Abstract
Tyrosine kinase inhibitors have revolutionized the oncology community and were pioneered by the use in HER2-targeted therapies. Improved outcomes were seen with the advent of trastuzumab, leading investigators to develop newer agents to target the HER2 pathway such as the novel monoclonal antibody pertuzumab. In this paper, we describe the attributes of pertuzumab including: mechanism of action, pharmacokinetics and metabolism, safety/cardiotoxicity, drug interactions, efficacy, and role in HER2-positive breast cancer management. Newly reviewed here versus previously published reviews on pertuzumab oriented therapy are data of pertuzumab monotherapy as it is used in combination with other anti-HER2 agents derived from preclinical research and ongoing clinical trials.
MATERIALS AND METHODS
A computer based literature search was carried out using PubMed data reported at international meetings (ASCO) up to September 2013 were included.
Keywords: pertuzumab, trastuzumab, HER2, breast cancer
Introduction
Inhibitors of tyrosine kinase-associated cell cycle activation protein are currently major players within the medical oncology arena. This burgeoning trend of treatment modalities is certainly present within the breast oncology community and their usage within these tumors is a bellwether for understanding the use of this class of medications on a grander scale. HER2, a well known oncogenic transmembrane protein, has been demonstrated to be over-expressed in 20–25% of breast cancers.1,2 Although the diagnosis of HER2-positive breast cancer heralds negative prognostic implications,1,2 clinical outcomes of this disease have changed with the institution of HER2-targeted therapy. Trastuzumab is the humanized monoclonal antibody to the HER2 extracellular domain that has revolutionized the treatment and clinical practice of HER2-amplified breast cancer since its Food and Drug Administration (FDA) approval in 1998.3 It has improved survival in both early and metastatic HER2-positive patients; the overall survival for these women is now comparable to patients with ER-positive, HER2-negative metastatic breast cancer.4
Despite the positive changes in HER2-positive patients with trastuzumab,5,6 recurrence and disease progression continue to occur. Nearly 15% of patients will relapse with distant disease within 12 months of treatment with trastuzumab.7,8 The HER2 oncogene appears to continue to be an important driver for tumor growth and survival despite progression on trastuzumab-based treatment.7,8 Current research is developing novel anti-HER2 agents to overcome trastuzumab resistance and efforts are ongoing to establish guidelines for the utility of these agents in the clinical arena.
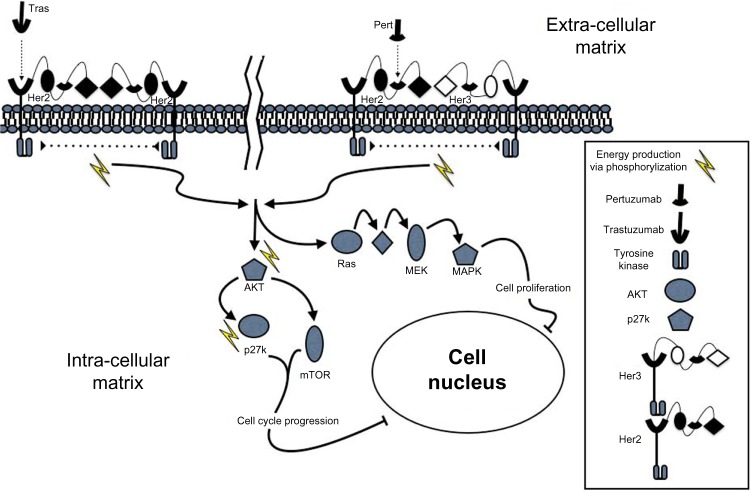
One of the most promising next-generation HER2-targeted interventions is pertuzumab, a humanized monoclonal antibody to the HER2 transmembrane protein targeting a separate different epitope on the HER2 protein from trastuzumab (Fig. 1). Pertuzumab was approved by the FDA in June 2012 for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer without prior treatment with anti-HER2 therapy for metastatic disease.9 Furthermore, in 2013, pertuzumab was expeditiously approved by the FDA for neoadjuvant therapy in HER2-positive patients with locally advanced, inflammatory, or early stage breast cancer. In this clinical scenario, pertuzumab is administered with trastuzumab and docetaxel with data indicating an improved pathologic complete response (pCR) when compared to trastuzumab and docetaxel alone pCR of 39 versus 21%, respectively.10 While pertuzumab has shown great promise, the oncologic community has only begun to understand its full potential. In this article, we will review the preclinical and clinical development of pertuzumab, as well as the clinical implications of this new therapy in every day practice.
Figure 1.
The complementary signaling cascade pathway inhibition of pertuzumab given in conjunction with trastuzumab. HER2 and HER3 are transmembrane proteins with associated tyrosine kinases. They each have multiple binding sites, which are denoted, only slightly disparate with regard to sub-units #2 and #4. Homodimerization and heterodimerization both result in tyrosine kinase activation with the subsequent signaling cascade. Trastuzumab binds to domain IV of HER2 that designates its major difference from Pertuzumab, which binds to domain II of HER3.
Mechanism of Action
Understanding the specific mechanisms behind the cytotoxic effects of HER2-directed therapy on breast cancer cells is critical to the advancement of further HER2-targeted treatment options. Originally described by Hynes and Stern in 1994, when HER2 is over-expressed in breast cancer cells, it undergoes ligand-independent dimerization resulting in constitutive activity.11 The HER2 receptor dimerizes leading to the phosphorylation of the intracellular domains setting of a cascade of signaling molecules. Trastuzumab binds to the subdomain-IV of the HER2 transmembrane protein sterically inhibiting its ability to dimerize.12 As HER2-over expressing tumors have been shown to have increased activation of the cellular protein kinase AKT, treatment with trastuzumab will downregulate AKT and thus allow the protein kinase p27 to enter the cellular nucleus and participate in cell cycle arrest as is its normal activity.13
One of the primary mechanisms of trastuzumab resistance is heterodimerization of HER2 with other HER family transmembrane proteins—most notably HER3.14,15 To overcome this mechanism of resistance, the novel recombinant humanized monoclonal antibody pertuzumab was developed to target domain II, the heterodimerization epitope of HER2.16 Pertuzumab sterically hinders the establishment of a HER2:HER3 heterodimer formation mediating antibody-dependent cytotoxicity17 and apoptosis.
Given their complimentary mechanisms of action, pertuzumab and trastuzumab appear to synergistically inhibit HER2-associated growth. HER2-overexpressing breast cancer cell lines demonstrate a decreased overall survival when given both trastuzumab and pertuzumab but have limited, if any, decreased survival when either agent was administered individually.15 This same study also asserted that in vivo xenograft HER2-over expressing tumor volume was diminished when pertuzumab was given in conjunction with trastuzumab.15
More recent data has led to further understanding of each anti-tumor effect pertuzumab has on cancerous cell lines. In vitro results published by Diessner et al showed radio-labeled cellular binding of trastuzumab and pertuzumab to HER2-positive cells increased when administered together compared to administration separately. Subsequent scatter plot data from the same 2013 publication by Diessner et al revealed NK-cell mediated killing of HER2-positive cells when trastuzumab and pertuzumab bind to the cell18 providing evidence for the antibody dependent cellular cytotoxic effects of pertuzumab. Ensuing efforts utilizing two different xenograph population models echoed the in vitro data.18 Combination therapy with trastuzumab plus pertuzumab showed improved anti-tumor activity in comparison to single-agent therapy.19,20
Pharmacokinetics and Metabolism
Adams et al. collected pharmacokinetic (PK) data from single-dose studies on both CD-1 mice and Sprague Dawley rats as well as multiple-dose studies on male and female cynomolgus monkeys. The authors report a biphasic distribution pattern, supporting a two-compartment PK model.21 Pertuzumab’s volume of distribution (Vd) was 27–58 mL/kg, with the Vd of the central compartment approximating the serum volume in all models (Table 1). The distribution phase was less than 24 hours and the terminal elimination half-life was approximately 10 days.21 Agus et al reported dose range data for 2.0–15.0 mg/kg with a steady state mean of 80.0 ± 28.2 mL/kg and an elimination of half-life mean of 18.9 ± 8 days.22 Yamamoto et al described a steady state Vd of 92.4 ± 15.2 mL/kg23 with constant pharmacokinetics at increasing doses of pertuzumab with proportional increases in Cmax and AUC (r = 0.914 and r = 0.808, respectively).23
Table 1.
Pharmacokinetics of pertuzumab.
| DISTRIBUTION PHASE | TERMINAL ELIMINATION HALF-LIFE | DISTRIBUTION PATTERN | RECOMMENDED FIXED DOSING | VOLUME DISTRIBUTION (STEADY STATE) | PERTUZUMAB CLEARANCE | TROUGH CONCENTRATION |
|---|---|---|---|---|---|---|
| ∼24 hours | ∼10 days21 | Biphasic21 | 840 mg × 1, followed by 420 mg24 | • 80.0 ± 28.2 mL/kg22 • 92.4 ± 15.2 mL/kg23 |
• 3.42 ± 1.20 mL/day/kg22 • 4.30 ± 1.72 mL/day/kg23 |
>20 ug/mL24 |
Ng et al incorporated data from one phase I and two phase II trials with a total of approximately 1,000 pertuzumab subjects receiving treatment every three weeks with either fixed dosing (840 mg × 1, followed by 420 mg), weight-based dosing (12.2 mg/kg × 1, followed by 6.1 mg/kg), or body surface area based dosing (485 mg/m2 × 1 followed by 242.5 mg/m2).24 All dosing regimens consistently kept serum trough concentration greater than the target 20 μg/mL more than 90% of the time. Dosing by weight and also dosing by body surface area resulted in serum trough concentrations lower than that of fixed dosing administration by 6.17% and 5.76% respectively. The percentage of patients with trough concentrations lower than 20 μg/mL was similar in patients weighing in either ≤10th or ≥90th percentile. No differences in the clearance of pertuzumab were seen at all dose levels with mean serum clearance levels of 0.214 L/day.24 Although serum albumin affected drug clearance and body surface area affected Vd of the central compartment, weight-based and body surface area based dosing did not improve steady state exposure to pertuzumab.24 Therefore, a fixed dosing regimen of pertuzumab every three weeks was recommended.
There is limited data regarding the exact site of pertuzumab metabolism and in fact it has not been formally studied. This is likely in part because of the historical difficulty in measuring the process of antibody metabolism. The data available includes evidence that IgG metabolism occurs prominently in the liver, and to a lesser extent in the kidneys and gastrointestinal tract.25 Additionally, murine radio-iodination models have demonstrated antibody clearance occurring predominantly in the gut (72.8%), followed by the liver (20.5%), and spleen (3.6%).26
Safety
Primary information regarding pertuzumab’s safety profile comes from two phase I studies with 21 and 18 patients, respectively, testing the safety of pertuzumab administered at 0.5–15 mg/kg (0.5, 2.0, 5.0, 10.0, and 15.0 mg/kg) every three weeks and no maximum tolerated dose was reported.22,23 From the first trial, the most common adverse events were asthenia (62%), vomiting (52%), nausea (48%), abdominal pain (48%), rash (43%), diarrhea (43%), pain (43%), and anemia (33%), most of which were considered NCI-CTC grade I or grade 2.22 In the second clinical trial, the most common adverse effects were diarrhea (61.1%), rash (50%), asymptomatic BNP increase (50%), and asymptomatic lymphopenia (38.9%) and were grade I or grade 2.23
The above two phase I trials were followed by multiple phase II studies and the results reveal a toxicity profile similar to that known to practitioners familiar with the toxicity profile of trastuzumab. Data from an open-label phase II study by Gianni et al. compared two different fixed-dose regimens (420 vs. 1050 mg every three weeks) of single agent pertuzumab in HER2-negative metastatic breast cancer patients.27 The most common adverse events were grade 1 and 2 diarrhea (43.9–45.9%), nausea (24.4–27%), fatigue (19.5–24.3%), rash (19.5–21.6%), and vomiting (12.2–16.2%). The only reported grade 3 adverse events were diarrhea (5.4–7.3%), fatigue (<3%), and vomiting (<3%).27 Cortes et al evaluated pertuzumab monotherapy in HER2-positive advanced breast cancer and toxicity results were similar to those previously reported: diarrhea (48.3%), nausea (34.5%), vomiting (24%), fatigue (17%), and asthenia (17%).28 This same study assessed the pertuzumab and trastuzumab therapy combination with the following toxicity profile: diarrhea (29%), nausea (29%), vomiting (24%), and fatigue (24%).28
The large phase II CLEOPATR A trial assessed the safety profile during which either pertuzumab or placebo was added to docetaxel and trastuzumab for first-line treatment of HER2-positive metastatic breast cancer. Toxicity profiles between the pertuzumab and placebo group were similar for grade 1 and 2 adverse events including diarrhea (46.3 vs. 66.8%), alopecia (60.5 vs. 60.9%), neutropenia (49.6 vs. 52.8%), nausea (41.6 vs. 42.3%), and fatigue (36.8 vs. 37.6%). For grade 3 or higher adverse events, neutropenia was the most common (45.8 vs. 48.9%), followed by febrile neutropenia (7.6 vs. 13.8%), leukopenia (14.6 vs. 12.3%), and diarrhea (5.0 vs. 7.9%). Deaths related to adverse effects of the treatment were 2.5% for the control group and 2.0% for the pertuzumab groups, with infection cited as the primary cause.29
A fourth phase II study dubbed the NeoSphere trial assessed the safety profile of pertuzumab in the neoadjuvant adjuvant setting in combination with trastuzumab and docetaxel for early stage HER2-positive breast cancer patients. There were four randomized groups in this trial: group A = trastuzumab plus docetaxel, group B = pertuzumab plus trastuzumab plus docetaxel, group C = pertuzumab plus trastuzumab, and group D = pertuzumab plus docetaxel. Considering all groups together, the most common adverse events were alopecia, neutropenia, diarrhea, nausea, fatigue, rash, and mucosal inflammation. The toxicity rates among groups that received a chemotherapy regimen (A, B, and D) were similar. Group C, which received a non-chemotherapy regimen demonstrated that alopecia, neutropenia, and mucosal inflammation occurred in ≤3% of patients. Febrile neutropenia occurred in 7–8% of patients in groups A, B, and D, compared to no events in group C. These results suggest that pertuzumab is generally well tolerated and that most of the adverse events experienced in this trial were related to the cytotoxic agent docetaxel.10
Despite no controlled studies of pertuzumab in pregnant women, embryogenic data were assessed in the offsprings of 36 pregnant cynomolgus monkeys administered pertuzumab during fetal organogenesis (gestation days, 20–50).30 Embryofetal spontaneous abortion was seen in 50% of the cynomolgus monkeys at a dose of 30 mg/kg by gestation day 70. Oligohydramnios was accompanied by delayed development of the fetal kidneys, as well as some external, visceral, and skeletal abnormalities, seen in nearly all subjects.30 These warnings are similar to those for trastuzumab as published on the package insert.31
Cardiotoxicity
Given trastuzumab’s propensity to induce cardiotoxicity, the effects on the heart were thoroughly evaluated in multiple phase I pertuzumab trials. The cardiac exclusion criteria for these trials were baseline left ventricular ejection fraction (LVEF) <50%.22,23 The results of the first phase I trial reported one patient who suffered a myocardial infarction during treatment, which was the suspected cause of left ventricular failure and three patients experienced asymptomatic decreases in baseline ejection fraction ranging from 5 to 14%.22 Asymptomatic BNP increase in 50% of the patients on a phase I study has been noted; however, no ejection fraction depression was measured in any of these patients.23
In two phase II trials evaluating pertuzumab monotherapy, 7–10% of patients experienced drops in ejection fraction ≥10%, which subsequently dropped these patients’ ejection fractions to <50%.27,28 In a phase II study of dual therapy combining pertuzumab with trastuzumab, 4.5% of patients experienced drops in ejection fraction ≥10%.29 Data reported by Portera et al demonstrated six patients (54%) had reduction in LVEF.32 However, only one patient had a symptomatic ejection fraction decrease or heart failure that required medical management.32 In the neoadjuvant NeoSphere phase II study that assessed treatment-naive women with HER2-positive breast cancer, there was no significant increased risk in LVEF dysfunction when pertuzumab was added to trastuzuamb.10 The prevalence of 10–15% decline in LVEF from baseline as well as a new LVEF <50% was not statistically significant: trastuzumab/docetaxel, 0.9%; pertuzumab/trastuzumab/docetaxel, 2.8%; pertuzumab/trastuzumab, 0.9%; and pertuzumab/docetaxel, 1.1%.29
In the CLEOPATRA trial, patients in the placebo group demonstrated a more pronounced drop in ejection fraction than those administered pertuzumab (all grades and reported respectively: 8.3 vs. 4.4%; grades 3–4 reported respectively: 2.8 vs. 1.2%).9 6.6% of patients in the control group and 3.8% in the pertuzumab group demonstrated a ejection fraction decline of 10% or more from baseline that resulted in a ejection fraction of less than 50%.9,33 On further assessment of the CLEOPATRA data set, Swain et al published the incidence of adverse cardiac events was slightly decreased in the pertuzumab arm at 14.5% compared to 16.4% in the control-placebo arm. Qualification of those adverse events demonstrated that the number of events reported as grade 3 (during which left ventricular systolic dysfunction was the most common) or higher was less in the study arm in comparison to the placebo arm (1.5 vs. 3.8% respectively).33
In a meta-analysis, 1,142 patients received combined anti-HER2 (pertuzumab plus trastuzumab or trastuzumab plus lapatinib) therapy with congestive heart failure (CHF) occurring at an incidence of 0.88 versus 1.49% for the 1,473 patients receiving monotherapy (lapatinib or trastuzumab or pertuzumab). Additionally, LVEF showed a decline of 3.1% in the dual therapy arm versus 2.9% in the monotherapy arm. These studies further were stratified by anti-HER2 treatment combinations and those listed in the publication, the combination of pertuzumab and trastuzumab had a CHF incidence of 0.8% and an LVEF decline of 2.9% versus a CHF incidence of 0.96% and an LVEF decline of 3.1% for the trastuzumab and lapatinib combination.34 Despite the initial concern of excessive cardiotoxicity, these data suggest that the risk to the heart from the addition of pertuzumab is likely similar to that of trastuzumab alone.
Drug Interactions
Limited data is available regarding drug–drug interactions with pertuzumab. Results from near-infrared fluorescence imagery and xenograft mice models show that both trastuzumab and pertuzumab are able to equally bind to HER2-positive tumors in vivo and the binding of one agent did not preclude the binding of the other to the same tumor cell.35 A phase 1b trial demonstrated no difference in the PK profile of pertuzumab or docetaxel when used in combination as compared to each agent alone.36 Further, published phase I data revealed that pertuzumab administration together with capecitabine resulted in no alteration in capecitabine’s pharmacokinetics, concentration-time, or toxicity profile.37 Pertuzumab was additionally studied in combination with the investigational antibody-drug conjugate trastuzumab-emtansine (T-DM1) with no alterations in either drugs pharmacokinetic profile.38
Most recent pharmacological data revealed that both trastuzumab and pertuzumab have similar net molecular charge at 19.77 and 19.74, respectively, thus minimizing any significant electrostatic interactions between medications when administered in the same IV normal saline bag. Additionally, an admixture bag of the two medications in normal saline did not induce any changes with regard to turbidity or UV spectroscopy indicating minimal presence of insoluble or visible particles. Still further, there were no differences in the sedimentation rates of the proteins in solo versus in combination, indicating no formation of molecular heterodimers that would have potentially changed their biological activity.39 The administration of pertuzumab and trastuzumab in the same infusion bag will be assessed for efficacy and safety in the VELVET clinical trial utilizing the dual anti-HER2 therapy in combination with Navelbine.
Efficacy
Owing to the complementary mechanisms of action of pertuzumab and trastuzumab in the inhibition of HER2 signaling, clinical research in HER2-positive breast cancer has primarily investigated the use of the two agents in combination. The single-arm phase II trial by Baselga et al assessed the efficacy and safety profile of the trastuzumab/pertuzumab combination in 66 HER2-positive patients with evidence of disease progression with trastuzumab. In this trial, trastuzumab was administered on either a weekly regimen (4 mg/kg loading dose followed by 2 mg/kg every week) or a every three-week regimen (8 mg/kg loading dose followed by 6 mg/kg every three weeks). The dosing schedule of pertuzumab was an 840 mg loading dose followed by 420 mg every three weeks. The objective response rate across the study population was 24.2% with a clinical benefit rate (clinical response plus stable disease) of 50% and a median progression free survival of 5.5 months.29
Following up on the clinical benefit seen with the trastuzumab and pertuzumab combination therapy, another phase II study by Cortes et al was conducted to specifically delineate the efficacy between dual anti-HER2 therapies compared to pertuzumab monotherapy. A cohort of 29 patients with HER2-positive breast cancer with prior exposure to a trastuzumab-containing regimen received the same pertuzumab dosing regimen as the Baselga 2010 cohort. Initially, pertuzumab produced an objective response rate and clinical benefit rate of 3.4 and 10.3%, respectively. All patients progressed on pertuzumab monotherapy, and at time of progressive disease, 17 patients were reintroduced to trastuzumab at either weekly or every three-week dosing, while continuing pertuzumab at the same dose. The subsequent objective response rate and clinical benefit rate increased to 17.6 and 41.2%, respectively, with combination therapy. Progression free survival was also longer with combination therapy (17.4 weeks) when compared with pertuzumab monotherapy (7.1 weeks). Despite the relatively small sample size, this study indicates that the efficacy of pertuzumab monotherapy may be limited, and the clinical utility of pertuzumab is best demonstrated when the agent is given in combination with another HER2-targeted agent.28
Pertuzumab has also been studied in conjunction with chemotherapy in the phase II study by Gianni et al during which 417 treatment-naive women with early stage HER2-overexpressing breast cancer were randomly assigned to receive one of four neoadjuvant cycles of varying combinations of trastuzumab (8 mg/kg loading dose followed by 6 mg/kg every three weeks), pertuzumab (840 mg loading dose followed by 420 mg every three weeks), and docetaxel (75 mg/m2, escalating, if tolerated, to 100 mg/m2 every three weeks). The combinations of therapy included: trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel. Patients who received pertuzumab and trastuzumab plus docetaxel had a significantly improved pathological complete response rate (45.8%) versus trastuzumab plus docetaxel (29.0%; P = 0.0141), pertuzumab plus docetaxel (24.0%; P = 0.003), and pertuzumab and trastuzumab (16.8%; P < 0.001).10 These data were the primary impetus for the expeditious approval by the FDA for this regimen in the neoadjuvant setting.
Similar results for combination therapy with docetaxel were seen in a first-line phase III CLEOPATRA trial in metastatic HER2-positive patients.29 In this study, investigators assessed the efficacy of pertuzumab when added to trastuzumab plus docetaxel. A total of 808 patients were randomly assigned to receive placebo plus trastuzumab plus docetaxel or pertuzumab plus trastuzumab plus docetaxel. Pertuzumab was given as an 840 mg loading dose, followed by 420 mg every three weeks, and trastuzumab was given as an 8 mg/kg loading dose, followed by 6 mg/kg every three weeks. The initial dose of docetaxel was 75 mg/m2, which could be increased to 100 mg/m2, if tolerated. The median progression free survival improved with the addition of pertuzumab to trastuzumab and docetaxel, with 18.5 months in the pertuzumab group versus 12.4 months in the placebo group (hazard ratio for progression or death, 0.62; 95% confidence interval, 0.51–0.75; P < 0.001). All predefined subgroups also demonstrated progression free survival benefit with the use of pertuzumab-trastuzumab-docetaxel therapy. This was most notable for the subgroup of patients who had received prior adjuvant or neoadjuvant chemotherapy with trastuzumab in which a 10.4 versus 16.9 month median progression free survival benefit was noted (HR of 0.62). Additionally, the subgroup who had received adjuvant or neoadjuvant chemotherapy without trastuzumab had a 12.6 versus 21.6 month median progression free survival benefit (HR 0.60).29 Furthermore, there was a strong trend toward an overall survival benefit with the addition of pertuzumab to trastuzumab and docetaxel as there have been fewer deaths in the pertuzumab group compared to the non-pertuzumab group (17.2 vs. 23.6%). The hazard ratio was reported as 0.64 (P = 0.005) with only 43% of the data mature of analysis at time of publication.9 In an updated analysis, Swain et al report a median overall survival of 37.6 months in the placebo group that, in fact, had not been reached in the study arm. This study reported on progression free survival, which improved from 12.4 to 18.7 months in the placebo versus the pertuzumab study arm.40 Given these dramatic improvements in patient outcome, the data from the CLEOPATRA trial led to accelerated FDA approval for pertuzumab as first-line therapy in combination with trastuzumab and docetaxel for metastatic HER2-amplified breast cancer.
Place in Therapy
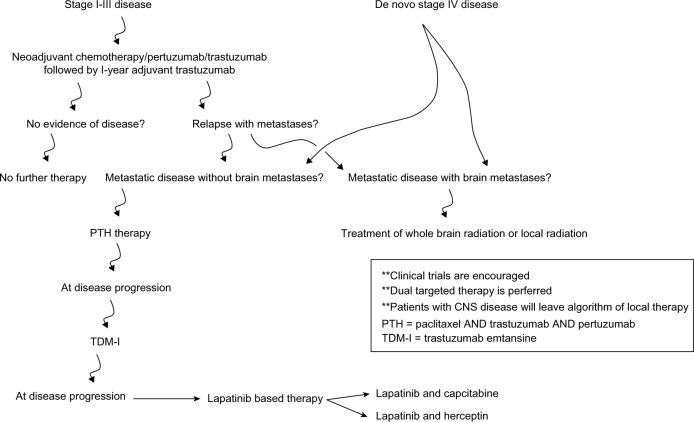
There are still many unanswered questions regarding the clinical use of pertuzumab. With the release of the CLEOPATRA data, pertuzumab in combination with trastuzumab is fundamentally used in the clinical setting as a first-line treatment for metastatic HER2-over expressing disease.9 This trial, in combination with other data supporting the use of dual-HER2-targeted approaches (such as those with trastuzumab and lapatinib), suggest that maximally inhibiting the HER2 oncogenic pathway results in most significant durable clinical responses. With a plethora of anti-HER2 agents in a medical oncologist’s armamentarium, the sequence and duration of therapy remains unclear. There have been other reviews in which the authors have outlined the clinical utility of pertuzumab in oncologic metastatic breast cancer and cited similar data to that reported here.41–44 With these reports in mind, in Figure 2, we have suggested an algorithm for anti-HER2 therapy administration. It is also noted that data on the use of these agents after progression remains scarce. While the exact response rate to anti-HER2 therapy after progression on pertuzumab is unknown, there is preliminary literature that suggest that patients will continue to have multiple responses to HER2-targeted therapy after progression on these second-line HER2-directed agents.4,45
Figure 2.
Recommended management algorithm for HER2-positive breast cancer patients.
Perhaps one of the most concerning issues is the suspicion that patients with brain metastases will not benefit from agents such as trastuzumab and pertuzumab. Trastuzumab and pertuzumab have an approximate molecular weight of 148 kDa.46 The majority of molecules greater than 500 Da do not cross the blood–brain barrier in concentrations needed to affect any CNS disease management.47 Data published from Olson et al revealed that adjuvant trastuzumab results in a harbor site for central nervous system metastases (CNS) of HER2-positive breast cancer. In this meta-analysis, among the 9,020 patients included, the incidence of CNS metastases as first site of disease recurrence was 2.56% compared with 1.94% in HER2-positive patients who did not receive adjuvant trastuzumab.48 With the use of pertuzumab in early stage disease and in first-line therapy in patients with metastatic HER2-positive breast cancer, the incidence of CNS progression is likely to increase.
In the footsteps of trastuzumab, pertuzumab is the next great breakthrough in the treatment of HER2-positive breast cancer. Pertuzumab has and will continue to transform the approach to HER2-positive disease, showing improved benefits when given in combination with trastuzumab. Dual anti-HER2 therapy maximally shuts down the HER2 oncogenic pathway and has revolutionized the medical approach to these patients. Further combination strategies incorporating pertuzumab and studies elucidating the most effective sequence of anti-HER2 therapy are in progress to tackle the next important questions in the development of HER2-targeted regimens.
Footnotes
Author Contributions
Conceived the concept: ERM. Analyzed the data: JJM, ERM. Wrote the first draft of the manuscript: JJM. Contributed to the writing of the manuscript: JJM. Agree with manuscript results and conclusions: JJM, ERM. Jointly developed the structure and arguments for the paper: JJM, ERM. Made critical revisions and approved final version: JJM. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Goberdhan Dimri, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post trastuzumab era. Breast. 2013;22(4):525–31. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 7.Nahta R, Hung M, Esteva FJ. The HER2-targeting anti-bodies trastuzumab and pertuzumab synergistically the survival of breast cancer cells. Cancer Res. 2004;64(7):2343–6. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 8.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–47. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomized multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 11.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:2–3. 165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 12.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 13.Tokunaga E, Kimura Y, Oki E, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–9. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 15.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 16.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 17.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 18.Diessner J, Bruttel V, Becker K, et al. Targeting breast cancer stem cells with HER2-specific antibodies and natural killer cells. Am J Cancer Res. 2013;3(2):211–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 20.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 21.Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55(6):717–27. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23(11):2534–43. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N, Yamada Y, Yutaka F. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2009;39(4):260–6. doi: 10.1093/jjco/hyp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng CM, Lum BL, Gimenez V, Kelsey S, Allison D. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res. 2006;23(6):1275–84. doi: 10.1007/s11095-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 25.Mariani G, Strober W. Immunoglobulin metabolism. In: Metzger H, editor. Fc Receptors and the Action of Antibodies. Washington, DC: American Society for Microbiology; 1990. pp. 94–177. [Google Scholar]
- 26.Zuckier L, Rodriguez L, Scharff M. Immunologic and Pharmacologic Concepts of Monoclonal Antibodies. Semin Nucl Med. 1989;19(3):166–86. doi: 10.1016/s0001-2998(89)80012-1. [DOI] [PubMed] [Google Scholar]
- 27.Gianni L, Llado A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(7):1131–7. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction for trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-postive breast cancer. J Clin Oncol. 2012;30(14):1594–600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28(7):1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega S, Arima A, Chihaya Y, et al. Embryo-fetal development study of pertuzumab administered by intravenous injection to pregnant cynomolgus monkey. Int J Toxicol. 2010;29(1):89–90. [Amer Coll Toxicol 30th Annu Meet (Nov 1–4, Palm Springs) 2009] (Abst P7) [Google Scholar]
- 31.Herceptin® [package insert] San Francisco, CA: Genetech; 2010. [Google Scholar]
- 32.Portera C, Walshe J, Rosing D, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14(9):2710–6. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain SM, Ewer MS, Cortés J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–64. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valachis A, Nearchou A, Poyzos NP, Lind P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer. 2013;133(9):2245–52. doi: 10.1002/ijc.28234. [DOI] [PubMed] [Google Scholar]
- 35.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 36.Attard G, Kitzen J, Blagden SP, et al. A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumors. Br J Cancer. 2007;97(10):1338–43. doi: 10.1038/sj.bjc.6604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albanell J, Montagut C, Jones ET, et al. A phase I study of safety and pharmacokinetics of the combination of pertuzumab (rhuMab) 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res. 2008;14(9):2726–31. doi: 10.1158/1078-0432.CCR-07-1980. [DOI] [PubMed] [Google Scholar]
- 38.Lu D, Burrris HA, III, Wang B, et al. Drug interactions potential of trastuzumab emtansine (T-DM1) combined with pertuzumab in patients with HER2-positive metastatic breast cancer. Curr Drug Metab. 2012;13(7):911–22. doi: 10.2174/138920012802138688. [DOI] [PubMed] [Google Scholar]
- 39.Glover Z, Gennaro L, Yadav S, Demeule B, Wong PY, Sreedhara A. Compatibility and stability of pertuzumab and trastuzumab admixtures in i.v. infusion bags for coadministration. J Pharm Sci. 2013;102:294–812. doi: 10.1002/jps.23403. [DOI] [PubMed] [Google Scholar]
- 40.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan CC Swain SM. Pertuzumab: evolving therapeutic strategies in the management of HER2-overexpressing breast cancer. Expert Opin Biol Ther. 2013;13(5):779–90. doi: 10.1517/14712598.2013.783007. [DOI] [PubMed] [Google Scholar]
- 42.Sendur MA, Aksoy S, Altundag K. Pertuzumab in HER2-positive breast cancer. Curr Med Res Opin. 2012;28(10):1709–16. doi: 10.1185/03007995.2012.728132. [DOI] [PubMed] [Google Scholar]
- 43.Capelan M, Pegliano L, De Azambuja E, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24(2):273–82. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 44.Zagouri F, Sergentanis TN, Chrysikos D, et al. Pertuzumab in breast cancer: a systematic review. Clin Breast Cancer. 2013;13(5):315–224. doi: 10.1016/j.clbc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Olson EM, Lin NU, DiPiro PJ, et al. Responses to subsequent anti-HER2 therapy after treatment with trastuzumab-DM1 in women with HER2-positive metastatic breast cancer. Ann Oncol. 2012;23:93–97. doi: 10.1093/annonc/mdr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drug Bank Genome Alberta & Genome Canada. Jun 13, 2005. Website. August 6, 2013.
- 47.Pardridge W. CNS drug design based on principles of blood–brain barrier transport. J Neurochem. 1998;70(50):1781–92. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 48.Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–33. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]