of interventions (Lando 2001) and concluded that multi-faceted approaches using strategies from each intervention were most likely to improve implementation.
There are a number of possible explanations for the limited effect in dissemination trials. Firstly, many of the studies that recruited individual women did not provide information on the number of women who were eligible for inclusion or were approached to take part in trials. The ‘participation rate’ would have provided useful information about the general ‘acceptability’ of the intervention, as well as the degree of ‘selection bias’ in the study population (Sedgwick 2013). Among those studies that did report the proportion approached and recruited from the total ‘eligible’ population, low participation rates were often reported. Therefore, some of the evidence in this review is from selective samples of the population of women who smoke during pregnancy. Women participating in studies (Mullen 1997) were more likely to be in contemplative and preparation stages of change, be ‘recent quitters’ and have a lower gestational age, compared to women not participating studies (Ruggiero 2003). The majority of women categorised as ‘Black’, ‘White’ and ‘Native American’ did enrol in the study, while women categorised as ‘Hispanic’ were less likely (51.6%) to enrol and the majority of Asian women did not enrol (Ruggiero 2003). Dissemination trials and ‘cluster trials’ that randomise clinics or providers are therefore likely to provide a more accurate estimate of the likely effect in a non-selective population of pregnant women.
Secondly, the implementation of interventions under conditions less stringent than an individually-randomised controlled trial may be reduced, which may limit exposure of the intervention group to the intervention, or components of the interventions (Walsh 2000). Several trials implemented in routine care settings by midwives (Moore 2002; DeVries 2006), doctors (Valbo 1994; Walsh 1997), and routine clinic staff (Kendrick 1995) reported difficulties with implementation. Some of the issues included: variable perceptions of smoking cessation as part of the providers’ role (DeVries 2006), stating they were too busy and did not have enough time to complete the intervention (Dunkley 1997; Haines 1998; Hajek 2001; Valanis 2001b; Leviton 2003), difficulty recruiting providers to the study (Lawrence 2003), providers reporting pessimism about the efficacy of the intervention (Moore 2002), and lack of acceptability of resources (Lowe 1998a; McBride 1999). Several studies reported positive ‘facilitators or enabling factors’ associated with implementation. Proposed criteria for interventions to be implemented into routine maternity care include: having program materials readily available; feasible provider time commitments; clear training requirements; minimal organisational and administrative barriers (Strand 2003); and program components that are acceptable to providers and women (Haynes 1998; Cabana 1999; Grol 1999; Walsh 2000; Cooke 2001a). Written resources, a written protocol to identify staff responsibilities, and reimbursement have also been suggested as other strategies to improve implementation (Hartmann 2007). A significant increase in both intervention delivery and smoking outcomes was seen in a cluster trial that supported staff with training based on national guidelines, a clinic management system, and establishment of program boards (Pbert 2004). Suggestions to overcome the barriers in a busy clinic setting included increasing the use of referral services and technology to reduce demand on clinicians’ time (Moore 2002). Subsequently, use of referral services such as ‘quitline’ (Williams 2010) and technology-driven interventions have gained popularity in the past five years (Tsoh 2010; Naughton 2012; Ondersma 2012). In the United Kingdom (UK), most services reported use of ‘quitline’ referral services (Williams 2010). One excluded (non-randomised) study in South Australia (Bowden 2010), describes positive experiences and perceptions of staff in implementing a ‘Smoke-free Pregnancy’ Project involving brief ‘5A’s’ intervention and referrals to ‘quitline’. While use of materials such as self-help materials and technological aids did not appear to significantly increase rates of smoking abstinence in this review, they may help to increase the feasibility and reduce the costs of delivering interventions.
A third possible explanation for the limited effect seen in implementation is that trials that involve broader implementation across the system and provision by usual care providers (effectiveness studies), may result in greater exposure of the comparison group to the intervention. While the difference was not significantly different, the pooled effect size was lower among trials that were assessed as having a high risk of contamination in this review. One study illustrated this effect by including a ‘historical control’ group, in which only 4% stopped smoking, compared to 10% who stopped in the randomised ‘concurrent control’ and 12% in the intervention group who stopped (Windsor 2011).
Institutionalisation, where interventions are part of routine care, is the final stage of the evidence-practice translation process. Australia, Canada, the UK and the United States (US) have developed guidelines recommending all pregnant women receive interventions to promote smoking cessation in pregnancy (Aveyard 2007; Fiore 2008). However, studies of clinicians practice in Canada, the US and Argentina suggest that while the majority (50% to 100%) ‘ask’ about smoking status, rates of assistance with effective strategies to support women to stop smoking are very low (11.5% to below 50%) (Floyd 2001; Hartmann 2007; Tong 2008; Mejia 2010; Okoli 2010). Strategies to address the deficiencies identified in these surveys are reported (Chapin 2004) and several studies in this review have trialled strategies to adapt these guidelines and improve implementation into routine settings (Tsoh 2010; Ondersma 2012). A recent survey suggests attitudes may be shifting in the UK about the provision of advice and support, but not the efficacy of the interventions (Beenstock 2012). A recent survey of women giving birth in Australia suggests there has been a significant increase in the provision of smoking advice and support in routine pregnancy care from 2000 to 2008, though half of smokers still did not receive the full complement of advice and support according to state guidelines, and there was marked variability according to where and from whom women received antenatal care (Perlen 2013).
Strategies to increase disclosure of smoking status
Barriers to implementation have been identified at each step of service provision in relation to support for smoking cessation in pregnancy. This includes detection of women who smoke so they can then be offered a supportive intervention (Tappin 2010). As previously noted, self-reported disclosure of smoking status can be variable. Disclosure is influenced by several factors, including the stigma and guilt associated with smoking in pregnancy, the relationship between the care provider and the way the woman is asked about smoking. In general, it appears that less direct questioning increases disclosure, for example, changing the question format from ‘yes’ or ‘no’ to a series of multiple choice questions and asking women to best describe their smoking status (Mullen 1991). There is some evidence from the literature around broader substance use in pregnancy, that asking about substance use of family members (e.g. secondhand smoke exposure) first (Chasnoff 2005; Chasnoff 2007), and leaving sensitive probing personal questions until later in the interview, when a rapport has been established. The rationale is that this provides an opportunity for the woman to gauge the response of the healthcare provider and feel more confident disclosing her smoking status. In the UK, ‘opt out’ carbon monoxide screening has been proposed to increase disclosure (Tappin 2010; Bauld 2012). Biochemical validation of smoking status is an understandable pre-requisite prior to receipt of contingent incentives, to provide feedback on cotinine levels as a motivational aid; or in the context of a smoking trial. However, the benefits and rationale for not accepting women’s disclosure outside these contexts is unclear and was not well received by women in this review (Thornton 1997). Furthermore, there are questions about the accuracy of carbon monoxide monitoring among women with high secondhand smoke exposure (McLaren 2010), and whether there are any adverse effects from routine screening, such as increased domestic violence or effects on mental health.
Adverse effects of interventions
While psychosocial interventions do not pose the same risks to fetal health as pharmacological agents in pregnancy, there are concerns about the potential unintended consequences of these interventions that aim to encourage pregnant women to stop smoking (Burgess 2009). The potential adverse effects identified in this review include: increased smoking; unhelpful peer or partner support; stigmatisation; and nicotine withdrawal.
Despite the number of studies reporting smoking reduction, only three studies reported rates of women who increased smoking by intervention group, and these showed mixed results (Hjalmarson 1991; Haug 1994; Tappin 2005). It would be helpful for studies to measure any increased smoking, particularly in light of recent qualitative evidence that suggests anti-smoking advice may increase resistance to smoking messages for some women (Bond 2012; Flemming 2013).
There has been an increasing focus on the partners and peers of pregnant women, with the additional aim of facilitating cessation by the women themselves (Stanton 2004; Gage 2007). In some cases this reflects cultural and demographic patterns of smoking, where smoking rates are still highest amongst men (Loke 2005; Kazemi 2012); in others, interest in environmental barriers that hinder smoking cessation has led to an understanding of the influence of a woman’s social networks on smoking behaviour (McBride 2004). Studies in this review suggest that there are both positive and negative aspects to partner and peer assistance with supporting women to stop smoking in pregnancy (McBride 2004; Hennrikus 2010). This legitimises concerns about the potential adverse effects on relationships and women’s position (Greaves 2007a). Therefore, these risks should be taken into consideration when developing interventions involving partners or peers, particularly in subpopulations or regions where protection for women’s rights are less than optimal. Pro-active measures to identify women at risk and ensure their safety should be implemented as part of interventions involving peer or partner support (Greaves 2007b).
No studies measured the impact of interventions on stigmatisation of women. However, studies of psychological impact do not suggest there are any negative effects, and individual psychological support may be beneficial (Stotts 2004; Bullock 2009; Cinciripini 2010). Nevertheless, public health professionals must remain ever vigilant when implementing population-based measures, as policies can disrupt highly complex systems and unintended consequences of tobacco policy may differentially impact on vulnerable population groups (Healton 2009). Stigmatisation research suggests that such policies may have unanticipated outcomes for vulnerable mothers, including decreased mental health; increased use of alcohol or cigarettes; avoidance or delay in seeking medical care; and poorer treatment by health professionals (Moore 2009). This stigmatisation may be compounded for some population groups, such as racial minority groups (Bond 2012; Flemming 2013). Few studies reported the effect of nicotine withdrawal, which is a gap given that these withdrawal effects may be more acute during pregnancy (Ussher 2012a; Ussher 2012b).
Overall completeness and applicability of evidence
Most of the included studies were carried out in high-income countries and it is not clear whether the results are applicable in other contexts. Given the rapidly evolving nature of the smoking epidemic in low- to middle-income countries, this is a major gap in the current body of evidence.
Many of the studies that recruited individual women did not provide information on the number of women who were eligible for inclusion or were approached to take part in trials (i.e. the participation rate), which would have provided useful information about the general ‘acceptability’ of the intervention, as well as the degree of ‘selection bias’ in the study population (Sedgwick 2013). Among those studies that did report the proportion approached and recruited from the total ‘eligible’ population, low participation rates were often reported. Therefore, some of the evidence in this review is from selective samples of the population of women who smoke during pregnancy and may affect the applicability of the evidence into routine settings.
The review includes a relatively large number of studies focusing on educational and counselling interventions but relatively few focusing on other approaches, such as the use of incentives and peer support. Furthermore, there are limited data for some outcomes (e.g. some perinatal outcomes, family functioning).
Quality of the evidence
The studies included in the review were of mixed quality and there is a substantial level of heterogeneity amongst the trial results (I2 often greater than 50%); hence, we would emphasise the need to consider the Risk of bias’ tables and urge caution when interpreting the combined effect of the interventions.
Potential biases in the review process
The timing of the final antenatal assessment of smoking status varied considerably among trials between the second and third trimester. This may affect the amount of time the participants were exposed to the intervention (if it involved ongoing support), as well as the number of those lost to follow-up and measurement of perinatal outcomes.
Agreements and disagreements with other studies or reviews
Agreements and disagreements with the previous review
There have been significant changes in the inclusion criteria for this update, with the ‘splitting’ of the previous review into pharmacological interventions (Coleman 2012b), and the exclusion of quasi-randomised trials. In this update we have changed the outcome from continued smoking (odds ratio), to quitting (risk ratio) so it is consistent with other Cochrane reviews from the Tobacco Addiction Group, and we have included ‘number needed to treat for benefit’ analyses, as this is likely to be of greater relevance to service providers. In this update we have also revised all data extraction to ensure that missing data and ‘Risk of bias’ assessments from all trials have been dealt with consistently across the five updates, so there are some minor amendments to some trial data from previous versions. However, the major findings from this review are similar to the previous review, with minor differences in effect size estimates, namely:
psychosocial interventions which include counselling, incentives and feedback support women to stop smoking in pregnancy are effective in supporting women to quit, reducing low birthweight infants and preterm births;
interventions including use of incentives continue to have the largest effect size estimate, but the sample size is very small so these results should be interpreted with caution.
The main differences from the previous review are that a significant effect was demonstrated in:·
continued abstinence in the postpartum period.
A significant effect was not demonstrated in:
a new subcategory of trials providing ‘health education’ only;
a new subcategory of trials using social support, although a significant effect was seen in the combined results of trials using targeted peer support, but not in the single trial using partner-assisted support.
Agreements and disagreements with other Cochrane reviews
See Appendix 1 for a full list of other reviews of smoking interventions.
Pharmacological interventions in pregnancy
A review of pharmacological interventions to support women to stop smoking in pregnancy (Coleman 2012b) did not report a significant effect (RR 1.33, 95% CI 0.93 to 1.91) http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010078/abstract.
Effects of types of interventions for the general population
Relapse prevention
The findings in this review of a significant effect on relapse prevention in the early postpartum period contrast to findings in another Cochrane review of relapse prevention (Hajek 2009). However, relapse prevention interventions for women who had spontaneously quit in this review did not demonstrate a significant effect, which is similar to the findings of Hajek 2009. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003999.pub3/abstract.
Enhanced partner support
The findings in this review were similar to findings in a review of enhanced partner support in the general population (Park 2012), which did not demonstrate a significant effect (RR 0.99, 95% CI 0.84 to 1.15). See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002928.pub3/abstract.
Stages of change
A systematic review of stage-based interventions concluded they are no more effective in general than interventions that do not tailor the intervention according to the stage of change (Riemsma 2003). http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004492.pub4/abstract This is similar to the findings in the previous version of this review.
Individual behavioural support
Our review findings for counselling interventions were similar to those reported by Lancaster 2005a in a review of individual interventions (RR 1.39, 95% CI 1.24 to 1.57), with little difference between intensive support and brief interventions. See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001292.pub2/abstract.
Self-help materials
Our review findings were different from a review of provision of self-help materials in the general population (Lancaster 2005b) that demonstrated a modest but significant effect (RR 1.21, 95% CI 1.05 to 1.39), particularly when the materials were tailored (RR 1.31, 95% CI 1.20 to 1.42). See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001118.pub2/abstract.
Competitions and incentives
The findings of our review contrast with findings of a review of incentives among the general population (Cahill 2011a) that showed no significant difference. See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004307.pub4/abstract. Given the subgroup analysis in our study is based on a very small number of studies and participants, our results should be viewed with caution.
Effects of interventions among other population groups
Psychosocial interventions among patients with coronary heart disease
The findings of this review are similar to findings of psychosocial interventions among patients with coronary heart disease (Barth 2008), another population with strong motivational factors to stop smoking (odds ratio (OR) 1.66, 95% CI 1.25 to 2.22), with high heterogeneity, and a reduced effect among validated smoking outcomes (OR 1.44, 95% CI 0.99 to 2.11).
Pre-operative interventions
The effect of brief smoking cessation interventions among the patients preparing for surgery was similar to our review (RR 1.41, 95% CI 1.22 to 1.63), although the effect of intensive interventions was significantly higher than in our review. See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002294.pub3/abstract.
Hospitalised patients
Our results were similar to those among hospitalised patients (RR 1.37, 95% CI 1.27 to 1.48). See http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001837.pub3/abstract.
Interventions in Indigenous populations
The findings of our review were in contrast to a review of four studies of non-pregnant Indigenous communities (Carson 2012) in New Zealand (2), United States (1) and Australia (1) that reported a modest but significant effect using psychosocial interventions, two of which were supplemented with pharmacological therapy.
AUTHORS’ CONCLUSIONS
Implications for practice
Psychosocial interventions can support women to stop smoking in pregnancy, and reduce preterm births and infants born low birthweight. Therefore, psychosocial support to stop smoking should be considered for women who are pregnant, or seeking to become pregnant. Contrary to concerns that women may be upset by offering support to stop smoking, studies in this review suggest women expect and appreciate the support, and interventions are more likely to improve women’s psychological wellbeing than worsen it. Qualitative evidence suggests this support should be positive, not punitive (Bond 2012), and is sensitive to potential feelings of guilt and worry, and concerns about the impact of quitting on women’s lives and their relationship with significant others (Flemming 2013). Burgess 2009 suggests it may help for healthcare providers to become aware of any of their own biases against mothers who smoke.
Evidence from this review suggests provision of health education and risk advice is not sufficient, and any psychosocial support should include multiple or tailored intervention components that provide help with strategies to quit, positive encouragement and other strategies, such as incentives, feedback or peer support. Partner support does not appear to be effective from the single study in this review, and care is needed when including peer or partner-support components, as some peer and/or partner-support behaviours may be unhelpful, and may potentially expose vulnerable women to increased risk. Inclusion of support for breastfeeding and prevention of weight gain should also be considered as part of smoking interventions for pregnant women, as obesity has overtaken smoking as a major cause of preterm births in high-income countries (Flenady 2011). Given the high co-morbidity with psychological symptoms and the potential to improve psychological wellbeing, interventions that include psychological support for women with symptoms should be considered. Studies in this review suggest many women resume smoking after pregnancy, so consideration should be given to messages that reinforce the benefits for the mother, rather than solely focusing on benefits for the infant.
There is limited evidence from this review that increasing the intensity of the intervention corresponds to an increased effect size. Therefore, consideration should be given to the quality of the intervention, and providing support that is convenient for women and does not unnecessarily overburden them. Consultation with women and local piloting of programs shown elsewhere to be effective may be a good place to begin to develop strategies suitable for each population. Additionally consultative processes that involve healthcare providers and organisational leaders should be another important consideration for implementation.
Given the clear difficulties which most women still smoking at the first antenatal visit have in stopping smoking, population-wide strategies for smoking control in the whole community are needed to reduce the initiation of smoking by young women: action to prevent sales of tobacco products to young people, prohibition of smoking in all public places, increases in tobacco taxation, workplace smoking cessation programs and bans on tobacco sponsorship (WHO 2008a). However, these interventions should incorporate strategies to reduce risks identified in this review, including stigmatisation, and negative effects on relationships; avoid singling out mothers and focus more broadly on ‘parents’; avoid depicting mothers who smoke as ‘harming’ their infants, but as women who are important in their own right; and assisting vulnerable women to develop alternative ‘coping’ strategies to deal with living in difficult circumstances (Burgess 2009). Given the strong association between social inequality and continued smoking by pregnant women shown in this review, there is a rationale to support WHO recommendations to reduce social inequalities in the wider community (WHO 2008b).
Implications for research
There is little doubt about ‘whether’ psychosocial interventions are effective in reducing smoking, preterm births or infants born with low birthweight. What is not clear is ‘which’ interventions are effective, ‘how’ these interventions work, ‘who for’ and ‘how’ should these interventions should be implemented, disseminated and institutionalised. As smoking rates have decreased in the general population in high-income countries, it is becoming increasingly recognised that smoking has become more closely correlated with entrenched social disadvantage and psychological co-morbidity (Shoff 2013). Studies are needed that refine interventions to address the specific needs of these subpopulations, without compounding problems of social alienation and low self-efficacy. Given the shifting demographics and burden of diseases from tobacco smoking from high- to low- and middle-income countries, more research is needed to develop strategies which are appropriate for these settings. In reflecting on whether the objectives of this review have been addressed, the authors feel that further research is needed into:
the feasibility and effectiveness of interventions in low- and middle-income countries, particularly given the aggressive tobacco marketing in these regions;
how to implement and disseminate interventions into routine care, and measures of whether they are effective when implemented at a population level;
the feasibility and effectiveness of the use of incentives to support pregnant women to quit smoking, including evaluation of any adverse effects or negative unforeseen circumstances for pregnant women or the broader community;
demonstrating effective interventions, including descriptions of how these were developed, to support ethnic and aboriginal women, and young women to stop smoking;
interventions to support women with mental illness to stop smoking, and whether interventions that improve mental health can also help women to quit smoking;
developing strategies to ensure that smoking interventions do not have a negative impact on breastfeeding, which would counteract some of the health benefits of quitting smoking for both the mother and her infant;
whether the timing of the psychosocial support is important, for instance, is more frequent support required in the early stages of quitting and less frequent support required later?
A WHO expert working group (Hunt 2012) recently recommended research in three areas to help reduce smoking during pregnancy:
social and cultural factors influencing pregnant women’s use of tobacco and exposure to secondhand smoke;
interventions to promote tobacco cessation and reduce secondhand smoke exposure during pregnancy in high-, low- and middle-income countries;
describing non-cigarette tobacco use by women and characterising the resulting risks for adverse pregnancy outcomes.
In 2009 the National Institute of Clinical Excellence developed guidance on Quitting smoking in pregnancy and following childbirth. Background documents for this guidance (Bauld 2010a; Williams 2010) identified a number of gaps in existing evidence, including:
whether the way the intervention is delivered influences the effect;
whether the site or setting influence the effect;
evidence of effective interventions for vulnerable population groups, including teenage mothers, disabled mothers, women with mental illness, and other women.
Future trials need to include the following elements:
number of potentially eligible women and number agreeing to participate, as this can help to assess the degree of selection bias in the trial and the potential acceptability and generalisability if implemented at a population level;
strategies to minimise contamination, as this appears to have an impact on the effect size;
a description of the intervention in sufficient detail for its replication even if the detail requires a separate paper;
process data as evidence of implementation;
women’s views of the intervention, particularly if partner or peer support are incorporated;
biochemical validation of non-smoking status;
nicotine withdrawal and adverse effects such as increased smoking, or disengagement with services;
the collection of perinatal outcome data on birthweight, preterm birth and perinatal deaths, particularly for nicotine replacement therapy trials;
collection of outcome data on breastfeeding, weight gain, operative delivery, maternal psychological wellbeing, and the perceived impact of the intervention on family functioning or other significant relationships;
subgroup analysis by vulnerabilities (to enable an equity analysis);
the impact factor or intra-cluster correlation needs to be reported, in order to assess the effect of clustering and include cluster-randomised trials in meta-analysis.
PLAIN LANGUAGE SUMMARY.
Psychosocial interventions for supporting women to stop smoking in pregnancy
Smoking during pregnancy increases the risk of the mother having complications during pregnancy and the baby being born with low birthweight and preterm (before 37 weeks). Tobacco smoking during pregnancy is relatively common, although the trend is towards it becoming less frequent in high-income countries and more frequent in low- to middle-income countries.
The review showed that psychosocial interventions to support women to stop smoking increased the proportion of women who stopped smoking in late pregnancy and reduced the number of low birthweight and preterm births. There did not appear to be any adverse effects from the psychosocial interventions, and three studies measured an improvement in women’s psychological wellbeing.
The review includes 86 randomised controlled trials, with data from seventy-seven trials (involving over 29,000 women). Nearly all studies were in high-income countries. The intervention that supported the most women to stop smoking in pregnancy appeared to be providing incentives. However, these results are based on only four trials with a small number of women (all in the US), and they only seemed to help women stop smoking when provided intensively (three trials). Counselling also appeared to be effective in supporting women to quit, but only when combined with other strategies (27 trials). The effectiveness of counselling was less clear when women in the control group received a less intensive smoking intervention (16 trials). Feedback also appeared to help women quit, but only when compared with usual care and combined with other strategies (two studies). It was unclear whether health education alone helped women quit, but the numbers of women involved in these trials were comparatively small. The evidence for social support was mixed; for instance, targeted peer support appeared to help women quit (five trials) but in one trial partner support did not. Women also reported that peer and partner support could be both helpful and unhelpful.
Increasing the frequency and duration of the intervention did not appear to increase the effectiveness. Interventions appeared to be as effective for women who were poor, as those who were not; but there is insufficient evidence that the interventions were effective for ethnic (five trials) and aboriginal women (two trials). Trials where the interventions became part of routine pregnancy care did not appear to help more women to quit, which suggests there are challenges to translating this evidence into practice.
ACKNOWLEDGEMENTS
This review represents decades of dedicated work led by Professor Judith Lumley to improve the health of women and children, up until her recent retirement. We thank all previous contributors (outlined below), and acknowledged in previous versions.
This update of the review was jointly funded by the World Health Organization, Australian Government Department of Health and Ageing, the National Health Service (UK), and the EPPI-Centre. This financial support has been greatly appreciated and enabled timely submission.
We thank Ms Josephine Kavanagh and Dr Katy Sutcliffe from the EPPI-Centre for extracting data for the update.
We thank the Cochrane Pregnancy and Childibirth Group’s editorial team for conducting the search and facilitating funding support for this review update. We thank Ms Sharon Kramer, from the Australasian Cochrane Centre for all her advice and assistance, including setting up the main comparisons.
We are very grateful to Professor Guadalupe X Ayala from the San Diego State University and Mrs Lorena Fromberg for translating a PhD thesis written in Spanish (Vilches 2009).
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | 3-armed randomised-controlled trial (pilot study) evaluated 2 different interventions provided to ‘pregnant teens’ to reduce smoking in pregnancy and relapse postpartum. The hypothesis was that an intervention including peer support would be more effective than the intervention alone. Study conducted in Pittsburgh, USA. Data collection dates not reported |
|
| Participants |
Inclusion criteria:
12 to 20 years of age; 4 to 28 weeks’ gestation; reported smoking at least 1 cigarette a day; single marital status; no previous live birth; able to read and write English. Exclusion criteria: Pregnancy complications preventing attendance at group sessions or participation in a home study program. Recruitment: Participants were recruited through local prenatal clinics and public schools. 84 women recruited (not known how many were eligible or approached) and randomised (C = 29, I1 = 29, I2 =26). Baseline characteristics: Mean cigarettes/day at first visit: C = 6.44; I1 (TFS) = 5.87; I2 (TFSB) = 6.81. 63% African-American heritage, 37% European-American heritage Progress+ coding: Coded as single (low social capital) and young age (less than 20) |
|
| Interventions |
Control: 30 minutes individual educational session with project nurse including information about the risks of smoking to the mother and the fetus and brochures on smoking and pregnancy. Intervention 1 (TFS): Cognitive behavioural group model designed specifically for adolescents based on problem-behaviour theory: eight modules to heighten awareness and attention to smoking messages; build and enhance smoking cessation skills; teach skills for maintenance of smoking control; includes experiential learning and round robin discussion. TFS was modified to include additional information on smoking and the fetus, body image changes and overall health. The intervention also included social activities, immediate rewards and adult modelling. Intervention 2 - TFS plus peer support (TFSB): Utilised all the components of TFS plus 1-to-1 support through a non-smoking peer (buddy) chosen by the young woman. Buddies were asked to attend all 8 sessions and to be available at other times for reinforcement of techniques learned and encouragement for continued cessation Main intervention strategy: Social support (multiple intervention) compared to less intensive intervention. TFSB compared with TFS and control in this review as outcomes only reported as combined figures Intensity rating: Frequency (C = 2, I = 6); Duration (C = 2, I = 6). Intervention provided by project staff:efficacy study. |
|
| Outcomes | Biochemically validated point prevalence abstinence at 4-6 weeks post baseline (late pregnancy*) Reduction in exhaled CO and self-reported mean cigarettes per day are reported as ‘reduction’ but actual post-intervention measures weren’t reported so are not included in this review. Baseline modified Fagerstrom Tolerance questionnaire for adolescents to assess nicotine dependence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as ‘randomly assigned’. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Only 46/84 had complete outcome data (high attrition rate = 45%),UC= 12 (41%), TFS = 13 (46%), TFSB = 13 (50%). No explanation for attrition. ITT analysis not mentioned. All those lost to follow-up were included as continuing smokers in this review |
| Selective reporting (reporting bias) | High risk | Only smoking outcomes reported and outcomes not reported separately for each of the control arms |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | CO level (>= 8 ppm) in exhaled air used to identify smokers. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Provider and participants unable to be blinded to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed there was a ‘significant drop out rate’ (45%) |
| Equal baseline characteristics in study arms | Unclear risk | Baseline smoking characteristics similar, but other baseline characteristics not reported |
| Contamination of control group | Low risk | Intervention provided by research project staff. |
| Methods | 3-armed randomised controlled trial evaluated the short- and long-term effects of 2 smoking cessation strategies tailored to support pregnant adolescents to attain abstinence in pregnancy and maintain abstinence postpartum The study was conducted in 5 hospital-based and 2 community-based prenatal clinics in Pittsburgh, Pennsylvania, USA. Years of data collection not reported |
|
| Participants |
Inclusion criteria:
‘Pregnant teens’ aged 14 to 19 years; 12 to 28 weeks’ gestation; able to read, write, and understand English; smoking at least 1 cigarette per day; single marital status; having no previous live births; and capable of being reached by telephone Exclusion criteria: pregnancy complications (i.e., bleeding or preterm labor) or required confinement to home by their physician Recruitment: During prenatal assessment, adolescents self-reporting smoking were invited to participate in study. Those expressing interest signed a consent form to allow the research team to contact them. Expressions of interest also advertised through flyers and brochures 470 screened; 142/224 (63%) eligible women randomised (C = 50; I1: (TFS) = 47; I2: (TFS + B) = 45. Baseline characteristics: Number of cigarettes per day before pregnancy: Control 15.75 (10.38); I1: (TFS) 14.08 (7.22); I2: (TFSB) 14.62 (9.72) Fagerstrom dependence score: Control 3.38 (2.05); I1: (TFS) 3.44 (1.79); I2: (TFSB) 3.68 (1.89) Progress + coding: Low SES, Low educational attainment, low social capital (single) and young age (< 20 years) |
|
| Interventions |
Control: Usual care that all teens would typically receive from a healthcare provider throughout their pregnancy. Smoking during pregnancy was addressed in the clinic by giving the teens educational materials on this subject during the initial prenatal visit. In this study, this material was explained and distributed to the participants by a research team member during the initial assessment. The meetings lasted 45-60 minutes and occurred at 1 of the antenatal clinics or centrally located community site. During the meeting, addresses and telephone numbers of the control group participants were updated after completion of the assessment. Prior to leaving the meeting, participants were informed of the date and time of their next assessment. Participants also received an attendance incentive (e.g. lipstick, nail polish). If the participant had delivered, the attendance incentive was a baby item Intervention 1 (TFS): The TFS intervention consisted of an 8 week group program designed to promote and maintain smoking abstinence based on the Cognitive Behavioral Theory, with modification that incorporated developmental components of Jessor’s Problem Behavior Theory, including a peer buddy and a peer co-leader for peer modelling and sanctioning on smoking. Information pertinent to pregnancy and smoking was provided at the beginning of the 8-week program. Intervention 2 (TFS-B): The TFS-B group received the same 8-week programming, but participants were required to bring a non-smoking female of a similar age as their buddy to the sessions. The role of the buddy was to reinforce smoking cessation strategies and to provide social support to the participant throughout the study Main intervention strategy: Social support (multiple intervention) compared to a less intensive intervention. The control group and TFS-B are compared in this review Intensity rating: Frequency (C = 2, I = 6); Duration (C = 3, I = 6). Provided by dedicated project staff: efficacy study. |
|
| Outcomes | Biochemically validated point prevalence abstinence 8 weeks (late pregnancy*) and 1 year (6-11 months post partum*) after the intervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consenting adolescents were assigned randomly to 1 of 3 group assignments (TFS, TFS-B, or control) by a computer algorithm with a permutated block design, stratified by entry site |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | High attrition: C = 60% (i.e. 40% did not complete 1 yr follow-up), TFS = 55%, TFS-B = 53%. Participants included in primary aim analysis pertaining to randomised treatment assignment, regardless of adherence to study treatment (ITT analysis) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of self-reported smoking status (point prevalence abstinence) using salivary cotinine (> 10 ng). Women reporting less than 1 cigarette per day with salivary cotinine 10-15 ng had salivary nicotine assessment to rule out environmental exposure, and were classified as smokers if that test was > 5 ng |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and providers unlikely to be blinded to this educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Blinding of outcome assessor not reported. |
| Incomplete implementation | High risk | Process evaluation showed poor implementation with almost 50% participants not completing study |
| Equal baseline characteristics in study arms | Low risk | Baseline characteristics appear equal. |
| Contamination of control group | Low risk | Intervention provided by research team. |
| Methods | A randomised controlled pilot study to evaluate whether medical advice had a effect on smoking cessation in pregnancy Study conducted in Bolton, England. Years of data collection not reported |
|
| Participants |
Inclusion criteria: Pregnant smokers at their first antenatal visit, less than 20 weeks’ gestation Exclusion criteria: Not reported. Recruitment: Women recruited from public antenatal clinic at Bolton and District General Hospital. 510 women screened, 142 eligible, 8 moved house and could not be followed up, and 24 women had spontaneously quit. 110 women randomised: control = 47, intervention = 63 Baseline characteristics: 89% heavy smokers and 75% had been smoking for 5 years or more 72% ‘working-class’ (majority low SES) and 75% had no educational qualifications Progress+ coding: Low SES and low educational attainment. |
|
| Interventions |
Control: Usual care, which was advice at the discretion of the doctor. Intervention: 1 to 1 counselling (‘a short interview’) from a senior medical student which involved discussion of the disadvantages of smoking during pregnancy: risk to the fetus; long-term risks of physical and intellectual impairment and possible reasons for this; possible effects on the mother’s own health; costs of smoking; special dangers of smoking in late pregnancy; various ways to help someone to stop smoking. Given strong encouragement to quit and to make a commitment to do so. If this was not agreed then reduction to less than 5 cigarettes a day. Half the intervention group were given a diary to record each cigarette smoked and a gift of a free smoking diary Main intervention strategy: Counselling (single intervention) compared with usual care. Intensity: Frequency (C = 0, I = 1); Duration: (C = 0, I = 1). Usual care intensity: Frequency = 1, duration = 1. Intervention conducted by existing staff (medical student): effectiveness study |
|
| Outcomes | Self-reported abstinence 11 weeks after baseline visit (late pregnancy*) Smoking reduction reported for whole cohort, not by intervention group, therefore not included in this review Discusses participants’ views of intervention. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as “randomly divided”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | There are some missing data in the tables. It is not clear if there was any overall loss to follow-up or whether missing data relate to specific outcomes only. All randomised women included in this review and those lost to follow-up were included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | No other outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Smoking outcomes were self-reported by participants during a visit at home. There was no biochemical validation |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention at first antenatal visit. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Medical student provided intervention (not usual care provider) |
| Methods | Randomised controlled trial of use of exhaled CO feedback for promoting smoking cessation in pregnancy Study conducted in Guildford County, North Carolina, USA over 6 months in 1981 |
|
| Participants |
Inclusion criteria: Women currently or recently smoking, attending public clinics Exclusion criteria: Not reported. All women attending antenatal care orientation sessions were randomly allocated to experimental or control groups Recruitment: 226 women entered prenatal program and 170 (75%) included in analyses. The authors compared those who did not participate and did not find any significant differences. 47% (79/170) were current smokers (C = 43, I = 36) Baseline characteristics: 43% had completed high school education, 56% were black, 80% classified as having no pregnancy risks other than smoking. 38% in the first trimester and 46% in the second trimester of pregnancy Progress+ coding: Low SES as all attending public prenatal clinic. |
|
| Interventions |
Control: Women were read a 135 script that described the relationship among cigarette smoking, CO, and the harmful consequences of smoking Intervention: Experimental group received same information as control group, and they provided breath specimen in which CO was measured, with feedback of the result Main intervention strategy: Feedback (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 1, I = 1); Duration (C = 1, I = 1). Implemented by regular health educators: effectiveness study |
|
| Outcomes | Biochemically validated abstinence 6 weeks after intervention (late pregnancy*) Exhaled CO (ppm), but no SD reported; unclear if ‘quantity of cigarettes’ is mean cigarettes per day; recency of smoking; depth of inhalation |
|
| Notes | Not clear whether this was a group intervention - in which case there was no adjustment for clustering | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear exactly how many women were randomised to each group, however we assume that those reported as ‘current smokers’ in table 1 are the baseline numbers, which were all included in this review |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of reported smoking behaviour for those followed up (CO >= 9 ppm in exhaled air) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Intervention was carried out by clinical staff, no participant blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | All women apparently received the intervention. |
| Equal baseline characteristics in study arms | Low risk | No difference between experimental and control arms on 12 variables measured |
| Contamination of control group | Low risk | Implemented by regular health educators at the maternity clinics |
| Methods | Randomised controlled trial of psychosocial support in pregnancy which aimed to improve maternal health, including reducing smoking during pregnancy Conducted in 4 countries in Latin America (Argentina, Brazil, Cuba, and Mexico) from January 1989 to March 1991 |
|
| Participants |
Inclusion criteria:
High-risk women whose antenatal care began at 15-22 weeks’ gestation, singleton pregnancy, 1 or more of the following: prior LBW infant; preterm birth; perinatal/infant death; < 18 years; body weight <= 50 kg; height <= 150 cm; low family income (local definitions applied); < 3 years school; crowded household (4 or more persons/bedroom); smoking; not living with husband or partner. Exclusion criteria: Heart or renal failure; diastolic BP > 100 mmHg; history of cervical cerclage; Rh negative; mental disease or any chronic disease that might interfere with pregnancy Recruitment: 2,235 women met eligibility criteria and gave consent (I = 1115-though 1110 in table, C = 1120) Baseline characteristics: Smokers (I = 23.9%, C = 21.8%), with variation between countries - Argentina (I = 21.9%, C = 20.6%), Brazil (I = 40.7%, C = 33.1%), Cuba (I = 27.4%, C = 28.9%), Mexico (I = 9%, C = 6.8%). Mean cigarettes per day at randomisation: C = 7.9, I = 7.5 Progress+ coding: Low SES based on place of residence (low family income 20% in Cuba, 52% in Mexico, 53% in Brazil and 100% in Argentina) |
|
| Interventions |
Control: Routine antenatal care, otherwise unspecified. Intervention: Flexible use of a standardised manual, based on site-specific ethnographic studies of needs, fears, expectations, social support networks, including detailed descriptions of situations likely to occur during home visits. 4 to 6 home visits of 1 to 2 hours with emphasis on psychosocial support, education on health habits including better nutrition, reducing smoking alcohol and other drugs, reducing their physical workload, recognition of alarm signs and symptoms, improved access to hospital facilities, reinforcement of health service utilisation. Additional components were a poster, a booklet, hotline to project office, guided tour of hospital, encouragement of family support and participation. Intervention was provided by specially trained female social workers or obstetric nurses with previous experience of childbirth Main intervention strategy: Social support (tailored) compared with usual care. Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 5). Usual care frequency and duration = 0 (unclear). Intervention provided by study team: efficacy study. |
|
| Outcomes | Self-reported point prevalence abstinence at 36 weeks’ gestation (late pregnancy*); Mean cigarettes per day.* Multiple perinatal and maternal health outcome data were collected, but not included in this review as other aspects of the intervention may have had an impact Baseline state anxiety score. |
|
| Notes | Sample size was planned for the primary trial objective. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centrally prepared, method not stated. |
| Allocation concealment (selection bias) | Low risk | Allocation was by opening sealed, opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition 202/2230 (9%): 101 in each arm. Unclear what attrition among smokers and no ITT analysis of drop-outs as continuing smokers, so not able to re-include smokers who dropped out in this review |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of reported smoking behaviour. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Home visitors were aware of group allocation. Social support intervention with home visits |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | The evaluation of the interventions was conducted by a team of independent professional interviewers who were not informed of the characteristics of the study |
| Incomplete implementation | Low risk | Most (83%) of the women randomly assigned to the intervention group received the planned number of home visits, and 90% were visited at least once |
| Equal baseline characteristics in study arms | Low risk | The distribution of risk factors was similar in the 2 groups and the 2 groups had similar demographic, obstetric, and psychological characteristics at baseline |
| Contamination of control group | Low risk | The clinic personnel were unaware of the identity of the women in the control group, and no attempts were made to inform them of which women were in the intervention group. Health educators providing intervention were separate from care providers |
| Methods | Randomised controlled trial of telephone support for improving maternal health outcomes, including smoking cessation during pregnancy Study conducted in a metropolitan city in the south island of New Zealand from March to December 1993 |
|
| Participants |
Inclusion criteria: Women with telephone access, who were either single or with an unemployed partner, less than 20 weeks’ gestation Exclusion criteria: None stated. Recruitment: Recruited in the outpatient department of a large maternity hospital, or its associated GP practices, or self-referral via an introductory letter, phone call, and full discussion of “Healthy Mothers/Healthy Babies” The eligible population was 221 women of whom 49 were never located, 23 were not interested, 10 refused after explanation, and 8 moved away, did not speak English or had a miscarriage. 131 (59%) participated (103 OPD, 22 from GPs, 6 self-referred) (C = 66, I = 65 randomised). Just over 50% were smokers (C = 35, I = 31). Baseline characteristics: Mean cigarettes per day at baseline = 6. 88% European, 10% Maori. 53% single. Progress+ coding: Low SES. |
|
| Interventions |
Control: Package of publicly available educational material on healthy behaviours during pregnancy. Intervention: Package plus weekly telephone call from trained volunteer with the aim of providing minimal support until 12 weeks after birth; aim “to be a friend and a good listener”; to ask about symptoms; signs; alcohol; drugs; smoking and meals in every call; to encourage attendance at antenatal clinic appointments and to ask about “feeling stressed”. Intervention provided by 19 female volunteers, trained for the project with a “case load” of 2 to 6 women each Main intervention strategy: Social support (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 6); Duration (C = 1, I = 4). Intervention provided by project staff: efficacy study. |
|
| Outcomes | Self-reported abstinence at 34/40 (late pregnancy*). Mean cigarettes per day*. Anxiety and depression scores at baseline and 34/40. There were other intervention components which might have influenced these outcomes |
|
| Notes | No process evaluation is reported. No sample size justification SDs for mean cigarettes per day were not reported, therefore we calculated a mean SD from 14 studies with available mean cigarette SDs (6.5) to include in this review, as recommended by the cochrane handbook |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random assignment to control or intervention in balanced blocks of 50 |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Data being reported were analysed on 122/131 of randomised women (control = 63/66, intervention = 59/65). 1 woman requested to be removed from the study, but there were 8 women who for various reasons had incomplete data. p477 4.5% control 9.2% intervention. Only a proportion were smokers (I = 31, C = 35), and the attrition among these is not reported so we were unable to re-include them in the analysis for this review |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of reported smoking behaviour. |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Caregiver blinded to allocation. Women not blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | No process evaluation. |
| Equal baseline characteristics in study arms | Unclear risk | Baseline psychosocial variables (stress; social support; self esteem; depression; anxiety) reported in Table 2. Demographic variables not reported |
| Contamination of control group | Unclear risk | Care providers blinded to allocation and not involved in intervention delivery |
| Methods | Randomised controlled trial (2 × 2 factorial design) evaluating nurse delivered telephone social support (“Baby BEEP”) to improve a range of maternal health outcomes, including smoking during pregnancy. Study conducted in 21 rural Women, Infant and Children Nutritional Supplement (WIC) clinics in a Midwestern state, USA, from January 2002 to July 2006 |
|
| Participants |
Inclusion criteria: Women attending rural WIC clinic who reported smoking at least 1 cigarette per day, spoke English, were 18 years or older, and less than 24 weeks’ gestation Exclusion criteria: Not further specified. Recruitment: When a woman attending a WIC clinic reported current smoking, staff explained the availability of a smoking cessation study and asked permission to provide her name and telephone number to the Baby BEEP research team. If the woman agreed, a nurse from the research team was assigned to contact her to arrange a face-to-face visit to explain the study and request written consent 1420 referrals from WIC clinics, 932 eligible, 695 (75%) randomised (C = 171; I1 (booklets) = 179; I2 (social support) = 175, I3 (social support+booklets) = 170. Baseline characteristics: > 90% ‘ready to quit this pregnancy’. Fagerstrom scores: C = 4.8, I1 (Booklets) = 5.0, I2 (SS) = 4.9, I3 (SS+booklets) = 4.7 Mean age: 22 years, 95% white, 63% high school diploma, 70% in relationship Psychosocial assessments indicated participants experienced high levels of perceived stress and depression and low levels of support generally and from partners Progress+ coding: Low SES as women recruited from WIC clinics. |
|
| Interventions |
Control: Quit Smoking for Good pamphlet from the American Heart Association and instructed that a member of the research team would call each month to arrange a saliva sample,measure exposure to tobacco smoke and ask some questions for 2more interviews Intervention (3 arms): I1 Serialised Pregnancy-Smoking Cessation Booklets (Booklets):Eight booklets comprised a program called “Stop Smoking! A Special Program for Pregnant Women” adapted to a 7th grade reading level. The first booklet was given to the woman at the recruitment visit without counselling, and the 7 remaining booklets were mailed at weekly intervals I2 Nurse-Delivered General Social Support (SS): scheduled weekly telephone call and 24-hour access to the nurse for any additional social support needed. The research nurse’s role on the calls was to use empathetic listening skills and provide social, emotional and/or informational support in response to each woman’s individual needs, such as stressors she was facing and ways she could manage her stress responses. The nurses kept logs of all conversations so that they would be able to follow-up on issues of importance on subsequent calls and as a measure of treatment integrity. All participants in these intervention study groups were encouraged to call the nurse any time they felt stressed or the need to talk, and they were also provided with a refrigerator magnet and a business card with their nurse’s first name and a toll-free number. The nurses received 40 h of training for the telephone support intervention. Each research nurse was given information about a variety of community resources available I3 SS+Booklets: This review included comparisons with the control group and I3 (SS+Booklets). Main intervention strategy: Social support (tailored) compared to a less intensive intervention Intensity: Frequency (C = 1, I = 6); Duration (C = 1, I = 4). Intervention provided by project staff: Efficacy study. |
|
| Outcomes | Biochemically validated point prevalence abstinence at 28-32 weeks’ gestation* (late pregnancy) and 6 weeks post-delivery (0-5 months postpartum*) Perceived stress scale, prenatal psychosocial profile, mental health index 5; readiness to stop smoking; Fagerstrom Test for Nicotine Dependence. Subgroup analysis for patterns of quitting and associations with partner smoking |
|
| Notes | Process evaluation to follow-up phone calls. Low attrition rate suggested as indicator of acceptability | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments were prepared individually for each nurse, were computer generated using SAS |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelope, prepared by the principle investigator that contained the study group assignment |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition: Nine had a spontaneous abortion (C = 2, I1 = 3, I2 = 3, I3 = 1) or non-viable infant (C = 0, I1 = 4, I2 = 1, I3 = 4) and were excluded from the analysis in this review. Those who dropped out and were lost to follow-up for other reasons were included in the final analysis as continuing smokers (C = 7, I1 = 11, I2 = 11, I3 = 7). However, 165 women were lost to lab error in analysing their saliva samples and were not included in analysis. Only 530/695 (76%) randomised participants were included in this analysis C = 126 and I3 = 124 included in this review. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | 165/695 sample lost. Self-reported abstinence in remaining women biochemically validated using salivary cotinine (30 ng/mL or less classified as non-smokers) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | The nurses who collected samples when they conducted the follow-up interviews in late pregnancy and 6-weeks postdelivery were aware of the study group assignment |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | The laboratory was blind to study group assignment while running the cotinine analyses. The assistants who collected the monthly saliva sample may or may not have been blinded to the study group but the rule was to treat all the women the same way |
| Incomplete implementation | High risk | Percent of calls completed in each of their caseloads ranged from 58% to 80% (p400) |
| Equal baseline characteristics in study arms | Low risk | Characteristics appear equal. |
| Contamination of control group | Low risk | Care-providers not involved in provision of the intervention |
| Methods | Randomised controlled trial of CO feedback and brief directive feedback to reduce smoking in pregnancy Study conducted in a large US municipal hospital antenatal clinic, over an 18-month study period (dates not specified) |
|
| Participants |
Inclusion criteria: Pregnant women, currently smoking, at any gestation, attending a clinic for ‘uncomplicated pregnancies’ Exclusion criteria: Very young age (not specified) or “complications” (not specified) Recruitment: All attending women were screened for smoking by questionnaire + CO breath measurement (>= 9 ppm) (over 50%were current smokers) and 139 women were randomly assigned (C = 69, I = 70) Baseline characteristics: An average of 12.7 cigarettes per day. The population consisted primarily poor and stable ‘working class’ Caucasian women. (52.4%), Black (44.6%) and Asian (3%) Progress+ coding: Low SES. |
|
| Interventions |
Control: Usual care, where a clinic nurse provided health education, including smoking. Intervention: A personal letter from the Chief (physician) of the prenatal clinic within 3 days of the visit, mentioning the CO test, discussing the risks of smoking to herself and the fetus and urging her to stop plus the American Cancer Society pamphlet (“Why start life under a cloud?”) about the negative effects of smoking and simple guidelines for self-directed smoking cessation Main intervention strategy: Health education (single intervention) compared to usual care. CO feedback was provided to both groups so not included as a feedback trial Intensity: Frequency (C = 0, I = 1), Duration (C = 0, I = 1). Usual care intensity: Frequency = 1, Duration = 1. Intervention provided by routine clinic staff: Effectiveness study |
|
| Outcomes | Biochemically validated point prevalence smoking cessation at 34 weeks’ gestation (late pregnancy*) | |
| Notes | Simple intervention so no process evaluation. Clinic-wide implementation so no consent sought. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No consent sought and no loss to followup apparent. |
| Selective reporting (reporting bias) | Unclear risk | None apparent. Primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of reported behaviour by exhaled CO (>= 9 ppm counted as smoking) |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | The authors state that clinic staff were unaware of group allocation. Women would not have been blind to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | All intervention participants provided with letter. No information regarding whether they read it or not |
| Equal baseline characteristics in study arms | Unclear risk | There were no significant baseline differences between 2 groups in terms of age, ethnicity, term of pregnancy, number of children, number of reported cigarettes smoked, or CO |
| Contamination of control group | Low risk | Intervention was a letter so unlikely to be sent to control group in error |
| Methods | This randomised controlled study aimed to evaluate the effectiveness of nurse counselling to reduce smoking in pregnancy. The study was conducted in 2 community-based obstetric clinics in Milwaukee (USA). Study dates unclear |
|
| Participants |
Inclusion criteria: Pregnant, ‘a current smoker’, English speaking, visually able to read 12 point typeset, being able to give free consent, and expecting to reside in Milwaukee following delivery Exclusion criteria: Not specified. Recruitment: 50% of patients enrolled in third trimester. 57 women randomised, but unclear how many to each group Baseline characteristics: Cigarette consumption mean at entry = 8.6 93% participants smoked fewer than 10 cigs per day. 79% Black, 16% had partner, 70% single, 77% unemployed, 32% < grade 12 education, 61% < $10,000 per year No coding as outcomes not able to be included in this review |
|
| Interventions |
Control: A smoking cessation booklet at 6th grade reading level or 11minute videotape. Intervention: Booklet or video Nurse counselling based on 4 As recommended by National Cancer Institute. The nurse intervention was a systematic tailored smoking cessation approach that was based on the 4 A (Ask, Advise, Assist, Arrange) approach by the National Cancer Institute Main intervention strategy and intensity not coded as not included in meta-analysis |
|
| Outcomes | Self-reported smoking status (20% had CO screening) 1 month after enrolment, in the ninth month of pregnancy, and 1 month postpartum. But not reported by intervention group so unable to include any outcomes in meta-analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Of the 57 participants enrolled in the study, 50 were available for 1 and 9 month followup, and 48 responded to the 1 month postpartum survey. All non-respondents were considered to be smokers at follow-up and considered to have made no quit attempts in the follow-up interval |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported by intervention group, but did not claim results were significant |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking status for 80% sample. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personal unlikely to be blinded in educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Home visits. |
| Methods | Cluster-randomised controlled trial which aimed to assess 2 methods of disseminating smoking cessation programmes to public antenatal clinics Study conducted in Newcastle, New South Wales, Australia. Data collection dates not reported |
|
| Participants |
Inclusion criteria: Public antenatal clinics with an antenatal clinic and more than 500 births per year (unit of randomisation). Women who attended the clinics and reported to be current smokers were the unit of analysis Exclusion criteria: Under 16 years of age, too sick, non-English speaking, illiterate, attendance was first visit Recruitment: 23/25 public hospitals agreed to participate 22 clinics randomised (C = 11, I = 11). Assume smoking prevalence identifies eligible smokers (2284 in control clinics and 2821 in intervention clinics). Included in post-dissemination assessment: C = 688, I = 781 Baseline characteristics: Smoking details not reported. Proportion more than high school: 22%; Language other than English at home: C = 35%, I = 33% Progress+ coding: Low SES as all attending a public pre-natal clinic. |
|
| Interventions | The cessation programme “Fresh Start for you and your baby”, developed by Windsor, based on CBT, was used. More details are described in Walsh 1997. Coded as a counselling (multi-modal) intervention. Control: Simple dissemination of programme to clinics which included mail out of written information on programme benefit and resources Intervention: Intensive dissemination of programme which included written information and feedback about programme benefits to managers, provision of programme resources, offers of visits to explain programme and provide training, sample smoking cessation policy, regular contacts to offer support, and computerised feedback on activities Main intervention strategy: Intensive dissemination vs less intensive dissemination. Intensity: Not coded as same intervention for women in both arms (counselling-tailored). This study is not included in intensity analysis Study provided by existing service providers: effectiveness study |
|
| Outcomes | Primary outcomes were the proportion of women whose smoking status was assessed and were provided smoking cessation advice Biochemically validated point prevalence smoking cessation at end of pregnancy* (The proportion of women who had been smokers when they first visited the clinic who had now quit, p99) was a secondary outcome for this study Provider views of interventions discussed. |
|
| Notes | No intracluster correlation or impact factor reported, so sensitivity analysis conducted using 4 ICCs and figures adjusting using ICC of 0.1 in outcome tables. See Table 2 for adjustment calculations. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random allocation not specified, but taken within strata based on clinic size and baseline smoking rates |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | One clinic excluded as did not report final data and some missing data for post-dissemination measures. No ITT of women dropping out of study. Only women completing study measures included in analysis. Unable to re-include in this review |
| Selective reporting (reporting bias) | Low risk | Smoking status and recall of intervention reported. |
| Other bias | High risk | There was a shorter recruitment period (1 week instead of 2 weeks) at post-dissemination for the 11 largest clinics (out of the 22 clinics involved), so the sample sizes have been adjusted to account for the shorter recruitment period for those clinics, by increasing the sample size to what they would have expected to have recruited if the period was over 2 weeks instead of 1. We have adjusted for these estimates in this review as outlined in Table 2. Also lower recruitment in control arms compared to intervention arms |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Exhaled CO >= 9 ppm. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention. Neither women nor providers would have been blind to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed good implementation in intervention group. However time constraints within clinics meant that training sessions could not be repeated. Although training permitted information about the programme to be provided to clinicians and the training videotape modelled smoking cessation skills, the time period was usually inadequate to provide skill development as originally planned. p100 |
| Equal baseline characteristics in study arms | Low risk | Patient population differences on nearly all 14 characteristics were minimal (less than 5%) |
| Contamination of control group | High risk | Similar proportions of control women received the specific risk information which indicated that midwives had increased the pre-study level of usual care advice |
| Methods | Randomised controlled trial evaluating provision of videotaped vignettes for promoting smoking cessation and relapse prevention during pregnancy Study conducted in a community-based university setting, Texas, USA. Data collection dates not reported |
|
| Participants |
Inclusion criteria: Volunteers who were willing to quit within 2 weeks. Exclusion criteria: Women smoking < 3 cigarettes per day; < 18 years; > 30 weeks’ pregnant; do not have a working video recorder (approximately 12% Americans); depressed Recruitment: Through local media, such as newspaper, radio, subscriber letters, community business flyers, waiting room posters 146 women screened and 82 women who met inclusion criteria were randomised (C = 40, I = 42) Baseline characteristics: Mean cigarettes/day at first visit: C = 14.5, I = 17.3. Progress+ coding: None. |
|
| Interventions |
Control: Received a quit calendar and tip guide. Intervention: As for control plus were mailed a video with 6 × 25-30 minute vignettes covering a range of topics and strategies from initial quitting to relapse prevention Main intervention strategy: Counselling (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 2), Duration (C = 1, I = 4). Intervention provided by study staff: efficacy study. |
|
| Outcomes | Biochemically validated point prevalence abstinence obtained within 2-3 days of quit date, 4-5 weeks after the quit date (late pregnancy)* and 1 month postpartum (0-5 months postpartum*). Participant evaluation of intervention materials. Associated references report association of quitting and depressive disorders. CES-D scores at baseline only |
|
| Notes | Authors say women in this study tend to be heavier smokers than described in previous studies | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Only 61% of participants completed all assessments. All those with missing data were treated as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | Pre-specified outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | All reports of abstinence were validated by measurement of salivary cotinine |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Video mailed to participants. Not clear if UC givers were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed only 53% of the intervention group viewed 1-3 of the 6 videos. 47% did not view them |
| Equal baseline characteristics in study arms | Low risk | No significant difference in socioeconomic variables between groups |
| Contamination of control group | Low risk | Video mailed out to participants only. |
| Methods | Randomised controlled trial to evaluate a depression-focused intervention which aims to promote smoking cessation during pregnancy Study conducted in Texas (USA) between January 2005 and January 2008 |
|
| Participants |
Inclusion criteria: >= 16 years of age, to be <= 32 weeks pregnant, to have smoked at least a puff or more during the past 7 days, to have a telephone, and to express a willingness to quit smoking during the study (i.e., women with a goal of only reducing cigarette consumption were not eligible) Exclusion criteria: Currently participating in psychotherapy or other smoking cessation treatment, had unstable medical conditions that would adversely affect attendance, or demonstrated psychological instability during the screening (e.g., high suicide risk, symptoms of cognitive disorder, or severe intellectual impairment) Recruitment: Through newspaper and television advertisements, and physician referrals. 730 women were screened for basic eligibility by telephone. 266/294 (90%) eligible women were randomised (C = 133, I = 133) Baseline characteristics: Smoking rate before finding out pregnant (mean cigarettes per day): I = 16.8 (8.7), C = 15.8 (9.1); Current smoking rate (mean cigarettes per day): I = 9.8 (7.1), C = 9.7 (6.7) Fagerstrom Test for Nicotine Dependence score I = 3.2 (2.1), C = 3.5 (2.0) 63% receiving medicaid or county health care, 54% African-American, 10% Hispanic, 33.5% Caucasian; 31.9% had less than high school education. 34.2%had family income < $10,000 75.5% had lifetime major depressive disorder (23.5% current major disorder) Progress+ coding: None. |
|
| Interventions | Ten individual counselling sessions were scheduled for 60 min. Each session consisted of 15 min of standard behavioural and motivational smoking cessation counselling (common to both groups). Counselling typically involved active efforts to prepare for quitting and maintaining abstinence using self-monitoring of their smoking prior to the quit date, identification of high-risk situations for smoking, and development of coping skills and support before and after the quit date. Therapists used motivational enhancement strategies based on techniques of motivational interviewing if resistant to quitting. The core features included exploration of participant ambivalence, use of open-ended questions, reflective listening, expressed empathy, rolling with resistance, and use of strategies to develop perceived discrepancy between smoking behaviour and important personal goals and values Control: The primary goal of the HW treatment was to educate women on ways to decrease stress, to respond to stressful events, and to take care of themselves physically during their pregnancies. The purpose was to provide a time- and attention-matched control for CBASP that was pregnancy relevant but instructional in nature-typical of health-education interventions. Participants chose from a list of discussion topics, including stress, pregnancy symptoms, sleep, exercise, yoga, relaxation training, time management, parenting tips, dealing with anger, negative thoughts and feelings, and postpartum depression. Intervention: CBASP was originally developed for the treatment of chronic depression. The primary CBASP treatment strategy is a social problem-solving exercise called Situational Analysis (SA), which is a technique used to create awareness of the contingent relationship between participants’ behaviour and outcomes in stressful interpersonal situations. Another CBASP treatment strategy involved increasing participants’ awareness of the contingent relationship between their behaviour and interpersonal outcomes within the therapeutic relationship and to apply this learning to relationships within the participants’ daily living arenas. The CBASP model assumes that repeated practice of SA within and outside of treatment and increased understanding of participants’ interpersonal impact on the therapist lead to acquisition of new perceptual and behavioural skills that improve interpersonal problem resolution. In turn, this is assumed to decrease interpersonal stress and depressive symptoms Main intervention strategy: Counselling (single intervention) compared to alternative intervention Intensity: Frequency (C = 6, I = 6); Duration (C = 6, I = 6). Intensity: Frequency (C = 6, I = 6); Duration (C = 6, I = 6). |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence at end of 10 weeks treatment (late pregnancy*); Smoking cessation 3 & 6 months after treatment, smoking cessation 3 (0-5*) & 6 (6-11*) months postpartum. Continuous and prolonged abstinence also reported Depression (CES-D scores) and probability of cessation 6 months post-treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Adaptive randomisation was used to stratify the groups on age, race, history of depression, baseline smoking rate, baseline depressive symptom severity (CES-D >= 16), and longest duration of last depressive episode |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: 3 months: C = 9/133, I = 22/133; 6months C = 42/133, I = 54/133. All analyses were carried out on the intent-to-treat sample, which included 128 participants in the Intervention group and 129 control - excluding only those who experienced a miscarriage during the study (5 participants in Intervention and 4 participants in control) |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of self-reported smoking status (7-day point prevalence only) using expired CO (< 4 ppm) throughout treatment and salivary cotinine (< 15 ng/mL) at follow-up contacts |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and providers unlikely to be blinded to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showed high levels of compliance with counselling standards in both groups. Participants attended an average of 8/10 sessions of approximately 58 mins |
| Equal baseline characteristics in study arms | Low risk | No significant differences noted. |
| Contamination of control group | Low risk | There is a potential risk with the same counsellors providing counselling for the intervention and control groups. However global competence ratings for CBASP, HW, and the smoking cessation counselling interventions were measured on a scale ranging from 1 (does not attempt intervention) to 4 (good use of intervention). No differences in competence between the groups were noted, averaging 3.8 (SD across conditions. Statistical agreement of competence ratings between primary and secondary raters was high, with a Cohen’s kappa (Landis & Koch, 1977) of .93 (95% CI 0.86 to 1.0) |
| Methods | Randomised controlled trial of counselling to support women to stop smoking during pregnancy in the USA. Location and dates of data collection not reported (abstract only available) | |
| Participants |
Inclusion criteria: Self-reported smokers presenting for prenatal care before 24 weeks’ gestation Exclusion criteria: Not specified. 150 women randomised. Data for only 43 women (C = 20, I = 23) who had delivered by the time of report are available. 2 women in control group had baseline cotinine levels consistent with abstinence so are not included (C = 18, I = 23) Baseline characteristics: Not reported. Progress+ coding: None. |
|
| Interventions |
Control: Discussion of smoking risks by a nutritionist and again by a resident physician at initial prenatal visit Intervention: Control + regular meetings with a smoking cessation counsellor and physician reinforcement at each visit. The women also received biochemical feedback from urine cotinine Main intervention strategy: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C=1, I=5); Duration (C=1, I=3). Estimates for intervention as little detail provided |
|
| Outcomes | Biochemically validated point prevalence abstinence at term or birth (late pregnancy*); >50% reduction in mean cotinine*; and mean birthweight* | |
| Notes | SDs for mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | One woman in the intervention group dropped out of the study and was not included in the original analysis but has been re-included as a continuing smoker in this review, but not included in the mean birthweight analysis |
| Selective reporting (reporting bias) | High risk | Preliminary results only available. Final results not reported and unable to be accessed |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation by urine cotinine but cut-off levels not reported |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible for participants and personnel to be blinded to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Baseline characteristics not reported (abstract only). |
| Contamination of control group | High risk | Appears that same physician provided advice to control and intervention women, and not clear if this was not repeated for control group |
| Methods | Randomised controlled trial evaluating effectiveness of feedback from a point-of-care cotinine test for supporting women to stop smoking during pregnancy Study conducted in Birmingham, UK. Dates of data collection not reported |
|
| Participants |
Inclusion criteria: ‘Current smokers’ (> 10 mg/L in preliminary urine cotinine result) Exclusion criteria: Not specified. Recruitment: Seen at initial antenatal visit and given brief explanation of test and aims of research, and asked to give verbal consent to participate in study. Women then had urine screened for cotinine and completed a questionnaire 745/856 (87%) eligible women agreed to participate and were randomised (C = 447, I = 298 in flow chart and 409 in results text). 280 women were smokers (C = 164, I = 116) Baseline characteristics: Average consumption of 11.8 cigarettes per day. Other characteristics not reported Progress+ coding: None |
|
| Interventions |
Control: Routine counselling from a doctor or midwife. Urine measured at initial visit but no feedback given to woman Intervention: Six-minute urine test completed in their presence. Results given as a number and graphic illustration. A specific quit date within the next 14 days was mutually agreed and the woman was given a printed leaflet containing practical advice on how to reduce their smoking measurement at each visit. A positive friendly attitude of providers - information, feedback, encouragement protocol was repeated whenever the patient returned to the clinic up to and including the 36 week visit, with measurement, questioning about changes in smoking, specific events on the quit date and reinforcement of advice Main intervention strategy: Feedback (multiple intervention) compared to usual care. Intensity: Frequency (C = 0, I = 5); Duration (C = 0, I = 3). Usual care intensity: F = 1, D = 1 Intervention provided by study staff: Efficacy study. |
|
| Outcomes | Biochemically validated point prevalence smoking cessation at 36 weeks’ gestation (late pregnancy*) Proportion with ‘some reduction*’ (20%-80% urine cotinine). Mean birthweight* and length. Preterm births* reported in attrition and re-included in both numerator and denominator for this outcome Gestation, type of delivery, and Apgar scores collected but results not reported Participants view of interventions reported. |
|
| Notes | SDs forv mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi-randomised: New referrals to 3 large inner-city hospital antenatal clinics were randomised on the basis of their allocated hospital unit number, even numbers being placed in the case or intervention group, or those who were provided with feedback from the smoking test at point of care. p675 |
| Allocation concealment (selection bias) | High risk | Group allocation could be anticipated. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Only 83/116 women in the control group and 109/164 women in the intervention group completed the study. Those who dropped out for medical reasons: miscarriage (C = 2, I = 3) or premature delivery (C = 6, I = 13), or transferred care (C = 3, I = 5) were excluded (C = 11, I = 21) from smoking outcome analysis. Those who failed to attend appointments, or refused further involvement were re-included as continuing smokers in this review (C = 18, I = 34), leaving a total sample of C = 101, I = 143 |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported. |
| Other bias | High risk | Clear financial conflict of interest declared by author (directorship of company producing feedback tests). |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Smoking status biochemically validated with urine cotinine (> 10 mg/L indicates active smoker) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Neither providers nor women were blind to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Contamination unlikely with provision of specific biochemical test |
| Methods | Randomised controlled trial of “Significant Other Supporter” (SOS) program, of social support and direct financial rewards to reduce smoking during pregnancy and postpartum Study conducted in Oregon WIC program sites, USA, between June 1996 and June 1997 | |
| Participants |
Inclusion criteria: Women smoking (even a puff in the last 7 days); less than 28 weeks’ gestation; over 15 years of age; literate in English Exclusion criteria: Not specified. Recruitment: 220/309 (71%) eligible women were randomised (C = 108, I = 112) Baseline characteristics: Mean salivary cotinine at baseline: I = 45.4; C = 45.7. Caucasian (I = 90%, C = 88%), household income < $20000 (I = 87%, C = 89%), Single (I = 47%, C = 42%), Mean age (I = 23.5, C = 24.0) Progress+ coding: Low SES. |
|
| Interventions |
Control: Verbal and written information on the importance of smoking cessation, a pregnancy specific smoking cessation self-help kit, and monthly telephone calls for self-reports on their smoking status. Intervention: As for the control group plus were asked to designate a social supporter (preferably a female non-smoker), and were advised both she and her supporter would receive an incentive: participants were given $50 voucher for each month biochemically confirmed as quit. Supporter received $50 voucher in first month and at 2 months postpartum, and $25 voucher for other months Main intervention strategy: Incentives (multiple intervention) compared with a less intensive intervention Intensity: Frequency (C = 2, I = 6),Duration (C = 1, I = 3)-estimated duration as limited information available The intervention was delivered by trained program staff or research staff: efficacy study |
|
| Outcomes | Biochemically validated point prevalence smoking cessation at 34 weeks’ gestation (late pregnancy*) and 2 (0-5*) months postpartum | |
| Notes | Data in outcome tables is inconsistent. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | High attrition rates I = 32%; C = 51.5% (reasons not specified), but all drop-outs included as continuing smokers in this analysis |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Reported quitting validated by salivary cotinine analysis (> 30 ng/mL considered to be smokers). Salivary thiocyanate also used (> 100 ug/mL considered to be smokers) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Neither providers nor women were blinded for this educational intervention with incentives |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | No process evaluation reported. |
| Equal baseline characteristics in study arms | Unclear risk | Preliminary analysis indicates no significant differences exist between randomised groups on baseline demographic characteristics |
| Contamination of control group | Low risk | Control group not reported clearly - however intervention given by trained research staff rather than usual care providers so unlikely that there was contamination |
| Methods | Randomised controlled trial of medical advice to stop smoking in pregnancy Study conducted in 3 public maternity units in the UK. Dates of data collection not stated |
|
| Participants |
Inclusion criteria: Pregnant women < 35 years; currently smoking >= 5 cigarettes/day and had been smoking >= 1/day at the onset of pregnancy; < 30 weeks’ gestation at first visit; no prior perinatal death; not seeking termination Exclusion criteria: Not further specified. Recruitment: Consecutive series of patients who contacted 3 maternity units regarding confinement were posted reply-paid questionnaires (including smoking questions), which were used to select eligible participants 588 women provided consent and were randomised. Baseline characteristics: Mean cigs/day at beginning of pregnancy (C = 17.6, I = 17.9); mean cigs/day at study entry (C = 15.2, I = 15.2), Mean age (C = 24.2, I = 23.8). Even distribution of social class categories Progress+ coding: None. |
|
| Interventions |
Control: ANC usually provided by the hospital, including any anti-smoking advice which may have been given routinely Intervention: Individualised medical advice by clinic doctor, (i) tell the woman the facts about smoking in pregnancy; (ii) encourage questions about these facts; (iii) once the woman has agreed to try, discuss how she may best give up; (iv) follow-up the advice at all later contacts. Medical records labelled asking other staff to reinforce advice Details of the intervention are in Donovan 1975. Main intervention strategy: Health education (single intervention) compared to usual care Intensity: Frequency (C = 0, I = 5); Duration: (C = 0, I = 2)-estimate. Usual care intensity: F = 1, I = 1 Intervention provided by existing service providers: effectiveness study |
|
| Outcomes | Self-reported mean cigarettes/day at 4 stages of pregnancy (late pregnancy*); mean birthweight*; low birthweight*; preterm birth* (< 36 weeks); perinatal deaths*. No data on smoking cessation | |
| Notes | Discussion of common problems identified when advising women to stop and on the contextual factors which encourage the continuation of smoking. Major inconsistency in smoking reports pre and post-birth is a problem in this trial Actual standard errors were able to be incorporated into software for this update (previously SD 500 used), so effect size estimates have altered slightly |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Twins (C = 2, I = 6) and miscarriages (C = 17, I = 11) not included in analysis. 552 women analysed (C = 289, I = 263). No further attrition reported |
| Selective reporting (reporting bias) | Unclear risk | Smoking cessation rates not reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of reported smoking behaviour. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Notes labelled. Caregivers asked to reinforce information. Educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation of the reinforcement of advice showed little difference between the groups in recall of advice being given |
| Equal baseline characteristics in study arms | Unclear risk | From table 2 characteristics appear to be equal - but there is no statement or statistic confirming this |
| Contamination of control group | High risk | Same providers offering intervention and control advice. Process evaluation of the reinforcement of advice showed little difference between the groups in recall of advice being given |
| Methods | Randomised controlled trial of counselling and telephone support to support women to stop smoking during pregnancy and post-partum Study conducted in Hartford, Connecticut (USA), between January 2001 and December 2002 |
|
| Participants |
Inclusion criteria: Pregnant women, over 18 years old, less than 30 weeks’ gestation, current smokers (recent quitters included in associated relapse prevention paper (Morasco 2006). Exclusion criteria: Recent history of abuse or dependence on alcohol or other non-nicotine substance, major psychiatric illness, no access to a telephone Recruitment: Study conducted in the prenatal clinic of a non-profit tertiary care community hospital. Written consent obtained. Unclear how many eligible women participated. 140 women enrolled in study. 33 spontaneously quit (C = 19, I = 14), 107 were randomised but 2 were excluded due to missing data, leaving 105 included in analysis (I = 53, C = 52) Baseline characteristics: 70.5% smoked less than 10 cigarettes per day at baseline. Mean 20.8 (12.37) pre-pregnancy 66% Hispanic, 17% Caucasian, 11% African American. 61% unemployed, 54% less than high school education, 60% single, 49% household income < $15000/yr, 52% 1 or more depression items and 19% all 4 items Progress+ coding: Low SES and minority ethnic group. |
|
| Interventions |
Control: Usual care according to standard smoking cessation guidelines, with providers offered 2 ×1h training sessions. Research study co-ordinator provided all participants with a booklet, inserted a chart prompt to remind providers to provide personalised quit messages at each visit, and audited charts to ensure the advice was documented Intervention: 1 90-minute psychotherapy session provided by masters-prepared mental health therapist trained in smoking cessation. The main goals were to assess readiness to quit, identify potential psychological or social problems that might pose as barriers to quitting, and set a quit date. This was followed by bi-monthly telephone calls from the therapist during pregnancy, and monthly calls after delivery Main intervention strategy: Counselling (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 5, I = 6), Duration (C = 2, I = 6). Intervention provided by study staff: efficacy study. |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence in late pregnancy* and 6 (6-11) months postpartum* Aggregated results by week of gestation to enter study. An associated study (Morasco 2006) reports abstinence rates for recent quitters (relapse prevention*) Cost-effectiveness of ‘cost per quitter’. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of methods of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 2/107 randomised women were excluded from analysis due to missing data and were unable to be re-included in this report as the group allocation is not reported. The remaining dropouts (18% at 6 months postpartum) are included as continuing smokers in this analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation with exhaled CO readings (cut off < 8 ppm but all participants less than 4 ppm) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention so blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed 17/53 did not receive the phone calls as planned |
| Equal baseline characteristics in study arms | Unclear risk | No significant differences in any of the baseline characteristic between the 2 groups |
| Contamination of control group | Low risk | Counselling and follow-up sessions provided by psychotherapist not involved in usual care |
| Methods | Randomised controlled trial of midwifery counselling to support women to stop smoking in pregnancy Study conducted in a large UK maternity service. Data collection dates not specified |
|
| Participants |
Inclusion criteria: Pregnant and booked for maternity care; <18 weeks’ gestation; currently smoking 1 or more cigarettes/day Practising midwives regularly attending antenatal clinic. 13 midwives selected for the intervention group and 13 for the control group Exclusion criteria: Not specified. Recruitment: All women identified as smokers in a busy teaching hospital with 3700 deliveries a year received a letter asking if they would like to participate. 100 women participated (described as ‘all 100 women contacted’) and were randomised (C = 50, I = 50) Baseline characteristics: ‘Contemplators’ (C = 70%, I = 60%), ‘pre-contemplators’ (C = 15%, I = 22%), ‘ready for action’ (C = 15%, I = 18%) No other baseline characteristics reported. Progress+ coding: None. |
|
| Interventions |
Control: Usual care. Intervention: Midwives were trained to assess the stages of change and provide a behavioural intervention, using the Health Education Authority material “Helping pregnant smokers quit: training for health professionals”, 1994 Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency (C = 0, I = 5), duration (C = 0, I = 2)-based on estimated brief contact (< 5min) at a standard number of antenatal visits (8), as very little information about intervention provided. Usual care intensity: F = 0, I = 0 Intervention provided by existing staff: effectiveness study |
|
| Outcomes | Self-reported smoking cessation at 37 weeks (late pregnancy)*; and at 4 weeks (0-5 months*) postpartum Reduction in cigarettes/day; “stage of change” at 11 to 18 weeks vs 37 weeks. No biochemical validation of smoking status. Care providers’ views discussed |
|
| Notes | No process evaluation reported. Abstract data used. States ‘after one year’ which is assumed to be of year of the study, at 37 weeks’ gestation, as reported in figure one. As there were no quitters in the control group, the relapse rates of 4% within 1 month postpartum are assumed to be from the treatment group only |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Described as ‘randomly allocated’. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 94 of 100 randomised women followed up (reasons for attrition not reported). No ITT analysis reported. However, all drop-outs re-included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of reported smoking status. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel unlikely to be blinded to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Midwives randomised so low risk of contamination. |
| Methods | Randomised controlled trial which aims to promote smoking cessation and relapse prevention during pregnancy and postpartum The study was conducted in 3 urban community-controlled health services in far north Queensland and Western Australia June 2005 and December 2009 |
|
| Participants |
Inclusion criteria: Pregnant Aboriginal or Torres Strait Islander women attending their first antenatal appointment at 1 of the Aboriginal community-controlled health services at or before 20 weeks’ gestation; were aged 16 years or older,were self-reported current smokers or recent quitters (quitting when they knew they were pregnant); and were residents of the local area Exclusion criteria: Women whose pregnancy was complicated by a mental illness or they were receiving treatment for chemical dependencies other than tobacco or alcohol use Recruitment: 1119/1180 women attending the antenatal clinic were assessed for eligibility. 263/379 (69%) eligible women agreed to participate (C = 115, I = 148) Baseline characteristics: Median cigarettes per day: C = 10 (4-15), I = 10 (5-15); Spontaneous quitting since pregnancy: C = 8, I = 24 100% Aboriginal and Torres Strait Islander women. Partner (C = 88%, I = 92%) Progress+ coding: Low SES and minority ethnic group. |
|
| Interventions |
Control: Usual care consisting of general advice from a GP about quitting smoking, based on existing brief intervention guidelines Intervention: Intervention developed after review of the literature and consultation with service providers and community members. At first antenatal visit women received a scripted invitation from the doctor to quit smoking and advised to quit ‘cold turkey’ and return to the clinic in 3-5 days and at 7-10 days. The woman received an appointment reminder card, fridge magnet, and a letter for other household members requesting their support. Women were asked to bring a partner or support person with them on their second visit. Women still smoking after 7-10 days were offered NRT if no contra-indications. Follow-up visits were conducted by female Aboriginal or Torres Strait Islander health workers and midwives who received training from a behavioural scientist and a GP, a study manual and a 1 page guide with scripted advice Main intervention strategy: Counselling (tailored) compared to usual care. Intensity: Frequency (C = 0, I = 4), Duration (C = 0, I = 3). Usual care intensity: F = 1, D = 1 Existing staff delivered intervention: effectiveness study. |
|
| Outcomes | Biochemically validated point prevalence smoking abstinence* and relapse prevention* at 36 weeks’ gestation (late pregnancy) Post-partum cessation (6 months) not reported due to very high rates of attrition |
|
| Notes | Cluster-randomisation by weeks but number of weeks not reported. No analysis for adjustment for clustering reported. Treated as individually randomised controlled trial in this review | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An Excel computer program was used to randomly allocate weeks to intervention or control for all clinics |
| Allocation concealment (selection bias) | High risk | Author notes lack of allocation concealment a methodological limitation of the study, which may account for unequal allocation in study arms |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | High rates of attrition (C = 37/115, I = 50/148) at end of pregnancy (reasons not reported). Very high attrition at 6 months post-partum. ITT analysis. Women lost to follow-up or with missing smoking status were classified as current smokers |
| Selective reporting (reporting bias) | Unclear risk | 6 months postpartum outcomes not reported due to high attrition |
| Other bias | High risk | Unequal numbers in each group with greater allocation to intervention groups |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported smoking cessation biochemically validated using urinary cotinine (< 250 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Clinic staff made aware of treatment allocation at beginning of each week and unlikely participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Outcome assessor blinding not reported. |
| Incomplete implementation | High risk | 64% doctors adhered to protocol and a lower proportion of nurses and health workers |
| Equal baseline characteristics in study arms | High risk | A slightly higher proportion of intervention group were in clinic 1, a slightly lower proportion had a partner, and had recently quit |
| Contamination of control group | High risk | Same antenatal care providers delivered intervention and control arms. High likelihood of contamination noted in discussion |
| Methods | This randomised controlled trial examines whether an integrated behavioural intervention improves pregnancy outcomes, including smoking cessation The study was conducted in 6 community-based clinical sites serving minority women (African-Americans and Hispanics) in Columbia, USA, from July 2001 to July 2004 |
|
| Participants |
Inclusion criteria: Women attending prenatal care in 6 community-based sites who self-identified as belonging to a minority group, being >= 18 years, < 29 weeks pregnant, a DC resident and English speaking. Had to have 1 risk factor (smoking, ETSE, depression, and IPV). Only women reporting smoking at baseline are included in this review Exclusion criteria: Suicidal women. Recruitment: 2913 women approached while waiting for prenatal appointments. 1044/1398 (75%) eligible women provided signed consent to participate in the study (C = 523, I = 521) 302 women reported smoking ‘1+ puff in the preceding 6 months and 198 reported ‘active’ smoking at baseline. These 198 ‘active’ smokers at baseline are included in this analysis (C = 92, I = 106) Baseline characteristics: 100% African American, 43.7% reliant on social housing, ~80% Medicaid recipients Progress+ coding: Minority ethnic group and low SES. |
|
| Interventions |
Control: Not reported-usual care. Intervention: The 10-session intervention was delivered during prenatal (eight sessions) and postpartum (2 booster sessions) care visits. 4 prenatal sessions were considered minimal adherence. The session duration was approximately 35 min. The smoking intervention was consistent with the Smoking Cessation or Reduction in Pregnancy Trial (SCRIPT) and the Counseling and Behavioral Interventions Work Group of the United States Preventive Services Task Force recommendations, a 5-step behavioral counselling approach. The intervention was tailored to the woman’s stage of change. Women were encouraged to avoid triggers and to use alternative coping and behavioural change strategies. The intervention included content to address both active smoking and ETSE, whether or not they met criteria for ETSE. Women with other risk factors (IPV, depression and drug or alcohol use) also received additional targeted interventions to address those issues Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency (C = 0, I = 5), Duration (C = 0, I = 4). Intervention provided by study staff: efficacy study. |
|
| Outcomes | Biochemically validated smoking cessation prior to delivery* (late pregnancy) and at 8-10 weeks (0-5 months*) postpartum. Mean urine cotinine* Outcomes also reported by intervention group for environmental tobacco smoke exposure, depression, intimate partner violence and illicit drug use Detailed pregnancy outcomes reported but not included in this analysis as they were not reported by smoking status at baseline, and these outcomes may be affected by several of the multi-modal interventions aimed at reducing risk factors other than smoking |
|
| Notes | Detailed participant satisfaction and intervention acceptability was reported in an associated reference (Katz 2008). | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Site- and risk-specific block randomisation to IG or UCG was conducted. A computer generated randomisation scheme considered all possible risk combinations within each of the recruitment sites |
| Allocation concealment (selection bias) | Low risk | Investigators and field workers were blinded to the block size. Recruitment staff at each site called in the details of the risk profile for a new recruit, and the assignment was generated centrally by the data co-ordinating centre |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition: 104/500 (21%) prior to delivery and 116/500 (23%) in the postpartum assessment. Participant data were analysed according to their care group assignment, regardless of whether they received any intervention sessions, using an ITT model |
| Selective reporting (reporting bias) | Unclear risk | Data on women spontaneously quitting before pregnancy were not reported |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Smoking cessation biochemically validated using salivary cotinine (< 10 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and providers not able to be blinded by dedicated intervention providers minimised risk of contamination of study arms |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | 4 research teams were allocated to ensure blinding of outcome assessors |
| Incomplete implementation | High risk | Process evaluation showed 16% women did not attend any sessions, 43% randomised women did not complete first follow-up interview and 31% did not complete 2nd follow-up interview |
| Equal baseline characteristics in study arms | Low risk | No significant differences noted. |
| Contamination of control group | Low risk | Persons delivering intervention were separate from care provider team |
| Methods | Randomised controlled trial of self-help booklets to support women to stop smoking in pregnancy Study conducted in 5 health centres of the same HMO in Los Angeles (USA), from 1985 to 87 |
|
| Participants |
Inclusion criteria: English-speaking women attending 1 of 5 health centres for prenatal care, < 18 weeks’ gestation; still smoking >= 7 cigarettes a week Exclusion criteria: Not specified further. Recruitment: 323 who self-reported still smoking >= 7 cigs/week were randomised (C = 158, I = 165). 242 included in final analysis (C = 116, I = 126). 228 women who had spontaneously quit also included (C = 108, I = 110) Baseline characteristics (smokers): Prepregnancy smoking: 27.3% 1-10 cigs/day, 14% 11-19 cigs/day, 58.7% 20+ cigs/day. At intake: 71.9% 1-10 cigs/day, 14.9% 11-19 cigs/day, 13.2%20+ cigs/day. Spontaneous quitters: mean pre-pregnancy cigarettes/day = 10.3 Smokers: 64% white, 73% had high school or some college education, 59.9% married Progress+ coding: None. |
|
| Interventions |
Control: 2-page pamphlet on hazards of smoking and on the need to quit; 2 minutes discussion with a health educator (within a 45 minutes individual conference); advised of free 5 session smoking cessation program available through the HMO. Coverage in antenatal classes remained unchanged. Intervention: As for the control group + first of series of 8 self-help booklets aimed to increase motivation for quitting; teach behavioural strategies for cessation and relapse prevention; 3 minutes introduction to these by health educator; asked to make a commitment to read the first 1 and list reasons for not smoking; others mailed weekly. Booklets were pregnancy-specific, multi-ethnic, and at a 9th Grade reading level Main intervention strategy: Counselling (single intervention) compared to less intensive intervention Intensity: Frequency (C = 6, I=6), Duration (C = 4, I = 4). Estimate based on uptake of optional HMO sessions × 5 approximately 20-40 mins Intervention provided by existing health staff: effectiveness study |
|
| Outcomes | Biochemically validated abstinence at 34 weeks’ gestation (late pregnancy*) Ershoff 1995 reports relapse prevention* among women who had spontaneously quit Ershoff 1990 reports birth outcomes (mean birthweight*; low birthweight*; preterm birth* (< 37 weeks); stillbirths*) and cost outcomes (economic evaluation) Associated reference (Mullen 1991) describes question structure’s to improve accurate disclosure of smoking status | |
| Notes | SDs for mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | High risk | The authors state that women had been randomised in advance of their visit. It was not clear how women were recruited to the study or gave consent for participation.The health educator turned over a ‘pre-assigned card’ to randomise women |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Smokers: Attrition I = 39/165, C = 44/158 not included in analysis. Losses due to termination (C = 11, I = 7); miscarriage (C = 13, I = 12); disenrolment or transfer to another HMO (C = 18, I = 20) Spontaneous quitters: Attrition 22% - Abortion (n = 5), miscarriage (n = 17), disenrolment from HMO or transfer (n = 25) Not re-included in analysis for this review as excluded for medical reasons or moving |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation by urinary cotinine levels. For participants reporting no smoking and low exposure to passive smoke urine cotinine had to be less than or equal to 10 ng/mL. For participants reporting a relapse and high exposure to passive smoke some values could be as high as 29 ng/mL though at least 1 sample had to be 10 ng/mL or less |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | The authors state that the health educator delivering the intervention was not aware of group allocation, but materials were provided to the experimental group at the clinic visit. Prenatal care providers were blinded to group assignment and no effort was made to modify their usual counselling practices |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation reports good implementation. |
| Equal baseline characteristics in study arms | Unclear risk | With the exception of partners smoking status. |
| Contamination of control group | Unclear risk | Prenatal care providers no involved in intervention so risk of contamination likely to be low |
| Methods | 3-armed randomised controlled trial of interactive computer program and telephone counselling to support women to stop smoking in pregnancy Study conducted in a large group model managed care organisation in Los Angeles, California (USA) with recruitment from November 1996 to June 1997 |
|
| Participants |
Inclusion criteria: Smokers were identified at first visit as women who self-report “smoking now”, “smoke but have cut down since pregnancy”, or “smoke from time to time” Exclusion criteria: < 18 years of age, > 26 weeks’ gestation, do not speak English, or smoked less than 7 cigarettes pre-pregnancy Recruitment: Researchers attempted to phone 931 women. 150 could not be contacted, 90 refused to be interviewed, 158 were not eligible and 34 were excluded as they experienced miscarriage (n = 34). 390/458 women (82%) agreed to participate (C = 131, I1 = 133, I2 = 126). Baseline characteristics: Pre-pregnancy mean cigs per day: C = 17.1 (9.7), I1 = 17.6 (9.8), I2 = 16.3 (7.6). Mean cigs per day at intake: C = 6.6(7.3), I1 = 6.7(6.5), I2 = 6.3 (6.5). 60% white, approximately 50% college educated, with a mean age of 29.4. Mean cigarette/day at first visit = 6.6 Progress+ coding: None. |
|
| Interventions | 3 interventions, based on stages of change model. Control: Received a 32-page self-help booklet “living smoke-free”. Intervention 1 (interactive computer program-IVR): received the same self-help booklet and had access to a computerised interactive telephone support system, which provided customised messages from a voice model. Participants responded to questions using a touch-tone keypad. Intervention 2 (motivational interviewing): received the same self-help booklet and 4-6 × 10-15 minute telephone counselling sessions by nurse educators trained in motivational interviewing. A personalised postcard sent to reinforce verbal communication Main intervention strategy: Counselling (single intervention) compared to a less intensive intervention (self-help booklet). Arms 1 and 3 only are compared in this review Intensity: Frequency (C = 2, I = 6), Duration (C = 1, I = 3). Intervention provided by study staff: efficacy study. |
|
| Outcomes | Biochemically validated smoking cessation at 34 weeks’ gestation (late pregnancy*). Mean cigarettes per day* Baseline mental health index and Cohen’s perceived stress scale. Number of quit attempts and movement in stages of change. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “random assignment” |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition 58/390 (14.87) due to abortion (n = 31), disenrolment from health plan (n = 22) and preterm birth less than 32 weeks (n = 5). Lost to follow-up not included as continuing smokers in analysis as attrition due to medical reasons and moving not reincluded in this review, and attrition from each study group not reported separately |
| Selective reporting (reporting bias) | Unclear risk | Results were difficult to interpret. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation by urinary cotinine levels (< 80 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Authors state that care providers were blind to group allocation. Educational intervention so blinding women not feasible |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported |
| Incomplete implementation | Low risk | Good process evaluation of each of the methods. 79.2% received at least 1 call. Mean 4 calls lasting 12 mins each |
| Equal baseline characteristics in study arms | Low risk | No significant differences reported. |
| Contamination of control group | High risk | 11% control group received individual smoking cessation counselling as they were classified as high risk patients |
| Methods | Randomised controlled trial of counselling and a self-help guide to support women to stop smoking during pregnancy Study conducted in Baltimore (USA). Study dates not reported |
|
| Participants |
Inclusion criteria: Pregnant women currently smoking (even 1 puff in the past 7 days), either African-American or white Exclusion criteria: > 28 weeks’ gestation; changing to another prenatal clinic or could not complete baseline interview Recruitment: 2,319 women assessed, 32% currently smoking by above definition. 72 were excluded for gestation, ethnicity or changing providers, leaving 662 eligible of whom 510 agreed to participate (77%). 25 quit prior to first visit, 18 did not wish to quit, leaving 467 (C = 235, I = 232) randomised Baseline characteristics: Mean cigarettes/day at intake I = 9.7, C = 7.5 (P = 0.01). 85% were on medical assistance. African American: I = 81% C = 89% Progress+ coding: Low SES and ethnic minority population. |
|
| Interventions |
Control: Usual clinic and inpatient smoking cessation: A brief discussion with a nurse/health counsellor about the risks of smoking; a recommendation to quit and pamphlets from the area’s voluntary agencies. Intervention: Peer health counsellors recruited from local communities, received 2 sessions training from PIs who explained content, rationale and how it was to be provided, then observed in practice by PIs with feedback to her. (i) A Pregnant Woman’s Guide to Quit Smoking (RA Windsor), 6th Grade level. (ii) 15 minutes 1:1 counselling session with peer health counsellor on how to use the Guide, showing how it is organised to be used daily, and discussing women’s thoughts and concerns about quitting, targeting cessation or relapse prevention, as appropriate. (iii) Educational materials for cessation support persons included with the Guide. (iv) Reinforcement at each clinic visit from doctors and nurses, written prescription to stop smoking provided directly from doctor to woman; 2 letters of encouragement (from the doctor and the counsellor) mailed to the woman 1-2 weeks after her first visit Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I=2). Usual care intensity F = 1, I = 1 Intervention provided by study staff: Efficacy study. |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence in hospital after delivery (late pregnancy*), 6 (6-11*) months postpartum abstinence, and >50%reduction in cotinine* from baseline to late pregnancy interview. Smoking cessation data collected at 3 months but not reported |
|
| Notes | Guide developed through needs assessment with pregnant women, constructs from the PRECEDE/PROCEED diagnosis and social learning theory, tested with focus groups, additional section on relapse prevention, and on passive smoking postpartum. Results show high rate of misclassification by self-report (I = 37%, C = 48%) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 16.3% attrition due to miscarriage, termination and change of care provider (C = 37, I = 34). 145/391 (37%) remaining women did not provide saliva samples and were treated as smokers in the analysis but those lost to follow-up for other reasons were excluded from the analysis in reports and in this review 6* months postpartum abstinence was collected and only small sample of 6-month data reported (C = 48, I = 46), however all missing data included as continuing smokers in this review |
| Selective reporting (reporting bias) | High risk | - month postpartum outcomes not reported and minimal follow-up for 6-month postpartum data |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-report of ‘not even a puff in past 7 days’ biochemically validated by salivary cotinine < 30 ng/mL |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showing good implementation. |
| Equal baseline characteristics in study arms | Unclear risk | Women in control group reported significantly fewer cigarettes per day and more likely to be African-American |
| Contamination of control group | High risk | Same care providers delivering intervention who were providing care to control group |
| Methods | This randomised controlled trial aimed to measure the effectiveness of home-based visiting from trained lay-persons to reduce low birthweight. The study was conducted in the prenatal clinic of a university hospital in Cleveland, USA, from March 1987 to September 1989 |
|
| Participants |
Inclusion criteria: Living within 5-mile radius of clinic, 17-28 weeks’ gestation, ‘low’ family function rating, at least 1 stressful life event during pregnancy, and additional risk factors such as smoking, low maternal weight-height ratio, aged over 27 years, or history of a previous premature baby Exclusion criteria: White patients, difficulty reading English. Recruitment: Every person registering at clinic was eligible to be screened. The first 105 screened participants were dropped from the study when it was found that they had difficulty reading the questions. 1326 women screened. 1022 ‘low risk, 190 ‘high risk’ women - of which 145 were randomised (I = 87, C = 58). 8.5% of low risk and 15% high risk women were smokers Baseline characteristics: Smoking characteristics not reported. Predominantly black, poor, inner city population. No progress plus coding as outcomes not able to be included in this review |
|
| Interventions |
Control: Routine care from obstetrical staff in the clinic. Intervention: 2 non-professional black women who demonstrated rapport with women served as home-visitors and were trained in childbirth education, community resources, and nutrition during pregnancy. 4 × 1 hour home visits occurred at 4-6 week intervals. The home visitors followed a protocol which included psychosocial support, efforts at stress reduction, information on health risks (especially smoking and drinking), nutrition education, and a small gift Main intervention strategy: Not coded as outcomes not included in this review. |
|
| Outcomes | Smoking outcomes were not able to be included in this review as it is unclear how many smokers were included in each study arm. Low birthweight was the primary outcome for this study, but was not included in this review, as aspects other than the smoking component of the intervention may have had an effect on birthweight. See Table 1 for summary of outcomes not able to be included in this meta-analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 24/87 dropped out and unclear if included in analysis. 7 refused intervention, 11 could not be contacted, 5 transferred care, 1 miscarried prior to visit Numbers reported as randomised different in abstract (154) and flow chart (145) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if selective reporting as smoking cessation was not the primary aim of the intervention |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Not applicable. Smoking outcomes not reported. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Women and home visitors not blinded, as would be expected in an educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed only 63/87 women received home visits |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Home visiting intervention so risk contamination of control group is low |
| Methods | Randomised controlled trial of providing feedback on cotinine to support women to stop smoking in pregnancy and reduce low birthweight Study conducted in physicians offices and clinic sites within Maine (USA) from 1984 to 1987 |
|
| Participants |
Inclusion criteria: Pregnant women with a singleton live pregnancy; having maternal serum AFP screening at 15-20 weeks’ gestation; who smoked >= 10 cigarettes a day Exclusion criteria: Not further specified. Recruitment: Physicians approached (no consent from women). 25,628 women completed maternal serum screening form, 97% answered question on smoking and 17% smoked >= 10 cigs/day. 2848 women were randomised (C = 1425, I = 1423) Baseline characteristics: Mean cigs/day at baseline: C = 16.3, I = 16.1 Maternal education (mean years): C = 11.8, I = 11.9. Progress+ coding: None. |
|
| Interventions |
Control: Standard medical care not otherwise specified. Intervention: Report on cotinine generated for her physician with interpretation relating smoking level to birthweight. Physician explained this to the woman and also gave her a copy of the report and a pregnancy-specific booklet about how to quit, using the cotinine information also + repeat measure 1 month later, 2 copies to physician, comparison of 1st and 2nd cotinine, report commenting on the change and its interpretation Main intervention strategy: Feedback (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 2). Usual care intensity: F = 0, I = 0 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | No smoking cessation data. Smoking data limited to comparability at first assessment and mean serum cotinine levels, which could not be included as they are disaggregated by low and high study site participation Mean birthweight*; low* and very low* birthweight; preterm birth* (< 37 weeks); stillbirths (> 20 weeks)*; neonatal deaths*; postneonatal deaths |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 2700/2848 (94.8%) included in analysis. 3% lost to follow-up and 2% multiple gestations or fetal deaths. Only 695/1343 (48%) women in the intervention groups provided repeat serum cotinine for comparison. No ITT analysis. No smoking outcomes reported and unable to re-include data for mean cotinine and birth outcomes |
| Selective reporting (reporting bias) | High risk | Results difficult to interpret. Smoking cessation not recorded |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Serum cotinine measurement at baseline for both the experimental and comparison groups but it was not clear that any follow-up measurements were made for the comparison group |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Caregivers aware of group allocation. Experimental group given feedback on serum cotinine levels |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed less than good implementation with differential impact on perinatal outcome by completeness with second blood samples taken for cotinine measurement |
| Equal baseline characteristics in study arms | Unclear risk | Intervention groups similar at trial entry. |
| Contamination of control group | Low risk | Intervention not provided by care provider. |
| Methods | Cluster-randomised controlled trial of a brief midwife-delivered intervention to support women to stop smoking in pregnancy Study conducted in nine hospital and community trusts in the UK. Years of data collection not reported |
|
| Participants | 290 midwives randomised to provide intervention or control care Inclusion criteria: Pregnant women currently smoking or stopped within the last 3 months Exclusion criteria: Not further specified. Recruitment: Women were recruited at first visit (approximately 12 weeks’ gestation). Estimated 8700 eligible women. Only 178/290 (61%) midwives (C = 86, I = 92) recruited any women. Financial incentives were paid to boost recruitment. 1287 women provided informed consent Baseline characteristics: Current smokers (C = 440, I = 441); Spontaneous quitters (C = 135, I = 114). 189 current smokers were assessed as ‘not motivated to stop’ therefore received no intervention. Mean cigs/day: Smokers (C = 9.7, I = 10.1), Ex-smokers (C = 10.9, I = 12.6) > 70%married, 26%-27% smokers and 10%-15% ex-smokers had no educational qualifications Progress+ coding: None. |
|
| Interventions |
Control: Midwives received 1 hour of training to discuss the study and were asked to provide usual care and any usual pamphlets Intervention: Midwives received 2 hours training which included using the CO monitor and providing ‘stage of change’ based advice, CO assessments. Intervention group also received written advice and motivational materials for current and recent smokers, including designating a ‘quit date’, a ‘quiz’ and the offer of ‘buddying’ to another pregnant smoker for support Main intervention strategy: Counselling (tailored) compared to usual care. Intensity: Frequency (C = 0, I = 5), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by routine midwives: Effectiveness study |
|
| Outcomes | Biochemically validated point prevalence abstinence at birth (late pregnancy*), relapse prevention*, and self-reported continuous abstinence at 6 (6-11) months postpartum among baseline smokers* and spontaneous quitters. Birthweight for smokers and ex-smokers reported, but not by intervention group so not included in this review Participants and midwives views of interventions reviewed. |
|
| Notes | Clustering effect not reported, so sensitivity analysis conducted using 4 ICCs and outcome figures adjusted using conservative intracluster correlation of 0.1. See Table 2 for adjustment calculations for cluster trials. Discussion of barriers includes 65% of midwives reporting the intervention could not be undertaken in the time they had available. Sample size justification |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster-randomisation of midwives adequate. Consecutive names on a list of midwives |
| Allocation concealment (selection bias) | Unclear risk | Midwives randomised. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 167/1287 (12.9%) (C = 83, I = 84) excluded from analysis due to moving away, being untraceable or deemed unsuitable for follow-up (e.g. miscarriage). 1120 in sample. 51/1287 non-responders were included as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation by expired CO < 10 ppm. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Midwives aware of allocation group. Educational intervention. Blinding women not feasible |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Blinding of outcome assessment not reported. Not blinded if performed by midwives |
| Incomplete implementation | High risk | Process evaluation showed poor implementation in some areas. |
| Equal baseline characteristics in study arms | High risk | Control group slightly more interested in quitting smoking and less nicotine dependent |
| Contamination of control group | Low risk | Cluster trial design to minimise risk of contamination. |
| Methods | Randomised controlled trial of self-help materials and health education to support women to stop smoking in pregnancy Study conducted in a teaching hospital (academic) clinic in North Carolina, USA from August 1991 to January 1993 |
|
| Participants |
Inclusion criteria: Pregnant women who smoke. Exclusion criteria: > 36 weeks’ gestation, psychiatric diagnosis. Recruitment: 842/846 (99%) women attending the clinic completed survey and 793/846 provided a CO breath sample.; 2 were excluded as > 36 weeks’ gestation; 1 for psychiatric diagnosis; leaving 266 (32%) eligible smokers (smoked at least once in the prior week). 12 refused, 4 were missed, 2 were not pregnant and 1 was a private patient. 247 women randomised Baseline characteristics: Mean cigs/day (C = 14.4, I = 13.5), Want to quit (C = 81%, I = 84%). Smokers in household (C = 75%, I = 78%) White (C = 74%, I = 78%), Single (C = 44%, I = 47%), < 12yrs education (C = 43%, I = 48%) Progress+ coding: Low SES. |
|
| Interventions | All 1-4 year residents given didactic and role play training for smoking cessation counselling, including self-assessment of current techniques and skills, which they were asked to continue with for the control group. Control: Standard care; residents reminded not to alter amount or time of this; help was provided if woman sought it and prenatal classes included discussion of substance abuse, including cigarettes. Intervention: (i) residents provided counselling at each visit, and a brief script aimed at setting a quit date or negotiated an alternative assignment such as a smoking diary at every contact; (ii) given Windsor’s self-directed 7-day smoking cessation guide; (iii) quit date patients given written prescription to quit, letter of support from doctor, contacted by volunteer smoking cessation counsellor to review the quit plan and encourage follow-through charts flagged, prompts with flow sheet, most recent CO and self-report included for care provider; (iv) successful quitters sent an encouraging postcard each week Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Biochemically validated abstinence at last prenatal visit (late pregnancy*). > 50% reduction in self-reported smoking*; Mean cigarettes per day* Cost-effectiveness data reported. |
|
| Notes | SDs for mean cigarettes per day were not reported, therefore we calculated a mean SD from 14 studies with available mean cigarette SDs (6.5) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random numbers. |
| Allocation concealment (selection bias) | High risk | State that neither the enrolling nurse nor the patient were aware of allocation, but experimental group notes were flagged |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition 40/247 (16%) (4 miscarriages first trimester, 3 miscarriages second trimester, 3 terminations, 15 moved to alternative care, and 12 lost to follow-up) 207 included in analysis (C = 100, I = 107). Those lost to follow-up not able to be re-included in analysis in this review as numbers not reported by study arm |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Exhaled CO measured at each visit for the experimental group and at 3 visits for the comparison group. < 5 ppm counted as non-smokers |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Case notes flagged. States patient not aware of randomisation status |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | No process evaluation reported. |
| Equal baseline characteristics in study arms | Low risk | No significant differences noted. |
| Contamination of control group | High risk | Concerns about residents having to treat similar/consecutive patients differently, and self-help manuals accidentally given to some controls. Discussion section reports evidence of contamination with self-help materials being given to controls |
| Methods | Cluster-randomised controlled trial of brief GP counselling to support women to stop smoking in pregnancy and prevent relapse postpartum Study conducted in Western Norway from November 1986 to November 1987 |
|
| Participants |
Inclusion criteria: No indications of serious social or medical problems, living with a partner, and smoking at least 5 cigarettes per day before pregnancy and still smoking at least 1 cigarette per day at the first checkup Exclusion criteria: Not further specified. Recruitment: All 398 GPs in western Norway were invited by mail to participate in the study. 187 participating GPs were asked to recruit 4 pregnant and 4 non-pregnant women for the study, at the first checkup in the first trimester. 1/3 pregnant and non-pregnant women ended up in control groups. The GPs who recruited pregnant women for the intervention groups recruited non-pregnant women for the control groups. 2379 pregnant women screened, 674 fulfilled inclusion criteria, 144 refused to participate (21%). 530 pregnant women were randomised (unclear how many each group) Baseline characteristics: Mean age starting smoking 27.6, mean cigs per day = 9.5. Mean age 25.9. 18-34 years of age, all living with a partner Progress+ coding: None. |
|
| Interventions |
Control: Ordinary control programme during pregnancy and for first year after delivery (usual care) Intervention: (i) < 15 mins GP consultation at initial visit about hazards of smoking, how to stop and how to avoid relapse; (ii) information about problems related to ‘the smoking fetus’; (iii) delivered with aid of a 5-page ‘flip-over’; (iv) 8-page booklet. Women invited to consult their GPs after 1, 6, 12 and 18 months to discuss their smoking habits Main intervention strategy: Counselling (multiple intervention) compared with usual care Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 1). Usual care intensity: F = 0, D = 0 Intervention provided by existing staff (GPs): Effectiveness study |
|
| Outcomes | Self-reported abstinence 6 months after study entry (late pregnancy*), biochemically validated at 12 months after study entry (0-5 months postpartum*), self-reported abstinence 15 (6-11 months postpartum*) and 18 months after study entry (12-17 months postpartum*) Sef-reported reduction and increase in smoking. An associated reference (Haug 1992) reports results of a survey of GPs delivering the intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | GPs described as randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 180/530 dropped out due to spontaneous abortions (24), serious complications (8), moved to another district (31) or for other unknown reasons (117). Only 350/530 (C = 98, I = 252) included in analysis and we were unable to re-include those lost to follow-up for other reasons in this review as they were not reported by group allocation. Further dropouts not explained (C = 97 and I = 244 in outcome tables-re-included in this review as continuing smokers) |
| Selective reporting (reporting bias) | High risk | Not clear if biochemically validated outcomes reported. |
| Other bias | High risk | Unequal recruitment to study arms (higher recruitment in intervention arms) |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Biochemical validation of smoking only at study entry and after 12 months (urinary thiocynate). Unclear if those who had high thiocynate levels were considered smokers. No cut-off levels reported |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | 59% residents did not document consultation. 1 component dropped |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | High risk | Same providers asked to provide control and intervention arms for pregnant and non-pregnant women |
| Methods | Randomised controlled trial of motivational enhancement therapy to support women to stop smoking in pregnancy Dates of research and location not stated. Assume USA from author affiliations |
|
| Participants |
Inclusion criteria: Opioid-dependent women, <= 26 weeks’ gestation, receiving methadone, currently smoking at least 5 cigarettes per day, enrolled in hospital prenatal program. Exclusion criteria: Not further specified. Recruitment: During first 48 hours of 7-day residential program. 77 women randomised. 14 women excluded from analysis due to miscarriage, abortion, premature delivery and miscalculated gestational age. 63 included in analysis (I = 30, C = 33) Baseline characteristics: Mean cigarettes per day 19.9 (SD 11.5). Approximately 50% had lifetime major depressive disorder, 32% were depressed in last month, and 39% had anxiety disorder. 84% African American, 79% single, 97% unemployed. 94% had less than high school education. Not coded for equity analysis as outcomes not able to be included in this review |
|
| Interventions |
Control: Health practitioner advice by trained research staff and printed materials from American Lung Association and American Cancer Society Intervention: As control + Motivational Enhancement therapy using ‘Project MATCH’ manual with modifications for nicotine dependence, provided over 4 sessions by masters level research associates Main strategy and intensity not coded as outcomes unable to be included in meta-analysis |
|
| Outcomes | Mean cigarettes per day, mean exhaled CO, mean cotinine, movement in stages of change were collected and authors report that there was no significant difference. However, not actual figures were provided to be able to include these outcomes in meta-analysis in this review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just states participants were ‘randomly assigned’ to 1 of 2 conditions |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Participant attrition was 14% (n = 9). Final figures not reported so unclear how many included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Actual smoking rates not reported, despite this being a primary outcome for the study. However, authors did not claim results were significant |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Cotinine and CO validation measured, but not reported. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Intervention providers and women not blinded as counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated. |
| Incomplete implementation | Unclear risk | Process evaluation not reported. |
| Equal baseline characteristics in study arms | Unclear risk | Intervention group had lower mean education levels, were more likely to be Caucasian, and had higher rates of pre-pregnancy cigarettes per day. Other factors equal |
| Contamination of control group | Low risk | Masters level research associates provided the intervention. |
| Methods | Quasi-randomised trial of counselling and optional nicotine replacement therapy, to support women to stop smoking in pregnancy Study conducted in a large midwifery centre in the Netherlands, with data collection from 1996 to 1998 |
|
| Participants |
Inclusion criteria: All pregnant women attending first prenatal visit. Exclusion criteria: Inability to speak Danish, age below 18 years, gestation of more than 22 weeks, verified psychiatric diseases, and alcohol or drug abuse Recruitment: 696/905 (77%) eligible women attending first antenatal clinic who smoked agreed to participate in study (informed consent) and were randomised (C = 347, I = 348). 647 included in final analysis (C = 320, I = 327) Baseline characteristics: Mean cigs/day = 11, Significant difference in partner smoking (I = 67%, C = 77%, P = 0.03), mean salivary cotinine (C = 141, I = 139) Mean age 29 yrs, > 12 yrs in school (C = 45%, I = 43%), mostly married Progress+ coding: None. |
|
| Interventions |
Control: Usual care, which included routine information about the risk of smoking in pregnancy and general advice on smoking cessation or reduction in a standard 30-minute consultation Intervention: (i) Extended initial consultation (from 30 to 40 minutes) which included a dialogue about smoking and motivation for cessation (ii) written information about risks of smoking and passive smoking (iii) invitation to join smoking cessation program, based on CBT. The program involved 9 appointments (individually or in a group) over a period of 14 weeks. 3 attendances prepared participants for quitting and 6 were used to maintain cessation and to hand out NRT. CO readings at each visit (iv) NRT offered to all women (2 mg gum or 15 mg patch × 16 h) for 11 weeks (v) encouragement at subsequent 5-6 antenatal visits. Main intervention strategy: Counselling (tailored) compared with usual care. Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 6). Usual care intensity: F = 1, D = 1 Intervention provided by specially trained midwife (study staff): Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation at 37 weeks’ gestation (late pregnancy*), mean birthweight*, low birthweight*. Preterm births* reported in attrition and re-included in both numerator and denominator for this outcome Regression analysis for passive smoke exposure, years of education reported |
|
| Notes | SDs for mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi-randomised by odd or even birth date. Included in review despite inadequate sequence generation as there is a low likelihood of interference with birthdate allocation |
| Allocation concealment (selection bias) | High risk | Quasi-randomised by odd or even birth date. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: 10 had miscarriage or stillbirth (C = 5, I = 5); 21 moved out of area (C = 12, I = 9); 17 had a premature delivery (C = 10, I = 7). These were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Smoking cessation validated by salivary cotinine <= 30 ng/mL |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Providers and participants not able to be blinded to educational intervention and NRT provision not blinded (no placebo) |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated. |
| Incomplete implementation | High risk | Only 87 women (27%) accepted participation: 81 in a group and 6 women accepted an individual smoking cessation program. 71 of 87 participants (82%) participated in 3 or more of a total of 9 meetings in the smoking cessation program. 75 (86%) of 87 women participating in the smoking cessation program were using nicotine substitution in the form of a 15 mg nicotine patch (16 h/day) or 2 mg nicotine chewing gum or a 15 mg nicotine patch (16 h/day) plus 2 mg nicotine chewing gum |
| Equal baseline characteristics in study arms | Unclear risk | Mostly equal except more women were exposed to passive smoking in the home in the intervention group (77%) than in the control group (67%) (P = 0.03) |
| Contamination of control group | Unclear risk | The strengths of the study include absence of treatment diffusion as all participants in the intervention group were seen by specially trained midwives as opposed to participants in the control group who were all consulting midwives without such training. The study enjoys a second advantage which is that intervention and control group participants were seen at different week days and hence could not easily share information. The secretaries summoning the pregnant women were continuously reminded about this allocation criterion to avoid treatment diffusion between the intervention and the control group. p814 |
| Methods | Randomised controlled trial of financial incentives to support women to stop smoking in pregnancy and prevent relapse postpartum Study conducted in Greater Burlington, Vermont (USA) with data collection from 2001 to 2003 |
|
| Participants |
Inclusion criteria: Self-reported smoking (even a puff in the last 7 days), gestational age less than 20 weeks, living within study clinic county and not planning to move until at least 6 months postpartum, and speaks English Exclusion criteria: Incarceration or previous participation in the study or living with anyone who has previously participated in the study Recruitment: Participants were recruited from 1 of 4 large obstetric practices in the Women, Infants and Children (WIC) program. 182 women were eligible for the study, and 82 (45%) agreed to participate. Mean gestation at recruitment (I = 8.9, C = 9.5). 77 included in analysis (C = 40, I = 37) Baseline characteristics: Pre-pregnancy cigarettes per day (I = 18.7, C = 18.4), Health insurance (I = 19%, C = 13%). Progress+ coding: Low SES as WIC program recipients. |
|
| Interventions |
Control (non-contingent voucher): Participants received voucher independent of smoking status. US$ 15.00 per antenatal visit and US$ 20.00 per postpartum visit, to result in comparable average earnings to the contingent group. Both groups received routine advice from the clinic Intervention (contingent voucher): participants chose a quit date, and reported daily to the clinic for CO monitoring for 5 days, then urine cotinine monitoring twice weekly for 7 weeks, weekly for 4 weeks, and then every 2 weeks for the remainder of the pregnancy. Vouchers were given dependent on biochemical validation, beginning at US$ 6.25 and escalated by US$ 1.25 to a maximum of US$ 45.00. Positive test results reset voucher back to original value, but 2 consecutive negative tests restored value to pre-reset value. It is unclear who delivered the intervention Main intervention strategy: Incentives (single intervention) compared to alternative intervention Intensity: Frequency (C = 6, I = 6), Duration (C = 6, I = 6). Intervention provided by study staff: efficacy study. |
|
| Outcomes | Biochemically validated smoking cessation at >= 28 weeks’ gestation (late pregnancy*), 12 weeks (0-5 months*) and 24 weeks’ (6-11 months*) postpartum. Reduction in mean cotinine Mean birthweight*, gestational age, fetal growth measures (US), and proportion of NICU admissions*, low birthweight* infants, and preterm births* Nicotine withdrawal symptoms reported in associated reference (Heil 2004). |
|
| Notes | Sample size justification. Some discussion of cost implications | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomisation stratified to clinics”. Details of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 5 women withdrew from the study due to fetal demise or termination of pregnancy and were not included in the final analysis (I = 3, C = 2) |
| Selective reporting (reporting bias) | Low risk | Detailed birth outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation using exhaled CO for 5 days (< 6 ppm) and then urine cotinine (< 80 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and providers not blinded as receiving incentives for participation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Compliance with periodic assessments was relatively high (83%-95%) |
| Equal baseline characteristics in study arms | Low risk | No significant differences in socio-demographics or smoking characteristics were noted |
| Contamination of control group | Low risk | Very unlikely - as clear voucher schemes for abstinence and non-abstinence |
| Methods | Randomised controlled trial of mobilising peer social networks to support pregnant women to stop smoking The study was conducted in urban Women, Infants and Children (WIC) clinics in Minnesota and an urban university outpatient obstetric clinic in Ohio, USA from 2005 to 2007 |
|
| Participants |
Inclusion criteria: Pregnant women in the first or second trimester, a current smoker, and at least 18 years old Exclusion criteria: Not further specified. Recruitment: Each eligible and consenting participant identified a woman in her social network to act as a supporter. 872 women screened in waiting areas. 82/156 (53%) eligible women and their supporters agreed to participate (C = 28, I = 54) Baseline characteristics: Median number of cigarettes smoked per day = 5 (range = 1-25) and 52% smoked their first cigarette within 30 min of waking. 52% of supporters were current smokers and 22% were former smokers. There were no significant differences between study arms 67% from racial minority groups, 65% had high school education or less. Median age = 24 Progress+ coding: Low SES as all WIC program recipients. |
|
| Interventions |
Control: 1 in-person counselling session for control and intervention participants designed to increase motivation to quit and provide information about community smoking cessation resources Intervention: Peer-supporters in the intervention group had 1 in-person visit and monthly telephone sessions. The primary goal was to develop strategies to help the participant quit smoking by identifying specific activities to support efforts to quit. Women and their supporters were given a pregnancy scrapbook that included pages related to smoking cessation tasks Main intervention strategy: Social support (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 4), Duration (C = 2, I = 5- estimated) Intervention provided by specific staff: Efficacy study. |
|
| Outcomes | Biochemically validated smoking status just prior to expected delivery date (late pregnancy*) and 3 (0-5*) months postpartum Women’s perceptions of peer support behaviours reported (both positive and negative) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked random allocation sequence |
| Allocation concealment (selection bias) | High risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: C = 25%, I = 11% by end of pregnancy. C = 19%, I = 32% by 3 months postpartum. Report ITT analysis for end of pregnancy validated quits. 7 women who had miscarriages were excluded from the analysis. All randomised participants included in the analysis in this review (dropouts included as continuing smokers) |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported smoking status biochemically validated using urinary cotinine (< 100 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and providers to this social support intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Outcome assessors blinded as ‘evaluation staff were blinded to group assignment’ |
| Incomplete implementation | High risk | Process evaluation showed over 90% supporters received at least 1 counselling session, but contacts with supporters occurred less frequently than the planned monthly intervals because of difficulty reaching supporters |
| Equal baseline characteristics in study arms | Unclear risk | Significantly more intervention participants had other children (78% vs. 57%, P = 0.052) and significantly fewer were white (22% vs. 54%, P = 0.016), but other characteristics equal |
| Contamination of control group | Low risk | Contamination unlikely with this intervention which required researchers to contact intervention group at home |
| Methods | Randomised controlled study of health education and feedback to support women to stop smoking Location and study dates unclear. Assume USA due to author affiliations | |
| Participants |
Inclusion criteria: Women enrolling for prenatal care. Exclusion criteria: Not further specified. Recruitment: 49 women randomised (I = 26, C = 23). Baseline characteristics: Not reported (abstract only). |
|
| Interventions |
Control: Usual prenatal care. Intervention: Education and at least 8 encounters with a program counsellor. Peak flow values and CO levels were obtained at each prenatal visit and shared with intervention group participants only Main intervention strategy and intensity not coded as outcomes not reported |
|
| Outcomes | Smoking cessation (biochemically validated) was collected but actual figures not reported so unable to include results in this meta-analysis. Peak flow values reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States ‘women were randomised into two groups’. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Data not reported. |
| Selective reporting (reporting bias) | High risk | Actual figures not reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of smoking status using urine cotinine and CO (cut-off levels not reported) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel unlikely to be blinded to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Low risk | Groups similar with maternal age, fagerstrom scores, initial peak flow values and initial urine cotinine levels |
| Contamination of control group | Unclear risk | Not stated who delivered intervention. |
| Methods | Quasi-randomised trial of a self-help manual to support women to stop smoking in pregnancy Study conducted in public health maternity clinics in Gothenburg, Sweden, with data collection from 1987 to 1988 |
|
| Participants |
Inclusion criteria: Pregnant women registered as daily smokers (at least 1 cigarette per day), gestational age less than 12 weeks, and speak Swedish Exclusion criteria: Not further specified. Recruitment: 13/14 public health clinics participated. Women born days 1-10 of each month were allocated to the control group and women born on days 11-31 were allocated to the intervention group. Unequal group sizes were allocated as it was expected more intervention women would refuse to participate. 723 eligible continuing smokers were randomised (C = 231, I = 492). 417/492 (85%) of the intervention group agreed to participate, and the control group were not asked for consent Baseline characteristics: Mean cigs/day 16.8. Mean age 28.4 years. Progress+ coding: None. |
|
| Interventions |
Control: Given an information sheet by their doctor with basic facts about smoking and pregnancy, as included in the last pages of the self-help manual Intervention: Given a self-help manual on stopping smoking, based on Windsor 1985. The manual was revised and pilot tested. The manual contained 2 phases, a preparatory (one week) and cessation phase. The smoker was given new assignments every day to the quit day and the tasks were based on the principle of behaviour therapy. The cessation period was followed for the first 5 days with new information daily Main intervention strategy: Health education (single intervention) compared to less intensive intervention Intensity: Frequency (C = 1, I=1), Duration (C = 1, I = 1). Intervention provided by existing staff (obstetrician provided self-help manual): Effectiveness study |
|
| Outcomes | Biochemically validated smoking cessation at 30-34 weeks’ gestation (late pregnancy*), 8 weeks postpartum (0-5 months), mean birthweight*, preterm births* (< 36 wks), low birthweight babies*, mean cigarettes per day at 30-34 weeks’ gestation among baseline smokers*. Mean cigarettes per day at baseline, week 12-14, week 30-34 among all randomised women, 8 weeks after delivery among baseline smokers and all randomised women | |
| Notes | SDs for mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by birth date is not random sequence. However, this study was included as interference is unlikely with birth dates |
| Allocation concealment (selection bias) | High risk | Allocation would not be concealed as allocated by birth dates (days 1-10 = control, days 11-31 = intervention) |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Loss to follow-up from miscarriage and moving out of district (C = 10% or 23, I = 11% or 46), not included in analysis. However, all other dropouts included as continuing smokers |
| Selective reporting (reporting bias) | Low risk | All primary outcomes appear to be reported. |
| Other bias | High risk | Unclear why there are 444 in intervention group and 209 in control group, when report states 10% of 231 were excluded and 11% of 492 were excluded |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of smoking status using serum thiocynate (100 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Manual given to all women who agreed to participate (85% of total assigned to intervention - i.e. 15% refused to participate) |
| Equal baseline characteristics in study arms | Unclear risk | Only age and mean no of cigarettes reported. |
| Contamination of control group | Low risk | Unlikely control group would accidentally be given the self-help manual |
| Methods | Randomised controlled trial of stage of change orientated motivational interviewing to support women to stop smoking in pregnancy The study was conducted in infertility and prenatal clinics in 3 hospitals in Ontario (Canada), with data collection from January 1996 to July 1999 |
|
| Participants |
Inclusion criteria: Newly referred infertile and pregnant patients who reported smoking more than 3 cigarettes in past 6 months Exclusion criteria: Women attending genetic counselling or with habitual abortion or who had previously been evaluated in consultation Recruitment: All women attending infertility and prenatal clinics who reported smoking were invited. Unclear how many were eligible. 110 pregnant women randomised (I = 56, C = 54) Baseline characteristics: Mean cigs/day = 12.19 (SD 6.81); (I = 13.43 +−7.07, C = 12 +− 6.69 |
|
| Interventions |
Control: Standard information that was already provided in the clinics about the impact of smoking on pregnancy Intervention: Scripted stage-based information and encouragement to quit at each prenatal visit by physicians, Stage-specific information booklet, optional referral for more in-depth counselling in a smoking cessation clinic Main intervention strategy: Counselling (tailored intervention) compared with usual care Intensity not coded as outcomes unable to be included in meta-analysis |
|
| Outcomes | Stage of change, biochemically validated cessation at 12 months post follow-up but data for intervention and control groups were combined so outcomes were unable to be included in this review. See Table 1 for description of outcomes. Relative value of intervention components reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer-generated, blocked schedule, administered through numbered, opaque, sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No attrition reported and not stated how, if any, dropouts were assessed |
| Selective reporting (reporting bias) | High risk | Smoking cessation outcomes not reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Biochemical validation with exhaled CO, but levels used to determine smoking status were not reported |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Providers and women not able to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated whether outcome assessors blinded. |
| Incomplete implementation | Unclear risk | Process evaluation not reported. |
| Equal baseline characteristics in study arms | Low risk | No significant differences noted. |
| Contamination of control group | High risk | Same care providers offering intervention and control interventions, therefore high risk of contamination |
| Methods | Cluster-randomised controlled trial to support women to stop smoking and prevent relapse during pregnancy and postpartum Study conducted in public prenatal and WIC clinics in Maryland, Colorado and Missouri (USA), with data collection from 1987 to 1991 |
|
| Participants |
Inclusion criteria: Smoking defined as “even a puff within the last 7 days before the women knew she was pregnant”, who were aggregated into ‘enrolment smokers’ (smoked within 7 days before study enrolment) and ‘recent quitters (smoked before they thought they were pregnant) Exclusion criteria: Not further specified. Recruitment: 1741/5262, 1936/6087 and 1895/4943 pregnant women screened in Colorado, Missouri and Maryland respectively, with nearly 50% of women in each state smoking. Participation rates ranged from 66% in Maryland to 79% in Missouri Baseline characteristics: Mean cigarettes/day at enrolment combined for smokers = 12 cigarettes/day High proportions were young, < 12 years education, white, unmarried and poor. Mean gestation at enrolment = 15.2 - 16.6 weeks Progress+ coding: Low SES. |
|
| Interventions |
Control: Usual care not otherwise specified by usual clinic staff. Intervention: Based on stages of change, but differed by State, locally adapted with some detailed development. Colorado: 1-5 minutes counselling; assessing smoking status; quitting tips; supportive statements by nurse-clinicians; healthcare providers’ Guide; 8 brochures for pregnant smokers; additional 1 for women postpartum. Maryland: brief clinic-based counselling program + self-help material focusing on the stages of quitting. Missouri: “becoming a life-long smoker” six minutes with clinic patient brochures, flip charts; 1-2 minutes at WIC clinics training staff, chart documentation and forms. All included effects of smoking on the fetus; benefits of quitting; quitting techniques; developing social support; preventing relapse and limiting exposure to environmental tobacco smoke. All materials were at 6th Grade reading level Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 2), Duration (C = 0, I = 1). Usual care intensity: F = 0, D = 0 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Biochemically validated point prevalence abstinence at 8 months gestation (late pregnancy*). Smoking outcomes for ‘recent quitters’ (relapse prevention) were not reported. Birthweight and proportion of low birthweight babies are not reported by intervention group so were unable to be included in meta-analysis | |
| Notes | Intracluster correlation of 0.003 reported and used for adjusting outcome figures in analysis. Substantial misclassification of self-report as non-smoking: 28% at enrolment; 35% at 8th month; 49% of self-reported quitters at intervention clinics; 32% of self-reported quitters at control clinics. Process evaluation suggested less difference between I and C clinics than might have been expected. Project staff felt that the use of existing staff to deliver the new interventions and to collect data affected the study negatively especially given the time needed to process questionnaires and urine samples. This led to less than full implementation and variable motivation to promote smoking cessation counselling among staff |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clinics stratified by size of clinic and also by prior low birthweight programme (Colorado) or % minority clients (Maryland), and randomly assigned to deliver either intervention or continue with standard care. No details of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | Cluster-randomised trial. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | In the 3 states combined, the reasons for loss to follow-up at the eighth month were early termination of pregnancy (7. 6%); enrolment after 32 weeks (6.1%); lost, moved, or unable to locate (27.7%) ; referred to another care provider (2.8%); and refused data collection (1.0%). The total number of enrolment smokers were not reported by intervention groups, and attrition rates were not reported by intervention groups, so we were unable to re-include data for respondents lost to follow-up. Report states loss to follow-up was balanced in experimental and control groups. Varying enrolment and attrition rates in different centres. No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | High rates of non-disclosure for smoking outcomes. |
| Other bias | Unclear risk | Uneven recruitment to study arms in Maryland, which affected the overall allocation (C = 1767, I = 1467) |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation by urinary cotinine (< 85 ng/mL indicates active smoker) |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Unclear whether participants and providers were aware of clinic allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation reported that implementation was less than ideal |
| Equal baseline characteristics in study arms | Low risk | Intervention and control sites were similar at enrolment, indicating that stratification and randomisation had been effective (data not shown) |
| Contamination of control group | Unclear risk | Many patients at control clinics also reported having received (non-SCIP) materials and counselling which indicated that usual care included exposure to smoking cessation messages |
| Methods | 3-armed cluster-randomised trial of self-help manuals and computer-generated advice to support women to stop smoking in pregnancy Study conducted in community midwife clinics in the West Midlands region of the UK, with data collection from July 1998 to March 2001 |
|
| Participants |
Inclusion criteria: Head midwife in every trust in region invited to participate and 16/19 agreed to participate. 204 potential midwifery practices identified, and 103 excluded by head midwife as those trusts were already involved in other regions or the practice crossed trust boundaries. Women were eligible if aged 16 years or over and a ‘current smoker’ at booking Exclusion criteria: Women not fluent in English. Recruitment: 72/101 practices were randomly sampled (C = 24, I1 = 24, I2 = 23). Further practices were later added to each arm due to slow recruitment, particularly in the control arm (C = 17, I1 = 12, I2 = 0), leaving active practices (C = 32, I1 = 30, I2 = 22). Participating midwives were asked to recruit all eligible women seen in routine antenatal appointments. Initial target of 1440 participants was reduced to 900 due to slow recruitment. Eligible smokers approached: C = 328/965 (34%),I1 (manuals) = 327/694 (47%), I2 (computer) = 397/529 (75%). Participation rate: C = 289/328 (88%), I1 = 305/327 (93%),I2 = 324/397 (82%). Baseline characteristics:Mean cigarettes per day at baseline were similar between groups (reported in 6 smoking categories). Majority (over 60%) smoked 5-20 cigarettes per day and over 50% had a partner who smoked. Median fagerstrom score 3 in all arms 63.6% of participants on < $300/week. Progress+ coding: Low SES. |
|
| Interventions |
Control: Standard care. Midwives received a half-day training on research protocol, and asked all midwives to give women the Health Education Authority booklet “Thinking about stopping” Intervention 1 (self-help booklets): Midwives received 2 and a half days training on theory of transtheoretical model. Participants received a set of 6 stage-based self-help manuals “Pro-Change programme for a healthy pregnancy”. The midwife assessed each participant’s stage of change and pointed the woman to the appropriate manual. No more than 15 minutes was spent on the intervention Intervention 2 (self-help booklets+computerised advice):Midwives received the same training as for I1, and participants received the same self-help manual and intervention as I1. Additionally, the participants used a computer programme, which consisted of questions and auto feedback of what stage they were in and what this meant, and a range of other concepts. It took about 20 minutes for the woman to complete. Printed information of the feedback was sent to the participant within a week of the intervention Main intervention strategy: Counselling (multiple intervention) compared with usual care. Intervention 2 were combined and compared with the control arm in this review Intensity: Frequency (C = 0, I = 3); Duration (C = 0, I = 3). Usual care intensity: F = 1, D = 1 Intervention provided by existing staff (Midwives providing self-help manuals): effectiveness study |
|
| Outcomes | Biochemically validated point prevalence abstinence at 28-30 weeks’ gestation (late pregnancy)* (T3) and 10 days post-birth* (T4) (0-5 months postpartum). Effect of midwife training (attitudes, expectations, confidence, concerns and routine practice) was assessed by pre-post training questionnaires Subsequent papers (Lawrence 2005b) measure and describe self-reported smoking cessation at 18 months postpartum, movement in stage of change, partner quitting, social support mobilisation, and the stress of receiving the intervention |
|
| Notes | Intracluster correlation of 0.003 reported and used for adjusting outcome data included in this meta-analysis (see Table 2). Sample size calculation given, but unable to recruit sufficient numbers | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised minimisation programme was used to stratify 72 eligible practices into 3 equal groups from101 available practices |
| Allocation concealment (selection bias) | High risk | Further practices were added to the sample because of slow recruitment - these were not randomly allocated |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Different rates of recruitment and followup in different arms of the trial. 272 (C= 1 04, I1 = 86, I2 = 82) women (22.5%) withdrew from the study or were lost to follow-up. Data on smoking status were only available for 67% of women. Where there was no urine sample available women were treated as continuing smokers. All randomised participants were included in the denominator in this analysis, with only those reported as confirmed non-smokers at T4 included as quitters |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | High risk | Slow recruitment to standard care arm, so additional practices needed to be added |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine analysis (< 1.5 ug/L). |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Neither providers nor women blinded to this educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported |
| Incomplete implementation | Low risk | 77% T4 questionnaires complete in I2. |
| Equal baseline characteristics in study arms | Low risk | There was little difference at recruitment between the midwives or recruited women in the 3 trial arms |
| Contamination of control group | Low risk | Cluster design to reduce risk of contamination. |
| Methods | A randomised controlled trial (RADIUS) of routine ultrasound screening to improve perinatal outcomes, including smoking in pregnancy The study was conducted in Missouri, USA, with data collection from November 1987 to May 1991 |
|
| Participants |
Inclusion criteria: Last menstrual period known within 1 week, gestational age < 18 weeks, no plans to change providers. All women enrolled in the RADIUS study who reported any smoking in the year before enrolment in the study were evaluated in the subgroup analysis Exclusion criteria:Medical or obstetric complications, planning an ultrasound for other reasons, twin pregnancy, not intending to continue pregnancy Recruitment: 53,367 pregnant women were screened for entry into RADIUS study; 32, 317 ineligible or excluded; leaving 21,050. 3163 refused (85% participation), 2357 had miscarriage or change of provider; leaving 15,530 randomised (C = 7718, I = 7812), 23. 8% (3,571) of whom were smokers in year before enrolment, and 1901 who were still smoking at enrolment. 3,571 smokers included in this analysis (C = 1803, I = 1768) Baseline characteristics: 95% aged 20-35, 95% white, Education: high school or less (C = 30%, I = 29%), some college (C = 29%, I = 30%), college graduation (C = 42%, I = 41%) Progress+ coding: None. |
|
| Interventions |
Control: Ultrasounds only if ordered by their physician for medical reasons Intervention: Ultrasound at 18-20 and 31-33 weeks, no details about feedback to the mother or others. No specific smoking intervention provided Main intervention strategy: Feedback (single intervention) as part of a broader intervention to improve maternal health compared to usual care Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 2). Usual care intensity: F = 0, D = 0 Intervention provided by study staff: efficacy study. |
|
| Outcomes | Mean number of cigarettes per day*. Self-reported smoking cessation recorded on birth certificate, but unable to determine how many smokers in each group so smoking outcomes not included in this review Mean birthweight, preterm births (< 36 weeks), very preterm birth (< 33 weeks), and adverse perinatal outcomes, but were not included in this review as other aspects of the intervention may have impacted on perinatal outcomes |
|
| Notes | SDs for mean cigarettes per day were not reported, therefore we calculated a mean SD from 14 studies with available mean cigarette SDs (6.5) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified computer randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Small loss to follow-up (approximately 2%). Miscarriage: C = 63, I = 64, records lost or moved: C = 121, I = 131, leaving C = 7534, I = 7617; Available case analysis but smoking cessation was not a primary outcome |
| Selective reporting (reporting bias) | Low risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation. |
| Blinding of participants and personnel (performance bias) All outcomes |
Low risk | Smoking status not revealed to sonographer. Intervention not explicitly about smoking cessation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | The mean number of sonograms obtained was 2.2 per woman in the ultrasound-screening group |
| Equal baseline characteristics in study arms | Low risk | Baseline characteristics appear equal. |
| Contamination of control group | Low risk | The mean number of sonograms obtained was 0.6 per woman in the control group and 55 percent had no sonograms. Only 2% of control group had 2 ultrasounds |
| Methods | A randomised controlled trial of counselling intervention to support women to stop smoking in pregnancy The study was conducted in an antenatal clinic in Newcastle Hospital (UK), from March to May 1982 |
|
| Participants |
Inclusion criteria: All pregnant women currently smoking >= 1 cigarette a day at the time of the first antenatal clinic under care of 4 consultant obstetricians Exclusion criteria: Women 28 weeks’ gestation or more. Recruitment: 156 smokers identified in clinics and 5 were excluded as over 28 weeks’ gestation. 151 randomised (C = 74, I = 77) Baseline characteristics: Mean cigarettes per day before pregnancy: C = 18.3, I = 18. 1. Mean cigs per day at booking: C = 14.4, I = 15.1. Mean age: C = 25 years, I = 22.7 years. Partner unemployment: C = 53%, I = 57% Progress + coding: Low SES as study in ‘deprived area’ and high partner unemployment |
|
| Interventions |
Control: Usual antenatal care with possible exposure to a concurrent television series (6 × 10-minute programme on stopping smoking in pregnancy). Intervention: (i) 10 minutes anti-smoking advice from SHO (Resident) based on Health Education Council Booklet “So you want to stop smoking for you and your baby”, an additional leaflet from the same source, and copies of the booklet for other family members; (ii) woman’s GP sent a letter describing the purpose of the study and a booklet, asked to reinforce the information at usual contacts; (iii) 2 weeks later a letter of reinforcement was sent to the woman; (iv) four weeks later there was a pre-planned home visit to provide anti-smoking advice with a letter of the same advice sent if the woman was not at home; (v) possible exposure to the concurrent TV series. Main intervention strategy: Health education (multiple intervention) compared to usual care Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 2) Estimate. Usual care intensity: F = 1. D = 1 Intervention provided by existing staff (resident): Effectiveness study |
|
| Outcomes | Self-reported smoking cessation 9-16 weeks after booking visit (late pregnancy*). Mean cigarettes per day* (the SD used in the analysis in this review was calculated from a P value of 0.05 given in the paper) | |
| Notes | Short interval between intervention and assessment. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as balanced “simple random allocation” in blocks. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Small loss to follow-up, some missing data but balanced across groups. Attrition 6/151 (4%, C = 3, I = 3): not pregnant (C = 1), 1 guilt over previous stillbirth (I = 1), and miscarriages or medical complications (C = 2, I = 2). 145 included in analysis (C = 73, I = 72) |
| Selective reporting (reporting bias) | Low risk | None apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of self-reported smoking cessation |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Neither women nor providers blinded to this educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | A home visit at 4 weeks was made to the remaining 76 test patients. 31 (41%) were found at home; 29 were given further antismoking advice; 45 (59%) were out and a letter of encouragement was left |
| Equal baseline characteristics in study arms | Unclear risk | Mean age of test mothers 22.7, controls 25. Report notes other variables were equal, but figures are not reported |
| Contamination of control group | Low risk | Main component home visit. |
| Methods | Cluster-randomised controlled trial of ‘Time for a Change’ behavioural intervention to support low income African American and Hispanic women to stop smoking and prevent relapse in pregnancy and prevent relapse postpartum Study conducted in 4Women, Infant, and Children (WIC) clinics in south and central Los Angeles (USA) from October 1990 to December 1992 |
|
| Participants |
Inclusion criteria: 4 clinic sites identified from similar neighbourhoods and pairmatched based on ethnic mix. Pregnant women at least 18 years of age who had smoked in the previous year Exclusion criteria: Not further specified. Recruitment: Clinics randomly assigned. All pregnant women were asked about smoking and participants in intervention sites were asked for informed consent. 8019 women screened (419 current smokers and 692 ex-smokers). 768/1102 (69%) current (410) or ex-smokers (692) entered the study. 18% refused (198), 12% (132) ineligible due to young age, early delivery or referral to a different clinic Baseline characteristics: Smoking: Current 40.5% (I = 51%, C = 36.5%); ex-smoker 59.5% (I = 49%, C = 63.5%) Mean age 26.8 (I = 27.3, C = 26.6). African American 53%, Hispanic 42.6% Progress+ coding: Low SES in this review as WIC clinic recipients, and ethnic minority population |
|
| Interventions |
Control: Usual care, including printed information about the risks of smoking during pregnancy and a group quit-smoking message as part of the initial WIC visit Intervention: (i) Assessment of smoking motivation and intention to quit. (ii) Bilingual health educators (Spanish and English) with bachelors degrees provided 15 minutes individual counselling that included risk information and quit messages or reinforcement. (iii) Self help guide ‘Time for a change’ with an explanation of how to use it and behavioural counselling.(iv) Explanation of how to win prizes by completing activity sheets (v) booster postcard 1 month after study entry Main intervention strategy: Counselling (multiple intervention) compared with usual care Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated study staff: efficacy study |
|
| Outcomes | Self-reported smoking cessation and relapse prevention at 9 months gestation (late pregnancy*), and 6 weeks postpartum (0-5 months postpartum*) Differential quite rates reported by African-American and Hispanic ethnic status Participants views of intervention. |
|
| Notes | Adjustment for clustering not reported. Adjustment in this review as per Table 2. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 4 participating clinics were identified from similar neighbourhoods and pair-matched based on ethnic mix. 2 clinics were ‘randomly assigned’ as control sites, and 2 clinics were assigned as intervention sites |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 28% attrition (213/768), C = 28%, I = 25%(not stated how many from each arm, so not able to be re-included in this review). Drop-outs due to inability to contact, miscarriage or discontinuance with the WIC program. 555 included in analysis (C = 400, I = 155) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported. |
| Other bias | High risk | Unequal recruitment to each study arm. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported abstinence only. Only able to obtain biochemical validation with salivary cotinine (cut-off 20 ng/mL) on 111/254 women who reported they were not smoking. High misclassification. Self-reported rates used in this review |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Providers and women not able to be blinded due to educational nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Only 12/155 women returned and completed 12 worksheets. |
| Equal baseline characteristics in study arms | High risk | Intervention group had a significantly higher proportion of smokers at baseline (51% vs 36%) and a significantly lower proportion of participants in the third trimester for the initial WIC visit (27% vs 36%) |
| Contamination of control group | Low risk | Cluster trial at service level with minimal contact with control organisations |
| Methods | Randomised controlled trial of interventions (individual and group), based on the ‘MRFIT’ trial, to support women to stop smoking during pregnancy Study conducted in 1 of 2 hospitals in the Kaiser Permanente HMO of Oregon (USA), with women recruited between July 1979 and September 1980 |
|
| Participants |
Inclusion criteria: Pregnant women who answered ‘yes’ to a questionnaire about whether they now smoked Exclusion criteria: Not further specified. Recruitment: 3856 pregnant women screened in first antenatal visit: 963 self-reported current smokers (25%) were randomised (C = 486, I = 477). All women in intervention group were invited to participate in study but high refusal rates (37%). After some changes to recruitment strategy refusal rate dropped to 30.6% Baseline characteristics: Partner smoking: 74.1%. Mean age 23.3 years. 66.2% married. 21% smokers in receipt of public assistance but only 7% of non-smokers Progress+ coding: None. |
|
| Interventions |
Control: Usual care: normal medical care for the duration of their pregnancy Intervention: (i) letter of invitation, reminder letter; (ii) group information meeting on programme for respondents with short information session by physician; (iii) individual session with trained smoking counsellor; (iv) 6 × 1.5 hour group sessions, once a week; (v) subsequent optional support groups, individual sessions and phone calls Main intervention strategy: Counselling (tailored intervention) compared with usual care Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 6). Usual care intensity: F = 0, D = 0 Intervention provided by dedicated project staff: efficacy study |
|
| Outcomes | Self-reported smoking cessation in late pregnancy*. Biochemically validated with cord blood thiocyanate in a subsample (C = 24, I = 29) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition rates high at all stages of this study. Approximately 45% lost to follow-up. I = 271/477 (56.8%) completed last questionnaire, with ‘similar numbers in control group’ (C = 276/486). However. all dropouts included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | Birth outcomes reported by smoking status, not intervention group |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Biochemical validation with urine thiocynate at delivery on a small subsample (C = 24, I = 29) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and providers not blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Very poor response to group sessions so intervention changed over the course of the trial to individual counselling, which also had very low participation overall: 18% active; 25.2% dropped out; 38% did not participate; 18% could not be contacted |
| Equal baseline characteristics in study arms | Unclear risk | Differences between intervention and control group not reported |
| Contamination of control group | Low risk | Usual care providers not delivering intervention. |
| Methods | A randomised controlled trial of brief counselling to support women who had recently quit smoking to prevent relapse during pregnancy and postpartum The study was conducted alongside a concurrent trial (Windsor 1993) to support women to stop smoking during pregnancy, relapse prevention among women who had stopped smoking since the beginning of pregnancy, in 4 public maternity clinics in Birmingham, Alabama (USA) from 1987 to 1989 |
|
| Participants |
Inclusion criteria: Pregnant women reporting as having quit within 3 months of first prenatal visit Exclusion criteria: Not further specified. Recruitment: 106/115 women who were invited agreed to participate (92%) and were randomised (C = 54, I = 52) Baseline characteristics: All recent quitters within 3 months of first visit. No other baseline characteristics reported, though report states there was no significant differences in age, race, gestation, or smoking history between intervention and control, or those lost to follow-up Progress+ coding: None. |
|
| Interventions |
Control: Usual prenatal care, including nurses’ advice to all women not to smoke. Intervention: i) 10-minute counselling by health educator using smoking relapse prevention materials on effects of smoking; benefits of maintaining cessation; possible problems; smoking triggers; solutions to smoking cues; strategies for staying quit, contract, and flip chart (5th grade reading material) ii) “stay quit buddy” encouragement, non-smoking gifts and pamphlets, iii) clinic reinforcement by prenatal staff through reminder form in the notes and to confirm abstinence, praise, encourage continuing cessation Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 5), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated relapse in late pregnancy*. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 3 had a miscarriage, 4 moved and 2 had babies for adoption, leaving C = 2/54, I = 7/52 included in analysis. Smoking status reported on 80% (C = 38, I = 40), but ITT analysis for main outcome, so those subsequently lost to follow-up treated as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Unclear what data were collected. Only smoking outcomes reported |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of non-smoking or reporting smoking less than or equal to 7 cigarettes since quitting with salivary thiocyanate analysis (cut-off levels not stated) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Notes flagged. Providers and women not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showed good implementation. |
| Equal baseline characteristics in study arms | Low risk | Figures not reported but author states there was no difference |
| Contamination of control group | High risk | Issues of possible ‘contamination’ in clinics with individual randomisation discussed |
| Methods | Cluster-randomised trial to evaluate dissemination of a behavioUrally-based program to support women to stop smoking in pregnancy Study conducted in Queensland (Australia). Data collection dates not stated |
|
| Participants |
Inclusion criteria: Public hospitals which provided antenatal and delivery care for 10 or more patients a year, had less than 50% Aboriginal and Torres Strait Islander population, and did not currently provide any antenatal smoking cessation care Exclusion criteria: Not further specified. Recruitment: Hospitals were matched on number of births, location of population centre (rural/metropolitan), and whether they had a specific antenatal clinic 80 (92% public hospitals) hospitals eligible. 10 omitted as they stopped providing antenatal care. 70 hospitals (35 pairs) included Baseline characteristics: Characteristics of individuals not reported. No outcomes included in study so not coded. |
|
| Interventions |
Control: Received ‘awareness’ phase of intervention based in Rogers’ Diffusion of Innovation theory. Flyers were distributed to all hospitals Intervention: Control +‘Persuasion’ phase, which included an educational workshop and presentation. ‘Implementation phase’ where each hospital conducted the recommended program Main intervention strategy: Intensive dissemination vs less intensive intervention. No outcomes to include in analysis Intensity: NA |
|
| Outcomes | Self-reported implementation of program at each hospital. Success was defined as the routine offer of an evidence-based smoking cessation program to at least 80% of the pregnant clients who smoke | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report states hospitals were randomised into intervention and control groups, within matched pairs |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Complete follow-up could not be obtained primarily due to the inability to contact either the medical superintendent or the director of nursing after a minimum of 3 attempts High attrition (37% hospitals), though those not responding were included in analysis as ‘not implemented’ |
| Selective reporting (reporting bias) | Unclear risk | Smoking cessation rates not reported, but not included as an aim of this dissemination study |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Smoking status not assessed in this dissemination study. |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Unclear whether control hospitals were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated. |
| Incomplete implementation | High risk | 37% reported as ‘not implemented’. |
| Equal baseline characteristics in study arms | Low risk | Matching of the hospitals was successful as there were no differences in number of births, rurality, and whether they had a specialised antenatal service at baseline |
| Contamination of control group | Low risk | Cluster design likely to minimise risk of contamination. |
| Methods | Randomised controlled trial of peer counselling to support women to stop smoking in pregnancy Study conducted in a large urban clinic in Hartford Hospital (USA), with recruitment from January 1998 to February 2000 |
|
| Participants |
Inclusion criteria: Pregnant women who smoke at least 1 cigarette per day in week before learning of pregnancy, less than 20 weeks’ gestation, literate in English or Spanish, 18 years of age or older, and intending to carry to term Exclusion criteria: Women using smokeless tobacco or nicotine replacement products, or who reported current substance abuse or dependence Recruitment: All pregnant women screened at first prenatal visit and invited if met criteria. Informed consent obtained. Participation rate not reported, but states high smoking prevalence in pregnancy (29%) and hospital had over 4000 deliveries per year, and only 142 women recruited to study (C = 75, I = 67) Baseline characteristics: Mean cigarettes/day at baseline significantly higher in intervention group: C = 11.2 (SD 8.4); I = 13.3 (SD 13.3). Baseline CO C = 7.25 (SD 8.4), I = 5.12 (SD 5.01). Short term Fagerstrom score: C = 3.8 (2.87), I = 4.2 (2.44) Mean age C = 26, I = 26. Approximately 40% 12 years education or above. > 85% single. 63% Black, 12%-13% Hispanic, 23%-24% white. ‘Low-income, uninsured women’. Progress+ coding: Low SES, ethnic minority, single population. |
|
| Interventions |
Control: Usual care, which included the program of “Ask, Advise, Arrange and Assist”, based on cognitive behaviour, described by Windsor 2000a, and provision of self-help materials, and smoking cessation counselling as per protocol as each visit Intervention: As for the control group + peer counselling from lay community health outreach workers (telephone or home visits). Peer counsellors received 2 × 3 hours of training Main intervention strategy: Social support (single intervention) compared to less intensive intervention Intensity: Frequency (C = 5, I = 6), Duration (C = 2, I = 5). Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated smoking abstinence*, and reduction (cigarettes/day) at 36 weeks’ gestation (late pregnancy). Mean exhaled CO Mean birthweight* and proportion of babies* born low birthweight were provided by the study authors (unpublished data) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated list. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | High attrition rates (C = 27/75 or 36%, I = 29/67 or 43%). ITT analyses for whole sample and for those remaining at follow-up |
| Selective reporting (reporting bias) | Unclear risk | Birth outcomes only reported by smoking status not intervention group |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine levels at baseline and at 36 weeks’ gestation (200ng/mL cut-off). Exhaled CO at each prenatal visit (< 8 ppm) |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | States that caregivers were masked but women may have discussed but educational/counselling support intervention that women may have discussed with caregivers |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Process evaluation suggests reasonable implementation (median 6 contacts for those who remained in study), but high attrition limits exposure to intervention |
| Equal baseline characteristics in study arms | High risk | The peer counselling group had a greater proportion of heavier smokers at baseline |
| Contamination of control group | High risk | Discussion notes that quit rate in control group higher than expected and that ‘usual care’ in this trial may be more comprehensive. Which is likely as prompts etc were provided as part of trial participation to remind providers to offer support as per guidelines. Providers were also given training about the guidelines from trial staff |
| Methods | Cluster-randomised controlled dissemination trial of “It’s Time” program, in 33 prenatal, family planning and paediatric clinics Study was conducted in Chicago (USA) between November 1994 and July 1996 |
|
| Participants |
Inclusion criteria: 33 prenatal, family-planning and well-child clusters at 12 public health clinics were included. Services were matched into pairs on type of public health clinic (health department, neighbourhood health centre, university clinic), location (urban/rural), and racial mix. 10 months baseline measures were taken. The intervention was randomly assigned to 6 intervention and 6 control public health clinics Exclusion criteria: Not further specified. Recruitment: 1495 smokers identified (21% of women screened). 77% (1112) women in intervention group and 85%(1045) women in the control group agreed to participate. 63% (516) women in intervention group and 61% (548) women in control group completed the follow-up assessments (T2) Baseline characteristics: Mean cigarettes per day: C = 10.96, I = 12.01, Black C = 68.3%, I = 81.2%, > high school ed C = 39.2%, I = 38.9% Not coded as no outcomes included in review. |
|
| Interventions |
Control: Not stated. Intervention: (i) Provider focused: Charts flagged with ‘smoker’ sticker, charts prepared with booklets and agreement form, documentation; (ii) Patient focused: motivational video played in waiting room, posters, brief provider advice, booklet, agreement form, letters reminding women of advice, 15-minute motivational interview Main intervention strategy: Counselling (multiple intervention) vs usual care. Intensity not coded as no outcomes able to be included in this review |
|
| Outcomes | Dissemination and smoking cessation outcomes reported, but not able to include in this review as we were unable to separate pregnant women from women attending family planning and paediatric clinics | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just states ‘randomly allocated’. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 37%-39% attrition (due mostly to lack of working telephones) and not clear how accounted for in analysis. Conducted analysis which suggests those lost to attrition did not differ significantly in race, cigarettes, stage of readiness, motivation, or confidence |
| Selective reporting (reporting bias) | Unclear risk | Actual outcomes for each service not reported so difficult to assess |
| Other bias | Low risk | No other bias detected |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking status, not biochemically validated. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Women and provider not able to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported, despite being a dissemination trial. |
| Equal baseline characteristics in study arms | Unclear risk | Smokers in intervention clinics slightly older and more likely to be African-American |
| Contamination of control group | Low risk | Low risk of contamination as cluster trial. |
| Methods | 3-armed randomised controlled trial comparing 2 smoking cessation interventions to support women to stop smoking in pregnancy Study conducted in WIC clinics in Grand Rapids, Michigan (USA), from 1985 to 86 |
|
| Participants |
Inclusion criteria: Pregnant women currently smoking (>= 1 cigarette/day). Exclusion criteria: Not further specified. Recruitment: 271/641 attending the clinics (42%) identified as smokers. 219/271 (81%) agreed to participate and were randomised (C = 77, I1 = 70, I2 = 72). Baseline characteristics: Mean cigarettes/day prior to pregnancy I = 19.9, C = 20.3. 75% white. 76.5% on medicaid. Progress+ coding: Low SES as WIC recipients. |
|
| Interventions |
Control: Usual care which included printed information about the risks of smoking in pregnancy. Intervention 1 (risk information): 10-minute discussion with a health educator using a flip chart and a brochure but with no behaviour change counselling or self-help manual. Intervention 2 (multi-component): 20-minute 1:1 counselling including risk information (“Because I Love My Baby” Am Lung Assoc, flip chart and brochure to take away), and behavioural change manual adapted from Windsor 1985 and the Am Lung Assoc “Freedom from Smoking” focusing on contracting and self-monitoring (CBT) Main intervention strategy: Counselling (multiple intervention) compared to usual care. Intervention 2 compared with control in this review Intensity: Frequency (C = 0, I = 2), Duration (C = 0, I= 2). Usual care intensity: F = 1, D= 1 Unclear whether intervention provided by existing staff or dedicated project workers |
|
| Outcomes | Self-reported smoking cessation at 9 months gestation (late pregnancy*) and approximately 4.7 weeks after birth (0-5 months postpartum*) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 15% attrition (33/219) at follow-up. All those lost to follow-up were treated as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | Not apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Biochemically validated with salivary thiocyanate in approximately a third of participants (n = 66), but no adjustment for misclassification |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Caregivers not blinded to this educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated. |
| Incomplete implementation | Unclear risk | No process evaluation. |
| Equal baseline characteristics in study arms | Unclear risk | Differences between study participants and refusals on variables available from the WIC record were relatively minor for important variables as were study group differences |
| Contamination of control group | Low risk | Health educator, not usual care provider, offering intervention |
| Methods | 3-armed randomised control trial of an intervention to support women to stop smoking and prevent relapse in pregnancy and postpartum The study was conducted at the Group Health Cooperative of Puget Sound (Seattle, USA) (HMO), and Park-Nicollet of Minnesota (USA), a multispecialty group practice. Years of data collection not stated |
|
| Participants |
Inclusion criteria: Women who had completed the baseline survey, were < 20 weeks of pregnancy, were currently smoking or had smoked in the 30 days before pregnancy but had quit at the time of the baseline survey Exclusion criteria: Unable to speak English. Recruitment: Women booked for a first prenatal visit were offered, by letter, study participation and unless they opted out were given a baseline telephone interview to assess smoking status. 9152 approached, 714 ineligible because of miscarriage, pregnancy termination, inability to speak English; 697 (8%) refused; 262 could not be reached by telephone after repeated attempts. 7479 (82%) completed survey. 1007/7479 (13%) were current smokers or recent quitters and were randomised: 897 participated (457 from Seattle, 440 from Minnesota), C = 297, I1 = 294, I2 = 306. Current smoker at baseline = 56% (C = 165, I1 = 176, I2 = 160). Baseline characteristics: Mean cigarettes/day before pregnancy = 14.9; Current mean cigarettes/day = 4.8. Mean age 27.7 years; Household income >= 30000 $US 67%; College graduates 17%; 88% white Progress+ coding: None. |
|
| Interventions | There were 3 stages of change based interventions, all delivered by mail or telephone without involving prenatal care providers. Control: Self-help booklet “Stop now for your baby”; 5th grade reading level; health effects of smoking during pregnancy; specific suggestions for quitting (setting date, enlisting support). For recent quitters: stress reduction techniques; suggestions for handling high-risk situations; pregnancy-appropriate behavioural alternatives to smoking. Intervention 1: High intensity interventions in pre and postpartum groups also received: (i) a personalised letter acknowledging baseline readiness for change, personal health concerns, motivation to quit, comparison with other pregnant women who had successfully quit. (ii) relapse prevention kit within 2 weeks of completing the 28 week follow-up survey. (iii) a booklet which discussed transition from pregnancy and factors that influence cessation and relapse; practical tips for high-risk situations, strategies for avoiding self-defeating reactions to slips, personal anecdotes from women who quit. (iv) 3 antenatal counselling phone calls: 2 weeks after the booklet and 1 and 2 months later. Calls were open-ended but with standardised protocol based on motivational interviewing and with stage-based objectives average 8.5 min. Intervention 2: The pre-post group received as for group 2 + an additional 3 counselling calls in the first 4 months after birth reinforcing themes from the Relapse Prevention booklet; 3 newsletters at 2, 6 and 12 months postpartum about health effects of environmental tobacco smoke and the importance of being a non-smoking parent Main intervention strategy: Counselling (multiple intervention) compared to less intensive intervention. Intervention 1 and 2 were only reported as combined outcomes in late pregnancy, and included in this review. Postpartum outcomes are reported by intervention group and combines smokers at baseline and spontaneous quitters Intensity: Frequency (C = 2, I = 6); Duration (C = 1, I = 3). Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Self-reported 7-day point prevalence abstinence at 28 weeks’ gestation (late pregnancy*), with sample biochemically validated. (combined I1&I2); Relapse prevention in late pregnancy (spontaneous quitters*); Abstinence at 8 weeks (0-5 months*); 6 months* (6-11 months); and 12 months (12-17 months) postpartum (combined baseline smokers and spontaneous quitters). Response rates were 92% at 28 weeks; 91% at 8 weeks’ postpartum; 89% at 6 months postpartum; 87% at 12 months postpartum A subsequent paper reports partner abstinence. |
|
| Notes | Process evaluation describes participation in specific intervention components, including relapse prevention | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. They were stratified by baseline smoking status |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 110/1007 (11%) attrition. 88 miscarried and 22 were sent wrong intervention material and were excluded from analysis. 897 women included in final analysis. For self-reported smoking status non-respondents were treated as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Smoking outcomes only reported and only combined outcomes for abstinence at 28 weeks’ gestation |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Salivary cotinine analysis. Salivary cotinine requested from all who reported abstaining for 7 days (< 20 ng/mL as cut-off). 64%-78% returned saliva samples and as there were no differences, outcomes reported are based on self-reported status |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind providers and women to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | All samples were analysed for cotinine at the American Health Foundation laboratory. The computer-assisted telephone surveys were implemented by trained interviewers who had no role in intervention activities |
| Incomplete implementation | Low risk | Over 90% in the intervention group recalled receiving the self-help booklet, relapse prevention kit, counselling calls and newsletters |
| Equal baseline characteristics in study arms | Unclear risk | There were some baseline differences reported in text. |
| Contamination of control group | Low risk | The intervention was delivered via mail and telephone without involving prenatal health care providers |
| Methods | 3-armed randomised controlled trial of counselling and social support interventions to support women to stop smoking during pregnancy and prevent relapse post-partum The study was conducted in Womack Army Medical Centre at Fort Bragg in Feyettville, North Carolina (USA) from 1996 to 2001 |
|
| Participants |
Inclusion criteria: <= 20 weeks pregnant, >= 18 years of age, current smokers or recent quitters (i.e., were smokers in the 30 days prior to pregnancy but not smoking at intake), living with an intimate partner, and willing to have the partner contacted for participation in the study Exclusion criteria: Not further specified. Recruitment: 6156 woman screened at first prenatal clinic appointments were sent introductory letters with a toll-free number to call to decline contact. 997 pregnant smokers or recent quitters underwent further screening and 625 eligible women were randomised Baseline characteristics: Active smokers (C = 91, I1 = 87, I2 = 89). Recent quitters (C = 107, I1 = 105, I2 = 104). Current mean cigarettes per day 6 (SD 5). 52% had a partner who smoked Mean age 24 years; Household income >= 20000 $US 44%; >high school 52%; 96% married; 77% white Progress+ coding: none. |
|
| Interventions |
Control: ‘Usual care’ where women received provider advice to quit smoking at the first prenatal visit and were mailed the American Cancer Society’s self-help guide, “Make Yours a Fresh Start Family,” written at the fifth-grade reading level and designed for pregnant women Intervention 1 (woman only): Control plus late pregnancy relapse-prevention kit (a booklet and gift items) and 6 counselling calls (3 in pregnancy and 3 in postpartum) initiated by a health advisor, who used a standardised protocol based on motivational interviewing techniques. All intervention contacts were completed by 4 months postpartum. Prenatal calls were timed to occur in each trimester and emphasised using self-help materials to take stage-appropriate steps towards cessation or to develop skills for remaining abstinent. Postpartum calls were timed to occur at monthly intervals and emphasised skills for remaining abstinent in the transition from pregnancy to parenting Intervention 2 (partner-assisted group): Woman only intervention plus a PA adjunct, in which the smoker described how her partner could be a coach to build and maintain the confidence she needed to quit smoking. An “It Takes Two” booklet and companion video were developed to guide couples in discussing support behaviours related to the woman’s smoking. Partners received 6 separate calls (3 in pregnancy and 3 postpartum) from the woman’s health advisor. These calls were made separately to the 2 individuals (pregnant woman and partner) and guided by a motivational interviewing protocol similar to that used for counselling the women. The second and fourth calls to the couple focused on developing a written agreement regarding helpful partner support behaviours. Partners who smoked were given self-help cessation guides, free nicotine patches if needed, and stage-appropriate counselling Main intervention strategy: Social support (multiple intervention) compared to a less intensive intervention. Intervention 2 compared to control in this review Intensity: Frequency (C = 2, I = 6); Duration (C = 1, I = 5). Estimate as duration of calls not reported Intervention provided by dedicated project staff: efficacy study |
|
| Outcomes | Self-reported point prevalence abstinence at 28 weeks pregnancy (late pregnancy*), relapse prevention at 28 weeks pregnancy (late pregnancy*), continued abstinence of combined spontaneous quitters and smokers at 2 (0-5*), 6 (6-11*) and 12 (12-17) months postpartum Partner cessation and perceived support were reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as ‘stratified by smoking status, partners smoking status and partners willingness to be involved and randomised to one of 3 conditions’ |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 42 (7%) women who miscarried were excluded resulting in a sample of 583 (C = 198, I1 = 192, I2 = 193). An ITT approach was used, in which all randomised women (other than those who had miscarried)were included in the final analysis as continuing smokers. Drop out rates did not differ significantly across groups |
| Selective reporting (reporting bias) | Low risk | All primary outcomes appear to be reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking status only. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants to social support intervention, requiring partner consent |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Partner participation decreased steadily throughout the trial |
| Equal baseline characteristics in study arms | Low risk | Baseline characteristics appear equal. |
| Contamination of control group | Low risk | Care providers not providing intervention. |
| Methods | 4-armed cluster-randomised trial (2×2) to support women to stop smoking in pregnancy and breastfeed postpartum Study conducted in the lower North Island, New Zealand, with recruitment from June 1999 to September 2000 |
|
| Participants |
Inclusion criteria: The midwifery team was the unit of randomisation, which were stratified by locality and randomised into 1 of 4 groups. All midwives in selected localities in the lower north island were invited to take part. Midwives asked all pregnant women who had smoked at the time they conceived to take part in the study Exclusion criteria: Not further specified. Recruitment: 93/121 (77%) midwives invited (from 62 midwifery teams), agreed to participate, and were randomised into 1 of 4 study arms (C = 23,I1 = 22,I2 = 22, I3 = 26). 61 midwives recruited women to the study (76%). 46/349 (13%) women approached declined to take part in the study, 6 were ineligible, and 297 were recruited (C=60, I1=60, I2=69, I3=108) Baseline characteristics: Partner smoking (C = 50%, I1 = 47%, I2 = 62%, I3 = 49%). Mean age: C = 24.9, I1 = 26.1, I2 = 27.3, I3 = 25.1. Maori: C = 42%. I1 = 36%. I2 = 20%, I3 = 27%. Over 50% in receipt of community services card. Progress+ coding: Low SES. |
|
| Interventions | Intervention developed with provider input and detailed discussion of provider views included Control: ‘Usual’ maternity care from a midwife, which ranged from asking about smoking, giving advice to quit and to providing more detailed smoking-cessation advice Intervention 1 (smoking education): Midwife training to implement education and support for smoking cessation and reduction Intervention 2 (breastfeeding): Midwife training and support to implement education and support for breastfeeding for women who smoked Intervention 3 (combined): Midwife training to implement smoking education and breastfeeding programmes Smoking education included motivational interviewing provided by a midwife (who was allocated an extra funded visit and given 4 hours training with a counsellor), flip-chart, video-tape Main intervention strategy: Counselling (single intervention) compared to usual care. Groups 1 and 3 compared to groups 2 and 4 in this review Intensity: Frequency (C = 0, I = 2), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by existing staff (midwives): Effectiveness study |
|
| Outcomes | Biochemically validated smoking cessation at 28 and 36 weeks’ gestation* (late pregnancy), and 6 weeks and 4 months postpartum* (0-5 months postpartum). Smoking reduction outcomes of self-reported ‘cut down a little’ or ‘cut down significantly’ are not included in this review as outcomes unclear Breastfeeding outcomes also reported. |
|
| Notes | Design effect for clustering reported, so outcome figures used | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation using excel for each stratum. |
| Allocation concealment (selection bias) | Low risk | Group allocation by external statistician. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Missing data for most outcomes, 28% attrition for 4 month postnatal follow-up. Only women who moved from the area were excluded from analysis in this review |
| Selective reporting (reporting bias) | Unclear risk | Smoking status only reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Serum cotinine samples provided by 108 women. 17/19 self-reported non-smokers had cotinine levels consistent with non-smoking, but outcomes not adjusted for misclassification. 15 ng/mL cut-off level |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Not possible to blind midwives to allocation group. Women were not aware of midwife group allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | There were problems with some midwives not recruiting any women to the study, but the degree of implementation among those women recruited is not reported |
| Equal baseline characteristics in study arms | High risk | When compared with control group, women in the smoking group were older and less likely to be Maori. Also the number of women recruited to the combined group was much larger than the other groups, which suggests potential issues with recruitment |
| Contamination of control group | Unclear risk | Cluster-study design to avoid contamination. |
| Methods | Cluster-randomised controlled trial to test the effectiveness of the ALA smoking in pregnancy intervention to support women to stop smoking in pregnancy Study conducted in 11 private obstetric practices in Michigan and Upper Wisconsin (USA), with recruitment from August 1985 to June 1986 |
|
| Participants |
Inclusion criteria: 24 physicians in 11 private practices participated in the study (12 family physicians and 12 obstetricians). Study practices randomised into ‘roughly equal groups’. Women smoking at first antenatal appointment, less than 28 weeks’ gestation were recruited to study Exclusion criteria: Not further specified. Recruitment: All women attending those clinics invited to participate. After giving informed consent, each woman was assigned a code number and had a questionnaire pack placed in her chart. 639 women screened (5 refusals), 206 smokers (32%), 69/209 had quit since becoming pregnant and 137 continuing smokers were included in the study (C = 70, I = 67) Baseline characteristics: Pre-pregnancy mean cigs per day = 20; current mean cigarettes per day = 11 98% white, 70% married, majority (80%) completed high school Progress+ coding: None. |
|
| Interventions |
Control: 3 counselling sessions with physician on risks, ashtrays removed from waiting rooms and staff asked not to smoke in front of patients Intervention: Control plus (i) use of ALA materials (because you love your baby flip chart; because you love your baby packets, because you love your baby poster) (ii) encouragement to send off for materials (freedom from smoking manual), (iii) slide tape presentation at each women’s first obstetrics visit Main intervention strategy: Counselling (multiple intervention) compared to less intensive intervention Intensity: Frequency (C = 3, I = 5), Duration (C = 1, I = 2). Intervention provided by existing staff (physicians): Effectiveness study |
|
| Outcomes | Self-reported smoking abstinence at 32-36 weeks’ gestation (late pregnancy*) and first postpartum visit (timing not specified but assumed is standard 6 weeks pp visit), 0-5 months pp* | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by size - and then assigned by coin toss. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed with coin toss randomisation. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: 7 miscarriages (C = 4, I = 3), 2 therapeutic abortions (C = 0, I = 2), 11 moved (C = 6, I = 5) and 8 had an incomplete dataset (C = 4, I = 4). Those with incomplete dataset were re-included as continuing smokers in this review (C = 60, I = 57) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of smoking status (self-report only) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind providers and women to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Exact rates not reported - but ‘only minor deviations’ suggests very high implementation |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Cluster-randomised by clinic - so unlikely to have ALA materials |
| Methods | Randomised controlled trial of nurse telephone support, which aimed to reduce infants born low birthweight and preterm, and included advice on smoking Study conducted in a community public clinic in the USA. Location and dates of data collection unclear |
|
| Participants |
Inclusion criteria: Women with a preterm labour risk score of at least 7 on the Wake Forest University School of Medicine risk assessment tool; English-speaking; access to telephone; 22-32 weeks’ gestation Exclusion criteria: Not further specified. Recruitment: 1850/3127 (59.2%) eligible women contacted. 1554 (84%) agreed to participate and were randomised (C = 779, I = 775) Baseline characteristics: 21.2% (n = 253) identified themselves as smokers. Black = 1113, White or other = 320. Progress+ coding: Not coded for this review as outcomes unable to be included |
|
| Interventions |
Control: Booklet about preventing preterm labour, available in regular clinic. $10 gift certificate for completing questionnaire at 34 weeks’ gestation Intervention: As control + instruction about signs of preterm labour, nurse telephone call schedule. 3 telephone calls per week which addressed: assessment of health status (including cigarette use); recommendations; and discussion of additional issues important to mother. $25 gift certificate at 37 weeks or after the birth of their baby if they returned their assessment and remained in contact with the nurse by telephone Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Not coded as outcomes not able to be included. |
|
| Outcomes | Low birthweight and preterm births. Outcomes not included in study as unclear what proportion of outcomes were related to smokers. Furthermore, other aspects of the intervention (other than smoking cessation) may have impacted on perinatal outcomes so not included in this review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment by biostatistician using computer randomisation table |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 7.8% attrition due to moving or multiple pregnancies, leaving 1433 included in birth outcome analysis. I = 718, C = 715 |
| Selective reporting (reporting bias) | Unclear risk | Smoking rates not reported, though not the primary aim of study |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking, but not reported as an outcome in this study |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Women and providers not able to be blinded to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Outcome assessor blinded. |
| Incomplete implementation | Low risk | Process evaluation not reported. |
| Equal baseline characteristics in study arms | Low risk | No significant differences between groups. |
| Contamination of control group | Unclear risk | Telephone intervention so unlikely calls were made to wrong women |
| Methods | Cluster-randomised trial of self-help booklets to support women to stop smoking and prevent relapse in pregnancy Study conducted in 3 NHS hospital trusts in England (UK), with recruitment from May 1998 to July 2000 |
|
| Participants |
Inclusion criteria: Midwives were the unit of randomisation. Women attending first visit; >= 16 years; < 17 weeks’ gestation; literate in English were eligible. Smokers counted as those who reported “I smoke now”, “I smoke now but have cut down since I thought I might be pregnant”, or “I have stopped smoking since I thought I might be pregnant” Exclusion criteria: Not further specified. Recruitment: All 128 community midwives in 3 trusts agreed to participate and were randomly allocated to 6 strata (C = 64, I = 64). Three midwives went on maternity leave and did not recruit any women (C = 64, I = 61). 8,586 women screened and 1527/1803 (85%) eligible women consented to participate (C = 803, I = 724) Baseline characteristics: Current smokers: C = 97, I = 97; Current but reduced since pregnancy: C = 464, I = 445 (All current smokers C = 561, I = 542); Recent quitters: C = 242, I = 182. Mean cigarettes per day before pregnancy: C = 15.1, I = 16. Mean cigarettes per day at baseline C = 5.5, I = 6.4 Maternal age: C = 26.7, I = 27.2. Left full time education by 16 years: C = 63.6%, I = 61%. Progress+ coding: Low SES. |
|
| Interventions |
Control: Midwives continued to give routine advice according to usual practice. Intervention: Midwives spent at least 5 minutes introducing a series of 5 self-help booklets “Stop for Good”, based on stages of change theory, and gave them a copy of the first booklet. Subsequent booklets were mailed directly to the woman Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 1). Usual care intensity: F = 1, D = 1 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | 7-day point prevalence abstinence at 26 weeks’ gestation (late pregnancy*), with 94% validated by urine cotinine (80 ng/mL). Self-reported mean cigarettes per day in late pregnancy*. Relapse prevention for recent quitters not reported separately so outcomes for smokers and recent quitters are combined in this analysis. Stillbirths or neonatal deaths (not included as unable to separate), and preterm births (< 27 weeks) not included as rates < 36-37 weeks not reported. Reported as ‘attrition’ |
|
| Notes | Reported intracluster correlation of 0.031 used to adjust outcome data for inclusion in outcome tables. Sample size justification | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random allocation by computer-generated random numbers. 118 midwives stratified according to workload and randomly allocated to provide intervention or control care |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 92/1527 (6%) excluded from analysis due to miscarriage or termination (C = 36, I = 40), stillbirth or neonatal death (C = 9, I = 6)-not included as unable to separate, preterm birth (C = 1). Those lost to further follow-up (C = 50, I = 68) were included as continuing smokers in this review, leaving 1435 (C = 757, I = 678) |
| Selective reporting (reporting bias) | High risk | Outcomes not reported separately for baseline smokers and spontaneous quitters |
| Other bias | Unclear risk | Some unequal recruitment in each arm |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine levels analysed (cut-off 60 ng/mL and 100 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Midwives randomised. Educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Outcome assessment blinding not reported. However, follow-up rates were high in both groups, and all data coding and cleaning was undertaken blind to treatment allocation |
| Incomplete implementation | High risk | Detailed qualitative and quantitative process analysis of participants’ and midwives’ views of the intervention, which suggested poor implementation in some areas |
| Equal baseline characteristics in study arms | High risk | There were some differences between the 2 treatment groups at baseline, most notably in the numbers of women who had stopped smoking before the booking appointment and in the quantity of cigarettes consumed before the pregnancy and at the time of booking |
| Contamination of control group | High risk | Some concerns about contamination of control group reported. |
| Methods | Pilot randomised controlled trial to evaluate the feasibility, acceptability and potential effectiveness of tailored leaflets and SMS text messaging self-help intervention (MiQuit) to support women to stop smoking in pregnancy Study conducted in 7 National Health Service Trusts in the south east, east and north east of England (UK), with recruitment between December 2008 and October 2009 |
|
| Participants |
Inclusion criteria: Pregnant women less than 21 weeks’ gestation, 16 years of age and over, smoked >= 7 cigarettes per week, owned or had regular use of a mobile phone, and could understand written English Exclusion criteria: Not further specified. Recruitment: 625 women were referred by midwives to the study and 207/512 (40%) eligible women agreed to participate and were randomised to the study (C = 105, I = 102) Baseline characteristics: Cigarettes per day before pregnancy and at enrolment reported by 6 categories and equal in both arms. Majority (over 60%) 11-20 cigs/day before pregnancy and approx 50% 4-10 cigarettes/day at enrolment Median age 26-27 years; 16% did not complete high school; 100% white Progress+ coding: None. |
|
| Interventions |
Control: Participants received a non-tailored self-help leaflet, which matched the tailored leaflet in format and style, and the same assessment texts as MiQuit participants but no intervention texts Intervention: Participants receive MiQuit tailored self-help leaflet by post. Thereafter automated tailored text message component of intervention is initiated. 80 texts sent out over 11 weeks. MiQuit participants could also request instant response supportive texts at any time of the day Main intervention strategy: Health education (multiple intervention) compared to less intensive intervention Intensity: Frequency: (C = 2, I = 5), Duration: (C = 1, I = 1). Technological intervention: Unclear whether efficacy or effectiveness study |
|
| Outcomes | Biochemically validated 7-day point prevalence at 3-month follow-up (late pregnancy)*, self-reported 4-week point prevalence, initiation and frequency of quit attempts and 7-day point prevalence at 3 and 7 weeks after enrolment; Self-efficacy (5-point scale), acceptability measures | |
| Notes | Process evaluation showed 98% intervention and 89% control participants received the leaflet and 87% intervention participants reported reading text messages at least once | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of the randomisation tables and allocation of participants were implemented in a computer programme and managed by SS who had no contact with participants or involvement in data collection or entry |
| Allocation concealment (selection bias) | Low risk | ‘The allocation sequence was concealed from other members of the research team, midwives, and participants’ (p570) |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Drop-outs due to miscarriage or stillbirth were excluded from the analysis (I = 6, C = 3). Reported as combined figure. 11% further attrition for other reasons (I = 10, C = 13), were included in analysis as continuing smokers (C = 96, I = 102) |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of self-reported smoking cessation with salivary cotinine (< 13 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Women unlikely to be blinded to educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | ‘FN undertook data collection and was blinded to group allocation until all data had been collected.’ (p570) |
| Incomplete implementation | Low risk | 90% MiQuit participants reported reading all the leaflet at least once |
| Equal baseline characteristics in study arms | Low risk | There were not differences between trial arms on baseline variables except that more participants in the control arm had smoked in a previous pregnancy (difference adjusted for in analyses) |
| Contamination of control group | Low risk | Technological intervention so low risk of contamination between study arms |
| Methods | 4-armed randomised controlled trial which aimed to improve the uptake of prenatal care and pregnancy outcomes (especially low birthweight), and included advice about smoking Study conducted in a semi-rural county of New York State (USA), with recruitment between April 1978 and September 1980 |
|
| Participants |
Inclusion criteria: Pregnant women with no prior live births + any of the following: < 19 years; single; low socio-economic status, and any other women with no prior live births who wished to participate in the program Exclusion criteria: > 25 weeks’ gestation (though some were enrolled at 25-29 weeks) Recruitment: Through private obstetricians’ offices, planned parenthood, public schools health department antenatal clinics and other health and human service agencies. 10% of target population entered prenatal care too late, 10% were not referred from private care. 500 women were interviewed and 400 enrolled (80%). Families were stratified by marital status, race, and 7 geographic regions (C = 90, I1 = 94, I2 = 100, I3 = 116). 141 smokers (C = 64, I = 77). Baseline characteristics: Mean cigarettes per day at intake: C = 6.94, I = 7.65. 47% < 19 years old, 62% single, 61% low SES (15% had none of these factors). Non-Whites (46) excluded because too few; serious maternal or fetal conditions (20) excluded Progress+ coding: Low SES. |
|
| Interventions |
Control: Health and developmental screening of the baby at 12 and 24 months; Intervention 1: Control + free transport to pregnancy and well-child visits (control); Intervention 2: 1+ nurse home visits during pregnancy (intervention); Intervention 3: 2+ nurse home visits in child’s first 2 years. The focus of the home visiting was individualised from a detailed curriculum dealing with information on fetal and infant development; improvement of maternal diet; monitoring weight gain; elimination of cigarettes, alcohol and drugs; identifying pregnancy complications; encouraging rest, exercise and hygiene; preparing for labour birth and early newborn care. The intervention was also described as enhancement of informal support systems (partners, family and friends) and linkage of parents to community services, including nutritional care, prenatal providers and other services Main intervention strategy: Social support (tailored intervention) compared to usual care. Intervention 2&3 (nurse-visiting arms) compared to control and intervention 1 arms (no nurse visiting) in this review. Intensity: Freqency (C = 0, I = 6), Duration (C = 0, I = 4). Usual care intensity: F = 0, D=0 Intervention provided by dedicated study team: Efficacy study |
|
| Outcomes | Cotinine levels taken in a subsample (n = 116), but no women reported smoking cessation at 32 weeks’ gestation (late pregnancy)*. Mean cigarettes per day at 32 weeks (late pregnancy*). No mean cotinine levels reported for inclusion. Self-reported reduction in cigarettes, but not reported as a mean for inclusion in this review. Birth outcomes were not included as aspects of the intervention, other than smoking cessation, may potentially improve birth outcomes | |
| Notes | SDs for mean cigarettes per day were not reported, therefore we calculated a mean SD from 14 studies with available mean cigarette SDs (6.5) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 6.5% attrition (C = 12, I = 14) due to moving or miscarriage. However outcomes for 307/400 women only reported. Outcomes for all smokers at intake reported |
| Selective reporting (reporting bias) | Low risk | Detailed range of outcomes reported. |
| Other bias | Unclear risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Serum cotinine analysis on subsample of 116. No self-reported cessation to validate |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Home visitation programme. Blinding of participants and personnel not viable |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | The interviewers and medical record reviewers hired by the research project did not know to which treatment the women had been assigned |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | High risk | Women assigned a nurse had less social support. |
| Contamination of control group | Low risk | Home visits. |
| Methods | 3-armed randomised controlled trial of home visiting during pregnancy by paraprofessionals and nurses to improve maternal and child health, and included advice about smoking The study was conducted in 21 prenatal clinics in Denver (USA) from March 1994 to June 1995 |
|
| Participants |
Inclusion criteria: Pregnant women with no previous live births and either qualified for Medicaid or had no private medical insurance Exclusion criteria: Not further specified. Recruitment: By written invite, and were not required to respond. 735/1135 eligible women participated in the study, 70 of whom were smokers (C = 25, I1 = 21, I2 = 24). Baseline characteristics: Not reported among smoking subgroup. |
|
| Interventions |
Control: Developmental screening and referral services for children at 6, 12, 15, 21 and 24 months old Intervention 1 (Paraprofessional): Screening and referral plus paraprofessional home visiting for first 2 years of infants life. Aimed to improve maternal and fetal health, improve health and development of child, and enhance parents personal development Intervention 2 (Nurse): Screening and referral plus nurse home visiting for first 2 years of infants life. Aimed to improve maternal and fetal health, improve health and development of child, and enhance parents personal development Main intervention strategy: Social support. Not coded or compared in this review as outcomes unable to be included |
|
| Outcomes | Outcomes not able to be included in meta-analysis, as only mean reduction in cotinine reported. See Table 1 for outcome summary. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation conducted in separate data centre. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear whether all randomised smokers were included in cotinine analysis |
| Selective reporting (reporting bias) | High risk | Smoking cessation rates not reported, but are not a primary outcome of this study |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Unclear whether all randomised women included in cotinine analysis |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Providers and women not able to be blinded as social support intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Outcome assessors blinded to allocation. Study team unaware of allocation, unless the participant told them |
| Incomplete implementation | Low risk | Paraprofessionals completed an average of 6.3 visits and nurses an average of 6.5 visits |
| Equal baseline characteristics in study arms | Unclear risk | Baseline characteristics of smokers not reported. But treatment groups similar with ‘few exceptions’ |
| Contamination of control group | Low risk | Home visits. |
| Methods | 4-armed (2× 2 factorial design) randomised controlled trial of a computer-delivered brief intervention (CD-5As) and incentives to support women to stop smoking in pregnancy The study was conducted in 4 prenatal care clinics in Detroit, MI (USA) with recruitment from July 2008 to November 2009, and final evaluation completed by January 2010 | |
| Participants |
Inclusion criteria: Pregnant women aged 18 years or older, being no further than 27 weeks’ gestation, and reporting smoking in the past week Exclusion criteria: Unable to understand spoken English. Recruitment: 1317 women were screened while in the clinic waiting area. 110/114 (96%) eligible women provided consent and were randomised (C = 26, I1: CD-5As only = 26, I2: CM-Lite only = 28, I3 = CM-Lite+CD 5As = 30). Baseline characteristics: Average cigarettes per day in week prior to recruitment: mean = 8 (SD 8.2). 70% lived with a smoker. 52.8% had a fagerstrom score >= 4 (nicotine dependence) Mean age 27.9 (6.4); 90% Black. K6 emotional distress 14.9. Progress+ coding: Low SES and ethnic minority. |
|
| Interventions |
Control: Usual Care from prenatal care from care-providers without influence from the research team Intervention 1 CD-5As only: Computer delivered brief intervention designed to be consistent with ‘5As national guidelines (USA)’ (Ask, Advise, Assess, Assist, Arrange) and-for those who are unwilling to set a quit goal-the 5Rs (with steps involving the highlighting of Relevance, Risks, Rewards, Roadblocks, and Repetition). The ‘Advice’ included a 5 minute video featuring a male Black Obstetrician and 3 testimonials from women of varying race, which was direct but designed to be positive and frame the benefits of quitting rather than the risks of smoking Intervention 2 CM-Lite (incentives) only: This modified version of ‘contingency management’ was designed for use with non-treatment-seeking persons in a health care setting with the presumption of (a) at least occasional repeat office visits and (b) limited ability of medical staff to monitor participants or participate in training. Thus, no proactive tracking was provided in CM-Lite: It was designed to be patient initiated, with staff checking eligibility if and when a patient asks to have their smoking status verified rather than relying on staff to check the eligibility of every incoming patient. CM-Lite calls for testing at prenatal care visits only and unlimited incentivisation attempts, but only up to a maximum of 5 episodes of reinforcement (in the form of retail gift cards worth $50), only at prenatal clinic visits, each at least a week apart. CM-Lite was delivered with the help of a website which facilitated the process of verifying eligibility of participants, provided step-by-step guidance in how to conduct a valid test for urinary cotinine, recorded the results of testing, and provided a record of all incentive attempts and their outcome Intervention 3 CD-5As + CM-Lite combined. Main intervention strategy: Incentives (tailored intervention) compared to usual care. Intervention 2 compared with control in this review Intensity: Frequency (C = 0, I = 5), Duration (C = 0, I = 1). Usual care intensity unclear: F = 0, D = 0 Technological intervention: unclear whether delivered by existing staff (Effectiveness study) or dedicated project staff (efficacy study) |
|
| Outcomes | Biochemically validated 7-day point prevalence at 10-week follow-up (late pregnancy*) with CO and urinary cotinine. Secondary help-seeking (Quitline), self-reported sustained abstinence in the past 30 days, Fagerstrom Test for nicotine dependence; K6 measure of overall emotional distress; Acceptability (satisfaction-related measures) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation of all participants into either CD-5As or time control conditions and after participants completed all computer-delivered content-research assistants used a predetermined list of computer-generated random numbers to further randomise half of all participants into the CM condition |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition 16/110 (14.5%) lost to follow-up. All analyses were on an intent-to-treat basis that analysed participants as allocated to condition without respect to completion of treatment elements. Only 2 women who withdrew due to miscarriage (one in combined arm and 1 in usual care arm) were excluded from the analysis in this review |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported 7-day abstinence biochemically validated with expired CO (< 4 ppm) and urinary cotinine (< 100 ng/mL)* |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Each intervention involved the same level of interaction with the computer and took the same approximate amount of time, thus keeping research assistants blind to computer-delivered intervention condition. Not feasible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | It is not stated whether outcome assessors were blinded. |
| Incomplete implementation | Unclear risk | Process evaluation showed all participants assigned to CD-5As condition completed the items and evaluations and gave high satisfaction ratings. Of the participants assigned to CM-Lite only 37.9% initiated testing of at least 1 urine sample (mean 3. 7, SD 1.9) |
| Equal baseline characteristics in study arms | Low risk | There were no significant differences between conditions on any of the baseline characteristics examined, although 1 variable (minority vs. non-minority race) was below P = .10 and so was controlled for in subsequent analyses |
| Contamination of control group | Low risk | The risk of contamination between study arms is low as interventions are all provided via technology |
| Methods | Randomised controlled trial of counselling interventions to support women to stop smoking in pregnancy Study conducted in a public antenatal clinic in Melbourne, Victoria, Australia. Data collected from April 1994 to June 1996 |
|
| Participants |
Inclusion criteria: Women who identified as “current smokers” at their first antenatal visit at approximately 12 weeks’ gestation (“even a puff in the last 7 days”) Exclusion criteria: >20 weeks’ gestation; twin pregnancy; not literate in English; drug dependency Recruitment: 9193 women screened, 1942 (21%) current smokers and 625 (7%) spontaneous quitters (not included in study but described in Panjari 1997). 1013/1942 smokers (52%) agreed to participate (929 refused or not eligible) and were randomised (C = 537, I = 476). Baseline characteristics: Mean cigarettes per day = 21 before pregnancy and 11 at time of first antenatal visit. 74% had a smoking partner Mean age 26 years. Progress+ coding: Low SES as authors note mostly low income women. |
|
| Interventions |
Control: Usual care, which included advice at the discretion of the caregiver, and 0 pamphlet “Smoking & Pregnancy” distributed during a group pregnancy information session Intervention: As for the control group plus 4 counselling sessions by a midwife specifically trained and employed to provide smoking cessation counselling, using CBT. Sessions included video presentation, interactive discussion and strong verbal messages. These were followed up with a 5 to 10 minute personalised counselling session Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 3). Usual care intensity: F = 1, D=1 Intervention provided by dedicated project staff: efficacy study |
|
| Outcomes | Self-reported smoking cessation biochemically validated with urine cotinine at 36 weeks’ gestation (late pregnancy*), 6 weeks postpartum (0-5 months)*, and 6 months (6-11 months*) postpartum*. Preterm births*, mean birthweight*, proportion LBW* (< 2500 g) Reduction in mean cigarettes/day* and mean urinary cotinine levels* Breastfeeding at 6 weeks and 6 months postpartum. General health assessment at first visit and 36 weeks General health questionnaire (including stress and depression measurement) at baseline and end of pregnancy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly allocated". |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 28% attrition (381/1013). 72/1013 (C = 35, I = 37) were excluded as they were over 20 weeks’ gestation, had a twin pregnancy or were transferred to the chemical dependency clinic. 209/1013 (C=109, I=100) excluded due to transfer to another hospital, miscarriage, termination of pregnancy and withdrawal from the study. The numbers of those who withdrew from the study were not reported separately in this group, therefore all were re-included as continuing smokers in this review (but were not included in mean outcome data) |
| Selective reporting (reporting bias) | Low risk | A detailed list of birth outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine levels measured at baseline and in late pregnancy (< 115/ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention delivered by clinic midwife. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed 71% women in the intervention group received the full intervention |
| Equal baseline characteristics in study arms | Low risk | There were no statistically significant differences between women allocated to the intervention and the control groups in terms of socio-demographic variables and smoking patterns |
| Contamination of control group | Low risk | Intervention provided by a research mid-wife, not usual care provider |
| Methods | 3-armed randomised controlled trial aimed to evaluate the feasibility, cost and effectiveness of a telephone counselling intervention to support women to stop smoking in pregnancy Study conducted at 22 urban prenatal care clinics in Rhode Island (Connecticut) and Massachusetts (USA). Study period not reported |
|
| Participants |
Inclusion criteria: Pregnant women who had smoked at least 1 puff of a cigarette within the past 30 days, no more than 26 weeks pregnant, had access to a telephone where she could be reached, and speak English or Spanish Exclusion criteria: Not further specified. Recruitment: 8526 pregnant women were assessed at their first or second visit. 1065/1582 eligible women (67%) agreed to participate and were randomly assigned to 3 conditions (C (self-help materials)=378; I1 (Self-help materials+quit and win contest) = 329; I2 (self-help materials + quit and win contest + motivational interviewing counselling calls = 358) Baseline characteristics: Strateifed by participation in calls: Mean cigarettes per day at baseline: 7.9 (6.3) to 8.7 (5.8). Baseline cotinine: 869 to 1239 mg/mL Majority white, 40% <= 11 years education. Progress+ coding: Low SES as 80% Medicaid recipients. |
|
| Interventions |
Control: Participants received self-help materials, which included a quit kit (A Smoker’s Guide to Quit Smoking) and a video (Commit to Quit), which had been shown to be effective in significantly reducing exposure or assisting pregnant women to quit smoking (SCRIPT trials) Intervention 1: Received the quit kit and were enrolled in a “Quit and Win” (Q&W) monetary incentive lottery program. Eligibility for the prize (US$100) was restricted to smokers who reported abstinence for at least 30 days and had their report confirmed by urinary cotinine. Intervention 2: Received the quit kit, the Q&W program, and up to 3 Motivational Interviewing telephone calls This review compares the control group and Intervention 2. Main intervention strategy: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C = 1, I = 4), Duration (C = 1, I = 3). Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Self-reported smoking cessation biochemically validated with urinary cotinine (< 80 ng/mL) at 32 weeks’ gestation (late pregnancy)*, 6 weeks and 6 months postpartum (outcomes not reported). Cost-effectiveness analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition: C = 101/378 (27%), I = 118/358 (33%) by 6 months postpartum (reasons not reported). All randomised women included in analysis |
| Selective reporting (reporting bias) | High risk | Smoking cessation at 6 weeks and 6 months postpartum not reported |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Biochemical validation of self-reported smoking status using urinary cotinine (<80ng/mL). Conference report states only 219 women with biochemically confirmed smoking status were included in report. But pg 1045 states “Samples were obtained from 114 women during the first prenatal visit, from 113 during the third trimester, and 23 during the 6 month postpartum visit. We were unable to contact the remainder of the women, and therefore did not have samples to confirm their self-reported smoking status” |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Not feasible for participants and personnel to be blinded to educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed researchers were unable to reach 14%, 86% received 1 call, 60% 2 calls and 46% 3 calls |
| Equal baseline characteristics in study arms | Low risk | The absence of significant differences for multiple salient predictors and other weaker predictors of smoking behaviour change strongly suggested that the call groups were comparable at baseline |
| Contamination of control group | Low risk | Specific counsellors providing intervention so low risk of contamination |
| Methods | Randomised controlled pilot study of a targeted intervention to support pregnant Alaskan Native women to stop smoking in pregnancy Study conducted in the Y-K Delta region in Western Alaska (USA), with recruitment from 2007 to 2008 |
|
| Participants |
Inclusion criteria: Pregnant Alaskan women ≥ 18 years, ≤ 24 weeks’ gestation, self-reported smoking or Iqmik/ST use in the last 7 days, planning to quit in the next 30 days, access to a telephone and VCR/DVD player, and willing to participate in all study procedures Exclusion criteria: Planning an abortion, current (past 3 months) participation in pharmacological or behavioural tobacco treatment, and another woman from her household had enrolled Recruitment: 293 women expressed an interest in the study and were referred to study coordinator. 81 did not attend screening appointment, 114 reported not smoking and 4 were ineligible. 35/94 (37%) of the remaining eligible women agreed to participate and were randomised (C = 18, I = 17) Baseline smoking characteristics: Current tobacco use (in past 7 days): Iqmik C = 44% (8), I = 47% (8); Commercial chew C = 22% (4), I = 18% (3); Cigarette smoking C = 33% (6), I = 35% (6). Spouse/partner uses tobacco: C = 78% (14), I = 54% (7). Smoking ban in the home C =89% (16), I= 88% (14). Chewing ban in the home C = 12% (2) , I = 19% (3) Baseline characteristics not reported. Progress+ coding: Low SES, ethnic minority population. |
|
| Interventions |
Control: Participants in the control arm received an intervention consistent with the 5-component treatment (5A’s) recommended for pregnant smokers by the Clinical Practice Guideline: Ask, Advise, Assess, Assist, and Arrange. At the first visit, participants in this condition received a brief (5-min) face-to-face intervention based on the 5A’s and 4 pregnancy and culturally specific brochures. The counsellor encouraged and assisted the participant to set a quit date. Participants requesting NRT or another medication from the counsellor were referred to the YKDRH clinical cessation program and enrolment in this program was tracked as part of this study Intervention: At the first visit women in the intervention group received: (i) a self-help guide adapted from the SCRIPT trials (Windsor 1999) and from culturally appropriate brochures developed and used by the YKDRH clinical cessation program (ii) 15-25 minutes of face-to-face counselling based on the 5A’s (iii) a video which was produced that included stories of Alaska Native women who stopped using tobacco during pregnancy. Focus groups suggested that story-telling was a potentially acceptable intervention component. The counsellor then discussed the video with the woman (iv) A further 4 × 10-15 minute proactive interactive sessions were provided by telephone, based on a counsellor manual which was developed based on completed evaluation research, at Weeks 1, 2, 4, and 6. These sessions provided opportunities for the counsellor to teach additional cessation skills and reinforce self-efficacy. The woman was encouraged to set a quit date at each contact, if she had not quit Main intervention strategies: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 6), Duration (C = 2, I = 3). Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Biochemically validated tobacco use in (salivary cotinine< 20n g/mL) 60 days post randomisation (late pregnancy*). Acceptability to women | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 35 participants were stratified by primary type of tobacco used (Iqmik, commercial ST, or cigarettes) and randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: C = 1/18 (6%), I = 5/17 (29%). 1 miscarriage in each study arm excluded from this analysis. All other drop outs counted as continuing smokers |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported tobacco use status biochemically validated using salivary cotinine (< 20 ng/mL). Some women were using NRT |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showed good treatment compliance and acceptability of intervention |
| Equal baseline characteristics in study arms | High risk | Level of education and spouse/partner smoking unequal. |
| Contamination of control group | High risk | Assessments and interventions provided by the same individual in each community |
| Methods | Cluster-randomised controlled trial of implementation of the “Quit Together” program which aims to support women to stop smoking and prevent relapse in pregnancy Study conducted WIC clinics in Massachusetts (USA) of implementation, with data collection from May 1997 to November 2000 | |
| Participants | Unit of randomisation was 6 community health centres with on-site WIC programs, prenatal services and paediatric services, and patients of diverse race and ethnicity. 1 control site was dropped due to low recruitment Inclusion criteria: Pregnant women, English or Spanish speaking, less than 32 weeks’ gestation, current smoker or spontaneous quitter, planning to remain in area for 6 months after delivery Exclusion criteria: Not further specified. Recruitment: 7853 women screened. 609/693 (88%) eligible smokers and ex-smokers consented, completed baseline interviews and were randomised (C = 300, I = 309) Baseline characteristics: Current smokers (C =72.3%, I = 70.2%), spontaneous quitters (C = 27.7%, I = 29.8%). Mean cigarettes per day before pregnancy: C = 18.43, I = 14. 89 Mean age 26 years. White (C = 78.6%, I = 22.8%), Black (C = 1.8%, I = 39%), Hispanic (C = 4.7%, I = 27.6%). Unmarried: C = 60.8%, I = 68.8%. Medicaid C = 63.1%, I = 65.5%. < High school C = 62.2%, I = 46.7% Progress+ coding: Low SES as high proportion of WIC recipients. |
|
| Interventions |
Control: Usual care condition, in which no training or intervention occurred Intervention: The dissemination intervention consisted of: (i) provider training based on national clinical practice guidelines (ii) an office practice management system for routine screening and follow-up reminders, and (iii) establishment of program boards. The intervention to women was based on motivational interviewing and the “4A’s” from the ‘SCRIPT trial’ conducted by Windsor 2000b. Main intervention strategy: Counselling (single intervention and intensive dissemination) compared to usual care Intensity: Frequency (C = 0, I = 2), Duration (C = 0, I = 1). Usual care intensity: F = 0, D=0 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Biochemically validated smoking cessation and relapse prevention at 1 month postpartum combined (late pregnancy*), and 3 (0-5*) and 6 (6-11*) months postpartum. 6-month figures not reported in text but estimated from Figure 3 to be I = 11%, C = 4% Mean cigarettes/day* estimated from figure 4. Associated references describe detailed organisational change and implementation processes for the clinic setting, subanalysis of a range of outcomes by socio-economic status; and clinical knowledge of nicotine dependence (Bonollo 2002). |
|
| Notes | No estimates of clustering effect reported, so sensitivity analysis conducted and intracluster correlation of 0.10 used to adjust data for inclusion in outcome tables (see table 2 for adjustment details) SDs for mean cigarettes per day were not reported, therefore we calculated a mean SD from 14 studies with available mean cigarette SDs (6.5) to include in this review, as recommended by the cochrane handbook |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 34/609 (6%) had a miscarriage and 12/609 (2%) transferred to another health service. 13 women excluded for other reasons (unexplained), but they are not reported by intervention group to be re-included and the figures reported in the flow chart are combined with drop-outs for other reasons. Also high loss to follow-up. 550/609 women included in this analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial part of a nutritional program, but only smoking outcomes in this report |
| Other bias | Unclear risk | One control site dropped due to low recruitment. Otherwise recruitment to study arms appears balanced |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | A woman was considered to be a smoker if she reported smoking in 30 days prior to 1 month postpartum interview. Salivary cotinine was analysed for women reporting abstinence in 7 days prior to the interview (<= 20 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Sites aware of allocation status. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Process evaluation not reported. |
| Equal baseline characteristics in study arms | Unclear risk | While no differences between SI and UC were statistically significant, some were large (e.g., race/ethnicity, education). This reflects the variability in size and race/ethnicity distributions among CHCs, the unit of randomisation |
| Contamination of control group | Low risk | Cluster design to avoid contamination. |
| Methods | 3-armed randomised controlled trial of self-help materials and counselling to support women to stop smoking and prevent relapse during pregnancy and postpartum Study conducted at a large Boston HMO (USA), with recruitment from March 1986 to September 1988 | |
| Participants |
Inclusion criteria: English-speaking literate women enrolling in prenatal care; who reported themselves as currently occasional or regular smokers or who had quit smoking in the previous 3 months Exclusion criteria: < 18 years of age; > 24 weeks’ gestation. Recruitment: 1442 women screened during early pregnancy class. 317 current smokers and recent quitters were identified. Participants from 3 centres were randomised to control and first intervention (I1) arms, and participants from a fourth arm were not randomly allocated and are not included in analysis ion this review. 93/317 attrition, leaving 224 included (C = 78, I1 = 71, I2 (not randomised) = 75). Baseline characteristics: Baseline smokers : 142 (C = 47, I1 = 43, I2 = 52) and baseline spontaneous quitters: 104 (C = 36, I1 = 34, I2 = 34) analysed at 6 months gestation. Majority 17-28 years, No participants less than high school, less than $US 20000/yr (C = 18.7%, I1 = 20%, I2 = 32.3%). Over 80% married and majority white. Progress+ coding: None. |
|
| Interventions |
Control: Routine obstetric care, including a mailed list of community-based smoking cessation resources other pregnancy-related health education materials. Brief repeated counselling by obstetricians and midwives for both groups as part of routine care. Intervention 1: Pregnancy-specific self-help manual (Am Lung Assoc and Harvard Community Health Plan (HMO)) and audiotape on safe aerobic exercise and pregnancy-related relaxation, mailed with other health-related education. Smoking component emphasised behavioural strategies for quitting, issues and concerns specific to pregnant women, non-smoking as part of a continuum of care in pregnancy; included a maintenance section for the postpartum period Intervention 2: As for I1 plus training for obstetrician and nurse practitioner to provide training, and support letters from physician Main intervention strategy: Health education (single intervention) compared to usual care. Intervention 1 and control compared in this review as the I2 group was not randomised. Intensity: Frequency (C = 0, I = 2), Duration (C = 0, I = 1). Usual care intensity: F = 3, D=2 Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Smoking cessation for smokers and spontaneous quitters at 6 months gestation (late pregnancy* and 8 weeks postpartum (0-5 months*) Description of costs. |
|
| Notes | Substantial misclassification of non-smoking self-report at 6 months gestation 24% controls 21% intervention (and 30% in clinic where the intervention was more intensive) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. Allocation to intervention arm 2 was not randomised but offered to all eligible enrollees at 1 clinic: therefore data from this intervention arm are not included in the review |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 93/317 (29%) were excluded from analyses due to miscarriage, therapeutic abortion, moving, or left the Harvard Health Plan, leaving 217 included. However, 246 (C = 83, I1 = 77, I2 = 86) ‘baseline smokers and spontaneous quitters’ included in analysis at 6 months gestation and 219 included in 8 weeks postpartum. It is not clear which randomised women are included in analysis |
| Selective reporting (reporting bias) | Unclear risk | None apparent but results were not simple to interpret. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation in 50% women. Those refusing urine test were coded as smoking |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | State that caregivers were blind as materials to the intervention group were mailed. Not feasible to blind women |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | All women received materials for intervention 1 used in this review. Some implementation problems noted with the counselling arm (I2), but that was not included in this review. |
| Equal baseline characteristics in study arms | High risk | Differences in educational attainment. |
| Contamination of control group | Low risk | Unlikely with mail out of materials. |
| Methods | Cluster-randomised trial of intervention to support women to stop smoking and prevent relapse in pregnancy and postpartum Study conducted in the Lodz district, Poland, with data collection from December 2000 to December 2001 |
|
| Participants | Unit of randomisation was maternity units, selected from 33 in district and stratified by size. Control = 1 small, 2 medium, 2 big; Intervention = 2 small, 4 medium, 4 big (as higher refusal expected in intervention arms Inclusion criteria: Current smokers or women who quit 1 month before the visit Exclusion criteria: Not further specified. Recruitment: 15/33 maternity units were allocated to intervention (10) or control (5) groups All pregnant women screened. 194/194 (100%) eligible women in control group and 216/275 (78.5%) eligible women in the intervention group agreed to participate Baseline characteristics: Current smokers: C = 156, I = 158. Spontaneous quitters: C = 38, I = 58. Cigarettes per day: < 5 (C = 8.8%, I = 10.3%), 5-50 (C = 54.7%, I = 46%), > 10 (C = 36.5%, I = 43.7%). Fagerstrom score 0-6 (C = 98.9%, I = 92.3%) Mean age: C = 25.9, I = 25.5; < 12 years education: C = 76.2%, I = 74.3%; Unmarried: C = 39.2%, I = 52.5% Progress+ coding: Low SES population as described by author. |
|
| Interventions |
Control: Received standard written information about health risks of smoking Intervention: Received 4-9 midwife home visits, based on a booklet translated from English (Ottawa) to Polish and adapted to Polish conditions: “How to talk about smoking with high risk pregnant smokers” Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 4). Usual care intensity: F = 1, D=1 Intervention provided by midwives, which appear to be existing staff, though this is not explicitly reported: coded as effectiveness study |
|
| Outcomes | Self-reported smoking cessation ‘shortly after delivery at home’ (0-5 months postpartum*) Relapse prevention rates* in text (p274). Mean birthweight* calculated by combined smokers and quitters in Table 6 An associated reference (Polanska 2005) reports relapse after 12 months* (12-17 months postpartum). All randomised from women from original study included as denominator and those not included in the follow-up analysis assumed to have relapsed in this review. Spontaneous quitters and smokers combined from Table 2 to calculate self-reported abstinence at 12 months |
|
| Notes | No estimates of clustering effect reported, so sensitivity analysis conducted and intra-cluster correlation of 0.10 used to adjust data for inclusion in outcome tables as shown in Table 2. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Notes random allocation, but no description of how this occurred. Only 15/33 eligible clinics allocated |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: Miscarriages: Smokers: I = 9/158 and C= 12/156. Spontaneous quitters: I = 2/58 and C= 1/38. Not included in analysis Those lost to follow-up: Smokers: (C = 6, I = 6) and Spontaneous quitters (C = 0, I = 2) are included in analysis of smoking outcomes |
| Selective reporting (reporting bias) | Unclear risk | Birthweight and relapse prevention outcomes difficult to interpret and unable to be included |
| Other bias | Unclear risk | Twice as many sites were allocated to the intervention arms as the control arms as it was assumed more women would refuse to participate in intervention activities. However recruitment to study arms was equal |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking status only. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel not blinded to this educational intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | No. of visits received not reported. |
| Equal baseline characteristics in study arms | High risk | Intervention group more likely to be married, have fewer children, and have a higher smoking addiction |
| Contamination of control group | Unclear risk | Cluster-design to minimise risk of contamination. |
| Methods | 3-armed randomised controlled trial of 2 brief interventions to support women to stop smoking in pregnancy Study conducted in an inner urban setting, Toledo, Ohio (USA), with recruitment from December 1987 to March 1989 |
|
| Participants |
Inclusion criteria: Not specified. Exclusion criteria: > 28 weeks’ gestation. Recruitment: All 1,164 patients screened, 486 current smokers (42%). 293 refused or were ineligible (40% participation). 193 smokers randomised to study (C = 71, I1 = 52, I2 = 70). Baseline characteristics: Baseline smoking not reported. Mean age=22.6 (5.6), ranging from 15-43 years. 58% single, 70% white, 87% had not graduated from high school. Author describes population as “Typically low income, single and poor” Progress+ coding: Low SES. |
|
| Interventions |
Control: Usual care not specified or assessed but “usual for physicians to address this issue with participants at least 1 prenatal visit”. Intervention 1: American Lung Association self-help booklet (with brief overview and explanation) emphasising behaviour modification skills, relation techniques and the support of significant others, and were given an opportunity to ask questions of the health educator. Progress reviewed with health educator at the second visit Intervention 2: Tailored educational videotape 6.5 minutes, potential fetal risks, benefits if mother quit + pamphlet on how to quit and opportunity to ask questions of the health educator. 1 month later they viewed a second 4 min video and the health educator was available to answer questions Main intervention strategy: Counselling (single intervention) compared to usual care. The control and intervention 2 (video-tape) are compared in this review Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 2). Usual care intensity: F = 1, D=1 Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation ‘two or three weeks prior to delivery’ (late pregnancy*). Smoking reduction* and mean cigarettes/day* | |
| Notes | Program was developed with input from a questionnaire (based on Health Belief Model) and open-ended questions about the advantages and disadvantages of smoking when pregnant from local population. Commentary on the contextual factors in the lives of indigent women which lead them to have different perceptions about the relative importance of smoking |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | High risk | Tossed die (allocation could therefore be changed). Method resulted in 3 unequal groups, so randomisation to only 2 groups for some of the study period, which was the control and intervention 2 (videotape) group, compared in this review |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition 44% (C = 46, I1 = 13, I2 = 25) . Reasons for attrition not reported. However all drop-outs treated as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Smoking cessation was biochemically validated using exhaled CO (<= 7 ppm cut-off) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | 44% did not receive intervention. |
| Equal baseline characteristics in study arms | Unclear risk | Not reported. |
| Contamination of control group | Low risk | Specific educators providing intervention (pregnancy care providers not involved) |
| Methods | Randomised controlled trial of ultrasound feedback on health beliefs and behaviours to improve maternal health, including smoking Study conducted in London, England (UK). Recruitment dates not specified |
|
| Participants |
Inclusion criteria: Caucasian origin, aged between 18 and 32 years, married or within a stable relationship, attending King’s College Hospital antenatal booking clinics Exclusion criteria: Women with a previous history of miscarriage, extended infertility investigations, or meet criteria for risk of congenital malformations Recruitment: Women ‘briefly informed that the study involved a continuing evaluation of aspects of obstetric care and that they would be seen on occasions throughout the pregnancy’. 6 women refused. 194 women recruited (see associated reference (Reading 1982), and were randomised to 3 arms: control (delayed ultrasound) = 55; I1 (low feedback) = 62; and I2 (high feedback = 67). The control arm was added during the course of recruitment and is not included in this review. 129 women included, 65 (50%) smokers at baseline (I1 = 26/62, I 2= 39/67). Baseline characteristics: Smoking characteristics not reported. Selective inclusion criteria: Pregnant women at 10-14 weeks’ gestation; 18 to 32 years; 85% had planned pregnancy, at low risk of complications; 86% nulliparous Progress+ coding: None. |
|
| Interventions |
Control: Women were assessed in the clinic following a delay interval Intervention 1 (low feedback): Routine ultrasound at 16 weeks’ gestation in which women were unable to view the monitor screen, did not receive specific visual or verbal feedback, and they received a global evaluation of the form “all is well”. Intervention 2 (high feedback): Women were shown the monitor screen and provided with standardized visual and verbal feedback as to fetal size, shape, and movement. No clear smoking cessation component Main intervention strategy: Feedback (single intervention) compared to usual care. Intervention 1 (low feedback) compared to Intervention 2 (high feedback) in this review. Control group details only reported in associated reference, so no smoking outcomes available Intensity: Frequency (C = 0, I=1), Duration (C = 0, I = 1). Usual care intensity: F = 0, D = 0 Unclear whether dedicated project staff delivered the intervention or not |
|
| Outcomes | Self-reported smoking cessation at 16 weeks’ gestation (late pregnancy*), without biochemical validation. Self-reported reduction in smoking* | |
| Notes | Cites evidence for the reliability of self-report. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “assigned at random”. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition: 3/129 (2%) from low feedback group in smoking outcomes. But considerable amounts of missing data for some variables. Those lost to follow-up not included in ITT analysis, and unclear whether they were smokers at baseline so not re-included |
| Selective reporting (reporting bias) | Unclear risk | Data collected not specified. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation of quitting. |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Intervention with verbal feedback, so not feasible to blind women. State that those providing care were not involved in the study |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | 3/62 low feedback group did not attend next visit at 16 weeks |
| Equal baseline characteristics in study arms | Low risk | Data in Tables 1 and 2 seem similar. |
| Contamination of control group | High risk | Assuming same ultrasonographer providing intervention for control and intervention groups |
| Methods | Randomised controlled trial of a telephone counselling intervention to support women to stop smoking and prevent relapse during pregnancy and postpartum Study conducted in a network-managed care organisation and a group of 65 community based prenatal care practices Massachusetts, New England (USA), with recruitment from September 2001 to July 2004 |
|
| Participants |
Inclusion criteria: Pregnant smokers (at least 1 cigarette in the past 7 days), at least 18 years of age, 26 weeks or less gestation, willing to consider altering smoking during pregnancy, reachable by telephone, English speaking and expected to live in New England for the next year Exclusion criteria: Not further specified. Recruitment: Smokers initially identified on ‘Obstetric Risk Assessment’ form, yielded low recruitment so 65/140 obstetric or family practices agreed to refer patients and 35 sent in 1 or more referral forms. 1444 pregnant smokers were referred to the study and 665 assessed as eligible. 442/446 (66%) agreed to participate and were randomised (C = 222, I = 220) Baseline characteristics: Mean cigarettes per day before pregnancy: C = 20.8, I = 20.9; Current mean cigarettes per day: C = 10, I = 10.4; Partner smoking: C = 62%, I = 71% Mean age: C = 28.1, I = 28.9; Mean years education: C = 13, I = 13.1; White: C = 87%, I = 88%; Private health insurance: C = 70%, I = 75%. Depression in last month: C = 1. 3%, I = 1.3% Progress+ coding: None. |
|
| Interventions |
Control: In addition to usual care, the control group were mailed a validated pregnancy-tailored smoking cessation booklet, and their prenatal care providers were sent the ACOG smoking cessation practice guideline, with a reminder to address smoking at the participant’s visits. The enrolment call concluded with a trained counsellor providing brief smoking counselling (less than 5 minutes). Smokers who requested further assistance were referred to the Massachusetts telephone quitline Intervention: The intervention group received as for the control group, plus a series of telephone calls accompanied by additional mailed written materials. Each participant had a dedicated counsellor who offered up to 90 minutes of counselling during pregnancy and up to 15 minutes over the 2 months postpartum. The trained counsellor tailored the call to the participant’s needs, consistent with the 5-step smoking cessation guideline, and drew on social learning theory and the transtheoretical model of change, the health belief model, and the principles of motivational interviewing Main intervention strategy: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 4), Duration (C = 1, I = 3). Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence at 28 weeks to term (late pregnancy*), and 3 (0-5) months postpartum*. Also measured reduction in smoking (proportion >50% reduction in cigarettes per day*), sustained abstinence at both time-points, and number of quit attempts Self-efficacy and social support at baseline and follow-up. Concerns about weight gain reported in an associated reference (Berg 2008). Women’s satisfaction with the intervention. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated. |
| Allocation concealment (selection bias) | Low risk | Stated that recruiters were not aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: 21/442 (5%) were excluded from the analysis due to miscarriage (C = 10/220, I = 11/222). 113 women did not have final assessment due to refusal (22%), baby born before assessment or lost to follow-up, but were included in the final analysis (ITT analysis) and in this review (C = 209, I = 212). Missing data (up to 30%) for outcomes measured in the postnatal period |
| Selective reporting (reporting bias) | High risk | Not clear if all outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Salivary cotinine (<= 20ng/mL cut-off) confirmation in 66%, and those refusing to provide a sample were included as continuing smokers |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | All providers and women sent smoking cessation practice guideline |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Mean number of calls received was 5. |
| Equal baseline characteristics in study arms | High risk | Both groups were similar, though the intervention group had a significantly higher proportion of women who had made a quit attempt this pregnancy and had social support to quit from partner and significant differences in parity, gestation, and partner smoking |
| Contamination of control group | Low risk | Trained counsellors delivering intervention not usual care givers |
| Methods | Randomised controlled trial of counselling to support women to stop smoking in pregnancy and postpartum Study conducted at the University of Vermont, Burlington (USA), with recruitment from May 1984 to June 1987 |
|
| Participants |
Inclusion criteria: Pregnant women less than 25 weeks’ gestation, smoking at least 1 cigarette a day Exclusion criteria: Not further specified. Recruitment: Women receiving prenatal care from obstetricians and nurse-midwives, or residents through Maternal, Infant & Child clinic for under-insured or non-insured women, were randomly assigned (23% Medicaid in study). 775/808 (96%) smokers invited agreed to participate. 175/775 women spontaneously quit before their first visit and were randomised into a separate study of relapse prevention (C = 86, I = 89) (Secker-Walker 1995). 600 smokers randomised (C = 300, I = 300). Baseline characteristics: Mean cigarettes per day pre-pregnancy C = 25.1, I = 24.4. Mean cigarettes per day at first prenatal visit: C = 12.4, I = 14.1 Mean age: 24 years; Less than high school: C = 30.7%, I = 28.2%; Medicaid recipient C = 23.2%, I = 25.3% (50% private insurance) Progress+ coding: Low SES due to high rates of women who hadn’t completed high school |
|
| Interventions |
Control: ‘Usual advice about smoking provided by obstetrician or midwife’. Intervention: Counselling from a trained health educator who: addressed concerns re smoking and pregnancy, health benefits of stopping, perception of the advantages and disadvantages of stopping, problem solving around those issues and coming to a decision. If agreeing to quit and formulating a plan, women were provided with skills rehearsal and a pregnancy-specific booklet. Follow-up at second antenatal clinic, 36 weeks and 6-week check (where infant health and parental role modelling was discussed) and re-encouraged to quit. Health educators given selected readings, discussion, rehearsal with psychologist + health educator (both former smokers) about smoking and smoking cessation counselling techniques + American Lung Association training group for class leaders + 4-week pilot The relapse prevention component was individualised but carried out within a defined protocol. Counselling about preventing relapse and a booklet. Follow-up at second antenatal clinic, 36 weeks and 6-week check (where infant health and parental role modelling was discussed) Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 3). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Smoking cessation at 36 weeks’ gestation (75% biochemically validated with cotinine) (late pregnancy*), Long-term quitting measured at 8-15 months’ pp (6-11 months pp*) , 16-24 pp (18 months postpartum), and 25-54 pp (self-reported) Relapse prevention* reported in associated reference (Secker-Walker 1995). Mean birthweight*, low birthweight*, other smoking-related complications (PPROM, placental abruption and placenta praevia) Reduction in mean cotinine/creatinine ratio at 36 weeks’ gestation |
|
| Notes | Sample size calculated for 10% increase (from 10% to 20%) in quitting. No adjustment for misclassification. Recall of advice about smoking. Separate paper (Secker-Walker 1992) evaluates training program for residents. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Unclear when randomisation took place. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Smokers: Attrition 39/600 (6.5%) due to miscarriage (27), fetal deaths (7), infant deaths (5), 48 transferred care (C = 24, I = 24), and were excluded from analysis, leaving C = 258, I = 255 Further losses were: 41 dropped out of study (C = 4, I = 37), and 59 were lost to follow-up (C = 28, I = 31), but were re-included in this review as continuing smokers, but are not included in mean birthweight and other birth outcomes analyses. Significant difference in pregnancy dropout rates for I (13% drop-out rate ) and C (1.4% drop-out rate). Those lost to followup smoked more Voluntary drop-outs treated as continuing smokers for some analyses Spontaneous quitters: attrition 8/175 (5%) due to miscarriage (5), abortion (1), fetal demise (1), and infant death (1) and lost records (2) were excluded from analysis, leaving C = 80, I = 85. Further attrition: transferred care (15)-not reported by study arm, dropped out of study (9), lost to follow- up (8), re-included in baseline as continuing smokers in this review Differential withdrawal in I and C groups a concern; good information collected on drop-outs being different |
| Selective reporting (reporting bias) | Unclear risk | Data collected not specified. Only smoking outcomes reported |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine/creatinine ratio levels measured at 36 weeks (< 80 ng/mg) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention in antenatal clinics. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | All but 9 intervention women not lost to follow-up received all 3 counselling sessions before 36 weeks, and 89% received the postpartum 1 |
| Equal baseline characteristics in study arms | High risk | Mostly similar but women in intervention group tended to smoke more cigarettes at time of their first visit |
| Contamination of control group | Low risk | A separate health educator provided intervention. |
| Methods | Randomised controlled trial of a videotape to support women to stop smoking in pregnancy Study conducted in the offices of ‘University Associates in Obstyetrics and Gynecology’, in Burlington, Vermont (USA), with recruitment from November 1992 to April 1993 |
|
| Participants |
Inclusion criteria: Pregnant women smoking ‘an average of one or more cigarettes per day’ Exclusion criteria: Not further specified. Recruitment: Women recruited through University prenatal clinics where obstetricians and nurse-midwives provide private prenatal care, and residents provide prenatal care for under-insured women. 60/67 (89%) smokers who were invited agreed to participate and were randomly assigned (C = 30, I = 30) Baseline characteristics: Mean cigarettes per day before pregnancy = 22.6. Mean age: 23 years; 30% married; 33% had less than high school education; 98% white Progress+ coding: Low SES in this review as participants recruited from a state-supported clinic for underinsured women |
|
| Interventions |
Control: Advice from an obstetrician or nurse-midwife (as per prompt sheet) and a booklet on quitting. The protocol for this advice has been described in Secker-Walker 1992. Intervention: As for control plus a 29-minute videotape of 4 women going through the process of quitting during pregnancy; talking about feelings; coping with weight gain; getting support, which could be borrowed and taken home. Based on social learning theory Main intervention strategy: Counselling (single intervention) compared to a less intensive intervention Intensity: Frequency (C = 1, I = 2), Duration (C = 1, I = 2). Unclear if technological intervention provided by existing staff or dedicated project staff |
|
| Outcomes | Smoking cessation in late pregnancy* (36/40), biochemically validated with exhaled CO measurements Process evaluation included perceptions of the videotape contents |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/60 (7%) women, all in the intervention had a miscarriage and 7 (C = 2, I = 5) moved to another care-provider, and were excluded from the analysis 3 (C = 1, I = 2) lost to follow-up but were re-included in this review, leaving C = 28, I = 21. Loss to follow-up not balanced, greater loss from the intervention group |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Exhaled CO (<8 ppm) used to validate self-reported smoking cessation |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | 53% viewed the videotape. 17% had no VCR, and 10% reported having no time |
| Equal baseline characteristics in study arms | High risk | Mean exhaled CO level was significantly lower in intervention group |
| Contamination of control group | Low risk | Video tape unlikely to be provided to women in control group |
| Methods | Randomised controlled trial of a counselling intervention to support women to stop smoking in pregnancy and prevent relapse postpartum The study was conducted in offices of the ‘University Associates in Obstetrics and Gynecology’ in Vermont (USA), with recruitment from October 1988 to October 1992 |
|
| Participants |
Inclusion criteria: Woman who reported smoking 1 or more cigarettes per day at onset of pregnancy Exclusion criteria: Not further specified. Recruitment: Women recruited through the state-supported (Maternal and Infant Care) prenatal clinic for underserved women or attending the Adolescent clinic for women 12 to 18 years. 524/544 (96%) women who were invited agreed to participate and were randomised. 399 current smokers (C = 202, I = 197); 125 spontaneous quitters (C = 63, I = 62) (separate paper). Baseline characteristics: Smokers: Mean cigarettes per day before pregnancy C = 25.1, I = 26.1; mean cigarettes per day at first prenatal visit: C = 11.8, I = 13.4. Another smoker in the household (C = 82.6%, I = 78.5%) Mean age: 23 years, < high school (C = 41%, I = 48%), 27% married; medicaid recipients (C = 73.1%, I = 71.9%); Adolescent clinic (C = 13.5%, I = 11.9%) Spontaneous quitters: Mean cigarettes per day before pregnancy (C = 14.1, I = 13.5). Other smokers in household (C = 64%, I = 70%) Mean age: C = 21.9, I = 20.9; < high school (C = 27%, I = 36%); 29% married; Medicaid recipients (C = 68.1%, I = 65.1%); adolescent clinic (C = 14.9%, I = 11.4%) Progress+ coding: Low SES. |
|
| Interventions |
Control: Physician acknowledged women’s smoking, gave a rationale for quitting, strong recommendation to quit and provided smoking cessation booklet designed for pregnant women. All participants received: baseline questionnaire, measurement of exhaled CO, and brief standardised health risk message from a research nurse about the effects of smoking on the fetus and pregnancy. Intervention: A structured smoking cessation protocol provided by physicians trained in its use (Secker-Walker 1992) at 1st, 2nd, 3rd and 5th visits: acknowledging the woman’s smoking, her exhaled CO level, any progress towards quitting, rationale for and unambiguous recommendation to quit, asking how she felt about quitting and acknowledging her response, asking how she could be helped and telling her about the counsellor, eliciting a commitment to change smoking behaviour before the next prenatal visit and referring her to the counsellor. The aim was to gain her agreement to set a quit date, a date when she would quit for 24 hours or a date when she would cut her consumption by half. Counsellor advised women on ways to accomplish the behaviour change. 2nd, 3rd, 5th and 7th visit included praise for those who had quit with referral to counsellor for help in staying quit. 36 week visits included a briefer protocol followed with referral for those who wanted to change, praise for success and referral to a nurse counsellor if smoking Main intervention strategy: Counselling (multiple intervention) compared to less intensive intervention Intensity: Frequency (C = 1, I = 5), Duration (C = 1, I = 3). Intervention provided by existing staff, with referral to a counsellor: Effectiveness study |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence at 36 weeks’ gestation (late pregnancy *) and 1 year postpartum*. Mean cigarettes per day at 36 weeks’ gestation* and 12 months postpartum. Mean birthweight*. Low birthweight* Relapse prevention at 36 weeks’ gestation (late pregnancy*) and 12 months postpartum reported in associated reference (Secker-Walker 1998b) Preterm births* are reported in attrition and are re-included in both numerator and denominator for this outcome |
|
| Notes | Methods included a detailed process evaluation of participants’ views and recall of provider advice. Sample size justification Separate paper reports relationship between exhaled CO and birthweight (Secker-Walker 1997b) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition. More than 25% lost to follow-up in pregnancy and more than 30% lost to longer-term follow-up Smokers: 109/399 (27% attrition) 24 (6%) women with miscarriage (14), fetal demise (5) and infant deaths (5) were excluded from analysis and are not reported by group allocation. Report states 376 women remain included (instead of 375) (C = 191, I = 185) 68 women transferred care (C = 34, I = 34) , 17 delivered before 36 weeks (C = 8, I = 9) and were not included in 36-week analysis 12 women withdrew from study (C = 5, I = 7) and 3 lost to follow-up (C = 3), and were re-included as continuing smokers in this review, but are not included in mean cigarettes per day or perinatal outcomes. 114 (I) and 110 (UC) were contacted 1 year after birth, including 16 (I) and 18 (UC) lost to follow-up during pregnancy. Women with adverse outcomes were not included in the analysis Spontaneous quitters: 33/125 (26%) attrition. Women with miscarriage (5), abortion (1), infant death (1), pregnancy loss (1), moving to another clinic or moving (22; C = 13, I = 9), delivering before 36 weeks (I = 2). All excluded from analysis leaving C = 48, I = 44 |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported cessation with biochemical validation by exhaled CO (<6 ppm) or urinary cotinine (<500 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Intervention by clinic staff. Notes flagged. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Methods included a detailed process evaluation of participants’ views and recall of provider advice and suggests ‘to a large extent the intervention was implemented as planned’ |
| Equal baseline characteristics in study arms | Unclear risk | No significant differences except for larger proportion of women in intervention group had not made a quit attempt in the past |
| Contamination of control group | Unclear risk | No women in cessation group received cessation counselling beyond the physician advice. Though the same physician provided advice so unclear if this was influenced by the intervention |
| Methods | Randomised controlled trial of a multifaceted intervention to support women to stop smoking in pregnancy Study conducted in a large university hospital obstetric clinic in Baltimore (USA) with enrolment over a 2.5 year period (dates not specified) |
|
| Participants |
Inclusion criteria: Pregnant women who were smoking >= 10 cigarettes/day immediately prior to pregnancy, <18 weeks’ gestation Exclusion criteria: Not further specified. Recruitment: Eligible women sought by a variety of methods but majority were attending 1 of 52 private obstetricians or a hospital antenatal clinic. Obstetric staff sought permission for study staff to contact women. 935 women recruited (participation rate unclear) (C = 472, I = 463). 157/935 had spontaneously quit (C = 17% or 80, I = 16% or 74, which only add up to 154). Smoking rates among spontaneous quitters not reported separately so all randomised women included in analyses Baseline characteristics: Mean cigarettes per day pre-pregnancy: C = 20.7, I = 20.9; mean cigarettes per day at randomisation: C = 11.7, I = 10.7 Mean age 24.9 years, Mean education 12.3 years, Black C = 41.3%, I = 40.3% Progress+ coding: None. |
|
| Interventions |
Control: Usual care, not further specified. Intervention: At least 1 personal visit, supplemented by frequent mail and telephone contacts (at least 1 visit and 1 call/month) from 1 of 2 health educators (MEd level, trained in pregnancy counselling and smoking intervention), providing information, support, practical guidance and behavioural strategies for quitting. Information on quitting and health risks of smoking was mailed every 2 weeks with “homework” linked to telephone calls; group sessions were also available. There was a monthly lottery and in the last year of the study a monthly newsletter. Hypnosis was offered by discontinued as poorly accepted Main intervention strategy: Counselling (tailored) compared to usual care. Intensity: Frequency (C = 0, I = 6), Duration (C = 0, I = 6). Usual care intensity: F = 0, I = 0 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Self-reported smoking at eight months gestation (late pregnancy*) Mean cigarettes per day* at 8 months gestation and mean thiocyanate* Mean birthweight*; low birthweight*; very low birthweight*, perinatal deaths*, neonatal deaths*, stillbirths* % Apgar scores <7 at 1 minute and 5 minutes; length and head circumference |
|
| Notes | Change of criteria for enrolment after the first 185 as 35% of these had smoked < 10/day and 71% of that group had quit spontaneously with little relapse. Detailed account of the intervention is in Nowicki 1984. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: 56/935 (6%), 35 miscarriages (C = 17/572, I = 18/463), 1 fetal death (C = 1), 20 stillbirths (C = 11, I = 9) excluded from analysis, leaving C = 443, I = 436. Women lost to follow-up included as continuing smokers in this review. Missing data for mean outcomes not included |
| Selective reporting (reporting bias) | High risk | Extensive range of outcomes reported. Outcomes not reported separately for spontaneous quitters |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking outcomes were not validated by salivary thiocyanate, despite it being collected. Mean thiocyanate for each group reported only |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Group sessions in the intervention were not readily accepted |
| Equal baseline characteristics in study arms | Low risk | Groups ‘similar’ at time of randomisation. |
| Contamination of control group | Low risk | Specific personnel employed to deliver intervention - not usual carers |
| Methods | Randomised controlled trial of telephone peer support to help women stop smoking in pregnancy Study conducted in a large obstetric practice in Burlington, Vermont (USA), with recruitment from 1996 to 1997 |
|
| Participants |
Inclusion criteria: Women reporting smoking at least 1 cigarette in the past week at their first antenatal visit Exclusion criteria: Not further specified. Recruitment: 151/186 (81%) women approached agreed to participate and were randomised (C = 74, I = 77) Baseline characteristics: Mean cigarettes/day before pregnancy: C = 20.2, I = 22.6; Mean cigarettes per day at first visit: C = 9.8, I = 10.5. Mean exhaled CO: C = 11.3, I = 11.3. Mean other smokers in household: C = 1.5, I = 1.3 Mean age C = 23.7, I = 23.1; Mean years education: C = 11.5, I = 11.7; White: C = 96%, I = 94.8%. Medicaid recipient: C = 74.6%, C = 77.5% Progress+ coding: Low SES. |
|
| Interventions |
Control: Received brief smoking cessation advice (including encouraging a quit date) from a midwife or obstetrician at each of the 3 prenatal visits and stage appropriate printed materials. Midwives and obstetricians were provided with a 45 minute training session and protocol prompt sheets were placed in charts at first prenatal visits Intervention: Received the same as the control group, plus any women in the experimental visit who reported they possibly, probably or definitely intended to quit smoking were offered telephone peer support by the obstetrician/midwife. The telephone peer support was provided by a female ex-smoker, who received 8 hours of training. The support person called the participant within several days of referral to provide support, encouragement and reinforcement of positive changes in smoking behaviour. Ongoing calls typically occurred on a weekly basis, but more frequently around a quit date. On average calls lasted 10 minutes Main intervention strategy: Social support (tailored intervention) compared to a less intensive intervention Intensity: Frequency: (C = 3, I = 6), Duration (C = 1, I = 4). Unclear whether intervention provided by dedicated or existing staff |
|
| Outcomes | Biochemically validated 7-day point prevalence abstinence at 28-34/40 gestation (late pregnancy*) Proportion of smoking reduction by more than 50%* was reported for a proportion (135 women) but unclear how many had dropped out of intervention and control groups. As report states ‘no significant difference’ in dropouts by intervention group (total n = 16) we have imputed 8 for each arm and calculated the number of reductions from a proportion of the remaining sample Movement in stages of change also reported for this group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States participants were randomised into either experimental or control condition |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 16/151 (11%) attrition at follow-up. Unclear how many from each arm, so outcomes (> 50% reduction and SOC movement) reported as a proportion of those remaining were not able to be included. All randomised women were included in the primary outcome of smoking cessation, with those lost to follow-up treated as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine assessment at 28-34 weeks used to confirm smoking status (cut-off <80 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to allocation. Medical charts flagged and referral for social support required by care providers |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Process evaluation showed 53% received the peer intervention. 9 (12%) had low intentions of quitting smoking during pregnancy and were never offered the peer support, 9 (12%) had no home telephone and were not referred, and 15 (19%) refused the offering, leaving 44 (57%) who were referred for peer support. Data from log sheets completed by the telephone support person revealed that 3 women referred were never reached; therefore, only 53% of the women in the experimental condition received the peer support intervention |
| Equal baseline characteristics in study arms | Low risk | Baseline comparisons of women in the experimental and control conditions revealed no significant differences in demographics, pregnancy history, or smoking information |
| Contamination of control group | Low risk | Unlikely telephone counselling would have been provided to control group in error |
| Methods | Randomised controlled trial of intensive late pregnancy intervention to support ‘resistant’ smokers to stop smoking in pregnancy Study conducted in 3 large multispecialty clinics in Houstan and Dallas metropolitan areas, Texas (USA). Enrolment over a 17-month period, dates not specified |
|
| Participants |
Inclusion criteria: Women were screened for eligibility into 2 concurrent studies: Pregnant women who smoked more than 5 cigarettes per week prior to pregnancy, fluent in English, over 18 years, less than 20 weeks’ gestation at first prenatal visit. Women who continue to smoke at 28 weeks’ gestation, after having counselling and 8 self-help booklets earlier in pregnancy care, and had telephone access, were eligible for this study Exclusion criteria: Women who had quit smoking at 28 weeks (continuous abstinence for 28 days),were enrolled in a large trial to prevent postpartum relapse (Project PANDA) Recruitment: 6956 (99%) women completed intake screening. 1255 current and recent smokers received brief intervention in early pregnancy as described by Ershoff 1989. 522/1255 (42%) had transferred care, had fetal demise or abortion, were over 34 weeks’ gestation, or could not be reached. All 269/733 (37%)who reported continuing to smoke at 28 weeks and were randomised to this study, as data collection and implementation were adopted as routine procedures, and required no formal written consent (C = 135, I = 134) Baseline characteristics: > 61 cigarettes/week before pregnancy: I = 57.9%, C = 43%; Partner smoking: C = 62.5%, I = 69.6% Mean age: C = 28.1, I = 28.6; Married: C = 71.1%, I = 65.7%, White: C = 76.3%, I = 81.3%. < high school: C = 11%, I = 9% Progress+ coding: None. |
|
| Interventions |
Control: All women smoking at intake (< 20 weeks), were provided with MI counselling (3-5 mins) and a series of 8 motivational self-help books (first given in person and 7 mailed weekly thereafter), based on “stage of change” program as described by Ershoff 1989. Intervention: The high intensity intervention group (and their partners) then received: (i) a 20-30 min MI telephone counselling call (conducted by trained counsellors and nurse health educators), (ii) a personalised, stages of change based feedback letter, (iii) a final MI-based telephone call conducted 4-5 days after the feedback letter was sent The MI counselling calls were adapted from the Motivational Enhancement Therapy developed for Project MATCH (Miller 1992). Main intervention strategy: Counselling (multiple intervention) compared to less intensive intervention Intensity: Frequency: (C = 6, I = 6), Duration: (C = 1, I = 3). Intervention provided by dedicated project staff: efficacy study |
|
| Outcomes | Biochemically validated smoking cessation at 34 weeks’ gestation (late pregnancy*) Self-reported smoking cessation at 6 weeks, 3 months* and 6 months* postpartum Movement in “stages of change”. Breastfeeding rates and general health behaviours obtained but not reported Discussion of provider views. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 35% attrition for cotinine testing: 175/269 provided cotinine subsample (C = 82, I = 84). 39% attrition for 6 weeks postpartum follow-up All women lost to follow-up for cotinine validated smoking status at 36/40 were included in this review as continuing smokers. Analysis includes all randomised women |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine analysis (cut-off 80 ng/mL) for a subset of the sample at 34 weeks’ gestation, but women without cotinine validation were included as continuing smokers. Postpartum outcomes self-reported |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel likely to have been aware of group allocation, though no formal consent requested |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Described as “single blind” (cotinine analysis performed blind) |
| Incomplete implementation | High risk | Only 55% of the experimental group received the full intervention (32% were never able to be reached). Implementation analysis suggested an effect in women who received full implementation: 43% vs 34% control group |
| Equal baseline characteristics in study arms | High risk | Group differences were found on number of cigarettes smoked per week at baseline, but no differences in demographic variables |
| Contamination of control group | Low risk | Specific counsellors delivered the intervention. |
| Methods | Randomised controlled trial (pilot study) of motivational interviewing intervention to support women to stop smoking in pregnancy Study conducted in a university-based, public obstetric/gynaecology clinic (USA). Exact location and recruitment dates not reported |
|
| Participants |
Inclusion criteria: Pregnant women who reported smoking in the past 7 days who were at least 16 years of age, fluent in English, less than 28 weeks’ gestation Exclusion criteria: Not further specified. Recruitment: Women attending a university-based, public obstetric/gynaecology clinic. Unclear how many women were approached or eligible, though author communication reports challenges with recruitment. 54 women randomised (C = 28, I = 21, from author communication) Baseline characteristics: Not reported but discussion describes women as ‘socio-economically disadvantaged pregnant smokers’ Progress+coding: Low SES. |
|
| Interventions |
Control: Usual care, which in this university-based prenatal clinic included physicians or nurses acknowledging a pregnant woman’s reported smoking and recommending that she quit Intervention: MI intervention over the course of 8 weeks: (i) 1 face-to-face MI session; (ii) 3 MI-based telephone counselling calls; and (iii) 1 personalised feedback letter providing assessment results. MI incorporated specific counselling strategies, including personalized and objective feedback, to create a supportive, non-confrontational environment through which clients can resolve ambivalence and initiate change Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 2). Usual care intensity F = 1, I = 1 2 masters-level counsellors delivered the intervention: Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation at post-treatment assessment (late pregnancy*) Stages of change, processes of change, self-efficacy, decisional balance, and depression scores also reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States women ‘were randomized’ into an intervention or usual care condition |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes reported as percentages. 5 women excluded from the analysis (as per author communication) for which there was no data (C = 2, I = 3), so abstinent percentages are based on C = 5/28 and I = 3/ 21. These women were included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported, author communication states low recruitment so focused on other outcomes in this pilot study |
| Other bias | Unclear risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemically validated smoking cessation with salivary cotinine (cut-off > 20 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Not reported. |
| Equal baseline characteristics in study arms | Low risk | Not reported but author states “ Initial comparisons of socio-demographic and smoking history variables revealed no differences between the MI and UC groups” |
| Contamination of control group | Low risk | Unlikely as intervention delivered by specific counsellors. |
| Methods | 3-armed randomised controlled trial of personalised feedback during ultrasound and counselling to support women to stop smoking in pregnancy The study was conducted in Women, Infant and Child (WIC) clinics in Houston and Harris County Area, University of Texas Houston Medical School obstetric clinics and the local community (USA). Recruitment years not reported |
|
| Participants |
Inclusion criteria: Pregnant women reporting having smoked a cigarette in the past 7 days; age 16 years and older; English speaking, and gestational age between 16 and 26 weeks (to recruit later-pregnancy continuing smokers who have had the most difficulty stopping smoking for the pregnancy) Exclusion criteria: Not further specified. Recruitment: Via routine prenatal screening and widely distributed advertisements. 4, 258 women were screened. 360/725 (49.6%) of eligible women agreed to participate and were randomly assigned to 3 conditions: C (BP) = 120, I1 (BP + US) = 120, I2 (MI + US) = 120. Baseline characteristics: Mean number of cigarettes per day: C = 11.72 (8.73), I1 = 11.78 (9.47), I2 = 11.03 (8.14). Partner smoking: C = 68 (68), I1 = 82 (79.6), I2 = 76 (72.4). Baseline cotinine: C = 117, I1 = 116, I2 = 131. Mean gestational age: C = 23.63, I2 = 22.48, I2 = 21.12; Mean age: 24.65, I1 = 25.45, I2 = 25.21; Mean years education: C = 11.40, I1 = 11.37, I 2= 11.63; White: C = 65. 22%, I1= 57.02%, I2 = 49.57% (remainder African-American and Hispanic); Income <$US15,000/yr: C = 49.58%, I1 = 55.85%, I2 = 56.67%. Progress+ coding: Low SES. |
|
| Interventions |
Control (BP): Best Practice or “BP” counselling based on the Agency for Healthcare Research Quality practice guidelines for identifying patients who smoke and intervening for smoking cessation (5A’s and 5R’s). Nurses trained and instructed to keep counselling to 10-15 minutes. Participants were also given American Cancer Society literature on prenatal smoking cessation and the toll-free number for the quit smoking hotline Intervention 1: BP+ Ultrasound feedback sessions lasting approximately 30 minutes . In addition to providing routine ultrasound results, the ultrasound session was designed to provide information regarding the effect of cigarette smoke on the fetus using a motivational style. The sonographers received 2 hours of training and a laminated prompt card. Smoking risk messages were incorporated into discussion Intervention 2: BP+US+ Motivational Interviewing consisting of 1 45- to 50-min, face-to-face, individual counselling session conducted immediately after the ultrasound; 1 personalised feedback letter mailed 1 week later; and 1 follow-up counselling session conducted via telephone 2 weeks subsequent to the initial session, provided by master’s level counsellors. Elements of the transtheoretical model were included and smoking in the household and social networks were also addressed Main intervention strategy: Feedback (multiple intervention) compared to a less intensive intervention Intensity: Frequency: (C = 2, I = 4), Duration: (C = 1, I = 3). Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation at 8 months gestation (late pregnancy*) ‘Predictors of abstinence’ including: Stages of change, depression (Beck’s Depression Inventory), baseline smoking, ethnicity, and social networks reported | |
| Notes | Concerns about potential distress with the ultrasounds intervention were considered in a pilot study of 30 women (Groff 2005) indicated no significant increase in anxiety post-ultrasound | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomisation method, using blocks of 6 (2 per condition), was used to generate 360 slots, 120 per intervention group |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition:16/360 (4.4%), C = 6, I1 = 5, I2 = 5 (reasons not reported). Analyses were conducted using an ITT approach with all randomised participants included in the baseline and those lost to follow-up treated as continued smoking |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Self-reported smoking status biochemically validated using salivary cotinine (< 20 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Outcome assessor blinding not reported. |
| Incomplete implementation | Unclear risk | Procecss evaluation not reported. |
| Equal baseline characteristics in study arms | Low risk | Treatment group differences only for gestational age at baseline |
| Contamination of control group | Low risk | Low risk of contamination as counselling provided by specialist counsellors, not accessible to the control group |
| Methods | Randomised controlled trial of computer generated messages to support women to stop smoking in pregnancy Study conducted in 2 university hospitals in North Carolina and Michigan (USA), with recruitment from December 1996 to December 1997 |
|
| Participants |
Inclusion criteria: Women who have “smoked 100 cigarettes in their lifetime and still smoking” or “had quit since becoming pregnant” Exclusion criteria: Not further specified. Recruitment: Unclear how many women screened during first prenatal visit. using a self-administered computer screening program. 173 women randomised (C = 85, I = 88) Baseline characteristics: Mean cigarettes per day before pregnancy: C = 18.7, I = 20.3; current mean cigarettes per day: C = 11.8, I = 12.9; Mean cotinine: C = 2597, I = 2701; Mean smokers in household: C = 1.1, I = 1.0 Mean age: C = 26.6, I = 25.5; Mean education: C = 12.5, I = 12.5; White: C = 81.2%, I = 87.4% Progress+ coding: None. |
|
| Interventions |
Control: Received “a pregnant woman’s guide to quit smoking” at the first visit Intervention: Entered personal data into a hand-held computer at antenatal visits, which subsequently generated personalised tailored messages, which were posted to the woman Main intervention strategy: Health education (single intervention) compared to less intensive intervention Intensity: Frequency (C = I, I = 6), Duration (C = 1, I = 2). Unclear if intervention provided by dedicated project or existing staff as technological intervention |
|
| Outcomes | Biochemically validated smoking cessation at 6 weeks postpartum* (0-5 months pp) Biochemically validated cessation at 24/40 gestation (‘mid-term’) and self-reported cessation 3 months postpartum but outcomes not reported Mean cigarettes per day and cotinine concentrations collected and reported as ‘not significant’ but actual figures not reported Participant evaluation of using hand-held computers and reactions to computerised materials |
|
| Notes | Numbers in paper inconsistent: I = 88, C = 85 in methods section, I = 104, C = 87 in results section. No justification for change of denominators | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer algorithm. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Outcome data (C = 87, I = 104) are inconsistent with figures reported as randomised in methods and baseline data table (C = 85, I = 88). If comparing outcome data using ITT and excluding those ‘lost to follow-up’ it appears that more than 30% of the control group (30/87) were lost to follow-up. In this review we have used the ITT data (C = 87, I = 104) as the denominator |
| Selective reporting (reporting bias) | High risk | Results are conflicting and actual figures for pregnancy (24/40) are not reported, nor are figures for mean cigarettes per day or cotinine concentrations |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine analysis at 24 weeks’ gestation and at 6 weeks postpartum (cut-off < 80ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel not blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Process evaluation not reported. |
| Equal baseline characteristics in study arms | Low risk | Baseline comparisons revealed no significant differences in age, race, education, number of cigarettes smoked before pregnancy, and baseline stage of change |
| Contamination of control group | Low risk | Technological intervention so contamination unlikely. |
| Methods | Randomised controlled trial (pilot study) of home based motivational interviewing to support women to stop smoking in pregnancy Study conducted in a Glasgow Hospital, Scotland (UK), with recruitment from March to May 1997 |
|
| Participants |
Inclusion criteria: Women who identified as smokers on a questionnaire at antenatal clinic booking Exclusion criteria: Not further specified. 133/393 (34%) women screened identified as smokers and 100/133 (75%) agreed to participate and were randomised (C =5 0, I = 50) Baseline characteristics: Mean cigarettes per day pre-pregnancy C = 18.1, I = 19.6; current mean cigarettes per day C = 13.2, I = 14.8; partner smoking: C = 82%, I = 90%; Mean cotinine C = 126 ng/mL, I = 136 ng/mL Mean age: C = 25.9, I = 26.6; 76% ‘severely deprived’ participants Progress+ coding: Low SES. |
|
| Interventions |
Control: Received usual advice from their prenatal providers, which should include information about smoking Intervention: Received 2-5 motivational interviewing sessions (mean 2.6 hours), based on stages of change, in the clients’ home conducted by a midwife with 3 weeks training in smoking cessation counselling Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 4). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation at >=27/40 (late pregnancy*) Mean birthweight*, preterm births*, stillbirths*. Ranking interviews measured movement around the ‘cycle of change’ Detailed evaluation of participant and midwifery views of interventions |
|
| Notes | SDs for mean birthweight were not reported, therefore we calculated a mean SD from 13 studies with available birthweight SDs (578) to include in this review, as recommended by the cochrane handbook | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers stratified by deprivation. |
| Allocation concealment (selection bias) | Low risk | Group allocation by telephone. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Low attrition (2%). Some missing data for cotinine validation. Smoking outcome results reported for all of those randomised, and those with missing data counted as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | Detailed outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Serum cotinine levels measured. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | Good process evaluation of implementation quality according to rating tool, showed 79% of women in the intervention group received at least 2 counselling sessions |
| Equal baseline characteristics in study arms | Low risk | No apparent difference. |
| Contamination of control group | Low risk | Specific counsellors provided intervention at home so contamination unlikely. Less than 20% of the control group recalled being given smoking information at the time of booking |
| Methods | Randomised controlled trial of home-based counselling to support women to stop smoking in pregnancy Study conducted in 2 hospitals in Glasgow, Scotland (UK), with recruitment from March 2001 to May 2003 |
|
| Participants |
Inclusion criteria: Women reporting smoking at prenatal booking visit and less than or equal to 24 weeks’ gestation Exclusion criteria: Not further specified. Recruitment: 762/1684 (45%) eligible women agreed to participate (C = 411, I = 351) Baseline characteristics: Current mean cigarettes per day: C = 11.3, I = 11.7; At least 1 other smoker in house: C = 66%, I = 65% Mean age: C = 26.9, I = 26.5; Most deprived social category (6-7): C = 73%, I = 69% Progress+ coding: Low SES. |
|
| Interventions |
Control: Midwives provided standard health promotion including information on smoking in pregnancy from a book given to all women in pregnancy in Scotland Intervention: Women also were offered 2-5 additional home visits of about 30 minutes duration from the same study midwife Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency: (C = 0, I = 4), Duration (C = 0, I = 4). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Biochemically validated and self-reported quitting soon after the routine 36 week antenatal visit (late pregnancy*), reduction (mean cotinine*, self-reported*, and biochemically validated, which was at least half baseline measurement*), and increased smoking, mean birthweight*, preterm delivery*, very low birthweight*, low birthweight*, neonatal death*, stillbirths*, and admission to NICU* Data collected on other adverse events including antenatal admissions, miscarriage, termination of pregnancy, and assisted delivery Discussion of participant and provider views of intervention and thorough process evaluation showed good implementation |
|
| Notes | Sample size calculated by recruitment to achieve sufficient power not able to be achieved | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified central randomisation. |
| Allocation concealment (selection bias) | Low risk | Group allocation provided by central administrator. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 29/762 (4%) women lost to follow-up: fetal loss = 6 (C = 2, I = 4) were excluded from this analysis; no late interview or cotinine = 10 (C = 5, I = 5), Not traceable 12 (C = 7, I = 5). Some missing data for cotinine validation All randomised participants (except fetal losses) included in smoking outcomes, and those with missing data counted as continuing smokers |
| Selective reporting (reporting bias) | Low risk | Detailed outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Serum cotinine (cut-off <13.7 ng/mL) or salivary cotinine (cut-off < 14.2 ng/mL) used to validate self-reported abstinence |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Midwife intervention, with caregivers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | ‘A second administrator, blind to the random allocation, established a primary outcome’ |
| Incomplete implementation | High risk | 26% of women did not have any home visits. |
| Equal baseline characteristics in study arms | Low risk | No apparent major difference noted. |
| Contamination of control group | Low risk | Research midwives provided the intervention. |
| Methods | Randomised controlled trial of counselling intervention to support women to stop smoking and prevent relapse in pregnancy Study conducted in a large public antenatal clinic, in Rotunda Ireland, with recruitment during 3 months in 1995 |
|
| Participants |
Inclusion criteria: Women who ‘currently smoke’ or had spontaneously quit since becoming pregnant Exclusion criteria: Non-viable pregnancy identified at first visit or intending to deliver at another hospital Recruitment: 967/524 (54%) women attending the public clinic were smokers. 418/518 (81%) eligible women agreed to participate and were randomised (C = 209, I = 209) Baseline characteristics: Current smoker: C = 192, I = 203; Spontaneous quitter: C = 17, I = 6; 34% smoked more than 20 cigarettes per day currently; Partner smoking: C = 74%, I = 69.9% < 21 years age C = 17%, I = 24%; Mean gestation at first visit I = 15.5, C = 15.3; Not living with partner C = 39.2%, I = 42.6%; age finished education C = 16.1, I = 16.0; Lower social class C = 71.5%, I = 70.9% Progress+ coding: Low SES. |
|
| Interventions |
Control: Routine prenatal advice on a range of health issues, from midwives and obstetricians Intervention: As for the control group + (i) structured 1 to 1 counselling by a trained facilitator (based on stages of change theory); (ii) partners invited to be involved in the program; (iii) an information pack (developed in collaboration with a focus group of women), which included a self-help booklet; (iv) and invited to join a stop smoking support group. A CO monitor was available for the intervention group, to quantify smoking habit and act as a motivational tool Main intervention strategy: Counselling (tailored) compared to usual care. Intensity: Frequency: (C = 0, I = 5); Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Biochemically validated smoking cessation* and relapse prevention* at delivery (late pregnancy) and 3 months postpartum among baseline smokers* and spontaneous quitter. Mean cigarettes per day at delivery*, reduction in daily cigarettes since first visit, quit attempts, comparisons of quitters and non quitters at various stages. Infant outcomes at birth (singleton births): mean birthweight*, proportion LBW(2500 g)*, preterm births*, stillbirths*, neonatal deaths*, NICU admissions*, delivery type, mean gestation Infant outcomes at 3 months postpartum: neonatal deaths, attendance at GP; attendance or admission to hospital |
|
| Notes | Detailed process analysis and participant feedback of program implementation | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number tables with restricted randomisation in groups of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 31/418 (7%) attrition at delivery (I = 13/209 or 6.2%, C 18/209 or = 8.6%). Miscarriage (7), delivered elsewhere (3), moved overseas (2), changed care provider (7) or never returned to Rotunda hospital after first visit (12), and were excluded from this analysis All other women lost to follow-up counted as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Exhaled CO measurement on 145/209 women on postnatal ward (cut-off < 4 ppm). Presume smoking outcomes reported are those biochemically validated although this is not explicitly stated |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and study personnel to counselling intervention. Intervention provided by trained facilitator, with staff unaware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Detailed process evaluation describes how women rarely initiated contact at subsequent visits and the groups sessions were poorly attended |
| Equal baseline characteristics in study arms | High risk | Intervention group were less likely to have spontaneously quit, or be employed |
| Contamination of control group | Low risk | Research facilitator provided intervention. |
| Methods | Randomised controlled trial of a computer-delivered brief intervention ‘Video Doctor’ to support women to stop smoking in pregnancy Study conducted as part of ‘Health in Pregnancy’ study in 5 community prenatal clinics in San Francisco Bay Area (USA), with recruitment from 2006 to December 2007 |
|
| Participants |
Inclusion criteria: Pregnant women ‘smoking in the past 30 days’ who were English-speaking, 18 years or older, and less than 26 weeks pregnant Exclusion criteria: Not further specified. Recruitment: 1208 women were screened for eligibility in the prenatal clinic waiting rooms and 114 refused (91% participation in screening). 42/410 (10%) eligible women identified as smokers on a risk assessment using a laptop computer via a low-literacy computerised interview with audio voiceover, and were randomised (C = 19, I = 23) Baseline characteristics: Current mean cigarettes per day I = 6.8, C = 6.7. Mean age C = 26.8, I = 27.5; White C = 31.6%, I = 17.4% (remaining Hispanic, Back or ‘other’); Less than high school C = 21.1%, I = 26.1%; Married C = 26.3%, I = 47. 8% Progress+ coding: None. |
|
| Interventions |
Control: Received the clinic’s usual care and did not interact with the ‘Video Doctor’ program. All participants received a gift card ($30-$50) for completing assessments Intervention: Participants received tailored advice from ‘Video Doctor’, a multimedia interactive intervention delivered on a laptop computer via a secure Internet connection. An actor-portrayed Video Doctor delivered interactive risk-reduction messages designed to simulate an ideal discussion with a prenatal health care provider who provided non-judgmental counselling following several key principles of motivational interviewing. At the conclusion of each intervention session, the program automatically printed 2 documents: (a) a cueing sheet for providers, which offered a summary of the patient’s risk profile and suggested risk-reduction counselling statements; and (b) an educational worksheet for participants with questions for self-reflection, harm reduction tips, and local resources. The cueing sheet was placed in the patient’s medical record for the provider’s use during the prenatal appointment Main intervention strategy: Counselling (multiple intervention) compared to usual care Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 2). Usual care intensity: F = 0, D = 0 Technological intervention which prompted usual care providers: Effectiveness study |
|
| Outcomes | Self-reported 30-day abstinence after 1 month and 2 months (late pregnancy*). Mean reduction in cigarettes smoked per day and days smoked | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women reporting risks were stratified by risk combination and randomly assigned by the computer to intervention or usual care groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition: I = 5/23 (22%), C = 5/19 (26%) at 1-month follow-up and I = 9/23 (39%) , C = 13/19 (32%) at 2-month follow-up (reasons not reported) All randomised participants included in analysis and women lost to follow-up treated as continuing smokers in this review |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | Self-reported smoking cessation outcomes only - no biochemical validation of smoking status |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel as intervention includes counselling component |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Blinding of outcome assessor not reported. |
| Incomplete implementation | Low risk | Only 3 women in the usual care group did not recall receiving provider advice |
| Equal baseline characteristics in study arms | Low risk | Similar baseline characteristics. |
| Contamination of control group | Unclear risk | Some risk of contamination between study arms as same provider delivering counselling to intervention and control groups. Process evaluation showed 77.8% intervention group received 2 provider advice sessions, compared to 21.4% control group |
| Methods | 3-armed randomised controlled trial of contingent incentives to support women to stop smoking in pregnancy Study conducted in the Center for Addiction and Pregnancy Treatment, at the Johns Hopkins Bayview Medical Center, Baltimore (USA), with recruitment from May 2005 to January 2009 |
|
| Participants |
Inclusion criteria: Requiring methadone during pregnancy, nicotine dependent or smoking 10 or more cigarettes daily, aged 18 years or older, <= 30 weeks’ gestation, and capable of providing informed consent Exclusion criteria: Nicotine replacement therapy. Recruitment: 1072/1181 women screened smoked (90.7%). 125/1072 were eligible, and 102/125 (82%) agreed to participate, and were randomised to 3 conditions (C = 32, I1 (non-contingent incentives) = 28, I2 (contingent incentives) = 42). Baseline characteristics: Current mean cigarettes per day = 18.0. Mean age 30.8 years; 65% Caucasian; 11.1 mean years education; 85.3% currently single. 94.7% unemployed Progress+ coding: Low SES. |
|
| Interventions |
Control: As part of usual care, inpatients at the centre are provided with specific information about the adverse effects associated with cigarette smoking for the mother and the infant. In addition, patients are provided with educational materials about risks of smoking during pregnancy. During follow-up obstetric appointments, patients are asked routinely about their cigarette smoking and commended on efforts to abstain. TAU participants were informed that they would be compensated for providing urine and breath samples, but that they would not earn incentives as part of their study participation Intervention 1 (non-contingent incentives): Participants were informed that they had the chance to earn vouchers, but whether they earned a voucher and the amount they earned was determined by an already generated schedule and thus was not linked to their own cigarette smoking. NCBI participants were required to leave CO and urine samples to receive any voucher earnings generated by the ‘yoked’ schedule, for 12 weeks or until delivery Intervention 2 (contingent incentives): Incentives contingent upon cigarette smoking reduction or abstinence for a period of 12 weeks or until delivery. Smoking targets were minimal during the initial weeks of intervention, and increased gradually to ensure adequate learning and reinforcement. Incentives could be earned for each sample left on Monday, Wednesday and Friday (3 samples per week) if the following reduction and abstinence targets were met: week 1: any reduction; weeks 2-4: 10% reduction; weeks 5-7: 25% reduction; weeks 8-9: 50% reduction; week 10-11: 75% reduction; and week 12 until delivery: abstinence (CO < 4 ppm.). Participants had the opportunity to earn a $7.50 voucher for the first smoking reduction target, and the value of the voucher increased by $1/day for each consecutive target met throughout the 12-week incentive period to amaximum of $41.50. If a contingent participant failed to meet the tobacco use reduction target during the 12-week incentive period, she earned $0 for that sample and the incentive schedule was reset to the original voucher value of $7.50. If the participant again met the target reduction on 5 consecutive occasions, she earned vouchers at the previously attained level Main intervention strategy: Incentives (single intervention) compared to usual care. Contingent incentives compared to usual care in this review Intensity: Frequency: (C = 0, I = 6), Duration (C = 0, I = 5). Usual care intensity: F = 3, D = 2 Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated point prevalence abstinence after 12 weeks of intervention (late pregnancy*); 75% cotinine reduction (> 50% reduction*); mean cotinine*; mean cigarettes per day 1 and three months post intervention* and 6 weeks postpartum Mean birthweight*, preterm births*, low birthweight*, NICU admissions* Spontaneous abortion, length of hospital stay, mean gestational age at delivery, mean 1-and 5-minute Apgars, urine toxicology and treatment for NAS Comparisons with non-contingent incentives (arm 2) are also reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States patients were ‘randomly assigned’ to 1 of 3 conditions |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 33% attrition (34/102) for pregnancy and birth outcomes and no explanation as to reasons for missing data. Unclear whether all women randomised were included in the outcome assessment, as percentage results only are reported. Assume all persons not meeting ‘nonsmoking targets’ (p1872) are counted as continuing smokers |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appear to be reported, except smoking outcomes postpartum |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | CO sampling to evaluate changes during in-patient treatment phase and urine cotinine (cut-off 200 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and personnel to incentives intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not stated if outcome assessment was blinded. |
| Incomplete implementation | Low risk | This was a well accepted intervention with high rates of participation among all 3 conditions |
| Equal baseline characteristics in study arms | Low risk | The conditions did not differ significantly on demographic, pre-treatment or baseline cigarette smoking measures |
| Contamination of control group | Low risk | Unlikely given the design of the study. |
| Methods | Randomised controlled trial of ultrasound feedback and cognitive-behavioural modification, to support women to stop smoking in pregnancy Study conducted in the National University Hospital, Oslo, Norway (Europe), with recruitment from June 1990 to October 1991 |
|
| Participants |
Inclusion criteria: Pregnant women attending antenatal clinic for 18 weeks for ultrasound, and still smoking 10 cigarettes per day or more (heavy smokers) Exclusion criteria: Not further specified. Recruitment: Not stated how many women approached or eligible (1800 births/year, study over 15 months). 112 women randomised (C = 56, I = 56) Baseline characteristics: Mean cigarettes per day at 18 weeks’ gestation: C = 14.8, I = 12.5. Smoking partner: C = 80%, I = 74% Mean age: C = 28.4, I = 20.2. Progress+ coding: None. |
|
| Interventions |
Control: Routine 18-week ultrasound and information on the negative effects of smoking and encouragement to quit, reinforced by a pamphlet, provided at the time of the ultrasound examination. Intervention: At the time of the 18 week ultrasound scan, offered the Windsor self-help manual (translated into Norwegian) describing a 10-day program which includes relapse prevention. During ultrasound (by midwife and obstetrician) women were given information about the negative effects of smoking. 2 weeks later women were sent an encouraging reminder and an appointment for an additional 32-week scan by an obstetrician, in which women were further encouraged to quit. A second reminder was sent 2 weeks later Main intervention strategy: Feedback (multiple intervention) compared to usual care. Intensity: Frequency (C = 0, I = 3), Duration (C = 0, I = 2). Usual care intensity: F = 1, D = 1 Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Self-reported abstinence at delivery (late pregnancy*); self-reported reduction in smoking at birth* mean cigarettes per day at birth*. Stillbirths* reported in attrition and re-included in both numerator and denominator for this outcome | |
| Notes | Process evaluation suggested that the acceptance of the manual was low (mean score 2. 6 on 7 point scale) and that it was staff involvement which had the most impact | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “consecutively randomised”. |
| Allocation concealment (selection bias) | High risk | Women consecutively randomised into 2 groups. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition: one stillbirth in intervention arm excluded from analysis. 7 women who did not return questionnaires (C = 6, I = 1)were not included in the study report but have been re-included as continuing smokers in this review (C = 56, I = 55) |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Unclear risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not feasible to blind participants and providers to educational intervention and ultrasound. Although it is unclear if consent was sought so participants may have been blind |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | No process evaluation reported but assume most women received manual and ultrasounds |
| Equal baseline characteristics in study arms | Unclear risk | Intervention group had significantly higher daily smoking on entry |
| Contamination of control group | High risk | Usual care providers offering intervention and control components |
| Methods | Randomised controlled trial of hypnosis to support women to stop smoking during pregnancy Study conducted in Buskerud Central Hospital in Oslo, Norway (Europe), with recruitment from January 1992 to June 1993 |
|
| Participants |
Inclusion criteria: Women still smoking at 18 week ultrasound visit. Exclusion criteria: Not further specified. Recruitment: Expected numbers of pregnant smokers were 630. 158 (25%) agreed to participate and were randomised (78, I = 80) Baseline characteristics: Mean cigarettes/day prior to pregnancy I = 15.6, C = 15.0; Mean cigarettes per day at 18 weeks’ gestation C = 9.7, I = 11.3; Partner smoking C = 73%, I = 71% Mean age C = 26.5, I = 27.9. Progress+ coding: None. |
|
| Interventions |
Control: “Routine pregnancy health care”. Intervention: Anaesthesiologist provided 2 × 45 minute sessions at 2 week interval of a protocol-based script (Handbook of the American Society of Clinical Hypnosis); the tape played after hypnosis was established emphasised the unpleasant effects of smoking, affirmed her wish to quit, encouraged her will and capacity to quit, and instructed her in meeting cravings with relaxation techniques and self-hypnosis, explained during the session. Second visit tape was different with more weight on her capacity and taking control. Both tapes avoided “moralizing about her responsibility for pregnancy outcome” Main intervention strategy: Counselling (single intervention) compared to usual care. Intensity: Frequency (C = 0, I = 4); Duration (C = 0, I = 3). Usual care intensity: F = 0, D = 0 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Self-reported abstinence at birth (late pregnancy*), mean cigarettes per day at birth*, Self-reported reduction in smoking* (The SD used in the analysis in this review was calculated from a P value = 0.2 given in the paper) and increase at end of pregnancy, Perinatal deaths*. |
|
| Notes | Process evaluation did not rate the intervention highly: mean score of 2.05/7 | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The numbers from 1 to 100 were set up in random order, and by drawing lot, the women willing to participate were randomised into the intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | Women allocated to groups by drawing lots (it was not clear when this took place) |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Of 80 allocated to intervention 13 did not receive an appointment in time, and 15 did not attend, and were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Unclear risk | Not other bias’ detected. |
| Biochemical validation of smoking abstinence (detection bias) | High risk | No biochemical validation. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Psychological intervention, authors state that usual caregivers were not aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | 28/80 women randomised did not receive the intervention |
| Equal baseline characteristics in study arms | High risk | Significantly more smokers in intervention group at entry. |
| Contamination of control group | Low risk | Dedicated hypnotist provided intervention. |
| Methods | 4-armed cluster-randomised controlled trial of counselling interventions to support women to stop smoking in pregnancy Study conducted in primary health care clinics in Malaga, southern Spain, with data collection from 2001-2003 |
|
| Participants |
Inclusion criteria: 12/23 community clinics selected to balance neighbourhood SES (low, medium, and high). Women included if less than 15 weeks’ gestation and smoked at least 1 cigarette since knowing they were pregnant Exclusion criteria: not further specified. Recruitment: 12 clinics ‘randomly selected’, stratified by SES status of neighbourhood. 3 randomly allocated to each study arm, based on SES status (3 levels, low, medium, high: so 1 level each study arm). Clinics balanced across study arms Women identified in 1999 in a preconceptual program (2,932 women screened in 23 clinics-38% were smokers). 719 eligible smokers from the 12 clinics were invited, of whom 455 agreed to participate (63% participation). 132 women spontaneously quit smoking after baseline and 27 had a spontaneous abortion; both were excluded from the study. 296 women were randomised (C = 54, I1 = 71, I2 = 47, I3 = 124). Baseline characteristics: Mean cigarettes per day before becoming pregnant 20.6 (9. 58); Fagerstrom score: 4.78 (SD 5.38) 97.7% married. Education: 4% did not complete junior high school, 45% completed junior level only (9 years), 33% 12 years school, 17% university level. SES: 4.8% high, 24.6% medium/high, 53.4% medium/low, 17.1% low SES Progress+ coding: None. |
|
| Interventions |
Control: Usual care. All 3 interventions were based on CBT, adapted to pregnant women taking into account factors important to women for smoking and quitting, but differ in intensity (frequency and duration). Intervention 1 (low intensity): 1 session 30 minutes by midwives who were trained in smoking cessation psychosocial education, provided with audiovisual materials and gave women a pamphlet. Delivered in 2nd trimester, usually before week 24. Included smokers and those who had spontaneously quit. Able to invite companions or people involved in pregnancy to session. Session covered basic smoking risks and benefits of quitting, motivational therapy and CBT for self-control to quit smoking, self-monitoring, developing alternative behaviours, stimulus control, setting a quit date and how to obtain social support. Intervention 2 (medium intensity): I1+ additional 3 group sessions × 90 mins over 4 weeks in 3rd trimester (weekly and then after 15 days) in clinic. Provided by midwife with additional training. Reviewed homework, introduced topic of day, set objectives and activity to complete before the following week. Recommended that by second week they abstain from tobacco. Only pregnant women invited to groups (6-10 women in each group), no partners. Audiovisual materials and self help guide to support sessions. Intervention 3 (high intensity): I1+5 × 90 mins weekly group sessions in 3rd trimester provided by clinical psychologist. Midwife present in sessions. Reviewed homework, set objectives and goals etc (similar to I2), counselled to quit smoking on 4th week of program. Used audiovisual equipment. CO monitoring and feedback provided in 2nd session with motivational interviewing. Included relapse prevention. Companions not included in group sessions Main intervention strategy: Counselling (multiple intervention) compared to usual care. Intervention 3 (high intensity) and control (usual care) compared in this review Intensity: Frequency (C = 0, I = 6); Duration (C = 0, I = 5). Usual care intensity: F = 0, D = 0 Intervention provided by dedicated study staff: Efficacy study |
|
| Outcomes | Self-reported mean cigarettes per day in late pregnancy*; Mean exhaled CO; Mean birthweight* Biochemically validated point prevalence abstinence rates not reported. Breastfeeding rates at 8 weeks postpartum reported |
|
| Notes | Report in Spanish. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clinics described as ‘randomly assigned’. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 455 consented and 132 excluded as they spontaneously quit smoking, and further 27 excluded due to spontaneous abortion. Substantial attrition in this study (92% in I3): 296 randomised, 204 started intervention and 142 completed intervention and used in the analysis. Not able to be re-included as mean outcomes only reported (e.g. mean cigs/day, mean CO). Randomised : C = 54, I1 = 71,I2 = 47, I3 = 124. Started intervention: C = 54,I1 = 71, I2 = 12, I3 = 67 Completed intervention and analysed: C = 54, I1 = 71, I2 = 8, I3 = 9. |
| Selective reporting (reporting bias) | High risk | Biochemically validated smoking cessation rates, proportion of preterm births, and stages of change outcomes stated as primary and secondary outcomes and not reported |
| Other bias | High risk | Tried to balance women across study arms and clinics (40 per arm per clinic) but were unable to achieve this |
| Biochemical validation of smoking abstinence (detection bias) | Unclear risk | Exhaled CO validation measured but biochemically confirmed smoking cessation rates not reported |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | States clinics were not aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Outcome assessors blinded. |
| Incomplete implementation | Unclear risk | Only 8% completed the high and medium intensity interventions (group sessions) |
| Equal baseline characteristics in study arms | Unclear risk | Baseline characteristics not reported by individual study arm |
| Contamination of control group | Unclear risk | Cluster-randomised trial design minimises risk of contamination |
| Methods | Randomised controlled trial of a counselling intervention to support women to stop smoking in pregnancy Study conducted in a public hospital antenatal clinic in Newcastle, Australia, with screening from January 1990 to May 1991 |
|
| Participants |
Inclusion criteria: Pregnant women attending their first antenatal clinic appointment who answered yes to ‘Are you a smoker?”, were less than 26 weeks’ gestation, ill or psychologically unwell Exclusion criteria: Not further specified. Recruitment: 1,909 pregnant women were screened by midwives, 725 smokers (38%). 293/538 (54%) eligible women agreed to participate and were randomised (C = 145, I = 148) Baseline characteristics: Not reported. Progress+ coding: None |
|
| Interventions |
Control: Doctor and midwife both informed women that smoking was an important cause of pregnancy problems and they should stop; Midwife provided a package (sticker, pamphlet on risks of smoking and 2-page cessation guide), none of which were specifically tailored to pregnant women. Intervention (CBT): (i) 2-3 minute standardised risk information from Doctor. (ii) 14 minute video on risk information rebuttal of barriers to quitting, cessation tips and 10-minute standardised information (iii) Counselling from midwife after the video, using a flip chart, with negotiation of a quit date whenever possible (iv) Self-help manual on risks, barriers and cessation plus 4 packets of confectionary gum (v) Lottery chance (4 prizes) for biochemically validated abstainers at the next visit (vi) Social support from accompanying adult (partner/friend/other) via support tip sheet, contract and form letter, chart, reminder sticker in the medical record, form-letter and sticker from 1st visit Midwife mailed within 10 days + 2nd visit and 34 to 36 week visit 5 minute counselling from Midwife and 1-2 minute risk advice from Doctor. Women still smoking at 34-36 weeks were advised to attend an external cessation course Main intervention strategy: Counselling (tailored) compared to a less intensive intervention Intensity: Frequency (C = 2, I = 3); Duration (C = 1, I = 2). Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Biochemically validated point prevalence abstinence at 34 weeks’ gestation (late pregnancy*) and 6-12 weeks’ postpartum*. Preterm births* are reported in attrition and re-included in both numerator and denominator for this outcome Program costs and time commitments. Discussion of provider views and implementation issues in associated reference (Walsh 2000). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated. |
| Allocation concealment (selection bias) | Low risk | Described as “precoded questionnaires in manila envelopes”. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition 14% due to: Leaving clinic (C = 7, I = 7), miscarriage or termination (C = 10, I = 10), and preterm birth (C = 3, I = 4), leaving 252 included in analysis (C = 125, I = 127) 25% lost to follow-up and further missing data for some variables including cotinine validation, however those with missing data were treated as continuing smokers in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Urinary cotinine was measured and revealed discrepancy with self-reported smoking status. biochemically validated with salivary cotinine (I = 86%, C = 78%) Cotinine data inconsistent with self-report were 52% in controls and 12% in the intervention group |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Educational intervention by usual care providers and notes flagged |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | High risk | Midwives involved in recruitment to the trial had variable ‘success’ in consent rates (9%-76%). Overall participation was quite low (54%) |
| Equal baseline characteristics in study arms | Low risk | Report states baseline characteristics were equal on 12 variables tested |
| Contamination of control group | Unclear risk | Same care providers for both groups. |
| Methods | 3-armed randomised trial controlled trial (SCRIPT trial I) of interventions to support women to stop smoking in pregnancy Study conducted in public health clinics in Birmingham, Alabama (USA), from October 1983 to September 1984 |
|
| Participants |
Inclusion criteria: Pregnant women presenting for their first prenatal visit who reported smoking at least 1 cigarette in the last 7 days Exclusion criteria: >= 32 weeks’ gestation. Recruitment: 460/1838 (25%) pregnant women screened were current smokers. 368/460 (80%) agreed to participate. Unclear exactly how many randomised to each group as attrition not reported by study arm Baseline characteristics: No baseline data on cigarettes/day. Mean age: 23.6; Black: 57%; Mean years education 11.5. Progress+ coding: Low SES as attending public clinics. |
|
| Interventions |
Control: Smoking cessation advice routinely given at prenatal visits: 2-3 minutes within a group prenatal education session at the 1st visit, when maternity clinic staff recommend quitting. Intervention 1:10 minute standardised counselling session from a health educator (B Comm H Ed) + ALA “Freedom from smoking” (ALA) manual (17 day self-directed plan for quitting) + “Because you love your baby” pamphlet on the dangers and risk of smoking and the benefits of quitting. Intervention 2: as for I1 except that the manual was “A pregnant woman’s self-help guide to quit smoking” (instead of the ALA manual) Main intervention strategy: Counselling (multiple intervention) compared to usual care. Control and Intervention 2 compared in this review Intensity: Frequency: (C = 0, I = 1); Duration: (C = 0, I = 1). Usual care intensity: F = 1, D = 1 Intervention provided by dedicated study staff (health educators): Efficacy study |
|
| Outcomes | Biochemically validated point prevalence abstinence at mid-pregnancy, and during last month of pregnancy or within 48 hours of birth (late pregnancy*); and number of women who self-reported reduction in smoking in late pregnancy* | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition 29/338 (9%) due to: leaving system or moved (9), miscarriage or termination (10), and 10 who went to poorly attended group discussions (this intervention abandoned), leaving 309 included in analysis (C = 104, I1 = 103, I2 = 102). All other women lost to follow-up were treated as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Only smoking outcomes reported. |
| Other bias | Low risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of self-reported smoking cessation using salivary thiocynate <100 ug/mL |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | Educational intervention by health educators in antenatal clinics. Participants unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Unclear risk | “Multiple attempts were made to bring pregnant smokers together for a peer-led, focused group discussion: not feasible in this setting”. Pre-trial assessment showed no nurses (n = 80) had smoking cessation training and less than 20% felt confident to advise women on how to stop |
| Equal baseline characteristics in study arms | Low risk | Characteristics in study arms appear equal. |
| Contamination of control group | Low risk | Administered by trained health educators, not involved in pregnancy care |
| Methods | Randomised controlled trial (SCRIPT trial II) of a cognitive behaviour therapy intervention to support women to stop smoking in pregnancy Study conducted in 4 public maternity clinics of the Jefferson County Health Department in Birmingham, Alabama (USA), with recruitment from September 1987 to November 1989 |
|
| Participants |
Inclusion criteria: Pregnant women who self-reported smoking during the first prenatal visit ‘at least one puff of one cigarette in the last 7 days’ Exclusion criteria: >= 32 weeks’ gestation, did not stay for visit or did not return, prisoners, or had difficulty reading the baseline questionnaire Recruitment: 1171/4352 (27%) of women screened at first prenatal visit were current smokers and 210 (3%) spontaneous quitters (who were included in a separate trial: Lowe 1997). 994/1061 (94%) eligible women agreed to participate and were randomised (C = 501, I = 493) Baseline characteristics: Mean cotinine 114 ng/mL. 45% had low cotinine levels (< 99 ng/mL) Mean age = 24.6 years; Mean education = 12.4 years; Black = 52% Progress+ coding: Low SES in this review as attending public maternity clinic |
|
| Interventions |
Control: 2-minute talk on smoking in 30 minute group session at first antenatal visit in which women were urged to quit and given 2 pamphlets: “Smoking and the two of you”’+ “Where to find help if you want to stop” including the name, contact phone number and cost of their local program. Intervention: Based on cognitive behaviour therapy: (i) 15-minute standardised cessation skills and risk counselling session from trained female health education counsellor + 7-day self-directed cessation guide on how to quit written at 6th Grade level (ii) Clinic reinforcement (chart sticker) + letter from Doctor within 7 days (iii) Social support in form of a ‘buddy’ letter, contract and buddy tip sheet + monthly newsletter with testimonials, cessation tips and additional information on risks Main intervention strategy: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C = 1, I = 4), Duration: (C = 1, I = 3). Intervention provided by dedicated project staff: Efficacy study |
|
| Outcomes | Biochemically validated point prevalence abstinence at 4-8 weeks after first visit (midpoint), 32 weeks’ gestation (late pregnancy*). “Significant” reduction* if cotinine at least 50% value of baseline cotinine* Cost estimates. Separate trial reports data on spontaneous quitters (Lowe 1997). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition 180/994 (18%) due withdrawal from the service, miscarriage or abortion (C = 87, I = 93) were not included in analysis, leaving C = 414, I = 400 Further 15% lost to follow-up survey or cotinine analysis included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | Data on gestation and birthweight were collected but the published analysis is by stopping smoking and the timing of cessation rather than by allocation, so not included in outcome tables |
| Other bias | Unclear risk | No other bias detected. |
| Biochemical validation of smoking abstinence (detection bias) | Low risk | Biochemical validation of smoking status using salivary cotinine (cut-off >= 30 ng/mL) |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Notes flagged. Educational intervention. |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showed 100% implementation of counselling and social support, and 88% for re-inforecement at subsequent visits |
| Equal baseline characteristics in study arms | Low risk | NS difference in baseline cotinine. |
| Contamination of control group | Low risk | Trained counsellor, not pregnancy care provider, delivered the intervention |
| Methods | Randomised controlled trial (SCRIPT Trial III) of counselling intervention provided by routine care staff (effectiveness study) to support women to stop smoking in pregnancy Study conducted in 16 /67 counties providing Medicaid care in Birmingham, Alabama (USA). Counties matched by number of smokers and percentage Black and White women, and 1 county per dyad (n=8) randomly selected to participate in study. There were 10 prenatal care clinics and 28 regular staff members in the 8 counties selected. Recruitment dates not reported, but study conducted over 5 years | |
| Participants |
Inclusion criteria: Pregnant women who reported ≥1 cigarette (‘even one puff’) in the last 7 days, or had a cotinine level ≥20 ng/mL Exclusion criteria: Not further specified. Recruitment: 6,514 women were screened at first antenatal visit and 1340/1736 (77%) eligible smokers agreed to participate. 1 trial site dropped out leaving 1,093 who were randomised (C=546, I=547) Baseline characteristics: Cigarettes per day: C= 9.8 (&10.3 among drop-outs), I=10. 4 (&12.0 among dropouts); Lives with smoker: C=69.8 (&75.3% among dropouts), I=73.7 (&66% among dropouts). Mean cotinine: C=163, I=181 Mean age: 22 years; Black C=15.7%, I=15.4%. Progress+ coding: Low SES as Medicaid clinics. |
|
| Interventions | Staff orientation and assessment, and 3 hours SCRIPT training for staff in intervention sites Control: All participants received 4 elements of the “5A’s” best practice guidelines (Ask-Advise-Remind) Intervention: Participants received (Assist) Procedures 4 through 8: (i) A 14 minute ‘Commit to Quit Smoking During and After Pregnancy’ video (ii) A ‘Pregnant Woman’s Guide to Quit Smoking’ written at 6th grade reading level and includes a 10 day self-help guide for cessation (Windsor 1985), and (iii) A ≤10-minute counselling session (MI) Main intervention strategy: Counselling (multiple intervention) compared to a less intensive intervention Intensity: Frequency (C=2, I=2), Duration (C=1, I=2). Intervention provided by existing staff: Effectiveness study |
|
| Outcomes | Biochemically validated point prevalence abstinence in late pregnancy* (>60 days after first visit, and <90 days postpartum) Number with a “significant reduction” in cotinine* (>50ng/mL at baseline and <50% at follow up, quitters not included as significant reducers) An additional ‘historical’ control group also provides comparison pre and post intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as ‘randomly selected’ counties. Then “Smokers were randomly assigned at each clinic to an experimental group or control group after screening, consent, and baseline assessment” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition: C=97/546 (17%) and I=95/547 (17%). Reasons for drop-out not reported. An intent-to-treat policy was used in the computation of impact rates and all dropouts included as continuing smokers in this review |
| Selective reporting (reporting bias) | Unclear risk | Unclear if there was 1 or 2 assessments (i. e. 1 assessment between >60 days after first visit and <90 days post partum; or 2 ‘assessments performed >60 days after first visit, and <90 days postpartum’). Only 1 assessment reported. |
| Other bias | High risk | Figures in Table 1 (baseline, C=546, I=547) conflict with the outcome denominator in Table 2, which is reported to include those lost to followup (C=549, I=544). Figures reported in Table 1 used for denominator and Table 2 for numerator in this report |
| Biochemical validation of smoking abstinence (detection bias) | High risk | 72% self-reported quitters validated with biochemical verification (salivary cotinine <20ng/mL). 10% non-disclosure of smoking detected |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Participants and personnel not blinded to counselling intervention |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | Not reported. |
| Incomplete implementation | Low risk | Process evaluation showed reasonable implementation (over 80%) |
| Equal baseline characteristics in study arms | Unclear risk | Equal on all variables apart from mean cotinine (ng/mL) |
| Contamination of control group | High risk | Process evaluation suggests there was significant contamination of the randomised control group with regular clinic staff providing the intervention to both study arms |
AFP: alpha fetoprotein
ALA: American lung association
AN: antenatal
BP: blood pressure
C: control group
CBT: cognitive behavioural therapy
CI: Confidence interval
CO: carbon monoxide
GP: general practitioner
HMO: Health Maintenance Organisation
I: intervention group
ICC: Intracluster correlation co-efficient
ITT:intention to treat
LBW: low birthweight
MI: motivational interviewing
min: minutes
MRFIT: randomised trial of health promotion carried out in the US
NICU: neonatal intensive care unit
NNTB: number needed to benefit
NRT: nicotine replacement therapy
OPD: out-patient department
Pls: principal investigators
ppm: parts per million
PPROM: preterm, prelabour rupture of the membranes
SD: Standard deviation
SES: socioeconomic status
SHO: senior house officer
TFS: teen fresh start
TFSB: teen fresh start + peer support
UC: usual care
UK: United Kingdom
US: ultrasound
USA: United States
vs: versus
WIC: Food program for Women, Infants and Children in the US
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Albrecht 2011 | Program description only, not a randomised controlled study. |
| Andrews 2007 | Women included were not-pregnant, plus quasi-randomised study design |
| Berlin 2008 | Double-blind study of nicotine replacement therapy. |
| Boshier 2003 | Cohort study, not a randomised study design. |
| Bowden 2010 | Cohort study only, no control or comparison group. |
| Brandon 2012 | Part of the intervention is provided during pregnancy but primary aim of the study is to prevent relapse after pregnancy and post-partum outcomes only reported |
| Britton 2006 | Quasi-experimental design. Control and experimental convenience samples collected consecutively |
| Chan 2005 | Controlled observational study of Bupropion for smoking cessation in pregnancy |
| Coleman 2007 | Randomised controlled trial of pharmacological intervention with equal psychosocial support in both arms |
| Culp 2007 | Controlled trial/evaluation of “The Community-Based Family Resource and Support” (CBFRS) Program. Control group not randomised |
| DeVries 2006 | Quasi-cluster-randomised study with inadequate sequence generation (40 practices selected with matched controls) |
| Disantis 2010 | Non-randomised postpartum intervention to promote smoking cessation and breastfeeding |
| Dixon 2009 | Longitudinal cohort study only. |
| Edwards 2009 | Evaluation of ‘SMART moms’ project, which has no control group |
| El-Mohandes 2013 | Randomised-controlled trial of pharmacological interventions (nicotine replacement therapy) with equal psychosocial support in both study arms |
| Emmons 2000 | Controlled trial/evaluation of the “Healthy Baby Second Hand Smoke Study” uses historical controls. Good documentation of implementation problems |
| Ershoff 1983 | The intervention took place in 1 HMO clinic with historical controls from the same clinic and concurrent controls from a second clinic. There was no randomisation of clinics and no adjustment of the data for clustering |
| Everett-Murphy 2010 | Evaluation of smoking cessation counselling using a historical control group only (pre-post study design, not randomised and no contemporary control group) |
| Ferguson 2012 | Pregnant women excluded from this study (non-pregnant study population) |
| Ferreira-Borges 2005 | Pre-test post-test control group design (not randomised). |
| Fish 2011 | Intervention aimed at partners of pregnant women only. Pregnant women not included in the intervention |
| French 2007 | Controlled clinical trial of postpartum relapse prevention. Excluded as not a trial during pregnancy, and not randomised |
| Gadomski 2011 | Evaluation of ‘The BABY and ME-Tobacco Free’ program for relapse prevention postpartum. Quasi-experimental design with non-randomised control group (matched randomly selected controls) |
| Gebauer 1998 | Study of effect of one 15-minute counselling session and a follow-up telephone call, performed 1994-95, using historical controls from 1993-1994 |
| Gillies 1987 | In this controlled clinical trial the intervention was carried out in 1 hospital with another hospital in the same city acting as a control, after a prior descriptive study which showed the similarity between the 2 in terms of social and demographic factors including smoking. There was no randomisation and recruitment differed substantially across the 2 sites. Data for smoking reduction and smoking cessation are combined in the paper with no separate data on cessation and no adjustment for clustering |
| Grange 2005 | Cohort study design. |
| Hahn 2005 | Controlled trial with a volunteer sample of non-pregnant contest registrants, compared with a randomly selected group of smokers not exposed to the campaign/contest. Context registrants not randomised and there is evidence of differences between groups |
| Hannover 2008 | Counselling intervention aimed at relapse prevention postpartum only. Screened for participation during birth admission |
| Herbert 2011 | Intervention to reduce ‘Environmental Tobacco Smoke’ exposure aimed at postpartum relapse prevention only |
| Higgins 2004 | Pilot study with 37/53 participants consecutively assigned (not randomised) |
| Hotham 2006 | Randomised controlled trial of pharmacotherapy (nicotine replacement therapy) with equal psychosocial support in both study arms |
| Hymowitz 2006 | Postpartum trial only which measures paediatrician implementation of smoking cessation and relapse prevention interventions |
| Jaakola 2001 | Controlled study, not randomised, of effects of a population-based smoking cessation programand its impact on smoking in pregnancy. Controls were matched on inclusion criteria from another district |
| Johnston 2011 | Cohort smoking data from a randomised controlled trial of maternal vaccines |
| Kaper 2006 | Non-pregnant population. |
| Kapur 2001 | Randomised controlled trial of pharmacotherapy with equal psychosocial support in both study arms |
| Karatay 2010 | Evaluation of a motivational interviewing intervention with no control group |
| Kazemi 2012 | Intervention aimed at partners of pregnant women only to reduce passive tobacco smoke exposure for pregnant women in Iran |
| Kientz 2005 | Unable to determine number allocated to each trial arm and unclear what happened if unequal flip of coin |
| Koren 2009 | Randomised controlled trial of pharmacotherapy with equal psychosocial support in both study arms |
| Langford 1983 | Prenatal classes, rather than individual women, were randomly allocated to provide the intervention or not. The intervention was provided in late pregnancy with no outcome data collected during pregnancy but only data 4 months after birth. There was no adjustment for cluster-randomisation in the analysis of the study findings |
| Lee 2008 | Intervention aimed at partners of pregnant women only to reduce passive tobacco smoke exposure for pregnant women in China |
| Loke 2005 | Intervention aimed at smoking cessation in men (partners of pregnant women) |
| Lowe 1998a | Quasi-randomised study with inadequate sequence generation (allocation by alternate clinic weeks) |
| Lowe 1998b | Quasi-randomised study with inadequate sequence generation (allocation by alternate clinic weeks) |
| MacArthur 1987 | Quasi-randomised study with inadequate sequence generation (allocation by date of clinic visit) |
| Mauriello 2011 | Formative research only for a non-randomised intervention with no control group |
| Miller 2003 | A pilot study of a pharmacological intervention (Bupropion). |
| Mullen 1997 | Study designed to promote postpartum smoking cessation (not antepartum or part of a trial conducted in pregnancy) |
| Murray 2008 | Intervention to promote smoking cessation among a general (not specifically pregnant) primary care population |
| O’Connor 1992 | Quasi-randomised study with inadequate sequence generation (alternate allocation according to day of week) |
| Oncken 2008 | Randomised controlled trial of pharmacotherapy (nicotine replacement therapy) with equal psychosocial support in both arms |
| Peden 2008 | Quasi-randomised study with sequential allocation to study arms |
| Phillips 2012 | Intervention aimed at post-partumrelapse prevention only. Mother’s were recruited during infant’s admission to NICU |
| Polanska 2011 | Observational cohort study only with no comparison group. |
| Pollak 2007 | Randomised controlled trial of pharmacotherapy (nicotine replacement therapy) and equal psychosocial support in both arms |
| Power 1989 | The intervention in this trial was unusual in that the focus was on anticipated benefits of smoking cessation to women themselves (not on harm to the fetus and infant), and on alternative coping strategies, with a designated midwife-facilitator to answer queries and provide friendly advice and encouragement. The intervention was carried out in 1 hospital with another being a comparison setting, after a prior study which showed the similarity between the 2 in social and demographic factors including smoking rates. There was no randomisation. Recruitment differed significantly across the 2 hospitals. Data for smoking cessation and smoking reduction are combined with no separate data on cessation and no adjustment for clustering |
| Ratner 1999 | Postpartum intervention only. No interventions in pregnancy. |
| Reitzel 2010 | Intervention aimed at postpartum relapse prevention only. |
| Rush 1992 | Quasi-experimental study with inadequate sequence generation (group allocation by alternate weeks) |
| Scott 2000 | This controlled clinical trial of the impact of using interactive software to promote smoking cessation, was excluded as it used historical controls |
| Shakespeare 1990 | Not a smoking in pregnancy intervention. |
| Stanton 2004 | Intervention aimed at partner’s of pregnant women only. Aim was to maximise potential of life-changing period for men too. Did not include pregnant women |
| Suplee 2004 | Randomised trial of relapse prevention counselling in the postpartum period only (not pregnancy) |
| Sutton 2007 | Intervention of tailored smoking cessation letters, self-help materials and counselling for the general population (not specifically pregnant women) |
| Valanis 2001 | This prospective controlled clinical trial design to test the effect of a low intensity intervention, used historical controls |
| Valbo 1991 | Quasi-experimental study with inadequate sequence generation (3 months consecutive recruitment for each arm) |
| Wadland 2007 | General study population (not pregnant). Implementation trial to change provider behaviour and increase referrals to quitline. Estimated smoking cessation outcome data only |
| Wiggins 2004 | Cluster-randomised controlled trial comparing 2 postnatal interventions to improve maternal health |
| Wilkinson 2010 | Quasi-experimental design with a non-randomised controlled pre-post test study design |
| Windsor 2000a | Quasi-experimental study with inadequate sequence generation (80% control group not randomly assigned) |
| Winickoff 2010 | Intervention aimed at postpartum relapse prevention only with women recruited during birth admission |
| Wisborg 1998 | This randomised study of the effect of midwifery training on smoking cessation intervention implementation and pregnancy outcomes, was excluded due to concerns about allocation concealment (clinic day allocation) |
| Wisborg 2000 | Randomised controlled trial of a pharmacological intervention (nicotine replacement therapy) and equal psychosocial support in both study arms |
| Yilmaz 2006 | Postnatal intervention in pediatric setting. |
HMO: Health Maintenance Organisation
NICU: neonatal intensive care unit
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Not stated. |
| Methods | Cluster-randomised controlled trial. |
| Participants | Pregnant women attending antenatal care in Argentina and Uruguay |
| Interventions | A multifaceted intervention to implement the “5A’s” strategy |
| Outcomes | Provision of smoking advice and smoking abstinence. |
| Starting date | Not stated. |
| Contact information | F. Althabe: Department of Mother and Child Health Research, Institute for Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina |
| Notes |
| Trial name or title | Not stated. |
| Methods | Randomised clinical trial. |
| Participants | Pregnant women smoking at least 1 cigarette each day attending 4 clinics in Madrid, Spain |
| Interventions | Brief counselling (3 to 5 minutes) on smoking cessation compared with a group intervention over 3 half-hour sessions |
| Outcomes | Not clear. |
| Starting date | Not clear. |
| Contact information | meliton65@eresmas.com No response from authors to written request for further trial information on 18/7/2012 |
| Notes | Original article in Spanish. Study report (2004) describes the study design. No papers including results have yet been identified |
| Trial name or title | Not stated. |
| Methods | Ongoing study of intervention to promote smoking cessation among men and women during pregnancy |
| Participants | Pregnant women and their partners. |
| Interventions | Not clear. |
| Outcomes | Not clear. |
| Starting date | Not clear. |
| Contact information | everettk@health.missouri.edu Minimal study information provided in response to email request sent 18/7/2012 |
| Notes |
| Trial name or title | Reducing ETS exposure of pregnant women and newborns. |
| Methods | Randomised 2-arm study in 6 prenatal clinics designed to develop and evaluate the efficacy of 5 tailored DVDs in reducing exposure to ETS among low-income pregnant/postpartum women |
| Participants | Pregnant women who attend first prenatal visit by 16 weeks’ gestation who are exposed to tobacco smoke daily. Exclusion criteria: women expecting complications or multiple births |
| Interventions | Provision of tailored DVDs to take home. |
| Outcomes | Salivary cotinine concentration of mother and baby. |
| Starting date | Feb 2006 |
| Contact information | Thomas M Lasater, Brown University, Rhode Island. email: thomas_lasater@brown.edu |
| Notes |
| Trial name or title | M-SCOPE |
| Methods | Randomised controlled trial which aims to test whether offering Greek pregnant smokers a high intensity intervention increases smoking cessation during pregnancy, when compared to a low intensity intervention |
| Participants | Pregnant women smoking more than 5 cigarettes per week recruited in the second trimester of pregnancy |
| Interventions | The control group will receive 5 mins of brief advice and a leaflet, while the intervention group will receive 30 minutes of counselling by a trained health professional (based on 5A’s) and a self-help manual |
| Outcomes | Biochemically validated smoking cessation at end of pregnancy and 6 months postpartum, infant birthweight, gestational age and other health-related complications in pregnancy |
| Starting date | November 2009 to June 2012. |
| Contact information | vardavas@hsph.harvard.edu |
| Notes | Preliminary results reported in an abstract published in ‘Chest’ were provided in response to written request for further trial information sent on 18/7/2012. However these outcomes were not reported in sufficient detail to be included in this review |
| Trial name or title | An RCT protocol of varying financial incentive amounts for smoking cessation among pregnant women |
| Methods | RCT (pilot). |
| Participants | 90 consenting pregnant women. |
| Interventions | 2 intervention arms will be assessed: (1) a $AUD20 incremental personal financial incentive; and (2) a $AUD40 incremental personal financial incentive. Women from both intervention groups will have an opportunity to receive a PFI at 8 study intervention sessions contingent upon smoking abstinence |
| Outcomes | (i) consent rates; (ii) loss to follow-up rates of study participants and (iii) participant compliance with saliva and hair cotinine analyses for biochemical validation of smoking status. Womens perceptions of the intervention will also be ascertained by 6 interview questions |
| Starting date | Not clear. |
| Contact information | marita.lynagh@newcastle.edu.au |
| Notes | Australian New Zealand Clinical Trials Registry (ANZCTR) number: ACTRN12612000399897 |
| Trial name or title | Nurse Family Partnership in Dutch preventive health care. |
| Methods | Randomised controlled trial. |
| Participants | High risk pregnant women. The VoorZorg program target’s women that definitely need support: most have 4 or more risk factors such as poverty, (sexual) violence in the past or present relationship, no support of a network and alcohol- or drug abuse |
| Interventions | VoorZorg: The primary aim is to reduce child abuse and other goals are to improve health outcomes in pregnancy. It is based on Bandura’s Self-Efficacy Theory; Brofenbrenner’s ecological model, and Bowlby’s Attachment theory. Similar to intervention by Olds 1984 in the USA. Voorzorg consists of approximately 10 nurse home visits during pregnancy, 20 during the first year of the child’s life and 20 during the second year of the child’s life. The duration for each visit in 1.5 hours and nurses use manuals. Incentives provided for participation in study |
| Outcomes | Smoking cessation. |
| Starting date | Not stated. |
| Contact information | crijnen@xs4all.nl No response to written request for further information sent to trial authors on 18/7/2012 |
| Notes |
| Trial name or title | Building Blocks - a trial of home visits for first time mothers |
| Methods | Individually randomised controlled trial. |
| Participants | First time pregnancy: 1. Women aged 19 years or under (at recruitment/consent) 2. Lives within the catchment area covered by the local family nurse partnership (FNP) team 3. First pregnancy confirmed by health services (including those expecting multiple birth) unless previous pregnancy ended in miscarriage, stillbirth or termination 4. Recruited no later than 24 weeks. 5. Gillick competent to provide adequate informed consent to research participation including competence in English at conversational level or higher |
| Interventions | This trial will assess the effectiveness of the FNP in England compared with existing universal services |
| Outcomes | Primary: 1. Changes in prenatal tobacco use (maternal measure), measured at baseline and 34 - 36 weeks’ gestation interviews 2. Birthweight (child measure), measured at birth (collected afterwards) 3. Emergency attendances/admissions within 2 years of birth, measured at all timepoints 4. Proportion of women with a second pregnancy within 2 years of first birth, measured at all timepoints Secondary: 1. Intention to breastfeed 2. Prenatal attachment 3. Injuries and ingestions 4. Breast feeding (initiation and duration) 5. Language development 6. Education 7. Employment 8. Income/benefits 9. Home (tenure) 10. Health status 11. Self-efficacy 12. Social support 13. Paternal involvement |
| Starting date | Not clear. |
| Contact information |
Dr Mike Robling: Associate Director South East Wales Trials Unit Department of Primary Care and Public Health 7th Floor Neuadd Meirionnydd Cardiff University Heath Park http://www.cardiff.ac.uk/medic/subsites/buildingblocks/index.html |
| Notes | ISRCTN23019866 |
| Trial name or title | Not stated. |
| Methods | Randomised controlled trial. |
| Participants | 302 low-income pregnant women less than 28 weeks pregnant, English or Spanish-speaking, and who were not receiving inpatient drug treatment were recruited from multiple obstetric sites in the Boston metropolitan area (USA). Current smokers or women smoking in the past 3 months (recent quitters) were included |
| Interventions | Motivational interviewing interventions to promote smoking cessation and reduce environmental tobacco smoke exposure provided during 3 home visits, with feedback provided about the household nicotine levels |
| Outcomes | Smoking cessation at end of pregnancy and relapse prevention; infant health outcomes; life-years and quality of life; primary cost data and economic analysis |
| Starting date | 1997-2000 |
| Contact information | jennifer.ruger@yale.edu |
| Notes | Written request for further trial information sent 18/7/2012, but advised that results were not yet available |
| Trial name or title | Cessation in Pregnancy Incentives Trial (CPIT). |
| Methods | Individually randomised controlled trial. |
| Participants | 600 pregnant smokers identified at maternity booking who, when contacted by specialist cessation services, agree to having their details passed to the NHS Smokefree Pregnancy Study Helpline to discuss the trial |
| Interventions | Standard care plus the additional offer of financial voucher incentives to engage with specialist cessation services and/or to quit smoking during pregnancy £50 for attending a face-to-face appointment with their NSPS adviser and setting a quit date; £50 if quit 4 weeks after their quit date corroborated by a carbon monoxide breath test result less than 10 ppm collected by a research nurse; £100 if quit after 12 weeks corroborated by a carbon monoxide breath test collected by a research nurse; £200 if they self-report quit for at least 2 months when contacted for primary outcome assessment by the Helpline at 34 to 38 weeks’ gestation |
| Outcomes | Self-reported smoking in late pregnancy verified by cotinine measurement |
| Starting date | Recruitment started in December 2011. On 9 June 2012, 199 of 600 were enrolled in the 12 month trial |
| Contact information | David Tappin: david.tappin@glasgow.ac.uk
Paediatric Epidemiology and Community Health Unit, Section of Child Health, Division of Developmental Medicine, Glasgow University, Yorkhill Campus, Glasgow G3 8SJ, Scotland, U.K |
| Notes | Current Controlled Trials ISRCTN87508788 |
| Trial name or title | Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial |
| Methods | Individually randomised controlled trial. |
| Participants | Pregnant women who smoke at least 1 cigarette a day (and at least 5 cigarettes a day before pregnancy), and are between 10 and 24 weeks pregnant |
| Interventions | Supervised exercise on a treadmill plus physical activity consultations |
| Outcomes | Self-reported and biochemically validated continuous abstinence from smoking between a specified quit date and the end of pregnancy |
| Starting date | The LEAP trial began recruiting patients in April 2009, and recruitment will close in November 2012 Data collection for the primary outcome is due to be completed in July 2013. As of October 2nd 2012, 768 women were recruited |
| Contact information | Michael Ussher: mussher@sgul.ac.uk
Division of Population Health Sciences and Education, St George’s University of London, Cranmer Terrace, London SW17 ORE, UK |
| Notes | ISRCTN48600346 |
| Trial name or title | Telephone intervention (California Smokers’ Helpline) or pregnant smokers |
| Methods | Randomised trial. |
| Participants | Pregnant smokers who called the helpline for services. |
| Interventions | Control group received a self-help quit kit of written materials, including the American Cancer Society booklet for pregnant smokers. Intervention group received the quit kit plus up to 7 counselling calls |
| Outcomes | Self-reported smoking cessation in third trimester. |
| Starting date | |
| Contact information | Shu-Hong Zhu 2004, University of California. szhu@ucsd.edu |
| Notes | Author emailed 2008, advised that results would not be available until publication. No response to written request for further trial information on 18/7/2012 |
ETS: environmental tobacco smoke
DATA AND ANALYSES
Comparison 1.
Smoking cessation interventions: counselling vs usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 27 | 11979 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [1.19, 1.75] |
| 1.1 Single interventions | 10 | 3753 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.89, 1.42] |
| 1.2 Multiple interventions | 11 | 4407 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [1.15, 2.21] |
| 1.3 Tailored interventions | 6 | 3819 | Risk Ratio (M-H, Random, 95% CI) | 1.49 [1.01, 2.20] |
| 2 Abstinence in late pregnancy: biochemically validated only | 18 | 9250 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [1.03, 1.50] |
| 2.1 Single interventions | 7 | 3413 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 2.2 Multiple interventions | 7 | 3860 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [0.94, 2.04] |
| 2.3 Tailored interventions | 4 | 1977 | Risk Ratio (M-H, Random, 95% CI) | 1.42 [0.84, 2.41] |
| 3 Continued abstinence (relapse prevention) in late pregnancy for spontaneous quitters | 8 | 688 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 3.1 Single interventions | 2 | 100 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.93, 1.07] |
| 3.2 Multiple interventions | 3 | 297 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.93, 1.26] |
| 3.3 Tailored interventions | 3 | 291 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.97, 1.46] |
| 4 Abstinence at 0 to 5 months postpartum | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Single interventions | 5 | 1164 | Risk Ratio (M-H, Random, 95% CI) | 1.52 [1.13, 2.05] |
| 4.2 Multiple interventions | 4 | 1097 | Risk Ratio (M-H, Random, 95% CI) | 2.32 [1.44, 3.72] |
| 4.3 Tailored interventions | 1 | 367 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.80, 0.97] |
| 5 Abstinence at 6 to 11 months postpartum | 6 | 2458 | Risk Ratio (M-H, Random, 95% CI) | 1.33 [1.00, 1.77] |
| 5.1 Single interventions | 2 | 776 | Risk Ratio (M-H, Random, 95% CI) | 1.34 [0.93, 1.92] |
| 5.2 Multiple interventions | 3 | 1055 | Risk Ratio (M-H, Random, 95% CI) | 1.47 [0.86, 2.52] |
| 5.3 Tailored interventions | 1 | 627 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.40, 2.46] |
| 6 Abstinence at 12 to 17 months postpartum | 2 | 431 | Risk Ratio (M-H, Random, 95% CI) | 2.20 [1.23, 3.96] |
| 6.1 Single interventions | 1 | 109 | Risk Ratio (M-H, Random, 95% CI) | 2.55 [1.05, 6.21] |
| 6.2 Multiple interventions | 1 | 322 | Risk Ratio (M-H, Random, 95% CI) | 1.97 [0.91,4.29] |
| 7 Abstinence at 18+ months postpartum | 2 | 934 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.57, 2.73] |
| 7.1 Multiple interventions | 2 | 934 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.57, 2.73] |
| 8 Reduction in late pregnancy: biochemically validated | 3 | 1311 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.54, 2.26] |
| 8.1 Single interventions | 1 | 657 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.34, 1.20] |
| 8.2 Multiple interventions | 2 | 555 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [0.71, 3.20] |
| 9 Reduction in late pregnancy: self reported (various definitions) | 2 | 323 | Risk Ratio (M-H, Random, 95% CI) | 1.61 [1.06, 2.43] |
| 9.1 Single interventions | 2 | 323 | Risk Ratio (M-H, Random, 95% CI) | 1.61 [1.06, 2.43] |
| 10 Biochemical measures in late pregnancy: mean cotinine | 3 | 1742 | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.14, 0.05] |
| 10.1 Single interventions | 2 | 1328 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.17, 0.05] |
| 10.2 Multiple interventions | 1 | 414 | Std. Mean Difference (IV, Random, 95% CI) | −0.01 [−0.21, 0.18] |
| 11 Mean cigarettes per day in late pregnancy | 9 | 3368 | Std. Mean Difference (IV, Random, 95% CI) | −0.25 [−0.46, −0.03] |
| 11.1 Single interventions | 5 | 1928 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.30, 0.18] |
| 11.2 Multiple interventions | 2 | 270 | Std. Mean Difference (IV, Random, 95% CI) | −0.60 [−1.02, −0.18] |
| 11.3 Tailored interventions | 2 | 1170 | Std. Mean Difference (IV, Random, 95% CI) | −0.43 [−0.83, −0.03] |
| 12 Low birthweight infants (< 2500 g) | 6 | 3836 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 12.1 Single interventions | 2 | 1460 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.56, 1.11] |
| 12.2 Multiple interventions | 1 | 414 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.45, 2.61] |
| 12.3 Tailored interventions | 3 | 1962 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.66, 1.32] |
| 13 Very low birthweight infants (< 1500 g) | 2 | 1666 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.60, 2.71] |
| 13.1 Single interventions | 1 | 731 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.32, 2.59] |
| 13.2 Tailored interventions | 1 | 539 | Risk Ratio (M-H, Random, 95% CI) | 1.83 [0.62, 5.43] |
| 14 Preterm births | 5 | 2653 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 14.1 Single interventions | 3 | 1571 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.60, 1.17] |
| 14.2 Tailored interventions | 2 | 1082 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.46, 2.80] |
| 15 Mean birthweight | 9 | 4846 | Mean Difference (IV, Random, 95% CI) | 36.72 [0.70, 72.74] |
| 15.1 Single interventions | 4 | 1880 | Mean Difference (IV, Random, 95% CI) | 45.65 [−10.17, 101.48] |
| 15.2 Multiple interventions | 2 | 624 | Mean Difference (IV, Random, 95% CI) | 84.65 [−95.37, 264.67] |
| 15.3 Tailored interventions | 3 | 2342 | Mean Difference (IV, Random, 95% CI) | 23.25 [−52.12, 98.62] |
| 16 Perinatal deaths | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 16.1 Single interventions | 1 | 130 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Tailored interventions | 1 | 539 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.52, 2.31] |
| 17 Stillbirths | 4 | 2212 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.51, 2.30] |
| 17.1 Single interventions | 2 | 859 | Risk Ratio (M-H, Random, 95% CI) | 2.58 [0.38, 17.48] |
| 17.2 Tailored interventions | 2 | 1353 | Risk Ratio (M-H, Random, 95% CI) | 0.92 [0.41, 2.10] |
| 18 Neonatal deaths | 3 | 2095 | Risk Ratio (M-H, Random, 95% CI) | 2.06 [0.61, 6.92] |
| 18.1 Single interventions | 1 | 762 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.07, 18.65] |
| 18.2 Tailored interventions | 2 | 1333 | Risk Ratio (M-H, Random, 95% CI) | 2.35 [0.61, 9.07] |
| 19 NICU admissions | 2 | 1140 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.52, 1.29] |
| 19.1 Single interventions | 1 | 762 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 19.2 Tailored interventions | 1 | 378 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.55, 2.46] |
Comparison 2.
Smoking cessation interventions: counselling vs less intensive intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 16 | 5247 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [1.00, 1.82] |
| 1.1 Single interventions | 5 | 735 | Risk Ratio (M-H, Random, 95% CI) | 1.51 [0.90, 2.54] |
| 1.2 Multiple interventions | 10 | 4260 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.84, 1.78] |
| 1.3 Tailored interventions | 1 | 252 | Risk Ratio (M-H, Random, 95% CI) | 2.39 [1.03, 5.56] |
| 2 Abstinence in late pregnancy: biochemically validated only | 12 | 2858 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [1.15, 1.85] |
| 2.1 Single interventions | 5 | 735 | Risk Ratio (M-H, Random, 95% CI) | 1.51 [0.90, 2.54] |
| 2.2 Multiple interventions | 6 | 1871 | Risk Ratio (M-H, Random, 95% CI) | 1.38 [1.05, 1.80] |
| 2.3 Tailored interventions | 1 | 252 | Risk Ratio (M-H, Random, 95% CI) | 2.39 [1.03, 5.56] |
| 3 Continued abstinence (relapse prevention) in late pregnancy (spontaneous quitters) | 4 | 692 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 3.1 Single interventions | 2 | 204 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.88, 1.18] |
| 3.2 Multiple interventions | 2 | 488 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.96, 1.17] |
| 3.3 Tailored interventions | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abstinence at 0 to 5 months postpartum | 6 | 1980 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.82, 1.66] |
| 4.1 Single interventions | 1 | 82 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.11, 3.60] |
| 4.2 Multiple interventions | 4 | 1646 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.99, 1.43] |
| 4.3 Tailored interventions | 1 | 252 | Risk Ratio (M-H, Random, 95% CI) | 12.80 [1.70, 96.35] |
| 5 Abstinence at 6 to 11 months postpartum | 3 | 1271 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.83, 1.40] |
| 5.1 Single interventions | 1 | 105 | Risk Ratio (M-H, Random, 95% CI) | 2.45 [0.50, 12.08] |
| 5.2 Multiple interventions | 2 | 1166 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.80, 1.38] |
| 6 Abstinence at 12 to 17 months postpartum | 2 | 1188 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.71, 2.20] |
| 6.1 Multiple interventions | 2 | 1188 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.71, 2.20] |
| 7 Reduction in late pregnancy: self-reported > 50% | 2 | 1235 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [1.07, 1.71] |
| 7.1 Multiple interventions | 2 | 1235 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [1.07, 1.71] |
| 8 Reduction in late pregnancy: biochemically validated | 2 | 758 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.98, 1.87] |
| 8.1 Multiple interventions | 2 | 857 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.98, 1.87] |
| 9 Mean cigarettes per day in late pregnancy | 2 | 397 | Std. Mean Difference (IV, Random, 95% CI) | −0.11 [−0.30, 0.09] |
| 9.1 Single interventions | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [−0.34, 0.37] |
| 9.2 Multiple interventions | 1 | 276 | Std. Mean Difference (IV, Random, 95% CI) | −0.16 [−0.40, 0.08] |
| 10 Low birthweight infants (< 2500 g) | 2 | 305 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.32, 1.04] |
| 10.1 Single interventions | 1 | 227 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 10.2 Multiple interventions | 1 | 276 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.25, 1.50] |
| 11 Preterm births | 3 | 794 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.47, 1.42] |
| 11.1 Single interventions | 1 | 227 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 11.2 Multiple interventions | 1 | 308 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.46, 2.95] |
| 11.3 Tailored interventions | 1 | 952 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [0.30, 5.71] |
| 12 Mean birthweight | 3 | 546 | Mean Difference (IV, Random, 95% CI) | 56.02 [−31.46, 143. 50] |
| 12.1 Single interventions | 1 | 227 | Mean Difference (IV, Random, 95% CI) | 57.00 [−93.50, 207. 50] |
| 12.2 Multiple interventions | 2 | 319 | Mean Difference (IV, Random, 95% CI) | 76.01 [−88.59, 240. 61] |
Comparison 3.
Smoking cessation interventions: health education vs usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 3 | 374 | Risk Ratio (M-H, Random, 95% CI) | 1.51 [0.64, 3.59] |
| 1.1 Single interventions | 2 | 229 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.49, 3.42] |
| 1.2 Multiple interventions | 1 | 145 | Risk Ratio (M-H, Random, 95% CI) | 4.06 [0.46, 35.41] |
| 2 Abstinence in late pregnancy: biochemically validated only | 2 | 229 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.49, 3.42] |
| 2.1 Single interventions | 2 | 229 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.49, 3.42] |
| 3 Mean cigarettes per day in late pregnancy | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Single interventions | 1 | 552 | Std. Mean Difference (IV, Random, 95% CI) | −0.72 [−0.89, −0.55] |
| 3.2 Multiple interventions | 1 | 135 | Std. Mean Difference (IV, Random, 95% CI) | −0.32 [−0.66, 0.02] |
Comparison 4.
Smoking cessation interventions: health education vs less intensive intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy: biochemically validated | 2 | 851 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [0.97, 2.31] |
| 1.1 Single interventions | 1 | 653 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [0.88, 2.43] |
| 1.2 Multiple interventions | 1 | 198 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [0.68, 3.73] |
| 2 Abstinence at 0 to 5 months postpartum | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Single interventions | 2 | 844 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.52, 3.22] |
Comparison 5.
Smoking cessation interventions: feedback vs usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 2 | 355 | Risk Ratio (M-H, Random, 95% CI) | 4.39 [1.89, 10.21] |
| 1.1 Multiple interventions | 2 | 355 | Risk Ratio (M-H, Random, 95% CI) | 4.39 [1.89, 10.21] |
| 2 Reduction in late pregnancy: various definitions | 2 | 355 | Risk Ratio (M-H, Random, 95% CI) | 1.69 [1.24, 2.31] |
| 2.1 Multiple interventions | 2 | 355 | Risk Ratio (M-H, Random, 95% CI) | 1.69 [1.24, 2.31] |
| 3 Preterm births | 2 | 3111 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.28, 1.29] |
| 3.1 Multiple interventions | 2 | 3111 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.28, 1.29] |
| 4 Mean birthweight | 2 | 3006 | Mean Difference (IV, Random, 95% CI) | 79.43 [−53.05, 211.91] |
| 4.1 Multiple interventions | 2 | 3006 | Mean Difference (IV, Random, 95% CI) | 79.43 [−53.05, 211.91] |
| 5 Stillbirths | 2 | 2960 | Risk Ratio (M-H, Random, 95% CI) | 1.28 [0.69, 2.39] |
| 5.1 Multiple interventions | 2 | 2960 | Risk Ratio (M-H, Random, 95% CI) | 1.28 [0.69, 2.39] |
Comparison 6.
Smoking cessation interventions: feedback vs less intensive intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy: biochemically validated | 2 | 319 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.45, 3.12] |
| 1.1 Single interventions | 1 | 79 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.16, 2.22] |
| 1.2 Multiple interventions | 1 | 240 | Risk Ratio (M-H, Random, 95% CI) | 1.69 [0.89, 3.20] |
Comparison 7.
Smoking cessation interventions: incentives vs usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy: biochemically validated | 2 | 129 | Risk Ratio (M-H, Random, 95% CI) | 3.59 [0.10, 130.49] |
| 1.1 Single interventions | 1 | 74 | Risk Ratio (M-H, Random, 95% CI) | 20.72 [1.28, 336.01] |
| 1.2 Tailored interventions | 1 | 55 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.25, 3.23] |
Comparison 8.
Smoking cessation interventions: social support vs less intensive intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy (peer and partner support) | 6 | 734 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.94, 1.78] |
| 1.1 Single interventions | 2 | 224 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.57, 3.18] |
| 1.2 Multiple interventions | 3 | 359 | Risk Ratio (M-H, Random, 95% CI) | 1.48 [0.74, 2.95] |
| 1.3 Tailored interventions | 1 | 151 | Risk Ratio (M-H, Random, 95% CI) | 1.22 [0.59, 2.52] |
| 2 Abstinence in late pregnancy: biochemically validated (peer support only) | 5 | 554 | Risk Ratio (M-H, Random, 95% CI) | 1.49 [1.01, 2.19] |
| 2.1 Single interventions | 2 | 224 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.57, 3.18] |
| 2.2 Multiple interventions | 2 | 179 | Risk Ratio (M-H, Random, 95% CI) | 2.26 [1.15, 4.46] |
| 2.3 Tailored interventions | 1 | 151 | Risk Ratio (M-H, Random, 95% CI) | 1.22 [0.59, 2.52] |
| 3 Abstinence at 0 to 5 months postpartum | 2 | 473 | Risk Ratio (M-H, Random, 95% CI) | 1.36 [0.46, 4.07] |
| 3.1 Single interventions | 1 | 82 | Risk Ratio (M-H, Random, 95% CI) | 5.8 [0.33, 101.27] |
| 3.2 Multiple interventions | 1 | 391 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.87, 1.41] |
| 4 Abstinence at 6 to 11 months postpartum | 2 | 486 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.83, 1.42] |
| 4.1 Multiple interventions | 2 | 486 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.83, 1.42] |
Comparison 9.
Maternal health intervention with smoking cessation component: social support (tailored) vs usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Self-reported | 1 | 492 | Risk Ratio (M-H, Random, 95% CI) | 1.83 [1.22, 2.73] |
| 1.2 Biochemically validated | 1 | 141 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Self-reported mean cigarettes per day in late pregnancy | 2 | 542 | Std. Mean Difference (IV, Random, 95% CI) | −0.28 [−0.45, −0.11] |
| 2.1 Self-reported | 1 | 401 | Std. Mean Difference (IV, Random, 95% CI) | −0.24 [−0.43, −0.04] |
| 2.2 Biochemically validated | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | −0.40 [−0.73, −0.06] |
Comparison 10.
Maternal health intervention with smoking cessation component: social support vs less intensive intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy | 2 | 316 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.46, 1.39] |
| 1.1 Single interventions | 1 | 66 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.09, 2.16] |
| 1.2 Tailored interventions | 1 | 250 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.48, 1.57] |
| 2 Abstinence in late pregnancy: biochemically validated | 1 | 250 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.48, 1.57] |
| 2.1 Tailored interventions | 1 | 250 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.48, 1.57] |
Comparison 11.
Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence in late pregnancy: self-reported and biochemically validated (non-winsorised) | 70 | 21948 | Risk Ratio (M-H, Random, 95% CI) | 1.45 [1.27, 1.64] |
| 1.1 Counselling | 45 | 17681 | Risk Ratio (M-H, Random, 95% CI) | 1.37 [1.17, 1.59] |
| 1.2 Health education | 5 | 1225 | Risk Ratio (M-H, Random, 95% CI) | 1.47 [1.02, 2.13] |
| 1.3 Feedback | 5 | 739 | Risk Ratio (M-H, Random, 95% CI) | 2.09 [1.17, 3.72] |
| 1.4 Incentives | 4 | 426 | Risk Ratio (M-H, Random, 95% CI) | 3.09 [1.34, 7.15] |
| 1.5 Social support | 10 | 1683 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.97, 1.73] |
| 1.6 Other | 1 | 194 | Risk Ratio (M-H, Random, 95% CI) | 1.63 [0.62, 4.32] |
| 2 Abstinence in late pregnancy: biochemically validated only (non-winsorised) | 49 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Counselling | 30 | 11924 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [1.11, 1.47] |
| 2.2 Health education | 4 | 1080 | Risk Ratio (M-H, Random, 95% CI) | 1.43 [0.98, 2.08] |
| 2.3 Feedback | 3 | 365 | Risk Ratio (M-H, Random, 95% CI) | 1.70 [0.71, 4.08] |
| 2.4 Incentives | 4 | 426 | Risk Ratio (M-H, Random, 95% CI) | 3.09 [1.34, 7.15] |
| 2.5 Social support | 7 | 549 | Risk Ratio (M-H, Random, 95% CI) | 1.31 [0.90, 1.91] |
| 2.6 Other | 1 | 194 | Risk Ratio (M-H, Random, 95% CI) | 1.63 [0.62, 4.32] |
| 3 Continued abstinence (Relapse prevention) in late pregnancy for spontaneous quitters | 14 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 3.1 Counselling | 12 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Health education | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Social support | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Abstinence at 0 to 5 months postpartum | 26 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 4.1 Counselling | 18 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Health education | 3 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Incentives | 2 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Social support | 3 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Abstinence at 6 to 11 months postpartum | 13 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 5.1 Counselling | 10 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Incentives | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Social support | 2 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Abstinence at 12 to 17 months postpartum | 5 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 6.1 Counselling | 4 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Social support | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Abstinence at 18+ months postpartum | 2 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 7.1 Counselling | 2 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Smoking reduction: numbers of women reducing smoking in late pregnancy | 15 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 8.1 Self-reported some reduction in smoking (various definitions) | 5 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Self-reported > 50% reduction in smoking | 4 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Biochemically validated reduction | 6 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Smoking reduction: biochemical measures in late pregnancy | 6 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Mean cotinine levels | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mean thiocynate level | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Smoking reduction: self-reported mean cigarettes per day measured in late pregnancy or at delivery | 20 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Counselling | 11 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Health education | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Feedback | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Incentives | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Social support | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Low birthweight (under 2500 g) | 14 | 8562 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 11.1 Counselling | 8 | 4339 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.68, 1.01] |
| 11.2 Health education | 2 | 1172 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.49, 1.55] |
| 11.3 Feedback | 1 | 2848 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 11.4 Incentives | 2 | 124 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.22, 0.93] |
| 11.5 Social support | 1 | 79 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.33, 2.99] |
| 12 Very low birthweight (under 1500 g) | 3 | 4366 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.62, 2.01] |
| 12.1 Counselling | 2 | 1666 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.60, 2.71] |
| 12.2 Feedback | 1 | 2700 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.35, 2.32] |
| 13 Preterm birth (under 37 weeks) | 14 | 7852 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.70, 0.96] |
| 13.1 Counselling | 8 | 3447 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.71, 1.20] |
| 13.2 Health education | 2 | 1170 | Risk Ratio (M-H, Random, 95% CI) | 0.92 [0.55, 1.56] |
| 13.3 Feedback | 2 | 3111 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.28, 1.29] |
| 13.4 Incentives | 2 | 124 | Risk Ratio (M-H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 14 Mean birthweight | 19 | 9859 | Mean Difference (IV, Random, 95% CI) | 40.78 [18.45, 63.10] |
| 14.1 Counselling | 12 | 5392 | Mean Difference (IV, Random, 95% CI) | 39.93 [9.12, 70.74] |
| 14.2 Health education | 2 | 1172 | Mean Difference (IV, Random, 95% CI) | 27.35 [−53.88, 108. 58] |
| 14.3 Feedback | 2 | 3006 | Mean Difference (IV, Random, 95% CI) | 79.43 [−53.05, 211. 91] |
| 14.4 Incentives | 2 | 147 | Mean Difference (IV, Random, 95% CI) | 213.78 [20.16, 407. 40] |
| 14.5 Social support | 1 | 142 | Mean Difference (IV, Random, 95% CI) | 28.0 [−152.48, 208. 48] |
| 15 Perinatal deaths | 4 | 4465 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| 15.1 Counselling | 2 | 1065 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.52, 2.31] |
| 15.2 Health education | 1 | 552 | Risk Ratio (M-H, Random, 95% CI) | 4.40 [0.49, 39.08] |
| 15.3 Feedback | 1 | 2848 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.59, 1.87] |
| 16 Stillbirths | 7 | 5414 | Risk Ratio (M-H, Random, 95% CI) | 1.22 [0.76, 1.95] |
| 16.1 Counselling | 5 | 2454 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.55, 2.33] |
| 16.2 Feedback | 2 | 2960 | Risk Ratio (M-H, Random, 95% CI) | 1.28 [0.69, 2.39] |
| 17 Neonatal deaths | 4 | 4905 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.44, 3.06] |
| 17.1 Counselling | 3 | 2095 | Risk Ratio (M-H, Random, 95% CI) | 2.06 [0.61, 6.92] |
| 17.2 Feedback | 1 | 2810 | Risk Ratio (M-H, Random, 95% CI) | 0.40 [0.08, 2.07] |
| 18 NICU admissions | 4 | 1264 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.59, 1.04] |
| 18.1 Counselling | 2 | 1140 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.52, 1.29] |
| 18.2 Incentives | 2 | 124 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.47, 1.21] |
Analysis 1.1. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 1 Abstinence in late pregnancy
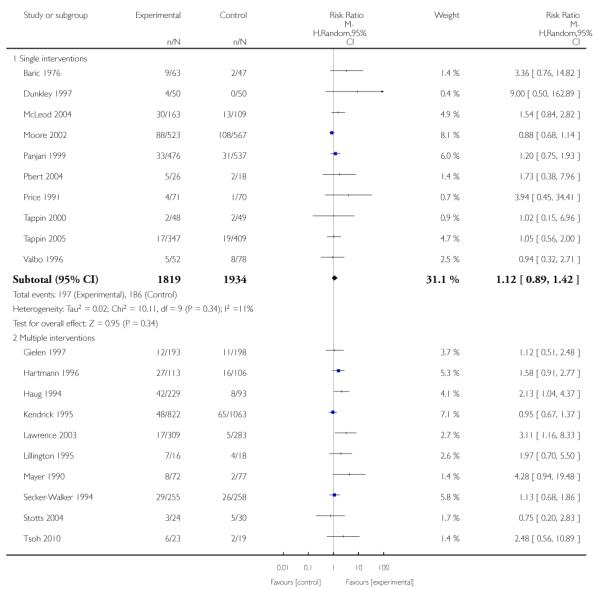
|
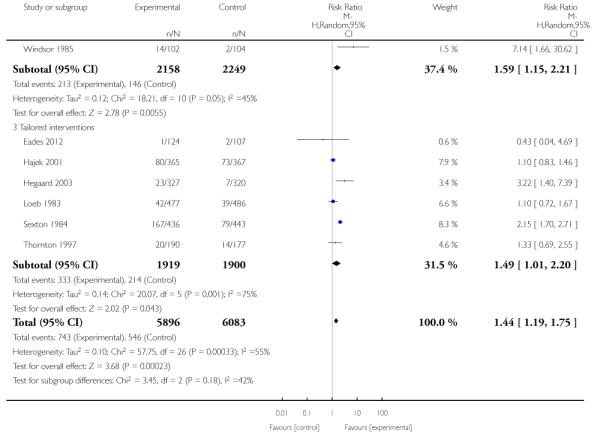
|
Analysis 1.2. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 2 Abstinence in late pregnancy: biochemically validated only
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 2 Abstinence in late pregnancy: biochemically validated only
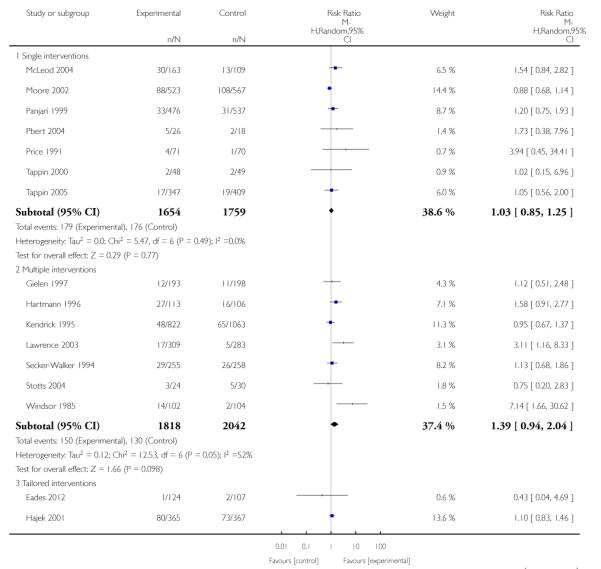
|
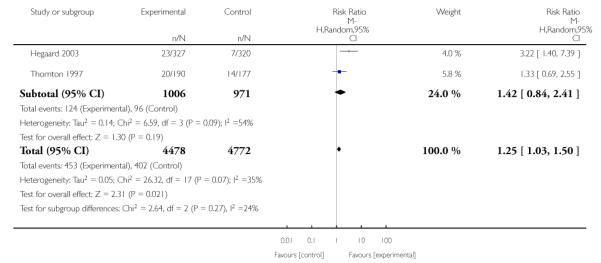
|
Analysis 1.3. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 3 Continued abstinence (relapse prevention) in late pregnancy for spontaneous quitters
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 3 Continued abstinence (relapse prevention) in late pregnancy for spontaneous quitters
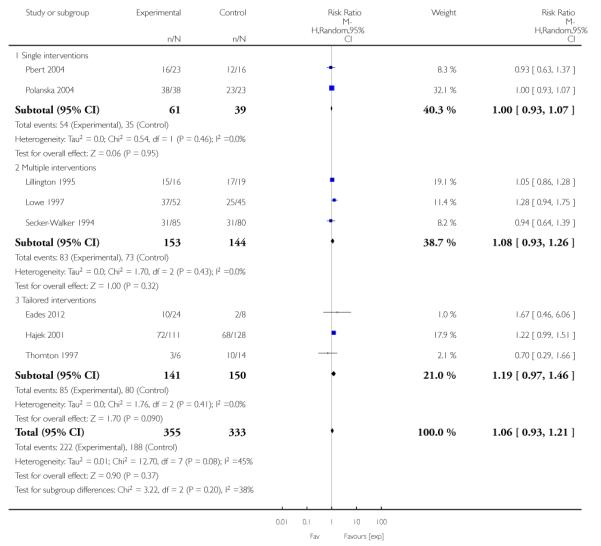
|
Analysis 1.4. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 4 Abstinence at 0 to 5 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 4 Abstinence at 0 to 5 months postpartum
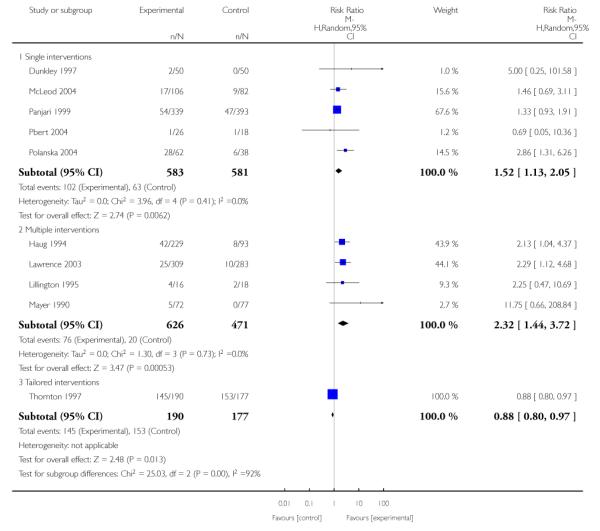
|
Analysis 1.5. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 5 Abstinence at 6 to 11 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 5 Abstinence at 6 to 11 months postpartum
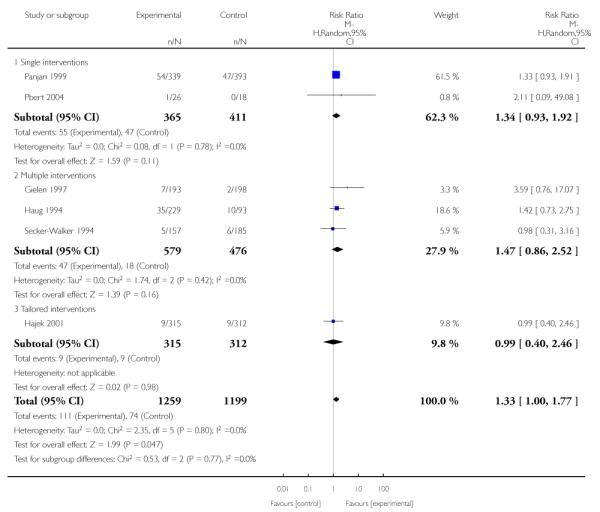
|
Analysis 1.6. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 6 Abstinence at 12 to 17 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 6 Abstinence at 12 to 17 months postpartum
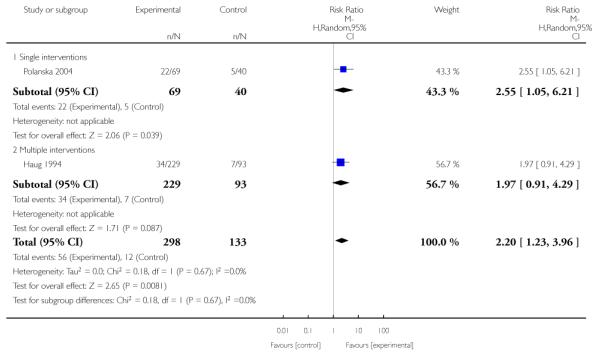
|
Analysis 1.7. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 7 Abstinence at 18+ months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 7 Abstinence at 18+ months postpartum
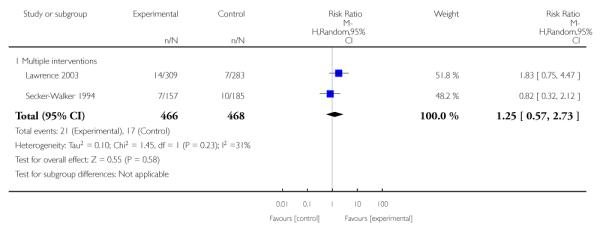
|
Analysis 1.8. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 8 Reduction in late pregnancy: biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 8 Reduction in late pregnancy: biochemically validated
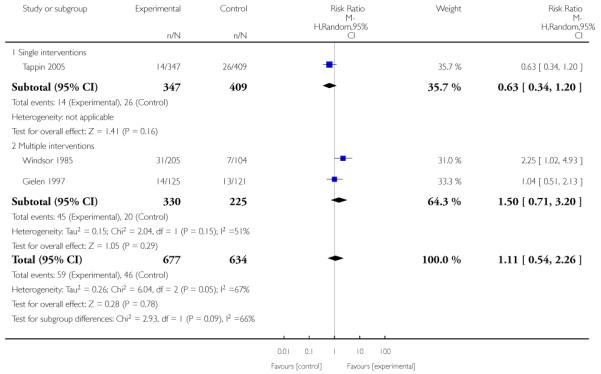
|
Analysis 1.9. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 9 Reduction in late pregnancy: self reported (various definitions)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 9 Reduction in late pregnancy: self reported (various definitions)
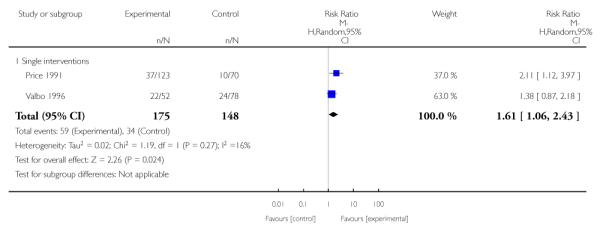
|
Analysis 1.10. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 10 Biochemical measures in late pregnancy: mean cotinine
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 10 Biochemical measures in late pregnancy: mean cotinine
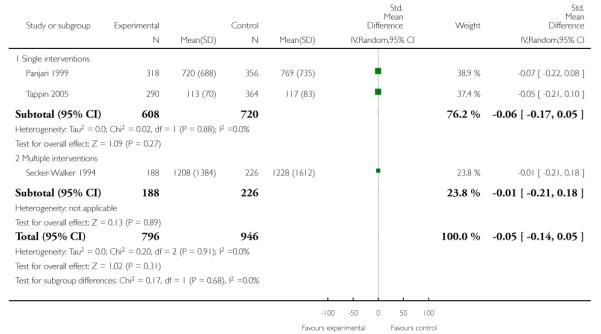
|
Analysis 1.11. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 11 Mean cigarettes per day in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 11 Mean cigarettes per day in late pregnancy
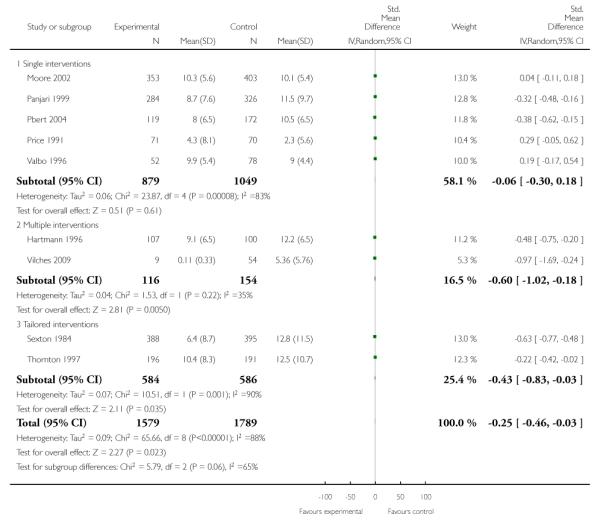
|
Analysis 1.12. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 12 Low birthweight infants (< 2500 g)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 12 Low birthweight infants (< 2500 g)
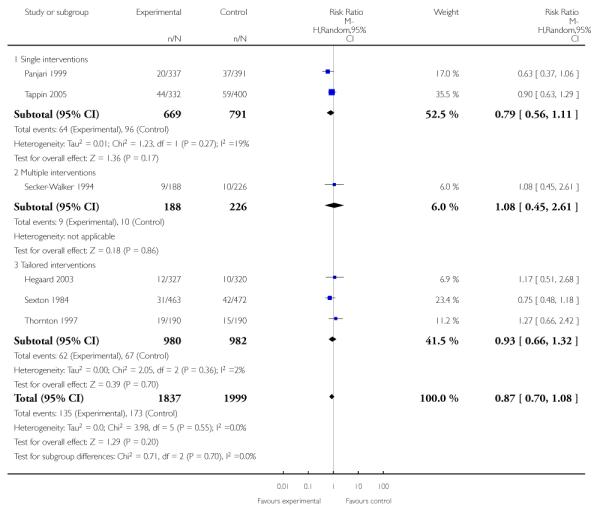
|
Analysis 1.13. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 13 Very low birthweight infants (< 1500 g)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 13 Very low birthweight infants (< 1500 g)
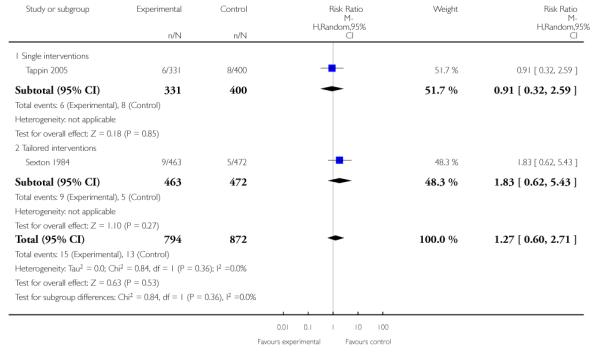
|
Analysis 1.14. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 14 Preterm births
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 14 Preterm births
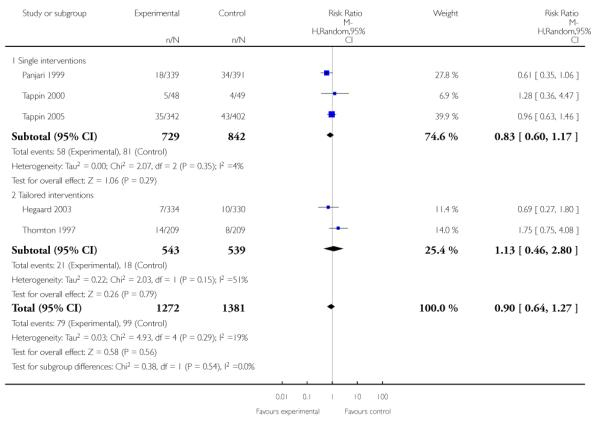
|
Analysis 1.15. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 15 Mean birthweight
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 15 Mean birthweight
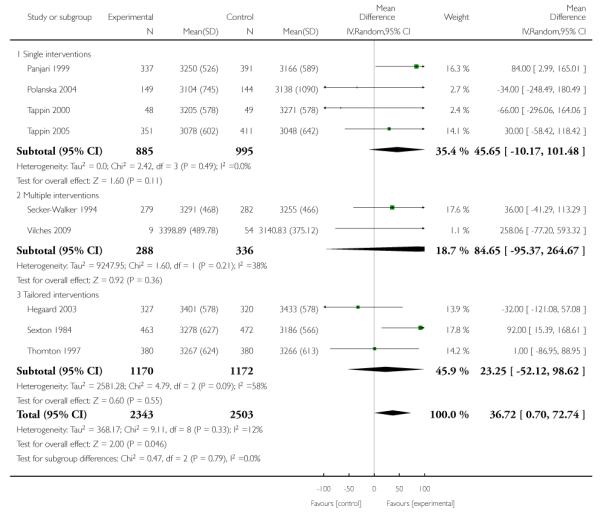
|
Analysis 1.16. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 16 Perinatal deaths
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 16 Perinatal deaths
|
Analysis 1.17. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 17 Stillbirths
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 17 Stillbirths
|
Analysis 1.18. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 18 Neonatal deaths
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 18 Neonatal deaths
|
Analysis 1.19. Comparison 1 Smoking cessation interventions: counselling vs usual care, Outcome 19 NICU admissions
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 1 Smoking cessation interventions: counselling vs usual care
Outcome: 19 NICU admissions
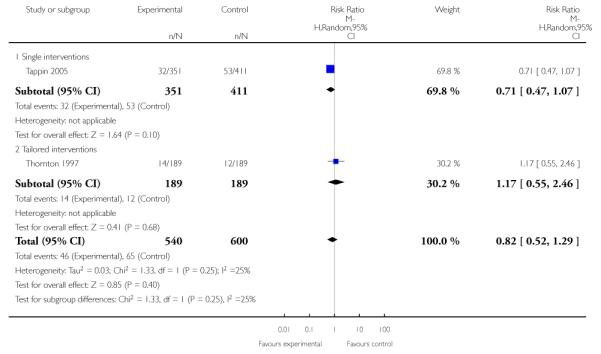
|
Analysis 2.1. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 1 Abstinence in late pregnancy
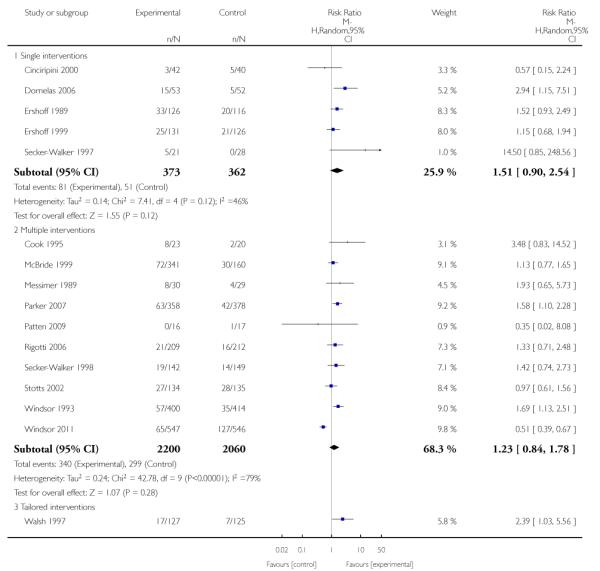
|
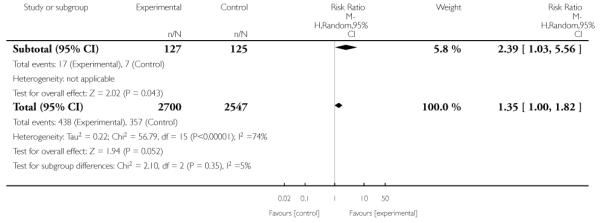
|
Analysis 2.2. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 2 Abstinence in late pregnancy: biochemically validated only
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 2 Abstinence in late pregnancy: biochemically validated only
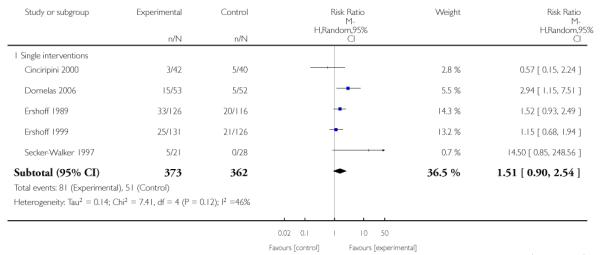
|
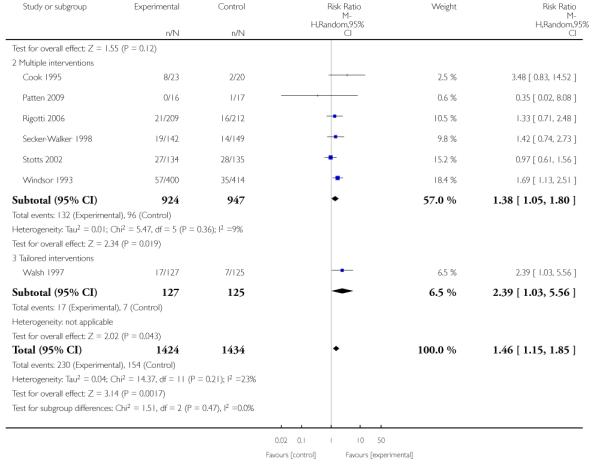
|
Analysis 2.3. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 3 Continued abstinence (relapse prevention) in late pregnancy (spontaneous quitters)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 3 Continued abstinence (relapse prevention) in late pregnancy (spontaneous quitters)
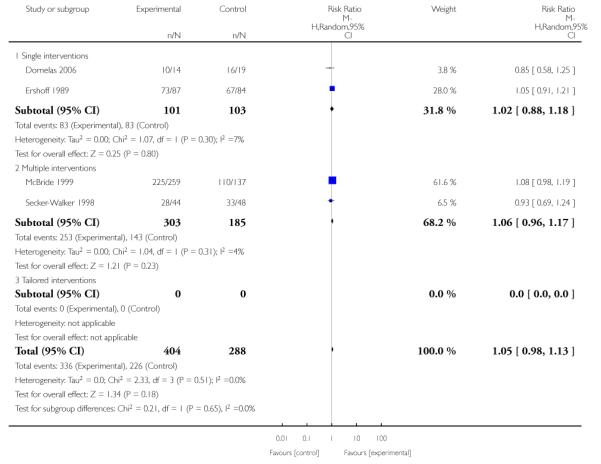
|
Analysis 2.4. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 4 Abstinence at 0 to 5 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 4 Abstinence at 0 to 5 months postpartum
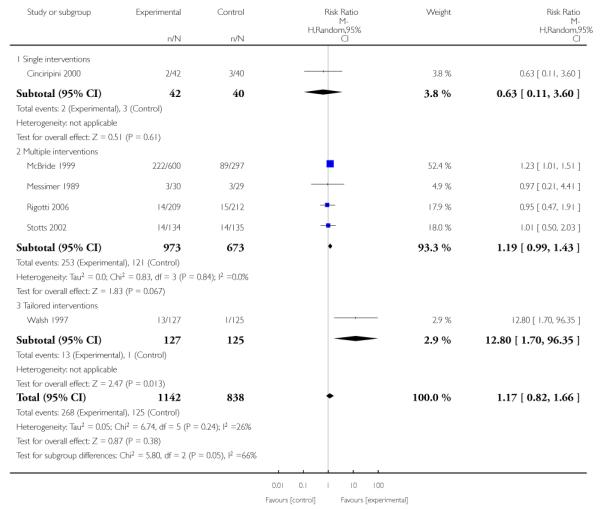
|
Analysis 2.5. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 5 Abstinence at 6 to 11 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 5 Abstinence at 6 to 11 months postpartum
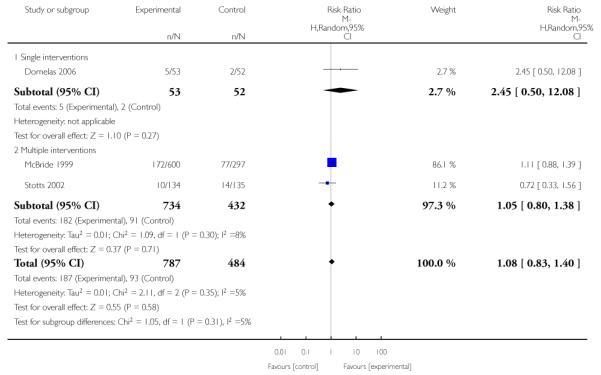
|
Analysis 2.6. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 6 Abstinence at 12 to 17 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 6 Abstinence at 12 to 17 months postpartum
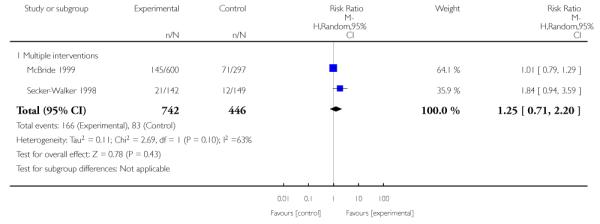
|
Analysis 2.7. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 7 Reduction in late pregnancy: self-reported > 50%
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 7 Reduction in late pregnancy: self-reported > 50%
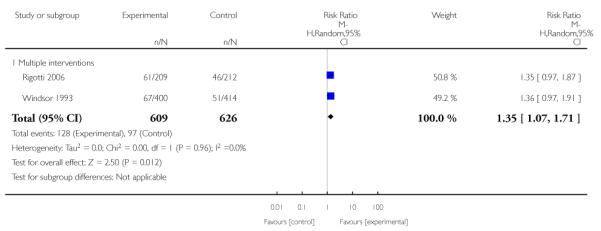
|
Analysis 2.8. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 8 Reduction in late pregnancy: biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 8 Reduction in late pregnancy: biochemically validated
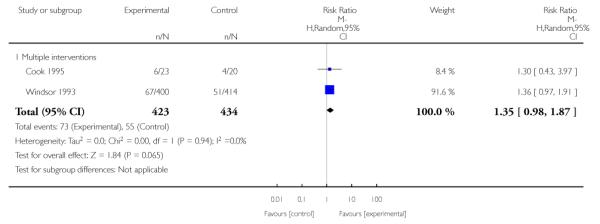
|
Analysis 2.9. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 9 Mean cigarettes per day in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 9 Mean cigarettes per day in late pregnancy
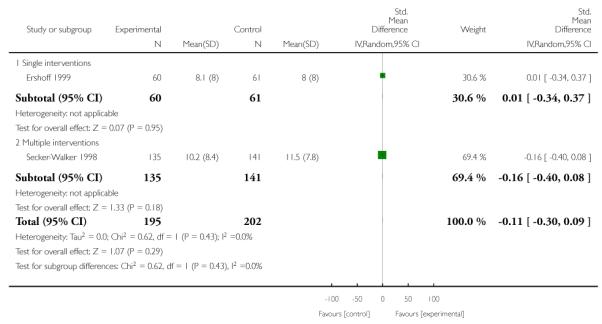
|
Analysis 2.10. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 10 Low birthweight infants (< 2500 g)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 10 Low birthweight infants (< 2500 g)
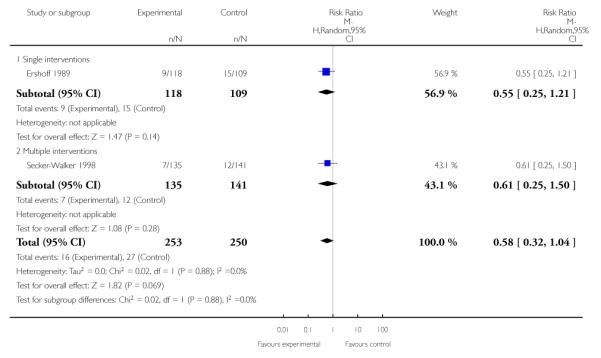
|
Analysis 2.11. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 11 Preterm births
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 11 Preterm births
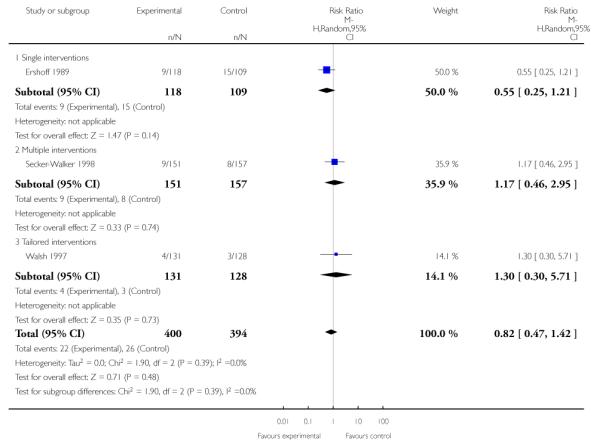
|
Analysis 2.12. Comparison 2 Smoking cessation interventions: counselling vs less intensive intervention, Outcome 12 Mean birthweight
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 2 Smoking cessation interventions: counselling vs less intensive intervention
Outcome: 12 Mean birthweight
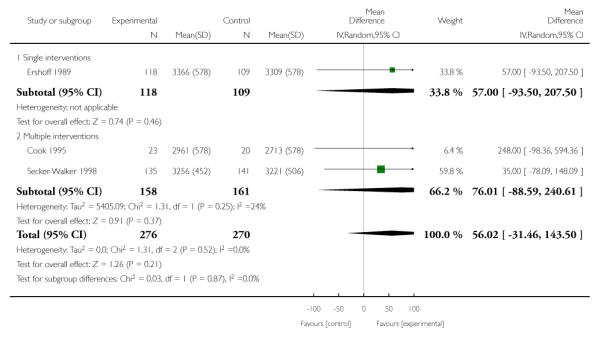
|
Analysis 3.1. Comparison 3 Smoking cessation interventions: health education vs usual care, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 3 Smoking cessation interventions: health education vs usual care
Outcome: 1 Abstinence in late pregnancy
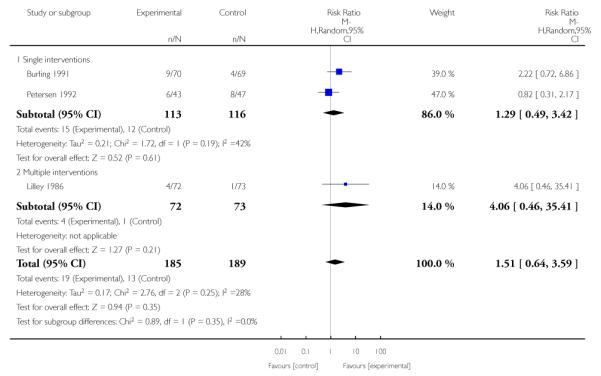
|
Analysis 3.2. Comparison 3 Smoking cessation interventions: health education vs usual care, Outcome 2 Abstinence in late pregnancy: biochemically validated only
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 3 Smoking cessation interventions: health education vs usual care
Outcome: 2 Abstinence in late pregnancy: biochemically validated only
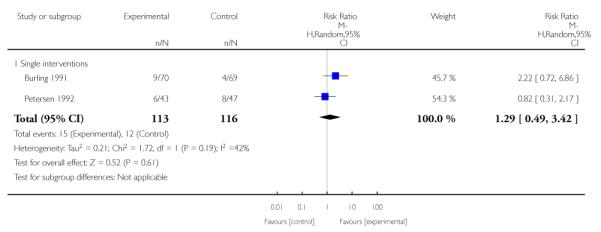
|
Analysis 3.3. Comparison 3 Smoking cessation interventions: health education vs usual care, Outcome 3 Mean cigarettes per day in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 3 Smoking cessation interventions: health education vs usual care
Outcome: 3 Mean cigarettes per day in late pregnancy
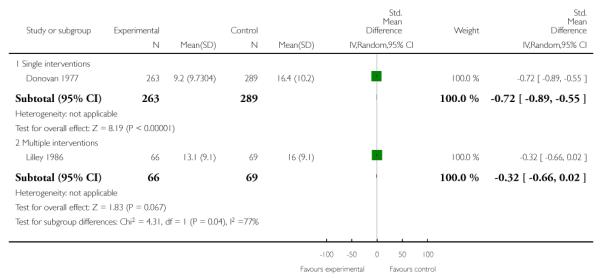
|
Analysis 4.1. Comparison 4 Smoking cessation interventions: health education vs less intensive intervention, Outcome 1 Abstinence in late pregnancy: biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 4 Smoking cessation interventions: health education vs less intensive intervention
Outcome: 1 Abstinence in late pregnancy: biochemically validated
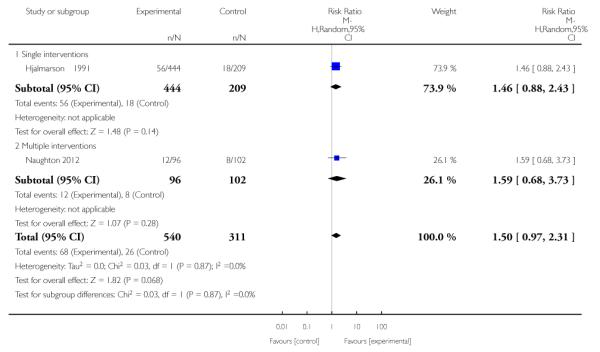
|
Analysis 4.2. Comparison 4 Smoking cessation interventions: health education vs less intensive intervention, Outcome 2 Abstinence at 0 to 5 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 4 Smoking cessation interventions: health education vs less intensive intervention
Outcome: 2 Abstinence at 0 to 5 months postpartum
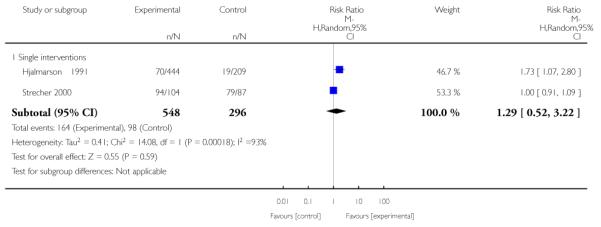
|
Analysis 5.1. Comparison 5 Smoking cessation interventions: feedback vs usual care, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 5 Smoking cessation interventions: feedback vs usual care
Outcome: 1 Abstinence in late pregnancy
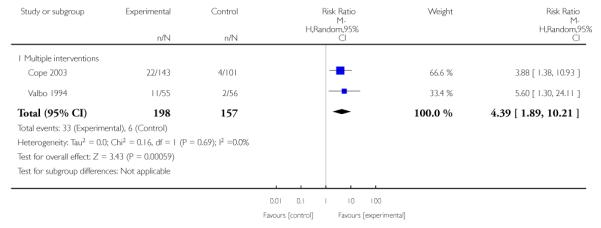
|
Analysis 5.2. Comparison 5 Smoking cessation interventions: feedback vs usual care, Outcome 2 Reduction in late pregnancy: various definitions
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 5 Smoking cessation interventions: feedback vs usual care
Outcome: 2 Reduction in late pregnancy: various definitions
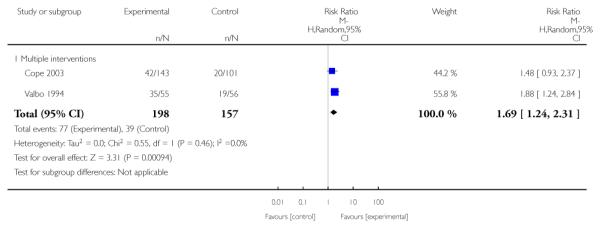
|
Analysis 5.3. Comparison 5 Smoking cessation interventions: feedback vs usual care, Outcome 3 Preterm births
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 5 Smoking cessation interventions: feedback vs usual care
Outcome: 3 Preterm births
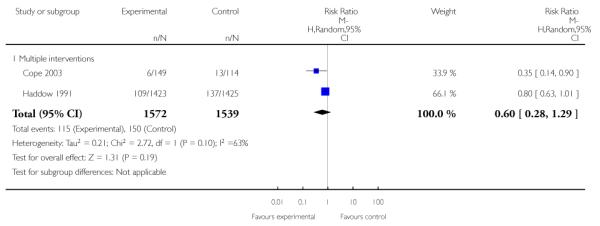
|
Analysis 5.4. Comparison 5 Smoking cessation interventions: feedback vs usual care, Outcome 4 Mean birthweight
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 5 Smoking cessation interventions: feedback vs usual care
Outcome: 4 Mean birthweight
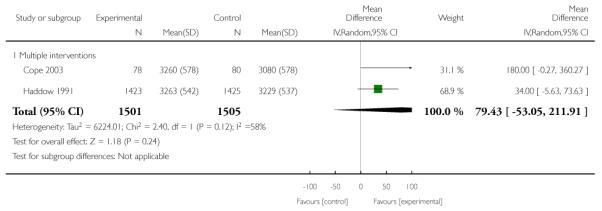
|
Analysis 5.5. Comparison 5 Smoking cessation interventions: feedback vs usual care, Outcome 5 Stillbirths
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 5 Smoking cessation interventions: feedback vs usual care
Outcome: 5 Stillbirths
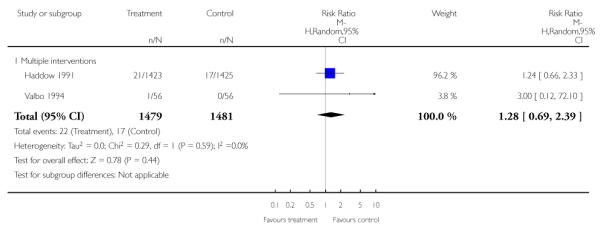
|
Analysis 6.1. Comparison 6 Smoking cessation interventions: feedback vs less intensive intervention, Outcome 1 Abstinence in late pregnancy: biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 6 Smoking cessation interventions: feedback vs less intensive intervention
Outcome: 1 Abstinence in late pregnancy: biochemically validated
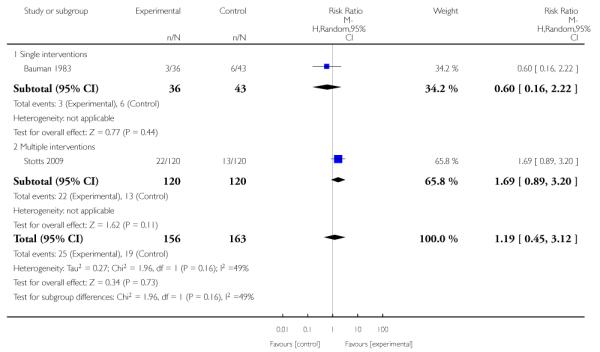
|
Analysis 7.1. Comparison 7 Smoking cessation interventions: incentives vs usual care, Outcome 1 Abstinence in late pregnancy:biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 7 Smoking cessation interventions: incentives vs usual care
Outcome: 1 Abstinence in late pregnancy:biochemically validated
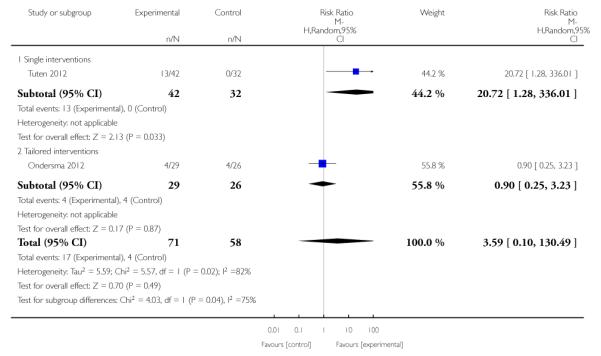
|
Analysis 8.1. Comparison 8 Smoking cessation interventions: social support vs less intensive intervention, Outcome 1 Abstinence in late pregnancy (peer and partner support)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 8 Smoking cessation interventions: social support vs less intensive intervention
Outcome: 1 Abstinence in late pregnancy (peer and partner support)
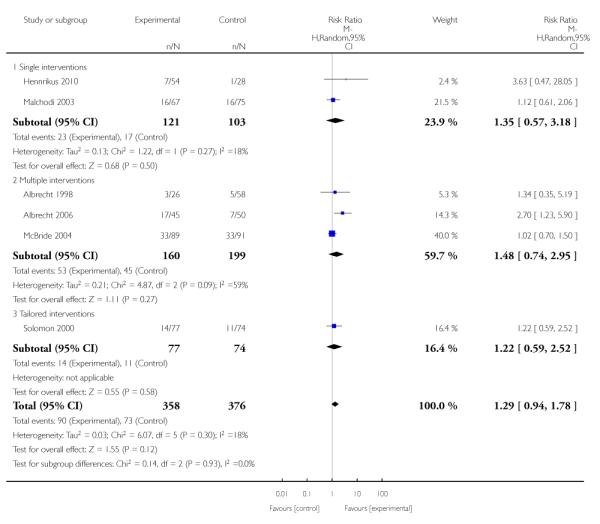
|
Analysis 8.2. Comparison 8 Smoking cessation interventions: social support vs less intensive intervention, Outcome 2 Abstinence in late pregnancy: biochemically validated (peer support only)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 8 Smoking cessation interventions: social support vs less intensive intervention
Outcome: 2 Abstinence in late pregnancy: biochemically validated (peer support only)
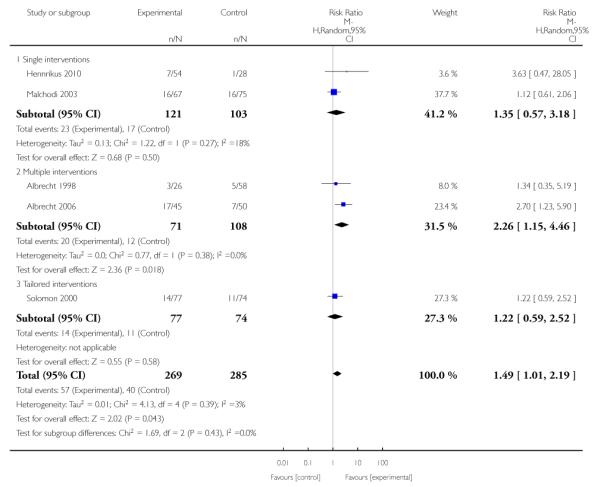
|
Analysis 8.3. Comparison 8 Smoking cessation interventions: social support vs less intensive intervention, Outcome 3 Abstinence at 0 to 5 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 8 Smoking cessation interventions: social support vs less intensive intervention
Outcome: 3 Abstinence at 0 to 5 months postpartum
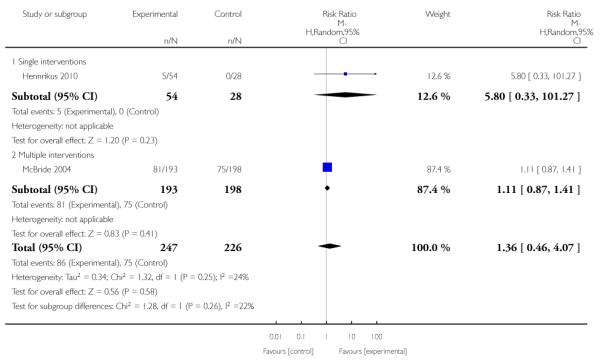
|
Analysis 8.4. Comparison 8 Smoking cessation interventions: social support vs less intensive intervention, Outcome 4 Abstinence at 6 to 11 months postpartum
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 8 Smoking cessation interventions: social support vs less intensive intervention
Outcome: 4 Abstinence at 6 to 11 months postpartum
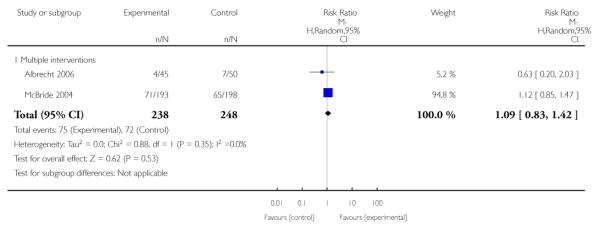
|
Analysis 9.1. Comparison 9 Maternal health intervention with smoking cessation component: social support (tailored) vs usual care, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 9 Maternal health intervention with smoking cessation component: social support (tailored) vs usual care
Outcome: 1 Abstinence in late pregnancy
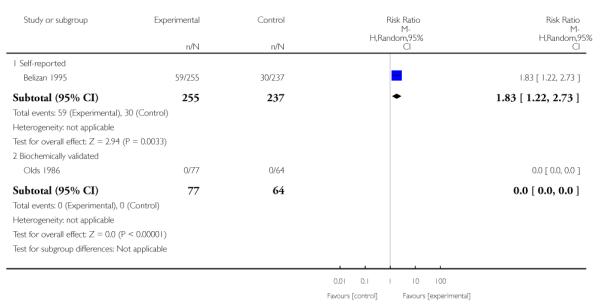
|
Analysis 9.2. Comparison 9 Maternal health intervention with smoking cessation component: social support (tailored) vs usual care, Outcome 2 Self-reported mean cigarettes per day in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 9 Maternal health intervention with smoking cessation component: social support (tailored) vs usual care
Outcome: 2 Self-reported mean cigarettes per day in late pregnancy
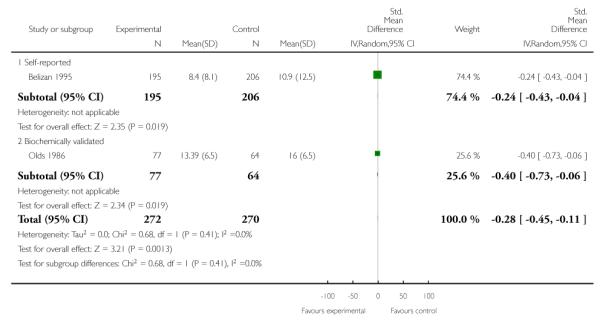
|
Analysis 10.1. Comparison 10 Maternal health intervention with smoking cessation component: social support vs less intensive intervention, Outcome 1 Abstinence in late pregnancy
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 10 Maternal health intervention with smoking cessation component: social support vs less intensive intervention
Outcome: 1 Abstinence in late pregnancy
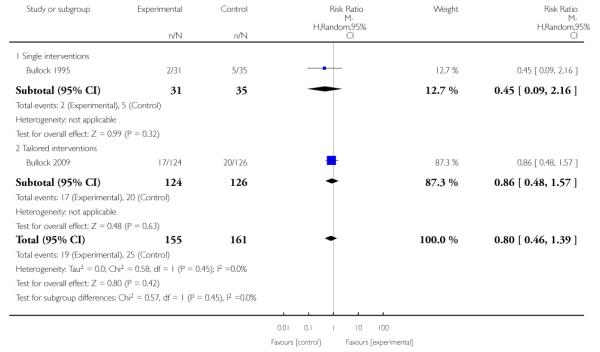
|
Analysis 10.2. Comparison 10 Maternal health intervention with smoking cessation component: social support vs less intensive intervention, Outcome 2 Abstinence in late pregnancy: biochemically validated
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 10 Maternal health intervention with smoking cessation component: social support vs less intensive intervention
Outcome: 2 Abstinence in late pregnancy: biochemically validated
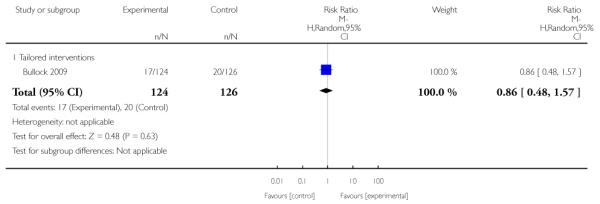
|
Analysis 11.1. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 1 Abstinence in late pregnancy: self-reported and biochemically validated (non-winsorised)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 1 Abstinence in late pregnancy: self-reported and biochemically validated (non-winsorised)
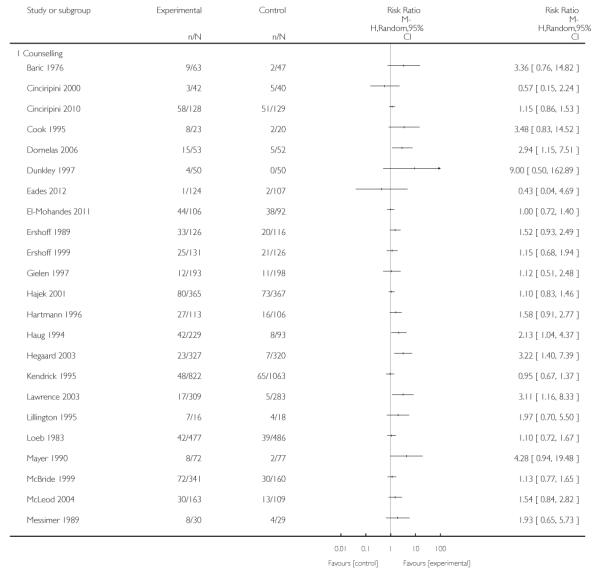
|
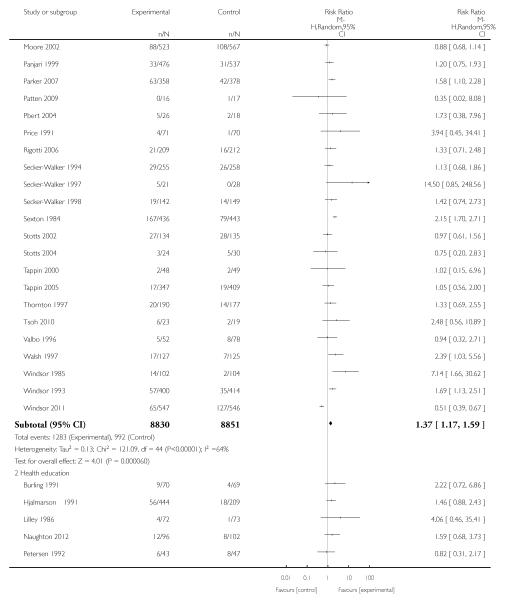
|
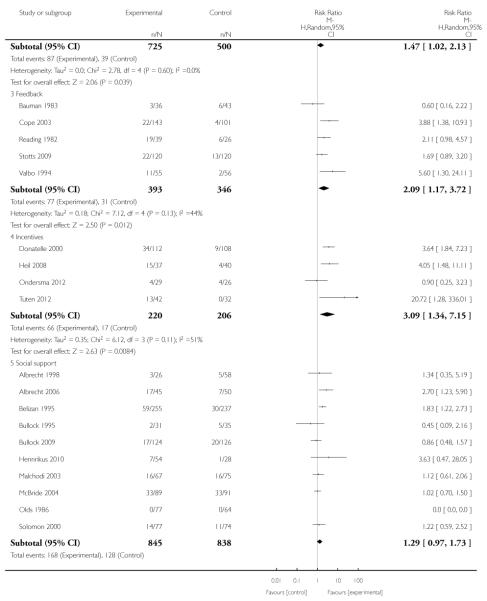
|
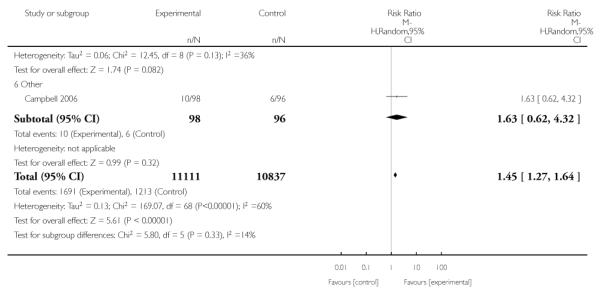
|
Analysis 11.2. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 2 Abstinence in late pregnancy: biochemically validated only (nonwinsorised)
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 2 Abstinence in late pregnancy: biochemically validated only (non-winsorised)
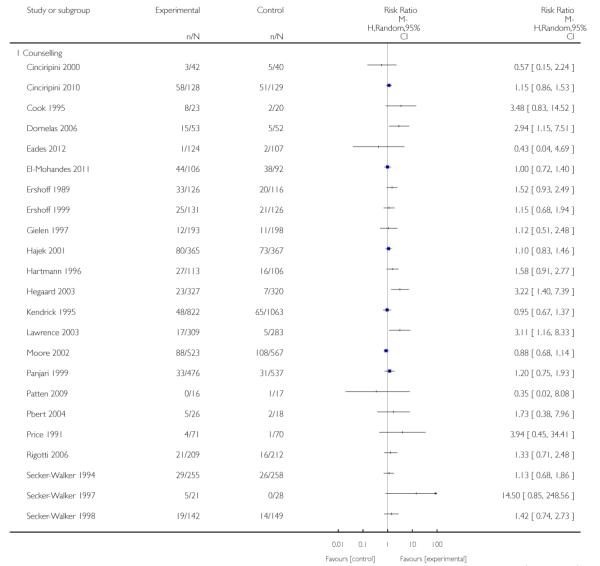
|

|
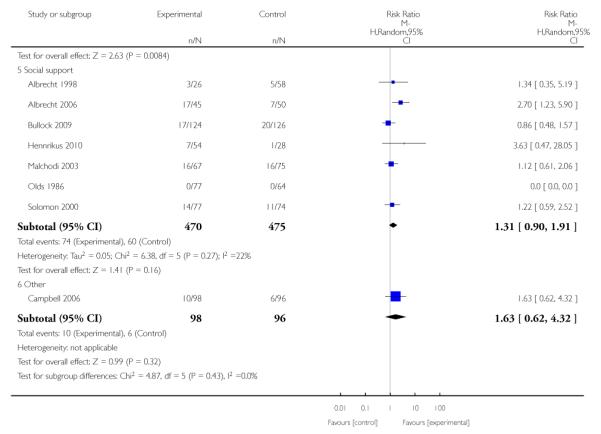
|
Analysis 11.3. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 3 Continued abstinence (Relapse prevention) in late pregnancy for spontaneous quitters.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 3 Continued abstinence (Relapse prevention) in late pregnancy for spontaneous quitters
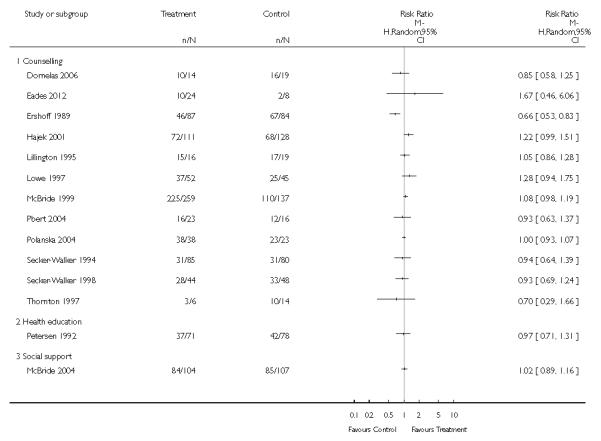
|
Analysis 11.4. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 4 Abstinence at 0 to 5 months postpartum.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 4 Abstinence at 0 to 5 months postpartum
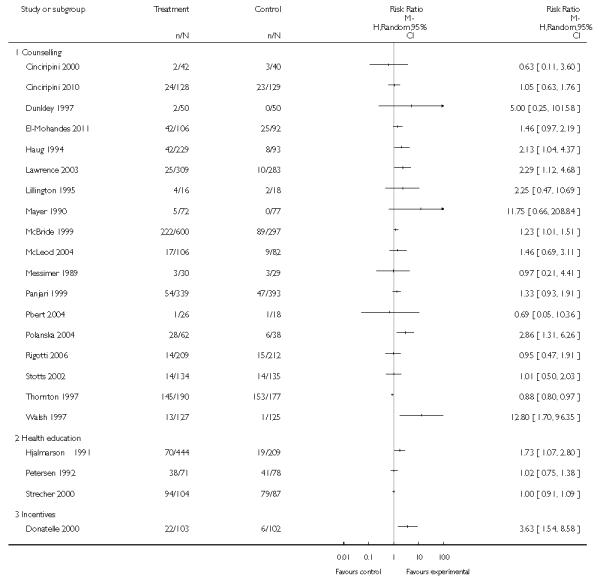
|
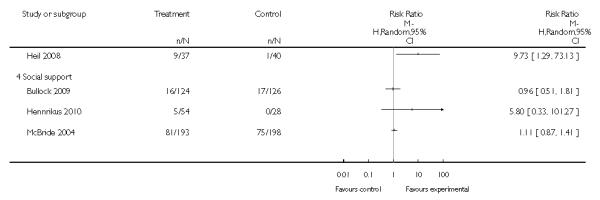
|
Analysis 11.5. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 5 Abstinence at 6 to 11 months postpartum.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 5 Abstinence at 6 to 11 months postpartum
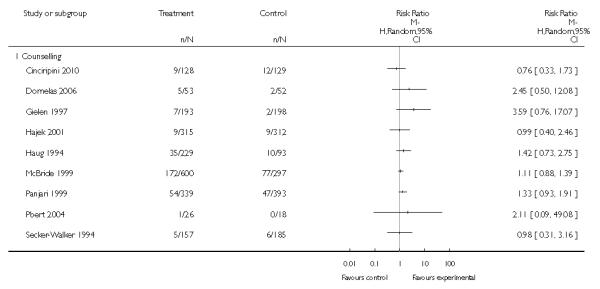
|
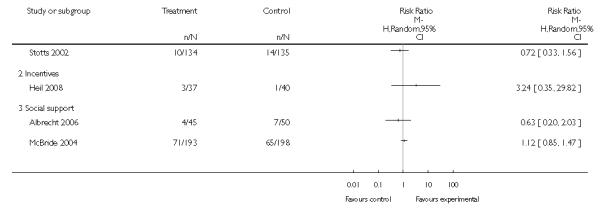
|
Analysis 11.6. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 6 Abstinence at 12 to 17 months postpartum.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 6 Abstinence at 12 to 17 months postpartum
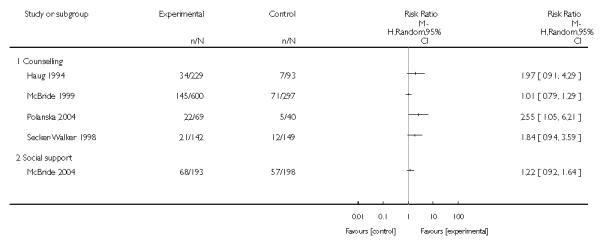
|
Analysis 11.7. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 7 Abstinence at 18+ months postpartum.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 7 Abstinence at 18+ months postpartum
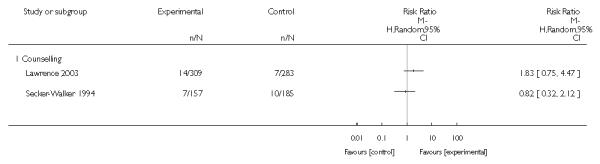
|
Analysis 11.8. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 8 Smoking reduction: numbers of women reducing smoking in late pregnancy.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 8 Smoking reduction: numbers of women reducing smoking in late pregnancy
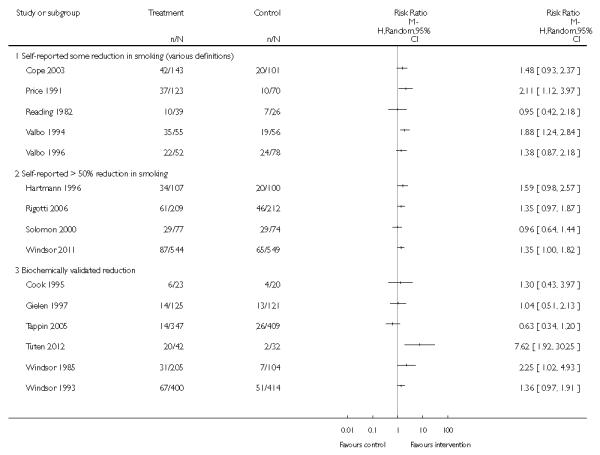
|
Analysis 11.9. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 9 Smoking reduction: biochemical measures in late pregnancy.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 9 Smoking reduction: biochemical measures in late pregnancy
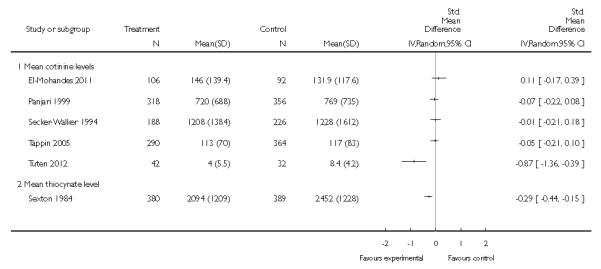
|
Analysis 11.10. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 10 Smoking reduction: self-reported mean cigarettes per day measured in late pregnancy or at delivery.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 10 Smoking reduction: self-reported mean cigarettes per day measured in late pregnancy or at delivery
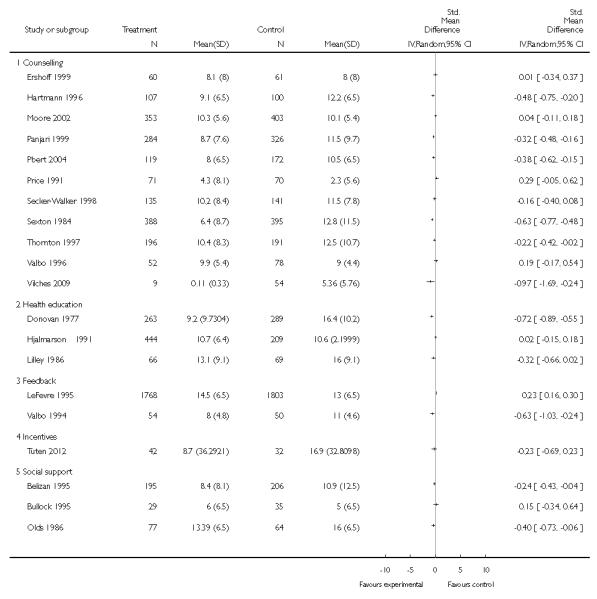
|
Analysis 11.11. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 11 Low birthweight (under 2500 g).
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 11 Low birthweight (under 2500 g)
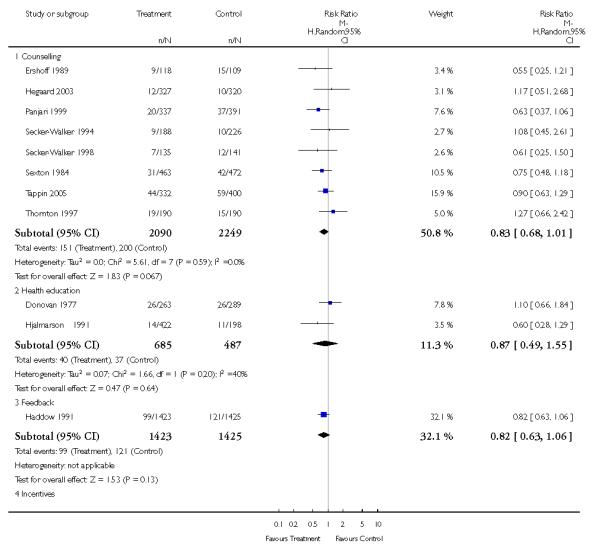
|
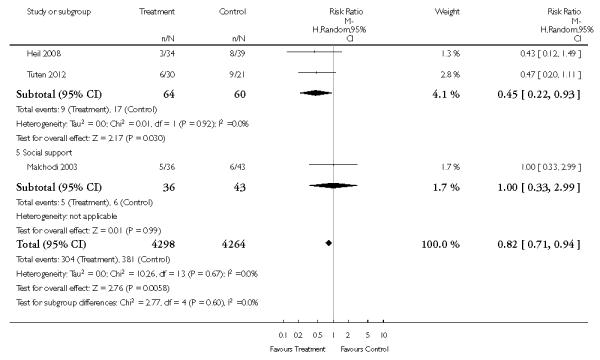
|
Analysis 11.12. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 12 Very low birthweight (under 1500 g).
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 12 Very low birthweight (under 1500 g)
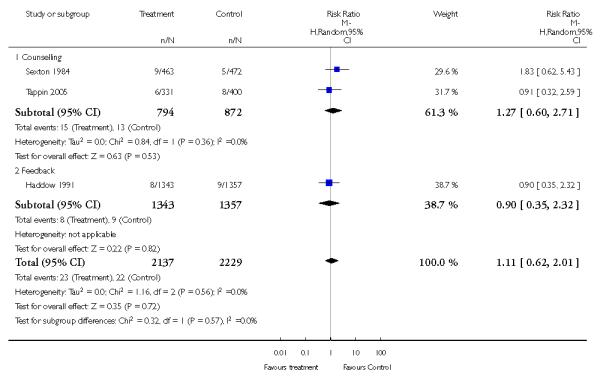
|
Analysis 11.13. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 13 Preterm birth (under 37 weeks).
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 13 Preterm birth (under 37 weeks)
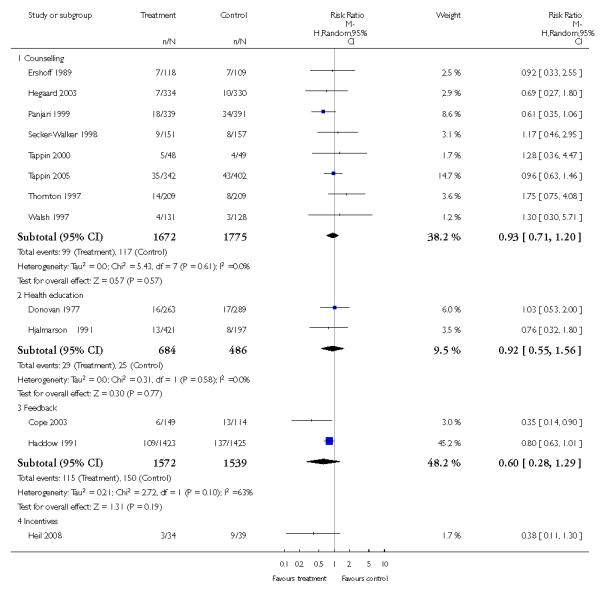
|
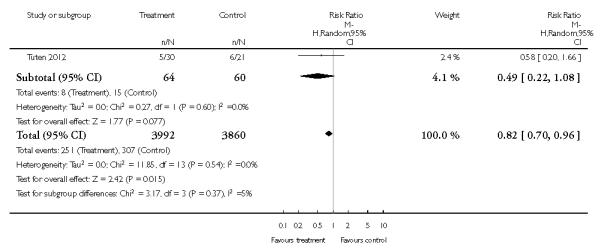
|
Analysis 11.14. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 14 Mean birthweight.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 14 Mean birthweight
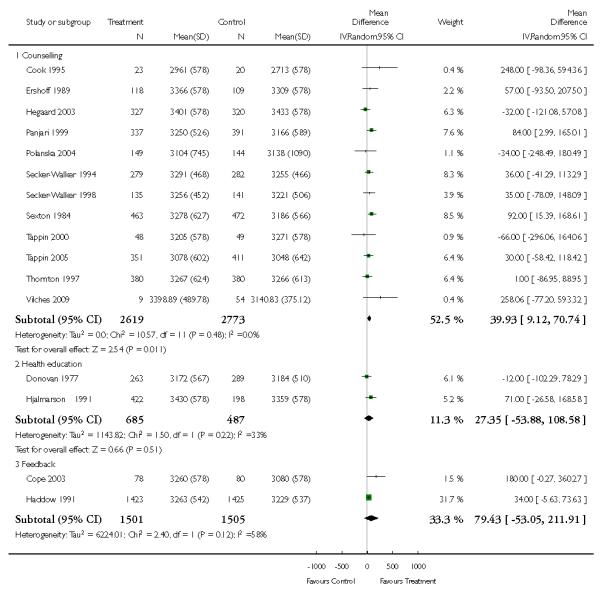
|
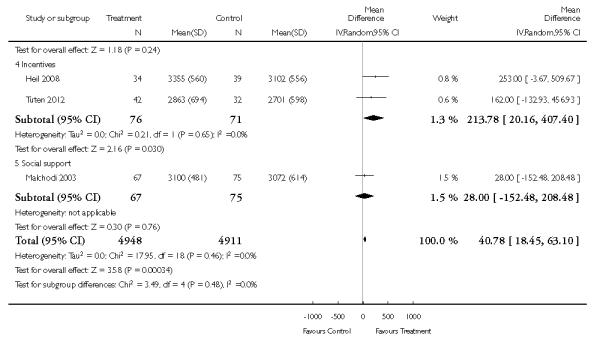
|
Analysis 11.15. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 15 Perinatal deaths.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 15 Perinatal deaths
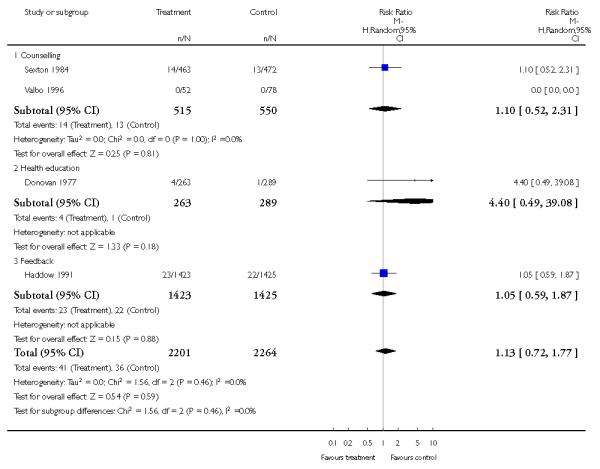
|
Analysis 11.16. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 16 Stillbirths.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 16 Stillbirths
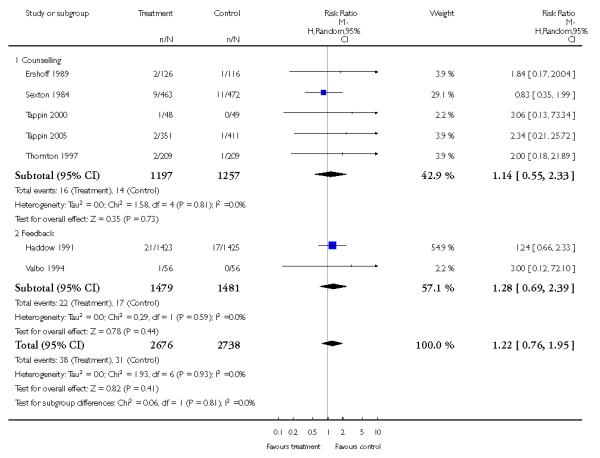
|
Analysis 11.17. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 17 Neonatal deaths.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 17 Neonatal deaths
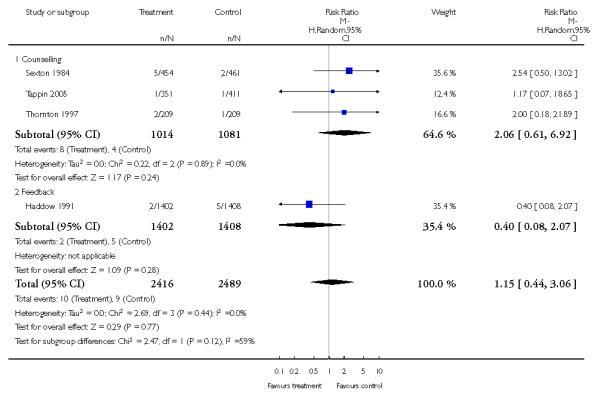
|
Analysis 11.18. Comparison 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy, Outcome 18 NICU admissions.
Review: Psychosocial interventions for supporting women to stop smoking in pregnancy
Comparison: 11 Interventions for smoking cessation in pregnancy versus control: subgrouped by main intervention strategy
Outcome: 18 NICU admissions
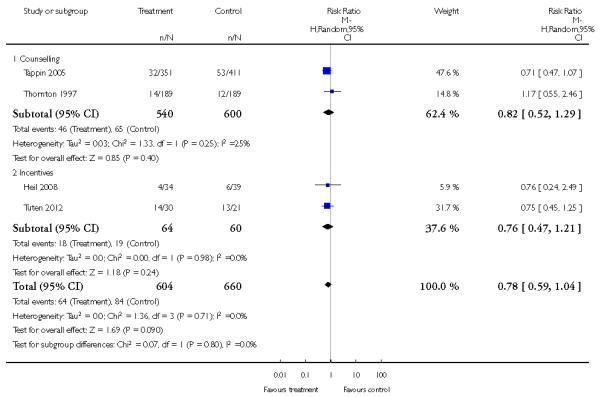
|
References to studies included in this review
- Albrecht 1998 [published data only] .Albrecht S, Cassidy B, Salamie D, Reynolds M. What’s happening. A smoking cessation intervention for pregnant adolescents: implications for nurse practitioners. Journal of American Academy of Nurse Practitioners. 1999;11(4):155–9. doi: 10.1111/j.1745-7599.1999.tb00555.x. [DOI] [PubMed] [Google Scholar]; Albrecht S, Cornelius M, Braxter B, Reynolds M, Stone C, Cassidy B. An assessment of nicotine dependence among pregnant adolescents. Journal of Substance Abuse Treatment. 1999;16(4):337–44. doi: 10.1016/s0740-5472(98)00074-9. [DOI] [PubMed] [Google Scholar]; * [published data only] Albrecht S, Stone CA, Payne L, Reynolds MD. A preliminary study of the use of peer support in smoking cessation programs for pregnant adolescents. Journal of the American Academy of Nurse Practitioners. 1998;10:119–25. doi: 10.1111/j.1745-7599.1998.tb01205.x. [DOI] [PubMed] [Google Scholar]; Albrecht SA, Higgins LW, Stone C. Factors relating to pregnant adolescents’ decisions to complete a smoking cessation intervention. Journal of Pediatric Nursing. 1999;14(5):322–8. doi: 10.1016/S0882-5963(99)80032-3. [DOI] [PubMed] [Google Scholar]
- Albrecht 2006 [published data only] .Albrecht SA, Caruthers D. Characteristics of inner-city pregnant Smoking teenagers. Journal of Obstetric Gynecologic and Neonatal Nursing. 2002;31:462–9. doi: 10.1111/j.1552-6909.2002.tb00069.x. [DOI] [PubMed] [Google Scholar]; * [published data only] Albrecht SA, Caruthers D, Patrick T, Reynolds M, Salamie D, Higgins LW, et al. A randomised controlled trial of a smoking cessation intervention for pregnant adolescents. Nursing Research. 2006;55(6):402–10. doi: 10.1097/00006199-200611000-00004. [DOI] [PubMed] [Google Scholar]; Albrecht SA, Higgins LW, Lebow H. Knowledge about the deletrious effects of smoking and its relationship to smoking cessation among pregnant adolescents. Adolescence. 2000;35(140):709–16. [PubMed] [Google Scholar]; Albrecht SA, Patrick T, Kim Y, Caruthers D. A randomised controlled trial of a smoking cessation intervention for pregnant adolescents. Society for Research on Nicotine and Tobacco 9th Annual Meeting; New Orleans, Louisiana. 2003 February 19-23.2003. p. 91. [Google Scholar]
- Baric 1976 [published data only] .Baric L, MacArthur C. Health norms in pregnancy. British Journal of Preventive and Social Medicine. 1977;31:30–8. doi: 10.1136/jech.31.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Baric L, MacArthur C, Sherwood M. A study of health education aspects of smoking in pregnancy. International Journal of Health Education. 1976;19(2 Suppl):1–17. [Google Scholar]
- Bauman 1983 [published data only] .Bauman KE, Koch GG, Dent CW, Bryan ES. The influence of observing carbon monoxide level on cigarette smoking by public prenatal patients. American Journal of Public Health. 1983;73:1089–91. doi: 10.2105/ajph.73.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizan 1995 [published data only] .* [published data only] Belizan JM, Villar J, Victora C, Farnot U, Langer A, Barros F. Impact of health education during pregnancy on behavior and utilization of health resources. American Journal of Obstetrics and Gynecology. 1995;173:894–9. doi: 10.1016/0002-9378(95)90362-3. [DOI] [PubMed] [Google Scholar]; Victora CG, Langer A, Barros F, Belizan J, Farnot U, Villar J, et al. The Latin American Multicenter Trial on psychosocial support during pregnancy: methodology and baseline comparability. Controlled Clinical Trials. 1994;15:379–94. doi: 10.1016/0197-2456(94)90034-5. [DOI] [PubMed] [Google Scholar]; Villar J, Farnot U, Barros F, Victora C, Langer A, Belizan JM. A randomized trial of psychosocial support during high-risk pregnancies. New England Journal of Medicine. 1992;327:1266–71. doi: 10.1056/NEJM199210293271803. [DOI] [PubMed] [Google Scholar]
- Bullock 1995 [published data only] .Bullock LF, Hornblow AR, Duff GB, Wells JE. Telephone support for pregnant women: outcome in late pregnancy. New Zealand Medical Journal. 1995;108:476–8. [PubMed] [Google Scholar]
- Bullock 2009 [published data only] .* [published data only] Bullock L, Everett KD, Mullen PD, Geden E, Longo DR, Madsen R. Baby BEEP: A randomized controlled trial of nurses’ individualized social support for poor rural pregnant smokers. Maternal and Child Health Journal. 2009;13(3):395–406. doi: 10.1007/s10995-008-0363-z. [DOI] [PubMed] [Google Scholar]; Bullock LF, Everett KD, Mullen PD. Baby beep: a randomized clinical trial of smoking cessation for low-income rural pregnant women using nurse-delivered social support. Annals of Behavioral Medicine. 2008;35:S99. [Google Scholar]
- Burling 1991 [published data only] .Burling TA, Bigelow GE, Robinson JC, Mead AM. Smoking during pregnancy: reduction via objective assessment and directive advice. Behavior Therapy. 1991;22:31–40. [Google Scholar]
- Byrd 1993 [published data only] .Byrd JC, Meade CD. Smoking cessation among pregnant women in an urban setting. Wisconsin Medical Journal. 1993;92:609–12. [PubMed] [Google Scholar]
- Campbell 2006 [published data only] .* [published data only] Campbell E, Walsh RA, Sanson-Fisher, Burrows S, Stojanovski E. A group randomised trial of two methods for disseminating a smoking cessation programme to public antenatal clinics: effects on patient outcomes. Tobacco Control. 2006;15(2):97–102. doi: 10.1136/tc.2004.011049. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cooke M, Mattick R, Campbell E. The influence of individual and organisational factors on the reported smoking intervention practices of staff in 20 antenatal clinics. Drug and Alcohol Review. 1998;17(2):175–85. doi: 10.1080/09595239800186981. [DOI] [PubMed] [Google Scholar]; Cooke M, Mattick RP, Campbell E. A description of the adoption of the ‘Fresh Start’ smoking cessation program by antenatal clinic managers. Australian Journal of Advanced Nursing. 2000;18(1):13–21. [PubMed] [Google Scholar]; Cooke M, Mattick RP, Campbell E. The dissemination of a smoking cessation program to 23 antenatal clinics: the predictors of initial program adoption by managers. Australian and New Zealand Journal of Public Health. 1999;23(1):99–103. doi: 10.1111/j.1467-842x.1999.tb01214.x. [DOI] [PubMed] [Google Scholar]; Cooke M, Mattick RP, Walsh RA. Differential uptake of a smoking cessation programme disseminated to doctors and midwives in antenatal in antenatal clinics. Addiction. 2001;96(3):495–505. doi: 10.1046/j.1360-0443.2001.96349512.x. [DOI] [PubMed] [Google Scholar]; Cooke M, Mattick RP, Walsh RA. Implementation of the ‘Fresh Start’ smoking cessation programme to 23 antenatal clinics: a randomized controlled trial investigating two methods of dissemination. Drug and Alcohol Review. 2001;20:19–28. [Google Scholar]
- Cinciripini 2000 [published data only] .Blalock JA, Fouladi RT, Wetter DW, Cinciripini PM. Depression in pregnant women seeking smoking cessation treatment. Addictive Behaviours. 2005;30(6):1195–208. doi: 10.1016/j.addbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]; Blalock JA, Robinson JD, Wetter DW, Cinciripini PM. Relationship of DSM-IV-Based depressive disorders to smoking cessation and smoking reduction in pregnant women. American Journal on Addictions. 2006;15(4):268–77. doi: 10.1080/10550490600754309. [DOI] [PubMed] [Google Scholar]; * [published data only] Cinciripini PM, McClure JB, Wetter DW, Perry J, Blalock JA, Cinciripini LG, et al. An evaluation of videotaped vignettes for smoking cessation and relapse prevention during pregnancy: The Very Important Pregnant Smokers (VIPS) Program. Tobacco Control. 2000;9(3):iii61–iii63. doi: 10.1136/tc.9.suppl_3.iii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini 2010 [published data only] .Cinciripini PM, Blalock JA, Minnix JA, Robinson JD, Brown VL, Lam C, et al. Effects of an intensive depression-focused intervention for smoking cessation in pregnancy. Journal of Consulting & Clinical Psychology. 2010;78(1):44–54. doi: 10.1037/a0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook 1995 [published data only] .Cook C, Ward S, Myers S, Spinnato J. A prospective, randomized evaluation of intensified therapy for smoking reduction in pregnancy. American Journal of Obstetrics and Gynecology. 1995;172:290. [Google Scholar]
- Cope 2003 [published data only] .Cope G, Nayyar P, Holder R, Gibbons J, Brunce R. A simple near patient test for nicotine and its metabolites in urine to assess smoking habit. Clinical Chimica Acta. 1996;256:135–49. doi: 10.1016/s0009-8981(96)06417-0. [DOI] [PubMed] [Google Scholar]; Cope GF. Smoking status and pregnancy: point of care cotinine test. BMJ. 2009;339:b5652. doi: 10.1136/bmj.b5652. [DOI] [PubMed] [Google Scholar]; * [published data only] Cope GF, Nayyar P, Holder R. Feedback from a point-of-care test for nicotine intake to reduce smoking during pregnancy. Annals of Clinical Biochemistry. 2003;40(Pt 6):674–9. doi: 10.1258/000456303770367289. [DOI] [PubMed] [Google Scholar]; Cope GF, Nayyar P, Holder R. Measurement of nicotine intake in pregnant women - associations to changes in blood cell count. Nicotine & Tobacco Research. 2001;3(2):119–22. doi: 10.1080/14622200110042645. [DOI] [PubMed] [Google Scholar]
- Donatelle 2000 [published data only] .Donatelle RJ, Hudson D. Using 5 A’s and incentives to promote prenatal smoking cessation. National Conference of Tobacco or Health; San Francisco, California, USA. 2002 November 19-21.2002. [Google Scholar]; Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine and Tobacco Research. 2004;6(S2):S163–S179. doi: 10.1080/14622200410001669196. [DOI] [PubMed] [Google Scholar]; * [published data only] Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Supporter (SOS) program. Tobacco Control. 2000;9(Suppl 3):iii67–iii69. doi: 10.1136/tc.9.suppl_3.iii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan 1977 [published data only] .* [published data only] Donovan J. Randomised controlled trial of anti-smoking advice in pregnancy. British Journal of Preventive and Social Medicine. 1977;31(1):6–12. doi: 10.1136/jech.31.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Donovan JW. Randomised controlled trial of anti-smoking advice in pregnancy. Journal of Epidemiology and Community Health. 1996;50(3):232–6. doi: 10.1136/jech.50.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]; Donovan JW, Burgess PL, Hossack CM, Yudkin GD. Routine advice against smoking in pregnancy. Journal of the Royal College of General Practitioners. 1975;25(153):264–8. [PMC free article] [PubMed] [Google Scholar]
- Dornelas 2006 [published data only] .* [published data only] Dornelas EA, Magnavita J, Beazoglou T, Fischer EH, Oncken C, Lando H, et al. Efficacy and cost-effectiveness of a clinic-based counseling intervention tested in an ethnically diverse sample of pregnant smokers. Patient Education and Counseling. 2006;64(1-3):342–9. doi: 10.1016/j.pec.2006.03.015. [DOI] [PubMed] [Google Scholar]; Morasco BJ, Dornelas EA, Fischer EH, Oncken C, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: a preliminary investigation. Addictive Behaviors. 2006;31(2):203–10. doi: 10.1016/j.addbeh.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Dunkley 1997 [published data only] .Dunkley J. Training midwives to help pregnant women stop smoking. Nursing Times. 1997;93(5):64–6. [PubMed] [Google Scholar]
- Eades 2012 [published data only] .* [published data only] Eades SJ, Sanson-Fisher RW, Wenitong M, Panaretto K, D’Este C, Gilligan C, et al. An intensive smoking intervention for pregnant Aboriginal and Torres Strait Islander women: a randomised controlled trial. Medical Journal of Australia. 2012;197(1):42–6. doi: 10.5694/mja11.10858. [DOI] [PubMed] [Google Scholar]; Gilligan C. A pilot randomised controlled trial to test the effectiveness of an intervention to help Aboriginal and Torres Strait Islander women to quit smoking during pregnancy: study design and preliminary results [thesis] University of Newcastle; Newcastle, Australia: 2008. [Google Scholar]; Gilligan C, Sanson-Fisher R, Eades S, Wenitong M, Panaretto K, D’Este C. Assessing the accuracy of self-reported smoking status and impact of passive smoke exposure among pregnant Aboriginal and Torres Strait Islander women using cotinine biochemical validation. Drug and Alcohol Review. 2010;29:35–40. doi: 10.1111/j.1465-3362.2009.00078.x. [DOI] [PubMed] [Google Scholar]; Gilligan C, Sanson-Fisher RW, D-Este C, Eades S, Wenitong M. Knowledge and attitudes regarding smoking during pregnancy among Aboriginal and Torres Strait Islander women. Medical Journal of Australia. 2009;190(10):557–61. doi: 10.5694/j.1326-5377.2009.tb02562.x. [DOI] [PubMed] [Google Scholar]; Panaretto KS, Mitchell MR, Anderson L, Gilligan C, Buettner P, Larkins SL, et al. Tobacco use and measuring nicotine dependence among urban Indigenous pregnant women. Medical Journal of Australia. 2009;191(10):554–7. doi: 10.5694/j.1326-5377.2009.tb03309.x. [DOI] [PubMed] [Google Scholar]
- El-Mohandes 2011 [published data only] .Blake S, El-Mohandes A, Schwartz D, El-Khorazaty N, Gantz M, Joseph J, et al. Promoting smoking cessation during pregnancy and preventing postpartum relapse [abstract]. Pediatric Academic Societies Annual Meeting; Washington DC, USA. 2005 May 14-17; 2005. Abstract no: 3074. [Google Scholar]; Blake S, Joseph J, Schwartz D, El-Khorazaty N, Gantz M, El-Mohandes A, et al. Preventing prenatal and postpartum environmental tobacco smoke (ETS) exposure [abstract]. Pediatric Academic Societies Annual Meeting; Washington DC, USA. 2005 May 14-17; Abstract no: 2353. [Google Scholar]; Blake SM, Murray KD, El-Khorazaty MN, Gantz MG, Kiely M, Best D, et al. Environmental tobacco smoke avoidance among pregnant African-American nonsmokers. American Journal of Preventive Medicine. 2009;36(3):225–34. doi: 10.1016/j.amepre.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; El-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AA, Subramanian S, Laryea HA, et al. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] El-Mohandes AA, El-Khorazaty MN, Kiely M, Gantz MG. Smoking cessation and relapse among pregnant African-American smokers in Washington, DC. Maternal & Child Health Journal. 2011;15(Suppl 1):S96–S105. doi: 10.1007/s10995-011-0825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; El-Mohandes AA, Kiely M, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to reduce environmental tobacco smoke exposure improves pregnancy outcomes. Pediatrics. 2010;125(4):721–8. doi: 10.1542/peds.2009-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]; El-Mohandes AA, Kiely M, Joseph JG, Subramanian S, Johnson AA, Blake SM, et al. An intervention to improve postpartum outcomes in African-American mothers: a randomized controlled trial. Obstetrics & Gynecology. 2008;112(3):611–20. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]; El-Mohandes AAE. A psycho-behavioral intervention on African American pregnant women with a history of intimate partner violence (IPV) improves birth weight distribution of their newborns [abstract]. Pediatric Academic Societies Annual Meeting; San Francisco, CA, USA. 2006 April 29-May 2. [Google Scholar]; El-Mohandes AAE. An integrated behavioral intervention reduces rates of moderate and extreme prematurity in African American (AA) mothers with a history of smoking during pregnancy [abstract]. Pediatric Academic Societies Annual Meeting; San Francisco, CA, USA. 2006 April 29-May 2. [Google Scholar]; El-Mohandes AAE, Kiely M, Gantz MG, El-Khorazaty N. A multiple risk factor behavioral intervention reduces environmental tobacco smoke exposure. Pediatric Academic Societies Annual Meeting; Toronto, Canada. 2007 May 5-8. [Google Scholar]; El-Mohandes AAE, for the NIH-DC initiative to reduce infant mortality An integrated psycho-behavioral intervention during pregnancy has significant effects in reducing risks during the post-partum period in African-American women. Pediatric Academic Societies Annual Meeting; Washington DC, USA. 2005 May 14-17; Abstract no: 39. [Google Scholar]; El-Mohandes AEE, Kiely M, Gantz MG, El-Khorazaty N. Very preterm birth is reduced in women receiving an integrated behavioural intervention: A randomized controlled trial. Maternal Child Health Journal. 2011;15:19–28. doi: 10.1007/s10995-009-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Joseph J, for NIH-DC initiative to reduce infant mortality Randomized trial to reduce 4 behaviors linked to adverse pregnancy outcomes among 1048 inner-city African American women [abstract]. Pediatric Academic Societies Annual Meeting; Washington DC, USA. 2005 May 14-17; Abstract no: 1701. [Google Scholar]; Joseph JG, El-Mohandes AA, Kiely M, El-Khorazaty MN, Gantz MG, Johnson AA, et al. Reducing psychosocial and behavioral pregnancy risk factors: results of a randomized clinical trial among high-risk pregnant African American women. American Journal of Public Health. 2009;99(6):1053–61. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]; Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, et al. The design, implementation and acceptability of an integrated intervention to address multiple behavioural and psychosocial risk factors among pregnant African American women. BMC Pregnancy and Childbirth. 2008;8(22) doi: 10.1186/1471-2393-8-22. [DOI: 10.1186/1471–2393-8-22] [DOI] [PMC free article] [PubMed] [Google Scholar]; Kiely M, El-Khorazaty MN, El-Mohandes AAE. Depression and smoking during pregnancy impact the efficacy of an integral behavioral intervention to resolve risks. Pediatric Academic Societies Annual Meeting; Toronto, Canada. 2007 May 5-8. [Google Scholar]; Subramanian S, Katz KS, Rodam M, Gantz MG, El-Khorazaty NM, Johnson A, et al. An integrated randomized intervention to reduce behavioural and psychosocial risks: Pregnancy and neonatal outcomes. Maternal Child Health Journal. 2012;16:545–54. doi: 10.1007/s10995-011-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tan S, Courtney LP, El-Mohandes AAE, Gantz MG, Blake SM, Thornberry J, et al. Relationships between self-reported smoking, household environmental tobacco smoke exposure and depressive symptoms in a pregnant minority population. Maternal Child Health Journal. 2011;15:S65–S74. doi: 10.1007/s10995-011-0876-8. [DOI] [PubMed] [Google Scholar]
- Ershoff 1989 [published data only] .Ershoff DH, Lairson DR, Mullen PD, Quinn VP. Pregnancy and medical cost outcomes of a self-help prenatal smoking cessation program in an HMO. Public Health Reports. 1990;105(4):340–7. [PMC free article] [PubMed] [Google Scholar]; * [published data only] Ershoff DH, Quinn VP, Mullen PD. A randomized trial of a serialized self-help smoking cessation program for pregnant women in an HMO. American Journal of Public Health. 1989;79(2):182–7. doi: 10.2105/ajph.79.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ershoff DH, Quinn VP, Mullen PD. Relapse prevention among women who stop smoking early in pregnancy: a randomized clinical trial of a self-help intervention. American Journal of Preventive Medicine. 1995;11(3):178–84. [PubMed] [Google Scholar]; Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. American Journal of Obstetrics and Gynecology. 1991;165:409–13. doi: 10.1016/0002-9378(91)90105-z. [DOI] [PubMed] [Google Scholar]; Quinn VP, Mullen PD, Ershoff DH. Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addictive Behaviour. 1991;16(1-2):29–40. doi: 10.1016/0306-4603(91)90037-i. [DOI] [PubMed] [Google Scholar]
- Ershoff 1999 [published data only] .* [published data only] Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking cessation trial. When more isn’t better, what is enough?. American Journal of Preventive Medicine. 1999;17(3):161–8. doi: 10.1016/s0749-3797(99)00071-9. [DOI] [PubMed] [Google Scholar]; Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking cessation trial: when more isn’t better, what is enough? Tobacco Control. 2000;9(Suppl 3):iii60. doi: 10.1136/tc.9.suppl_3.iii60. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ershoff DH, Solomon LJ, Dolan-Mullen P. Predictors of intentions to stop smoking early in prenatal care. Tobacco Control. 2000;9(3):41. doi: 10.1136/tc.9.suppl_3.iii41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen 1997 [published data only] .Gielen AC, Windsor R, Faden RR, O’Campo P, Repke J, Davis M. Evaluation of a smoking cessation intervention for pregnant women in an urban prenatal clinic. Health Education Research. 1997;12(2):247–54. doi: 10.1093/her/12.2.247. [DOI] [PubMed] [Google Scholar]
- Graham 1992 [published data only] .Graham AV, Reeb KG, Kitson GC, Zyzanski SJ, Frank SH. A clinical trial to reduce the rate of low birth weight in an inner-city black population. Family Medicine. 1992;24:439–46. [PubMed] [Google Scholar]
- Haddow 1991 [published data only] .Haddow JE, Polomak JE, Sepulveda D. Smoking cessation during routine public prenatal care. American Journal of Public Health. 1995;85(10):1451–2. doi: 10.2105/ajph.85.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Haddow JE, Wald NJ, Palomaki GE, Kloza EM, Knight GJ. Cotinine-assisted intervention in pregnancy to reduce smoking and low birthweight delivery. British Journal of Obstetrics and Gynaecology. 1991;98(9):859–65. doi: 10.1111/j.1471-0528.1991.tb13506.x. [DOI] [PubMed] [Google Scholar]
- Hajek 2001 [published data only] .Hajek P, West R, Lee A, Foulds J, Owen L, Eiser JR, et al. Randomized controlled trial of a midwife-delivered brief smoking cessation intervention in pregnancy. Addiction. 2001;96(3):485–94. doi: 10.1046/j.1360-0443.2001.96348511.x. [DOI] [PubMed] [Google Scholar]
- Hartmann 1996 [published data only] .Hartmann K, Thorp J, Pahel-Short L, Koch M. A randomized controlled trial of smoking cessation intervention in pregnancy. American Journal of Obstetrics and Gynecology. 1995;172:287. doi: 10.1016/0029-7844(95)00492-0. [DOI] [PubMed] [Google Scholar]; * [published data only] Hartmann KE, Koch MA, Pahel-Short L, Thorp JM. A randomized controlled trial of smoking cessation intervention in pregnancy in an academic clinic. Obstetrics & Gynecology. 1996;87:621–6. doi: 10.1016/0029-7844(95)00492-0. [DOI] [PubMed] [Google Scholar]
- Haug 1994 [published data only] .Haug K, Fugelli P, Aaro LE. Recruitment and participation of General Practitioners in a multipractice study of smoking cessation. Scandinavian Journal of Primary Health Care. 1992;10(3):206–10. doi: 10.3109/02813439209014062. [DOI] [PubMed] [Google Scholar]; * [published data only] Haug K, Fugelli P, Aaro LE, Foss OP. Is smoking intervention in general practice more successful among pregnant than non-pregnant women? Family Practice. 1994;11:111–6. doi: 10.1093/fampra/11.2.111. [DOI] [PubMed] [Google Scholar]
- Haug 2004 [published data only (unpublished sought but not used)] .Haug NA, DiClemente C, Svikis DS. Motivational enhancement therapy for nicotine dependence in methadone-maintained pregnant women. Psychology of Addictive Behaviours. 2004;18(3):289–92. doi: 10.1037/0893-164X.18.3.289. [DOI] [PubMed] [Google Scholar]
- Hegaard 2003 [published data only] .* [published data only] Hegaard H, Hjaergaard H, Moller L, Wachmann H, Ottesen B. Multimodel intervention raises smoking cessation rate during pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2003;82:813–9. doi: 10.1034/j.1600-0412.2003.00221.x. [DOI] [PubMed] [Google Scholar]; Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnanct non-smokers. Acta Obstetricia et Gynecologica. 2007;86:401–6. doi: 10.1080/00016340601147517. [DOI] [PubMed] [Google Scholar]; Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Long-term nicotine replacement therapy. British Journal of Midwifery. 2004;12(4):214–20. [Google Scholar]
- Heil 2008 [published data only] .Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birthweight. Obstetrics & Gynecology. 2005;106(5 Pt 1):986–91. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]; Heil SH, Higgins ST. Characterizing nicotine withdrawal and craving in pregnant cigarette smokers. 66th Annual Scientific Meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. 2004 June 12-17.2004. [Google Scholar]; * [published data only] Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–18. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heil SH, Higgins ST, Solomon LJ, Lynch ME, McHale L, Dumeer A, et al. Voucher-based incentives for abstinence from cigarette smoking in pregnant and postpartum women. Society for Research on Nicotine and Tobacco 13th Annual Meeting; Austin, Texas. 2007 Feb 21-24; 2007. p. 25. Abstract no: PA6-1. [Google Scholar]; Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: effects on abstinence and withdrawal. Nicotine and Tobacco Research. 2003;5(2):205–13. doi: 10.1080/1462220031000074864. [DOI] [PubMed] [Google Scholar]; Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105:2023–30. doi: 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Higgins ST, Heil SH, Badger GJ, Mongeon JA, Solomon LJ, McHale L, et al. Biochemical verification of smoking status in pregnant and recently postpartum women. Experimental and Clinical Psychopharmacology. 2007;15(1):58–66. doi: 10.1037/1064-1297.15.1.58. [DOI] [PubMed] [Google Scholar]; Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug and Alcohol Dependence. 2006;85:138–41. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]; Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6(6):1015–20. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]; Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine & Tobacco Research. 2010;12(5):483–8. doi: 10.1093/ntr/ntq031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviours. 2009;34:705–8. doi: 10.1016/j.addbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]; Solomon LJ, Higgins ST, Heil SH, Badger GJ, Thomas CS, Bernstein IM. Predictors of postpartum relapse to smoking. Drug and Alcohol Dependence. 2007;90:224–7. doi: 10.1016/j.drugalcdep.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Washio Y, Higgins ST, Heil SH, Badger GJ, Skelly J, Bernstein IM, et al. Examining maternal weight gain during contingency-management treatment for smoking cessation among pregnant women. Drug and Alcohol Dependence. 2011;114(1):73–6. doi: 10.1016/j.drugalcdep.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15(2):176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- Hennrikus 2010 [published data only] .Hennrikus D, Pirie P, Hellerstedt W, Lando HA, Steele J, Dunn C. Increasing support for smoking cessation during pregnancy and postpartum: results of a randomized controlled pilot study. Preventive Medicine. 2010;50(3):134–7. doi: 10.1016/j.ypmed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Hiett 2000 [published data only] .Hiett A, Brazus S, Hedberg J, Brown H. Smoking cessation program effectiveness during pregnancy. American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 2):S150. [Google Scholar]
- Hjalmarson 1991 [published data only] .* [published data only] Hjalmarson AIM, Svanberg B, Hahn L. Stopping smoking in pregnancy: effect of a self-help manual in a controlled trial. British Journal of Obstetrics and Gynaecology. 1991;98:260–4. doi: 10.1111/j.1471-0528.1991.tb13390.x. [DOI] [PubMed] [Google Scholar]; Svanberg B. Smoking during pregnancy: possibilities of prevention in antenatal care. International Journal of Technology Assessment in Health Care. 1992;8(Suppl 1):96–100. doi: 10.1017/s0266462300012976. [DOI] [PubMed] [Google Scholar]
- Hughes 2000 [published data only] .* [published data only] Hughes E, Lamont D, Beecroft M, Wilson D. Randomized trial of a “stage-of-change” orientated smoking cessation intervention in infertile and pregnant women. Fertility and Sterility. 2000;74(3):498–503. doi: 10.1016/s0015-0282(00)00687-7. [DOI] [PubMed] [Google Scholar]; Hughes EG, Beecroft ML, Lamont D, Rice S, Wilson D, Freebury M, et al. A randomised controlled trial of a “State-of Change” smoking cessation intervention for subfertile and pregnant patients. Fertility and Sterility. 1999;72(3 Suppl 1):S61–S62. doi: 10.1016/s0015-0282(00)00687-7. [DOI] [PubMed] [Google Scholar]
- Kendrick 1995 [published data only] .England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]; Kendrick JS, Metzger RW, Sexton M, Spierto FW, Floyd RL, Gargiullo PM, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. American Journal of Public Health. 1995;85:217–22. doi: 10.2105/ajph.85.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sierto FW, Hannon WH, Kendrick JS, Bernert JT, Pirkle J, Gargiullo PM. Urinary cotinine levels in women enrolled in a smoking cessation study during and after pregnancy. Journal of Smoking-Related Disease. 1994;5:65–76. [Google Scholar]
- Lawrence 2003 [published data only] .Aveyard P, Lawrence T, Cheng KK, Griffin C, Croghan E, Johnson C. A randomized controlled trial of smoking cessation for pregnant women to test the effect of a transtheoretical model-based intervention on movement in stage and interaction with baseline stage. British Journal of Health Psychology. 2006;11:263–78. doi: 10.1348/135910705X52534. [DOI] [PubMed] [Google Scholar]; Aveyard P, Lawrence T, Croghan E, Evans O, Cheng KK. Is advice to stop smoking from a midwife stressful for pregnant women who smoke? Data from a randomized controlled trial. Preventive Medicine. 2004;40:575–82. doi: 10.1016/j.ypmed.2004.07.012. [DOI] [PubMed] [Google Scholar]; Aveyard P, Lawrence T, Evans O, Cheng KK. The influence of in-pregnancy smoking cessation programmes on partner quitting and women’s social support mobilization: a randomized controlled trial. BMC Public Health. 2005;5:80. doi: 10.1186/1471-2458-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aveyard P, West R. Managing smoking cessation. BMJ. 2007;335:37–41. doi: 10.1136/bmj.39252.591806.47. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking advice in pregnancy result in long-term quitters? 18-month post-partum follow up of a randomised controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting; Prague. 2005 March 20-23; Czech Republic; 2005. [DOI] [PubMed] [Google Scholar]; Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking cessation advice in pregnancy result in long term quitters? 18-month postpartum follow-up of a randomized controlled trial. Addiction. 2005;100:107–16. doi: 10.1111/j.1360-0443.2005.00936.x. [DOI] [PubMed] [Google Scholar]; Lawrence T, Aveyard P, Croghan E. What happens to women’s self-reported cigarette consumption and urinary cotinine levels in pregnancy? Addiction. 2003;98:1315–20. doi: 10.1046/j.1360-0443.2003.00485.x. [DOI] [PubMed] [Google Scholar]; * [published data only] Lawrence T, Aveyard P, Evans O, Cheng KK. A cluster randomised controlled trial of smoking cessation in pregnant women comparing interventions based on the transtheoretical (stages of change) model to standard care. Tobacco Control. 2003;12:168–77. doi: 10.1136/tc.12.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre 1995 [published data only] .Ewigman B, Crane J, Frigoletto F, LeFevre M, Bain R, McNellis D. Effect of prenatal ultrasound screening on perinatal outcome. New England Journal of Medicine. 1993;329:821–9. doi: 10.1056/NEJM199309163291201. [DOI] [PubMed] [Google Scholar]; LeFevre ML, Ewigman B, Evans JK. Is smoking an indication for prenatal ultrasonography? RADIUS Study Group. Archives of Family Medicine. 1995;4:120–3. doi: 10.1001/archfami.4.2.120. [DOI] [PubMed] [Google Scholar]
- Lilley 1986 [published data only] .Lilley J, Forster DP. A randomised controlled trial of individual counselling of smokers in pregnancy. Public Health. 1986;100:309–15. doi: 10.1016/s0033-3506(86)80053-1. [DOI] [PubMed] [Google Scholar]
- Lillington 1995 [published data only] .Lillington L, Chlebowski R, Ruvalcaba M, Novak D, Royce J. Evaluation of a smoking cessation program for pregnant minority smokers. Cancer Practice. 1995;3(3):157–63. [PubMed] [Google Scholar]
- Loeb 1983 [published data only] .Bailey JW, Loeb BK, Waage G. A randomized trial of smoking intervention during pregnancy. Proceedings of the American Public Health Association 111th Annual Meeting; Dallas, Texas, USA. 1983 Nov 15.1983. p. 58. [Google Scholar]; * [published data only] Loeb BK, Waage G, Bailey J. Smoking intervention in pregnancy. Proceedings of the Fifth World Conference on Smoking and Health; Winnipeg, Canada. Ottawa. 1983 July; Canadian Council on Smoking and Health; 1983. pp. 389–95. [Google Scholar]
- Lowe 1997 [published data only] .Lowe JB, Windsor R, Balanda K, Woodby L. Smoking relapse prevention methods for pregnant women: a formative evaluation. American Journal of Health Promotion. 1997;11:244–6. doi: 10.4278/0890-1171-11.4.244. [DOI] [PubMed] [Google Scholar]
- Lowe 2002 * [published data only] .Lowe JB, Balanda KP, Stanton WR, Del Mar C, O’Connor V. Dissemination of an efficacious antenatal smoking cessation program in public hospitals in Australia: a randomised controlled trial. Health Education & Behavior. 2002;29(5):608–19. doi: 10.1177/109019802237028. [DOI] [PubMed] [Google Scholar]; Stanton WR, Lowe JB, Moffatt J, DelMar CB. Randomised control trial of a smoking cessation intervention directed at men whose partners are pregnant. Preventive Medicine. 2004;38:6–9. doi: 10.1016/j.ypmed.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Malchodi 2003 [published data only] .Malchodi CS, Oncken C, Dornelas EA, Caramanica L, Gregonis E. The effects of peer counselling on smoking cessation and reduction. Obstetrics & Gynecology. 2003101(3):504–10. doi: 10.1016/s0029-7844(02)03070-3. [DOI] [PubMed] [Google Scholar]
- Manfredi 1999 [published data only] .* [published data only] Manfredi C, Crittenden KS, Warnecke R, Engler J, Cho YI, Shaligram C. Evaluation of a motivational smoking cessation intervention for women in public health clinics. Preventive Medicine. 1999;28:51–60. doi: 10.1006/pmed.1998.0377. [DOI] [PubMed] [Google Scholar]; Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: the impact of timevarying pregnancy status, healthcare messages, stress and health concerns. Addictive Behaviors. 2007;32:1347–66. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manfredi C, Cho YI, Warnecke R, Saunders S, Sullivan M. Dissemination strategies to improve implementation of the PHS smoking cessation guideline in MCH public health clinics: experimental evaluation results and contextual factors. Health Education Research. 2011;26(2):348–60. doi: 10.1093/her/cyr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer 1990 [published data only] .Mayer JP, Todd R, Hawkins B. A randomised evaluation of smoking cessation interventions for pregnant women at a WIC clinic. American Journal of Public Health. 1990;80:76–7. doi: 10.2105/ajph.80.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride 1999 [published data only] .Curry SJ, McBride C, Grothus L, Lando H, Pirie P. Motivation for smoking cessation among pregnant women. Psychology of Addictive Behaviors. 2001;15(2):126–32. doi: 10.1037//0893-164x.15.2.126. [DOI] [PubMed] [Google Scholar]; Lando HA, Valanis BG, Lichtenstein E, Curry SJ, McBride CM, Pirie PL, et al. Promoting smoking abstinence in pregnant and postpartum patients: a comparison of 2 approaches. American Journal of Managed Care. 2001;7:685–93. [PubMed] [Google Scholar]; McBride CM, Curry SJ, Grothaus LC, Nelson JC. Partner smoking status and pregnant smokers’ perception of support for and likelihood of smoking cessation. Health Psychology. 1998;17:63–9. doi: 10.1037//0278-6133.17.1.63. [DOI] [PubMed] [Google Scholar]; McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. American Journal of Public Health. 1999;89:706–11. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride 2004 [published data only] .McBride CM, Baucom DH, Peterson BL, Pollak KI, Palmer C, Westman E, et al. Prenatal and postpartum smoking abstinence: a partner assisted approach. American Journal of Preventive Medicine. 2004;27(3):232–8. doi: 10.1016/j.amepre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McLeod 2004 [published data only] .McLeod D, Benn C, Pullon S, Viccars A, White S, Cookson T, et al. The midwife’s role in facilitating smoking behaviour change during pregnancy. Midwifery. 2003;19(4):285–97. doi: 10.1016/s0266-6138(03)00038-x. [DOI] [PubMed] [Google Scholar]; * [published data only] McLeod D, Pullon S, Benn C, Cookson T, Dowell A, Viccars A, et al. Can support and education for smoking cessation and reduction be provided effectively by midwives within primary maternity care? Midwifery. 2004;20:37–50. doi: 10.1016/S0266-6138(03)00051-2. [DOI] [PubMed] [Google Scholar]; Pullon S, McCleod D, Benn C, Viccars A, White S, Cookson T, et al. Smoking cessation in New Zealand: education and resources for use by midwives for women who smoke during pregnancy. Health Promotion International. 2003;18(4):315–24. doi: 10.1093/heapro/dag405. [DOI] [PubMed] [Google Scholar]
- Messimer 1989 [published data only] .Messimer SR, Henry RC, Hickner JM. A comparison of two antismoking interventions among pregnant women in eleven primary care practices. Journal of Family Practice. 1989;28(3):283–8. [PubMed] [Google Scholar]
- Moore 1998 [published data only] .* [published data only] Moore ML, Meis PJ, Ernest JM, Wells HB, Zaccaro DJ, Terrell T. A randomized trial of nurse intervention to reduce preterm and low birth weight births. Obstetrics & Gynecology. 1998;91:656–61. doi: 10.1016/s0029-7844(98)00012-x. [DOI] [PubMed] [Google Scholar]; Moore ML, Zaccaro DJ. Cigarette smoking, low birth weight, and preterm births in low-income African-American women. Journal of Perinatology. 2000;3:176–80. doi: 10.1038/sj.jp.7200336. [DOI] [PubMed] [Google Scholar]
- Moore 2002 [published data only] .Moore LO, Campbell R, Whelan A, Mills N, Lupton P, Misselbrook E, et al. Self help smoking cessation in pregnancy: cluster randomised controlled trial. BMJ. 2002;325:1383–6. doi: 10.1136/bmj.325.7377.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton 2012 [published data only] .Naughton F, Prevost AT, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self-help intervention for pregnant smokers (MiQuit) Nicotine and Tobacco Research. 2012;14(5):569–77. doi: 10.1093/ntr/ntr254. [DOI] [PubMed] [Google Scholar]
- Olds 1986 [published data only] .Olds DL, Henderson CR, Chamberlin R, Tatelbaum R. Preventing child abuse and neglect: a randomized trial of nurse home visitation. Pediatrics. 1986;78:65–78. [PubMed] [Google Scholar]; Olds DL, Henderson CR, Tatelbaum R. Prevention of intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:228–33. [PubMed] [Google Scholar]; * [published data only] Olds DL, Henderson CR, Tatelbaum R, Chamberlin R. Improving the delivery of prenatal care and outcomes of pregnancy: a randomized trial of nurse home visitation. Pediatrics. 1986;77:16–28. [PubMed] [Google Scholar]
- Olds 2002 [published data only] .Olds D, Robinson J, O’Brien R, Luckey D, Pettit L, Henderson C, et al. Home visiting by paraprofessionals and by nurses: a randomized, controlled trial. Pediatrics. 2002;110(3):486–96. doi: 10.1542/peds.110.3.486. [DOI] [PubMed] [Google Scholar]
- Ondersma 2012 [published data only] .Ondersma S, Svikis DS, Beatty JR, Lockhart N. A randomized clinical trial of a computer delivered brief intervention for post-partum drug, alcohol, and tobacco use: three-month outcomes. Proceedings of the 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence; Hollywood, Florida. 2011 June 18-23; [Accessed 22/8/2013:134]. 2011. http://www.cpdd.vcu.edu/Pages/Meetings/Meetings.PDFs/2011Programbook.pdf. Abstract no: 534. [Google Scholar]; Ondersma SJ. Computer-assisted Intervention for smoking during pregnancy (HPP) ClinicalTrials.gov. 2009. ; Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment. 2005;28:305–12. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine & Tobacco Research. 2012;Vol. 14(issue 3):351–60. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjari 1999 [published data only] .Panjari M, Bell R, Astbury J, Bishop S, Dalais F, Rice G. Women who spontaneously quit smoking in early pregnancy. Australian New Zealand Journal of Obstetrics and Gynaecology. 1997;37(3):271. doi: 10.1111/j.1479-828x.1997.tb02407.x. [DOI] [PubMed] [Google Scholar]; * [published data only] Panjari M, Bell R, Bishop S, Astbury J, Rice G, Doery J. A randomized controlled trial of a smoking cessation intervention during pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1999;39(3):312–7. doi: 10.1111/j.1479-828x.1999.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Parker 2007 [published data only] .Parker DR, Roberts MB, Windsor RA, Lasater TM. Telephone-based smoking cessation interventions effective in high-risk, underserved pregnant women. Joint Conference of SRNT and SRNT-Europe; Dublin, Ireland. 2009 April 27-30.2099. [Google Scholar]; * [published data only] Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost, and cost-effectiveness of a telephone-based motivational intervention for underserved pregnant smokers. Nicotine and Tobacco Research. 2007;9(10):1043–51. doi: 10.1080/14622200701591617. [DOI] [PubMed] [Google Scholar]
- Patten 2009 [published data only] .Patten CA. Tobacco cessation intervention during pregnancy among Alaska Native women. Journal of Cancer Education. 2012;27(Supp 1):S86–90. doi: 10.1007/s13187-012-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Patten CA. [accessed 21 June 2007];Tobacco cessation treatment for pregnant Alaska natives. ClinicalTrials.gov. http://clinicaltrials.gov/ [Google Scholar]; * [published data only] Patten CA, Windsor RA, Renner CC, Enoch C, Hochreiter A, Nevak C, et al. Feasibility of a tobacco cessation intervention for pregnant Alaska Native women. Nicotine & Tobacco Research. 2009;12(2):79–87. doi: 10.1093/ntr/ntp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pbert 2004 [published data only] .Bonollo DP, Zapka JG, Stoddard AM, Ma Y, Pbert L, Ockene JK. Treating nicotine dependence during pregnancy and postpartum: understanding clinician knowledge and performance. Patient Education and Counselling. 2002;48:265–74. doi: 10.1016/s0738-3991(02)00023-x. [DOI] [PubMed] [Google Scholar]; Ma Y, Goins KV, Pbert L, Ockene JK. Predictors of smoking cessation in pregnancy and maintenance postpartum in low-income women. Maternal and Child Health Journal. 2005;9(4):393–402. doi: 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]; * [published data only] Pbert L, Ockene JK, Zapka J, Ma Y, Goins KV, Oncken C, et al. A community health center smoking cessation intervention for pregnant and postpartum women. American Journal of Preventive Medicine. 2004;26(5):377–85. doi: 10.1016/j.amepre.2004.02.010. [DOI] [PubMed] [Google Scholar]; Zapka J, Goins KV, Pbert L, Ockene JK. Translating efficacy research into effectiveness studies in practice: lessons from research to promote smoking cessation in community health centers. Health Promotion Practice. 2004;5(3):245–55. doi: 10.1177/1524839904263713. [DOI] [PubMed] [Google Scholar]; Zapka JG, Pbert L, Stoddard AM, Ockene JK, Goins KV, Bonollo D. Smoking cessation counseling with pregnant and postpartum women: a survey of community health center providers. American Journal of Public Health. 2000;90:78–84. doi: 10.2105/ajph.90.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen 1992 [published data only] .Peterson L, Rosen A, Podedworny T, Kotch J, Handel J. Smoking reduction during pregnancy by a program of self-help and clinical support. Obstetrics & Gynecology. 1992;79:924–30. [PubMed] [Google Scholar]
- Polanska 2004 [published data only] .Polanska K, Hanke W, Sobala Characteristic of the smoking habit among pregnant women on the base of the test “Why am I a smoker?” [Charakterystyka nagogu palenia papierosow wsrod kobiet ciezarnych na podstawie testu Dlaczego pale?] Przeglad Lekarski. 2005;62(10):1095–8. [PubMed] [Google Scholar]; Polanska K, Hanke W, Sobala W. Smoking relapse one year after delivery among women who quit smoking during pregnancy. International Journal of Occupational Medicine and Environmental Health. 2005;18(2):159–65. [PubMed] [Google Scholar]; * [published data only] Polanska K, Hanke W, Sobala W, Lowe JB. Efficacy and effectiveness of the smoking cessation program for pregnant women. International Journal of Occupational Medicine and Environmental Health. 2004;17(3):369–77. [PubMed] [Google Scholar]
- Price 1991 [published data only] .Price JH, Snyder FF, Roberts SM, Losh DP, Desmond SM, Krol RA. Comparison of three antismoking interventions among pregnant women in an urban setting: a randomized trial. Psychological Reports. 1991;68:595–604. doi: 10.2466/pr0.1991.68.2.595. [DOI] [PubMed] [Google Scholar]
- Reading 1982 [published data only] .Reading AE, Cox DN. The effects of ultrasound on maternal anxiety. Journal of Behavioral Medicine. 1982;5(2):237–47. doi: 10.1007/BF00844812. [DOI] [PubMed] [Google Scholar]; * [published data only] Reading AE, Sledmere CM, Cox DNB, Campbell S. Health beliefs and health care behaviour in pregnancy. Psychological Medicine. 1982;12:379–83. doi: 10.1017/s0033291700046717. [DOI] [PubMed] [Google Scholar]
- Rigotti 2006 [published data only] .Berg CJ, Park ER, Chang Y, Rigotti NA. Is concern about post-cessation weight gain a barrier to smoking cessation among pregnant women? Nicotine & Tobacco Research. 2008;10(7):1159–63. doi: 10.1080/14622200802163068. [DOI] [PubMed] [Google Scholar]; Park ER, Quinn VP, Chang Y, Regan S, Loudin B, Cummins S, et al. Recruiting pregnant smokers into a clinical trial: using a network-model managed care organization versus community-based practices. Preventive Medicine. 2007;44:223–9. doi: 10.1016/j.ypmed.2006.10.008. [DOI] [PubMed] [Google Scholar]; Rigotti N, Park E, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of proactive telephone counseling for pregnant smokers: a randomized trial. Society for Research on Nicotine and Tobacco 12th Annual Meeting; Orlando, Florida, USA. 2006 February 15-18.2006. p. 22. [Google Scholar]; Rigotti N, Park E, Regan S, Chang Y, Perry K, Loudin B, et al. The efficacy of telephone couseling for pregnant smokers: a randomized controlled trial [abstract]. 13th World Conference on Tobacco or Health; Washington DC, USA. 2006 July 12-15; 2006. [DOI] [PubMed] [Google Scholar]; * [published data only] Rigotti N, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers. Obstetrics & Gynecology. 2006;108(1):83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]; Rigotti NA, Park ER, Chang Y, Regan S. Smoking cessation medication use among pregnant and postpartum smokers. Obstetrics & Gynecology. 2008;111(2 Pt 1):348–55. doi: 10.1097/01.AOG.0000297305.54455.2e. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 1994 [published data only] .* [published data only] Secker-Walker RH, Mead PB, Goodwin GD, Lepage SS, Skelly JM, Flynn BS, et al. Individualised smoking cessation counseling during prenatal and early postnatal care. American Journal of Obstetrics and Gynecology. 1994;71:1347–55. doi: 10.1016/0002-9378(94)90159-7. [DOI] [PubMed] [Google Scholar]; Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Lepage SS, Goodwin GD, et al. Smoking relapse prevention counseling during prenatal and early postnatal care. American Journal of Preventive Medicine. 1995;11(2):86–93. [PubMed] [Google Scholar]
- Secker-Walker 1997 [published data only] .Secker-Walker RH, Solomon LJ, Flynn BS, LePage SS, Crammond JE, Worden JK, et al. Training obstetric and family practice residents to give smoking cessation advice during prenatal care. American Journal of Obstetrics and Gynecology. 1992;166:1356–63. doi: 10.1016/0002-9378(92)91604-9. [DOI] [PubMed] [Google Scholar]; * [published data only] Secker-Walker RH, Solomon LJ, Geller BM, Flynn BS, Worden JK, Skelly JM, et al. Modeling smoking cessation: exploring the use of a videotape to help pregnant women quit smoking. Women & Health. 1997;25:23–35. doi: 10.1300/J013v25n01_02. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 1998 [published data only] .Secker-Walker RH, Solomon LJ, Flynn BS, LePage SS, Crammond JE, Worden JK, et al. Training obstetric and family practice residents to give smoking cessation advice during prenatal care. American Journal of Obstetrics and Gynecology. 1992;166:1356–63. doi: 10.1016/0002-9378(92)91604-9. [DOI] [PubMed] [Google Scholar]; * [published data only] Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Reducing smoking during pregnancy and postpartum: physician’s advice supported by individual counseling. Preventive Medicine. 1998;27:422–30. doi: 10.1006/pmed.1998.0287. [DOI] [PubMed] [Google Scholar]; Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Smoking relapse prevention during pregnancy. A trial of coordinated advice from physicians and individual counseling. American Journal of Preventive Medicine. 1998;15:25–31. doi: 10.1016/s0749-3797(98)00029-4. [DOI] [PubMed] [Google Scholar]; Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Estimated gains in birth weight associated with reductions in smoking during pregnancy. Journal of Reproductive Medicine. 1998;43(11):967–74. [PubMed] [Google Scholar]; Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstetrics and Gynecology. 1997;89:648–53. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]; Solomon LJ, Secker-Walker RH, Skelly JM, Flynn BS. Stages of change in smoking during pregnancy in low risk women. Journal of Behavioral Medicine. 1996;19:333–4. doi: 10.1007/BF01904760. [DOI] [PubMed] [Google Scholar]
- Sexton 1984 [published data only] .Fox NL, Sexton M, Hebel JR, Thompson B. The reliability of self-reports of smoking and alcohol consumption by pregnant women. Addictive Behaviors. 1989;14(2):187–95. doi: 10.1016/0306-4603(89)90047-6. [DOI] [PubMed] [Google Scholar]; Fox NL, Sexton MJ, Hebel JR. Alcohol consumption among pregnant smokers: effects of a smoking cessation intervention program. American Journal of Public Health. 1987;77:211–3. doi: 10.2105/ajph.77.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hamilton BH. Estimating treatment effects in randomized clinical trials with non-compliance: the impact of maternal smoking on birthweight. Health Economics. 2001;10(5):399–410. doi: 10.1002/hec.629. [DOI] [PubMed] [Google Scholar]; Hebel JR, Sexton M, Nowicki P. The effect of antismoking intervention during pregnancy: an assessment of interactions with maternal characteristics. American Journal of Epidemiology. 1985;122:135–48. doi: 10.1093/oxfordjournals.aje.a114073. [DOI] [PubMed] [Google Scholar]; Nowicki P, Gintzig L, Hebel JR, Lathem R, Miller V, Sexton M. Effective smoking intervention during pregnancy. Birth. 1984;11:217–24. doi: 10.1111/j.1523-536x.1984.tb00787.x. [DOI] [PubMed] [Google Scholar]; * [published data only] Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA. 1984;251:911–5. [PubMed] [Google Scholar]; Sexton M, Nowicki P, Hebel JR. Verification of smoking status by thiocyanate in unrefrigerated, mailed saliva samples. Preventive Medicine. 1986;15(1):28–34. doi: 10.1016/0091-7435(86)90033-2. [DOI] [PubMed] [Google Scholar]
- Solomon 2000 [published data only] .Solomon LJ, Secker-Walker RH, Flynn BS, Skelly JM, Capeless EL. Proactive telephone peer support to help pregnant women stop smoking. Tobacco Control. 2000;9(Suppl 3):iii72–iii74. doi: 10.1136/tc.9.suppl_3.iii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts 2002 [published data only] .Stotts AL, Schmitz JM, Shipley SL, Delaune KA, Grabowski J. Impact of a motivational interviewing intervention on mechanisms of change in low-income pregnant smokers (POS4-32) [abstract]. Society for Research on Nicotine and Tobacco 9th Annual Meeting; New Orleans, Louisiana. 2003 February 19-22.2003. p. 89. [Google Scholar]; * [published data only] Stotts S, DiClemente CC, Dolan-Mullen P. One-to-One: a motivational intervention for resistant pregnant smokers. Addictive Behaviors. 2002;27:275–92. doi: 10.1016/s0306-4603(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Stotts 2004 [published data only] .Stotts AL, DeLaune KA, Schmitz JM, Grabowski J. Impact of a motivational intervention on mechanisms of change in low-income pregnant women. Addictive Behaviors. 2004;29(8):1649–57. doi: 10.1016/j.addbeh.2004.02.063. [DOI] [PubMed] [Google Scholar]
- Stotts 2009 [published data only] .Groff J, Stotts A, Velasquez M, Benjamin-Garner R, Green C, Mastrobattista J. Ultrasound and motivational enhancement for prenatal smoking cessation. Annual Meeting of the Society for Behavioural Medicine; Boston, MA. 2005 April 13-16.2005. [Google Scholar]; Groff JY. [accessed March 2006];Ultrasound and motivational enhancement for prenatal smoking cessation. ClinicalTrials.gov. http://clinicaltrials.gov/; * [published data only] Stotts AL, Groff JY, Velasquez MM, Benjamin-Garner R, Green C, Carbonari JP, et al. Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine & Tobacco Research. 2009;11(8):961–8. doi: 10.1093/ntr/ntp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher 2000 [published data only] .Strecher VJ, Bishop KR, Bernhardt J, Thorp JM, Cheuvrout B, Potts P. Quits for keeps: tailored smoking cessation guides for pregnancy and beyond. Tobacco Control. 2000;9(Suppl 3):iii78–iii79. doi: 10.1136/tc.9.suppl_3.iii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappin 2000 [published data only] .* [published data only] Tappin DM, Lumsden MA, McIntyre D, McKay C, Gilmour WH, Webber R, et al. A pilot study to establish a randomized trial methodology to test the efficacy of a behavioural intervention. Health Education Research. 2000;15(4):491–502. doi: 10.1093/her/15.4.491. [DOI] [PubMed] [Google Scholar]; Tappin DM, Lumsden MA, McKay C, McIntyre D, Gilmour H, Webber R, et al. The effect of home-based motivational interviewing on the smoking behaviour of pregnant women: a pilot randomised controlled efficacy study. Ambulatory Child Health. 2000;6(Suppl 1):34–5. [Google Scholar]
- Tappin 2005 [published data only] .Tappin DM, Lumsden MA, Gilmour WH, Crawford F, McIntyre D, Stone DH, et al. Randomised controlled trial of home based motivational interviewing by midwives to help pregnant smokers quit or cut down. BMJ. 2005;331:373–7. doi: 10.1136/bmj.331.7513.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton 1997 [published data only] .* [published data only] Thornton L. Smoking and pregnancy: feasibility and effectiveness of a smoking intervention programme among pregnant women [thesis] Dept of Public Health; Dublin: 1997. [Google Scholar]; Thornton L, Gogan C, McKenna P. The rotunda stop smoking programme [abstract] Irish Journal of Medical Science. 1998;167(Suppl 9):28. [Google Scholar]
- Tsoh 2010 [published data only] .Calderón SH, Gilbert P, Jackson R, Kohn MA, Gerbert B. Cueing prenatal providers: effects on discussions of intimate partner violence. American Journal of Preventive Medicine. 2008;34(2):134–7. doi: 10.1016/j.amepre.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gilbert P. [accessed 20 February 2008];The health in pregnancy (HIP) study. ClinicalTrials.gov. 2008 http://clinicaltrials.gov/ [Google Scholar]; * [published data only] Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trial. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):515–23. doi: 10.3109/00016341003678419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuten 2012 [published data only] .Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction. 2012;107(10):1868–77. doi: 10.1111/j.1360-0443.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbo 1994 [published data only] .Valbo A, Nylander G. Smoking cessation in pregnancy: intervention among heavy smokers. Acta Obstetricia et Gynecologica Scandinavica. 1994;73:215–9. doi: 10.3109/00016349409023442. [DOI] [PubMed] [Google Scholar]
- Valbo 1996 [published data only] .Valbo A, Eide T. Smoking cessation in pregnancy: the effect of hypnosis in a randomized study. Addictive Behaviors. 1996;21:29–35. doi: 10.1016/0306-4603(95)00033-x. [DOI] [PubMed] [Google Scholar]
- Vilches 2009 [published and unpublished data] .Aranda Regules JM, Mateos Vilchez P, Gonzalez Villalba A, Sanchez F, de Dios Luna del Castillo J. Validity of smoking measurements during pregnancy: specificity, sensitivity and cut-off points. Revista Espanola de Salud Publica. 2008;82(5):535–45. doi: 10.1590/s1135-57272008000500008. [DOI] [PubMed] [Google Scholar]; * [published data only] Vilches P. Doctoral Thesis. Univesidad di Malaga; 2009. Consumption of tobacco in pregnant women: Proposal of a psychological intervention model in the public health system of Andalucia. [Consumo de tabaco en mujeres gestantes: propuesta de un modelo de intervención psicológica en el sistema sanitario público de Andalucía] [Google Scholar]
- Walsh 1997 [published data only] .* [published data only] Walsh RA, Melmeth A, Byrne JM, Brinsmead MW, Redman S. A smoking cessation program at a public antenatal clinic. American Journal of Public Health. 1997;87:1201–4. doi: 10.2105/ajph.87.7.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]; Walsh RA, Redman S, Byrne JM, Melmeth A, Brinsmead MW. Process measures in an antenatal smoking cessation trial: another part of the picture. Health Education Research. 2000;15(4):469–83. doi: 10.1093/her/15.4.469. [DOI] [PubMed] [Google Scholar]
- Windsor 1985 [published data only] .Windsor RA. The efficacy and cost-effectiveness of smoking cessation methods for pregnant women. Southern Medical Journal. 1986;79:34. [Google Scholar]; * [published data only] Windsor RA, Spanos D, Samuelsson C, Bartlett EE, Manzella B, Reese Y, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: a randomized trial. American Journal of Public Health. 1985;75:1389–92. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]; Windsor RA, Warner KE, Cutter GR. A cost-effectiveness analysis of self-help smoking cessation methods for pregnant women. Public Health Reports. 1988;103(1):83–8. [PMC free article] [PubMed] [Google Scholar]
- Windsor 1993 [published data only] .Windsor RA, Contreras L, Artz L, Lowe JB. Smoking cessation and pregnancy intervention trial: preliminary mid-trial results. Progress in Clinical and Biological Research. 1990;339:107–17. [PubMed] [Google Scholar]; Windsor RA, Li CQ, Boyd NR, Hartmann KE. The use of significant reduction rates to evaluate health education methods for pregnant smokers: a new harm reduction behavioural indicator. Health Education and Behavior. 1999;26:648–61. doi: 10.1177/109019819902600506. [DOI] [PubMed] [Google Scholar]; * [published data only] Windsor RA, Lowe JB, Perkins LL, Smith-Yoder D, Artz L, Crawford M, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. American Journal of Public Health. 1993;83:201–6. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor 2011 [published data only] .Windsor R, Woodby L, Miller T, Hardin M. Effectiveness of Smoking Cessation and Reduction in Pregnancy Treatment (SCRIPT) methods in Medicaid-supported prenatal care: Trial III. Health Education & Behavior. 2011;38(4):412–22. doi: 10.1177/1090198110382503. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Albrecht 2011 [published data only] .Albrecht S, Kelly-Thomas K, Osborne JW, Ogbagaber S. The SUCCESS program for smoking cessation for pregnant women. Journal of Obstetric Gynecologic and Neonatal Nursing. 2011;40:520–31. doi: 10.1111/j.1552-6909.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- Andrews 2007 [published data only] .Andrews JO, Bentley G, Crawford S, Pretlow L, Tingen MS. Using community-based participatory research to develop a culturally sensitive smoking cessation intervention with public housing neighbourhoods. Ethnicity and Disease. 2007;17:326–31. [PubMed] [Google Scholar]; * [published data only] Andrews JO, Felton G, Wewers ME, Waller J, Tingen M. The effect of a multi-component smoking cessation intervention in African American women residing in public housing. Research in Nursing and Health. 2007;30:45–60. doi: 10.1002/nur.20174. [DOI] [PubMed] [Google Scholar]
- Berlin 2008 [published data only] .Berlin I. Study of Nicotine Patch in Pregnancy (SNIPP) ClinicalTrials.gov. 2008.
- Boshier 2003 [published data only] .Boshier A, Wilton LV, Shakir SAW. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. European Journal of Clinical Pharmacology. 2003;59:767–73. doi: 10.1007/s00228-003-0693-0. [DOI] [PubMed] [Google Scholar]
- Bowden 2010 [published data only] .Bowden JA, Oag DA, Smith KL, Miller CI. An integrated brief intervention to address smoking in pregnancy. Acta Obstetricia et Gynecologica. 2010;89:496–504. doi: 10.3109/00016341003713869. [DOI] [PubMed] [Google Scholar]
- Brandon 2012 [published and unpublished data] .* [published data only] Brandon TH, Simmons VN, Meade CD, Quinn GP, Lopez Khoury EN, Sutton SK. Self-help booklets for preventing postpartum smoking relapse: a randomized trial. American Journal of Public Health. 2012;102(11):2109–15. doi: 10.2105/AJPH.2012.300653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lopez E, Simmons MAV, Meade C, Quinn G, Pedraza J, Brandon T. The elusive pregnant ex-smoker: lessons from recruitment for a clinical trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting; Prague, Czech Republic. 2005 20-23 March.2005. [Google Scholar]; Lopez EN, Simmons VN, Quinn GP, Meade CD, Chirikos TN, Brandon TH. Clinical trials and tribulations: lessons learned from recruiting pregnant ex-smokers for relapse prevention. Nicotine & Tobacco Research. 2008;10(1):87–96. doi: 10.1080/14622200701704962. [DOI] [PMC free article] [PubMed] [Google Scholar]; Quinn G, Ellison BB, Meade C, Roach CN, Lopez E, Albrecht T, et al. Adapting smoking relapse-prevention materials for pregnant and postpartum women: formative research. Maternal and Child Health Journal. 2006;10(3):235–45. doi: 10.1007/s10995-005-0046-y. [DOI] [PubMed] [Google Scholar]; Simmons VN, Lopez Khoury EN, Segall Koltz EJ, Quinn G, Meade CD, Unrod M, et al. Preventing smoking relapse among pregnant and postpartum women: A randomised clinical trial. Joint conference of SRNT and SRNT-EUROPE; Dublin, Ireland. April 27–30, 2009.pp. POS2–16. [Google Scholar]
- Britton 2006 [published data only] .Britton GRA, Brinthaupt J, Stehle JM, James GD. The effectiveness of a nurse-managed perinatal smoking cessation program implemented in a rural county. Nicotine and Tobacco Research. 2006;8(1):13–28. doi: 10.1080/14622200500431536. [DOI] [PubMed] [Google Scholar]
- Chan 2005 [published data only] .Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. Journal of Addictive Diseases. 2005;24(2):19–23. doi: 10.1300/J069v24n02_02. [DOI] [PubMed] [Google Scholar]
- Coleman 2007 [published data only] .Coleman T. National Research Register; [accessed 6 July 2006]. Double-blind, randomised, placebo-controlled trial of nicotine replacement therapy in pregnancy (ongoing trial) www.nrr.nhs.uk. [Google Scholar]; Coleman T, Antoniak M, Britton J, Thornton J, Lewis S, Watts K. Recruiting pregnant smokers for a placebo-randomised controlled trial of nicotine replacement therapy. BMC Health Services Research. 2004;4:29. doi: 10.1186/1472-6963-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Coleman T, Thornton J, Britton J, Lewis S, Watts K, Coughtrie MW, et al. Protocol for the smoking, nicotine and pregnancy (snap) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy. BMC Health Services Research. 2007;7:2. doi: 10.1186/1472-6963-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp 2007 [published data only] .Culp AM, Culp RE, Anderson JW, Carter S. Health and safety intervention with first time mothers. Health Education Research. 2007;22(2):285–94. doi: 10.1093/her/cyl079. [DOI] [PubMed] [Google Scholar]
- DeVries 2006 [published data only] .Bakker M. Pregnancy: a window of opportunity to quit smoking! The development, implementation and evaluation of a minimal intervention strategy for pregnant women and partners [thesis] Maastricht Health Research Institute for Prevention and Care; Netherlands: 2001. [Google Scholar]; Bakker MJ, De Vries H, Ausems EMGT. Effectiveness of a multi media smoking cessation and relapse prevention program for pregnant women. 10th World Conference on Tobacco or Health; Beijing, China. 1997 August 24-28.1997. p. 111. [Google Scholar]; Bakker MJ, Dolan-Mullen P, de Vries H, van Breukelen G. Feasibility of implementation of a Dutch smoking cessation and relapse prevention protocol for pregnant women. Patient Education and Counseling. 2003;49(1):35–43. doi: 10.1016/s0738-3991(02)00038-1. [DOI] [PubMed] [Google Scholar]; Bakker MJ, de Vries H, Dolan Mullen P, Kok G. Predictors of perceiving smoking cessation counselling as a midwife’s role: a survey of Dutch midwives. European Journal of Public Health. 2005;15(1):39–42. doi: 10.1093/eurpub/cki109. [DOI] [PubMed] [Google Scholar]; * [published data only] de Vries H, Bakker M, Dolan Mullen P, van Breukelen G. The effects of smoking cessation counseling by midwives on Dutch pregnant women and their partners. Patient Education and Counseling. 2006;63(1-2):177–87. doi: 10.1016/j.pec.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Disantis 2010 [published data only] .Disantis KI, Collins BN, McCoy AC. Associations among breastfeeding, smoking relapse, and prenatal factors in a brief postpartum smoking intervention. Acta Obstetricia et Gynecologica. 2010;89:582–6. doi: 10.3109/00016341003678435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon 2009 [published data only] .Dixon LE, Aimer P, Fletcher L, Guilliland K, Hendry C. Smoke free outcomes with maternity lead maternity carers: An analysis of smoking during pregnancy from the New Zealand College of Midwives Midwifery database 2004 -2007. New Zealand College of Midwives Journal. 2009;40:13–9. [Google Scholar]
- Edwards 2009 [published data only] .Edwards MJ, Geiser T, Chafin C, Weatherby NL, Smith CM. S.M.A.R.T. Mothers are Resisting Tobacco: Prenatal smoking cessation in WIC mothers. Journal of Allied Health. 2009;38(3):170–6. [PubMed] [Google Scholar]
- El-Mohandes 2013 [published data only] .El-Mohandes AA, Windsor R, Tan S, Perry DC, Gantz MG, Kiely M. A randomized clinical trial of trans-dermal nicotine replacement in pregnant african-american smokers. Maternal Child Health Journal. 2013;17(5):897–906. doi: 10.1007/s10995-012-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons 2000 [published data only] .Emmons K, Sorensen G, Klar N, Digianni L, Barclay G, Schmidt K, et al. Healthy baby second-hand smoke study. Tobacco Control. 2000;9(Suppl 3):iii58–iii60. doi: 10.1136/tc.9.suppl_3.iii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershoff 1983 [published data only] .Ershoff DH, Aaronson NK, Danaher BG, Wasserman FW. Behavioral, health and cost outcomes of an HMO-based prenatal health education program. Public Health Reports. 1983;98:536–47. [PMC free article] [PubMed] [Google Scholar]
- Everett-Murphy 2010 [published data only] .* [published data only] Everett-Murphy K, Steyn K, Mathews C, Petersen Z, Odendaal H, Gwebushe N, et al. The effectiveness of adapted, best practice guidelines for smoking cessation couseling with disadvantaged, pregnant smokers attending public sector antenatal clinics in Cape Town, South Africa. Acta Obstetricia et Gynecologica. 2010;89:478–89. doi: 10.3109/00016341003605701. [DOI] [PubMed] [Google Scholar]; Petersen Z, Steyn K, Lombard C, Everett K, Emmelin M. Smoking cessation intervention among pregnant women in South Africa. African Journal of Midwifery and Women’s Health. 2009;3(4):181–6. [Google Scholar]
- Ferguson 2012 [published data only] .Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, Boyd KA, et al. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ. 2012;Vol. 344(issue 7854) doi: 10.1136/bmj.e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Borges 2005 [published data only] .Ferreira-Borges C. Effectiveness of a brief counseling and behavioral intervention for smoking cessation in pregnant women. Preventive Medicine. 2005;41(1):295–302. doi: 10.1016/j.ypmed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fish 2011 [published data only] .* [published data only] Fish LJ, Lyna P, Denman S, Gordon K, Rocha P, Pollak K. Recruitment and retention of Latinos in a couples-based smoking cessation trial. Annals of Behavioral Medicine. 2011;41(Suppl 1):S118. [Google Scholar]; Pollak KI, Denman S, Coop Gordon K, Lyna P, Rocha P, Brouwer RN, et al. Is pregnancy a teachable moment for smoking cessation among US Latino expectant fathers? A pilot study. Ethnicity & Health. 2010;15(1):47–59. doi: 10.1080/13557850903398293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French 2007 [published data only] .French GM, Groner JA, Wewers ME, Ahijevych K. Staying smoke free: an intervention to prevent postpartum relapse. Nicotine and Tobacco Research. 2007;9(6):663–70. doi: 10.1080/14622200701365277. [DOI] [PubMed] [Google Scholar]
- Gadomski 2011 [published data only] .Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Maternal Child Health Journal. 2011;15:188–97. doi: 10.1007/s10995-010-0568-9. [DOI] [PubMed] [Google Scholar]
- Gebauer 1998 [published data only] .Gebauer C, Chung-Ying K, Haynes E, Wewers ME. A nurse-managed smoking cessation intervention during pregnancy. Journal of Obstetric, Gynecologic & Neonatal Nursing. 1998;21:47–53. doi: 10.1111/j.1552-6909.1998.tb02590.x. [DOI] [PubMed] [Google Scholar]
- Gillies 1987 [published data only] .Gillies PA, Power FL, Turner ID, Madely R. Successful antismoking interventions in pregnancy-behaviour change, “clinical indicators” or both?. Proceedings of the 7th World Conference on Tobacco & Health; 1990; Perth, Australia. Perth. Health Department of Western Australia; 1990. pp. 952–8. [Google Scholar]
- Grange 2005 [published data only] .Grange G, Borgne A, Ouazana A, Valensi P, L’Hullier JP, Aubin JP. Obstetrical ultrasound screening and motivation to stop smoking [Echographies obstetricales et motivation du tabac] Journal de Gynécologie, Obstétrique et Biologie de la Reproduction. 2005;34(7 Pt 1):674–8. doi: 10.1016/s0368-2315(05)82900-x. [DOI] [PubMed] [Google Scholar]
- Hahn 2005 [published data only] .Hahn EJ, Rayens MK, Warnick TA, Chirila C, Rasnake RT, Paul TP, et al. A controlled trial of a quit and win contest. American Journal of Health Promotion. 2005;20(2):117–26. doi: 10.4278/0890-1171-20.2.117. [DOI] [PubMed] [Google Scholar]
- Hannover 2008 [published data only] .* [published data only] Hannover W, Thyrian JR, Roske K, Grempler J, Rumpf HJ, John U, et al. Smoking cessation and relapse prevention for postpartum women: results from a randomized controlled trial at 6, 12, 18 and 24 months. Addictive Behaviors. 2008;34:1–8. doi: 10.1016/j.addbeh.2008.07.021. [DOI] [PubMed] [Google Scholar]; Röske K, Hannöver W, Grempler J, Thyrian JR, Rumpf HJ, John U, et al. Post-partum intention to resume smoking. Health Education Research. 2006;21(3):386–92. doi: 10.1093/her/cyh069. [DOI] [PubMed] [Google Scholar]; Röske K, Schumann A, Hannöver W, Grempler J, Thyrian JR, Rumpf HJ, et al. Postpartum smoking cessation and relapse prevention intervention: A structural equation modeling application to behavioral and non-behavioral outcomes of a randomized controlled trial. Journal of Health Psychology. 2008;13(4):556–68. doi: 10.1177/1359105308088528. [DOI] [PubMed] [Google Scholar]; Thyrian JR, Freyer-Adam J, Hannover W, Roske K, Mentzel F, Kufeld C, et al. Population-based smoking cessation in women post partum: adherence to motivational interviewing in relation to client characteristics and behavioural outcomes. Midwifery. 2010;26(2):202–10. doi: 10.1016/j.midw.2008.04.004. [DOI] [PubMed] [Google Scholar]; Thyrian JR, Hannover W, Grempler J, Roske K, Ulrich J, Hapke U. An intervention to support postpartum women to quit smoking or remain smoke-free. Journal of Midwifery & Women’s Health. 2006;51:45–50. doi: 10.1016/j.jmwh.2005.07.002. [DOI] [PubMed] [Google Scholar]; Thyrian JR, Hannover W, Roske K, Rumpf HJ, John U, Hapke U. Postpartum return to smoking: identifying different groups to tailor interventions. Addictive Behaviours. 2006;31:1785–96. doi: 10.1016/j.addbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Herbert 2011 [published data only] .Herbert RJ, Gagnon AJ, O’Loughlin JL, Rennick JE. Testing an empowerment intervention to help parents make homes smoke-free: A randomized controlled trial. Journal of Community Health. 2011;36:650–7. doi: 10.1007/s10900-011-9356-8. [DOI] [PubMed] [Google Scholar]
- Higgins 2004 [published data only] .Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6(6):1015–20. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hotham 2006 [published data only] .Atkinson E, Hotham L, Baghurst P. Nicotine replacement therapy as an adjunct to smoking cessation counselling in pregnancy - a randomised study to evaluate efficacy in an antenatal clinic setting. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2003;43:177. [Google Scholar]; * [published data only] Hotham ED, Gilbert AL, Atkinson ER. A randomised-controlled pilot study using nicotine patches with pregnant women. Addictive Behaviors. 2006;31(4):641–8. doi: 10.1016/j.addbeh.2005.05.042. [DOI] [PubMed] [Google Scholar]; Hotham ED, Gilbert AL, Atkinson ER. Case studies of three pregnant smokers and their use of nicotine replacement therapy. Midwifery. 2005;21:224–32. doi: 10.1016/j.midw.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Hymowitz 2006 [published data only] .Hymowitz N, Schwab J, Eckholdt H. Pediatric residency training on tobacco. Pediatrics. 2001;108(1):e8. doi: 10.1542/peds.108.1.e8. [DOI] [PubMed] [Google Scholar]; * [published data only] Hymowitz N, Schwab J, Haddock CK, Pyle S, Meshberg S. The pediatric resident training on tobacco project: interim findings. Journal of the National Medical Association. 2006;98(2):190–203. [PMC free article] [PubMed] [Google Scholar]; Hymowitz N, Schwab J, Haddock CK, Pyle S, Moore G, Meshberg S. The pediatric resident training on tobacco project: baseline findings from the parent/guardian tobacco survey. Preventive Medicine. 2005;41(1):334–41. doi: 10.1016/j.ypmed.2004.11.019. [DOI] [PubMed] [Google Scholar]; Hymowitz N, Schwab M, McNerney C, Schwab J, Eckholdt H, Haddock K. Postpartum relapse to cigarette smoking in inner city women. Journal of the National Medical Association. 2003;95(6):461–74. [PMC free article] [PubMed] [Google Scholar]
- Jaakola 2001 [published data only] .Jaakola N, Zahlsen K, Jaakola J. Effects of a population based smoking cessation programme on smoking in pregnancy. European Journal of Public Health. 2001;11:446–9. doi: 10.1093/eurpub/11.4.446. [DOI] [PubMed] [Google Scholar]
- Johnston 2011 [published data only] .Johnston V, Thomas DP, McDonnell J, Andrews RM. Maternal smoking and smoking in the household during pregnancy and postpartum: findings from an Indigenous cohort in the Northern Territory. Medical Journal of Australia. 2011;194(10):556–9. doi: 10.5694/j.1326-5377.2011.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Kaper 2006 [published data only] .Kaper J, Wagena EJ, Willemsen MC, van Schayck CP. A randomized controlled trial to assess the effects of reimbursing the costs of smoking cessation therapy on sustained abstinence. Addiction. 2006;101:1656–61. doi: 10.1111/j.1360-0443.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- Kapur 2001 [published data only] .Kapur B, Hackman R, Selby P, Klein J, Koren G. Randomized, double blind, placebo-controlled trial of nicotine replacement therapy in pregnancy. Current Therapeutic Research. 2001;62(4):274–8. [Google Scholar]
- Karatay 2010 [published data only] .Karatay G, Kublay G, Emiroglu ON. Effect of motivational interviewing on smoking cessation in pregnant women. Journal of Advanced Nursing. 2010;66(6):1328–37. doi: 10.1111/j.1365-2648.2010.05267.x. [DOI] [PubMed] [Google Scholar]
- Kazemi 2012 [published data only] .Kazemi A. [accessed 6 December 2010];The effect of education on reducing passive smoking in pregnant women. IRCT Iranian Registry of Clinical Trials. 2010 www.irct.ir.; * [published data only] Kazemi A, Ehsanpour S, Nekoei-Zahraei NS. A randomized trial to promote health belief and to reduce environmental tobacco smoke exposure in pregnant women. Health Education Research. 2012;27(1):151–9. doi: 10.1093/her/cyr102. [DOI] [PubMed] [Google Scholar]
- Kientz 2005 [published data only] .Kientz E, Kupperschmidt B. KICCS: a successful strategy to promote smoking cessation in women during and post pregnancy. Oklahoma Nurse. 2005;50(4):27–30. [PubMed] [Google Scholar]
- Koren 2009 [published data only] .Koren G. [accessed 4 January 2009];Study of nicotine replacement therapy in pregnancy. ClinicalTrials.gov. 2009 http://clinicaltrials.gov/ [Google Scholar]
- Langford 1983 [published data only] .Langford ER, Thompson EG, Tripp SC. Smoking and health education during pregnancy: evaluation of a program for women in prenatal classes. Canadian Journal of Public Health. 1983;74:285–9. [PubMed] [Google Scholar]
- Lee 2008 [published data only] .Lee AH. A pilot intervention for pregnant women in Sichuan, China on passive smoking. Patient Education and Counseling. 2008;71:396–401. doi: 10.1016/j.pec.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke 2005 [published data only] .Loke AY, Lam TH. A randomized controlled trial of the simple advice given by obstetricians in Guangzhou, China, to non-smoking pregnant women to help their husbands quit smoking. Patient Education and Counseling. 2005;59(1):31–7. doi: 10.1016/j.pec.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Lowe 1998a [published data only] .Lowe JB, Balanda KP, Clare G. Evaluation of antenatal smoking cessation programs for pregnant women. Australian and New Zealand Journal of Public Health. 1998;22:55–9. doi: 10.1111/j.1467-842x.1998.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Lowe 1998b [published data only] .Lowe JB, Balanda KP, Clare G. Evaluation of antenatal smoking cessation programs for pregnant women. Australian and New Zealand Journal of Public Health. 1998;22:55–9. doi: 10.1111/j.1467-842x.1998.tb01145.x. [DOI] [PubMed] [Google Scholar]
- MacArthur 1987 [published and unpublished data] .MacArthur C, Knox EG. The Needs of Parents and Infants: Proceedings of a Symposium. The Health Promotion Trust; Cambridge: 1989. Stopping smoking in pregnancy and birthweight; pp. 41–55. [Google Scholar]; MacArthur C, Knox EG, Lancashire RJ. Effects at the age of nine of maternal smoking in pregnancy: experimental and observational findings. BJOG: an international journal of obstetrics and gynaecology. 2001;108(1):67–73. doi: 10.1111/j.1471-0528.2001.00006.x. [DOI] [PubMed] [Google Scholar]; * [published data only] MacArthur C, Knox EG, Newton JR. Effect of anti-smoking health education on infant size at birth: a randomized controlled trial. British Journal of Obstetrics and Gynaecology. 1987;94:295–300. doi: 10.1111/j.1471-0528.1987.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Mauriello 2011 [published data only] .Mauriello L, Dyment S, Prochaska J, Gagliardi A, Weingrad-Smith J. Acceptability and feasibility of a multiple-behaviour, computer-tailored intervention for underserved pregnant women. Journal of Midwifery and Women’s Health. 2011;56:75–80. doi: 10.1111/j.1542-2011.2010.00007.x. [DOI] [PubMed] [Google Scholar]
- Miller 2003 [published data only (unpublished sought but not used)] .Miller H, Ranger-Moore J, Hingten M. Bupropion sr for smoking cessation in pregnancy: a pilot study [abstract] American Journal of Obstetrics and Gynecology. 2003;189(6 Suppl 1):S133. [Google Scholar]
- Mullen 1997 [published data only] .Mullen PD, DiClemente CC, Bartholomew LK. Project PANDA: The importance of theory and context in a program to help women who stop smoking remain abstinent after pregnancy. In: Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, editors. Intervention Mapping: A Process for Designing Theory- and Evidence-based Health Promotion Programs. Mayfield: McGraw-Hill; Palo Alto, CA: 2001. pp. 453–477. [Google Scholar]; Mullen PD, DiClemente CC, Carbonari JP, Micol L, Sockrider MM, Richardson M, et al. Project PANDA maintenance of prenatal smoking abstinence postpartum at 6 weeks, and 3, 6, and 12 months. Annals of Behavioural Medicine. 1997;Vol. 19:130. [Google Scholar]; Mullen PD, Quinn VP, Ershoff DH. Maintenance of nonsmoking postpartum by women who stopped smoking during pregnancy. American Journal of Public Health. 1990;80(8):992–4. doi: 10.2105/ajph.80.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Mullen PD, Richardson MA, Quinn VP, Ershoff DH. Postpartum return to smoking: who is at risk and when. American Journal of Health Promotion. 1997;11(5):323–30. doi: 10.4278/0890-1171-11.5.323. [DOI] [PubMed] [Google Scholar]; Ruggiero L, Webster K, Peipert JF, Wood C. Identification and recruitment of low-income pregnant smokers. Who are we missing? Addictive Behaviours. 2003;28:1497–505. doi: 10.1016/s0306-4603(02)00269-1. [DOI] [PubMed] [Google Scholar]; Sockrider MM, Hudmon KS, Addy R, Dolan Mullen P. An exploratory study of control of smoking in the home to reduce infant exposure to environmental tobacco smoke. Nicotine and Tobacco Research. 2003;5(6):901–10. doi: 10.1080/14622200310001615240. [DOI] [PubMed] [Google Scholar]
- Murray 2008 [published data only] .Murray RL, Coleman T, Antoniak M, Stocks J, Fergus A, Brfitton J, et al. The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: a cluster-randomized trial. Addiction. 2008;103(6):998–1006. doi: 10.1111/j.1360-0443.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- O’Connor 1992 [published data only] .O’Connor AM, Benzie RJ, McBride BH, Nadon C, Buhler PL, Dulberg CS, et al. Effectiveness of a pregnancy smoking cessation program. Journal of Obstetric, Gynecologic & Neonatal Nursing. 1992;21:385–92. doi: 10.1111/j.1552-6909.1992.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Oncken 2008 [published data only] .Oncken C. [accessed 31 July 2009];Pilot study of nicotine replacement for smoking cessation during pregnancy. ClinicalTrials.gov. 2009 http://clinicaltrials.gov. [Google Scholar]; Oncken C, Campbell W, Chan G, Hatsukami D, Kranzler HR. Effects of nicotine patch or nasal spray on nicotine and cotinine concentrations in pregnant smokers. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22(9):751–8. doi: 10.3109/14767050902994515. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Feinn R, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstetrics & Gynecology. 2008;112(4):859–67. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Kranzler HR. Nicotine replacement treatment for pregnant smokers. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. 2008 June 14-19.2008. p. 143. [Google Scholar]; Oncken C, Morris B, Dornelas E, Kranzler H, Walsh S, Greene J. The efficacy and safety of a fixed versus titrated dosage regimen of nicotine gum for smoking cessation or reduction in pregnancy. American Journal of Obstetrics and Gynecology. 2006;195(6 Suppl 1):S89. [Google Scholar]
- Peden 2008 [published data only] .Peden AR, Rayens MK, Hall LA, Hahn E, Riker C, Ashford K, et al. Nicotine addiction in pregnancy: preliminary efficacy of a mental health intervention. Addictive Disorders and their Treatment. 2008;7(4):179–89. [Google Scholar]
- Phillips 2012 [published data only] .* [published data only] Phillips RM, Merritt TA, Goldstein MR, Deming DD, Slater LE, Angeles DM. Prevention of postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. Journal of Perinatology. 2012;32(5):374–80. doi: 10.1038/jp.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phillips RM, Merritt TA, Goldstein MR, Job JS, Rudatsikira EM. Prevention of postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. Pediatric Academic Societies’ 2010 Annual Meeting; Vancouver, Canada. 2010 May 1-4; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska 2011 [published data only] .Polanska K, Hanke W, Sobala W, Lowe JB, Jaakkola JJK. Predictors of smoking relapse after delivery: Prospective study in Central Poland. Maternal Child Health Journal. 2011;15:579–86. doi: 10.1007/s10995-010-0639-y. [DOI] [PubMed] [Google Scholar]
- Pollak 2007 [published data only] .Fish LJ, Peterson BL, Namenek Brouwer RJ, Lyna P, Oncken CA, Swamy GK, et al. Adherence to nicotine replacement therapy among pregnancy smokers. Nicotine and Tobacco Research. 2009;11(5):514–8. doi: 10.1093/ntr/ntp032. [DOI] [PMC free article] [PubMed] [Google Scholar]; Myers E. [accessed 21 June 2007];Testing pharmacological therapies for pregnant smokers. ClinicalTrials.gov. http://clinicaltrials.gov/ [Google Scholar]; Pollak KI, Oncken C, Lipkus IM, Peterson BL, Swamy GK, Pletsch PK, et al. Effectiveness of adding nicotine replacement therapy to cognitive behavioral therapy for smoking cessation in pregnant smokers: the Baby Steps trial. [Abstract no: PA6-3]. Society for Research on Nicotine and Tobacco 13th Annual Meeting; Austin, Texas. 2007 Feb 21-24.2007. p. 25. [Google Scholar]; * [published data only] Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Plettsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. American Journal of Preventive Medicine. 2007;33(4):297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pollak KI, Oncken CA, Lipkus IM, Peterson BL, Swany GK, Pletsch PK, et al. Challenges and solutions for recruiting pregnant smokers into a nicotine replacement therapy trial. Nicotine and Tobacco Research. 2006;8(4):547–54. doi: 10.1080/14622200600789882. [DOI] [PubMed] [Google Scholar]; Swamy GK, Roelands JJ, Peterson BL, Fish LJ, Oncken CA, Pletsch PK, et al. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. American Journal of Obstetrics & Gynecology. 2009;201(4):354, e1–7. doi: 10.1016/j.ajog.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power 1989 [published data only] .Power FL, Gillies PA, Madeley R, Abbott M. Research in an antenatal clinic - the experience of the Nottingham Mothers’ Stop Smoking Project. Midwifery. 1989;5(3):106–12. doi: 10.1016/s0266-6138(89)80024-5. [DOI] [PubMed] [Google Scholar]
- Ratner 1999 [published data only] .Ratner PA, Johnson JL, Bottorff JL. Smoking relapse and early weaning among postpartum women: is there an association? Birth. 1999;26(1):76–82. doi: 10.1046/j.1523-536x.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- Reitzel 2010 [published data only] .Heppner WL, Ji L, Reitzel LR, Castro Y, Correa-Fernandez V, Vidrine JI, et al. The role of prepartum motivation in the maintenance of postpartum smoking abstinence. Health Psychology. 2011;30(6):736–45. doi: 10.1037/a0025132. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Vidrine JI, et al. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine & Tobacco Research. 2010;12(10):983–8. doi: 10.1093/ntr/ntq132. [DOI] [PMC free article] [PubMed] [Google Scholar]; * [published data only] Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Costello TJ, Li Y, et al. Preventing postpartum smoking relapse among diverse low-income women: a randomized clinical trial. Nicotine & Tobacco Research. 2010;12(4):326–35. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush 1992 [published data only] .King J, Eiser JR. A strategy for counselling pregnant smokers. Health Education Journal. 1981;40:66–8. [Google Scholar]; * [published data only] Rush D, Butler NR, Eiser JR, King J, Orme J. A trial of health education aimed to reduce cigarette smoking. Paediatric and Perinatal Epidemiology. 1992;6:285–97. doi: 10.1111/j.1365-3016.1992.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Scott 2000 [published data only] .Scott WJ, McIlvain H. Interactive software: an educational/behavioural approach to smoking cessation for pregnant women and their families. Tobacco Control. 2000;9(Suppl 3):III56–III57. doi: 10.1136/tc.9.suppl_3.iii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare 1990 [published data only] .Shakespeare R. The development and evaluation of a smoking cessation counselling training programme for midwives. Proceedings of the 7th World Conference on Tobacco & Health; 1990; Perth; Australia. Perth. Health Department of Western Australia; 1990. pp. 950–1. [Google Scholar]
- Stanton 2004 [published data only] .Stanton WR, Lowe JB, Moffatt J, Del Mar CB. Randomised control trial of a smoking cessation intervention directed at men whose partners are pregnant. Preventive Medicine. 2004;38:6–9. doi: 10.1016/j.ypmed.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Suplee 2004 [published data only] .Suplee PD. The importance of providing smoking relapse counseling during the postpartum hospitalization. Journal of Gynecological and Neonatal Nursing. 2005;34(6):703–12. doi: 10.1177/0884217505281861. [DOI] [PubMed] [Google Scholar]
- Sutton 2007 [published data only] .Sutton S, Gilbert H. Effectiveness of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self-help materials: randomized controlled trial. Addiction. 2007;102:994–1000. doi: 10.1111/j.1360-0443.2007.01831.x. [DOI] [PubMed] [Google Scholar]
- Valanis 2001 [published data only] .Valanis B, Lichtenstein E, Mullooly JP, Labuhn K, Brody K, Severson H, et al. Maternal smoking cessation and relapse prevention during health care visits. American Journal of Preventive Medicine. 2001;20(1):1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- Valbo 1991 [published data only] .Valbo A, Schioldborg P. Smoking cessation in pregnancy: mode of intervention and effect. Acta Obstetricia et Gynecologica Scandinavica. 1991;70:309–13. doi: 10.3109/00016349109007878. [DOI] [PubMed] [Google Scholar]
- Wadland 2007 [published data only] .Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Annals of Family Medicine. 2007;5(2):135–42. doi: 10.1370/afm.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins 2004 [published data only] .Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al. The social support and family health study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas. Health Technology Assessment. 2004;8(32):1–120. doi: 10.3310/hta8320. [DOI] [PubMed] [Google Scholar]
- Wilkinson 2010 [published data only] .Wilkinson SA, Miller YD, Watson B. The effects of a woman-focused, woman-held resource on preventive health behaviours during pregnancy: the pregnancy pocketbook. Women and Health. 2010;50:342–58. doi: 10.1080/03630242.2010.498756. [DOI] [PubMed] [Google Scholar]
- Windsor 2000a [published data only] .Crawford MA, Woodby LL, Russell TV, Windsor RA. Using formative evaluation to improve a smoking cessation intervention for pregnant women. Health Communication. 2005;17(3):265–81. doi: 10.1207/s15327027hc1703_4. [DOI] [PubMed] [Google Scholar]; Hahn EJ, Rayens MK, Warnick TA, Chrila C, Rasnake RT, Paul TP, et al. A controlled trial of a quit and win contest. American Journal of Health Promotion. 2005;20:117–26. doi: 10.4278/0890-1171-20.2.117. [DOI] [PubMed] [Google Scholar]; * [published data only] Windsor RA, Woodby L, Miller T, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of agency for health care policy and research clinical practice guideline and patient education methods for pregnant smokers in medicaid maternity care. American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 1):68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]; Windsor RA, Woodby LL, Crawford MA, Hardin JM. Effectiveness and cost benefit of the smoking cessation or reduction in pregnancy treatment model in Medicaid maternity care. Society for Research on Nicotine and Tobacco 7th Annual Meeting; Seattle, Washington. 2001 March 20-23.2001. [Google Scholar]
- Winickoff 2010 [published data only] .Winickoff JP, Healey EA, Regan S, Park ER, Cole C, Friebely J, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics. 2010;125(3):518–25. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- Wisborg 1998 [published data only] .Wisborg K, Henriksen TB, Secher NJ. A prospective intervention study of stopping smoking in pregnancy in a routine antenatal care setting. British Journal of Obstetrics and Gynaecology. 1998;105:1171–6. doi: 10.1111/j.1471-0528.1998.tb09970.x. [DOI] [PubMed] [Google Scholar]
- Wisborg 2000 [published data only] .Wisborg K. Nicotine patches to pregnant smokers - a randomised study. 1st International Conference of the Society for Research on Nicotine and Tobacco; Copenhagen, Denmark. 1998 August 22-23.1998. [Google Scholar]; * [published data only] Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: a randomized controlled study. Obstetrics & Gynecology. 2000;96(6):967–71. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- Yilmaz 2006 [published data only] .Yilmaz G, Karacan C, Yoney A, Yilmaz T. Brief intervention on maternal smoking: a randomized controlled trial. Child: Care, Health and Development. 2006;32(1):73–9. doi: 10.1111/j.1365-2214.2006.00570.x. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Althabe 2012 [published data only] .Althabe F, Berrueta M, Mazzoni A, Morello P, Aleman A, Colomar M. Prenatal tobacco cessation intervention for women in Latin America. International Journal of Gynecology and Obstetrics. 2012;119(Suppl 3):S165. [Google Scholar]
- Blasco Oliete 2004 [published data only] .Blasco Oliete M, Sanz Cuesta T, Girbés Fontana M, Pascual Malanda M, Ortiz Valdepeñas J, Garcia Lopez L. Effectiveness of two health interventions to get pregnant women to give up smoking [Efectividad de dos intervenciones sanitarias para conseguir el abandono del consumo de tabaco.] Atencion Primaria. 2004;33(5):277–83. doi: 10.1016/S0212-6567(04)79414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett 2008 [published data only] .Everett KD, Debnam KJ, Gwede CK. Smoking behaviors of women and men in a smoking cessation clinical trial during pregnancy. Annals of Behavioral Medicine. 2008;35(Suppl 1):S199. [Google Scholar]
- Lasater 2007 [published data only] .Lasater T. [accessed 21 March 2006];Reducing ETS exposure of pregnant women and newborns. ClinicalTrials.gov. http://clinicaltrials.gov/; Lasater TM. [accessed 21 June 2007];Tailored videos to reduce tobacco smoke exposure among pregnant women and newborns. ClinicalTrials.gov. http://clinicaltrials.gov/
- Loukopoulou 2011 [published data only] .Loukopoulou AN, Vardavas CI, Farmakides G, Rossolymos C, Chrelias C, Tzatzarakis MN, et al. Design and study protocol of the maternal smoking cessation during pregnancy study, (M-SCOPE) BMC Public Health. 2011;11:903. doi: 10.1186/1471-2458-11-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh 2012 [published data only] .Lynagh M, Bonevski B, Sanson-Fisher R, Symonds I, Scott A, Hall A, et al. An RCT protocol of varying financial incentive amounts for smoking cessation among pregnant women. BMC Public Health. 2012;12:1032. doi: 10.1186/1471-2458-12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejdoubi 2011 [published data only] .Mejdoubi J, van den Heijkant S, Struijf E, van Leerdam F, HiraSing R, Crijnen A. Addressing risk factors for child abuse among high risk pregnant women: design of a randomised controlled trial of the nurse family partnership in Dutch preventive health care. BMC Public Health. 2011;11:823. doi: 10.1186/1471-2458-11-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling 2012 [published data only] .Robling M. Evaluating the family nurse partnership programme in England: a randomised controlled trial. 2012 http://www.controlled-trials.com/ISRCTN23019866.ISRCTN23019866.
- Ruger 2008 [published data only] .Ruger JP, Emmons KM, Kearney MH, Weinstein MC. Measuring the costs of outreach motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women. BMC Pregnancy and Childbirth. 2009;9:46. doi: 10.1186/1471-2393-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM. Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: a randomized controlled trial. Value in Health. 2008;11(2):191–8. doi: 10.1111/j.1524-4733.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappin 2012 [published data only] .Tappin DM, Bauld L, Tannahill C, de L, Radley A, McConnachie A, et al. The Cessation in Pregnancy Incentives Trial (CPIT): study protocol for a randomized controlled trial. Trials. 2012;13:113. doi: 10.1186/1745-6215-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher 2012 [published data only] .Ussher M, Aveyard P, Manyonda I, Lewis S, West R, Lewis B, et al. Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial. Trials. 2012;13(1):186. doi: 10.1186/1745-6215-13-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu 2004 [published data only] .Zhu SH, Cummins S, Anderson C, Tedeschi G, Rosbrook B, Gutierrez-Terrell E. Telephone intervention for pregnant smokers: a randomized trial. Society for Research on Nicotine and Tobacco 10th Annual Meeting; Phoenix, Arizona. 2004 February 18-21.2004. [Google Scholar]
Additional references
- Abatemarco 2007 .Abatemarco DJ, Steinberg MB, Delnevo CD. Midwives’ knowledge, perceptions, beliefs, and practice supports regarding tobacco dependence treatment. Journal of Midwifery & Women’s Health. 2007;52:451–7. doi: 10.1016/j.jmwh.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Abdullah 2004 .Abdullah ASM, Husten CG. Promotion of smoking cessation in developing countries: a framework for urgent public health interventions. Thorax. 2004;59:623–30. doi: 10.1136/thx.2003.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams 1998 .Adams K, Melvin C. Costs of maternal conditions attributable to smoking during pregnancy. American Journal of Preventive Medicine. 1998;15(3):212–9. doi: 10.1016/s0749-3797(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Adams 2002 .Adams EK, Miller VP, Ernst C, Nishimura BK, Melvin C, Merritt R. Neonatal health care costs related to smoking during pregnancy. Health Economics. 2002;11:193–206. doi: 10.1002/hec.660. [DOI] [PubMed] [Google Scholar]
- Adams 2011 .Adams EK, Melvin CL, Raskind-Hood C, Joski PJ, Galactionova E. Infant delivery costs related to maternal smoking: an update. Nicotine & Tobacco Research. 2011;13:627–37. doi: 10.1093/ntr/ntr042. [DOI] [PubMed] [Google Scholar]
- Adams 2012 .Adams EK, Markowitz S, Kannan V, Dietz PM, Tong VT, Malarcher AM. Reducing prenatal smoking: the role of state policies. American Journal of Preventive Medicine. 2012;43:34–40. doi: 10.1016/j.amepre.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Al-Sahab 2010 .Al-Sahab B, Saqib M, Hauser G, Tamim H. Prevalence of smoking during pregnancy and associated risk factors among Canadian women: a national survey. BMC Pregnancy and Childbirth. 2010;10:24. doi: 10.1186/1471-2393-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligne 1997 .Aligne CA, Stoddard JJ. Tobacco and Children: an economic evaluation of the medical effects of parental smoking. Archives of Pediatrics and Adolescent Medicine. 1997;151:648–53. doi: 10.1001/archpedi.1997.02170440010002. [DOI] [PubMed] [Google Scholar]
- Altman 1998 .Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Legacy Foundation 2012 .American Legacy Foundation [Accessed 23/8/2013];Priority populations initiative: breaking new ground and building capacity in cultural tailoring. 2012 http://www.legacyforhealth.org/content/download/687/7898/version/2/file/Priority.Populations.Initiative-.Breaking.New.Ground.and.Building.Capacity.in.Cultural.Tailoring.pdf.
- Amir 2001a .Amir LH. Maternal smoking and reduced duration of breastfeeding: a review of possible mechanisms. Early Human Development. 2001;64:45–67. doi: 10.1016/s0378-3782(01)00170-0. [DOI] [PubMed] [Google Scholar]
- Amir 2002a .Amir LH, Donath SM. Does maternal smoking have a negative physiological effect on breastfeeding? The epidemiological evidence. Birth. 2002;29:112–23. doi: 10.1046/j.1523-536x.2002.00152.x. [DOI] [PubMed] [Google Scholar]
- Apollonio 2012 .Apollonio D, Philipps R, Bero L. Interventions for tobacco use cessation in people in treatment for or recovery from substance abuse. Cochrane Database of Systematic Reviews. 2012;(Issue 12) doi: 10.1002/14651858.CD010274. [DOI: 10.1002/14651858.CD010274] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq 2008 .Ashfaq M, Channa MA, Malik MA, Khan D. Morphological changes in human placenta of wet snuff users. Journal of Ayub Medical College Abbottabad. 2008;20:110–3. [PubMed] [Google Scholar]
- Aveyard 2004 .Aveyard P, Lawrence T, Croghan E, Evans O. Cheng KK. Is advice to stop smoking from a midwife stressful for pregnant women who smoke? Data from a randomized controlled trial. Preventive Medicine. 2004;40:575–82. doi: 10.1016/j.ypmed.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Aveyard 2007 .Aveyard R, West R. Managing smoking cessation. BMJ. 2007;335:37–41. doi: 10.1136/bmj.39252.591806.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayadi 2006 .Ayadi MF, Adams EK, Melvin CL, Rivera CC, Gaffney CA, Pike J, et al. Costs of a smoking cessation counseling intervention for pregnant women: comparison of three settings. Public Health Reports. 2006;121(2):120–6. doi: 10.1177/003335490612100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba 2012 .Baba S, Wikström AK, Stephansson O, Cnattingius S. Influence of smoking and snuff cessation on risk of preterm birth. European Journal of Epidemiology. 2012;27:297–304. doi: 10.1007/s10654-012-9676-8. [DOI] [PubMed] [Google Scholar]
- Bala 2008 .Bala M, Strzeszynski L, Cahill K. Mass media interventions for smoking cessation in adults. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD004704.pub2. [DOI: 10.1002/14651858.CD004704.pub2] [DOI] [PubMed] [Google Scholar]
- Balfour 2004 .Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine & Tobacco Research. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Barnes 2010 .Barnes J, Dong CY, McRobbie H, Walker N, Mehta M, Stead LF. Hypnotherapy for smoking cessation. Cochrane Database of Systematic Reviews. 2010;(Issue 10) doi: 10.1002/14651858.CD001008.pub2. [DOI: 10.1002/14651858.CD001008.pub2] [DOI] [PubMed] [Google Scholar]
- Barraclough 1999 .Barraclough S. Women and tobacco in Indonesia. Tobacco Control. 1999;8:327–32. doi: 10.1136/tc.8.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth 2008 .Barth J, Critchley JA, Bengel J. Psychosocial interventions for smoking cessation in patients with coronary heart disease. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD006886. [DOI: 10.1002/14651858.CD006886] [DOI] [PubMed] [Google Scholar]
- Bartholomew 2011 .Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, Fernandez ME. Planning Health Promotion Programs: An Intervention Mapping Approach. 3rd Edition Jossey-Bass; San Francisco: 2011. [Google Scholar]
- Bartlett 1994 .Bartlett JC, Miller LS, Rice DP, Max WB. Medical-care expenditures attributable to cigarette smoking--United States, 1993. Morbidity and Mortality Weekly Report. 1994;43:469–72. [PubMed] [Google Scholar]
- Bauld 2010 .Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. Journal of Public Health. 2010;32:71–82. doi: 10.1093/pubmed/fdp074. [DOI] [PubMed] [Google Scholar]
- Bauld 2010a .Bauld L, Coleman T. [Accessed 30/5/2013];The effectiveness of smoking cessation interventions during pregnancy: a briefing paper. 2010 http://www.nice.org.uk/nicemedia/live/13023/49422/49422.pdf. [Google Scholar]
- Bauld 2012 .Bauld L, Hackshaw L, Ferguson J, Coleman T, Taylor G, Salway R. Implementation of routine biochemical validation and an ‘opt out’ referral pathway for smoking cessation in pregnancy. Addiction. 2012;107:53–60. doi: 10.1111/j.1360-0443.2012.04086.x. [DOI] [PubMed] [Google Scholar]
- Baum 2009 .Baum F. Reducing health inequities requires a new national health research agenda. Health Promotion Journal of Australia. 2009;20:163–4. doi: 10.1071/he09163. [DOI] [PubMed] [Google Scholar]
- Beenstock 2012 .Beenstock J, Sniehotta F, White M, Bell R, Milne EMG, Araujo-Soares V. What helps and hinders midwives in engaging with pregnant women about stopping smoking? A cross-sectional survey of perceived implementation difficulties among midwives in the North East of England. Implementation Science. 2012;7:36. doi: 10.1186/1748-5908-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg 2008 .Berg CJ, Park ER, Chang Y, Rigotti NA. Is concern about post-cessation weight gain a barrier to smoking cessation among pregnant women? Nicotine and Tobacco Research. 2008;10:1159–63. doi: 10.1080/14622200802163068. [DOI] [PubMed] [Google Scholar]
- Bize 2012 .Bize R, Burnand B, Mueller Y, Rège-Walther M, Camain J-Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 12) doi: 10.1002/14651858.CD004705.pub4. [DOI: 10.1002/14651858.CD004705.pub4] [DOI] [PubMed] [Google Scholar]
- Blalock 2005 .Blalock JA, Fouladi RT, Wetter DW, Cinciripini PM. Depression in pregnant women seeking smoking cessation treatment. Addictive Behaviors. 2005;30(6):1195–208. doi: 10.1016/j.addbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bloch 2008 .Bloch M, Althabe F, Onyamboko M, Kaseba-Sata C, Castilla EE, Freire S, et al. Tobacco use and secondhand smoke exposure during pregnancy: an investigative survey of women in 9 developing nations. American Journal of Public Health. 2008;98:1833–40. doi: 10.2105/AJPH.2007.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood-Siegfried 2010 .Blood-Siegfried J, Rende EK. The long-term effects of prenatal nicotine exposure on neurologic development. Journal of Midwifery and Women’s Health. 2010;55:143–52. doi: 10.1016/j.jmwh.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard 2012 .Bombard JM, Farr SL, Dietz PM, Tong VT, Zhang L, Rabius V. Telephone smoking cessation quitline use among pregnant and non-pregnant women. Maternal Child Health Journal. 2012;17(6):989–95. doi: 10.1007/s10995-012-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond 2005 .Bond C. A culture of ill health: public health or Aboriginality? Medical Journal of Australia. 2005;183:39–41. doi: 10.5694/j.1326-5377.2005.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Bond 2012 .Bond C, Brough M, Spurling G, Hayman N. ‘It had to be my choice’ Indigenous smoking cessation and negotiations of risk, resistance and resilience. Health, Risk & Society. 2012;14:565–81. [Google Scholar]
- Bonollo 2002 .Bonollo DP, Zapka JG, Stoddard AM, Ma Y, Pbert L, Ockene JK. Treating nicotine dependence during pregnancy and postpartum: understanding clinician knowledge and performance. Patient Education and Counselling. 2002;48:265–74. doi: 10.1016/s0738-3991(02)00023-x. [DOI] [PubMed] [Google Scholar]
- Borenstein 2009 .Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. John Wiley & Sons, Ltd; Chichester, UK: 2009. [Google Scholar]
- Boyle 2011 .Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database of Systematic Reviews. 2011;(Issue 12) doi: 10.1002/14651858.CD008743.pub2. [DOI: 10.1002/14651858.CD008743.pub2] [DOI] [PubMed] [Google Scholar]
- Brinn 2010 .Brinn MP, Carson KV, Esterman AJ, Chang AB, Smith BJ. Mass media interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews. 2010;(Issue 11) doi: 10.1002/14651858.CD001006.pub2. [DOI: 10.1002/14651858.CD001006.pub2] [DOI] [PubMed] [Google Scholar]
- Burgess 2009 .Burgess DJ, Fu SS, vanRyn M. Potential unintended consequences of tobacco-control policies on mothers who smoke: a review of the literature. American Journal of Preventive Medicine. 2009;37:S151–S158. doi: 10.1016/j.amepre.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Bush 2003 .Bush J, White M, Kai J, Rankin J, Bhopal R. Understanding influences on smoking in Bangladeshi and Pakistani adults: community based, qualitative study. BMJ. 2003;326:962. doi: 10.1136/bmj.326.7396.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana 1999 .Cabana C, Rand C, Powel N. Why don’t physicians follow clinical practice guidelines? JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- Cahill 2008 .Cahill K, Moher M, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(Issue 4) doi: 10.1002/14651858.CD003440.pub3. [DOI: 10.1002/14651858.CD003440.pub3] [DOI] [PubMed] [Google Scholar]
- Cahill 2008c .Cahill K, Perera R. Quit and Win contests for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(Issue 4) doi: 10.1002/14651858.CD004986.pub3. [DOI: 10.1002/14651858.CD004986.pub3] [DOI] [PubMed] [Google Scholar]
- Cahill 2010 .Cahill K, Lancaster T, Green N. Stage-based interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2010;(Issue 11) doi: 10.1002/14651858.CD004492.pub4. [DOI: 10.1002/14651858.CD004492.pub4] [DOI] [PubMed] [Google Scholar]
- Cahill 2011 .Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database of Systematic Reviews. 2011;(Issue 3) doi: 10.1002/14651858.CD005353.pub4. [DOI: 10.1002/14651858.CD005353.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill 2011a .Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews. 2011;(Issue 4) doi: 10.1002/14651858.CD004307.pub4. [DOI: 10.1002/14651858.CD004307.pub4] [DOI] [PubMed] [Google Scholar]
- Cahill 2012 .Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 4) doi: 10.1002/14651858.CD006103.pub2. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
- Cahill 2013 .Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews. 2013;(Issue 5) doi: 10.1002/14651858.CD009329.pub2. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callinan 2010 .Callinan JE, Clarke A, Doherty K, Kelleher C. Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews. 2010;(Issue 4) doi: 10.1002/14651858.CD005992.pub2. [DOI: 10.1002/14651858.CD005992.pub2] [DOI] [PubMed] [Google Scholar]
- Campion 1994 .Campion P, Owen L, McNeill A, McGuire C. Evaluation of a mass media campaign on smoking and pregnancy. Addiction. 1994;89:1245–54. doi: 10.1111/j.1360-0443.1994.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Carr 2012 .Carr AB, Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews. 2012;(Issue 6) doi: 10.1002/14651858.CD005084.pub3. [DOI: 10.1002/14651858.CD005084.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson 2011 .Carson KV, Brinn MP, Labiszewski NA, Esterman AJ, Chang AB, Smith BJ. Community interventions for preventing smoking in young people. Cochrane Database of Systematic Reviews. 2011;(Issue 7) doi: 10.1002/14651858.CD001291.pub2. [DOI: 10.1002/14651858.CD001291.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson 2012 .Carson KV, Brinn MP, Labiszewski NA, Peters M, Chang AB, Veale A, et al. Interventions for tobacco use prevention in Indigenous youth. Cochrane Database of Systematic Reviews. 2012;(Issue 8) doi: 10.1002/14651858.CD009325.pub2. [DOI: 10.1002/14651858.CD009325.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Carson 2012b .Carson KV, Brinn MP, Peters M, Veale A, Esterman AJ, Smith BJ. Interventions for smoking cessation in Indigenous populations. Cochrane Database of Systematic Reviews. 2012;(Issue 1) doi: 10.1002/14651858.CD009046.pub2. [DOI: 10.1002/14651858.CD009046.pub2] [DOI] [PubMed] [Google Scholar]
- Carson 2012c .Carson KV, Verbiest MEA, Crone MR, Brinn MP, Esterman AJ, Assendelft WJJ, et al. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 5) doi: 10.1002/14651858.CD000214.pub2. [DOI: 10.1002/14651858.CD000214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates 2002 .Cates CJ. Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Medical Research Methodology. 2002;2:1. doi: 10.1186/1471-2288-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates 2008 .Cates C. [accessed 22 August 2013];Visual Rx. (version 3). 2008 2008 http://www.nntonline.net/visualrx/
- CDCP 2002 .Centers for Disease Control and Prevention Women and smoking: a report of the Surgeon General. Executive Summary. Morbidity and Mortality Weekly Report. 2002;51(RR-12):i–iv. 1–13. [PubMed] [Google Scholar]
- CDCP 2013 .Centers for Disease Control and Prevention [accessed 31 May 2013];WHO Guidelines for the Management of Tobacco, Alcohol, and Illicit Drug Dependence. http://www.cdc.gov/reproductivehealth/TobaccoUsePregnancy/cdc.activities.htm.
- Chan 2001 .Chan A, Keane RJ, Robinson JS. The contribution of maternal smoking to preterm birth, small for gestational age and low birth weight among aboriginal and non-aboriginal births in South Australia. Medical Journal of Australia. 2001;174(8):389–93. doi: 10.5694/j.1326-5377.2001.tb143339.x. [DOI] [PubMed] [Google Scholar]
- Chang 2012 .Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2012;381(9862):223–34. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin 2004 .Chapin J, Root W. Improving obstetrician-gynecologist implementation of smoking cessation guidelines for pregnant women: An interim report of the American College of Obstetricians and Gynecologists. Nicotine & Tobacco Research. 2004;6:S253–S257. doi: 10.1080/14622200410001669123. [DOI] [PubMed] [Google Scholar]
- Chapman 2010 .Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7:e1000216. doi: 10.1371/journal.pmed.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman 2012 .Chapman S, Wakefield M. Smoking cessation strategies: Time to be more realistic in our expectations of interventions to help quitters. BMJ. 2012;344:e1732. doi: 10.1136/bmj.e1732. [DOI] [PubMed] [Google Scholar]
- Chasnoff 2005 .Chasnoff IJ, McGourty RF, Bailey GW, Hutchins E, Lightfoot SO, Pawson LL, et al. The 4P’s Plus screen for substance use in pregnancy: Clinical application and outcomes. Journal of Perinatology. 2005;25:368–74. doi: 10.1038/sj.jp.7211266. [DOI] [PubMed] [Google Scholar]
- Chasnoff 2007 .Chasnoff IJ, Wells AM, McGourty RF, Bailey LK. Validation of the 4P’s Plus© screen for substance use in pregnancy. Journal of Perinatology. 2007;27:744–8. doi: 10.1038/sj.jp.7211823. [DOI] [PubMed] [Google Scholar]
- Chomba 2010 .Chomba E, Tshefu A, Onyamboko M, Kaseba-Sata C, Moore J, McClure EM, et al. Tobacco use and secondhand smoke exposure during pregnancy in two African countries: Zambia and the Democratic Republic of Congo. Acta Obstetricia et Gynecologica. 2010;89:531–9. doi: 10.3109/00016341003605693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civljak 2010 .Civljak M, Sheikh A, Stead LF, Car J. Internet-based interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2010;(Issue 9) doi: 10.1002/14651858.CD007078.pub3. [DOI: 10.1002/14651858.CD007078.pub3] [DOI] [PubMed] [Google Scholar]
- Cnattingius 2004 .Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6(S2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Cochran 1954 .Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- Coleman 2004 .Coleman T. The use of simple advice and behavioural support. BMJ. 2004;328:397–9. doi: 10.1136/bmj.328.7436.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman 2012b .Coleman T, Chamberlain C, Davey M-A, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2012;(Issue 9) doi: 10.1002/14651858.CD010078. [DOI: 10.1002/14651858.CD010078] [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger 2012 .Coleman-Cowger VH. Smoking cessation intervention for pregnant women: a call for extension to the postpartum period. Maternal-Child Health Journal. 2012;16:937–40. doi: 10.1007/s10995-011-0837-2. [DOI] [PubMed] [Google Scholar]
- Cooke 2001 .Cooke M, Mattick RP, Walsh RA. Differential uptake of a smoking cessation programme disseminated to doctors and midwives in antenatal in antenatal clinics. Addiction. 2001;96:495–505. doi: 10.1046/j.1360-0443.2001.96349512.x. [DOI] [PubMed] [Google Scholar]
- Cooke 2001a .Cooke M, Mattick RP, Walsh RA. Implementation of the ‘Fresh Start’ smoking cessation programme to 23 antenatal clinics: a randomized controlled trial investigating two methods of dissemination. Drug and Alcohol Review. 2001;20:19–28. [Google Scholar]
- Coppo 2012 .Coppo A, Galanti MR, Buscemi D, Giordano L, Faggiano F. School policies for preventing smoking among young people. Cochrane Database of Systematic Reviews. 2012;(Issue 7) doi: 10.1002/14651858.CD009990.pub2. [DOI: 10.1002/14651858.CD009990] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox 2013 .Cox B, Martens E, Nemery B, Nawrot TS. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMJ. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford 2008 .Crawford JT, Tolosa JE, Goldenberg RL. Smoking cessation in pregnancy: why, how, and what next. Clinical Obstetrics and Gynecology. 2008;51(2):419–35. doi: 10.1097/GRF.0b013e31816fe9e9. [DOI] [PubMed] [Google Scholar]
- Creative Spirits 2013 . [accessed 2 October 2013];Creative Spirits. How to name Aboriginal people? http://www.creativespirits.info/aboriginalculture/people/how-to-name-aboriginal-people.
- Critchley 2012 .Critchley JA, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database of Systematic Reviews. 2012;(Issue 2) doi: 10.1002/14651858.CD003041.pub3. [DOI: 10.1002/14651858.CD003041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden 2007 .Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: the impact of time-varying pregnancy status, healthcare messages, stress and health concerns. Addictive Behaviours. 2007;32:1347–66. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypher 2012 .Cypher RL. Reducing recurrent preterm births: best evidence for transitioning to predictive and preventative strategies. Journal of Perinatal & Neonatal Nursing. 2012;26(3):220–9. doi: 10.1097/JPN.0b013e3182611b9e. [DOI] [PubMed] [Google Scholar]
- David 2006 .David S, Lancaster T, Stead LF, Evins AE. Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD003086.pub2. [DOI: 10.1002/14651858.CD003086.pub2] [DOI] [PubMed] [Google Scholar]
- Disantis 2010b .Disantis KI, Collins BN, McCoy AC. Associations among breastfeeding, smoking relapse, and prenatal factors in a brief postpartum smoking intervention. Acta Obstetricia et Gynecologica. 2010;89:582–6. doi: 10.3109/00016341003678435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon 2009b .Dixon L, Aimer P, Fletcher L, Guilliland K, Hendry C. Smoke free outcomes with midwife lead maternity carers: an analysis of smoking during pregnancy from the New Zealand College of Midwives Midwifery database 2004-2007. New Zealand College of Midwives Journal. 2009;40:13–9. [Google Scholar]
- Donath 2004 .Donath SM, Amir LH, the ALSPAC Study Team The relationship between maternal smoking and breastfeeding duration after adjustment for maternal infant feeding intention. Acta Paediatrica. 2004;93(11):1514–8. doi: 10.1080/08035250410022125. [DOI] [PubMed] [Google Scholar]
- Donovan 1975 .Donovan JW, Burgess PL, Hossack CM, Yudkin GD. Routine advice against smoking in pregnancy. Journal of the Royal College of General Practitioners. 1975;25:264–8. [PMC free article] [PubMed] [Google Scholar]
- Duckworth 2012 .Duckworth AL, Chertok IR. Review of perinatal partner-focused smoking cessation interventions. MCN American Journal of Maternal Child Nursing. 2012;37:174–81. doi: 10.1097/NMC.0b013e31824921b4. [DOI] [PubMed] [Google Scholar]
- Dwyer 2008 .Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Research Part C - Embryo Today: Reviews. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Ebbert 2011 .Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews. 2011;Issue(2) doi: 10.1002/14651858.CD004306.pub4. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
- Ebert 2007 .Ebert LM, Fahy K. Why do women continue to smoke in pregnancy? Women and Birth. 2007;20:161–8. doi: 10.1016/j.wombi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ebert 2009 .Ebert L, Fahy K. What do midwives need to understand/know about smoking in pregnancy? Women and Birth. 2009;22:35–40. doi: 10.1016/j.wombi.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Einarson 2009 .Einarson A, Riordan S. Smoking in pregnancy and lactation: A review of risks and cessation strategies. European Journal of Clinical Pharmacology. 2009;65:325–30. doi: 10.1007/s00228-008-0609-0. [DOI] [PubMed] [Google Scholar]
- England 2001 .England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- England 2010 .England LJ, Kim SY, Tomar SL, Ray CS, Gupta PC, Eissenberg T, et al. Non-cigarette tobacco use among women and adverse pregnancy outcomes. Acta Obstetricia et Gynecologica. 2010;89:454–64. doi: 10.3109/00016341003605719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson 1996 .Eriksson KM, Salvesen KA, Haug K, Eik-Nes SH. Smoking habits among pregnant women in a Norwegian county 1987-94. Acta Obstetricia et Gynecologica Scandinavica. 1996;75:355–9. doi: 10.3109/00016349609033331. [DOI] [PubMed] [Google Scholar]
- Eriksson 1998 .Eriksson KM, Haug K, Salvesen KA, Nesheim BI, Nylander G, Rasmussen S, et al. Smoking habits among pregnant women in Norway 1994-95. Acta Obstetricia et Gynecologica Scandinavica. 1998;77:159–64. [PubMed] [Google Scholar]
- Ershoff 1990 .Ershoff DH, Lairson DR, Mullen PD, Quinn VP. Pregnancy and medical cost outcomes of a self-help prenatal smoking cessation program in an HMO. Public Health Reports. 1990;105(4):340–7. [PMC free article] [PubMed] [Google Scholar]
- Ershoff 1995 .Ershoff DH, Quinn VP, Mullen PD. Relapse prevention among women who stop smoking early in pregnancy: a randomized clinical trial of a self-help intervention. American Journal of Preventive Medicine. 1995;11(3):178–84. [PubMed] [Google Scholar]
- Fang 2004 .Fang WL, Goldstein AO, Butzen AY, Hartsock SA, Hartmann KE, Helton M, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. Journal of American Board of Family Practice. 2004;17:264–75. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- FAO 2003 .Food, agriculture organization of the United Nations [accessed 23 August 2013];Projections of tobacco production, consumption and trade to the year 2010. 2003 ftp://ftp.fao.org/docrep/fao/006/y4956e/y4956e00.pdf.
- Farley 2012 .Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 1) doi: 10.1002/14651858.CD006219.pub3. [DOI: 10.1002/14651858.CD006219.pub3] [DOI] [PubMed] [Google Scholar]
- Farrimond 2006 .Farrimond HR, Joffe H. Pollution, peril and poverty: a British study of the stigmatization of smokers. Journal of Community and Applied Social Psychology. 2006;16:481–502. [Google Scholar]
- Fernander 2010 .Fernander A, Moorman G, Azuoru M. Race-related stress and smoking among pregnant African-American women. Acta Obstetricia et Gynecologica Scandinavica. 2010;89:558–64. doi: 10.3109/00016340903508676. [DOI] [PubMed] [Google Scholar]
- Fiore 2008 .Fiore M. [accessed 23 August 2013];Treating tobacco use and dependence: Clinical practice guideline. Centers for Disease Control and Prevention. 2008 http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/treating.tobacco.use08.pdf.
- Flemming 2013 .Flemming K, Graham H, Heirs M, Fox D, Sowden A. Smoking in pregnancy: a systematic review of qualitative research of women who commence pregnancy as smokers. Journal of Advanced Nursing. 2013;69:1023–36. doi: 10.1111/jan.12066. [DOI] [PubMed] [Google Scholar]
- Flenady 2005 .Flenady V, New K, MacPhail J, for the Cessation for the Clinical Practice Guideline Working Party on Smoking in Pregnancy . Clinical Practice Guideline for Smoking Cessation in Pregnancy. Mater Health Services; Centre for Clinical Studies; Brisbane: [Accessed 23/8/2013]. 2005. http://www.stillbirthalliance.org.au/guideline2.htm. [Google Scholar]
- Flenady 2011 .Flenady V, Koopmans L, Middleton P, Frøen J, Smith G, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- Floyd 2001 .Floyd RL, Belodoff B, Sidhu J, Schulkin J, Ebrahim SH, Sokol RJ. A survey of obstetrician-gynecologists on their patients’ use of tobacco and other drugs during pregnancy. Prenatal and Neonatal Medicine. 2001;6:201–7. [Google Scholar]
- Fox 1989 .Fox NL, Sexton M, Hebel JR, Thompson B. The reliability of self-reports of smoking and alcohol consumption by pregnant women. Addictive Behaviors. 1989;14(2):187–95. doi: 10.1016/0306-4603(89)90047-6. [DOI] [PubMed] [Google Scholar]
- Frost 1994 .Frost FJ, Cawthorn ML, Tollestrup K, Kenny FW, Schrager LS, Nordlund DJ. Smoking prevalence during pregnancy for women who are and women who are not Medicaid-funded. American Journal of Preventive Medicine. 1994;10:91–6. [PubMed] [Google Scholar]
- Gage 2007 .Gage JD, Everett KD, Bullock L. A review of research literature addressing male partners and smoking during pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2007;36(6):574–80. doi: 10.1111/j.1552-6909.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Gilbert 2005 .Gilbert E. Contextualising the medical risks of cigarette smoking: Australian young women’s perceptions of anti-smoking campaigns. Health Risk and Society. 2005;7:227–45. [Google Scholar]
- Gilligan 2008 .Gilligan C. A pilot randomised controlled trial to test the effectiveness of an intervention to help Aboriginal and Torres Strait Islander women to quit smoking during pregnancy: study design and preliminary results [thesis] University of Newcastle; Newcastle, Australia: 2008. [Google Scholar]
- Gilligan 2009 .Gilligan C, Sanson-Fisher RW, D-Este C, Eades S, Wenitong M. Knowledge and attitudes regarding smoking during pregnancy among Aboriginal and Torres Strait Islander women. Medical Journal of Australia. 2009;190:557–61. doi: 10.5694/j.1326-5377.2009.tb02562.x. [DOI] [PubMed] [Google Scholar]
- Gilmore 2004 .Gilmore AB, McKee M. Tobacco and transition: an overview of industry investments, impact and influence in the former Soviet Union. Tobacco Control. 2004;13:136–42. doi: 10.1136/tc.2002.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino 2007 .Giovino GA. The tobacco epidemic in the United States. American Journal of Preventive Medicine. 2007;33(6 Suppl):S318–S326. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Glanz 2008 .Glanz K, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. 4th Edition Jossey-Bass; San Francisco: 2008. [Google Scholar]
- Gluckman 2008 .Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in-utero and early life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay 2004 .Gourlay SG, Stead LF, Benowitz N. Clonidine for smoking cessation. Cochrane Database of Systematic Reviews. 2004;(Issue 3) doi: 10.1002/14651858.CD000058.pub2. [DOI: 10.1002/14651858.CD000058.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham 1977 .Graham H. Smoking in pregnancy: the attitudes of pregnant mothers. Social Science and Medicine. 1977;10:399–405. doi: 10.1016/0037-7856(76)90097-4. [DOI] [PubMed] [Google Scholar]
- Graham 1996 .Graham H. Smoking prevalence among women in the European community 1950-1990. Social Science and Medicine. 1996;43:243–54. doi: 10.1016/0277-9536(95)00369-x. [DOI] [PubMed] [Google Scholar]
- Graham 2006 .Graham H, Francis B, Inskip HM, Harman J. SWS study team. Socioeconomic lifecourse influences on women’s smoking status in early adulthood. Journal of Epidemiology and Community Health. 2006;60:228–33. doi: 10.1136/jech.2005.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham 2009 .Graham H. Australian Health Inequities Program lecture. Melbourne, Victoria, Australia: Mar 13, 2009. 2009. Disadvantage across the lifecourse. [Google Scholar]
- Graham 2010 .Graham H, Hawkins SS, Law C. Lifecourse influences on women’s smoking before, during and after pregnancy. Social Science and Medicine. 2010;70:582–7. doi: 10.1016/j.socscimed.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Greaves 2007a .Greaves L, Kalaw C, Bottorff JL. Case studies of power and control related to tobacco use during pregnancy. Women’s Health Issues. 2007;17:325–32. doi: 10.1016/j.whi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Greaves 2007b .Greaves L, Tungohan E. Engendering tobacco control: Using an international public health treaty to reduce smoking and empower women. Tobacco Control. 2007;16:148–50. doi: 10.1136/tc.2006.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green 2005a .Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of the Dimes. American Journal of Obstetrics and Gynecology. 2005;193:626–35. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- Green 2005b .Green LW, Kreuter MW. Health Program Planning: An Educational and Ecological Approach. 4th Edition McGraw-Hill; New York: 2005. [Google Scholar]
- Greenhalgh 2004 .Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw 2006 .Grimshaw G, Stanton A. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD003289.pub4. [DOI: 10.1002/14651858.CD003289.pub4] [DOI] [PubMed] [Google Scholar]
- Groff 2005 .Groff J, Stotts A, Velasquez M, Benjamin-Garner R, Green C, Mastrobattista J. Ultrasound and motivational enhancement for prenatal smoking cessation. Paper presented at the Annual meeting of the Society for Behavioural Medicine; Boston, MA. 2005. [Google Scholar]
- Grol 1999 .Grol R, Grimshaw J. Evidence-based implementation of evidence-based medicine. Journal of Quality Improvement. 1999;25:503–13. doi: 10.1016/s1070-3241(16)30464-3. [DOI] [PubMed] [Google Scholar]
- Gupta 2004 .Gupta PC, Sreevidya S. Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1217 women in Mumbai, India. BMJ. 2004;328:1538. doi: 10.1136/bmj.38113.687882.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta 2006 .Gupta PC, Subramoney S. Smokeless tobacco use and risk of stillbirth: a cohort study in Mumbai, India. Epidemiology. 2006;17:47–51. doi: 10.1097/01.ede.0000190545.19168.c4. [DOI] [PubMed] [Google Scholar]
- Gupta 2012 .Gupta PC. Smokeless tobacco use in pregnancy: Adverse reproductive outcomes. International Journal of Gynecology and Obstetrics. 2012;119:S195. [Google Scholar]
- Haines 1998 .Haines A, Donald A. Making better use of research findings. BMJ. 1998;317:72–5. doi: 10.1136/bmj.317.7150.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek 2001b .Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database of Systematic Reviews. 2001;(Issue 3) doi: 10.1002/14651858.CD000546.pub2. [DOI: 10.1002/14651858.CD000546.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek 2009 .Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.CD003999.pub3. [DOI: 10.1002/14651858.CD003999.pub3] [DOI] [PubMed] [Google Scholar]
- Hammoud 2005 .Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. American Journal of Obstetrics and Gynecology. 2005;192:1856–63. doi: 10.1016/j.ajog.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Hartmann 2007 .Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstetrics and Gynecology. 2007;110:765–70. doi: 10.1097/01.AOG.0000280572.18234.96. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce 2012 .Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 8) doi: 10.1002/14651858.CD007072.pub2. [DOI: 10.1002/14651858.CD007072.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug 1992 .Haug K, Fugelli P, Aaro LE. Recruitment and participation of General Practitioners in a multipractice study of smoking cessation. Scandinavian Journal of Primary Health Care. 1992;10(3):206–10. doi: 10.3109/02813439209014062. [DOI] [PubMed] [Google Scholar]
- Haynes 1998 .Haynes B, Haines A. Barriers and bridges to evidence based clinical practice. BMJ. 1998;317:273–6. doi: 10.1136/bmj.317.7153.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healton 2009 .Healton CG, Vallone D, Cartwright J. Unintended consequences of tobacco policies: implications for public health practice. American Journal of Preventive Medicine. 2009;37:S181–S182. doi: 10.1016/j.amepre.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Heath 2006 .Heath D, Panaretto K, Manessis V, Larkins S, Malouf P, Reilly E, et al. Factors to consider in smoking interventions for Indigenous women. Australian Journal of Primary Health. 2006;12(2):131–6. [Google Scholar]
- Hegaard 2007 .Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnanct non-smokers. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:401–6. doi: 10.1080/00016340601147517. [DOI] [PubMed] [Google Scholar]
- Heil 2004 .Heil SH, Higgins ST. Characterizing nicotine withdrawal and craving in pregnant cigarette smokers. 66th Annual Scientific Meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. 2004 June 12-17.2004. [Google Scholar]
- Hemsing 2012 .Hemsing N, Greaves L, O’Leary R, Chan K, Okoli C. Partner support for smoking cessation during pregnancy: a systematic review. Nicotine & Tobacco Research. 2012;14:767–76. doi: 10.1093/ntr/ntr278. [DOI] [PubMed] [Google Scholar]
- Herbert 2005 .Herbert RD, Bo K. Analysis of quality of interventions in systematic reviews. BMJ. 2005;331:507–9. doi: 10.1136/bmj.331.7515.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann 2008 .Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Current Opinion in Pediatrics. 2008;20:184–90. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Higgins 2006b .Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug and Alcohol Dependence. 2006;85:138–41. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; [updated February 2008]. 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins 2008b .Higgins ST, Silverman K, Heil SH, editors. Contingency Management in Substance Abuse Treatment. The Guilford Press; New York: 2008. [Google Scholar]
- Higgins 2010a .Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105:2023–30. doi: 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2010b .Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine & Tobacco Research. 2010;12(5):483–8. doi: 10.1093/ntr/ntq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2012 .Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine. 2012;55:27. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott 2010 .Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Social Science and Medicine. 2010;70(5):769–78. doi: 10.1016/j.socscimed.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Hollands 2011 .Hollands GJ, Vogt F, McDermott M, Parsons AC, Aveyard P. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database of Systematic Reviews. 2011;(Issue 6) doi: 10.1002/14651858.CD009164.pub2. [DOI: 10.1002/14651858.CD009164] [DOI] [PubMed] [Google Scholar]
- Horta 1997 .Horta BL, Barros FC, Memezes AM, Victora CG. Environmental tobacco smoke and breastfeeding duration. American Journal of Epidemiology. 1997;146:128–33. doi: 10.1093/oxfordjournals.aje.a009243. [DOI] [PubMed] [Google Scholar]
- Hotham 2002 .Hotham ED, Atkinson ER, Gilbert AL. Focus groups with pregnant smokers: barriers to cessation, attitudes to nicotine patch use and perceptions of cessation counselling by care providers. Drug and Alcohol Review. 2002;21(2):163–8. doi: 10.1080/09595230220139064. [DOI] [PubMed] [Google Scholar]
- Hotham 2008 .Hotham E, Ali R, White J, Robinson J. Pregnancy-related changes in tobacco, alcohol and cannabis use reported by antenatal patients at two public hospitals in South Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48:248–54. doi: 10.1111/j.1479-828X.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- Howard 2013 .Howard LM, Bekele D, Rowe M, Demilew J, Bewley S, Marteau TM. Smoking cessation in pregnant women with mental disorders: a cohort and nested qualitative study. BJOG: an international journal of obstetrics and gynaecology. 2013;120:362–70. doi: 10.1111/1471-0528.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston 1994 .Hueston WJ, Mainous AG, Farrell JB. A cost-benefit analysis of smoking cessation programs during the first trimester of pregnancy for the prevention of low birthweight. Journal of Family Practice. 1994;39:353. [PubMed] [Google Scholar]
- Hughes 2000b .Hughes JR, Stead LF, Lancaster T. Anxiolytics for smoking cessation. Cochrane Database of Systematic Reviews. 2000;(Issue 4) doi: 10.1002/14651858.CD002849. [DOI: 10.1002/14651858.CD002849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes 2007 .Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD000031.pub3. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]
- Hunt 2012 .Hunt J. Research to prevent tobacco use and secondhand smoke exposure in pregnancy. International Journal of Gynecology and Obstetrics. 2012;119:S251. [Google Scholar]
- Hurt 2005 .Hurt RD, Renner CC, Patten CA, Ebbert J, Offord KP, Schroeder DR. Iqmik-a form of smokeless tobacco used by pregnant Alaska natives: nicotine exposure in their neonates. Journal of Maternal-Fetal and Neonatal Medicine. 2005;17:281–9. doi: 10.1080/14767050500123731. [DOI] [PubMed] [Google Scholar]
- Johnston 2011b .Johnston V, Thomas DP, McDonnell J, Andrews RM. Maternal smoking and smoking in the household during pregnancy and postpartum: findings from an Indigenous cohort in the Northern Territory. Medical Journal of Australia. 2011;194:556–9. doi: 10.5694/j.1326-5377.2011.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Johnston 2012 .Johnston V, Liberato S, Thomas D. Cochrane Database of Systematic Reviews. issue 10. John Wiley & Sons, Ltd; 2012. Incentives for preventing smoking in children and adolescents. [DOI: 10.1002/14651858.CD008645.pub2] [DOI] [PubMed] [Google Scholar]
- Johnston 2013 .Johnston V, Westphal DW, Glover M, Thomas DP, Segan C, Walker N. Reducing smoking among indigenous populations: new evidence from a review of trials. Nicotine & Tobacco Research. 2013;15(8):1329–38. doi: 10.1093/ntr/ntt022. [DOI] [PubMed] [Google Scholar]
- Kadir 2010 .Kadir MM, McClure EM, Goudar SS, Garces AL, Moore J, Onyamboko M, et al. Exposure of pregnant women to indoor air pollution: a study from nine low and middle income countries. Acta Obstetricia et Gynecologica Scandinavica. 2010;89:540–8. doi: 10.3109/00016340903473566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen 2001 .Kallen K. The impact of maternal smoking during pregnancy on delivery outcome. European Journal of Public Health. 2001;11:329–33. doi: 10.1093/eurpub/11.3.329. [DOI] [PubMed] [Google Scholar]
- Kaplan 1997 .Kaplan SD, Lanier AP, Merritt RK, Siegel PZ. Prevalence of tobacco use among Alaska natives: a review. Preventive Medicine. 1997;26:460–5. doi: 10.1006/pmed.1997.0165. [DOI] [PubMed] [Google Scholar]
- Katz 2008 .Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, et al. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy and Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman 2001 .Kaufman N, Nichter M. The marketing of tobacco to women: global perspectives. In: Samet JM, Yoon SY, editors. Women and the Tobacco Epidemic. World Health Organization; Geneva: 2001. pp. 69–98. [Google Scholar]
- Khanna 2012 .Khanna P, Clifton A, Banks D, Tosh G. Smoking cessation advice for people with serious mental illness. Cochrane Database of Systematic Reviews. 2012;(Issue 3) doi: 10.1002/14651858.CD009704.pub2. [DOI: 10.1002/14651858.CD009704] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim 2009a .Kim SY, England L, Dietz PM, Morrow B, Perham-Hester KA. Prenatal cigarette smoking and smokeless tobacco use among Alaska native and white women in Alaska, 1996-2003. Maternal and Child Health Journal. 2009;13:652–9. doi: 10.1007/s10995-008-0402-9. [DOI] [PubMed] [Google Scholar]
- Kim 2009b .Kim SY, England LJ, Kendrick JS, Dietz PM, Callaghan WM. The contribution of clinic-based interventions to reduce prenatal smoking prevalence among US women. American Journal of Public Health. 2009;99:893–8. doi: 10.2105/AJPH.2008.144485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim 2010 .Kim SY, England L, Dietz PM, Morrow B, Perham-Hester KA. Patterns of cigarette and smokeless tobacco use before, during, and after pregnancy among Alaska native and white women in Alaska, 2000-2003. Maternal and Child Health Journal. 2010;14(3):365–72. doi: 10.1007/s10995-009-0444-7. [DOI] [PubMed] [Google Scholar]
- Klebanoff 1998 .Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. American Journal of Epidemiology. 1998;148:259–62. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- Kong 2008 .Kong GW, Tam WH, Sahota DS, Nelson EA. Smoking pattern during pregnancy in Hong Kong Chinese. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2008;48:280–5. doi: 10.1111/j.1479-828X.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Kramer 1987 .Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bulletin of the World Health Organization. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Lai 2010 .Lai DTC, Cahill K, Qin Y, Tang J-L. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews. 2010;(Issue 1) doi: 10.1002/14651858.CD006936.pub2. [DOI: 10.1002/14651858.CD006936.pub2] [DOI] [PubMed] [Google Scholar]
- Lambe 2007 .Lambe M. Swedish snus for tobacco harm reduction. Lancet. 2007;370:1206–7. doi: 10.1016/S0140-6736(07)61531-1. [DOI] [PubMed] [Google Scholar]
- Lancaster 1998 .Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database of Systematic Reviews. 1998;(Issue 2) doi: 10.1002/14651858.CD001009. [DOI: 10.1002/14651858.CD001009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster 2005a .Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2005;(Issue 3) doi: 10.1002/14651858.CD001118.pub2. [DOI: 10.1002/14651858.CD001118.pub2] [DOI] [PubMed] [Google Scholar]
- Lancaster 2005b .Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD001292.pub2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
- Lancaster 2012 .Lancaster T, Stead LF. Silver acetate for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 9) doi: 10.1002/14651858.CD000191.pub2. [DOI: 10.1002/14651858.CD000191.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando 2001 .Lando HA, Valanis BG, Lichtenstein E, Curry SJ, McBride CM, Pirie Pl, et al. Promoting smoking abstinence in pregnant and postpartum patients: a comparison of 2 approaches. American Journal of Managed Care. 2001;7:685–93. [PubMed] [Google Scholar]
- Lanting 2012 .Lanting CI, van Wouwe JP, van den Burg I, Segaar D, van der Pal-de Bruin KM. Smoking during pregnancy: trends between 2001 and 2010 [Roken tijdens de zwangerschap: trends in de periode 2001–2010.] Nederlands Tijdschrift Voor Geneeskunde. 2012;156(46):A5092. [PubMed] [Google Scholar]
- Lawrence 2005a .Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking cessation advice in pregnancy result in long term quitters? 18-month postpartum follow-up of a randomized controlled trial. Addiction. 2005;100:107–16. doi: 10.1111/j.1360-0443.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- Lawrence 2005b .Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking advice in pregnancy result in long-term quitters? 18-month post-partum follow up of a randomised controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting; Prague, Czech Republic. 2005 March 20-23.2005. [Google Scholar]
- Lawson 1994 .Lawson EJ. The role of smoking in the lives of low-income pregnant adolescents: a field study. Adolescence. 1994;29(113):61–79. [PubMed] [Google Scholar]
- Levine 2006 .Levine MD, Marcus MD, Kalarchian MA, Weissfeld L, Qin L. Weight concerns affect motivation to remain abstinent from smoking postpartum. Annals of Behavioral Medicine. 2006;32(2):147–53. doi: 10.1207/s15324796abm3202_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton 2003 .Leviton L, Orleans C. Promoting the uptake of evidence in clinical practice: a prescription for action. Clinics in Perinatolgy. 2003;30:403–17. doi: 10.1016/s0095-5108(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Lightwood 1999 .Lightwood JM, Phibbs CS, Glantz SA. Short-term health and economic benefits of smoking cessation: low birth weight. Pediatrics. 1999;104(6):1312–20. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- Linares 2009 .Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors. 2009;34:705–8. doi: 10.1016/j.addbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindson-Hawley 2012 .Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database of Systematic Reviews. 2012;(Issue 11) doi: 10.1002/14651858.CD008033.pub3. [DOI: 10.1002/14651858.CD008033.pub3] [DOI] [PubMed] [Google Scholar]
- Lipsey 2001 .Lipsey MW, Wilson DB. Applied Social Research Methods Series. 9th Edition SAGE; Thousand Oaks, CA: 2001. Practical Meta-Analysis. [Google Scholar]
- Lopez 1994 .Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control. 1994;3:242. [Google Scholar]
- Lorencatto 2012 .Lorencatto F, West R, Michie S. Specifying evidence-based behavior change techniques to aid smoking cessation in pregnancy. Nicotine and Tobacco Research. 2012;14(9):1019–26. doi: 10.1093/ntr/ntr324. [DOI] [PubMed] [Google Scholar]
- Lovato 2011 .Lovato C, Watts A, Stead LF. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. Cochrane Database of Systematic Reviews. 2011;(Issue 10) doi: 10.1002/14651858.CD003439.pub2. [DOI: 10.1002/14651858.CD003439.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi 2011 .Manfredi C, Cho YI, Warnecke R, Saunders S, Sullivan M. Dissemination strategies to improve implementation of the PHS smoking cessation guideline in MCH public health clinics: experimental evaluation results and contextual factors. Health Education Research. 2011;26:348–60. doi: 10.1093/her/cyr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzari 2012 .Mantzari E, Vogt F, Marteau T. The effectiveness of financial incentives for smoking cessation during pregnancy: is it from being paid or from the extra aid? BMC Pregnancy and Childbirth. 2012;12:24. doi: 10.1186/1471-2393-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano 2012 .Marcano BJS, Bruggeling MN, Gunn LH, Brusamento S, Car J. Interventions for recruiting smokers into cessation programmes. Cochrane Database of Systematic Reviews. 2012;(issue 12) doi: 10.1002/14651858.CD009187.pub2. [DOI: 10.1002/14651858.CD009187.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz 2008 .Maritz GS. Nicotine and lung development. Birth Defects Research Part C - Embryo Today: Reviews. 2008;84:45–53. doi: 10.1002/bdrc.20116. [DOI] [PubMed] [Google Scholar]
- Massey 2013 .Massey SH, Compton MT. Psychological differences between smokers who spontaneously quit during pregnancy and those who do not: A review of observational studies and directions for future research. Nicotine & Tobacco Research. 2013;15:307–19. doi: 10.1093/ntr/nts142. [DOI] [PubMed] [Google Scholar]
- Maziak 2007 .Maziak W, Ward KD, Eissenberg T. Interventions for waterpipe smoking cessation. Cochrane Database of Systematic Reviews. 2007;(Issue 4) doi: 10.1002/14651858.CD005549.pub2. [DOI: 10.1002/14651858.CD005549.pub2] [DOI] [PubMed] [Google Scholar]
- McBride 2003 .McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Education Research. 2003;18(2):156–70. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McDermott 2006 .McDermott E, Graham H. Young mothers and smoking: evidence of an evidence gap. Social Science and Medicine. 2006;63(6):1546–9. doi: 10.1016/j.socscimed.2006.03.025. [DOI] [PubMed] [Google Scholar]
- McLaren 2010 .McLaren DJ, Conigrave KM, Robertson JA, Ivers RG, Eades S, Clough AR. Using breath carbon monoxide to validate self-reported tobacco smoking in remote Australian Indigenous communities. Population Health Metrics. 2010;8:2. doi: 10.1186/1478-7954-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan 2000 .McLellan A, Lewis D, O’Brien C, Kleber H. Drug dependence, a chronic medical illness. JAMA. 2000;284(13):1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McRobbie 2012 .McRobbie H, Bullen C, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews. 2012;(Issue 11) doi: 10.1002/14651858.CD010216.pub2. [DOI: 10.1002/14651858.CD010216] [DOI] [PubMed] [Google Scholar]
- Meghea 2010 .Meghea CI, Rus D, Rus IA, Summers Holtrop J, Roman L. Smoking during pregnancy and associated risk factors in a sample of Romanian women. European Journal of Public Health. 2010;22(2):229–33. doi: 10.1093/eurpub/ckq189. [DOI] [PubMed] [Google Scholar]
- Mejia 2010 .Mejia R, Martinez VG, Gregorich SE, Pacrez-Stable EJ. Physician counseling of pregnant women about active and secondhand smoking in Argentina. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):490–5. doi: 10.3109/00016341003739567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant 2001 .Merchant KM, Villar J, Kestler E. Maternal height and newborn size relative to risk of intrapartum caesarean delivery and perinatal distress. BJOG: an international journal of obstetrics and gynaecology. 2001;108:689–96. doi: 10.1111/j.1471-0528.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- Merlo 2005 .Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. Journal of Epidemiology and Community Health. 2005;60:290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie 2008 .Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Applied Psychology: An International Review. 2008;57(4):660–80. [Google Scholar]
- Michie 2012 .Michie S, Johnston M. Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychology Review. 2012;6(1):1–6. doi: 10.1080/17437199.2014.912538. [DOI] [PubMed] [Google Scholar]
- Miller 1992 .Miller W, Zweden A, DiClemente C, Rychtarik R. Motivational Enhancement Therapy Manual. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1992. [Google Scholar]
- Miller 2001 .Miller DP, Villa KF, Hogue SL, Sivapathasundaram D. Birth and first-year costs for mothers and infants attributable to maternal smoking. Nicotine and Tobacco Research. 2001;3:25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]
- Ministry of Health 2007 .Ministry of Health [accessed 23 August 2013];New Zealand Smoking Cessation Guidelines. 2007 http://www.health.govt.nz/publication/newzealand-smoking-cessation-guidelines.
- Mirahmadizadeh 2008 .Mirahmadizadeh A, Nakhaee N. Prevalence of waterpipe smoking among rural pregnant women in Southern Iran. Medical Principles and Practice. 2008;17:435–9. doi: 10.1159/000151563. [DOI] [PubMed] [Google Scholar]
- Moore 2009 .Moore RS, McLellan DL, Tauras JA, Fagan P. Securing the health of disadvantaged women: a critical investigation of tobacco-control policy effects on women worldwide. American Journal of Preventive Medicine. 2009;37:S117–S120. doi: 10.1016/j.amepre.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Suarez-Varela 2006 .Morales-Suarez-Varela MM, Bille C, Christensen K, Olsen J. Smoking habits, nicotine use, and congenital malformations. Obstetrics & Gynecology. 2006;107(1):51–7. doi: 10.1097/01.AOG.0000194079.66834.d5. [DOI] [PubMed] [Google Scholar]
- Morasco 2006 .Morasco BJ, Dornelas EA, Fischer EH, Oncken C, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: a preliminary investigation. Addictive Behaviors. 2006;31(2):203–10. doi: 10.1016/j.addbeh.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Mullen 1991 .Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. American Journal of Obstetrics and Gynaecology. 1991;165:409–13. doi: 10.1016/0002-9378(91)90105-z. [DOI] [PubMed] [Google Scholar]
- Mullen 1999 .Mullen PD. Maternal smoking during pregnancy and evidence-based intervention to promote cessation. Primary Care. 1999;26(3):577–89. doi: 10.1016/s0095-4543(05)70118-4. [DOI] [PubMed] [Google Scholar]
- Murthy 2010 .Murthy P, Subodh BN. Current developments in behavioural interventions for tobacco cessation. Current Opinion in Psychiatry. 2010;23(2):151–6. doi: 10.1097/YCO.0b013e328336653f. [DOI] [PubMed] [Google Scholar]
- Nguyen 2012a .Nguyen KH, Subramanian SV, Sorensen G, Tsang K, Wright RJ. Influence of experiences of racial discrimination and ethnic identity on prenatal smoking among urban black and Hispanic women. Journal of Epidemiology and Community Health. 2012;66:315–21. doi: 10.1136/jech.2009.107516. [DOI] [PubMed] [Google Scholar]
- Nguyen 2012b .Nguyen SN, Von Kohorn I, Schulman-Green D, Colson ER. The importance of social networks on smoking: Perspectives of women who quit smoking during pregnancy. Maternal and Child Health Journal. 2012;16(6):1312–8. doi: 10.1007/s10995-011-0896-4. [DOI] [PubMed] [Google Scholar]
- NICE 2010 .National Institute of Clinical Excellence [accessed 23 August 2013];Quitting smoking in pregnancy and following childbirth. 2010 http://publications.nice.org.uk/quitting-smoking-in-pregnancy-and-following-childbirth-ph26.
- Nichter 2010 .Nichter M, Padmawati RS, Ng N. Developing a smoke free household initiative: an Indonesian case study. Acta Obstetricia et Gynecologica Scandinavica. 2010;89:578–81. doi: 10.3109/00016340903578893. [DOI] [PubMed] [Google Scholar]
- NICS 2003 .National Institute of Clinical Studies . Evidence-Practice Gap Reports. NICS; Melbourne: 2003. Smoking Cessation Interventions in Pregnancy. [Google Scholar]
- Noar 2005 .Noar SM, Zimmerman RS. Health behavior theory and cumulative knowledge regarding health behaviors: are we moving in the right direction? Health Education Research. 2005;20(3):275–90. doi: 10.1093/her/cyg113. [DOI] [PubMed] [Google Scholar]
- Nowicki 1984 .Nowicki P, Gintzig L, Hebel JR, Lathem R, Miller V, Sexton M. Effective smoking intervention during pregnancy. Birth. 1984;11:217–24. doi: 10.1111/j.1523-536x.1984.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Nutbeam 2006 .Nutbeam D, Bauman A. Evaluation in a Nutshell: A Practical Guide to the Evaluation of Health Promotion Programs. 1st Edition Vol. 1. McGraw-Hill; Sydney: 2008. [Google Scholar]
- Okoli 2010 .Okoli CT, Greaves L, Bottorff JL, Marcellus LM, Okoli Chizimuzo TC, Greaves L, et al. Health care providers’ engagement in smoking cessation with pregnant smokers. JOGNN - Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2010;39(1):64–77. doi: 10.1111/j.1552-6909.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Oliver 2001 .Oliver S, Oakley L, Lumley J, Waters E. Smoking cessation programmes in pregnancy: systematically addressing development, implementation, women’s concerns and effectiveness. Health Education Journal. 2001;60:362–70. [Google Scholar]
- Oliver 2008a .Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al. Methodology Programme. University of Birmingham; [accessed 2008]. RCTs for policy interventions? a review of reviews and meta-regression. http://www.pcpoh.bham.ac.uk/publichealth/methodology/projects/RM03.JH09.SO.shtml. [DOI] [PubMed] [Google Scholar]
- Oliver 2008b .Oliver S, Kavanagh J, Caird J, Lorenc T, Oliver K, Harden A, et al. [accessed 4 October 2013];Health promotion, inequalities and young people’s health. A systematic review of research. 2008 http://eppi.ioe.ac.uk/cms/Default.aspx?tabid=2410.
- Oncken 2009 .Oncken CA, Kranzler HR. What do we know about the role of pharmacotherapy for smoking cessation before or during pregnancy? Nicotine & Tobacco Research. 2009;11:1265–73. doi: 10.1093/ntr/ntp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr 2012 .Orr ST, Blazer DG, Orr CA. Maternal prenatal depressive symptoms, nicotine addiction, and smoking-related knowledge, attitudes, beliefs, and behaviors. Maternal Child Health Journal. 2012;16:973–8. doi: 10.1007/s10995-011-0822-9. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2007a .Ortendahl M. Predicting lapse when stopping smoking among pregnant and non-pregnant women. Journal of Obstetrics and Gynaecology. 2007;27(2):138–43. doi: 10.1080/01443610601113862. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2007b .Ortendahl M, Nasman P. Somatic, psychological and social judgments related to smoking among pregnant and non-pregnant women. Journal of Addictive Diseases. 2007;26(4):69–77. doi: 10.1300/J069v26n04_09. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2007c .Ortendahl M, Nasman P. Use of coping techniques as a predictor of lapse when quitting smoking among pregnant and non-pregnant women. American Journal on Addictions. 2007;16(3):238–43. doi: 10.1080/10550490701375582. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2008a .Ortendahl M. Coping mechanisms actually and hypothetically used by pregnant and non-pregnant women in quitting smoking. Journal of Addictive Diseases. 2008;27(4):61–8. doi: 10.1080/10550880802324804. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2008b .Ortendahl M, Nasman P. Quitting smoking is perceived to have an effect on somatic health among pregnant and non-pregnant women. Journal of Maternal-Fetal and Neonatal Medicine. 2008;21(4):239–46. doi: 10.1080/14767050801924829. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2008c .Ortendahl M, Nasman P. Judgments of risk for consequences of continuing or quitting smoking - A study of pregnant and nonpregnant women intending and not intending to quit. American Journal of Drug and Alcohol Abuse. 2008;34(2):225–33. doi: 10.1080/00952990701877169. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2009a .Ortendahl M, Nasman P. Factors affecting continuation of smoking by pregnant and nonpregnant women. Substance Abuse. 2009;30(2):150–7. doi: 10.1080/08897070902802075. [DOI] [PubMed] [Google Scholar]
- Ortendahl 2009b .Ortendahl M, Uttermalm A, Simonsson B, Nasman P, Wallsten T. Estimated time for occurrence of smoking-related consequences among pregnant and non-pregnant women. International Journal of Environmental Research and Public Health. 2009;6(5):1665–75. doi: 10.3390/ijerph6051665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea 2008 .Ostrea EM, Jr, Villanueva-Uy E, Ngerncham S, Punnakanta L, Batilando MJ, Agarwal P, et al. An epidemiologic study comparing fetal exposure to tobacco smoke in three Southeast Asian countries. International Journal of Occupational & Environmental Health. 2008;14(4):257–62. doi: 10.1179/oeh.2008.14.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palipudi 2009 .Palipudi KM, Asma S, Gupta PC, Dhirendra S. Tobacco use during pregnancy: An investigation from a National Household Survey. Proceedings of the 57th Session of the International Statistical Institute; Durban, South Africa. 2009 August 16-22.2009. [Google Scholar]
- Panjari 1997 .Panjari M, Bell R, Astbury J, Bishop S, Dalais F, Rice G. Women who spontaneously quit smoking in early pregnancy. Australian New Zealand Journal of Obstetrics and Gynaecology. 1997;37(3):271. doi: 10.1111/j.1479-828x.1997.tb02407.x. [DOI] [PubMed] [Google Scholar]
- Park 2012 .Park EW, Tudiver FG, Campbell T. Enhancing partner support to improve smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 7) doi: 10.1002/14651858.CD002928.pub3. [DOI: 10.1002/14651858.CD002928.pub3] [DOI] [PubMed] [Google Scholar]
- Parsons 2009 .Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.CD006219.pub2. [DOI: 10.1002/14651858.CD006219.pub2] [DOI] [PubMed] [Google Scholar]
- Passey 2012 .Passey ME, D’Este CA, Stirling JM, Sanson-Fisher RW. Factors associated with antenatal smoking among Aboriginal and Torres Strait Islander women in two jurisdictions. Drug and Alcohol Review. 2012;31:608–16. doi: 10.1111/j.1465-3362.2012.00448.x. [DOI] [PubMed] [Google Scholar]
- Patrick 1994 .Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. American Journal of Public Health. 1994;84(7):1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlen 2013 .Perlen S, Brown S, Yelland J. Have guidelines about smoking cessation support in pregnancy changed practice in Victoria, Australia? Birth. 2013;40(2):81–7. doi: 10.1111/birt.12036. [DOI] [PubMed] [Google Scholar]
- Petticrew 2012 .Petticrew M, Tugwell P, Kristjansson E, Oliver S, Ueffing E, Welch V. Damned if you do, damned if you don’t: subgroup analysis and equity. Journal of Epidemiology and Community Health. 2012;66(1):95–8. doi: 10.1136/jech.2010.121095. [DOI] [PubMed] [Google Scholar]
- Pettiti 1981 .Pettiti DB, Friedman ED, Kahn W. Accuracy of information on smoking habits provided on self-administered research questionnaires. American Journal of Public Health. 1981;71(3):308–11. doi: 10.2105/ajph.71.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett 2009 .Pickett KE, Wilkinson RG, Wakschlag LS. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. Journal of Epidemiology and Community Health. 2009;63(6):474–80. doi: 10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling 2010 .Pilling S, Hesketh K, Mitcheson L. Psychosocial Interventions for drug misuse: A framework and toolkit for implementing NICE-recommended. Gateway approval reference: 11486. The National Treatment Agency for Substance Misuse (NHS); London: 2010. [Google Scholar]
- Polanska 2005 .Polanska K, Hanke W, Sobala Characteristic of the smoking habit among pregnant women on the base of the test “Why am I a smoker?” [Charakterystyka nagogu palenia papierosow wsrod kobiet ciezarnych na podstawie testu Dlaczego pale?] Przeglad Lekarski. 2005;62(10):1095–8. [PubMed] [Google Scholar]
- Pomerleau 2000 .Pomerleau CS, Brouwer RJ. Weight concerns in women smokers during pregnancy and postpartum. Addictive Behaviors. 2000;25(5):759–67. doi: 10.1016/s0306-4603(00)00086-1. [DOI] [PubMed] [Google Scholar]
- Potter 1996 .Potter A, Lumley J, Watson L. The ‘new’ risk factors for SIDS: is there an association with ethnic and place of birth differences in incidence in Victoria? Early Human Development. 1996;45:119–31. doi: 10.1016/0378-3782(96)01726-4. [DOI] [PubMed] [Google Scholar]
- Pratinidhi 2010 .Pratinidhi A, Gandham S, Shrotri A, Patil A, Pardeshi S. Use of ‘Mishri’ A smokeless form of tobacco during pregnancy and its perinatal outcome. Indian Journal of Community Medicine. 2010;35(1):14–8. doi: 10.4103/0970-0218.62547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest 2008 .Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews. 2008;(Issue 4) doi: 10.1002/14651858.CD001746.pub2. [DOI: 10.1002/14651858.CD001746.pub2] [DOI] [PubMed] [Google Scholar]
- Prochaska 1992 .Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. American Psychology. 1992;47(9):1102–14. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Prochaska 2007 .Prochaska JM. The transtheoretical model applied to the community and the workplace. Journal of Health Psychology. 2007;12(1):198–200. doi: 10.1177/1359105307071754. [DOI] [PubMed] [Google Scholar]
- Quinn 1991 .Quinn VP, Mullen PD, Ershoff DH. Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addictive Behaviour. 1991;16(1-2):29–40. doi: 10.1016/0306-4603(91)90037-i. [DOI] [PubMed] [Google Scholar]
- Rattan 2013 .Rattan D, Mamun A, Najman JM, Williams GM, Doi SA. Smoking behaviour in pregnancy and its impact on smoking cessation at various intervals during follow-up over 21 years: a prospective cohort study. BJOG: an international journal of obstetrics and gynaecology. 2013;120(3):288–96. doi: 10.1111/1471-0528.12027. [DOI] [PubMed] [Google Scholar]
- Reda 2012 .Reda AA, Kotz D, Evers SMAA, van Schayck CP. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database of Systematic Reviews. 2012;(Issue 6) doi: 10.1002/14651858.CD004305.pub4. [DOI: 10.1002/14651858.CD004305.pub4] [DOI] [PubMed] [Google Scholar]
- Rice 2008 .Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD001188.pub3. [DOI: 10.1002/14651858.CD001188.pub3] [DOI] [PubMed] [Google Scholar]
- Richmond 2003 .Richmond R. You’ve come a long way baby: women and the tobacco epidemic. Addiction. 2003;98(5):553–7. doi: 10.1046/j.1360-0443.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- Riemsma 2003 .Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. BMJ. 2003;326:1175–7. doi: 10.1136/bmj.326.7400.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti 2012 .Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. 2012;(issue 5) doi: 10.1002/14651858.CD001837.pub3. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers 1998 .Rogers I, Emmett P, Baker D, Golding J, ASLPAC Study Team Financial difficulties, smoking habits, composition of the diet and birthweight in a population of pregnant women in the South West of England. European Journal of Clinical Nutrition. 1998;52(4):251–60. doi: 10.1038/sj.ejcn.1600544. [DOI] [PubMed] [Google Scholar]
- Rogers 2009 .Rogers JM. Tobacco and pregnancy. Reproductive Toxicology. 2009;28(2):152–60. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Ruggiero 2003 .Ruggiero L, Webster K, Peipert JF, Wood C. Identification and recruitment of low-income pregnant smokers. Who are we missing? Addictive Behaviours. 2003;28(8):1497–505. doi: 10.1016/s0306-4603(02)00269-1. [DOI] [PubMed] [Google Scholar]
- Ryan 1980 .Ryan P, Booth R, Coates D, Chapman A, Healy P. Pregnant Pause Campaign. Health Commission of New South Wales, Division of Drug and Alcohol Services; Sydney: 1980. Experiences of Pregnancy. [Google Scholar]
- Salihu 2007 .Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Human Development. 2007;83(11):713–20. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Samet 2001 .Samet JM, Yoon SY. Women and the Tobacco Epidemic: Challenges For the 21st Century. Institute for Global Tobacco Control, Johns Hopkins School of Public Health; WHO: 2001. [Google Scholar]
- Sayers 1995 .Sayers G, Burke M, Corcoran R, Thornton L. Influences on breast feeding initiation and duration. Irish Journal of Medical Science. 1995;164(4):281–4. doi: 10.1007/BF02967205. [DOI] [PubMed] [Google Scholar]
- Schmidt 2004 .Schmidt S. Nicotine addiction. Journal of Addictive Nursing. 2004;15(2):15. [Google Scholar]
- Schneider 2008 .Schneider S, Schutz J. Who smokes during pregnancy? A systematic literature review of population-based surveys conducted in developed countries between 1997 and 2006. European Journal of Contraceptive and Reproductive Health Care. 2008;13(2):138–47. doi: 10.1080/13625180802027993. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 1992 .Secker-Walker RH, Solomon LJ, Flynn BS, LePage SS, Crammond JE, Worden JK, et al. Training obstetric and family practice residents to give smoking cessation advice during prenatal care. American Journal of Obstetrics and Gynecology. 1992;166(5):1356–63. doi: 10.1016/0002-9378(92)91604-9. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 1995 .Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Lepage SS, Goodwin GD, et al. Smoking relapse prevention counseling during prenatal and early postnatal care. American Journal of Preventive Medicine. 1995;11(2):86–93. [PubMed] [Google Scholar]
- Secker-Walker 1997b .Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstetrics and Gynecology. 1997;89:648–53. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 1998b .Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Estimated gains in birth weight associated with reductions in smoking during pregnancy. Journal of Reproductive Medicine. 1998;43(11):967–74. [PubMed] [Google Scholar]
- Secker-Walker 2002a .Secker-Walker RH, Vacek PM. Infant birth weight as a measure of harm reduction during smoking cessation trials in pregnancy. Health Education and Behavior. 2002;29(5):557–69. doi: 10.1177/109019802237024. [DOI] [PubMed] [Google Scholar]
- Secker-Walker 2002b .Secker-Walker R, Gnich W, Platt S, Lancaster T. Community interventions for reducing smoking among adults. Cochrane Database of Systematic Reviews. 2002;(Issue 2) doi: 10.1002/14651858.CD001745. [DOI: 10.1002/14651858.CD001745] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick 2013 .Sedgwick P. Selection bias versus allocation bias. BMJ. 2013;346:f3345. [Google Scholar]
- Serra 2008 .Serra C, Bonfill X, Pladevall VM, Cabezas PC. Interventions for preventing tobacco smoking in public places. Cochrane Database of Systematic Reviews. 2008;(Issue 3) doi: 10.1002/14651858.CD001294.pub2. [DOI: 10.1002/14651858.CD001294.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton 2009 .Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoff 2013 .Shoff C, Yang TC. Understanding maternal smoking during pregnancy: Does residential context matter? Social Science & Medicine. 2013;78:50–60. doi: 10.1016/j.socscimed.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair 2004 .Sinclair HK, Bond CM, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD003698.pub2. [DOI: 10.1002/14651858.CD003698.pub2] [DOI] [PubMed] [Google Scholar]
- Slade 2006 .Slade P, Laxton-Kane M, Spiby H. Smoking in pregnancy: the role of the transtheoretical model and the mother’s attachment to the fetus. Addictive Behaviors. 2006;31:743–57. doi: 10.1016/j.addbeh.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Slotkin 2008 .Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicology and Teratology. 2008;30(1):1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Small 2000 .Small R. Mothers in a New Country (MINC) [thesis] LaTrobe University; Victoria: 2000. [Google Scholar]
- Solomon 1996 .Solomon LJ, Secker-Walker RH, Skelly JM, Flynn BS. Stages of change in smoking during pregnancy in low risk women. Journal of Behavioral Medicine. 1996;19:333–4. doi: 10.1007/BF01904760. [DOI] [PubMed] [Google Scholar]
- Solomon 2006 .Solomon LJ, Higgins ST, Heil SH, Badger GJ, Mongeon JA, Bernstein IM. Psychological symptoms following smoking cessation in pregnant smokers. Journal of Behavioral Medicine. 2006;29(2):151–60. doi: 10.1007/s10865-005-9041-4. [DOI] [PubMed] [Google Scholar]
- Spangler 2001 .Spangler JG, Michielutte R, Bell R, Knick S, Dignan MB, Summerson JH. Dual tobacco use among Native American adults in southeastern North Carolina. Preventive Medicine. 2001;32(6):521–8. doi: 10.1006/pmed.2001.0835. [DOI] [PubMed] [Google Scholar]
- Stead 2005a .Stead LF, Lancaster T. Interventions for preventing tobacco sales to minors. Cochrane Database of Systematic Reviews. 2005;(Issue 1) doi: 10.1002/14651858.CD001497.pub2. [DOI: 10.1002/14651858.CD001497.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2005b .Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD001007.pub2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2006a .Stead LF, Lancaster T. Nicobrevin for smoking cessation. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD005990. [DOI: 10.1002/14651858.CD005990] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead 2006b .Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD002850.pub2. [DOI: 10.1002/14651858.CD002850.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2007 .Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD005231.pub2. [DOI: 10.1002/14651858.CD005231.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2008 .Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(Issue 2) doi: 10.1002/14651858.CD000165.pub3. [DOI: 10.1002/14651858.CD000165.pub3] [DOI] [PubMed] [Google Scholar]
- Stead 2012a .Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 11) doi: 10.1002/14651858.CD000146.pub4. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
- Stead 2012b .Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 12) doi: 10.1002/14651858.CD009670.pub2. [DOI: 10.1002/14651858.CD009670.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2012c .Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 10) doi: 10.1002/14651858.CD008286.pub2. [DOI: 10.1002/14651858.CD008286.pub2] [DOI] [PubMed] [Google Scholar]
- Stead 2012d .Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 2) doi: 10.1002/14651858.CD000124.pub2. [DOI: 10.1002/14651858.CD000124.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne 2001 .Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology. 2001;54(10):1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Steyn 2006 .Steyn K, de Wet T, Saloojee Y, Nel H, Yach D. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth To Ten Study. Paediatric Perinatal Epidemiology. 2006;20(2):90–9. doi: 10.1111/j.1365-3016.2006.00707.x. [DOI] [PubMed] [Google Scholar]
- Stotts 1996 .Stotts AL, DiClemente CC, Carbonari JP, Mullen PD. Pregnancy smoking cessation: a case of mistaken identity. Addictive Behaviors. 1996;21:459–71. doi: 10.1016/0306-4603(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Strand 2003 .Strand M, Phelan K, Donovan E. Promoting the uptake and use of evidence: an overview of the problem. Clinics in Perinatology. 2003;30:389–402. doi: 10.1016/s0095-5108(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Strauss 1997 .Strauss KF, Mokdad A, Ballew C, Mendlein JM, Will JC, Goldberg HI, et al. The health of Navajo women: findings from the Navajo Health and Nutrition Survey, 1991-1992. Journal of Nutrition. 1997;127(10 Suppl):2128S–2133S. doi: 10.1093/jn/127.10.2128S. [DOI] [PubMed] [Google Scholar]
- Subramoney 2008 .Subramoney S, Gupta PC. Anemia in pregnant women who use smokeless tobacco. Nicotine & Tobacco Research. 2008;10(5):917–20. doi: 10.1080/14622200802027206. [DOI] [PubMed] [Google Scholar]
- Tabachnick 2001 .Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th Edition Allyn & Bacon; Needham Heights, MA: 2001. [Google Scholar]
- Tamim 2008 .Tamim H, Yunis KA, Chemaitelly H, Alameh M, Nassar AH. Effect of narghile and cigarette smoking on newborn birthweight. BJOG: an international journal of obstetrics and gynaecology. 2008;115(1):91–7. doi: 10.1111/j.1471-0528.2007.01568.x. [DOI] [PubMed] [Google Scholar]
- Tappin 1996 .Tappin DM, Ford RP, Nelson KP, Wild CJ. Prevalence of smoking in early pregnancy by census area, measured by anonymous cotinine testing of residual antenatal blood samples. New Zealand Medical Journal. 1996;109(1018):101–3. [PubMed] [Google Scholar]
- Tappin 2010 .Tappin DM, MacAskill S, Bauld L, Eadie D, Shipton D, Galbraith L. Smoking prevalence and smoking cessation services for pregnant women in Scotland. Substance Abuse: Treatment, Prevention, and Policy. 2010;5 doi: 10.1186/1747-597X-5-1. [DOI: 10.1186/1747–597X-5-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor 2009 .Taylor M. York Health Economics Consortium for National Institute for Health and Clinical Excellence. [accessed 22 August 2013]. 2009. Economic analysis of interventions for smoking cessation aimed at pregnant women: supplementary report. http://www.nice.org.uk/nicemedia/live/13023/49421/49421.pdf. [Google Scholar]
- Thomas 2007 .Thomas RE, Baker PRA, Lorenzetti D. Family-based programmes for preventing smoking by children and adolescents. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD004493.pub2. [DOI: 10.1002/14651858.CD004493.pub2] [DOI] [PubMed] [Google Scholar]
- Thomas 2008 .Thomas S, Fayter D, Misso K, Ogilvie D, Petticrew M, Sowden A, et al. Population tobacco control interventions and their effects on social inequalities in smoking: systematic review. Tobacco Control. 2008;17(4):230–7. doi: 10.1136/tc.2007.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas 2010 .Thomas J, Brunton J, Graziosi S. EPPI–Centre Software. Social Science Research Unit, Institute of Education; London: 2010. EPPI-Reviewer 4.0: software for research synthesis. [Google Scholar]
- Thomas 2013 .Thomas RE, McLellan J, Perera R. School-based programmes for preventing smoking. Cochrane Database of Systematic Reviews. 2013;(Issue 4) doi: 10.1002/14651858.CD001293.pub3. [DOI: 10.1002/14651858.CD001293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson 2007a .Thompson L, Pearce J, Ross Barnett J. Moralising geographies: stigma, smoking islands and responsible subjects. Area. 2007;39(4):508–17. [Google Scholar]
- Thomsen 2010 .Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews. 2010;(Issue 7) doi: 10.1002/14651858.CD002294.pub3. [DOI: 10.1002/14651858.CD002294.pub3] [DOI] [PubMed] [Google Scholar]
- Todd 2001 .Todd S, LaSala K, Neil-Urban S. An integrated approach to prenatal smoking cessation interventions. MCN, American Journal of Maternal Child Nursing. 2001;26:185–90. doi: 10.1097/00005721-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Tong 2008 .Tong VT, England LJ, Dietz PM, Asare LA, Tong VT, England LJ, et al. Smoking patterns and use of cessation interventions during pregnancy. American Journal of Preventive Medicine. 2008;35(4):327–33. doi: 10.1016/j.amepre.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Tong 2009 .Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy risk assessment monitoring system (PRAMS), United States, 31 sites, 2000-2005. Morbidity and Mortality Weekly Report. 2009;58:1–29. [PubMed] [Google Scholar]
- Tong 2011 .Tong VT, Dietz PM, England LJ, Farr SL, Kim SY, D’Angelo D, et al. Age and racial/ethnic disparities in prepregnancy smoking among women who delivered live births. Preventing Chronic Disease. 2011;8:A121. [PMC free article] [PubMed] [Google Scholar]
- Troe 2008 .Troe EJ, Raat H, Jaddoe V, Hofman A, Steegers E, Verhulst F, et al. Smoking during pregnancy in ethnic populations: the Generation R study. Nicotine and Tobacco Research. 2008;10:1373–84. doi: 10.1080/14622200802238944. [DOI] [PubMed] [Google Scholar]
- Tsoi 2013 .Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews. 2013;(Issue 2) doi: 10.1002/14651858.CD007253.pub3. [DOI: 10.1002/14651858.CD007253.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueffing 2009 .Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E, for the Cochrane Health Equity Field [Version 2012-10-04];Equity Checklist for Systematic Review Authors. 2009 http://equity.cochrane.org/our-publications.
- US DHHS 2004 .U.S. Department of Health and Human Services . 2004 Surgeon General’s Report. U.S. Department of Health and Human Services; 2004. The Health Consequences of Smoking. [Google Scholar]
- Ussher 2004 .Ussher M, West R, Hibbs N. A survey of pregnant smokers’ interest in different types of smoking cessation support. Patient Education and Counseling. 2004;54(1):67–72. doi: 10.1016/S0738-3991(03)00197-6. [DOI] [PubMed] [Google Scholar]
- Ussher 2012a .Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 1) doi: 10.1002/14651858.CD002295.pub4. [DOI: 10.1002/14651858.CD002295.pub4] [DOI] [PubMed] [Google Scholar]
- Ussher 2012b .Ussher M, Etter J-F, Giatras N, Coleman T. Tobacco withdrawal symptoms and urges to smoke in pregnant versus non-pregnant smokers. Addictive Behaviors. 2012;37(12):1353–7. doi: 10.1016/j.addbeh.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Valanis 2001b .Valanis B, Lichtenstein E, Mullooly JP, Labuhn K, Brody K, Severson H, et al. Maternal smoking cessation and relapse prevention during health care visits. American Journal of Preventive Medicine. 2001;20:1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- van der Meer 2001 .van der Meer RM, Wagena E, Ostelo RWJG, Jacobs AJE, van SCP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2001;(Issue 1) doi: 10.1002/14651858.CD002999. [DOI: 10.1002/14651858.CD002999] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer 2006 .van der Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD006102.pub2. [DOI: 10.1002/14651858.CD006102] [DOI] [PubMed] [Google Scholar]
- Vardavas 2010 .Vardavas CI, Chatzi L, Patelarou E, Plana E, Sarri K, Kafatos A, et al. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. European Journal of Pediatrics. 2010;169:741–8. doi: 10.1007/s00431-009-1107-9. [DOI] [PubMed] [Google Scholar]
- Venditti 2012 .Venditti CC, Smith GN. Self-reported cigarette smoking status imprecisely quantifies exposure in pregnancy. Open Journal of Obstetrics and Gynecology. 2012;2:56–61. [Google Scholar]
- Verma 1983 .Verma RC, Chansoriya M, Kaul KK. Effect of tobacco chewing by mothers on fetal outcome. Indian Pediatrics. 1983;20(2):105–11. [PubMed] [Google Scholar]
- Wakschlag 2003 .Wakschlag LS, Pickett KE, Middlecamp MK, Walton LL, Tenzer P, Leventhal BL. Pregnant smokers who quit, pregnant smokers who don’t: does history of problem behavior make a difference? Social Science and Medicine. 2003;56(12):2449–60. doi: 10.1016/s0277-9536(02)00248-4. [DOI] [PubMed] [Google Scholar]
- Walsh 2000 .Walsh RA, Redman S, Byrne JM, Melmeth A, Brinsmead MW. Process measures in an antenatal smoking cessation trial: another part of the picture. Health Education Research. 2000;15:469–83. doi: 10.1093/her/15.4.469. [DOI] [PubMed] [Google Scholar]
- Wanless 2004 .Wanless D. Securing Good Health for the Whole Population. TSO; London: 2004. [Google Scholar]
- Washio 2011 .Washio Y, Higgins ST, Heil SH, Badger GJ, Skelly J, Bernstein IM, et al. Examining maternal weight gain during contingency-management treatment for smoking cessation among pregnant women. Drug and Alcohol Dependence. 2011;114(1):73–6. doi: 10.1016/j.drugalcdep.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt 2012 .Watt TT. Alcohol use and cigarette smoking during pregnancy among American Indians/Alaska Natives. Journal of Ethnicity in Substance Abuse. 2012;11(3):262–75. doi: 10.1080/15332640.2012.701570. [DOI] [PubMed] [Google Scholar]
- Webb 2010 .Webb TL, Sniehotta FF, Michie S. Using theories of behaviour change to inform interventions for addictive behaviours. Addiction. 2010;105(11):1879–92. doi: 10.1111/j.1360-0443.2010.03028.x. [DOI] [PubMed] [Google Scholar]
- Welch 2012 .Welch V, Petticrew M, Tugwell P, Moher D, O’Neill J, Waters E, et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333. doi: 10.1371/journal.pmed.1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland 2008 .Wendland E, Pinto M, Duncan B, Belizn J, Schmidt M. Cigarette smoking and risk of gestational diabetes: a systematic review of observational studies. BMC Pregnancy and Childbirth. 2008;8:53. doi: 10.1186/1471-2393-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West 2005 .West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- White 2011 .White AR, Rampes H, Liu JP, Stead LF, Campbell J. Acupuncture and related interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2011;(Issue 1) doi: 10.1002/14651858.CD000009.pub3. [DOI: 10.1002/14651858.CD000009.pub3] [DOI] [PubMed] [Google Scholar]
- Whittaker 2012 .Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(Issue 11) doi: 10.1002/14651858.CD006611.pub3. [DOI: 10.1002/14651858.CD006611.pub3] [DOI] [PubMed] [Google Scholar]
- WHO 2008a .World Health Organization [accessed 2008];Report on the Global Tobacco Epidemic 2008 - the mpower package. http://www.who.int/tobacco/mpower/en/index.html.
- WHO 2008b .World Health Organization [accessed 2008];Closing the gap in a generation: health equity through action on the social determinants of health. http://www.who.int/social.determinants/en/
- Wiemann 1994 .Wiemann CM, Berenson AB, San Miguel VV. Tobacco, alcohol and illicit drug use among pregnant women: age and racial/ethnic differences. Journal of Reproductive Medicine. 1994;39(10):769–76. [PubMed] [Google Scholar]
- Wigginton 2012 .Wigginton B, Lee C. [accessed October 2013];A story of stigma: Australian women’s accounts of smoking during pregnancy. Critical Public Health. 2012 http://www.tandfonline.com/doi/pdf/10.1080/09581596.2012.753408.
- Williams 2010 .Williams N, Kathrecha P, Williams R. [accessed 23 August 2013];Consultation on NICE draft recommendations on quitting smoking in pregnancy and after childbirth: Report to the National Institute for Health and Clinical Excellence. 2010 http://guidance.nice.org.uk/PH26/SupportingEvidence/FieldworkReport/pdf/English.
- Wilson 2005 .Wilson DB. Meta-analysis macros for SPSS version 2005.05.23. SPSS; 2005. [Google Scholar]
- Windsor 1998 .Windsor RA, Boyd NR, Orleans CT. A meta-evaluation of smoking cessation intervention research among pregnant women: improving the science and art. Health Education Research. 1998;13(3):419–38. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- Windsor 1999 .Windsor RA, Li CQ, Boyd NR, Hartmann KE. The use of significant reduction rates to evaluate health education methods for pregnant smokers. Health Education & Behavior. 1999;26(5):648–62. doi: 10.1177/109019819902600506. [DOI] [PubMed] [Google Scholar]
- Windsor 2000b .Windsor RA, Whiteside HP, Solomon LJ, Donatelle RJ, Cinciripini PM, McIlvain HE. A process evaluation for patient education programs for pregnant smokers. Tobacco Control. 2000;9(Suppl 3):iii28–iii35. doi: 10.1136/tc.9.suppl_3.iii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong 2011 .Wong S, Ordean A, Kahan M. Substance use in pregnancy: SOGC clinical practice guideline. International Journal of Obstetrics and Gynecology. 2011;114(2):190–202. doi: 10.1016/j.ijgo.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Wood 2008 .Wood L, France K, Hunt K, Eades S, Slack-Smith L. Indigenous women and smoking during pregnancy: Knowledge, cultural contexts and barriers to cessation. Social Science and Medicine. 2008;66(11):2378–89. doi: 10.1016/j.socscimed.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Woodby 1999 .Woodby LL, Windsor RA, Snyder SW, Kohler CL, Diclemente CC. Predictors of smoking cessation during pregnancy. Addiction. 1999;94(2):283–92. doi: 10.1046/j.1360-0443.1999.94228311.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization 2001 .World Health Organization . Women and the Tobacco Epidemic. World Health Organization; Geneva: 2001. [Google Scholar]
- Yang 2010 .Yang L, Tong EK, Mao Z, Hu T. Exposure to secondhand smoke and associated factors among non-smoking pregnant women with smoking husbands in Sichuan province, China. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):549–57. doi: 10.3109/00016341003713851. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Lumley 1995a .Lumley J. Advice as a strategy for reducing smoking in pregnancy. [revised 02 October 1993] Pregnancy and Childbirth Module. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration. Issue 2. Update Software; Oxford: 1995. [Google Scholar]
- Lumley 1995b .Lumley J. Behavioural strategies for reducing smoking in pregnancy. [revised 27 September 1993] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration. Issue 2. Update Software; Oxford: 1995. [Google Scholar]
- Lumley 1995c .Lumley J. Counselling for reducing smoking in pregnancy. [revised 02 October 1993] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration. Issue 2. Update Software; Oxford: 1995. [Google Scholar]
- Lumley 1995d .Lumley J. Feedback as a strategy for reducing smoking in pregnancy. [revised 27 September 1993] Pregnancy and Childbirth Module. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration. Issue 2. Update Software; Oxford: 1995. [Google Scholar]
- Lumley 1995e .Lumley J. Strategies for reducing smoking in pregnancy. [revised 02 October 1993] Pregnancy and Childbirth Module. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] The Cochrane Collaboration. Issue 2. Update Software; Oxford: 1995. [Google Scholar]
- Lumley 1999 .Lumley J, Oliver S, Waters E. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 1999;(Issue 4) doi: 10.1002/14651858.CD001055. [DOI: 10.1002/14651858.CD001055] [DOI] [PubMed] [Google Scholar]
- Lumley 2004 .Lumley J, Oliver S, Chamberlain C, Oakley L. Interventions to promote smoking cessation in pregnancy. Cochrane Database of Systematic Reviews. 2004;(Issue 4) doi: 10.1002/14651858.CD001055.pub2. [DOI: 10.1002/14651858.CD001055.pub2] [DOI] [PubMed] [Google Scholar]
- Lumley 2009 .Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Cochrane Database of Systematic Reviews. issue 3. John Wiley & Sons, Ltd; 2009. Interventions for promoting smoking cessation during pregnancy. [DOI: 10.1002/14651858.CD001055.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Indicates the major publication for the study