Abstract
Purpose
To investigate the extent to which clinicians avoid well-established drug-drug interactions associated with warfarin. We hypothesised that clinicians would avoid combining non-steroidal anti-inflammatory drugs (NSAIDs), tramadol and sulfamethoxazole with warfarin.
Methods
A cross-sectional analysis of nationwide dispensing data was performed in Swedish individuals 18 years or older (n = 7 563 649). Odds ratios of interacting NSAIDs, tramadol and sulfamethoxazole versus respective prevalence of comparator drugs codeine, and ciprofloxacin in patients co-dispensed interacting warfarin versus patients unexposed was calculated.
Results
The odds of receiving an interacting NSAID versus the comparator codeine was markedly lower in patients with warfarin than in the remaining population (adjusted OR 0.21; 95% CI 0.20 – 0.22). Also, the interacting drugs tramadol and sulfamethoxazole were less common among patients dispensed warfarin as compared to the remaining population, although the decrease was much more modest (adjusted OR 0.83; CI 0.80–0.87 and 0.81; CI 0.73 – 0.90).
Conclusions
In conclusion, Swedish doctors in the vast majority of cases refrain from prescribing NSAIDs to patients already on warfarin. Tramadol and sulfamethoxazole are however rarely avoided.
Introduction
Warfarin, a vitamin K antagonist, offers an effective means of thrombosis prevention. However, it has a narrow therapeutic range [1] which explains its association with frequently occurring serious adverse reactions such as gastrointestinal and cerebral bleeding. Drug-drug interactions are a major risk factor in this regard [2]–[5] especially among the elderly due to a greater exposure to multiple drug use [6].
Non-steroidal anti-inflammatory drugs (NSAIDs) work by inhibiting the synthesis of inflammatory prostaglandins, and may therefore impair the aggregation of thrombocytes [7]. In addition, they damage the gastrointestinal mucosa [8], and increase the sensitivity to warfarin treatment [9], factors all of which contribute to a substantially increased risk of severe bleeding [5], [10], [11]. The interaction between warfarin and NSAIDs is one of the most prevalent clinically relevant drug-drug interactions, and in a large US prescription study 24% of warfarin-treated patients received an NSAID during a two-year follow-up [12], [13].
The analgesic effect of tramadol derives from opioid receptor agonism in combination with norepinephrine and serotonin reuptake inhibition. It increases the sensitivity to warfarin treatment [14]–[16] and inhibits thrombocyte aggregation [17] which may result in an increased risk of gastrointestinal bleeding [18], [19]. For a patient already on warfarin, an alternative analgesic such as codeine should therefore be considered. The prevalence of the warfarin-tramadol interaction is not well-documented, but with more than 100 tramadol prescriptions per 1000 adults annually in Sweden, the interaction is likely to concern a large number of patients [20].
Sulfamethoxazole is a widely used antibiotic. Combined with warfarin, it greatly increases the risk for gastrointestinal bleeding, due to cytochrome P450 enzyme (CYP) 2C9 inhibition [21]–[24]. For warfarin-treated patients in need of treatment of urinary tract infections ciprofloxacin may therefore be a better alternative [25], [26]. Despite the well-documented interaction risk, sulfamethoxazole prescription is common in warfarin-treated patients. For example, a recent US study showed that sulfamethoxazol (in combination with trimethoprim) accounts for 12% of all antibiotic prescription in ambulatory patients taking warfarin, indicating that the interaction could be of significant clinical importance [27].
In Sweden, the SFINX prescribing support software automatically alerts doctors when they are about to co-prescribe a potentially dangerous combination [28]. Guidance is hereby provided on how to handle the specific drug-drug interaction. SFINX is available to approximately 80% of the doctors [29].
Table 1 present in detail information regarding respective interaction provided by SFINX as well as on the labelling of respective study drug according to The Swedish summary of product characteristics [30].
Table 1. Rationale for the choice of study drugs.
| Labelled indications for groups of drugs according to FASS1 | Drugs (ATC code2) | Role of study drug | Rationale | Recommendations according to SFINX with regard to combining warfarin with respective study drug | Labelling according to FASS1 with regard to interactions leading to an increased risk of bleeding |
| Vitamin-K-epoxide reductase inhibitor The only anticoagulant used in Sweden. Small therapeutic window. Indications include the treatment and prevention of venous thrombosis, to prevent thromboembolism in patients with atrial fibrillation | warfarin (B01AA03) | Interacting drug | Has a narrow therapeutic range and the dose needed for sufficient anticoagulation is close to that which may cause bleeding [1], [44] | NA | NSAIDs3 incresase the effect of warfarin pharmacokinectally and pharmacodynamically. Combining with inhibitors of CYP2C94 should be avoided. |
| Analgesics Sharing indications for treatment of acute moderate pain. NSAID3 used for conditions associated with inflammation | NSAID3 (MA01A)5 | Interacting analgesic | Impair the aggregation of thrombocytes [7], damage the gastrointestinal mucosa [8], and increase the sensitivity to warfarin treatment [9] leading to a substantially increased risk of severe bleedings [10], [11]. | Co-dispension with warfarin may cause severe bleedings. The combination should be avoided. | May increase the risk of bleeding when used together with warfarin. Close monitoring is warranted. |
| tramadol (N02AX02) | Interacting analgesic | Increases the sensitivity to warfarin treatment [14]–[16] and inhibits thrombocyte aggregation [17] which may result in an increased risk of gastrointestinal bleeding [18], [45]. | The effect of warfarin may increase when used concomitantly. The combination should be avoided. | May increase the anticoagulant effect of warfarin. Increased monitoring is warranted. | |
| codeine (R05DA04, N02AA59) | Comparator analgesic | Opiod analgesic. No evidence of interaction with warfarin. | No warnings issued regarding the coadministration with warfarin. | No warnings issued regarding the coadministration with warfarin. | |
| Anti-infectives Sharing indications for treatment of gram-negative bacteria and used for the treatment of urinary tract infections. | Sulfa-methoxazole (J01EE01) | Interacting anti-infective | Substantially increased risk for gastrointestinal bleeding on the basis of cytochrome CYP 2C94 inhibition [21]–[24]. | The effect of warfarin and bleeding is markedly increased. The combination should be avoided. | May significantly increase the effect of warfarin. Close monitoring is warranted. |
| ciprofloxacin (J01MA02) | Comparator anti-infective | Some data suggest an increase of the anticoagulant effect of warfarin due to an uncertain mechanism although the evidence is conflicting [25], [26], . | May increase the anticoagulant effect of warfarin. Careful monitoring of warfarin is warranted. | May increase the anticoagulant effect of warfarin. Increased monitoring of warfarin is warranted. |
The Swedish summary of product characteristics.
Anatomical Therapeutic Chemical code.
Non steroidal anti-inflammatory drugs.
Cytochrome P450 enzyme (CYP) 2C9.
Include all acetylsalicylic acid drugs beginning with MA01, glucosamine (M01AX05) excluded.
The aim of the current study was to investigate the compliance to guidelines on drug-drug interactions with the potential to cause warfarin induced bleeding. We hypothesized that doctors in Sweden would avoid the combined use of warfarin and interacting drugs.
Methods
Ethics statement
This was a database study that included data on the entire Swedish population 18 years or older. Hence we did not interfere with the treatment of these individuals nor in any other way. Since the data was anonymized and none of the individuals were identifiable, the integrity of the individuals was not judged to be violated. This view was also supported by the Regional Ethics Committee in Stockholm, Karolinska Institute, which waived the need for written informed consent from the participants and approved the study as a whole.
Study design
The study design was a retrospective, cross-sectional analysis of patients being dispensed prescription drugs in Sweden during the period from August 15 to December 15, 2011. The choice of a four-month-study-period was based on the Swedish regulation and experience that most patients on long-term/chronic treatment repeat their drug-dispensing every third to fourth month. We selected all individuals, 18 years or older, that were dispensed any of the drugs presented in Table 1. The cohort was established on data obtained from the Swedish Prescribed Drug Register (SPDR) [31], [32].
Data source
The Swedish Prescribed Drug Register contains data with unique patient identifiers for all dispensed prescriptions covering the whole population of Sweden. The data collection is administered by the National Corporation of Swedish Pharmacies, a state-owned company responsible for the provision of pharmaceutical services at a nationwide level. Data on all dispensed prescriptions is transferred monthly to the National Board of Health and Welfare. The drugs are classified according to the Anatomical Therapeutic Chemical (ATC) classification system. From this register, we selected all individuals, 18 years and older.
Variables
We hypothesised that physicians in Sweden would avoid the combined use of warfarin and interacting drugs. If so, the odds ratio between the prevalences of interacting drugs to comparator drug users would be lower among patients co-dispensed warfarin as compared with patients without warfarin, a methodology used previously [33]–[35].
Thus, in the analgesic area, corresponding outcomes were the odds ratio of being prescribed a NSAID (vs codeine) and that of being prescribed tramadol (vs codeine). NSAIDs include acetylsalicylic acid and all drugs with Anatomical Therapeutic Chemical (ATC) codes beginning with M01A, glucosamine (M01AX05) excluded, i.e. celecoxib, dexibuprofen, dexketoprofen, diclofenac, etoricoxib, ibuprofen, indomethacin, ketoprofen, ketorolac, lornoxicam, meloxicam, nabumetone, naproxen, parecoxib, piroxicam, tenoxicam. The grouping of individual NSAIDs into a single exposure category was justified by the fact that COX inhibitors are expected to have similar effects on the thrombocyte function and gastric mucosa [7], [8], [36], and the interaction is generally considered a class-effect of NSAIDs. Consequently, SFINX recommends that prescribers abstain from combining warfarin with any NSAID without differentiating between individual substances [28].
For anti-infectives, the outcome measure was the odds ratio of being prescribed the interacting drug sulfamethoxazole (rather than ciprofloxacin) comparing warfarin users to non-users of warfarin.
In the statistical analysis, factors considered potential effect modifiers were age, gender, number of drugs and medical setting, all treated as categorical variables. The variable age was divided into four groups: young adults, adults, geriatric patients and the oldest (18–44, 45–64, 65–79 and ≥ 80) and the variable number of drugs was divided into three groups (<5, 5–9 and >9). Information on medical setting was based on the variable “Prescribers'working place” in the SPDR and whether the interacting drug was being prescribed from a primary or specialist care unit. Primary care was defined as care provided by health care professionals that often play a role in the local community and act as a first point of consultation for all patients within the health care system. Secondary care was defined as care provided by medical specialists often associated with a hospital such as cardiologists, endocrinologists or other internists.
Analysis
To study associations between warfarin and the interacting drugs and to control for potential effect modifiers we used multivariate logistic regression. The associations are presented as odds and odds ratios (OR) with 95% confidence intervals (CI). The departure from 1 (no association) is statistically significant at the 5% level, two-tailed, if the 95% CI does not include 1. All statistical calculations were performed in IBM SPSS Statistics 22.0 (SPSS Inc., Chicago, IL, USA).
Selection of study population
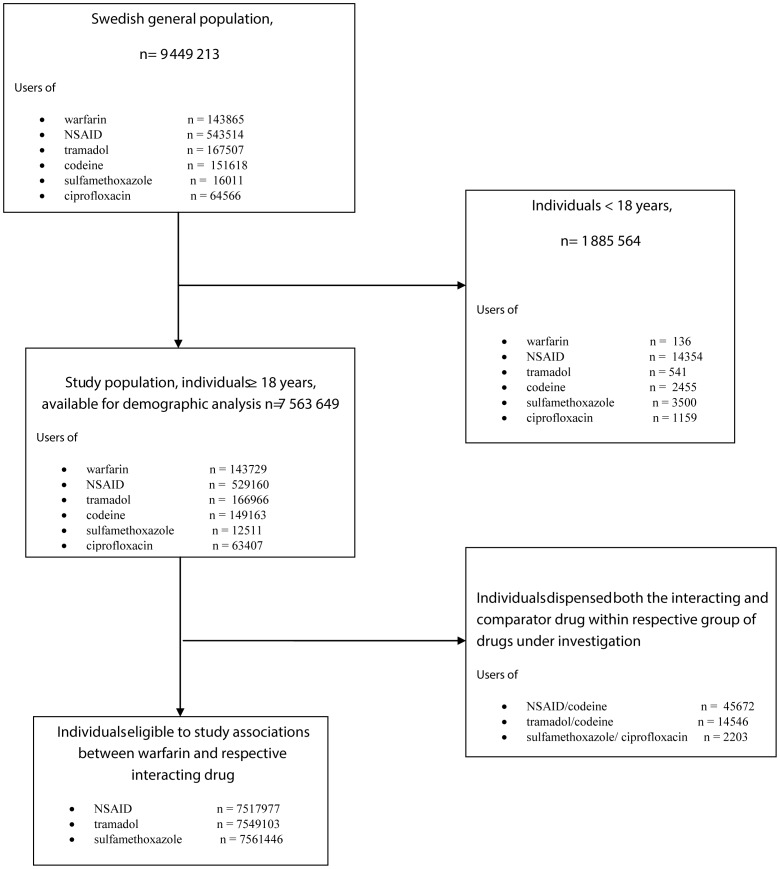
Individuals in the Swedish population 18 years or older (n = 7 563 649) were included in the study [20]. To minimize the possible bias of patients who changed interacting drugs and comparator drugs within the 4-month-study period, associations between different classes of drugs was based on the individuals who had been dispensed no more than one of the drugs in each therapeutic area. Those few individuals who had been dispensed both an interacting drug and a comparator drug were thus excluded (Figure 1).
Figure 1. Patient flow chart.
Results
The mean age was 49 years, and 51% were women. The prevalence of the use of study drugs in the Swedish study population is given in Table 2 along with corresponding demographics. Warfarin was dispensed to 1.9 percent of the study population (Table 2).
Table 2. Prevalence of study drugs used in the adult Swedish population (≥18 years of age) and corresponding demographics, 15th August to 15th December, 2011.
| n | n/1000 individuals | Mean age (SD1) | Women (%) | Individuals ≥ 65 years (%) | Percentage of drugs2 prescribed from primary care | Mean number of drugs (SD1) | |
| warfarin | 143729 | 19 | 73 (12) | 41 | 81 | 65 | 7.4 (4.1) |
| Warfarin interacting drugs | |||||||
| NSAID3 | 529160 | 70 | 54 (17) | 58 | 29 | 73 | 5.3 (4.0) |
| tramadol | 166966 | 22 | 59 (17) | 58 | 40 | 72 | 7.5 (4.7) |
| sulfamethoxazole | 12511 | 1.7 | 61 (18) | 42 | 49 | 34 | 8.8 (5.5) |
| Comparator drugs | |||||||
| codeine | 149163 | 20 | 55 (18) | 60 | 33 | 61 | 6.7 (4.7) |
| ciprofloxacin | 63407 | 8.4 | 61 (19) | 39 | 48 | 58 | 7.2 (5.2) |
Standard Deviation
Defined as a seven-digit Anatomical Therapeutic Chemical (ATC) code. The remaining proportions were prescribed from a specialist care setting.
Non steroidal anti-inflammatory drugs include all drugs beginning with M01A, glucosamine (M01AX05) excluded.
Table 3 shows the number of individuals in the different groups of patients under comparison. The number of patients dispensed warfarin in combination with NSAID, tramadol and sulfamethoxazole was 4273, 6650 and 464 respectively. Figure 2 shows odds ratios of receiving interacting drugs in patients dispensed warfarin as compared to the remaining population. The odds of receiving an interacting NSAID versus the comparator codeine was markedly lower in patients with warfarin than in the remaining population (adjusted OR 0.21; 95% CI 0.20 – 0.22). The interacting drugs tramadol and sulfamethoxazole were also less common among patients dispensed warfarin as compared to the remaining population (adjusted OR 0.83; CI 0.80 – 0.87 and 0.81; CI 0.73 – 0.90).
Table 3. The number of individuals with interactive drugs and comparator drugs among individuals with or without co-dispensed warfarin under the study period 15 August to 15 December, 2011.
| Interactive drugs | Comparator drugs | |
| NSAID | codeine | |
| warfarin | 4273 | 5516 |
| no warfarin | 479215 | 97975 |
| sulfamethoxazole | ciprofloxacin | |
| warfarin | 464 | 3114 |
| no warfarin | 9844 | 58090 |
| tramadol | codeine | |
| warfarin | 6650 | 5472 |
| no warfarin | 145770 | 129145 |
Figure 2. Odds ratios of exposure to an interacting rather than a non-interacting drug in warfarin-treated individuals vs individuals without warfarin.
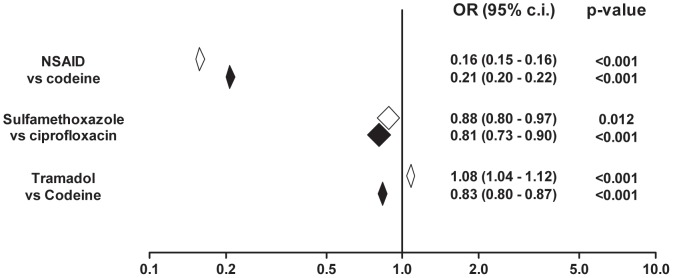
White diamonds represent 95% c.i. of unadjusted ORs, filled diamonds represent ORs adjusted for gender, age, number of drugs and clinical setting.
Table 4 shows the adjusted odds ratios of being dispensed interacting drugs in primary care settings, specialist care settings, individuals < 65 respectively ≥65 years of age, in males and in females. The results in the investigated subgroups were mostly consistent with those for the general population. However some differences were noted. The inverse association between tramadol and warfarin was stronger in primary care (adjusted OR 0.80; CI 0.76–0.84) compared to specialized care (adjusted OR 0.91; CI 0.85–0.98). On the contrary, the inverse association between sulfamethoxazole and warfarin was not evident in primary care patients (adjusted OR 0.87; CI 0.74–1.02) while being pronounced in individuals receiving their prescriptions in a specialized care setting (adjusted OR 0.77; CI 0.67–0.88). Among patients 65 years or older the decreased association between interacting tramadol and sulfamethoxazole respectively and warfarin was stronger as compared to the population as a whole (adjusted OR 0.82; CI 0.78–0.86 and 0.80; CI 0.71–0.90). In younger patients this association was very weak and statistically non-significant (adjusted OR 0.98; CI 0.90–1.06 and 0.90; CI 0.71–1.14).
Table 4. Associations between study drugs, in patients with or without dispensed warfarin dispensed in primary care setting, specialist care setting, and in males under the study period 15 August to 15 December, 2011.
| Study drugs | Individuals prescribed from primary care, adjusted1 odds ratios (95% CI2) | Individuals prescribed from specialised care, adjusted1 odds ratios (95% CI2) | <65 years (n = 2752666), adjusted1 odds ratios (95% CI2) | ≥65 years (n = 1547787), adjusted1 odds ratios (95% CI2) | Males (n = 1781475), adjusted3 odds ratios (95% CI2) | Females (n = 2518978), adjusted3 odds ratios (95% CI2) |
| Interacting drugs: NSAID4 | 0.20 (0.19 – 0.21) | 0.24 (0.22 – 0.26) | 0.20 (0.18–0.22) | 0.21 (0.20 – 0.23) | 0.23 (0.22 – 0.25) | 0.19 (0.17–0.20) |
| Comparator drug: codeine (reference group) | ||||||
| Interacting drugs: tramadol | 0.80 (0.76 – 0.84) | 0.91 (0.85 – 0.98) | 0.98 (0.90–1.06) | 0.82 (0.78– 0.86) | 0.86 (0.83 – 0.93) | 0.89 (0.84–0.93) |
| Comparator drug: codeine (reference group) | ||||||
| Interacting drug: sulfamethoxazole | 0.87 (0.74 – 1.02) | 0.77 (0.67 – 0.88) | 0.90 (0.71–1.14) | 0.80 (0.71 – 0.90) | 0.79 (0.71– 0.88) | 0.82 (0.68–0.99) |
| Comparator drug: ciprofloxacin (reference group) |
Estimates adjusted for gender and age.
Confidence Intervals.
Estimates adjusted for age and medical setting.
Non steroidal anti-inflammatory drugs.
Discussion
Using complete data on all prescription drugs dispensed during a four-month period in Sweden, we demonstrate that prescribers to a large extent avoid combining warfarin with the potentially interacting NSAIDs. In contrast, sulfamethoxazole and tramadol are rarely avoided in warfarin-exposed individuals, despite the risk of drug-drug interactions.
An obvious explanation for this discrepancy could be that physicians are more aware of the interaction between NSAIDs and warfarin. For example, education on warfarin drug-drug interactions may have put greater emphasis on the NSAIDs, since their use is much more widespread [12], [13] compared to that of tramadol [20] and, in particular, sulfamethoxazole [27]. Also, the NSAIDs' well-known propensity to cause gastrointestinal bleeding per se could make the risk of combining them with warfarin more intuitive [7], [8], [37]. On the contrary, tramadol and sulfamethoxazole are prone to cause hemorrhage primarily in patient co-medicating with warfarin [21], [24].
An alternative explanation is that the prescribers are aware of the potential interaction risks, but conscientiously decide to combine warfarin with tramadol or sulfamethoxazole. The documentation of the warfarin-tramadol interaction may be considered too scarce to justify the choice of an alternative analgesic why clinicians instead choose to employ close INR monitoring [14]–[17]. The reason for the quite modest decrease in association between sulfamethoxazole and warfarin may not only represent a lack of knowledge regarding the potential risks of this specific drug-drug interaction, but could also reflect a tendency to prioritize adherence to available guidelines for microbial usage.
The study has important strengths. Most importantly, the large number of prescriptions analysed provided it with sufficient power for very precise effect estimates. In addition, the use of complete data on all drugs dispensed in Sweden during a defined time period means that the results should not be influenced by selection bias regarding prescribers, patients or drug prescriptions.
The study also has some potential weaknesses. Most importantly, the observational design means that the results could have been influenced by various types of bias. Using available register data, we statistically controlled for the influence of age, gender, medical care setting, and number of prescribed drugs, but other potential sources of bias could not be accounted for. For example, we did not have information on treatment indication and although the pairs of interacting and non-interacting drugs were meticulously chosen to represent plausible treatment alternatives, the prescribers' choice could have been influenced by a range of factors other than the risk of warfarin interactions. For example, regarding choice of urinary tract infection antibiotic the antimicrobial resistance pattern may have prevented the prescriber from choosing freely between the two study drugs. In the individual case, a second best option may be to prescribe sulfamethoxazole despite the interaction risk and then employ close monitoring of INR, perhaps in combination with a proactive reduction of the warfarin dose, a strategy that could not be evaluated in the current study. Furthermore, although the data have the advantage of being based on information on dispensed rather than prescribed drugs, it was not possible to ascertain whether the medication was actually consumed. Another uncertainty about the dispensing data relates to the employment of a fixed time window to estimate the use of drug combinations. Although generally regarded valid, applying a time window may be associated with both under- and overestimation of exposure [32]–[34]. An alternative method that sometimes may be associated with less bias is the assessment of concomitantly used drugs at a fixed time point is the “legend time” method, where treatment duration is calculated from the amount of drug dispensed. However, for assessing exposure to drugs used on an “as needed” basis such as analgesics, the validity of this method is questionable being based on an assumption of regular intake [38], [39].
The current study was limited to prescription drugs and over the counter drugs (OTCs) were thus not included. The prevalence of patients on warfarin that were co-dispensed an NSAID (n = 4273) may therefore be underestimated. Although the present study focus on prescribed drugs it cannot be excluded that some physician tells the patient to go and buy an NSAID over-the-counter which is a limitation of the study.
The results from investigating the population as a whole were mostly consistent with the results of the subgroup analyses. However, some differences were noted. The decreased association between interacting tramadol and sulfamethoxazole and warfarin was more pronounced in patients ≥65 years as compared to younger patients. This may indicate an increased effort to avoid warfarin associated interactions in the more vulnerable geriatric population [1]. When comparing the two medical settings the results deviated from the population as a whole as well but in different directions for the two therapeutic areas. The tendency to avoid combining tramadol with warfarin was more pronounced in the primary care as compared to individuals prescribed from specialist care. On the contrary, the decreased association between sulfamethoxazole and warfarin was more marked in the specialist care. The significance of these findings remains unclear.
A few previous studies have investigated prescriber awareness of drug-drug interactions using similar methods [33]–[35]. These studies have indicated that prescribers to some extent do take drug-drug interactions into account when prescribing well-known inhibitors of drug-metabolizing enzymes. However, the extent to which drug-drug interactions have been avoided has generally been small compared to the effective avoidance of NSAID prescription in warfarin-treated patients seen in our study (adjusted OR 0.21). Interestingly, the only previous example of a drug-drug interaction awareness of this magnitude did also involve warfarin. Using prescription data from Ireland, Williams et al. showed that interacting H2 blockers are effectively avoided in warfarin-treated patients (OR 0.21 for prescription of an interacting rather than a non-interacting drug) [33]. Inversely, drug interactions potentially resulting in loss of therapeutic effect were very rarely taken into account by Swedish prescribers in a study by Mannheimer et al. [35]. For these interactions, odds ratios were approximately 1, matching those of warfarin-tramadol and warfarin-sulfametoxazole in the present study.
Although the odds receiving an NSAID vs. codeine are about five times lower in patients receiving warfarin, 4273 patients received co-prescriptions of warfarin with NSAIDs. This is likely to represent a considerable clinical problem and is clearly against available guidelines.
The present study indicates a need for improved compliance with drug label recommendations as well as a need for continuous medical education about the basic pharmacology of commonly used drugs. In Sweden, the SFINX prescribing support software provides guidance on how to handle drug-drug interactions, including the ones studied herein [28], [29]. Apparently the Swedish physicians often fail to take advantage of this tool. Prescribers' tendency to override DDI alerts is a well-known problem described from several clinical contexts [40]–[43]. The present study further emphasizes the need to overcome this barrier.
In conclusion, Swedish doctors in the vast majority of cases refrain from prescribing NSAIDs to patients already on warfarin. Tramadol and sulfamethoxazole are however rarely avoided.
Funding Statement
The work was supported financially by the Swedish Society for Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fitzmaurice DA, Blann AD, Lip GY (2002) Bleeding risks of antithrombotic therapy. BMJ 325: 828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pirmohamed M, James S, Meakin S, Green C, Scott AK, et al. (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. Bmj 329: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budnitz DS, Lovegrove MC, Shehab N, Richards CL (2011) Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 4. Gasse C, Hollowell J, Meier CR, Haefeli WE (2005) Drug interactions and risk of acute bleeding leading to hospitalisation or death in patients with chronic atrial fibrillation treated with warfarin. Thromb Haemost 94: 537–543. [DOI] [PubMed] [Google Scholar]
- 5. Jonsson AK, Spigset O, Jacobsson I, Hagg S (2007) Cerebral haemorrhage induced by warfarin - the influence of drug-drug interactions. Pharmacoepidemiol Drug Saf 16: 309–315. [DOI] [PubMed] [Google Scholar]
- 6. Johnell K, Klarin I (2007) The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf 30: 911–918. [DOI] [PubMed] [Google Scholar]
- 7. Schafer AI (1995) Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol 35: 209–219. [DOI] [PubMed] [Google Scholar]
- 8. Langman MJ, Weil J, Wainwright P, Lawson DH, Rawlins MD, et al. (1994) Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet 343: 1075–1078. [DOI] [PubMed] [Google Scholar]
- 9. Diana FJ, Veronich K, Kapoor AL (1989) Binding of nonsteroidal anti-inflammatory agents and their effect on binding of racemic warfarin and its enantiomers to human serum albumin. J Pharm Sci 78: 195–199. [DOI] [PubMed] [Google Scholar]
- 10. van Dijk KN, Plat AW, van Dijk AA, Piersma-Wichers M, de Vries-Bots AM, et al. (2004) Potential interaction between acenocoumarol and diclofenac, naproxen and ibuprofen and role of CYP2C9 genotype. Thromb Haemost 91: 95–101. [DOI] [PubMed] [Google Scholar]
- 11. Delaney JA, Opatrny L, Brophy JM, Suissa S (2007) Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ 177: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagne JJ, Maio V, Rabinowitz C (2008) Prevalence and predictors of potential drug-drug interactions in Regione Emilia-Romagna, Italy. J Clin Pharm Ther 33: 141–151. [DOI] [PubMed] [Google Scholar]
- 13. Malone DC, Hutchins DS, Haupert H, Hansten P, Duncan B, et al. (2005) Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm 62: 1983–1991. [DOI] [PubMed] [Google Scholar]
- 14. Juel J, Pedersen TB, Langfrits CS, Jensen SE (2013) Administration of tramadol or ibuprofen increases the INR level in patients on warfarin. Eur J Clin Pharmacol 69: 291–292. [DOI] [PubMed] [Google Scholar]
- 15. Scher ML, Huntington NH, Vitillo JA (1997) Potential interaction between tramadol and warfarin. Ann Pharmacother 31: 646–647. [DOI] [PubMed] [Google Scholar]
- 16. Sabbe JR, Sims PJ, Sims MH (1998) Tramadol-warfarin interaction. Pharmacotherapy 18: 871–873. [PubMed] [Google Scholar]
- 17. Klotz U (2003) Tramadol—the impact of its pharmacokinetic and pharmacodynamic properties on the clinical management of pain. Arzneimittelforschung 53: 681–687. [DOI] [PubMed] [Google Scholar]
- 18. de Abajo FJ, Rodriguez LA, Montero D (1999) Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ 319: 1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, et al. (2003) Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding - A population-based cohort study. Archives of Internal Medicine 163: 59–64. [DOI] [PubMed] [Google Scholar]
- 20.Statistiska Centralbyrån (SCB), Statistics Sweden, available from: http://www.scb.se/Pages/List____250612.aspx. Access date November 2011.
- 21. Hassall C, Feetam CL, Leach RH, Meynell MJ (1975) Letter: Potentiation of warfarin by co-trimoxazole. Lancet 2: 1155–1156. [DOI] [PubMed] [Google Scholar]
- 22. Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, et al. (2008) Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther 84: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer HD, Juurlink DN, Mamdani MM, Kopp A, Laupacis A (2010) Hemorrhage during warfarin therapy associated with cotrimoxazole and other urinary tract anti-infective agents: a population-based study. Arch Intern Med 170: 617–621. [DOI] [PubMed] [Google Scholar]
- 24. Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ (2002) Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos 30: 631–635. [DOI] [PubMed] [Google Scholar]
- 25. Washington C, Hou SY, Hughes NC, Campanella C, Berner B (2007) Ciprofloxacin prolonged-release tablets do not affect warfarin pharmacokinetics and pharmacodynamics. J Clin Pharmacol 47: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 26. Israel DS, Stotka J, Rock W, Sintek CD, Kamada AK, et al. (1996) Effect of ciprofloxacin on the pharmacokinetics and pharmacodynamics of warfarin. Clin Infect Dis 22: 251–256. [DOI] [PubMed] [Google Scholar]
- 27. Lane MA, Devine ST, McDonald JR (2012) High-risk antimicrobial prescriptions among ambulatory patients on warfarin. J Clin Pharm Ther 37: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bottiger Y, Laine K, Andersson ML, Korhonen T, Molin B, et al. (2009) SFINX-a drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol 65: 627–633. [DOI] [PubMed] [Google Scholar]
- 29.Marie Eliasson PlPPc (2011) Project leader PASCAL. Personal communication, October, 2011.
- 30.FASS (the Swedish Physicians' Desk Reference), publisher: Läkemedelsindustriföreningen (LIF), access date December 2011.
- 31. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, et al. (2007) The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16: 726–735. [DOI] [PubMed] [Google Scholar]
- 32. Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, et al. (2010) The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol 106: 86–94. [DOI] [PubMed] [Google Scholar]
- 33. Williams D, Kelly A, Feely J (2000) Drug interactions avoided-a useful indicator of good prescribing practice. Br J Clin Pharmacol 49: 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Settergren J, Eiermann B, Mannheimer B (2013) Adherence to drug label recommendations for avoiding drug interactions causing statin-induced myopathy-a nationwide register study. PLoS One 8: e69545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mannheimer B, Wettermark B, Lundberg M, Pettersson H, von Bahr C, et al. (2010) Nationwide drug-dispensing data reveal important differences in adherence to drug label recommendations on CYP2D6-dependent drug interactions. Br J Clin Pharmacol 69: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Battistella M, Mamdami MM, Juurlink DN, Rabeneck L, Laupacis A (2005) Risk of upper gastrointestinal hemorrhage in warfarin users treated with nonselective NSAIDs or COX-2 inhibitors. Arch Intern Med 165: 189–192. [DOI] [PubMed] [Google Scholar]
- 37. Chan FK, Wong VW, Suen BY, Wu JC, Ching JY, et al. (2007) Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet 369: 1621–1626. [DOI] [PubMed] [Google Scholar]
- 38. Bjerrum L, Rosholm JU, Hallas J, Kragstrup J (1997) Methods for estimating the occurrence of polypharmacy by means of a prescription database. Eur J Clin Pharmacol 53: 7–11. [DOI] [PubMed] [Google Scholar]
- 39. Lau HS, de Boer A, Beuning KS, Porsius A (1997) Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol 50: 619–625. [DOI] [PubMed] [Google Scholar]
- 40. Mannheimer B, Ulfvarson J, Eklof S, Bergqvist M, von Bahr C (2008) A clinical evaluation of the Janus Web Application, a software screening tool for drug-drug interactions. Eur J Clin Pharmacol 64: 1209–1214. [DOI] [PubMed] [Google Scholar]
- 41. Glassman PA, Belperio P, Simon B, Lanto A, Lee M (2006) Exposure to automated drug alerts over time: effects on clinicians' knowledge and perceptions. Med Care 44: 250–256. [DOI] [PubMed] [Google Scholar]
- 42. Glassman PA, Simon B, Belperio P, Lanto A (2002) Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care 40: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 43. Ahearn MD, Kerr SJ (2003) General practitioners' perceptions of the pharmaceutical decision-support tools in their prescribing software. Med J Aust 179: 34–37. [DOI] [PubMed] [Google Scholar]
- 44. Rane A, Lindh JD (2010) Pharmacogenetics of anticoagulants. Hum Genomics Proteomics 2010: 754919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, et al. (2003) Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 163: 59–64. [DOI] [PubMed] [Google Scholar]
- 46. Ghaswalla PK, Harpe SE, Tassone D, Slattum PW (2012) Warfarin-antibiotic interactions in older adults of an outpatient anticoagulation clinic. Am J Geriatr Pharmacother 10: 352–360. [DOI] [PubMed] [Google Scholar]