Background: Genomic duplications involving the smooth muscle myosin heavy chain gene, MYH11, are associated with increased risk for acute aortic dissections.
Results: MYH11 overexpression causes increased turnover of contractile proteins through increased autophagy.
Conclusion: MYH11 duplications may predispose to aortic disease through increased turnover of contractile proteins and disruption of contractile signaling.
Significance: Increased protein turnover may be an important mechanism by which genomic duplications cause human disease.
Keywords: Autophagy, ER Stress, Myosin, Protein Turnover, Unfolded Protein Response, Vascular Smooth Muscle Cells, Thoracic Aortic Aneurysms and Dissections
Abstract
Duplications spanning nine genes at the genomic locus 16p13.1 predispose individuals to acute aortic dissections. The most likely candidate gene in this region leading to the predisposition for dissection is MYH11, which encodes smooth muscle myosin heavy chain (SM-MHC). The effects of increased expression of MYH11 on smooth muscle cell (SMC) phenotypes were explored using mouse aortic SMCs with transgenic overexpression of one isoform of SM-MHC. We found that these cells show increased expression of Myh11 and myosin filament-associated contractile genes at the message level when compared with control SMCs, but not at the protein level due to increased protein degradation. Increased expression of Myh11 resulted in endoplasmic reticulum (ER) stress in SMCs, which led to a paradoxical decrease of protein levels through increased autophagic degradation. An additional consequence of ER stress in SMCs was increased intracellular calcium ion concentration, resulting in increased contractile signaling and contraction. The increased signals for contraction further promote transcription of contractile genes, leading to a feedback loop of metabolic abnormalities in these SMCs. We suggest that overexpression of MYH11 can lead to increased ER stress and autophagy, findings that may be globally implicated in disease processes associated with genomic duplications.
Introduction
Genomic deletions and duplications, or copy number variants, are associated with a number of human diseases (1, 2). Although it is easy to understand why gene deletions leading to haploinsufficiency and decreased protein levels can cause disease, the mechanisms linking an extra copy of a gene with pathology are less obvious. Recurrent copy number variants at the chromosomal locus 16p13.1 have been implicated in the pathogenesis of neuropsychiatric disorders, and we have recently reported that duplications but not deletions of this same locus are enriched 11-fold in patients with sporadic thoracic aortic aneurysms and dissections (TAAD)2 (3). Although the size of the duplication can vary, nine genes are commonly duplicated in all TAAD patients, including MYH11. This gene encodes the smooth muscle (SM)-specific myosin heavy chain (MHC) and is the most likely candidate gene for the predisposition to TAAD associated with the 16p13.1 duplication because heterozygous mutations in MYH11 cause a dominantly inherited predisposition to TAAD (4, 5).
Myosin heavy chain molecules require a specific molecular chaperone to ensure proper protein folding. Folding of myosin is critical as monomers of myosin interact with each other as well as the regulatory and essential light chains to form first hexamers containing two of each molecule, and then filaments. Increased expression of myosin heavy chain due to three copies of the gene could reduce access of individual myosin molecules to the required chaperone protein, potentially resulting in misfolded monomers that could disrupt proper myosin filament formation. Unc45 is a myosin heavy chain-specific chaperone that was first described in the nematode Caenhorabditis elegans (6). Mammals have two Unc45 genes, encoding two distinct isoforms of the chaperone called Unc45a and Unc45b (7). Unc45b is specifically expressed in striated muscle and acts as the chaperone for cardiac and skeletal muscle myosins. Unc45a is ubiquitously expressed, and is the canonical chaperone for nonmuscle and smooth muscle myosins. Early work performed on this chaperone in the C. elegans model indicates that the ratio of myosin heavy chain protein levels to Unc45 is critical for proper muscle function. Worms either over- or underexpressing Unc45 show accelerated degradation of myosin heavy chains via the ubiquitin-proteasome system, supporting the hypothesis that the correct ratio between myosin heavy chain and its chaperone is important for stable myosin filament formation (8).
In C. elegans, myosin turnover is accomplished by the ubiquitin-proteasome system of protein degradation (8). Both the ubiquitin-proteasome system and autophagy have also been implicated in degradation of contractile proteins in cardiac and skeletal muscle cells during muscular atrophy (9–11). However, thus far no studies have examined turnover of contractile proteins in mammalian smooth muscle cells (SMCs). Unlike striated muscle cells, SMCs are not terminally differentiated and can de-differentiate and decrease cellular contractile protein levels in response to environmental cues, such as platelet-derived growth factor. Due to the more dynamic nature of SMC contractile structures compared with those in cardiac or skeletal muscle cells, the pathways leading to degradation in SMCs may differ from other muscle cells.
The MYH11 gene encodes four distinct transcripts leading to four isoforms of the SM-MHC protein, two of which are expressed in the aorta (12). Transgenic mouse models overexpressing each of those two isoforms were previously generated and characterized. Despite evidence of successful transgene expression using a tag on the myosin protein, neither transgenic expression of SM1 nor SM2 altered the ratio between the two isoforms in aortic tissue (13). These results suggest that overexpression of myosin leads to selective degradation that normalizes the total amount and isoform ratio of myosin in the cell. In this study, we demonstrate that overexpression of the SM1 isoform of Myh11 in SMCs leads to autophagic turnover of contractile proteins. Surprisingly, autophagy in these cells is not associated with decreased mammalian target of rapamycin (mTOR) signaling, but rather is driven by signaling downstream of the unfolded protein response.
EXPERIMENTAL PROCEDURES
Vascular Smooth Muscle Cell Culture
To isolate primary mouse aortic SMCs, the thoracic descending aorta from the left subclavian branch to the diaphragm was removed under sterile conditions from nontransgenic (NTG) and transgenic (SM1) mice. Cells were explanted and maintained as previously described (14). The identity of these cells as SMCs was verified by mRNA expression of Myh11 and staining for smooth muscle α-actin (mouse monoclonal antibody; Sigma) at each passage (95% of cells stained positive for smooth muscle α-actin). All studies were performed on SMCs at passage 4 or earlier. For all experiments, cells were serum starved in 1% FBS for 24 h prior to the start of the experiment.
Materials
Where indicated, cell cultures were treated with cycloheximide (Sigma), bortezomib (Selleck Chemicals), bafilomycin (Sigma), and 4-phenylbutyric acid (Sigma).
Immunoblot Analyses
Frozen aortic tissue sections from patients or cultured cells were homogenized and lysed in RIPA buffer supplemented with protease inhibitor mixture (Sigma) and phosphatase inhibitor mixture (Sigma). Protein (5 μg) for each sample was separated by SDS-PAGE, transferred to PVDF membrane, and probed with antibodies. Immunoblots were quantitated with ImageJ.
Immunofluorescence in Explanted Aortic SMCs
After reaching confluence, cells were seeded onto coverslips in 6-well plates with the density of 13.15 cells/mm2 for 24 h prior to serum starvation. After 24 h of culture in serum-free SMC medium, cells were stimulated with 10 ng/ml of TGF-β1 for 72 h. Immunofluorescence was carried out as described previously (15). All analyses of immunofluorescent images were performed using the Nikon NIS Elements software. Individual focal adhesion size was calculated for at least 30 adhesions per cell on at least 10 cells per genotype. For MRTF-A analysis, green fluorescent intensity was calculated for nuclear areas (as defined by DAPI staining) compared with cytoplasmic areas.
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted from frozen tissue sections or cultured SMCs and measured by two-step quantitative RT-PCR. Experiments were performed in triplicate. GAPDH was used as the endogenous control.
Radiolabeled Pulse-Chase Assay (16)
Confluent cells were seeded into 35-mm dishes (100,000 cells per dish) and allowed to attach overnight. Media was changed to 1% serum containing medium for 24 h before being switched to pulse media (1% serum containing media plus 0.1 μCi/ml of 14C-labeled phenylalanine). At specified time points throughout the pulse period, media was removed, and cells were lysed in 1 ml of 10% trichloroacetic acid and stored at 4 °C until the end of the experiment. At the end of the pulse period (48 h), the media was changed on the remaining plates to chase media (1% serum containing media plus excess unlabeled phenylalanine, 2 mm). At specified time points during the chase period, a 500-μl aliquot of media was removed and combined with 500 μl of 20% trichloroacetic acid. These aliquots were also stored at 4 °C until the end of the experiment. At the end of the experiment, all lysates were combined with scintillation fluid and analyzed on a 1900TR liquid scintillation analyzer (Packard Instruments). Protein synthesis rates were calculated using the incorporation of 14C into cellular protein over the pulse period, whereas protein degradation rates were calculated using the rate of 14C release into the media over the chase period.
Infection with RFP-GFP-LC3 Lentivirus
Cells were seeded and serum starved as for other immunofluorescence protocols, then treated with 1% serum containing media supplemented with 1 μl/ml (8 μg/ml) of Polybrene and viral titers at a multiplicity of infection of 5, determined through initial dose-dependent experiments, with or without 4-phenyl butyric acid (1 mg/ml). After 72 h of incubation with the virus, cells were fixed in 4%, and coverslips were mounted in Vectashield mounting media with a DAPI counterstain.
Collagen Contraction Assays
Cells were separated into 250,000 cell aliquots and seeded within a matrix of type I collagen from rat tail (BD Biosciences). The collagen mixture was prepared according to the manufacturer's specifications at a final concentration of 1 mg/ml of collagen. Gels were allowed to polymerize at room temperature for at least 1 h, then immediately treated with 1% serum containing media with or without the addition of Rho kinase inhibitor, Y-27632 (Calbiolchem) or myosin light chain kinase inhibitor, ML-7 (Calbiolchem). Gels were allowed to incubate for 5 days to accumulate tension, and then released from attachment to the cell culture plastic to induce contraction. Gels were photographed 10 min after release, and measurements were performed using ImageJ software.
Calcium Imaging
Cells were seeded onto 15-mm coverslips at a density of 5,000 cells per coverslip and allowed to attach overnight. Cells were then incubated in 1% serum media with or without the addition of 1 mg/ml of 4-phenylbutyric acid for 24 h. Coverslips were pre-treated with 4 μm Fura-2AM (Teflabs) for 30 min at 37 °C. Cells were washed with standard extracellular buffer (in mm): 140 NaCl, 5 KCl, MgCl2, 10 HEPES, 10 glucose, 2 CaCl2 (pH 7.4 with NaOH) and then transferred to calcium-free buffer (in mm): 140 NaCl, 5 KCl, MgCl2, 10 HEPES, 10 glucose, 0.2 EGTA (pH 7.4 with NaOH), at room temperature for the remainder of the experiment. Coverslips were mounted for viewing on a Nikon TE200 microscope, and imaging was performed with Incytim2 software (Intracellular Imaging, Inc.). Measurements were taken using excitation wavelengths of 343/380 nm and emission wavelength of 520 nm. Baseline measurements were taken for 1 min, followed by treatment with 1 μm thapsigargin. Measurements of cytoplasmic calcium continued for 6 min to allow maximum endoplasmic reticulum (ER) calcium release from slow responding cells.
G-LISA
RhoA and Rac1 activation were quantified using G-LISA (Cytoskeleton) performed according to the manufacturer's specifications. Briefly, cells were serum-starved in 1% FBS for 24 h, then treated with or without lypophosphatidic acid (Sigma) for 15 min. Cells were lysed in the provided lysis buffer and lysates were snap frozen. Protein (0.5 μg) was loaded per well of the provided ELISA plate, and activated Rac1 and RhoA were quantified by ELISA.
F/G-actin Assay
Polymerization of actin was assayed using an F/G-actin assay (Cytoskeleton) performed according to the manufacturer's specifications. Briefly, after serum starvation cells were lysed and homogenized in the provided lysis buffer. Samples were pelleted in an ultracentrifuge (Optima TLX Ultracentrifuge, Rotor TLA-110, Beckman Coulter) at 55,000 rpm for 1 h at 37 °C. Pellets and supernatant fractions were separately processed and processed by SDS-PAGE for subsequent immunoblot analysis.
Replication and Statistical Analysis
Each experiment included in this article was performed at least four independent times. For quantitative PCR, data from three replicates generated from one set of experiments are included in the figures. For all other quantitative experiments, including densitometry quantitation of immunoblot results, pooled data across all replicates is shown. Where applicable, statistical analysis comparing two groups was performed by Student's t test, and analysis comparing four groups was performed by one-way analysis of variance. p values less than 0.05 were considered significant, and are denoted with asterisks (*) in the figures. Time courses shown in Fig. 1, G and H, were analyzed by linear regression analysis, and the p values are reported in the text. All other time courses were analyzed by two-way analysis of variance.
FIGURE 1.
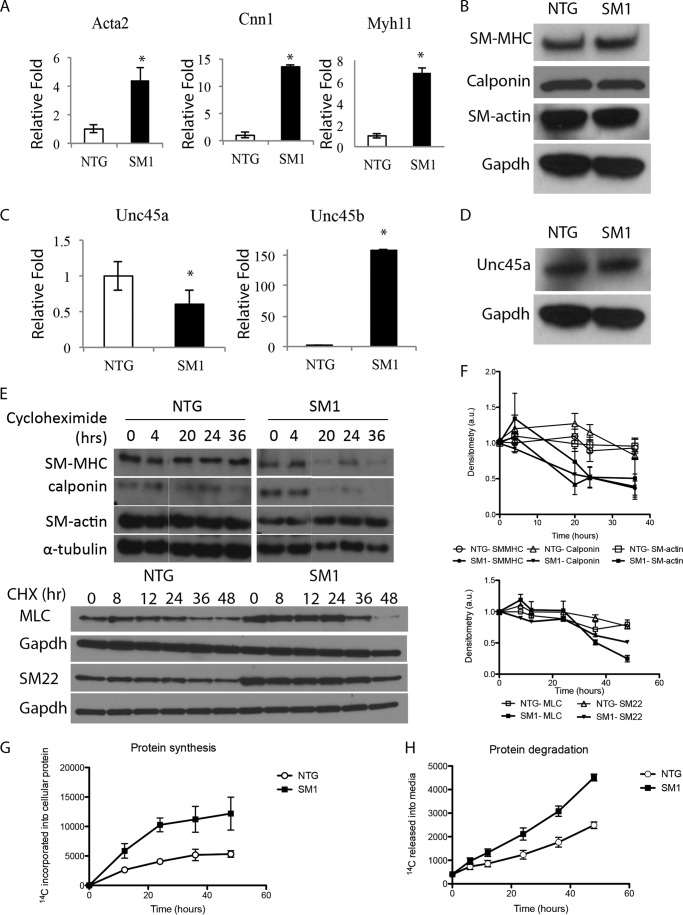
Increased protein turnover in SM1 transgenic cells. A and B, contractile protein expression is increased at the RNA level (A) but unchanged at the protein level (B) in SM1 cells. C and D, Unc45a expression is significantly decreased at both the RNA (C) and protein (D) levels, and ectopic expression of Unc45b RNA is observed (C). E and F, cycloheximide chase assay shows highly stable contractile proteins in NTG cells but degradation of contractile proteins in SM1 cells by 12 h (E). Blots from four experiments are quantified by densitometry in F: p < 0.05 at 12 h and beyond for SM-MHC and SM22; 20 h and beyond for calponin; and 48 h for MLC; p > 0.05 for SM-actin. G and H, radioactive pulse-chase with [14C]phenylalanine shows that SM1 cells have increased incorporation of [14C] into cellular protein during the pulse (G) and increased release of [14C] into the media during the chase (H).
RESULTS
Increased Protein Turnover in Systems Overexpressing Myosin
SMCs were explanted from the thoracic descending aorta of NTG and SM1 transgenic mice to assess the effects of smooth muscle myosin overexpression on SMC phenotype. Initial characterization of the transgenic SM1 mouse SMCs (termed SM1) revealed a significant increase in mRNA expression of MYH11 relative to nontransgenic cells. Surprisingly, there was a similar increase in the expression of other contractile genes (Acta2 and Cnn1) (Fig. 1A). However, there was no concomitant increase in SM-MHC, SM-actin, or calponin cellular protein levels (Fig. 1B). These results suggested either decreased translation or increased degradation of these contractile proteins.
A specific chaperone called Unc45a helps to fold the smooth muscle and nonmuscle myosin heavy chain isoforms. In SM1 cells, mRNA expression of Unc45a was significantly decreased compared with control cells, and protein accumulation also appears lower (Fig. 1, C and D). However, expression of Unc45b mRNA, typically seen only in cardiac and skeletal muscle cells, was increased compared with expression in NTG cells (Fig. 1C). Unfortunately, an Unc45b-specific antibody is not available so we were unable to assess levels of Unc45b protein in the cells.
The increased expression of contractile genes at the message level but not the protein level suggested increased protein turnover. Cycloheximide, a protein translation inhibitor that is well tolerated by SMCs and does not cause cell death (17, 18), was administered to block protein elongation, and contractile proteins levels were followed over time. In NTG cells, the contractile proteins remain highly stable for up to 48 h of exposure to cycloheximide (Fig. 1, E and F), which is consistent with the previously reported extended half-lives of contractile proteins in muscle cells (19, 20). However, in SM1 SMCs, the levels of several contractile proteins including SM-MHC, calponin, myosin light chain (MLC), and SM22 begin to decay significantly as early as 12 h after exposure to cyclohexamide (Fig. 1, E and F). However, SM-actin was not similarly degraded in the SM1 cells (no significant p values). Because cycloheximide can also disrupt protein translation, increased protein degradation in the SM1 SMCs was confirmed using a pulse-chase assay to assess global total protein synthesis and degradation. Labeled [14C]phenylalanine, which is readily incorporated into cellular proteins and not metabolized by muscle (21), was introduced into the culture media. Protein synthesis was assessed by the accumulation of 14C in the cellular protein lysates, whereas protein degradation was assessed by the release of 14C into the media during the chase period (16). There was increased protein synthesis (p = 0.007) and degradation (p < 0.001) in SM1 cells when compared with the NTG cells, thus supporting the conclusion that contractile protein turnover was significantly increased in SM1 cells (Fig. 1, G and H).
Protein Turnover in SM1 Cells Is Driven by Autophagy and Not by Proteasomal Degradation
To assess the contribution of the proteasome to contractile protein turnover in SM1 cells, SM1 and NTG SMCs were treated with the proteasome inhibitor bortezomib. Treatment with bortezomib did not lead to increased accumulation of contractile proteins in SM1 SMCs (Fig. 2, A and B). To confirm that the unchanged protein levels were due to changes in protein degradation rather than changes in mRNA expression, we assessed gene expression of contractile genes during bortezomib treatment. Six hours after the initiation of bortezomib treatment, expression of Acta2, Cnn1, and Myh11 all decreased significantly in NTG cells (Fig. 2C). Despite this decrease in expression, there was no significant change in the protein level of SM-MHC, SM-actin, or calponin (Fig. 2, A and B), suggesting that the proteasome may be responsible for regulating contractile protein turnover in NTG cells. By contrast, in SM1 cells expression of contractile genes actually increased at early time points of bortezomib treatment, and expression of Myh11 and Cnn1 did not decrease for up to 24 h of treatment (Fig. 2C). Therefore, the lack of increase in protein levels suggests that the ubiquitin-proteasome system is not responsible for degrading contractile proteins in SM1 cells.
FIGURE 2.
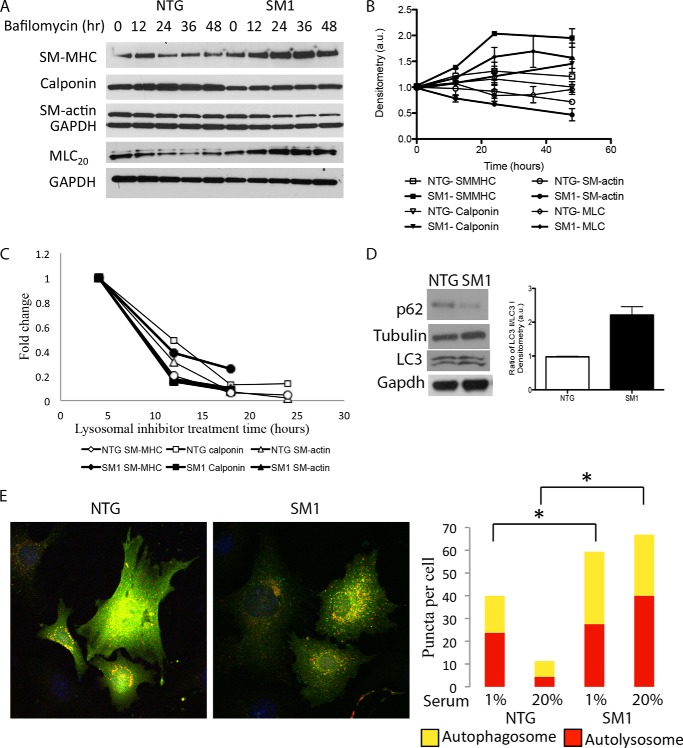
The proteasome degrades contractile proteins in NTG, but not SM1 cells. A and B, treatment with the proteasome inhibitor bortezomib does not lead to accumulation of contractile proteins in SM1 cells (A). Blots from four experiments are quantified by densitometry in B; there are no significant p values. C, bortezomib reduces transcript levels of contractile genes in NTG cells, but not in SM1 cells. Data shown are derived from a single replicate, but are representative of results from three independent experiments.
We next assessed whether autophagy was responsible for the degradation of contractile proteins in SM1 cells by using treatment with bafilomycin to block autophagy. Unlike the results with bortezomib, bafilomycin lead to a significant accumulation of contractile proteins in SM1 SMCs over 48 h of treatment, but not in NTG SMCs, supporting the notion that autophagy is involved in the degradation of these proteins (Fig. 3, A and B). As with cycloheximide, SM-actin was an exception to the trend of increased contractile protein levels after bafilomycin treatment in SM1 SMCs (no significant difference, p > 0.05). The corresponding transcript levels for Myh11 and Cnn1 significantly decreased by 12 h after initiation of treatment with the lysosomal inhibitors in both genotypes, indicating that stabilization of the protein levels was not due to increased transcript levels (Fig. 3C).
FIGURE 3.
Autophagy degrades contractile proteins in SM1 cells. A and B, treatment with the autophagy inhibitor bafilomycin induces accumulation of contractile proteins in SM1 cells (A). Blots from four experiments are quantified by densitometry in B: p < 0.05 at 24 h and beyond for SM-MHC and MLC, and at 48 h for calponin; p > 0.05 for SM-actin. C, quantitative RT-PCR results show that transcript levels of contractile genes decrease following lysosomal inhibitor treatment in both NTG and SM1 cells. D, Western blotting shows increased LC3 processing and decreased levels of p62 in SM1 cells. Quantitation shows the ratio of LC3 II to LC3 I. E, SM1 cells infected with RFP-GFP-LC3 lentivirus have increased numbers of red and yellow puncta compared with NTG-infected cells under both high-serum and low-serum media conditions. Yellow puncta represent autophagasomes, whereas red puncta represent autophagolysosomes. Immunofluorescent quantitations reflect four independent experiments, *, p < 0.05.
To further assess whether autophagy is increased in SM1 cells, we performed immunoblot analysis to determine whether the LC3 II to LC3 I ratio, a marker of autophagic activity, was increased. The LC3 II to LC3 I ratio was significantly increased in SM1 compared with NTG cells (p = 0.015) (Fig. 3D). Likewise, p62 levels (decreased levels are associated with increased rates of autophagy) were lower in the SM1 SMCs than in NTG cells, suggesting increased autophagy in the transgenic SMCs (Fig. 3D) (22). Last, we used an RFP-GFP-LC3 expressing lentivirus to assess autophagic flux in the cells. At early stages of autophagy, LC3 is tagged with both RFP and GFP. During initial autophagosome formation, LC3 staining becomes punctate and the dots appear yellow. As the autophagosome fuses with the lysosome to form an autolysosome, the pH of the compartment decreases and GFP is destabilized, leaving only RFP expression, changing the LC3 fluorescence to red (23). Based on quantitation of yellow and red puncta in fixed cells, SM1 cells have significantly more autophagosomes and autolysosomes than NTG cells, suggesting an increase in autophagic flux in the SM1 SMCs (Fig. 3E).
ER Stress Response and Autophagy in SM1 Cells
We sought to identify the cellular signaling pathway driving increased autophagy in the SM1 cells. The canonical signaling pathway leading to increased autophagy is decreased mTOR signaling (24). Current data indicate that AMP-associated protein kinase (AMPK) and mTOR are the primary regulators of autophagy initiation, with AMPK activating the process and mTOR inhibiting it (25). AMPK and mTOR play opposing roles in regulating cellular metabolism, and AMPK can directly inhibit mTOR activation during cellular stress (26). Thus, under normal conditions, when nutrient levels are high, AMPK is not activated, and mTOR remains active to drive protein synthesis. Under conditions of starvation or cellular stress, AMPK is activated, mTOR gets turned off, protein synthesis decreases, and autophagy increases in an effort to conserve energy. There is a moderate increase in SM1 cells in phosphorylation of ACC, the downstream target of AMPK (Fig. 4A). However, SM1 cells also exhibit increased rates of protein synthesis. Markers of increased mTOR signaling, phosphorylation of p70S6 kinase and its downstream target the ribosomal protein S6, were increased in SM1 cells (Fig. 4B). Therefore, increased autophagy in the SM1 cells was not due to decreased mTOR signaling.
FIGURE 4.
ER stress response occurs without mTOR inhibition and is upstream of autophagy in SM1 cells. A, AMPK related signaling is increased in SM1 cells compared with NTG cells. B, mTOR related signaling is increased in SM1 cells compared with NTG cells. C, ER stress signaling and markers are increased in SM1 cells compared with NTG cells. D, treatment with the small molecule chaperone 4-PBA reduces red and yellow puncta in RFP-GFP-LC3-infected SM1 and NTG cells. Quantitations reflect three independent experiments, *, p < 0.05. E, treatment with 4-PBA reduces LC3 processing in SM1 cells. Quantitation represents three independent experiments, *, p < 0.05.
Another cellular process linked with autophagy is the induction of ER stress pathways (27–29). Nascent translated proteins are processed in either the cytoplasm or the ER for proper protein folding and post-translational modifications. However, if the protein assembly system is slowed down for any reason, the ER initiates signals to drive transcription and translation of chaperone proteins, and temporally slows down protein synthesis and induces autophagy. Because of the increase in global protein synthesis, as well as the imbalance in expression of SM-MHC and its Unc45 chaperone, we hypothesized that ER stress might be increased in the SM1 cells. Phosphorylation levels of PERK and its downstream target eIF2α were increased in the SM1 SMCs, indicating activation of one of the three major signaling pathways initiated by ER stress (30). Grp78, Grp94, and Erp72 are all chaperone proteins that are up-regulated to help relieve ER stress (31), and all three of these proteins were increased in the SM1 cells compared with NTG cells (Fig. 4C). Furthermore, we treated the SM1 SMCs with 4-phenylbutyric acid (4-PBA), a small molecule drug that acts as a chemical chaperone to relieve ER stress (32), and determined that the number of autophagosomes and autolysosomes were significantly decreased in SM1 cells as assessed by immunofluorescence (Fig. 4D). The LC3 II to LC3 I ratio also decreased in SM1 cells after treatment with 4-PBA, supporting the conclusion that ER stress pathways directly up-regulate autophagy in SM1 cells (Fig. 4E).
Contractile Filaments Are Intact in SM1 SMCs
Although the contractile proteins are turned over more rapidly in the SM1 cells, it is unclear what effect this increased turnover has on filament formation and stability. To assess filament architecture, we initially used immunofluorescence to visualize the filaments in paraformaldehyde-fixed cells. Staining for SM-actin revealed thin, stretched out filaments across the cell body of NTG SMCs (Fig. 5A). However, the filament structure in SM1 cells appeared less organized and the staining appears brighter. SM-actin specific staining (green) co-localized with phalloidin staining (red) in both NTG and SM1 cells, indicating that most SM-actin was polymerized. To confirm, we performed an ultracentrifugation-based assay to separate polymerized from unpolymerized actin. There was no difference in the ratio of polymerized to unpolymerized actin between SM1 and NTG cells (Fig. 5B). These results suggest that whereas filaments could potentially be less stable in SM1 cells, the actual number of actin filaments present in the cell at any given time is comparable with the NTG SMCs.
FIGURE 5.
Contractile filament formation in SM1 cells. A, immunofluorescent staining for smooth muscle-specific α-actin (SM-actin, green), and all polymerized actin (phalloidin, red) show intact but disorganized filaments in SM1 SMCs. B, ultracentrifugation assay shows similar ratios of polymerized (P, pellet): unpolymerized (S, supernatant) actin in SM1 and NTG cells. Densitometry results are representative of at least three independent experimental replicates, p > 0.05. C, immunofluorescent staining for vinculin shows larger individual focal adhesions in SM1 cells. Quantification to the right reflects three independent staining procedures and 10 cells quantitated per experimental replicate, **, p < 0.01. D, G-LISA show decreased Rac1 activation at baseline and increased RhoA activation after 5 min of treatment with lysophosphatidic acid (LPA) in SM1 cells, *, p < 0.05. E, immunofluorescent staining for MRTF-A (green) shows increased nuclear accumulation in SM1 cells. Quantification to the right reflects three independent staining procedures and 10 random fields quantitated per experimental replicate, *, p < 0.05.
Actin polymerization in SMCs is driven by activation of the small GTPase RhoA (33). As force is generated across the SMC, protein complexes at the cell membrane called focal adhesions mature both by increasing in size and changing in composition (34). The composition changes from predominantly activators of Rac1 to activators of RhoA (35). As RhoA is activated at these focal adhesions, it drives actin polymerization (33). Immunofluorescent staining for the focal adhesion protein vinculin indicated that individual focal adhesions were larger in SM1 cells than in NTG cells (Fig. 5C). Furthermore, Rac1 activation was significantly decreased, and RhoA activation was increased following treatment with lysophosphatidic acid (Fig. 5D). These results suggest that SM1 cells have more mature focal adhesions, which in turn drive polymerization of actin.
Formation of SM-actin filaments is linked to transcription of SMC-specific contractile genes via the myocardin-related transcription factor:serum response factor (MRTF:SRF) axis. MRTFs are transcriptional coactivators that facilitate binding of serum response factor to the CarG boxes present in the promoters of SMC-specific genes like Acta2 and Cnn1 (36). Unpolymerized actin sequesters MRTFs in the cytoplasm, preventing transcription of these genes (37). SM1 cells had increased nuclear intensity of MRTF-A staining, suggesting a decrease in pools of monomeric actin in these cells (Fig. 5E). Importantly, the accumulation of nuclear MRTF-A in SM1 cells may drive the increased Acta2 and Cnn1 transcript levels described above.
Increased Contraction and Calcium Signaling in SM1 Cells
A recent study linked increased ER stress response with increased contractility in the aorta (38). Intriguingly, SM1 transgenic mice exhibit increased aortic contractility in vivo (13). To confirm increased contractility of the SM1 SMCs in culture, we used a collagen gel contraction assay: after incubation in 1% serum media for 4 days, the gels were released and allowed to contract. Gels populated with SM1 cells contracted significantly more than gels populated with NTG cells (Fig. 6A). As described above, the percentage of polymerized actin is equivalent in SM1 and NTG cells, so an alternative mechanism must drive increased cellular contraction. Previous studies have shown that increased contractility can be driven by increased calcium release from the ER as a result of ER stress (38, 39). Therefore, we sought to determine whether baseline cytosolic calcium concentrations were increased in SM1 cells and, if so, would the cells respond aberrantly to thapsigargin, a drug that inhibits calcium reuptake into the ER. After fura2-AM loading, cells were imaged before and after addition of thapsigargin in calcium-free buffer. SM1 cells had a higher baseline intracellular calcium concentration than NTG cells (Fig. 6, B and C). After thapsigargin treatment, SM1 cells reached peak calcium levels faster, indicating that release of calcium from the ER occurs faster in these cells, and the percentage increase in calcium is lower, indicating lower reserves of calcium in the ER of SM1 cells (Fig. 6, B and C). These changes are consistent with the hypothesis that ER stress is causing an increased release of calcium from ER stores in SM1 cells. To confirm that these changes are downstream of ER stress, cells were pretreated for 24 h with 4-PBA. 4-PBA completely rescued the aberrant calcium signaling phenotype in SM1 cells (Fig. 6, B and C).
FIGURE 6.
Increased contractility in SM1 cells is a result of increased cytosolic Ca2+. A, SM1 cells plated within collagen gels affect greater contraction of the gels than NTG cells, resulting in a significant decrease in gel area. Quantifications reflect three independent platings with two gels per plating per treatment group, *, p < 0.05. B, Ca2+ imaging reveals higher baseline Ca2+ levels in the cytoplasm of SM1 cells, and a more rapid but lower amplitude peak of Ca2+ levels following treatment with the SERCA-pump inhibitor thapsigargin. These changes are all rescued by treatment with 4-PBA. Experiments were performed on at least five separate coverslips per condition, with at least 10 cells quantitated per coverslip, *, p < 0.05. C, representative traces of single cells from calcium imaging experiments reflect the changes observed in SM1 cells and confirm rescue with 4-PBA. Black arrows indicate the addition of thapsigargin. D and E, phosphorylation of MLC20 is increased in SM1 cells compared with NTG cells by both immunofluorescence (D) and Western blot (E). Blot quantitations reflect three independent experiments, *, p < 0.05.
Increased cytosolic calcium concentrations led to increased activation of the myosin light chain, which is the primary determinant of SMC contractility (40). SM1 cells showed increased phosphorylation of the myosin light chain at serine 19 by immunoblot analysis (Fig. 6E). Additionally, fluorescent staining for total myosin light chain shows more intense staining in SM1 cells; however, the filament structure looks more disorganized in SM1 cells (Fig. 6D). Taken together, these data support the model that ER stress in SM1 cells is responsible for both increased contractility and autophagic degradation of contractile proteins.
Increased Rho and Rho kinase activation can also impact phosphorylation of the myosin light chain both by inhibiting the myosin light chain phosphatase and sensitizing the smooth muscle cells to calcium signaling (41). To assess whether Rho kinase or myosin light chain kinase plays a larger role in the dysregulated contractile phenotype of SM1 SMCs, we used specific inhibitors for each molecule in the collagen gel contraction assay. Surprisingly, an inhibitor of myosin light chain kinase, ML-7, did not rescue the increased contractility of SM1 cells, whereas an inhibitor of Rho kinase, Y-27632, did (Fig. 6A). Because Rho kinase also contributes to contraction by driving actin filament formation and stabilization (33), these results may suggest that excessive MLC phosphorylation in SM1 cells alone is insufficient to explain the increased contractility. Alternatively, these results may show that the activity of the light chain phosphatase is more critical than the activity of the light chain kinase in influencing myosin light chain phosphorylation and smooth muscle contraction.
Tissue from Patients with 16p13.1 Duplications Recapitulates Findings in SM1 Cells
We sought to determine whether some of the cellular changes observed in the SM1 SMCs were also present in the aortic tissue from patients with 16p13.1 duplications that lead to three copies of MYH11. Proteins were harvested from frozen aortic medial tissue samples from patients with 16p13.1 duplications and immunoblot analysis was used to assess expression and protein levels. Similar to the phenotype seen in SM1 SMCs, the aortic tissue showed increased contractile gene expression with no change in contractile protein levels compared with aortic medial samples from unaffected individuals (Fig. 7, A–C). In addition, the aortic tissue from 16p13.1 duplication demonstrated decreased Unc45a expression and ectopic expression of Unc45b (Fig. 7D). Although limited analyses could be done on these samples, these aortic tissues from TAAD patients with 16p13.1 duplications show similar changes in gene expression to those observed in the SM1 cells.
FIGURE 7.
Frozen aortic tissue from patients with 16p13.1 duplication shows similar phenotypes to SM1 cells. A, increased expression of contractile genes at the mRNA level in a 16p13.1 duplication patient tissue compared with controls (n = 4 per group, *, p < 0.05). B, no change was seen in the levels of contractile proteins in patients compared with controls, but Unc45a levels were decreased. C, densitometry quantitation shows the average of three control and three patient tissue samples. D, decreased Unc45a mRNA expression and ectopic Unc45b mRNA expression is observed in 16p13 duplication patient tissues compared with controls (n = 4 per group), *, p < 0.05.
DISCUSSION
SMCs overexpressing myosin heavy chain have a unique phenotype characterized by increased turnover of contractile proteins in conjunction with increased cellular contractility. Mouse SMCs with overexpression of Myh11 driven by a transgene exhibit increased expression of multiple contractile genes when compared with nontransgenic cells without a corresponding increase in contractile protein levels. Both protein synthesis and degradation are globally increased in SM1 cells, and turnover of many contractile proteins is specifically accelerated. Specifically, proteins that associate directly with myosin thick filaments including SM-MHC, MLC, and SM22 are turned over more rapidly in SM1 cells than in NTG cells. Although calponin is predominantly studied as an actin-binding protein, a previous study reported direct binding of calponin to the regulatory light chain of myosin (42). These data may explain why calponin is turned over along with other thick filament components. Although previous work in C. elegans indicated that any alteration in the ratio of Unc45 to myosin heavy chain expression led to ubiquitination and degradation of myosin heavy chain (8), treatment with a proteasome inhibitor did not induce accumulation of contractile proteins in SM1 cells. Instead, inhibition of autophagy blocked degradation of contractile proteins in the SM1 cells. Surprisingly, activation of autophagy in these cells is associated with increased mTOR signaling and our data support the conclusion that the ER stress response drives autophagy. In addition to autophagy-driven protein turnover, the ER stress response in the SM1 cells also contributes to an increase in cellular contractility through release of intracellular calcium stores. SM1 mice were also previously shown to have increased aortic contractility in vivo (13). Taken together, these results show a phenotypic shift in both the metabolic status and the contractile status of the myosin heavy chain overexpressing SMCs, SM1 cells, compared with NTG cells (Fig. 8).
FIGURE 8.

Working model of molecular changes in SM1 cells. See text for more details.
The cellular pathway identified in the majority of studies to be responsible for autophagy induction is inhibition of mTOR signaling (24, 25), however, in SM1 cells, both mTOR signaling and autophagy are activated. Instead, signaling due to increased ER stress drives autophagy independent of the mTOR pathway in these cells. The unfolded protein response has previously been linked to induction of autophagy in disease contexts like cancer and neurodegenerative diseases (43, 44). Signaling through both the PERK- and IRE-dependent arms of the unfolded protein response has been linked with induction of autophagy (for review see Ref. 45). A transcription factor downstream of PERK signaling directly transcriptionally activates autophagy-related genes, including the gene encoding LC3 (46). Activation of JNK downstream of IRE has been shown to induce autophagy by phosphorylating Beclin1 (47). Last, calcium release from the ER during ER stress leads to autophagy through both an AMPK- and mTOR-dependent mechanism and a PKCθ-dependent mechanism (48, 49). SM1 cells have both increased cytosolic calcium levels and AMPK activity, but mTOR activation is also increased compared with wild type cells. Therefore, the pathway linking ER stress response to autophagy in SM1 cells appears to be independent of decreased levels of mTOR signaling. It is interesting to note that a recent study found that inhibition of nonsense-mediated mRNA decay in cells activates autophagy (50). Although the cellular pathway for the increased autophagy was not investigated, protein production from the mutant transcript may have induced ER stress and autophagy in a similar manner that overexpression of myosin heavy chain does in the SM1 cells.
Simultaneous activation of mTOR and autophagy in cells has rarely been reported in the literature (51). A recent study proposed that a protein complex called Ragulator, after the Rag G-proteins, is involved in coupling autophagy to mTOR activation through a pathway that involves increases in localized amino acid concentrations (52). This model may explain why mTOR activation does not necessarily lead to inhibition of autophagy: the localization of activated mTOR within specialized complexes at the site of amino acid release prevents mTOR from interacting with and inhibiting Atg1 (53, 54). Similarly, microdomains of activation may be occurring in SM1 cells, allowing activation of mTOR and an increase in global protein synthesis to coexist with autophagy, AMPK activation, and ER stress response.
Chemical induction of ER stress using either tunicamycin or MG-132 has recently been linked with increased contractility in SMCs (38). Increased cytoplasmic calcium levels due to ER stress lead to calcium-calmodulin complexes, which bind and activate myosin light chain kinase, leading to increased myosin regulatory light chain phosphorylation and SMC contraction (40). Our data support the conclusion that the increased contractility of SM1 SMCs is partially a result of ER stress-dependent calcium release. Prior work on the SM1 mouse showed increased aortic contractility in vivo, suggesting that ER stress may be increasing contractility of SMCs in the aorta (13). The SM2 transgenic mouse also described in that report showed decreased contractility, but SMCs explanted from the SM2 transgenic mouse showed a 500-fold increase in Myh11 expression (data not shown). Therefore, this model is not as physiologically relevant as the SM1 model in which Myh11 expression is increased only 5-fold. Increased contractility due to ER stress induced by in vivo tunicamycin infusion also leads to increased systolic blood pressures (38). Although the SM1 mouse strain was lost prior to documentation of blood pressures, it is interesting to note that many of the patients with 16p13.1 duplications are hypertensive (3).
Mutations causing aortic disease have previously been reported in both the myosin light chain kinase (MYLK) and the nitric oxide responsive, GMP-dependent protein kinase that controls smooth muscle cell relaxation (PRKG1) (55, 56). However, in both contexts, mechanistic data showed that the mutations led to decreased phosphorylation of myosin light chain, and are therefore predicted to decrease aortic contractility. Mutations in smooth muscle-specific actin (ACTA2) and myosin (MYH11) are also predicted to decrease aortic contractility through disruption of thin and thick filament formation, respectively (57). It is therefore surprising that the 16p13.1 duplications implicated in aortic disease may result in increased contractility. Both contraction and increased metabolic stress could lead to increased ATP turnover in SM1 cells, potentially driving increased production of reactive oxygen species, which have been previously associated with aortic disease (58, 59). Alternatively, vascular SMCs typically retain a large reserve of unphosphorylated myosin light chain to respond rapidly to changes in biomechanical forces. The reduction of this reserve in SM1 cells, as well as the depleted ATP stores and metabolic reserve that would result from constant and excessive protein turnover, may leave these cells less able to respond appropriately to biomechanical forces in the ascending aorta.
The results of this study show that increased ER stress response can result from an extra genetic copy of a single gene, suggesting this mechanism may be more globally applicable to genes involved in genomic duplications. These data raise the possibility that small molecule chemical chaperones, such as those approved for treatment of urea cycle disorders, may be useful to prevent disease in patients with 16p13.1 duplications and possibly for other disease-causing genomic duplications in which increased expression of a gene leads to increased ER stress (60, 61). Duplication of 16p13.1 is the most common genetic alteration associated with acute aortic dissections, and a specific therapy to prevent these deadly dissections would be beneficial for this at risk population.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1HL62594, P50HL083794-01 and P01HL110869-01 (to D. M. M.) and a grant from the Richard T. Pisani Fund (to D. M. M.).
- TAAD
- thoracic aortic aneurysms and dissections
- ER
- endoplasmic reticulum
- MLC
- myosin light chain
- SMC
- smooth muscle cell
- SM-MHC
- smooth muscle myosin heavy chain
- mTOR
- mammalian target of rapamycin
- NTG
- nontransgenic
- AMPK
- AMP-associated protein kinase
- 4-PBA
- 4-phenylbutyric acid
- MRTF
- myocardin-related transcription factor.
REFERENCES
- 1. Morrow E. M. (2010) Genomic copy number variation in disorders of cognitive development. J. Am. Acad. Child. Adolesc. Psychiatry 49, 1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vissers L. E., Stankiewicz P. (2012) Microdeletion and microduplication syndromes. Methods Mol. Biol. 838, 29–75 [DOI] [PubMed] [Google Scholar]
- 3. Kuang S. Q., Guo D. C., Prakash S. K., McDonald M. L., Johnson R. J., Wang M., Regalado E. S., Russell L., Cao J. M., Kwartler C., Fraivillig K., Coselli J. S., Safi H. J., Estrera A. L., Leal S. M., Lemaire S. A., Belmont J. W., Milewicz D. M., and GenTAC Investigators (2011) Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genet. 7, e1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L., Vranckx R., Khau Van Kien P., Lalande A., Boisset N., Mathieu F., Wegman M., Glancy L., Gasc J. M., Brunotte F., Bruneval P., Wolf J. E., Michel J. B., Jeunemaitre X. (2006) Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 38, 343–349 [DOI] [PubMed] [Google Scholar]
- 5. Pannu H., Tran-Fadulu V., Papke C. L., Scherer S., Liu Y., Presley C., Guo D., Estrera A. L., Safi H. J., Brasier A. R., Vick G. W., Marian A. J., Raman C. S., Buja L. M., Milewicz D. M. (2007) MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor I and angiotensin II. Hum. Mol. Genet. 16, 2453–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U., Epstein H. F. (2002) Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669–671 [DOI] [PubMed] [Google Scholar]
- 7. Price M. G., Landsverk M. L., Barral J. M., Epstein H. F. (2002) Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 115, 4013–4023 [DOI] [PubMed] [Google Scholar]
- 8. Landsverk M. L., Li S., Hutagalung A. H., Najafov A., Hoppe T., Barral J. M., Epstein H. F. (2007) The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J. Cell Biol. 177, 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatnagar S., Mittal A., Gupta S. K., Kumar A. (2012) TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J. Cell. Physiol. 227, 1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ochala J., Gustafson A. M., Diez M. L., Renaud G., Li M., Aare S., Qaisar R., Banduseela V. C., Hedström Y., Tang X., Dworkin B., Ford G. C., Nair K. S., Perera S., Gautel M., Larsson L. (2011) Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms. J. Physiol. 589, 2007–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Portbury A. L., Willis M. S., Patterson C. (2011) Tearin' up my heart: proteolysis in the cardiac sarcomere. J. Biol. Chem. 286, 9929–9934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Babu G. J., Warshaw D. M., Periasamy M. (2000) Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microsc. Res. Tech. 50, 532–540 [DOI] [PubMed] [Google Scholar]
- 13. Martin A. F., Bhatti S., Pyne-Geithman G. J., Farjah M., Manaves V., Walker L., Franks R., Strauch A. R., Paul R. J. (2007) Expression and function of COOH-terminal myosin heavy chain isoforms in mouse smooth muscle. Am. J. Physiol. Cell Physiol. 293, C238–C245 [DOI] [PubMed] [Google Scholar]
- 14. Cao J., Gong L., Guo D. C., Mietzsch U., Kuang S. Q., Kwartler C. S., Safi H., Estrera A., Gambello M. J., Milewicz D. M. (2010) Thoracic aortic disease in tuberous sclerosis complex: molecular pathogenesis and potential therapies in Tsc2+/− mice. Hum. Mol. Genet. 19, 1908–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo D. C., Pannu H., Tran-Fadulu V., Papke C. L., Yu R. K., Avidan N., Bourgeois S., Estrera A. L., Safi H. J., Sparks E., Amor D., Ades L., McConnell V., Willoughby C. E., Abuelo D., Willing M., Lewis R. A., Kim D. H., Scherer S., Tung P. P., Ahn C., Buja L. M., Raman C. S., Shete S. S., Milewicz D. M. (2007) Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 39, 1488–1493 [DOI] [PubMed] [Google Scholar]
- 16. Baskin K. K., Taegtmeyer H. (2011) AMP-activated protein kinase regulates E3 ligases in rodent heart. Circ. Res. 109, 1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Croons V., Martinet W., Herman A. G., Timmermans J. P., De Meyer G. R. (2007) Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J. Pharmacol. Exp. Ther. 320, 986–993 [DOI] [PubMed] [Google Scholar]
- 18. Croons V., Martinet W., Herman A. G., De Meyer G. R. (2008) Differential effect of the protein synthesis inhibitors puromycin and cycloheximide on vascular smooth muscle cell viability. J. Pharmacol. Exp. Ther. 325, 824–832 [DOI] [PubMed] [Google Scholar]
- 19. Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. (1977) Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J. Biol. Chem. 252, 3422–3429 [PubMed] [Google Scholar]
- 20. Zak R., Martin A. F., Prior G., Rabinowitz M. (1977) Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J. Biol. Chem. 252, 3430–3435 [PubMed] [Google Scholar]
- 21. Katsanos C. S., Chinkes D. L., Sheffield-Moore M., Aarsland A., Kobayashi H., Wolfe R. R. (2005) Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am. J. Physiol. Endocrinol. Metab. 289, E1064-E1070 [DOI] [PubMed] [Google Scholar]
- 22. Bjørkøy G., Lamark T., Pankiv S., Øvervatn A., Brech A., Johansen T. (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 452, 181–197 [DOI] [PubMed] [Google Scholar]
- 23. Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung C. H., Ro S. H., Cao J., Otto N. M., Kim D. H. (2010) mTOR regulation of autophagy. FEBS Lett. 584, 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egan D., Kim J., Shaw R. J., Guan K. L. (2011) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoki K., Zhu T., Guan K. L. (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 27. Ciechomska I. A., Gabrusiewicz K., Szczepankiewicz A. A., Kaminska B. (2013) Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine A-induced cell death. Oncogene 32, 1518–1529 [DOI] [PubMed] [Google Scholar]
- 28. Salazar M., Hernández-Tiedra S., Torres S., Lorente M., Guzmán M., Velasco G. (2011) Detecting autophagy in response to ER stress signals in cancer. Methods Enzymol. 489, 297–317 [DOI] [PubMed] [Google Scholar]
- 29. Schröder M., Kaufman R. J. (2005) The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 [DOI] [PubMed] [Google Scholar]
- 30. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 31. Kuznetsov G., Chen L. B., Nigam S. K. (1994) Several endoplasmic reticulum stress proteins, including ERp72, interact with thyroglobulin during its maturation. J. Biol. Chem. 269, 22990–22995 [PubMed] [Google Scholar]
- 32. Ayala P., Montenegro J., Vivar R., Letelier A., Urroz P. A., Copaja M., Pivet D., Humeres C., Troncoso R., Vicencio J. M., Lavandero S., Díaz-Araya G. (2012) Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp. Mol. Pathol. 92, 97–104 [DOI] [PubMed] [Google Scholar]
- 33. Mack C. P., Somlyo A. V., Hautmann M., Somlyo A. P., Owens G. K. (2001) Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 276, 341–347 [DOI] [PubMed] [Google Scholar]
- 34. Geiger B., Spatz J. P., Bershadsky A. D. (2009) Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 [DOI] [PubMed] [Google Scholar]
- 35. Kuo J. C., Han X., Hsiao C. T., Yates J. R., 3rd, Waterman C. M. (2011) Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parmacek M. S. (2007) Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 100, 633–644 [DOI] [PubMed] [Google Scholar]
- 37. Mack C. P., Hinson J. S. (2005) Regulation of smooth muscle differentiation by the myocardin family of serum response factor co-factors. J. Thromb. Haemost. 3, 1976–1984 [DOI] [PubMed] [Google Scholar]
- 38. Liang B., Wang S., Wang Q., Zhang W., Viollet B., Zhu Y., Zou M. H. (2013) Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-α2 in vivo. Arterioscler. Thromb. Vasc. Biol. 33, 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabas I., Ron D. (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding H. L., Ryder J. W., Stull J. T., Kamm K. E. (2009) Signaling processes for initiating smooth muscle contraction upon neural stimulation. J. Biol. Chem. 284, 15541–15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 389, 990–994 [DOI] [PubMed] [Google Scholar]
- 42. Szymanski P. T., Goyal R. K. (1999) Calponin binds to the 20-kilodalton regulatory light chain of myosin. Biochemistry 38, 3778–3784 [DOI] [PubMed] [Google Scholar]
- 43. Hart L. S., Cunningham J. T., Datta T., Dey S., Tameire F., Lehman S. L., Qiu B., Zhang H., Cerniglia G., Bi M., Li Y., Gao Y., Liu H., Li C., Maity A., Thomas-Tikhonenko A., Perl A. E., Koong A., Fuchs S. Y., Diehl J. A., Mills I. G., Ruggero D., Koumenis C. (2012) ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Invest. 122, 4621–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vidal R. L., Hetz C. (2012) Crosstalk between the UPR and autophagy pathway contributes to handling cellular stress in neurodegenerative disease. Autophagy 8, 970–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rouschop K. M., van den Beucken T., Dubois L., Niessen H., Bussink J., Savelkouls K., Keulers T., Mujcic H., Landuyt W., Voncken J. W., Lambin P., van der Kogel A. J., Koritzinsky M., Wouters B. G. (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Invest. 120, 127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 30, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madaro L., Marrocco V., Carnio S., Sandri M., Bouché M. (2013) Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J. 27, 1990–2000 [DOI] [PubMed] [Google Scholar]
- 49. Høyer-Hansen M., Jäättelä M. (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 14, 1576–1582 [DOI] [PubMed] [Google Scholar]
- 50. Wengrod J., Martin L., Wang D., Frischmeyer-Guerrerio P., Dietz H. C., Gardner L. B. (2013) Inhibition of nonsense-mediated RNA decay activates autophagy. Mol. Cell. Biol. 33, 2128–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. She P., Zhang Z., Marchionini D., Diaz W. C., Jetton T. J., Kimball S. R., Vary T. C., Lang C. H., Lynch C. J. (2011) Molecular characterization of skeletal muscle atrophy in the R6/2 mouse model of Huntington's disease. Am. J. Physiol. Endocrinol. Metab. 301, E49–E61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Narita M., Inoki K. (2012) Rags connect mTOR and autophagy. Small GTPases 3, 111–114 [DOI] [PubMed] [Google Scholar]
- 53. Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S., Sabatini D. M. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 141, 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Young A. R., Narita M., Narita M. (2011) Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy 7, 1387–1388 [DOI] [PubMed] [Google Scholar]
- 55. Wang L., Guo D. C., Cao J., Gong L., Kamm K. E., Regalado E., Li L., Shete S., He W. Q., Zhu M. S., Offermanns S., Gilchrist D., Elefteriades J., Stull J. T., Milewicz D. M. (2010) Mutations in myosin light chain kinase cause familial aortic dissections. Am. J. Hum. Genet. 87, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo D. C., Regalado E., Casteel D. E., Santos-Cortez R. L., Gong L., Kim J. J., Dyack S., Horne S. G., Chang G., Jondeau G., Boileau C., Coselli J. S., Li Z., Leal S. M., Shendure J., Rieder M. J., Bamshad M. J., Nickerson D. A., GenTAC Registry Consortium, National Heart, Lung, and Blood Institute Grand Opportunity Exome Sequencing Project, Kim C., Milewicz D. M. (2013) Recurrent gain of function mutation in cGMP-dependent protein kinase (PRKG1) causes thoracic aortic aneurysms and acute aortic dissections. Am. J. Hum. Genet. 93, 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Milewicz D. M., Guo D. C., Tran-Fadulu V., Lafont A. L., Papke C. L., Inamoto S., Kwartler C. S., Pannu H. (2008) Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet. 9, 283–302 [DOI] [PubMed] [Google Scholar]
- 58. Ejiri J., Inoue N., Tsukube T., Munezane T., Hino Y., Kobayashi S., Hirata K., Kawashima S., Imajoh-Ohmi S., Hayashi Y., Yokozaki H., Okita Y., Yokoyama M. (2003) Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc. Res. 59, 988–996 [DOI] [PubMed] [Google Scholar]
- 59. Xiong W., Mactaggart J., Knispel R., Worth J., Zhu Z., Li Y., Sun Y., Baxter B. T., Johanning J. (2009) Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis. 202, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lichter-Konecki U., Diaz G. A., Merritt J. L., 2nd, Feigenbaum A., Jomphe C., Marier J. F., Beliveau M., Mauney J., Dickinson K., Martinez A., Mokhtarani M., Scharschmidt B., Rhead W. (2011) Ammonia control in children with urea cycle disorders (UCDs): phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol. Genet. Metab. 103, 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mimori S., Okuma Y., Kaneko M., Kawada K., Hosoi T., Ozawa K., Nomura Y., Hamana H. (2012) Protective effects of 4-phenylbutyrate derivatives on the neuronal cell death and endoplasmic reticulum stress. Biol. Pharm. Bull. 35, 84–90 [DOI] [PubMed] [Google Scholar]