Abstract
In the halophilic archaea Haloferax volcanii, the surface (S)-layer glycoprotein can be modified by two distinct N-linked glycans. The tetrasaccharide attached to S-layer glycoprotein Asn-498 comprises a sulfated hexose, two hexoses and a rhamnose. While Agl11-14 have been implicated in the appearance of the terminal rhamnose subunit, the precise roles of these proteins have yet to be defined. Accordingly, a series of in vitro assays conducted with purified Agl11-Agl14 showed these proteins to catalyze the stepwise conversion of glucose-1-phosphate to dTDP-rhamnose, the final sugar of the tetrasaccharide glycan. Specifically, Agl11 is a glucose-1-phosphate thymidylyltransferase, Agl12 is a dTDP-glucose-4,6-dehydratase and Agl13 is a dTDP-4-dehydro-6-deoxy-glucose-3,5-epimerase, while Agl14 is a dTDP-4-dehydrorhamnose reductase. Archaea thus synthesize nucleotide-activated rhamnose by a pathway similar to that employed by Bacteria and distinct from that used by Eukarya and viruses. Moreover, a bioinformatics screen identified homologues of agl11-14 clustered in other archaeal genomes, often as part of an extended gene cluster also containing aglB, encoding the archaeal oligosaccharyltransferase. This points to rhamnose as being a component of N-linked glycans in Archaea other than Hfx. volcanii.
Introduction
N-glycosylation, the covalent attachment of oligosaccharides to select asparagine residues, is performed by members of all three domains of life [1]–[5]. Still, understanding of the archaeal version of this protein-processing event remains relatively limited. In the last decade, however, substantial progress has been realized in deciphering pathways of N-glycosylation in several archaeal species, including the halophile Haloferax volcanii [5].
In Hfx. volcanii, the surface (S)-layer glycoprotein, a well-studied glycoprotein and the sole component of the protein-based shell surrounding the cell, is modified by a pentasaccharide comprising a hexose, two hexuronic acids, a methyl ester of hexuronic acid and mannose. Through a series of genetic and biochemical studies, a series of Agl (archaeal glycosylation) proteins involved in the assembly and the attachment of this glycan to the S-layer glycoprotein Asn-13 and Asn-83 positions was described [6]–[12]. Most recently, a second glycan composed of a sulfated hexose, two hexoses and a rhamnose was shown to be N-linked to position Asn-498 of the S-layer glycoprotein [13]. Moreover, whereas the Asn-13- and Asn-83-linked pentasaccharide was identified when cells were grown across a range of NaCl concentrations, the novel Asn-498-bound tetrasaccharide was observed when cells were grown in 1.75 M but not 3.4 M NaCl-containing medium.
Relying on bioinformatics, gene deletions and mass spectrometry, Agl5-Agl15 have been identified as components of the pathway responsible for the assembly of the so-called ‘low salt’ tetrasaccharide N-linked to S-layer glycoprotein Asn-498 [14]. Based on these studies, Agl11-Agl14 were deemed to be involved in the appearance of the final sugar of the ‘low salt’ tetrasaccharide, rhamnose, on the dolichol-phosphate carrier upon which the glycan is initially assembled.
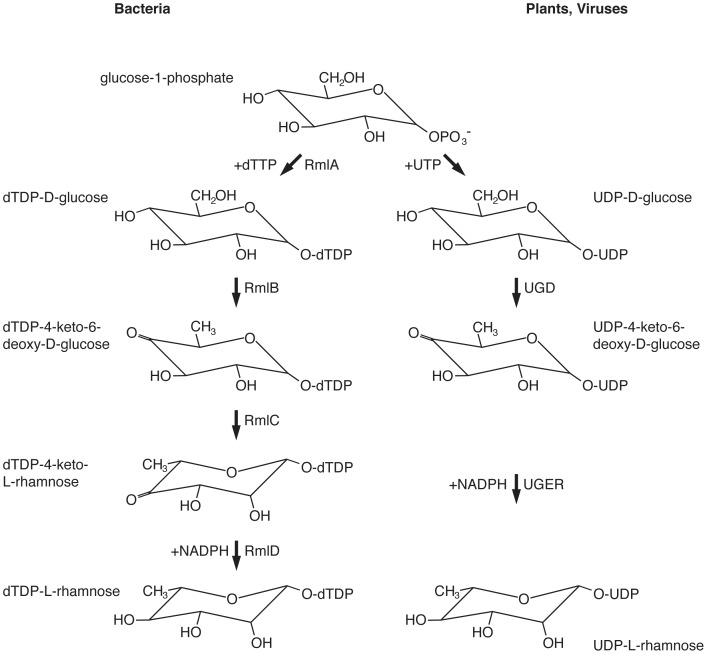
Rhamnose, a naturally occurring deoxy-hexose, is found in the L- rather than the D-configuration assumed by most other sugars. In Bacteria, plants and fungi, rhamnose is a common component of the cell wall [15]–[17], and was also recently found in viruses [18]. At present, two pathways for synthesizing nucleotide-activated rhamnose are known. In Bacteria, RmlA, RmlB, RmlC and RmlD act sequentially to convert glucose-1-phosphate and deoxy-thymidine triphosphate (dTTP) into thymidine diphosphate (dTDP)-rhamnose [19], [20]. Specifically, RmlA, the first enzyme of the pathway, is a glucose-1-phosphate thymidylyltransferase that combines thymidine monophosphate with glucose-1-phosphate to create dTDP-glucose. RmlB, a dTDP-glucose-4,6-dehydratase, then catalyzes the oxidation and dehydration of dTDP-glucose to form dTDP-4-keto 6-deoxy-glucose. RmlC, a dTDP-4-dehydro-6-deoxy-glucose-3,5-epimerase, next performs a double epimerization at the C3 and C5 positions of the sugar. Finally, RmlD, a dTDP-4-dehydrorhamnose reductase, catalyzes the last step of the pathway, namely reduction of the C4 keto group of the sugar to yield dTDP-rhamnose. In plants, uridine diphosphate (UDP)-rhamnose rather than dTDP-rhamnose is generated by RHM (UDP-L-rhamnose synthase), a single polypeptide that contains all of the enzymatic activities required [21]. Here, UDP-glucose is converted to UDP-4-keto-6-deoxy-glucose by an enzymatic activity similar to bacterial RmlB. Next, and in contrast to the bacterial process, whereby RmlC and RmlD operate sequentially to generate dTDP-rhamnose, plants instead rely on nucleotide-rhamnose synthase/epimerase-reductase, a bifunctional enzyme mediating both the epimerization and reduction reactions that lead to the biosynthesis of UDP-rhamnose [21]–[23]. More recently, the same pathway was shown to catalyze UDP-rhamnose biogenesis in large DNA viruses [18]. The two pathways for nucleotide-activated rhamnose biosynthesis are depicted in Fig 1.
Figure 1. Pathways of nucleotide-activated rhamnose biogenesis.
Schematic depiction of pathways of dTDP-rhamnose biosynthesis in Bacteria and of UDP-rhamnose biosynthesis in plants and viruses. UGD, UDP-D-glucose-4,6-dehydratase; UGER, UDP-4-keto-6-deoxy-D-glucose-3,5-epimerase/4-reductase.
Although rhamnose has been identified in several archaeal species [13], [24], [25], studies addressing rhamnose biosynthesis in Archaea are few. In Sulfolobus tokodaii, one of three RmlA homologues was shown to possess sugar-1-phosphate nucleotydyltransferase activity using either glucose-1-phosphate or N-acetylglucosamine-1-phosphate and all four deoxyribonucleoside triphosphates or UTP as substrates [26], while S. tokodaii RmlB and RmlD were reported to be functionally identical to their bacterial counterparts [27]. At the same time, the crystal structure of Methanobacter thermautotrophicus RmlC has been reported [28], as have those of S. tokodaii RmlC and RmlD (PDB 2B9U and 2GGS, respectively). Still, it remains to be determined whether rhamnose is used for glycosylation by these species. Thus, to better understand the biosynthesis of this deoxy-hexose in Archaea, the present study addressed the involvement of Hfx. volcanii Agl11-Agl14 in the biogenesis of nucleotide-activated rhamnose. In addition, the presence and genomic distribution of homologues of genes involved in such activity across the Archaea were considered.
Methods and Materials
Chemicals
DNaseI, Glucose-1-phosphate, dTTP, dTDP-D-glucose, malachite green reagent, NADPH, phenylmethanesulfonyl fluoride (PMSF), pyrophosphatase, UTP and UDP-glucose were obtained from Sigma-Aldrich (St. Louis MO), dTDP-4-keto-6-deoxy-glucose came from Carbosynth (Berkshire, UK), novobiocin and ampicillin were obtained from Duchefa Biochemie (Haarlem, The Netherlands), while restriction endonucleases were purchased from Promega (Madison, WI).
Strains and growth conditions
Hfx. volcanii WR536 (H53) parent strain cells were grown in complete medium containing 1.75 M NaCl, 0.15 M MgSO4•7H20, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3% (w/v) yeast extract, 0.5% (w/v) tryptone, 50 mM Tris-HCl, pH 7.2, at 42°C [14]. Escherichia coli were grown in Luria-Bertani medium at 37°C. Strains transformed to express plasmid-encoded versions of Agl11-Agl14 containing an N-terminally fused Clostridium thermocellum cellulose-binding domain (CBD) were supplemented with 100 µg/ml of ampicilin (for E. coli) or 1 µg/ml of novobiocin (for Hfx. volcanii).
Plasmid construction
To generate a plasmid encoding CBD-Agl11, the agl11 gene was PCR-amplified using primers designed to introduce NdeI and KpnI restriction sites at the 5′ and 3′ ends of the gene, respectively (primers listed in Table 1). The amplified fragment was digested with NdeI and KpnI and ligated into plasmid pWL-CBD, previously digested with the same restriction enzymes, to produce plasmid pWL-CBD-Agl11. Plasmid pWL-CBD-Agl11 was then introduced into Hfx. volcanii cells. Plasmids encoding CBD-Agl12, CBD-Agl13 and CBD-Agl14 were similarly generated, using the primers listed in Table 1, and also introduced into Hfx. volcanii parent strain cells.
Table 1. Primers used in this study.
| Primer | Sequence1 |
| agl11-NdeI-F | gggcatATGAAAGGCGTACTTCTCTCAGGAGG |
| agl11-KpnI-R | cccggtaccTTAGAGTTTCAGTTGGGAGTTCTC |
| agl12-NdeI-F | gggcatATGGACGTACTCGTTACTGGTGGTG |
| agl12-KpnI-R | cccggtaccCTATTCGTCGTCACCGAGGTAG |
| agl13-NdeI-F | gggcatATGCCAAACATCCACGATGTCG |
| agl13-KpnI-R | cccggtaccTTAGCCGTGGATTTCCGCGTTC |
| agl14-NdeI-F | gggcatATGTACGCATTCGTCACCGGC |
| agl14-KpnI-R | cccggtaccCTACGAGCTGTAATCGCTGAACG |
| agl13F | ATGCCAAACATCCACGATGTCG |
| agl11R | TTAGAGTTTCAGTTGGGAGTTCTCG |
Genomic sequence in capital letters.
Protein purification
To purify the CBD-tagged proteins, 1 ml aliquots of Hfx. volcanii cells transformed to express CBD-Agl11, CBD-Agl12, CBD-Agl13 or CBD-Agl14 were grown to mid-logarithmic phase, harvested and resuspended in 1 ml solubilization buffer (1% Triton X-100, 1.75 M NaCl, 50 mM Tris-HCl, pH 7.2) containing 3 µg/ml DNaseI and 0.5 µg/ml PMSF. The solubilized mixture was nutated for 20 min at 4°C, after which time 50 µl of a 10% (w/v) solution of cellulose was added. After a 120 min nutation at 4°C, the suspension was centrifuged (5,000 rpm for 5 min), the supernatant was discarded and the cellulose pellet was washed four times with wash buffer containing 1.75 M NaCl, 50 mM Tris-HCl, pH 7.2. After the final wash, the cellulose beads were centrifuged (5,000 rpm for 5 min), the supernatant was removed and the pellet, containing cellulose beads linked to CBD-tagged Agl11, Agl12, Agl13 or Agl14, was either subjected to further in vitro assays or resuspended in SDS-PAGE sample buffer, boiled for 5 min, centrifuged (5,000 rpm for 5 min) and subjected to SDS-PAGE and Coomassie Brilliant Blue staining.
Agl11 activity assay
Cellulose-bound CBD-Agl11 were resuspended in reaction buffer containing 1.75 M NaCl, 5 mM MgCl2, 50 mM Tris-HCl, pH 7.2 and incubated with 5 mM glucose-1-phosphate and 5 mM dTTP (or UTP) at 42°C. As controls, glucose-1-phosphate, dTTP (or UTP) or both were omitted from the reaction. Aliquots were removed immediately following substrate addition and at several time points up to 40 min and incubated for 10 min at room temperature (RT) with 1 U/µl of pyrophosphatase. The extent of phosphate release was determined using a malachite green-based assay [29]. Briefly, 10 µl aliquots were incubated for 5 min at RT with 850 µl of a Malachite green solution followed by addition of 100 µl of 34% citric acid and incubation for an additional 40 min at RT. Phosphate concentration was calculated using a standard curve based on the 660 nm absorbance of a 0-1000 µM phosphate solution.
Thin layer chromatography
To perform TLC, 10 µl of the products generated in the Agl11 assay described above were spotted onto a Partisil K6 silica gel plate (Whatman, Maidstone, UK). In addition, 10 µl of 2 mM glucose-1-phosphate and dTDP-D-glucose solutions were applied to the same plate as standards. The plates were developed in 95% ethanol/1 M acetic acid (5∶2, pH 7.5). The separated spots were detected by spraying the plate with orcinol monohydrate solution (0.1% in 5% H2SO4 in ethanol) and then heating the plate for 10 min at 120°C.
Agl12 activity assay
The dTDP-D-glucose 4,6-dehydratase of Agl12 activity was assayed as described previously [30]. Briefly, cellulose-bound CBD-Agl12 was resuspended in reaction buffer containing 1.75 M NaCl, 5 mM MgCl2, 50 mM Tris-HCl, pH 7.2 and incubated at 42°C with 4 mM dTDP-D-glucose or UDP-D-glucose. Aliquots were removed immediately following substrate addition and at several time points up to 40 min and mixed with 750 µl of 100 mM NaOH. Each mixture was incubated for 20 min at 42°C and absorbance at 320 nm was measured.
Combined Agl13 and Agl14 activity assay
Cellulose-bound CBD-Agl13 and CBD-Agl14 were resuspended in reaction buffer containing 1.75 M NaCl, 50 mM Tris-HCl, pH 7.2 and incubated with 4 mM dTDP-4-keto-6-deoxy-glucose and 10 mM NADPH at 42°C for 20 h. As controls, CBD-Agl13, CBD-Agl14 or NADPH was omitted. After incubation, the mixtures were centrifuged (5,000 rpm for 5 min), and the supernatant was examined by nano- ESI/MS analysis. For nano-ESI/MS analysis, a 10 µl aliquot was dried using a SpeedVac apparatus, resuspended in 10 µl methanol:water (1∶1; v/v) containing 10 mM ammonium acetate and injected into a LTQ Orbitrap XL mass spectrometer using static medium NanoES Spray capillaries (Thermo Fisher Scientific, Bremen, Germany). Mass spectra were obtained in the negative mode.
Reverse transcriptase polymerase chain reaction (RT-PCR)
RT-PCR performed as previously described [31]. Briefly, RNA from Hfx. volcanii cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was prepared for each sequence from the corresponding RNA (2 µg) using random hexamers (150 ng) in a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). The single-stranded cDNA was then used as PCR template in a reaction containing forward and reverse primers to sequences within agl13 and agl11, respectively (Table 1). In control reactions, genomic DNA or RNA served as template, or no nucleic acid was added to the reaction. The generation of PCR products was assessed by electrophoresis in 1% agarose followed by detection using ethidium bromide.
Bioinformatics analysis
Predicted archaeal RmlABCD proteins were identified using Hfx. volcanii Agl11, Agl12, Agl13 and Agl14 as query in a BLAST search of the Joint Genome Institute Database for Integrated Microbial Genomes – Expert Review (https://img.jgi.doe.gov/cgi-bin/er/main.cgi), using the terms ‘EC 2.7.7.24’, ‘EC 4.2.1.46’, ‘EC 5.1.3.13’ and ‘EC 1.1.1.133’ to search for RmlA, RmlB, RmlC and RmlD homologues, respectively. Archaeal RmlA-, RmlB-, RmlC- and RmlD-encoding genes were deemed as being clustered with the oligosaccharyltransferase-encoding aglB gene based upon the presence of these genes within previously identified aglB-based glycosylation gene clusters [32], or when rmlA, rmlB, rmlC and rmlD were clustered and found 10 genes or less away from clusters containing aglB and other glycosylation- or sugar processing-related genes.
Results
Agl11 is a glucose-1-phosphate thymidylyltransferase/uridylyltransferase
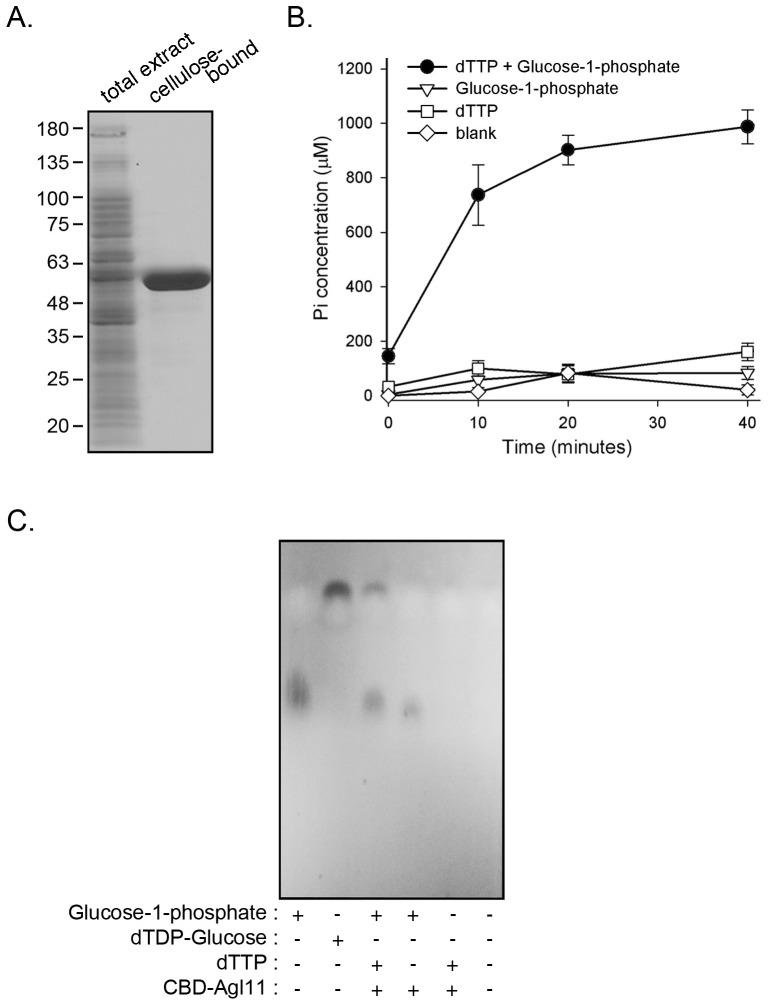
As a first step towards defining the precise function of Agl11, a BLAST homology-based search was conducted using Hfx. volcanii Agl11 as query. This revealed the homology of Agl11 to RmlA, the bacterial glucose-1-phosphate thymidylyltransferase (EC 2.7.7.24) that catalyzes the formation of dTDP-glucose from dTTP and glucose 1-phosphate [33]. For instance, Agl11 shared 53% identity, with 100% coverage and an E-value of 8e-120, to RmlA from the bacterium Sulfobacillus acidophilus TPY. To biochemically confirm that Agl11 indeed acts as does RmlA, Hfx. volcanii cells were transformed with a plasmid encoding Agl11 bearing an N-terminally-fused CBD tag [34]. The presence of the CBD tag allows for cellulose-based purification compatible with the hypersaline conditions in which Hfx. volcanii grow. PCR amplification using DNA extracted from the transformed strain as template, together with forward and reverse primers directed against regions within the CBD and agl11 sequences, respectively, confirmed uptake of the plasmid (not shown). Cellulose-based purification of an extract prepared from the transformed cells captured a single 55 kDa protein, corresponding to the predicted molecular mass of the 17 kDa CBD moiety and the 38 kDa Agl11 protein (Fig 2A).
Figure 2. Agl11 is a glucose-1-phosphate thymidylyltransferase.
A. Hfx. volcanii cells transformed to express CBD-Agl11 were subjected to cellulose-based chromatography. A cell extract and cellulose-bound proteins were separated on 10% SDS-PAGE and Coomassie-stained. A cellulose-bound ∼55 kDa protein band corresponding to CBD-Agl11 is observed. The positions of molecular weight markers are shown on the left. B. Cellulose-bound CBD-Agl11 or cellulose beads alone (blank) were resuspended in reaction buffer and incubated in the presence of dTTP and glucose-1-phosphate, with each substrate separately or without both substrates. Aliquots removed immediately after substrate addition and up to 40 min later were incubated with pyrophosphatase and the extent of phosphate release was measured 29]. The results represent average of triplicates ± standard deviation for one of three repeats of the experiment. C. The assay products obtained after a 5 h incubation at 42°C were separated by TLC, along with glucose-1-phosphate and dTDP-glucose standards, as described in Experimental Procedures.
The predicted glucose-1-phosphate thymidylyltransferase activity of purified Agl11 was next considered. Glucose-1-phosphate thymidylyltransferase, like RmlA, transfers the deoxy-thymidine monophosphate (dTMP) group of dTTP to glucose-1-phosphate to yield dTDP-glucose and pyrophosphate. Hence, the actions of Agl11 as a glucose-1-phosphate thymidylyltransferase was tested using a malachite green-based assay to detect the formation of phosphate following the conversion of pyrophosphate into inorganic phosphate upon addition of pyrophosphatase [29]. The assay revealed that Agl11 was able to generate phosphate only when incubated with dTTP and glucose-1-phosphate but not with either substrate alone or without both substates (Fig 2B). Thin layer chromatography (TLC) was also employed to further confirm the glucose-1-phosphate thymidylyltransferase activity of Agl11. In these experiments, the product generated upon incubation of Agl11 with dTTP and glucose-1-phosphate migrated to the same position as a dTDP-glucose standard (Fig 2C). Similar results were obtained when UTP was used in place of dTTP (not shown). As such, Agl11 acts as a glucose-1-phosphate thymidylyltransferase and a glucose-1-phosphate uridylyltransferase, namely the first enzyme in the biosynthesis of nucleotide activated-rhamnose in bacteria and in plants, respectively.
Agl12 is a dTDP-glucose-4,6-dehydratase
The function of Agl12 was next addressed. A BLAST homology-based search revealed the homology of Hfx. volcanii Agl12 to both dTDP-glucose-4,6-dehydratase (RmlB) (EC 4.2.1.46), the bacterial enzyme catalyzing the second step of the dTDP-rhamnose biosynthetic pathway, i.e, the conversion of dTDP-glucose to dTDP-4-keto-6-deoxy-glucose [35], and to UDP-glucose-4-epimerase (or UDP-galactose-4-epimerase; EC 5.1.3.2), the enzyme catalyzing the reversible conversion of UDP-galactose to UDP-glucose, the final step in the Leloir pathway of galactose metabolism [36]. Specifically, Agl12 shared 58% identity, with 98% coverage and an E-value of 5e-125, to RmlB from the bacterium Caldithrix abyssi. Indeed, Agl12 contains the GxxGxxG (7GGAGFIG13) and YxxxK (146YSATK150) motifs characteristic of RmlB proteins [23].
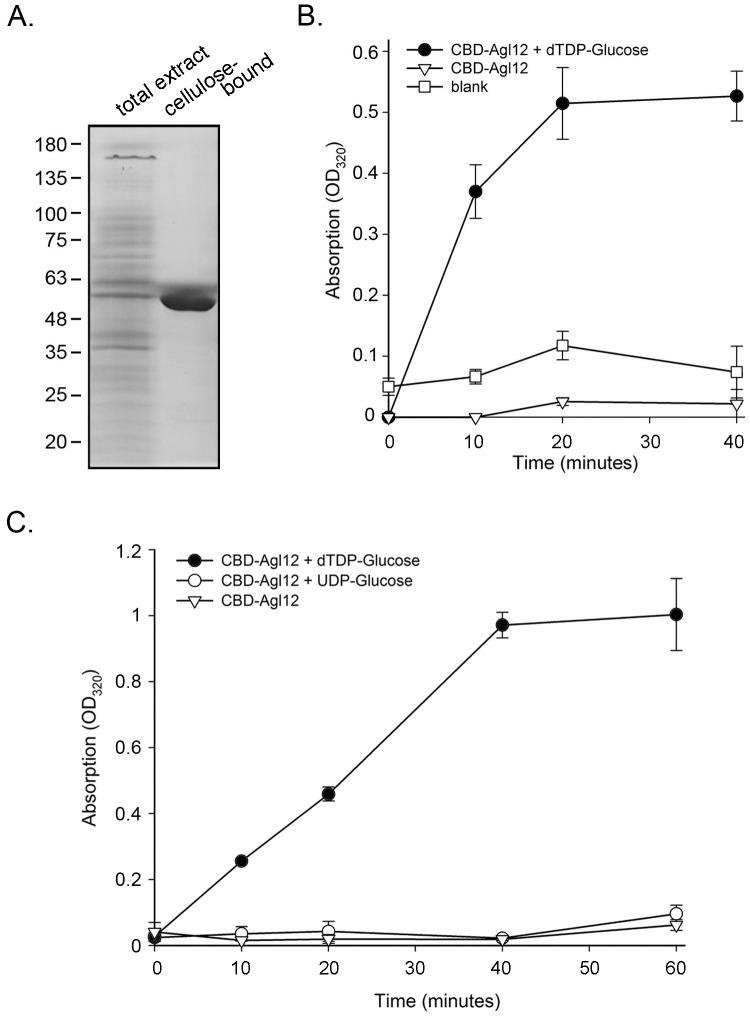
To test whether Agl12 indeed acts as a dTDP-glucose-4,6-dehydratase, working downstream to Agl11 in the biosynthesis of dTDP-rhamnose, Hfx. volcanii cells were transformed to express CBD-tagged Agl12. Again, successful transformation was verified by PCR amplification using DNA from the transformed strain as template, together with forward and reverse primers directed against regions within the CBD and agl12 sequences, respectively (not shown). Cellulose-based purification of an extract prepared from Hfx. volcanii cells transformed to express CBD-Agl12 captured a 51 kDa species, corresponding to the predicted molecular mass of the 17 kDa CBD moiety and the 34 kDa Agl12 protein (Fig 3A).
Figure 3. Agl12 functions as dTDP-D-glucose-4,6-dehydratase.
A. Hfx. volcanii cells transformed to express CBD-Agl12 were subjected to cellulose-based capture. A cell extract and cellulose-bound protein were separated on 10% SDS-PAGE and Coomassie-stained. Protein band corresponding to CBD-Agl11 (∼51 kDa) is observed. The positions of molecular weight markers are shown on the left of the gel. B. Cellulose-bound CBD-Agl12 or cellulose beads alone (blank) was resuspended in reaction buffer and incubated in the presence of dTDP-glucose or no substrate. Immediately after substrate addition and up to 40 min of incubation at 42°C, the samples were incubated with 0.1 M NaOH for 20 min at 42°C and the increase of absorbance at 320 nm was measured. C. The same assay was repeated for up to 60 min using dTDP-glucose or UDP-glucose as substrate or with no substrate. The assay results presented in B and C represent averages of triplicates ± standard deviation obtained in one of two repeats of the experiment.
Cellulose-purified CBD-Agl12 was incubated in the absence or presence of dTDP-glucose, the product of the Agl11-catalyzed reaction, and the formation of dTDP-4-keto-6-deoxy-glucose was assessed spectrophotometrically, following the increase in absorption at 320 nm, indicative of the formation of the product keto group. dTDP-4-keto-6-deoxy-glucose was only generated when Agl12 was combined with dTDP-glucose, confirming that Agl12 is indeed a dTDP-glucose-4,6-dehydratase, like RmlB (Fig 3B). When CBD-Agl12 was combined with UDP-glucose, the product generated when UDP-rhamnose serves as substrate, no UDP-4-keto-6-deoxy-glucose was formed (Fig 3C).
Agl13 and Agl14 are homologues of RmlC and RmlD, respectively
Next, the functions of Agl13 and Agl14 were addressed. The homologies of Hfx. volcanii Agl13 to dTDP-4-dehydrorhamnose-3,5-epimerase (RmlC) (EC 5.1.3.13) and of Agl14 to dTDP-4-dehydrorhamnose reductase (RmlD) (EC 1.1.1.133), bacterial enzymes respectively catalyzing the isomerization of dTDP-4-keto-6-deoxy-glucose to dTDP-4-keto-L-rhamnose and its dehydrogenation to yield dTDP-rhamnose [36], were revealed by BLAST searches. Specifically, Agl13 shared 51% identity, with 97% coverage and an E-value of 9e-45, to RmlC from Nitrolancea hollandica, while Agl14 shared 37% identity, with 96% coverage and an E-value of 1e-46, to RmlD from Ammonifex degensii KC4. Moreover, while Agl14 contains the GxxGxxG (7GANGLLG13) and YxxxK (135YGRSK139) motifs characteristic of RmlD proteins [37].
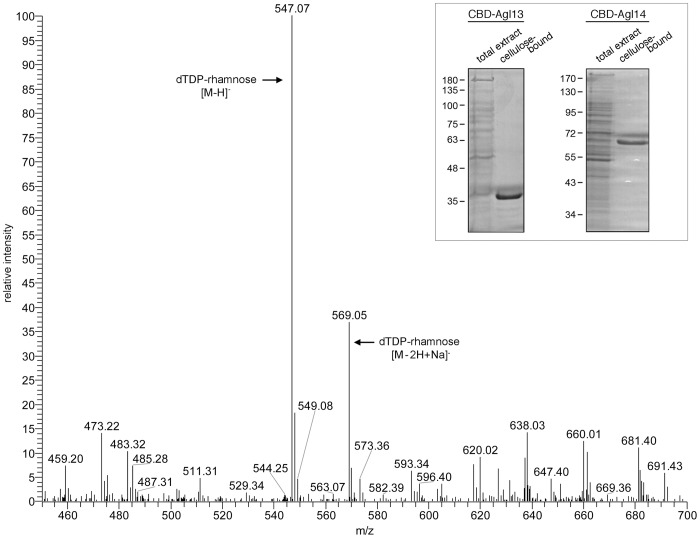
To determine whether Agl13 and Agl14 indeed participate in the biosynthesis of dTDP-rhamnose by acting as RmlC and RmlD, respectively, Hfx. volcanii cells were transformed to express CBD-tagged versions of Agl13 and Agl14. Here as well, each transformation was verified by PCR amplification using DNA from the transformed strain as template, together with forward and reverse primers directed against regions within the CBD and agl13, or the CBD and agl14 sequences, respectively (not shown). Following transformation, cellulose-based purification of extracts prepared from Hfx. volcanii cells transformed to express either CBD-Agl13 or CBD-Agl14 was conducted. Following SDS-PAGE separation of cellulose-captured proteins, only bands corresponding to CBD-Agl13 or CBD-Agl14 were observed (Fig 4 inset, left and right panels, respectively).
Figure 4. Agl13 and Agl14 together convert dTDP-4-keto-6-deoxy-glucose into dTDP-rhamnose.
Cellulose-bound CBD-Agl13 and CBD-Agl14 were combined with dTDP-4-keto-6-deoxy-glucose and NADPH and the soluble fraction was examined by nano- ESI/MS analysis. Peaks corresponding to dTDP-rhamnose and the sodium adduct are indicated. Inset: Purification of CBD-Agl13 and CBD-Agl14. Cell extracts and cellulose-bound protein from Hfx. volcanii cells transformed to express CBD-Agl13 (left) or CBD-Agl14 (right) were separated on 10% SDS-PAGE and Coomassie-stained. Protein bands corresponding to CBD-Agl13 and CBD-Agl14 are observed in each lane of cellulose-bound material. The positions of molecular weight markers are shown on the left of each gel.
The ability of Agl13 and Agl14 to act as RmlC and RmlD respectively in the production of dTDP-rhamnose was next considered in a combined assay. Briefly, dTDP-4-keto-6-deoxy-glucose was incubated together with CBD-tagged Agl13 and Agl14, along with NADPH, the substrate for the dehydrogenase reaction putatively catalyzed by Agl14. The appearance of dTDP-rhamnose was revealed by nano-electrospray ionization mass spectrometry (nano-ESI/MS) [18], since initial attempts to detect dTDP-rhamnose formation spectrophotometrically as previously described [38], [39] were unsuccessful. Nano-ESI/MS analysis revealed the formation of a m/z 547.07 peak corresponding to dTDP-rhamnose (m/z 547.07 calculated [M-H]− mass) and a peak at m/z 569.05 corresponding to the sodium adduct (m/z 569.05 calculated [M-2H+Na]− mass) (Fig 4). In the absence of CBD-Agl13, CBD-Agl14 or NADPH, peaks corresponding to dTDP-4-keto-6-deoxy-glucose were observed (m/z 545.06 calculated [M-H]− mass); no peaks corresponding to dTDP-rhamnose were seen (Fig S1A-C, respectively). In the absence of dTDP-4-keto-6-deoxy-glucose, no peaks corresponding to either sugar were detected (Fig S1D).
agl11 and agl13 are co-transcribed
To obtain further insight into the actions of Agl11-Agl14, the transcription of each gene was addressed. Specifically, given that agl11 is found adjacent to agl13 in the Hfx. volcanii genome and that both are similarly oriented (Fig 5A), the co-transcription of these genes was considered. Accordingly, RT-PCR amplification was performed using primers directed at regions corresponding to the beginning of agl13 and the end of agl11 together with cDNA produced from RNA isolated from Hfx. volcanii cells. A PCR product of approximately 1500 bp, consistent with the genomic sizes of agl13 (471 bp) and agl11 (1074 bp), was observed (Fig 5B).
Figure 5. agl11 and agl13 are co-transcribed.
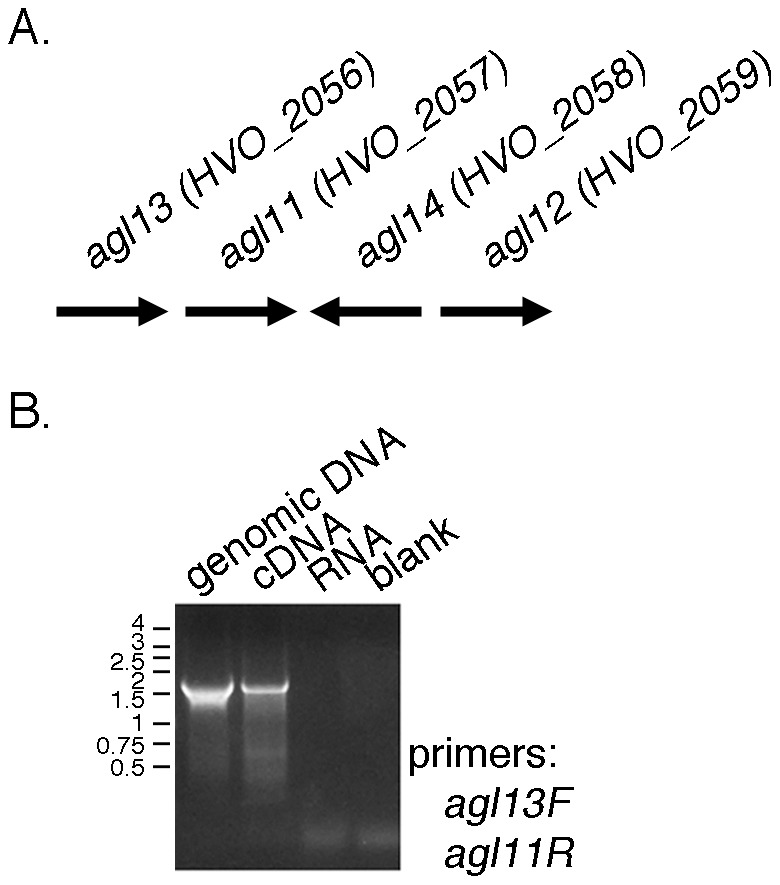
A. Schematic depiction of the position and orientation of agl11-agl14 in the Hfx. volcanii genome. The length of each gene is arbitrarily drawn. B. PCR amplifications were performed using a forward primer against a region within agl11 and a reverse primer against a region within agl13, together with genomic DNA or RNA isolated from Hfx. volcanii cells, cDNA prepared from the same RNA or no nucleic acid (blank) as template. The positions of Kbp markers are shown on the left.
Genome scanning reveals the clustering of rmlABCD in other Archaea
Given the identification of an rmlABCD gene cluster in Hfx. volcanii, similar clusters were sought in other available archaeal genomes. Towards this aim, the 166 completed archaeal genomes listed at the Joint Genome Institute Database for Integrated Microbial Genomes (January, 2014) were subjected to a BLAST search seeking homologues of Hfx. volcanii Agl11, Agl12, Agl13 and Agl14. In addition, these genomes were also scanned for genes encoding proteins listed as EC 2.7.7.24 (RmlA), EC 4.2.1.46 (RmlB), EC 5.1.3.13 (RmlC) or EC 1.1.1.133 (RmlD). In this manner, 69 genomes were shown to encode an rmlABCD gene cluster, including Hfx. volcanii. Of these, 16 included rmlABCD as part of a previously defined larger cluster anchored by aglB, encoding the archaeal oligosaccharyltransferase [32] (Table 2). In addition, 19 species were found to encode partial rmlABCD gene clusters, where two or three of these genes are clustered (Table S1). Of these species, four (Methanobrevibacter ruminantium, Methanosarcina acetivorans, Sulfolobus islandicus Y.G.57.14 and Sulfolobus solfataricus P2) also encode a complete rmlABCD gene cluster.
Table 2. Archaea where rmlABCD are clustered.
| Species | rmlA | rmlB | rmlC | rmlD | aglB 1 |
| Acidilobus saccharovorans | ASAC_0660 | ASAC_0661 | ASAC_0658 | ASAC_0659 | |
| Aciduliprofundum boonei | Aboo_0257 | Aboo_0256 | Aboo_0255 | Aboo_0254 | |
| Aeropyrum pernix | APE1181 | APE1180 | APE1178 | APE1179 | |
| Archaeoglobus fulgidus 7324 | AFULGI_00003220 | AFULGI_00003210 | AFULGI_00003190 | AFULGI_00003200 | AFULGI_00003280 |
| Archaeoglobus fulgidus VC-16 | AF00325 | AF00324 | AF00323a | AF00323b | AF00329 |
| Archaeoglobus profundus | Arcpr_1197 | Arcpr_1198 | Arcpr_1202 | Arcpr_1201 | Arcpr_1194 |
| Archaeoglobus veneficus | Arcve_0544 | Arcve_0545 | Arcve_0551 | Arcve_0546 | Arcve_0568 |
| Caldisphaera lagunensis | Calag_0942 | Calag_0943 | Calag_0941 | Calag_0944 | |
| Candidatus Caldiarchaeum subterraneum | Kcr_0845 | Kcr_0846 | Kcr_0848 | Kcr_0847 | |
| Candidatus Methanomethylophilus alvus | MMALV_00940 | MMALV_00960 | MMALV_00950 | MMALV_00970 | |
| Candidatus Methanoregula boonei | Mboo_1752 | Mboo_1749 | Mboo_1751 | Mboo_1750 | |
| Candidatus Nitrosopumilus sp. AR2 | NSED_08565,08570 | NSED_08580 | NSED_08585 | NSED_08575 | |
| Desulfurococcus mucosus | Desmu_1148 | Desmu_1145 | Desmu_1143 | Desmu_1144 | |
| Haloferax volcanii | Agl11 | Agl12 | Agl13 | Agl14 | |
| Halogeometricum borinquense | Hbor_31500 | Hbor_31470 | Hbor_31510 | Hbor_31480 | |
| Halomicrobium mukohataei | Hmuk_1214 | Hmuk_1217 | Hmuk_1213 | Hmuk_1216 | |
| Halophilic archaeon sp. DL31 | Halar_0604 | Halar_0607 | Halar_0603 | Halar_0606 | |
| Halorhabdus utahensis | Huta_2143 | Huta_2146 | Huta_2142 | Huta_2145 | |
| Metallosphaera cuprina | Mcup_0924 | Mcup_0925 | Mcup_0922 | Mcup_0923 | |
| Methanobacterium sp. SWAN-1 | MSWAN_0553 | MSWAN_0550 | MSWAN_0551 | MSWAN_0552 | |
| Methanobrevibacter ruminantium | mru_0108 | mru_0110 | mru_0109 | mru_0107 | |
| Methanobrevibacter smithii PS | Msm_1307 | Msm_1309 | Msm_1308 | Msm_1304 | |
| Methanocella arvoryzae | MRE50lv_2499 | MRE50lv_2497 | MRE50lv_2496 | MRE50lv_2498 | |
| Methanocella conradii | Mtc_0188,185 | Mtc_0186,189 | Mtc_0190 | Mtc_0187 | |
| Methanocella paludicola | MCPlv_2757,2760 | MCPlv_2756,2759 | MCPlv_2755 | MCPlv_2758 | MCPlv_2762 |
| Methanococcoides burtonii | Mbur_2230 | Mbur_2232 | Mbur_2233 | Mbur_2231 | |
| Methanococcus aeolicus | Maeo_0379 | Maeo_0380 | Maeo_0383 | Maeo_0381 | |
| Methanococcus maripaludis C5 | MmarC5_1315 | MmarC5_1314 | MmarC5_1316 | MmarC5_1313 | |
| Methanococcus maripaludis C6 | MmarC6_0591 | MmarC6_0590 | MmarC6_0592 | MmarC6_0589 | |
| Methanococcus maripaludis X1 | GYY_01860 | GYY_01865 | GYY_01855 | GYY_01875 | |
| Methanoculleus marisnigri | Memar_0188 | Memar_0186 | Memar_0185 | Memar_0187 | Memar_0175 |
| Methanolobus psychrophilus | Mpsy_2402 | Mpsy_2400 | Mpsy_2399 | Mpsy_2401 | |
| Methanosaeta concilii | MCON_2594 | MCON_2593 | MCON_2590 | MCON_2591 | |
| Methanosaeta harundinacea | Mhar_1098 | Mhar_1099 | Mhar_1097 | Mhar_1100 | Mhar_1091 |
| Methanosaeta thermophila | Mthe_0954 | Mthe_0956 | Mthe_0955 | Mthe_0953 | |
| Methanosarcina acetivorans | MA3777 | MA3779 | MA3780 | MA3778 | MA3752,3753,3754 |
| Methanosarcina barkeri | Mbar_A0233 | Mbar_A0231 | Mbar_A0230 | Mbar_A0232 | Mbar_A0242,A024 |
| Methanosarcina mazei | MM1169 | MM1167 | MM1166 | MM1168 | |
| Methanosphaerula palustris | Mpal_2406 | Mpal_2404 | Mpal_2403 | Mpal_2405 | |
| Methanospirillum hungatei | Mhun_3075 | Mhun_3072 | Mhun_3074 | Mhun_3073 | Mhun_3066 |
| Methanothermobacter marburgensis | MTBMA_c03630 | MTBMA_c03610 | MTBMA_c03620 | MTBMA_c03640 | |
| Methanothermobacter thermoautotrophicus | MTH1791 | MTH1789 | MTH1790 | MTH1792 | |
| Methanothermus fervidus | Mfer_0286 | Mfer_0280 | Mfer_0281 | Mfer_0285 | |
| Methanotorris formicicus | Metfo_1922 | Metfo_1923 | Metfo_1926 | Metfo_1925 | |
| Methanotorris igneus | Metig_0176 | Metig_0177 | Metig_0179 | Metig_0178 | |
| Picrophilus torridus | PTO0307 | PTO0310,0312 | PTO0311 | PTO0308 | |
| Pyrococcus abyssi | PAB0784 | PAB0785 | PAB0787 | PAB0789 | PAB1586 |
| Pyrococcus horikoshii | PH0417 | PH0414 | PH0413 | PH0417 | |
| Pyrococcus sp. ST04 | Py04_0479 | Py04_0480 | Py04_0481 | Py04_0482 | Py04_0456 |
| Pyrococcus yayanosii | PYCH_17780 | PYCH_17770 | PYCH_17740 | PYCH_17690 | PYCH_17920 |
| Pyrolobus fumarli | Pyrfu_0681 | Pyrfu_0680 | Pyrfu_0677 | Pyrfu_0679 | |
| Sulfolobus acidocaldarius 98-3 | Saci_1703 | Saci_1704 | Saci_1706 | Saci_1705 | |
| Sulfolobus acidocaldarius N8 | SacN8_08270 | SacN8_08275 | SacN8_08285 | SacN8_08280 | |
| Sulfolobus acidocaldarius Ron12/l | SacRon12l_08280 | SacRon12l_08285 | SacRon12l_08295 | SacRon12l_08290 | |
| Sulfolobus islandicus LAL14/1 | Sil_0867 | Sil_0868 | Sil_0865 | Sil_0866 | |
| Sulfolobus islandicus L.D.8.5 | LD85_1121 | LD85_1117 | LD85_1123 | LD85_1122 | |
| Sulfolobus islandicus REY15A | SiRe_0841 | SiRe_0840 | SiRe_0843 | SiRe_0842 | |
| Sulfolobus islandicus Y.G.57.14 | YG5714_0665 | YG5714_0664 | YG5714_0667 | YG5714_0666 | |
| Sulfolobus solfataricus P2 | SSO0831 | SSO0830 | SSO0833 | SSO0832 | |
| Thermococcus barophilus | TERMP_02079 | TERMP_02080 | TERMP_02084 | TERMP_02089 | TERMP_02078 |
| Thermococcus onnurineus | TON_1842 | TON_1843 | TON_1848 | TON_1851 | TON_1820 |
| Thermococcus sibiricus | TSIB_2044 | TSIB_2045 | TSIB_2047 | TSIB_2048 | TSIB_0007 |
| Thermogladius cellulolyticus | TCELL_0180 | TCELL_0179 | TCELL_0177 | TCELL_0178 | |
| Thermogladius shockii | Des1633_00001920 | Des1633_00001910 | Des1633_00001890 | Des1633_00001900 | |
| Thermoproteus tenax | TTX_1336 | TTX_1335 | TTX_1333 | TTX_1334 | |
| Thermosphaera aggregans | Tagg_0563 | Tagg_0562 | Tagg_0560 | Tagg_0561 |
Clustering with aglB is defined as occurring when rmlABCD are part of a gene cluster containing aglB as described in ref. [39] or ≤10 genes away from such aglB–based clusters.
Discussion
In addition to the pentasaccharide linked to select Asn residues of the Hfx. volcanii S-layer glycoprotein, it was recently shown that at least one additional Asn can be modified by a novel tetrasaccharide [14]. While many of the enzymes involved in the assembly of the N-linked pentasaccharide have been characterized biochemically [9], [10], [12], [40], virtually nothing is known of the enzymes responsible for the assembly of the N-linked tetrasaccharide. As such, this study reports the first biochemical analysis of enzymes contributing to this novel N-glycosylation pathway. The results reveal that Agl11 is a glucose-1-phosphate thymidylyltransferase, Agl12 is a dTDP-glucose-4,6-dehydratase, Agl13 is a dTDP-4-dehydro-6-deoxy-glucose-3,5-epimerase and Agl14 is a dTDP-4-dehydrorhamnose reductase.
While rhamnose is a common component of both the bacterial and the plant cell wall, different biosynthetic pathways are employed in each case, leading to the generation of differentially nucleotide-activated species. At the same time, it is not clear which of these strategies Archaea employ for nucleotide-activated rhamnose biogenesis. Indeed, numerous examples of Archaea relying on the same biochemical pathways as used by either their bacterial or eukaryal counterparts have been reported, as have examples of archaeal pathways comprising selected aspects of the parallel bacterial and eukaryal processes or even biosynthetic pathways unique to this form of life [41]–[50]. In the case of Hfx. volcanii, the current study revealed that Agl11-Agl14 are homologous to RmlA-D, enzymes that catalyze the conversion of glucose-1-phosphate to dTDP-rhamnose in Bacteria [19], [20]. Indeed, examination of available archaeal genomes detected the presence of RmlA-D in numerous species, pointing to Archaea and Bacteria as relying on the same route for nucleotide-activated rhamnose generation. At the same time, no gene encoding a homologue of the bifunctional nucleotide-rhamnose synthase/epimerase-reductase used in eukaryal UDP-rhamnose biosynthesis was detected in Archaea. Still, the fact that several archaeal species encode only a partial rmlABCD cluster (Table S1) raises the possibility that those enzymes present are recruited for the synthesis of molecules other than dTDP-rhamnose.
In addition to determining the route of nucleotide-activated rhamnose biosynthesis in Hfx. volcanii, the present study also represents the first biochemical characterization of components of a second N-glycosylation pathway recently identified in this species [14]. Based on earlier work revealing the presence of one or more genes encoding AglB, the oligosaccharyltransferase of the archaeal N-glycosylation machinery, in all but two of 168 genomes considered, it would appear that this protein-processing event is common in Archaea [40]. Yet, the diverse composition of the few N-linked archaeal glycans characterized to date points to archaeal N-glycosylation as largely relying on species-specific pathways [5]. The finding that some species contain rmlABCD homologues as part of a larger gene cluster containing aglB and other sugar-related genes implies that as in Hfx. volcanii, rhamnose is a component of N-linked glycans in these other Archaea as well. Continued investigation into archaeal protein glycosylation will test this prediction.
Finally, the simultaneous modification of the same protein by two completely different N-linked glycans has only been reported to date in two haloarchaeal species, namely Halobacterium salinarum and Hfx. volcanii [13], [51]. Of these, it is only in Hfx. volcanii that two N-glycosylation pathways have been identified [14]. Moreover, it was shown that N-glycosylation by both pathways occurs as a function of salt levels in the growth medium [13]. At present, it is not clear why the Hfx. volcanii S-layer glycoprotein is modified by two distinct N-linked glycans in 1.75 M NaCl-containing medium but not when cells are grown at higher salinity, nor what advantages such differential N-glycosylation offer the cell. The results obtained in this study will help answer these and other outstanding questions related to Hfx. volcanii N-glycosylation.
Supporting Information
Agl13, Agl14 and NADPH are required for the conversion of dTDP-4-keto-6-deoxy-glucose into dTDP-rhamnose. Reactions were conducted as described in the legend to Figure 4, albeit in the absence of cellulose-bound CBD-Agl13 (A), CBD-Agl14 (B) or NADPH (C). In each case, nano-ESI/MS analysis detected peaks corresponding to dTDP-4-keto-6-deoxy-glucose (m/z 545.06 calculated [M-H]− mass) but not peaks corresponding to dTDP rhamnose (m/z 547.07 calculated [M-H]− mass). When the reaction was conducted in the absence of dTDP-4-keto-6-deoxy-glucose (D), no peaks corresponding to either sugar were detected. As standards, 10 µl of 1 mM dTDP-4-keto-6-deoxy-glucose (E) and dTDP rhamnose (F) solutions were examined by nano-ESI/MS.
(TIF)
Archaea encoding partial rmlABCD clusters.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
J.E. is supported by the Israel Science Foundation (grant 8/11) and the U. S. Army Research Office (W911NF-11-1-520). L.K. is the recipient of a Negev-Zin Associates Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nothaft H, Szymanski CM (2010) Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol 8: 765–778. [DOI] [PubMed] [Google Scholar]
- 2. Schwarz F, Aebi M (2011) Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol 21: 576–582. [DOI] [PubMed] [Google Scholar]
- 3. Aebi M (2013) N-linked protein glycosylation in the ER. Biochim Biophys Acta 1833: 2430–2437. [DOI] [PubMed] [Google Scholar]
- 4. Nothaft H, Szymanski CM (2013) Bacterial protein N-glycosylation: new perspectives and applications. J Biol Chem 288: 6912–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eichler J (2013) Extreme sweetness: protein glycosylation in archaea. Nat Rev Microbiol 11: 151–156. [DOI] [PubMed] [Google Scholar]
- 6. Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, et al. (2007) Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J Mol Biol 374: 1224–1236. [DOI] [PubMed] [Google Scholar]
- 7. Abu-Qarn M, Giordano A, Battaglia F, Trauner A, Hitchen PG, et al. (2008) Identification of AglE, a second glycosyltransferase involved in N-glycosylation of the Haloferax volcanii S-layer glycoprotein. J Bacteriol 190: 3140–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yurist-Doutsch S, Abu-Qarn M, Battaglia F, Morris HR, Hitchen PG, et al. (2008) AglF, aglG and aglI, novel members of a gene island involved in the N-glycosylation of the Haloferax volcanii S-layer glycoprotein. Mol Microbiol 69: 1234–1245. [DOI] [PubMed] [Google Scholar]
- 9. Magidovich H, Yurist-Doutsch S, Konrad Z, Ventura VV, Dell A, et al. (2010) AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. . Mol Microbiol 76: 190–199. [DOI] [PubMed] [Google Scholar]
- 10. Yurist-Doutsch S, Magidovich H, Ventura VV, Hitchen PG, Dell A, et al. (2010) N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol Microbiol 75: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 11. Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, et al. (2010) AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J Bacteriol 192: 5572–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen-Rosenzweig C, Yurist-Doutsch S, Eichler J (2012) AglS, a novel component of the Haloferax volcanii N-glycosylation pathway, is a dolichol phosphate-mannose mannosyltransferase. J Bacteriol 194: 6909–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guan Z, Naparstek S, Calo D, Eichler J (2012) Protein glycosylation as an adaptive response in Archaea: growth at different salt concentrations leads to alterations in Haloferax volcanii S-layer glycoprotein N-glycosylation. Environ Microbiol 14: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaminski L, Guan Z, Yurist-Doutsch S, Eichler J (2013) Two distinct N-glycosylation pathways process the Haloferax volcanii S-Layer glycoprotein upon changes in environmental salinity. MBio 4: e00716–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mäki M, Renkonen R (2004) Biosynthesis of 6-deoxyhexose glycans in bacteria. Glycobiology 14: 1R–15R. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Ji Q, Jiang L, Shen S, Fan Y, et al. (2009) Overexpression of a cytosol-localized rhamnose biosynthesis protein encoded by Arabidopsis RHM1 gene increases rhamnose content in cell wall. Plant Physiol Biochem 47: 86–93. [DOI] [PubMed] [Google Scholar]
- 17. Martinez V, Ingwers M, Smith J, Glushka J, Yang T, et al. (2012) Biosynthesis of UDP-4-keto-6-deoxyglucose and UDP-rhamnose in pathogenic fungi Magnaporthe grisea and Botryotinia fuckeliana . J Biol Chem 287: 879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parakkottil Chothi M, Duncan GA, Armirotti A, Abergel C, Gurnon JR, et al. (2010) Identification of an L-rhamnose synthetic pathway in two nucleocytoplasmic large DNA viruses. J Virol 84: 8829–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glaser L, Kornfeld S (1961) The enzymatic synthesis of thymidine linked sugars. II. Thymidine diphosphate L-rhamnose. J Biol Chem 236: 1795–1799. [PubMed] [Google Scholar]
- 20. Giraud MF, Naismith JH (2000) The rhamnose pathway. Curr Opin Struct Biol 10: 687–696. [DOI] [PubMed] [Google Scholar]
- 21. Oka T, Nemoto T, Jigami Y (2007) Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J Biol Chem 282: 5389–5403. [DOI] [PubMed] [Google Scholar]
- 22. Kamsteeg J, Van Brederode J, Van Nigtevecht G (1978) The formation of UDP-L-rhamnose from UDP-D-glucose by an enzyme preparation of red campion (Silene dioica (L) Clairv) leaves. FEBS Lett. 91: 281–284. [DOI] [PubMed] [Google Scholar]
- 23. Watt G, Leoff C, Harper AD, Bar-Peled M (2004) A bifunctional 3,5-epimerase/4-keto reductase for nucleotide-rhamnose synthesis in Arabidopsis . Plant Physiol 134: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sprott GD, Shaw KM, Jarrell KF (1983) Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei . J Biol Chem 258: 4026–4031. [PubMed] [Google Scholar]
- 25. Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R (1997) Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii . Glycobiology 7: 897–904. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Z, Tsujimura M, Akutsu J, Sasaki M, Tajima H, et al. (2005) Identification of an extremely thermostable enzyme with dual sugar-1-phosphate nucleotidylyltransferase activities from an acidothermophilic archaeon, Sulfolobus tokodaii strain 7. J Biol Chem 280: 9698–9705. [DOI] [PubMed] [Google Scholar]
- 27.Teramoto M, Zhang Z, Shizuma M, Kawasaki T, Kawarabayasi Y, et al. (2012) The thermostable enzyme genes of the dTDP-L-Rhamnose synthesis pathway (rmlBCD) from a thermophilic archaeon. In: Petre M, editor. Advances in Applied Biotechnology, InTech. DOI: 10.5772/29196. Available from: http://www.intechopen.com/books/advances-in-applied-biotechnology/the-thermostable-enzyme-genes-of-the-dtdp-l-rhamnose-synthesis-pathway-rmlbcd-from-a-thermophilic-ar
- 28. Christendat D, Saridakis V, Dharamsi A, Bochkarev A, Pai EF, et al. (2000) Crystal structure of dTDP-4-keto-6-deoxy-D-hexulose 3,5-epimerase from Methanobacterium thermoautotrophicum complexed with dTDP. J Biol Chem 275: 24608–24612. [DOI] [PubMed] [Google Scholar]
- 29. Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100: 95–97. [DOI] [PubMed] [Google Scholar]
- 30. Okazaki R, Okazaki T, Strominger JL, Michelson AM (1962) Thymidine diphosphate 4-keto-6-deoxy-d-glucose, an intermediate in thymidine diphosphate L-rhamnose synthesis in Escherichia coli strains. J Biol Chem 237: 3014–3026. [PubMed] [Google Scholar]
- 31. Abu-Qarn M, Eichler J (2006) Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol Microbiol 61: 511–525. [DOI] [PubMed] [Google Scholar]
- 32. Kaminski L, Lurie-Weinberger MN, Allers T, Gophna U, Eichler J (2013) Phylogenetic- and genome-derived insight into the evolution of N-glycosylation in Archaea. Mol Phylogenet Evol 68: 327–339. [DOI] [PubMed] [Google Scholar]
- 33. Pazur JH, Shuey EW (1961) The enzymatic synthesis of thymidine diphosphate glucose and its conversion to thymidine diphosphate rhamnose. J Biol Chem 236: 1780–1785. [PubMed] [Google Scholar]
- 34. Plavner N, Eichler J (2008) Defining the topology of the N-glycosylation pathway in the halophilic archaeon Haloferax volcanii . J Bacteriol 190: 8045–8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snipes CE, Brillinger GU, Sellers L, Mascaro L, Floss HG (1977) Stereochemistry of the dTDP-glucose oxidoreductase reaction. J Biol Chem 252: 8113–8117. [PubMed] [Google Scholar]
- 36. Holden HM, Rayment I, Thoden JB (2003) Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem 278: 43885–43888. [DOI] [PubMed] [Google Scholar]
- 37. Graninger M, Nidetzky B, Heinrichs DE, Whitfield C, Messner P (1999) Characterization of dTDP-4-dehydrorhamnose 3,5-epimerase and dTDP-4-dehydrorhamnose reductase, required for dTDP-L-rhamnose biosynthesis in Salmonella enterica serovar Typhimurium LT2. J Biol Chem 274: 25069–25077. [DOI] [PubMed] [Google Scholar]
- 38. Melo A, Glaser L (1968) The Mechanism of 6-deoxyhexose synthesis. II. Conversion of deoxythymidine diphosphate 4-keto-6-deoxy-D-glucose to deoxythymidine diphosphate L-rhamnose. J Biol Chem 243: 1475–1478. [PubMed] [Google Scholar]
- 39. Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T (1997) Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans . J Bacteriol 179: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaminski L, Eichler J (2010) Identification of residues important for the activity of Haloferax volcanii AglD, a component of the archaeal N-glycosylation pathway. Archaea 2010: 315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell SD, Jackson SP (2001) Mechanism and regulation of transcription in archaea. Curr Opin Microbiol 4: 208–213. [DOI] [PubMed] [Google Scholar]
- 42. Sakuraba H, Goda S, Ohshima T (2004) Unique sugar metabolism and novel enzymes of hyperthermophilic archaea. Chem Rec 3: 281–287. [DOI] [PubMed] [Google Scholar]
- 43. Siebers B, Schönheit P (2005) Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. Curr Opin Microbiol 8: 695–705. [DOI] [PubMed] [Google Scholar]
- 44. Grochowski LL, Xu H, White RH (2006) Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J Bacteriol 188: 3192–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phillips G, Chikwana VM, Maxwell A, El-Yacoubi B, Swairjo MA, et al. (2010) Discovery and characterization of an amidinotransferase involved in the modification of archaeal tRNA. J Biol Chem 285: 12706–12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benelli D, Londei P (2011) Translation initiation in Archaea: conserved and domain-specific features. Biochem Soc Trans 39: 89–93. [DOI] [PubMed] [Google Scholar]
- 47. Sato T, Atomi H (2011) Novel metabolic pathways in Archaea. Curr Opin Microbiol 14: 307–314. [DOI] [PubMed] [Google Scholar]
- 48. Nishimura H, Azami Y, Miyagawa M, Hashimoto C, Yoshimura T, Hemmi H (2013) Biochemical evidence supporting the presence of the classical mevalonate pathway in the thermoacidophilic archaeon Sulfolobus solfataricus . J Biochem 153: 415–420. [DOI] [PubMed] [Google Scholar]
- 49. Bräsen C, Esser D, Rauch B, Siebers B (2014) Carbohydrate metabolism in archaea: current insights into unusual enzymes and pathways and their regulation. Microbiol Mol Biol Rev 78: 89–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vannice JC, Skaff DA, Keightley A, Addo J, Wyckoff GJ, et al. (2014) Identification in Haloferax volcanii of phosphomevalonate decarboxylase and isopentenyl phosphate kinase as catalysts of the terminal ezymatic reactions in an archaeal alternate mevalonate pathway. J Bacteriol 196: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lechner J, Wieland F (1989) Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem 58: 173–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Agl13, Agl14 and NADPH are required for the conversion of dTDP-4-keto-6-deoxy-glucose into dTDP-rhamnose. Reactions were conducted as described in the legend to Figure 4, albeit in the absence of cellulose-bound CBD-Agl13 (A), CBD-Agl14 (B) or NADPH (C). In each case, nano-ESI/MS analysis detected peaks corresponding to dTDP-4-keto-6-deoxy-glucose (m/z 545.06 calculated [M-H]− mass) but not peaks corresponding to dTDP rhamnose (m/z 547.07 calculated [M-H]− mass). When the reaction was conducted in the absence of dTDP-4-keto-6-deoxy-glucose (D), no peaks corresponding to either sugar were detected. As standards, 10 µl of 1 mM dTDP-4-keto-6-deoxy-glucose (E) and dTDP rhamnose (F) solutions were examined by nano-ESI/MS.
(TIF)
Archaea encoding partial rmlABCD clusters.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.