Abstract
Objective
Quantified risks of congenital Toxoplasma gondii infection and abnormal pregnancy outcomes following primary maternal infection were evaluated with meta- analysis based on published studies.
Methods
The related literatures were searched in multiple literature databases regardless of languages. Odds ratio (OR) and 95% confidence interval (CI) were used to evaluate the risks of vertical transmission of Toxoplasma gondii and abnormal pregnancy outcomes following primary maternal infection with meta-analysis.
Results
53 of the 2632 searched literatures were included in our analysis. The incidence of abnormal pregnancy outcomes in T. gondii infected pregnant women (infected group) was significantly higher than that in the uninfected pregnant women (control group) (OR = 5.10; 95% CI, 3.85–6.75). Toxoplasma gondii infection rate in the abnormal-pregnancy-outcome group was significantly higher than in the normal-pregnancy group (OR = 3.71; 95% CI, 3.31–4.15). The pooled rate of vertical transmission was 20% (95% CI, 15%–26%) in maternal infection of T. gondii. The incidences of vertical transmission in women who were infected in the first, second or third trimester of pregnancy were 5% (95%CI, 2%–16%), 13% (95%CI, 7%–23%), and 32% (95%CI, 24%–41%), respectively. The rates of vertical transmission in women who were treated with spiramycin-only, PSF (pyrimethamine + sulfadiazine + folinic acid) or PS (pyrimethamine + sulfadiazine) combined with spiramycin, or other untypical treatments were 13% (95%CI, 7%–22%), 13%(95%CI, 7%–25%), and 24%(95%CI, 18%–32%), respectively.
Conclusions
Toxoplasma gondii infection can result in adverse pregnancy outcomes in pregnant women. The pooled rate of vertical transmission was 20% in maternal infection and the incidences of vertical transmission increased in the first, second or third trimester of pregnancy. The pooled rates of transmission in groups treated with spiramycin-only, PSF or PS combined with spiramycin, or other untypical treatments were not significantly different.
Introduction
Toxoplasma gondii is an intracellular protozoan parasite which is highly prevalent in humans and animals [1]. A wide variety of warm-blooded animals, including humans, can serve as the intermediate hosts of T. gondii, but its definitive host is limited to domestic cats and other felids [1], [2]. People become infected by ingestion of T. gondii tissue cysts in infected meat or by ingestion of infective oocysts shed by cats in contaminated food or water [3]. Primary infection of T. gondii in pregnant women can cause vertical transmission of the parasite and result in miscarriage, stillbirth, premature birth, malformations and other adverse pregnancy outcomes. Children with congenital toxoplasmosis may exhibit clinical signs of hydrocephalus, mental retardation, eye disease and other severe sequelae [4], [5]. Currently, congenital toxoplasmosis is believed to be the second most common seen fetal intrauterine infection [6]. Additionally, according to Torgerson and Mastroiacovo's study, the global annual prevalence of congenital toxoplasmosis was estimated to be 190 100 cases (95% confidence interval, CI: 179 300–206 300), which means the global burden of congenital toxoplasmosis was 1.20 million disability-adjusted life years (DALYs) (95% CI: 0.76–1.90) [7]. Hence, the poor health condition of children with congenital toxoplasmosis contributes to the heavy global health burden of children.
Women are usually symptomless when they acquire T. gondii infection in pregnancy. If maternal infection is detected, the mother usually receives treatment for toxoplasmosis and the fetus will face the risk of congenital infection. For treatment of T. gondii infection in pregnant women, the most commonly used drug is spiramycin because it can be absorbed efficiently and has little side effects to the fetus [8]. It is generally recommended to treat Toxoplasma infection with spiramycin in early trimesters, then change to PSF in the later trimesters [9].
Several studies have investigated the relationship between T. gondii infection and adverse pregnancy outcomes and the vertical transmission rate of T. gondii, but the parameters and methods used varied greatly in these studies. Because this is an extremely important health care issue, we used meta-analysis to evaluate the risks of vertical transmission and abnormal pregnancy outcomes in women experiencing primary infection with T. gondii during pregnancy.
Materials and Methods
Search strategy
Our study was performed according to the recommendations of the PRISMA Statement [10], which is available in (Checklist S1). We searched Pubmed, Embase, Google scholar, ScienceDirect, and CNKI database, Chongqing VIP database, Wanfang academic journal full-text database for papers published up to May 2013. Studies were identified using combinations of the following search terms regardless of languages: “Toxoplasma OR gondii OR toxoplasmosis” AND “pregnancy infection” AND “adverse pregnancy outcome OR abortion OR stillbirth OR abnormality OR fetal growth restriction OR FGR OR intrauterine growth retardation OR IUGR”.
Literature citation inclusion and exclusion criteria
The literature citations were screened according to the following criteria. Inclusion criteria: (i) a case-control or cohort study or a survey with cases collected from clinical notes that related to our theme; (ii) the women in the control group were non-Toxoplasma-infected pregnant women and they were located in the same area as the women in the case group; (iii) the diagnosis of maternal T. gondii infection was based on seroconversion, parasite observation from cell culture or mouse ascites after inoculation of maternal blood, or PCR test of parasite DNA during gestation; (iv) the diagnosis of congenital Toxoplasma infection met one of the following standards: A. persistence of specific IgG in the child beyond 12 months or reappearance of IgG antibodies after cessation of antibiotic therapy, B. Toxoplasma specific IgM and/or IgA in cord blood and/or in neonatal blood (the purity of fetal blood was ascertained or the positive results were confirmed at least 7–10 days later), C. presence of parasite in amniotic fluid, placenta or fetal blood confirmed by inoculation to mice ascites, cell culture, or by PCR test. Literatures were excluded in the studies if (i) the paper was a review or a descriptive study; (ii) its subjects were not human beings but animals; (iii) the data was duplicate or the study only presented the final result without the raw data; (iv) the sample contained less than 40 participants or the number of participants in different groups was less than 10.
Data extraction
The following information was extracted from each study: first author, publication year, location of the study, demographic characteristics, the number of cases and controls, diagnostic methods of cases, treatment regimes of the infected women, pregnancy outcomes, and gestational age of infection. In some studies, not all of the data were extracted because a portion of the data had already been reported. And for the republished studies, only the most complete or recent study was included. Two reviewers independently collected the data and reached a consensus after a discussion on the literatures which were controversial.
Statistical analysis
The risk of T. gondii infection and various adverse pregnancy outcomes was estimated by odds ratio (OR) with the corresponding 95% confidence interval (95%CI). The pooled proportion of vertical transmission of toxoplasmosis with the corresponding 95%CI was calculated as well. It was considered statistically significant when P<0.05. In the forest plots, OR>1 represented a risk effect and OR<1 represented a protective effect. Statistical heterogeneity of results was appraised using a χ2-based Q test and I2 statistic [11]. Only when P>0.10 and I2<50% was the heterogeneity considered not significant. The fixed-effects model was used when literature heterogeneity not existed; otherwise, the random-effects model was employed. Sensitivity analysis was conducted by modification of the inclusion criteria of this meta-analysis. The pooled proportion of vertical transmission of toxoplasmosis was calculated by Meta-Analysis Beta 3.13 software (Tufts Medical Center, Boston, MA). The other analyses were conducted using Stata software version 11.0 (Stata Corporation, College Station, TX, USA) and the publication bias was considered significant when P value was less than 0.05 in either Begg's test or Egger's test [12].
Results
Studies characteristics
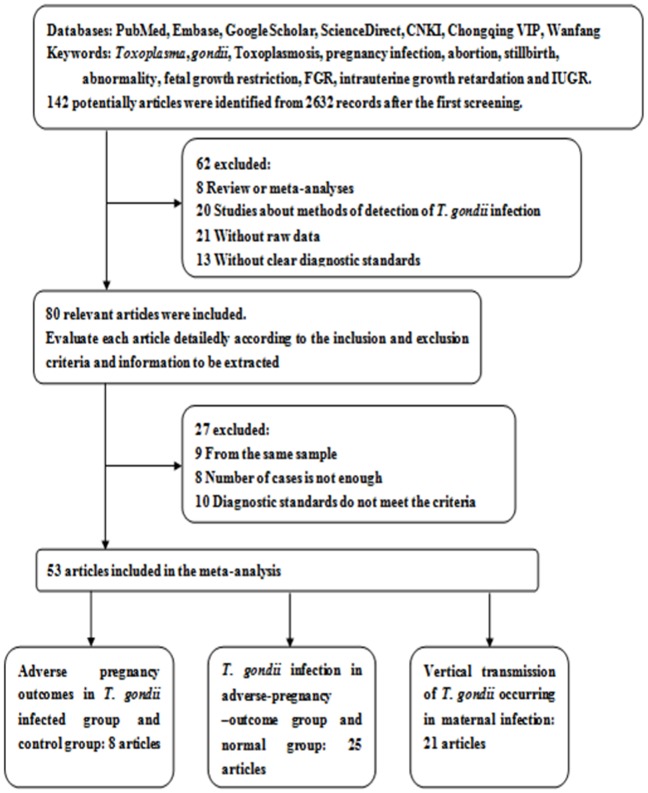
From the 2632 searched literatures, 53 were included and the results from these literatures were weighted [13]–[64],[74], including 8 studies about adverse pregnancy outcomes when the mother was infected with T. gondii and with control groups for each [13]–[20], 25 studies about infection rate in adverse outcomes and normal groups [21]–[44], [74], 21 studies about vertical transmission of the parasite [15], [45]–[64] (Figure 1). Further, 7 papers provided the detailed information about the gestation age when the woman was infected [46], [48], [49], [55], [58], [61], [64]. Additionally, two papers involved mothers that gave birth to twins [51], [53]. Some women received prenatal treatment in some studies [45], [47], [48], [50], [51], [53]–[55], [58], [59], [61], [64]. Details about the first author, published year, area, diagnostic standard, number of cases and controls and treatment regimes in each literature were listed in Tables 1, 2, 3 and 4.
Figure 1. Flow diagram of the selection of the studies.
Table 1. Studies about abnormal pregnancy outcomes in T.gondii infected groups and control groups.
| First author | Year | Area | Cases/Controls# | Diagnosis of Maternal Infection | Abortion* | Premature Birth* | Fetal Anomaly* | FGR* | Stillbirth* | Reference |
| Su CK | 2002 | Guangxi | 64/932 | Positive IgM | - | 0.06/0.02 | 0.08/0.01 | - | 0.03/0.01 | [13] |
| Wen LZ | 2003 | East China | 95/117 | Positive IgM | 0.13/0.03 | 0.04/0.02 | 0.03/0.01 | 0.04/0.02 | 0.05/0.01 | [14] |
| Liu J | 2004 | Shanxi | 76/986 | Positive IgM and/or PCR | 0.11/0.02 | 0.04/0.05 | - | 0.05/0.01 | 0.07/0.01 | [15] |
| Yan Q | 2006 | Guangdong | 64/932 | Positive IgM and PCR | - | 0.06/0.02 | 0.05/0.01 | - | 0.13/0.01 | [16] |
| Yuan WY | 2009 | Hebei | 325/147 | Positive IgM | 0.07/0.02 | 0.09/0.01 | 0.07/0.02 | - | 0.06/0.01 | [17] |
| Suo QL | 2011 | Hubei | 775/629 | Positive IgM | 0.07/0.01 | 0.02/0.01 | 0.03/0.01 | 0.03/0.01 | 0.03/0.01 | [18] |
| Wang J | 2011 | Liaoning | 149/5537 | Positive IgM | 0.09/0.01 | - | 0.08/0.01 | - | 0.03/0.01 | [19] |
| Fang L | 2012 | Heilongjiang | 273/496 | Positive IgM | 0.18/0.02 | 0.29/0.04 | - | - | - | [20] |
Notes: #Cases, Toxoplasma-infected pregnant women, Controls, Non-infected pregnant women;* the data before and after the slash represent the rate of adverse pregnancy outcome in T.gondii infection groups and uninfected groups; - no statistics; “FGR”, fetal grown restriction.
Table 2. Studies about T.gondii infection rate in abnormal pregnancy and normal pregnancy.
| First author | Year | Area | Diagnosis of Maternal Infection | Cases/Controls# | Infection rate* | Reference |
| Sahwi SY | 1995 | Bristol | Positive IgM and/or IgA | 100/40 | 0.19/0.08 | [42] |
| Moyo SR | 1995 | Zimbabwe | Positive culture | 104/96 | 0.36/0.13 | [41] |
| Zhang Y | 2002 | Tianjing | Positive PCR | 1135/7141 | 0.01/0.00 | [21] |
| Yang QF | 2003 | Guizhou | Positive IgM | 86/100 | 0.07/0.02 | [22] |
| Laila N | 2004 | Grenoble | Positive PCR | 148/100 | 0.14/0 | [44] |
| Cao MG | 2004 | Shandong | Positive IgM | 1546/3568 | 0.09/0.01 | [23] |
| Hu CM | 2004 | Guangdong | Positive IgM | 101/1282 | 0.15/0.08 | [25] |
| Chen HM | 2004 | Hubei | Positive IgM | 476/562 | 0.13/0.05 | [24] |
| Wei SZ | 2005 | Fujian | Positive IgM | 117/1695 | 0.13/0.05 | [26] |
| Yang AJ | 2005 | Shandong | Positive PCR | 380/152 | 0.21/0.04 | [27] |
| Ye HZ | 2005 | Guangdong | Positive IgM | 93/944 | 0.03/0.00 | [28] |
| Chen MR | 2006 | Shandong | Positive IgM | 1546/3568 | 0.15/0.03 | [29] |
| Li BY | 2006 | Guangdong | Positive IgM | 48/48 | 0.33/0.04 | [30] |
| Xie DC | 2006 | Guangxi | Positive IgM | 502/400 | 0.14/0.06 | [31] |
| Chen XJ | 2007 | Jilin | Positive IgM | 200/1805 | 0.24/0.07 | [32] |
| Guo EP | 2008 | Hubei | Positive IgM | 71/819 | 0.14/0.03 | [33] |
| Zhan HY | 2008 | Jiangsu | Positive IgM | 197/200 | 0.10/0.02 | [34] |
| Weng H | 2009 | Zhejiang | Positive IgM | 89/102 | 0.20/0.05 | [35] |
| Janak K | 2011 | Lucknow | Positive IgM | 60/29 | 0.08/0 | [43] |
| Long C | 2011 | Hubei | Positive IgM | 402/3449 | 0.03/0.00 | [36] |
| Qiu JZ | 2011 | Hunan | Positive IgM | 193/512 | 0.06/0.01 | [37] |
| Wang JY | 2011 | Hebei | Positive IgM | 102/102 | 0.13/0.12 | [38] |
| Wang KB | 2012 | Sichuan | Positive IgM | 126/1430 | 0.13/0.04 | [40] |
| Munmun DS | 2012 | India | Positive IgM | 105/105 | 0.22/0.03 | [39] |
| Aljumaili ZKM | 2013 | Iraq | Positive IgM | 293/245 | 0.02/0 | [74] |
Notes: # the data before and after the slash represent the sample in abnormal pregnancy group and normal pregnancy group;* the data before and after the slash represent the T.gondii infection rate in abnormal pregnancy group and normal pregnancy group.
Table 3. Studies about the rate of vertical transmission when mother got infected in pregnancy.
| First author | Year | Area | Diagnostic Standards | Rate* | Reference | |
| Mother | Baby# | |||||
| Berrebi A | 1994 | Toulouse | Seroconversion | Positive IgM, PCR or culture, clinical signs | 0.17 | [56] |
| Pratlong F | 1994 | Montpellier | Seroconversion, high-titre IgG with IgM | Positive IgM and IgA, culture | 0.11 | [50] |
| Hohlfeld P | 1994 | Paris | Seroconversion | Positive IgM, PCR or culture | 0.07 | [54] |
| Dar FK | 1997 | UAE | High-titre IgM | Positive IgM | 0.38 | [52] |
| Jenum A | 1998 | Norway | Seroconversion | Persistent IgG beyond 12 months, positive PCR or culture | 0.23 | [64] |
| Gratzl R | 1998 | Austria | Seroconversion, high-titre IgG and IgM | Persistent IgG beyond 12 months, positive PCR | 0.22 | [51] |
| Foulon W | 1999 | France | Seroconversion | Persistent IgG beyond 12 months, reappearance of IgG after therapy | 0.44 | [57] |
| Robert-Gangneux F | 1999 | Paris | Seroconversion | Persistent IgG beyond 12 months, positive PCR or culture | 0.25 | [59] |
| Naessens A | 1999 | America | Seroconversion | Persistent IgG beyond 12 months, reappearance of IgG after therapy | 0.32 | [57] |
| Lebech M | 1999 | Denmark | Seroconversion | Persistent IgG beyond 12 months, positive IgM and/or IgA | 0.19 | [49] |
| Gilbert R | 2001 | EUR,Austria | Seroconversion | Persistent IgG beyond 12 months, positive PCR or culture | 0.24 | [62] |
| Antsaklis A | 2002 | Athens | Seroconversion | Positive IgM, PCR or culture | 0.19 | [60] |
| Logar J | 2002 | Ljubljana | High-titre IgG, high-titre IgM and/or IgA | Positive IgM and IgA | 0.11 | [61] |
| Ricci M | 2003 | Italy | Seroconversion, high-titre IgG and IgM | Persistent IgG beyond 12 months | 0.11 | [55] |
| Mombro M | 2003 | Italy | Seroconversion, positive cultures | Persistent IgG beyond 12 months, reappearance of IgG after therapy, specific IgM and/or IgA | 0.22 | [46] |
| Liu J | 2004 | China | Positive PCR, high-titre IgM | Positive PCR | 0.37 | [15] |
| Di Carlo P | 2005 | Italy | Seroconversion | Persistent IgG beyond 12 months, positive PCR | 0.19 | [58] |
| Buffolano W | 2005 | Campania | Seroconversion | Persistent IgG beyond 12 months | 0.34 | [63] |
| Berrébi A | 2010 | Toulouse | Seroconversion | Persistent IgG beyond 12 months | 0.17 | [53] |
| Hotop A | 2012 | Germany | Seroconversion | Persistent IgG beyond 12 months, positive PCR | 0.05 | [48] |
| Wallon M | 2013 | Lyon | Seroconversion, high-titre IgG and IgM | Persistent IgG beyond 12 months, positive culture | 0.25 | [45] |
Notes:# For the positive IgM/IgA results, the purity of fetal blood was ascertained or the positive results were confirmed at least 7-10 days later; * Rate stands for vertical transmission rate caused by T.gondii infection.
Table 4. Studies about the rate of vertical transmission when infected mother got treatment in pregnancy.
| First Author | Year | Treatment | Infected Mother | Infected Baby | Rate | Reference |
| Pratlong F | 1994 | Spir-only | 190 | 20 | 0.11 | [50] |
| Hohlfeld P | 1994 | Spir-only | 2632 | 194 | 0.07 | [54] |
| Gratzl R | 1998 | Spir-only | 12 | 1 | 0.08 | [51] |
| PSF/Spir | 37 | 10 | 0.27 | [51] | ||
| Jenum A | 1998 | PS/Spir | 47 | 11 | 0.23 | [64] |
| Robert-Gangneux F | 1999 | Spir-only | 110 | 27 | 0.25 | [59] |
| Naessens A | 1999 | Others1 | 294 | 93 | 0.32 | [47] |
| Logar J | 2002 | PSF/Spir | 100 | 11 | 0.11 | [61] |
| Ricci M | 2003 | PSF/Spir | 141 | 16 | 0.11 | [55] |
| Buffolano W | 2005 | Spir-only | 74 | 12 | 0.16 | [58] |
| Berrébi A | 2010 | Others2 | 666 | 112 | 0.17 | [53] |
| Hotop A | 2012 | PSF/Spir | 685 | 33 | 0.05 | [48] |
| Wallon M | 2013 | Others3 | 2048 | 513 | 0.25 | [45] |
Notes: Spir-only, spiramycin only; PS/Spir, PS in combination with spiramycin; PSF/Spir, PSF in combination with spiramycin; Others, other untypical treatment, 1 only 75% of infected women were administered to antibiotic treatment, the rest were conducted with other medicine; 2 80% of infected women were administered to pyrimethamine-sulfadoxine, 20% were taken with spiramycin; 3 PS alternated every 3 weeks with spiramycin before 1996, and then PS was taken continually.
Quantitative synthesis and heterogeneity analysis
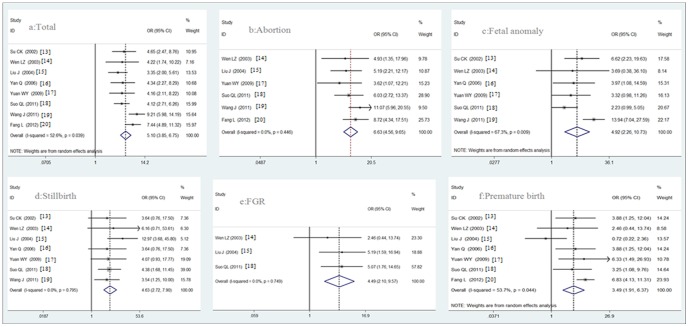
1. Comparison of the abnormal pregnancy chances between T. gondii infected and uninfected pregnant women. The prevalence of abnormal pregnancy outcomes in T. gondii infected pregnant women (infected group) was significantly higher than in the uninfected pregnant women (control group) (P<0.05); the OR was 5.10 (95% CI, 3.85–6.75) analyzed with the random-effects model. Among these abnormal pregnancy outcomes, the prevalence of abortion, fetal anomaly, stillbirth, FGR (fetal growth restriction), and premature birth were all significantly higher in the infected group than that in the control group (P<0.05), with OR and 95% CI of 6.63 (4.56–9.65), 4.92 (2.26–10.73), 4.63 (2.72–7.90), 4.49 (2.10–9.57), and 3.49 (1.91–6.37), respectively (Figure 2). The detail analysis results were shown in Table 5.
Figure 2. Forest plot of the relationship between T.gondii infection and adverse pregnancy outcomes.
a, The odds ratio of the total abnormal pregnancy chance between Toxoplasma infected and uninfected pregnant women; b-f, The odds ratio of the different abnormal pregnancy outcomes between Toxoplasma infected and uninfected pregnant women. Scale: for value of odds ratio.
Table 5. Analysis results of the relationship between maternal T.gondii infection and adverse pregnancy outcomes.
| Outcomes | Test of risk | Test of heterogeneity | Model | Reference | |||
| Odds Ratio (95%CI) | P | Q | P | I2 (%) | |||
| Abortion | 6.63 (4.56 to 9.65) | p<0.0001 | 4.76 | 0.04 | <0.01 | Fixed-effects model | [14], [15], [17]–[20] |
| Fetal anomaly | 4.92 (2.26 to 10.73) | p<0.0001 | 15.30 | 0.01 | 67.3 | Random-effects model | [13], [14], [16]–[19] |
| Stillbirth | 4.63 (2.72 to 7.90) | p<0.0001 | 3.11 | 0.80 | <0.01 | Fixed-effects model | [13]–[19] |
| FGR | 4.49 (2.10 to 9.57) | p<0.0001 | 0.58 | 0.75 | <0.01 | Fixed-effects model | [14], [15], [18] |
| Premature birth | 3.49 (1.91 to 6.37) | p<0.0001 | 12.95 | 0.04 | 53.7 | Random-effects model | [13]– |
| Total | 5.10 (3.85 to 6.75) | p<0.0001 | 14.76 | 0.04 | 52.6 | Random-effects model | [13]–[20] |
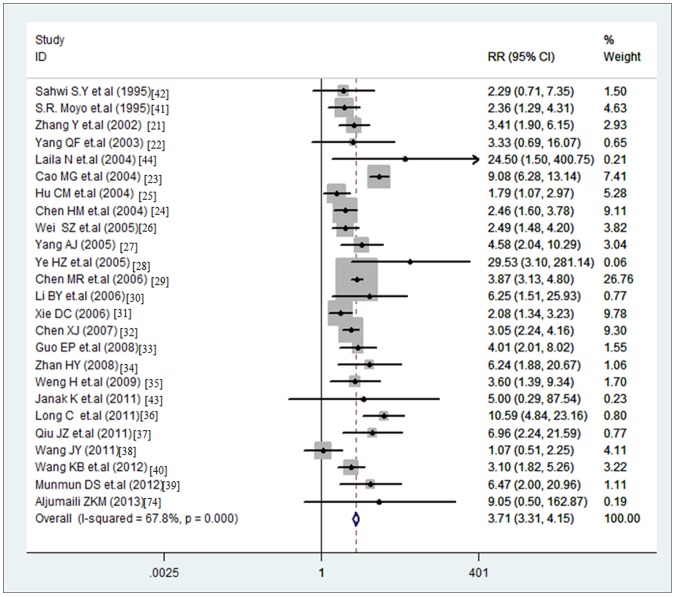
2. Comparison of T. gondii infection rate between abnormal pregnancy and normal pregnancy groups. The Toxoplasma gondii infection rate of abnormal-pregnancy-outcome group was significantly higher than in the normal-pregnancy group (P<0.05), with an OR of 3.71 (95% CI, 3.31–4.15) analyzed with the random-effects model (Q = 74.62, p<0.0001, I2 = 67.8%) (Figure 3).
Figure 3. Odds ratio of Toxoplasma infection rate between abnormal pregnancy and normal pregnancy.
Scale: for value of odds ratio.
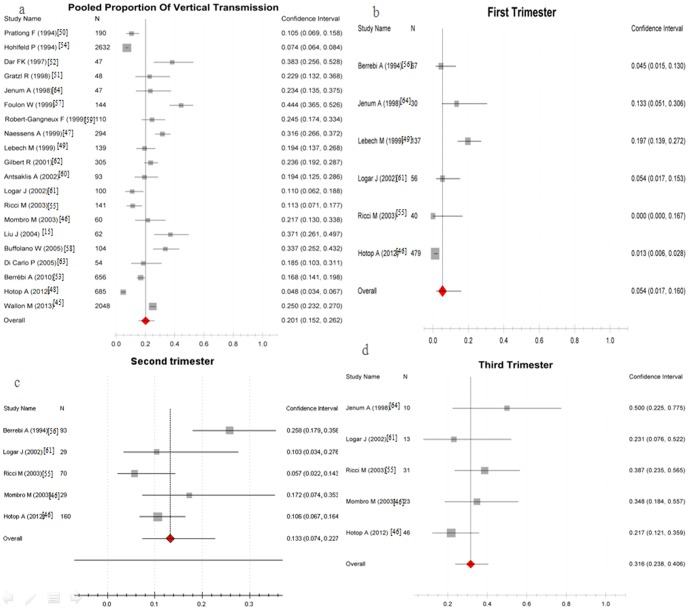
3. Chance of congenital T. gondii transmission occurring in maternal infection. The rate of congenital transmission of T. gondii in maternal infection was 20% (95% CI, 15%–26%), which suggested that about 20% of infected mothers would transmit the parasite to fetus. The rate of vertical transmission in women who were infected in the first, second or third trimesters of pregnancy were 5% (95%CI, 2%–16%), 13% (95%CI, 7%–23%), and 32% (95%CI, 24%–41%), respectively (Figure 4). The detailed analysis results are shown in Table 6.
Figure 4. Proportion of congenital toxoplasmosis happening by mother infection.
a, The rate of vertical transmission when mother got infected in pregnancy; b-d, The rate of vertical transmission in different pregnancy trimester. Scale: incidence of congenital toxoplasmosis.
Table 6. Analysis results of the rate of vertical transmission when mother got infected in different trimester.
| Time | Test of risk | Test of heterogeneity | Model | Reference | |||
| Pooled Proportion (95%CI) | P | Q | P | I2 (%) | |||
| First trimester | 0.05 (0.02 to 0.16) | <0.0001 | 0.979 | <0.001 | 47.2 | Random-effects model | [48], [49], [55], [56], [61], [64] |
| Second trimester | 0.13 (0.07 to 0.28) | <0.0001 | 0.939 | 0.004 | 42.5 | Random-effects model | [46], [48], [55], [56], [61] |
| Third trimester | 0.32 (0.24 to 0.41) | <0.0001 | 0.827 | 0.237 | 13.9 | Fixed-effects model | [46], [48], [55], [61], [64] |
| Total pregnancy | 0.20 (0.15 to 0.26) | <0.0001 | 0.998 | <0.001 | 49.0 | Random-effects model | [15], [45]–[64] |
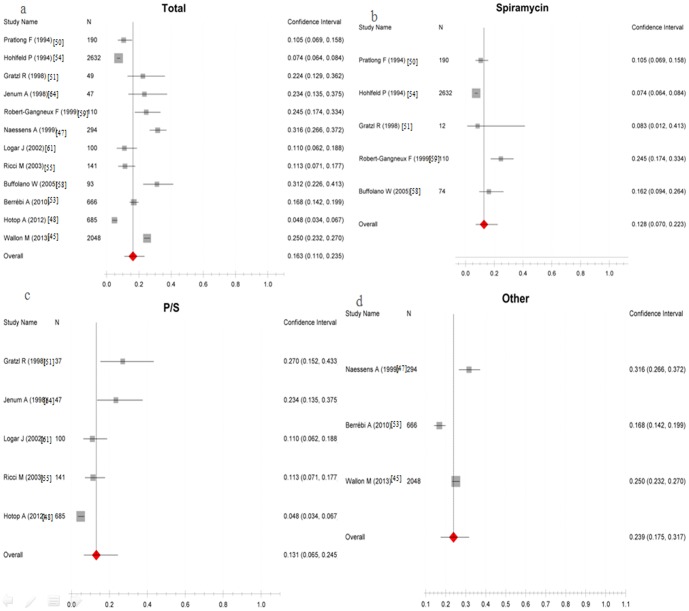
The pooled rate of congenital transmission of T. gondii occurring in women who received treatment was 16% (95% CI, 11%–24%), which suggested that about 16% of treated infected mothers would transmit the parasite to fetus. The rate of vertical transmission in women who received Spiramycin-only, PSF or PS in combination with spiramycin, or other untypical treatment were 13%(95%CI, 7%–22%), 13%(95%CI, 7%–25%), and 24%(95%CI, 18%–32%), respectively (Figure 5). The detailed analysis results are shown in Table 7.
Figure 5. Proportion of congenital toxoplasmosis happening when infected mother received prenatal treatment.
a, The total rate of vertical transmission when mother received treatment; b-d, The rate of vertical transmission when mother received different treatment regimes. Scale: incidence of congenital toxoplasmosis.
Table 7. Analysis results of the vertical transmission rate when infected mother got treatment in pregnancy.
| Treatment | Test of risk | Test of heterogeneity | Model | Reference | |||
| Pooled Proportion (95%CI) | P | Q | P | I2 (%) | |||
| Spir-only | 0.128 (0.070 to 0.223) | <0.0001 | 0.977 | <0.001 | 47.5 | Random-effects model | [50], [51], [54], [58], [59] |
| P/S | 0.131 (0.065 to 0.245) | <0.0001 | 0.975 | <0.001 | 47.3 | Random-effects model | [48], [51], [55], [61], [64] |
| Others | 0.239(0.175 to 0.317) | <0.0001 | 0.967 | <0.001 | 48.2 | Random-effects model | [45], [47], [53] |
| Total | 0.163 (0.110 to 0.235) | <0.0001 | 0.997 | <0.001 | 49.3 | Random-effects model | [45], [47], [48], [50], [51], [53]–[55], [58], [59], [61], [64] |
Notes: Spir-only, spiramycin only; P/S, PS or PSF in combination with spiramycin; Others, other untypical treatment.
Sensitivity analysis
A sensitivity analysis was conducted to ascertain whether modification of the inclusion criteria of this meta-analysis affected the final results. On the analysis of the association between T. gondii infection and the abnormal pregnancy outcomes, the sensitivity analysis was carried out by excluding one single study each time and limiting the meta-analysis to studies with sample size more than 100. All the results were not materially altered.
Publication bias
For prevalence of abortion between infected groups and uninfected groups and the T. gondii infection rate between abnormal pregnancy and normal pregnancy, the publication bias showed statistical significance (Begg's test, p = 0.060.0.059; Egger's test, p = 0.025,0.516) (Figure 6). In the other analysis, no publication bias was suggested.
Figure 6. Funnel plot showing publication bias.
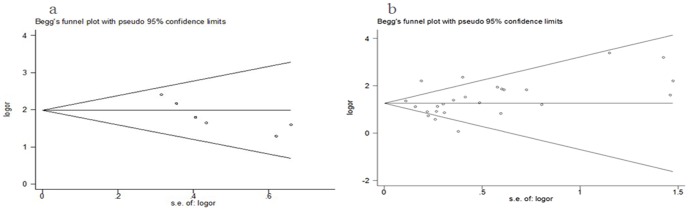
a, in group of abortion and T. gondii infection; b, in group of infection rate in normal and abnormal pregnancy outcomes.
Discussion
Several studies have investigated the relationship between maternal infection with T. gondii and the adverse pregnancy outcomes including miscarriage, stillbirth, premature birth, and malformations. Our meta-analysis results confirmed this relationship and showed that miscarriage was the highest risk (OR = 6.63; 95% CI, 4.56–9.66) among the adverse pregnancy outcomes. Furthermore, a population-based study on the effects of congenital toxoplasmosis found out that infected babies were born or delivered earlier than uninfected babies, but the mechanism leading to a shorter length of gestation is unknown [65]. Additional studies are needed to determine whether adverse pregnancy outcomes after acquisition of T. gondii infection are related to a consequence of fetal infection or an effect of maternal infection. Additionally, mechanism of T. gondii causes placental inflammation and infects the fetus remains unknown.
Our study also showed that later infection during pregnancy was more likely to result in congenital infection, which was consistent with Dunn's and Foulon's studies, but the 30% vertical transmission rate in the third trimester of pregnancy in our meta analysis was much lower than that of 60% in their studies [57], [66], which possibly resulted from the small sample in their studies. Children with congenital Toxoplasma infection had more severe clinical symptoms when the mother acquired acute T. gondii infection during the first trimester than in the third trimester [54], [67], [68]. This may be due to the placental trophoblast, which is not conductive to the propagation of T. gondii and could prevent the parasite from crossing the placenta in early gestation [69]. But in later trimesters, the parasite is more likely to get through the placental barrier, so transmission is more frequent in later pregnancy than in earlier pregnancy. If the infection occurred in the first trimester, owing to the immature development and the low resistance of the fetus, the prevalence of sequelae may be higher than the infection happened in a later trimester [69], [70].
The rate of vertical transmission in women who were treated with spiramycin only, PSF or PS in combination with spiramycin, were 13% (95%CI, 7%–22%), and 13% (95%CI, 7%–25%), respectively. Comparing to Lebech M's study, the transmission rate of untreated pregnant women was 19% (95%CI, 13%–27%) [49], so we speculated that there was a low risk of vertical transmission in treated women with Toxoplasma infection during pregnancy. However, the effect of the prenatal treatment remains vague as there was no clear evidence from biological studies that prenatal treatment would reduce the risk of congenital infection. To prove whether the treatment regimes have a significant impact on pregnancy outcome, a clinical study with a large sample and an untreated comparison group is needed.
To avoid unnecessary drug therapy and pregnancy termination, much effort had been put to find an effective, quick, safe and cheap method for prenatal diagnosis of maternal infection. Now it is available through PCR on amniotic fluid, which was confirmed to be the most reliable method with high sensitivity and high specificity [54], [71]. Moreover, in Austria, apart from the routine prenatal maternal T. gondii serology screening, the identification of T. gondii infection is significantly improved by the additional maternal and/or fetal serological testing at birth [72]. Many countries have adopted the prenatal screening program and it has been proved to be effective in France at reducing the rate of congenital infection [73].
In order to provide precise and updated information for T. gondii infected pregnant women with clinical counseling, this study adopted the strict diagnostic criteria to screen the cases in each literature citation. However, our meta-analysis still has several limitations. First, on analyses the risks of T. gondii infected women with abnormal pregnancy outcomes, most studies involved are from China owing to few equivalent foreign studies. Second, as only a few studies provided the exact gestation age at maternal infection, so the pooled vertical transmission rate of congenital toxoplasmosis was calculated based on the trimester of pregnancy rather than weeks. Third, the diagnostic methods of infected mother/fetal in different literature citations were not adopted uniformly, which may increase the source of the heterogeneity.
Conclusions
This meta-analysis confirms the previous results that primary maternal infection of T. gondii during gestation plays a crucial role in adverse pregnancy outcomes. The incidences of abortion, fetal anomaly, stillbirth, fetal growth restriction, and premature birth were significantly higher in the infected group than in the control group, and showed in declining Odds Ratios. Reversely, Toxoplasma gondii infection rate in the abnormal-pregnancy-outcome group was significantly higher than in the normal-pregnancy group. The pooled rate of vertical transmission was 20% in maternal infection and the incidences of vertical transmission increased with the pregnancy time (the first, second or third trimester of pregnancy). Compared to the vertical transmission rate of 32% in the third trimester, the rate (5%) was much lower when the infection occurred in the first trimester. The pooled rate of vertical transmission in maternal infection received treatment was 16%, and the rates of treatment with spiramycin-only, PSF or PS combined with spiramycin, or other untypical treatments were not significantly different.
Supporting Information
PRIMSA checklist of this meta-analysis.
(DOC)
Funding Statement
Supported by the funding of National Natural Science Foundation of China (No. 81071377, 81271866), the Research Fund for the Doctoral Program of Higher Education of China (20104433120014), Guangdong Province talent introduction of special funds (2011-67), Guangdong provincial key scientific and technological project (2011B010500003), the Guangdong Province College Students Renovation Experimental Program (1212111020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dubey JP (2010) Toxoplasmosis of Animals and Humans, Second Edition. Taylor & Francis.
- 2. Dubey JP (2009) History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol 39: 877–882. [DOI] [PubMed] [Google Scholar]
- 3. Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363: 1965–1976. [DOI] [PubMed] [Google Scholar]
- 4. Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R (2001) Congenital toxoplasmosis: a review. Obstet Gynecol Surv 56: 296–305. [DOI] [PubMed] [Google Scholar]
- 5. Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG (2011) Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 30: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 6. Bojar I, Szymanska J (2010) Environmental exposure of pregnant women to infection with Toxoplasma gondii—state of the art. Ann Agric Environ Med 17: 209–214. [PubMed] [Google Scholar]
- 7. Torgerson PR, Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desmonts G, Couvreur J (1974) Toxoplasmosis in pregnancy and its transmission to the fetus. Bull N Y Acad Med 50: 146–159. [PMC free article] [PubMed] [Google Scholar]
- 9. Robert Koch Institute (2007) Guideline toxoplasmosis. Epidemiol Bull 42: 390–4. [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097 doi:10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 12. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su CK, Wu ZB, Guo XB, Su PJ, Ma G (2002) The effects of toxoplasma infection on pregnancy in woman. Chinese journal of birth health & heredity 10: 78–79. [Google Scholar]
- 14. Wen LZ, Liu LQ, Ao LM, Chen SH, Zeng WJ, et al. (2003) Effect of toxoplasma infection during pregnancy to the development of fetus and infant. Chin J Obstet Gynecol 38: 331–333. [PubMed] [Google Scholar]
- 15. Liu J, Zhang L, Zhao XL, Ye GL, Cai XN, et al. (2004) Study on fetuses and infants' abnormalities induced by toxoplasmosis infection in pregnancy. Chinese journal of child health care 12: 313–315. [Google Scholar]
- 16. Yan Q, Zhang HY (2006) The effects of toxoplasma infection during pregnancy on fetus. Modern Medicine & Health 22: 1654–1655. [Google Scholar]
- 17. Yuan WY, Liu XH, Yu CY, Zhao S, Zhao Q, et al. (2009) Follow up and observation on effect of toxoplasma gondii infection during pregnancy on pregnant outcomes and live—born infants. Maternal & child health care of China 24: 4416–4417. [Google Scholar]
- 18. Suo QL, Liu SW, Yao T (2011) Study of abnormal pregnancy outcome among toxoplasma IgM-positive pregnant women in Wuhan. Modern preventive medicine 38:863–865, 868 [Google Scholar]
- 19. Wang J, Yang JC, Ding XP (2011) The relation between toxoplasma infection and poor pregnancy outcomes in pregnant women. Journal of Shenyang Medical College 13: 81–83. [Google Scholar]
- 20. Fang L, Li PL, Cui R (2012) An investigation on the influence of pregnant women infected with Toxoplasma gondii during the early pregnancy. Chinese journal of birth health & heredity 20: . [Google Scholar]
- 21. Zhang Y, Li Y, Zhang XL, Shi KZ, Liu CJ, et al. (2002) Incidence of toxoplasmosis in 10114 generative women investigated and clinical trial. Journal of Tianjin Medical University 8: 469–471. [Google Scholar]
- 22. Yang QF, Zhang H, Xiong P, Feng XF, Zhou YZ (2003) Serum epidemiological surveillance of TORCH infection in pregnant women in Zunyi. Chinese journal of Epidemiology 24: 853. [Google Scholar]
- 23. Cao MG, Cao L (2004) Investigation and Analysis of Examination Result of Toxoplasma Gondii in 9638 Married Women at Reproductive Ages. Qilu journal of medical laboratory science 15: 30–31. [Google Scholar]
- 24. Chen HM, Chai H, Le ZP, Liao HF (2004) Investigation of Toxoplasma infection in spontaneous abortion females. Journal of public health and preventive medicine 15: 14–15, 19. [Google Scholar]
- 25. Hu CM, Yang X, Luo J (2004) Detection and analysis of the five infection indicators in pregnant women of North Guangdong. Practical Preventive Medicine 11: 356–357. [Google Scholar]
- 26. Wei SZ, Lu SL, Xiao XH, Qi YY, Liu GH (2005) The investigation and analysis of TORCH infection in pregnant women of Ningde district. Chinese journal of birth health & heredity 13: 108–109. [Google Scholar]
- 27. Yang AJ (2005) Detection of Toxoplasmosis infection in pregnant women. Practical Preventive Medicine 12: 1434–1435. [Google Scholar]
- 28. Ye HZ, Gu SD, Huang CQ (2005) TORCH screenings in gestational period and outcomes of fetus from the pregnant women. Chinese primary health care 19: 12–13. [Google Scholar]
- 29. Chen MR, Han YJ (2006) Investigation of infection rate of Cytomegalo Virus,Rubella Virus and Toxoplasma Gondii in married women at reproductive ages. Medcine Industry Information 3: 34–35. [Google Scholar]
- 30. Li BY, Zhou P, Liu XY, Zhou LP, Zhou W (2006) Relationship between poor pregnancy outcome and TORCH infection in pregnant women. Maternal & Child health care of China 21: 1628–1630. [Google Scholar]
- 31. Xie DC (2006) Analysis on the result of TORCH infection in 502 women with adverse pregnancy outcomes. Journal of Guangxi University of Chinese Medicine 9: 42–43. [Google Scholar]
- 32. Chen XJ (2007) Study on toxoplasma infection among pregnant women in Changchun district. Journal of Changchun University of Traditional Chinese Medicine 23: 92. [Google Scholar]
- 33. Guo EP, Zhang Z, Li JD, Wang SJ, Song MH (2008) Study on the relationship between TORCH infection and abnormal pregnancy in pregnant women. Modern Preventive Medicine 35: 3823–3824. [Google Scholar]
- 34. Zhan HY (2008) Serum epidemiological surveillance of TORCH infection in pregnant women of Wuxi. Chinese journal of birth health & heredity 16: 72. [Google Scholar]
- 35. Weng H, Chen J (2009) Analysis of the relationship between Toxoplasma IgM and women with adverse pregnancy outcomes in Ningbo. Chinese journal of birth health & heredity 17: 78. [Google Scholar]
- 36. Long C, Fan W, Zhang JJ, Liu XZ (2011) Analysis on the relationship between TORCH infection and abnormal pregnancy. J Trop Med 11: 1281–1282. [Google Scholar]
- 37. Qiu JZ, Zhou Y, Xu NW, Deng XB, Huang X (2011) Serological survey of Toxoplasma infection among pregnant women in Zhuzhou city. Chinese Journal of Clinical Rational Drug Use 04: 100–101. [Google Scholar]
- 38. Wang JY (2011) Analysis of the specific TORCH antibody in pregnant women. Chinese Journal of Clinical Rational Drug Use 04: 97–98. [Google Scholar]
- 39. Sarkar MD, Anuradha B, Sharma N, Roy RN (2012) Seropositivity of toxoplasmosis in antenatal women with bad obstetric history in a tertiary-care hospital of Andhra Pradesh, India. J Health Popul Nutr 30: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang KB, Zhang HW, Zhang J, Li XF (2012) Retrospective analysis of TORCH test in Bazhong area during 2009–2011. Laboratory Medicine and Clinic 9: 1449–1452. [Google Scholar]
- 41. Moyo SR, Tswana SA, Nystrom L, Bergstrom S, Blomberg J, et al. (1995) Intrauterine death and infections during pregnancy. Int J Gynaecol Obstet 51: 211–218. [DOI] [PubMed] [Google Scholar]
- 42. Sahwi SY, Zaki MS, Haiba NY, Elsaid OK, Anwar MY, et al. (1995) Toxoplasmosis as a cause of repeated abortion. J Obstet Gynaecol (Tokyo 1995) 21: 145–148. [DOI] [PubMed] [Google Scholar]
- 43. Janak Kishore, Richa Misra, Abhiruchi Paisal, Yashodhra Pradeep (2011) Adverse reproductive outcome induced by Parvovirus B19 and TORCH infections in women with high-risk pregnancy. J Infect Dev Ctries 5(12): 868–873. [DOI] [PubMed] [Google Scholar]
- 44. Laila Nimri, Herve Pelloux, Layla Elkhatib (2004) Detection of Toxoplasma gondii DNA and specific antibodies in high-risk pregnant women. Am. J. Trop. Med. Hyg. 71(6) pp 831–835. [PubMed] [Google Scholar]
- 45. Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, et al. (2013) Congenital toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 56: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 46. Mombro M, Perathoner C, Leone A, Buttafuoco V, Zotti C, et al. (2003) Congenital toxoplasmosis: assessment of risk to newborns in confirmed and uncertain maternal infection. Eur J Pediatr 162: 703–706. [DOI] [PubMed] [Google Scholar]
- 47. Naessens A, Jenum PA, Pollak A, Decoster A, Lappalainen M, et al. (1999) Diagnosis of congenital toxoplasmosis in the neonatal period: A multicenter evaluation. J Pediatr 135: 714–719. [DOI] [PubMed] [Google Scholar]
- 48. Hotop A, Hlobil H, Gross U (2012) Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 54: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 49. Lebech M, Andersen O, Christensen NC, Hertel J, Nielsen HE, et al. (1999) Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Danish Congenital Toxoplasmosis Study Group. Lancet 353: 1834–1837. [DOI] [PubMed] [Google Scholar]
- 50. Pratlong F, Boulot P, Issert E, Msika M, et al. (1994) Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenat Diagn 14: 191–198. [DOI] [PubMed] [Google Scholar]
- 51. Gratzl R, Hayde M, Kohlhauser C, Hermon M, Burda G, et al. (1998) Follow-up of infants with congenital toxoplasmosis detected by polymerase chain reaction analysis of amniotic fluid. Eur J Clin Microbiol Infect Dis 17: 853–858. [DOI] [PubMed] [Google Scholar]
- 52. Dar FK, Alkarmi T, Uduman S, Abdulrazzaq Y, Grundsell H, et al. (1997) Gestational and neonatal toxoplasmosis: regional seroprevalence in the United Arab Emirates. Eur J Epidemiol 13: 567–571. [DOI] [PubMed] [Google Scholar]
- 53. Berrebi A, Assouline C, Bessieres MH, Lathiere M, et al. (2010) Long-term outcome of children with congenital toxoplasmosis. Am J Obstet Gynecol 203: 551–552. [DOI] [PubMed] [Google Scholar]
- 54. Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F, et al. (1994) Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med 331: 695–699. [DOI] [PubMed] [Google Scholar]
- 55. Ricci M, Pentimalli H, Thaller R, Rava L, Di Ciommo V (2003) Screening and prevention of congenital toxoplasmosis: an effectiveness study in a population with a high infection rate. J Matern Fetal Neonatal Med 14: 398–403. [DOI] [PubMed] [Google Scholar]
- 56. Berrebi A, Kobuch WE, Bessieres MH, Bloom MC, Rolland M, et al. (1994) Termination of pregnancy for maternal toxoplasmosis. Lancet 344: 36–39. [DOI] [PubMed] [Google Scholar]
- 57. Foulon W, Villena I, Stray-Pedersen B, Decoster A, Lappalainen M, et al. (1999) Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Am J Obstet Gynecol 180: 410–415. [DOI] [PubMed] [Google Scholar]
- 58. Buffolano W, Beghetto E, Del PM, Spadoni A, Di Cristina M, et al. (2005) Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J Clin Microbiol 43: 5916–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robert-Gangneux F, Gavinet MF, Ancelle T, Raymond J, Tourte-Schaefer C, et al. (1999) Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J Clin Microbiol 37: 2893–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Antsaklis A, Daskalakis G, Papantoniou N, Mentis A, Michalas S (2002) Prenatal diagnosis of congenital toxoplasmosis. Prenat Diagn 22: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 61. Logar J, Petrovec M, Novak-Antolic Z, Premru-Srsen T, Cizman M, et al. (2002) Prevention of congenital toxoplasmosis in Slovenia by serological screening of pregnant women. Scand J Infect Dis 34: 201–204. [DOI] [PubMed] [Google Scholar]
- 62. Gilbert R, Dunn D, Wallon M, Hayde M, Prusa A, et al. (2001) Ecological comparison of the risks of mother-to-child transmission and clinical manifestations of congenital toxoplasmosis according to prenatal treatment protocol. Epidemiol Infect 127: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paola Di Carlo, Angela Mazzola, Amelia Romano, Maria Gabriella Schimmenti, Paola Colicchia, et al. (2005) Postnatal follow-up of infants born to mothers with certain Toxoplasma gondii infection: evaluation of prenatal management.Le Infezioni in Medicina, n. 2, 72–78. [PubMed]
- 64. Jenum A, Stray-Pedersen B, Melby KK, Kapperud G, Whitelaw A, et al. (1998) Incidence of Toxoplasma gondii Infection in 35,940 Pregnant Women in Norway and Pregnancy Outcome for Infected Women. J Clin Microbiol 36: 2900–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freeman K, Oakley L, Pollak A, Buffolano W, Petersen E, et al. (2005) Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG 112: 31–37. [DOI] [PubMed] [Google Scholar]
- 66. Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, et al. (1999) Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 67. Holliman RE: Congenital to xoplasmosis (1995) prevention, screening and treatment. J Hosp Infect 30 Suppl:179–190 [DOI] [PubMed] [Google Scholar]
- 68. Desmonts G, Couvreur J (1974) Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med 290: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 69. Xue CL (2000) Diagnosis, treatment and prevention of toxoplasma infection during pregnancy. Chinese journal of parasitology and parasitic diseases 18: 55–57. [PubMed] [Google Scholar]
- 70. Wang SP, Yang XY, Jiang SH, Chen JP, Huang SG, et al. (2008) The immune characteristics of intrauterine vertical transmission of Toxoplasma gondii infection. Journal of Tropical Medicine 8: 505–508. [Google Scholar]
- 71. Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, et al. (1999) Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. Am J Obstet Gynecol 181: 843–847. [DOI] [PubMed] [Google Scholar]
- 72. Prusa AR, Kasper DC, Olischar M, Husslein P, Pollak A, et al. (2013) Evaluation of serological prenatal screening to detect Toxoplasma gondii infections in Austria. Neonatology 103: 27–34. [DOI] [PubMed] [Google Scholar]
- 73. Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, et al. (2013) Congenital toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 56: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 74. Aljumaili ZKM, Alsamarai AM (2013) Risk factors for bad obstetric history in Kirkuk women, Iraq. Int J Infect Microbiol 2(3): 70–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRIMSA checklist of this meta-analysis.
(DOC)