Background: Tissue ischemia stimulates production of secreted factors that regulate angiogenesis.
Results: Neuron-derived neurotrophic factor (NDNF) is up-regulated in endothelial cells in ischemic limbs of mice. NDNF stimulates endothelial cell function and promotes ischemia-induced revascularization through NOS-dependent mechanisms.
Conclusion: NDNF functions as a novel modulator that stimulates revascularization processes.
Significance: NDNF represents a novel therapeutic target for ischemic vascular diseases.
Keywords: Akt, Angiogenesis, Apoptosis, Endothelial Cell, Ischemia, Nitric Oxide Synthase, Neuron-derived Neurotrophic Factor
Abstract
Strategies to stimulate revascularization are valuable for cardiovascular diseases. Here we identify neuron-derived neurotrophic factor (NDNF)/epidermacan as a secreted molecule that is up-regulated in endothelial cells in ischemic limbs of mice. NDNF was secreted from cultured human endothelial cells, and its secretion was stimulated by hypoxia. NDNF promoted endothelial cell network formation and survival in vitro through activation of Akt/endothelial NOS (eNOS) signaling involving integrin αvβ3. Conversely, siRNA-mediated knockdown of NDNF in endothelial cells led to reduction of cellular responses and basal Akt signaling. Intramuscular overexpression of NDNF led to enhanced blood flow recovery and capillary density in ischemic limbs of mice, which was accompanied by enhanced phosphorylation of Akt and eNOS. The stimulatory actions of NDNF on perfusion recovery in ischemic muscles of mice were abolished by eNOS deficiency or NOS inhibition. Furthermore, siRNA-mediated reduction of NDNF in muscles of mice resulted in reduction of perfusion recovery and phosphorylation of Akt and eNOS in response to ischemia. Our data indicate that NDNF acts as an endogenous modulator that promotes endothelial cell function and ischemia-induced revascularization through eNOS-dependent mechanisms. Thus, NDNF can represent a therapeutic target for the manipulation of ischemic vascular disorders.
Introduction
Cardiovascular disease is a major cause of death worldwide (1). Obesity-linked metabolic disorders, including type 2 diabetes, contribute to impaired collateralization and vascular insufficiency under ischemic conditions, thereby leading to exacerbation of cardiac dysfunction and tissue injury (2–4). It is well established that the enhancement of collateral vessel development can represent a promising therapeutic strategy for cardiovascular diseases.
A number of growth factors and cytokines regulate the recruitment and development of blood vessels in autocrine, paracrine, and endocrine manners. Tissue ischemia or hypoxia can stimulate angiogenesis to restore blood flow to ischemic lesions by regulating various secretory factors, including VEGF family of proteins and angiopoietins (5–7). A model of hind limb ischemia is widely used to evaluate angiogenic responses and processes (8–10). In this model, limb ischemia leads to enhanced expression of angiogenesis-related genes (11–13).
To identify the secreted factors involved in ischemia-induced vessel growth, we performed microarray analyses by comparing gene expression profiles of non-ischemic and ischemic muscle tissues in wild-type C57BL/6J mice subjected to hind limb ischemia. After screening of the differentially regulated genes by bioinformatics analysis, we focused on neuron-derived neurotrophic factor (NDNF)5/epidermacan as a potential secretory protein that is up-regulated in ischemic muscle. NDNF is a glycosylated and disulfide-bonded secreted protein with a fibronectin type III domain (14). NDNF is expressed in mouse brain and spinal cord and promotes migration and neurite growth of mouse hippocampal neurons (14). However, nothing is known about the functional role of NDNF in vascular responses. Here we investigated whether NDNF modulates the phenotypic changes in cultured endothelial cells and tested whether NDNF affects blood vessel recruitment under conditions of ischemia.
EXPERIMENTAL PROCEDURES
Materials
Mouse CD31 antibody was purchased from BD Biosciences (San Jose, CA). Antibodies of phosphorylated eNOS (Ser-1177), Akt (Ser-473), P42/44 ERK1/2 (Thr-202/Tyr-204), AMP-activated protein kinase (AMPK) (Thr-172), P42/44 ERK, eNOS, Akt, AMPK, HA, Bcl-2, and tubulin were purchased from Cell Signaling Technology (Beverly, MA). Anti-NDNF antibody was purchased from Abgent (San Diego, CA). Antibody of human von Willebrand factor was purchased from DakoCytomation (Denmark). NG-nitro-l-arginine methyl ester (l-NAME) was purchased from Sigma. YC-1 and cobalt chloride (CoCl2) were purchased from Wako. Dimethylated glycine was purchased from Cayman Chemical. Recombinant human VEGF protein was purchased from Peprotech (Rocky Hill, NJ). The GRGDSP and GRGESP peptides were purchased from AnaSpec (Fremont, CA). Anti-αvβ3 neutralizing antibody was purchased from Millipore (Billerica, MA). Control mouse IgG was purchased from Santa Cruz Biotechnology.
Microarray Analysis
Total RNA from ischemic and non-ischemic adductor muscle of the WT mouse following femoral artery resection was analyzed by Agilent array (SurePrint G3 Mouse Gene Expression 8 × 60K). Among transcripts that are increased in ischemic skeletal muscle, we analyzed amino acid sequences for assessment of the presence of signal sequences by using SignalP software (13, 15, 16). SOSUI signal beta version software was also used to predict proteins that lack transmembrane domain (13, 15, 16).
Construction of Adenoviral Vectors
The adenoviral vectors expressing β-galactosidase (Ad-β-gal) or HA-tagged dominant negative mutant Akt (Ad-dn-Akt) were constructed under the control of the CMV promoter (13, 17). Full-length mouse NDNF cDNA was subcloned into a pShuttle expression vector that encodes as a fusion to FLAG epitope at the C terminus and was transformed into Escherichia coli with the adenoviral backbone plasmid pAdEasy-1. The resultant recombinant pAdEasy-1 was transfected into HEK 293 cells to generate the adenoviral vector expressing NDNF (Ad-NDNF).
Mouse Model of Hind Limb Ischemia
Male wild-type (C57BL/6J) or eNOS knockout (eNOS-KO) (The Jackson Laboratory) mice at the ages of 8–11 weeks were subjected to unilateral hind limb surgery to remove the left femoral artery and vein under anesthesia (18–21). Ad-β-gal at 4 × 107 pfu or Ad-NDNF at 4 × 107 pfu or 1 × 108 pfu was injected into five different sites of the adductor muscle in the left limb 3 days prior to the surgery, as described previously (18, 22). Hind limb blood flow was measured by a laser Doppler blood flow analyzer (Moor LDI, Moor Instruments) immediately before surgery and on postoperative days 3, 7, and 14. To avoid data variations caused by ambient light and temperature, hind limb blood flow was expressed as the ratio of left (ischemic) to right (non-ischemic) LDBF. In some experiments, l-NAME, a NOS inhibitor, was added to the drinking water at 1 mg/ml, whereas the animals without l-NAME received plain drinking water (23).
Capillary density within thigh adductor muscle was analyzed by immunohistochemistry (18, 22). Muscle samples were embedded in optimum cutting temperature compound (Miles, Elkhart, IN) and snap-frozen in liquid nitrogen. Tissue slices (5-μm in thickness) were stained with anti-CD31 antibodies (BD Biosciences). Fifteen randomly chosen microscopic fields from three different sections in each tissue block were examined for the presence of CD31-positive capillary endothelial cells. Capillary density was expressed as the number of CD31-positive cells per muscle fiber or per high-power field. Study protocols were approved by the Institutional Animal Care and Use Committee at Nagoya University.
Mouse Adductor Muscle Endothelial Cell Isolation
Mouse adductor muscle endothelial cells were isolated from wild-type mice on which a hind limb ischemia operation had been performed. Briefly, the mouse adductor muscles were excised, minced, and digested with 0.1% collagenase in phosphate-buffered saline for 30 min. Endothelial cells were isolated by immunoselection with CD31-conjugated (BD Biosciences) magnetic beads (Invitrogen).
Preparation of Recombinant Mouse NDNF Protein
COS-7 cells were transfected with the pShuttle vector expressing full-length mouse NDNF cDNA tagged with FLAG at the C terminus. The culture supernatants were collected and incubated with anti-FLAG M2 affinity gel (Sigma) for 16 h. NDNF protein was eluted by incubation with 3× FLAG peptide (Sigma) and dialyzed with PBS.
Quantification of mRNA Levels
Gene expression levels were quantified by real-time PCR. Total RNA was extracted from skeletal muscle tissues using an RNeasy fibrous tissue mini kit (Qiagen) and from HUVECs and isolated adductor muscle endothelial cells using an RNeasy mini kit (Qiagen). RNA that had an A260:A280 ratio of 1.8 or greater was used for the reverse transcription reaction. cDNA was produced from 0.5 μg of total RNA using a SuperScript III first-strand synthesis system (Invitrogen). 3 ng of cDNA was used for the PCR. PCR was performed with a Bio-Rad real-time PCR detection system using POWER SYBR Green as a double-standard, DNA-specific dye, as described previously (22, 24). RT-PCR signals were quantitated by the relative standard curve method (25) and normalized to 36B4 as an internal standard by dividing the value of NDNF by that of 36B4 in each sample. Triplicate measurements of each sample were performed.
Primers were 5′-TGCCTCCTGTTACCACTCACTTC-3′ and 5′-TTCCTTATCCCGGATCTGCAT-3′ for mouse NDNF, 5′-GCTCCAAGCAGATGCAGCA-3′ and 5′-CCGGATGTGAGGCAGCAG-3′ for mouse 36B4, 5′-AGCCTTTGACAAGCTCCGTA-3′ and 5′-TCTGCAGGTTTTGACTGTGG-3′ for human NDNF, and 5′-GAGTGATGTGCAGCTGATCAAGAC-3′ and 5′-GGATGACCAGCCCAAAGGA-3′ for human 36B4.
Cell Culture
Human umbilical endothelial cells (HUVECs) were cultured in endothelial cell growth medium 2 (Lonza). HUVECs were infected with Ad-NDNF or Ad-β-gal at a multiplicity of infection of 25 for 24 h before the experiments. HUVECs were also cultured in the presence or absence of recombinant NDNF protein (200 ng/ml) for the indicated lengths of time. In some experiments, HUVECs were treated with Ad-dn-Akt or Ad-β-gal at a multiplicity of infection of 1 for 24 h (18, 26, 27). Hypoxic conditions were generated using an AnaeroPack (Mitsubishi GAS Chemical). In some experiments, HUVECs were pretreated with YC-1 (10 μm) or vehicle (dimethyl sulfoxide) for 60 min before the induction of hypoxia. In some experiments, HUVECs were treated with dimethylated glycine (100 μm), CoCl2 (250 μm) or vehicle (dimethyl sulfoxide) for the indicated lengths of time. In some experiments, HUVECs were transfected with siRNAs targeting NDNF or unrelated siRNAs at 100 nm (Thermo Scientific, ON-TARGET plus SMARTpool) by using Lipofectamine 2000 reagent (Invitrogen) according to the instructions of the manufacturer. In some experiments, HUVECs were pretreated with GRGDSP or GRGESP peptides (100 μm) before stimulation with NDNF protein. In some experiments, HUVECs were pretreated with anti-integrin αvβ3 antibody (20 μg/ml) and control IgG (20 μg/ml) prior to the addition of NDNF protein.
Assessment of Endothelial Cell Differentiation
The formation of vascular-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as described previously (20, 27). Differentiation was quantified by measuring the area of the “tube-like” networks that form in three randomly chosen fields from each well. Each experiment was repeated three times. Protein expression was determined by Western blot analysis.
Analysis of Apoptotic Activity
Apoptosis was assayed by a TUNEL staining method with a commercial kit (Roche) (13, 20). The mean number of apoptotic (TUNEL-positive) cells from three random fields (magnification ×40) in each well was calculated. Nucleosome fragmentation was assessed by ELISA using a cell death detection kit (Roche) according to the protocol of the manufacturer. Eight wells were used for one group, and assays were repeated three times.
Matrigel Plug Assay
Matrigel plug assay was also performed as described previously (27). Briefly, 450 μl of Matrigel containing Ad-NDNF (1 × 108 pfu) or Ad-β-gal (1 × 108 pfu) was injected subcutaneously into the abdomen of wild-type C57BL/6J mice. Mice were sacrificed on day 14 after the injection. The Matrigel plugs with adjacent subcutaneous tissues were fixed in 4% paraformaldehyde, dehydrated with 30% sucrose, and embedded in optimum cutting temperature compound (Miles, Elkhart, IN) in liquid nitrogen. Infiltration of microvessels was evaluated by immunohistostaining for CD31. CD31-positive capillaries were counted in four randomly chosen, low-power (×100) microscopic fields.
Magnetofection-mediated Silencing of NDNF in Vivo
To evaluate the physiological role of NDNF in revascularization in vivo, siRNAs targeting NDNF or unrelated siRNAs were injected into the left adductor muscle of wild-type mice at the time of hind limb ischemic surgery by using an in vivo PolyMag magnetofection kit (OZ Bioscience). Briefly, 30 μg of siRNA was gently mixed with 30 μl of in vivo PolyMag reagent. After 20 min of incubation, the complexes were slowly injected into five different sites of the adductor muscle in the left limb. During the procedure, a magnet was placed on the left adductor muscle for 20 min.
Western Blot Analysis
Tissue samples were homogenized in lysis buffer containing 1 mm PMSF (Cell Signaling Technology). Immunoblot analysis was performed with antibodies at a 1:1000 dilution, followed by incubation with a secondary antibody conjugated with horseradish peroxidase at a 1:5000 dilution. An ECL Plus Western blotting detection kit (GE Healthcare) was used.
Statistical Analysis
Data are presented as mean ± S.E. Differences between groups were evaluated by Student's t test or analysis of variance with Fisher's protected least significant difference test. p < 0.05 denoted the presence of a statistically significant difference.
RESULTS
NDNF Is Up-regulated in Endothelial Cells of Muscles in Response to Ischemia
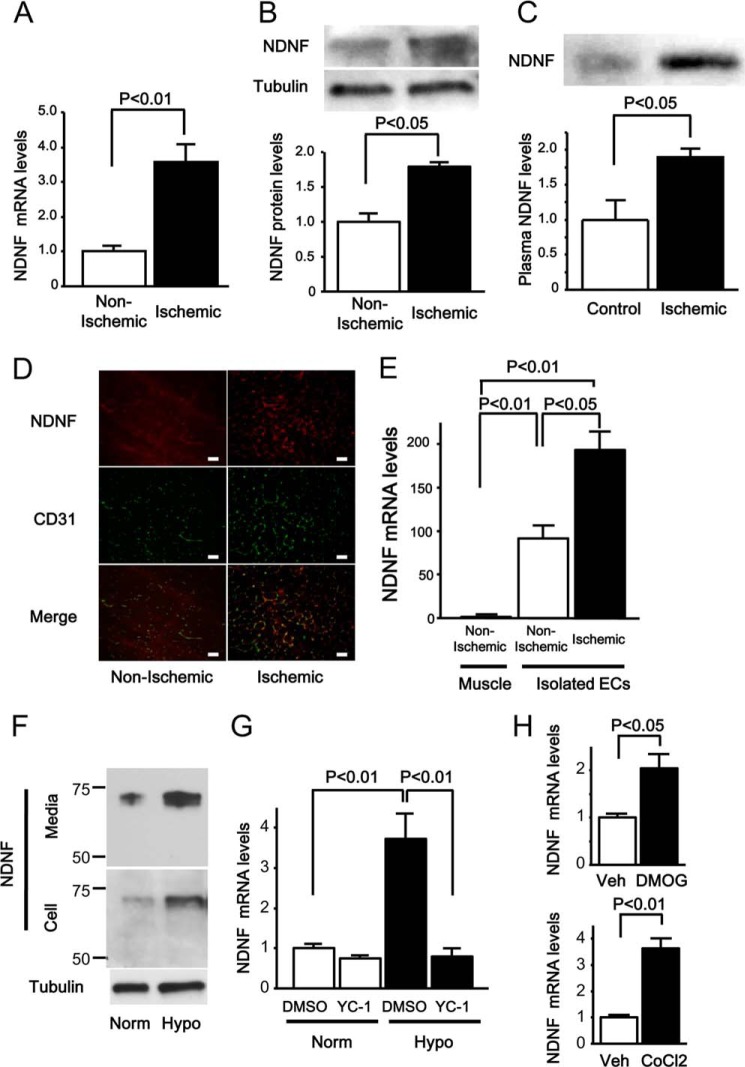
We analyzed the gene expression profiles of ischemic adductor muscle of C57BL/6J mice on day 14 after hind limb ischemic surgery compared with those of non-ischemic muscle by microarray analysis. Among the differentially regulated genes, we selected predicted secretory proteins by analysis of the presence of signal sequence and the absence of transmembrane domains using SignalP and SOSUI, respectively (13, 15, 16). On the basis of these screening strategies, we focused on NDNF/epidermacan because muscle ischemia up-regulated NDNF transcripts by a factor of 3.0. Consistent with these data, NDNF mRNA levels were increased significantly in ischemic muscle tissue by a factor of 4.6 ± 0.4 compared with those in non-ischemic muscle by quantitative real-time PCR analysis using the relative standard curve method (Fig. 1A). NDNF mRNA levels were normalized to 36B4. The protein levels of NDNF were also increased in the ischemic muscle compared with non-ischemic muscle, as assessed by Western blot analysis (Fig. 1B). Furthermore, plasma levels of NDNF were increased significantly following ischemic surgery compared with a sham operation, as determined by Western blot analysis (Fig. 1C).
FIGURE 1.
NDNF expression is increased in the endothelium of muscle tissue after hind limb ischemic surgery. A, NDNF mRNA levels in non-ischemic and ischemic adductor muscle of WT mice on day 14 after hind limb ischemic surgery (n = 4), as measured by real-time PCR. NDNF mRNA levels were expressed relative to levels of 36B4 mRNA and normalized to non-ischemic muscle controls. B, NDNF protein expression in non-ischemic and ischemic skeletal muscle on day 14 after operation, as determined by Western blot analysis. Bottom panel, quantitative analysis of the NDNF protein levels relative to tubulin as evaluated by the ImageJ program. n = 4 in each group. C, plasma NDNF levels on day 14 after hind limb ischemic or sham (Control) operation, as determined by Western blot analysis. Bottom panel, quantitative analysis of plasma NDNF expression. n = 6 in each group. D, localization of NDNF in non-ischemic and ischemic limbs by immunohistochemical analysis. Tissue sections of non-ischemic and ischemic limbs were stained with an antibody against NDNF (red) and an antibody against CD31 (green), and merged images are shown. Scale bars = 50 μm. E, NDNF mRNA levels in whole non-ischemic muscle and endothelial cells isolated from non-ischemic or ischemic muscle tissue. n = 4 in each group. NDNF mRNA levels were expressed relative to levels of 36B4 mRNA and normalized to non-ischemic muscle controls. EC, endothelial cells. F, NDNF is secreted from cultured endothelial cells. HUVECs were cultured under conditions of normoxia (Norm) or hypoxia (Hypo) for 24 h. NDNF protein expression was determined in medium and cell lysates by Western blot analysis. G, NDNF mRNA levels in HUVECs treated with YC-1 (10 μm) or vehicle (dimethyl sulfoxide (DMSO)) under normoxic or hypoxic conditions as determined by real-time PCR. NDNF mRNA levels were expressed relative to levels of 36B4 mRNA and normalized to dimethyl sulfoxide controls under normoxic conditions. n = 4 in each group. H, NDNF mRNA levels in HUVECs after treatment with dimethylated glycine (DMOG) (100 μm), CoCl2 (250 μm), or vehicle (Veh) for 6 h. NDNF mRNA levels were expressed relative to levels of 36B4 mRNA and normalized to vehicle controls. n = 4 in each group.
To dissect the localization of NDNF in muscle tissues, the tissue sections of non-ischemic and ischemic limbs of mice were stained with an antibody against NDNF (red). NDNF protein was detectable in both non-ischemic and ischemic muscles, and the more intense signal of NDNF was observed in ischemic tissues (Fig. 1D). Tissue sections were also stained with an antibody against the endothelial cell marker CD31 (green). Dual immunofluorescence staining demonstrated that NDNF was colocalized with endothelial cells (yellow) in the non-ischemic and ischemic limbs. To determine the source of NDNF in muscle tissues in vivo, endothelial cells were isolated from skeletal muscle tissues by treatment with magnet beads conjugated with an antibody against an endothelial cell marker, CD31. The mRNA expression of muscle creatine kinase, which is specifically expressed in myocytes, was detected in skeletal muscle tissues but not in isolated endothelial cells (data not shown). The mRNA levels of NDNF were markedly higher in endothelial cells isolated from muscle tissues than in whole muscle tissues, as quantified by real-time PCR (Fig. 1E). Moreover, the mRNA levels of NDNF were significantly higher in isolated endothelial cells from ischemic muscles compared with those from non-ischemic muscles. These data suggest that NDNF is mainly expressed in endothelial cells of skeletal muscles and that NDNF expression is enhanced in response to tissue ischemia.
To test whether NDNF is produced by cultured endothelial cells, NDNF protein expression was assessed in the cell lysate and media in HUVECs by Western blot analysis. NDNF protein was detected in both the cell lysate and medium of cultured HUVECs (Fig. 1F). NDNF protein was also detected in medium of cultured microvascular endothelial cells (data not shown). Exposure of HUVECs to 24 h of hypoxia resulted in a marked increase in NDNF protein level in the cell lysate and medium (Fig. 1F).
Because hypoxia leads to activation of hypoxia-inducible factor (HIF) (28), we assessed the possible contribution of HIF to hypoxic induction of NDNF in HUVECs. Pretreatment with the HIF inhibitor YC-1 suppressed the stimulatory effects of hypoxia on NDNF mRNA expression in HUVECs, as measured by real-time PCR analysis using the relative standard curve method (Fig. 1G). NDNF mRNA levels were normalized to 36B4. In line with these observations, treatment of HUVECs with the HIF-hydroxylase inhibitor dimethylated glycine, which up-regulates HIF, enhanced NDNF mRNA expression (Fig. 1H). Another hypoxia-mimicking agent, CoCl2, also increased NDNF mRNA expression in HUVECs (Fig. 1H). These data suggest that NDNF is an endothelial cell-derived secretory factor that is up-regulated in response to ischemia or hypoxia in a HIF-dependent fashion.
NDNF Promotes Endothelial Cell Network Formation and Survival in Vitro
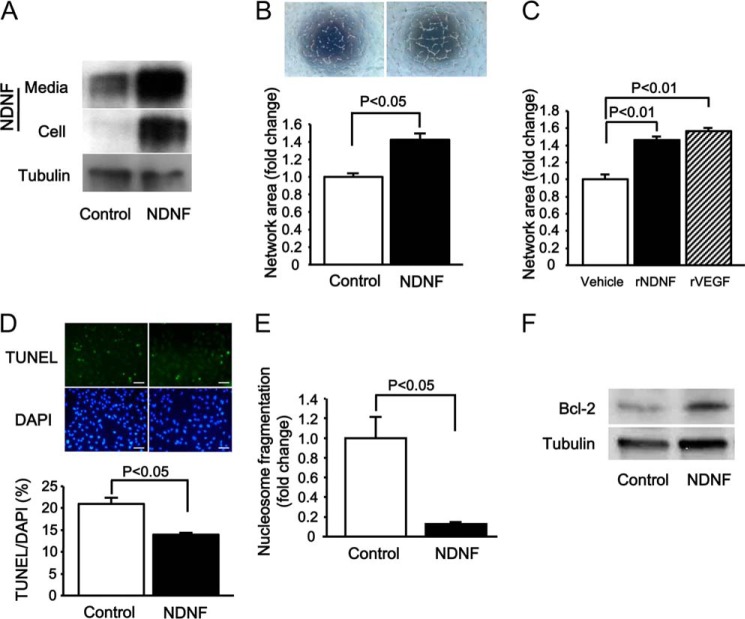
To examine whether NDNF affects endothelial cell function, HUVECs were transduced with an adenoviral vector expressing NDNF (Ad-NDNF) or Ad-β-gal as a control and cultured on a Matrigel matrix. Transduction with Ad-NDNF increased NDNF protein levels in both the cell lysate and medium compared with Ad-β-gal treatment (Fig. 2A). Quantitative analysis of network areas revealed that Ad-NDNF treatment significantly promoted HUVEC network formation compared with Ad-β-gal (Fig. 2B). To assess whether NDNF modulates endothelial cell network formation in a paracrine manner, HUVECs were treated with recombinant NDNF protein or vehicle. Treatment with NDNF protein enhanced the network formation of HUVECs (Fig. 2C). The effect of NDNF on the formation of network structure was comparable with that of VEGF.
FIGURE 2.
NDNF promotes endothelial cell network formation and survival in vitro. A, NDNF protein expression in medium and cell lysates from HUVECs as determined by Western blot analysis. HUVECs were transduced with adenoviral vectors encoding NDNF (Ad-NDNF, NDNF) or β-galactosidase (Ad-β-gal, Control) (multiplicity of infection of 25 each). B, HUVEC network formation in response to NDNF. After 24 h of transduction with Ad-NDNF and control Ad-β-gal, HUVECs were seeded on Matrigel-coated dishes for 16 h. Top panel, representative photographs of network formation. Bottom panel, quantitative analysis of network area. n = 4 in each group. C, HUVEC network formation in response to 16 h-treatment with recombinant NDNF protein (rNDNF)(200 ng/ml), recombinant VEGF protein (rVEGF)(50 ng/ml), or vehicle. n = 4 in each group. D, effect of NDNF on the frequency of TUNEL-positive HUVECs. HUVECs were transduced with Ad-NDNF and Ad-β-gal (Control) for 24 h, followed by subjection to serum deprivation for 48 h. Top panel, representative images of HUVECs stained with TUNEL (green) and DAPI (blue). Bottom panel, quantitative analysis of TUNEL-positive HUVECs. n = 4 in each group. Scale bars = 50 μm. E, effect of NDNF on nucleosome fragmentation of HUVECs. Nucleosome fragmentation of HUVECs following 48 h of serum deprivation was assessed by ELISA. n = 4 in each group. F, Bcl-2 protein expression in HUVECs after 24 h of treatment with Ad-NDNF or control Ad-β-gal as determined by Western blot analysis.
To test the effect of NDNF on endothelial apoptosis, HUVECs were transduced with Ad-NDNF or Ad-β-gal, cultured in serum-deprived medium for 48 h, and stained with TUNEL. Treatment with Ad-NDNF significantly reduced the number of TUNEL-positive cells (Fig. 2D). Consistent with these findings, Ad-NDNF significantly reduced the extent of nucleosome fragmentation under serum-deprived conditions (Fig. 2E). Because Bcl-2 plays an important role in the regulation of endothelial cell apoptosis (29, 30), we measured the protein expression of Bcl-2 in HUVECs after transduction with Ad-NDNF or Ad-β-gal. Ad-NDNF significantly increased the protein levels of Bcl-2 by a factor of 1.96 ± 0.22 (n = 3 in each group, p < 0.05) in HUVECs (Fig. 2F).
NDNF Stimulates the Phosphorylation of Akt and eNOS
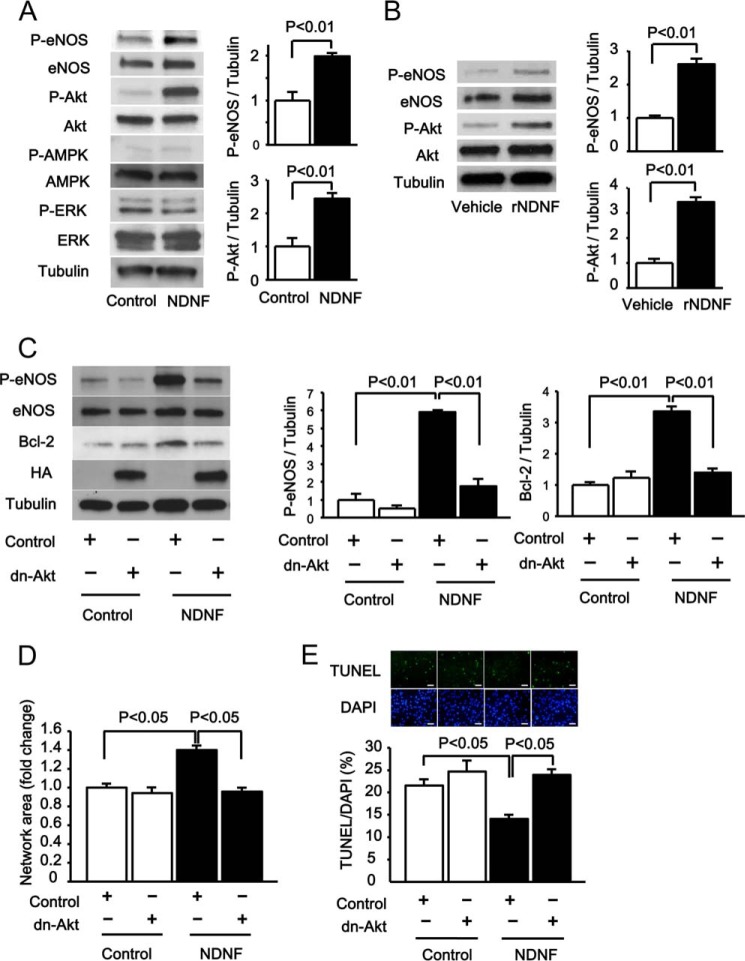
Because Akt is a key regulator of endothelial cell function and survival (26, 27, 30, 31), we tested whether NDNF affects Akt signaling in endothelial cells. Transduction with Ad-NDNF significantly increased the activating phosphorylation of Akt at Ser-473, as evaluated by Western blot analysis (Fig. 3A). Because eNOS functions downstream of Akt in endothelial cells (31–33), the phosphorylation status of eNOS at Ser-1177 was assessed by Western blot analysis. Treatment of HUVECs with Ad-NDNF also significantly enhanced eNOS phosphorylation (Fig. 3A). In contrast, Ad-NDNF had no effects on the phosphorylation levels of AMPK at Thr-172 and ERK at Thr-202/Tyr-204 in HUVECs (Fig. 3A). Furthermore, treatment of HUVECs with NDNF protein led to an increase in phosphorylation of Akt and eNOS (Fig. 3B).
FIGURE 3.
NDNF promotes endothelial cell responses via an Akt-dependent mechanism. A, NDNF-stimulated phosphorylation signals in HUVECs. Changes in the phosphorylation of eNOS (P-eNOS), Akt (P-Akt), AMPK (P-AMPK), and ERK (P-ERK) after 24 h of treatment with Ad-NDNF or Ad-β-gal (Control) were determined by Western blot analysis. Right panel, quantitative analyses of phosphorylation levels of eNOS and Akt relative to tubulin as evaluated by the ImageJ program. n = 3 in each group. B, phosphorylation levels of eNOS and Akt in HUVECs treated with recombinant NDNF protein (rNDNF) (200 ng/ml) or vehicle for 30 min. Right panel, quantitative analyses of phosphorylation levels of eNOS and Akt relative to tubulin as assessed by the ImageJ program. n = 3 in each group. C, involvement of Akt in NDNF-stimulated increases in eNOS activation and Bcl-2 expression. HUVECs were transduced with adenoviral vectors expressing dominant negative mutant of Akt1 tagged with HA (Ad-dn-Akt, dn-Akt) or β-gal (Control) along with Ad-NDNF or Ad-β-gal (Control) for 24 h. Right panel, quantitative analyses of eNOS phosphorylation and Bcl-2 expression relative to tubulin as evaluated by the ImageJ program. n = 3 in each group. D, role of Akt in NDNF-stimulated endothelial cell network formation. HUVECs were transduced with Ad-dn-Akt or Ad-β-gal (Control) along with Ad-NDNF or Ad-β-gal (Control) for 24 h. After 24 h of serum deprivation, Matrigel assays were performed. The quantitative analysis of the network area is shown. n = 4 in each group. E, involvement of Akt in NDNF-stimulated endothelial cell survival. After transduction with Ad-dn-Akt or Ad-β-gal (Control) along with Ad-NDNF or Ad-β-gal (Control) for 24 h, cells were incubated in serum-deprived medium for 48 h. HUVEC apoptosis was evaluated by TUNEL staining. n = 4 in each group. Scale bars = 50 μm.
To examine the role of Akt in the regulation of NDNF-induced eNOS phosphorylation, HUVECs were infected with an adenoviral vector producing dominant negative mutant forms of Akt tagged with HA (Ad-dn-Akt) or Ad-β-gal. Transduction of HUVECs with Ad-dn-Akt significantly abolished NDNF-induced eNOS phosphorylation (Fig. 3C). Transduction with Ad-dn-Akt also significantly abolished NDNF-stimulated expression of Bcl-2 in HUVECs (Fig. 3C). These data indicate that NDNF promotes eNOS activation and Bcl-2 induction via activation of Akt.
Akt Is Required for the Endothelial Cell Response to NDNF
To investigate whether Akt is involved in NDNF-induced endothelial cell function and viability, HUVECs were transduced with Ad-dn-Akt or Ad-β-gal, and endothelial cell responses were assessed. Transduction with Ad-dn-Akt abolished NDNF-stimulated network formation of HUVECs (Fig. 3D). Ad-dn-Akt treatment also restored the suppressive actions of NDNF on the frequency of TUNEL-positive cells (Fig. 3E). These observations indicate that the actions of NDNF on endothelial cell behavior are mediated partly through its ability to activate Akt.
Role of Integrin in NDNF-stimulated Endothelial Cell Function
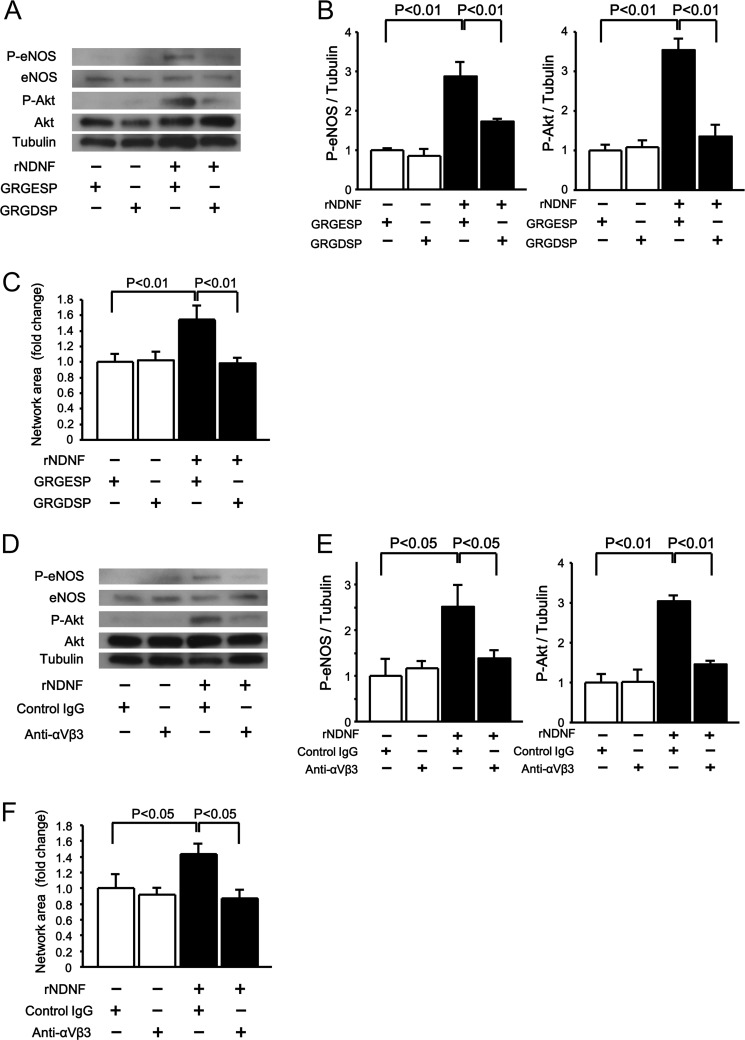
NDNF has the fibronectin type III domains, and this allowed us to speculate that NDNF modulates integrin-dependent signals. To further elucidate the upstream mechanism underlying NDNF-stimulated Akt activation and endothelial cell function, HUVECs were treated with integrin-blocking RGD-based peptides (GRGDSP) or control peptides (GRGESP). Pretreatment with GRGDSP peptides blocked the stimulatory actions of NDNF protein on the phosphorylation of Akt and eNOS compared with GRGESP peptides (Fig. 4, A and B). GRGDSP peptides also reversed the NDNF-enhanced network formation of HUVECs (Fig. 4C). In accordance with these findings, pretreatment with a neutralizing antibody against integrin αvβ3 suppressed NDNF-induced increase in Akt and eNOS phosphorylation and network formation of HUVECs compared with control antibody (Fig. 4, D–F).
FIGURE 4.
NDNF promotes endothelial cell function and phosphorylation signaling pathways via integrin αvβ3. A–C, effect of integrin-blocking peptides on NDNF-stimulated phosphorylation signals and endothelial cell network formation. HUVECs were pretreated with integrin-blocking RGD-based peptides (GRGDSP) or control peptides (GRGESP) (100 μm each), followed by stimulation with recombinant NDNF protein (rNDNF) (200 ng/ml) or vehicle. Phosphorylation levels of eNOS (P-eNOS) and Akt (P-Akt) were determined by Western blot analysis (A and B). B, phosphorylation levels of eNOS and Akt were expressed relative to tubulin levels and normalized to controls. n = 3 in each group. C, Matrigel assays were performed. n = 4 in each group. D–F, effect of anti-integrin αvβ3 antibody on the NDNF-induced increase in phosphorylation of eNOS and Akt (D and E) and network formation (F) of HUVECs. HUVECs were pretreated with anti-integrin αvβ3 antibody (20 μg/ml) and control IgG (20 μg/ml), and treated with recombinant NDNF protein (200 ng/ml) or vehicle. Levels of P-eNOS and P-Akt were determined by Western blot analysis. E, phosphorylation levels of eNOS and Akt were expressed relative to tubulin levels and normalized to controls. n = 3 in each group. F, Matrigel assays were performed. n = 4 in each group.
Role of Endogenous NDNF in Endothelial Cell Behavior in Vitro
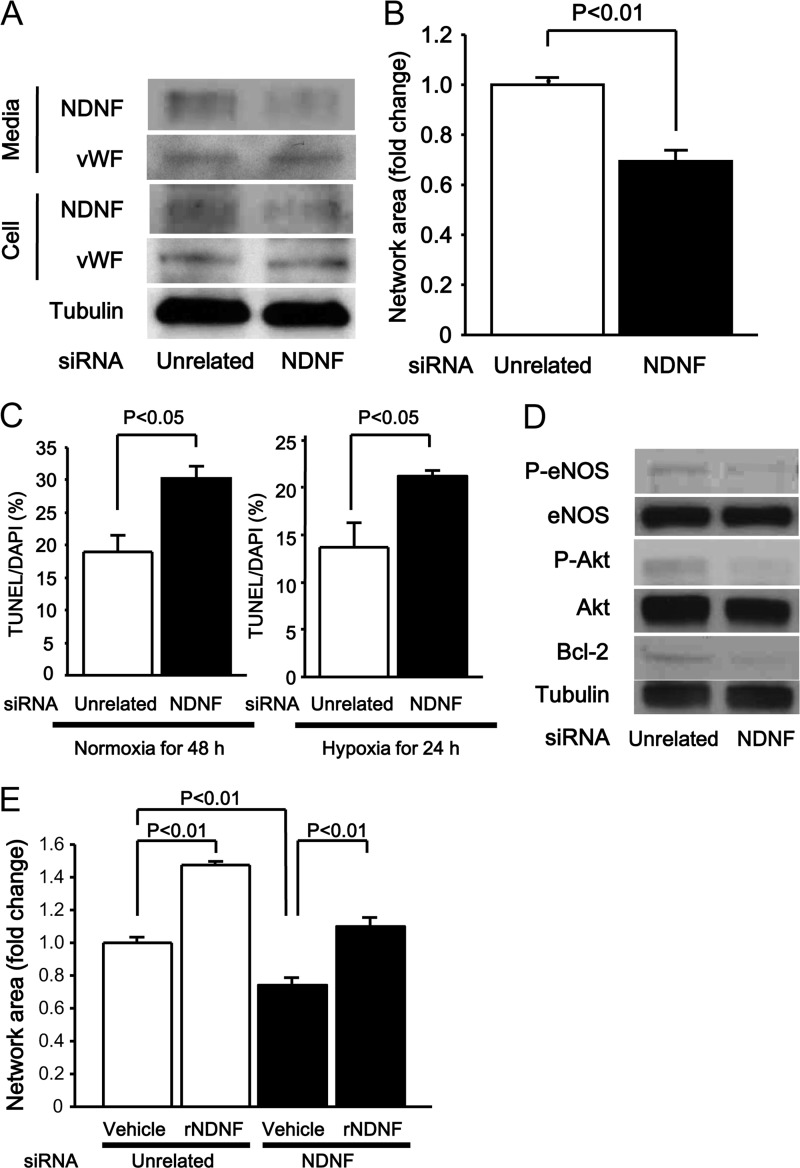
To test whether endogenous NDNF affects endothelial cell behavior, HUVECs were treated with siRNAs against NDNF or unrelated siRNAs. Treatment of HUVECs with siRNAs for NDNF reduced NDNF protein levels by a factor of 0.39 ± 0.05 (n = 3 in each group, p < 0.05) in cell lysate and by a factor of 0.52 ± 0.01 (n = 3 in each group, p < 0.05) in culture medium (Fig. 5A). On the other hand, knockdown of NDNF did not affect the protein levels of von Willebrand factor in cell lysate and culture medium. Knockdown of NDNF significantly attenuated the formation of network structures (Fig. 5B). Deletion of NDNF also enhanced the frequency of TUNEL-positive cells in response to serum starvation under conditions of normoxia for 48 h or hypoxia for 24 h (Fig. 5C). Moreover, knockdown of NDNF led to reduction of the basal phosphorylation of Akt by a factor of 0.26 ± 0.02 (n = 3 in each group, p < 0.05), eNOS by a factor of 0.32 ± 0.08 (n = 3 in each group, p < 0.05), and Bcl-2 by a factor of 0.12 ± 0.04 (n = 3 in each group, p < 0.01) in HUVECs (Fig. 5D). Reduction of HUVEC network formation caused by NDNF deletion was restored significantly by treatment with NDNF protein (Fig. 5E).
FIGURE 5.
Role of endogenous NDNF in endothelial cell responses and signals. A, NDNF protein expression in medium and cell lysates from HUVECs. HUVECs were transfected with siRNA targeting NDNF or unrelated siRNAs. Protein levels of NDNF and von Willebrand factor (vWF) were determined in medium and cell lysates from HUVECs by Western blot analysis. B, effect of NDNF knockdown on HUVEC network formation. After transfection with siRNA targeting NDNF or unrelated siRNAs, HUVECs were seeded on Matrigel-coated dishes. The quantitative analysis of the network area is shown. n = 4 in each group. C, effect of NDNF ablation on HUVEC apoptosis. HUVECs were transfected with siRNA targeting NDNF or unrelated siRNAs for 24 h, followed by subjection to serum deprivation under conditions of normoxia for 48 h or under conditions of hypoxia for 24 h. Apoptosis was assessed by TUNEL staining. The quantitative analysis of TUNEL-positive HUVECs is shown. n = 4 in each group. D, effect of NDNF knockdown on phosphorylation signals and Bcl-2 expression in HUVECs as determined by Western blot analysis. E, reversal effect of NDNF on HUVEC network formation induced by NDNF ablation. HUVECs were cultured on Matrigel-coated dishes in the presence of recombinant NDNF protein (rNDNF) (200 ng/ml) or vehicle for 16 h after transfection with siRNA targeting NDNF or unrelated siRNAs. n = 4 in each group. P-eNOS, phosphorylated eNOS at Ser-1777; P-Akt, phosphorylated Akt at Ser-473.
Overexpression of NDNF Accelerates Ischemia-induced Revascularization in Vivo
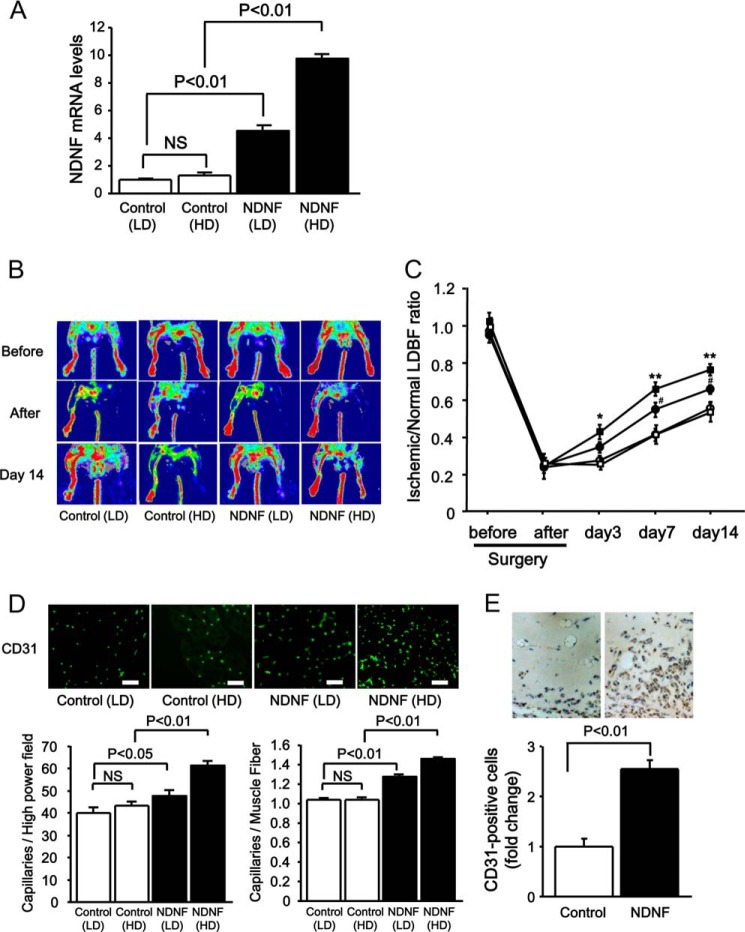
To evaluate the effects of NDNF on revascularization in vivo, C57BL/6J mice were treated intramuscularly with Ad-NDNF (low dose (LD), 4 × 107 pfu/mouse and high dose (HD), 1 × 108 pfu/mouse) or Ad-β-gal (LD, 4 × 107 pfu/mouse and HD, 1 × 108 pfu/mouse), followed by subjection to hind limb ischemia. Intramuscular injection of Ad-NDNF dose-dependently increased NDNF mRNA expression in ischemic skeletal muscles compared with Ad-β-gal treatment, as assessed by quantitative real-time PCR analysis using the relative standard curve method (Fig. 6A). All signals were normalized to 36B4. Ad-NDNF-treated mice (NDNF (LD) and NDNF (HD)) showed increased blood flow recovery in ischemic limbs at day 3, 7, or 14 after operation compared with Ad-β-gal-treated mice (Control (LD) and Control (HD)), as evaluated by laser Doppler blood flow analysis (Fig. 6, B and C).
FIGURE 6.
NDNF promotes blood vessel formation in vivo. Adenoviral vectors producing NDNF (Ad-NDNF, NDNF at an LD of 4 × 107 pfu or at an HD of 1 × 108 pfu) or β-galactosidase (Ad-β-gal, control at an LD of 4 × 107 pfu or at an HD of 1 × 108 pfu) were injected into five different sites in the adductor muscles of WT mice 3 days prior to ischemic surgery. A, NDNF mRNA expression in ischemic muscle 17 days after injection of Ad-NDNF or control Ad-β-gal. n = 3 in the Control (LD) and Control (HD) group as measured by real-time PCR. n = 4 in the NDNF (LD) and NDNF (HD) groups. NDNF mRNA levels were expressed relative to levels of 36B4 mRNA and normalized to the values of Control (LD). B and C, effect of NDNF on blood flow recovery in the ischemic limb. B, representative LDBF images of limb blood flow in Ad-NDNF-treated and control Ad-β-gal-treated mice before surgery, immediately after surgery, and on day 14 after surgery. White to red indicates a high perfusion signal, whereas blue shows a low perfusion signal. C, quantitative analysis of the ischemic/non-ischemic LDBF ratio of NDNF-treated (●, LD; ■, HD) and control-treated (○, LD; □, HD) mice (n = 8 in each group). *, p < 0.05 versus control; **, p < 0.01 versus control. D, effect of NDNF on capillary density in the ischemic limb. Shown is the representative immunostaining of ischemic muscle tissues with anti-CD31 antibody (green) on postoperative day 14. Bottom panel, quantitative analyses of capillary density in ischemic muscles of Ad-NDNF-treated (NDNF (LD) and NDNF (HD)) and control-treated (Control (LD) and Control (HD)) mice on postoperative day 14 (n = 8 in each group). Capillary density was expressed as the number of capillaries per high-power field (left panel) and per muscle fiber (right panel). Scale bars = 50 μm. E, effect of NDNF on capillary formation in Matrigel plugs. Matrigel plugs containing Ad-NDNF or control Ad-β-gal (1 × 108 pfu each) were injected subcutaneously into mice. On day 14 after injection, plugs were stained with CD31. Quantitative analysis of CD31-positive cells is shown. n = 6 in each group.
To assess the extent of revascularization at a microcirculatory level, capillary density in ischemic skeletal muscles was measured by staining with CD31. The number of CD31-positive cells was significantly higher in Ad-NDNF-treated mice (NDNF (LD) and NDNF (HD)) than in Ad-β-gal-treated mice (Control (LD) and Control (HD)) (Fig. 6D).
To further examine the effects of NDNF on the angiogenic response in vivo, we performed a mouse Matrigel plug assay by injecting Matrigel with Ad-NDNF or Ad-β-gal as a control. Endothelial cell infiltration into the plug was assessed by immunohistochemical staining with CD31. Quantitative analysis revealed that the plugs containing NDNF had a higher frequency of CD31-positive cells compared with the control (Fig. 6E).
NDNF Promotes Blood Flow Recovery through an eNOS-dependent Mechanism
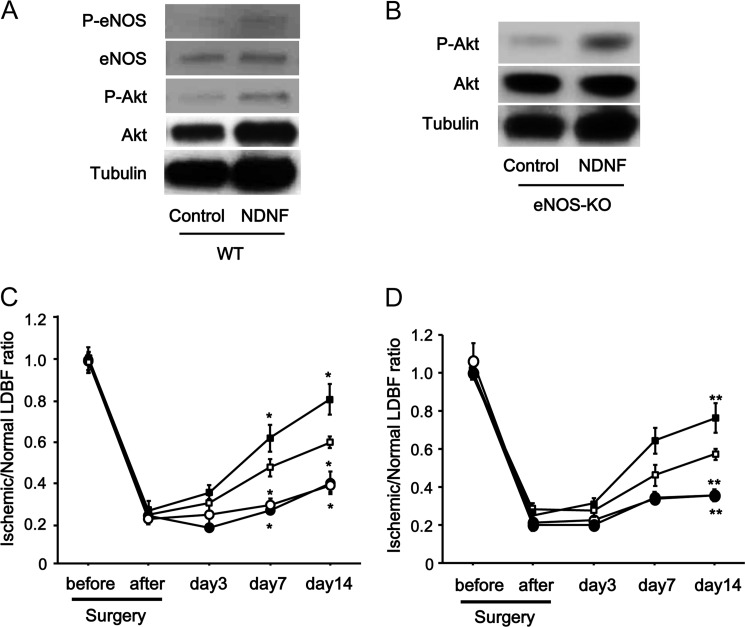
To investigate the contribution of eNOS signaling to the NDNF-mediated revascularization process in vivo, the phosphorylation levels of eNOS and Akt in ischemic muscle were evaluated by Western blot analysis. Ad-NDNF treatment enhanced eNOS phosphorylation at Ser-1177 in ischemic adductor muscles in WT mice by a factor of 1.97 ± 0.26 (n = 3 in each group, p < 0.05) compared with Ad-β-gal (Fig. 7A). Akt phosphorylation in ischemic muscles was also increased by a factor of 1.76 ± 0.17 (n = 3 in each group, p < 0.05) by Ad-NDNF treatment.
FIGURE 7.
Role of eNOS in NDNF-mediated revascularization in ischemic limbs. A, NDNF promotes phosphorylation of eNOS and Akt in ischemic limbs of WT mice. Phosphorylation of eNOS (P-eNOS) and Akt (P-Akt) in ischemic muscles of Ad-NDNF-treated or control Ad-β-gal-treated WT mice (4 × 107 pfu each) was analyzed by Western blotting. B, NDNF stimulates Akt phosphorylation in ischemic limbs of eNOS-KO mice. Phosphorylation of Akt in ischemic muscles of Ad-NDNF-treated or control Ad-β-gal-treated eNOS-KO mice (4 × 107 pfu each) was determined by Western blot analysis. C, effect of NDNF on perfusion recovery in eNOS-KO and WT mice. Quantitative analysis of the ischemic/non-ischemic LDBF ratio in eNOS-KO and WT mice treated with Ad-NDNF (●, eNOS-KO; ■, WT) or Ad-β-gal (○, eNOS-KO; □, WT) (4 × 107 pfu each) is shown. n = 5 or 6 in each group. *, p < 0.05 for Ad-β-gal-treated WT mice. D, effect of NDNF on blood flow recovery under conditions of NOS inhibition. Shown is the quantitative analysis of the ischemic/non-ischemic LDBF ratio in Ad-NDNF-treated (●, l-NAME; ■, vehicle) or control-treated (○, l-NAME; □, vehicle) WT mice (4 × 107 pfu each) receiving the NOS inhibitor l-NAME or vehicle. n = 5 or 6 in each group. **, p < 0.01 for vehicle and Ad-β-gal-treated WT mice.
To assess the causal role of eNOS in NDNF-stimulated revascularization in response to ischemia in vivo, we investigated the effect of NDNF on perfusion recovery of ischemic limbs in eNOS-KO mice. Ad-NDNF stimulated the phosphorylation of Akt in ischemic limbs in eNOS-KO mice by a factor of 1.99 ± 0.16 (n = 3 in each group, p < 0.01), as measured by Western blot analysis (Fig. 7B). In contrast to WT mice, Ad-NDNF had no effects on the perfusion of ischemic limbs in eNOS-KO mice compared with Ad-β-gal, as evaluated by laser Doppler blood flow analysis (Fig. 7C). We also investigated the effect of NDNF on the blood flow of ischemic muscles in WT mice receiving the NOS inhibitor l-NAME. No significant differences in blood flow recovery were observed between Ad-β-gal-treated and Ad-NDNF-treated WT mice during treatment with l-NAME (Fig. 7D). These data suggest that the actions of NDNF on ischemia-driven revascularization in vivo are attributed to eNOS activation.
Role of Endogenous NDNF in Ischemia-induced Revascularization in Vivo
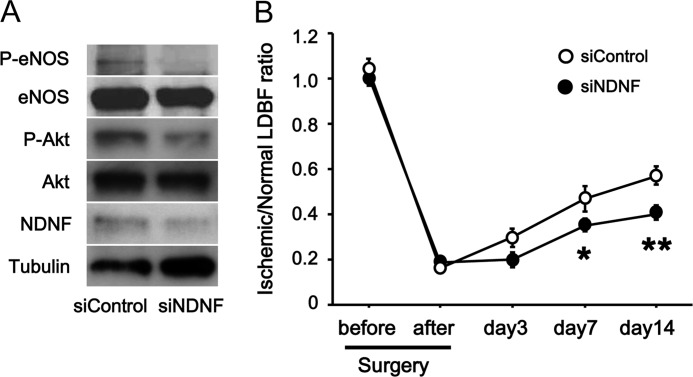
To examine whether endogenous NDNF modulates the revascularization processes under conditions of ischemia in vivo, we injected siRNAs attached to magnetic nanoparticles into adductor muscles of WT mice subjected to hind limb ischemia. Intramuscular delivery of siRNAs targeting NDNF led to a 60% reduction of NDNF protein levels compared with treatment with unrelated siRNAs, which was accompanied by the reduced phosphorylation of Akt and eNOS in ischemic limbs (Fig. 8A). Furthermore, knockdown of NDNF resulted in impaired flow recovery in ischemic muscle of WT mice at days 7 and 14 after hind limb ischemic surgery, as assessed by laser Doppler blood flow analysis (Fig. 8B).
FIGURE 8.
Contribution of endogenous NDNF to revascularization in response to ischemia in vivo. WT mice were treated intramuscularly with magnetic nanoparticle-attached siRNAs targeting NDNF or control siRNAs, followed by subjection to hind limb ischemia. A, NDNF expression and phosphorylation of eNOS (P-eNOS) and Akt (P-Akt) in the ischemic limb after transfection with siRNAs targeting NDNF (siNDNF) or unrelated siRNAs (siControl). B, blood flow recovery in ischemic muscle following intramuscular delivery of siRNAs targeting NDNF or unrelated siRNAs. Quantitative analysis of the ischemic/non-ischemic LDBF ratio in WT mice treated with siRNAs against NDNF or unrelated siRNAs is shown. n = 6 in each group. *, p < 0.05 versus control; **, p < 0.01 versus control.
DISCUSSION
In this study, we found, for the first time, that NDNF functions to promote endothelial cell function and enhance blood vessel recruitment. NDNF was up-regulated in the endothelium of ischemic muscle and that its secretion was enhanced in hypoxic endothelial cells. Treatment of endothelial cells with NDNF led to an increase in network formation and survival in vitro. Conversely, ablation of NDNF resulted in a reduction of endothelial cell network formation and viability. Intramuscular overexpression of NDNF enhanced perfusion recovery and capillary formation in a mouse model of hind limb ischemia. The proangiogenic activity of NDNF was also documented in the mouse Matrigel plug assay. Furthermore, deletion of NDNF in skeletal muscle led to impaired flow recovery in ischemic limbs of mice. Therefore, multiple lines of evidence suggest that NDNF serves as an ischemia-inducible hormonal factor secreted mainly from endothelial cells that can modulate vascular responses.
eNOS plays a crucial role in the maintenance of vascular homeostasis (32–34). Our data showed that adenovirus-mediated overexpression of NDNF enhances eNOS phosphorylation in ischemic muscle tissues and cultured endothelial cells. In contrast, siRNA-mediated ablation of NDNF contributed to reduced phosphorylation of eNOS in ischemic limbs and endothelial cells. Furthermore, NDNF-induced enhancement of blood flow in ischemic muscle was abolished under conditions of eNOS deficiency or NOS inhibition in vivo. Thus, NDNF can act as a positive regulator of eNOS activity in endothelial cells, and it can promote revascularization in response to ischemia via an eNOS-dependent mechanism.
It is well established that Akt activation leads to stimulation of endothelial cell function and neovascularization (30, 31, 35). In this study, NDNF treatment promoted the activating phosphorylation of Akt in ischemic muscle and endothelial cells. Inhibition of Akt activation blocked the NDNF-induced increase in endothelial cell network formation and survival. Therefore, Akt signaling is essential for the vascular effects of NDNF. Furthermore, blockade of Akt activity abrogated NDNF-induced phosphorylation of eNOS in endothelial cells. Collectively, these data suggest that the NDNF-Akt-eNOS signaling axis plays a pivotal role in regulating endothelial cell response and blood vessel growth during ischemia.
Integrin αvβ3 binds and recognizes both RGD-containing and non-RGD-containing ligands (36, 37). NDNF contains the fibronectin type III domain but not the RGD motif, which is typically present in αvβ3 ligands such as fibronectin. Several αvβ3 ligands, including platelet endothelial cell adhesion molecule, MMP-2, and Cyr61, have no RGD sequence, and the interaction of these proteins with integrin αvβ3 is inhibited by the RGD peptides (38–40). In line with these observations, an RGD-containing peptide and an anti-integrin αvβ3 function-blocking antibody effectively blocked the effects of NDNF on endothelial cell phenotypic changes and signal transduction pathways. Thus, NDNF can modulate the endothelial cell response, at least in part, through integrin αvβ3-dependent mechanisms.
Endothelial cell apoptosis is a key feature in various vascular disorders, including atherosclerosis (41). Our data showed that adenovirus-mediated overexpression of NDNF can protect endothelial cells from apoptosis under conditions of serum starvation. Knockdown of NDNF exacerbated endothelial cell apoptosis in response to serum starvation under normoxic or hypoxic conditions. Given that NDNF was up-regulated in ischemic endothelium or hypoxic endothelial cells, NDNF can serve as a positive feedback loop to prevent endothelial cell apoptosis, or it can function to reduce the apoptosis of nearby endothelial cells. Out data also indicated that the stimulatory effects of NDNF on survival and Bcl-2 expression of endothelial cells were dependent, at least in part, on its ability to activate Akt signaling. Furthermore, NDNF knockdown resulted in attenuation of Bcl-2 expression and Akt phosphorylation. Because the Akt-Bcl-2 regulatory axis participates in cellular survival (29, 30), the protective actions of NDNF on endothelial cell death may result from the induction of this prosurvival signaling pathway. Taken together, it is conceivable that NDNF functions as an endogenous modulator that confers resistance to endothelial cell death under pathological conditions, thereby contributing to the vascular protection.
NDNF has been identified as a neurotrophic factor that can modulate neurite growth (14). Several neurotrophic factors have been reported to stimulate angiogenic responses in vascular endothelial cells (42–45). For example, brain-derived neurotrophic factor induces the survival and migration of endothelial cells and stimulates angiogenesis (42, 43). Similarly, nerve growth factor promotes the proliferation of endothelial cells and stimulates angiogenesis in vivo (44, 45). Furthermore, secretoneurin is a neuropeptide that promotes the angiogenic signaling pathways, including Akt, and stimulates the proliferation and network formation of endothelial cells in vitro (46). Secretoneurin has also been reported to stimulate the revascularization responses in a mouse model of hind limb ischemia by a nitric oxide-dependent mechanism (47). Similar to these neuron-derived factors or neuropeptides, NDNF exerts prosurvival and proangiogenic properties in vitro and in vivo.
A number of proangiogenic growth factors, including VEGF, are up-regulated in ischemic muscle (6, 11–13). Hypoxic conditions increase the expressions of VEGF via activation of HIF (28). In this study, limb ischemia led to up-regulation of NDNF, and hypoxia enhanced the expression of NDNF in cultured endothelial cells via HIF-dependent mechanisms. Thus, tissue ischemia can stimulate angiogenic responses and maintain blood flow through modulation of HIF-inducible angiogenic factors, including VEGF and NDNF. It has been shown that VEGF enhances revascularization in response to tissue ischemia via activation of Akt-eNOS-dependent signaling in endothelial cells (32, 48, 49). Our pilot data showed that overexpression of NDNF had no effect on the expression levels of VEGF in ischemic muscle tissue (data not shown). Thus, it is likely that the effects of NDNF on revascularization are mediated through its ability to directly activate angiogenic signaling within endothelial cells.
In conclusion, this study is the first demonstration that NDNF is endothelium-derived secreted factor that plays an important role in the process of revascularization in vitro and in vivo. Our data suggest that NDNF could be a potentially useful therapeutic target for treatment of ischemic cardiovascular disease.
Acknowledgment
We thank Yoko Inoue for technical assistance.
This work was supported by a grant-in-aid for scientific research; a grant-in-aid for challenging exploratory research; and grants from the Akeda Science Foundation, the Uehara Memorial Foundation, the Daiichi-Sankyo Foundation of Life Science, and the SENSHIN Medical Research Foundation (to N. O.).
- NDNF
- neuron-derived neurotrophic factor
- AMPK
- AMP-activated protein kinase
- eNOS
- endothelial NOS
- l-NAME
- NG-nitro-l-arginine methyl ester
- Ad
- adenovirus
- dn
- dominant negative
- LDBF
- laser Doppler blood flow
- HUVEC
- human umbilical vein endothelial cell
- HIF
- hypoxia-inducible factor
- LD
- low dose
- HD
- high dose.
REFERENCES
- 1. Roger V. L., Go A. S., Lloyd-Jones D. M., Adams R. J., Berry J. D., Brown T. M., Carnethon M. R., Dai S., de Simone G., Ford E. S., Fox C. S., Fullerton H. J., Gillespie C., Greenlund K. J., Hailpern S. M., Heit J. A., Ho P. M., Howard V. J., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Makuc D. M., Marcus G. M., Marelli A., Matchar D. B., McDermott M. M., Meigs J. B., Moy C. S., Mozaffarian D., Mussolino M. E., Nichol G., Paynter N. P., Rosamond W. D., Sorlie P. D., Stafford R. S., Turan T. N., Turner M. B., Wong N. D., Wylie-Rosett J. (2011) Heart disease and stroke statistics: 2011 update: a report from the American Heart Association. Circulation 123, e18-e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy S. M., Brewer H. B., Jr., Cleeman J. I., Smith S. C., Jr., Lenfant C. (2004) Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler. Thromb. Vasc. Biol. 24, e13–18 [DOI] [PubMed] [Google Scholar]
- 3. Yilmaz M. B., Biyikoglu S. F., Akin Y., Guray U., Kisacik H. L., Korkmaz S. (2003) Obesity is associated with impaired coronary collateral vessel development. Int. J. Obes. Relat. Metab. Disord. 27, 1541–1545 [DOI] [PubMed] [Google Scholar]
- 4. Turhan H., Yasar A. S., Erbay A. R., Yetkin E., Sasmaz H., Sabah I. (2005) Impaired coronary collateral vessel development in patients with metabolic syndrome. Coron. Artery Dis. 16, 281–285 [DOI] [PubMed] [Google Scholar]
- 5. Losordo D. W., Dimmeler S. (2004) Therapeutic angiogenesis and vasculogenesis for ischemic disease: part I: angiogenic cytokines. Circulation 109, 2487–2491 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 7. Simons M. (2005) Angiogenesis: where do we stand now? Circulation 111, 1556–1566 [DOI] [PubMed] [Google Scholar]
- 8. Couffinhal T., Silver M., Zheng L. P., Kearney M., Witzenbichler B., Isner J. M. (1998) Mouse model of angiogenesis. Am. J. Pathol. 152, 1667–1679 [PMC free article] [PubMed] [Google Scholar]
- 9. Emanueli C., Minasi A., Zacheo A., Chao J., Chao L., Salis M. B., Straino S., Tozzi M. G., Smith R., Gaspa L., Bianchini G., Stillo F., Capogrossi M. C., Madeddu P. (2001) Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation 103, 125–132 [DOI] [PubMed] [Google Scholar]
- 10. Goukassian D. A., Qin G., Dolan C., Murayama T., Silver M., Curry C., Eaton E., Luedemann C., Ma H., Asahara T., Zak V., Mehta S., Burg A., Thorne T., Kishore R., Losordo D. W. (2007) Tumor necrosis factor-α receptor p75 is required in ischemia-induced neovascularization. Circulation 115, 752–762 [DOI] [PubMed] [Google Scholar]
- 11. Schiekofer S., Galasso G., Sato K., Kraus B. J., Walsh K. (2005) Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler. Thromb. Vasc. Biol. 25, 1603–1609 [DOI] [PubMed] [Google Scholar]
- 12. Asano T., Kaneko E., Shinozaki S., Imai Y., Shibayama M., Chiba T., Ai M., Kawakami A., Asaoka H., Nakayama T., Mano Y., Shimokado K. (2007) Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ. J. 71, 405–411 [DOI] [PubMed] [Google Scholar]
- 13. Ouchi N., Oshima Y., Ohashi K., Higuchi A., Ikegami C., Izumiya Y., Walsh K. (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283, 32802–32811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuang X. L., Zhao X. M., Xu H. F., Shi Y. Y., Deng J. B., Sun G. T. (2010) Spatio-temporal expression of a novel neuron-derived neurotrophic factor (NDNF) in mouse brains during development. BMC Neurosci. 11, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouchi N., Higuchi A., Ohashi K., Oshima Y., Gokce N., Shibata R., Akasaki Y., Shimono A., Walsh K. (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oshima Y., Ouchi N., Sato K., Izumiya Y., Pimentel D. R., Walsh K. (2008) Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117, 3099–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enomoto T., Ohashi K., Shibata R., Higuchi A., Maruyama S., Izumiya Y., Walsh K., Murohara T., Ouchi N. (2011) Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J. Biol. Chem. 286, 34552–34558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 19. Galasso G., Schiekofer S., Sato K., Shibata R., Handy D. E., Ouchi N., Leopold J. A., Loscalzo J., Walsh K. (2006) Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ. Res. 98, 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohashi K., Ouchi N., Sato K., Higuchi A., Ishikawa T. O., Herschman H. R., Kihara S., Walsh K. (2009) Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol. Cell. Biol. 29, 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohashi K., Ouchi N., Higuchi A., Shaw R. J., Walsh K. (2010) LKB1 deficiency in Tie2-Cre-expressing cells impairs ischemia-induced angiogenesis. J. Biol. Chem. 285, 22291–22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouchi N., Shibata R., Walsh K. (2005) AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 96, 838–846 [DOI] [PubMed] [Google Scholar]
- 23. Takemura Y., Fukuo K., Yasuda O., Inoue T., Inomata N., Yokoi T., Kawamoto H., Suhara T., Ogihara T. (2004) Fas signaling induces Akt activation and upregulation of endothelial nitric oxide synthase expression. Hypertension 43, 880–884 [DOI] [PubMed] [Google Scholar]
- 24. Ogura Y., Ouchi N., Ohashi K., Shibata R., Kataoka Y., Kambara T., Kito T., Maruyama S., Yuasa D., Matsuo K., Enomoto T., Uemura Y., Miyabe M., Ishii M., Yamamoto T., Shimizu Y., Walsh K., Murohara T. (2012) Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical animal models. Circulation 126, 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cikos S., Bukovská A., Koppel J. (2007) Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol. Biol. 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagata D., Mogi M., Walsh K. (2003) AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 27. Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greer S. N., Metcalf J. L., Wang Y., Ohh M. (2012) The updated biology of hypoxia-inducible factor. EMBO J. 31, 2448–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pugazhenthi S., Nesterova A., Sable C., Heidenreich K. A., Boxer L. M., Heasley L. E., Reusch J. E. (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 275, 10761–10766 [DOI] [PubMed] [Google Scholar]
- 30. Fujio Y., Walsh K. (1999) Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 274, 16349–16354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiojima I., Walsh K. (2002) Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 90, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 32. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 34. Luo Z., Fujio Y., Kureishi Y., Rudic R. D., Daumerie G., Fulton D., Sessa W. C., Walsh K. (2000) Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J. Clin. Invest. 106, 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kureishi Y., Luo Z., Shiojima I., Bialik A., Fulton D., Lefer D. J., Sessa W. C., Walsh K. (2000) The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 6, 1004–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 37. Plow E. F., Haas T. A., Zhang L., Loftus J., Smith J. W. (2000) Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 [DOI] [PubMed] [Google Scholar]
- 38. Kireeva M. L., Lam S. C., Lau L. F. (1998) Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J. Biol. Chem. 273, 3090–3096 [DOI] [PubMed] [Google Scholar]
- 39. Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin α v β 3. Cell 85, 683–693 [DOI] [PubMed] [Google Scholar]
- 40. Piali L., Hammel P., Uherek C., Bachmann F., Gisler R. H., Dunon D., Imhof B. A. (1995) CD31/PECAM-1 is a ligand for α v β 3 integrin involved in adhesion of leukocytes to endothelium. J. Cell Biol. 130, 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabas I. (2010) The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107, 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuda S., Fujita T., Kajiya M., Takeda K., Shiba H., Kawaguchi H., Kurihara H. (2012) Brain-derived neurotrophic factor induces migration of endothelial cells through a TrkB-ERK-integrin αVβ3-FAK cascade. J. Cell. Physiol. 227, 2123–2129 [DOI] [PubMed] [Google Scholar]
- 43. Kermani P., Hempstead B. (2007) Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc. Med. 17, 140–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cantarella G., Lempereur L., Presta M., Ribatti D., Lombardo G., Lazarovici P., Zappalà G., Pafumi C., Bernardini R. (2002) Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 16, 1307–1309 [DOI] [PubMed] [Google Scholar]
- 45. Emanueli C., Salis M. B., Pinna A., Graiani G., Manni L., Madeddu P. (2002) Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106, 2257–2262 [DOI] [PubMed] [Google Scholar]
- 46. Kirchmair R., Gander R., Egger M., Hanley A., Silver M., Ritsch A., Murayama T., Kaneider N., Sturm W., Kearny M., Fischer-Colbrie R., Kircher B., Gaenzer H., Wiedermann C. J., Ropper A. H., Losordo D. W., Patsch J. R., Schratzberger P. (2004) The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 109, 777–783 [DOI] [PubMed] [Google Scholar]
- 47. Schgoer W., Theurl M., Jeschke J., Beer A. G., Albrecht K., Gander R., Rong S., Vasiljevic D., Egger M., Wolf A. M., Frauscher S., Koller B., Tancevski I., Patsch J. R., Schratzberger P., Piza-Katzer H., Ritsch A., Bahlmann F. H., Fischer-Colbrie R., Wolf D., Kirchmair R. (2009) Gene therapy with the angiogenic cytokine secretoneurin induces therapeutic angiogenesis by a nitric oxide-dependent mechanism. Circ. Res. 105, 994–1002 [DOI] [PubMed] [Google Scholar]
- 48. Connolly D. T., Heuvelman D. M., Nelson R., Olander J. V., Eppley B. L., Delfino J. J., Siegel N. R., Leimgruber R. M., Feder J. (1989) Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 84, 1470–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 [DOI] [PubMed] [Google Scholar]