Abstract
The neuropeptides kisspeptin (encoded by Kiss1) and RFamide-related peptide-3 (also known as GnIH; encoded by Rfrp) are potent stimulators and inhibitors, respectively, of reproduction. Whether kisspeptin or RFRP-3 might act directly on each other’s neuronal populations to indirectly modulate reproductive status is unknown. To examine possible interconnectivity of the kisspeptin and RFRP-3 systems, we performed double label in-situ hybridization (ISH) for RFRP-3’s receptors, Gpr147 and Gpr74, in hypothalamic Kiss1 neurons of adult male and female mice, as well as double-label ISH for kisspeptin’s receptor, Kiss1r, in Rfrp-expressing neurons of the hypothalamic dorsal-medial nucleus (DMN). Only a very small proportion (5–10%) of Kiss1 neurons of the anteroventral periventricular region expressed Gpr147 or Gpr74 in either sex, whereas higher co-expression (~25%) existed in Kiss1 neurons in the arcuate nucleus. Thus, RFRP-3 could signal to a small, primarily arcuate, subset of Kiss1 neurons, a conclusion supported by the finding of ~35% of arcuate kisspeptin cells receiving RFRP-3--immunoreactive fibre contacts. In contrast to the former situation, no Rfrp neurons co-expressed Kiss1r in either sex, and Tacr3, the receptor for neurokinin B (NKB; a neuropeptide co-expressed with arcuate kisspeptin neurons) was found in <10% of Rfrp neurons. Moreover, kisspeptin-immunoreactive fibres did not readily appose RFRP-3 cells in either sex, further excluding the likelihood that kisspeptin neurons directly communicate to RFRP-3 neurons. Lastly, despite abundant NKB in the DMN region where RFRP-3 soma reside, NKB was not co-expressed in the majority of Rfrp neurons. Our results suggest that RFRP-3 may modulate a small proportion of kisspeptin-producing neurons in mice, particularly in the arcuate nucleus, whereas kisspeptin neurons are unlikely to have any direct reciprocal actions on RFRP-3 neurons.
Keywords: RFRP-3, GnIH, Gpr147, Gpr74, Gpr54, Kisspeptin, Kiss1, Kiss1r, Tacr3, Tac2, Neurokinin B, reproduction, hypothalamus
Introduction
Neuropeptides of the arginine-phenylalanine-amide (RFamide) family have been demonstrated to have potent modulatory effects on a variety of physiological functions, including reproduction (1). Two members of this family, kisspeptin (encoded by Kiss1) and RFamide-related peptide 3 (RFRP-3, encoded by Rfrp), have been shown to regulate mammalian reproductive function through central mechanisms, but have opposing effects on the reproductive axis in mice, with kisspeptin stimulating and RFRP-3 inhibiting reproduction, respectively.
The kisspeptin system, which includes kisspeptin and its receptor, Kiss1r (formerly known as Gpr54), is considered stimulatory and essential for reproductive function. Human patients or rodents lacking functional Kiss1 or Kiss1r genes suffer from impaired puberty and hypogonadotropic hypogonadism, presenting with low levels of gonadotropins and sex steroids, underdeveloped gonads, impaired sexual development, and infertility (2–5). Exogenous kisspeptin administration potently stimulates the secretion of luteinizing hormone (LH) and follicle stimulating hormone (5–9), working centrally through a gonadotropin-releasing hormone (GnRH)-dependent mechanism (10, 11). Kisspeptin can directly activate GnRH neurons, as determined via c-fos induction (a marker of neuronal activation) in GnRH cells (6, 11) and stimulation of electrical firing of GnRH neurons in brain explants (12, 13). Anatomical support for a direct kisspeptin effect on GnRH cells includes the presence of kisspeptin neuronal fibres appositions on GnRH neurons (14–16) and high Kiss1r expression in the majority of GnRH neurons (5, 11, 12). Within the rodent brain, kisspeptin/Kiss1 mRNA somata are found in two primary populations: the rostral hypothalamic continuum of the anteroventral periventricular nucleus and neighboring rostral periventricular nucleus (AVPV/PeN), and the arcuate nucleus (ARC) (10, 14). In the ARC, kisspeptin neurons highly co-express both neurokinin B (NKB, encoded by the Tac2 gene) (17) and dynorphin, giving rise to the terminology KNDy neurons, but exact roles of these co-transmitters are still being elucidated.
In contrast to kisspeptin, RFRP-3 has potent inhibitory actions on both GnRH neuronal activity and LH secretion in most rodent species (18–20). RFRP-3 is produced from a precursor peptide encoded by the Rfrp gene (21) and is the mammalian orthologue of avian gonadotropin-inhibiting hormone (GnIH) (22, 23). Through immunohistochemical assessment, RFRP-3-immunoreactive (ir) cells are found exclusively in the dorsal-medial nucleus of the hypothalamus (DMN) of rodents (23, 24), mirroring the selective expression of Rfrp mRNA in this region, as determined by in-situ hybridization (ISH) (25, 26). In rodents, some GnRH neurons are contacted by RFRP-3 axonal fibres (23, 27, 28) and a subset of GnRH neurons express Gpr147, a high affinity receptor for RFRP-3 (26, 28). In addition, RFRP-3 can bind to a second G-protein coupled receptor, Gpr74, with lower affinity (21, 25), but this receptor is not expressed in GnRH neurons (26), and its relevance for the reproductive actions of RFRP-3 is currently unknown.
While both kisspeptin and RFRP-3 appear to modulate the reproductive axis in part by direct effects on GnRH, it is possible that these two neuropeptides may also influence reproductive status via indirect pathways. To this end, it is currently unclear if there is modulatory cross-talk between these two neuropeptide populations. In addition to projecting to some GnRH cells, RFRP-3-ir fibres also project to a variety of brain regions that do not have GnRH neurons, including the AVPV, lateral hypothalamic area, paraventricular nucleus, and ARC (23, 24, 27–29), and appositions of RFRP-3 fibres on some kisspeptin cells in the AVPV/PeN have been observed in female mice (28). Moreover, RFRP-3’s receptors, Gpr147 and Gpr74 are also expressed in several hypothalamic non-GnRH regions, including the periventricular nucleus, paraventricular nucleus, and ARC (26, 28, 30, 31). Additionally, RFRP-3 has been functionally shown to inhibit the electrical firing of some ARC kisspeptin neurons (32), suggesting that RFRP-3 may in fact be able to directly regulate this kisspeptin population. However, whether ARC kisspeptin neurons actually express RFRP-3 receptors in animals of either sex has not been addressed. Likewise, the possibility of kisspeptin neurons regulating RFRP-3 neurons, either through kisspeptin itself or one of its co-transmitters, such as NKB, has not yet been explored. Indeed, kisspeptin fibres have been observed in the DMN, and some Kiss1r expression has also been reported in this area (33), as has Tacr3 (the receptor for NKB, a co-transmitter of ARC kisspeptin neurons) (34). Thus, there may be unilateral or bilateral communication between the RFRP-3 and kisspeptin populations to fine-tune each other’s actions on the reproductive axis, but this has not yet been thoroughly examined.
To begin to address the possible anatomical interconnectivity of the kisspeptin and RFRP-3 systems, we used double-label ISH and immunocytochemistry to determine 1) if one or both of RFRP-3’s receptors are expressed in Kiss1 cells of either the AVPV/PeN or the ARC of males and females, 2) if the kisspeptin or NKB receptors are co-expressed with Rfrp neurons in the DMN, 3) if kisspeptin axonal fibres are found apposing RFRP-3 cells in the DMN, and lastly, 4) if Rfrp neurons co-express Tac2 (the gene encoding NKB) which is also known to be highly expressed in the DMN.
Materials and Methods
Animals, Gonadectomies, and Tissue Collection
Adult C57BL6 mice of both sexes were housed on a 12-12 light-dark cycle (lights off at 1800h) with food and water available ad libitum. For some experiments, mice were anesthetized and bilaterally gonadectomized (GDX) one week prior to sacrifice, as previously described (35, 36). For in-situ hybridization studies, GDX mice or gonadal-intact mice (females in diestrus, as determined by vaginal smears) were anesthetized with isoflurane and sacrificed by rapid decapitation. Brains were collected, frozen on dry ice, and stored at −80°C. Five coronal series of 20 µm brain sections were cut on a cryostat, thaw-mounted onto Superfrost-plus slides, and stored at −80°C until use in in-situ hybridization. For immunohistochemistry experiments, gonadal-intact male and GDX male and female mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer for brain collection. Coronal (30 µm thick) sections throughout the caudal hypothalamus, containing the entire ARC and DMN, were cut from each brain on a sliding microtome with a freezing stage.
For all experiments, each group consisted of 4–6 animals. All experiments were conducted in accordance with the NIH Animal Care and Use Guidelines and with approval of the Animal Care and Use Committee of the University of California, San Diego and the University of Otago Animal Ethics Committee.
Double-label In-Situ Hybridization (ISH)
The following cRNA ISH riboprobes have been previously described and validated: Rfrp, Kiss1r, Gpr74, Gpr147 (26); Kiss1 (10); Tac2 (17, 37); Gnrh (38). Tacr3 was cloned from adult mouse hypothalamic cDNA into pBluscript II SK(−) transcription plasmid (Stratagene, CA) as described previously (26) and corresponds to bases 286 to 691 of the mouse Tacr3 sequence (NM_021382).
Double-label ISH assays were performed as previously described (17, 39). For double-label assays studying ARC KNDy cells, we used Tac2 as a designator for KNDy neurons, as pilot studies indicated that Tac2 expression per cell was stronger than Kiss1, allowing for better detection with the fluorescent DIG probe. Briefly, slide-mounted brain sections encompassing the hypothalamus were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2X SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air-dried. Radio-labeled (33P) antisense Gpr147, Gpr74, Kiss1r, Tac2, or Tacr3 (0.05 pmol/ml) and digoxigenin (DIG)-labeled Rfrp, Kiss1, Tac2 or Gnrh riboprobes (Roche digoxigenin labeling kit, 1:500) were combined with tRNA, denatured by boiling, and dissolved together in hybridization buffer. The probe mix was then applied to slides (100 µl/slide), and slides hybridized at 55°C overnight. Slides were cover-slipped and placed in a 55°C humidity chamber overnight. The slides were then washed in 4X SSC and placed into RNAse A treatment for 30 min at 37°C, then in RNAse buffer without RNase at 37°C for 30 min. After washing in 2X SSC at room temperature, slides were washed in 0.1X SSC at 62°C for 1 hour. Slides were then incubated in 2X SSC with 0.05% Triton X-100 containing 3% sheep serum (NSS) for 75 min at room temperature and then incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase [(Roche) diluted 1:500 in Buffer 1 containing 1% NSS and 0.3% Triton X-100]. The next day, slides were washed with Buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Labs) for 1 h at room temperature. The slides were then air-dried, dipped in Kodak NTB emulsion, stored at 4°C, and developed and cover-slipped 9–11 days later.
ISH slides were analyzed with an automated image processing system (Dr. Don Clifton, University of Washington) by a person unaware of the treatment group of each slide (40). DIG-containing cells (Kiss1, Tac2, Rfrp or Gnrh cells) were identified under fluorescence microscopy and the grain-counting software was used to quantify silver grains (representing Gpr147, Gpr74, Kiss1r, Tacr3 or Tac2 mRNA) overlying each cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was >3.
Immunohistochemistry for kisspeptin and RFPR-3 in the DMN and ARC
For dual label immunohistochemistry of kisspeptin and RFRP-3, all steps were separated by four 10 minute washes in 50 mM tris-buffered saline containing 0.5% Triton X-100 (TBS-TX). After blocking in TBS-TX containing 1% BSA and 1% normal donkey serum, sections were incubated overnight at 4 °C in sheep anti-mouse kisspeptin-52 (AC053, kindly provided by Dr Alain Caraty, National Institute for Agronomic Research, France; 1:2000 dilution), and rabbit anti-sparrow GnIH (PAC 123/124, kindly provided by Dr George Bentley, University of California, Berkeley; 1:5000) in blocking solution. Sections were then incubated for 2 hours at room temperature in biotinylated donkey anti-sheep (1:500 dilution; Jackson ImmunoResearch) and Alexa Fluor 488 donkey anti-rabbit (1:500 dilution; Molecular Probes, Life Technologies). Following this, sections were incubated for 1 hour in Alexa Fluor 568-streptavidin (1:500 dilution; Molecular Probes). Staining was observed with a Zeiss LSM 710 confocal microscope using a 63 X objective lens and laser excitation lines and filters for 488 nm or 543 nm. Stacks of images, collected at intervals of 600 nm, were analyzed offline using ImageJ software (National Institutes of Health, Bethesda, MD). In the DMN, twenty RPRP-3 cell bodies were visualized per mouse and all kisspeptin-ir contacts recorded. In the ARC, 29–50 kisspeptin cell bodies were visualized per mouse and all RFRP-3 contacts were recorded. Contacts were defined as no black pixel between the fibre and the soma. Omission of any of the primary antibodies resulted in complete absence of staining.
Statistical Analysis
All data are expressed as the mean ± SEM for each group. In all experiments, differences were analyzed by Student’s t-test or by 2-way ANOVA, followed by post-hoc comparisons for individual sex/treatment groups via Fisher’s (protected) LSD. Statistical significance was set at p<0.05. All analyses were performed in Statview 5.0.1 (SAS Institute, Cary, NC).
Results
Experiment 1: Only a small proportion of AVPV Kiss1 neurons express Gpr147 or Gpr74
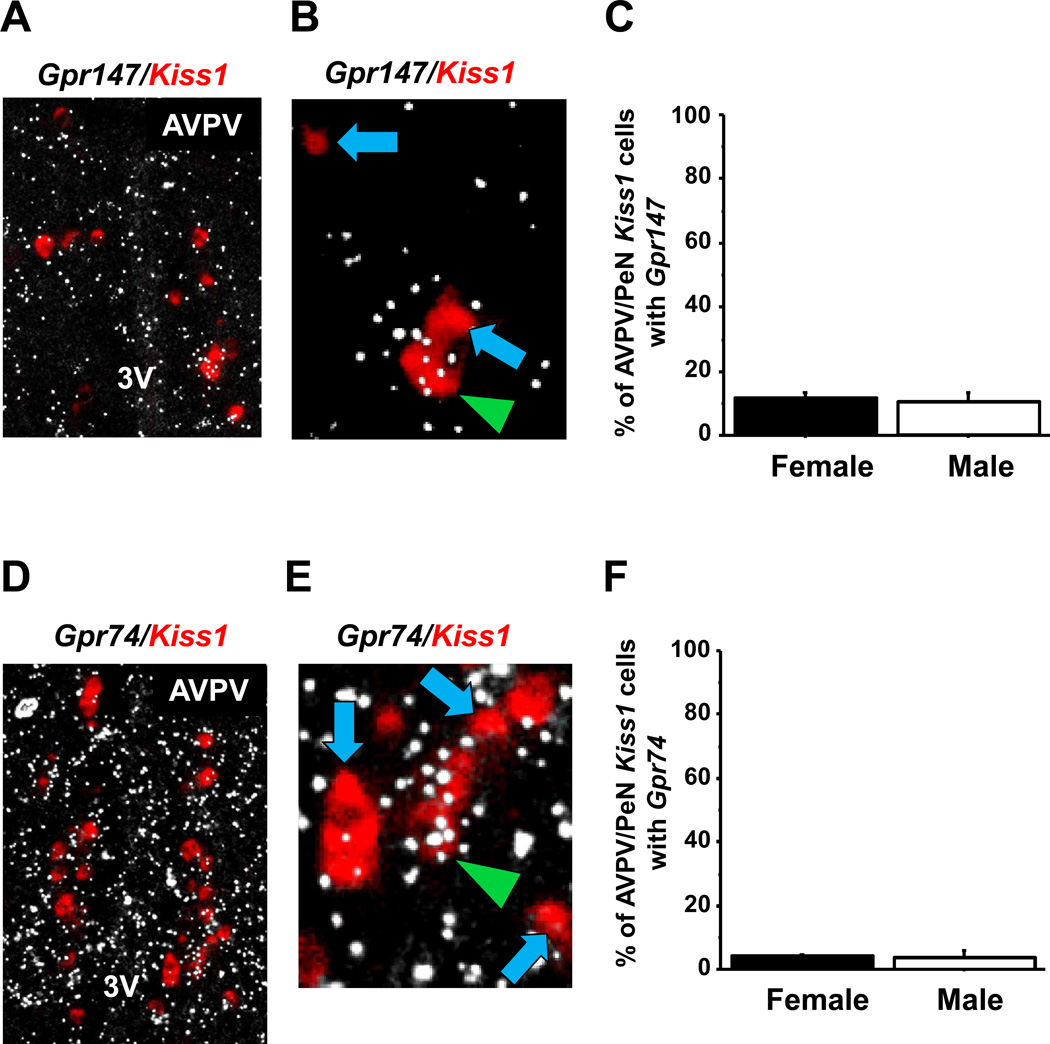
We previously reported that 12–15% of Kiss1 neurons in the AVPV/PeN of female mice express Gpr147, both in ovary-intact (dioestrus) and oestradiol-treated conditions (28). However, it is unknown if a similar proportion of AVPV/PeN Kiss1 neurons in male mice express Gpr147. Additionally, the co-expression of Gpr74 with Kiss1 in the AVPV/PeN in either sex has not previously been determined. In the present experiment, we used double-label ISH to determine coexpression of Gpr147 or Gpr74 mRNA in Kiss1 neurons in the AVPV/PeN of gonadally-intact female (diestrous) and male mice. Such coexpression was not also examined in GDX mice, as Kiss1 expression is nearly undetectable in the AVPV/PeN in the GDX state (35). As expected, there was a pronounced sex difference in the number of detectable AVPV/PeN Kiss1 neurons, with females having several fold more Kiss1 neurons than males (data not shown) (41). In terms of RFRP-3 receptors, we found an overall low abundance of both Gpr147 and Gpr74 expression in the AVPV/PeN region, unlike other regions such as the paraventricular nucleus and thalamus where Gpr147 and Gpr74 mRNAs, respectively, were more highly expressed. In agreement with our previous study (28), quantitatively, only 12% of AVPV/PeN Kiss1 neurons expressed Gpr147 in females, and a similar proportion was observed in males (Figure 1). An even smaller proportion (5–6%) of AVPV/PeN Kiss1 neurons co-expressed Gpr74 in either sex (Figure 1). There were no statistical differences between the sexes for co-expression of either RFRP-3 receptor with Kiss1 in the AVPV/PeN. The relative amount of Gpr147 or Gpr74 mRNA per Kiss1 cell, reflected by the number of silver grains in each Kiss1 cell, also did not differ between sexes (not shown).
Figure 1.
Expression of Gpr147 and Gpr74 in AVPV/PeN Kiss1 neurons by double label in-situ hybridization. [A] Representative photomicrographs of double label in-situ hybridization of Kiss1 (red fluorescence) and Gpr147 (silver grains) in an intact male. 3V, third ventricle [B] Kiss1 neurons co-expressing Gpr147 (green arrowhead) and Kiss1 neurons with no co-expression of Gpr147 (blue arrows). [C] Quantification of the percent co-expression of Gpr147 in AVPV/PeN Kiss1 neurons between gonadally-intact females (F) and males (M). There were no significant differences in co-expression between any of the groups. [D] Representative photomicrographs of double label in-situ hybridization of Kiss1 (red fluorescence) and Gpr74 (silver grains) in a dioestrous female. [E] Kiss1 neurons co-expressing Gpr74 (green arrowhead) and Kiss1 neurons with no co-expression of Gpr147 (blue arrows). [F] Quantification of the percent co-expression of Gpr147 in Kiss1 neurons between gonadally-intact females (F) and males (M). There were no statistical differences between the sex or gonadal state.
Experiment 2: A moderate proportion of KNDy neurons in the ARC express Gpr147 or Gpr74 and receive contacts from RFRP-3 fibres
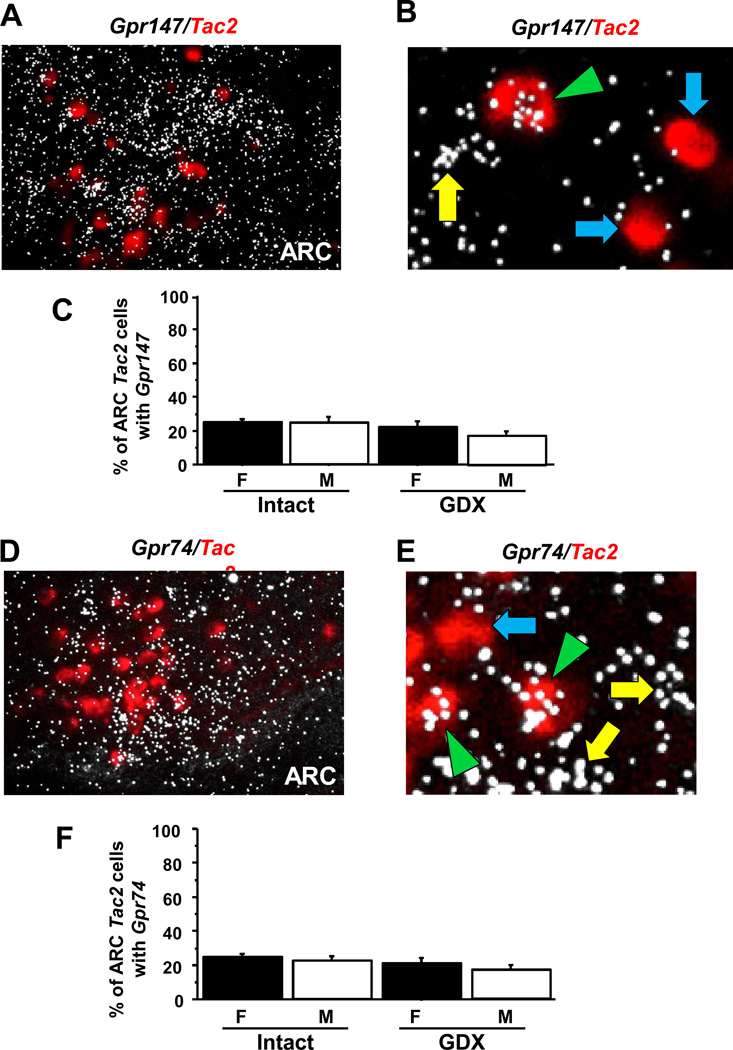
The ARC Kiss1 population highly co-expresses Tac2 (which encodes NKB) and is referred to as the KNDy neuron population. Here, we determined if either Gpr147 or Gpr74 is co-expressed in ARC KNDy neurons of adult male and female mice. We found that Gpr147 mRNA was moderately expressed in approximately 25% of KNDy neurons in gonadally-intact female (diestrous) and male mice (Figure 2A–C). Similar levels of Gpr147 co-expression were found in GDX mice of both sexes (Figure 2). Like Gpr147, 23–25% of ARC KNDy neurons in gonadally-intact male and female mice co-expressed Gpr74 mRNA, with slightly lower but not significantly different coexpression in GDX mice (Figure 2D–E). For both assays, GDX mice had considerably more KNDy neurons than gonadally-intact mice (p<0.05; data not shown), as expected due to known stimulatory effects of GDX on KNDy cells in rodents (42, 43). There were no statistical differences between sexes in the degree of Gpr147 or Gpr74 co-expression in ARC KNDy neurons and no group differences were observed in the relative amount of receptor mRNA per KNDy neuron (silver grains per double-labeled cell; not shown). The coexpression for the two RFRP-3 receptors within KNDy neurons was evenly dispersed throughout the ARC and not noticeably different between in any anatomical subregion within the KNDy neuron population.
Figure 2.
Expression of Gpr147 and Gpr74 in ARC KNDy neurons by double label in-situ hybridization. [A] Representative photomicrographs of double label in-situ hybridization of Tac2 (red fluorescence) and Gpr147 (silver grains) in the ARC of a dioestrous female. [B] ARC Tac2 neurons co-expressing Gpr147 (green arrowhead) and Tac2 neurons with no co-expression of Gpr147 (blue arrows). [C] Quantification of the percent co-expression of Gpr147 in Tac2 neurons between gonadally-intact females (F) and males (M) and gonadectomized (GDX) M and F. There were no significant differences in co-expression between any of the groups. [D] Representative photomicrographs of double label in-situ hybridization of Tac2 (red fluorescence) and Gpr74 (silver grains) in the ARC of a dioestrous female. [E] Tac2 neurons co-expressing Gpr74 (green arrowhead) and a Gpr74 neuron that is not expressing Tac2 (yellow arrow). [F] Quantification of the percent co-expression of Gpr147 in Tac2 neurons between gonadally-intact females (F) and males (M) and gonadectomized (GDX) M and F. These experimental groups were not statistically difference.
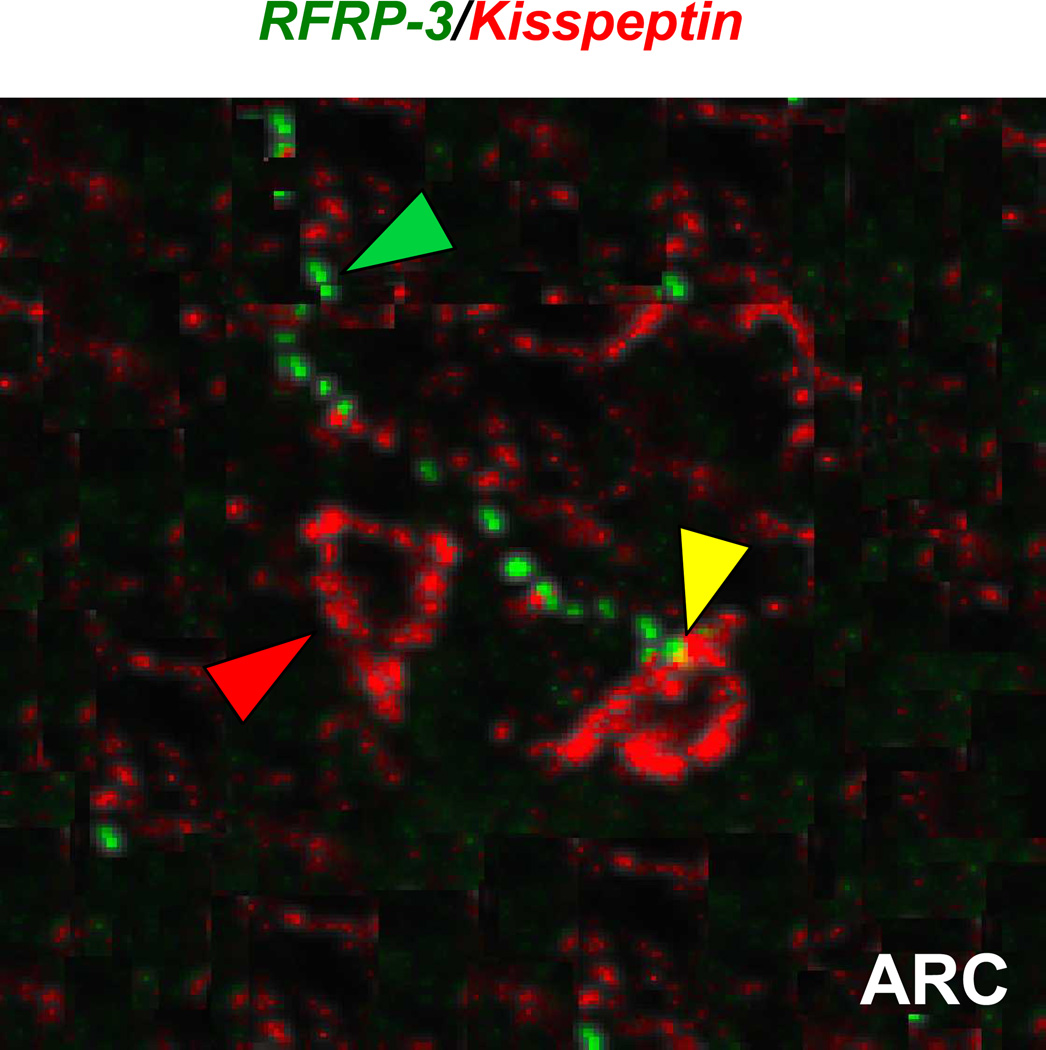
To further examine possible RFRP-3 neuron to kisspeptin neuron interactions, we next used double-label IHC to assess potential RFRP-3 fibre contacts on kisspeptin neurons in the ARC of female mice. For this analysis, GDX females were used, as this gonadal state allows for identification of kisspeptin cell bodies in the ARC, unlike gonadal-intact mice in which the dense kisspeptin fibre network obscures cell bodies. Supporting our receptor coexpression data above, RFRP-3 fibres were observed to appose a moderate proportion of ARC kisspeptin cells. Quantification determined that ~35% of kisspeptin soma in the ARC received RFRP-3 fibre contacts (Figure 3).
Figure 3.
Representative photomicrograph of RFRP-3 immunoreactive fibre (green fluorescence) in apposition with ARC kisspeptin neuron (red fluorescence) in a female mouse. Immunohistochemical analysis revealed RFRP-3 contacts with ~35% of kisspeptin cell bodies in the ARC of GDX female mice. Figure is a collapsed stack of several confocal optical sections. Green triangle denotes example RFRP-3 fibre immunoreactivity. Red triangle denotes an ARC kisspeptin cell not receiving RFRP-3 input. Yellow triangle denotes RFRP-3 fibre contacting an ARC kisspeptin cell.
Experiment 3: Rfrp neurons do not highly express Kiss1r or Tacr3 or receive axonal contacts from kisspeptin neurons
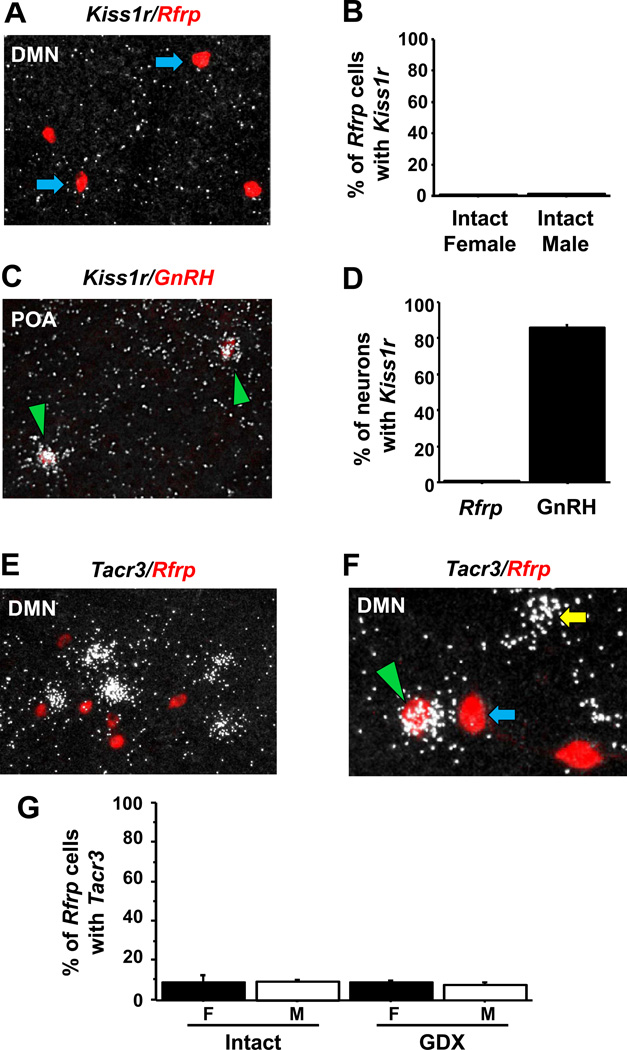
Experiments 1 and 2 indicated that a small population of kisspeptin neurons in the AVPV/PeN, and more so in the ARC, could be responsive to RFRP-3 signaling. Next, we determined if the reciprocal relationship might also exist: could kisspeptin neurons also act on Rfrp neurons? To address this, we measured the degree of Kiss1r co-expression in Rfrp neurons and also determined the degree to which kisspeptin fibres contact RFRP-3 neurons in the DMN. Double label ISH for Kiss1r in Rfrp neurons in adult male and female mice revealed that essentially all Rfrp neurons (>99%) lacked Kiss1r (Figure 4A). In fact, Kiss1r was surprisingly absent from the DMN, despite previous ISH data demonstrating high expression of Kiss1r in this nucleus (33). As a positive control, pronounced Kiss1r mRNA expression was observed in the habenula, a known region of Kiss1r expression (not shown). To ensure that lack of Kiss1r in Rfrp neurons was not due to technical reasons, a second set of slides from the rostral hypothalamus of adult females was concurrently assayed for Kiss1r expression in Gnrh neurons (Figure 4C, D) along with Kiss1r in Rfrp neurons. Whereas >85% of GnRH neurons expressed Kiss1r, no Rfrp neurons expressed Kiss1r (Figure 4C, D), consistent with the previous assay.
Figure 4.
Expression of Kiss1r in Rfrp and GnRH neurons and expression of Tacr3 in Rfrp neurons by double label in-situ hybridization. [A] Representative photomicrographs of double label in-situ hybridization of Rfrp (red fluorescence) and Kiss1r (silver grains) in the DMN of a dioestrous female. Rfrp neurons lacking Kiss1r are marked with blue arrows [B] Quantification of the percent co-expression of Kiss1r in Rfrp neurons between gonadally-intact females and males. There were no significant differences in co-expression between sexes. [C] Representative photomicrographs of double label in-situ hybridization of Kiss1r (silver grains) and GnRH (red fluorescence) in the preoptic area (POA) of an intact male. Green arrowheads identify double-labeled cells [D] Quantification of the percent co-expression of Kiss1r in Rfrp or GnRH neurons in dioestrous females and intact males (percentages averaged across all animals). [E] Representative photomicrographs of double label in-situ hybridization of Rfrp (red fluorescence) and Tacr3 (silver grains) in the DMN of an intact male mouse. [F] Rfrp neurons co-expressing Tacr3 (green arrowhead) and a cell expressing Tacr3 without Rfrp (yellow arrow). [G] Quantification of the percent co-expression of Tacr3 in Rfrp neurons between gonadally-intact females (F) and males (M) and gonadectomized (GDX) M and F. There were no significant differences in co-expression between any of the groups.
In the ARC, kisspeptin neurons co-express NKB, which could potentially be used by ARC KNDy neurons to communicate with RFRP-3 neurons via Tacr3 signaling. Indeed, Tacr3 (the NKB receptor) is highly expressed in the DMN region, along with NKB fibres (34, 44), but it is unknown if this specifically includes RFRP-3 neurons. Using double-label ISH for Tacr3 and Rfrp, we observed robust staining for both mRNAs in the DMN of mice of both sexes (Figure 4E, F). However, quantitative analysis determined that Tacr3 mRNA was absent in most Rfrp neurons. Less than 10% of Rfrp neurons expressed Tacr3 in gonadally-intact males and females (Figure 4G), and similar low coexpression levels were quantified in GDX mice of both sexes, with Tacr3 being detected in only ~8% of Rfrp neurons (Figure 4G). There were no statistical differences between sexes or gonadal state in the proportion of cells expressing Tacr3 or the relative Tac3r mRNA level per Rfrp cell.
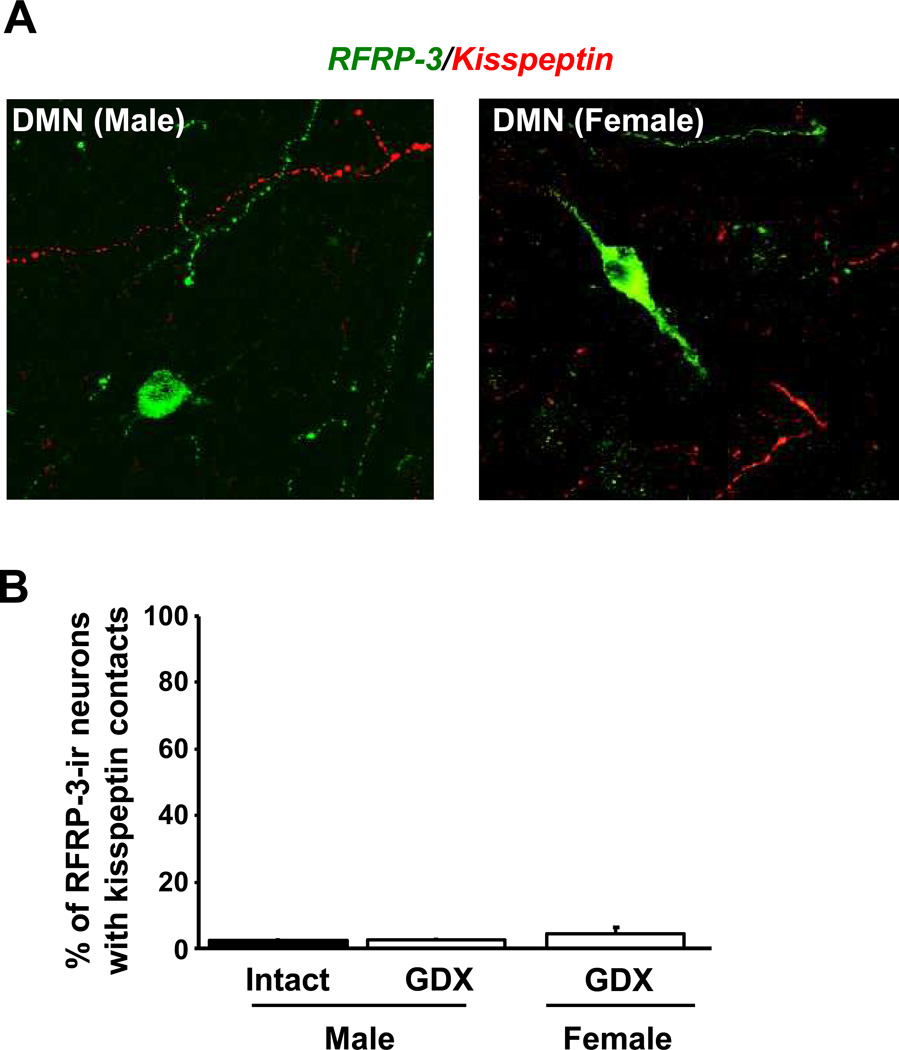
In a complementary experiment, we used double-label IHC to assess potential kisspeptin fibre contacts, which could arise from either the AVPV/PeN kisspeptin population and/or ARC kisspeptin/NKB (KNDy) cells, on RFRP-3 neurons in the DMN. Matching the receptor coexpression data, virtually no RFRP-3 cells were observed with contacts from kisspeptin fibres (Figure 5A). Quantification of the staining revealed that, on average, just 4% of RFRP-3-immunoreactive cells received apparent contacts from kisspeptin-containing fibres in gonadally-intact adult male mice (Figure 5B). Similar results were observed in GDX mice, with only ~3% and ~7% of RFRP-3 cells receiving kisspeptin fibre appositions in males and females, respectively (Figure 5B). There were no statistical differences in the degree of kisspeptin-RFRP-3 contacts between intact and GDX mice or between sexes.
Figure 5.
Immunohistochemistry for kisspeptin fibres and RFRP-3 cell bodies in the mouse DMN. [A] Representative images of RFRP-3 cell bodies and fibres (green fluorescence) and kisspeptin fibres (red fluorescence) in the DMN of a gonadally-intact male (left) and GDX female (right). [B] Quantification of the percent of RFRP-3 neurons receiving contacts from kisspeptin fibres in the DMN of intact and gonadectomized (GDX) male and female mice. Almost no RFRP-3 cells were contacted and there was no statistical difference between gonadal states.
Experiment 4: Is NKB a co-neuropeptide with RFRP-3?
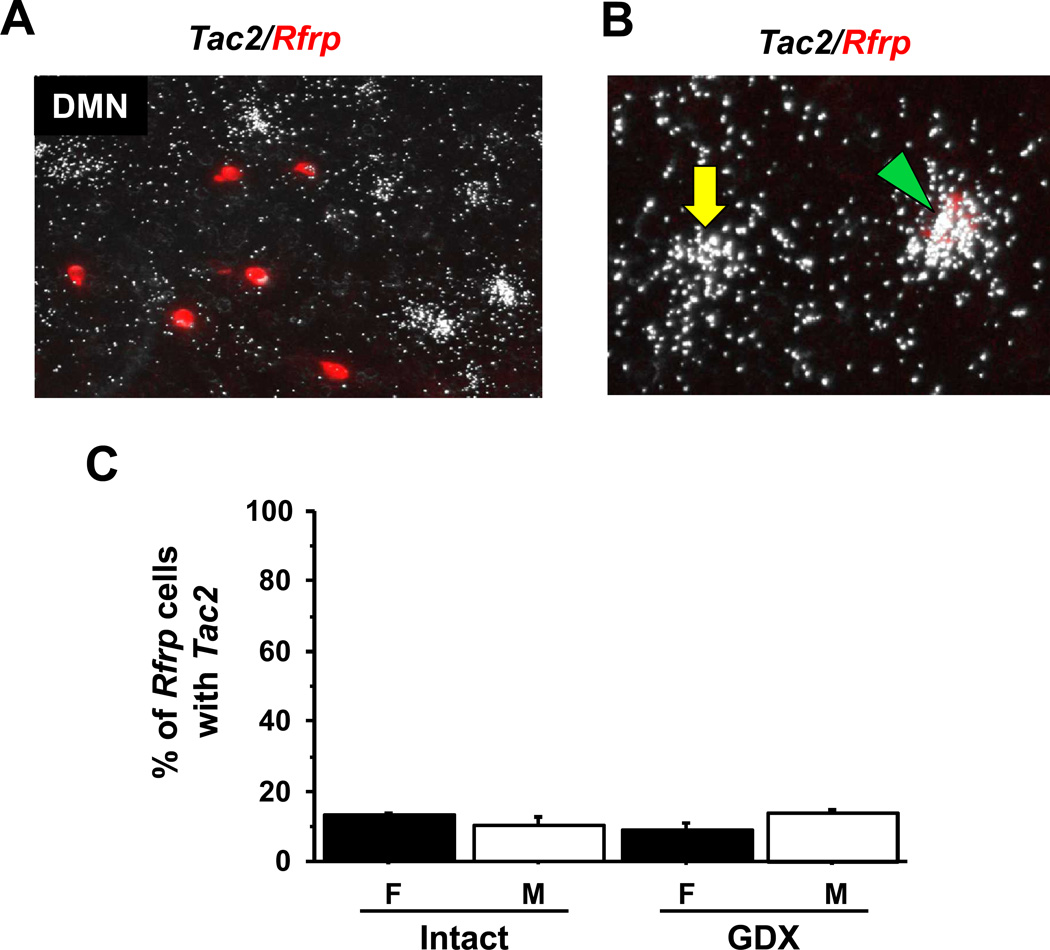
Tac2 mRNA, which codes for NKB, is known to be highly expressed in the DMN (45), but its co-expression in RFRP-3 neurons is unknown. We used double-label ISH to determine if Rfrp neurons are in fact the same or an overlapping population of DMN cells as those expressing Tac2. However, despite strong expression of both genes in the DMN region, we found that Rfrp and Tac2 neurons in the DMN are mostly distinct populations, with relatively low levels of co-expression (Figure 6A, B). Quantitatively, approximately 12% of Rfrp neurons co-express Tac2 in adult mice of both sexes (Figure 6C) under both gonadal-intact and GDX conditions. There were no statistical differences in the proportion of Rfrp neurons co-expressing Tac2 between sexes or treatment group (Figure 6C) and there were no differences in the grains per cell representing relative Tac2 mRNA levels in the double-labeled cells (not shown). We did not attempt to quantify the degree of reciprocal co-localization of DMN Tac2 neurons expressing Rfrp. However, in general, we consistently noted significantly more total Tac2 neurons than Rfrp neurons in the DMN region, indicating that the proportion of Tac2 cells co-expressing Rfrp would be notably lower than the 12% of Rfrp neurons found to co-express Tac2.
Figure 6.
Expression Tac2 in Rfrp neurons in the DMN by double label in-situ hybridization. [A] Representative photomicrographs of double label in-situ hybridization of Rfrp (red fluorescence) and Tac2 (silver grains) in the DMN of a dioestrous female. [B] Higher magnification of Rfrp neurons co-expressing Tac2 (green arrowhead) and a Tac2 neuron that is not expressing Rfrp (yellow arrow). [C] Quantification of the percent co-expression of Tac2 in Rfrp neurons between gonadally-intact females (F) and males (M) and gonadectomized (GDX) M and F. These experimental groups were not statistically different.
Discussion
Despite the potent and reciprocal activities of kisspeptin and RFRP-3 on the reproductive axis, comprehensive interconnectivity of these two neuropeptide systems has not been thoroughly investigated. Here, we sought to determine if the receptors for RFRP-3, Gpr147 and Gpr74, were expressed in either population of hypothalamic kisspeptin neurons and whether kisspeptin’s receptor, or that for NKB, was expressed in RFRP-3 cells. We found that the majority of AVPV/PeN Kiss1 neurons do not express either of the receptors known to mediate the actions of RFRP-3, whereas a moderate percentage of kisspeptin cells in the ARC do co-express RFRP-3 receptors. Conversely, Kiss1r (kisspeptin receptor) was absent in virtually all Rfrp neurons and almost no RFRP-3 neurons receive appositions from kisspeptin axonal fibres. Moreover, Tacr3, the receptor for ARC kisspeptin’s co-transmitter NKB, was not highly expressed in most RFRP-3 cells. Overall, our anatomical data suggest that the kisspeptin and RFRP-3 neuronal systems likely act independently on the GnRH-pituitary axis and may only have notable communication with each other at the level of RFRP-3 signaling to ARC kisspeptin cells.
The various mechanisms by which RFRP-3 neurons might regulate the reproductive axis are not fully elucidated. A good part of RFRP-3’s reproductive modulation appears to occur through the inhibition of GnRH release, and antagonizing the GnRH receptor abolishes the stimulatory effect of an RFRP-3 antagonist, RF9, on LH secretion (28). These data suggest that RFRP-3 provides an inhibitory tone upstream of GnRH signaling, since blockade of RFRP-3 signaling is only effective at stimulating LH when GnRH signaling pathways are functional. However, using several different techniques, we previously found only low to moderate co-expression of the RFRP-3 receptors, Gpr147 and Gpr74, in GnRH neurons, with a majority of GnRH cells not expressing either receptor (26, 28), matching the finding that only a subset of GnRH neurons changing their firing rate after RFRP-3 treatment (19). The ability of RFRP-3 to inhibit GnRH and LH despite a majority of GnRH cells not expressing RFRP-3 receptors could indicate that some RFRP-3-mediated inhibition on GnRH may occur indirectly. While the possibility of RFRP-3 acting on the pituitary has been hypothesized, RFRP-3 neurons of rodents are not hypophysiotropic, since they are unable take up peripherally administered retrograde tracers (46). These data exclude the possibility of RFRP-3 acting directly on the pituitary of rodents, which differs from the ovine model, where RFRP-3 can be measured in portal blood (47).
Given that only a subset of GnRH neurons expresses RFRP-3 receptors, we speculated that RFRP-3 may also regulate the GnRH axis through an intermediate neuropeptide population(s), such as kisspeptin neurons. Kisspeptin is a potent stimulator of GnRH release (10), but the “upstream” circuitry that regulates the synthesis and secretion of kisspeptin is poorly understood. Thus, we hypothesized that RFRP-3 may be an upstream factor that negatively modulates kisspeptin neurons to thereby reduce GnRH activation. This possibility was supported by data indicating that RFRP-3 fibres appose some AVPV/PeN kisspeptin neurons in female mice (28) and are also present in the ARC where kisspeptin neurons also reside (23, 24, 29). However, based on our present findings, it appears that a large majority of kisspeptin neurons, in both the AVPV/PeN and the ARC, are lacking receptors for RFRP-3. This was especially apparent in the AVPV/PeN, suggesting that kisspeptin neurons in that nucleus are unlikely to be significantly regulated by direct RFRP-3 signaling. In the ARC, however, a moderate proportion (~25%) of kisspeptin cells co-expressed Gpr147 or Gpr74, and nearly 35% of ARC kisspeptin neurons receive RFRP-3 fibre contacts, indicating that there could be some functional regulation of kisspeptin neurons by RFRP-3 in this specific brain region. Even so, it is not clear what the functional significance of such communication would be, given the lack of RFRP-3 receptors and fibre contacts in such a large proportion of these ARC KNDy cells. Since we could not perform triple labeling experiments, we do not know if the same ARC kisspeptin cells that express Gpr147 also express Gpr74, or if different kisspeptin cells express each of the two RFRP-3 receptors. If the latter scenario, then not only would a larger proportion of ARC kisspeptin cells than what we observed (~25%) actually be responsive to RFRP-3 signals, but also the differing affinities of RFRP-3 for these receptor subtypes might enable graded or differing responses of different kisspeptin cells to the same RFRP-3 stimulus. Whereas the maximum percent of ARC kisspeptin neurons directly modulated by RFRP-3 signaling is likely capped at ~ 1/3, due to the proportion of KNDy neurons with RFRP-3 fibre appositions and RFRP-3 receptors, it remains possible that such RFRP-3 signaling may still affect the entire KNDy population indirectly via the reciprocally interconnected nature of the KNDy neuron network. Of note, we used Tac2 expression to represent KNDy neurons, since in our hands, nearly all Tac2 neurons (>95%) coexpress Kiss1 in the ARC of mice (A.S. Kauffman, unpublished observation). Thus, Tac2 expression in the mouse ARC faithfully reflects Kiss1 expressing neurons.
One interesting possible role for RFRP-3-kisspeptin interactions that has been hypothesized is the regulation of the preovulatory GnRH/LH surge, an event driven by kisspeptin and suppressed by RFRP-3 (18, 48). RFRP-3 neuronal activity declines at the time of the LH surge (29), as does the hypothalamic concentration of RFRP-3 peptide (M.Z. Rizwan and G.M. Anderson, unpublished data). It is conceivable that this decline reduces inhibitory RFRP-3 tone on kisspeptin neurons, allowing increased kisspeptin drive to the trigger the GnRH/LH surge(15). Such speculation would be consistent with previous reports of RFRP-3 causing suppression of cellular activity as well as reduced kisspeptin neuronal firing rate in the AVPV (18, 19), a key brain region implicated in the LH surge event. However, this is less consistent with our present finding of minimal RFRP-3 receptors in AVPV kisspeptin neurons. Indeed, most RFRP-3 receptors in kisspeptin neurons were located in the ARC rather than the AVPV, and the former brain region is not implicated in the LH surge in rodents. Thus, our findings suggest that any effects of RFRP-3 on AVPV kisspeptin neurons to govern the LH surge would likely be indirect on those neurons.
Initial ISH studies targeting Kiss1r suggested it was highly expressed in the DMN (33), and kisspeptin fibres have been observed in the DMN (49), supporting the possibility that kisspeptin signaling may interface with RFRP-3 neurons. Our present results, however, strongly exclude the possibility of kisspeptin acting on RFRP-3 neurons through Kiss1r, as no Rfrp neurons expressed the mRNA for this receptor. In fact, contrary to the initial report (33), we find no evidence of significant Kiss1r mRNA in the DMN area, at least under our conditions examined. This was not due to poor sensitivity of our Kiss1r ISH, since we observed high Kiss1r expression in GnRH neurons and other brain regions, as expected. To complement this receptor expression data, we also determined if kisspeptin-containing fibres apposed RFRP-3 cells. In agreement with the ISH data, these immunohistochemistry results also excluded the likelihood of kisspeptin neurons targeting RFRP-3 neurons, as virtually all RFRP-3 neurons were devoid of kisspeptin fibre appositions. Importantly, these fibre apposition data also indicate that, due to the lack of physical connectivity, it is highly unlikely that kisspeptin neurons utilize other co-neuropeptides, such NKB or dynorphin, to act directly on Rfrp neurons. This was supported by our finding that Tacr3, the receptor for NKB (a co-transmitter with kisspeptin from ARC cells), was absent in the vast majority of Rfrp neurons, despite robust Tacr3 expression elsewhere nearby in the DMN. Any NKB interaction on the small subset of RFRP-3 neurons expressing Tacr3 would likely arise from non-ARC NKB neurons, since almost no kisspeptin fibres appose RFRP-3 neurons (ARC KNDy neuron fibers would contain kisspeptin as well as NKB).
Most hypothalamic neuropeptide populations tend to produce more than one neuropeptide or neurotransmitter. Yet, the potential co-transmitters that may also be expressed in and released by RFRP-3 neurons are unknown. In the present study we therefore also examined if NKB was co-expressed with RFRP-3, as many neurons in the DMN highly express Tac2. However, despite a little degree of overlap, we found that Tac2 was not expressed in the large majority of Rfrp neurons. These data suggest that the RFRP-3 and NKB neuronal populations residing in the DMN are, for the most part, distinct and separate neuropeptide populations. Thus, it presently remains unknown if RFRP-3 neurons also highly secrete additional co-transmitters or not.
In summary, the data presented here exclude the likelihood of RFRP-3 acting on any sizable proportion of kisspeptin neurons in the AVPV/PeN in either sex, but suggest that RFRP-3 may potentially provide some direct regulation to a moderate subset of ARC KNDy cells. Additionally, the possibility of a reciprocal action of direct kisspeptin or NKB on RFRP-3 neurons was strongly excluded, as no Rfrp neurons express Kiss1r and virtually no RFRP-3-ir neurons receive kisspeptin appositions or express Tacr3. Lastly, the majority of Rfrp neurons lack Tac2, indicating that NKB is not a co-expressed with RFRP-3 and that these are likely two separate neuronal populations in the DMN. These data demonstrate that kisspeptin is acting independently of, and in parallel with, the RFRP-3 system to govern the reproductive axis, whereas some effects of RFRP-3 on reproduction may potentially be derived via actions on a subset of ARC kisspeptin cells. Whether RFRP-3 also acts elsewhere in the brain to indirectly modulate reproduction remains unexplored, but could possibly include other regions such as paraventicular nucleus, lateral hypothalamus, thalamus, and amygdala where RFRP-3 fibres have been reported (24) and where Gpr147 or Gpr74 mRNAs are notably expressed (50).
Acknowledgements
This research was supported by NSF grant IOS-1025893 and NIH grant R01 HD065856. Additional support was provided by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) grant U54-HD012303 (U.C. San Diego). The authors thank Sheila J. Semaan and Kristen P. Tolson for their technical assistance and comments on the manuscript.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010;22(7):716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 3.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27(33):8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 8.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146(4):1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 9.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 10.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 11.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 12.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- 16.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of GnRH secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–1840. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 19.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2(10):703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 23.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003;982(2):156–167. doi: 10.1016/s0006-8993(03)02877-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276(40):36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- 26.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, Sex Steroid Regulation, and Phenotypic Characterization of RFamide-Related Peptide (Rfrp) Gene Expression and RFamide Receptors in the Mouse Hypothalamus. Endocrinology. 2012;153(4):1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, Kauffman AS, Anderson GM. RFamide-Related Peptide-3 Receptor Gene Expression in GnRH and Kisspeptin Neurons and GnRH-Dependent Mechanism of Action. Endocrinology. 2012;153(8):3770–3779. doi: 10.1210/en.2012-1133. [DOI] [PubMed] [Google Scholar]
- 29.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quennell JH, Rizwan MZ, Relf HL, Anderson GM. Developmental and steroidogenic effects on the gene expression of RFamide related peptides and their receptor in the rat brain and pituitary gland. J Neuroendocrinol. 2010;22(4):309–316. doi: 10.1111/j.1365-2826.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- 31.Son YL, Ubuka T, Millar RP, Kanasaki H, Tsutsui K. Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LbetaT2 cells. Endocrinology. 2012;153(5):2332–2343. doi: 10.1210/en.2011-1904. [DOI] [PubMed] [Google Scholar]
- 32.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O'Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 34.Allen Mouse Brain Atlas Resources [Internet] Allen Institute for Brain Science. 2009 [Google Scholar]
- 35.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 37.Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. Sex Differences in the Regulation of Kiss1/NKB Neurons in Juvenile Mice: Implications for the Timing of Puberty. Am J Physiol Endocrinol Metab. doi: 10.1152/ajpendo.00461.2009. 200900461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finn PD, Steiner RA, Clifton DK. Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci. 1998;18(2):713–719. doi: 10.1523/JNEUROSCI.18-02-00713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-kiss1r and GnRH signaling. Endocrinology. 2012;153(2):782–793. doi: 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowen JA, Clifton DK. Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci. 1991;51:37–58. [Google Scholar]
- 41.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 42.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 43.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. J Comp Neurol. 1992;317(4):341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- 46.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 47.Smith JT, Young IR, Veldhuis JD, Clarke IJ. Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology. 2012;153(7):3368–3375. doi: 10.1210/en.2012-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan AR, Kauffman AS. The Role of Kisspeptin and RFRP-3 Neurons in the Circadian-Timed Preovulatory Luteinizing Hormone Surge. J Neuroendocrinol. 2012;24(1):131–143. doi: 10.1111/j.1365-2826.2011.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- 50.Gouarderes C, Puget A, Zajac JM. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: a comparative autoradiographic study. Synapse. 2004;51(4):249–269. doi: 10.1002/syn.10305. [DOI] [PubMed] [Google Scholar]