Background: EMP2 is a tetraspan protein linked with aggressive disease.
Results: EMP2 correlates with activated Src in patients with GBM. Using intracranial mouse models, EMP2 promotes tumor cell invasiveness. Antibodies to EMP2 reduce GBM tumor load.
Conclusion: EMP2 is a novel therapeutic target in GBM.
Significance: The clinical outcome for patients with GBM remains poor, and thus new targeted therapies are needed.
Keywords: Cell Invasion, Glioblastoma, Immunotherapy, Tetraspanins, Tumor Marker, EMP2
Abstract
Despite recent advances in molecular classification, surgery, radiotherapy, and targeted therapies, the clinical outcome of patients with malignant brain tumors remains extremely poor. In this study, we have identified the tetraspan protein epithelial membrane protein-2 (EMP2) as a potential target for glioblastoma (GBM) killing. EMP2 had low or undetectable expression in normal brain but was highly expressed in GBM as 95% of patients showed some expression of the protein. In GBM cells, EMP2 enhanced tumor growth in vivo in part by up-regulating αvβ3 integrin surface expression, activating focal adhesion kinase and Src kinases, and promoting cell migration and invasion. Consistent with these findings, EMP2 expression significantly correlated with activated Src kinase in patient samples and promoted tumor cell invasion using intracranial mouse models. As a proof of principle to determine whether EMP2 could serve as a target for therapy, cells were treated using specific anti-EMP2 antibody reagents. These reagents were effective in killing GBM cells in vitro and in reducing tumor load in subcutaneous mouse models. These results support the role of EMP2 in the pathogenesis of GBM and suggest that anti-EMP2 treatment may be a novel therapeutic treatment.
Introduction
Despite recent advances in molecular classification, surgery and radiotherapy, and targeted therapies, the clinical outcome of patients with malignant brain tumors remains extremely poor. The prognosis for patients with glioblastoma (GBM),3 the most common and aggressive form of brain tumors, yields only a median survival of 12 months and a 5-year survival of 5% (1). The rapid and deadly course of the disease is due, in large part, to the highly invasive nature of these malignant cells. In most patients, GBM cells migrate into the surrounding brain parenchyma, thus making complete surgical resection difficult (2).
GBMs are characterized by two spatial and temporal events, uncontrolled proliferation and abnormal cell migration (3). These events are disassociated as tumor cores contain highly proliferative populations that are distinct from more invasive cells at the periphery, which show slower proliferation rates (4). With regard to invasion, it is believed that integrins, a family of heterodimeric proteins that link the cytoskeleton to the extracellular matrix, play an important role. Integrin adhesion and invasion activate focal adhesion kinase (FAK), a nonreceptor cytoplasmic tyrosine kinase that is found to be up-regulated in both anaplastic astrocytomas and GBM (2, 5). FAK phosphorylation activates Src kinase, which is part of the family of kinases that regulate the translation of extracellular signals with intracellular signaling (6). Dysregulated Src signaling has been shown in many cancers, including GBM (7).
A new protein implicated in the activation of FAK is the oncogenic protein EMP2 (8, 9). EMP2 is a member of the growth arrest-specific gene 3/peripheral myelin protein-22 (GAS3/PMP22) group of tetraspan proteins, and its expression is up-regulated in ovarian, breast, and endometrial malignancies (10–12). Within these tumors, EMP2 has been shown to be a prognostic indicator as its expression correlates with poor survival and/or advanced disease (12, 13).
To date, little is known about the role of EMP2 in the central nervous system. Although there have been no reports of EMP2 in normal brain, a recent Affymetrix study revealed up-regulation of its mRNA in GBM (14). Hence, in this study, we generate preliminary evidence as to the protein expression and role of EMP2 in GBM. Specifically, we provide principal data that suggest that EMP2 promotes a more aggressive disease phenotype and that it may ultimately serve as a novel therapeutic target for antibody therapy.
MATERIALS AND METHODS
Cell Lines and Reagents
Human GBM cell lines U87MG, U138, and U373 (ATCC, Manassas, VA) were cultured in DMEM supplemented with 10% fetal bovine serum, 1% glutamine, 1% penicillin and streptomycin, and 1% sodium pyruvate in humidified 5% CO2 at 37 °C. Primary human cells GM1, GM2, GM3, GM4, GM5, GM6, and GM97 were derived from patient tumors and cultured as described previously (15). U87MG cells that overexpress an in-frame deletion of amino acids 6–273 in the EGFR gene termed EGFR VIII (U87/EGFR VIII) have been previously described and were cultured in complete DMEM as above (16, 17). Cell lines were used within 2 months after resuscitation of frozen aliquots and were authenticated based on viability, recovery, growth, morphology, and isoenzymology by the supplier. Cells were passaged every 2–4 days. EMP2 expression was stably overexpressed using a retroviral vector encoding both EMP2 and GFP genes under the control of the CMV promoter, with the GFP gene translated from an internal ribosomal entry sequence or through the use of a human EMP2-GFP fusion (46 kDa) protein (8). EMP2 expression was reduced using the Mission pLKO.1 puro shRNA lentiviral vector (shRNA; Sigma) (18) or through the previously described use of an EMP2-specific ribozyme (RIBO) (19). Vector control cell lines were generated using empty shRNA or control GFP vectors (V). In some experiments, scrambled or EMP2-specific siRNA vectors (Thermo Scientific, Pittsburgh, PA) were used to transiently reduce EMP2 expression as described previously (19–21). To create tumors for intracranial models, U87/EMP2, U87/V, and U87/shRNA cells were stably infected with an HIV-1-based lentiviral vector containing the firefly luciferase gene under the control of the CMV promoter (U87/Luc) by the UCLA Vector Core and Shared Resource as described previously (22).
Immunohistochemistry
A GBM array containing 0.6-mm cores (two tumors, one normal) from 110 patients has been previously described (14). The array was stained with human EMP2 antisera or a preimmune control. Briefly, for antigen retrieval, sections were incubated at 95 °C for 20 min in 0.1 m citrate, pH 6.0. EMP2 was detected using rabbit human EMP2 antisera at a dilution of 1:400 as described previously (13) followed by visualization using the Vector ABC kit (Vector Labs, Burlingame, CA) according to the manufacturer's instructions. Diaminobenzidine or deNOVO Red was used for visualization, and staining was quantified by two independent pathologists (P. M.) and (A. I.) on a 0–3 histological score.
Preparation of Xenografts
Ethical Treatment of Animals Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The protocol was approved by the Animal Research Committee at UCLA. All efforts were made to minimize animal suffering.
Four-6-week-old nude BALB/c female mice were obtained from Charles River Laboratories (Wilmington, MA) and maintained in the vivarium at UCLA. Animals were inoculated subcutaneously with 1 × 106 U373/EMP2, U373/V, or U373/RIBO or 5 × 105 U87/EMP2, U87/V, or U87/shRNA cells. The number of mice used per group is indicated in the figure legends. Tumors were measured twice a week, and tumor volumes were calculated by the formula (length × width2)/2 (23). Data are expressed as mean ± S.E. Student's t test was used to evaluate overall differences in the means between groups versus the control at a given time, and significance was defined as p < 0.05.
To create an intracranial model for GBM, 1 × 105 U87/EMP2/Luc, U87/V/Luc, and U87/shRNA/Luc were stereotactically implanted into the right frontal lobe of 6–8-week-old female BALB/c nude mice (24, 25). Animal health was evaluated for the development of behavioral and neurological signs and weight loss. Mice were euthanized if weight loss exceeded 10%. Tumor loads were monitored by bioluminescence imaging. Briefly, mice received an intraperitoneal injection of 100 μl of d-luciferin (30 mg/ml), and 30 min after injection, mice were anesthetized with ketamine/xylazine (100 and 10 mg/kg) and placed on the imaging stage. The bioluminescence signals were captured using an IVIS-200 (Xenogen Corp., Alameda, CA). The data were analyzed using maximum photon flux emission (photons/s) in the regions of interest. A one-way analysis of variance was used to evaluate differences between the different experimental groups, with significance defined as p < 0.05.
To determine the therapeutic potential for EMP2 antibodies in GBM, U87/EGFR VIII or U373 tumors were created subcutaneously on the shoulder of BALB/c nude mice. Anti-EMP2 diabodies and control diabodies have been detailed previously (11, 26), and the variable regions were recently cloned to produce a fully human IgG1 (12). Both cell lines were also tested for murine pathogens, including mycoplasma by the Division of Laboratory Animal Medicine at UCLA prior to injection. When tumors approached 4 mm3, they were injected twice a week with intratumoral injections of the anti-EMP2 diabodies at 1 mg/kg during week 1 and then 2 mg/kg during week 2. To test the full-length EMP2 IgG1, tumors were created using the wild type U373 cell line, and mice were treated weekly through intraperitoneal injections using 3 mg/kg anti-EMP2 IgG1 or control antibodies. Tumors were measured twice a week. Following treatment, tumors were excised, fixed in formalin, and then processed for hematoxylin and eosin staining by the Tissue Procurement Laboratory at UCLA.
Proliferation Assays
Cellular proliferation was monitored using a BrdU cell proliferation assay (EMD Chemicals, Gibbstown, NJ) as per the manufacturer's instructions. Briefly, 104 cells were cultured in a 96-well plate. Triplicate wells were used for each condition. Cells were incubated in DMEM + 0.5% FCS overnight to arrest the cells and then were released in complete media containing BrdU for 2 or 24 h. Cells were fixed and permeabilized, and the DNA was denatured. A detector anti-BrdU monoclonal antibody was added and ultimately detected using a horseradish peroxidase (HRP)-conjugated goat anti-mouse. To determine the amount of incorporated BrdU, a fluorogenic substrate was added, and the absorbance was quantified at dual wavelengths of 450 and 595 nm.
Wound Healing
105 GBM cells with modulated EMP2 expression were plated on 35-mm tissue culture dishes. When cells were confluent, a “wound” was created using a 100-μl pipette tip as described (9, 27). Wound healing was monitored over 48 h with a ×10 phase contrast objective, and images were collected using a Power Shot S80 camera (Canon, Lake Success, NY). Quantification of the wound healing was determined by measuring the remaining wound diameter. Wound healing was calculated as a percent of the closed wound divided by the original scratch area. Three independent experiments were performed, and the results were averaged.
Invasion
Transwell inserts of 24-well plates were coated with fibronectin or collagen I (BD Biosciences) for the in vitro cell invasion assays. Equivalent numbers (5 × 103 cells) of GBM cells with modified EMP2 levels were added to the top chamber of the transwell, and complete DMEM was added to the bottom of the well. Cells were allowed to invade for 6 h at 37 °C. The filters were then fixed and stained with 0.1% crystal violet in 20% methanol. The invasive cells were visualized using bright-field microscopy. Cells were enumerated by counting four random fields per transwell. The experiment was repeated three times, with the data averaged. In some experiments, cells were pretreated with anti-EMP2 IgG1 or anti-αvβ3 integrin antibodies for 2 h at 4 °C.
SDS-PAGE/Western Blotting Analysis
Cells were resuspended in Laemmli sample buffer (62.5 mm Tris-Cl, pH 6.8, 10% glycerol, 2% SDS, 0.01% bromphenol blue, 2% β-mercaptoethanol). As EMP2 contains multiple glycosylation sites, N-linked glycans were cleaved using peptide N-glycanase (New England Biolabs, Beverly, MA) as described previously (28). Lysates were treated as per the manufacturer's instructions at 37 °C for 2 h. Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane (Amersham Biosciences), and stained with Ponceau S (Sigma) to determine transfer efficiency. Membranes were blocked with 10% low fat milk in TBS containing 0.1% Tween 20 and probed with EMP2 antisera (1:1000), anti-p-FAK (Tyr-576/577) (Santa Cruz Biotechnology), anti-total FAK (BD Biosciences), anti-p-Src (Tyr-416) (Cell Signaling, Danvers, MA), anti-total Src (Cell Signaling), or β-actin (Sigma). Protein bands were visualized using HRP-conjugated secondary antibodies (BD Biosciences and Southern Biotechnology Associates, Birmingham, AL) followed by chemiluminescence (ECL; Amersham Biosciences). Band intensities were quantified using ImageJ (29). At least three independent experiments were performed, and the results were evaluated for statistical significance using Student's t test (unpaired, two-tailed test).
Cellular Viability
2 × 105 U87MG, U87/EGFR VIII, U373, T98, and GM5 cells were plated in triplicate. Cells were incubated with a vehicle control (PBS) or molar equivalents of the anti-EMP2 antibodies (20 μg/ml anti-EMP2 diabody or 60 μg/ml anti-EMP2 IgG1). After 72 h, cells were enumerated using the trypan blue exclusion assay. In some experiments, to validate changes in viability, T98 or GM5 cells were treated as above, harvested, and stained with an annexin V-propidium iodide detection kit as per manufacturer's instructions (BD Biosciences). Flow cytometry analysis was performed with a FACScan Analytic Flow Cytometer (BD Biosciences) at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility.
Statistical Analysis
All values in the text were mean ± S.E. Differences between means were evaluated using a two-tailed Student's t test or analysis of variance as indicated. Significant differences were taken at the p < 0.05 level.
RESULTS
EMP2 Is Expressed in Most GBMs
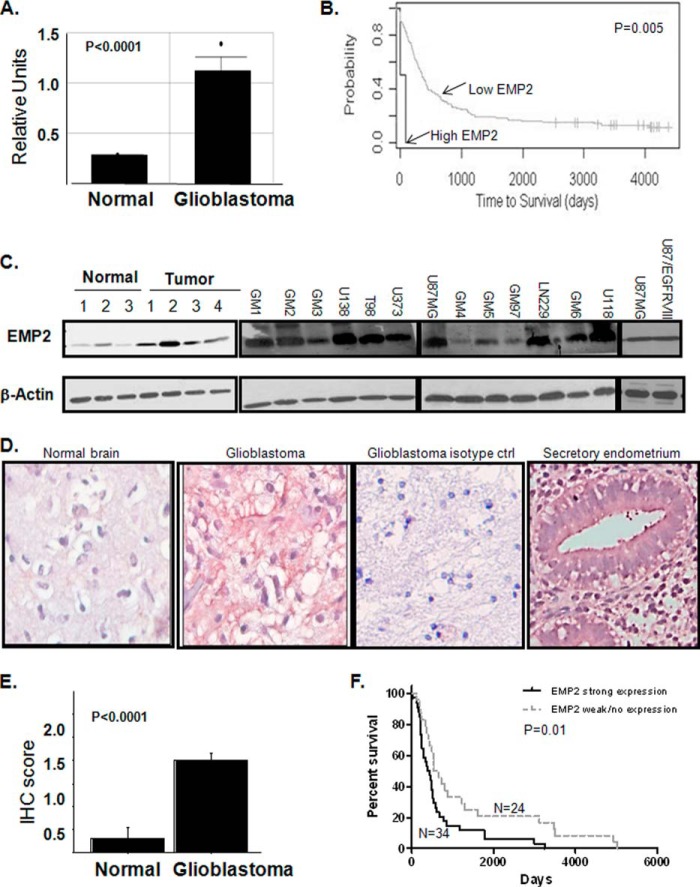
In a previously published Affymetrix gene chip dataset (14), we were intrigued to find that EMP2 expression was significantly increased in 50 human GBM compared with 24 normal brain samples (Fig. 1A). In that study, when EMP2 mRNA was dichotomized into high or low expression levels, high EMP2 mRNA expression predicted early death and thus was an independent prognostic indicator (Fig. 1B). To translate this expression, we tested EMP2 levels by Western blot analysis in a small panel of GBM tumors and normal brain as well as in a panel of primary and established GBM cell lines (Fig. 1C). Using whole tumor or normal brain homogenates, EMP2 expression was elevated in tumor lysates. Concordantly, EMP2 expression was detectable at various levels in all primary and established GBM cell lines. As EGFR gene amplification and mutation are a particularly striking feature of GBM (17), we tested whether EMP2 levels were altered in U87MG cells harboring an intragenic rearrangement termed EGFR VIII (30). However, this mutation did not detectably alter EMP2 protein levels.
FIGURE 1.
EMP2 expression is increased in GBM. A, EMP2 mRNA expression (Affymetrix microarray) was increased in GBM compared with normal brain. *, p < 0.001. B, survival data for high and low EMP2 mRNA expression. C, left, EMP2 expression was increased in GBM tumors compared with normal regions. Right, EMP2 expression was evaluated in a panel of GBM cells lines and in lines derived from patients by Western blotting analysis. β-Actin expression was used as a loading control. D and E, GBM tissue arrays containing 329 cores from 110 patients were stained for EMP2 expression. D, EMP2 protein expression was determined in normal brain, in GBM, and in secretory endometrium (positive control) using an EMP2 polyclonal antibody. To detail nonspecific staining, rabbit preimmune serum was used. Staining was visualized using deNovo Red. Nuclei were counter-stained using hematoxylin. E, EMP2 expression was quantitated on a 0–3 histological scale by two independent pathologists, and the average IHC score is shown. F, EMP2 expression was dichotomized based on high (histological score, ≥2) or low (histological score, ≥1) expression. High EMP2 expression correlated with a poor survival.
To extend the expression of EMP2 in GBM on a population basis, a tissue microarray consisting of 329 cores from 110 patients was stained using anti-EMP2 antisera as described under “Materials and Methods.” Preimmune serum was used as isotype control. Using the EMP2 antisera, significant EMP2 expression was observed in tumors compared with nonmalignant brain tissue (Fig. 1D). The staining pattern observed was similar to what has previously been reported in secretory endometrium with EMP2 localization on both the membrane and within the cytoplasm of cells. When the expression was quantitated, the vast majority of tumors from GBM patients (95%) expressed some EMP2 compared with adjacent nonmalignant brain tissues (Fig. 1E), with 53% of tumors expressing high levels (histological score ≥2) of EMP2. Although the mean survival period for most individuals with GBM is relatively short, EMP2 was a negative prognostic indicator as higher levels of EMP2 (≥2) predicted a poor outcome compared with tumors with lower levels of EMP2 (Fig. 1F), and notably this was concordant with the prior association between EMP2 mRNA and survival (Fig. 1B).
EMP2 Accelerates GBM Tumor Growth
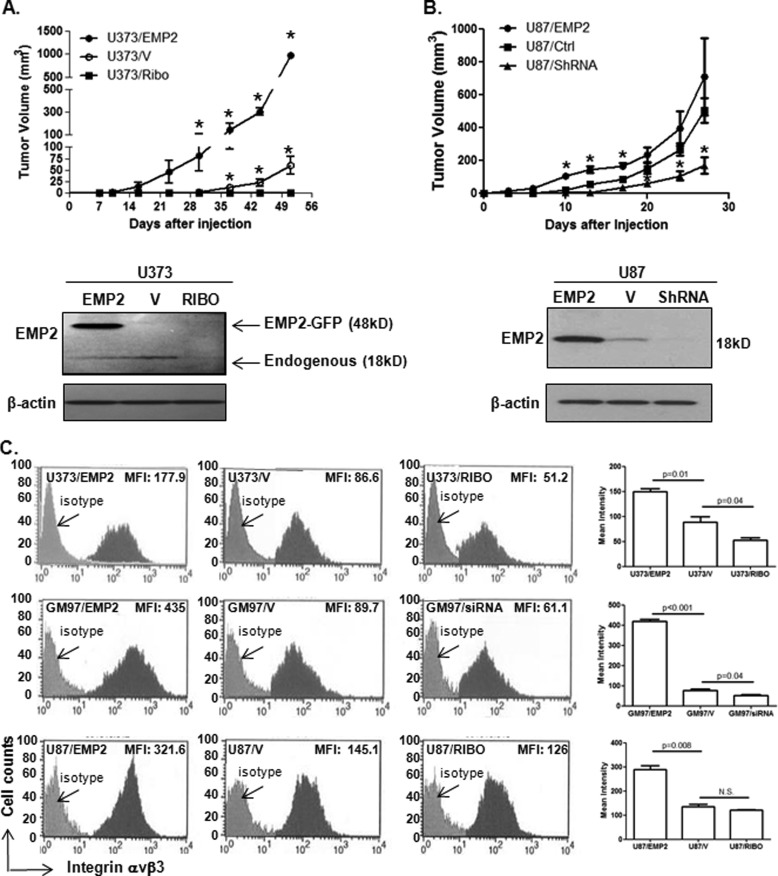
To characterize the effects of EMP2 in GBM, we initially determined whether EMP2 expression was necessary for tumorigenicity. Equivalent numbers of U373 and U87MG GBM cells with modified EMP2 levels were inoculated into athymic nude mice subcutaneously. Tumor growth kinetics of EMP2 overexpressing U373 and U87MG GBM xenografts were accelerated in athymic nude mice, compared with control vector-expressing U373 and U87MG xenografts. Furthermore, reduction of EMP2 through either an shRNA or ribozyme in U87MG and U373, respectively, significantly decreased tumor size (Fig. 2, A and B). These results suggested that EMP2 was an oncogenic protein in GBM and promoted tumorigenesis.
FIGURE 2.
EMP2 expression promotes GBM tumorigenicity. A, U373 modified cell lines were created that overexpress EMP2, express a vector control, or express reduced levels of the protein. U373/EMP2, U373/V, and U373/RIBO cells were then inoculated subcutaneously into athymic nude mice. Tumor volume was calculated twice a week for 50 days. n = 4 per group. *, comparison of U373/EMP2 with U373/V or U373/V with U373/RIBO by Student's t test, p < 0.05. Bottom, EMP2 expression was determined using Western blot analysis in U373/EMP2, U373/V, and U373/RIBO cells. Overexpression of EMP2 in these cells is via a GFP-EMP2 fusion protein (48 kDa). B, U87/EMP2, U87/V, and U87/shRNA cells were created and injected as above. Tumor volume was monitored for 27 days. n = 4 per group. *, p < 0.05 by Student's t test. Bottom, expression of EMP2 in the U87/EMP2, U87/V, and U87/shRNA cells was determined by Western blot analysis. In U87MG cells, EMP2 was overexpressed using a bicistronic vector (18 kDa). C, EMP2 expression was modified in U373, U87MG, and GM97 cells. To reduce EMP2 levels, GM97 were transiently transfected with a control or EMP2-specific siRNAs. In U373 and U87MG cells, EMP2 expression was stably reduced using a ribozyme (RIBO). αvβ3 integrin surface expression was measured using flow cytometry, and a representative histogram is shown. Right, values represent the average mean fluorescent intensity (MFI) ± S.E. from three experiments. N.S., not significant.
EMP2 Increases αvβ3 Integrin Surface Expression
Previously, we have shown that EMP2 up-regulates αvβ3 integrin surface expression in endometrial cancer cells (19). To determine whether EMP2 could alter integrin expression in GBM, cells were created with modified EMP2 levels. In U373, GM97, and U87MG cells, forced overexpression of EMP2 significantly increased αvβ3 integrin surface expression, whereas a reduction in EMP2 decreased it in both U373 and GM97 (Fig. 2C). The reduction in αvβ3 levels was also observed in U87MG cells stably transfected with a ribozyme, although this reduction was not significant. The effects of EMP2 on αvβ3 integrin expression appear to be specific as no significant changes in αvβ5 integrin expression were observed in any of the three cell lines (data not shown).
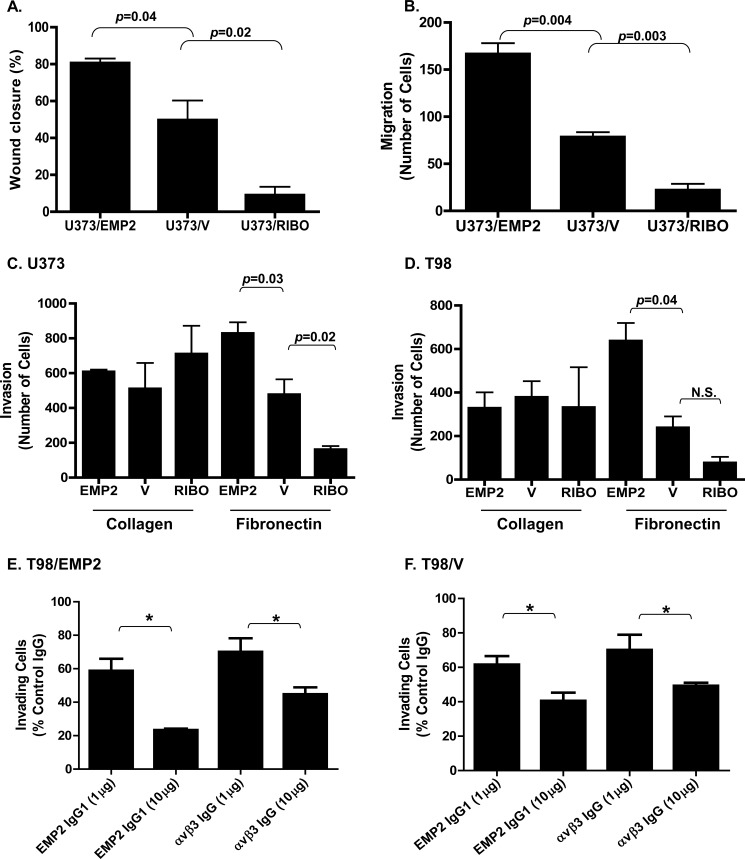
Integrins are known to transmit signals enhancing cancer cell proliferation and invasion (31); hence, we first examined whether EMP2 levels altered GBM proliferation. BrdU incorporation assays over 24 h revealed that cell proliferation was unaffected by overexpression or reduction in EMP2 in both U373 and T98 cells (data not shown). We next focused on tumor cell migration and invasion. Using U373 cells, ectopic overexpression of EMP2 increased wound healing (Fig. 3A) and cell migration (Fig. 3B) compared with vector control-expressing cells. Concordantly, a reduction of EMP2 expression using ribozymes decreased wound healing and cell migration, suggesting a role of EMP2 in the regulation of GBM cellular motility. Integrins typically show specificity for select extracellular matrices. It is known that αvβ3 integrin adheres to vitronectin and fibronectin (32, 33), but it does not have an affinity for collagen. To determine whether EMP2-mediated changes in integrin expression alter the cell's affinity for its ligand, transwells were coated with either fibronectin or collagen. U373 and T98 cells with modified levels of EMP2 were incubated for 6 h and allowed to invade through the matrix. EMP2 up-regulated fibronectin-mediated cell invasion in U373 (Fig. 3C) and T98 cells (Fig. 3D), but it had no effect on collagen-mediated cell invasion using this assay.
FIGURE 3.
EMP2 promotes GBM invasion. A, U373/EMP2, U373/V, or U373/RIBO cells were grown to form a monolayer. A scratch was then created, and closure of the wound was measured after 24 h. Experiments were performed at least three times, and the results were averaged. B, equal numbers of U373/EMP2, U373/V, and U373/RIBO were plated into the top of the transwell. After 6 h, cells that had migrated through the transwell were fixed, stained with crystal violent, and counted. Values are averages of three independent experiments (±S.E.). U373/EMP2, U373/V, or U373/RIBO cells (C) or T98/EMP2, T98/V, or T98/RIBO cells (D) were added to transwells coated with collagen I or fibronectin. Cells that had invaded through the transwell were determined as above. The experiment was repeated three times, with the data presented as the mean ± S.E. A–D, Student's t test was used to determine significant differences between groups with specific p values indicated in the figure; N.S., not significant. T98/EMP2 (E) or T98/V (F) cells were preincubated with varying concentrations of an anti-EMP2 IgG1, anti-αvβ3 integrin, or isotype control antibody. Cells were then plated onto a fibronectin-precoated transwell, and percent invasion relative to the isotype control was determined. Results represent averaged results from three independent experiments ± S.E. *, p < 0.05.
To further confirm the role of EMP2 and αvβ3 integrin in GBM motility, a full-length IgG1 to target EMP2 (12) and commercial antibodies to αvβ3 integrin (34, 35) were tested for their ability to functionally inhibit EMP2-mediated integrin activation. Cells were preincubated with either an EMP2 IgG1 or αvβ3 integrin-specific antibody, and invasion through fibronectin-coated transwells was monitored. EMP2/integrin-mediated cell invasion was significantly impaired by specific antibodies against EMP2 or αvβ3 integrin in a dose-dependent manner in both T98/EMP2 (Fig. 3E) and control T98/V cells (Fig. 3F). Collectively, these results suggest that EMP-regulated αvβ3 integrin surface expression modulated GBM cell migration and invasion.
EMP2 Activates FAK and Src
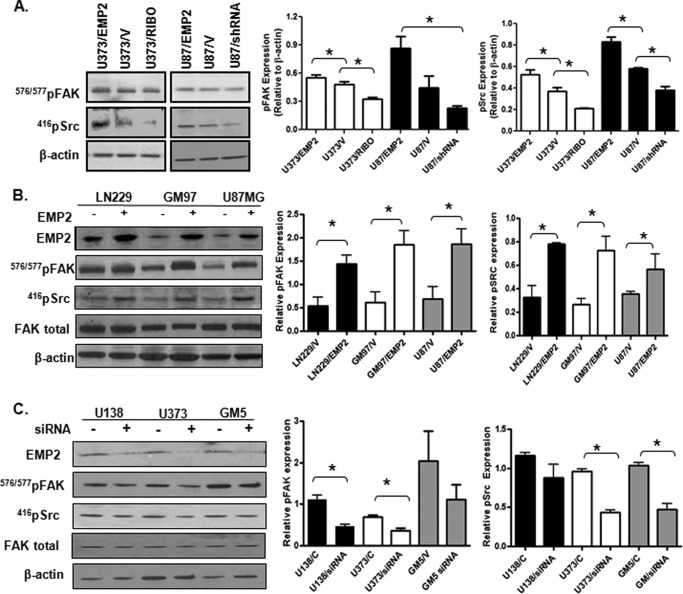
One consequence of integrin activation is to alter cellular behavior through the recruitment of FAK and Src kinase (36, 37). Panels of U373 and U87MG were plated for 24 h, and levels of activated FAK and Src kinase were measured. EMP2 levels significantly correlated with both FAK and Src activation in these cells (Fig. 4A). To confirm these results, EMP2 was overexpressed in GBM lines LN229 and GM97 and compared with U87MG cell lines. Overexpression of EMP2 activated FAK and Src by increasing phosphorylation levels at Tyr-576/577 and Tyr-416, respectively, compared with vector control-expressing cells (Fig. 4B). This increase was significant in all three cell lines (LN229, U87MG, and GM97), further suggesting that this may be a direct consequence of EMP2 up-regulation. To confirm that a reduction in EMP2 could produce a reciprocal effect, GBM cells with high endogenous levels of EMP2 were transiently transfected with an EMP2 siRNA. Similar to the shRNA knockdown, siRNA vectors to EMP2 decreased FAK and Src phosphorylation in U138, U373, and GM5 cells (Fig. 4C) with significant effects observed in U373 and U118 cells. These results collectively suggest that EMP2 promotes activation of the integrin-FAK-Src signaling pathway.
FIGURE 4.
EMP2 expression promotes activation of FAK and Src. A, panels of U373 or U87MG cells were created to overexpress EMP2 or down-regulate its expression through the use of a ribozymes (RIBO) or shRNA vector. Cells were plated, harvested, and probed for activated FAK (Tyr-576/577) and activated Src (Tyr-416). *, p < 0.05, comparison by Student's t test. B, vector control LN229, GM97, and U87 or cells which overexpress EMP2 were plated for 24 h, harvested, and then probed for activated FAK and Src, total FAK, and β-actin. Left, representative Western blots. Right, semi-quantitative analysis of activated FAK and Src levels from three independent experiments; comparison by Student's t test, *, p < 0.05. C, U118, U373, and GM5 cells were transfected with an EMP2 siRNA or control siRNA, and cells were incubated for 48 h, then harvested and probed as above. Left, representative Western blots. Right, semi-quantitative analysis of pFAK and pSrc after correction for β-actin levels from three independent experiments.
To correlate the in vitro data with clinical data, tissue microarrays were probed by IHC for both EMP2 and activated Src (Table 1). Analysis of 87 patients showed a Spearman's rank correlation coefficient of r = 0.54, p < 0.01 between EMP2 and anti-p-Src (Tyr-416) expression, where 98% (49 out of 50) of tissues positive for p-Src (Tyr-416) showed intense staining for EMP2.
TABLE 1.
Correlation of EMP2 and pSRC expression in GBM patient samples
Spearman's rank correlation coefficient is r + 0.54, p < 0.01.
| n + 87 | EMP2 positive | EMP2 negative | Total |
|---|---|---|---|
| pSRC positive | 49 | 1 | 50 |
| pSRC negative | 20 | 17 | 37 |
Increased EMP2 Expression Increases GBM Cell Invasion in Vivo
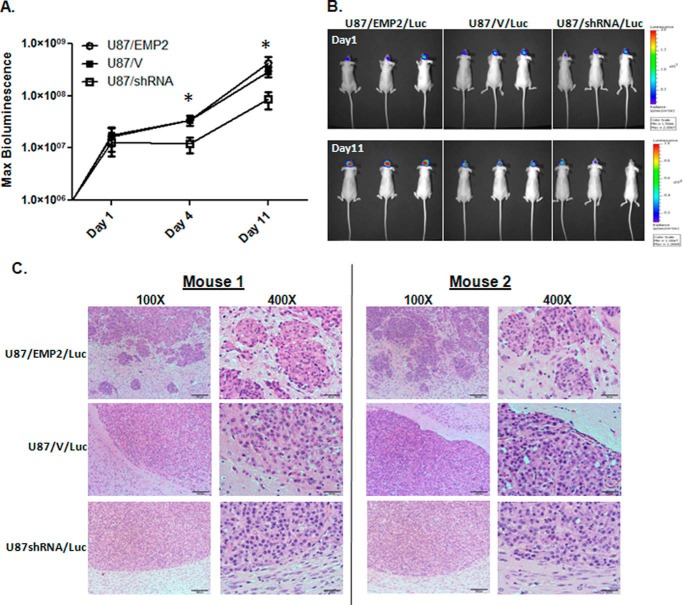
To determine whether EMP2 expression altered GBM tumor growth in the brain, U87/Luc cells with modified EMP2 levels were stereotactically implanted into the right frontal lobe of athymic nude mice, and tumor load was monitored using bioluminescence (Fig. 5, A and B). Although an increase in EMP2 expression did not significantly increase tumor growth compared with control animals, it did increase tumor invasion into the surrounding parenchyma (Fig. 5C). In contrast, a reduction of EMP2 through a specific shRNA significantly inhibited tumor growth compared with control mice, suggesting that targeting EMP2 expression may have a therapeutic benefit. Importantly, the rate of growth between the tumor lines is consistent with the U87MG subcutaneous model created above.
FIGURE 5.
EMP2 expression promotes tumor migration in vivo. A, U87/EMP2/Luc, U87/V/Luc, or U87/shRNA/Luc cells were stereoscopically implanted into the right frontal lobe of athymic nude mice. Tumor load was monitored using bioluminescence imaging; n = 6 per group. *, p < 0.05 by one-way analysis of variance. B, representative bioluminescence images of mice from each group on day 1 and day 11. C, representative low and high magnification images from two U87/EMP2, U87/V, and U87/shRNA tumors. The high magnification image centers on the brain-tumor margin. Magnification: left, ×100; right, ×400.
Antibodies Targeting EMP2 Inhibit GBM Tumor Growth in Vitro and in Vivo
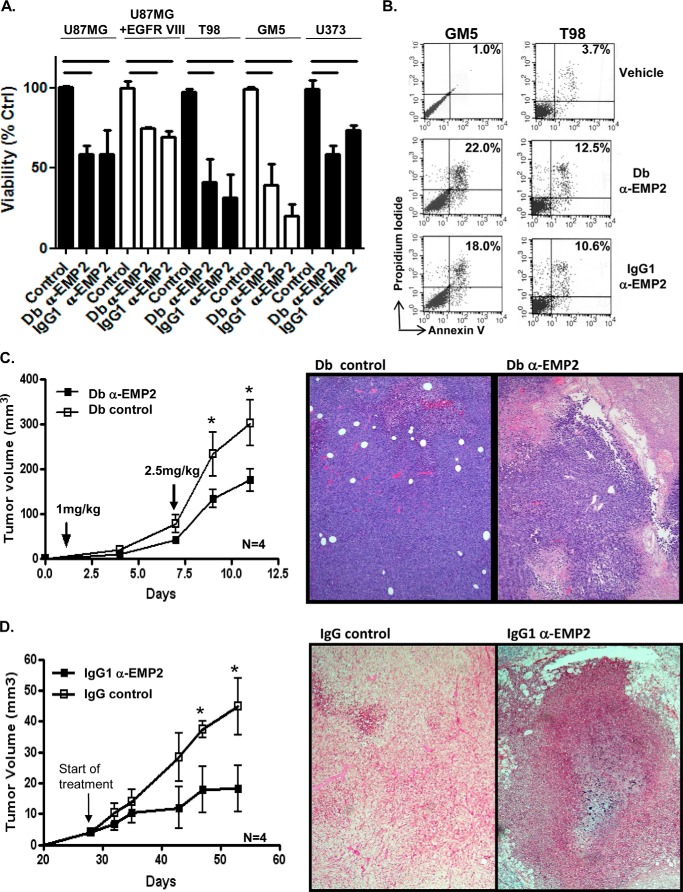
We have recently shown that anti-EMP2 antibodies are a novel therapeutic option for endometrial, breast, and ovarian cancers in preclinical models using a panel of recombinant immunoglobulin-based reagents (11, 12, 26). These reagents include high affinity diabodies (bivalent scFv dimers, 55 kDa) as well as a full-length IgG1 (150 kDa). The rationale for constructing different sized immunoglobulin reagents was to create a panel of reagents with properties tailored for tumor infiltration and serum half-life (38). In contrast to native IgG1, diabodies biodistribute more quickly, penetrate target tissues efficiently, and clear more rapidly from circulation. Both diabody and native IgG1 reagents bind to the second extracellular loop of EMP2 (26), and these immunoglobulin variants show cross-reactivity for mouse and human EMP2 as detected by flow cytometry and IHC (26). To determine whether the anti-EMP2 diabody or IgG1 could induce cell death, a panel of GBM cells such as U87MG, U87/EGFR VIII, U373, T98, and GM5 were incubated with EMP2-specific immunoglobulin reagents or a vehicle control. Both EMP2-specific reagents significantly reduced viable cell numbers in all GBM cell lines tested (Fig. 6A). To confirm that the reduction in cell number translated into an induction of cellular death, T98 and GM5 cells were incubated with EMP2 IgG1, EMP2 diabodies, or a vehicle control, and after 72 h, they were stained with annexin V and propidium iodide. Compared with the control, both EMP2 IgG1 and anti-EMP2 diabodies increased the percentage of cell death (Fig. 6B).
FIGURE 6.
EMP2 antibodies reduced cellular viability and tumor load. A, 2 × 105 GM5, U87MG, U87/EGFR VIII, T98, and U373 cells were incubated for 72 h with a vehicle control (PBS) or molar equivalents of the anti-EMP2 diabody or anti-EMP2 IgG1. Cellular viability was enumerated using trypan blue exclusion. Data represent viability as a percentage of control from three independent experiments. B, GM5 and T98 cells were treated as above, with cellular viability determined using annexin V and propidium iodide staining. The experiment was repeated three times, and a representative graph is shown. C, U87/EGFR VIII cells were inoculated subcutaneously in BALB/c nude mice. When tumors reached 4 mm3, they were treated twice weekly (1st week, 1.0 mg/kg; 2nd week, 2.5 mg/kg), with anti-EMP2 diabody or control diabody. Left, tumor size (arrow indicates start of treatment). Right, tumor histology after treatment. Magnification, ×100. n = 6. *, p < 0.05 as determined by Student's t test. D, U373 cells were subcutaneously injected into athymic nude mice. When tumors reached 4 mm3, mice were treated systemically (intraperitoneally) with anti-EMP2 IgG1 or control IgG (3 mg/kg). Left, tumor growth. Right, tumor histology at day 65. Magnification, ×100. n = 6. *, p < 0.05 as determined by Student's t test.
To determine whether recombinant anti-EMP2 antibodies could be effective in treating GBM tumors in vivo, U87/EGFR VIII cells were inoculated subcutaneously to the flanks of athymic nude mice. When tumors reached 4 mm3, mice were treated intratumor injections of either the anti-EMP2 or control diabody. Mice were injected with 1 mg/kg twice in the 1st week followed by 2.5 mg/kg twice in the week. Tumor growth was reduced when treated with anti-EMP2 diabody (Fig. 6C, left panel), and the residual tumors showed marked necrosis compared with the control diabody (Fig. 6C, right panel), suggesting that the anti-EMP2 diabody retarded tumor growth by inducing tumor cell death.
Similar results were observed using systemic treatment of anti-EMP2 IgG1 (Fig. 6D). U373 xenografts were implanted subcutaneously into the shoulder of athymic nude mice. When the tumors reached 4 mm3, mice were treated intraperitoneally with full-length anti-EMP2 or control IgG1 weekly at 3 mg/kg. Anti-EMP2 IgG1 retarded U373 tumor growth compared with control IgG-treated mice (Fig. 6D, left panel), with tumors exhibiting significant necrosis throughout the tumor (Fig. 6D, right panel).
DISCUSSION
The current repertoire of chemotherapy, surgical options, and targeted therapies has not significantly enhanced the survival profile for patients with GBM, and it remains the most common and aggressive form of brain tumors with a median survival time of 12 months (39). In this study, we identify a membrane protein EMP2 as an important contributor to GBM tumorigenicity as well as a novel target for GBM killing. Several properties and characteristics of EMP2 make it a potentially attractive therapeutic target. First, EMP2 is expressed in most GBM tumors and cell lines examined to date. Importantly, EMP2 is low in nonmalignant adjacent brain tissue. Second, EMP2 has prognostic value as higher levels suggest a more rapid course of the disease for GBM, and this effect can be reproduced using human xenograft models. Third, we have developed a therapeutic approach to target GBM cells in vitro and in vivo using specific anti-EMP2 antibody reagents. These reagents are effective in killing GBM cells in vitro and in reducing tumor load in mouse model systems.
How does EMP2 contribute to GBM tumorigenicity? It appears that in GBM EMP2 enhances tumor growth in part or exclusively by modulating αvβ3 integrin surface expression. The importance of αvβ3 integrin in glioma has been well documented, and it is thought to play a variety of roles in tumorigenesis (2, 40, 41). One role is the involvement of αvβ3 integrin in cellular migration and invasion. Recruitment of αvβ3 to focal adhesions within the leading edge of the migratory tumor cells has been observed using patient samples (42, 43). In this study, we have shown that the increase in αvβ3 integrin expression correlates with increased activation of FAK and Src kinases and an increase in cell migration and invasion in vitro. In vivo, increased EMP2 promotes tumor cell invasion using intracranial models, and EMP2 and activated Src are correlated in patient samples. In concert, these results suggest that regulation of the integrin-FAK-Src nexus is at least one of the pathways by which EMP2 significantly contributes to pathogenicity.
How does this regulation of αvβ3 integrin expression occur? Although the mechanism of integrin regulation by EMP2 is not known in glioma, previous studies suggest that integrin expression is downstream of EMP2 (9, 19, 44). Notably, in endometrial cancer, EMP2 promotes β3 integrin transcription and helps traffic this integrin pair to the plasma membrane (19). Additional experiments will be needed to decipher its regulation in GBM as well as determine whether αvβ3 integrin expression can cross-regulate EMP2 expression.
Notably, both EMP2 diabodies and IgG1 induced tumor cell death in vitro and in vivo. Although modulation of EMP2 levels did not affect GBM cell proliferation in vitro, several possibilities exist to explain how EMP2 may be affecting cell survival. First, anti-EMP2 antibodies may down-regulate αvβ3 integrin. As αvβ3 integrin is thought to be important for GBM progression, invasion, and survival, inactivation or suppression of this integrin may be sufficient to induce cell death (45). Consistent with the observed cytotoxicity of anti-EMP2 antibodies, down-regulation of αvβ3 integrin by tumistatin or RGD peptides has been shown to induce apoptosis (46, 47). In the case of tumistatin, apoptosis is induced via dampening of AKT signaling, and whether anti-EMP2 therapy has a similar effect on AKT signaling is currently being studied by our group. Another possibility for how anti-EMP2 antibodies induce cell death is that they may modulate the tumor microenvironment and alter tumor angiogenesis. GBM is known for being highly hemorrhagic, and gliomas express important pro-angiogenic molecules such as vascular endothelial growth factor (VEGF) at elevated levels (48). Recent studies from our laboratory suggest that EMP2 may be important for regulating VEGF expression in endometrial cancer, and it is possible that a similar effect is observed in GBM (18). Hence, anti-EMP2 antibodies may inhibit VEGF levels in the tumor and thus indirectly suppress tumor growth in vivo.
An important premise in oncology is that cancers can be classified and treated according to their molecular phenotype. In addition to identifying targeting reagents, a particular challenge in GBM is delivery because many molecules, such as antibodies, fail to cross the blood-brain barrier (1, 49). Multiple methods have been developed to enhance antibody delivery to the central nervous system, including direct injection, mechanical or biochemical disruption of the blood-brain barrier, and more recently, stem cell-mediated antibody delivery (49). As an initial proof of principle, we have evaluated diabody and IgG1 forms of anti-EMP2 antibody as each offers distinct advantages for in vivo therapy. Although clearance through the blood-brain barrier may be an issue for both immunoglobulin reagents, we predict that diabodies may have a distinct advantage for brain tumors as their small size allows them to access tissues that are poorly accessible by intact antibodies (49). In contrast, intact IgG1 antibodies can elicit antibody-dependent cellular cytotoxicity, which may improve their in vivo efficacy (50, 51), and preliminary data suggest that the anti-EMP2 IgG1 is able to elicit such an effect (12). However, additional experiments will be needed to fully elucidate the desired molecular format for GBM as well the optimized delivery strategy.
Our results suggest that EMP2 may be a promising molecular therapeutic target in GBM, and additional work will be needed to determine whether anti-EMP2 antibodies can be combined with standard chemotherapy or molecularly targeted treatments. Although it may be difficult to overcome the permeability issues in the brain using an antibody or antibody fragments, studies here indicate that EMP2 has an important role in GBM tumorigenesis and tumor progression. Moreover, a reduction of EMP2 in GBM cells was sufficient to inhibit tumor growth in vivo, lending support to the idea that anti-EMP2 treatment may be therapeutically beneficial.
This work was supported, in whole or in part, by National Institutes of Health Grants CA-86366 from NCI Early Detection Research Network (to L. G.), R01 CA163971 (to M. W.), CA 143931CDU/UCLA Cancer Center Partnership U54 (to M. W.), Grant P30 CA016042 to Jonsson Comprehensive Cancer Center, and Grant 5P30 AI028697 from CFAR. This work was also supported by a Stein Oppenheimer seed grant (to L. G.).
- GBM
- glioblastoma
- FAK
- focal adhesion kinase
- EMP2
- epithelial membrane protein-2
- EGFR
- epithelial growth factor receptor
- RIBO
- ribozyme
- IHC
- immunohistochemistry
- V
- vector.
REFERENCES
- 1. Rolle C. E., Sengupta S., Lesniak M. S. (2010) Challenges in clinical design of immunotherapy trials for malignant glioma. Neurosurg. Clin. N. Am. 21, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teodorczyk M., Martin-Villalba A. (2010) Sensing invasion: cell surface receptors driving spreading of glioblastoma. J. Cell. Physiol. 222, 1–10 [DOI] [PubMed] [Google Scholar]
- 3. Wang S. D., Rath P., Lal B., Richard J. P., Li Y., Goodwin C. R., Laterra J., Xia S. (2012) EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 31, 5132–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giese A., Loo M. A., Tran N., Haskett D., Coons S. W., Berens M. E. (1996) Dichotomy of astrocytoma migration and proliferation. Int. J. Cancer 67, 275–282 [DOI] [PubMed] [Google Scholar]
- 5. Zagzag D., Lukyanov Y., Lan L., Ali M. A., Esencay M., Mendez O., Yee H., Voura E. B., Newcomb E. W. (2006) Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab. Invest. 86, 1221–1232 [DOI] [PubMed] [Google Scholar]
- 6. de Groot J., Milano V. (2009) Improving the prognosis for patients with glioblastoma: the rationale for targeting Src. J. Neurooncol. 95, 151–163 [DOI] [PubMed] [Google Scholar]
- 7. Wick W., Weller M., Weiler M., Batchelor T., Yung A. W., Platten M. (2011) Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro-Oncology 13, 566–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu M., Rao R., Sudhakar D., Hogue C. P., Rutta Z., Morales S., Gordon L. K., Braun J., Goodglick L., Wadehra M. (2011) Epithelial membrane protein-2 promotes endometrial tumor formation through activation of FAK and Src. PLoS One 6, e19945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Morales S. A., Mareninov S., Wadehra M., Zhang L., Goodglick L., Braun J., Gordon L. K. (2009) FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest. Ophthalmol. Vis. Sci. 50, 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habeeb O., Goodglick L., Soslow R. A., Rao R. G., Gordon L. K., Schirripa O., Horvath S., Braun J., Seligson D. B., Wadehra M. (2010) Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer 116, 4718–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu M., Maresh E. L., Soslow R. A., Alavi M., Mah V., Zhou Q., Iasonos A., Goodglick L., Gordon L. K., Braun J., Wadehra M. (2010) Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin. Cancer Res. 16, 3954–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu M., Maresh E. L., Helguera G., Kiyohara M., Qin Y., Ashki N., Daniels-Wells T. R., Aziz N., Gordon L. K., Braun J., Elshimali Y., Soslow R. A., Penichet M. L., Goodglick L., Wadehra M. (2014) Rationale and preclinical efficacy of a novel anti-EMP2 antibody for the treatment of invasive breast cancer. Mol. Cancer Ther., in press 10.1158/1535-7163.MCT-13-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadehra M., Natarajan S., Seligson D. B., Williams C. J., Hummer A. J., Hedvat C., Braun J., Soslow R. A. (2006) Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer 107, 90–98 [DOI] [PubMed] [Google Scholar]
- 14. Freije W. A., Castro-Vargas F. E., Fang Z., Horvath S., Cloughesy T., Liau L. M., Mischel P. S., Nelson S. F. (2004) Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 64, 6503–6510 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y., Zhu S., Cloughesy T. F., Liau L. M., Mischel P. S. (2004) p53 disruption profoundly alters the response of human glioblastoma cells to DNA topoisomerase I inhibition. Oncogene 23, 1283–1290 [DOI] [PubMed] [Google Scholar]
- 16. Wang M. Y., Lu K. V., Zhu S., Dia E. Q., Vivanco I., Shackleford G. M., Cavenee W. K., Mellinghoff I. K., Cloughesy T. F., Sawyers C. L., Mischel P. S. (2006) Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 66, 7864–7869 [DOI] [PubMed] [Google Scholar]
- 17. Kim K., Brush J. M., Watson P. A., Cacalano N. A., Iwamoto K. S., McBride W. H. (2008) Epidermal growth factor receptor vIII expression in U87 glioblastoma cells alters their proteasome composition, function, and response to irradiation. Mol. Cancer Res. 6, 426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon L. K., Kiyohara M., Fu M., Braun J., Dhawan P., Chan A., Goodglick L., Wadehra M. (2013) EMP2 regulates angiogenesis in endometrial cancer cells through induction of VEGF. Oncogene 32, 5369–5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wadehra M., Forbes A., Pushkarna N., Goodglick L., Gordon L. K., Williams C. J., Braun J. (2005) Epithelial membrane protein-2 regulates surface expression of αvβ3 integrin in the endometrium. Dev. Biol. 287, 336–345 [DOI] [PubMed] [Google Scholar]
- 20. Wadehra M., Dayal M., Mainigi M., Ord T., Iyer R., Braun J., Williams C. J. (2006) Knockdown of the tetraspan protein epithelial membrane protein-2 inhibits implantation in the mouse. Dev. Biol. 292, 430–441 [DOI] [PubMed] [Google Scholar]
- 21. Mellinghoff I. K., Wang M. Y., Vivanco I., Haas-Kogan D. A., Zhu S., Dia E. Q., Lu K. V., Yoshimoto K., Huang J. H., Chute D. J., Riggs B. L., Horvath S., Liau L. M., Cavenee W. K., Rao P. N., Beroukhim R., Peck T. C., Lee J. C., Sellers W. R., Stokoe D., Prados M., Cloughesy T. F., Sawyers C. L., Mischel P. S. (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 [DOI] [PubMed] [Google Scholar]
- 22. Cronin M., Akin A. R., Collins S. A., Meganck J., Kim J.-B., Baban C. K., Joyce S. A., van Dam G. M., Zhang N., van Sinderen D., O'Sullivan G. C., Kasahara N., Gahan C. G., Francis K. P., Tangney M. (2012) High resolution in vivo bioluminescent imaging for the study of bacterial tumour targeting. PLoS One 7, e30940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agus D. B., Scher H. I., Higgins B., Fox W. D., Heller G., Fazzari M., Cordon-Cardo C., Golde D. W. (1999) Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and -independent human xenograft models. Cancer Res. 59, 4761–4764 [PubMed] [Google Scholar]
- 24. Wang W., Zhu N. L., Chua J., Swenson S., Costa F. K., Schmitmeier S., Sosnowski B. A., Shichinohe T., Kasahara N., Chen T. C. (2005) Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J. Neurosurg. 103, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 25. Huang T. T., Hlavaty J., Ostertag D., Espinoza F. L., Martin B., Petznek H., Rodriguez-Aguirre M., Ibañez C. E., Kasahara N., Gunzburg W., Gruber H. E., Pertschuk D., Jolly D. J., Robbins J. M. (2013) Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 20, 544–551 [DOI] [PubMed] [Google Scholar]
- 26. Shimazaki K., Lepin E. J., Wei B., Nagy A. K., Coulam C. P., Mareninov S., Fu M., Wu A. M., Marks J. D., Braun J., Gordon L. K., Wadehra M. (2008) Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin. Cancer Res. 14, 7367–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Acconcia F., Barnes C. J., Kumar R. (2006) Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology 147, 1203–1212 [DOI] [PubMed] [Google Scholar]
- 28. Wadehra M., Sulur G. G., Braun J., Gordon L. K., Goodglick L. (2003) Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp. Mol. Pathol. 74, 106–112 [DOI] [PubMed] [Google Scholar]
- 29. Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 30. Fan Q.-W., Cheng C. K., Gustafson W. C., Charron E., Zipper P., Wong R. A., Chen J., Lau J., Knobbe-Thomsen C., Weller M., Jura N., Reifenberger G., Shokat K. M., Weiss W. A. (2013) EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 24, 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hood J. D., Cheresh D. A. (2002) Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91–100 [DOI] [PubMed] [Google Scholar]
- 32. Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. (1990) The vitronectin receptor αvβ3 binds fibronectin and acts in concert with α5β1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 111, 2795–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiao Y., Feng X., Zhan Y., Wang R., Zheng S., Liu W., Zeng X. (2012) Matrix metalloproteinase-2 promotes αvβ3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One 7, e41591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerr J. S., Slee A. M., Mousa S. A. (2000) Small molecule αv integrin antagonists: novel anticancer agents. Expert Opin. Investig. Drugs 9, 1271–1279 [DOI] [PubMed] [Google Scholar]
- 35. Rooprai H. K., Vanmeter T., Panou C., Schnüll S., Trillo-Pazos G., Davies D., Pilkington G. J. (1999) The role of integrin receptors in aspects of glioma invasion in vitro. Int. J. Dev. Neurosci. 17, 613–623 [DOI] [PubMed] [Google Scholar]
- 36. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 37. Hehlgans S., Haase M., Cordes N. (2007) Signalling via integrins: implications for cell survival and anticancer strategies. Biochim. Biophys. Acta 1775, 163–180 [DOI] [PubMed] [Google Scholar]
- 38. Beckman R. A., Weiner L. M., Davis H. M. (2007) Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer 109, 170–179 [DOI] [PubMed] [Google Scholar]
- 39. Buonerba C., Di Lorenzo G., Marinelli A., Federico P., Palmieri G., Imbimbo M., Conti P., Peluso G., De Placido S., Sampson J. H. (2011) A comprehensive outlook on intracerebral therapy of malignant gliomas. Crit. Rev. Oncol. Hematol. 80, 54–68 [DOI] [PubMed] [Google Scholar]
- 40. Petrás M., Hutóczki G., Varga I., Vereb G., Szöllosi J., Bognár L., Ruszthi P., Kenyeres A., Tóth J., Hanzély Z., Scholtz B., Klekner A. (2009) Expression pattern of invasion-related molecules in brain tumors of different origin. Magy. Onkol. 53, 253–258 [DOI] [PubMed] [Google Scholar]
- 41. Kanamori M., Kawaguchi T., Berger M. S., Pieper R. O. (2006) Intracranial microenvironment reveals independent opposing functions of host αVβ3 expression on glioma growth and angiogenesis. J. Biol. Chem. 281, 37256–37264 [DOI] [PubMed] [Google Scholar]
- 42. D'Abaco G. M., Kaye A. H. (2007) Integrins: molecular determinants of glioma invasion. J. Clin. Neurosci. 14, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 43. Gladson C. L., Cheresh D. A. (1991) Glioblastoma expression of vitronectin and the αvβ3 integrin. Adhesion mechanism for transformed glial cells. J. Clin. Invest. 88, 1924–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wadehra M., Iyer R., Goodglick L., Braun J. (2002) The tetraspan protein epithelial membrane protein-2 interacts with β1 integrins and regulates adhesion. J. Biol. Chem. 277, 41094–41100 [DOI] [PubMed] [Google Scholar]
- 45. Lorger M., Felding-Habermann B. (2012) in Integrin Signaling in Angiogenesis and Metastatic Cancer Progression in the Brain Signaling Pathways and Molecular Mediators in Metastasis (Fatatis A., ed) pp. 311–329, Springer, Netherlands [Google Scholar]
- 46. Kawaguchi T., Yamashita Y., Kanamori M., Endersby R., Bankiewicz K. S., Baker S. J., Bergers G., Pieper R. O. (2006) The PTEN/Akt pathway dictates the direct αVβ3-dependent growth-inhibitory action of an active fragment of tumstatin in glioma cells in vitro and in vivo. Cancer Res. 66, 11331–11340 [DOI] [PubMed] [Google Scholar]
- 47. Chang M.-W., Lo J.-M., Juan H.-F., Chang H.-Y., Chuang C.-Y. (2012) Combination of RGD compound and low-dose paclitaxel induces apoptosis in human glioblastoma cells. PLoS One 7, e37935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kerbel R. S. (2008) Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frank R. T., Aboody K. S., Najbauer J. (2011) Strategies for enhancing antibody delivery to the brain. Biochim. Biophys. Acta 1816, 191–198 [DOI] [PubMed] [Google Scholar]
- 50. McEarchern J. A., Oflazoglu E., Francisco L., McDonagh C. F., Gordon K. A., Stone I., Klussman K., Turcott E., van Rooijen N., Carter P., Grewal I. S., Wahl A. F., Law C.-L. (2007) Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood 109, 1185–1192 [DOI] [PubMed] [Google Scholar]
- 51. Fukai J., Nishio K., Itakura T., Koizumi F. (2008) Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 99, 2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]