Summary
Miniature neurotransmission is the transsynaptic process where single synaptic vesicles spontaneously released from presynaptic neurons induce miniature postsynaptic potentials. Since their discovery over 60 years ago, miniature events have been found at every chemical synapse studied. However, the in vivo necessity for these small-amplitude events has remained enigmatic. Here, we show that miniature neurotransmission is required for the normal structural maturation of Drosophila glutamatergic synapses in a developmental role that is not shared by evoked neurotransmission. Conversely, we find that increasing miniature events is sufficient to induce synaptic terminal growth. We show that miniature neurotransmission acts locally at terminals to regulate synapse maturation via a Trio guanine nucleotide exchange factor (GEF) and Rac1 GTPase molecular signaling pathway. Our results establish that miniature neurotransmission, a universal but often-overlooked feature of synapses, has unique and essential functions in vivo.
Highlights
-
•
Miniature, but not evoked, neurotransmission is required for synapse development
-
•
Miniature neurotransmission bidirectionally regulates synaptic terminal maturation
-
•
Miniature events signal locally through the GEF Trio and the GTPase Rac1
-
•
Miniature neurotransmission has unique and essential functions in vivo
Miniature events (or “minis”) are a universal feature of all chemical synapses, but their function in vivo has remained enigmatic. Here, Choi et al. show that miniature neurotransmission is essential for synaptic terminal maturation in a role not shared by evoked neurotransmission.
Introduction
Two forms of neurotransmission (NT) occur at fast chemical synapses: evoked NT and the much less studied process of miniature NT. During evoked NT, action potentials trigger the release of multiple synaptic vesicles inducing the synchronous activation of many postsynaptic receptors, thereby allowing information to be transmitted across the synaptic cleft. Evoked NT is absolutely essential to brain function and is considered to be the primary carrier for neurochemical communication between neurons. The second form, miniature NT, often called “minis,” occurs via the spontaneous release of single synaptic vesicles from presynaptic neurons activating a small number of postsynaptic receptors. Miniature NT is a general property of every fast chemical synapse studied since their discovery by Katz (Fatt and Katz, 1952). However, in contrast to evoked neurotransmission, the in vivo necessity for miniature events has remained a conundrum and they have been often dismissed as a stochastic byproduct of evoked NT (Otsu and Murphy, 2003; Ramirez and Kavalali, 2011; Sutton and Schuman, 2009; Zucker, 2005).
Recent studies, however, have begun to question the notion that miniature events are simply superfluous “noise” derived from the process of evoked NT. First, a number of synaptic vesicle fusion molecules, such as vSNAREs, that are necessary for evoked NT are not essential for miniature NT and vice versa (Kavalali and Monteggia, 2012). Second, specialized synaptic Ca2+-sensing molecules can regulate the frequency of miniature events independently of evoked NT (Walter et al., 2011). Third, some evidence suggests that the synaptic vesicle pools that mediate miniature NT and evoked NT may be distinct, though this remains the subject of active debate (Ramirez and Kavalali, 2011). Fourth, though most active zones at Drosophila synapses have both forms of NT, some have recently been shown to produce exclusively miniature or evoked events (Melom et al., 2013; Peled et al., 2014). These studies suggest that miniature events have some properties that are different from evoked NT, prompting the hypothesis that minis could have unique functions at the synapse. Consistent with this idea, in cultured mammalian neurons, miniature NT has been found to influence synaptic scaling, stabilize spine structure, change the activity of postsynaptic kinases, and affect local protein synthesis (Otsu and Murphy, 2003; Sutton and Schuman, 2009; Turrigiano, 2012). However, as of yet, an in vivo function for miniature neurotransmission has not been demonstrated.
One in vivo process that can be disrupted by the depletion of both evoked and miniature NT is synaptic structural development. In mammals, the absence of vesicular NT does not appear to disrupt initial pre- and postsynaptic assembly (Verhage et al., 2000). Nonetheless, when both forms of NT are depleted at neuromuscular synapses, subsequent aspects of synaptic structural development and maturation are perturbed (Kummer et al., 2006; Witzemann et al., 2013). However, the individual contribution of evoked or miniature neurotransmission to these phenotypes was not dissected in these studies.
A tractable model to investigate synaptic structural development is Drosophila glutamatergic larval neuromuscular junction (NMJ) synapses (Collins and DiAntonio, 2007). Like synapses in other systems, Drosophila terminals undergo a growth and development phase subsequent to initial synaptic assembly. This process involves a 10-fold expansion of the synaptic terminal area through the iterative addition and enlargement of synaptic varicosities or boutons over 4 days of larval development (Schuster et al., 1996). Like mammalian synapses, the initial assembly of Drosophila terminals is not perturbed when both evoked and miniature neurotransmission are abolished (Daniels et al., 2006); however, the effect of a similar depletion on subsequent phases of synaptic development has not been described.
Here, we have investigated the necessity for evoked and miniature neurotransmission during Drosophila larval synaptic growth. We found that inhibition of both forms of NT caused characteristic defects in terminal morphology, bouton growth, and ultrastructure. Surprisingly, by manipulating each form of NT independently, we found these defects were caused by the specific loss of miniature NT and not evoked NT. Moreover, we found that increasing miniature NT could promote synaptic growth. We show that miniature NT regulates local synaptic terminal growth by activating a Trio guanine nucleotide exchange factor (GEF), Rac1 GTPase signaling pathway in presynaptic neurons. Our results establish that miniature neurotransmission, an often-overlooked universal feature of all chemical synapses, has a unique and essential role during synaptic development in vivo.
Results
Neurotransmission Is Required for Drosophila Larval Synaptic Terminal Development
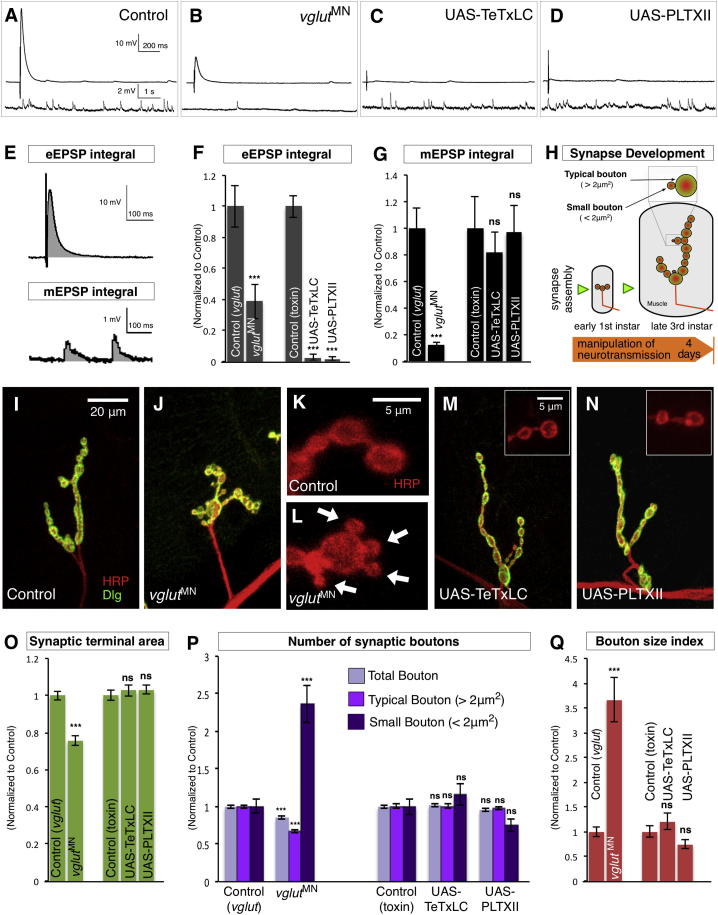
To determine if neurotransmission is necessary for Drosophila larval NMJ synapse development, we sought to inhibit synaptic transmission without perturbing other cellular processes. Vesicular glutamate transporters (Vgluts) are required for the uptake of glutamate into synaptic vesicles (Daniels et al., 2006). Drosophila has a single vglut gene that completely abolishes all NT at glutamatergic NMJ terminals when eliminated. Importantly, removal of Vglut does not impede either exo/endocytosis (Daniels et al., 2006), which can disrupt synaptic development independently of effects on NT (Dickman et al., 2006). vglut null mutants die as embryos, but formation of their synaptic terminals appears normal (Daniels et al., 2006). In order to strongly deplete NT during larval stages (Figure 1H), we combined hypomorphic vglut mutants (Daniels et al., 2006; Mahr and Aberle, 2006) with transgenic Vglut-RNAi expressed in motor neurons (MNs) to generate vglutMN. In this mutant combination, the amplitude of evoked excitatory postsynaptic potentials (eEPSPs) was reduced by 66% (p < 0.001) compared to controls (Figures 1A and 1B; Figure S1A available online). To determine the total amount of evoked NT, we measured the eEPSP integral (Stuart and Sakmann, 1995) (normalized area under the eEPSP above the baseline resting membrane potential [RMP]) (Figure 1E). We found that vglutMN had a 61% (p < 0.001) decrease in the eEPSP integral compared to controls (Figure 1F). We also measured miniature excitatory postsynaptic potential (mEPSP) frequency, amplitude, and the mEPSP integral (normalized average area under the mEPSP above the baseline RMP) (Figure 1E). In vglutMN mutants, we found an 89% reduction (p < 0.001) in mEPSP frequency (Figures 1B and S1B) but no change in mEPSP amplitude (Figures 1B and S1C), consistent with other vglut alleles (Daniels et al., 2006), leading to an 88% (p < 0.001) reduction in the mEPSP integral compared to controls (Figure 1G). Thus, in vglutMN mutants, both evoked and miniature NT was inhibited.
Figure 1.
Neurotransmission Is Required for Larval Synaptic Terminal Development
(A–D) Representative traces of eEPSPs (above) and mEPSPs (below) from (A) control (CS), (B) vglutMN (vglutHypo/Df,UAS-Vglut-RNAiKK;D42-Gal4/UAS-Vglut-RNAiJF), (C) UAS-TeTxLC (OK319-Gal4/UAS-TeTxLC), and (D) UAS-PLTXII (OK6-Gal4/+;+/UAS-PLTXII).
(E) Representation of measurement of eEPSP integral (above) and mEPSPs integral (below).
(F and G) Quantification of (F) eEPSP integral (n ≥ 6) and (G) mEPSPs integral (n ≥ 9) of the indicated genotypes.
(H) Schematic of Drosophila larval neuromuscular junction synaptic terminal development during the experimental period.
(I–N) Representative NMJ terminals and individual boutons (K and L, insets of M and N) labeled with the postsynaptic marker Dlg (green) and the neuronal membrane marker horseradish peroxidase (HRP) (red). Arrows indicate small boutons (L).
(O–Q) Quantification of the morphological features of NMJ synaptic terminals including (O) synaptic terminal area, (P) synaptic bouton number, and (Q) the bouton size index (n ≥ 30) of the indicated genotypes.
All quantification data are normalized to control (Control [vglut]; vglutDf/+); (Control [toxin]; CS). Scale is identical in (I), (J), (M), and (N); in (K) and (L); and in the insets of (M) and (N). All error bars indicate ±SEM. ∗p < 0.05, ∗∗∗p < 0.001. See also Figures S1 and S2.
When we examined the terminals of vglutMN mutants at the third-instar larval stage (Figure 1H), we found severe morphological defects compared to controls. vglutMN mutants had reduced synaptic terminal area (Figures 1I, 1J, and 1O), but the most striking change we observed was an alteration of individual synaptic bouton sizes (Figures 1K and 1L). In wild-type third-instar larvae, less than 10% of all synaptic boutons are smaller than 2 μm2, while the majority of boutons are larger than this (Figure S1D). In vglutMN mutants, we observed a dramatic increase in the proportion of boutons smaller than 2 μm2 (small boutons), while the number of synaptic boutons larger than this (typical boutons) in addition to the total number of boutons was reduced compared to controls (Figure 1P; Table S1). To quantify this shift of bouton sizes, we calculated the ratio of small to typical boutons (bouton size index) and found a 366% (p < 0.001) increase in vglutMN mutants compared to controls (Figure 1Q). These phenotypes were observed with multiple vglut RNAi lines or hypomorphic vglut mutants and could be rescued by transgenic Vglut (Figures S1E–S1H). Similar to typical synaptic boutons, the small boutons in vglutMN mutants had correctly localized markers for active zones, periactive zones, synaptic vesicles, postsynaptic membranes, and postsynaptic receptor fields (Figure S2A). Therefore, vglut mutants have synapses with reduced terminal area concomitant with a disproportionally large amount of small synaptic boutons. This result established that even though synaptic transmission is not required for initial embryonic synapse assembly in Drosophila (Daniels et al., 2006), it is necessary for the subsequent phase of synaptic terminal growth.
Evoked Neurotransmission Is Not Necessary for Normal Synaptic Terminal Development
In vglutMN mutants, both evoked and miniature forms of neurotransmission are inhibited. Because the majority of NT at Drosophila NMJ terminals is via evoked release (Kurdyak et al., 1994), we next used genetically encoded peptide toxins to specifically block this form of NT and dissect its contribution to synapse development. Transgenic tetanus toxin light chain (UAS-TeTxLC) cleaves the vSNARE n-Synaptobrevin, which is essential for evoked, but not miniature, synaptic vesicle release (Sweeney et al., 1995). Expression of TeTxLC in a subset of MNs eliminated the ability of these NMJ terminals to produce evoked release when the axon was stimulated (Figures 1C, 1F, and S2B). In contrast, miniature NT was unaffected (Figures 1C, 1G, S2C, and S2D). As a second independent method of inhibiting evoked release, we generated a transgenic membrane-tethered version of Plectreurys toxin II (UAS-PLTXII), which blocks the Drosophila synaptic N-type voltage-gated calcium channel Cacophony that is essential for evoked release (Wu et al., 2008). Similar to TeTxLC, expression of PLTXII in MNs dramatically reduced evoked release but did not significantly alter miniature NT (Figures 1D, 1F, 1G, and S2B–S2D). We assessed the effects of expression of both of these toxins on synaptic terminal development (Figures 1M and 1N). We found no change of synaptic terminal area, the number of synaptic boutons, or the bouton size index at these terminals compared to controls (Figures 1O–1Q). Therefore, using these criteria, evoked neurotransmission is not required for normal synaptic structural development.
Miniature Neurotransmission Is Required for Synaptic Terminal Development
Our results indicated that while the inhibition of both evoked and miniature neurotransmission in vglutMN mutants perturbed synaptic development, blocking evoked release alone was not detrimental. We therefore hypothesized that miniature NT could be particularly required for synapse development or alternatively that synapse development relied upon the total amount of NT regardless of whether it was derived from evoked or miniature events. To discriminate between these hypotheses, we sought genetic conditions where miniature NT could be preferentially reduced versus evoked NT. To do this, we took advantage of the phenomena of synaptic homeostasis that occurs at both Drosophila and mammalian synapses (Davis, 2013; Turrigiano, 2012). When postsynaptic ionotropic glutamate receptors (iGluRs) are reduced at Drosophila NMJ synapses, presynaptic terminals increase the number of synaptic vesicles released (quantal content) per action potential in order to maintain synaptic strength (Frank et al., 2006; Petersen et al., 1997). We exploited this process in mutant combinations where iGluR function was severely inhibited to specifically reduce miniature NT.
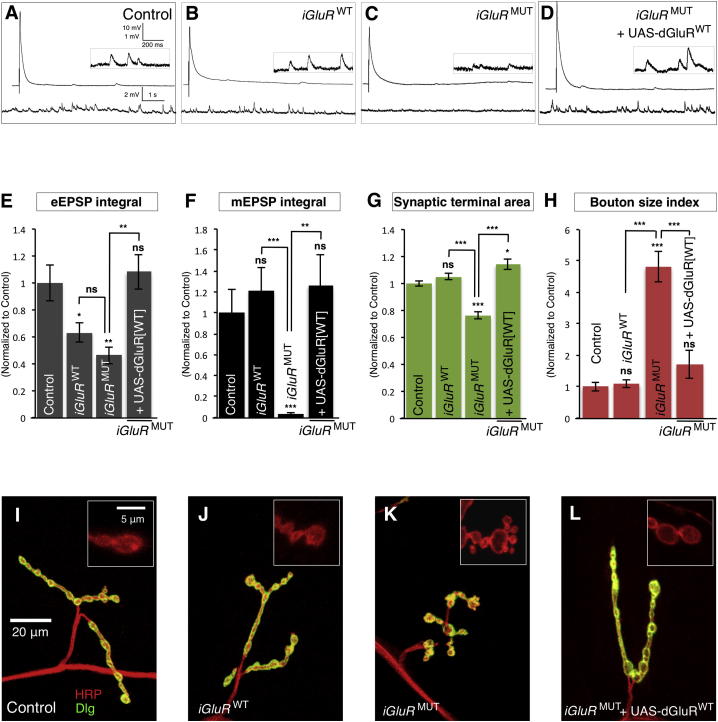
As a starting point, we employed iGluR mutants (Schmid et al., 2006) where the expression levels of endogenous glutamate receptor subunits were severely depleted (Figure S3A). In order to avoid disrupting the synaptic scaffolding functions of iGluRs, we combined these mutants with genomic promoter-driven rescuing transgenes. These transgenes produced either a wild-type glutamate receptor subunit (iGluRWT combination) or a subunit where the glutamate binding region was mutated (Schmid et al., 2006), rendering the receptor nonfunctional (iGluRMUT combination) (Figure S3A). Synaptic levels of both iGluRWT and iGluRMUT receptor clusters were similar when measured using an independent obligate iGluR subunit (dGluRIIC) (Figures S3B–S3D). We then measured NT in these mutants. iGluRWT terminals had similar miniature NT to controls (Figures 2A, 2B, 2F, S3F, and S3G). In contrast, iGluRMUT terminals had severely reduced miniature NT with a 96% (p < 0.001) reduction of the mEPSP integral (Figures 2C, 2F, S3F, and S3G) compared to controls. Miniature NT defects in iGluRMUT mutants were fully rescued by postsynaptic expression of a wild-type iGluR subunit (UAS-dGluRWT) (Figures 2D and 2F). Though both iGluRWT and iGluRMUT had reduced evoked NT compared to background controls, importantly, they had similar evoked NT to each other (Figures 2A–2C, 2E, and S3E). As predicted, this was due to an increase in quantal content at iGluRMUT terminals compared to iGluRWT terminals (Figure S3H). To determine if this homeostatic compensation occurred throughout larval synaptic development, we also measured NT of iGluRWT and iGluRMUT first-instar larval terminals. Just as in later animals, we found that evoked NT was similar while miniature NT was reduced in iGluRMUT mutants compared to iGluRWT (Figures S3I–S3N). Therefore, during synaptic development, miniature NT is specifically reduced at iGluRMUT terminals compared to iGluRWT terminals, while evoked NT remains similar.
Figure 2.
Miniature Neurotransmission Is Required for Normal Synaptic Terminal Development
(A–D) Representative traces of eEPSPs (above) and mEPSPs (below and inset) from (A) control (dglurIIA+/−,IIBDf/−), (B) iGluRWT (dglurIIAHypo/−,IIBDf/−;+/genomic-dglurIIAWT), (C) iGluRMUT (dglurIIAHypo/−,IIBDf/−;+/genomic-dglurIIAE783A), and (D) iGluRMUT+UAS-dGluRWT (dglurIIAHypo/−,IIBDf/−,G14-Gal4;UAS-dglurIIAWT/genomic-dglurIIAE783A).
(E and F) Quantification of (E) eEPSP integral (n ≥ 8) and (F) mEPSP integral (n ≥ 8).
(G and H) Quantification of the NMJ (G) synaptic terminal area and (H) bouton size index (n ≥ 22). All quantification data are normalized to control.
(I–L) Representative NMJ terminals and boutons (inset) labeled with Dlg (green) and HRP (red). Scale is the same in (I)–(L) and in the insets of (I)–(L).
Error bars indicate ±SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figures S3 and S4.
We next examined the synaptic terminal morphology of iGluRMUT and iGluRWT combinations. We found that iGluRMUT mutants had aberrant terminals with decreased synaptic terminal area and dramatic 443% increase (p < 0.001) of the bouton size index (Figures 2G, 2H, 2J, and 2K) compared to iGluRWT terminals. iGluRWT terminal morphology was similar to controls (Figures 2G–2J). The synaptic defects of iGluRMUT terminals were strikingly similar to those of vglutMN mutants (Figure 1L) and were rescued by postsynaptic expression of UAS-dGluRWT (Figures 2G, 2H, and 2L). In addition, though homeostatic compensation was active at iGluRMUT terminals, their aberrant morphology was unaltered by the postsynaptic activation or inhibition of the homeostasis regulator CamKII (Figures S4A and S4B) (Haghighi et al., 2003), indicating these morphological defects were not dependent upon synaptic homeostasis mechanisms. Therefore, the specific synaptic morphology defects of iGluRMUT mutants compared to iGluRWT supported the hypothesis that miniature events had a unique role in synapse development.
The Role of Miniature Neurotransmission in Synapse Development Is Independent of Evoked Neurotransmission
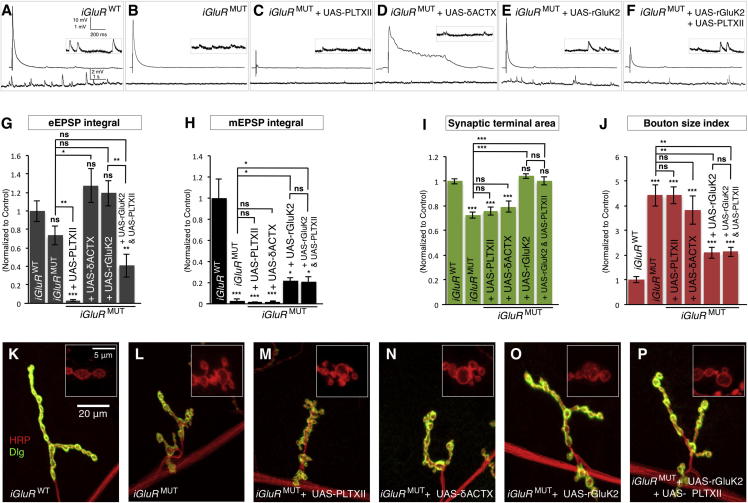
To further investigate the specific role of miniature neurotransmission in synapse development, we next asked if the phenotypes induced by the loss of miniature events were independent of the amount of evoked NT. To do this, we first blocked evoked release together with miniature NT by MN expression of PLTXII in iGluRMUT mutants. This did not further alter miniature NT but, as expected, strongly inhibited evoked release (Figures 3A–3C, 3G, 3H, and S4D–S4F). In spite of this, the synaptic morphology in these animals was unchanged compared to iGluRMUT mutants alone (Figures 3I–3M). Expression of PLTXII in the MNs of iGluRWT also induced no morphological phenotypes (data not shown). Therefore, depleting evoked release in addition to miniature NT did not further disrupt synaptic morphology.
Figure 3.
The Requirement of Miniature Neurotransmission for Synapse Development Is Independent of Evoked Neurotransmission and Requires Ionotropic Glutamate Receptor Activity
(A–F) Representative traces of eEPSPs (above) and mEPSPs (below and inset) from (A) iGluRWT (dglurIIAHypo/−,IIBDf/−;+/genomic-dglurIIAWT), (B) iGluRMUT (dglurIIAHypo/−,IIBDf/−;+/genomic-dglurIIAE783A), (C) iGluRMUT + UAS-PLTXII (dglurIIAHypo/−,IIBDf/−,OK319-Gal4;UAS-PLTXII/genomic-dglurIIAE783A), (D) iGluRMUT + UAS-δACTX (dglurIIAHypo/−,IIBDf/−,OK319-Gal4;UAS-δACTX/genomic-dglurIIAE783A), (E) iGluRMUT + UAS-rGluK2 (dglurIIAHypo/−,IIBDf/−,G14-Gal4;UAS-rGluK2/genomic-dglurIIAE783A), and (F) iGluRMUT + UAS-rGluK2 + UAS-PLTXII (dglurIIAHypo/−,IIBDf/−,G14-Gal4,OK319-Gal4;UAS-rGluK2 /genomic-dglurIIAE783A,UAS-PLTXII).
(G and H) Quantification of (G) eEPSP integral (n ≥ 8) and (H) mEPSP integral (n ≥ 8).
(I and J) Quantification of the NMJ (I) synaptic terminal area and (J) bouton size index (n ≥ 27). All quantification data are normalized to control (iGluRWT).
(K–P) Representative NMJ terminals and boutons (inset) labeled with Dlg (green) and HRP (red). Scale is the same in (K)–(P) and in the insets of (K)–(P).
Error bars indicate ±SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S4.
In a converse experiment, we asked if increasing evoked release could compensate for the decreased miniature NT in iGluRMUT mutants. Evoked NT, unlike miniature NT, depends upon action potentials, which are induced by voltage-gated sodium channels. To specifically increase evoked NT without affecting miniature NT, we generated a transgenic membrane-tethered version of the Australian funnel-web spider peptide toxin delta-ACTX-Hv1a (UAS-δACTX), which prolongs the activation of the Drosophila voltage-gated sodium channel Para by inhibiting its inactivation (Wu et al., 2008). Expression of δACTX in the MNs of control animals increased the amount of evoked NT by prolonging the duration of eEPSPs (Figure S4C). When we expressed δACTX in the MNs of iGluRMUT mutants, we also observed prolonged eEPSPs (Figure 3D) resulting in a 78% (p < 0.05) increase of evoked NT but no change of miniature NT (Figures 3G, 3H, and S4D–S4F). Nonetheless, when we examined the synaptic terminals of iGluRMUT mutants expressing δACTX, we observed no change of synaptic terminal area or the bouton size index compared to iGluRMUT mutants alone (Figures 3I, 3J, and 3N). This indicated that increasing evoked NT could not rescue synaptic defects induced by the depletion of miniature events. Congruously, we also found no change of synapse morphology when evoked NT was specifically increased or decreased in vglut hypomorphic mutants (Figures S4I–S4L). We conclude, therefore, that the role of miniature neurotransmission in synapse development is distinct from and cannot be compensated by evoked release.
Ionotropic Glutamate Receptor Activity Induced by Miniature Events Is Essential for Synapse Development
Presynaptic depletion of vesicular glutamate transporters or postsynaptic disruption of glutamate binding to receptors perturbs synapse morphology. This suggested that the release or detection of glutamate from miniature events was critical for normal synaptic development. To determine if the subsequent ionotropic activity of receptors in response to glutamate was also required, we sought to restore mEPSPs independently of endogenous receptors. To do this, we generated a Drosophila transgene of the rat kainate-type ionotropic glutamate receptor subunit GluR6/GRIK2/GluK2 (UAS-rGluK2). This mammalian receptor can form functional homotetrameric channels in heterologous systems (Egebjerg et al., 1991; Kauwe and Isacoff, 2013). We found that rGluK2 localizes to the Drosophila postsynapse when expressed in muscle, though it was not concentrated at active zones unlike endogenous receptors (Figures S4M and S4N). Postsynaptic expression of rGluK2 in control animals did not disrupt synapse morphology (data not shown). When we expressed rGluK2 in the postsynapse of iGluRMUT mutants, we found this increased the mEPSP integral >7-fold (p < 0.05) compared to iGluRMUT mutants alone (Figures 3E and 3H). The eEPSP integral was also increased, though this did not reach significance (Figures 3E and 3G). When we examined the synaptic terminals of these animals, we found that the synaptic terminal area was fully restored to control size and the aberrant bouton size index was reduced by 53% (p < 0.01) (Figures 3I, 3J, and 3O). This result indicated that ionotropic activity of glutamate receptors was essential for synapse development.
To establish if the rescue ability of rGluK2 specifically depended upon miniature NT, we coexpressed PLTXII in MNs in iGluRMUT mutants together with postsynaptic rGlurK2. This combination did not alter miniature NT but strongly reduced evoked release (p < 0.01) (Figures 3F–3H) compared to iGluRMUT mutants expressing rGluK2 alone. However, the inhibition of evoked NT did not inhibit any aspect of the morphological rescue of iGluRMUT mutants by rGluK2 (Figures 3I, 3J, 3O, and 3P). Expression of PLTXII or rGluK2 in either the pre- or postsynapse of controls did not alter terminal morphology (Figures S4G and S4H; data not shown). This finding further supported a singular requirement for miniature NT in terminal development. We conclude that the ionotropic activity of postsynaptic glutamate receptors, triggered by miniature events, is required for synapse growth.
Increasing Miniature Neurotransmission Promotes Synaptic Terminal Expansion
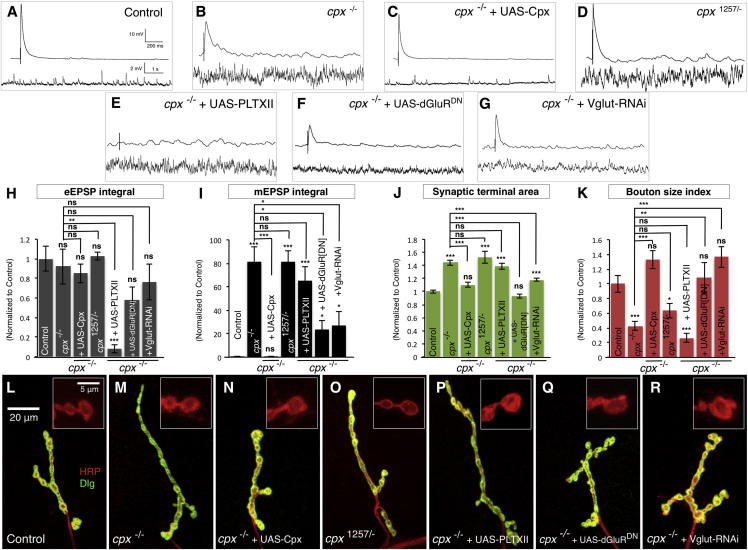
Because our results established that reduction of miniature neurotransmission inhibited synaptic development, we next investigated if increasing these events could also change synapse morphology. Complexin proteins bind to neuronal SNARE complexes and regulate neurotransmitter release (Brose, 2008). Mutants of Drosophila complexin (cpx) have a dramatic increase in spontaneous synaptic vesicle release and have increased numbers of synaptic boutons (Huntwork and Littleton, 2007). We hypothesized that these two phenotypes could be causally related through increased miniature NT. To test this idea, we first measured evoked and miniature NT in cpx null mutants. We found no change in the eEPSP integral (Figures 4A, 4B, and 4H) in these mutants, although eEPSP amplitudes were reduced compared to controls (Figure S5A), consistent with previous studies (Huntwork and Littleton, 2007; Iyer et al., 2013). In contrast, cpx mutants had a dramatic 81-fold increase (p < 0.001) in miniature NT (Figures 4A, 4B, and 4I). Expression of a complexin transgene (UAS-Cpx) in MNs rescued cpx mutants, restoring miniature NT to control levels (Figures 4C and 4I). When we measured the terminal morphology of cpx mutants, we observed a 44% increase (p < 0.001) in terminal area (Figures 4J, 4L, and 4M) accompanied by a 32% increase (p < 0.001) in typical bouton numbers but a 47% (p < 0.01) decrease in the number of small boutons (Figures S5B and S5C). This lead to a 64% decrease (p < 0.001) of the bouton size index (Figure 4K). As with neurotransmission, rescue of cpx mutants with transgenic complexin restored terminal area and the bouton size index (Figures 4J, 4K, and 4N). Therefore, cpx mutants have larger synaptic terminals with a decreased fraction of small boutons, the inverse of vglutMN and iGluRMUT mutant phenotypes.
Figure 4.
Increasing Miniature Neurotransmission Promotes Synaptic Terminal Expansion
(A–G) Representative traces of eEPSPs (above) and mEPSPs (below) from (A) control (cpxDf/+), (B) cpx−/− mutant (cpxDf/−), (C) cpx−/− + UAS-Cpx (UAS-Cpx/+;OK6-Gal4/+;cpxDf/−), (D) cpx1257/− mutant (cpxDf/1257), (E) cpx−/− + UAS-PLTXII (UAS-PLTXII/OK319-Gal4;cpxDf/−), (F) cpx−/− + UAS-dGluRDN (G14-Gal4/+;cpxDf/-,UAS-dglurIIAE783A), and (G) cpx−/− + Vglut-RNAi (UAS-Vglut-RNAiKK/OK6-Gal4;cpxDf/−).
(H and I) Quantification of (H) eEPSP integral (n ≥ 8) and (I) mEPSP integral (n ≥ 11).
(J and K) Quantification of the NMJ (J) synaptic terminal area and (K) bouton size index (n ≥ 23). All quantification data are normalized to control.
(L–R) Representative NMJ terminals and boutons (inset) labeled with Dlg (green) and HRP (red). Scale is the same in (L)–(R) and in the insets of (L)–(R).
Error bars indicate ±SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S5.
We next wished to determine if evoked NT contributed to cpx mutant terminal phenotypes. We first analyzed the cpx1257 mutant allele, which has normal eEPSP amplitudes and kinetics (Iyer et al., 2013) (Figure S5A) but has similarly increased miniature NT to cpx null alleles (Figures 4B, 4D, and 4I). We found that cpx1257 mutants had increased terminal areas with a decreased bouton size index not significantly different from cpx null alleles (Figures 4J, 4K, and 4O). This indicated that the aberrant terminal overgrowth of cpx mutants was not due to abnormal evoked release. As a second test, we expressed PLTXII in MNs of cpx null mutants. As expected, this strongly inhibited evoked NT without significantly altering miniature events (Figures 4E, 4H, and 4I). When we measured the terminal morphology of these animals, we found no change compared to cpx mutants alone (Figures 4J, 4K, and 4P). Therefore, evoked NT is not required for the synaptic overgrowth of cpx mutants.
We next asked if increased miniature events are necessary for cpx mutant terminal overgrowth. We reduced miniature NT by expressing either a dominant-negative glutamate receptor subunit (UAS-dGluRDN) (Schmid et al., 2006) in postsynaptic muscles or an RNAi against vglut (RNAi-Vglut) in the presynaptic MNs of cpx mutants (Figures 4F and 4G) and controls (Figures S5D and S5E). Both manipulations did not significantly alter evoked NT in cpx mutants but did decrease miniature NT (Figures 4F–4I). In both conditions, we found that the aberrant synaptic terminal area and bouton size indexes of cpx mutants were suppressed (Figures 4J, 4K, 4Q, and 4R). Thus, the inhibition of miniature NT suppressed the terminal overgrowth of cpx mutants while the depletion of evoked NT did not. Therefore, increased miniature neurotransmission, as found in complexin mutants, is sufficient to promote synaptic terminal growth.
Individual Bouton Expansion Is Bidirectionally Regulated by Miniature Neurotransmission
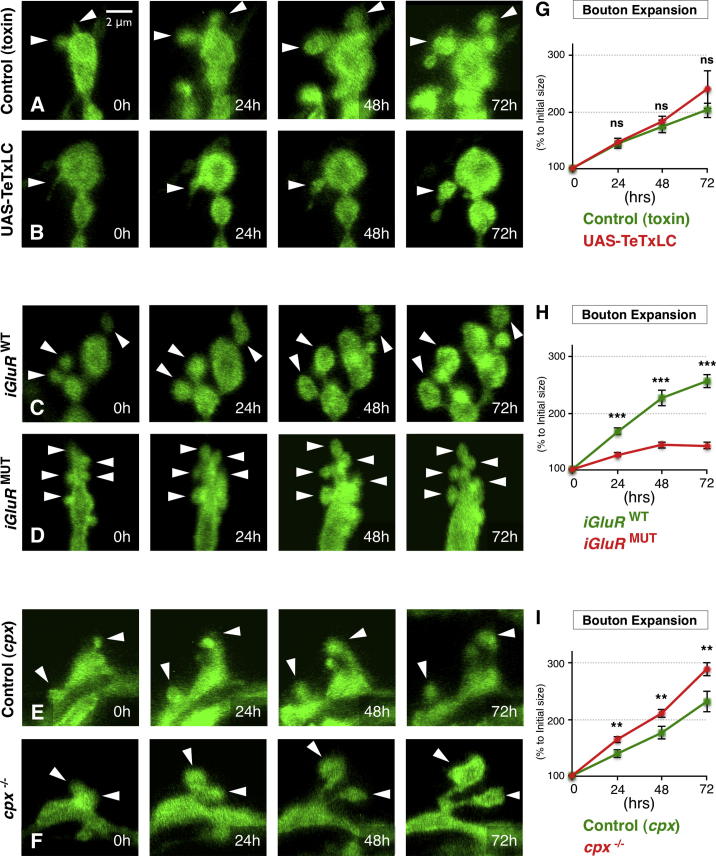
cpx mutants had opposing synaptic morphological changes to vglutMN and iGluRMUT mutants. This was most apparent in the bidirectional effect upon bouton size, with vglutMN and iGluRMUT mutants having an increase in the proportion of small boutons and cpx mutants oppositely having a decreased fraction of these boutons. During terminal development, new synaptic boutons are added and then expand and may also be eliminated (Koch et al., 2008; Zito et al., 1999). A defect in one or more of these steps could potentially result in the changes to bouton sizes we observed when miniature NT was altered. We sought therefore to visualize the development of individual synaptic boutons by time-lapse live imaging through the transparent cuticle of intact larvae. To do this, we utilized the LexA binary system to express a membrane-localized GFP in the presynaptic terminals of both control and miniature NT mutants. Beginning 24 hr after hatching, we anesthetized animals every 24 hr for 4 days during larval development and imaged their synaptic terminals, returning them to food media between imaging periods.
Using this technique, we found that new synaptic boutons formed continuously throughout the imaging period in control and NT mutant backgrounds at the same rate (Figures S6A and S6B). In control animals, 94.4% (34/36) of newly formed small boutons (<2 μm2) then became progressively larger over time to become typical-sized boutons (>2 μm2) during the imaging period (Figures 5A and 5G). This expansion in size was not perturbed by inhibiting evoked NT using TeTxLC (Figures 5B and 5G). However, in iGluRMUT mutants, where miniature NT was reduced, we found that the enlargement of small boutons was severely retarded compared to iGluRWT animals (Figures 5C, 5D, and 5H) and only 19.6% (10/51) of small boutons ever expanded to become typical-sized boutons. In contrast, in cpx mutants where miniature NT was increased, the rate of expansion of small boutons to typical boutons was accelerated compared to controls (Figures 5E, 5F, and 5I). The effect on individual bouton growth of increasing or decreasing miniature NT was similar regardless of whether new boutons formed at early, intermediate, or late stages during the 4-day imaging period (Figures S6C–S6H). Finally, we saw no change in the low frequency of elimination of existing boutons in any NT mutant compared to controls (Figures S6I and S6J). Thus, the enlargement of individual synaptic boutons was stalled when miniature NT was inhibited and conversely was accelerated when miniature NT was increased. This modification of the growth properties of individual boutons by altering miniature NT was consistent with the changes we observed of bouton size indexes. These results established that the growth process of individual synaptic boutons was discretely regulated by miniature neurotransmission.
Figure 5.
Individual Bouton Expansion Is Bidirectionally Regulated by Miniature Neurotransmission
(A–F) Representative time-lapse images from live animals of synaptic boutons labeled with membrane tagged GFP at muscles 1 or 9 captured every 24 hr during larval development beginning 24 hr after hatching. Small boutons formed before 0 hr and tracked over the imaging period are indicated by arrowheads. Genotypes (A) control(toxin) (Vglut-lexA,LexOp-CD8-GFP/+), (B) UAS-TeTxLC (OK319-Gal4/UAS-TeTxLC;Vglut-lexA,LexOp-CD8-GFP/+), (C) iGluRWT (dglurIIAHypo/-,IIBDf/-;Vglut-lexA,LexOp-CD8-GFP/genomic-dglurIIAWT), (D) iGluRMUT (dglurIIAHypo/−,IIBDf/−;Vglut-lexA,LexOp-CD8-GFP/genomic-dglurIIAE783A), (E) control (cpx) (Vglut-lexA,LexOp-CD8-GFP,cpxDf/+), and (F) cpx−/− mutant (cpxDf/−,Vglut-lexA,LexOp-CD8-GFP). Scale is the same for all images.
(G–I) Quantification of the bouton size expansion during the time-lapse imaging period (n ≥ 35 boutons for G, n ≥ 51 boutons for H, and n ≥ 42 boutons for I, from n ≥ 7 NMJs).
All data are normalized to initial bouton size. Error bars indicate ±SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S6.
Synapse Maturation Is Regulated by Miniature Neurotransmission
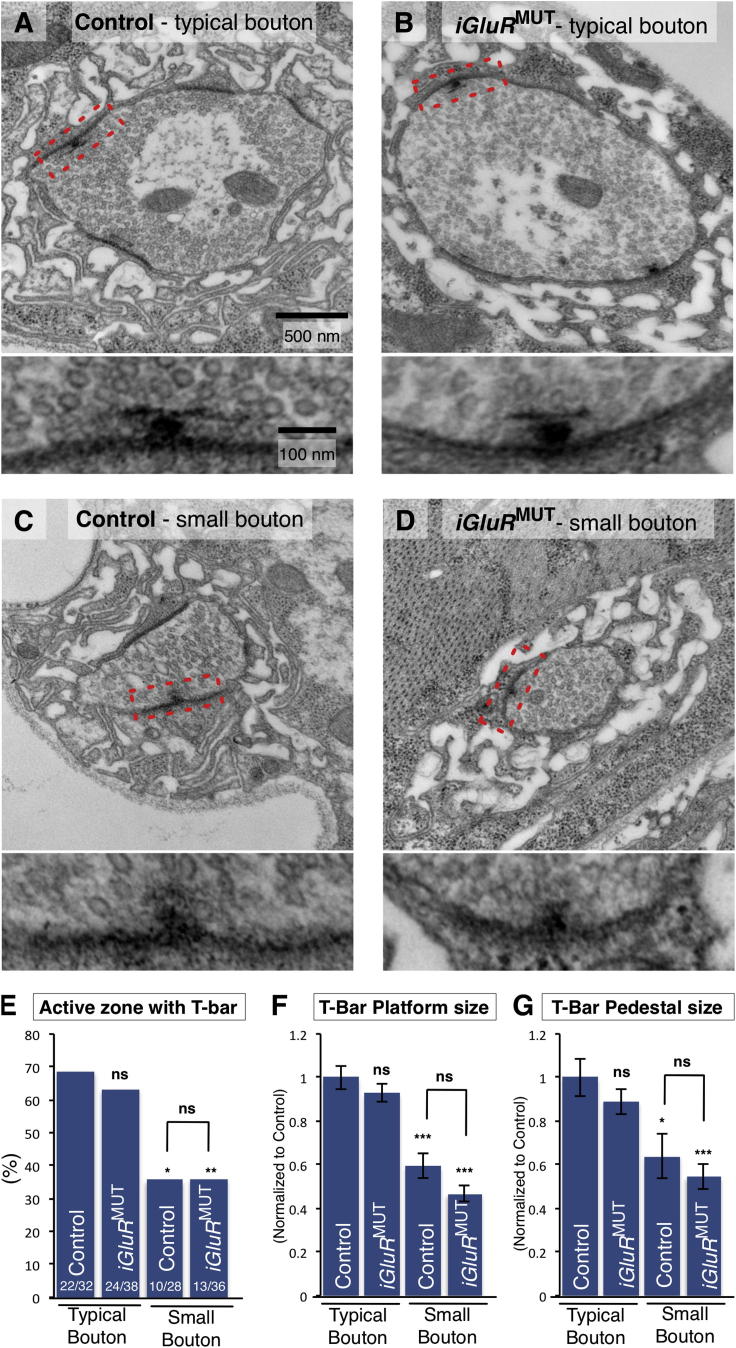
In control animals, ∼95% of all small boutons expand to become larger, and our data demonstrated a failure of this process in the majority of boutons when miniature neurotransmission was depleted. We speculated that this morphological change of boutons could be associated with other important features of synaptic maturation. To investigate this, we first compared the synaptic ultrastructure of small (<2 μm2) and typical boutons (>2 μm2) in wild-type animals. We found that both bouton categories were grossly similar with clearly discernable synaptic hallmarks including mitochondria, presynaptic vesicle clusters, synaptic clefts, and postsynaptic elaborations (Figures 6A and 6C). However, we found that T-bars, the electron-dense presynaptic specializations required for efficacy of evoked release at Drosophila synapses (Kittel et al., 2006), were different between the active zones of typical and small boutons. While in typical boutons 69% of active zones had a T-bar present, only 36% of active zones in small boutons had an electron-dense presynaptic structure (Figure 6E). Furthermore, the structures present at small bouton active zones were primitive, irregularly shaped (Figures 6A and 6C, insets), and smaller than those at the active zones of typical boutons (Figures 6F and 6G). These results indicated that synaptic ultrastructure is less developed in small boutons compared to typical boutons in wild-type animals.
Figure 6.
Synaptic Active-Zone Maturation Is Regulated by Miniature Neurotransmission
(A and B) Representative micrographs of synaptic boutons (above) and active zones (box above, inset below) from a typical-size bouton of (A) control (CS) and (B) iGluRMUT mutants.
(C and D) Representative micrographs of small boutons from (C) control and (D) iGluRMUT.
(E–G) Quantification of (E) the active-zone occurrence, (F) T-bar platform size, and (G) T-bar pedestal size (n ≥ 10). Quantification data are normalized to control (F and G).
Scale is the same in (A)–(D) and in the insets of (A)–(D). Error bars indicate ±SEM. ∗p < 0.05, ∗ p < 0.01, ∗∗∗p < 0.001.
We then examined the synaptic ultrastructure in iGluRMUT mutant terminals and compared them to controls. We found no ultrastructure differences between the typical boutons of iGluRMUT mutants and the typical boutons of controls, including the features of active zones and T-bars (Figures 6A, 6B, and 6E–6G). However, we found that the numerous small boutons in iGluRMUT mutants had immature active-zone features similar to those of small boutons in wild-type animals, including reduced T-bar frequency, rudimentary T-bar structure, and reduced T-bar size (Figures 6C–6G). These data, combined with live-imaging results, suggest that inhibition of miniature NT interrupts synaptic development resulting in boutons becoming stalled in an immature state, reminiscent of the normally transient small boutons of wild-type animals. These results are consistent with miniature neurotransmission also being necessary for synaptic ultrastructural maturation in addition to morphological expansion.
Miniature Neurotransmission Acts Locally to Regulate Synapse Development
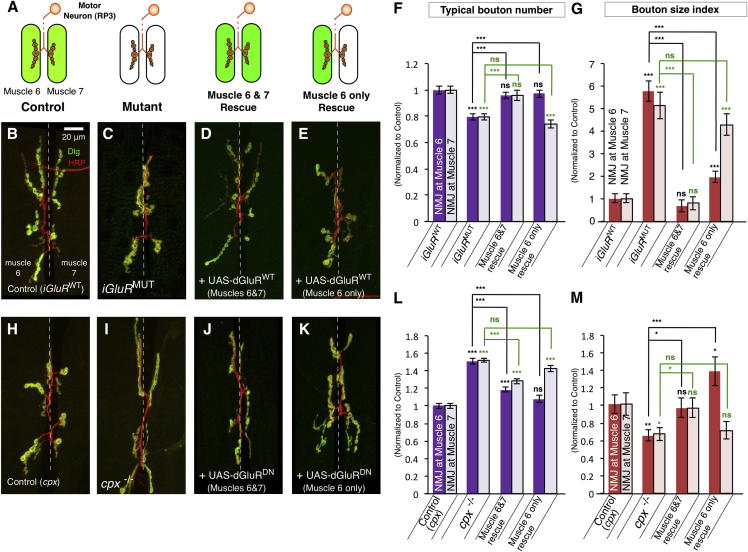
We next sought to establish the nature of the developmental signal induced by miniature neurotransmission. Synaptic structure can be influenced by factors that act locally at the individual terminal level (e.g., synaptic adhesion factors; Davis and Goodman, 1998) or throughout the whole neuron (e.g., transcriptional regulation; McCabe et al., 2003). Action potentials and evoked NT affect the entire synaptic terminal. In contrast, we surmised that the effects of miniature NT might be spatially restricted to individual active zones and therefore could act locally to regulate bouton maturation. To test this hypothesis, we examined the synaptic terminals generated by the type Ib motor neuron RP3 (Hoang and Chiba, 2001). The single axon of this neuron bifurcates to produce synaptic terminals concurrently on two postsynaptic targets: muscle 6 and muscle 7 (Figure 7A). The ratio of synaptic boutons produced at each muscle is stereotyped (Davis and Goodman, 1998), and these muscles are not electrically coupled with each other (Ueda and Kidokoro, 1996), facilitating independent manipulation of NT. We used Gal4 drivers expressed either in muscle 6 alone (but not muscle 7) (Figures 7A and S7A) or in both muscles to dissect if miniature NT signaling acts locally at terminals or throughout the neuron.
Figure 7.
Miniature Neurotransmission Acts Locally to Regulate Bouton Development
(A) Schematic of the two separate synaptic terminals on muscles 6 and 7 generated by the single motor neuron RP3. Muscle Gal4 lines allow rescue of mutants at either both terminals at muscles 6 and 7 or the terminal at muscle 6 only.
(B–E and H–K) Representative NMJ terminals at muscles 6 and 7, segment A3, labeled with Dlg (green) and HRP (red) from (B) iGluRWT, (C) iGluRMUT, (D) iGluRMUT + UAS-dGluRWT (muscles 6 and 7) (dglurIIAHypo/−,IIBDf/−;C57-Gal4/genomic-dglurIIAE783A,UAS-dglurIIAWT), (E) iGluRMUT + UAS-dGluRWT (muscle 6 only) (dglurIIAHypo/−,IIBDf/−;H94-Gal4,nSyb-Gal80/genomic-dglurIIAE783A,UAS-dglurIIAWT), (H) control(cpx) (cpxDf/+), (I) cpx−/− mutant, (J) cpx−/− + UAS-dGluRDN (muscles 6 and 7) (G14-Gal4/+;cpxDf/−,UAS-dglurIIAE783A), and (K) cpx−/− + UAS-dGluRDN (muscle 6 only) (cpxDf/−,UAS-dglurIIAE783A,H94-Gal4,nSyb-Gal80).
(F, G, L, and M) Quantification of the typical bouton number (F and L) and bouton size index (G and M) from terminals at muscles 6 and 7 (n ≥ 32). Statistical comparisons are labeled in black for terminal 6 and green for terminal 7.
All quantification data are normalized to control. Scale is the same for all images. Error bars indicate ±SEM. ∗p < 0.05, ∗∗∗p < 0.001. See also Figure S7.
Similar to other synapses, reduction of miniature NT by iGluRMUT reduced typical bouton numbers (Figures 7B, 7C, and 7F) and increased the fraction of small boutons (Figure 7G) at both of the RP3 MN terminals on muscles 6 and 7 compared to controls, though the area of these terminals could not be accurately measured due their complex spatial arrangement. When we overexpressed a wild-type iGluR transgene (UAS- dGluRWT) in both postsynaptic muscles of iGluRMUT mutants, we restored normal miniature NT at both terminals (Figure S7B). This also fully rescued bouton numbers and the bouton size index at both terminals (Figures 7D, 7F, and 7G). We next expressed UAS-dGluRWT only in muscle 6 of iGluRMUT mutants. This increased miniature NT at muscle 6 terminals without altering NT at muscle 7 (Figure S7B). When we examined the morphology of both terminals, we found that bouton numbers and bouton size were restored at terminals at muscle 6 (Figures 7E–7G). In contrast, however, the terminals at muscle 7 were not rescued (Figures 7E–7G). Because both terminals are produced by a single neuron, this result suggested that the effect of reduced miniature NT on synaptic bouton maturation is localized to individual terminals.
In a complementary experiment, we examined the suppression of cpx mutants using a similar strategy. cpx mutant terminals on both muscles 6 and 7 are expanded and the bouton size index was reduced compared to controls (Figures 7H, 7I, 7L, and 7M). When we expressed dominant-negative UAS-dGluRDN in both postsynaptic muscles of these mutants, the excessive miniature NT at both terminals was strongly inhibited (Figure S7C). In both terminals, the aberrant number and size ratio of synaptic boutons were also suppressed (Figures 7J, 7L, and 7M). In contrast, when UAS-dGluRDN was expressed only in muscle 6 of cpx mutants, miniature NT, bouton number, and bouton size index were only suppressed at the terminal on this muscle and not at the terminal on muscle 7 (Figures 7K–7M and S7C). Together, these experiments demonstrated that the effect on synapse maturation of increasing or decreasing miniature neurotransmission is via a mechanism that acts locally at synaptic terminals.
Miniature Neurotransmission Regulates Synapse Maturation through the GEF Trio and the GTPase Rac1
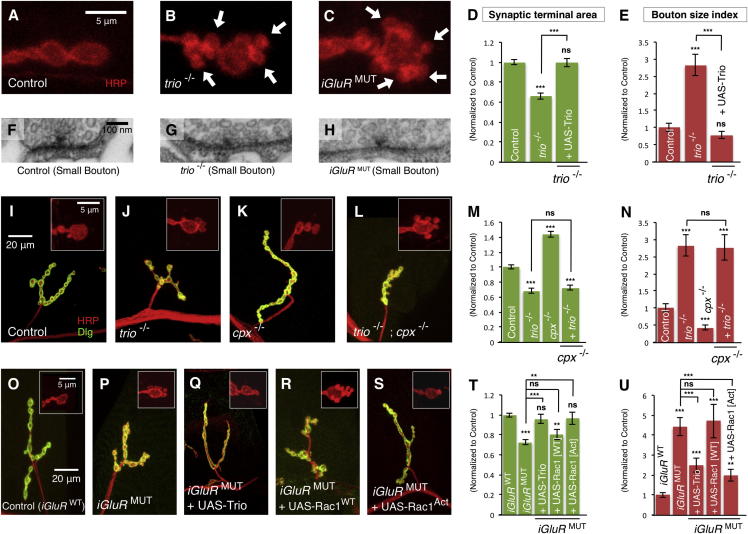
To determine the molecular mechanism through which miniature neurotransmission regulates bouton maturation, we next carried out a candidate mutant screen of molecules that were (1) linked to synapse morphological development and (2) likely to have localized activity at terminals. Among these candidates was Trio, a member of the evolutionarily conserved Dbl homology family of GEFs (Miller et al., 2013). trio mutants had previously been reported to have defective synaptic terminal growth (Ball et al., 2010), and Trio has been linked to the local regulation of the neuronal cytoskeleton (Miller et al., 2013). We confirmed that trio mutants had reduced numbers of synaptic boutons (Ball et al., 2010) (Figure S8A). We additionally found that trio mutants had reduced terminal area accompanied by large increase in the proportion of small boutons (Figures 8A, 8B, 8D, and 8E) very reminiscent of synaptic terminals when miniature NT is reduced (Figure 8C). All of these trio mutant synaptic phenotypes were fully rescued by presynaptic expression of transgenic Trio (UAS-Trio) (Figures 8D and 8E). When we examined the ultrastructure of the abundant small boutons in trio mutants, we found rudimentary T-bar structures (Figure 8G) of reduced size similar to those observed in the small boutons of miniature NT mutants (Figures 8H, S8C, and S8D). However, when we measured miniature NT in these mutants, we found it was unchanged compared to controls (Figure S8E), consistent with previous reports (Ball et al., 2010). This indicated that the synaptic terminal phenotypes in trio mutants did not originate from defective NT. However, the similarity of trio mutant synaptic morphology phenotypes to miniature NT mutant phenotypes suggested that Trio could be part of a molecular pathway triggered by miniature events.
Figure 8.
Miniature Neurotransmission Regulates Bouton Maturation through the GEF Trio and the Small GTPase Rac1
(A–C) Representative boutons of (A) control (CS), (B) trio−/− mutant, and (C) iGluRMUT mutants.
(F–H) Representative micrographs of the active zone of small boutons of the indicated genotypes.
(I–L and O–S) Representative NMJ terminals and boutons (inset) from (I) control (CS), (J) trio−/− mutant, (K) cpx−/− mutant, (L) trio−/− ; cpx−/− mutants, (O) iGluRWT, (P) iGluRMUT, (Q) iGluRMUT + UAS-Trio (dglurIIAHypo/−,IIBDf/−,OK319-Gal4;UAS-Trio/genomic-dglurIIAE783A), (R) iGluRMUT + UAS-Rac1WT (dglurIIAHypo/−,IIBDf/−,OK319-Gal4;UAS-Rac1WT/genomic-dglurIIAE783A), and (S) iGluRMUT + UAS-Rac1Act (dglurIIAHypo/−,IIBDf/−,OK319-Gal4;UAS-Rac1V12/genomic-dglurIIAE783A).
(D, E, M, N, T, and U) Quantification of the NMJ (D, M, and T) synaptic terminal area and (E, N, and U) bouton size index (n ≥ 23) of the indicated genotypes normalized to controls (CS for trio−/− and trio−/− + UAS-Trio), (cpxDf/+ for cpx−/− and trio−/−;cpx−/− mutants), (iGluRWT for iGluRMUT).
Scale is the same in (A)–(C), in (F)–(H), in (I)–(S), in the insets of (I)–(L), and in the insets of (O)–(S). Error bars indicate ±SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S8.
Pursuing this hypothesis, we next tested the genetic interaction of miniature NT mutants with trio mutants. We first examined if Trio is required for the terminal overgrowth and bouton size alteration of cpx mutants. Double null mutants of cpx and trio had similarly increased miniature NT to cpx mutants alone (Figure S8F). However, when we examined the morphology of these double-mutant terminals, we found that synaptic terminal area and bouton size ratio were not different from trio mutants alone (Figures 8L–8N). Therefore, cpx mutant synaptic overgrowth was completely suppressed by the removal of trio. Furthermore, when we examined the terminal area and bouton size index of double mutants of iGluRMUT and trio, they were also not different from trio mutant terminals alone (Figures S8G and S8H). Therefore, iGluRMUT phenotypes are not genetically additive with trio mutant synaptic phenotypes. These results were consistent with Trio and miniature NT acting in a common molecular pathway regulating bouton development.
Building upon this result, we next examined if overexpression of Trio could rescue the effects of loss of miniature NT. When we overexpressed Trio in the MNs of iGluRMUT mutants, we found no alteration of miniature NT compared to these mutants alone (Figure S8E). Nonetheless, when we examined the terminals of these animals, we found the synaptic terminal area was fully rescued to control levels and that the aberrant increased ratio of small boutons was suppressed by 44% (p < 0.001) (Figures 8O–8Q, 8T, and 8U). Overexpression of Trio in the presynapse of control animals caused a small increase in terminal area but no alteration of the bouton size ratio (Figures S8G and S8H). These results indicated that Trio acted as an essential “downstream” mediator of miniature NT in the regulation of bouton development.
Trio has previously been shown to activate the small GTPase Rac1 to modify the neuronal cytoskeleton (Ball et al., 2010; Miller et al., 2013). We therefore investigated if Rac1 also mediated the effects of miniature NT on synaptic development. Overexpression of either a transgenic wild-type Rac1 (UAS-Rac1WT) or a GEF-independent activated mutant of Rac1 (UAS-Rac1ACT) in the presynapse of controls induced a small change of terminal area and increased the bouton size index (Figures S8G and S8H). We then tested if these constructs could rescue the effects of reduced miniature NT. Presynaptic overexpression of UAS-Rac1WT in iGluRMUT mutants did not alter either synaptic terminal area or the bouton size index compared to iGluRMUT mutants alone (Figures 8R, 8T, and 8U). However, presynaptic overexpression of UAS-Rac1ACT in iGluRMUT mutants fully rescued synaptic terminal area to control levels and reduced the aberrant bouton size index by 55% (p < 0.001) (Figures 8S–8U). This was comparable to rescue by presynaptic overexpression of Trio (Figures 8T and 8U). These results are consistent with Rac1 being activated by Trio in response to miniature NT in order to modulate synaptic development. Our results support a mechanism where miniature neurotransmission acts locally at synaptic terminals through a Trio-Rac1 signaling pathway to modify the synaptic cytoskeleton and promote structural maturation.
Discussion
In vertebrates, initial synaptic assembly appears to occur normally in the absence of all vesicular neurotransmission (Verhage et al., 2000), though subsequent aspects of structural development at some synapses are perturbed (Kummer et al., 2006; Witzemann et al., 2013). Similarly, we have found that depletion of both evoked and miniature NT disrupts Drosophila synaptic terminal development, particularly of the size of individual synaptic boutons. Surprisingly, however, we found that the specific abolishment of evoked NT using two different transgenic toxins had no effect on synaptic morphology. In contrast, synaptic development was disrupted when miniature NT was specifically depleted by manipulation of postsynaptic glutamate receptors. These phenotypes could be rescued by wild-type receptors, including mammalian glutamate receptors, but were unaltered by manipulating evoked NT. Oppositely, we found that increasing miniature NT is sufficient to induce synaptic terminal overgrowth. Using live imaging, we observed that enlargement of synaptic boutons is bidirectionally responsive to changes in miniature NT, and we found that this process was coupled with the ultrastructural maturation of synaptic active zones. We determined that miniature NT acts locally at synaptic terminals to regulate bouton maturation via a Trio GEF and Rac1 GTPase molecular signaling pathway. Our data therefore reveal a unique and specific requirement for miniature events in the development of synaptic terminals that is not shared with and cannot be compensated by evoked NT. These results indicate that miniature neurotransmission, often dismissed as superfluous “noise” from evoked release, has essential and independent functions in vivo in the nervous system.
Miniature Neurotransmission Is Uniquely Required for Synapse Development
Our data reveal a surprisingly distinct requirement for miniature NT for normal synaptic development. Like many chemical synapses, the majority of neurotransmitter released at Drosophila NMJ terminals is via evoked NT. Not only is the amplitude of eEPSPs approximately 50-fold larger than mEPSPs at this terminal, but also evoked release occurs during endogenous activity as frequent rhythmic bursts (Kurdyak et al., 1994). Despite this, when evoked NT was completely abolished at these terminals, we observed no defects in morphological development, consistent with other studies (Dickman et al., 2006). Dissection of miniature NT from evoked release was made possible by exploiting synaptic homeostasis (Davis, 2013; Petersen et al., 1997), which we show occurs throughout the development of this terminal when postsynaptic glutamate receptors (iGluRs) are inhibited. Replacement of endogenous iGluRs by mutant subunits resulted in conditions where evoked NT was similar to controls, due to a relative increase in the number of synaptic vesicles released per action potential, but miniature NT was dramatically decreased. In these mutants, where miniature NT is depleted far more severely than in previous reports (e.g., dGluRIIA mutants; Petersen et al., 1997; data not shown), synaptic maturation was specifically perturbed. These defects were not reliant upon the activation of synaptic homeostasis because they were unaffected by manipulation of CamKII (Haghighi et al., 2003). Furthermore, very similar defects in synaptic development occur when presynaptic miniature neurotransmitter release is diminished by vglut mutations. Therefore, inhibition of the production or detection of postsynaptic miniature events results in developmental defects consistent with a transsynaptic signal. Moreover, additionally increasing or decreasing evoked release, when miniature NT is depleted, does not further alter synaptic development. In contrast, restoring miniature NT in iGluR mutants with either Drosophila or mammalian receptors can rescue normal terminal morphology. These results indicate that it is the discrete contribution of miniature NT rather than the total quantity of vesicular NT that is the critical factor necessary for normal synapse development. Therefore, the role of small miniature events during synapse development is qualitatively rather than quantitatively different from the function of larger evoked events. Miniature neurotransmission thus seems to act as a parallel second layer of synaptic communication with a unique and essential role in promoting normal synaptic structural development.
Synapse Maturation Requires Miniature Neurotransmission
Depletion of miniature NT results in terminals with aberrantly large numbers of small boutons. Two lines of evidence suggest that these small boutons are stalled in an immature phase of a normal growth process. First, live imaging revealed that when miniature NT is depleted, new boutons form at normal frequency but then fail to subsequently expand, unlike wild-type boutons. Second, small boutons in miniature NT mutants have synapse marker and ultrastructure features that appear identical to the small boutons of wild-type animals. These stalled boutons appear different from the aberrant small “satellite boutons” that occur when endocytosis is disrupted and have different synaptic marker and ultrastructure characteristics to normal boutons (Dickman et al., 2006). Therefore, our data support that miniature NT is critical for the normal progression of synaptic maturation. Since miniature NT is also a component of synaptic activity, it is intriguing to speculate that miniature events could contribute activity-dependent synaptic structural plasticity.
Localized Signaling by Miniature Neurotransmission
The discrete effect of altering miniature NT on individual bouton maturation coupled with the spatially restricted nature of these small events suggested a localized signaling activity. This was supported by our demonstration that miniature NT can regulate the development of individual synaptic terminals within a single neuron independently of each other. Interestingly, in cultured mammalian neurons, that activity of miniature NT on synaptic scaling also acts the levels of individual dendritic branches (Sutton et al., 2006), consistent with localized molecular signaling induced by miniature events in both paradigms. In Drosophila, we have identified Trio and Rac1 as essential components of the miniature NT signaling mechanism. trio mutants perturb synapse maturation in a manner similar to loss of miniature events, and activation of Trio or Rac1 can rescue miniature NT mutants. Trio and Rac1 have been implicated in actin dynamics in multiple contexts, including axonal growth cones and synapses (Ball et al., 2010; Miller et al., 2013), and GTPases can act as spatially confined “switches” inducing local cytoskeletal rearrangements. Interestingly, Trio is also transcriptionally regulated by the synaptotrophic BMP pathway (Ball et al., 2010) offering a potential molecular “node” to integrate local fine-tuning of maturation by miniature NT with global synaptic growth regulation. While our data support that Trio and Rac1 mediate the effects of miniature NT on presynaptic neurons, multiple intercellular signaling molecules can interact with Trio (Miller et al., 2013), requiring further investigation to establish how postsynaptic miniature events interact with this presynaptic pathway.
Discriminating between Miniature and Evoked Neurotransmission
Our studies beg the question of how miniature NT can be differentiated from evoked NT. The effects of miniature NT on developing synaptic boutons are both specific and localized. In mammalian cultured neurons, it has been suggested that miniature NT can target populations of postsynaptic receptors spatially separated to those activated by evoked neurotransmitter release (Ramirez and Kavalali, 2011). Consistent with this, it has also been directly observed that subpopulations of active zones at Drosophila synapses are specialized for the release of either miniature or evoked events (Melom et al., 2013; Peled et al., 2014). Therefore, miniature and evoked NT may activate spatially distinct postsynaptic signaling mechanisms. An alternative possibility is that differences in the release kinetics between evoked and miniature NT could allow postsynaptic mechanisms to detect and differentiate between them. For example, local or global Ca2+ signaling through voltage-gated Ca2+ channels can be distinguished by calmodulin (Tadross et al., 2008). Unsynchronized activation of glutamate receptors through miniature events could also trigger downstream signaling mechanisms that are not activated by the synchronized activation of receptors by evoked release.
Reconsideration of Minis
In the past, miniature events were often dismissed as synaptic epiphenomena related to the requirement for a high fidelity of synaptic vesicle release during evoked NT (Sutton and Schuman, 2009; Zucker, 2005). Several studies over the last decade, however, have challenged this view. For example, miniature synaptic vesicle release has recently been found to be regulated by specialized Ca2+ sensors (Walter et al., 2011). mEPSPs can influence the firing rates of cerebellar interneurons, affect synaptic homeostasis, and at elevated levels trigger spiking of hippocampal neurons (Frank et al., 2006; Otsu and Murphy, 2003; Sutton and Schuman, 2009). In cultured neurons, miniature NT can stabilize spine structure and influence the activity of postsynaptic CamKII and is required for synaptic facilitation (Jin et al., 2012; Otsu and Murphy, 2003). Miniature NT can also alter local protein translation in dendrites and has been recently implicated as a potential mechanism of action of some fast-acting antidepressants (Kavalali and Monteggia, 2012; Sutton et al., 2006). Our data now demonstrate an in vivo role for miniature neurotransmission in the regulation of synapse development. Therefore, miniature events, a universal but often-overlooked feature of all chemical synapses, may be critical for many aspects of brain development and function.
Experimental Procedures
See also Supplemental Experimental Procedures.
Drosophila Stocks
Motor neuron Gal4 drivers were OK319-Gal4 (Beck et al., 2012), OK6-Gal4 (Aberle et al., 2002), or D42-Gal4 (Yeh et al., 1995). Muscle Gal4 drivers were G14-Gal4 (Aberle et al., 2002), C57-Gal4 (Budnik et al., 1996), or H94-Gal4 (Davis and Goodman, 1998). Further details and descriptions of transgenic lines, mutant combinations, and transgenes are described in Supplemental Experimental Procedures.
Electrophysiology
Intracellular recordings were performed as previously described (McCabe et al., 2003) at physiological Ca2+ conditions (1.5 mM). eEPSP and mEPSP amplitudes, frequencies, and integrals were measured using the peak detection feature of the MiniAnalysis program (Synaptosoft). All events were verified manually while blinded to genotype. The amplitude, frequency, and integrals of mEPSPs were calculated from continuous recordings in the absence of stimulation (50–100 s). For animals expressing UAS-δACTX, unstimulated spontaneous multiquantal events occurred (data not shown), so mEPSP amplitude, frequency, and integrals were measured in the presence of tetrodotoxin (TTX) (4 μM final concentration), which did not affect miniature NT in control conditions. In cpx mutants, mEPSPs were so frequent that conventional measurements of frequency and amplitude were precluded, and the insect ionotropic glutamate receptor antagonist Philanthatoxin-343 (PhTox, Sigma) (Frank et al., 2006) was employed to establish the RMP baseline (4 μM final concentration).
Immunohistochemistry
Third-instar larvae of comparable size at the ∼2 hr wandering stage time window were collected, dissected, and stained as previously described (McCabe et al., 2003). See Supplemental Experimental Procedures for details of the antibodies employed.
Morphological Analysis
All morphological analysis was done in maximum projections of z stacks from confocal images (Zeiss) of muscle 4 (Figures 1, 2, 3, 4, and 8) or muscles 6 and 7 (Figure 7) of segment A3, type Ib terminals only, identified by Dlg staining. All quantifications were performed while blinded to genotype. Synaptic terminal area was measured as the area of HRP-labeled presynaptic membrane surrounded by Dlg using MetaMorph (Molecular Devices). Typical boutons were counted as type Ib synaptic axonal varicosities with a size of >2 μm2. Small boutons were counted as type Ib small (<2 μm2) boutons labeled by Dlg. We restricted our analysis to small boutons that were clearly discernable. This may underestimate the actual number of small boutons, because small boutons partially occluded by surrounding typical boutons were excluded. The bouton size index was calculated by dividing the number of small boutons by the number of typical boutons per terminal.
Time-Lapse Live Imaging
Presynaptic motor neurons were labeled with membrane localized LexOp-CD8-GFP expressed by vglut-LexA (Baek et al., 2013) in both control and mutant backgrounds. Only images from animals that survived the entire 4-day imaging procedure were included in analysis. For bouton size expansion in live images, the size of each bouton was measured using the round regional tool of MetaMorph while blinded to genotype. Further details are in Supplemental Experimental Procedures.
Electron Microscopy
Electron microscopy and ultrastructure quantification were previously described (Jiao et al., 2010). Only type Ib boutons, identified by postsynaptic subsynaptic reticulum structure, were selected for quantification. Small boutons were defined as having the longest axis among serial section <1.6 μm, based on a 2-dimensional projection area of <2 μm2. All small boutons were identified using serial sections. Frequency of T-bar per active zone was verified by serial section images around the active zone. T-bar size was measured at middle images of serial sections where the T-bar size was largest.
Data Analysis
Statistical significance for all morphological and electrophysiological data were determined using a Kruskal-Wallis test followed by a Dunn’s post hoc test when multiple comparisons were required. Otherwise, we employed Mann-Whitney-Wilcoxon test (Instat, GraphPad) except for Figures 6E and S6J, where Fisher’s exact test was used.
Acknowledgments
We are grateful to Rafael Yuste, Amy McDermott, George Mentis, and Erin Beck for critical reading of the manuscript. We thank Stephan Sigrist, Aaron DiAntonio, Troy Littleton, Fumiko Kawasaki, Hermann Aberle, Pejmun Haghighi, Cahir O’Kane, Rachel Kraut, Vivian Budnik, Richard Mann, Corey Goodman, Robert Oswald, and the Bloomington and the Vienna stock centers for stocks, advice, and reagents. We thank Tim Crawley, Liyan McCurdy, and Mitchell Hayes for technical assistance. B.J.C. was supported by NIH 5T32HL08774, and Y.W. was supported by F32NS055527. Work in the laboratory of R.A.B. is funded by the Wellcome Trust (090798/Z/09/Z). Work in the laboratory of M.N.N. is funded by NIH NS055035, NS056443, NS058330, and GM098931. Work in the laboratory of B.D.M. was supported by NIH NS075572, AG08702, the DANA foundation, the Gatsby Initiative in brain circuitry and the New York Presbyterian Seizure Disorders Fund.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Aberle H., Haghighi A.P., Fetter R.D., McCabe B.D., Magalhães T.R., Goodman C.S. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Baek M., Enriquez J., Mann R.S. Dual role for Hox genes and Hox co-factors in conferring leg motoneuron survival and identity in Drosophila. Development. 2013;140:2027–2038. doi: 10.1242/dev.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R.W., Warren-Paquin M., Tsurudome K., Liao E.H., Elazzouzi F., Cavanagh C., An B.-S., Wang T.-T., White J.H., Haghighi A.P. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–549. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Beck E.S., Gasque G., Imlach W.L., Jiao W., Jiwon Choi B., Wu P.-S., Kraushar M.L., McCabe B.D. Regulation of Fasciclin II and synaptic terminal development by the splicing factor beag. J. Neurosci. 2012;32:7058–7073. doi: 10.1523/JNEUROSCI.3717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N. For better or for worse: complexins regulate SNARE function and vesicle fusion. Traffic. 2008;9:1403–1413. doi: 10.1111/j.1600-0854.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- Budnik V., Koh Y.H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.A., DiAntonio A. Synaptic development: insights from Drosophila. Curr. Opin. Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Daniels R.W., Collins C.A., Chen K., Gelfand M.V., Featherstone D.E., DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G.W. Homeostatic signaling and the stabilization of neural function. Neuron. 2013;80:718–728. doi: 10.1016/j.neuron.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G.W., Goodman C.S. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Dickman D.K., Lu Z., Meinertzhagen I.A., Schwarz T.L. Altered synaptic development and active zone spacing in endocytosis mutants. Curr. Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Fatt P., Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Frank C.A., Kennedy M.J., Goold C.P., Marek K.W., Davis G.W. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi A.P., McCabe B.D., Fetter R.D., Palmer J.E., Hom S., Goodman C.S. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hoang B., Chiba A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 2001;229:55–70. doi: 10.1006/dbio.2000.9983. [DOI] [PubMed] [Google Scholar]
- Huntwork S., Littleton J.T. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Iyer J., Wahlmark C.J., Kuser-Ahnert G.A., Kawasaki F. Molecular mechanisms of COMPLEXIN fusion clamp function in synaptic exocytosis revealed in a new Drosophila mutant. Mol. Cell. Neurosci. 2013;56:244–254. doi: 10.1016/j.mcn.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W., Shupliakov A., Shupliakov O. A semi-correlative technique for the subcellular localization of proteins in Drosophila synapses. J. Neurosci. Methods. 2010;185:273–279. doi: 10.1016/j.jneumeth.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Jin I., Puthanveettil S., Udo H., Karl K., Kandel E.R., Hawkins R.D. Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia. Proc. Natl. Acad. Sci. USA. 2012;109:9131–9136. doi: 10.1073/pnas.1206914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauwe G., Isacoff E.Y. Rapid feedback regulation of synaptic efficacy during high-frequency activity at the Drosophila larval neuromuscular junction. Proc. Natl. Acad. Sci. USA. 2013;110:9142–9147. doi: 10.1073/pnas.1221314110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali E.T., Monteggia L.M. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kittel R.J., Wichmann C., Rasse T.M., Fouquet W., Schmidt M., Schmid A., Wagh D.A., Pawlu C., Kellner R.R., Willig K.I. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Koch I., Schwarz H., Beuchle D., Goellner B., Langegger M., Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Kummer T.T., Misgeld T., Sanes J.R. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kurdyak P., Atwood H.L., Stewart B.A., Wu C.F. Differential physiology and morphology of motor axons to ventral longitudinal muscles in larval Drosophila. J. Comp. Neurol. 1994;350:463–472. doi: 10.1002/cne.903500310. [DOI] [PubMed] [Google Scholar]
- Mahr A., Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- McCabe B.D., Marqués G., Haghighi A.P., Fetter R.D., Crotty M.L., Haerry T.E., Goodman C.S., O’Connor M.B. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Melom J.E., Akbergenova Y., Gavornik J.P., Littleton J.T. Spontaneous and evoked release are independently regulated at individual active zones. J. Neurosci. 2013;33:17253–17263. doi: 10.1523/JNEUROSCI.3334-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.B., Yan Y., Eipper B.A., Mains R.E. Neuronal Rho GEFs in synaptic physiology and behavior. Neuroscientist. 2013;19:255–273. doi: 10.1177/1073858413475486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y., Murphy T.H. Miniature transmitter release: accident of nature or careful design? Sci. STKE. 2003;2003:pe54. doi: 10.1126/stke.2112003pe54. [DOI] [PubMed] [Google Scholar]
- Peled E.S., Newman Z.L., Isacoff E.Y. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr. Biol. 2014;24:484–493. doi: 10.1016/j.cub.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.A., Fetter R.D., Noordermeer J.N., Goodman C.S., DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Ramirez D.M.O., Kavalali E.T. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr. Opin. Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Qin G., Wichmann C., Kittel R.J., Mertel S., Fouquet W., Schmidt M., Heckmann M., Sigrist S.J. Non-NMDA-type glutamate receptors are essential for maturation but not for initial assembly of synapses at Drosophila neuromuscular junctions. J. Neurosci. 2006;26:11267–11277. doi: 10.1523/JNEUROSCI.2722-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C.M., Davis G.W., Fetter R.D., Goodman C.S. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Stuart G., Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Schuman E.M. Partitioning the synaptic landscape: distinct microdomains for spontaneous and spike-triggered neurotransmission. Sci. Signal. 2009;2:pe19. doi: 10.1126/scisignal.265pe19. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Ito H.T., Cressy P., Kempf C., Woo J.C., Schuman E.M. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sweeney S.T., Broadie K., Keane J., Niemann H., O’Kane C.J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Tadross M.R., Dick I.E., Yue D.T. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 2008;133:1228–1240. doi: 10.1016/j.cell.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A., Kidokoro Y. Longitudinal body wall muscles are electrically coupled across the segmental boundary in the third instar larva of Drosophila melanogaster. Invert. Neurosci. 1996;1:315–322. doi: 10.1007/BF02211911. [DOI] [PubMed] [Google Scholar]
- Verhage M., Maia A.S., Plomp J.J., Brussaard A.B., Heeroma J.H., Vermeer H., Toonen R.F., Hammer R.E., van den Berg T.K., Missler M. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Walter A.M., Groffen A.J., Sørensen J.B., Verhage M. Multiple Ca2+ sensors in secretion: teammates, competitors or autocrats? Trends Neurosci. 2011;34:487–497. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Chevessier F., Pacifici P.G., Yampolsky P. The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface. Mech. Dev. 2013;130:402–411. doi: 10.1016/j.mod.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Wu Y., Cao G., Pavlicek B., Luo X., Nitabach M.N. Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin. PLoS Biol. 2008;6:e273. doi: 10.1371/journal.pbio.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Gustafson K., Boulianne G.L. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc. Natl. Acad. Sci. USA. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K., Parnas D., Fetter R.D., Isacoff E.Y., Goodman C.S. Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 1999;22:719–729. doi: 10.1016/s0896-6273(00)80731-x. [DOI] [PubMed] [Google Scholar]
- Zucker R.S. Minis: whence and wherefore? Neuron. 2005;45:482–484. doi: 10.1016/j.neuron.2005.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.