Abstract
Macrophage death plays a role in several physiological and inflammatory pathologies such as sepsis and arthritis. In our previous work, we showed that simvastatin triggers cell death in LPS-activated RAW 264.7 mouse macrophage cells through both caspase-dependent and independent apoptotic pathways. Here, we show that the nuclear orphan receptor NR4A1 is involved in a caspase-independent apoptotic process induced by LPS and simvastatin. Simvastatin-induced NR4A1 expression in RAW 264.7 macrophages and ectopic expression of a dominant-negative mutant form of NR4A1 effectively suppressed both DNA fragmentation and the disruption of mitochondrial membrane potential (MMP) during LPS- and simvastatin-induced apoptosis. Furthermore, apoptosis was accompanied by Bcl-2-associated X protein (Bax) translocation to the mitochondria. Our findings suggest that NR4A1 expression and mitochondrial translocation of Bax are related to simvastatin-induced apoptosis in LPS-activated RAW 264.7 macrophages.
Keywords: NR4A1, Apoptosis, LPS, Simvastatin, Macrophages
INTRODUCTION
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the mevalonate pathway (1). Statins have been shown to improve survival in patients with cardiovascular diseases through inhibiting the prenylation of small GTPases such as Ras and Rho/Rac (2). Although the beneficial effects of statins are attributed to their lipid lowering ability, statins are now believed to exert pleiotropic effects that include decreased inflammation, improved endothelial function, stabilization of atherosclerotic plaques, and induction of apoptosis (3,4).
The inflammatory response can be controlled in many ways. Statins lower the level of C-reactive protein (5), suppress IFNγ-inducible expression of MHC class II on monocyte-derived macrophages and endothelial cells (6), and bind directly to β2 integrin LFA-1, blocking LFA-1-mediated adhesion and co-stimulation of lymphocytes (7). In addition, statins inhibit Rho-family G proteins (2), T cell activation (8), and the activation and maturation of macrophages and dendritic cells (6,9). Statins are also known to induce cell death in vivo and in vitro. In macrophages, statin-induced cell death is mediated through the depletion of isoprenoids such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which serve as important lipid moieties for protein isoprenylation (10,11). Statin-mediated inhibition of isoprenylation targets Rac1 and Cdc42, and in turn inhibition of their downstream signaling molecules such as c-Jun N-terminal kinases, is responsible for apoptosis in macrophage cells (11). The death of activated macrophages can also be advantageous in the control of inflammation levels.
NR4A1 (also known as Nur77, TR3, and NGFI-B) is an orphan member of the steroid/thyroid/retinoid receptor superfamily and an immediate-early response gene (12,13,14). Various lines of evidence have suggested a role for NR4A1 in the control of inflammation and apoptosis, including the negative selection of thymocytes (15,16,17,18,19). In macrophages, NR4A1 is induced rapidly by multiple stimuli including LPS, TNF-α, and oxidized low-density lipoprotein (20,14). NR4A1 is also involved in activation-induced cell death in macrophages (21). However, the role of the NR4A1 in the death of LPS- and simvastatin-induced macrophages has not been studied in detail.
Previously, we showed that simvastatin triggers apoptosis in LPS-stimulated macrophages by both caspase-dependent and independent apoptotic pathways (10), and induces the translocation of Bax to mitochondria in MethA fibrosarcoma (22,23). However, the precise signaling pathways involved in this process have not been elucidated. In this study, we further analyzed this pathway by investigating the involvement of the nuclear orphan receptor NR4A1 in the apoptosis pathway induced by LPS and simvastatin. We report that NR4A1 expression is involved in LPS- and simvastatin-induced apoptosis in RAW 264.7 macrophage cells.
MATERIALS AND METHODS
Cell lines, reagents, and cell transfection
Cells of the murine monocyte/macrophage cell line, RAW 264.7, were maintained in DMEM (Jeil Biotech Services, Daegu, Korea) supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100µg/ml streptomycin, 1 mM sodium pyruvate, 2 mM glutamine, and 5 mM HEPES (complete DMEM). LPS from Escherichia coli 0127:B8 was obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in PBS. Simvastatin (SMD Korea, Ansan, Korea) was reconstituted in absolute ethanol and stored at -20℃. Benzyloxycarbonyl-Val-Ala-DL-Asp(O-methyl)-fluoromethylketone (Z-VAD-FMK), a pan-caspase inhibitor, was obtained from AG Scientific (San Diego, CA, USA). FTI-276 (farnesyl transferase inhibitor), GGTI-286 (geranylgeranyl transferase inhibitor), and Y-27632 (Rho kinase inhibitor) was obtained from Calbiochem (La Jolla, CA). Anti-Bax Ab was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), HRP-conjugated anti-mouse and rabbit IgG Abs were obtained from Sigma-Aldrich, and HRP-conjugated anti-goat IgG Ab was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The construct used to express a dominant-negative form of NR4A1 (NR4A1-DN; provided by Dr. Heung-Sik Choi) (24) was introduced into RAW 264.7 cells by using electroporation (25). After 48 h of incubation in complete DMEM, transfected cells were selected with 1.2 mg/ml G418 for 2 weeks, and drug-resistant isolates were maintained in medium containing 0.12 mg/ml G418.
RT-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Approximately 2µg of total RNA was reverse-transcribed using MMLV reverse transcriptase and oligo(dT) primers (Promega; Madison, WI) at 42℃ for 4 h. Semi-quantitative PCR of the synthetic cDNA was performed using Taq polymerase pre-mixture (Genotech, Daejeon, Korea) in a 96-well MyCycler (Bio-Rad, Hercules, CA, USA). Sequences of the primers for mouse NR4A1 and GAPDH were as follows: NR4A1 forward primer, 5'-CTCGCCATCTACACCCAACT-3'; NR4A1 reverse primer, 5'-AGCCTTAGGCAACTGCTCTG-3'; GAPDH forward primer 5'-TGTTGCCATCAATGACCCCTT-3'; and GAPDH reverse primer 5'-CTCCACGACGTACTCAGCG-3'.
Immunoblotting
The fractionation of mitochondrial and cytosolic protein extracts was performed using the Mitochondrial Isolation Kit (Pierce, Rockford, IL, USA) as previously described (22). Briefly, whole cell extracts were prepared by incubating cells with radio-immunoprecipitation assay buffer (10 mM Tris, 150 mM NaCl, 0.5% NP-40, 0.1% SDS, 0.1% deoxycholate, 1 mM PMSF, and 1× protease inhibitor cocktail from Sigma-Aldrich) on ice for 20 min. To separate nuclear and cytosolic fractions, RAW 264.7 cells (5×106 cells/dish) were washed with ice-cold PBS and mixed with a hypotonic buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 1× protease inhibitor cocktail) for 10 min. The cytosolic faction was obtained by microcentrifugation at 1,000×g for 15 min. To prepare the nuclear extracts, the pellet was resuspended and incubated in 150µl high-salt buffer (20 mM HEPES, 400 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, and protease inhibitor cocktail) for 2 h. Protein was quantified using the Bradford method. Equal amounts of protein were subjected to electrophoresis on a 10% pre-mixed SDS-PAGE gel (Elpisbiotech, Daejeon, Korea) and blotted onto a PVDF membrane (Millipore, Billerica, MA, USA) using a semi-dry transferring apparatus (Sigma-Aldrich). Blots were blocked in 3% BSA fraction V (Sigma-Aldrich) in TTBS (10 mM Tris, 100 mM NaCl, and 0.05% Tween-20) overnight and incubated with the indicated primary Ab at 4℃ for 6 h. After incubation with HRP-conjugated secondary Ab at 4℃ for 3 h, protein bands were visualized with an ECL detection kit (Pierce). Immunoblots of tubulin and heat shock protein 60 were used as loading controls.
Propidium iodide staining of genomic DNA
To analyze the fragmentation of genomic DNA in apoptotic cells, cells (1×106 cells/ml) were incubated with reagents for 12 h. The cells were harvested, washed with PBS, and fixed using 1 ml of ice-cold 70% ethanol at 4℃ overnight. Fixed cells were labeled with 500µg/ml propidium iodide (PI) at 37℃ for 1 h. Genomic DNA was analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) as previously described (26,27). All data were calculated and displayed using the Cell Quest analysis software provided by Becton Dickinson.
Mitochondrial membrane potential measurements
RAW 264.7 cells (1×106 cells/ml) were incubated with reagents at 37℃ for 10~12 h. The cells were harvested, washed in ice-cold PBS, and resuspended in 500µl of 4µg/ml rhodamine-123 solution (Sigma-Aldrich) at 37℃ for 20 min. The rhodamine-123-labeled cells were then incubated with 500µl of 0.1 mg/ml PI solution for 3 min. Incorporation of rhodamine-123 and PI was analyzed using the FACSCalibur flow cytometer. The fluorescence of rhodamine-123 (green) and PI (red) were detected using FL1 and FL2 sensors, respectively. All data were analyzed using Cell Quest software.
RESULTS
LPS and simvastatin induce NR4A1 expression
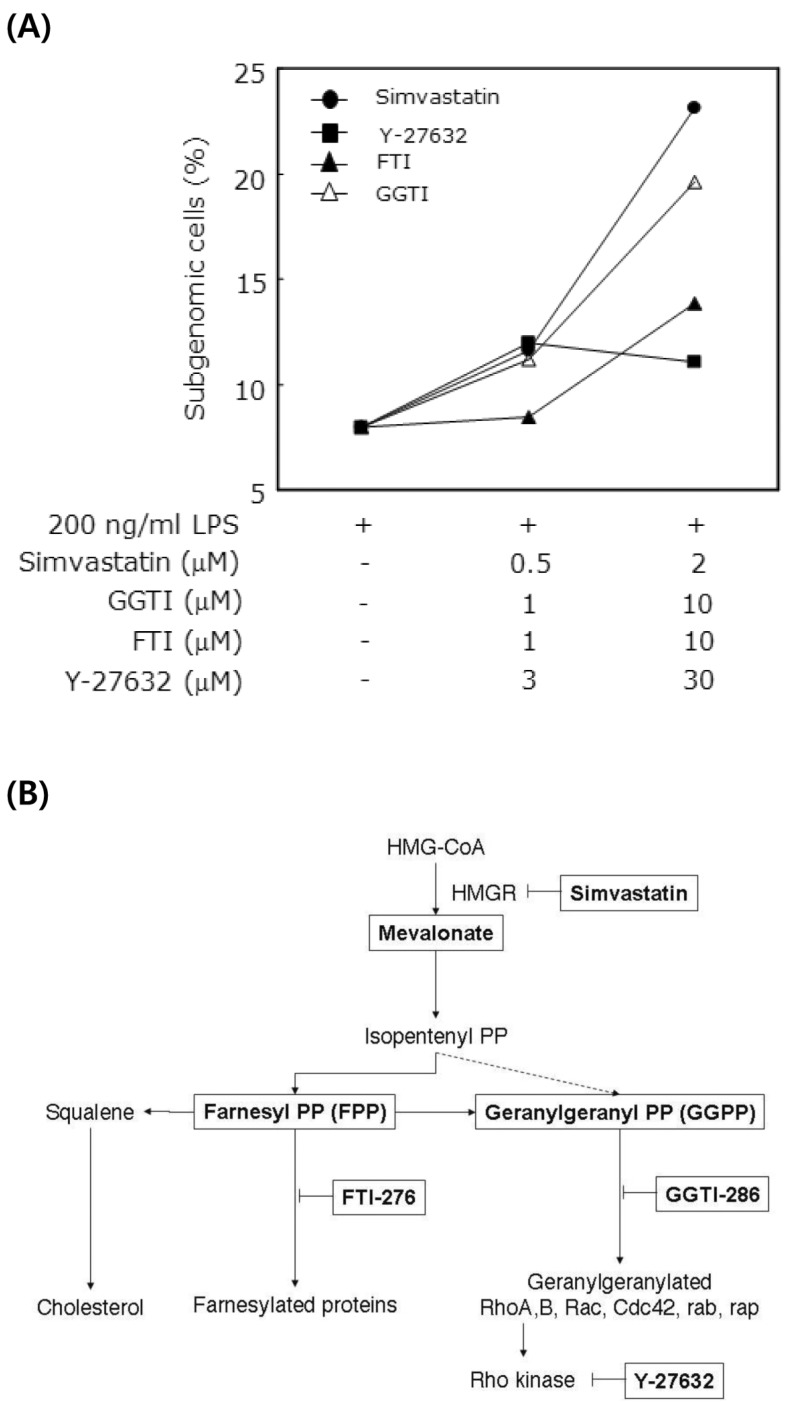
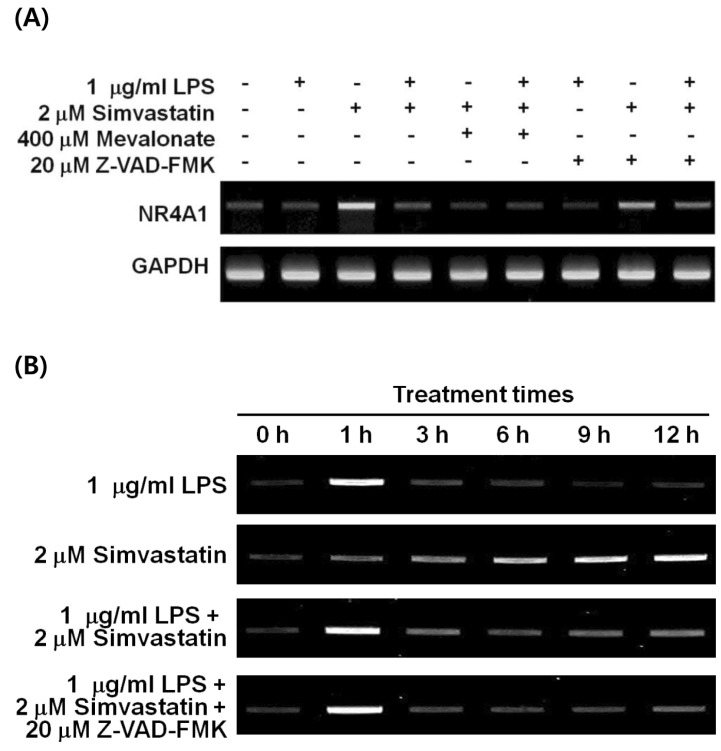
We previously reported the involvement of a caspase-independent pathway in the apoptosis of RAW 264.7 cells induced by LPS and simvastatin (10). Apoptosis was not induced by the sole treatment of 1µg/ml LPS or 2µM simvastatin; however, cotreatment of LPS and simvastatin at the same doses synergistically induced apoptosis partly through a caspase-independent pathway (Fig. 1A). Moreover, farnesyl transferase inhibitor (FTI-276) significantly suppressed the LPS and simvastatin-induced apoptosis among FTI-276, GGTI-286, and Y-27632 (Fig. 1B), suggesting that certain farnesylated proteins are mainly responsible for the apoptosis. Because NR4A1 is implicated in caspase-independent apoptosis in LPS-stimulated macrophages (21), we investigated the expression pattern of NR4A1 mRNA in LPS and simvastatin-treated RAW 264.7 macrophage cells. Expression of NR4A1 was significantly up-regulated 12 h after simvastatin treatment (Fig. 2A). Moreover, simvastatin-induced NR4A1 expression was greatly inhibited by mevalonate, suggesting the involvement of prenylation in NR4A1 induction. As shown in our previous work, LPS- and simvastatin-induced apoptosis can be suppressed by FPP and GGPP supplementation (10). Apoptosis was not markedly suppressed by the pan-caspase inhibitor Z-VAD-FMK, suggesting the apoptotic pathway is caspase-independent.
Figure 1.
Induction of apoptosis by LPS and simvastatin. (A) Raw264.7 cells were treated with LPS and simvastatin with FTI-276, GGTI-286, Y-27632 for 14 h, and subgenomic contents were analyzed by PI staining and flow cytometry. (B) Mevalonate pathway and inhibitors used in this study. The enzymatic target steps of simvastatin (HMG-CoA reductase inhibitor), FTI-276 (farnesyl transferase inhibitor), GGTI-286 (geranylgeranyl transferase inhibitor), and Y-27632 (Rho kinase inhibitor) were displayed.
Figure 2.
Induction of NR4A1 mRNA by LPS and simvastatin. (A) RAW 264.7 cells were treated with LPS and/or simvastatin alone or in combination with mevalonate or benzyloxycarbonyl-Val-Ala-DL-Asp(O-methyl)-fluoromethylketone (Z-VAD-FMK) for 12 h and the level of nuclear orphan receptor NR4A1 mRNA was measured using RT-PCR. Total RNA was extracted and amplified as described in the Materials and Methods. GAPDH was used to normalize the quantity of amplified NR4A1. (B) For the kinetic analysis of NR4A1 expression, cells were treated with 1µg/ml LPS or 2µM simvastatin in combination with 20µM Z-VAD-FMK for indicated times (up to 12 h) and were analyzed using RT-PCR.
Because NR4A1 expression is known to be an early response to stimuli, we further explored the effect of LPS and simvastatin on expression of NR4A1 in RAW 264.7 cells at various time points up to 12 h (Fig. 2B). In response to LPS, the pattern of early induction and extinction of NR4A1 mRNA was a typical to that of an early response gene. In contrast, simvastatin caused a gradual increase in NR4A1 transcript over the course of 12 h. Cotreatment of LPS and simvastatin had an additive effect on the expression of NR4A1. The response to LPS or simvastatin was not significantly changed by Z-VAD-FMK treatment. These results suggest a possible role of NR4A1 transcriptional regulation in the caspase-independent apoptotic pathway that is induced by LPS and simvastatin treatments.
Expression of dominant-negative NR4A1 suppresses LPS- and simvastatin-induced apoptosis in macrophages
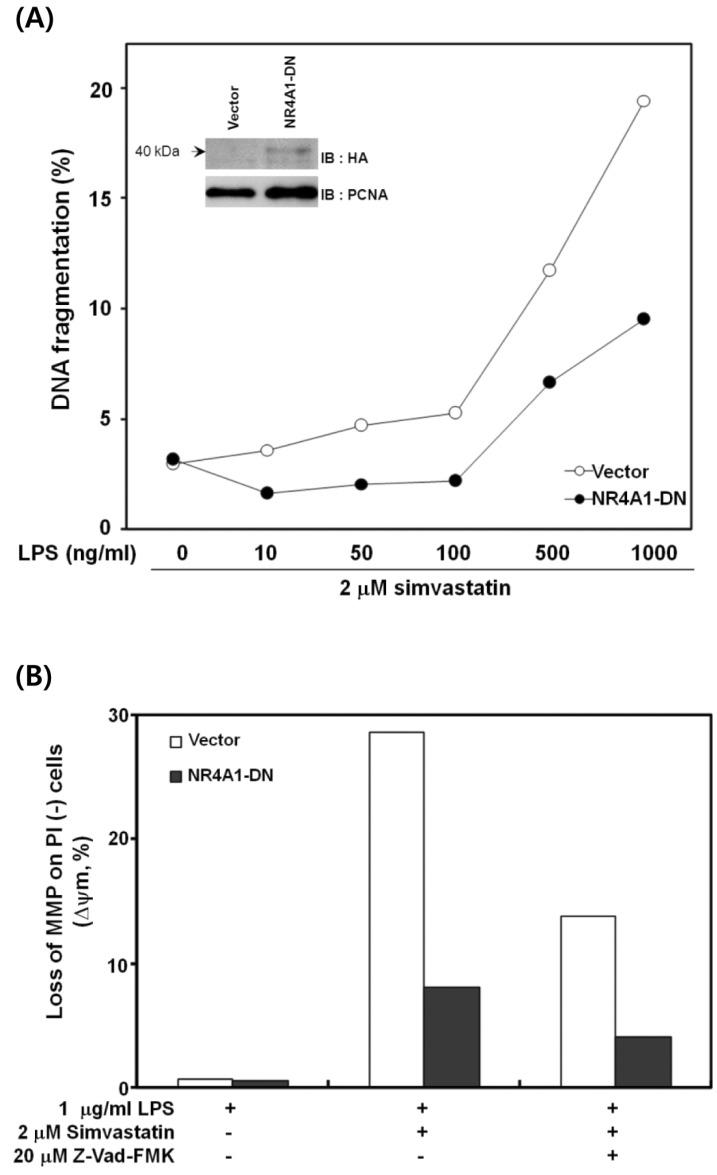
To investigate whether the NR4A1 plays a role in LPS- and simvastatin-induced apoptosis, RAW 264.7 cells were transfected with vectors encoding a dominant-negative form of NR4A1 (NR4A1-DN). The transfected cells were analyzed for DNA fragmentation and changes in MMP. As shown in Fig. 3A, LPS- and simvastatin-induced DNA fragmentation in cells expressing NR4A1-DN was reduced significantly (~50%). LPS- and simvastatin-induced apoptosis in RAW 264.7 cells is associated with a dramatic disruption of MMP (10). Therefore, we examined whether the expression of the dominant-negative NR4A1 mutant also blocked the disruption of MMP. As expected, the disruption of MMP was decreased by ~70% in cells expressing NR4A1-DN (Fig. 3B). Together, these results indicate that NR4A1 is actively involved in the induction of apoptosis by LPS and simvastatin in RAW 264.7 cells.
Figure 3.
The effect of dominant-negative NR4A1 expression on apoptosis. (A) RAW 264.7 cells were transfected with control or dominant-negative NR4A1 (NR4A1-DN) vectors and treated with various concentrations of LPS and 2µM simvastatin for 12 h. Using flow cytometry, the percentage of subgenomic DNA content was measured in propidium iodide (PI)-stained cells to evaluate DNA fragmentation. The inset shows the presence of NR4A1-DN in the nucleus of transfected cells. (B) RAW 264.7 cells transfected with control or NR4A1-DN vectors were incubated in LPS or simvastatin alone or in combination with Z-VAD-FMK for 12 h and stained using rhodamine-123. The mitochondrial membrane potential (MMP) of the stained cells was analyzed using flow cytometry. A representative result of four experiments is shown.
Bax translocates to mitochondria in apoptotic cells treated with LPS and simvastatin
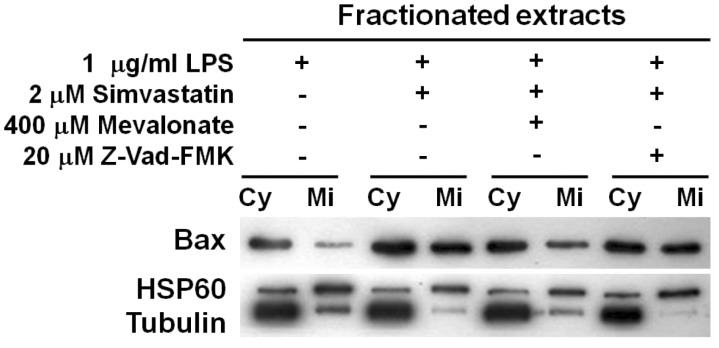
Disruption of MMP is a key apoptotic event that activates caspase-dependent and independent cell death. Several proapoptotic and anti-apoptotic molecules including B-cell lymphoma (Bcl)-extra-large, Bcl-2-associated death promoter, and Bax participate in MMP destruction. To further understand the mechanism of LPS- and simvastatin-induced apoptosis, the behavior of the pro-apoptotic Bax protein was analyzed as was done previously in cancer cells (22). Whole protein extracts were separated into cytoplasmic and mitochondrial fractions, and immunoblotting for Bax protein was performed (Fig. 4). Bax proteins had significantly translocated from cytoplasm to mitochondria in response to LPS and simvastatin treatments. The Bax translocation to mitochondria was partly inhibited by mevalonate, but not markedly inhibited by the pan-caspase inhibitor. These results strongly suggest that the translocation of Bax protein to mitochondria causes the disruption of MMP in cells undergoing LPS and simvastatin-induced apoptosis.
Figure 4.
Translocation of Bax to mitochondria during simvastatin-triggered apoptosis in LPS-activated cells. RAW 264.7 cells were incubated with LPS or simvastatin in combination with mevalonate or Z-VAD-FMK for 11 h. Cell extracts were separated into cytosolic and mitochondrial fractions. For SDS-PAGE separation, 4µg and 2µg of cytosolic and mitochondrial protein of cytosolic and mitochondrial protein, respectively, were loaded and Bax protein in each fraction was detected by western blot. Cy, cytosolic protein; Mi, mitochondrial protein.
DISCUSSION
In this study, we evaluated the molecular mechanism of apoptosis induced by simvastatin and LPS in RAW 264.7 macrophage cells. LPS and/or simvastatin treatments induced the expression of a nuclear orphan receptor, NR4A1. The expression of a dominant-negative mutant form of NR4A1 effectively suppressed the cardinal signs of apoptosis, DNA fragmentation, and disruption of MMP. Apoptosis was accompanied by Bax translocation to mitochondria. To our knowledge, this is the first report revealing that NR4A1 is linked to LPS- and simvastatin-induced apoptosis in macrophages.
As shown in Fig. 2, LPS and simvastatin increased the levels of NR4A1 mRNA in macrophages. However, LPS and simvastatin had differential effects on the expression pattern NR4A1. LPS-induced NR4A1 mRNA appeared early and rapidly dissipated, similar to the pattern of a typical immediate-early gene. In contrast, simvastatin induced a gradual accumulation of NR4A1 mRNA that increased for 12 h. Recent studies have shown that NR4A1 is rapidly induced in macrophages following LPS stimulation (20,21,28). Interestingly, IFNγ also increases the expression of the NR4A1, but in a slower manner than LPS, peaking NR4A1 expression 16~24 h after IFNγ exposure (28). Previous studies reported that co-treatment of LPS and IFNγ induced cell death, but did not do so in single treatments (29). Recently, prolonged NR4A1 expression was shown to induce apoptosis more effectively than shorter periods of expression (30). Fenretinide, a synthetic retinoid, induces apoptosis of hepatocellular carcinoma (HCC) cells through time-dependent expression and intracellular localization of NR4A1. This result suggests that the time-dependent expression of NR4A1 may correlate with the sensitivity of the HCC cells to fenretinide-induced apoptosis. In our study, we identified the prolonged expression of NR4A1 during LPS- and simvastatin-mediated cell death.
To address the role of NR4A1 in the apoptosis, we expressed NR4A1-ND in RAW 264.7 cells and found that it effectively suppressed both DNA fragmentation and the disruption of MMP induced by LPS and simvastatin (Fig. 3). We did not identify the precise role of induced NR4A1 during apoptosis; however, several mechanisms have been proposed by others. For example, NR4A1 is proposed to translocate to mitochondria (17), convert Bcl-2 to a killer form (31), and associate with 14-3-3 zeta (32) or small heterodimer partner proteins (24). NR4A1 also directly induced Fas ligand, TRAIL, and Nur77 downstream gene 1/2(NDG-1/2), factors involved in apoptosis (33). Further work is required to elucidate the functional mechanism of NR4A1 in LPS- and simvastatin-induced apoptosis in macrophages.
LPS and simvastatin treatments also induced the mitochondrial translocation of Bax, a pro-apoptotic member of the Bcl-2 family. The mitochondrial translocation of Bax was inhibited by adding mevalonate, the product of HMG-CoA reductase, but not fully inhibited in the presence of Z-VAD-FMK (Fig. 4). Previously, we demonstrated that simvastatin induces p53 stabilization and mitochondrial translocation of p53 and Bax in MethA fibrosarcoma cells. Stabilization and translocation of p53 to mitochondria is linked to the translocation of Bax during simvastatin-induced apoptosis (22,23). An apoptotic mechanism involving NR4A1, Bax, and p53 had not been investigated. In this study, we demonstrated that LPS and simvastatin have an apoptotic effect on RAW264.7 macrophage cells through induction of NR4A1 expression and Bax translocation to mitochondria. To our knowledge, we are the first to report that NR4A1 is involved in the LPS- and simvastatin-induced apoptotic process in macrophages. Previously, we report that activated macrophages have increased sensitivity to statins (10). We present data supporting the theory that the therapeutic effect of statins in atherosclerosis may involve the induction of the NR4A1 nuclear orphan receptor.
ACKNOWLEDGEMENTS
This work was supported by grants from the Korea Healthcare Technology R&D project, Ministry and Welfare, Republic of Korea (A084773), and partly from the Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology (2013013202).
Abbreviations
- Bax
Bcl-2-associated X protein
- Bcl
B-cell lymphoma
- Z-VAD-FMK
benzyloxycarbonyl-Val-Ala-DL-Asp (O-methyl)-fluoromethylketone
- MMP
mitochondrial membrane potential
- NR4A1-DN
nuclear receptor 4A1 dominant-negative
- PI
propidium iodide
Footnotes
The authors have no financial conflict of interest.
References
- 1.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 2.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280:34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 3.Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction? Lancet. 1996;347:102–103. doi: 10.1016/s0140-6736(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Moye LA, Davis BR, Cole TG, Rouleau JL, Nash DT, Pfeffer MA, Braunwald E. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation. 1998;97:1446–1452. doi: 10.1161/01.cir.97.15.1446. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 6.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 7.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 8.Ghittoni R, Patrussi L, Pirozzi K, Pellegrini M, Lazzerini PE, Capecchi PL, Pasini FL, Baldari CT. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005;19:605–607. doi: 10.1096/fj.04-2702fje. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, Ludwig J, Berger T, Steinkasserer A, Daniel WG, Garlichs CD. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004;172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Kim YC, Song SB, Lee MH, Kang KI, Lee H, Paik SG, Kim KE, Kim YS. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006;339:1007–1014. doi: 10.1016/j.bbrc.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 11.Liang SL, Liu H, Zhou A. Lovastatin-induced apoptosis in macrophages through the Rac1/Cdc42/JNK pathway. J Immunol. 2006;177:651–656. doi: 10.4049/jimmunol.177.1.651. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 15.He YW. Orphan nuclear receptors in T lymphocyte development. J Leukoc Biol. 2002;72:440–446. [PubMed] [Google Scholar]
- 16.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269:532–535. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 18.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109(Suppl):S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 19.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 20.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 21.Kim SO, Ono K, Tobias PS, Han J. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med. 2003;197:1441–1452. doi: 10.1084/jem.20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SK, Kim YS. Phosphorylation of eIF2alpha attenuates statin-induced apoptosis by inhibiting the stabilization and translocation of p53 to the mitochondria. Int J Oncol. 2013;42:810–816. doi: 10.3892/ijo.2013.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SK, Kim YC, Song SB, Kim YS. Stabilization and translocation of p53 to mitochondria is linked to Bax translocation to mitochondria in simvastatin-induced apoptosis. Biochem Biophys Res Commun. 2010;391:1592–1597. doi: 10.1016/j.bbrc.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 24.Yeo MG, Yoo YG, Choi HS, Pak YK, Lee MO. Negative cross-talk between Nur77 and small heterodimer partner and its role in apoptotic cell death of hepatoma cells. Mol Endocrinol. 2005;19:950–963. doi: 10.1210/me.2004-0209. [DOI] [PubMed] [Google Scholar]
- 25.Jin BR, Kim SJ, Lee JM, Kang SH, Han HJ, Jang YS, Seo GY, Kim PH. Alum Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype. Immune Netw. 2013;13:10–15. doi: 10.4110/in.2013.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim H, Do SA, Park SM, Kim YS. Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8(+) T Cells. Immune Netw. 2013;13:63–69. doi: 10.4110/in.2013.13.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim HY, Ju HY, Chung HY, Kim YS. Antitumor effects of a tumor cell vaccine expressing a membrane-bound form of the IL-12 p35 subunit. Cancer Biol Ther. 2010;10:336–343. doi: 10.4161/cbt.10.4.12310. [DOI] [PubMed] [Google Scholar]
- 28.Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19:2466–2477. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- 29.Dijkmans R, Van Damme J, Cornette F, Heremans H, Billiau A. Bacterial lipopolysaccharide potentiates gamma interferon-induced cytotoxicity for normal mouse and rat fibroblasts. Infect Immun. 1990;58:32–36. doi: 10.1128/iai.58.1.32-36.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Bushue N, Bu P, Wan YJ. Induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem Pharmacol. 2010;79:948–954. doi: 10.1016/j.bcp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 32.Masuyama N, Oishi K, Mori Y, Ueno T, Takahama Y, Gotoh Y. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276:32799–32805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 33.Rajpal A, Cho YA, Yelent B, Koza-Taylor PH, Li D, Chen E, Whang M, Kang C, Turi TG, Winoto A. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003;22:6526–6536. doi: 10.1093/emboj/cdg620. [DOI] [PMC free article] [PubMed] [Google Scholar]