Abstract
Purpose
Vertebral fractures due to osteoporosis are a potential complication of childhood acute lymphoblastic leukemia (ALL). To date, the incidence of vertebral fractures during ALL treatment has not been reported.
Patient and Methods
We prospectively evaluated 155 children with ALL during the first 12 months of leukemia therapy. Lateral thoracolumbar spine radiographs were obtained at baseline and 12 months. Vertebral bodies were assessed for incident vertebral fractures using the Genant semi-quantitative method, and relevant clinical indices such as spine bone mineral density (BMD), back pain and the presence of vertebral fractures at baseline were analyzed for association with incident vertebral fractures.
Results
Of the 155 children, 25 (16%, 95% Confidence Interval (CI) 11% to 23%) had a total of 61 incident vertebral fractures, of which 32 (52%) were moderate or severe. Thirteen of the 25 children with incident vertebral fractures (52%) also had fractures at baseline. Vertebral fractures at baseline increased the odds of an incident fracture at 12 months by an odds ratio of 7.3 (95% CI 2.3 to 23.1, p = 0.001). In addition, for every one standard deviation reduction in spine BMD Z-score at baseline, there was 1.8-fold increased odds of incident vertebral fracture at 12 months (95% CI 1.2 to 2.7, p = 0.006).
Conclusion
Children with ALL have a high incidence of vertebral fractures after 12 months of chemotherapy, and the presence of vertebral fractures and reductions in spine BMD Z-scores at baseline are highly associated clinical features.
Keywords: acute lymphoblastic leukemia, children, osteoporosis, vertebral fractures
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most frequent pediatric cancer, with risk-targeted treatment regimens curing ALL in more than 80% of patients.1 Fractures due to osteoporosis are an important complication of childhood leukemia at diagnosis, as well as during and after ALL therapy.2–10 We recently found that 16% of children with recently diagnosed ALL had vertebral fractures, and that half of the children manifested moderate or severe fractures. Furthermore, each standard deviation (SD) reduction in spine bone mineral density (BMD) Z-score was associated with an 80% increased odds of vertebral fractures.11
To date, the incidence of vertebral fractures following the initiation of chemotherapy has not been reported. This is an important consideration since children with ALL receive osteotoxic medications such as glucocorticoids and methotrexate, both of which may further compromise bone strength.12 In the present study, we describe the incidence of vertebral fractures in children with ALL in the year after chemotherapy initiation and evaluate the associated clinical factors.
SUBJECTS AND METHODS
Patients and Study Design
Patients were recruited through pediatric oncology clinics in 10 children’s hospitals across Canada as part of the STeroid-associated Osteoporosis in the Pediatric Population (STOPP) research program. Children from one month to 17 years of age with ALL were enrolled (N = 188) from 2005 to 2007, with the baseline bone health assessment initiated within 30 days of glucocorticoid therapy.11 The study was approved by the Ethics Board in each institution and informed consent/assent were obtained, as appropriate. Children were excluded from the study if they had received treatment with a bisphosphonate or calcium and vitamin D supplementation that exceeded the Dietary Reference Intake for age.11
Clinical Data
All children were treated according to Children’s Oncology Group (nine sites) or the Dana Farber Cancer Institute (one site) protocols. Clinical data were obtained at baseline and every three months following the baseline assessment for a total of 12 months. Height, weight and pubertal staging according to Marshall and Tanner, 13,14 were determined as previously described.11 Height, weight, and body mass index (weight (kg) divided by height (meters2)) raw values were transformed into age- and gender-matched Z-scores according to the United States Centers for Disease Control National Center for Health Statistics normative database15; for children younger than two years, body mass index Z-scores were calculated according to the World Health Organization child growth standards.16 The presence or absence of back pain reported by the participant was recorded at each study visit, and the spine was palpated for tenderness at baseline and at 12 months. For non-verbal children, the history of back pain was obtained from the caregiver.
Dietary calcium and vitamin D intake were assessed by a validated food frequency questionnaire.17 Daily intake (diet plus supplement) was expressed as the percent of the Adequate Intake value based on Dietary Reference Intakes.18 The percentage of adequate intake scores were then classified as <50% of the age-related Dietary Reference Intake, ≥50 and < 100%, or ≥100% of the Reference Intake. Physical activity was assessed through the Habitual Activity Estimation Scale.11,19,20
Quantification of Glucocorticoid and Methotrexate Exposure
The dose of systemic glucocorticoid therapy (oral and intravenous) received during the 12 month observation period was converted into prednisone equivalents expressed as:21–23 (1) cumulative glucocorticoid dose, the amount in prednisone equivalents (mg/m2) received during the observation period; (2) average glucocorticoid dose, the cumulative dose in prednisone equivalents divided by the total number of days during the observation period; and (3) glucocorticoid dose intensity, the cumulative dose in prednisone equivalents, divided by the number of days in receipt of steroids during the observation period. Methotrexate was quantified by summing the cumulative dose over the observation period.
Vertebral Fracture Assessment
Lateral thoracolumbar spine radiographs were obtained at baseline and 12 months, with vertebral fracture assessment based on the Genant semi-quantitative method from T4 to L4.24 Vertebral bodies were graded according to the extent of the difference in height ratios from 100% when the anterior vertebral height was compared to the posterior height, the middle height to the posterior height, and the posterior height to the posterior height of adjacent vertebral bodies. The scores corresponded to the following differences in height ratios: Grade 0: 20% or less (normal); Grade 1 fracture (mild): > 20 to 25%; Grade 2 fracture (moderate): > 25 to 40%; Grade 3 fracture (severe): > 40%. Minimal physiological rounding of vertebral bodies in the mid-thoracic region of the spine, as can be seen in normal children, was assigned a Grade 0 score.25,26 An incident vertebral fracture was defined as an increase in the Genant grade of at least one compared with baseline.
Lumbar Spine BMD by Dual-Energy X-Ray Absorptiometry, Bone Age and Second Metacarpal Morphometry
BMD was measured in the anterior-posterior direction at the lumbar spine (L1-L4) by dual-energy x-ray absorptiometry using either Hologic (Hologic, Bedford, MA; QDR 4500, three centers; Discovery, two centers; Delphi, one center) or Lunar Prodigy (GE Lunar Corporation, Madison, WI; four centers) systems at baseline and at 6 and 12 months. Machines were cross-calibrated as previously described.11 Data were converted to Hologic units and Z-scores were generated using the Hologic 12.4 normative database, a database provided by the manufacturer that spans the age ranges included in this study. Radiographs of the left hand and wrist were obtained at baseline and 12 months to determine bone age and second metacarpal percent cortical area, as previously reported.11
Statistical Analyses
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and standard deviation (SD) or median (25th percentile, 75th percentile or minimum, maximum). The 95% Confidence Intervals (CI) for the proportions of patients with vertebral deformities were calculated using the Wilson score method.27
Differences between those with and without incident vertebral fracture were assessed using a chi-square or Fisher’s exact test for categorical variables and a Student’s t-test or a non-parametric (Wilcoxon Mann-Whitney) test for continuous variables, as appropriate. Multivariable logistic regression was performed to identify clinical variables significantly associated with incident vertebral fracture after adjusting for the variables in the model. To address overfitting of models, the variables included in the models were identified using a two step process. First, the variables that were statistically significant based on univariate testing were assessed. This first step considered at least 5 events per variable, an approach supported by Vittinghoff and McCulloch. 28 The second step was to add non-significant but nevertheless clinically important variables, to demonstrate that such covariates were considered in the models. Vittinghoff and McCullogh28 found a low risk of bias with 5 to 9 events per outcome variable in logistic regression models and this formed the basis for the sample size used for the models in this article.
For the models that included vertebral fractures in children at baseline as a predictor variable, covariance analysis was used. Model discrimination was assessed using the c statistic (equivalent to the area under the receiver operating characteristic curve), which refers to the ability of the model to distinguish between those children having a higher risk of sustaining incident vertebral fractures versus those with lower risk.29 The c statistic between 0.7 and 0.8 is consistent with acceptable model discrimination; 0.8 to 0.9 is an indication of excellent discrimination. Reported p values are two-sided and a p-value < 0.05 was considered statistically significant. Analyses were conducted using SPSS 18.0 (SPSS Inc., Chicago IL).
RESULTS
Clinical Characteristics of the Cohort
A total of 368 children were approached for participation in the study; 161 declined and 19 were excluded because of failure to undergo a bone health evaluation within the baseline timeline. Of these 188 children, 155 completed follow-up to 12 months (Table 1). The reasons for lack of available data on 33 children at 12 months are presented in Figure 1. The clinical profile at baseline for the 33 children without data at 12 months did not differ significantly from those with complete follow-up (data not shown).
Table 1.
Clinical Characteristics of the Children with Acute Lymphoblastic Leukemia 12 Months After the Initiation of Therapy
| Clinical Characteristics | N=155 * |
|---|---|
| Demographic Data | |
| Male, N (%) | 91 (59) |
| Age at 12 months (years), median (min, max) | 6.4 (2.2, 18.0) |
| Bone Age at 12 months (years), median (min, max) | 5.8 (2.0, 18.5) |
| Diagnosis, N (%) | |
| Pre-B-cell acute lymphoblastic leukemia | 141 (91) |
| T-cell acute lymphoblastic leukemia | 14 (9) |
| Leukemia protocol, N (%) | |
| Dana Farber | 33 (21) |
| Children’s Oncology Group | 122 (79) |
| Leukemia risk category, N (%) | |
| Standard Risk | 96/153 (63) |
| High Risk | 57/153 (37) |
| Anthropometry, mean (SD) | |
| Δ Height Z-score from baseline to 12 months | −0.4 (0.5) |
| Δ Weight Z-score from baseline to 12 months | 0.1 (0.7) |
| Δ BMI** Z-score from baseline to 12 months | 0.5 (1.0) |
| Lumbar Spine Bone Mineral Density, mean (SD) | |
| LS BMD Z-score at baseline | −1.2 (1.3) |
| Δ LS BMD Z-score from baseline to 12 months | 0.1 (0.9) |
| Vitamin D and Calcium Intake | |
| Average daily vitamin D intake***, N (%) | |
| < 50 % | 25/123 (20) |
| 50 – <100 % | 63/123 (51) |
| >= 100% | 35/123 (29) |
| Average daily calcium intake***, N (%) | |
| < 50 % | 5/124 (4) |
| 50 – <100 % | 11/124 (9) |
| >= 100% | 108/124 (87) |
For data expressed as percentages, the denominator is 155 children unless otherwise specified
BMI = Body mass index
Combined dietary plus supplemental intake, expressed as the % of the Dietary Reference Intake for age
Figure 1.
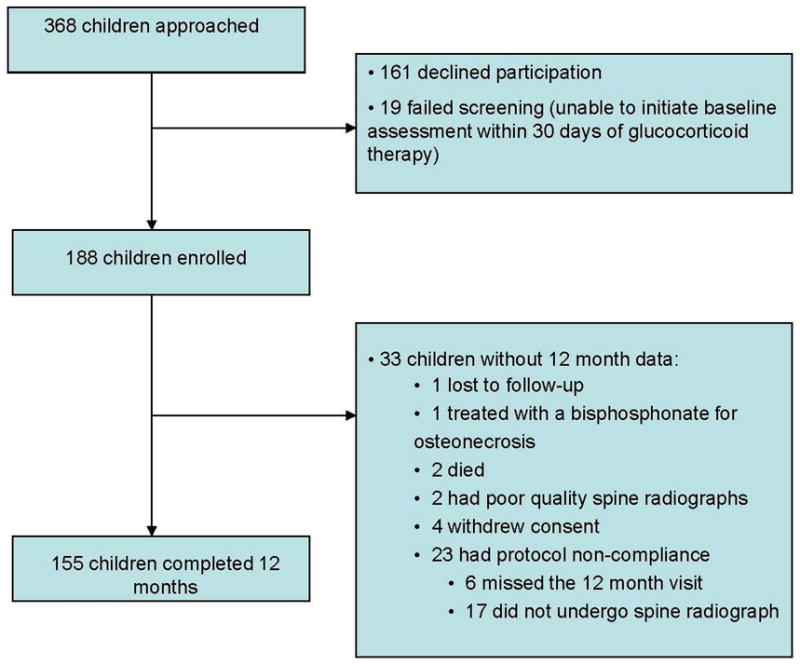
Disposition of patients from baseline to 12 months with reasons for lack of available data on 33 children at 12 months.
For the 155 children who completed 12 month data collection, the baseline spine BMD was carried out at a median of 15 days from glucocorticoid initiation (inter-quartile range (IQR): 5, 22) and the baseline spine radiograph was carried out at a median of 20 days (IQR: 10.5, 25.5). Children with prevalent vertebral fractures at baseline (N = 25) had a similar number of days between glucocorticoid initiation and the spine radiograph (median 20 days; IQR 6.5, 26) compared with those without baseline vertebral fractures (median 19.5 days, IQR 11.25, 25, p = 0.642). Similarly, the cumulative glucocorticoid dose up until the time of the baseline spine radiograph was similar for children with baseline vertebral fractures (median 890 mg/m2, IQR 200, 1064) compared with those without (median 877 mg/m2, IQR 514, 1134; p = 0.369). The percentage of children in Tanner Stage 1 pubertal development at 12 months was 76%, with 24% in Stages 2 to 5. Mean height Z-scores were lower at 12 months (mean ± SD = −0.1± 1.2) compared with baseline (0.3 ± 1.2, p < 0.001) whereas weight Z-scores were higher (0.5 ± 1.2 at 12 months versus 0.4 ± 1.3 at baseline, p = 0.03), resulting in increased body mass index Z-scores (0.9 ± 1.1 at 12 months versus 0.4 ± 1.5 at baseline, p < 0.001).
Vertebral Fractures
Twenty-five of the 155 children (16%, 95% CI 11% to 23%; 14 boys) sustained 61 incident vertebral fractures. None reported trauma. Fifty-two (85%) of the 61 incident vertebral fractures were in previously normal vertebral bodies, whereas nine were worsening of existing fractures. Twelve children (48%) had mild fractures as the worst grade, eight (32%) had moderate fractures and five (20%) sustained severe incident fractures. Representative fractures are shown in Figures 2A and 2B. Thirteen children (52%) had a single vertebral fracture, seven had two to three fractures (28%) and five children had four to ten incident fractures (20%). These five children had 31 (51%) of the total number of incident fractures. Fractures were clustered in the mid-thoracic region (38% from T5 to T7) and thoracolumbar junction (21% from T12 to L2) and 52% of the incident vertebral fractures were moderate or severe.
Figure 2.
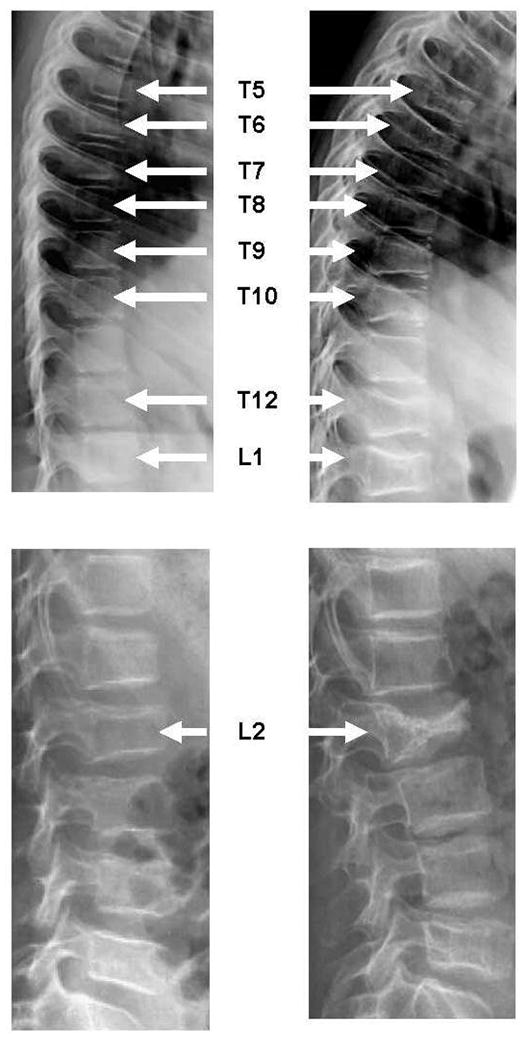
Representative incident vertebral fractures at 12 months following initiation of therapy for pediatric ALL. (A) left panel shows a 9 year old girl at baseline with a normal spine radiograph. At 12 months (right panel), she has multiple incident vertebral fractures (a severe fracture at T5, moderate fractures at T10, T12 and L1, and mild fractures at T6, T7, T8 and T9). (B) left panel shows a 9 year old boy at baseline with a moderate L2 fracture. At 12 months (right panel), the L2 deformity has progressed to a severe fracture.
Differences in the Clinical Profiles of Children With and Without Incident Vertebral Fractures (Table 2)
Table 2.
Comparison of Children With and Without Incident Vertebral Fractures at 12 Months According to the Genant Semi-Quantitative Method
| Clinical Characteristics | Children without Incident Vertebral Fractures at 12 Months | Children with Incident Vertebral Fractures at 12 Months | p |
|---|---|---|---|
| N=130* | N=25* | ||
| Demographic Data | |||
| Male, N (%) | 77 (59) | 14 (56) | 0.76A |
| Age at 12 months (years), median (min, max) | 6.2 (2.2, 18.0) | 6.7 (3.1, 15.5) | 0.69B |
| Anthropometry, mean (SD) | |||
| Height Z-score at baseline | 0.3 (1.2) | 0.1 (1.2) | 0.37C |
| Δ Height Z-score from baseline to 12 months | −0.4 (0.4) | −0.4 (0.6) | 0.98C |
| Weight Z-score at baseline | 0.5 (1.3) | 0.1 (1.2) | 0.14C |
| Δ Weight Z-score from baseline to 12 months | 0.1 (0.6) | 0.3 (0.9) | 0.23C |
| BMI** Z-score at baseline | 0.4 (1.5) | 0.0 (1.6) | 0.17C |
| Δ BMI Z-score from baseline to 12 months | 0.5 (0.9) | 0.7 (1.4) | 0.44C |
| History of Prevalent Vertebral Fractures | |||
| Children with vertebral fractures at baseline, N (%) | 12 (9) | 13 (52) | <0.001A |
| Back Pain | |||
| Back pain by report at baseline (yes), N (%) | 30 (23) | 13 (52) | 0.003A |
| Back pain by report after baseline (yes), N (%) | 53 (41) | 15 (60) | 0.08A |
| Back pain by palpation after baseline (yes), N (%) | 3/121 (3) | 2/24 (8) | 0.19A |
| Lumbar Spine BMD, mean (SD) | |||
| Δ Spine BMD Z-score from baseline to 6 months | 0.1 (0.8) | 0.1 (0.9) | 0.95C |
| Δ Spine BMD Z-score from 6 to 12 months | 0.0 (0.6) | 0.3 (0.5) | 0.02C |
| Δ Spine BMD Z-score from baseline to 12 months | 0.0 (0.8) | 0.3 (1.1) | 0.19C |
| Glucocorticoid Exposure (Total and Dexamethasone Alone) | |||
| Cumulative glucocorticoid dose (mg/m2), median (IQR) | 4017 (3455, 4446) | 3984 (3371, 4407) | 0.90B |
| Average glucocorticoid dose (mg/m2/day), median (IQR) | 10.2 (9.0, 11.3) | 10.3 (8.6, 11.4) | 0.90B |
| Glucocorticoid dose intensity (mg/m2/day), median (IQR) | 45.5 (42.9, 53.9) | 45.0 (39.7, 49.9) | 0.25B |
| Cumulative dexamethasone dose (mg/m2), median (IQR) | 3419 (2187, 4229) | 3197 (1047, 4141) | 0.15B |
| Methotrexate Exposure | |||
| Cumulative methotrexate dose (mg/m2), median (IQR) | 1330 (705, 5619) | 1243 (721, 5488) | 0.99B |
For data expressed as percentages, the denominators are 130 for children without incident vertebral fractures and 25 for children with incident fractures unless otherwise indicated.
BMI = body mass index.
Statistical significance determined by Chi-square or Fisher’s Exact test;
Statistical significance determined by Wilcoxon Mann-Whitney test;
Statistical significance determined by Student’s t-test.
The two groups did not differ significantly in age, gender, anthropometry, methotrexate or glucocorticoid exposure, leukemia immunophenotype or risk category, white blood count, physical activity, second metacarpal percent cortical area Z-score, or total calcium and vitamin D intake. However, over 50% of children with incident vertebral fractures had fractures at baseline. Furthermore, the 13 children with both incident fractures as well as prevalent fractures at baseline carried most of the 12-month incident fracture burden, harbouring 45/61 (74%) of the incident fractures. The percentage of children with incident fractures was highest among those with more severe fractures at baseline: 12/130 children (9%) without fractures at baseline had incident vertebral fractures at 12 months compared with 5/11 children (45%) with Grade 1 fractures, 4/9 children (44%) with Grade 2 fractures and 4/5 children (80%) with Grade 3 fractures (p < 0.001).
The mean spine BMD Z-score was significantly lower in children with incident vertebral fractures compared with those without at baseline and at 6 and 12 months (Figure 3). The mean change in spine BMD Z-score was similar between the two groups from baseline to 12 months (Table 2); however, children with incident vertebral fractures had greater increases in spine BMD Z-scores between six and 12 months.
Figure 3.
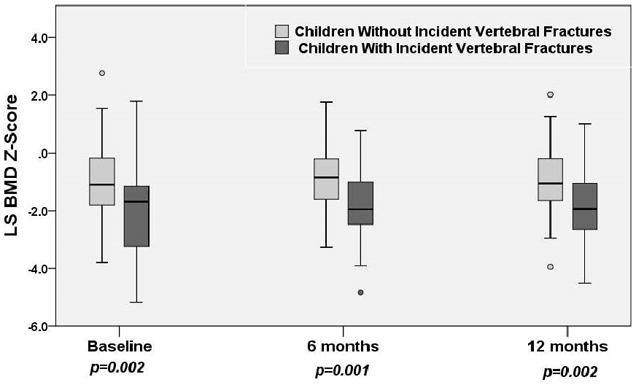
The median (with 25th and 75th percentiles) lumbar spine BMD Z-scores at baseline, 6 and 12 months post-initiation of therapy for children with and without incident vertebral fractures.
Clinical Variables Associated with Increased Odds of Incident Vertebral Fracture
Logistic regression models were fit to examine factors associated with increased odds of incident vertebral fracture at 12 months for the entire cohort (Models 1 through 3; n=155) and for the subset of children who did not have vertebral fractures at baseline (Model 4; n=130). For all models, the dependent variable was a yes/no indicator defined as yes for children who had at least one incident vertebral fracture at 12 months.
The results of Model 1 (Figure 4) show the presence of at least one vertebral fracture at baseline was highly associated with increased odds of a child sustaining at least one incident vertebral fracture at 12 months (c statistic = 0.78, 95% CI 0.67 to 0.90). Other variables included in this model were spine BMD Z-score, back pain and age (all at baseline) as well as gender and cumulative glucocorticoid dose. Model 2 (c statistic = 0.74, 95% CI 0.63 to 0.85) was generated by excluding vertebral fractures at baseline from Model 1. In this model, baseline spine BMD Z-score was associated with increased odds of incident vertebral fracture, as follows: for every one SD reduction in spine BMD Z-score at baseline, the odds of incident vertebral fracture increased 1.8-fold (95% CI 1.2 to 2.7, p = 0.006). In Model 3 (c statistic = 0.78, 95% CI 0.67 to 0.90), the presence of vertebral fractures at baseline was replaced with the highest Genant Grade at baseline. Both the presence of Grade 1 vertebral fractures (odds ratio 7.6, 95% CI 1.8 to 31.8, p = 0.006) and the presence of Grade 2/3 fractures (odds ratio 7.0, 95% CI 1.6 to 30.2, p = 0.009) were associated with an increased odds of sustaining at least one incident vertebral fracture.
Figure 4.
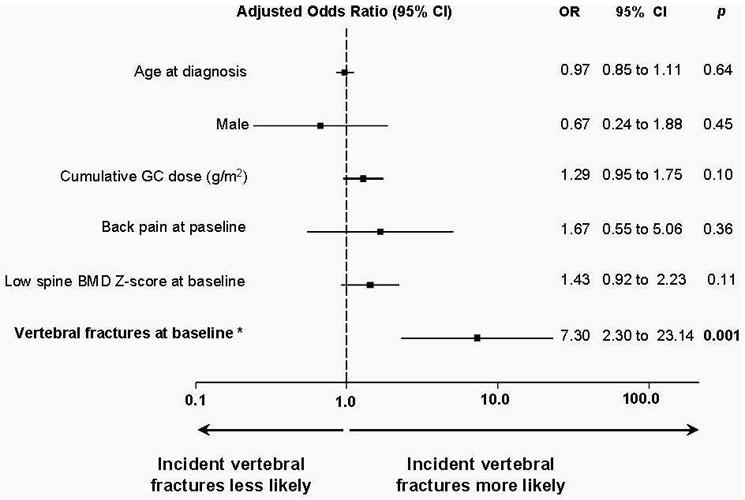
Results of logistic regression model 1 showing that the presence of at least one vertebral fracture at baseline was highly associated with increased odds of a child sustaining at least one incident vertebral fracture at 12 months. BMD, bone mineral density; GC, glucocorticoid; OR, odds ratio.
The fourth model (c statistic = 0.81, 95% CI 0.68 to 0.94) assessing factors associated with incident vertebral fracture in the 130 children who did not have vertebral fractures at baseline showed that among spine BMD Z-score at baseline, change in body mass index from baseline to 12 months, back pain reported after baseline, age, gender and cumulative glucocorticoid dose, back pain was associated with 9.2-fold increased odds of incident vertebral fractures (95% CI 1.6 to 51.7, p = 0.012). Children with incident vertebral fractures in the absence of fractures at baseline had greater increases in body mass index Z-scores from baseline to 12 months compared with those without fractures at either time point (mean ± SD = +1.1 ± 1.5 versus +0.5 ± 0.9, p = 0.043).
DISCUSSION
We found that 16% of children with ALL developed incident vertebral fractures 12 months following the initiation of therapy. The presence of low spine BMD Z-score or vertebral fractures of any grade were associated with significantly increased odds of incident vertebral fractures. That an initial vertebral fracture is associated with increased odds of a subsequent fracture has not been previously reported in children. However, the phenomenon is well-known among adults,30,31 described as the so called “vertebral fracture cascade”.32 Among post-menopausal women, vertebral fracture at an initial timepoint is associated with five-fold increased relative risk for incident vertebral fracture 12 months later.31 Our observation that every one SD reduction in spine BMD Z-score at baseline was associated with an 80% increased odds of incident vertebral fracture is also aligned with adult studies.33,34
Our results did not show a relationship between glucocorticoid or methotrexate dose and incident vertebral fracture. Retrospective studies in pediatric ALL have shown a relationship between these agents and skeletal morbidity after five to ten years of follow-up,3,6,35 including an increase in long-bone fractures.4 Interestingly, in our study the children with incident vertebral fractures in the absence of fractures at baseline had greater increases in body mass index over the 12 month period, an observation that suggests clinically important variability in glucocorticoid sensitivity mediated by differences in glucocorticoid pharmacokinetics or pharmacogenomics among patients.36 The absence of a link to glucocorticoid or methotrexate dose in our study could also reflect the relatively small number of children with vertebral fractures, particularly in view of the standardized chemotherapy protocols. Similarly, we did not find any differences in leukemia variables (such as white blood count) for children with incident vertebral fractures compared with those without, an observation that could also have been impacted by sample size.
That a sub-set of children had a disproportionate number of prevalent fractures at baseline plus incident fractures at 12 months raises the possibility of genetic susceptibility to bone fragility in these particular children. Our data also suggest that the determinants of compromised bone strength leading to fracture may be different early in the course of leukemia therapy compared to later. At ALL diagnosis, bone fragility has been linked to excessive skeletal resorption through liberation of osteoclast-activating factors by the leukemic cells (including interleukins 6 and 8).37 This observation is supported by our data showing excessive osteolysis on the second metacarpal endosteal surface among children with vertebral fractures and recently diagnosed ALL, resulting in reduced metacarpal cortical width.11 Vassilopoulou-Sellin and Ramirez 38 reported a young ALL patient with back pain and subtle vertebral changes at diagnosis followed by severe vertebral fractures just two months later. A trans-iliac bone biopsy showed signs of prior, accelerated resorption that had resolved by 2 months. Our data showing that children with incident vertebral fractures at 12 months had greater increases in spine BMD Z-scores between 6 and 12 months also suggests the 12-month incident vertebral fractures may have occurred early in the observation period, and were then followed by a degree of recovery manifesting as enhanced BMD accrual. In contrast, studies that have shown a relationship between glucocorticoid exposure and skeletal morbidity after five to ten years of follow-up3,6,35 suggest that medication-induced osteotoxicity may take time to manifest clinically.
A key clinical question is whether children with ALL and vertebral fractures in the first year of therapy should be treated with osteoporosis agents such as bisphosphonates. To date, there have been no randomized, placebo-controlled trials in pediatric ALL to provide adequate safety and efficacy data and thereby justify the use of such agents as standard of care. It is possible that bisphosphonates could treat the low BMD and also stabilize vertebral fractures in this context by inflencing the early ALL effects on the skeleton, just as bisphosphonates are effective in the cytokine-induced bone disease of multiple myeloma.39 If later adverse bone manifestations in ALL are chemotherapy-related, bisphosphonates might also be effective, as their benefits are well-documented in the treatment of adult glucocorticoid-induced osteoporosis.39 It will be important that any future osteoporosis treatment studies in pediatric ALL consider that the mechanisms underlying bone disease may be different early after disease presentation compared with later or even after completion of chemotherapy.
Acknowledgments
This study was primarily funded by an operating grant from the Canadian Institutes for Health Research. Additional funding for this work has been provided to Dr. Leanne Ward by the Canadian Institutes for Health Research New Investigator Program, the Canadian Child Health Clinician Scientist Career Enhancement Program, and a University of Ottawa Research Chair Award. This work was also supported by the Children’s Hospital of Eastern Ontario Research Institute and the University of Alberta Women and Children’s Health Research Institute.
The Canadian STOPP Consortium would like to thank the following individuals:
The children and their families who participated in the study and without whom the STOPP study would not have been possible.
Research Associates who managed the study at the co-ordinating center (the Children’s Hospital of Eastern Ontario Ottawa, Ontario): Elizabeth Sykes (STOPP Project Manager), Maya Scharke (STOPP Data Analyst and Database Manager), Monica Tomiak (Statistical Analyses), Victor Konji (STOPP Publications and Presentations Committee Liaison and hand morphometry measurements), Steve Anderson (Children’s Hospital of Eastern Ontario Pediatric Bone Health Program Research Manager), Catherine Riddell (STOPP National Study Monitor); Research Associates who took care of the patients from the following institutions: Alberta Children’s Hospital, Calgary, Alberta: Eileen Pyra; British Columbia Children’s Hospital, Vancouver British Columbia: Terry Viczko, Angelyne Sarmiento, Jacqueline Page; Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Heather Cosgrove, Josie MacLennan, Catherine Riddell; Children’s Hospital, London Health Sciences Centre, London, Ontario: Vinolia ArthurHayward, Leila MacBean, Mala Ramu; McMaster Children’s Hospital, Hamilton, Ontario: Susan Docherty-Skippen; IWK Health Center, Halifax, Nova Scotia: Aleasha Warner; Montréal Children’s Hospital, Montréal, Québec: Valérie Gagné, Diane Laforte, Maritza Laprise; Ste. Justine Hospital, Montréal, Québec: Claude Belleville, Natacha Gaulin Marion; Stollery Children’s Hospital, Edmonton, Alberta: Ronda Blasco, Germaine, McInnes, Amanda Mullins, Toronto Hospital for Sick Children, Toronto, Ontario: Michele Petrovic; Winnipeg Children’s Hospital, Winnipeg, Manitoba: Dan Catte, Erika Bloomfield. The Research Nurses, Support Staff and all the STOPP collaborators from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
Funding: Primary Funding Source: The Canadian Institutes of Health Research Operating Grants Program. Additional Funding Sources: The Canadian Child Health Clinician Scientist Program; The Canadian Institutes for Health Research New Investigator Program; The Children’s Hospital of Eastern Ontario Research Institute, University of Ottawa; The Women and Children’s Health Research Institute, University of Alberta
Abbreviations
- ALL
Acute lymphoblastic leukemia
- BMD
Bone mineral density
- CI
Confidence interval
- IQR
Inter-quartile range
- SD
Standard deviation
The Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium (a pan-Canadian, pediatric bone health working group)
Co-ordinating Center
Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Leanne M. Ward#,*,§ (Study Principal Investigator), Ciaran Duffy (Rheumatology), Janusz Feber*,§ (Nephrology), Jacqueline Halton*,§ (Oncology), Roman Jurencak (Rheumatology), MaryAnn Matzinger (Radiology, Central Radiograph Analyses), Johannes Roth (Rheumatology), Nazih Shenouda§ (Radiology, Central Radiograph Analyses)
Ottawa Hospital Research Institute, Ottawa Methods Centre Ottawa, Ontario: David Moher*,§ (Research Methods), Tim Ramsay (Statistics)
Participating Centers
Alberta Children’s Hospital, Calgary, Alberta: David Stephure (Site Principal Investigator), Reinhard Kloiber (Radiology), Victor Lewis (Oncology), Julian Midgley (Nephrology), Paivi Miettunen (Rheumatology)
British Columbia Children’s Hospital, Vancouver, British Columbia: David Cabral* (Site Principal Investigator), David B. Dix (Oncology), Kristin Houghton (Rheumatology), Helen R. Nadel (Radiology)
British Columbia Women’s Hospital and Health Sciences Center, Vancouver, British Columbia: Brian C. Lentle§ (Radiology)
Brock University, Faculty of Applied Health Sciences, St. Catharines, Ontario: John Hay§ (Physical Activity Measurements)
Children’s Hospital, London Health Sciences Centre, University of Western Ontario, London, Ontario: Robert Stein (Site Principal Investigator), Elizabeth Cairney (Oncology), Cheril Clarson (Bone Health), Guido Filler (Nephrology)§, Joanne Grimmer (Nephrology), Keith Sparrow (Radiology), Scott McKillop (Radiology)
IWK Health Center, Halifax, Nova Scotia: Elizabeth Cummings (Site Principal Investigator), Conrad Fernandez (Oncology), Adam M. Huber§ (Rheumatology), Bianca Lang*,§ (Rheumatology), Kathy O’Brien (Radiology)
McMaster Children’s Hospital, Hamilton, Ontario: Stephanie Atkinson*,§ (Site Principal Investigator), Steve Arora (Nephrology), Ronald Barr§ (Oncology), Craig Coblentz (Radiology), Peter B. Dent (Rheumatology), Maggie Larché (Rheumatology), Colin Webber* (DXA Methodology),
Montreal Children’s Hospital, Montréal, Québec: Celia Rodd§ (Site Principal Investigator), Sharon Abish (Oncology), Lorraine Bell (Nephrology), Claire LeBlanc (Rheumatology), Rosie Scuccimarri (Rheumatology)
Shriners Hospital for Children, Montréal, Québec: Frank Rauch*,§ (Co-Chair, Publications and Presentations Committee and Ancillary Studies Committee), Francis Glorieux*
Ste. Justine Hospital, Montréal, Québec: Nathalie Alos* (Site Principal Investigator), Josée Dubois (Radiology), Caroline Laverdière (Oncology), Véronique Phan (Nephrology), Claire Saint-Cyr (Rheumatology)
Stollery Children’s Hospital, Edmonton, Alberta: Robert Couch* (Site Principal Investigator), Janet Ellsworth (Rheumatology), Maury Pinsk (Nephrology), Kerry Siminoski§ (Radiology), Beverly Wilson (Oncology)
Toronto Hospital for Sick Children, Toronto, Ontario: Ronald Grant* (Site Principal Investigator), Martin Charron (Radiology), Diane Hebert (Nephrology)
Université de Sherbrooke, Department of family medicine, Sherbrooke, Québec: Isabelle Gaboury*,§ (Biostatistics)
Winnipeg Children’s Hospital, Winnipeg, Manitoba: Shayne Taback§ (Site Principal Investigator), Tom Blydt-Hansen (Nephrology), Sara Israels (Oncology), Kiem Oen (Rheumatology), Martin Reed (Radiology)
Footnotes
Principal Investigator;
Publications and Presentations Committee Member
Executive Committee Member;
Previous Presentation of the Study Findings: The study findings were presented in part as a poster at the International Society of Paediatric Oncology Meeting, Auckland, New Zealand, October 26–30, 2011, as a plenary poster at the Annual Meeting of the American Society for Bone and Mineral Research, September 16–20, 2011, San Diego, California, USA and as an oral presentation at the Society for Pediatric Radiology Meeting, April 16–20, 2012, San Francisco, California, USA
References
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Rogalsky RJ, Black GB, Reed MH. Orthopaedic manifestations of leukemia in children. J Bone Joint Surg. 1986;68-A:494–501. [PubMed] [Google Scholar]
- 3.Elmantaser M, Stewart G, Young D, et al. Skeletal morbidity in children receiving chemotherapy for acute lymphoblastic leukaemia. Archives of disease in childhood. 2010;95:805–9. doi: 10.1136/adc.2009.172528. [DOI] [PubMed] [Google Scholar]
- 4.Hogler W, Wehl G, van Staa T, et al. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: comparison of fracture risk with the General Practice Research Database. Pediatr Blood Cancer. 2007;48:21–7. doi: 10.1002/pbc.20701. [DOI] [PubMed] [Google Scholar]
- 5.Sinigaglia R, Gigante C, Bisinella G, et al. Musculoskeletal manifestations in pediatric acute leukemia. Journal of pediatric orthopedics. 2008;28:20–8. doi: 10.1097/BPO.0b13e31815ff350. [DOI] [PubMed] [Google Scholar]
- 6.Strauss AJ, Su JT, Dalton VM, et al. Bony morbidity in children treated for acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3066–72. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 7.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, et al. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141:204–10. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro RC, Pui CH, Schell MJ. Vertebral compression fracture as a presenting feature of acute lymphoblastic leukemia in children. Cancer. 1988;61:589–92. doi: 10.1002/1097-0142(19880201)61:3<589::aid-cncr2820610328>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr. 1995;126:557–64. doi: 10.1016/s0022-3476(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 10.Halton JM, Atkinson SA, Fraher L, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res. 1996;11:1774–83. doi: 10.1002/jbmr.5650111122. [DOI] [PubMed] [Google Scholar]
- 11.Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–34. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593–8. doi: 10.1016/j.bone.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. pp. 229–300. [Google Scholar]
- 17.Musgrave KO, Giambalvo L, Leclerc HL, et al. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc. 1989;89:1484–8. [PubMed] [Google Scholar]
- 18.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, Vitamin D, fluoride. Washington, D.C: National Academy Press; 1997. (note this is the reference that was in place at the time the study protocol was developed) [PubMed] [Google Scholar]
- 19.Hay J. Development and validation of the Habitual Activity Estimation Scale (HAES) In: Welsman J, Armstrong N, Kilby B, editors. Children and Exercise XIX (II) Washington Singer; Exeter, UK: 1997. pp. 125–129. [Google Scholar]
- 20.Wells GD, Wilkes DL, Schneiderman-Walker J, et al. Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43:345–53. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 21.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–87. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–6. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 23.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–30. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 25.Keats TE, Smith TH. An Atlas of Normal Developmental Anatomy, Year Book of Medical Publishers. Chicago, IL, USA: 1977. [Google Scholar]
- 26.Gaca AM, Barnhart HX, Bisset GS., 3rd Evaluation of wedging of lower thoracic and upper lumbar vertebral bodies in the pediatric population. AJR Am J Roentgenol. 2010;194:516–20. doi: 10.2214/AJR.09.3065. [DOI] [PubMed] [Google Scholar]
- 27.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American journal of epidemiology. 2007;165:710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley and Sons, Inc; 2000. [Google Scholar]
- 30.Delmas PD, Genant HK, Crans GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–32. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA : the journal of the American Medical Association. 2001;285:320–3. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen BA, Bouxsein ML. Biomechanics of vertebral fractures and the vertebral fracture cascade. Current osteoporosis reports. 2010;8:198–204. doi: 10.1007/s11914-010-0031-2. [DOI] [PubMed] [Google Scholar]
- 33.Ross PD, Davis JW, Epstein RS, et al. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Annals of internal medicine. 1991;114:919–23. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar S, Mitlak BH, Wong M, et al. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17:1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Mandel K, Atkinson S, Barr RD, et al. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. 2004;22:1215–21. doi: 10.1200/JCO.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 36.Lovas K, Gjesdal CG, Christensen M, et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160:993–1002. doi: 10.1530/EJE-08-0880. [DOI] [PubMed] [Google Scholar]
- 37.Jayanthan A, Miettunen PM, Incoronato A, et al. Childhood acute lymphoblastic leukemia (ALL) presenting with severe osteolysis: a model to study leukemia-bone interactions and potential targeted therapeutics. Pediatric hematology and oncology. 2010;27:212–27. doi: 10.3109/08880011003663382. [DOI] [PubMed] [Google Scholar]
- 38.Vassilopoulou-Sellin R, Ramirez I. Severe osteopenia and vertebral compression fractures after complete remission in an adolescent with acute leukemia. Am J Hematol. 1992;39:142–3. doi: 10.1002/ajh.2830390213. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. The New England journal of medicine. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]


