Abstract
OBJECTIVE
To assess whether the addition of an omega-3 long-chain polyunsaturated fatty acid supplement would reduce preterm birth in women with at least one prior spontaneous preterm birth receiving 17α-hydroxyprogesterone caproate.
METHODS
We conducted a randomized, double-masked, placebo-controlled trial in 13 centers. Women with a history of prior spontaneous singleton preterm birth and a current singleton gestation were assigned to either a daily omega-3 supplement (1,200 mg eicosapentaenoic acid and 800 mg docosahexaenoic acid) or matching placebo from 16–22 through 36 weeks of gestation. All participants received weekly intramuscular 17α-hydroxyprogesterone caproate (250 mg). The primary study outcome was delivery before 37 weeks of gestation. A sample size of 800 was necessary to have 80% power to detect a 30% reduction in the primary outcome from 30%, assuming a type I error two-sided of 5%.
RESULTS
A total of 852 women were included, and none was lost to follow up. Delivery before 37 weeks of gestation occurred in 37.8% (164/434) of women in the omega-3 group and 41.6% (174/418) in the placebo group (relative risk 0.91, 95% confidence interval 0.77–1.07).
CONCLUSION
Omega-3 long-chain polyunsaturated fatty acid supplementation offered no benefit in reducing preterm birth among women receiving 17α-hydroxyprogesterone caproate who have a history of preterm delivery.
In 2005, 12.7% of all births in the United States were preterm.1 Prematurity is now the leading cause of neonatal mortality and the rate continues to increase.2,3 One of the strongest predictors of preterm delivery is history of a preterm delivery.4 In the previous National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network trial reported by Meis et al weekly 17α–hydroxyprogesterone caproate injections significantly reduced the rate of repeat preterm birth in women with at least one prior preterm delivery; however, the rate of preterm birth in the active treatment group was still 36.3%.5 Omega-3 long chain polyunsaturated fatty acid supplementation has also been reported to reduce significantly the rate of recurrent preterm birth in a randomized trial.6 Differences in the metabolic derivatives of omega-6 and omega-3 polyunsaturated fatty acids and the relative amounts of these essential fatty acids in the typical western diet provide biological plausibility for the hypothesis that increased omega-3 intake could prolong gestation and delay parturition.7–15 Whether omega-3 long-chain polyun-saturated fatty acid supplementation offers additional benefit in reducing repeat preterm birth among women receiving 17α-hydroxyprogesterone caproate has not previously been examined.
We conducted a randomized, double-masked, placebo control trial to test the hypothesis that among women with at least one prior spontaneous preterm delivery receiving weekly 17α-hydroxyprogesterone caproate the addition of an omega-3 supplement would further reduce the rate of recurrent preterm birth. The dosage and composition of the supplement were chosen after review of those trials included in the meta-analyses by Horvath and Szajewska.16,17 We assessed dietary fish intake at baseline to examine its interaction with supplementation.
MATERIALS AND METHODS
Women presenting for prenatal care at one of the 13 participating network centers were screened for eligibility between January 2005 and October 2006. Inclusion criteria were a documented history of at least one prior singleton preterm delivery between 20 0/7 and 36 6/7 weeks of gestation after spontaneous preterm labor or premature rupture of the membranes, and a current singleton pregnancy between 16 and 21 6/7 weeks of gestation. Exclusion criteria were evidence of a major fetal anomaly, intake of a fish oil supplement in excess of 500 mg per week at any time during the preceding month, allergy to fish, anticoagulation therapy, hypertension, White's classification D or higher diabetes, drug or alcohol abuse, seizure disorder, uncontrolled thyroid disease, clotting disorder, current or planned cerclage, or a plan to deliver either elsewhere or before 37 weeks of gestation. An ultrasound examination was required between 14 weeks of gestation and enrollment to screen for major anomalies. Gestational age at randomization was determined according to a previously described algorithm on the basis of the last menstrual period and earliest ultrasound examination and was not revised after the woman was assigned to a group.18
Women who were eligible after initial screening were approached by a research nurse who explained the study and asked for signed consent to obtain the medical records of the previous pregnancy ending in preterm delivery. If the preceding preterm delivery was due to spontaneous preterm labor or preterm premature rupture of the fetal membranes and if no criteria for exclusion were present, the woman was invited to participate. The study was approved by the institutional review board of each clinical site and of the data coordinating center. All participants gave written informed consent before enrollment.
Consenting eligible women received an injection of 250 mg of 17α-hydroxyprogesterone caproate and a 7-day supply of placebo capsules. Those who either did not return after five days and before 21 6/7 weeks of gestation or had taken less than half of the placebo capsules were not allowed to participate. Women passing the compliance run-in were randomly assigned to receive either a daily supplement containing 1,200 mg of eicosapentaenoic acid (EPA, 20:5n-3) and 800 mg of docosahexaenoic acid (DHA, 22:6n-3), for a total of 2,000 mg of omega-3 long-chain polyunsaturated fatty acids, divided into four capsules, or matching placebo capsules, which contained only a minute amount of inert mineral oil. The women were encouraged to use a daily dosing schedule that worked best for them, either once daily or spread out over the day. All women received weekly injections of 17α-hydroxyprogesterone caproate (250 mg) as described in the trial by Meis et al5 The capsules and 17α-hydroxyprogesterone caproate injections were supplied by a company that manages investigational drugs (Eminent Services, Frederick, MD). The source of the omega-3 long-chain polyunsaturated fatty acids was deep ocean fish. Each capsule contained 10 international units of vitamin E as a preservative. Chromatographic analysis of a sample of the omega-3 capsules was performed by a laboratory independent from the supplier and confirmed the content of 300 mg of EPA and 200 mg of DHA per capsule and no detectable levels of environmental contaminants. The data coordinating center at George Washington University used the simple urn method of randomization19 with stratification according to clinical center to create a randomization sequence for each center. Personnel at the data coordinating center had no contact with participants and no other role in recruit ment. Boxes containing a woman's entire supply of capsules in blister packs were sequentially numbered according to the predetermined randomization sequence, and on enrollment a woman was assigned the next number in sequence. Study group assignment was not known by study participants, their health care providers, or the research personnel. Blister packs were dispensed monthly at which time compliance and side effects were assessed. Study drug and 17α-hydroxyprogesterone caproate injections were continued until delivery or 36 6/7 weeks of gestation, whichever occurred first. Maternal blood was obtained at the enrollment visit and again between 25 and 28 weeks of gestational age for analysis of plasma fatty acids in a central laboratory (Dr. Mary Harris, Colorado State University) using methods previously described.20 A validated four item food frequency questionnaire designed to assess intake of fish was administered at baseline. The four items are darkmeat fish, canned tuna, other fish and shellfish.21,22 Participants received no dietary advice as part of the study and otherwise received usual clinical care.
The primary study outcome was delivery before 37 completed weeks of gestation (259 days). Prespecified secondary outcomes included delivery before 35 weeks and before 32 weeks, spontaneous preterm delivery (defined as preterm birth in which the initiating event was spontaneous labor or membrane rupture), medically indicated preterm delivery, and delivery after 40 completed weeks. The occurrences of gestational hypertension and preeclampsia were based on documentation of the diagnosis in the medical chart. Postpartum hemorrhage was defined as an estimated blood loss of at least 500 mL after vaginal delivery or at least 1,000 mL after cesarean delivery or receipt of blood transfusion. Prespecified fetal and neonatal outcomes included birth weight percentile for gestational age,23 admission to a neonatal intensive care unit (NICU) or intermediate care unit, and duration of NICU or intermediate care unit stay. Retinopathy was diagnosed by ophthalmologic examination. Intraventricular hemorrhage was graded according to the most severe radiologic finding before hospital discharge. Documentation in the medical record of medical or surgical treatment for patent ductus arteriosus was considered evidence of patent ductus arteriosus. Necrotizing enterocolitis was defined by the unequivocal presence of intramural air or perforation on abdominal radiography or the development of stricture or abscess after an episode of suspected necrotizing enterocolitis. Proven sepsis was defined by positive blood, cerebral spinal fluid, or urine cultures or, in the absence of positive cultures, either cardiovascular collapse or radiographic evidence of infection in a neonate with a clinical diagnosis of sepsis. Compliance with study drug was assessed by capsule count and calculated as the number taken divided by the expected number. Compliance with 17α-hydroxyprogesterone caproate injections was calculated as the number received divided by the expected number.
After delivery, records of the participants and their newborns were reviewed by study personnel, unaware of treatment assignment, who abstracted delivery date, birth weight, occurrence of maternal or neonatal complications and interventions.
The preterm delivery rate in the progesterone plus placebo arm was estimated to be 30%. A sample size of 800 women was estimated to detect a 30% reduction in the risk of repeat preterm delivery with two-sided alpha of .05 and at least 80% power.
The data were analyzed according to the intention-to-treat principle. Categorical variables were compared with the use of χ2 or Fisher exact test as appropriate. Continuous variables were compared with the use of the Wilcoxon rank-sum test. The Breslow–Day test was used to assess the interaction of fish consumption at baseline with treatment group.24 The proportions of patients remaining undelivered in each group were compared using survival analysis adjusting for staggered gestational age at entry.25 Maternal plasma levels of DHA, EPA, and arachidonic acid were reported as percent weight of total fatty acids. The Wilcoxon test was used to compare changes between samples (level between 25 and 28 weeks of gestation minus level at enrollment) and per week of study drug exposure between the omega-3 and placebo groups. An independent data and safety monitoring committee monitored the trial and reviewed the interim results using a prespecified plan employing the group sequential method of Lan and DeMets with the modified O'Brien-Fleming spending function for adjustment of the significance level at both interim and final analyses.26 One interim analysis was performed and thus in the final analysis of the primary outcome, a two-tailed p value of less than 0.047 was considered significant. However, because the adjustment is minimal, 95% not 95.3% confidence intervals (CIs) are reported. For secondary outcomes, two-tailed p values of less than 0.05 were considered to indicate significance and no adjustments were made for multiple comparisons.
RESULTS
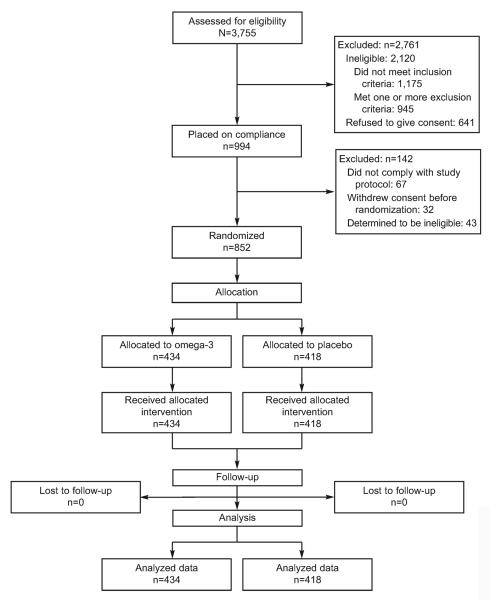
A total of 3,755 women were screened; 994 were placed on compliance, and 852 were randomly as signed to treatment (Fig. 1). Of those assigned to treatment, 434 were randomly assigned to the omega-3 supplement arm and 418 to the placebo arm. The primary outcome was available for all 852 women.
Fig. 1.
Screening, randomization, and follow-up of study participants.
Harper. Omega-3 for Repeat Preterm Birth Prevention. Obstet Gynecol 2010.
The two groups were similar with respect to baseline demographics, risk factors for recurrent preterm delivery and dietary fish intake (Table 1). Compliance with 17α-hydroxyprogesterone caproate injections was not different between the two groups, 90.6% in the omega-3 group and 90.9% in the placebo group (P=.78). The mean compliance rates for study capsules was 85.1% in the omega-3 group and 84.8% in the placebo group (P=.33). Fatty acid analyses from both the enrollment visit and the 25- to 28-week visit were available for 512 women (261 in the omega-3 group and 251 in the placebo group). There were significant differences in the change in plasma levels of EPA, DHA, and arachidonic acid between the omega-3 and placebo groups, all P<.001 (Fig. 2). Accounting for differences in length of study drug exposure by determining change per week did not alter these results (all P<.001, data not shown).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Omega-3 Group (n=434) | Placebo Group (n=418) |
|---|---|---|
| No. of previous preterm deliveries | 1 (1–2) | 1 (1–2) |
| Cestational age of earliest spontaneous preterm delivery (wk) | 32 (27–34) | 31 (26–34) |
| More than 1 previous preterm delivery | 131 (30.2) | 116 (27.8) |
| More than 1 previous term delivery | 160 (36.9) | 136 (32.5) |
| Maternal age (y) | 28 (23–32) | 27 (24–32) |
| Cestational age at randomization (wk) | 19.6 (17.9–20.9) | 19.6 (18.0–21.0) |
| Race | ||
| African American | 148 (34.1) | 145 (34.9) |
| White | 245 (56.5) | 240 (57.7) |
| Asian | 13 (3.0) | 5 (1.2) |
| Other | 28 (6.5) | 26 (6.3) |
| Hispanic/Latina ethnicity | 64 (14.7) | 57 (13.6) |
| Marital status | ||
| Married | 309 (71.2) | 278 (66.5) |
| Divorced, separated, or widowed | 16 (3.7) | 25 (6.0) |
| Never married | 109 (25.1) | 115 (27.5) |
| Education level (y) | 13 (12–16) | 13 (12–16) |
| Prepregnancy body mass index | 25.1 (21.5–30.3) | 24.6 (21.5–30.3) |
| Smoking during pregnancy | 64 (14.7) | 72 (17.2) |
| Servings of fish/wk | 1.0 (0.0–1.5) | 0.5 (0.0–1.5) |
| At least 2 servings of fish/wk | 76 (17.5) | 78 (18.7) |
| At least 1 serving of fish/mo | 310 (71.4) | 289 (69.1) |
Data are median (interquartile range) or n (%).
Fig. 2.
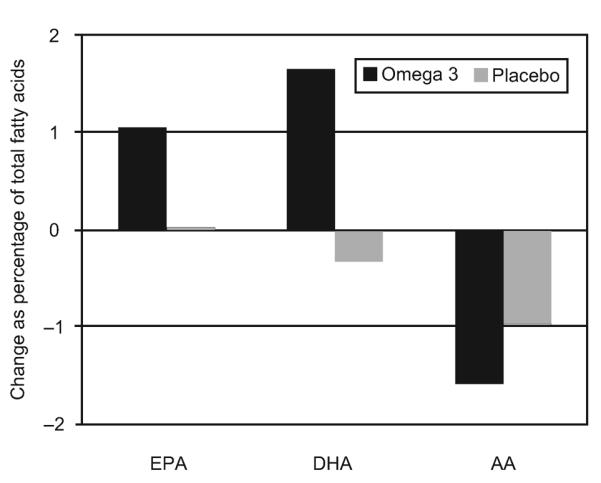
Change in percent weight of total fatty acids from enrollment to 25–28 weeks of gestation. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid. All P<.001.
Harper. Omega-3 for Repeat Preterm Birth Prevention. Obstet Gynecol 2010.
The risk of delivery before 37 weeks was not different between the omega-3 (164/434, 37.8%) and placebo groups (174/418, 41.6%) (relative risk [RR] 0.91, 95% CI 0.77–1.07). The risk of spontaneous preterm delivery before 37 weeks was also not different between the omega-3 (143/434, 32.9%) and placebo groups (149/418, 35.6%) (RR 0.92, 95% CI 0.77–1.11). The rates of early preterm delivery before 35 weeks and before 32 weeks were similar. Gestational age at delivery was on average 2 days longer in the omega-3 group, but this difference was not significant (P=.26) (Table 2). The proportion of patients remaining undelivered at each week of gestation also was not different between groups (P=.37). The rate of preeclampsia or gestational hypertension was 4.6% in the omega-3 group and 4.8% in the placebo group (P=.9). Gestational diabetes developed in 7.4% of women in the omega-3 group and in 5.5% of those in the placebo group (P=.27). The rate of postpartum hemorrhage was 13.8% in the omega-3 group and 12.5% in the placebo group (P=.56).
Table 2.
Pregnancy Outcome According to Treatment Assignment
| Outcome | Omega-3 (n=434) | Placebo (n=418) | Relative Risk (95% CI) |
|---|---|---|---|
| Delivery before 37 wk of gestation | 164 (37.8) | 174 (41.6) | 0.91 (0.77–1.07) |
| Spontaneous | 143 (32.9) | 149 (35.6) | 0.92 (0.77–1.11) |
| Medically indicated | 21 (4.8) | 25 (6.0) | 0.81 (0.46–1.42) |
| Delivery before 35 wk of gestation | 82 (18.9) | 83 (19.9) | 0.95 (0.72–1.25) |
| Delivery before 32 wk of gestation | 43 (9.9) | 45 (10.8) | 0.92 (0.62–1.37) |
| Delivery after 40 wk of gestation | 11 (2.5) | 8 (1.9) | 1.32 (0.54–3.25) |
| Pregnancy loss or neonatal death | 16 (3.7) | 17 (4.1) | 0.90 (0.46–1.77) |
| Cestational age at delivery (wk)* | 37.7 (36.0–39.0) | 37.4 (35.7–38.7) |
CI, confidence interval.
Data are n (%) or median (interquartile range) unless otherwise specified.
P=.26.
Dietary fish consumption was condensed into two categories based on results from two randomized trials that showed that the effect of omega-3 supplementation on length of gestation was modified by dietary fish intake.27,28 At baseline, two hundred fifty three or 29.7% of women in the study reported eating no fish or less than one serving per month and five hundred ninety-nine or 70.3% reported eating at least one fish meal per month. The effect of omega-3 supplementation was similar among women who consumed no fish or less than 1 fish meal per month (RR 0.96, 95% CI 0.74–1.24) and those who consumed at least one fish meal per month (RR 0.89, 95% CI 0.72–1.10, P for interaction=.74.)
The distribution of fetal and neonatal outcomes was similar between the two groups. Median birth weight was 2,990 g (interquartile range 2,585–3,330 g) in the omega-3 group and 2,923 g (interquartile range 2,389–3,317) in the placebo group (P=.13). There was no difference in the proportion of neonates with birth weight less than 1,500 g, less than 2,500 g, or the proportion that were small for gestational age or large for gestational age (Table 3). The average length of stay in days in the NICU was 5.8 (standard deviation ±16.0) for the omega-3 group and 5.1 (standard deviation ±14.2) for the placebo group (P=.82). Among the 828 liveborn neonates who survived to be admitted to the nursery, we did observe a statistically significantly increased rate of respiratory distress syndrome (RDS) in the omega-3 group compared with the placebo group (P=.019); however, there were no significant differences in other respiratory outcomes (Table 4). We found no association between maternal plasma levels of arachidonic acid and the outcome of RDS after controlling for gender, gestational age at delivery and administration of prenatal steroids, P=.49. Women in the omega-3 group experienced significantly more burping (21.0% compared with 5.5%, P<.001), vomiting (4.4% compared with 1.2%, P=.005) and bad taste (2.3% compared with 0%, P=.002). Swelling or other reaction at the 17α-hydroxyprogesterone caproate injection site occurred in 64.3% of women in the omega-3 group and 58.6% of women in the placebo group, P=.09.
Table 3.
Outcomes for Liveborn Neonates According to Maternal Treatment Assignment
| Outcome | Omega-3 (n=427) | Placebo (n=410) | Relative Risk (95% CI) |
|---|---|---|---|
| Birth weight less than 2,500 g | 94 (22.0) | 112 (27.3) | 0.81 (0.64–1.02) |
| Birth weight less than 1,500 g | 26 (6.1) | 29 (7.1) | 0.86 (0.52–1.44) |
| Small for gestational age less than 10th percentile | 35 (8.2) | 41 (10.0) | 0.82 (0.53–1.26) |
| Large for gestational age more than 90th percentile | 21 (4.9) | 15 (3.7) | 1.34 (0.70–2.57) |
| Admission to intensive/intermediate care nursery* | 110 (25.9) | 99 (24.6) | 1.05 (0.83–1.33) |
| Retinopathy of prematurity* | 5 (1.2) | 4 (1.0) | 1.18 (0.32–4.37) |
| Intraventricular hemorrhage* | |||
| Any grade | 10 (2.4) | 9 (2.2) | 1.05 (0.43–2.57) |
| Grade 3 or 4 | 5 (1.2) | 3 (0.7) | 1.58 (0.38–6.57) |
| Patent ductus arteriosus* | 11 (2.6) | 7 (1.7) | 1.49 (0.58–3.81) |
| Necrotizing enterocolitis* | 3 (0.7) | 4 (1.0) | 0.71 (0.16–3.16) |
| Proven sepsis* | 5 (1.2) | 3 (0.7) | 1.58 (0.38–6.57) |
CI, confidence interval.
Data are n (%) unless otherwise specified.
Two neonates in the omega-3 group and seven in the placebo group died before admission to the neonatal intensive care unit and are not included.
Table 4.
Respiratory Outcomes for Liveborn Neonates According to Maternal Treatment Assignment
| Outcome | Omega-3 (n=425) | Placebo (n=403) | Relative Risk (95% CI) |
|---|---|---|---|
| Respiratory distress syndrome | 59 (13.9) | 35 (8.7) | 1.60 (1.08–2.37) |
| Received surfactant | 38 (8.9) | 29 (7.2) | 1.24 (0.78–1.98) |
| Bronchopulmonary dysplasia | 9 (2.1) | 6 (1.5) | 1.42 (0.51–3.96) |
| Transient tachypnea | 31 (7.3) | 24 (6.0) | 1.22 (0.73–2.05) |
| Supplemental oxygen* (d) | 2.2±8.9 | 1.9±9.4 | |
| Ventilator support† (d) | 0.8±5.6 | 0.5±4.0 |
CI, confidence interval.
Data are n (%) or mean±standard deviation unless otherwise specified. Medians and interquartile ranges were all zero.
Respiratory distress syndrome (RDS) was defined as a clinical diagnosis of type I RDS and oxygen therapy (FiO2 more than 0.40) for more than 24 hours or by death before 24 hours in a neonate who met the other criteria for RDS. Bronchopulmonary dysplasia was defined as the requirement for supplemental oxygen at 36 weeks corrected age for neonates born before 34 weeks. Transient tachypnea was defined as the requirement for supplemental oxygen with or without mechanical ventilation during the first 24 hours of life in a neonate in whom there were no other causes of respiratory distress.
P=.16.
P=.28.
DISCUSSION
Daily supplementation with 1,200 mg of EPA and 800 mg of DHA resulted in significant increases in maternal plasma levels of these omega-3 long-chain polyunsaturated fatty acids and a decrease in the omega-6 long-chain polyunsaturated fatty acid, arachidonic acid, but did not reduce the rate of repeat preterm birth before 37 weeks, before 35 weeks, or before 32 weeks among high risk women receiving 17α-hydroxyprogesterone caproate. The rates of preterm delivery were similar to those in the progesterone arm of the original trial by Meis et al5 These results suggest that omega-3 long-chain polyunsaturated fatty acid supplementation offers no additional protection from preterm birth in women already receiving 17α-hydroxyprogesterone caproate. Omega-3 long-chain polyunsaturated fatty acid supplementation had no effect on birth weight.
The rate of RDS was higher in the omega-3 supplemented group. This adverse effect has not been reported with any other prenatal omega-3 trials and we observed no difference between groups in other measures of RDS morbidity or receipt of surfactant. It is possible that this appeared to be different by chance, given the multiple comparisons; however, another consideration is the lower maternal plasma levels of arachidonic acid in the omega-3 group. In a study of human preterm neonates stratified by birth weight, Lane et al observed significantly lower cord-blood levels of arachidonic acid in those with RDS compared with those without RDS in the subgroups of birth weights between 1,500 and 1,999 g and between 1,000 and 1,499 g.29 Studies in animal models and tissue cultures have produced mixed results. Clarke et al observed an increase in the phosphatidylcholine fraction in whole fetal lungs when pregnant rats were fed a diet with omega-3s as the sole source of fatty acids suggesting that omega-3 long-chain polyunsaturated fatty acids are beneficial for surfactant synthesis and this benefit is not dependent on a balance with omega-6 long-chain polyunsaturated fatty acids.30 Prostaglandin E2, which is the predominant cyclooxygenase metabolite of arachidonic acid in type II alveolar cells, has been shown to stimulate pulmonary surfactant secretion31; however, arachidonic acid has been shown to inhibit glucocorticoid binding in type II alveolar cells, which could affect responsiveness to betamethasone in the preterm fetus.32 We found no association between maternal plasma levels of arachidonic acid and the outcome of RDS.
This double-masked, placebo-controlled trial has several strengths. Women were enrolled before 22 weeks of gestation, and the primary outcome was available for all included patients. Plasma fatty acid analysis demonstrated significant differences in the changes in levels of DHA, EPA, and arachidonic acid in the supplemented group compared with the placebo group suggesting good compliance in the active treatment group and limited if any self-medicating with omega-3 in the placebo group. There are several weaknesses as well. Cord blood was not collected for fatty acid levels. We did not include an omega-3 without 17α-hydroxyprogesterone caproate group. Because 17α-hydroxyprogesterone caproate had been shown previously to reduce the rate of recurrent preterm birth in a similar population in the Meis trial,5 we believed it would not be appropriate to randomly assign women to a treatment arm that did not include 17α-hydroxyprogesterone caproate.
Clinical trials and cohort studies of the potential benefits of omega-3 in pregnancy have included both low risk and high risk women. Trials in low risk women and women with risk factors other than history of a preterm delivery have reported mixed results and systematic reviews of these trials suggest no benefit for preterm birth prevention.16,17,33,34 However, in the European Multicenter Trial in women with prior preterm delivery,6 those randomly assigned to receive an omega-3 supplement (1,300 mg EPA and 900 mg DHA) had a significantly lower rate of recurrent preterm delivery before 37 weeks (21.3% compared with 33.3%, odds ratio 0.54, 95% CI 0.30–0.98) and before 34 weeks (4.6% compared with 13.3%, odds ratio 0.32, 95% CI 0.11–0.89). The discrepancy in results between this trial and ours may be the result of the addition of 17 OHPC in our trial. We also used a different placebo. Mineral oil was selected because it is not absorbed and does not affect the metabolic cascade of omega-6 or omega-3 long-chain polyunsaturated fatty acids to the 2 and 3 series eicosanoids. The placebo for the European trial was olive oil, a combination of oleic acid (18:1 n–9) and linoleic acid (18:2 n–6). Linoleic acid is the essential fatty acid that is elongated to form omega-6 long-chain polyunsaturated fatty acids including arachidonic acid. The authors state in the discussion that the olive oil supplement “could theoretically interfere with prostanoid metabolism in a way opposite to the omega-3 fatty acids.”6 We observed nonsignificant increases in median length of gestation of 2 days and median birth weight of 67 g in the omega-3 group compared with the placebo group. These results are consistent with those from two independent systematic reviews of omega-3 long-chain polyunsaturated fatty acid supplementation in low risk pregnancies.17,34 This suggests that women with prior preterm delivery receiving 17α-hydroxyprogesterone caproate respond to omega-3 supplementation similarly to low risk women. Although none of the differences were significant, the rate of preterm birth before 37, before 35, and before 32 weeks as well as the rate of spontaneous preterm birth before 37 weeks were all lower in the omega-3 compared with placebo group. The effect of omega-3 long-chain polyunsaturated fatty acid supplementation on length of gestation in a Danish trial of healthy women and the European Multicenter Trial of high risk women was influenced by dietary intake of fish at baseline.27,28 We did not find this to be the case in our trial. Interestingly, 29.7% of women in our trial reported eating either no fish or less than one meal per month. This is higher than the rate of low fish intake in the Danish study (19.3%, 103/533).27
In summary, an omega-3 long-chain polyunsaturated fatty acid supplement provided no additional benefit for prevention of recurrent preterm birth among women receiving 17α-hydroxyprogesterone caproate. This is in disagreement with an earlier trial of omega-3 alone in women with prior preterm birth. There are several possible explanations for our results. First, it is suggested that both 17α-hydroxyprogesterone caproate and omega-3 long-chain polyunsaturated fatty acids can reduce gap junction formation and production of pro-inflammatory cytokines and so they may share a common mechanism in the delay of parturition among women with prior preterm delivery.8,35,36 Second, omega-3 long-chain polyunsaturated fatty acid supplementation may have only a modest effect on length of gestation with or without progesterone and thus not affect the rate of preterm delivery. Review of other trials and systematic reviews would favor the second explanation. Our finding of an increased rate of RDS in the omega-3 group is of interest but has not been reported in previous trials.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD27860, HD27917, HD40560, HD34208, HD40485, HD21410, HD27915, HD40500, HD40512, HD40544; MO1-RR-000080; HD34136; HD27869; HD40545; HD36801 and HD19897).
The authors thank Catherine Y. Spong, MD, for oversight, protocol development, and manuscript preparation; Paul J. Meis, MD, for protocol development; Julia Zachary for protocol and data management and statistical analysis; and Karen Dorman, RN, MS, and Melissa Swain, RN, for development of manual of operations and coordination between clinical research centers.
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00135902.
Footnotes
Presented at the Annual Scientific Meeting of the Society for Maternal-Fetal Medicine, January 31, 2008, Dallas, Texas.
Financial Disclosure The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 2.Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep. 2003;52:1–85. [PubMed] [Google Scholar]
- 3.Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):7–16. doi: 10.1046/j.1365-3016.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 4.Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178:1035–40. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 5.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 6.Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Br J Obstet Gynaecol. 2000;107:382–95. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 7.Norwitz ER, Robinson JW, Challis JRG. The control of labor. N Engl J Med. 1999;341:660–6. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- 8.Allen KGD, Harris MA. The role of n-3 fatty acids in gestation and parturition. Exp Biol Med. 2001;226:498–506. doi: 10.1177/153537020122600602. [DOI] [PubMed] [Google Scholar]
- 9.Karim SM. The role of prostaglandins in human parturition. Proc R Soc Med. 1971;64:10–2. doi: 10.1177/003591577106400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravett MG. Causes of preterm delivery. Semin Perinat. 1984;8:246–57. [PubMed] [Google Scholar]
- 11.Hagve TA, Christophersen BO. Linolenic acid desaturation and chain elongation and rapid turnover of phospholipids (n-3) fatty acids in isolated rat liver cells. Biochem Biophys Acta. 1983;753:339–49. doi: 10.1016/0005-2760(83)90057-7. [DOI] [PubMed] [Google Scholar]
- 12.Broadhurst CL, Cunnane SC, Crawford MA. Rift valley lake fish and shellfish provided brain specific nutrition in early Homo. Br J Nutr. 1998;79:3–21. doi: 10.1079/bjn19980004. [DOI] [PubMed] [Google Scholar]
- 13.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 14.Henderson RA, Jensen RG, Lammi-Keefe CJ, Ferris AM, Dardick KR. Effect of fish oil on the fatty acid composition of human milk and maternal and infant erythrocytes. Lipids. 1992;27:863–9. doi: 10.1007/BF02535865. [DOI] [PubMed] [Google Scholar]
- 15.Jensen RG, Bitman J, Carlson SE, Couch SC, Hamosh M, Newburg DS. Milk lipids A. Human milk lipids. In: Jensen RG, editor. Handbook of milk composition. Academic Press; San Diego (CA): 1995. pp. 495–542. [Google Scholar]
- 16.Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr. 2007;98:253–9. doi: 10.1017/S0007114507709078. [DOI] [PubMed] [Google Scholar]
- 17.Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–44. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- 18.Dombrowski MP, Schatz M, Wise R, Momirova V, Landon M, Mabie W, et al. Asthma during pregnancy. Obstet Gynecol. 2004;103:5–12. doi: 10.1097/01.AOG.0000103994.75162.16. [DOI] [PubMed] [Google Scholar]
- 19.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trial. Control Clin Trials. 1988;9:345–64. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 20.Reece MS, McGregor JA, Allen KGD, Harris MA. Maternal and perinatal long-chain fatty acids: possible roles in preterm birth. Am J Obstet Gynecol. 1997;176:907–14. doi: 10.1016/s0002-9378(97)70620-3. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 23.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Mater Child Health J. 1999;3:225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 24.Agresti A. An introduction to categorical data analysis. Wiley; New York (NY): 1996. [Google Scholar]
- 25.Cnaan A, Ryan L. Survival analysis in natural history studies of disease. Stat Med. 1989;8:1255–68. doi: 10.1002/sim.4780081009. [DOI] [PubMed] [Google Scholar]
- 26.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 27.Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, et al. Randomized trial of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–7. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 28.Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomized clinical trial with fish oil. Eur J Clin Nutr. 2007;61:976–85. doi: 10.1038/sj.ejcn.1602609. [DOI] [PubMed] [Google Scholar]
- 29.Lane DM, McConathy WJ, McCaffree MA, Hall M. Cord serum lipid and apolipoprotein levels in preterm infants with the neonatal respiratory distress syndrome. J Matern Fetal Neonatal Med. 2002;11:118–25. doi: 10.1080/jmf.11.2.118.125. [DOI] [PubMed] [Google Scholar]
- 30.Clarke SD, Benjamin L, Phinney SD. Fetal growth and fetal lung phospholipid content in rats fed safflower oil, menhaden oil, or hydrogenated coconut oil. Am J Clin Nutr. 2008;47:828–35. doi: 10.1093/ajcn/47.5.828. [DOI] [PubMed] [Google Scholar]
- 31.Morsy MA, Isohama Y, Miyata T. Prostaglandin E(2) increases surfactant secretion via the EP(1) receptor in rat alveolar type II cells. Eur J Pharmacol. 2001;426:21–4. doi: 10.1016/s0014-2999(01)01211-0. [DOI] [PubMed] [Google Scholar]
- 32.Viscardi RM, Max SR. Unsaturated fatty acid modulation of glucocorticoid receptor binding in L2 cells. Steroids. 1993;58:357–61. doi: 10.1016/0039-128x(93)90038-o. [DOI] [PubMed] [Google Scholar]
- 33.Bulstra-Ramakers MT, Huisjes HJ, Visser GH. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. Br J Obstet Gynaecol. 1995;102:123–6. doi: 10.1111/j.1471-0528.1995.tb09064.x. [DOI] [PubMed] [Google Scholar]
- 34.Makrides M, Duley L, Olsen SF. The Cochrane Database of Systematic Reviews. Issue 3. 2006. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Art. No.: CD003402. DOI: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz N, Xue X, Elovitz MA, Dowling O, Metz CN. Progesterone suppresses the fetal inflammatory response ex vivo. Am J Obstet Gynecol. 2009;201:211.e1–9. doi: 10.1016/j.ajog.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Facchinetti F, Vaccaro V. Pharmacologycal use of progesterone and 17-alfa-hydroxyprogesterone caproate in the prevention of preterm delivery. Minerva Ginecol. 2009;61:401–9. [PubMed] [Google Scholar]