Abstract
Purpose
In the metastatic process, interactions between circulating tumor cells (CTCs) and the extracellular matrix or surrounding cells are required. β1-Integrin may mediate these interactions. The aim of this study was to investigate whether β1-integrin is associated with the detection of CTCs in colorectal cancer.
Methods
We enrolled 30 patients with colorectal cancer (experimental group) and 30 patients with benign diseases (control group). Blood samples were obtained from each group, carcinoembryonic antigen (CEA) mRNA for CTCs marker and β1-integrin mRNA levels were estimated by using reverse transcription-polymerase chain reaction, and the results were compared between the two groups. In the experimental group, preoperative results were compared with postoperative results for each marker. In addition, we analyzed the correlation between the expressions of β1-integrin and CEA.
Results
CEA mRNA was detected more frequently in colorectal cancer patients than in control patients (P = 0.008). CEA mRNA was significantly reduced after surgery in the colorectal cancer patients (P = 0.032). β1-Integrin mRNA was detected more in colorectal cancer patients than in the patients with benign diseases (P < 0.001). In colorectal cancer patients, expression of β1-integrin mRNA was detected more for advanced-stage cancer than for early-stage cancer (P = 0.033) and was significantly decreased after surgery (P < 0.001). In addition, expression of β1-integrin mRNA was significantly associated with that of CEA mRNA in colorectal cancer patients (P = 0.001).
Conclusion
In conclusion, β1-integrin is a potential factor for forming a prognosis following surgical resection in colorectal cancer patients. β1-Integrin may be a candidate for use as a marker for early detection of micrometastatic tumor cells and for monitoring the therapeutic response in colorectal cancer patients.
Keywords: β1-Integrin, Circulating tumor cells, Carcinoembryonic antigen, Colorectal cancer
INTRODUCTION
Colorectal cancer is one of the most common cancers worldwide, and is well known to be the main cause of the cancer death [1, 2]. Early diagnosis and development of its treatment options have improved the treatment outcome continuously; however, it still ranks as the 4th and the 3rd highest mortality rates among men and women, respectively [2]. Such high mortality rates are due to recurrence and metastasis of the cancer, which are shown in 50% of the patients [3]. Thus, providing a proper plan for patients with colorectal cancer is necessary in order to improve the mortality rate by early detection of the metastasis of cancer.
The concept of circulating tumor cells was derived from such a background. Circulating tumor cells are tumor cells that circulate in the peripheral blood of the patients, and they are originally derived from a primary cancer. The presence of circulating tumor cells is one of the steps that are necessary to provoke distant metastasis. This process includes detachment of cancer cells from the primary lesion and their implantation at target organs through peripheral circulation, thus establishing the distant metastasis [2]. Recently, many methods had been tried in order to extract circulating tumor cells effectively, and carcinoembryonic antigen (CEA) has been used as the typical marker for circulating tumor cells [2, 4, 5]. However, practical usage of circulating tumor cells in clinical situations is still limited due to the facts that they are extracted in small amounts and that the method of extraction is yet to be standardized. Consequently, further development of a new marker that not only reflects the expression of circulating tumor cells but also is involved in the metastatic process of colon cancers is necessary in the future.
Cellular adhesion molecules are major molecules that are involved in the metastatic process of circulating tumor cells. In order for the circulating tumor cells to cause metastasis, they need to attach to the vascular wall and penetrate into surrounding tissues while they are circulating in the peripheral blood. During this process, an interaction between the tumor cells and the surrounding extracellular matrix near the target organ is required. At this very moment, many types of cellular adhesion molecules are expressed abnormally, especially, integrin. Integrin is a major cellular adhesion molecule that plays an important role in the migration and the invasion of the tumor cells [6, 7]. Among these molecules, β1-integrin is known to be overexpressed in many cancers, especially in metastatic colorectal cancer [6].
Many articles have reported on circulating tumor cell or β1-integrin in colorectal cancer. Unfortunately, such previous investigations were mainly reporting the functions of each molecule. So far, very few articles have attempted to reveal the interactions between the molecules. Cancer metastasis is a process of several mechanisms, in which we assume that a relation may exist between the number of circulating tumor cells and the expression of the β1-integrin. In this study, we evaluated the expression level of β1-integrin in relation to different clinical characteristics of patients with colorectal cancer. The expression level of β1-integrin was compared to that of CEA, which is a marker for circulating tumor cells, in order to analyze their relationship. In this way, we aimed to investigate the function of β1-integrin, which might be utilized as a new marker to predict the presence of micrometastasis of the cancer and the prognosis for the patient.
METHODS
This study consisted of 2 groups of 30 patients each, for a total of 60 patients. The experimental group included patients who were diagnosed with colorectal cancer at the Surgery Department of Ewha Woman's University Mokdong Hospital and were to go surgery. The patients of the experimental group were diagnosed with an adenocarcinoma of the colorectum through tissue biopsy, and they had no history of cancer of other organs, metastasis that is accompanied by cancer of other organs, surgery, chemotherapy, radiotherapy, or any other procedures that were part of the treatment for cancer. The control group included patients suffering from benign diseases who had no history of cancer. Written informed consent was obtained for all the enrolled patients. The protocol of this study was confirmed by clinical audit committee of our institution (confirmation number: ECT12-10B-25).
Peripheral venous blood samples were taken from the experimental group during preoperative state under anesthesia and on the 7th postoperative day. In the control group, the blood samples were only taken before the surgery while the patients were under sedation. When the peripheral blood was sampled, an angio-needle was inserted, and first 5 mL of the sample was discarded so that contamination of the epithelial cells might be prevented. An additional 10 mL of venous blood was sampled and stored in an ethylenediaminetetraacetic acid bottle.
At room temperature, the blood was centrifuged for 10 minutes at 2,500 rpm, and the serum was removed. The buffy coat layer was separated, and RNA was extracted according to a conventional method [8]. One microgram of the separated RNA was stored at 42℃ for 15 minutes, at 95℃ for 5 minutes, and at 4℃ for 5 minutes by using a reverse transcription system (Promega Co., Madison, WI, USA) consisting of a reverse transcription 1 × buffer, 5-mM MgCl2, 10-mM deoxynucleotide (dNTP), 40 unit/µL recombinant RNasin ribonuclease inhibitor, 25 unit/µL avian myeloblastosis virus reverse transcriptase, and 0.5 µg of oligo dT 15 primer in order to reverse transcribe into cDNA. Primers of CEA, which is a marker for circulating tumor cells, β1-integrin, which is a cell adhesion molecule, and β-actin, which is an internal control, were produced, and then polymerase chain reaction (PCR) was performed (Table 1). GoTaq DNA polymerase, 5 × green GoTaq reaction buffer, dNTP mix (Promega Co.), each primer mixture, and distilled water were mixed together. Four microliter of CEA cDNA, 2.7 µL of β1-integrin cDNA, and 1 µL of β-actin cDNA were mixed together to produce the PCR mixture. The amplification of the primer was processed through denaturation of CEA at 95℃ for 5 minutes, and then at different temperatures of 95℃, 55℃, and 72℃ for 30 seconds each for 35 cycles. Lastly, the mixture was stored for 5 minutes at 72℃. In the case of β1-integrin, each mixture that contained primer pairs was denatured at 95℃ for 5 minutes and at 95℃, 57℃, and 72℃ for 1 minute each for 35 cycles. The last process included storage at 72℃ for 5 minutes, after which the amplifying process was completed.
Table 1.
Primers used for amplification
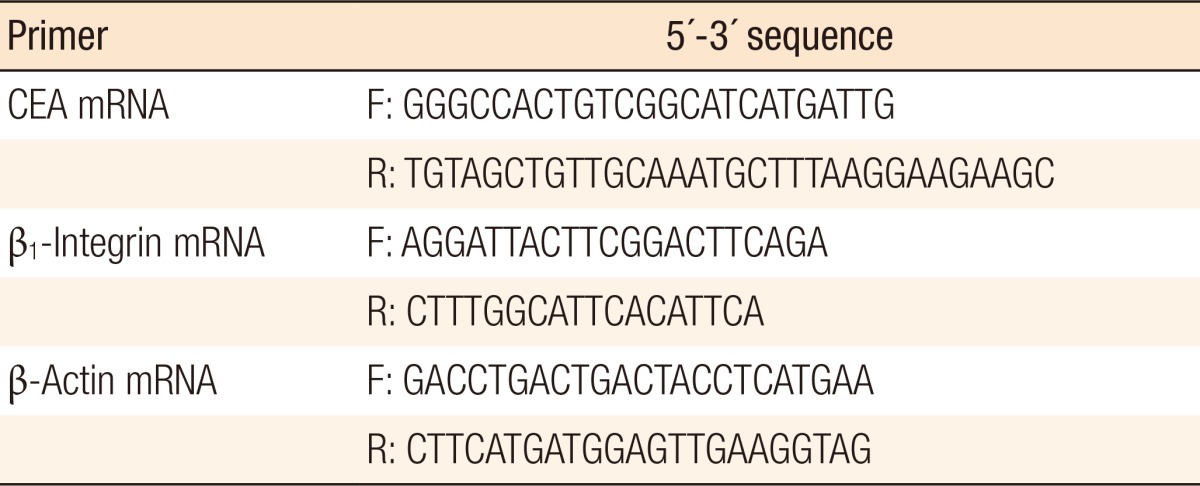
CEA, carcinoembryonic antigen; F, forward; R, reverse.
From the electrophoretic reverse transcription-polymerase chain reaction (RT-PCR) results that were obtained from each sample, we could assess the expression of CEA and β1-integrin. In order to semiquantify their expression levels, we measured the density of the observed bands of electrophoresis with the help of UN-SCAN-IT gel ver. 6.1 (Silk Scientific Inc., Orem, UT, USA). The results of the procedure were comparatively analyzed using the histological characteristics of the patients, and the expression levels of CEA and β1-integrin, along with their relationship, were analyzed at the same time.
The analysis of the clinical characteristics of the patients was done by using descriptive statistics, and we compared the positivities of CEA and β1-integrin by using the Mann-Whitney the analysis of variance, and the chi-square tests. In the experimental group, the comparison of each marker before and after surgery was completed with the paired t-test and the chi-square test. The comparison of the experimental and the control groups was done by using the Student t-test and the chi-square test. Also, the analysis of the correlation between the positivity of CEA and that of β1-integrin was done by using Pearson correlation coefficient and the chi-square test in order to identify the statistical meaning of the results. The program used for this analysis was SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), and we considered the results to be significant when the P-value was less than 0.05.
RESULTS
Characteristics of patients
A total of 30 patients were included in the experimental group: 16 males (53.3%) and 14 females (46.7%). Their mean age was 61.26 ± 12.24 years (range, 34-6 years); 16 patients (53.3%) were under the age of 60, and 14 patients (46.7%) were above the age of 60. They all went through a curative resection of colorectal cancer, and after the surgery, they were histopathologically diagnosed with an adenocarcinoma of the colorectum. Sixteen patients (53.5%) had lesions in their colon, and 14 patients (46.7%) had lesions in their rectum. Among these patients, 7 patients (23.3%) showed pathological stage I, 12 patients (40.0%) showed stage II, and 11 patients (36.7%) showed stage III cancer. We observed lymph-node metastasis among 11 patients (36.7%) while vascular invasion and lymphatic invasion were observed in 5 (16.7%) and 9 patients (30.0%), respectively (Table 2).
Table 2.
Patients' characteristics
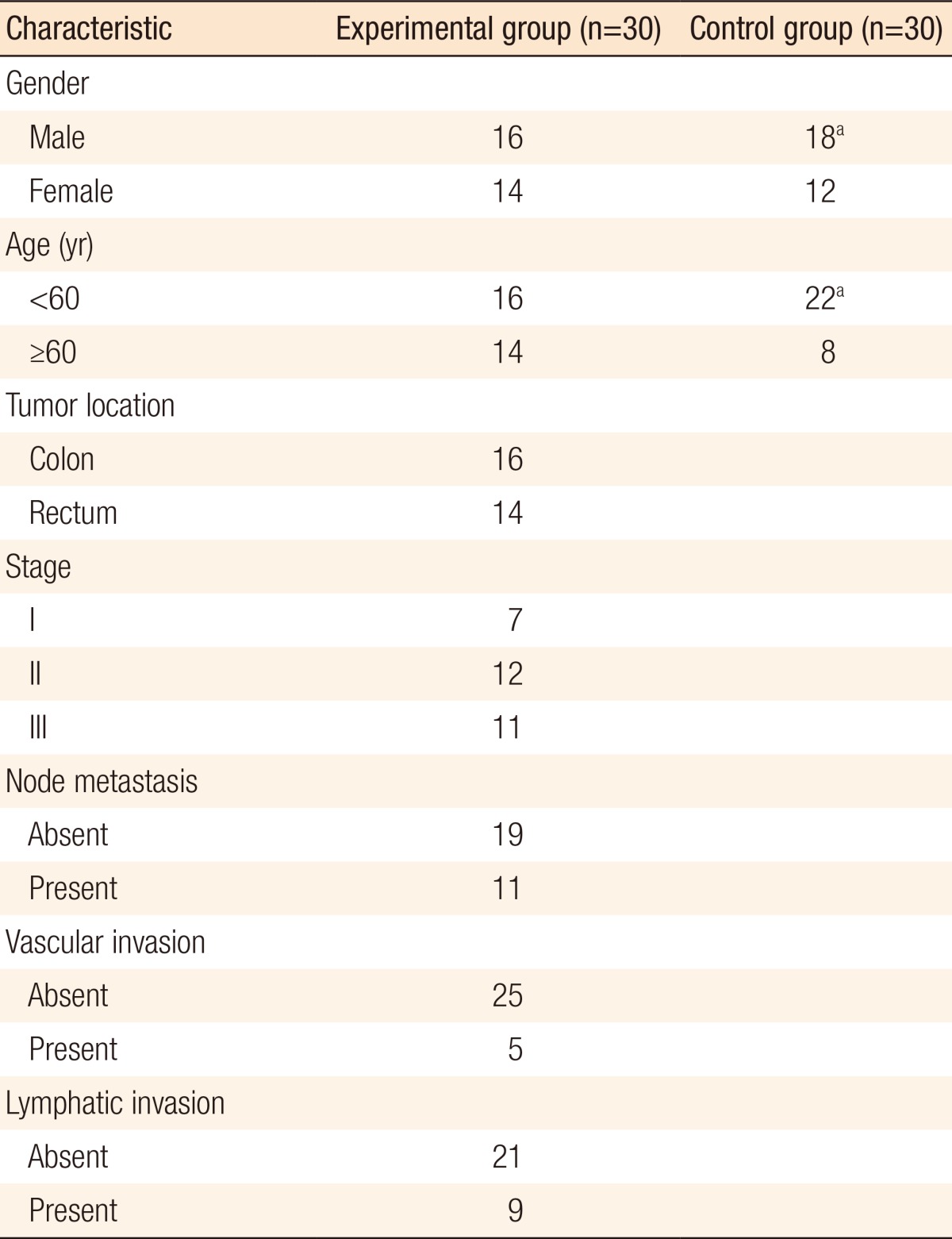
aNo statistically significant difference between the experimental and the control groups.
The control group consisted of 30 patients: 18 males (60.0%) and 12 females (40.0%). Their mean age was 56.17 ± 10.45 years (range, 43-4 years), and patients under and above the age of 60 were 22 (77.3%) and 8 (26.7%), respectively. These groups showed no significant difference in regards to age distribution (P = 0.088). The control group underwent surgery for benign disease. Among these patients, hemorrhoids were observed in 9 patients, inguinal hernias in 7, anal fistulae in 4, abdominal hernias in 3, appendicitis in 2, diverticulitis or rectal prolapse in 2, and varicose veins in 1.
CEA mRNA expression
According to the findings of the analysis of the expression level of CEA, which depends on the clinicopathological characteristics of the experimental group, no significant difference in expression level was discovered. The density of the band that was observed as a result of electrophoresis seemed to be higher in women (P = 0.034), but no other factor showed any significant difference (Table 3).
Table 3.
Expression of CEA according to the clinicopathologic findings in the experimental group
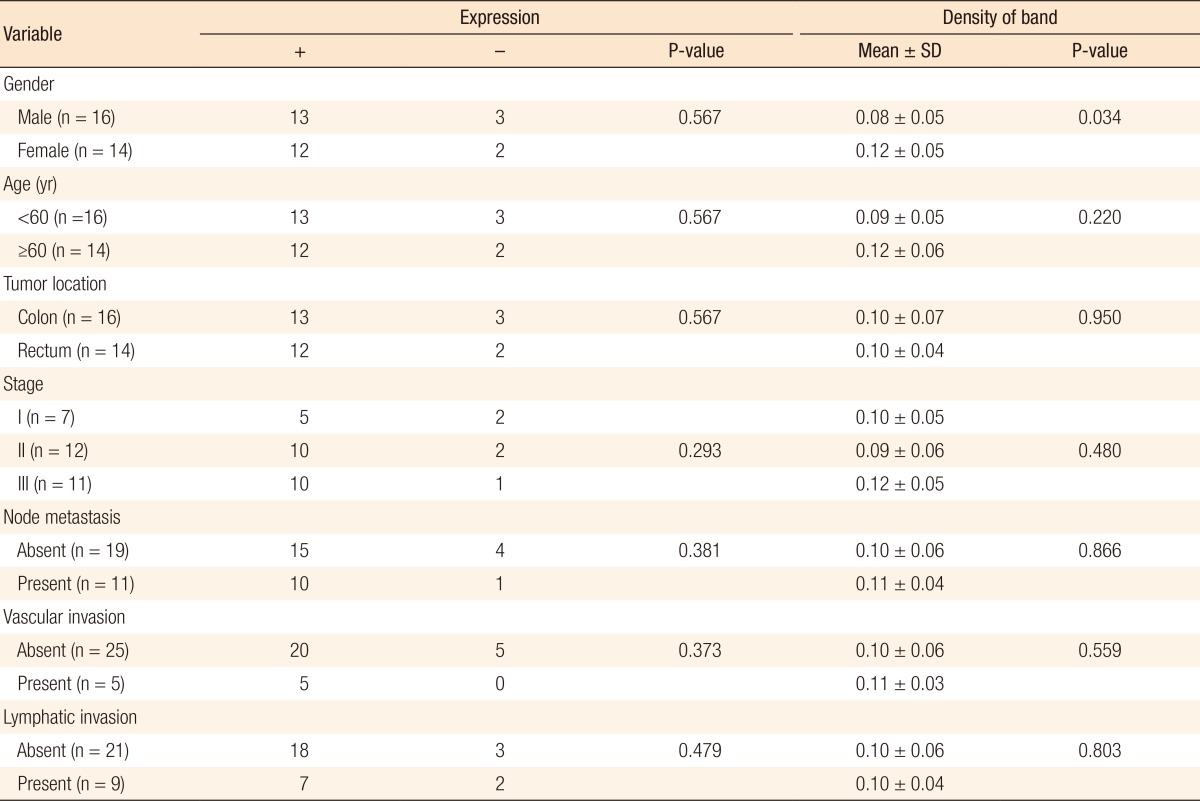
SD, standard deviation.
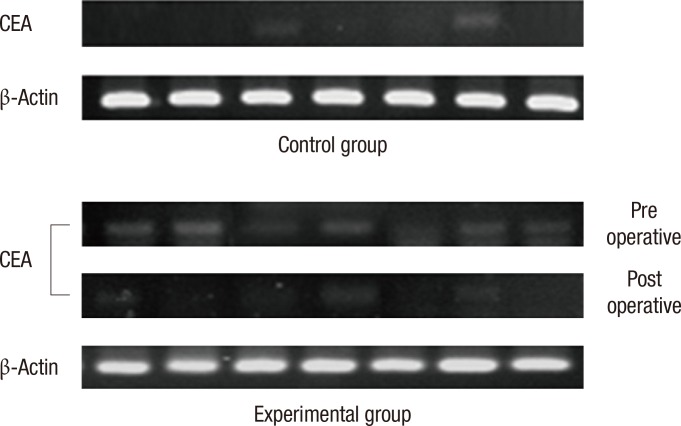
We comparatively analyzed the expression level of CEA mRNA in each patient group. Preoperative positive CEA expression was shown in 25 patients in the experimental group, which is a higher value than that of a control group, in which only 9 patients showed positivity (P = 0.008). There was significant difference in the density of the electrophoretic band (0.10 ± 0.06 vs. 0.03 ± 0.03, P < 0.001) (Table 4, Fig. 1). Moreover, when the expression levels of CEA that were measured before and after surgery in the experimental group were compared to each other, positivity was found to be extensively reduced after surgery (n = 25 vs. n = 13, P = 0.032). The density of the electrophoretic band was lower after sugery (0.10 ± 0.06 vs. 0.03 ± 0.04, P < 0.001) (Table 4, Fig. 1).
Table 4.
Expression of carcinoembryonic antigen (CEA) in the control and the experimental groups
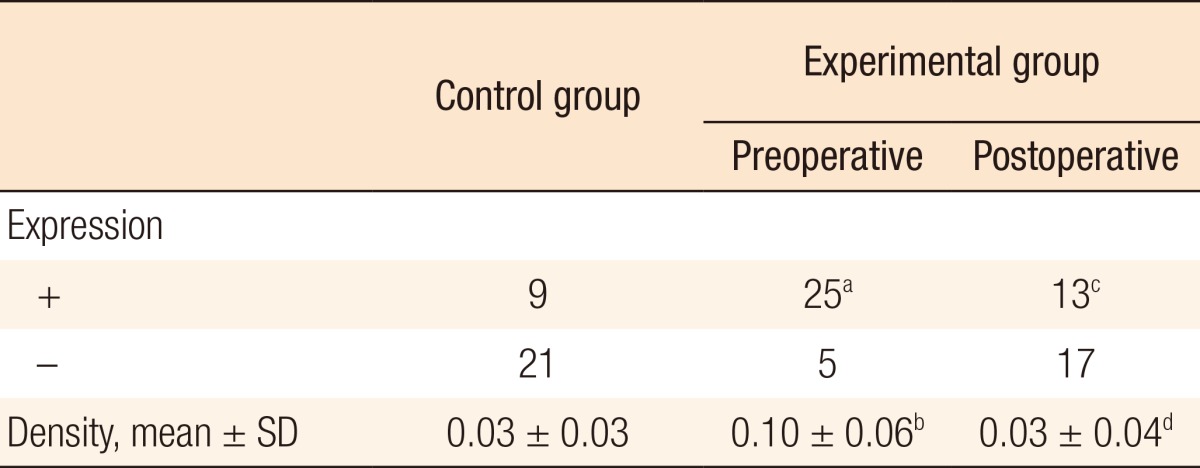
SD, standard deviation.
aExpression of CEA was detected more in the experimental group than in the control group (P = 0.008). bPreoperative density of CEA was higher in the experimental group than in the control group (P < 0.001). cPositive expression of CEA was significantly decreased in the experimental group after surgery (P = 0.032). dDensity of CEA in the experimental group was significantly decreased after surgery (P < 0.001).
Fig. 1.
Detection of carcinoembryonic antigen (CEA) in the blood of each group by using reverse transcription-polymerase chain reaction. CEA was detected more frequently in colorectal cancer patients than in the controls (P = 0.008). In colorectal cancer patients, expression of CEA was significantly decreased after surgey (P = 0.032). β-actin is an internal control.
β1-Integrin mRNA expression
We analyzed the expression level of β1-integrin mRNA according to the clinicopathological characteristics of the experimental group. For higher histological stages, the positivity of β1-integrin seemed to be higher (P = 0.033). No specific differences of other factors were discovered. No significant difference in the density of bands of β1-integrin was observed, either (Table 5).
Table 5.
Expression of β1-integrin in the experimental group according to the clinicopathologic findings
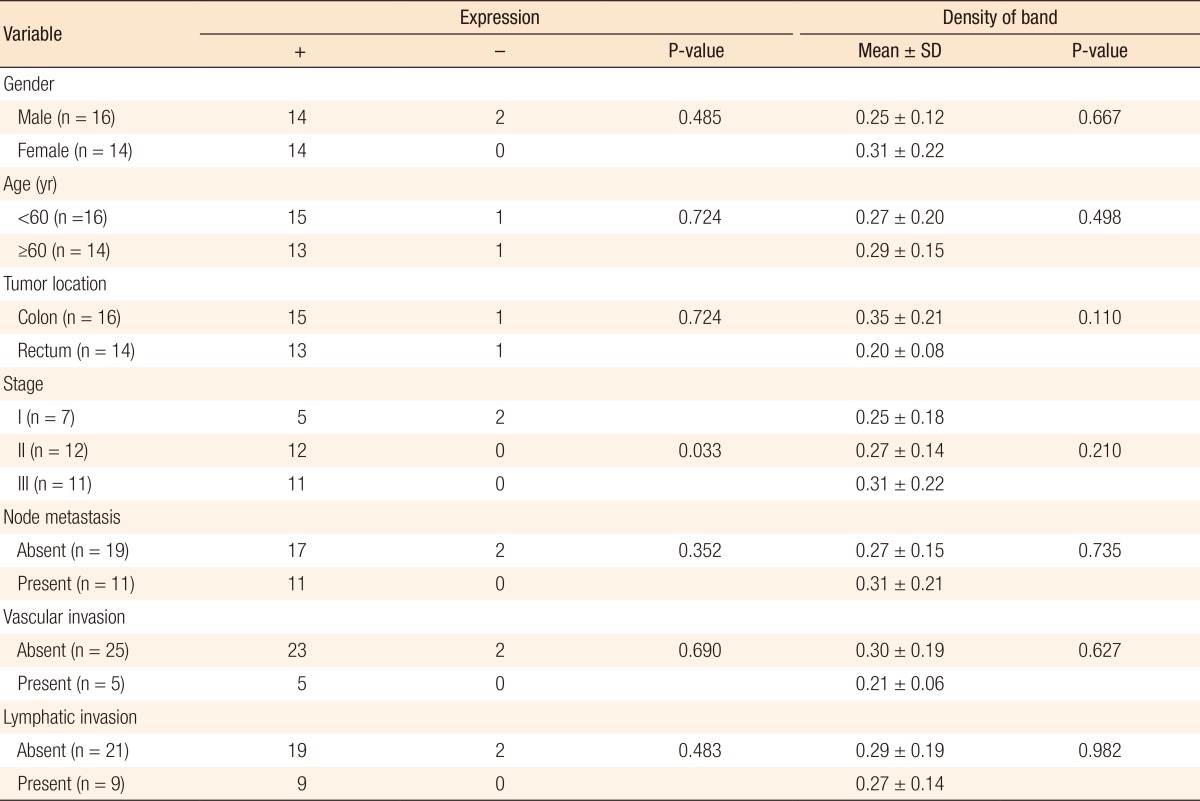
SD, standard deviation.
When the positivity of β1-integrin mRNA was compared between the experimental group and the control group, the followings were observed: In the experimental group, 28 patients showed preoperative positive results, and 2 patients showed preoperative negative results. In the control group, 8 patients showed positive results and 22 patients showed negative results. Consequently, the number of patients showing preoperative positive expression of β1-integrin was significantly higher in the experimental group (P < 0.001). Regarding the measurement of the preoperative density of a band, the experimental group showed a higher value (0.32 ± 0.17) compared to the control group (0.07 ± 0.08) (P < 0.001) (Table 6, Fig. 2).
Table 6.
Expression of β1-integrin in the control and the experimental groups
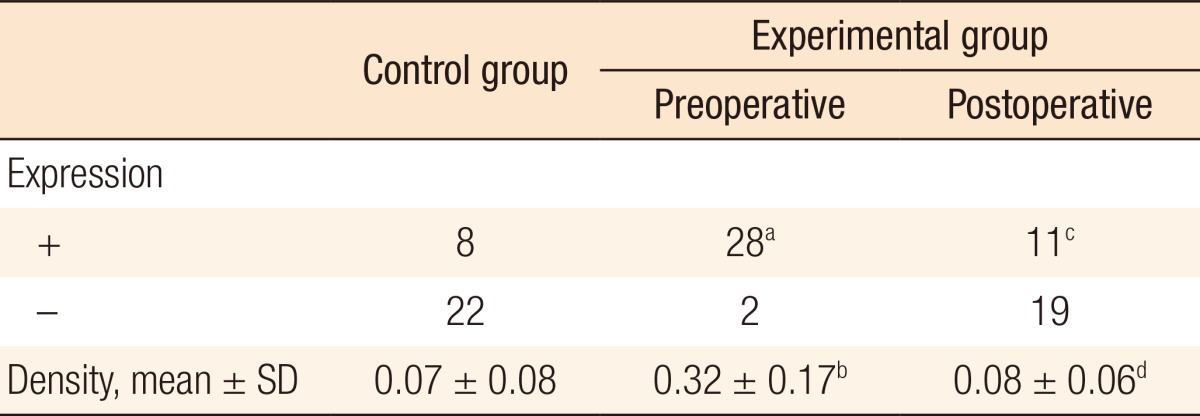
SD, standard deviation.
aExpression of β1-integrin was detected more in the experimental group than in the control group (P < 0.001). bPreoperative density of β1-integrin was higher in the experimental group than in the control group (P < 0.001). cExpression of β1-integrin was significantly decreased after surgery (P < 0.001). dDensity of β1-integrin was significantly decreased after surgery (P < 0.001).
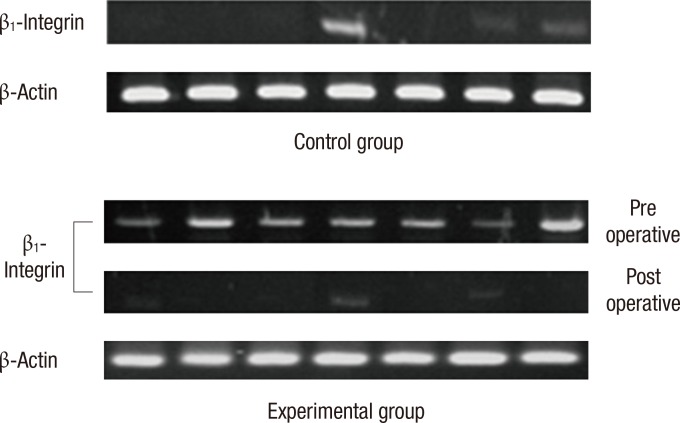
Fig. 2.
Detection of β1-integrin in the blood of each group by using reverse transcription-polymerase chain reaction. β1-Integrin was detected more frequently in colorectal cancer patients than in the controls (P < 0.001). In colorectal cancer patients, expression of β1-integrin was significantly decreased after surgey (P < 0.001). β-actin is an internal control.
Next, we analyzed the results in regards to β1-integrin mRNA before and after surgery in the experimental group. A total of 28 patients were found to be positive to β1-integrin before surgery, and that number decreased to 11 after surgery (P < 0.001). Also, the density of β1-integrin that was observed during the preoperative state (0.32 ± 0.17) was lower after surgery (0.08 ± 0.06) (P < 0.001) (Table 6). Such results are also shown quite well in the pictures taken from electrophoresis. Whether or not the band of β1-integrin that was present before surgery was expressed after surgery, its density was much lower after surgery (Fig. 2).
Analysis of the correlation between β1-integrin and CEA mRNA
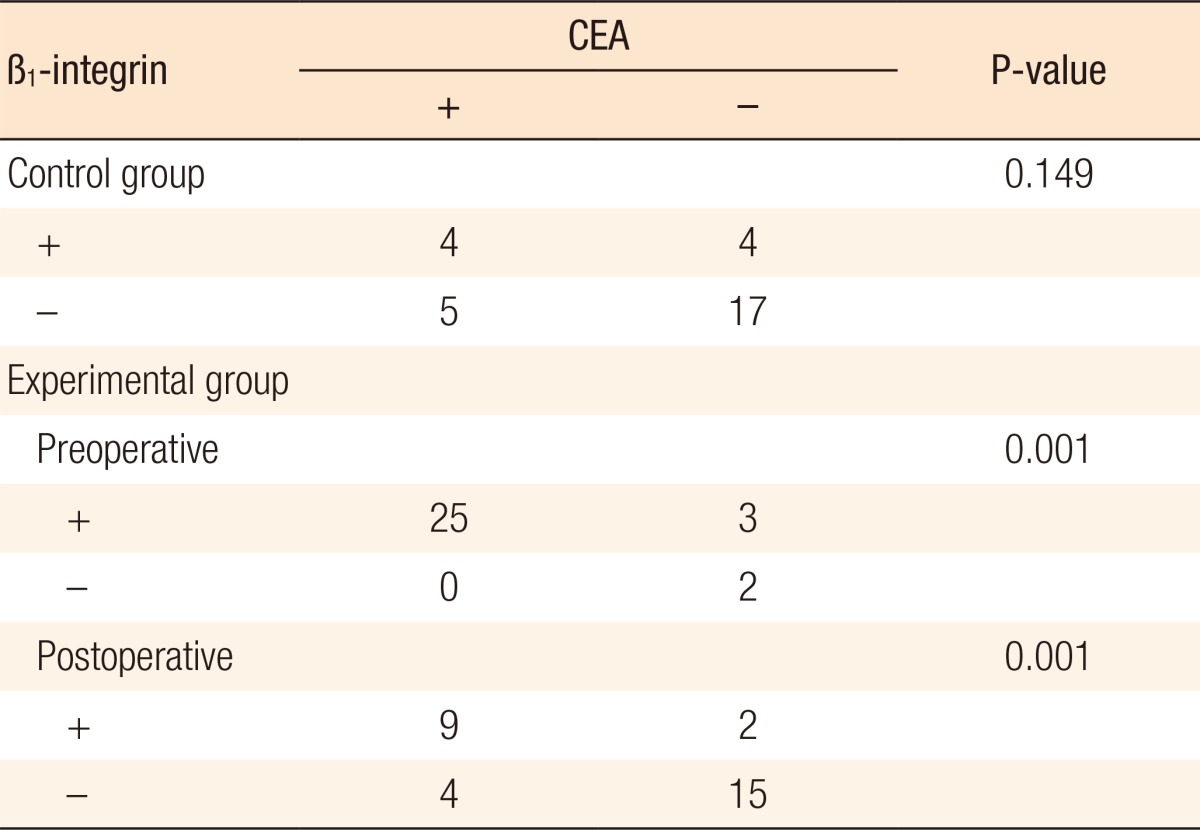
We analyzed the results of RT-PCR of β1-integrin and CEA mRNA in each patient group in order to understand the correlation between β1-integrin and circulating tumor cells. Regarding the control group, 4 patients showed positivity in both β1-integrin and CEA, and 17 patients showed negativity in both, which means that there's no close relationship between the expression levels (P = 0.149). However, a moderate-positive correlation was shown with a correlation coefficient of 0.446 regarding the densities of β1-integrin and CEA. Such an observed result proves that a significant relationship exists between the expression tendencies of β1-integrin and CEA (P = 0.014) (Fig. 3A).
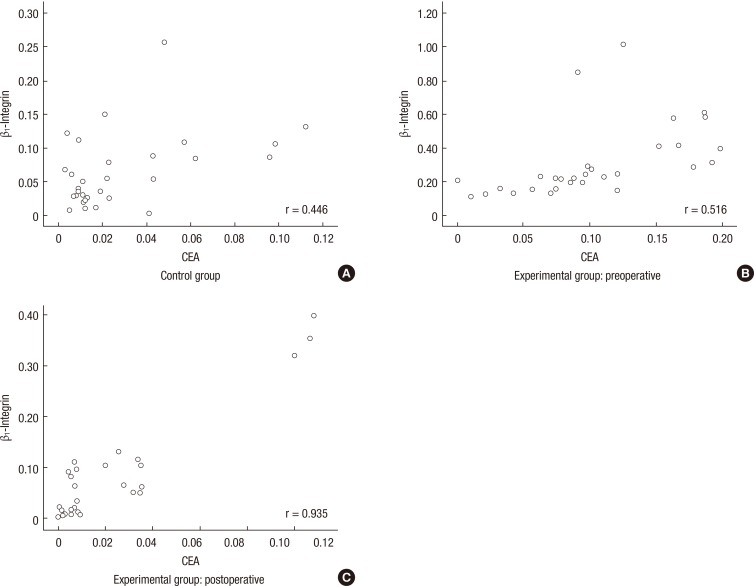
Fig. 3.
Correlation of β1-integrin and carcinoembryonic antigen (CEA). Expression of β1-integrin was significantly associated with that of CEA. (A) In the control group, a moderate-positive correlation existed between β1-integrin and CEA (r = 0.446, P = 0.014). (B) In the preoperative experimental group, a moderate positive correlation existed between β1-integrin and CEA (r = 0.516, P = 0.003). (C) In the postoperative experimental group, a high positive correlation existed between β1-integrin and CEA (r = 0.935, P < 0.001).
We also analyzed each result that was obtained before and after surgery in order to understand the correlation between β1-integrin and CEA in the experimental group. There was a significant correlation between the expressions of β1-integrin and CEA in the blood samples that were collected before surgery (P = 0.001) (Table 7). In addition, a moderate positive correlation existed in the density of bands (r = 0.516, P = 0.003) (Fig. 3B). In postoperative results, there were similar results on the expressions of β1-integrin and CEA (P = 0.001) (Table 7), and the density of bands (r = 0.935, P < 0.001) (Fig. 3C).
Table 7.
Correlation of β1-integrin and carcinoembryonic antigen (CEA)
DISCUSSION
Various diagnostic methods and treatment options are being developed in order to improve the survival rate of patients with colorectal cancer. Moreover, many markers are used to evaluate the progression of the cancer in the patients and to predict the prognosis. So far, many investigations had been done, but only the extraction of CEA within the blood has been found to be useful clinically [9]. Accordingly, new markers that will allow us to predict the spread and the recurrence of the cancer and to evaluate the reaction of the patient to the treatment need to be developed in order to improve the survival rate of the patients.
Since the theory of circulating tumor cells was introduced by Ashworth [10] in 1869, its clinical importance has been emphasized and has been the subject of many investigations. Circulating tumor cells are produced at the beginning of the metastatic process, and they are rarely observed in a healthy individual [4]. They are known to be able to not only metastasize to distant organs, but also able to return to the primary organ and self-seed the cancer [2]. The circulating tumor cells play an important role in diagnosing the cancer and predicting recurrence and metastasis, so they may be used to evaluate the reaction of the patient to the treatment. The extraction of circulating tumor cells may be a new way to predict the prognosis of the patient [2]. According to many articles written about circulating tumor cells, they were expressed at higher levels in patients with colorectal cancer than in normal control patients [8, 11], and the expression level tended to be higher for higher histological stage of the cancer [8, 12]. Moreover, metastasis and recurrence were shown to occur at an earlier time in a patient who showed expression of circulating tumor cells [3, 8], and their survival rate was reported to be lower as well [3, 13]. According to the study of Wong et al. [14], among the patients suffering from colorectal cancer with the presence of circulating tumor cells, 88% showed decreased levels of such cells after surgery. Similar results were observed in this study. The marker that was used to detect the circulating tumor cells was expressed especially at higher levels in patients with colorectal cancer, and the expression level was shown to be significantly decreased after surgery.
The molecular extraction of circulating tumor cells was tried for the first time by Smirnov et al. [15] in 2005 in patients with colorectal cancer, and similar investigations have been reported since then. The markers that are most commonly used to detect the circulating tumor cells in colorectal cancer are CEA, cytokeratin 19, cytokeratin 20, epithelial cell adhesion molecule (EpCAM), human telomerase reverse transcriptase, etc., and among these, CEA is the most standardized marker [2, 4]. Many methods for extracting circulating tumor cells have been tried; unfortunately, many of them have not yet been standardized. Thus, the usefulness of the various methods is a subject of debate [5]. Among the enlisted methods, Cell Search System [5] utilizes a semi-automatic system that dyes EpCAM immunologically, and its use is increasing these days. Its pitfall is low sensitivity, and the system cannot be used in cells that show EpCAM negativity. What's most important is that it costs approximately 800 US dollars per each sample to be tested and is difficult to use on a clinical basis [4, 16]. Therefore, we selected RT-PCR [2, 5, 8], which is widely used to extract circulating tumor cells, as our extraction method due to its easy operation and high sensitivity compared to the other methods.
Every day, approximately 106 cells are produced and excreted from 1 g of primary cancer into the circulatory system. Through the process called anoikis, a process of cellular degeneration that is caused by the loss of cell-matrix interaction, circulating tumor cells survive within the blood [2, 17]. Unfortunately, the number of such circulating cells that are measured in reality is quite low (1 in 105-107), and this phenomenon is due to the dissolution of many tumor cells by the shear force [2]. Eventually less than 0.1% of the cells will survive 24 hours after generation, and the percentage of circulating tumor cells that actually contribute to metastasis is reported to be less than 0.01% [17]. Many efforts have been made to extract circulating tumor cells; however as described above, limitations on extracting the cells effectively still exist due to the very small number of cells and the unsettled extraction method. Accordingly, developing a new marker for patients that may show higher extraction level of circulating tumor cells compared to CEA, an ability to reflect the presence of circulating tumor cells and their involvement in the metastatic process of colorectal cancer, would be clinically meaningful.
The circulating tumor cells were reported to be related to factors such as tissue inhibitor of metalloproteinase 1, carbonic anhydrase IX, and epidermal growth factor receptor [18, 19]; however, we assumed that research on a substance that is involved in the cell-matrix interaction, which may influence the metastasis more directly, seemed important. One such important substance is integrin. Integrin is a heterodimeric cell surface receptor that mediates the interaction between the cell and the extracellular matrix, and it is included in the glycoprotein family. It consists of 18 α-subunits and 8 β-subunits, and it forms 24 heterodimers through combinations of different bonds [6]. Very little integrin is observed in most normal tissues, and its expression level is increased abnormally in tumor cells [6]. Integrin enables tumor cells to move to target tissue by acting as a driving force with an adhesion function, and it accelerates the degradation of the basement membrane, easing the invasion by tumor cells. It accelerates the process of mobility and invasion of the cell through modification of many signaling pathways, such as the focal adhesion kinase and the src family kinase pathways [6, 7, 20]. Integrin is also known for its ability to maintain the shape of the cell and induce cellular survival and proliferation, and it is involved in gene transcription, as well [20]. Recently, thanks to such various functions of integrin, many researchers have been turning their attention to the development of new treatments that involve integrin as a target. Their argument includes the power of integrin to prevent the growth and metastasis of the cancer by injecting the antagonists of integrin, thus inhibiting the movement of and the invasion by tumor cells. Actually, many authors have suggested that growth of the cancer can be inhibited by blocking integrin [7, 21]. In an investigation that utilized human breast cancer cell lines, monoclonal antibody against α5β1-integrin was observed to inhibit the growth of tumor cells [22]. In a similar mouse model in which liver metastasis of colorectal cancer was induced, the sizes of the primary cancer and the liver metastatic lesion were greatly decreased with the injection of both monoclonal antibody to α5β1-integrin and fluorouracil, and this reduction seemed to be greater than it was in other groups that received fluorouracil only [23].
The interaction between the cell and the matrix as mediated by integrin is organ-specific compared to that of other cell adhesion molecules [2, 6], and β1-integrin seems to be expressed at higher level in colorectal cancer [24, 25]. Especially, it is reported to be related to metastatic colorectal cancers [26]. β1-integrin promotes the growth, survival, and invasion of many different types of tumor cells involving colorectal cancer by its attachment to different α-subunits. According to Yang et al. [27], α5β1-integrin was expressed at higher levels in patients suffering from colorectal cancer than in normal patients, and the expression level was higher in cases of lower differentiation level and higher histological stage of the cancer. Moreover, the 5-year survival rate was significantly reduced in patients with high expression levels of α5β1-integrin. In the investigation of Bartolome et al. [28] who used a mouse model, the results proved that liver metastasis of colorectal cancer might be caused by activation of α5β1-integrin. According to many reports of previous years, β1-integrin is closely related to the progression of colorectal cancer, and it is regarded as a factor that worsens the prognosis of the patients [6, 7, 25].
A small number of articles have reported that researchers tried to extract tumor cells from locations other than the bloodstream, such as lymph nodes, bone marrow, and peritoneal fluid. Their results showed a similar correlation to the prognosis for the patients [2, 29, 30]. Wong et al. [14, 29] reported that tumor cells were extracted in molecular tests even in patients with colorectal cancer and no metastasis to lymph nodes, and their survival rate was reduced due to a more frequent development of metastasis. The level of β1-integrin was observed at higher levels in patients with colorectal cancer than in those with benign diseases (26.7% vs. 93.3%), and the expression level was higher for higher stages of colorectal cancer. Even in cases with the absence of lymphatic metastasis, vascular invasion, and lymphatic invasion, the expression level of β1-integrin was quite high, but with no statistical significance.
When the expression levels that were measured before and after surgery in patients with colorectal cancer were compared, the expression level was significant reduced after surgery. This result proves the theory that the amount of β1-integrin tends to decrease significantly as the tumor burden within the body becomes less. The expression level of β1-integrin in patients with colorectal cancer is 93.3%, which is higher than the expression level of CEA, 73.3%. An analysis of the relationship between β1-integrin and CEA showed a significant correlation between both patients with colorectal cancer and patients with benign diseases. Despite the lesser amount of β1-integrin compared to that of CEA, which is specific of tumor cells, β1-integrin can be expressed in endothelial cells, epithelial cells, white blood cells, platelets, and normal cells [6]. Because the RNA of β1-integrin was obtained from only the buffy coat layer, we cannot clearly confirm that the relationship we have discovered from our result proves whether the actual presence of β1-integrin reflects the presence of circulating tumor cells or not. Consequently, additional investigations that use tumor tissue or lymph nodes other than blood samples e thought to be necessary in order to obtain more meaningful results.
This is a case-control study, which shows better utilization in regards to clinical application of the results that were observed in the investigation, and the significant results that were acquired from comparisons of the two markers seem to be very meaningful considering that no research articles have ever reported the relationship between β1-integrin and circulating tumor cells. Additionally, the measurement of β1-integrin by using RT-PCR is very simple, noninvasive, and cheap, and it can predict the presence of micrometastasis at an earlier stage compared to computed tomography and magnetic resonance imaging in patients with colorectal cancer. Thus, it seems to be a method that may be used repeatedly and usefully in such patients. In our opinion, the results of this study may be used during the follow-up of patients. In this way, patients with higher expression of β1-integrin can be selected, recurrence and metastasis in those patients can be discovered at an earlier stage, and more intensified treatment can be considered while planning treatment options. We also suggest that the results of the study be used for monitoring both the reaction of the patient to treatment and the progression of the disease after surgery. Furthermore, we think that new treatment methods can be investigated, with β1-integrin as a new target.
The expression level of β1-integrin was higher in patients with colorectal cancer, and that level was significantly reduced after surgery. Moreover, it showed a close relationship with the expression of CEA, which serves as a marker for circulating tumor cells. In conclusion, β1-integrin was shown to be a new potential marker that can be used in patients with colorectal cancer to predict the progression of the cancer and the prognosis after surgery and that, along with circulating tumor cells, can be used to detect micrometastasis earlier.
ACKNOWLEDGMENTS
This research was supported by The Korean Society of Coloproctology Boryung Research Grant 2012.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Steinert G, Scholch S, Koch M, Weitz J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbecks Arch Surg. 2012;397:535–542. doi: 10.1007/s00423-012-0917-9. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Qian J, Feng JG, Ju HX, Zhu YP, Feng HY, et al. Detection of circulating tumor cells in peripheral blood of colorectal cancer patients without distant organ metastases. Cell Oncol (Dordr) 2013;36:43–53. doi: 10.1007/s13402-012-0112-6. [DOI] [PubMed] [Google Scholar]
- 4.Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer: a review. Eur J Surg Oncol. 2011;37:459–465. doi: 10.1016/j.ejso.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods. 2010;50:289–297. doi: 10.1016/j.ymeth.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Haier J, Nasralla M, Nicolson GL. Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg. 2000;231:11–24. doi: 10.1097/00000658-200001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinka R, Kapoor R, Sistla PG, Raj TA, Pande G. Alterations in Cell-Extracellular Matrix Interactions during Progression of Cancers. Int J Cell Biol. 2012;2012:219196. doi: 10.1155/2012/219196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, et al. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis. World J Surg. 2006;30:1007–1013. doi: 10.1007/s00268-005-0485-z. [DOI] [PubMed] [Google Scholar]
- 9.Bolocan A, Ion D, Ciocan DN, Paduraru DN. Prognostic and predictive factors in colorectal cancer. Chirurgia (Bucur) 2012;107:555–563. [PubMed] [Google Scholar]
- 10.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust. 1869;14:146–147. [Google Scholar]
- 11.Raeisossadati R, Farshchian M, Ganji A, Tavassoli A, Velayati A, Dadkhah E, et al. Quantitative analysis of TEM-8 and CEA tumor markers indicating free tumor cells in the peripheral blood of colorectal cancer patients. Int J Colorectal Dis. 2011;26:1265–1270. doi: 10.1007/s00384-011-1230-8. [DOI] [PubMed] [Google Scholar]
- 12.Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:420–428. doi: 10.1093/annonc/mds336. [DOI] [PubMed] [Google Scholar]
- 14.Wong SC, Chan CM, Ma BB, Hui EP, Ng SS, Lai PB, et al. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res. 2009;15:1005–1012. doi: 10.1158/1078-0432.CCR-08-1515. [DOI] [PubMed] [Google Scholar]
- 15.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Hayashi N, Imamura Y, Tanaka Y, Kinoshita K, Kurashige J, et al. Usefulness of transcription-reverse transcription concerted reaction method for detecting circulating tumor cells in patients with colorectal cancer. Ann Surg Oncol. 2012;19:2060–2065. doi: 10.1245/s10434-011-1889-7. [DOI] [PubMed] [Google Scholar]
- 17.Wai Wong C, Dye DE, Coombe DR. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol. 2012;2012:340296. doi: 10.1155/2012/340296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res. 2011;13:R71. doi: 10.1186/bcr2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zieglschmid V, Hollmann C, Mannel J, Albert W, Jaeschke-Melli S, Eckstein B, et al. Tumor-associated gene expression in disseminated tumor cells correlates with disease progression and tumor stage in colorectal cancer. Anticancer Res. 2007;27(4A):1823–1832. [PubMed] [Google Scholar]
- 20.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 21.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–188. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, et al. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
- 24.Nam JM, Chung Y, Hsu HC, Park CC. beta1 integrin targeting to enhance radiation therapy. Int J Radiat Biol. 2009;85:923–928. doi: 10.3109/09553000903232876. [DOI] [PubMed] [Google Scholar]
- 25.Huang WS, Chen CN, Sze CI, Teng CC. Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J Cell Physiol. 2013;228:1017–1024. doi: 10.1002/jcp.24248. [DOI] [PubMed] [Google Scholar]
- 26.Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, et al. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Gao J, Rao Z, Shen Q. Clinicopathological and prognostic significance of α5β1-integrin and MMP-14 expressions in colorectal cancer. Neoplasma. 2013;60:254–261. doi: 10.4149/neo_2013_034. [DOI] [PubMed] [Google Scholar]
- 28.Bartolome RA, Barderas R, Torres S, Fernandez-Acenero MJ, Mendes M, Garcia-Foncillas J, et al. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014;33:1658–1669. doi: 10.1038/onc.2013.117. [DOI] [PubMed] [Google Scholar]
- 29.Wong CS, Cheung MT, Ma BB, Pun Hui E, Chan AC, Chan CK, et al. Isolated tumor cells and circulating CK20 mRNA in pN0 colorectal cancer patients. Int J Surg Pathol. 2008;16:119–126. doi: 10.1177/1066896907311901. [DOI] [PubMed] [Google Scholar]
- 30.Koyanagi K, Bilchik AJ, Saha S, Turner RR, Wiese D, McCarter M, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res. 2008;14:7391–7396. doi: 10.1158/1078-0432.CCR-08-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]