Abstract
Context
The Caregiver Pain Medicine Questionnaire is designed to measure caregiver agreement with statements regarding pain management. However, little testing has been done to determine its reliability and validity.
Objectives
The objective of the study was to test the factorial validity of scores from the Caregiver Pain Medicine Questionnaire as hypothesized by the original study authors.
Methods
Confirmatory factor analysis was conducted to assess whether the subscales postulated by the instrument authors could be replicated in external data.
Results
Fit statistics reveal an unsatisfactory fit between the hypothesized model and the observed data.
Conclusion
The theoretical model hypothesized by the original study authors was not confirmed. Results lead us to conclude that the instrument is poor and should not be used. Further research is needed to define content domains and validate the items developed to assess them.
Keywords: Caregiver Pain Medicine Questionnaire, factor analysis, hospice, pain management
Introduction
Cancer is the second leading cause of death,1 and prevalence of pain in patients with terminal cancer is estimated to be between 59% and 75%.2 Although nearly half of the patients enrolled in hospice programs are cancer patients,3 terminally ill patients with other diseases also experience pain. In fact, pain is a major problem for most hospice patients.4–6 With 66% of the hospice patients dying in their place of residence,3 the day-to-day implementation of pain management plans is accomplished by informal family caregivers. These untrained caregivers are ill prepared for the struggles associated with managing pain in the terminally ill and their former caregiving experiences influence their perception and management of their loved ones’ pain experience.7,8
Barriers to effective pain management arise from knowledge, beliefs, and attitudes of patients and family caregivers.9 A recent review of the literature found that the pharmacological management of pain is challenging for caregivers across cultures and within numerous contexts of care.10 This review concluded that there is a need for targeted educational interventions to address the myths held by caregivers and strategies to increase self-efficacy for caregivers faced with the burden of managing pain.10 It is critical to have reliable and valid scores from instruments both to understand these caregiver perceptions and to evaluate the effectiveness of potential interventions. One such instrument, the Caregiver Pain Medicine Questionnaire (CPMQ), was created in 2004;11 however, little testing has been done to determine the reliability and validity of its scores, especially in the hospice population.
Caregiver Pain Medicine Questionnaire
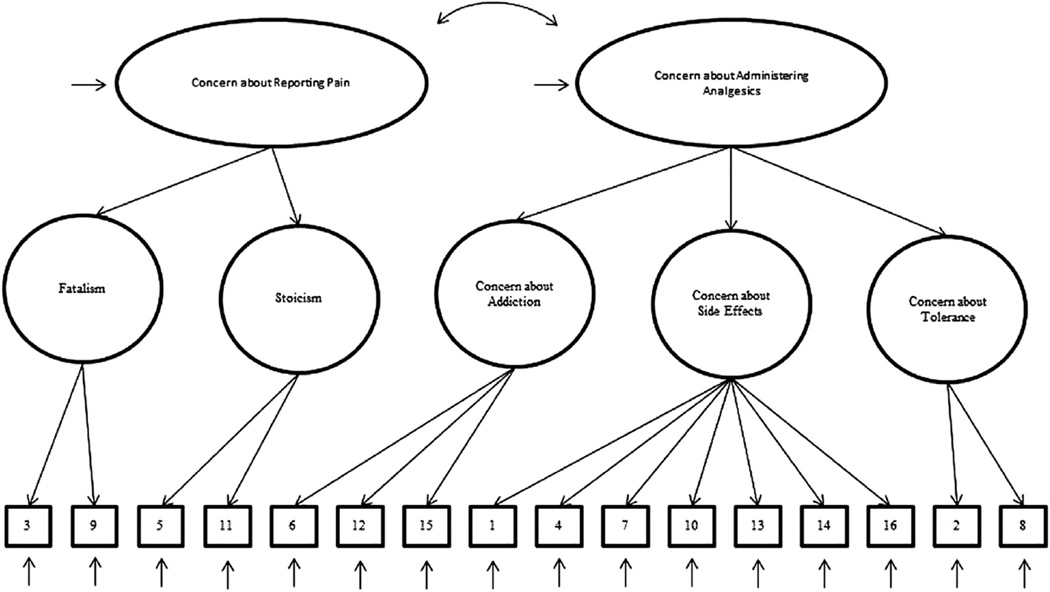
The CPMQ is a 16-item self-report instrument that measures informal caregivers’ agreement with statements regarding pain management, with a few additional questions about medication administration/adherence.11 The factor structure proposed by the original instrument authors11 is hierarchical and includes two second-order factors (“Concern about Reporting Pain” and “Concern about Administering Analgesics”) and five first-order factors (“Fatalism,” “Stoicism,” “Concern about Addiction,” “Concern about Side Effects,” and “Concern about Tolerance”). Hierarchical factor structures represent the associations between multiple observed variables or indicators in terms of a smaller set of associated latent variables or factors, which themselves can be represented by a higher order latent variable or factor.
The original study authors11 supported their decision to include the second-order factors by citing the 1994 Clinical Practice Guidelines for the Management of Cancer Pain,12 which identified the factors as two (of the eight) problems related to patients that are potential barriers to effective cancer pain management. “Concern about Reporting Pain” was delimited to include two first-order factors, namely “Fatalism” and “Stoicism.” “Fatalism” was defined as the belief that pain is inevitable and untreatable.11 “Stoicism” was defined as the belief that pain is to be tolerated and not complained about.11 “Concern about Administering Analgesics” also was delimited to include “Concern about Addiction,” “Concern about Side Effects,” and “Concern about Tolerance.” These factors were not defined. Additional questions about medication administration/adherence were included but the rationale is not documented. The content validity of the CPMQ, however, was tested by having six experts apply the index of content validity,13 resulting in the retention of all 16 items (Table 1 shows a complete list of these items).
Table 1.
Items in the Caregiver Pain Medicine Questionnaire
| Proportion of Responses |
||||||
|---|---|---|---|---|---|---|
| Second-Order Factor, First-Order Factor, Item | 1 | 2 | 3 | 4 | 5 | |
| Concern about reporting pain | ||||||
| Fatalism | ||||||
| 3 | Pain medicine cannot really control pain. | 0.009 | 0.049 | 0.103 | 0.517 | 0.322 |
| 9 | It is not realistic to expect that pain can be relieved. | 0.003 | 0.060 | 0.078 | 0.534 | 0.325 |
| Stoicism | ||||||
| 5 | People in pain should not complain about their pain. | 0.014 | 0.026 | 0.009 | 0.322 | 0.629 |
| 11 | People in pain should be strong; they should take the medicine only when the pain is extreme. | 0.003 | 0.075 | 0.020 | 0.460 | 0.443 |
| Concern about administering analgesics | ||||||
| Concern about addiction | ||||||
| 6 | It is dangerous if hospice patients become addicted to pain medicine. | 0.026 | 0.109 | 0.089 | 0.402 | 0.374 |
| 12 | It is not a good idea for people to take pain medicine regularly because they can get addicted to it. | 0.009 | 0.147 | 0.092 | 0.437 | 0.316 |
| 15 | It a hospice patient becomes addicted to pain medicine, it is one more problem to have to deal with. | 0.020 | 0.218 | 0.118 | 0.385 | 0.259 |
| Concern about side effects | ||||||
| 1 | People should take less than the prescribed dose of pain medication to avoid side effects. | 0.023 | 0.103 | 0.124 | 0.460 | 0.290 |
| 4 | It is easier to put up with pain than with the side effects that come from pain medicine. | 0.006 | 0.043 | 0.144 | 0.483 | 0.325 |
| 7 | Taking pain medication can cause a person to lose control. | 0.017 | 0.204 | 0.279 | 0.353 | 0.147 |
| 10 | Taking too much pain medicine can hasten a person’s death. | 0.023 | 0.201 | 0.250 | 0.330 | 0.195 |
| 13 | It is better to have a good bowel movement than to get constipated with the pain medicine. | 0.086 | 0.310 | 0.224 | 0.256 | 0.124 |
| 14 | It is better to be alert and have pain than to be drowsy with the pain medicine. | 0.011 | 0.075 | 0.141 | 0.506 | 0.267 |
| 16 | When people who take pain medicine have nausea, it is really distressing. | 0.055 | 0.529 | 0.184 | 0.167 | 0.066 |
| Concern about tolerance | ||||||
| 2 | It is not good when people need to take more of the pain medication as time goes on. | 0.017 | 0.144 | 0.103 | 0.457 | 0.279 |
| 8 | It is better to wait to take pain medication until it is really needed or else it will not work later. | 0.009 | 0.141 | 0.118 | 0.437 | 0.296 |
1 = strongly agree; 2 = agree; 3 = undecided; 4 = disagree; 5 = strongly disagree.
Objective and Hypotheses
The CPMQ was developed with a priori hypotheses of the relationships among the variables. It follows that a validity investigation should use confirmatory factor analysis (CFA) to test these relationships. The objective of our study was to test the model of the CPMQ hypothesized by the original study authors.11 The model tests that 1) responses to the CPMQ can be explained by five first-order factors (“Fatalism,” “Stoicism,” “Concern about Addiction,” “Concern about Side Effects,” and “Concern about Tolerance”) and two second-order factors (“Concern about Reporting Pain” and “Concern about Administering Analgesics”); 2) each item has a non-zero loading on the first-order factor it was designed to measure, and zero loadings on the other four first-order factors; 3) residuals associated with each item are uncorrelated; and 4) covariation among the five first-order factors is explained fully by their regression on the second-order factors. Fig. 1 is a diagrammatic representation of the model. We did not test the additional questions about medication administration/ adherence because our pilot study found that 43% of the caregivers did not administer medication, which resulted in high amounts of missing data and increased the likelihood of respondent burden.24
Fig. 1.
Hypothesized second-order model of factorial structure of the Caregiver Pain Medicine Questionnaire (CPMQ). Numbers in the blocks represent CPMQ items.
Methods
Participants
We have CPMQ data from two studies: the ongoing Assessing Caregivers for Team Intervention through Video Encounters (ACTIVE) study (R01NR011472)14 and the pilot study for ACTIVE (R21CA120179).23,24 The ACTIVE study enrolls caregivers of patients in three Midwestern hospices; two Midwestern hospices participated in the pilot study. The present study sample comprises CPMQ data for 352 informal caregivers of hospice patients collected at the time of enrollment, 283 from ACTIVE and 69 from the pilot study. Full study details are provided in another article.14 The health sciences institutional review board of each participating institution approved the study. The sample of 352 caregivers (77% females) was largely married (70%), white (94%), older (M = 59.45, SD = 13.43), and adult children of the patients (50%). Table 2 presents complete caregiver characteristics.
Table 2.
Caregiver Characteristics (N = 352)
| Characteristics | n (%) |
|---|---|
| Age, yrs, mean (SD) | 59.45 (13.43) |
| Sex (female) | 272 (77) |
| Marital status | 350 (100) |
| Never married | 32 (9) |
| Married | 245 (70) |
| Separated | 8 (2) |
| Divorced | 43 (12) |
| Widowed | 21 (6) |
| Other | 1 (1) |
| Education | 346 (100) |
| Some high school | 33 (10) |
| High school/GED | 81 (23) |
| Some college | 95 (27) |
| Undergraduate degree | 69 (20) |
| Graduate degree | 60 (17) |
| Other | 8 (3) |
| Race | 352 (100) |
| American Indian | 2 (1) |
| African American | 20 (5) |
| Caucasian | 329 (93) |
| Other | 1 (1) |
| Employment status | 344 (100) |
| Not employed | 171 (49) |
| Part-time | 36 (11) |
| Full-time | 99 (28) |
| Volunteers | 5 (2) |
| Other | 33 (10) |
| Relationship to patient | 349 (100) |
| Spouse | 91 (26) |
| Adult child | 176 (50) |
| Other | 82 (24) |
SD = standard deviation; GED = general educational development.
Measure
The CPMQ is a 16-item self-report instrument that measures informal caregiver agreement with statements related to pain management. Individual items are scored from one (“strongly agree”) to five (“strongly disagree”) and summed. When informal caregivers agree with any CPMQ statement, it is considered to represent a potential barrier to effective pain management. Thus, lower scores on the overall scale indicate more problematic attitudes toward pain management.
Statistical Analysis
Casewise deletion was applied to missing data. We calculated the sample size needed to obtain accurate parameter estimates to be 153, using recommendations for the analysis of categorical data.15 We evaluated the assumption of multivariate normality by reviewing Mahalanobis distances. We used the means- and variance-adjusted weighted least squares estimator because it performs well in the CFA modeling of categorical data.16,17
The CFA was conducted to test the factor structure postulated by the original authors.11 We used several recommended16,18,19 fit indices and cutoff values to test the model, namely comparative fit index of 0.95 or higher, root mean square error of approximation of 0.06 or lower, and weighted root mean square residual of 0.90 or lower. All analyses were conducted with Mplus 7.20
Given that we have five first-order factors, we have 15 ([5 × 6]/2) pieces of information; the number of estimable parameters is 10 (five factor loadings and five residual variances), thereby resulting in an overidentied model.21 The first factor-loading path for each congeneric set of parameters is automatically constrained to 1.0 and requires no specification. These parameters (i.e., Items 1, 2, 3, 5, and 6) serve as the reference indicator variables in the model related to the first-order factors. The parameter specifications for the second-order factor model constrain the variance of both (i.e., Report and Admin) to 1.0 so that the higher order factor loading paths are allowed to freely estimate.
Results
Data Screening
Table 3 presents a correlation table with means, standard deviations, skewness, and kurtosis of the CPMQ items. Table 1 presents a list of the items on the CPMQ and the summary of the proportions of sample respondents who endorsed each of the five response categories. For example, 46% of the caregivers disagreed and 29% strongly disagreed with Item 1, which elicits a response to “People should take less than the prescribed dose of pain medication to avoid side effects.” This suggests that it is not a strong barrier to pain management. A review of this information for all 16 items reveals that for 13 of the 16 items, most respondents selected Category 4, thereby indicating minimal evidence for barriers to pain management. This is undesirable in an instrument and raises questions about the underlying premise of the instrument.
Table 3.
Correlation Coefficients, Means, Standard Deviations, Skewness, and Kurtosis of the CPMQ Items
| Item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation between items | ||||||||||||||||
| 1 | 1.00 | |||||||||||||||
| 2 | 0.59 | 1.00 | ||||||||||||||
| 3 | 0.45 | 0.54 | 1.00 | |||||||||||||
| 4 | 0.52 | 0.52 | 0.58 | 1.00 | ||||||||||||
| 5 | 0.40 | 0.43 | 0.41 | 0.44 | 1.00 | |||||||||||
| 6 | 0.58 | 0.59 | 0.45 | 0.56 | 0.50 | 1.00 | ||||||||||
| 7 | 0.36 | 0.31 | 0.25 | 0.39 | 0.31 | 0.44 | 1.00 | |||||||||
| 8 | 0.51 | 0.49 | 0.41 | 0.56 | 0.41 | 0.56 | 0.36 | 1.00 | ||||||||
| 9 | 0.48 | 0.48 | 0.53 | 0.51 | 0.52 | 0.51 | 0.29 | 0.58 | 1.00 | |||||||
| 10 | 0.31 | 0.32 | 0.30 | 0.38 | 0.30 | 0.37 | 0.43 | 0.39 | 0.38 | 1.00 | ||||||
| 11 | 0.54 | 0.50 | 0.47 | 0.47 | 0.62 | 0.59 | 0.33 | 0.65 | 0.58 | 0.41 | 1.00 | |||||
| 12 | 0.56 | 0.55 | 0.49 | 0.58 | 0.54 | 0.68 | 0.42 | 0.58 | 0.62 | 0.42 | 0.71 | 1.00 | ||||
| 13 | 0.39 | 0.36 | 0.32 | 0.36 | 0.32 | 0.51 | 0.38 | 0.43 | 0.30 | 0.30 | 0.36 | 0.49 | 1.00 | |||
| 14 | 0.45 | 0.48 | 0.44 | 0.48 | 0.44 | 0.54 | 0.35 | 0.54 | 0.51 | 0.44 | 0.54 | 0.59 | 0.50 | 1.00 | ||
| 15 | 0.53 | 0.53 | 0.94 | 0.51 | 0.44 | 0.68 | 0.40 | 0.55 | 0.48 | 0.44 | 0.54 | 0.63 | 0.457 | 0.570 | 1.00 | |
| 16 | 0.21 | 0.098 | 0.104 | 0.194 | 0.081 | 0.210 | 0.243 | 0.193 | 0.144 | 0.199 | 0.135 | 0.236 | 0.390 | 0.262 | 0.330 | 1.00 |
| Mean | 3.89 | 3.84 | 4.10 | 4.08 | 4.55 | 3.99 | 3.40 | 3.87 | 4.12 | 3.48 | 4.26 | 3.91 | 3.01 | 3.95 | 3.66 | 2.64 |
| SD | 1.009 | 1.044 | 0.831 | 0.826 | 0.747 | 1.073 | 1.021 | 1.023 | 0.810 | 1.082 | 0.856 | 1.023 | 1.186 | 0.892 | 1.135 | 1.026 |
| Skewness | −0.927 | −0.800 | −1.061 | −0.847 | −2.397 | −1.032 | −0.145 | −0.744 | −1.038 | −0.197 | −1.445 | −0.785 | 0.103 | −0.902 | −0.478 | 0.789 |
| Kurtosis | 0.350 | −0.184 | 1.461 | 0.764 | 7.465 | 0.275 | −0.814 | −0.337 | 1.24 | −0.932 | 2.118 | −0.333 | −1.016 | 0.747 | −0.957 | −0.264 |
SD = standard deviation.
Model Fit
The model fit statistics (χ2(98) = 283.73, P < 0.0001, comparative fit index = 0.974, root mean square error of approximation = 0.074, weighted root mean square residual = 0.996) reveal an unsatisfactory fit between the model and the observed data. This means that the factor structure hypothesized by the original study authors11 does not fit our data. Table 4 presents the unstandardized parameter estimates that represent the amount of change in the latent variable as a function of a single unit change in the variable (observed or latent) causing it. The first-and second-order factor loadings reported in Table 5 are all statistically significant at P < 0.05 and almost all of the standardized factor loadings (except for Item 16) are greater than 0.40, suggesting adequate convergent validity.22 Convergent validity is the extent to which items of a specific factor share a high proportion of variance in common. The high correlation of the second-order factors (0.963), however, suggests poor discriminant validity.22 Discriminant validity is the extent to which a factor is different from other factors. Table 6 presents the internal reliabilities of the second- and first-order factors of the CPMQ. The internal reliability coefficients for “Fatalism” (α = 0.59), “Stoicism” (α = 0.57), and “Concern about Tolerance” (α = 0.56) are poor and suggest that the items do not consistently measure these factors.
Table 4.
Unstandardized Parameter Estimates
| Model Results | ||||
|---|---|---|---|---|
| Factor, Item | Estimate | SE | Estimate/SE | Two-Tailed P-Value |
| Fatalism by | ||||
| Item 3 | 1.000 | 0.000 | 999.000 | 999.000 |
| Item 9 | 1.123 | 0.063 | 17.719 | <0.001 |
| Stoicism by | ||||
| Item 5 | 1.000 | 0.000 | 999.000 | 999.000 |
| Item 11 | 1.269 | 0.090 | 14.124 | <0.001 |
| Addiction by | ||||
| Item 6 | 1.000 | 0.000 | 999.000 | 999.000 |
| Item 12 | 1.040 | 0.033 | 31.582 | <0.001 |
| Item 15 | 0.944 | 0.034 | 27.506 | <0.001 |
| Side effects by | ||||
| Item 1 | 1.000 | 0.000 | 999.000 | 999.000 |
| Item 4 | 1.015 | 0.047 | 21.625 | <0.001 |
| Item 7 | 0.731 | 0.056 | 13.108 | <0.001 |
| Item 10 | 0.758 | 0.059 | 12.814 | <0.001 |
| Item 13 | 0.826 | 0.060 | 13.834 | <0.001 |
| Item 14 | 1.014 | 0.048 | 20.911 | <0.001 |
| Item 16 | 0.429 | 0.066 | 6.476 | <0.001 |
| Tolerance by | ||||
| Item 2 | 1.000 | 0.000 | 999.000 | 999.000 |
| Item 8 | 1.054 | 0.052 | 20.249 | <0.001 |
| Concern about reporting pain by | ||||
| Fatalism | 0.642 | 0.035 | 18.410 | <0.001 |
| Stoicism | 0.633 | 0.044 | 14.478 | <0.001 |
| Concern about administering analgesics by | ||||
| Addiction | 0.795 | 0.022 | 36.129 | <0.001 |
| Side effects | 0.696 | 0.028 | 25.026 | <0.001 |
| Tolerance | 0.712 | 0.025 | 25.696 | <0.001 |
| Reporting with administering | 0.963 | 0.023 | 42.452 | <0.001 |
| Variances | ||||
| Reporting | 1.000 | 0.000 | 999.000 | 999.000 |
| Administering | 1.000 | 0.000 | 999.000 | 999.000 |
| Residual variances | ||||
| Fatalism | 0.064 | 0.031 | 2.061 | 0.039 |
| Stoicism | 0.083 | 0.028 | 3.007 | 0.003 |
| Addiction | 0.045 | 0.016 | 2.752 | 0.006 |
| Side effects | 0.034 | 0.015 | 2.222 | 0.026 |
| Tolerance | –0.046 | 0.027 | –1.672 | 0.095 |
SE = standard error.
Table 5.
Standardized Parameter Estimates
| Model Results | ||||
|---|---|---|---|---|
| Estimate | SE | Estimate/SE | Two-Tailed P-Value | |
| Fatalism by | ||||
| Item 3 | 0.690 | 0.036 | 19.320 | <0.001 |
| Item 9 | 0.774 | 0.031 | 25.023 | <0.001 |
| Stoicism by | ||||
| Item 5 | 0.696 | 0.036 | 19.212 | <0.001 |
| Item 11 | 0.884 | 0.035 | 25.479 | <0.001 |
| Addiction by | ||||
| Item 6 | 0.823 | 0.020 | 41.062 | <0.001 |
| Item 12 | 0.856 | 0.020 | 43.622 | <0.001 |
| Item 15 | 0.777 | 0.023 | 34.344 | <0.001 |
| Side effects by | ||||
| Item 1 | 0.719 | 0.029 | 25.112 | <0.001 |
| Item 4 | 0.730 | 0.028 | 26.156 | <0.001 |
| Item 7 | 0.526 | 0.039 | 13.418 | <0.001 |
| Item 10 | 0.545 | 0.038 | 14.193 | <0.001 |
| Item 13 | 0.594 | 0.038 | 15.727 | <0.001 |
| Item 14 | 0.730 | 0.029 | 25.542 | <0.001 |
| Item 16 | 0.309 | 0.045 | 6.849 | <0.001 |
| Tolerance by | ||||
| Item 2 | 0.679 | 0.035 | 19.591 | <0.001 |
| Item 8 | 0.715 | 0.031 | 23.208 | <0.001 |
| Concern about reporting pain by | ||||
| Fatalism | 0.930 | 0.032 | 28.629 | <0.001 |
| Stoicism | 0.910 | 0.032 | 28.307 | <0.001 |
| Concern about administering analgesics by | ||||
| Addiction | 0.967 | 0.012 | 78.705 | <0.001 |
| Side effects | 0.967 | 0.014 | 67.326 | <0.001 |
| Tolerance | 1.049 | 0.031 | 33.479 | <0.001 |
| Reporting with administering | 0.963 | 0.023 | 42.452 | <0.001 |
| Variances | ||||
| Reporting | 1.000 | 0.000 | 999.000 | 999.000 |
| Administering | 1.000 | 0.000 | 999.000 | 999.000 |
| Residual variances | ||||
| Fatalism | 0.135 | 0.060 | 2.233 | 0.026 |
| Stoicism | 0.172 | 0.058 | 2.944 | 0.003 |
| Addiction | 0.066 | 0.024 | 2.770 | 0.006 |
| Side effects | 0.065 | 0.028 | 2.330 | 0.020 |
| Tolerance | −0.100 | 999.000 | 999.000 | 999.000 |
SE = standard error.
Table 6.
Internal Consistency of the CPMQ
| Scalesa | Number of Itemsb | Mean (SD)c | Cronbach’s Alpha |
|---|---|---|---|
| Total | 16 | 60.84 (9.66) | 0.80 |
| Concern about reporting pain | 4 | 17.05 (2.32) | 0.65 |
| Fatalism | 2 | 8.23 (1.38) | 0.59 |
| Stoicism | 2 | 8.82 (1.33) | 0.57 |
| Concern about administering analgesics | 12 | 43.73 (7.93) | 0.78 |
| Concern about addiction | 3 | 11.55 (2.69) | 0.78 |
| Concern about side effects | 7 | 24.46 (4.49) | 0.75 |
| Concern about tolerance | 2 | 7.71 (1.72) | 0.56 |
CPMQ = Caregiver Pain Medicine Questionnaire; SD = standard deviation.
Each measures the average response to the items.
Each item is scored from one (strongly agree) to five “strongly disagree.”
Means and SDs of raw scores.
Table 7 presents the reliability estimates and residual variances for each of the items and the reliability estimates for the five first-order factors. The residual variances for the items reveal that the factor, “Concern about Side Effects,” does not explain 72% (0.724) of the variance of Item 7, 70% (0.702) of the variance of Item 10, or 91% (0.905) of the variance of Item 16, suggesting that the items do a poor job of operationalizing the factor. The reliability estimates reveal that seven of the 16 items are weak (R2 < 0.5). These seven items have weak factor-indicator relationships, which is another indicator of poor model fit and suggests that four of the first-order factors (“Fatalism,” “Stoicism,” “Concern about Side Effects,” and “Concern about Tolerance”) could be better operationalized. Additionally, the factor, “Concern about Tolerance,” is undefined, which is not surprising given that the residual variance of the factor is negative (known as a Heywood case, which indicates that the data do not fit the model) and that there are only two items (a minimum of three items per factor is generally recommended). This also suggests a problematic fit between the model and data. Finally, the model modification indices (available on request) indicate multiple misspecifications (e.g., cross-loading factors and covarying residuals) suggesting poor model fit to the data.
Table 7.
Reliability Estimates
|
R2 | |||||
|---|---|---|---|---|---|
| Estimate | SE | Estimate/SE | Two-Tailed P-Value | Residual Variance | |
| Observed variables | |||||
| Item 1 | 0.518 | 0.041 | 12.556 | <0.001 | 0.482 |
| Item 2 | 0.461 | 0.047 | 9.795 | <0.001 | 0.539 |
| Item 3 | 0.476 | 0.049 | 9.660 | <0.001 | 0.524 |
| Item 4 | 0.534 | 0.041 | 13.078 | <0.001 | 0.466 |
| Item 5 | 0.485 | 0.050 | 9.606 | <0.001 | 0.515 |
| Item 6 | 0.677 | 0.033 | 20.531 | <0.001 | 0.323 |
| Item 7 | 0.276 | 0.041 | 6.709 | <0.001 | 0.724 |
| Item 8 | 0.512 | 0.044 | 11.604 | <0.001 | 0.488 |
| Item 9 | 0.600 | 0.048 | 12.512 | <0.001 | 0.400 |
| Item 10 | 0.298 | 0.042 | 7.096 | <0.001 | 0.702 |
| Item 11 | 0.781 | 0.061 | 12.740 | <0.001 | 0.219 |
| Item 12 | 0.732 | 0.034 | 21.811 | <0.001 | 0.268 |
| Item 13 | 0.353 | 0.045 | 7.863 | <0.001 | 0.647 |
| Item 14 | 0.532 | 0.042 | 12.771 | <0.001 | 0.468 |
| Item 15 | 0.604 | 0.035 | 17.172 | <0.001 | 0.396 |
| Item 16 | 0.095 | 0.028 | 3.424 | <0.001 | 0.905 |
| Latent variables | |||||
| Fatalism | 0.865 | 0.060 | 14.314 | <0.001 | |
| Stoicism | 0.828 | 0.058 | 14.154 | <0.001 | |
| Addiction | 0.934 | 0.024 | 39.353 | <0.001 | |
| Side effects | 0.935 | 0.028 | 33.663 | <0.001 | |
| Tolerance | Undefined | ||||
SE = standard error.
Discussion
Our results suggest that both the second-order factors (“Concern about Reporting Pain” and “Concern about Administering Analgesics”) and one first-order factor (“Concern about Tolerance”) should be eliminated in future modeling of the scores from the CPMQ. The items from this first-order factor (Items 2 and 8) should be included in the operationalization of the “Concern about Side Effects” factor. Items 7, 10, 13, and 16 should be eliminated completely. Additionally, the “Fatalism” and “Stoicism” factors are unlikely to have much psychometric robustness as demonstrated by the low reliability coefficients presented in Table 6.
In a qualitative analysis of caregiver interviews from the larger study, only two of the CPMQ factors were found as themes in the caregiver comments.25 Although caregiver themes were consistent with “Concern about Administering Analgesics” and “Concern about Side Effects,” there were no caregiver comments to provide evidence related to “Concern about Reporting Pain” or “Concern about Tolerance.” Additionally, we identified strong themes related to challenges in communication and challenges with assessing pain. The study also found instances of unrelieved pain as pain management protocols were not followed by the caregivers. Finally, for some caregivers who followed prescribed protocols, concerns that they may have (or the nurse administering the final doses may have) facilitated the death of the patient were mentioned.25
Hospice caregivers do hold beliefs and experiences that impact both how they make sense of pain and their willingness and ability to manage pain during the hospice experience. Studies show us that these beliefs and experiences are impacted by previous experiences with pain; thus, both unrelieved and inaccurate representations of pain within the hospice experience can impact future decision making. These challenges with pain management are not unique to the U.S. hospice experience, but to the death and dying experience globally.26–29 The unique aspect for U.S. hospice caregivers is that most care for these patients occurs in a residential setting, and untrained family caregivers are the ones primarily responsible for carrying out the pain management strategies.30
It is clear that hospice providers need validated instruments to help assess and measure these family beliefs as well as a clear understanding of the former experiences that may impede the hospice teams’ ability to control patients’ pain as caregivers implement the plans developed by the hospice staff. Theoretically informed and psychometrically robust instruments that are easy to administer and interpret could be very valuable as hospice nurses and physicians develop plans of care to address patients’ pain. Although patients’ pain ratings are an important ingredient of pain management strategies, so are the beliefs of the caregivers charged with implementing those strategies. Caregivers believing that a prescribed dose of morphine may kill their loved one will be hesitant to give that medication as prescribed, or will be left with the guilt that they may have hastened the death of their loved one.
Conclusions
The need to measure caregivers’ beliefs of pain management is significant and the original authors’ intentions to do so with the CPMQ were pioneering; however, our results lead us to conclude that the instrument is poor and should not be used. Although some qualitative evidence7 exists for the barriers to pain management measured by the CPMQ, the results of our study suggest the definition of the content domain and the relationship between that domain and the content of the CPMQ is problematic. We recognize the significant need for such an instrument and, therefore, recommend the development of a new instrument to measure caregivers’ beliefs of pain management in hospice settings that is informed by a theoretical rationale, with congruent conceptual and operational definitions, and is easily translated into a useable form for bedside hospice providers.
Acknowledgments
This project was supported by Award Number R01NR011472 from the National Institute of Nursing Research and Award Number R21CA120179 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, the National Cancer Institute, or the National Institutes of Health. The ClinicalTrials.gov registration number for ACTIVE is NCT01211340.
Footnotes
Disclosures
There are no other financial relationships associated with these results that may reflect a conflict of interest or be perceived to reflect a conflict of interest.
References
- 1.AfHRa. National health disparities report. Rockville, MD: AHRQ; 2005. [Google Scholar]
- 2.Swarm R, Abernathy A, Angehelescu D, et al. Adult cancer pain. J Natl Compr Canc Netw. 2010;8:1046–1086. doi: 10.6004/jnccn.2010.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Hospice and Palliative Care Organization. [Accessed April 5, 2013]; Available from www.nhpco.org.
- 4.Sahlberg-Blom E, Ternestedt B, Johansson J. Is good ‘quality of life’ possible at the end of life? an explorative study of the experiences of a group of cancer patients in two different care cultures. J Clin Nurs. 2001;10:550–561. doi: 10.1046/j.1365-2702.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- 5.Hermann C, Looney S. The effectiveness of symptom management in hospice patients during the last seven days of life. J Hosp Palliat Nurs. 2001;3:88–96. [Google Scholar]
- 6.Kutner JS, Kassner CT, Nowels DE. Symptom burden at the end of life: hospice providers’ perceptions. J Pain Symptom Manage. 2001;21:473–480. doi: 10.1016/s0885-3924(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 7.Parker Oliver D, Wittenberg-Lyles E, Washington K, et al. Hospice caregivers’ concerns with pain: “I’m not a doctor and I don’t know if I helped her go faster or slower”. J Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2013.02.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker Oliver D, Wittenberg-Lyles E, Demiris G, et al. Barriers to pain management: caregiver perception and pain talk by hospice interdisciplinary teams. J Pain Symptom Manage. 2008;36:374–382. doi: 10.1016/j.jpainsymman.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A, Schonberg M, Fisher J, et al. Emergency department experiences of acutely symptomatic patients with terminal illness and their family caregivers. J Pain Symptom Manage. 2010;39:972–981. doi: 10.1016/j.jpainsymman.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meeker M, Finnell D, Othman A. Family caregivers and cancer pain management: a review. J Fam Nurs. 2011;17:29–60. doi: 10.1177/1074840710396091. [DOI] [PubMed] [Google Scholar]
- 11.Letizia M, Creech S, Norton E, Shanahan M, Hedges L. Barriers to caregiver administration of pain medication in hospice care. J Pain Symptom Manage. 2004;27:114–124. doi: 10.1016/j.jpainsymman.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Jaycox A, Carr D, Payne R, et al. Clinical Practice Guideline. Rockville, MD: Agency for Health Care Policy and Research, U.S. Department of Health and Human Services; 1994. Management of cancer pain. No. 9 AHCPR Publication No. 94–0592. [Google Scholar]
- 13.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382–386. [PubMed] [Google Scholar]
- 14.Kruse RL, Parker Oliver D, Wittenberg-Lyles E, Demiris G. Conducting the ACTIVE randomized trial in hospice care: keys to success. Clin Trials. 2013;10:160–169. doi: 10.1177/1740774512461858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joreskog KG, Sorbom D. LISREL 8: User’s reference guide. Chicago, IL: Scientific Software International; 1996. [Google Scholar]
- 16.Confirmatory factor analysis for applied research. New York, NY: Guilford; 2006. Brown TA. [Google Scholar]
- 17.Beauducel A, Herzberg PY. On the performance of maximum likelihood versus means and variance adjusted weighted least squares estimation in CFA. Struct Equ Modeling. 2006;13:186–203. [Google Scholar]
- 18.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 19.Yu C-Y, Muthen B. Evaluation of model fit indices for latent variable models with categorical and continuous outcomes. [Accessed April 5, 2013];Technical report. Available from http://www.statmodel.com/download/Yudis sertation.pdf.
- 20.Muthen LK, Muthen B. Mplus version 7 base program and combination add-on. Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- 21.Harrington D. Confirmatory factor analysis. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 22.Canrera-Nguyen P. Author guidelines for reporting scale development and validation results in the Journal of the Society for Social Work and Research. J Soc Social Work Res. 2010;1:99–103. [Google Scholar]
- 23.Parker Oliver D, Porock D, Demiris G, Courtney K. Patient and family involvement in hospice interdisciplinary teams. J Palliat Care. 2005;21:270–276. [PubMed] [Google Scholar]
- 24.Parker Oliver D, Demiris G, Wittenberg-Lyles E, et al. Caregiver participation in hospice interdisciplinary team meetings via videophone technology: a pilot study to improve pain management. Am J Hosp Palliat Care. 2010;27:465–473. doi: 10.1177/1049909110362402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker Oliver D, Albright DL, Washington K, et al. Hospice caregiver depression: the evidence surrounding the greatest pain of all. J Soc Work End Life Palliat Care. doi: 10.1080/15524256.2013.846891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Rosa M, Kofahl C, McKee K. A typology of caregiving situations and service use in family carers of older people in six European countries: the EUROFAMCARE study. GeroPsych. 2011;24:5–18. [Google Scholar]
- 27.Perner A, Kohler N, Brahler E, Gorze H. Quality of life and satisfaction of family caregivers in palliative care—results of postmortem interviews with bereaved family members[Article in German] Z Psychosom Med Psychother. 2012;58:267–281. doi: 10.13109/zptm.2012.58.3.267. [DOI] [PubMed] [Google Scholar]
- 28.Csikós Á, Nagy L, Busa C, Kállai J. Important aspects of end-of-life care. Survey of patients visiting the primary care office. [Article in Hungarian] Orv Hetil. 2011;152:1082–1090. doi: 10.1556/OH.2011.29139. [DOI] [PubMed] [Google Scholar]
- 29.Andruccioli J, Russo M, Bruschi A. Death representation of caregivers in hospice. Am J Hosp Palliat Care. 2012;29:531–535. doi: 10.1177/1049909111432623. [DOI] [PubMed] [Google Scholar]
- 30.Lau DT, Berman R, Halpern L, et al. Exploring factors that influence informal caregiving in medication management for home hospice patients. J Palliat Med. 2010;13:1085–1090. doi: 10.1089/jpm.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]