Abstract
The objective of the work reported in this paper is to determine if saliva addition has an effect on the rheology of xanthan gum solutions. The reasons for the interest was that it has been previously reported that flavour release from high viscosity xanthan thickened foods is not reduced in the same way as foods thickened by other hydrocolloids at comparable viscosities. It was previously postulated that this could be due to an interaction between saliva and xanthan that could change the microstructure and rheology of xanthan solutions. In this work the effect of saliva on the rheology of CMC and xanthan solutions was compared. Solutions of molecularly dissolved xanthan gum and CMC mixed with water or human whole saliva at a ratio of 5:1 showed little impact of the presence of saliva on steady shear or dynamic viscosity for the two hydrocolloids. In filament thinning experiments saliva addition significantly increased filament break-up time for xanthan gum while it had little effect on the break-up time of the CMC filament. Also, filament thinning appeared a lot less even and was not as reproducible in the case of xanthan gum. Addition of CMC and hydroxypropyl methylcellulose (HPMC) to xanthan gum solutions showed a similar increase in break-up time to saliva, but to see this effect the viscosity of the added CMC or HPMC solution had to be very much higher than the viscosity of saliva. The results are discussed in the context of the structure of xanthan gum and the reported extensional rheology of saliva.
Keywords: Saliva, Xanthan, Rheology, Filament break-up, Oral processing
Graphical abstract
Highlights
-
•
Shear rheology of xanthan gum and CMC not affected by saliva.
-
•
Filament break-up time shorter for xanthan than CMC at comparable viscosity.
-
•
Saliva increases filament break-up time for xanthan but not for CMC.
-
•
Rigid rod conformation of xanthan promotes interaction with saliva mucin fraction.
1. Introduction
For many years there has been substantial interest in the taste and flavour perception from viscous solutions. It is universally observed that increase in viscosity inhibits taste and flavour perception due to increasingly poor mixing with saliva and therefore slow transport of tastant and flavour molecules to the taste buds (Baines & Morris, 1987; Morris, 1993). Mixing efficiency has also been linked with the good taste and flavour release from starches, which retain their granular form when swollen, compared to a molecular solution of hydroxypropyl methylcellulose (HPMC) that was of the same shear viscosity at 50 s−1 (Ferry et al., 2006). Rheologically, the two types of solution differ in droplet break-up behaviour as measured rheooptically (Desse, Mitchell, Wolf, & Budtova, 2011) and by mixing a solution containing a marker dye with water (Ferry et al., 2006). However, a recent study on model soups thickened with swollen particles of physically modified xanthan gum and molecularly dissolved xanthan gum revealed that superior mixing behaviour and shorter filament break-up times did not coincide with enhanced taste and flavour release (Abson et al., 2014). The sensory panel was also not able to distinguish taste and flavour perception between the tomato flavour intensity in xanthan and modified starch thickened tomato soup. Morris (1993) found that xanthan gum gives better taste perception at high viscosity than solutions thickened with other molecularly dissolved polysaccharides. The cited observations could suggest that predicting the sensory properties of hydrocolloids from measurements in water could be misleading and that the appropriate fluid to predict sensory behaviour based on flow properties would be saliva.
In this paper the rheological properties of xanthan gum solutions following mixing with water and saliva were analysed in steady shear, oscillatory shear and in uniaxial extensional flow. For the scope of this initial study behaviour in extensional flow was quantified by filament break-up times acquired in a capillary break-up extensional rheometer. The results were compared to solutions of carboxymethyl cellulose (CMC). This was chosen partly because like xanthan it is not only a polyelectrolyte with a cellulose backbone, but also because it is frequently used in the formulation of artificial saliva (Hahnel, Behr, Handel, & Buergers, 2009). Unusual strand-like phase separation has been found for xanthan polyelectrolyte blends, but is not seen in mixtures of xanthan with non-polyelectrolytes (Boyd et al., 2009). Thus, the addition of solutions of CMC and the uncharged hydroxypropyl methylcellulose (HPMC) to xanthan gum solutions was included to see if either hydrocolloid gave similar effects to saliva addition.
2. Materials and methods
2.1. Materials
Xanthan gum (KELTROL®-T) and carboxymethyl cellulose (CEKOL®-20000) with a degree of carboxymethyl substitution in the range 0.75–0.85 (manufacturers data) were provided by CP Kelco UK Ltd (Leatherhead, UK). Hydroxypropyl methylcellulose (METHOCEL™ K4M) was obtained from Dow Wolff Cellulosics (Bomlitz, D). The methoxyl degree of substitution and the hydroxypropyl molar substitution were 1.4 and 0.21 respectively (manufacturers data). The aqueous solutions were prepared with double distilled water and also contained sodium chloride (Sigma Aldrich, Gillingham, UK) and the antimicrobial sodium azide (Sigma Aldrich, Gillingham, UK) at a level of 0.2%w/w and 0.05%w/w respectively. Human whole saliva was used following collection from a healthy volunteer. All protocols involving saliva were previously approved by the local ethics committee and the biological safety officer.
2.2. Polysaccharide solution preparation
Each polysaccharide was added in appropriate quantity to an aqueous solution of 0.2%w/w NaCl and 0.05%w/w sodium azide to make a 1%w/w stock solution while mixing on a magnetic stirrer hot plate at 70 °C. The dispersion was stirred continuously for 1 h and kept at room temperature until further use but at least overnight. The desired concentration of each polysaccharide solution was obtained by mixing the stock solution with 0.2%w/w sodium chloride solution on a roller mixer for at least 2 h at room temperature.
2.3. Saliva sample preparation
Mechanically stimulated saliva was collected from a healthy volunteer between 10 am and 12 pm. The volunteer was asked to not eat and drink, with the exception of water, for 2 h prior to donation. The donor chewed on a small piece of laboratory self-sealing film (Parafilm, Bermis Flexible Packaging, Neenah, USA) (approximately 5 cm × 5 cm) to stimulate flow of saliva over a period of 10 min. Saliva was expectorated every 30 s into an ice-chilled container whereby the saliva from the first 30 s was discarded as it was likely to contain food and other debris that may have been present in the oral cavity. The collected saliva sample was gently swirled, transferred to a centrifuge tube and centrifuged at 10,000g for 30 min at 4 °C to remove cell debris. The supernatant of the centrifuged sample was stored in a −80 °C freezer and used for experiments within two weeks of collection.
2.4. Mixing of polysaccharide solution with saliva
The frozen saliva was defrosted in an ice-chilled water bath just before experiments. 1 g of saliva was added to 5 g of 0.5%w/w xanthan gum solution or 0.65%w/w CMC solution in a 50 mL conical tube. These solutions were prepared by diluting the stock solution with 0.2% NaCl. Mixing was carried out on a roller mixer for 2 h at 4 °C. Control samples were prepared by replacing saliva with double distilled water under the same conditions.
2.5. Steady shear and oscillatory shear rheology
All rheological measurements were carried out in duplicate and at 20 °C with a stress controlled rotational rheometer (MCR 301, Anton Paar, Graz, A) using a cone and plate geometry (0.5° angle, 50 mm diameter). Steady shear viscosity data were collected at shear rates between 0.1 and 1000 s−1. Strain sweeps were performed from 0.1 to 1000% strain at the angular frequency of 1.59 Hz. Frequency sweeps were conducted from 10 Hz to 0.1 Hz at 0.5% strain which was in the linear viscoelastic domain.
2.6. Filament break-up
Extensional rheology tests were carried out at 25 °C using a capillary break-up extensional rheometer (CaBER-1, Thermo Haake GmbH, Karlsruhe, D) equipped with 6 mm diameter plates. Samples were carefully loaded to ensure the absence of air bubbles between the upper and lower plates with an initial gap of 3 mm. The upper plate was moved up rapidly (within 20 ms; shorter times were not feasible as then the sample would de-wet the upper plate) to a pre-set height of 19 mm to form a filament. The thinning of the filament diameter was monitored at the mid-point of the filament length which corresponds to the standard set-up of the equipment used. Due to the difficulty in loading the sample in the shape of a perfect cylinder and almost certain inhomogeneity of the samples at molecular level, at least 20 replicate measurements were performed and representative results were chosen for analysis.
2.7. Experimental design and data analysis
The concentration of the xanthan gum solution was set to 0.5%w/w to correspond to a value typically found in thickened foods. Similarly, the concentration of sodium chloride of 0.2%w/w is found in popular brands of tomato soup. To gain insight into the role charge has to play in the interaction with saliva, CMC was chosen as a negatively charged food hydrocolloid as it is often used as food thickener. A 0.65%w/w concentration of the CMC solution was selected to be shear viscosity matched at a shear rate close to 50 s−1 at 20 °C with 0.5%w/w xanthan gum following dilution of five parts of polysaccharide solution with one part of water. The shear rate of 50 s−1 was chosen as the shear rate often used to assess the viscosity of liquid foods in correlation with sensory behaviour (Christensen, 1980; Ferry et al., 2006; Koliandris et al., 2010; Moskowitz & Arabie, 1970) and it corresponds to the value applied in the paper motivating this study. The mixing ratio of 5:1 was chosen based on the maximum flow rate of stimulated saliva reported by Humphrey and Williamson (2001), an estimated serving size of 10 mL of liquid food and an in-mouth residence time of roughly 15 s.
To aid the discussion of the main study data further solutions of CMC at lower concentration and HPMC solutions at two concentrations typically found in food products were included. HPMC is uncharged and mixing xanthan solution with either CMC or HPMC, maintaining the mixing ratio of five parts of xanthan to one part of diluent, filament break-up experiments of these two types of mixtures were conducted. The aim was to gain insight into the role of viscosity and charge of the diluent. Shear viscosity of these CMC and HPMC solutions was also measured and viscosity at 50 s−1 is reported.
All shear rheological data are reported in form of graphs showing one data trace from two independent measurements which would have overlapped on the scale chosen for the graphs. Data correction was not required as a cone and plate geometry was used. Shear viscosity at 50 s−1 is reported as the average of the two measurements. Filament break-up data are reported as evolution of the normalised diameter, calculated as the actual filament diameter divided by the diameter of the filament before onset of thinning, with time. Full analysis of the thinning data for extension rate and viscosity was outside the scope of this preliminary study on the impact of saliva on the flow properties of hydrocolloid solutions. This would require certainty that the filament thins in the middle where the diameter is monitored in our set-up. Break-up times are reported as average over at least 15 measurements. Analysis of variances (ANOVA) followed by Tukey's post hoc test was used to investigate if there were any significant differences between the filament break-up times of samples (P > 0.05).
3. Results and discussion
3.1. Shear rheology properties
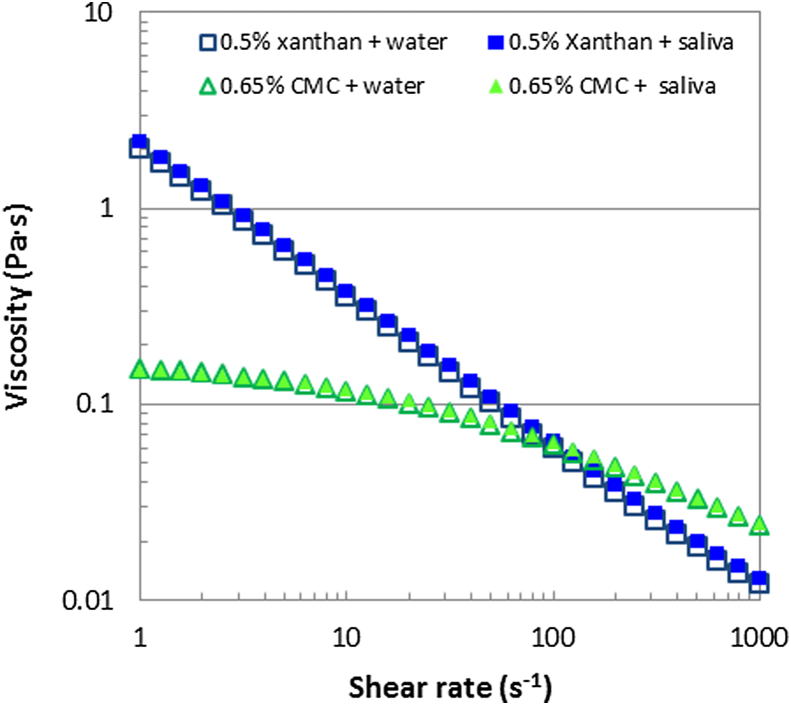
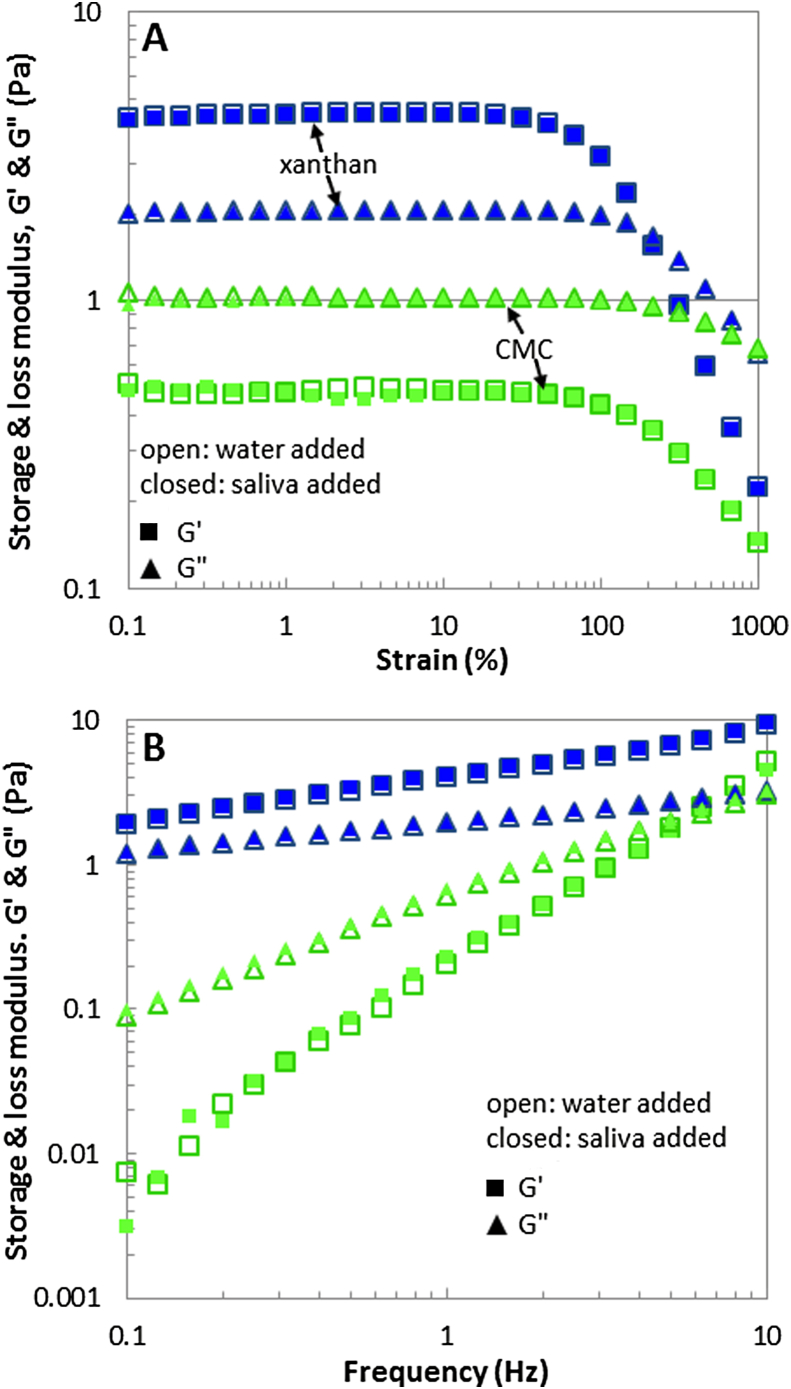
Figs. 1 and 2 display the effect of saliva addition on the steady shear viscosity and the small deformation viscoelastic moduli of xanthan gum and CMC solutions. For both hydrocolloids the general form of this relationship is consistent with many previous studies (Sworn, 2009). The addition of saliva as opposed to water shows no difference in the large and small deformation shear rheological responses.
Fig. 1.
Effect of saliva on steady shear rheology of xanthan and CMC solutions showing one data trace for each sample (CV < 2%). One part of saliva or water was added to five parts of hydrocolloid solution.
Fig. 2.
Effect of saliva on small deformation viscoelastic moduli of xanthan gum and CMC solutions. One part of saliva or water was added to five parts of hydrocolloid solution. (A): Amplitude sweep at 1.59 Hz, (B): Frequency sweep at 0.5% strain.
3.2. Filament break-up behaviour
In contrast to the shear rheological properties, saliva addition does have an effect on the filament thinning behaviour. Figs. 3(A) and (B) compare the changes in normalised filament diameter with time until break-up for xanthan gum and CMC solutions showing five selected data traces for each sample. In contrast to CMC which showed smooth curves xanthan filaments thinned more unevenly and less reproducibly. This was the motivation for conducting the large number of replicate measurements. Immediately obvious from the graphs is that mixing with saliva, compared with water, has an impact on filament thinning for xanthan gum. Mixing with saliva imparts a longer filament life time. For CMC the impact of the use of water or saliva as diluent is much less pronounced. Filament break-up times are reported in Table 1. Standard deviation for xanthan gum mixed with water is >10% and around 5% or less for the other samples. Analysis of the data for extensional viscosity was outside the scope of this short communication in part due to the extent of the data scatter and the limitation that the CaBER records filament diameters at filament mid-point which may not correspond to the thinnest diameter.
Fig. 3.
Normalised filament diameter as a function of time for xanthan (A) and CMC (B) solutions. Five representative data sets are depicted for each sample.
Table 1.
Filament break-up times for the different hydrocolloid solutions diluted with water or saliva (mean and standard deviation). Superscripts (letters) indicate the results of statistical analysis of all samples containing xanthan gum. Samples with same superscript are not statistically different from each other.
| Samples | Shear viscosity of added solutions at 50 s−1 |
Hydrocolloid concentrations in the mixture |
Filament break-up time (ms)a | |||
|---|---|---|---|---|---|---|
| Added solution | Viscosity (mPa s) | Xanthan (%) | CMC (%) | HPMC (%) | ||
| 0.5% Xanthan + water (5:1) | Waterb | 1 | 0.42 | – | – | 57 ± 5A |
| 0.5% Xanthan + saliva (5:1) | Salivac | 1–2 | 0.42 | – | – | 70 ± 3D |
| 0.65% CMC + water (5:1) | Water | 1 | – | 0.54 | – | 75 ± 2 |
| 0.65% CMC + saliva (5:1) | Saliva | 1–2 | – | 0.54 | – | 82 ± 3 |
| 0.5% Xanthan + 0.1% CMC (5:1) | 0.1% CMC | 4 | 0.42 | 0.02 | – | 62 ± 3B |
| 0.5% Xanthan + 0.25% CMC (5:1) | 0.25% CMC | 19 | 0.42 | 0.04 | – | 66 ± 3C |
| 0.5% Xanthan + 0.5% CMC (5:1) | 0.5% CMC | 74 | 0.42 | 0.08 | – | 69 ± 3CD |
| 0.5% Xanthan + 0.35% HPMC (5:1) | 0.35% HPMC | 19 | 0.42 | – | 0.06 | 77 ± 4E |
| 0.5% Xanthan + 0.65% HPMC (5:1) | 0.65% HPMC | 74 | 0.42 | – | 0.11 | 88 ± 6F |
Averaged over at least 15 measurements.
Literature value of water at 20 °C (Mezger, 2006).
Literature value of centrifuged saliva (Bongaerts, Rossetti, & Stokes, 2007).
While this study was concerned with the difference in interaction with water or saliva and the impact on the flow properties and less so with the differences between the two hydrocolloids, it is worth mentioning that the shorter break-up time for xanthan gum based samples is not surprising. The two types of hydrocolloid solution were shear viscosity matched at 50 s−1. Over the whole shear rate range (Fig. 1), the xanthan gum systems have a higher viscosity at lower shear rates and lower viscosity at higher shear rate which is due to the exceptional shear thinning nature of xanthan gum solutions. CMC on the other hand is a flexible polymer showing a zero shear plateau followed by shear thinning which is less pronounced than for xanthan gum. The filament thinning experiment follows a rapid stretch of the sample in which the rod-shaped xanthan gum molecules align in stretch direction whereas the flexible CMC molecules will be entangled. Thus, break-up time is shorter for the xanthan gum system in agreement with literature reports on the impact of molecular confirmation on filament break-up behaviour (Haward, Sharma, Butts, McKinley, & Rahatekar, 2012; Jones, 1990). It has also been reported previously that increased viscoelasticity, which is the case here for the xanthan samples compared to the CMC samples, will lead to quick thinning and breaking of a fast stretched filament (Chan et al., 2009). Another factor which could partially explain the shorter break-up time of xanthan along with its uneven thinning might be the more particulate structure of “molecularly” dissolved xanthan. This is probably not only due to the large size of individual xanthan molecules which have an end to end length of ∼1 μm, but also the more complex structures found in reordered materials including microgels (Capron, Alexandre, & Muller, 1998; Gulrez, Al-Assaf, Fang, Phillips, & Gunning, 2012; Morris et al., 2001).
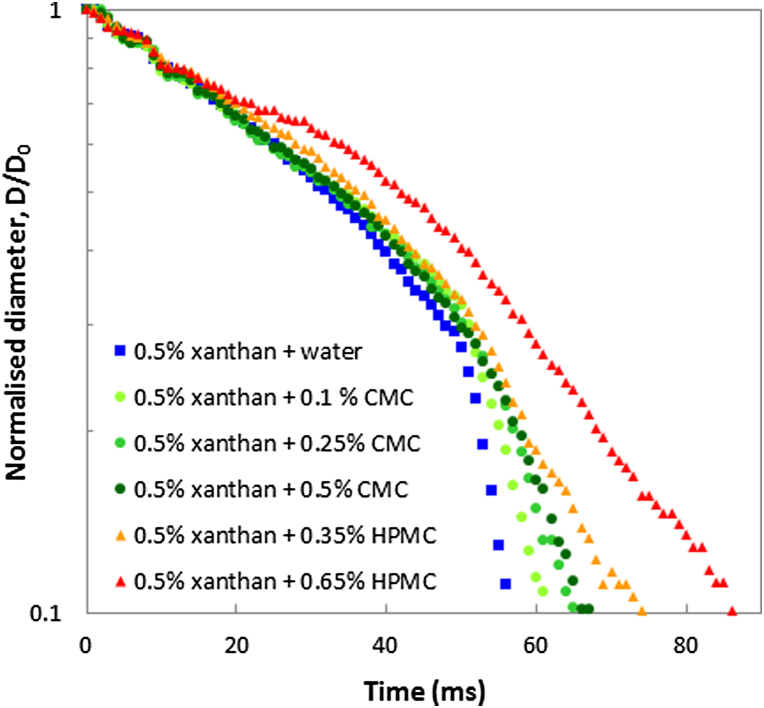
To gain further preliminary insight into the mechanism by which saliva protects xanthan against filament break-up, CMC and HPMC solutions were used as charged and uncharged diluent, respectively. Fig. 4 shows representative data traces for the normalised diameter for a range of diluent concentrations mixed with xanthan gum solution. Filament break-up times for these mixtures have been included in Table 1. An obvious hypothesis is that shear viscosity impacts on filament break-up kinetics so also shown in Table 1 is the viscosity of the added solution (water, saliva, or hydrocolloid).
Fig. 4.
Effect of added hydrocolloids on filament thinning of xanthan. One part of hydrocolloid solution or water was added to 5 parts of xanthan solution.
It is clear from this data that the delay in filament break-up upon addition of saliva is not related to its shear viscosity. To produce a similar effect on filament break-up to saliva the viscosity of an added CMC solution has to be about 40 times higher than that of saliva. The extensional viscosity of centrifuged saliva has recently been measured with a stagnation point extensional flow device and Trouton ratios as high as 100 were reported (Haward et al., 2012). Several other studies including measurements of filament break-up (Stokes & Davies, 2007; Zussman, Yarin, & Nagler, 2007) have shown that saliva is an elastic fluid due to the presence of high molecular weight mucins of high persistence length in particularly MUC5B (Haward, Odell, Berry, & Hall, 2011). The concentration of this component in human saliva is ∼0.02% (Rayment, Liu, Offner, Oppenheim, & Troxler, 2000). Using plausible values for the molecular weight of MUC5B (Haward et al., 2012) calculated values for the overlap concentration c∗ may be as low as 0.006%. So here where saliva was diluted fivefold with hydrocolloid solution, its concentration is above c∗ and its elasticity still impacts on the behaviour in an extensional flow situation. It manifests itself as a protection against filament break-up. This effect was more pronounced for xanthan gum than for CMC which may be explained by the rigid rod versus flexible polymer conformation as well as charge repulsion in case of CMC. There is scope for more extensive work including physicochemical analysis of the donated saliva as saliva rheology including filament thinning behaviour will depend on the source, age of donor, time and methods of collection and whether or not the saliva samples have been centrifuged to remove cell debris (Stokes & Davies, 2007). However, the objective of this short study to gain some preliminary insight if saliva has an effect on the rheological behaviour of xanthan gum has been achieved. The original hypothesis (Abson et al., 2014) that xanthan will mix more efficiently with water than with saliva due to phase separation involving the mucin polyelectrolytes is not supported by this data. The filament thinning data suggest that xanthan solutions in saliva will have somewhat longer textures than in water, rather than the shorter textures related to good mixing. The addition of the non-polyelectrolyte HPMC induces a greater delay in filament break-up than CMC at comparable viscosities of added hydrocolloid. In this context it may be of some relevance that HPMC droplets are extremely difficult to break-up in a counter rotating shear device (Desse et al., 2011).
4. Conclusions
Inspection of the filament break-up behaviour of xanthan gum has revealed an impact of saliva on its extensional flow properties that is more pronounced than for CMC. The presence of saliva had no impact on the shear rheological properties both in large and small deformation. The research thus far allows concluding that the interaction behaviour between the rigid rod molecule xanthan gum and saliva differs from the behaviour of other flexible or semi-flexible hydrocolloid molecules classically included in taste perception studies. In terms of the saliva specific components responsible for this interaction behaviour it can be hypothesised that it is the large molecular weight mucin fraction. Further fundamental studies including purified systems and hydrodynamic interaction studies are part of future work.
Acknowledgements
This work was carried out as part of the Advanced Food Manufacturing LINK Programme titled Development of Physically Modified Hydrocolloids and Starches for Enhanced Salt Perception (FQI30/FT1715), supported by BBSRC and DEFRA.
References
- Abson R., Gaddipati S.R., Hort J., Mitchell J.R., Wolf B., Hill S.E. A comparison of the sensory and rheological properties of molecular and particulate forms of xanthan gum. Food Hydrocolloids. 2014;35:85–90. doi: 10.1016/j.foodhyd.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines Z.V., Morris E.R. Flavour/taste perception in thickened systems: the effect of guar gum above and below c∗. Food Hydrocolloids. 1987;1(3):197–205. [Google Scholar]
- Bongaerts J.H.H., Rossetti D., Stokes J.R. The lubricating properties of whole saliva. Tribology Letters. 2007;27(3):277–287. [Google Scholar]
- Boyd M.J., Hampson F.C., Jolliffe I.G., Dettmar P.W., Mitchell J.R., Melia C.D. Strand-like phase separation in mixtures of xanthan gum with anionic polyelectrolytes. Food Hydrocolloids. 2009;23(8):2458–2467. [Google Scholar]
- Capron I., Alexandre S., Muller G. An atomic force microscopy study of the molecular organisation of xanthan. Polymer. 1998;39(23):5725–5730. [Google Scholar]
- Chan P.S.K., Chen J.S., Ettelaie R., Alevisopoulos S., Day E., Smith S. Filament stretchability of biopolymer fluids and controlling factors. Food Hydrocolloids. 2009;23(6):1602–1609. [Google Scholar]
- Christensen C.M. Effects of solution viscosity on perceived saltiness and sweetness. Perception & Psychophysics. 1980;28(4):347–353. doi: 10.3758/bf03204394. [DOI] [PubMed] [Google Scholar]
- Desse M., Mitchell J., Wolf B., Budtova T. Droplet deformation and break-up under shear: hydrocolloid solution vs. suspension of starch granules. Food Hydrocolloids. 2011;25(3):495–502. [Google Scholar]
- Ferry A.L., Hort J., Mitchell J.R., Cook D.J., Lagarrigue S., Pamies B.V. Viscosity and flavour perception: why is starch different from hydrocolloids? Food Hydrocolloids. 2006;20(6):855–862. [Google Scholar]
- Gulrez S.K.H., Al-Assaf S., Fang Y., Phillips G.O., Gunning A.P. Revisiting the conformation of xanthan and the effect of industrially relevant treatments. Carbohydrate Polymers. 2012;90(3):1235–1243. [Google Scholar]
- Hahnel S., Behr M., Handel G., Buergers R. Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Supportive Care in Cancer. 2009;17(11):1331–1343. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- Haward S.J., Odell J.A., Berry M., Hall T. Extensional rheology of human saliva. Rheologica Acta. 2011;50(11–12):869–879. [Google Scholar]
- Haward S.J., Sharma V., Butts C.P., McKinley G.H., Rahatekar S.S. Shear and extensional rheology of cellulose/ionic liquid solutions. Biomacromolecules. 2012;13(5):1688–1699. doi: 10.1021/bm300407q. [DOI] [PubMed] [Google Scholar]
- Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. Journal of Prosthetic Dentistry. 2001;85(2):162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Jones W.M. Comparison of the behavior of filaments of xanthan solutions with that of filaments of polyacrylamide solutions. Journal of Non-Newtonian Fluid Mechanics. 1990;35(1):77–81. [Google Scholar]
- Koliandris A.L., Morris C., Hewson L., Hort J., Taylor A.J., Wolf B. Correlation between saltiness perception and shear flow behaviour for viscous solutions. Food Hydrocolloids. 2010;24(8):792–799. [Google Scholar]
- Mezger T.G. Hannover, D; Vincentz: 2006. The rheology handbook. [Google Scholar]
- Morris E.R. Rheological and organoleptic properties of food hydrocolloids. Food Hydrocolloids. 1993;24(8):201–210. [Google Scholar]
- Morris V.J., Mackie A.R., Wilde P.J., Kirby A.R., Mills E.C.N., Gunning A.P. Atomic force microscopy as a tool for interpreting the rheology of food biopolymers at the molecular level. Lebensmittel-Wissenschaft und-Technologie/Food Science and Technology. 2001;34(1):3–10. [Google Scholar]
- Moskowitz H.R., Arabie P. Taste intensity as a function of stimulus concentration and solvent viscosity. Journal of Texture Studies. 1970;1:502–510. doi: 10.1111/j.1745-4603.1970.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Rayment S.A., Liu B., Offner G.D., Oppenheim F.G., Troxler R.F. Immunoquantification of human salivary mucins MG1 and MG2 in stimulated whole saliva: factors influencing mucin levels. Journal of Dental Research. 2000;79(10):1765–1772. doi: 10.1177/00220345000790100601. [DOI] [PubMed] [Google Scholar]
- Stokes J.R., Davies G.A. Viscoelasticity of human whole saliva collected after acid and mechanical stimulation. Biorheology. 2007;44(3):141–160. [PubMed] [Google Scholar]
- Sworn G. Xanthan gum. In: Phillips G.O., Williams P.A., editors. Handbook of hydrocolloids. Woodhead Publishing; Cambridge, UK: 2009. pp. 186–203. [Google Scholar]
- Zussman E., Yarin A.L., Nagler R.M. Age- and flow-dependency of salivary viscoelasticity. Journal of Dental Research. 2007;86(3):281–285. doi: 10.1177/154405910708600316. [DOI] [PubMed] [Google Scholar]