Abstract
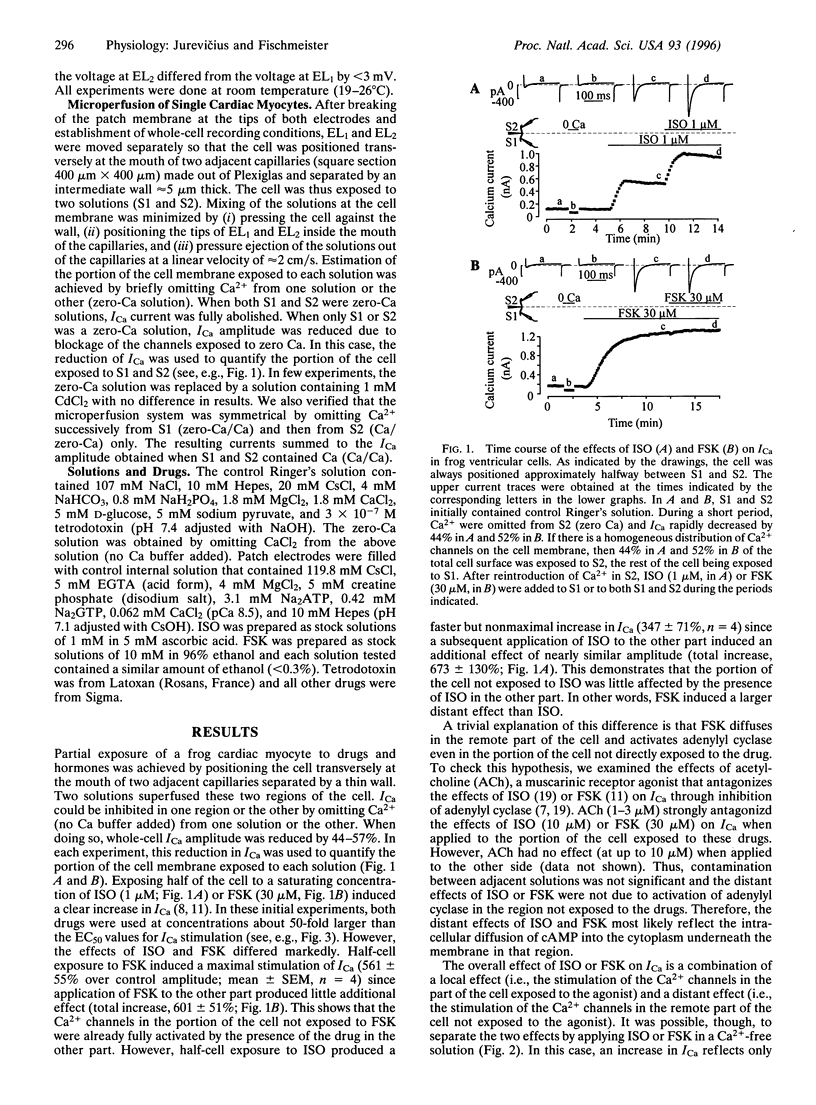
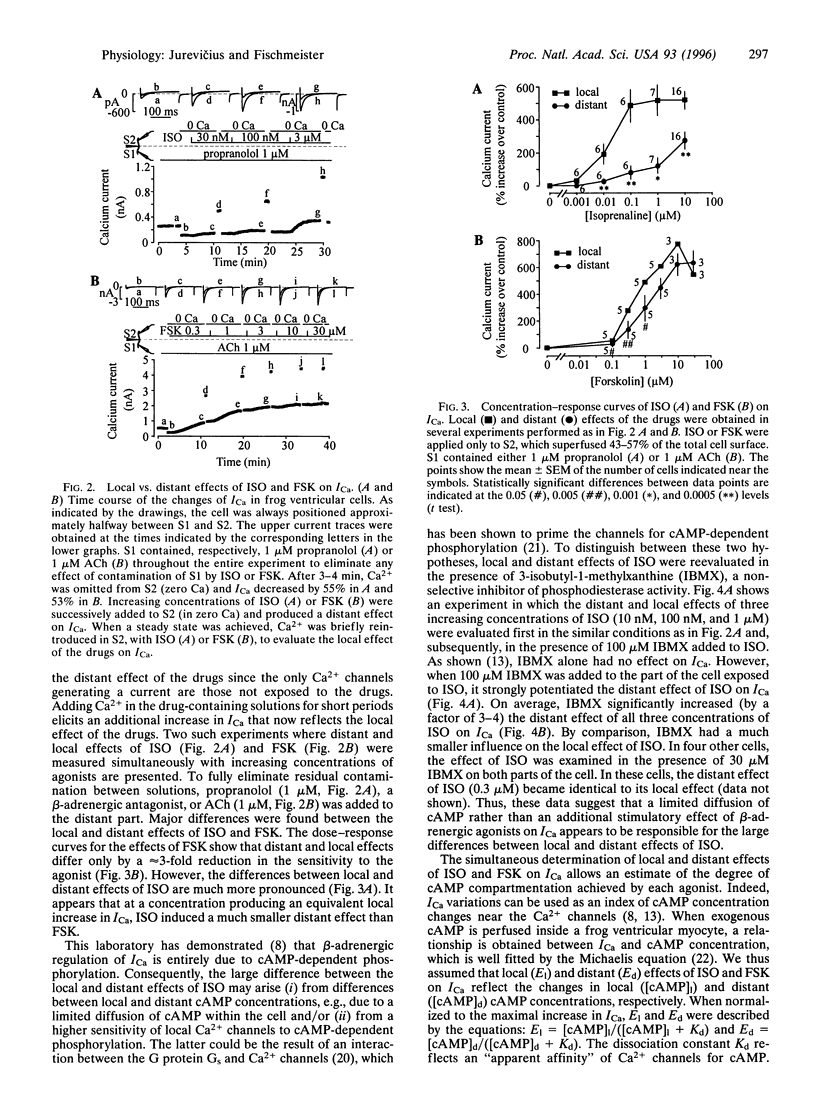
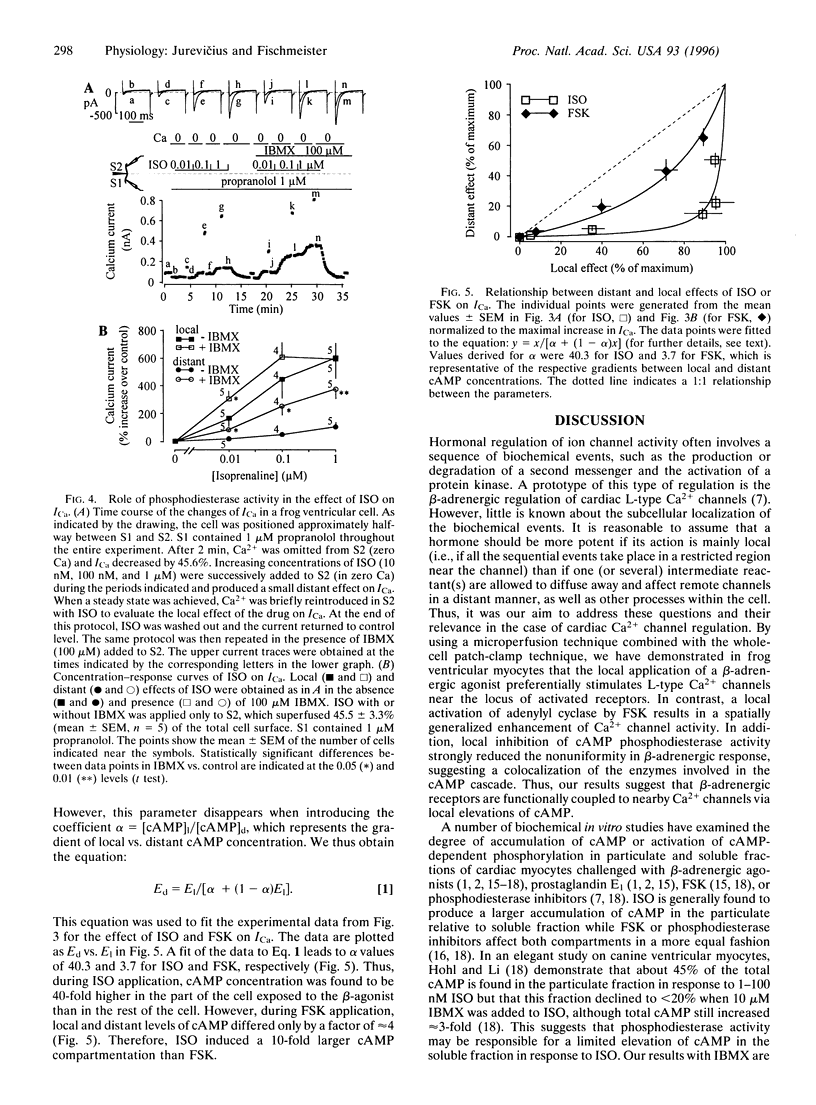
The role of cAMP subcellular compartmentation in the progress of beta-adrenergic stimulation of cardiac L-type calcium current (ICa) was investigated by using a method based on the use of whole-cell patch-clamp recording and a double capillary for extracellular microperfusion. Frog ventricular cells were sealed at both ends to two patch-clamp pipettes and positioned approximately halfway between the mouths of two capillaries that were separated by a 5-micron thin wall. ICa could be inhibited in one half or the other by omitting Ca2+ from one solution or the other. Exposing half of the cell to a saturating concentration of isoprenaline (ISO, 1 microM) produced a nonmaximal increase in ICa (347 +/- 70%; n = 4) since a subsequent application of ISO to the other part induced an additional effect of nearly similar amplitude to reach a 673 +/- 130% increase. However, half-cell exposure to forskolin (FSK, 30 microM) induced a maximal stimulation of ICa (561 +/- 55%; n = 4). This effect was not the result of adenylyl cyclase activation due to FSK diffusion in the nonexposed part of the cell. To determine the distant effects of ISO and FSK on ICa, the drugs were applied in a zero-Ca solution. Adding Ca2+ to the drug-containing solutions allowed us to record the local effect of the drugs. Dose-response curves for the local and distant effects of ISO and FSK on ICa were used as an index of cAMP concentration changes near the sarcolemma. We found that ISO induced a 40-fold, but FSK induced only a 4-fold, higher cAMP concentration close to the Ca2+ channels, in the part of the cell exposed to the drugs, than it did in the rest of the cell. cAMP compartmentation was greatly reduced after inhibition of phosphodiesterase activity with 3-isobutyl-methylxanthine, suggesting the colocalization of enzymes involved in the cAMP cascade. We conclude that beta-adrenergic receptors are functionally coupled to nearby Ca2+ channels via local elevations of cAMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aass H., Skomedal T., Osnes J. B. Increase of cyclic AMP in subcellular fractions of rat heart muscle after beta-adrenergic stimulation: prenalterol and isoprenaline caused different distribution of bound cyclic AMP. J Mol Cell Cardiol. 1988 Sep;20(9):847–860. doi: 10.1016/s0022-2828(88)80009-9. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Boutjdir M., Méry P. F., Hanf R., Shrier A., Fischmeister R. High affinity forskolin inhibition of L-type Ca2+ current in cardiac cells. Mol Pharmacol. 1990 Dec;38(6):758–765. [PubMed] [Google Scholar]
- Brodde O. E. Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991 Jun;43(2):203–242. [PubMed] [Google Scholar]
- Brunton L. L., Hayes J. S., Mayer S. E. Hormonally specific phosphorylation of cardiac troponin I and activation of glycogen phosphorylase. Nature. 1979 Jul 5;280(5717):78–80. doi: 10.1038/280078a0. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983 Sep 10;258(17):10233–10239. [PubMed] [Google Scholar]
- Cavalié A., Allen T. J., Trautwein W. Role of the GTP-binding protein Gs in the beta-adrenergic modulation of cardiac Ca channels. Pflugers Arch. 1991 Nov;419(5):433–443. doi: 10.1007/BF00370785. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Regulation of calcium current by low-Km cyclic AMP phosphodiesterases in cardiac cells. Mol Pharmacol. 1990 Sep;38(3):426–433. [PubMed] [Google Scholar]
- Frace A. M., Méry P. F., Fischmeister R., Hartzell H. C. Rate-limiting steps in the beta-adrenergic stimulation of cardiac calcium current. J Gen Physiol. 1993 Mar;101(3):337–353. doi: 10.1085/jgp.101.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol. 1987 Nov;32(5):639–645. [PubMed] [Google Scholar]
- Hartzell H. C., Méry P. F., Fischmeister R., Szabo G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature. 1991 Jun 13;351(6327):573–576. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. S., Brunton L. L., Mayer S. E. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980 Jun 10;255(11):5113–5119. [PubMed] [Google Scholar]
- Hohl C. M., Li Q. A. Compartmentation of cAMP in adult canine ventricular myocytes. Relation to single-cell free Ca2+ transients. Circ Res. 1991 Nov;69(5):1369–1379. doi: 10.1161/01.res.69.5.1369. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P., Ambrose J. R., Irving P. E. The relative efficiency of beta adrenoceptor coupling to myocardial inotropy and diastolic relaxation: organ-selective treatment for diastolic dysfunction. J Pharmacol Exp Ther. 1991 Jun;257(3):1189–1197. [PubMed] [Google Scholar]
- Levi R. C., Alloatti G. Histamine modulates calcium current in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1988 Jul;246(1):377–383. [PubMed] [Google Scholar]
- Méry P. F., Brechler V., Pavoine C., Pecker F., Fischmeister R. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature. 1990 May 10;345(6271):158–161. doi: 10.1038/345158a0. [DOI] [PubMed] [Google Scholar]
- Rapundalo S. T., Solaro R. J., Kranias E. G. Inotropic responses to isoproterenol and phosphodiesterase inhibitors in intact guinea pig hearts: comparison of cyclic AMP levels and phosphorylation of sarcoplasmic reticulum and myofibrillar proteins. Circ Res. 1989 Jan;64(1):104–111. doi: 10.1161/01.res.64.1.104. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Wolff A. A., Levi R. Histamine and cardiac arrhythmias. Circ Res. 1986 Jan;58(1):1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- Xenopoulos N. P., Applegate R. J. Effects of vasoactive intestinal peptide on myocardial performance. Am J Physiol. 1994 Feb;266(2 Pt 2):H399–H405. doi: 10.1152/ajpheart.1994.266.2.H399. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]



