Abstract
The epithelium, including the respiratory system, acts as a selective gate between the outside environment and underlying tissue. Epithelial cells are polarized due to the formation of the apical junctional complex, which includes adherent junctions and tight junctions. Endothelial cells are one of the most important cellular constituents of blood vessels. Endothelial junctional proteins play important roles in tissue integrity as well as in vascular permeability, leukocyte extravasation, and angiogenesis. This review focuses on the apical junctional complex in respiratory diseases.
Keywords: Epithelium, Permeability, Tight junctions
INTRODUCTION
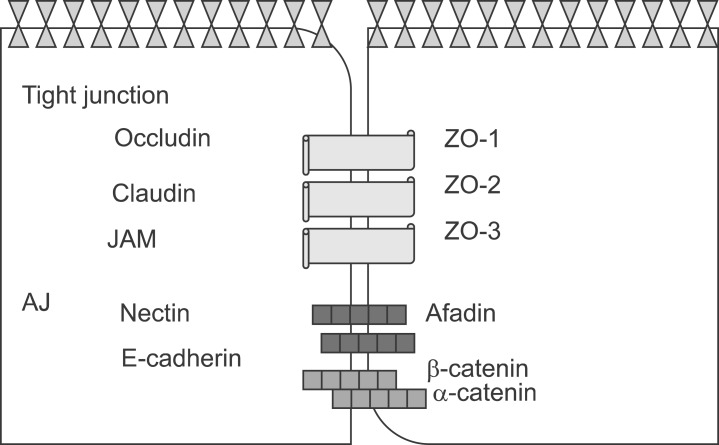
The epithelium, including the respiratory system and other organs, acts as a selective gate between the outside environment and underlying tissue.1 Epithelial cells are polarized by the formation of specialized cell-cell junctions, which are referred to as the apical junctional complex (AJC) (Fig. 1). The AJC consists of adherent junctions (AJs) and tight junctions (TJs).1
FIG. 1.
Apical junctional complexes. The AJC includes adherent junctions (AJs) and tight junctions (TJs).
Endothelial cells are one of the important cellular constituents of blood vessels. Endothelial junctional proteins play important roles in tissue integrity as well as in vascular permeability, leukocyte extravasation, and angiogenesis. Inter-endothelial junctions play pivotal roles in tissue integrity, barrier function, and cell-cell communication. 2 Cell-cell junctions are the sites of attachment between endothelial cells but also function as signaling structures that communicate cell position, limit growth and apoptosis, and regulate vascular homeostasis.2
Some proteins3 are particular to endothelial cells [e.g., VE-cadherin and claudin (CLDN)-5], whereas others are common to epithelial cells [e.g., occludin, junctional adhesion molecule (JAM)-A, nectins, claudins, and connexins], blood cells (e.g., PECAM/CD31, endothelial cell selective adhesion molecule, JAM-A, JAM-C, CD99), smooth muscle cells (e.g., S-endo-1/CD146), and mesangial/trophoblast cells (e.g., protocadherin12/VE-cadherin-2). These proteins may be components of organized junctional structures, such as VE-cadherin in AJs, claudins and occludins in TJs, or connexins in gap junctions. However, others are independent, such as PECAM, CD99, and S-endo-1.3,4
Junctional complexes start off intracellular signaling in various ways. Signaling can be directly activated by engaging signaling proteins or growth-factor receptors or can be indirectly activated by tethering and retaining transcription factors at the cell membrane, thereby limiting their nuclear translocation.5,6,7,8
ADHERENT JUNCTIONS
The important AJ transmembrane proteins belong to the cadherin family.9 E-cadherin is expressed primarily in epithelium. The cytoplasmic tail of E-cadherin is linked to the actin cytoskeleton and other signaling elements through many peripheral membrane proteins, including catenins, vinculin, and α-actinin.10 Through their cytoplasmic tail, junctional adhesion proteins bind to cytoskeletal and signaling proteins, which permits anchoring of the adhesion proteins to actin microfilaments and the transfer of intracellular signals inside the cell.10 The association with actin is required for stabilization of the junctions as well as for dynamic regulation of junction opening and closure. In addition, the interaction of junctional adhesion proteins with the actin cytoskeleton might be relevant for maintaining cell shape and polarity.11 In addition to acting as adaptors that mediate the binding of adhesion proteins to actin, some intracellular junctional proteins, upon release from junctions, translocate to the nucleus and modulate transcription. 12 Another characteristic of some junctional proteins is their function as scaffolds and binding of several effector proteins, which makes their reciprocal interaction easy. A typical example is the TJ component zona occludens-1 (ZO1), which can associate with many transmembrane proteins, including claudins, occludin, or JAMs, as well as with cytoskeletal binding proteins, such as cortactin, cingulin, α-CATENIN, and indirectly with vinculin and α-actinin.13,14 ZO1 also associates with other PDZ-DOMAIN-containing proteins such as ZO2 and with signaling mediators such as ZO1-associated nucleic-acid binding (ZONAB).13,14 VE-cadherin is the transmembrane component of endothelial AJs.15 The VE-cadherin complex also transiently or permanently associates with signaling partners, as well as with a specific apical-basal polarity complex, through direct interaction with PAR-3 and PAR-6.15 VE-cadherin is located at the junctions of all endothelial subtypes. β-Catenin is often noticed in the nucleus and cytoplasm of endothelial cells during pathological angiogenesis or vascular remodeling.15 Nectins are Ca2+-independent Ig-like CAMs.15 Nectin-based cell-cell adhesions set up and maintain AJCs, both independently and by working together with the cadherin-based AJs.15 Afadin binds to both nectins and α-catenin and recruits the cadherin-β-catenin complex to the nectin-based cell-cell adhesion site to form AJs.14,15
TIGHT JUNCTIONS
TJs are the apical-most constituents of the AJC in vertebrate epithelial cell sheets.1 They are also observed in vascular endothelial cells and mesothelial cells. TJs function as a semipermeable gate for the paracellular transport of ions, solutes, water, and cells and are considered to function as a fence that divides the apical and basolateral domains of plasma membranes.1 TJs coordinate a variety of signaling and trafficking molecules that regulate cell differentiation, proliferation, and polarity.1 These functions enable epithelial and endothelial cell sheets to establish distinct tissue compartments within the body and to maintain homeostasis.16,17 Moreover, disturbance of TJ function likely causes or contributes to a variety of pathological conditions, such as inflammatory bowel disease, infection, cancer, vasogenic edema, and blood-borne metastasis.16,17 The barrier function of the TJ also restricts drug delivery to underlying tissues.16,17 Therefore, determining how to overcome paracellular barriers, such as the blood-brain barrier, is critical for the treatment of human disease.16,17 Occludin is an approximately 60-kDa tetraspan membrane protein with two extracellular loops, a short intracellular turn, and N-and C-terminal cytoplasmic domains.18 Claudins19 are 18- to 27-kDa tetraspan proteins with short cytoplasmic N-termini, two extracellular loops, and a C-terminal cytoplasmic domain. They do not show any sequence similarity to occludin. Claudins are capable of forming TJ strands and thus are the backbone of the TJ.19 The claudin family consists of 24 members in mice and humans and exhibits distinct tissue- and cell-type-specific expression patterns.19 Many claudin species are generally expressed in most epithelial cell types. Cldn2, Cldn3, Cldn4, Cldn7, Cldn8, Cldn12, and Cldn15 are abundantly expressed in the duodenum, jejunum, ileum, and colon.19 The segment- or axis-specific expression of different claudins has also been reported in several other epithelial tissues, including the liver lobule, nephron, and inner ear.
JAMs contain two extracellular Ig-like domains, a single transmembrane region, and a C-terminal cytoplasmic domain.20 The JAM family is divided into two subgroups based on their sequence similarities.20 The first subgroup, JAM-A, JAM-B, and JAM-C, has a class II PDZ domainbinding motif at the C-terminal end and directly interacts with ZO-1 and PAR-3.20 In contrast, members of the second subgroup, coxsackie and adenovirus receptor, endothelial cell-selective adhesion molecule (ESAM), and JAM4, contain a class I PDZ domain-binding motif at their C-terminus.20 CAR and JAM4 associate with Ligand-of-Numb protein X1, and JAM4 and ESAM bind the membrane-associated guanylate kinase protein.21
RESPIRATORY DISEASES
Epithelial cells form a tight barrier against environmental stimuli via TJs and AJs. Defects in TJ and AJ proteins may cause changes in epithelial morphology and integrity and potentially result in more rapid trafficking of inflammatory cells through the epithelium. House dust mite (HDM) allergens are important factors in the increasing prevalence of asthma. The lung epithelium forms a barrier that allergens must cross before they can cause sensitization. The cysteine proteinase allergen Der p 1 from fecal pellets of the HDM Dermatophagoides pteronyssinus disrupts intercellular TJs, which are the principal components of the epithelial paracellular permeability barrier. These data suggest that opening of TJs by environmental proteinases may be the initial step in the development of asthma in response to a variety of allergens.22
Lipoxin A4 (LXA4) is a biologically active eicosanoid produced in human airways that displays anti-inflammatory properties. LXA4 plays an important role in the regulation of TJ formation, and stimulation of ZO-1 localization and expression at the plasma membrane occurs through a mechanism involving the LXA4 receptor.23
HDM peptidase is capable of increasing epithelial permeability and thereby creating conditions that favor transepithelial allergen delivery.24,25 Lower epithelial α-catenin, E-cadherin, and/or ZO-1 expression in patients with atopic asthma contributes to defective airway epithelial barriers and higher influx of eosinophils into the epithelium. Der p 1 could contribute to sensitization and allergic responses by degrading airway epithelial barrier function.24 Pollen peptidases directly or indirectly disrupt epithelial tight junctions, and this activity should be considered a possible mechanism for facilitating allergen delivery across epithelia.26
In the normal lung, bronchiolar epithelial cells predominantly express CLDN 1, 3, 4, 7, and 18, whereas alveolar type II epithelial cells predominantly express CLDN 3, 4, 7, and 18.27 In epithelial cells, transgenic expression of CLDN1 with CLDN3 increases transepithelial resistance and decreases paracellular permeability, whereas CLDN4 confers a selective ion transport function without affecting paracellular solute permeability.28 CLDN4 inhibition decreases transepithelial resistance without altering paracellular permeability in primary rat and human epithelial cells.29 CLDN5, although expressed weakly in the epithelium, is strongly expressed in normal lung endothelium and is very highly expressed in interstitial pneumonia endothelium.30 Newborn gene-targeted Cldn5(-/-) mice die within 10 hours of birth, possibly as the result of altered permeability of the blood-brain barrier.31 When CLDN5 is transfected into airway epithelial cells, paracellular permeability increases even in the presence of excessive CLDN1 and CLDN3.32 Moreover, inducing CLDN5 expression in leaky rat lung endothelial cells can enhance paracellular barrier function against large, but not small, molecules.33
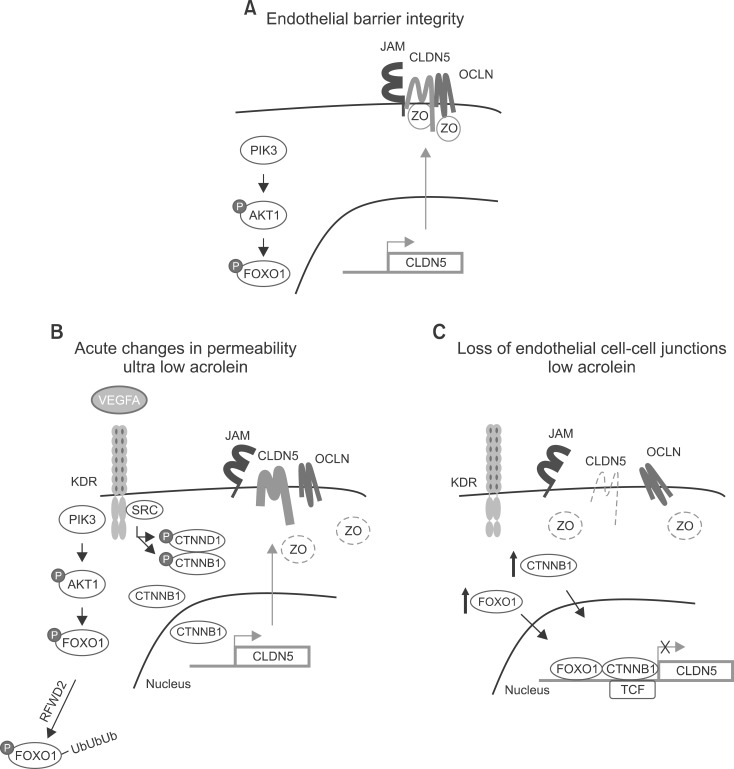
An integral membrane protein, CLDN5 (Fig. 2), is a critical component of the endothelial TJs that control pericellular permeability. The breach of endothelial barriers is a key event in the development of pulmonary edema during acute lung injury. A major irritant in smoke, acrolein, can induce acute lung injury, perhaps by altering CLDN5 expression. The phosphorylation status of the FOXO1 and CTNNB1 transcription factors is consistent with the observed alteration in CLDN5 expression.34 Thus, preservation of endothelial CLDN5 may be a novel clinical approach to treatment of acute lung injury.34
FIG. 2.
Acrolein can induce acute lung injury through changes in claudin 5 expression. (A) Endothelial barrier integrity. (B) Acute changes in permeability. (C) Loss of endothelial cell-cell junctions.
AJC MODULATORS
Safe and effective routes for drug delivery continue to be widely studied. Tissue barriers associated with such non-invasive delivery pose a considerable challenge, particularly for macromolecules.35 This challenge can be met by employing permeation enhancers to facilitate paracellular transport.36 Permeation enhancers can be selected empirically from among currently acceptable excipients for pharmaceutical use, or novel TJM excipients can be developed rationally.36 The growing knowledge of TJ biology, the development of high-throughput cell/tissue-based assay systems, and the use of diverse molecular libraries have led to the identification of promising TJ-modulating compounds that safely and reversibly open TJs to enhance tissue permeability and drug transport.37 New-generation peptide and lipid compounds, identified as TJ modulators, are amenable to optimization by structure-activity analyses and detailed mechanistic studies. This will continue to improve bioavailability and extend the molecular weight range of drugs that can be delivered effectively for chronic applications. The dynamic nature of TJs and the involvement of specific endocytic pathways and intracellular signaling mechanisms that regulate their function offer new possibilities for the development of drugs with more specific mechanisms of action, including the ability to restore normal barrier properties to TJs that are dysregulated during respiratory disease.
In conclusion, the AJC proteins, such as occludin, claudins, and JAMs, have substantially contributed to our understanding of the molecular mechanism of the AJC. Although knowledge regarding AJC membrane proteins has accumulated, several unsolved questions remain. In particular, the significance of AJC proteins in respiratory diseases should be examined further.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2005465).
Footnotes
None declared.
References
- 1.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 2.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 3.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 6.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 7.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 8.Bazzoni G, Dejana E, Lampugnani MG. Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol. 1999;11:573–581. doi: 10.1016/s0955-0674(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 9.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 10.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 11.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–L422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 12.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanning AS, Anderson JM. Protein modules as organizers of membrane structure. Curr Opin Cell Biol. 1999;11:432–439. doi: 10.1016/S0955-0674(99)80062-3. [DOI] [PubMed] [Google Scholar]
- 15.Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, et al. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 2006;7:1239–1246. doi: 10.1038/sj.embor.7400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullin JM, Agostino N, Rendon-Huerta E, Thornton JJ. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today. 2005;10:395–408. doi: 10.1016/S1359-6446(05)03379-9. [DOI] [PubMed] [Google Scholar]
- 17.Landau D. Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens. 2006;15:425–429. doi: 10.1097/01.mnh.0000232883.43093.76. [DOI] [PubMed] [Google Scholar]
- 18.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 20.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res. 2004;300:121–133. doi: 10.1016/j.yexcr.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–294. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 23.Grumbach Y, Quynh NV, Chiron R, Urbach V. LXA4 stimulates ZO-1 expression and transepithelial electrical resistance in human airway epithelial (16HBE14o-) cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L101–L108. doi: 10.1152/ajplung.00018.2008. [DOI] [PubMed] [Google Scholar]
- 24.Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, et al. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000;30:685–698. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 25.de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105–112. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]
- 26.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 27.Koval M. Tight junctions, but not too tight: fine control of lung permeability by claudins. Am J Physiol Lung Cell Mol Physiol. 2009;297:L217–L218. doi: 10.1152/ajplung.00196.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaarteenaho-Wiik R, Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009;57:187–195. doi: 10.1369/jhc.2008.951566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 33.Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, et al. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300:202–212. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar TR, Soppimath K, Nachaegari SK. Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol. 2006;7:261–276. doi: 10.2174/138920106777950852. [DOI] [PubMed] [Google Scholar]
- 37.Romeo VD, deMeireles JC, Gries WJ, Xia WJ, Sileno AP, Pimplaskar HK, et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Deliv Rev. 1998;29:117–133. doi: 10.1016/s0169-409x(97)00064-1. [DOI] [PubMed] [Google Scholar]