Background: PTEN localizes to the nucleus, whose molecular regulation remains unknown.
Results: Plk1 phosphorylates PTEN on Ser-380 and regulates its function in mitosis.
Conclusion: Plk1 is an important regulator of PTEN during the cell cycle.
Significance: PTEN plays a key role in regulating mitotic progression.
Keywords: Cell Cycle, Cell Signaling, Chromatin, Mitosis, PTEN, Kinase, Phosphorylation, Plk1
Abstract
PTEN is a well known tumor suppressor through the negative regulation of the PI3K signaling pathway. Here we report that PTEN plays an important role in regulating mitotic timing, which is associated with increased PTEN phosphorylation in the C-terminal tail and its localization to chromatin. Pulldown analysis revealed that Plk1 physically interacted with PTEN. Biochemical studies showed that Plk1 phosphorylates PTEN in vitro in a concentration-dependent manner and that the phosphorylation was inhibited by Bi2635, a Plk1-specific inhibitor. Deletional and mutational analyses identified that Plk1 phosphorylated Ser-380, Thr-382, and Thr-383, but not Ser-385, a cluster of residues known to affect the PTEN stability. Interestingly, a combination of molecular and genetic analyses revealed that only Ser-380 was significantly phosphorylated in vivo and that Plk1 regulated the phosphorylation, which was associated with the accumulation of PTEN on chromatin. Moreover, expression of phospho-deficient mutant, but not wild-type PTEN, caused enhanced mitotic exit. Taken together, our studies identify Plk1 as an important regulator of PTEN during the cell cycle.
Introduction
Phosphatase and tensin homolog (PTEN)2 functions as a tumor suppressor because it is frequently mutated in a variety of cancer cells, and inherited PTEN mutations cause cancer-susceptibility conditions including Cowden syndrome (1–4). Biochemically, PTEN dephosphorylates the lipid second-messenger phosphatidylinositol 3,4,5-trisphosphate to generate phosphatidylinositol 3,4-bisphosphate and, by doing so, antagonizes the PI3K/Akt signaling pathway. The PTEN level, as well as its activity, profoundly influences cell growth, survival, and tumor susceptibility (5, 6). Extensive research in the past has revealed that the PTEN tumor suppressor is a central negative regulator of the PI3K/PDK1/Akt signaling axis that controls multiple cellular functions including cell growth, survival, proliferation, and angiogenesis (5).
PTEN is subjected to modifications by several types of the post-translational mechanisms including phosphorylation, oxidation, ubiquitination, and acetylation (7). PTEN is extensively phosphorylated at the C-tail region by several protein kinases. Phosphorylation of several serine/threonine residues (e.g. Ser-370, Thr-382, Thr-383, and Ser-385) in the C-tail region of PTEN by casein kinase 2 (CK2) and Plk3 is essential for the tail-dependent regulation of stability as phospho-defective mutant proteins exhibit decreased stability compared with the wild-type PTEN (8–10). Glucose synthase kinase 3β phosphorylates PTEN at Ser-362 and Thr-366 (8). PTEN is also phosphorylated on tyrosine residues by Rak, and this phosphorylation stabilizes PTEN as well (11).
Plk1 is an important member of the POLO kinase family (12–14). Extensive investigations in the past have uncovered a variety of cellular processes and molecular pathways that are regulated by Plk1, including DNA-damage response and repair (15–19), tRNAs and ribosome RNA transcription (20), mitotic onset and progression (21–25), microtubule dynamics (26), and centrosome duplication and maturation (27–30). Although Plk1 expression is associated with the development of a wide spectrum of malignancies evidence also exists suggesting that Plk1 may possess additional functions that are not related to its growth promotion. For example, mutations in PLK1 have been detected in several types of tumor cells (31), and haploinsufficiency of PLK1 results in enhanced tumorigenesis (32). Moreover, Plk3 and Plk4, both members of the POLO kinase family bearing a close structural similarity to Plk1, function to suppress cell and tumor growth (33, 34). Therefore, to gain a full appreciation of Plk1 function in the regulation of cell division, it is necessary to further identify and characterize new molecular components that are targeted by Plk1.
Given that PTEN also has a nuclear function and is involved in regulating chromosomal stability (35, 36), we investigated whether Plk1 might directly target PTEN by phosphorylation. Our extensive biochemical and molecular analyses revealed that Plk1 phosphorylates PTEN in vitro and regulates its phosphorylation in vivo. We also obtained evidence that Plk1-mediated phosphorylation was important for PTEN binding to chromatin, as well as for normal mitotic progression.
MATERIALS AND METHODS
Cell Culture and Transfection
HeLa and HEK-293T cell lines obtained from the American Type Culture Collection were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen) and antibiotics (100 μg/ml penicillin and 50 μg/ml streptomycin sulfate; Invitrogen) at 37 °C under 5% CO2. Murine embryonic fibroblasts (MEFs) were obtained from embryos through crossing wild-type and PLK1+− mice (a gift from Dr. Xiaochun Lu, Michigan University). Transfection of HeLa cells was achieved with either FuGENE HD (Roche Diagnostics) or Lipofectamine 2000 (Invitrogen) following the manufacturers' protocol. Transfection efficiency was estimated to be between 80 and 100% in all cases through transfecting a GFP-expressing plasmid.
Antibodies and Reagents
Antibodies to PTEN, phospho-PTENSer-380/Thr-382/Thr-383 (p-PTENS/T/T), p-PTENSer-380, cyclin E, cyclin B1, α-tubulin, PARP, HA, and phospho-histone H3 were purchased from Cell Signaling Technology. Antibody to GFP was purchased from Santa Cruz Biotechnology. Antibody to phospho-PTENThr-366/Thr-370 (p-PTENT/S) was purchased from Epitomics. Plk1 antibody was obtained from Millipore. Antibodies to GST and GFP were purchased from Santa Cruz Biotechnology. CK2 inhibitor (4,5,6,7-tetrabromobenzimidazole abbreviated as TBBz) was purchased from Sigma-Aldrich. Plk1 inhibitor (Bi2536) was purchased from Selleck Chemicals.
RNA Interference
Human ON-TARGETplus SMARTpool siRNA oligonucleotides that specifically target PTEN (L-003023-00-0010) and PLK1 (L-003290-00-0010) were purchased from Dharmacon. Each set of siRNA oligonucleotides contains four sequences as follows: PTEN (5′-GAUCAGCAUACACAAAUUA-3′, 5′-GACUUAGACUUGACCUAUA-3′, 5′-GAUCUUGACCAAUGGCUAA-3′, and 5′-CGAUAGCAUUUGCAGUAUA-3′) and PLK1 (5′-GCACAUACCGCCUGAGUCU-3′, 5′-CCACCAAGGUUUUCGAUUG-3′, 5′-GCUCUUCAAUGACUCAACA-3′, and 5′-UCUCAAGGCCUCCUAAUAG-3′). Individual sets of siRNAs were transfected into HeLa cells with Dharmafect I according to the protocol provided by the supplier. Briefly, cells seeded at 50% confluence in an antibiotic-free culture medium were transfected with siRNA duplexes at a final concentration of 100 nm for 24 h. Small interfering RNAs targeting firefly (Photinus pyralis) luciferase (5′-UUCCTACGCTGAGTACTTCGA-3′, GL-3 from Dharmacon) were used as negative control for transfection.
Plasmids
Plasmids encoding HA-tagged PTEN (pSG5L-HA-PTEN) and its phosphorylation mutants including T366A, S370A, S380A, T382A, and T383A were obtained from Addgene. The plasmid constructs expressing EGFP-N3 and EGFP-Plk1 were kindly provided by Dr. Xiaoqi Liu (Purdue University). To make plasmids encoding GST-PTEN fusion proteins, PCR products encoding human PTEN residues of 1–403, 1–347, 1–186, 186–347, 186–403, or 347–403 were inserted into the BamHI and EcoRI sites of pGEX-3X (GE Healthcare). Individual GST-PTEN mutants were obtained using a QuikChange Site-directed Mutagenesis kit (Agilent Technologies). PTEN cDNA and its deletion fragments were subcloned into pGEX-3X plasmid vector to create GST-PTEN fusion constructs. GST-PTEN mutants were generated using the QuikChange Lightning Multi Site-directed Mutagenesis kit (Agilent Technologies) according to the manufacturer's instructions. Individual deletion fragments of GST-PTEN cDNA, as well as wild-type GST-PTEN cDNA, were also subcloned into pcDNA3 plasmid, a mammalian expression vector.
Cell Cycle Synchronization
HeLa cells were synchronized at the G1/S boundary by double-thymidine blocks. Briefly, cells were treated with 2 mm thymidine for 18 h followed by a 9- h release; these cells were treated with 2 mm thymidine for another 18 h and then released into the cell cycle for various times. MEF cells synchronization at the G1/S junction was achieved by treatment with 2 mm thymidine for 18 h followed by a 9-h release; these cells were then treated with 2 μg/ml aphidicolin for another 18 h. Mitotic shake-off cells were obtained from gentle tapping of either exponentially growing rounded-up cells or cells treated with nocodazole (40 ng/ml; Sigma-Aldrich) for 14 h.
Preparation of Protein Extracts and Immunoblotting
Total cell lysates were prepared in a buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% IGEPAL, 0.1% SDS, and 0.5% sodium deoxycholate) supplemented with a mixture of protease and phosphatase inhibitors. Chromatin and cytosolic/soluble extracts were obtained as described previously (37). In brief, cell extracts were prepared in the harvest buffer (10 mm HEPES (pH 8.0), 50 mm NaCl, 0.5 m sucrose, 0.1 m EDTA, 0.5% Triton X-100) containing both protease inhibitors (1 mm dithiothreitol (DTT), 2 mg/ml pepstatin, 4 mg/ml aprotinin, 100 mm PMSF) and phosphatase inhibitors (10 mm tetrasodium pyrophosphate, 100 mm NaF, 17.5 mm β-glycerophosphate). The low speed supernatant (500 × g) containing cytoplasmic proteins was collected, and nuclear extracts were made by vortexing the nuclei for 15 min at 4 °C in a buffer containing 20 mm HEPES (pH 7.9), 400 mm NaCl, 1 mm EDTA, 1 mm EGTA, 0.1% IGEPAL CA-630, and protease inhibitors. Protein concentrations were measured using the bicinchoninic acid protein assay reagent kit (Pierce Chemical Co). Equal amounts of protein lysates from various samples were used for SDS-PAGE analysis followed by immunoblotting. Specific signals on immunoblots (polyvinylidene difluoride) were visualized using enhanced chemiluminescence (Super-Signal; Pierce Chemical Co.).
GST Pulldown Assay
To investigate the interaction between PTEN and Plk1, HeLa cells treated with nocodazole for 14 h were lysed in TBSN buffer (20 mm Tris-Cl (pH 8.0), 150 mm NaCl, 0.5% Nonidet P-40, 5 mm EGTA, 1.5 mm EDTA, 0.5 mm Na3VO4, and 20 mm β-glycerol phosphate). The cell lysates were clarified by centrifugation at 15,000 × g for 20 min at 4 °C. Cleared lysates (1 mg) were added to either bead-bound GST (5 μg) or bead-bound GST-PTEN fusion protein (5 μg) followed by incubation in the TBSN buffer for 3 h at 4 °C. After incubation, proteins bound to each resin were washed extensively with the binding buffer, eluted in the SDS-PAGE sample buffer, and analyzed by SDS-PAGE followed by immunoblotting with anti-GST or Plk1 antibody.
Expression and Purification of Fusion Proteins
Overnight bacterial cultures (5 ml) of individual clones expressing GST-tagged PTEN protein or its mutants were inoculated into 100 ml of LB medium containing ampicillin (100 μg/ml) followed by incubation at 37 °C for 4 h in a shaking incubator. Protein expression was induced by the addition of isopropyl-1-thio-β-d-galactopyranoside (0.1 mm). After incubation at room temperature for 2 h, cells were harvested by centrifugation and resuspended in 2 ml of sonication buffer (50 mm Tris (pH 7.5) 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Nonidet P-40, 200 μg/ml lysozyme, 1 mm DTT). Cells were lysed with sonication. Cellular debris was removed by centrifugation, and the supernatants were incubated with glutathione-Sepharose beads (GE Healthcare) on a rotating platform at 4 °C overnight. The beads were washed three times with wash buffer A (50 mm Tris (pH 7.5), 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, and 1 mm DTT) and three times with wash buffer B (50 mm Tris (pH 7.5), 100 mm NaCl, and 1 mm DTT). Glutathione beads were resuspended in 50 μl of a buffer (50 mm Tris (pH 7.4), 150 mm NaCl, 1 mm DTT and 10 mm reduced glutathione) for 30 min at room temperature to elute GST fusion proteins. The quantity of the isolated fusion proteins was assessed by SDS-PAGE and Coomassie Blue staining.
Kinase Assays
Plk1 kinase assays were carried out essentially using the protocol described (10). Briefly, recombinant His6-Plk1 protein (Sigma) was added to the kinase assay buffer (20 mm HEPES (pH 7.4), 2 mm EGTA, 10 mm MgCl2, 5 mm MnCl2, 1 mm DTT). Substrates (PTEN or casein) were then added to each reaction mixture to achieve a total volume of 20 μl. Kinase reactions were initiated by the addition of 5 μl of either the radioactive ATP mix (50 mm MgCl2, 0.1 mm ATP, 5 μCi of [γ-32P]ATP) or the cold ATP mix (50 mm MgCl2, 5 mm ATP). After incubation for 30 min at 30 °C, SDS sample buffer was supplemented to each reaction. Kinase assay samples were fractionated on SDS-polyacrylamide gels, and the fractionated proteins were transferred to PVDF membranes followed by autoradiography or immunoblotting. To detect phosphorylation of PTEN (p-PTENT/S or p-PTENS/T/T) by Plk1, His-tagged PTEN recombinant protein was first dephosphorylated using λ-phosphatase (New England Biolabs) according to the protocol provided by the supplier. Five hundred μl of immunoprecipitation buffer (20 mm HEPES (pH 7.4), 120 mm NaCl, 1% Triton X-100, 2 mm EGTA, 10% glycerol, 1 mm DTT, 500 μm PMSF, 2 μm pepstatin A, 1 μg/ml aprotinin) and 40 μl of nickel-nitrilotriacetic acid resin (50:50) was then added to the reaction. The mixture was incubated with rotation at 4 °C for 3 h. After incubation, the resin was washed three times with the immunoprecipitation buffer, three times with the kinase buffer (20 mm HEPES (pH 7.2), 2 mm EGTA, 10 mm MgCl2, 5 mm MnCl2, 1 mm DTT, 1 mm Na3VO4), and then resuspended in the kinase buffer as a 50:50 (v/v) slurry. The suspension was then used as substrate for the kinase assay containing Plk1. After the reaction, the reaction mixtures were subjected to the immunoblotting analysis with antibodies to p-PTENThr-366/Ser-370, p-PTENSer-380/Thr-382/Thr-383, or total PTEN.
Genotyping of PLK1 Knockout Mice
MEFs were isolated from 13.5-day-old embryos. Genomic DNA was isolated from mouse tails mice and embryos, as well as from MEF cells. Genotyping was performed by polymerase chain reaction (PCR) using three primers including the sense primer for both wild-type (WT) and knock-out (KO) alleles (5′-TATTTCCGCAATTACATGAGTGAGC-3′), the antisense primer for WT allele (5′-TAGCAGAGTGAAGGGGCACCAGTCC-3′), and the antisense primer for KO allele (5′-CTCCTACATAGTTGGCAGTGTTTGG-3′). PCR products were analyzed on agarose gels.
RESULTS
PTEN Has a Role in Mitosis
Cytoplasmic PTEN has a well known function as a negative regulator of the PI3K/Akt signaling axis (5). Recent studies have shown that PTEN is also implicated in regulating APC/C activity (36) and maintaining chromosomal stability (4, 36). We first asked whether PTEN had a clear mitotic role. HeLa cells transfected with PTEN siRNAs, or control siRNAs, were arrested in mitosis after nocodazole treatment. Mitotic cells were then released into the cell cycle. Down-regulation of PTEN significantly accelerated the degradation of cyclin B, as well as the disappearance of phosphorylated histone H3 (p-H3) during mitotic release (Fig. 1A). The half-life of cyclin B was about 1.5 h in cells with PTEN down-regulation whereas it was close to 3 h in control cells (Fig. 1B). These observations strongly suggest that PTEN has an important function in regulating mitotic progression.
FIGURE 1.
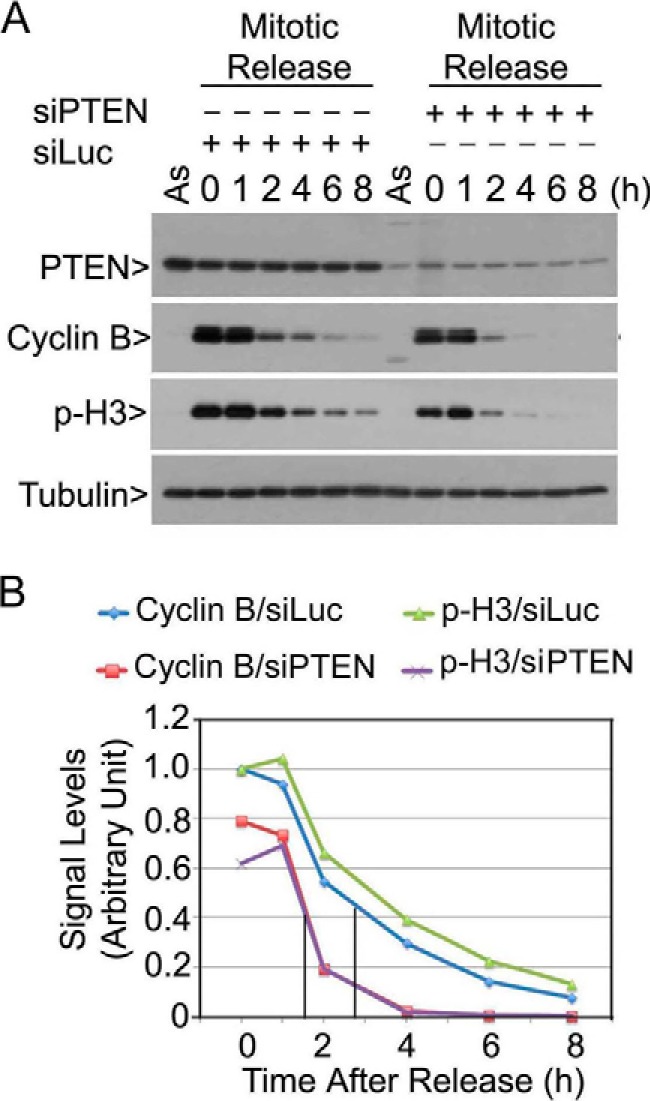
PTEN is important for regulating mitotic timing. A, HeLa cells transfected with PTEN siRNAs (siPTEN) or luciferase siRNA (siLuc) for 1 day were treated with nocodazole for 14 h. Mitotic cells collected by shake-off were released into the cell cycle. At various times after release, cells were collected, and equal amounts of cell lysates, along with lysates from asynchronized cells (As), were prepared and blotted for PTEN, cyclin B, p-H3, and α-tubulin. Experiments were repeated at least three times, and representative data are shown. B, cyclin B and p-H3 signals in cells transfected with siPTEN or siLuc, as shown in A, were quantified, and the summarized data were plotted.
Plk1 Phosphorylates PTEN in Vitro
Given that PTEN is heavily phosphorylated at the C terminus (8, 38), we attempted to identify a mitotic kinase(s) that might play a role in the regulation of PTEN phosphorylation and its function during mitosis. When HeLa cells synchronized at the G1/S junction were released into the cell cycle, we observed that total PTEN and phosphorylated PTEN (epitope Ser-380/Thr-382/Thr-383, abbreviated as S/T/T) exhibited a gradual increase during the release, plateauing around 11 h after release (Fig. 2A). Phosphorylated PTEN detected by the antibody to the second epitope (Thr-366/Ser-370, abbreviated as T/S) was also up-modulated during the release. Oscillations in the levels of cyclin E (S phase), cyclin B (M phase), and Plk1 (M phase) confirmed that the cell cycle release was efficient.
FIGURE 2.
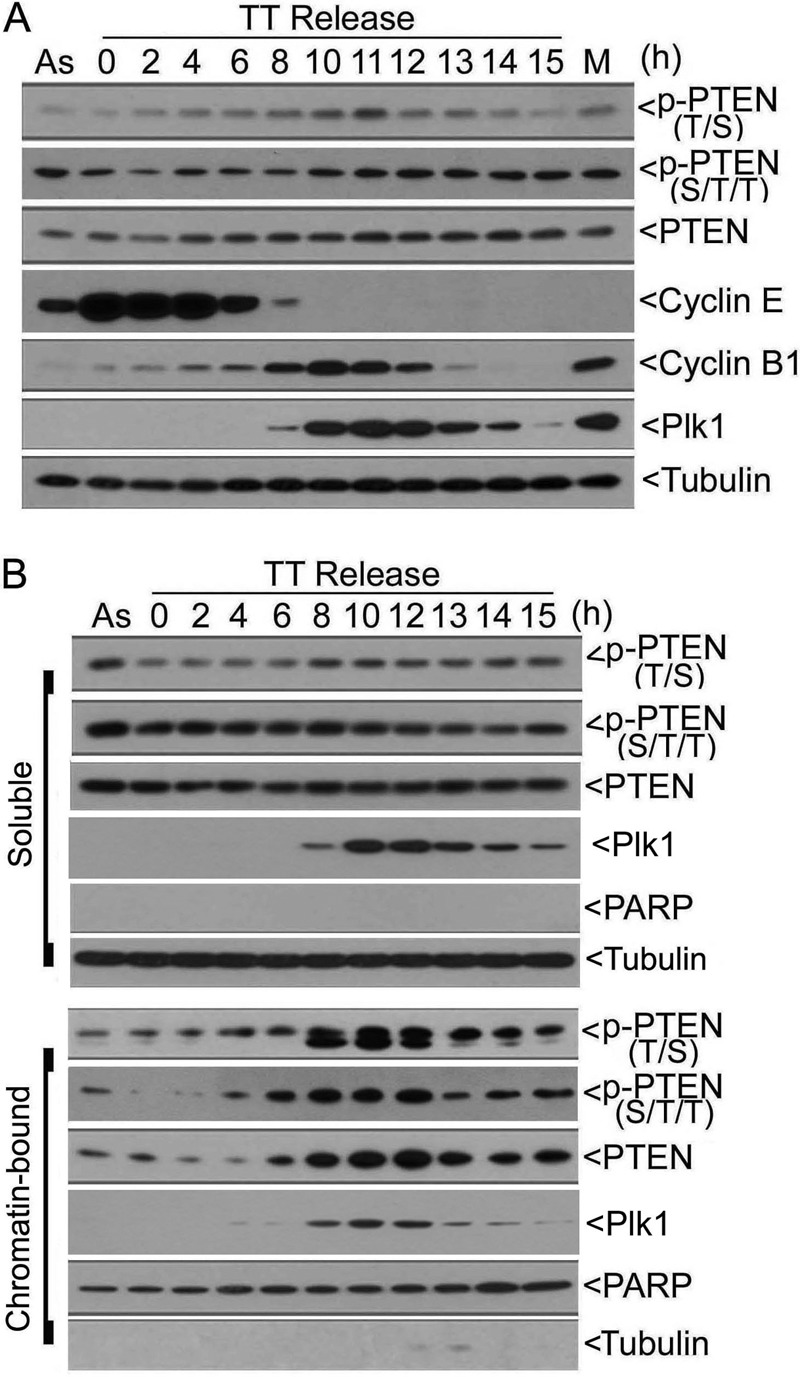
PTEN phosphorylation is closely correlated with its chromatin association during mitosis. A, HeLa cells synchronized at the G1/S junction by the double-thymidine (TT) treatment were released into the cell cycle. At various times of release, cells were collected, and equal amounts of lysates were blotted with antibodies to phosphorylated PTEN on two separate epitopes p-PTENT/S and p-PTENS/T/T, total PTEN, cyclin E, cyclin B, Plk1, and α-tubulin. B, HeLa cells synchronized at the G1/S junction by the double-thymidine block were released into the cell cycle. At various times of release, cells were collected and fractionated into soluble and chromatin-bound fractions. Equal amounts of lysates from both fractions were blotted for phospho-PTEN, total PTEN, Plk1, PARP, and α-tubulin.
We then examined phosphorylated PTEN, as well as the total PTEN, in both soluble and chromatin-bound fractions during the release. Neither total PTEN nor phosphorylated PTEN underwent significant changes in the soluble fraction. However, total PTEN in the chromatin-bound fraction, as well as its phosphorylated counterparts, exhibited a time-dependent increase (Fig. 2B). Whereas p-PTENT/S signals peaked around 8–10 h after release, p-PTENS/T/T signals reached the plateau at about 12 h of the release, roughly paralleling the mitotic increase of the total PTEN and Plk1 in the chromatin fraction. The observations that the signals of two discrete phospho-epitopes peaked at different times also suggest the involvement of separate protein kinases in regulating PTEN. Blotting with antibodies to PARP (primarily chromatin) and α-tubulin (primarily soluble) indicated that the cellular fractionation process was efficient.
Given that Plk1 is a major mitotic kinase whose increase correlated with PTEN accumulation in the chromatin fraction (Fig. 2B), we first determined the physical interaction between PTEN and Plk1. Pulldown analysis revealed that GST-PTEN, but not GST alone, was capable of binding to Plk1 from HeLa cell lysates (Fig. 3A). We next determined whether PTEN was a direct substrate of Plk1. In vitro protein kinase assays showed that Plk1 catalyzed a concentration-dependent incorporation of 32P into recombinant PTEN and α-casein (Fig. 3B), the latter being a well known substrate for kinases of the POLO family. Inclusion of Plk1 in the kinase assay greatly increased p-PTENS/T/T signals (Fig. 3C), suggesting that Plk1 phosphorylates one or more residue(s) within the cluster. Moreover, incorporation of 32P into PTEN catalyzed by Plk1 was inhibited by Bi2536, a Plk1-specific inhibitor (39), and the inhibition was concentration-dependent (Fig. 3D). However, the incorporation of 32P into PTEN catalyzed by Plk3 was largely unaffected by Bi2536. Immunoblotting showed that Plk1 primarily phosphorylated the epitope of S/T/T and that its phosphorylation was inhibited by Bi2536 (Fig. 3E). Consistent with an earlier study (10), Plk3 primarily phosphorylated the T/S epitope, and its phosphorylation was not inhibited by Bi2536 (Fig. 3E).
FIGURE 3.
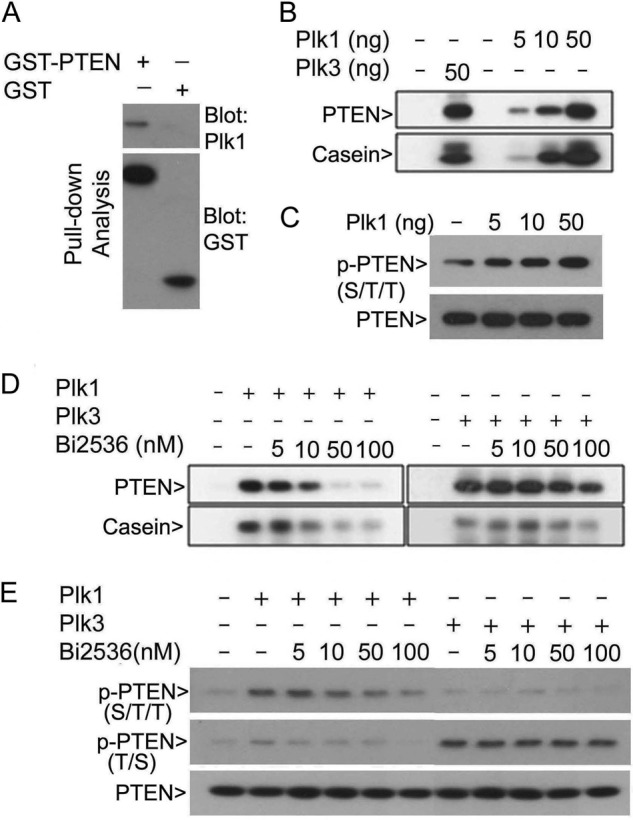
Plk1 specifically phosphorylates PTEN in vitro. A, purified GST or GST-PTEN immobilized onto glutathione resin was incubated with HeLa cell lysates. Proteins specifically bound to the resin were eluted and blotted for both Plk1 and GST. B, purified recombinant PTEN was incubated with various concentrations of Plk1 in the kinase reaction buffer containing [γ-32P]ATP. After the reaction, kinase assay samples were fractioned by SDS-PAGE followed by autoradiography. Plk3 and α-casein were used as positive controls for the kinase assay. C, dephosphorylated recombinant PTEN obtained via pretreatment with λ-phosphatase was incubated with (or without) various concentrations of Plk1 in the kinase reaction containing cold ATP. After the reaction, the assay samples were fractionated by SDS-PAGE and blotted for p-PTENS/T/T as well as PTEN. D, purified recombinant PTEN or α-casein was incubated with Plk1 or Plk3 in the kinase reaction buffer containing [γ-32P]ATP and various concentrations of Bi2536. After the reaction, the assay samples were fractioned by SDS-PAGE followed by autoradiography. E, dephosphorylated recombinant PTEN was incubated with (or without) Plk1 or Plk3 in the kinase reaction buffer containing various concentrations of Bi2536. After the reaction, the assay samples were fractionated by SDS-PAGE and blotted for p-PTENS/T/T, p-PTENT/S, and PTEN.
Plk1 Phosphorylates PTEN on Ser-380
To systematically identify the site(s) of PTEN phosphorylation by Plk1, we made a series of PTEN deletion constructs (constructs A–C), as well as full-length PTEN constructs harboring individual clusters of mutations within the C-terminal tail (constructs C1–C5) (Fig. 4A). The individual deletion or mutation constructs of PTEN were expressed and purified as GST fusion proteins. Protein kinase assays revealed that Plk1 phosphorylated deletion constructs of D and E, as well as the full-length PTEN (Fig. 4B, Kinase Assay panel), indicating that the phosphorylation site resides in the C-tail of PTEN. Blotting the same gels with the anti-GST antibody revealed that similar amounts of recombinant GST-PTEN and its deletion mutant proteins were used for the assay (Fig. 4B, Anti-GST panel). Additional kinase assays showed that Plk1 significantly phosphorylated full-length GST-PTEN and various full-length mutant proteins except for C4 which harbored mutations of four residues (Fig. 4C, Kinase Assay panel). Given that equal amounts of recombinant GST-PTEN or its mutant proteins were used for the assay (Fig. 4C), this result strongly suggests that Plk1 directly phosphorylates one or more residues with the C4 cluster (Ser-380/Thr-382/Thr-383/Ser-385).
FIGURE 4.
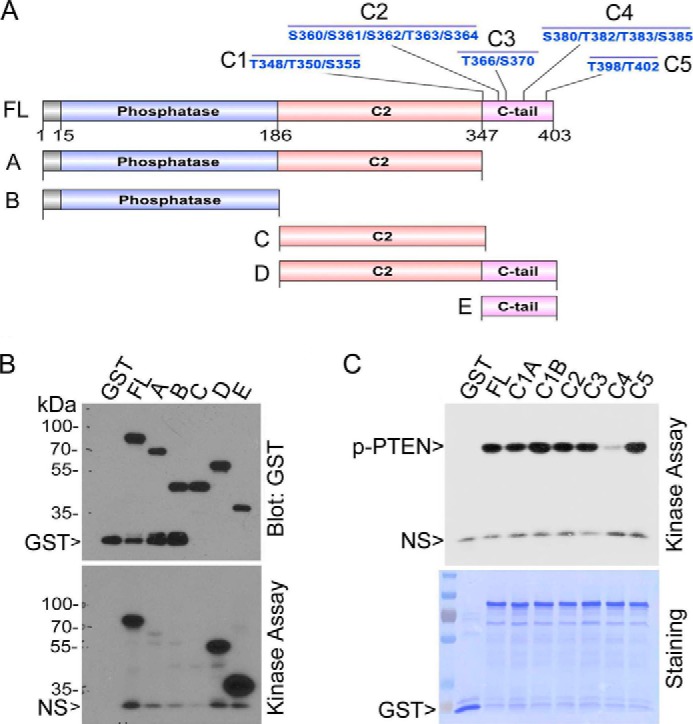
Plk1 phosphorylates PTEN in the C-tail region. A, schematic presentations show various deletion and mutation constructs of PTEN. B, full-length PTEN and various deletion counterparts were expressed and purified as GST fusion proteins. Purified proteins, along with purified GST, were incubated with Plk1 in the assay reaction containing [γ-32P]ATP. After the reaction, the assay samples were fractionated by SDS-PAGE followed by autoradiography (lower panel). The same blot was also probed with the antibody to GST (upper panel). NS denotes a nonspecific band. C, full-length PTEN and various mutational counterparts in the C-tail domain as described in A were expressed and purified as GST fusion proteins. Purified proteins, along with purified GST, were incubated with Plk1 in the kinase reaction containing [γ-32P]ATP. After the reaction, the assay samples were fractionated by SDS-PAGE followed by autoradiography (upper panel). The same blot was also stained with Coomassie Blue (lower panel). NS denotes a nonspecific band.
We then made and purified additional GST-PTEN fusion proteins with mutations in one or more sites within the C4 cluster. Mutations of Ser-380, Thr-382, or Thr-383, but not Ser-385, greatly reduced the incorporation of 32P (Fig. 5A), strongly suggesting that Plk1 may target these three sites in vitro. Consistent with early studies (10), mutations in all four sites (4A) also greatly reduced the Plk1-mediated phosphorylation in vitro.
FIGURE 5.
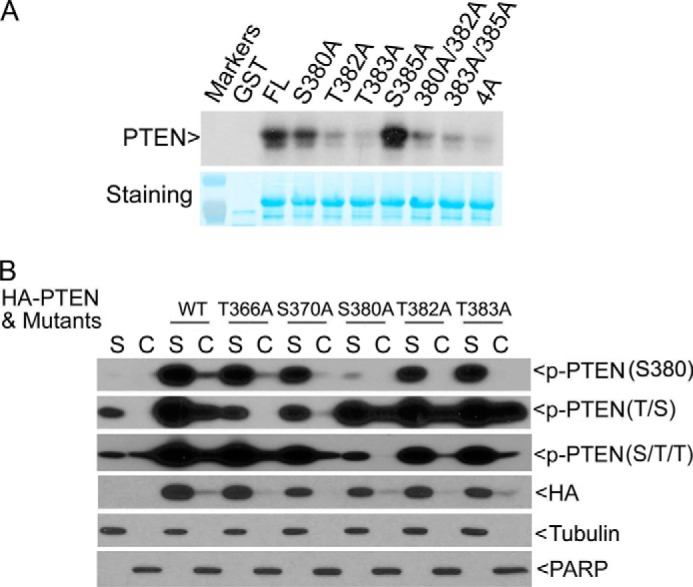
Ser-380 of PTEN is the major site of phosphorylation in vivo. A, full-length PTEN and various mutational counterparts as indicated were expressed and purified as GST fusion proteins. Purified proteins, along with GST, were incubated with Plk1 in the kinase buffer containing [γ-32P]ATP. After the reaction, assay samples were fractionated by SDS-PAGE followed by autoradiography (upper panel). The same blot was also stained with Coomassie Blue (lower panel). B, PTEN mutants harboring individual point mutations at the designated sites were made by replacing serine or threonine residue with alanine. HA-tagged full-length PTEN or its corresponding mutants were transfected into HeLa cells for 2 days. Soluble (S) and chromatin (C) fractions of cell lysates were obtained from each transfection, and equal amounts of proteins were blotted for p-PTENSer-380, p-PTENS/T/T, p-PTENT/S, HA tag, α-tubulin, and PARP.
PTEN Ser-380 Is Phosphorylated in Vivo
To determine which residue(s) within the C4 cluster was the major site of phosphorylation in vivo, we made and transfected HA-tagged constructs expressing full-length PTEN or individual mutants with single amino acid substitutions into HeLa cells (which express wild-type Plk1). Transfected cells were fractionated into soluble and chromatin fractions, which were subsequently blotted for PTEN phosphorylation, as well as for ectopically expressed total PTEN. Mutation of Ser-380 alone greatly reduced the signals detected by the phospho-specific antibody against the p-PTENSer-380 epitope or against the S/T/T epitope (Fig. 5B), indicating that Ser-380 is a major site of phosphorylation in vivo. Intriguingly, mutations of either Thr-382 or Thr-383 did not significantly reduce the amount of signals detected by the phospho-antibody against the S/T/T epitope, suggesting that Thr-382 and Thr-383 may not be major sites of phosphorylation in vivo. Thr-382 or Thr-383 mutation also had a weak affect on reducing p-PTENSer-380 on chromatin compared with WT control. Mutation of either Thr-366 or Ser-370 diminished the phospho-signals (the T/S epitope) compared with ectopically expressed wild-type PTEN; intriguingly, mutation of either residue, but not any other residues, essentially abolished p-PTENT/S signals in the nuclear fraction (Fig. 5B), suggesting that their phosphorylation may regulate nuclear entry or retention of PTEN.
Plk1 Regulates PTEN Phosphorylation and Chromatin Association
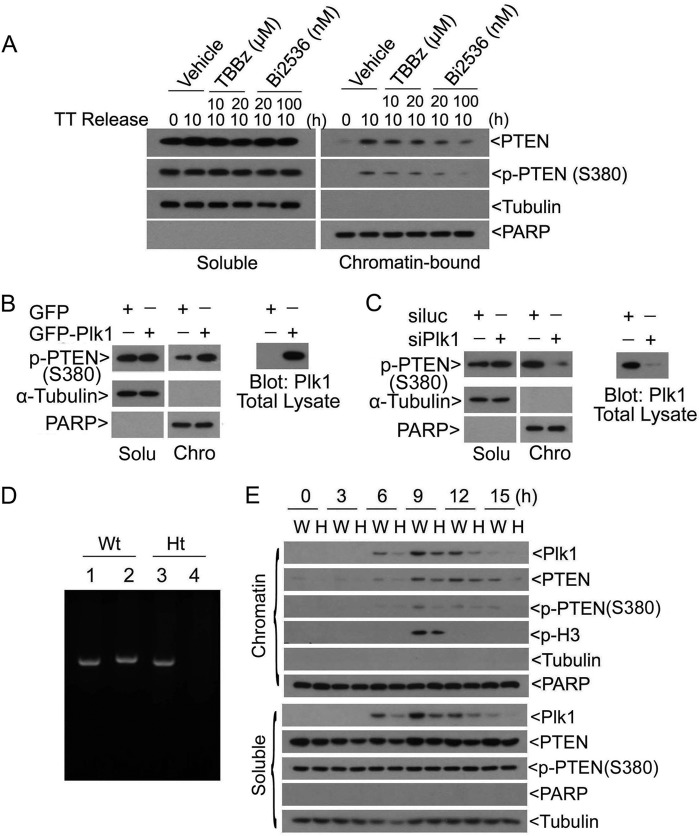
To determine whether Plk1 regulated PTEN phosphorylation on Ser-380 in vivo, we determined PTEN phosphorylation in cells with altered Plk1 activities. We first treated cells with Bi2536, a Plk1-specific inhibitor, at two different concentrations. As a control, we also treated cells with a CK2 inhibitor, TBBz, as CK2 is reported to phosphorylate several residues including Ser-380 in the C-tail region (8, 9). Because chromatin-bound PTEN peaks at approximately 10 h after double-thymidine release (roughly at the peak of mitosis), we collected both soluble and chromatin fractions from cells treated with Plk1 inhibitor, CK2 inhibitor, or vehicle for 10 h. Immunoblotting revealed that Bi2536, but not TBBz, significantly reduced both total PTEN and its Ser-380 phosphorylation in the chromatin fraction (Fig. 6A). No significant changes in PTEN levels, or Ser-380 phosphorylation, in cells treated with either CK2 or Plk1 inhibitor were observed in the soluble fraction.
FIGURE 6.
Plk1 regulates PTEN phosphorylation on Ser-380 in vivo. A, HeLa cells synchronized at the G1/S junction by the double-thymidine (TT) treatment were released into the cell cycle. Cells were treated with or without CK2 or Plk1 inhibitor or vehicle for 10 h immediately after release. At the end of the treatment, cells were collected and fractionated into soluble (S) and chromatin (C) fractions. Equal amounts of lysates from both fractions were blotted for p-PTENSer-380, total PTEN, PARP, and α-tubulin. B, HeLa cells transfected with GFP-Plk1 or GFP alone for 2 days were collected and fractionated into soluble and chromatin fractions. Equal amounts of proteins from both fractions were blotted for p-PTENSer-380, α-tubulin, and PARP. The total cell lysates were also blotted for Plk1 (right panel). C, HeLa cells transfected with PLK1 siRNA (siPlk1) or luciferase siRNA (siLuc) for 2 days were collected and fractionated into soluble (Solu) and chromatin (Chro) fractions. Equal amounts of proteins from both fractions were blotted for p-PTENSer-380, α-tubulin, and PARP. The total cell lysates were also blotted for Plk1 (right panel). D, PLK1 genotyping was carried out using the PCR approach. Lanes 1 and 2 represent PLK1 heterozygous mouse genotyping. Lanes 3 and 4 represent wild-type mouse genotyping. Samples in lanes 1 and 3 were performed with wild-type and knock-out sense primers and a wild-type antisense primer. Samples in lanes 2 and 4 were from PCRs using wild-type and knock-out sense primers and a knock-out antisense primer. Primer information is as described under “Materials and Methods.” E, MEFs isolated from both wide-type (W) and PLK1 haploinsufficient (H) embryos were synchronized at the G1/S junction by thymidine-aphidicolin block. These cells were then released into the cell cycle by culturing in fresh medium. At various times after release, cells were collected and fractionated into cytoplasmic and chromatin fractions. Equal amounts of proteins from both fractions were blotted for Plk1, total PTEN, p-PTENSer-380, phospho-H3, α-tubulin, and PARP.
To confirm that Plk1 regulated PTEN phosphorylation in vivo, we transfected HeLa cells with a plasmid expressing GFP-Plk1. Ectopic expression of GFP-Plk1, but not GFP alone,specifically enhanced p-PTENSer-380 signals in the chromatin fraction but not in the soluble fraction (Fig. 6B). Expression of GFP-Plk1 was confirmed with immunoblotting. Consistent with this observation, transfection of PLK1 siRNAs greatly reduced p-PTENSer-380 signals in the chromatin fraction but not in the soluble fraction (Fig. 6C). Depletion of endogenous Plk1 was also confirmed with immunoblotting.
To further confirm that Plk1 regulated phosphorylation of PTEN on Ser-380 in vivo, we obtained primary MEFs with deletion of one PLK1 allele (Fig. 6D) (32). Paired MEFs were first blocked at the G1/S junction after double-thymidine treatment. Haploinsufficiency of PLK1 resulted in significant reduction of total PTEN, as well as p-PTENSer-380 signals, in both soluble and chromatin fractions (Fig. 6E). When MEFs were released into the cell cycle, Plk1 accumulation was closely associated with the amount of chromatin PTEN that peaked at mitosis as revealed by p-H3 signals. Significantly, levels of chromatin-bound PTEN and its Ser-380 phosphorylation were closely correlated with the PLK1 status. Combined, these results strongly suggest that Plk1 specifically phosphorylates PTEN on Ser-380 in vivo and that the phosphorylation may positively regulate chromatin association of PTEN.
PTEN Phosphorylation on Ser-380 Is Important for Its Role in Mitosis
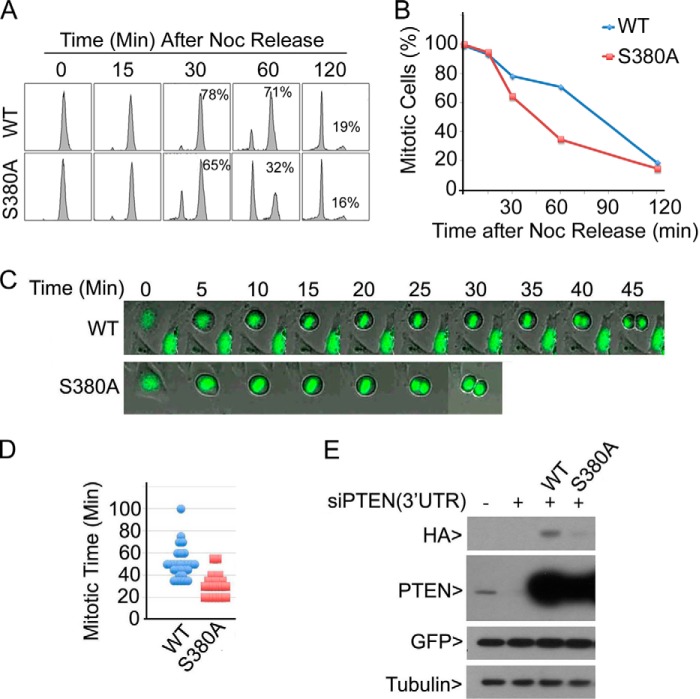
To determine whether p-PTENSer-380 alone plays a major role in regulating mitotic progression, cells were co-transfected with PTEN siRNAs (3′-UTR) and an HA-PTEN (WT) or HA-PTENS380A expression construct and treated with nocodazole. Mitotic cells were collected and then released into the cell cycle. Flow cytometric analysis revealed that PTENS380A expression led to significant reduction of mitotic cells, starting at approximately 30 min after release, which was correlated with an increase in G1 population (Fig. 7, A and B). This observation thus suggests that Ser-380 phosphorylation is important for the PTEN role in normal mitotic progression.
FIGURE 7.
Ser-380 phosphorylation of PTEN is important for normal mitotic progression. A, HeLa cells were co-transfected with PTEN siRNAs (corresponding to 3′-untranslated regions (3′UTR)) and a plasmid expressing HA-PTEN (WT) or HA-PTENS380A for 24 h followed by treatment with nocodazole (Noc) for 16 h. Mitotic cells were collected, washed, and released into the cell cycle for various times. Cells were then processed for flow cytometry. Percentages of mitotic cells are indicated. Experiments were repeated three times, and representative data are shown. B, mitotic cells from each time as shown in A were quantified, and the summarized data are plotted. C, HeLa cells seeded in chamber slides were co-transfected with 3′-UTR PTEN siRNAs and expression plasmids of HA-PTEN (or HA-PTENS380A) and GFP-H2B (at 1/10 of concentration) for 24 h. Mitotic cells were monitored by time lapse confocal microscopy. At least 50 mitotic cells were monitored for each transfection. Representative cells are shown. D, quantification of mitotic times of cells spent in mitosis. The summarized data are plotted. E, equal amounts of cell lysates from experiments as shown in A and C were blotted with antibodies to HA, PTEN, GFP, and α-tubulin.
We further analyzed mitotic progression of cells with depletion of endogenous PTEN but expressing transfected HA-PTEN or HA-PTENSer-380 via time lapse confocal microscopy. We observed that cells expressing PTENS380A underwent rapid mitotic progression and exit (Fig. 7C). The average mitotic time for cells expressing the mutant was 35 min whereas cells expressing PTEN spent approximately 55 min in mitosis (Fig. 7D). Blotting analysis revealed that knocking down of endogenous PTEN and expression of transfected PTEN plasmid constructs were efficient (Fig. 7E). We consistently observed that PTENS380A was expressed at a lower level than that of WT PTEN, suggesting that Ser-380 phosphorylation may play a role in stabilizing PTEN during mitosis.
DISCUSSION
Our current study reveals a previously unrecognized role of Plk1 in the negative regulation of cell proliferation through phosphorylation of PTEN. To date, Plk1 has been largely considered as an oncogene, promoting cell proliferation and malignant transformation (13). However, there are several reports in the literature that are consistent with a potential inhibitory role of Plk1 in cell growth. Plk3 and Plk4, members of the POLO family sharing significant sequence homology with Plk1, function both as negative regulators for cell proliferation and as suppressors for tumor development (33, 34). Moreover, mutations in PLK1 are reported in HepG2, A431, MKN74, and A549 (31), all of which are tumor-derived cell lines, suggesting that mutations confer certain advantages for the transformed phenotype. Furthermore, haploinsufficiency of PLK1 leads to enhanced development of tumors in mice (32). Therefore, the biological activities of Plk1 are likely to be more complicated than originally thought.
Research in the past has identified several protein kinases that phosphorylate PTEN in the C-tail region (2, 7). Glucose synthase kinase 3β phosphorylates Ser-362 and Thr-366 whereas CK2 phosphorylates Ser-370 and Ser-385 (8). Although one study reported that CK2 also phosphorylates Ser-380 (9) the data that support this claim are rather weak in that study. We have directly compared the effect of Plk1 and CK2 on phosphorylating Ser-380 of PTEN and found that Plk1 is the primary protein kinase that phosphorylates Ser-380 residue and regulates chromatin-association of PTEN because Plk1 inhibitor, but not the CK2 inhibitor, compromises PTEN association with chromatin (Fig. 6A). Plk3 also phosphorylates Ser-370, and its phosphorylation stabilizes PTEN in vivo (10). Intriguingly, Plk1 and Plk3 exhibit rather mutually exclusive substrate specificity even through both kinases target PTEN. Plk1 does not significantly phosphorylate Ser-370; similarly, Plk3 catalyzes little phosphorylation of Ser-380 (Fig. 3E). Several previous reports show that the C-terminal domain of PTEN is important for its stability as well as the phosphatase activity (9, 10, 40). Deletion of C-terminal region or mutations of phosphorylation sites within the region including Thr-366, Ser-370, and 4A cluster (Ser-380, Thr-382, Thr-383, Ser-385) all lead to reduced stability of PTEN (9, 10, 40). Our current study reveals that Ser-380 is a key residue, phosphorylation of which stabilizes chromatin PTEN. During the cell cycle, Plk1 is regulated primarily at the protein level although it is subjected to regulation by phosphorylation (14, 22). During mitosis, Plk1 undergoes dramatic subcellular translocation. Plk1 associates with centrosomes in prophase and then is enriched at kinetochores from prometaphase to metaphase. Subsequently, Plk1 translocates to the central spindle and then accumulates in the midbody during late mitosis. Given that PTEN also exhibits kinetochore/centromere localization (41), it is tempting to speculate that Plk1 may directly interact and phosphorylate PTEN on centromeres.
Amino acid residues of Ser-380, Thr-382, Thr-383, and Ser-385 of PTEN consist of an epitope whose phosphorylation can be detected by a specific antibody commercially available (Cell Signaling; 9549). Mutational analysis followed by in vitro kinase assays revealed that Plk1 displayed a weakened activity toward PTENS380A compared with wild-type PTEN (Fig. 5A). Intriguingly, Plk1 also exhibited a compromised activity toward PTENT382A and PTENT383A in in vitro kinase assays (Fig. 5A). However, ectopically expressed PTENT382A and PTENT383A, but not PTENS380A, in HeLa cells remained significantly phosphorylated as detected by the phospho-specific antibody to the S/T/T epitope (Fig. 5B), suggesting that Thr-382 and Thr-383 may not be major sites of phosphorylation in vivo. Therefore, it is possible that the compromised activity of Plk1 toward PTENT382A and PTENT383A in vitro could be due to an altered structure of these mutant proteins rather than the lack of targeted sites. Obviously, future studies should focus on the function of Thr-382 and Thr-383 in regulating PTEN activity in vivo.
Our current study also confirms that PTEN has a significant role in regulating mitotic progression and that Ser-380 phosphorylation by Plk1 mediates PTEN mitotic function. Although the mechanism by which PTEN regulates mitotic progresses remains unclear, several studies suggest that nuclear/chromatin PTEN may be involved in modulating activities of genes involved in homologous recombination (41, 42). Therefore, it would be interesting to determine whether PTEN has a function in mediating mitotic recombination.
Acknowledgments
We thank Dr. Dazhong Xu for valuable discussions; Xianan Liu for assistance during the course of the work; Dr. Kyung Lee at the NCI, National Institutes of Health, and Xiaoqi Liu, Purdue University, for providing the Plk1 protein; and Dr. Xiaochun Yu, University of Michigan, for PLK1 knock-out mice.
This work was supported, in whole or in part, by United States Public Service Awards CA090658 and CA150512 (to W. D.) and National Institutes of Health NIEHS Center Grant ES000260 and Grants R01-GM057587 and R21-AG032560 (to M. P.).
- PTEN
- phosphatase and tensin homolog
- C-tail
- C-terminal tail
- CK2
- casein kinase 2
- MEF
- murine embryonic fibroblast
- p-H3
- phosphorylated histone H3
- p-PTENS/T/T
- phospho-PTENSer-380/Thr-382/Thr-383
- p-PTENT/S
- phospho-PTENThr-366/Thr-370
- PARP
- poly(ADP-ribose) polymerase
- TBBz
- 4,5,6,7-tetrabromobenzimidazole.
REFERENCES
- 1. Di Cristofano A., Pandolfi P. P. (2000) The multiple roles of PTEN in tumor suppression. Cell 100, 387–390 [DOI] [PubMed] [Google Scholar]
- 2. Salmena L., Carracedo A., Pandolfi P. P. (2008) Tenets of PTEN tumor suppression. Cell 133, 403–414 [DOI] [PubMed] [Google Scholar]
- 3. Trimboli A. J., Cantemir-Stone C. Z., Li F., Wallace J. A., Merchant A., Creasap N., Thompson J. C., Caserta E., Wang H., Chong J. L., Naidu S., Wei G., Sharma S. M., Stephens J. A., Fernandez S. A., Gurcan M. N., Weinstein M. B., Barsky S. H., Yee L., Rosol T. J., Stromberg P. C., Robinson M. L., Pepin F., Hallett M., Park M., Ostrowski M. C., Leone G. (2009) PTEN in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin Y., Shen W. H. (2008) PTEN: a new guardian of the genome. Oncogene 27, 5443–5453 [DOI] [PubMed] [Google Scholar]
- 5. Carracedo A., Pandolfi P. P. (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27, 5527–5541 [DOI] [PubMed] [Google Scholar]
- 6. Berger A. H., Pandolfi P. P. (2011) Haplo-insufficiency: a driving force in cancer. J. Pathol. 223, 137–146 [DOI] [PubMed] [Google Scholar]
- 7. Wang X., Jiang X. (2008) PTEN: a default gate-keeping tumor suppressor with a versatile tail. Cell Res. 18, 807–816 [DOI] [PubMed] [Google Scholar]
- 8. Al-Khouri A. M., Ma Y., Togo S. H., Williams S., Mustelin T. (2005) Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3β. J. Biol. Chem. 280, 35195–35202 [DOI] [PubMed] [Google Scholar]
- 9. Torres J., Pulido R. (2001) The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276, 993–998 [DOI] [PubMed] [Google Scholar]
- 10. Xu D., Yao Y., Jiang X., Lu L., Dai W. (2010) Regulation of PTEN stability and activity by Plk3. J. Biol. Chem. 285, 39935–39942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yim E. K., Peng G., Dai H., Hu R., Li K., Lu Y., Mills G. B., Meric-Bernstam F., Hennessy B. T., Craven R. J., Lin S. Y. (2009) Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell 15, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barr F. A., Silljé H. H., Nigg E. A. (2004) Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5, 429–440 [DOI] [PubMed] [Google Scholar]
- 13. Strebhardt K., Ullrich A. (2006) Targeting polo-like kinase 1 for cancer therapy. Nat. Rev. Cancer 6, 321–330 [DOI] [PubMed] [Google Scholar]
- 14. Strebhardt K. (2010) Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 9, 643–660 [DOI] [PubMed] [Google Scholar]
- 15. Liu X., Erikson R. L. (2003) Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smits V. A., Klompmaker R., Arnaud L., Rijksen G., Nigg E. A., Medema R. H. (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2, 672–676 [DOI] [PubMed] [Google Scholar]
- 17. Liu X. S., Li H., Song B., Liu X. (2010) Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery. EMBO Rep. 11, 626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassermann F., Frescas D., Guardavaccaro D., Busino L., Peschiaroli A., Pagano M. (2008) The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134, 256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Vugt M. A., Brás A., Medema R. H. (2004) Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell 15, 799–811 [DOI] [PubMed] [Google Scholar]
- 20. Fairley J. A., Mitchell L. E., Berg T., Kenneth N. S., von Schubert C., Silljé H. H., Medema R. H., Nigg E. A., White R. J. (2012) Direct regulation of tRNA and 5S rRNA gene transcription by polo-like kinase 1. Mol. Cell 45, 541–552 [DOI] [PubMed] [Google Scholar]
- 21. Liu X., Erikson R. L. (2002) Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. U.S.A. 99, 8672–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petronczki M., Lénárt P., Peters J. M. (2008) Polo on the rise: from mitotic entry to cytokinesis with Plk1. Dev. Cell 14, 646–659 [DOI] [PubMed] [Google Scholar]
- 23. Eldridge A. G., Loktev A. V., Hansen D. V., Verschuren E. W., Reimann J. D., Jackson P. K. (2006) The evi5 oncogene regulates cyclin accumulation by stabilizing the anaphase-promoting complex inhibitor emi1. Cell 124, 367–380 [DOI] [PubMed] [Google Scholar]
- 24. Jackman M., Lindon C., Nigg E. A., Pines J. (2003) Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5, 143–148 [DOI] [PubMed] [Google Scholar]
- 25. Wang X., Yang Y., Duan Q., Jiang N., Huang Y., Darzynkiewicz Z., Dai W. (2008) sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev. Cell 14, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johmura Y., Soung N. K., Park J. E., Yu L. R., Zhou M., Bang J. K., Kim B. Y., Veenstra T. D., Erikson R. L., Lee K. S. (2011) Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc. Natl. Acad. Sci. U.S.A. 108, 11446–11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soung N. K., Park J. E., Yu L. R., Lee K. H., Lee J. M., Bang J. K., Veenstra T. D., Rhee K., Lee K. S. (2009) Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Dev. Cell 16, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soung N. K., Kang Y. H., Kim K., Kamijo K., Yoon H., Seong Y. S., Kuo Y. L., Miki T., Kim S. R., Kuriyama R., Giam C. Z., Ahn C. H., Lee K. S. (2006) Requirement of hCenexin for proper mitotic functions of polo-like kinase 1 at the centrosomes. Mol. Cell. Biol. 26, 8316–8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loncarek J., Hergert P., Khodjakov A. (2010) Centriole reduplication during prolonged interphase requires procentriole maturation governed by Plk1. Curr. Biol. 20, 1277–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsou M. F., Wang W. J., George K. A., Uryu K., Stearns T., Jallepalli P. V. (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell 17, 344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simizu S., Osada H. (2000) Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat. Cell Biol. 2, 852–854 [DOI] [PubMed] [Google Scholar]
- 32. Lu L. Y., Wood J. L., Minter-Dykhouse K., Ye L., Saunders T. L., Yu X., Chen J. (2008) Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol. Cell. Biol. 28, 6870–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Y., Bai J., Shen R., Brown S. A., Komissarova E., Huang Y., Jiang N., Alberts G. F., Costa M., Lu L., Winkles J. A., Dai W. (2008) Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1α under hypoxic conditions. Cancer Res. 68, 4077–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko M. A., Rosario C. O., Hudson J. W., Kulkarni S., Pollett A., Dennis J. W., Swallow C. J. (2005) Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat. Genet. 37, 883–888 [DOI] [PubMed] [Google Scholar]
- 35. Baker S. J. (2007) PTEN enters the nuclear age. Cell 128, 25–28 [DOI] [PubMed] [Google Scholar]
- 36. Song M. S., Carracedo A., Salmena L., Song S. J., Egia A., Malumbres M., Pandolfi P. P. (2011) Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144, 187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu P. D., Jousse C., Marciniak S. J., Zhang Y., Novoa I., Scheuner D., Kaufman R. J., Ron D., Harding H. P. (2004) Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 23, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller S. J., Lou D. Y., Seldin D. C., Lane W. S., Neel B. G. (2002) Direct identification of PTEN phosphorylation sites. FEBS Lett. 528, 145–153 [DOI] [PubMed] [Google Scholar]
- 39. Steegmaier M., Hoffmann M., Baum A., Lénárt P., Petronczki M., Krssák M., Gürtler U., Garin-Chesa P., Lieb S., Quant J., Grauert M., Adolf G. R., Kraut N., Peters J. M., Rettig W. J. (2007) BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 17, 316–322 [DOI] [PubMed] [Google Scholar]
- 40. Odriozola L., Singh G., Hoang T., Chan A. M. (2007) Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J. Biol. Chem. 282, 23306–23315 [DOI] [PubMed] [Google Scholar]
- 41. Shen W. H., Balajee A. S., Wang J., Wu H., Eng C., Pandolfi P. P., Yin Y. (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 [DOI] [PubMed] [Google Scholar]
- 42. Choi B. H., Chen Y., Dai W. (2013) Chromatin PTEN is involved in DNA damage response partly through regulating Rad52 sumoylation. Cell Cycle 12, 3442–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]