Background: The crosstalk between Hgf and Wnt signaling pathways is not well defined.
Results: Hgf was found to stimulate Gsk3-dependent Lrp5/6 phosphorylation, increase nuclear β-catenin and reduce apoptosis in an Lrp5/6-dependent fashion.
Conclusion: Hgf transactivates canonical Wnt signaling.
Significance: This pathway is involved in the survival of renal epithelial cells and is activated in vivo after renal ischemia.
Keywords: Beta-catenin, Glycogen synthase kinase 3, Hepatocyte growth factor, Kidney, Wnt signaling
Abstract
While Wnt and Hgf signaling pathways are known to regulate epithelial cell responses during injury and repair, whether they exhibit functional cross-talk is not well defined. Canonical Wnt signaling is initiated by the phosphorylation of the Lrp5/6 co-receptors. In the current study we demonstrate that Hgf stimulates Met and Gsk3-dependent and Wnt-independent phosphorylation of Lrp5/6 at three separate activation motifs in subconfluent, de-differentiated renal epithelial cells. Hgf treatment stimulates the selective association of active Gsk3 with Lrp5/6. In contrast, Akt-phosphorylated inactive Gsk3 is excluded from this association. Hgf stimulates β-catenin stabilization and nuclear accumulation and protects against epithelial cell apoptosis in an Lrp5/6-dependent fashion. In vivo, the increase in Lrp5/6 phosphorylation and β-catenin stabilization in the first 6–24 h after renal ischemic injury was significantly reduced in mice lacking Met receptor in the renal proximal tubule. Our results thus identify Hgf as an important transactivator of canonical Wnt signaling that is mediated by Met-stimulated, Gsk3-dependent Lrp5/6 phosphorylation.
Introduction
Renal tubular epithelial cells (TEC)2 are highly susceptible to injury during episodes of ischemia, and yet must serve as the progenitor cells for tubule repair (1, 2). This repair process involves survival, dedifferentiation, migration, and proliferation of sub-lethally injured TECs, followed by redifferentiation to a fully functional phenotype. Two signaling cascades that are activated following acute kidney injury (AKI) and are known to regulate epithelial cell differentiation and survival are the hepatocyte growth factor (Hgf)/Mesenchymal-epithelial transition factor (Met) pathway and the integration/wingless (Wnt)/β-catenin (canonical) pathway (3–7).
Wnts bind to their membrane co-receptors Frizzled (Fzd) and the low density lipoprotein receptor-related proteins (Lrp) 5 and 6 (8), leading to glycogen synthase kinase 3 (Gsk3) inhibition and β-catenin stabilization and translocation to the nucleus. Hgf initiates a signaling cascade after binding to its plasma membrane receptor Met leading to downstream activation of a number of kinases including Akt (protein kinase B) (9). These pathways are known to cross-talk at the level of Gsk3 which they both inhibit, albeit in different ways. While Hgf-stimulated Met activation is known to result in Akt mediated inhibitory phosphorylation of Gsk3β at serine 9 (10), the mechanism by which Gsk3 is inhibited as a result of Wnt signaling is still under debate. The conventional hypothesis suggests that Gsk3 is directly inhibited by the C terminus of Lrp5/6 (11) while a more recent study suggests that Gsk3 is sequestered into multi-vesicular bodies and hence prevented from phosphorylating (and degrading) β-catenin (12). β-catenin activates gene transcription leading to various cellular processes including epithelial cell survival and morphologic changes.
A critical early event in canonical Wnt signaling is the phosphorylation of the highly homologous Wnt co-receptors Lrp5 and 6. The intracellular domain (ICD) of Lrp 6 consists of five PPS/TP repeats (S1490, T1530, T1572, S1590, S1607) juxtaposed with sites phosphorylated by casein kinase-1 (CK1). Together these sites are called PPSPXS motifs, and they are highly conserved among species and between the Lrp5 and 6 proteins (13). It has been shown that these motifs act synergistically to initiate downstream Wnt signaling and that phosphorylation of one site alone is not enough (14).
Lrp6 phosphorylation can be mediated by multiple kinases depending on the activating stimulus and cellular context (13). Zeng et al. identified Gsk3 and casein kinase 1 (CK1) as dual kinases that mediate Wnt-dependent Lrp6 phosphorylation (15). They showed that different cellular pools of Gsk3 exist with an active membrane pool that stimulates Lrp6 phosphorylation, and a cytosolic pool that is subsequently inhibited to prevent β-catenin phosphorylation and ubiquitination. In addition to Gsk3 and CK1, G protein-coupled receptor kinases (Grk5/6) have also been shown to phosphorylate PPS/TPXS motifs in a Wnt-dependent fashion (16), while protein kinase A (PKA) and PFTAIRE protein kinase 1 (Pftk1) can phosphorylate these motifs in a Wnt-independent fashion (17, 18). Recently, mitogen-activated protein kinases (MAPKs) have also been shown to phosphorylate Lrp6 following fibroblast growth factor (Fgf) stimulation. (19).
We have previously demonstrated that Hgf stimulation of subconfluent renal TECs leads to Akt-mediated inhibition of Gsk3 (10). In the current study, we further investigated the cross-talk between these pathways and its significance. Our data show that Hgf stimulates Lrp5/6 phosphorylation at three activation sites in a Wnt-independent manner. This phosphorylation is mediated by Gsk3 utilizing a previously unidentified mechanism in which active Gsk3 is selectively recruited to Lrp5/6. This phosphorylation leads to β-catenin stabilization and nuclear accumulation, and is required for the anti-apoptotic effects of Hgf. Consistent with these in vitro findings, in vivo studies show that there is a Met-dependent increase in Lrp5/6 phosphorylation and β-catenin stabilization after renal ischemia/reperfusion (I/R) injury in mice. Our results highlight a novel mechanism of Hgf-dependent transactivation of canonical Wnt signaling that identifies a Wnt-independent pathway for in vitro and in vivo Lrp5/6 activation.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Mouse inner medullary collecting duct-3 (mIMCD-3 (20)) and mouse proximal tubule (MPT (21)) epithelial cells were maintained using standard cell culture techniques in Dulbecco's modified Eagle's medium (DMEM)-F12 containing 10% fetal bovine serum (FBS). Antibodies to β-actin, Lrp6, Met, phospho-Met (Tyr-1234), Lamin A/C, and Gsk3-β were obtained from Santa Cruz Biotechnology. Antibodies to Lrp6, Lrp5, phospho-Lrp6 Ser-1490, total Erk1/2, phospho-Erk1/2 (Thr-202/Tyr-204), phospho-Gsk3-β Ser-9, and HSP-90 antibodies were obtained from Cell Signaling. Antibodies to phospho-Lrp6 Thr-1572 and Ser-1607 were obtained from Millipore, while anti-phospho-Gsk3-β-Tyr-216 and anti-E-cadherin antibodies were from BD Biosciences.
Recombinant mouse Wnt3a and Dickkopf-related protein 1 (Dkk-1) were purchased from R&D Systems. Recombinant human HGF and lithium chloride were purchased from Sigma Aldrich. The Gsk-3 inhibitor BIO IX and Met kinase inhibitor-II were purchased from Calbiochem. Met inhibitor PHA-665752 and Gsk3 inhibitor CHIR-99021 were purchased from Selleck Chemicals. For Western blotting after immunoprecipitation experiments, light-chain-specific anti-mouse and anti-rabbit IgG secondary antibodies were obtained from Jackson Immunoresearch. For all other experiments secondary antibodies were purchased from Invitrogen. EDTA-free protease and phosphatase inhibitor mixture was obtained from Thermo Scientific.
Cell Density
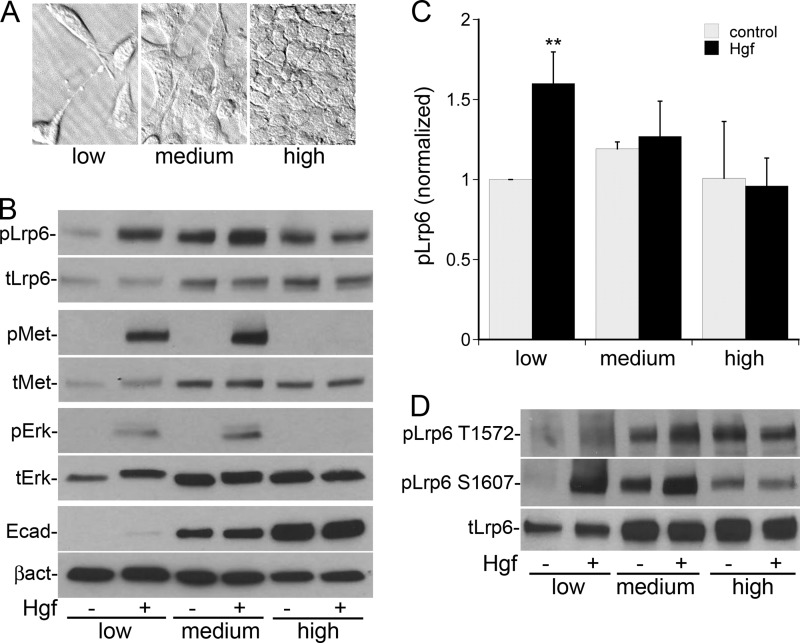
Cells were counted using a hemocytometer. Low, medium and high confluency cells were plated at 2.1 × 103/cm2, 4.2 × 104/cm2, and 4.2 × 105 cells/cm2, respectively. Experiments were performed 24 h after plating (cell morphology as shown in Fig. 2A).
FIGURE 2.
Hgf-stimulated phosphorylation of Lrp5/6 is dependent on cell confluency. A, ×40 bright field microscopy images of morphology of MPT cells plated at low (2. 1 × 103 cells/cm2), medium (4.2 × 104/cm2), and high (4.2 × 105/cm2) confluency. B, immunoblot images of MPT cells plated at the indicated confluencies ±Hgf stimulation for 10 min showing relatives changes in the levels of total Lrp6, pLrp6 Ser-1490, total Met, pMet, total Erk1/2, pErk1/2, E-cadherin, and β-actin. C, quantification of the relative phosphorylation of pLrp6 Ser-1490 as in B normalized to total Lrp6 (n = 3, **, p < 0.01 versus control). D, MPT cells treated as in B were immunoblotted for pLrp6 Thr-1572 and Ser-1607.
Immunoprecipitation and Western Analysis
Cells were serum starved for 24 h, followed by Hgf (40 ng/ml) or Wnt3a (50 ng/ml) stimulation for the indicated time. Cells were lysed in RIPA buffer (50 mm Tris-Hcl, 150 mm NaCl, 1.0% deoxycholic acid, 0.1% SDS, 1.0% Triton X-100, and 2 mm EDTA), insoluble material removed by centrifugation, and the supernatant protein content determined using the Bradford assay. For co-immunoprecipitation experiments, cell were lysed in Nonidet P-40 buffer (50 mm Tris-Hcl, 150 mm NaCl, 5 mm EDTA, and 1% Nonidet P-40) containing protease and phosphatase inhibitors, pre-cleared with protein G-PLUS or protein A-agarose beads (1:1 slurry in PBS; Santa Cruz) for 45 min. 0.5–1.0 mg of cell lysate was immunoprecipitated with the appropriate antibody overnight, washed ×3 in ice-cold Nonidet P-40 buffer and precipitated proteins separated using 7.5–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrophoretically transferred to Immobilon-P membranes (Millipore), immunoblotted with the appropriate antibody overnight, and visualized by enhanced chemiluminescence (Amersham Biosciences, Inc.) or West Femto Maximum Sensitivity Substrate (Thermo Scientific). Quantification of ECL signals was performed using NIH Image J software.
For protein lysates from mice, kidneys were harvested at 6 and 24 h after IRI surgery and immediately flash frozen in liquid nitrogen. Tissue was homogenized in RIPA buffer containing protease and phosphatase inhibitors using a Dounce homogenizer while being kept on ice (4 °C) throughout.
Reverse Transcriptase-Polymerase Chain Reaction
MPT cells were lysed and total RNA isolated using the RNeasy kit (Qiagen Valencia, CA). One microgram of RNA was reverse transcribed using random hexamer primers (SuperScript II, Invitrogen), and PCR was performed using REDTaq DNA Polymerase (Sigma) followed by visualization of amplified DNA through agarose gel electrophoresis.
Nuclear and Cytoplasmic Protein Extraction
Cells were trypsinized, washed with ice-cold PBS, centrifuged, and the manufacturer's protocol (NE-PER Nuclear and Cytoplasmic Extraction Reagents, Thermo Scientific) was followed. Fresh protease and phosphatase inhibitors were added to the manufacturer's reagents. The cytoplasmic and nuclear extracts were analyzed by Western blot.
Apoptosis Assay
To induce apoptosis, hydrogen peroxide (H2O2) at 250 mm concentration (22) in serum-free media was added to the cells after 90 min of stimulation with either vehicle or Hgf. Cells were cultured for 2 h followed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining based on the manufacturer's protocol (Roche). Briefly the cells were washed, fixed in 4% paraformaldehyde, and stained. TUNEL-positive cells were imaged using a Nikon microscopy system and counted in eight randomly selected fields per well at ×40. Results are expressed as the percentage of total (DAPI-positive) cells counted that were apoptotic (TUNEL-positive). An average of the 10 fields per condition in three experiments was used for quantification.
Stable Lrp5 and 6 Knockdown in MPT Cells
Lentivirus containing shRNA against the Lrp5 and 6 proteins and control (scrambled) shRNA were obtained from Santa Cruz Biotechnology. MPT cells were plated on 48-well dishes 1 day before infection. Polybrene (Santa Cruz Biotechnology) at 5 μg/ml and lentivirus particles were added into FBS/antibiotic-free DMEM and left for overnight culture. A concentration of 100 infectious units (IFU) of virus per cell was used. On day 2 following transduction, the medium was replaced with DMEM supplemented with FBS and 10 μg/ml of puromycin (Santa Cruz Biotechnology). Untransduced dead cells were removed on day 3, and live cells were further expanded and maintained to generate stable cell lines.
Generation of Conditional Met Knock-out Mice and I/R Surgery
Metfl/fl mice (23, 24) were mated to γGT-Cre mice to induce Met knock-out in the renal proximal tubule (3). 8–10-week old γGT-Cre;Metfl/fl mice or γGT-Cre;Met+/+ (WT) controls were subjected to 25 min of warm left kidney ischemia with contralateral nephrectomy, followed by reperfusion as previously described (3). Sham animals underwent midline ventral incision without clamping. The mice were sacrificed after 6 h or 1 day for kidney harvest. All mouse experiments were conducted under a protocol approved by the Yale IACUC.
Statistical Methods
The data between two groups were compared using the Student's two-tailed t test. Significance was determined at p < 0.05, and the error bars represent standard deviations.
RESULTS
Hgf Induces Rapid Phosphorylation of the Wnt Co-receptor Lrp5/6 in Subconfluent MPT Cells
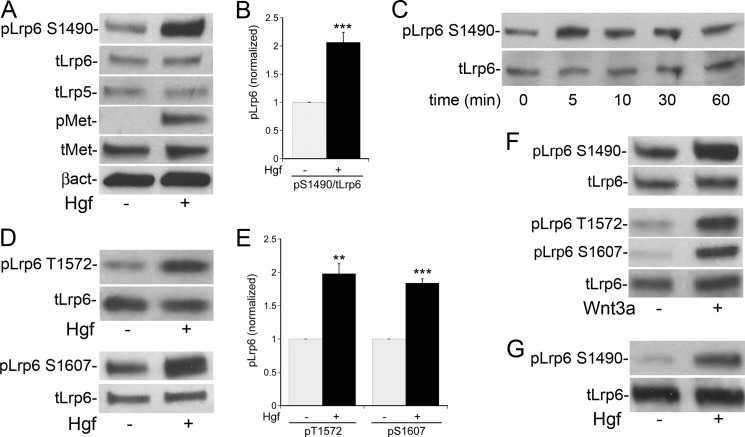
Treatment of non-confluent cultured MPT cells with Hgf (40 ng/ml) for 10 min lead to activation of the Met receptor and an approximately 2-fold increase in phosphorylation of Lrp6 at Ser-1490, with no change in the total amount of Lrp5 and -6 (Fig. 1A, quantification in Fig. 1B). The phosphorylation peaked within 5–10 min of stimulation and declined over the next 60 min (Fig. 1C). Similarly, Met activation resulted in phosphorylation of Lrp6 at Thr-1572 and Ser-1607 (Fig. 1D, quantified in E) at this early time point. Of note, PCR revealed that MPT cells express the mRNA for both Lrp5 and Lrp6 (data not shown), and the commercial antibodies used do not distinguish between the phosphorylated forms of the two proteins. Individual knockdown of Lrp6 or Lrp5 suggests that the homologous phosphorylation motifs on the Lrp5 protein (Ser-1502, Thr-1577, and Ser-1608) are also activated by Hgf (data not shown).
FIGURE 1.
Hgf induces rapid phosphorylation of the Wnt co-receptor Lrp5/6. A, MPT cells were stimulated ±Hgf for 10 min, and the lysates immunoblotted using a phosphospecific Lrp6 antibody against Ser-1490 in Lrp6 (and potentially Ser-1502 in Lrp5). B, quantification of Hgf-stimulated pLrp5/6 in MPT cells normalized to total Lrp6 (n = 4). C, time course of Lrp5/6 phosphorylation in Hgf-stimulated MPT cells. D, lysates from Hgf-stimulated MPT cells were immunoblotted using phosphospecific Lrp6 antibodies that recognize Thr-1572 and Ser-1607 in Lrp6 (and potentially Thr-1577 and Ser-1608 in Lrp5). E, quantification of pLrp5/6 as in C, D normalized to total Lrp6 (n = 3). F, Wnt3a stimulation of MPT cells for 10 min with immunoblots using pLrp6 antibodies to the three phosphorylation motifs (Ser-1490, Thr-1572, and Ser-1607). G, mIMCD-3 cells stimulated ±Hgf for 10 min followed by immunoblotting for pLrp6 S1490. (**, p < 0.01 versus control; ***, p < 0.001 versus control).
As expected, treatment of MPT cells with Wnt3a (50 ng/ml for 10 min) induced phosphorylation of these same motifs on Lrp6 (Fig. 1F). Our in vivo data have shown that renal collecting duct cells also express high levels of the Met receptor (25). Similar to MPT cells, Hgf stimulation of collecting duct derived mIMCD-3 cells induced Lrp5/6 phosphorylation within 10 min (Fig. 1G).
Hgf-stimulated Phosphorylation of Lrp5/6 Is Dependent on Cell Confluency
We have previously shown that both Hgf signaling and expression of epithelial differentiation markers are regulated by cell confluency (10, 26). To determine the impact of confluency on Hgf-stimulated phosphorylation of Lrp5/6, MPT cells were plated at low, medium, and high confluency (representative images in Fig. 2A). At low confluency the cells express low levels of E-cadherin and Lrp6, but respond in a robust fashion to Hgf with Met activation, Erk activation and Lrp6 phosphorylation at Ser-1490 (Fig. 2B, quantified in C). At medium confluency, both total Lrp5/6 expression and phosphorylation are increased in unstimulated cells, with no significant increase in the ratio of phosphorylated Lrp5/6 to total Lrp5/6 following Hgf stimulation. At high confluency Hgf-stimulated Met activation and signaling were completely suppressed, with no change in Lrp5/6 phosphorylation. Hgf-mediated phosphorylation of Lrp6 at Thr-1572 and Ser-1607 demonstrate a similar confluency-dependent pattern (Fig. 2D).
These findings, coupled with our previous demonstration that Hgf-mediated inhibition of cytosolic Gsk3 occurs only in subconfluent cells (10), suggest that Hgf/Met signaling specifically transactivates Wnt signaling in low confluency, de-differentiated epithelial cells, reminiscent of sub-lethally injured cells after AKI. This pathway is apparently suppressed as cells become more differentiated and confluent, resembling the healthy, uninjured epithelial cells of the renal tubule. Subsequent cell-based experiments were performed under low confluency conditions.
Hgf-stimulated Phosphorylation of Lrp5/6 Is Independent of Wnt
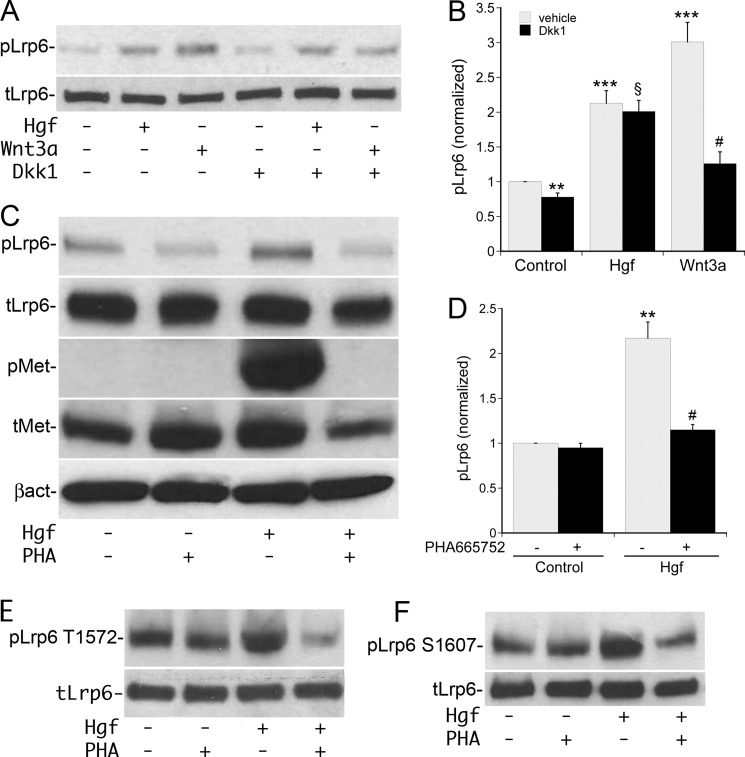
The rapid increase in Lrp5/6 phosphorylation following Hgf stimulation suggested that Wnt expression/secretion may not be required for this pathway of Lrp5/6 activation. To confirm this, serum starved MPT cells were incubated with the extracellular Wnt-antagonist Dickkopf-1 (Dkk-1, 0.1 μg/ml) followed by stimulation with Hgf or Wnt3a. Treatment with exogenous Wnt3a resulted in the expected increase in Lrp5/6 phosphorylation, with Dkk-1 pretreatment inhibiting this effect by ∼70% (Fig. 3A, quantified in B). In contrast, Hgf-stimulated Lrp5/6 phosphorylation was not inhibited by Dkk-1 (Fig. 3A, quantified in B). Of note, incubation of control cells with Dkk-1 resulted in a modest but significant decrease in basal Lrp5/6 phosphorylation.
FIGURE 3.
Hgf-stimulated phosphorylation of Lrp5/6 is independent of Wnt. A, MPT cells were pre-incubated with Dkk1 or vehicle, followed by stimulation with vehicle, Wnt3a, or Hgf for 10 min followed by immunoblotting for pLrp6 Ser-1490 and total Lrp6. B, densitometric quantification of three separate experiments in A using pLrp6 Ser-1490 normalized to total Lrp6. (**, p < 0.01 for control + Dkk1 versus control; ***, p < 0.001 for Hgf versus control and Wnt3a versus control; $, p = NS for Hgf + Dkk1 versus Hgf; #, p < 0.01 for Wnt + Dkk1 versus Wnt alone). C, MPT cells were pre-incubated with the Met inhibitor PHA-665752 or vehicle, followed by stimulation ±Hgf for 10 min followed by immunoblotting for pLrp6 Ser-1490, total Lrp6, pMet, total Met, and β-actin. D, densitometric quantification of four separate experiments as in C with pLrp6 Ser-1490 normalized to total Lrp6 (**, p < 0.01 for Hgf versus control and #, p < 0.01 for Hgf + PHA-665752 versus Hgf alone). E and F, immunoblots of pLrp6 Thr-1572 and pLrp6 Ser-1607 from cells treated as in C.
To determine if Hgf-stimulated Lrp5/6 phosphorylation required activation of the Met receptor, MPT cells were preincubated with the specific Met kinase inhibitor PHA-665752 (0.2 μm) followed by stimulation with Hgf. Met inhibition reduced Hgf-stimulated Lrp6 phosphorylation at Ser-1490, Thr-1572, and Ser-1607 by greater than 90% (Fig. 3, C–F). These findings were reproduced with a separate Met inhibitor, Met inhibitor-II (0.5 μm, data not shown) and demonstrate that Hgf stimulation of Lrp5/6 is Wnt-independent and requires activation of the Met receptor.
Hgf Induces Gsk3-dependent Lrp5/6 Phosphorylation by Selective Association of Lrp5/6 with Active Gsk3
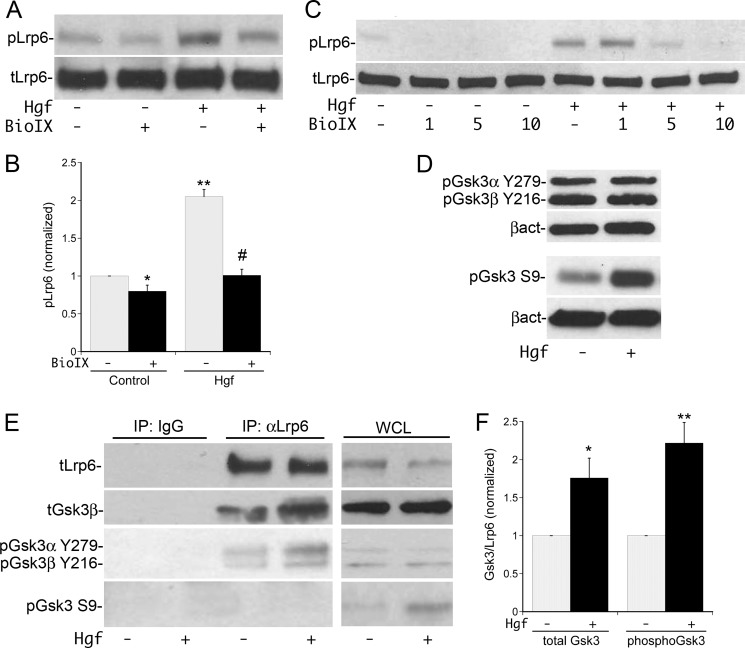
We subsequently proceeded to define the mechanism of this phosphorylation. We did not detect co-immunoprecipitation of Met with Lrp5/6. In light of the evidence that Gsk3 can phosphorylate Lrp5/6 (15), MPT cells were stimulated with Hgf ± pre-incubation with the specific Gsk3 inhibitor BioIX (27). Hgf-stimulated Lrp6 phosphorylation at Ser-1490 was inhibited by BIO IX (Fig. 4A, quantified in B) in a dose-dependent manner (Fig. 4C). BioIX also reduced the basal phosphorylation of Lrp5/6 by ∼25% (Fig. 4, A, C, quantified in B). This is similar to the effect seen with Dkk-1 (Fig. 3A), and consistent with the possibility that MPT cells express a Wnt ligand that weakly activates Gsk3-dependent Lrp5/6 phosphorylation at baseline. Taking into account the inhibition of basal phosphorylation, there was an 80% decrease in the Hgf-stimulated phosphorylation of Lrp5/6 following BIO IX treatment (10 μm). To confirm that Hgf-mediated phosphorylation of Lrp5/6 was dependent on active Gsk3, two other Gsk3 inhibitors, CHIR-99021 and lithium chloride (LiCl), were tested and also found to inhibit Hgf-stimulated phosphorylation of Ser-1490 in a dose-dependent fashion (data not shown). Gsk3 inhibition also reduced Hgf-dependent Lrp6 phosphorylation at Thr-1572 and Ser-1607 to a comparable degree (data not shown).
FIGURE 4.
Hgf-stimulated phosphorylation of Lrp5/6 is dependent on Gsk3. A, MPT cells were pre-incubated with the Gsk3 inhibitor BioIX (10 μm) or vehicle, followed by treatment ± Hgf for 10 min and immunoblotting for pLrp6 Ser-1490. B, densitometric quantification of pLrp6 S1490 as in A using total Lrp6 for normalization (n = 4; *, p < 0.05 BioIX versus control; **, p < 0.01 Hgf versus control; #, p < 0.001 Hgf +BioIX versus Hgf alone). C, dose response of pLrp6 S1490 phosphorylation by Hgf along with the indicated doses of Bio IX (in μm). D, MPT cells were stimulated ±Hgf for 10 min, followed by immunoblotting of whole cell lysates for the inhibitory pS9 site on Gsk3β and activating pY279/216 sites on Gsk3α and -β, respectively. E, MPT cells were stimulated ±Hgf for 10 min, the lysates immunoprecipitated with anti-Lrp6 or control IgG and then immunoblotted for total Lrp6, total Gsk3, pGsk3α/β Y279/216, and pGsk3β Ser-9. Immunoblots from whole cell lysates (WCL) prior to IP are shown on right. F, densitometric quantification of the amount of total Gsk3 and pGsk3α/β Thr-279/216 that coimmunoprecipitated with Lrp5/6 normalized to the amount of immunoprecipitated Lrp6. (total Gsk3: n = 3,*, p < 0.05 Hgf versus control; pGsk3α/β Y279/216: n = 5, **, p < 0.01 Hgf versus control).
Gsk3 is believed to be constitutively active, with substrate specificity regulated by both phosphorylation and cellular compartmentalization. Activation of Gsk3 can occur via Src family kinase phosphorylation of Gsk3β at tyrosine 216 (28, 29). In contrast, stimulation with Hgf has been shown to inhibit Gsk3 via PI3K/Akt dependent phosphorylation at serine 9 (10), similar to that described downstream of insulin receptor signaling (30). Neither of these sites are thought to be utilized during canonical Wnt signaling (31, 32). Examination of the phosphorylation state of Gsk3 in whole cell lysates confirmed that inhibitory phosphorylation at serine 9 was significantly increased by Hgf whereas activating phosphorylation of Gsk3β at Tyr-216 and Gsk3α at Tyr-279 was unchanged (Fig. 4D).
In light of the studies demonstrating separate membrane and cytosolic pools of Gsk3 (15), Lrp5/6 was immunoprecipitated from MPT cell lysates to assess the degree of Gsk3 association and the activation state of the associated Gsk3. These experiments revealed basal association of Lrp5/6 and Gsk3, with a significant increase in association following stimulation with Hgf (Fig. 4E, quantified in F). Surprisingly, despite the increase in serine 9 phosphorylation of Gsk3 detected in Hgf-stimulated whole cell lysates, there was no detectable serine 9 phosphorylated Gsk3 found in Hgf-stimulated Lrp5/6 immunoprecipitates. In contrast, Tyr-216 phosphorylated Gsk3β and Tyr-279 phosphorylated Gsk3α were readily detected in Lrp5/6 immunoprecipitates, and increased in a similar fashion to total Gsk3 following Hgf treatment (Fig. 4E, quantified in F). These findings demonstrate that Hgf selectively recruits the active form of Gsk3 to Lrp5/6 while suppressing recruitment of the inhibited form.
Hgf-mediated β-Catenin Stabilization and Anti-apoptotic Activity Is Dependent on Lrp5/6 Phosphorylation
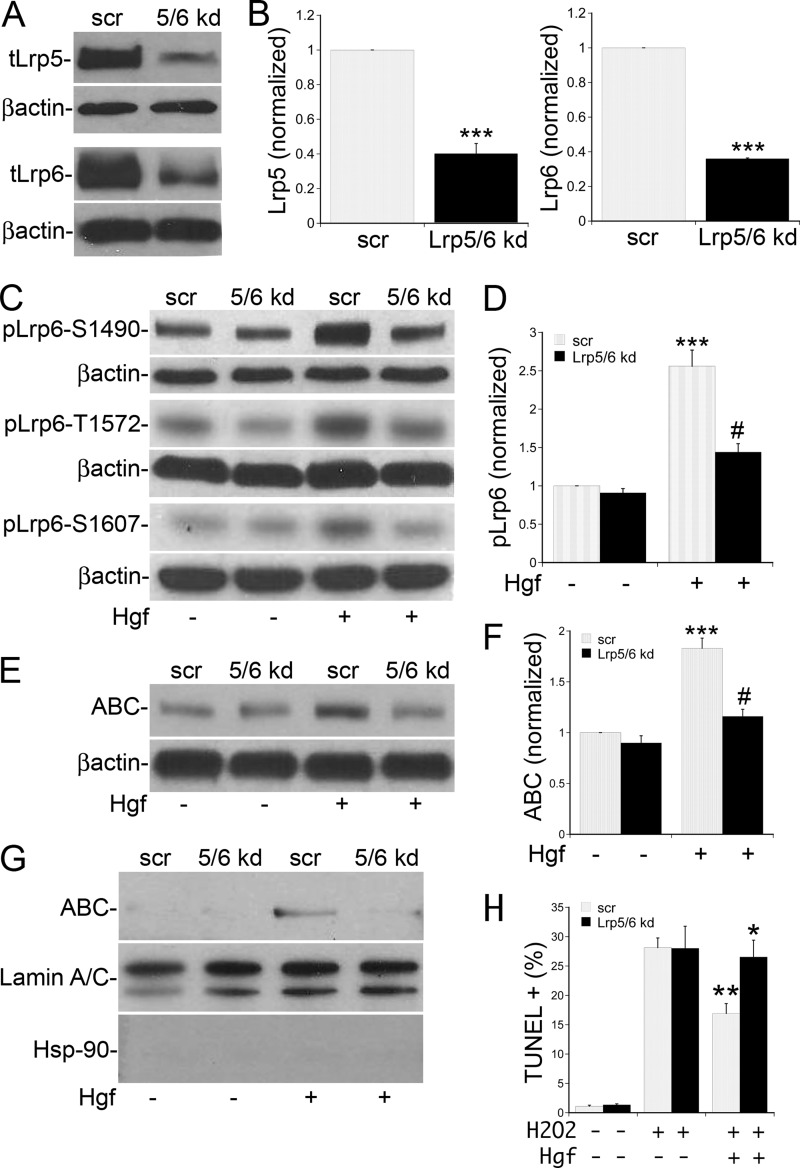
To determine the significance of Hgf-stimulated Lrp5/6 phosphorylation in MPT cells, stable cell lines were created with simultaneous knockdown of both Lrp5 and Lrp6 proteins. MPT cells were infected with lentivirus containing both shRNA against Lrp5 and 6 or the scrambled (scr) control and stable cell mixtures expressing the appropriate shRNA selected on puromycin. We were able to achieve a 60% knockdown of Lrp5 and 65% knockdown of Lrp6 compared with control (Fig. 5A, quantified in B), correlating with a 60–65% reduction in Hgf-stimulated Lrp5/6 phosphorylation at the Gsk3 phosphorylation sites (Fig. 5C, quantified in D). Analysis of the effects of Hgf treatment on control MPT cell β-catenin revealed an increase in the active, non-Gsk3 phosphorylated form of β-catenin (ABC) in both whole cell lysates (Fig. 5E) and nuclear fractions (Fig. 5G) at 90 min after Hgf stimulation. In Lrp5/6 knock-down cells, the stabilization of β-catenin was significantly reduced in both the whole cell lysates (Fig. 5E, quantified in F) and isolated nuclear fractions (Fig. 5G).
FIGURE 5.
Hgf-mediated β-catenin stabilization and anti-apoptotic activity is dependent on Lrp5/6 phosphorylation. A, lysates of MPT cells expressing shRNA against both Lrp5 and 6 proteins (5/6 kd) or a scrambled shRNA control (scr) were immunoblotted for Lrp5, Lrp6, and β-actin. B, densitometric quantification as in A using β-actin for normalization (n = 4; ***, p < 0.001 Lrp5/6 kd versus scr for both Lrp5 and -6). C, 5/6 kd or scr cells were stimulated ±Hgf for 10 min and lysates immunoblotted for pLrp6 Ser-1490, Thr-1572, Ser-1607, and β-actin. D, densitometric quantification of pLrp6 Ser-1490 from four separate experiments normalized to β-actin (***, p < 0.001 for Hgf versus control in scr cells; #, p < 0.01 for Hgf in 5/6kd cells versus Hgf in scr cells). E, Lrp5/6 kd or scr cells were stimulated ±Hgf for 90 min, and the lysates immunoblotted for active β-catenin (ABC) and β-actin. F, densitometric quantification of four separate experiments as in E using ABC normalized to β-actin (***, p < 0.001 for Hgf versus control in scr cells; #, p < 0.01 for Hgf in 5/6kd cells versus Hgf in scr cells). G, Lrp5/6 kd or scr cells were stimulated ±Hgf for 90 min followed by isolation of nuclear fractions and immunoblotting for ABC, HSP-90 (to detect cytosolic contamination) and Lamin A/C (to detect nuclear fractions). H, Lrp5/6 kd or scr cells were stimulated ±Hgf for 90 min followed by culture in 250 mm H202 for 2 h. Control cells were cultured in the same media without H202, The cells were fixed and processed for TUNEL staining. The quantification represents five experiments. (**, p < 0.01 for Hgf+H2O2 in scr control versus H2O2 alone; *, p < 0.05 for Hgf+H2O2 in scr versus Hgf+H2O2 in 5/6 kd).
Because both Hgf/Met (3) and canonical Wnt signaling (6) have been shown to mediate anti-apoptotic effects on renal epithelial cells after injury, we tested the importance of Lrp5/6 phosphorylation in mediating the Hgf-dependent anti-apoptotic activity. MPT cells were treated with Hgf or vehicle for 90 min followed induction of apoptosis with hydrogen peroxide (H2O2) based on previously published protocols (22). Treatment with Hgf reduced the H2O2-induced apoptosis by 40% in control cells, but failed to prevent apoptosis in the Lrp5/6 knock-down cells (Fig. 5H).
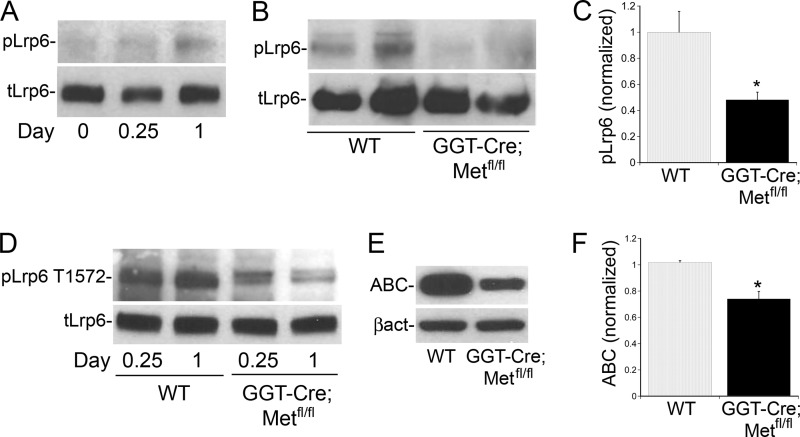
Lrp5/6 Phosphorylation Early After Ischemic Kidney Injury Is Met Dependent
It has been previously shown that Lrp6 phosphorylation and subsequent canonical Wnt signaling are activated following acute kidney injury (33), so this model was used to assess the relevance of Hgf-stimulated transactivation of this pathway in vivo. Consistent with the published data, phosphorylation of Lrp5/6 was found to be increased in kidney lysates from wild type mice on the first day after renal ischemia-reperfusion (I/R) injury (Fig. 5A). To test the importance of the Hgf/Met signaling pathway in this process, we utilized GGT-Cre;Metfl/fl mice in which the Met receptor has been conditionally knocked out in the renal proximal tubule (3). These mice develop normally, exhibit no Met expression in the proximal renal tubule, but continue to express Met in other kidney cell types and have increased tubular cell injury and apoptotic cell death as compared with WT mice after I/R injury (3). Western analysis of kidney lysates from GGT-Cre;Metfl/fl mice revealed a significant reduction in I/R-induced Lrp5/6 phosphorylation at both Ser-1490 and Thr-1572 at 1 day after I/R injury as compared with wild-type mice (Fig. 5, B--D). Consistent with the in vitro findings, this decrease in Met-dependent Lrp5/6 phosphorylation in I/R injured GGT-Cre;Metfl/fl mice correlated with a decrease in active, non-phosphorylated β-catenin (ABC, Fig. 5E, quantified in 5F) compared with the control (wild type) mice. These data suggest that Lrp5/6 phosphorylation and β-catenin stabilization early after ischemic kidney injury is in part dependent on Met receptor activation in the proximal tubule.
DISCUSSION
Recent studies have highlighted the cross-talk between growth factor and Wnt signaling pathways. Fgf has been shown to mediate Erk-dependent phosphorylation of the Wnt co-receptor Lrp5/6 (19) and contribute to downstream signaling in both a β-catenin-dependent (19, 34) and independent (35) fashion. Wnt, MAPK, and BMP pathways have shown to interact through the regulation of Smad1 (36). In the current study, we have identified a distinct mechanism of cross talk of Hgf with the Wnt signaling pathway in which the activated Met receptor leads to phosphorylation of the Wnt co-receptor Lrp5/6 in renal epithelial cells (Fig. 1). The observation that Hgf stimulates phosphorylation of 3 of the 5 known Lrp5/6 activation sites and that this pathway is activated or suppressed depending on the differentiation status of epithelial cells (Fig. 2), suggested that it was likely to be functionally important. The reproduction of these findings in another cell line (m-IMCD3) suggests that this was not a cell line restricted phenomenon. Hence we pursued the mechanism and significance of this phosphorylation.
The phosphorylation occurs rapidly (within 5–10 min), is not blocked by Dkk-1 (which antagonizes Wnt-dependent Lrp5/6 phosphorylation by competing with Wnt for cell surface binding to Lrp5/6 (37)) and is almost completely blocked by Met inhibition, suggesting that Met activation induces transphosphorylation of Lrp5/6 in a Wnt-independent manner (Fig. 3). Since the Hgf-dependent Lrp5/6 phosphorylation was rapid, did not involve direct association of Met and Lrp5/6, and correlated with an increase in the association of Gsk3, we investigated the possibility that Gsk3 was responsible for Met activated Lrp5/6 phosphorylation. Using three separate kinase inhibitors, we show that Gsk3 is the kinase responsible for the majority of Hgf-stimulated Lrp5/6 phosphorylation (Fig. 4).
Gsk3 is a serine/threonine kinase with two mammalian isoforms, α and β, that are highly homologous in their kinase domains but differ in the N and C termini. While the α and β isoforms can function independently in some systems (38), they were found to be functionally redundant for Wnt signaling (39). Gsk3 appears to play multiple roles in canonical Wnt signaling which are primarily mediated by its cellular localization. A membrane pool of Gsk3 has been shown to be responsible for Wnt-mediated Lrp5/6 phosphorylation (15). We find that Gsk3 increases its association with Lrp5/6 in response to Hgf (Fig. 4), suggesting that Hgf can either recruit cytosolic Gsk3 to the membrane or selectively target membrane-associated Gsk3 to Lrp5/6.
While canonical Wnts, such as Wnt3a, appear to regulate Gsk3 activity by either sequestration into multivesicles or direct inhibition by phosphorylated Lrp6, tyrosine kinase activating growth factors such as Hgf regulate Gsk3 activity via a different mechanism, namely phosphorylation at inhibitory and/or activating sites that are not believed to be important for Wnt-dependent Gsk3 regulation. Hgf induces Akt-dependent phosphorylation of Gsk3β at Ser-9 and Gsk3α at Ser-21, resulting in a conformational change that promotes the N terminus to become a pseudo-substrate that blocks the phospho-binding site and inhibits Gsk3 kinase activity (10, 40). In the current study, we show that stimulation of cells with Hgf results in the selective exclusion of this Ser-9 phosphorylated pool of Gsk3β from association with Lrp5/6, even though there is a marked increase in Ser-9-phosphorylated Gsk3β at the whole cell level. Instead, the pool of Gsk3 that is recruited to Lrp5/6 following Hgf stimulation includes Gsk3β that is phosphorylated at Tyr-216 and Gsk3α phosphorylated at Tyr-279 (Fig. 4). These residues are on the activating T-loop and their phosphorylation has been shown to result in a conformational change in the enzyme that facilitates substrate binding to the catalytic site and thus increases kinase activity (41). Our data suggest that Hgf treatment does not directly stimulate the phosphorylation at these sites at the whole cell level, but instead mediates the recruitment of this active form of Gsk3 to Lrp5/6 (Fig. 4). How Met activation leads to the recruitment of active Gsk3β to Lrp5/6 while excluding pS9 Gsk3β remains unclear. One possibility is that Met induces a priming kinase to phosphorylate Lrp5/6 and selectively increase the affinity for pY216 Gsk3 and not pS9 Gsk3. However this possibility is not yet experimentally proven.
To study the significance of this pathway for downstream signaling, Lrp5 and -6 proteins were knocked down in stable pooled cells. This lead to a reduction in Hgf-dependent Lrp5/6 phosphorylation by almost 65% (Fig. 5) and significantly suppressed the Hgf-stimulated increase in the active, non-phosphorylated form of β-catenin in both whole cell lysates and the nucleus. These results show that Hgf-dependent Lrp5/6 phosphorylation leads to inhibition of the destruction complex of β-catenin (likely via downstream inhibition of Gsk3, similar to Wnt), allowing β-catenin to be translocated to the nucleus. The functional importance of this signaling pathway for Hgf-mediated cell survival was demonstrated by our finding that the anti-apoptotic activity of Hgf was significantly reduced in Lrp5/6 knockdown cells (Fig. 5). β-Catenin signaling has been previously reported to be anti-apoptotic (42, 43), with potential targets that include activation of the anti-apoptotic genes Bcl-2, c-Jun, c-Myc, and survivin (44, 45) and/or inhibition of pro-apoptotic molecules such as Bax (6).
The finding that Hgf-dependent Lrp5/6 phosphorylation is maximal at low cell confluency is consistent with our previous data (10), and argues that this pathway is specifically activated in dedifferentiated cells. Renal ischemic injury leads to rapid cell loss and dedifferentiation of the surviving cells. Therefore, the in vivo relevance of this pathway was assessed using I/R injury in a genetic mouse model with specific deletion of the Met receptor in the proximal tubule of the kidney (GGT-Cre Met fl/fl). We have recently shown that GGT-Cre Met fl/fl mice have an increase in proximal tubule apoptosis compared with WT mice early after ischemic kidney injury that correlates with decreased Akt activation (3). We now show that Lrp5/6 phosphorylation and β-catenin stabilization are detectable in the first day after ischemic kidney injury and are also dependent on Hgf/Met signaling (Fig. 6). This suggests that the early anti-apoptotic effects of Hgf that are critical for tubular cell survival and ultimate tubule repair may be dependent on coordinated signals derived from both Lrp5/6 and Akt activation. Of note, Wnt-stimulated activation of Lrp5/6 phosphorylation has been shown to occur 2–7 days after injury, and is also required for normal kidney repair (33). The current findings suggest that Hgf signaling transactivates this pathway prior to Wnt, thus potentially initiating the expression of downstream cell survival signals and contribute to the repair process. Combined with our previous studies (3, 10), this work identifies a dual mechanism through which Hgf/Met signaling cross-talks with canonical Wnt signaling; upstream activation of Lrp5/6 phosphorylation by Gsk3 and downstream inhibition of Gsk3 by Akt, both potentially contributing to β-catenin activation and regulation of apoptosis of renal epithelial cells. Parathyroid hormone has been shown to also stimulate canonical Wnt signaling via 2 separate mechanisms, similarly involving proximal phosphorylation of Lrp5/6 (17) and distal inhibition of Gsk3 (46).
FIGURE 6.
Lrp5/6 phosphorylation and β-catenin stabilization immediately after ischemic kidney injury are Met dependent. A, immunoblot of kidney lysates from the wild type (WT) mouse kidney before (0), 6 h after (0.25) and 1 day after I/R injury showing relative amounts of pLrp6 Ser-1490 and total Lrp6. B, representative immunoblot from kidney lysates from 2 GGT-Cre;Metfl/fl mice and 2 WT mice 1 day after I/R injury showing relative amounts of pLrp6 Ser-1490 and total Lrp6. C, densitometric quantification of pLrp6 Ser-1490 1 day after I/R as in B normalized to total Lrp6. n = 5 mice in each group, *, p < 0.05 GGT-Cre;Metfl/fl versus WT. D, kidney lysates from GGT-Cre;Metfl/fl and WT mice 6 h and 1 day after I/R injury immunoblotted for pLrp6 T1572. E, representative immunoblot from kidney lysates from GGT-Cre;Metfl/fl mice and WT mice 1 day after I/R injury showing relative amounts of ABC and β-actin. F, densitometric quantification of ABC 1 day after I/R as in E normalized to β-actin. n = 5 mice in each group, *, p < 0.05 GGT-Cre;Metfl/fl versus WT.
In summary, our present study identifies a novel pathway in de-differentiated renal epithelial cells through which Hgf stimulates phosphorylation of the Wnt co-receptor Lrp5/6 by selective recruitment of the active form of Gsk3 to Lrp5/6 in a Wnt-independent fashion leading to β-catenin stabilization and anti-apoptotic effects.
This work was supported, in whole or in part, by NIDDK, National Institutes of Health Grant DK65109 (to L. G. C.) and an American Society of Nephrology (Ben J. Lipps) Research Fellowship (to F. M. K.).
- TEC
- tubular epithelial cells
- Hgf
- hepatocyte growth factor
- Lrp
- lipoprotein receptor-related protein
- Met
- mesenchymal-epithelial transition factor
- ICD
- intracellular domain
- CK
- casein kinase
- I/R
- ischemia/reperfusion
- ABC
- active β-catenin
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- scr
- scrambled
- MPT
- mouse proximal tubule.
REFERENCES
- 1. Humphreys B. D., Valerius M. T., Kobayashi A., Mugford J. W., Soeung S., Duffield J. S., McMahon A. P., Bonventre J. V. (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 [DOI] [PubMed] [Google Scholar]
- 2. Ishibe S., Cantley L. G. (2008) Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr. Opin. Nephrol. Hypertens. 17, 379–385 [DOI] [PubMed] [Google Scholar]
- 3. Mason S., Hader C., Marlier A., Moeckel G., Cantley L. G. (2014) Met activation is required for early cytoprotection after ischemic kidney injury. J. Am. Soc. Nephrol. 25, 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou D., Tan R. J., Lin L., Zhou L., Liu Y. (2013) Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 84, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou D., Li Y., Lin L., Zhou L., Igarashi P., Liu Y. (2012) Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 82, 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z., Havasi A., Gall J. M., Mao H., Schwartz J. H., Borkan S. C. (2009) β-catenin promotes survival of renal epithelial cells by inhibiting Bax. J. Am. Soc. Nephrol. 20, 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cantley L. G., Barros E. J., Gandhi M., Rauchman M., Nigam S. K. (1994) Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am. J. Physiol. 267, F271–F280 [DOI] [PubMed] [Google Scholar]
- 8. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trusolino L., Bertotti A., Comoglio P. M. (2010) MET signalling: principles and functions in development, organ regeneration and cancer. Nature Rev. Mol. Cell Biol. 11, 834–848 [DOI] [PubMed] [Google Scholar]
- 10. Ishibe S., Haydu J. E., Togawa A., Marlier A., Cantley L. G. (2006) Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a β-catenin-dependent manner. Mol. Cell Biol. 26, 9232–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mi K., Dolan P. J., Johnson G. V. (2006) The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J. Biol. Chem. 281, 4787–4794 [DOI] [PubMed] [Google Scholar]
- 12. Taelman V. F., Dobrowolski R., Plouhinec J. L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D., De Robertis E. M. (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niehrs C., Shen J. (2010) Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 67, 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacDonald B. T., Yokota C., Tamai K., Zeng X., He X. (2008) Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J. Biol. Chem. 283, 16115–16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen M., Philipp M., Wang J., Premont R. T., Garrison T. R., Caron M. G., Lefkowitz R. J., Chen W. (2009) G Protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J. Biol. Chem. 284, 35040–35048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan M., Yang C., Li J., Wu X., Yuan H., Ma H., He X., Nie S., Chang C., Cao X. (2008) Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 22, 2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson G., Shen J., Huang Y. L., Su Y., Karaulanov E., Bartscherer K., Hassler C., Stannek P., Boutros M., Niehrs C. (2009) Cell cycle control of wnt receptor activation. Dev. Cell 17, 788–799 [DOI] [PubMed] [Google Scholar]
- 19. ervenka I., Wolf J., Mašek J., Krejci P., Wilcox W. R., Kozubík A., Schulte G., Gutkind J. S., Bryja V. (2011) Mitogen-activated protein kinases promote WNT/β-catenin signaling via phosphorylation of LRP6. Mol. Cell. Biol. 31, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rauchman M. I., Nigam S. K., Delpire E., Gullans S. R. (1993) An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am. J. Physiol. 265, F416–F424 [DOI] [PubMed] [Google Scholar]
- 21. Sinha D., Wang Z., Price V. R., Schwartz J. H., Lieberthal W. (2003) Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am. J. Physiol. 284, F488–F497 [DOI] [PubMed] [Google Scholar]
- 22. Liu J., Wang Y., Du W., Liu W., Liu F., Zhang L., Zhang M., Hou M., Liu K., Zhang S., Yu B. (2013) Wnt1 inhibits hydrogen peroxide-induced apoptosis in mouse cardiac stem cells. PloS one 8, e58883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh C. G., Factor V. M., Sánchez A., Uchida K., Conner E. A., Thorgeirsson S. S. (2004) Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. U. S. A. 101, 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma H., Saenko M., Opuko A., Togawa A., Soda K., Marlier A., Moeckel G. W., Cantley L. G., Ishibe S. (2009) Deletion of the Met receptor in the collecting duct decreases renal repair following ureteral obstruction. Kidney Int. 76, 868–876 [DOI] [PubMed] [Google Scholar]
- 25. Ishibe S., Karihaloo A., Ma H., Zhang J., Marlier A., Mitobe M., Togawa A., Schmitt R., Czyczk J., Kashgarian M., Geller D. S., Thorgeirsson S. S., Cantley L. G. (2009) Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hader C., Marlier A., Cantley L. (2010) Mesenchymal-epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene 29, 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 [DOI] [PubMed] [Google Scholar]
- 28. Hartigan J. A., Xiong W. C., Johnson G. V. (2001) Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem. Biophys. Res. Commun. 284, 485–489 [DOI] [PubMed] [Google Scholar]
- 29. Hagen T., Di Daniel E., Culbert A. A., Reith A. D. (2002) Expression and characterization of GSK-3 mutants and their effect on β-catenin phosphorylation in intact cells. J. Biol. Chem. 277, 23330–23335 [DOI] [PubMed] [Google Scholar]
- 30. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 31. Ding V. W., Chen R. H., McCormick F. (2000) Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275, 32475–32481 [DOI] [PubMed] [Google Scholar]
- 32. Shaw M., Cohen P., Alessi D. R. (1997) Further evidence that the inhibition of glycogen synthase kinase-3β by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 416, 307–311 [DOI] [PubMed] [Google Scholar]
- 33. Lin S. L., Li B., Rao S., Yeo E. J., Hudson T. E., Nowlin B. T., Pei H., Chen L., Zheng J. J., Carroll T. J., Pollard J. W., McMahon A. P., Lang R. A., Duffield J. S. (2010) Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. U. S. A. 107, 4194–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krejci P., Aklian A., Kaucka M., Sevcikova E., Prochazkova J., Masek J. K., Mikolka P., Pospisilova T., Spoustova T., Weis M., Paznekas W. A., Wolf J. H., Gutkind J. S., Wilcox W. R., Kozubik A., Jabs E. W., Bryja V., Salazar L., Vesela I., Balek L. (2012) Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PloS one 7, e35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lyu J., Joo C. K. (2004) Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development 131, 1813–1824 [DOI] [PubMed] [Google Scholar]
- 36. Fuentealba L. C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E. M., De Robertis E. M. (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131, 980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B. M., Delius H., Hoppe D., Stannek P., Walter C., Glinka A., Niehrs C. (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 417, 664–667 [DOI] [PubMed] [Google Scholar]
- 38. Cho J., Rameshwar P., Sadoshima J. (2009) Distinct roles of glycogen synthase kinase (GSK)-3α and GSK-3β in mediating cardiomyocyte differentiation in murine bone marrow-derived mesenchymal stem cells. J. Biol. Chem. 284, 36647–36658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doble B. W., Patel S., Wood G. A., Kockeritz L. K., Woodgett J. R. (2007) Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaidanovich-Beilin O., Woodgett J. R. (2011) GSK-3: Functional Insights from Cell Biology and Animal Models. Frontiers Mol. Neurosci. 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Medina M., Wandosell F. (2011) Deconstructing GSK-3: The Fine Regulation of Its Activity. Int. J. Alzheimer's Dis. 2011, 479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 [DOI] [PubMed] [Google Scholar]
- 43. Yi H., Patel A. K., Sodhi C. P., Hackam D. J., Hackam A. S. (2012) Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PloS one 7, e36560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Q., Shen K., Zhao Y., Ma C., Liu J., Ma J. (2013) MiR-92b inhibitor promoted glioma cell apoptosis via targeting DKK3 and blocking the Wnt/β-catenin signaling pathway. J. Translat. Med. 11, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang T., Otevrel T., Gao Z., Gao Z., Ehrlich S. M., Fields J. Z., Boman B. M. (2001) Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 61, 8664–8667 [PubMed] [Google Scholar]
- 46. Suzuki A., Ozono K., Kubota T., Kondou H., Tachikawa K., Michigami T. (2008) PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3β in osteoblastic Saos-2 cells. J. Cell. Biochem. 104, 304–317 [DOI] [PubMed] [Google Scholar]