Background: Lutropin and choriogonadotropin are essential for human fertility.
Results: Human lutropin and choriogonadotropin assemble by two separate folding pathways.
Conclusion: The α-subunit of lutropin has a key role in β-subunit folding.
Significance: The unique folding properties of LH were retained by having an evolutionary advantage for most vertebrate fertility.
Keywords: Glycoprotein Hormones, Mutagenesis, Protein Chimeras, Protein Cross-linking, Protein Folding, Human Choriogonadotropin, Human Follitropin, Human Lutropin, Human Thyrotropin
Abstract
The glycoprotein hormones are all structurally related heterodimers consisting of an α-subunit and a ligand-specific β-subunit that confers their unique biological activity. Crystal structures showed how the β-subunit surrounds a part of the α-subunit, and we showed the existence of the two mechanisms responsible for that assembly. In human choriogonadotropin, the β-subunit is folded before the subunits dock, and the α-subunit becomes incorporated into the dimer by a mechanism we termed “threading,” passing between parts of the preassembled β-subunit. Here, we show that the human lutropin β-subunit is not folded completely prior to its interaction with the α-subunit and show that docking of the subunits enables the α-subunit to serve as a chaperone to the β-subunit. Based on data described here, we propose that the α-subunit facilitates formation of the human lutropin β-subunit by two mechanisms. First, the cystine knot of the α-subunit potentiates formation of the β-subunit cystine knot, and second, contacts between α-subunit loop 2 and a hydrophobic tail in the β-subunit facilitate formation of the seatbelt latch disulfide, which stabilizes the heterodimer. The primary influence of the α-subunit was seen when the hydrophobic tail was present or absent, but the secondary mechanism was required only when the hydrophobic tail of the β-subunit was present. During the evolution of human choriogonadotropin, neither of these α-subunit roles was necessary for folding of the β-subunit. The complex mechanism for lutropin assembly may be required to provide an additional control on its positive feedback function in vertebrate reproduction.
Introduction
The vertebrate pituitary glycoprotein hormones, lutropin, follitropin, and thyrotropin, regulate the activities of the gonads and thyroid gland (1). Some mammals also have a placental gonadotropin known as CG2 that regulates ovarian steroidogenesis during pregnancy. All four hormones are αβ-heterodimers composed of subunits that are divided into three large loops by cystine knots. The formation of the cystine knot is critical to the formation of a fully functional subunit. During heterodimer assembly, α-subunit loop 2 becomes surrounded by residues in the β-subunit “core” on one side and by 20–22 β-subunit residues on the other side that have been likened to a “seatbelt” (2). The C terminus of the seatbelt is “latched” by a disulfide bond to a cysteine in loop 1 of the β-subunit. Earlier studies suggested that heterodimer assembly occurred by a “wraparound” mechanism in which the seatbelt became latched after the subunits docked (3). Contrary to these studies, we reported that the assembly of human choriogonadotropin (hCG), human follitropin (hFSH), and human thyrotropin (hTSH) occurred in the endoplasmic reticulum only after the seatbelt was latched (4, 5). Assembly of these heterodimers is driven by contacts between the subunits that allow for the formation of a parallel β-sheet composed of residues from the β-subunit and α-subunit loop 2 (2, 6, 7). Following the formation of this sheet, α-subunit loop 2, along with its carbohydrate, is pulled stepwise through the hole in the β-subunit in a method that we termed “threading,” to distinguish it from a mechanism where the seatbelt is “wrapped around” α-subunit loop 2 prior to latching the docked subunits (5). We also found that threading was facilitated by disruption of a disulfide that stabilizes a small loop in the N-terminal end of the seatbelt that functions as a “tensor” (8). When the tensor disulfide is disrupted, the seatbelt is elongated, and the hole in the β-subunit is increased substantially. Although the tensor disulfide does not need to be disrupted to permit assembly of heterodimers, particularly after the α-subunit loop 2 oligosaccharide has been removed, disruption of the tensor disulfide facilitates assembly markedly (8). Glycoprotein hormones with structures similar to that of FSH in many teleosts can form only by a wraparound mechanism (9). In these heterodimers, the seatbelt is latched to a cysteine in the N-terminal end of the β-subunit. This makes the β-subunit hole too small for threading and dramatically increases the stability of teleost FSH. In this case, assembly has to occur by a wraparound mechanism in which the disulfide that latches the seatbelt to the β-subunit forms after the subunits dock.
In the case of glycoprotein hormone assembly, it is difficult to distinguish assembly that occurs by a threading or wraparound pathway because both would lead to assembly of a heterodimer having the same disulfides. Our strategy for distinguishing these two methods of assembly involved adding an extra cysteine to the α-subunit, removing the latch cysteine of the β-subunit and determining whether the subunits were able to form disulfide cross-links (9, 10). This permitted us to determine if assembly can proceed by a wraparound pathway because it would trap the formation of a disulfide cross-link between the α- and β-subunits, which could not be formed by a threading mechanism. To distinguish the relative importance of the threading and wraparound pathways, we used a strategy in which both mechanisms could compete with one another. If we observed that the pathway in which both threading and wraparound components could lead to heterodimer formation and a mechanism in which the wraparound pathway was essential, then we would be able to distinguish which pathway was occurring in the cell. In the case of hCG, hFSH, and hTSH, we observed that the wraparound mechanism was outcompeted nearly exclusively by the threading mechanism, and we did not observe any heterodimer cross-linking. In the present study, we found that hLH is predominantly formed by a wraparound mechanism, which outcompeted the threading mechanism, as will be described. This was surprising due to the similarity of hLH and hCG. We suggest that this observation has important implications for differences in the physiological properties of hLH.
During the evolution of choriogonadotropins, notably hCG, factors that limit assembly of the type seen in hLH were modified. The hCG β-subunit can fold independently of the α-subunit, and the hCG β-subunit can be secreted as a monomer as well as an αβ-heterodimer (11). The heterodimer is required for maintenance for early human pregnancy, but the role of the free β-subunit is unknown.
EXPERIMENTAL PROCEDURES
The rationale for studying assembly used in these studies has been described earlier (5). An alternative method for studying assembly is by utilizing a pulse-chase strategy in which the assembly process is blocked and intermediates are measured as a function of time. This approach is difficult after transfecting both α- and β-subunits of the gonadotropins because we do not have antibodies capable of distinguishing multiple intermediates. Therefore, we used an alternative approach, which was successful for monitoring the assembly of hCG, hFSH, and hTSH. Briefly, as noted above, we made use of the observation that the seatbelt of hCG can be latched to a cysteine added to the α-subunit when the cysteine in the normal β-subunit latch site is replaced by alanine. Because the seatbelt cannot be latched in this type of heterodimer until after its α- and β-subunits have docked, assembly that takes place requires a wraparound mechanism. By comparing the ability of an α-subunit analog with an additional cysteine at position 43 (αS43C) to compete for formation of heterodimers with β-subunit analogs either lacking or retaining the normal seatbelt latch site, we determined the relative efficiencies of the threading and wraparound pathways. This is why we chose this method to monitor the assembly of hLH although this would limit us to monitoring the overall process and not enable us to monitor intermediates directly.
A limitation of this assay is that it depends on the presence of an α-subunit analog that is capable of binding the seatbelt latch disulfide. For hormones such as hCG in which the β-subunit cystine knot forms naturally, the presence of the α-subunit analog in these assays does not influence the folding mechanism, which is centered around formation of the latch disulfide. In studies of the assembly of hLH, the presence of the α-subunit is required for formation of the β-subunit. This complicates our understanding of the role of the α-subunit in assembly. To counter this limitation, we modified the assay to determine how the α-subunit might influence another component of the folding mechanism, the formation of the cystine knot. In this analysis, alternate latch cysteines were added to an hCG β-subunit analog containing four N-terminal substitutions unique to hLH, two of which are adjacent to the first cysteine in the β-subunit cystine knot of hLH. This strategy enabled us to determine that the formation of the cystine knot was influenced by the presence of residues unique to the hLHβ-subunit by determining its ability to interfere with folding of the hCG analog relative to that of hCG.
Constructs produced by standard polymerase chain reaction and cassette mutagenesis methods (12) were sequenced prior to use. They were transfected into COS-7 cells obtained from ATCC (Manassas, VA) using a calcium phosphate procedure (13). Secreted analogs were harvested 3 days after transfection and analyzed in monoclonal antibody sandwich immunoassays (14) employing antibodies A113 and B401, obtained from Dr. William Munroe (Hybritech Inc., a division of Beckman Coulter, Inc., San Diego, CA) and B110 produced as described (15). Antibody A113 recognizes conformation-dependent α-subunit epitopes in the heterodimer. Antibody B110 recognizes conformation-dependent epitopes formed when β-subunit loops 1 and 3 are adjacent in hCGβ and hCG. Antibody B401 recognizes hLH heterodimers and heterodimers in which the N-terminal end of the β-subunit contained the 15 residues derived from hLHβ. This made it useful for studying the influence of this region of the β-subunit on assembly. All of these antibodies have little, if any, ability to detect the β-subunit prior to formation of the cystine knot, a key step in folding of the β-subunit core. Antibodies used for detection were radioiodinated to a specific activity of ∼50 μCi/μg using IODO-GEN (Pierce). The acid stability of the heterodimers was tested in 0.4-ml samples by reducing the pH to 2 by the addition of microliter aliquots of 2 m HCl while monitoring the pH, incubating acidified samples for 30 min at 37 °C, readjusting the pH to 7.5 by the addition of sufficient aliquots of a mixture of 10 n NaOH, 1 m Tris buffer, pH 7.5 (1:2) and then quantifying them by a sandwich immunoassay. A cDNA encoding the hLHβ-subunit gene as well as pure recombinant hCG, used as a standard, was kindly donated by Dr. Robert Campbell (Serono Reproductive Biology Institute, Rockland, MA). The hCG β-subunit used as a standard was purified from this hCG by high performance liquid chromatography on a C-18 resin using an acetonitrile gradient in water containing 0.1% trifluoroacetic acid. Procedures to monitor assembly in vitro have been described (5). All sandwich assay estimates were determined statistically using Prism (GraphPad Software, San Diego, CA). hLH α- and β-subunit assembly was modeled based on the published x-ray structure of hCG (Protein Data Bank code 1HRP). An α-subunit of hLH was made from an α-subunit of hCG by replacing residues not seen in the crystal structure. The β-subunit of hLH was created by homology modeling (sequence of lutropin β-subunit from the Uniprot data bank, accession number P01229) from an hCG β-subunit. Missing carbohydrates were added in MOE 2012 (Chemical Computing Group, Montreal, Canada). Structures were refined with molecular dynamics in the Amber 12 molecular dynamics package.
RESULTS
We utilized the strategy that had been successful in identifying threading and wraparound mechanisms for the other gonadotropins to hLH. Remarkably, we observed that hLH was not formed by a threading mechanism but was assembled by a wraparound mechanism. This was completely unexpected due to the fact that hLH is considered an ancestral predecessor of hCG that was created by a genetic read-through (16) and differs from hCG in only 15 amino acids within their conserved regions. The data in Table 1, row 2, show that hCG is produced very efficiently and that 10% of the dimer formed is stable at low pH. This is typical during this type of transfection with an α-subunit that contains an extra cysteine at position 43 because it forms a disulfide with a tensor cysteine (10, 17). hLH is produced much less efficiently (Table 1, row 1), and a somewhat larger fraction was acid-stable. Rows 3 and 4 show that both hCG and hLH can become cross-linked to a cysteine added to the α-subunit when they are prevented from assembling to the normal seatbelt latch disulfide. This shows that each of them can form a heterodimer when they are prevented from being latched to cysteine 26 of the β-subunit. To test the efficiency, to determine which of these hormones can be assembled by a wraparound pathway, we compared their abilities to be latched normally and to the β-subunits lacking the seatbelt latch disulfide (Table 1, rows 5 and 6). The data in row 5 show the result of a competition experiment in which native hLHβ-subunit and an analog of the hLHβ-subunit lacking the ability to latch its disulfide normally were cotransfected with the α-subunit having a cysteine at residue 43. Approximately 70% of it became acid-stable, indicating that it had been cross-linked to the α-subunit, whereas only a fraction of the native subunit was produced. Although the total material produced was reduced in amount, most of it was produced by a threading pathway (compare rows 1 and 5). Conversely, we repeated the same competition experiment with the hCG β-subunit and its analog that cannot be latched normally. Almost all of the hormone that was formed occurred by a threading mechanism, and only 12% was acid-stable (row 6). This 12% was probably the result of a cross-link to a cysteine in the tensor loop, as shown previously (8). Thus, under the same conditions, hLH appears to be formed primarily by a wraparound pathway, and hCG is formed by a threading pathway. We repeated this study under conditions in which hLH was allowed to compete with an analog of hCG that could not latch its seatbelt normally (row 7) and observed that the amount of heterodimer produced was reduced substantially, with 50% of it being acid-stable. This showed that, under these conditions, hCG was not formed efficiently and that its formation was reduced due to the presence of the hLH β-subunit. Conversely, when we repeated the same experiment when hCG was produced normally and hLH was forced to bind by a wraparound pathway (row 8), very little of the heterodimer was acid-stable. This indicated that hCG dominated the assembly pathway. Together, the data in rows 7 and 8 show that the presence of hLH appears to slightly reduce the efficiency of hCG assembly.
TABLE 1.
Comparison of competition experiments between hCGβ, hLHβ, and LC β chimaeras
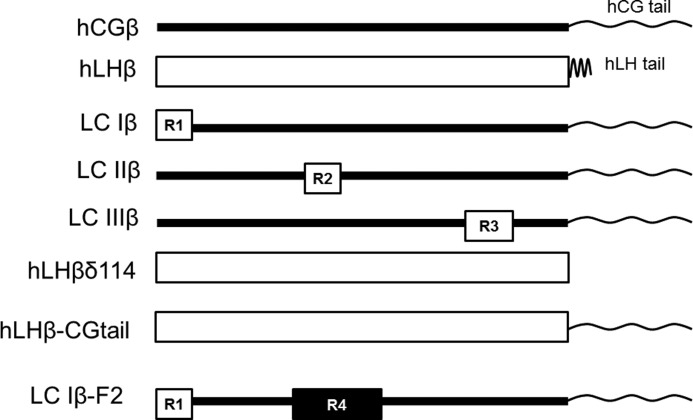
As diagrammed in Fig. 1, LC Iβ is an analog containing 4 residues of hLHβ (residues 2, 8, 10, and 15) with the remaining components hCG. LC IIβ is similar to LC Iβ except that it contains 4 different hLHβ residues (residues 42, 47, 51, and 58). LC IIIβ is similar to LC Iβ and LC IIβ except that it contains 7 different hLHβ residues (residues 77, 82, 83, 89, 91, 92, and 98). The α-subunit contains a cysteine in place of serine 43 in all assays. Analogs were co-transfected into COS-7 cells and quantified in A113/125I-B110 assays. In this assay, antibody A113 binds to a region of the α-subunit in loop 1, near residues 19–21, with high affinity for hCG and hLH but not bovine LH. Antibody B110 binds many mammalian LH and CG analogs in loop 3, near residues 70–80. We have not measured it by individual site-specific mutation. “Total dimer” represents the total amount of dimer detected. “Cross-linked dimer” represents the amount of heterodimer that remained after pH 2 treatment, 30 min at 37 °C. “% X-linked dimer” is the ratio of cross-linked dimer to total dimer. In all analogs that contain βC26A, the β-subunit has the cysteine at position 26 replaced by alanine.
| Row no. | Analogs | Total dimer | X-linked dimer | % X-linked dimer |
|---|---|---|---|---|
| % | ||||
| 1 | hLHβ | 1.56 ± 0.03 | 0.35 ± 0.02 | 22 |
| 2 | hCGβ | 11.81 ± 0.5 | 1.21 ± 0.03 | 10 |
| 3 | hLHβ C26A | 0.78 ± 0.04 | 0.65 ± 0.01 | 83 |
| 4 | hCGβ C26A | 8.90 ± 0.06 | 7.29 ± 0.44 | 83 |
| 5 | hLHβ + hLHβ C26A | 0.61 ± 0.02 | 0.43 ± 0.02 | 71 |
| 6 | hCGβ + hCGβ C26A | 14.18 ± 0.50 | 1.68 ± 0.25 | 12 |
| 7 | hLHβ + hCGβ C26A | 3.74 ± 0.30 | 1.95 ± 0.15 | 52 |
| 8 | hCGβ + hLHβ C26A | 6.83 ± 1.4 | 0.45 ± 0.03 | 7 |
| 9 | LC Iβ | 16.25 ± 1.69 | 1.28 ± 0.05 | 8 |
| 10 | LC Iβ + hCGβ C26A | 14.57 ± 1.04 | 3.24 ± 0.22 | 22 |
| 11 | LC IIβ | 11.73 ± 0.41 | 0.46 ± 0.01 | 4 |
| 12 | LC IIβ + hCGβ C26A | 17.20 ± 0.07 | 1.48 ± 0.10 | 9 |
| 13 | LC IIIβ | 10.33 ± 0.20 | 0.71 ± 0.05 | 7 |
| 14 | LC IIIβ + hCGβ C26A | 11.40 ± 0.57 | 2.10 ± 0.14 | 18 |
The core of hLH differs from hCG by 15 amino acids and can be divided into three regions that contain 4, 4, and 7 differences, respectively (Fig. 1, R1, R2, and R3). To identify which of these regions had the most influence on hLH assembly, we expressed each of them using the competition assay. LC Iβ differs from hCG at residues 2, 8, 10, and 15. When we replaced these residues with their hCG counterparts to obtain LC Iβ and expressed it along with the αS43C analog, we observed that expression of the heterodimer was high (Table 1, row 9), and very little cross-linked analog was formed. When we competed LC Iβ with the hCG analog lacking its latch disulfide, we observed high expression, and only a small fraction of the heterodimer was cross-linked (row 10), but it was more than double that from row 9. This may be due to the fact that this analog of hCG does not compete well with LC Iβ, which contains only 4 residues specific to the hLH β-subunit. To examine the second region, we monitored the expression of LC IIβ, which contains hCG residues 42, 47, 51, and 58 (row 11). LC IIβ, expressed with the αS43C analog, also expressed well, but when we coexpressed it with the hCGβ analog lacking its latch disulfide, we observed only minimal cross-linking (row 12). This again may be due to the fact that it contained only 4 hLHβ-specific residues. LC IIIβ contains hCG residues 77, 82, 83, 89, 91, 92, and 98. When we expressed LC IIIβ along with the αS43C analog, we observed that it expressed well (row 13). When it was expressed along with the hCG analog lacking its latch disulfide, only a small fraction of cross-linking was observed (row 14). When we used the entire hLH β-subunit in competition with hCGβ C26A (row 7), we observed a higher cross-linking, which suggested that more than one region of hLHβ influences its ability to compete with the hCG analog.
FIGURE 1.
Comparison of β-subunit sequences used in these studies. These illustrations represent the analogs used in this work and their relative sizes. hCGβ is displayed as a dark line and contains a large tail as illustrated. hLHβ is displayed as an open bar and has a short tail of 7 residues that is later removed. LC Iβ is an analog containing 4 residues of hLHβ (residues 2, 8, 10, and 15 in region 1, R1) with the remaining components hCG. LC IIβ is similar to LC Iβ except that it contains 4 different hLHβ residues (residues 42, 47, 51, and 58 in region 2, R2). LC IIIβ is similar to LC Iβ and LC IIβ except that it contains 7 different hLHβ residues (residues 77, 82, 83, 89, 91, 92, and 98 in region 3, R3). δ114 analogs are missing the 7 hydrophobic residues at the hLH β-subunit tail. hLHβ-CG tail, hLH β-subunit containing hCG tail residues in place of the 7 hLH β-subunit tail residues. LC Iβ-F2, LC Iβ containing FSH β-subunit residues from loop 2 (in region 4, R4) in place of those in LC Iβ.
Until now, we focused on the major differences of hLH and hCG observed in the core regions of these β-subunits. As an internal control, we repeated studies that are shown in Table 1, which showed that the differences between hLH and hCG are reproducible and are typical for several experiments (Table 2, rows 1–6). We observed in Table 1 that the presence of hCG β-subunit gave higher levels of expression, and in one case, the presence of hLH β-subunit tended to reduce the expression seen for hCG (Table 1, row 8). This may have been caused by the presence of the hydrophobic tail on the hLH β-subunit, which differs dramatically in the two β-subunits. To determine the influence on expression and assembly by this tail, we removed it or replaced it with the tail of hCG. Row 7 (Table 2) shows that the removal of the C terminus of hLH did not disrupt the assembly of heterodimer as compared with the intact hLH β-subunit seen in row 1. Indeed, the amount of expression we observed was significantly greater. This suggested that the tail of the hLH β-subunit had an inhibitory influence on assembly but did not prevent threading because it did not result in cross-linking. Furthermore, studies with the intact hLH β-subunit revealed significant cross-linking of the heterodimer to the α-subunit (row 1 in both Tables 1 and 2). However, as noted earlier in the Introduction, it is not possible to distinguish threading and wraparound pathways in the absence of competition; therefore, we are not able to distinguish the assembly mechanism from the data in row 7 alone. To determine if the absence of the hLH β-subunit tail would permit cross-linking to the α-subunit, we removed the natural latch site in the analog lacking the tail (row 8). In this case, we observed that assembly occurred by a wraparound mechanism, which indicated that the absence of the tail did not prevent cross-linking. This analog had reduced efficiency for expression, however, and was present in an amount similar to that observed in the native hLH β-subunit (row 1). In the competition experiment (row 9), we observed that most of the assembly occurred by a wraparound mechanism although both analogs lacked the hLH tail. This result suggested that the C-terminal tail of the hLH β-subunit has a significant influence on its structure and that it also contributes to its assembly process. Again, the amount of dimer produced was similar to that observed in the intact hLH β-subunit (row 1).
TABLE 2.
Comparison of competition experiments between hCGβ, hLHβ, and LC Iβ chimeras
As diagrammed in Fig. 1, hLHβδ114 represents the human LHβ-subunit truncated at position 114. hLHβ-CGtail represents the human LHβ-subunit with amino acids from 114 to the C terminus replaced with those from hCG. LC Iβ-F2 is an analog containing 4 residues of hLHβ (residues 2, 8, 10, and 15) with the remaining components hCG except for residues 38–56 (where hCG residues have been replaced with those from hFSH). The α-subunit contains a cysteine in place of serine 43 in all assays. Analogs were co-transfected into COS-7 cells and quantified in A113/125I-B110 assays. “Total dimer” represents the total amount of dimer detected. “Cross-linked dimer” represents the amount of heterodimer that remained after pH 2 treatment, 30 min at 37 °C. “% cross-linked dimer” is the ratio of cross-linked dimer to total dimer. In all analogs that contain βC26A, the β-subunit has the cysteine at position 26 replaced by alanine.
| Row no. | Analogs | Total dimer | X-linked dimer | % X-linked dimer |
|---|---|---|---|---|
| % | ||||
| 1 | hLHβ | 0.98 ± 0.04 | 0.21 ± 0.01 | 21 |
| 2 | hCGβ | 5.89 ± 0.61 | 0.67 ± 0.10 | 11 |
| 3 | hLHβ C26A | 0.47 ± 0.02 | 0.50 ± 0.02 | 100 |
| 4 | hCGβ C26A | 3.61 ± 0.17 | 3.41 ± 0.32 | 94 |
| 5 | hLHβ + hLHβ C26A | 0.40 ± 0.06 | 0.31 ± 0.04 | 78 |
| 6 | hCGβ + hCGβ C26A | 6.45 ± 0.62 | 1.26 ± 0.10 | 20 |
| 7 | hLHβδ114 | 4.53 ± 0.18 | 0.43 ± 0.02 | 9 |
| 8 | hLHβδ114 C26A | 0.89 ± 0.07 | 0.94 ± 0.03 | 100 |
| 9 | hLHβδ114 + hLHβδ114 C26A | 0.81 ± 0.05 | 0.65 ± 0.05 | 80 |
| 10 | hLHβ-CGtail | 3.56 ± 0.20 | 0.46 ± 0.04 | 13 |
| 11 | hLHβ-CGtail C26A | 3.35 ± 0.48 | 3.53 ± 0.51 | 100 |
| 12 | hLHβ-CGtail + hLHβ-CGtail C26A | 3.16 ± 0.25 | 2.26 ± 0.30 | 72 |
| 13 | LC Iβ-F2 | 0.89 ± 0.03 | 0.30 ± 0.01 | 34 |
| 14 | LC Iβ-F2 C26A | 1.12 ± 0.04 | 1.11 ± 0.02 | 99 |
| 15 | LC Iβ-F2 + LC Iβ-F2 C26A | 0.47 ± 0.02 | 0.37 ± 0.04 | 79 |
Next, to determine if these results were a function of the hCG C-terminal tail, we replaced the hLH tail (residues 115–121) with the hCG tail (residues 115–145). As shown in Table 2, row 10, we observed that the hLH analog was produced nearly as well as the hLH analog lacking its tail (row 7). When we removed the cysteine latch (C26A), we observed that all of the assembly went by the wraparound pathway, as shown by the formation of a cross-link to the α-subunit analog (row 11). But when we did the competition experiment (row 12), where we permitted the dimer to form by either the threading or wraparound pathways, 72% of it was formed by the wraparound pathway, and it became cross-linked. These results confirmed that the hLH β-subunit C-terminal tail reduced dimer formation with the α-subunit analog (18, 19) (compare rows 1, 7, and 10). Therefore, these observations suggest that the C terminus of hLH has a negative influence on hLH expression.
We did not know why the few residues in LC IIβ appeared to have less of an influence on assembly than LC 1β or LC IIIβ (Table 1, rows 9–14). To learn if loop 2 of the β-subunit had an influence on assembly, we combined loop 2 from the hFSH β-subunit with LC Iβ and monitored its influence on assembly in the competition assay. The relative positions of these residues are shown in Fig. 1 (LC Iβ-F2). This region of hFSHβ differed from hLHβ by the presence of 14 unique residues and, as seen in Table 2, rows 13–15, had a significant effect on the assembly of LC Iβ. Thus, although it was less evident in Table 1, loop 2 of the β-subunit can have an influence on the mechanism of assembly.
Data in Tables 1 and 2 show that hLH is bound to the α-subunit analog when it is blocked from binding to the normal latch site (row 5 in both tables), but there is some binding to the wild type hLH β-subunit. This is unlike what we observe for hCG, which binds to the wild type form preferentially and most likely exclusively (Tables 1 and 2, row 6). Because antibody A113 was used as a capture agent for these analogs, we were concerned that it might have a preference for the α-subunit of hCG relative to hLH. Therefore, we repeated a portion of these experiments using an hLH-specific antibody to monitor binding to the β-subunit of hLH in the presence of the αS43C analog. Table 3, rows 1, 2, and 4, shows that antibody B401 interacts with hLHβ and LC Iβ, both of which contain hLHβ residues at their N termini. It does not interact with LC IIβ or hCGβ, which do not have N-terminal residues of hLHβ (row 3). hLHβ is outcompeted by an hCGβ subunit analog that lacks its latch cysteine and has very high affinity for the α-subunit containing a cysteine in place of residue 43. In contrast, LC Iβ competes better with the same analog of hCGβ (row 2). This suggests that hCG residues in a downstream region of its β-subunit are more active than wild type hLH β-subunit residues in this assay. The LC II β-subunit was not active in this assay because it is not recognized by antibody B401 (row 3). Confirming what was shown earlier (Tables 1 and 2, row 6), hCGβ outcompetes the analog lacking its latch disulfide in this assay (Tables 1 and 2, row 6) and was much more active than an hLH β-subunit analog that was forced to assemble by a wraparound pathway (row 4) but is not seen because hCG does not bind to this antibody. These observations show that the hCG β-subunit is more active than the full-length hLH β-subunit possibly because all of the hLH β-subunit analogs used in this assay contained the 7 hydrophobic terminal residues. Elimination of these 7 residues increased assembly, as seen in Table 2, row 4. We will propose a rationale for this under “Discussion.”
TABLE 3.
Comparison of competition experiments using an antibody specific for hLHβ N terminus
LC Iβ is an analog containing 4 residues of hLHβ (residues 2, 8, 10, and 15) with the remaining components hCG. LC IIβ is similar to LC Iβ except that it contains 4 different hLHβ residues (residues 42, 47, 51, and 58). The α-subunit contains a cysteine in place of serine 43 in all assays. Analogs were co-transfected into COS-7 cells and quantified in A113/125I-B401 assays. Antibody B401 binds to the hLH β-subunit N terminus. Total dimer refers to the total amount of dimer detected. Cross-linked dimer refers to the amount of heterodimer that remained after pH 2 treatment, 30 min at 37 °C. In all analogs that contain βC26A, the β-subunit has the cysteine at position 26 replaced by alanine.
| Row no. | Analogs | Total dimer | X-linked dimer |
|---|---|---|---|
| 1 | hLHβ + hCGβ C26A | 0.27 ± 0.01 | 0.05 ± 0.01 |
| 2 | LC Iβ + hCGβ C26A | 3.88 ± 0.03 | 0.18 ± 0.01 |
| 3 | LC IIβ + hCGβ C26A | 0.03 ± 0.01 | 0.05 ± 0.01 |
| 4 | hCGβ + hLHβ C26A | 0.04 ± 0.00 | 0.03 ± 0.01 |
As described under “Experimental Procedures,” a limitation of the assay used in Tables 1 and 2 is that we are unable to determine if the LH β-subunit folding is influenced by the presence of the α-subunit because the α-subunit is required for hLH folding. Therefore, to determine if the assembly of the hLH β-subunit cystine knot requires the presence of the α-subunit, we monitored the ability of alternate latch cysteines added to the β-subunits of both hCG (Table 4, rows 1–8) and an hCG β-subunit analog containing 4 residues specific to hLH (Table 4, rows 9–16) to compete for the latch disulfide. Two of the four substitutions are adjacent to the first cysteine in the hLH β-subunit cystine knot. With the addition of αS43C, we then monitored the competition for assembly of both hCG analogs with their latch cysteines available or disrupted (C26A). In the case of hCG (10), disrupting the latch site did not disrupt assembly of a cross-linked heterodimer, as seen here as a control (Table 4, row 2), which is forced to assemble by a wraparound pathway. When we added a cysteine in place of hCG β-subunit residues Asn-77, Phe-64, and Ala-83, we did not disrupt assembly of the heterodimer that contained a normal seatbelt latch site although it contained a cysteine at αSer-43 (Table 4, compare rows 1, 3, 5, and 7). In contrast, when we added these same cysteines to an hCG β-subunit analog that lacked its normal latch site (C26A), these β-subunit cysteines disrupted its assembly with the α-subunit cysteine (S43C), as seen in rows 2, 4, 6, and 8.
TABLE 4.
Internal competition experiments for hCGβ and LC Iβ single and double mutants
LC Iβ is an analog containing 4 residues of hLHβ (residues 2, 8, 10, and 15) with the remaining components hCG. β-Subunit mutations include N77C, F64C, and A83C. In all analogs that contain βC26A, the β-subunit has the cysteine at position 26 replaced by alanine. The α-subunit contains a cysteine in place of serine 43 in all assays. Analogs were co-transfected into COS-7 cells and quantified in A113/125I-B110 assays. “Total dimer” represents the total amount of dimer detected. “Cross-linked dimer” represents the amount of heterodimer that remained after pH 2 treatment, 30 min at 37 °C. ND, not determined.
| Analogs | Total dimer | X-linked dimer | % X-linked dimer | |
|---|---|---|---|---|
| % | ||||
| 1 | hCGβ | 9.73 ± 1.79 | 0.70 ± 0.06 | 7.2 |
| 2 | hCGβ C26A | 5.20 ± 0.71 | 5.98 ± 0.24 | 100 |
| 3 | hCGβ N77C | 8.70 ± 0.60 | 0.64 ± 0.14 | 7.4 |
| 4 | hCGβ N77C C26A | 0.15 ± 0.00 | 0.15 ± 0.00 | ND |
| 5 | hCGβ F64C | 6.89 ± 1.40 | 0.62 ± 0.05 | 9.0 |
| 6 | hCGβ F64C C26A | 0.24 ± 0.02 | 0.22 ± 0.03 | ND |
| 7 | hCGβ A83C | 8.41 ± 0.70 | 0.55 ± 0.06 | 6.5 |
| 8 | hCGβ A83C C26A | 0.13 ± 0.00 | 0.11 ± 0.02 | ND |
| 9 | LC Iβ | 14.23 ± 0.45 | 0.94 ± 0.06 | 6.6 |
| 10 | LC Iβ C26A | 13.33 ± 3.28 | 13.17 ± 0.91 | 98.8 |
| 11 | LC Iβ N77C | 3.54 ± 0.99 | 0.29 ± 0.01 | 8.2 |
| 12 | LC Iβ N77C C26A | 1.27 ± 0.06 | 1.36 ± 0.02 | 100 |
| 13 | LC Iβ F64C | 2.90 ± 0.47 | 0.37 ± 0.03 | 12.8 |
| 14 | LC Iβ F64C C26A | 2.66 ± 0.46 | 2.88 ± 0.26 | 100 |
| 15 | LC Iβ A83C | 2.58 ± 0.27 | 0.15 ± 0.00 | 5.8 |
| 16 | LC Iβ A83C C26A | 0.70 ± 0.01 | 0.64 ± 0.04 | 91.4 |
These observations showed that there is a natural tendency for the hCG β-subunit to attach to its normal seatbelt latch site. It can, however, be latched to a cysteine in loop 2 of the α-subunit when the normal latch site is removed. When cysteines were substituted for residues in hCG, in which its normal seatbelt latch site had been substituted by an alanine residue to block it, the hCG assembly mechanism was blocked (Table 4, rows 4, 6, and 8). This showed that when the normal hCG β-subunit latch site is blocked, the added cysteines are used preferentially to disrupt assembly of the heterodimer. These studies showed that there is a rapid formation of the hCG cystine knot such that any additional cysteines added to the β-subunit are unable to interfere with its formation. Observations with hLH analogs differed significantly. In this case, we used the LC I β-subunit so that we could retain the rest of the hCG molecule. Removal of the normal seatbelt latch site did not prevent assembly with a cysteine in αS43C (Table 4, rows 9 and 10). However, the presence of additional cysteines at Asn-77, Phe-64, and Ala-83 reduced assembly of all of the analogs (rows 11, 13, and 15), indicating that there was no tendency for latching to the natural cysteine latch site but a small tendency for latching to the cysteine added to the α-subunit. This observation was remarkable because there are only 4 residues in this analog of hLHβ that differ from hCG. Additionally, there was a small tendency for these cysteine substitutions to promote assembly by a wraparound mechanism, which led to a small increase in assembly that was significantly different from what we observed when we added identical cysteines to hCG (compare rows 4, 6, and 8 with rows 12, 14, and 16, respectively). This observation showed that the presence of the 4 hLH residues in the β-subunit of this analog reduced substantially the ability of the cystine knot to be formed regardless of where the β-subunit substituted cysteines were introduced.
DISCUSSION
The glycoprotein hormones evolved from invertebrate precursors (20). The initial steps in this process appear to have involved thyrostimulin, which is active in TSH receptors but lacks the seatbelt (21). In addition, choriogonadotropin, equine LH, and marmoset LH appear to have evolved from an LH precursor by the addition of a C-terminal tail (22, 23). There is good evidence that human chorionic gonadotropin evolved from an LH molecule that underwent a read-through that replaced its hydrophobic tail with a long disordered tail (16) in addition to the changes that facilitated the formation of its cystine knot. LH, FSH, TSH, and hCG all contain a similar seatbelt latch disulfide, which appears to stabilize the heterodimer, although it can be cross-linked to the α-subunit without disrupting the activity of hCG (9). The formation of the seatbelt latch disulfide appears to have a substantial influence on the assembly of LH and may have enabled the unique function of LH relative to those of the other glycoprotein hormones. LH is the only hormone that is both stored and has a positive feedback role in vertebrate fertility. Our studies have shown that regulation of hLH assembly is highly controlled by the hydrophobic tail and the assembly of the cystine knot, the latter of which appears to be dominant.
All LH β-subunits contain 12 cysteines, including a seatbelt latch disulfide. Additionally it seems highly unlikely that the six disulfides of the hLH β-subunit would be located in an arrangement different from those in hCGβ (2, 6). One way that we found to distinguish differences in the way these disulfides were formed was to incorporate a cysteine into the α-subunit that would enable us to monitor β-subunit folding and heterodimer assembly (5). Unlike hCG, hFSH, and hTSH, it appears that formation of the hLH disulfides differs significantly based on the abilities of the hLH seatbelt latch to interact with the α-subunit. It is not yet clear, however, how the assembly of the hLH heterodimer has a role in its physiology. There are two observations that suggest that hLH is assembled differently than hCG aside from the observations we made by replacing α-subunit Ser-43 with cysteine. The first of these is that it appears to be impossible to fold the hLH β-subunit in the absence of the α-subunit (24). This suggests that the α-subunit has a critical role in β-subunit folding in the endoplasmic reticulum because this portion of the β-subunit remains attached to BiP, implying that it is malfolded (24, 25). The second observation is that the hCG α-subunit can be found in human plasma (26), suggesting that it is folded and secreted in the absence of the β-subunit. Therefore, we suggest that the α-subunit has a critical role for folding of the human LH β-subunit. Even in the presence of the α-subunit, the assembly of the cystine knot in hLH is retarded, as seen when additional cysteines added to the β-subunit interfere with assembly of an hLH analog but not hCG (Table 4). This shows that it is difficult to form the cystine knot in the hLH β-subunit, a phenomenon that we postulate to be a major regulator of LH assembly. Furthermore, we have found that the assembly of hCG heterodimers is facilitated by the presence of a small amount of reducing agent in vitro, due to the fact that the tensor disulfide becomes disrupted (5). We anticipate that the reducing equivalent of the endoplasmic reticulum may facilitate the disruption of α-subunit cysteines 7 and 31, because these are among the most easily disrupted cysteines in the α-subunit (27). As a consequence of our observations in Table 4, we expect that the presence of a reducing agent, such as the cysteine substituted for α-subunit residue 43, may have an influence on the assembly mechanism in these studies. We summarize the differences between hCG and hLH assembly in Fig. 2. A1 shows that hCG is assembled by a threading mechanism as published (8). Fig. 2, A2, shows the mechanism for assembly of hCG heterodimers, in which an α-subunit containing an additional disulfide is cross-linked to the β-subunit, in which the latch disulfide is disrupted. These two mechanisms form the basis of several studies in this paper. In contrast, we propose that the β-subunit of hLH is much more difficult to fold than the hCG β-subunit (Fig. 2, B1). Folding of the hLH β-subunit is dependent on the α-subunit acting as a chaperone (24). Based on the observations made in Table 4, we anticipate that the β-subunit seatbelt latch or other portions of the β-subunit are not completely folded in the absence of the α-subunit and that, as a result, much of the assembly process takes place by a wraparound mechanism. In contrast to this mechanism, when the seatbelt latch of the hLH β-subunit is disrupted, the presence of the α-subunit is still required for β-subunit folding, and the presence of an additional cysteine in the α-subunit facilitates the wraparound process that leads to cross-linked gonadotropin (Fig. 2, B2).
FIGURE 2.
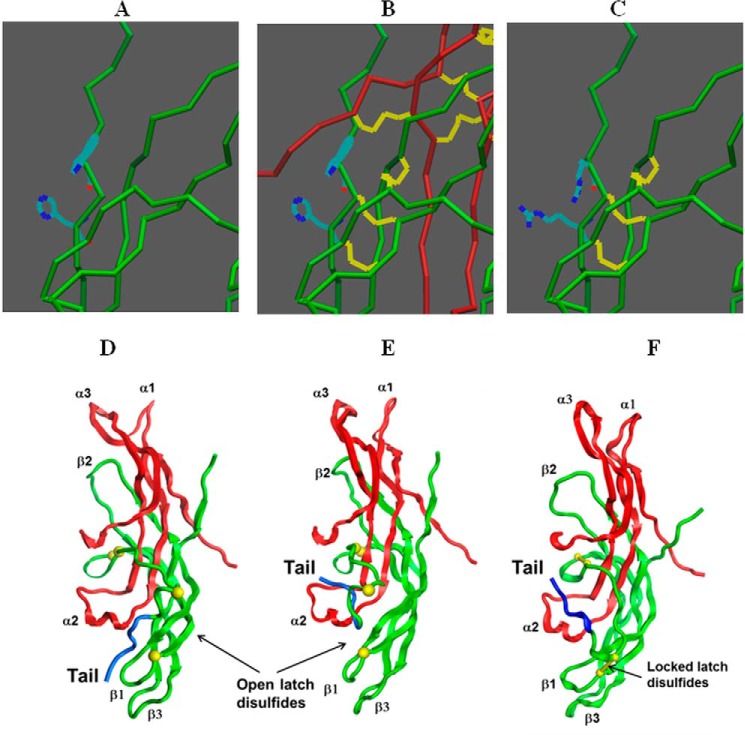
Comparison of hCG and full-length hLH assembly. In the α-subunit shown in all figures, the only disulfide that we anticipate to be easily reduced in the endoplasmic reticulum is that at residues 7–31 (27). In the experiments done in this paper, we substituted an additional cysteine in the α-subunit at residue Ser-43. This is shown only in A2 and B2. This is omitted in A1 and B1, which refer to the normal assembly process, in vivo. The hCG β-subunit is folded before it combines with the α-subunit (8). In vivo formation of the hCG heterodimer depends on a process in which the α-subunit is threaded between portions of the β-subunit that is facilitated by transient disruption of a disulfide (termed the “tensor disulfide”) between hCG residues 93 and 100 that expands this region of the molecule and permits loop 2 to pass through the seatbelt (A1). In contrast (B1), folding of the hLH β-subunit requires the presence of the α-subunit, which serves as a chaperone and may require disruption of the easily reduced α-subunit disulfide. Subsequent assembly of the hLH heterodimer takes place after the β-subunit has folded by a “wraparound” mechanism. As described under “Experimental Procedures,” we distinguished these differences in heterodimer assembly by adding a cysteine to the α-subunit (αS43C), removing a cysteine in the β-subunit (βC26A), and observing how this enabled the two subunits to be cross-linked by a disulfide bond (A2 and B2). Single open circles, free cysteine residues; double open circles, disulfide-bonded cysteines. Red, α-subunit; green, β-subunit. Boxes with dotted lines refer to a transient intermediate. A1, relative positions of the α- and β-subunit loops as well as the β-subunit tail. This is consistent for all illustrations in this figure.
To further illustrate differences between hLH and hCG, we built a model of the influence of the α-subunit on the formation of the cystine knot (Fig. 3, A–C). These figures describe the positions of some selected residues of the β-subunit cystine knot in hLH with and without the α-subunit as well as that in hCG without the α-subunit. Fig. 3A shows that the hLH β-subunit cystine knot does not form unless the α-subunit is present (as shown in Fig. 3B). Fig. 3C shows that the cystine knot in the hCG β-subunit forms when the α-subunit is not present.
FIGURE 3.
Differences in assembly mechanisms of hLH and hCG. A–C, differences between the initial steps in assembly of hLH and hCG. In A–C, the red lines represent the α-subunit, the green lines represent the β-subunit, and the yellow lines represent disulfides. A illustrates the cystine knot in the β-subunit of hLH that does not form in the absence of the α-subunit. The two residues adjacent to cysteine 9 of the cystine knot of the hLH β-subunit are a tryptophan at position 8 and a histidine at position 10. B illustrates the positions of the formed cystine knots of both α- and β-subunits. The α-subunit cystine knot forms before the β-subunit cystine knot. Until the β-subunit cystine knot is formed, folding of the β-subunit is indeterminate. Following the formation of the β-subunit cystine knot, the remainder of the hLH β-subunit is folded, including the latch disulfide. This delay is responsible for the observations seen in Tables 1, 2, and 4. C shows that the hCG β-subunit cystine knot forms independently of the α-subunit, and as a consequence, the remainder of the β-subunit, including the seatbelt latch disulfide, forms before the α-subunit binds. This explains why additional cysteines in the hCG β-subunit do not interfere with its assembly unless formation of the latch disulfide is blocked. D–F, assembly of the hLH tail. The colors illustrated here are identical to those in Fig. 3, A–C, with the LH β-subunit 7-residue tail shown in blue. The oligosaccharides are shown with sequences typical of those added in the endoplasmic reticulum. The LH β-subunit tail starts the final step of the assembly pathway, a mechanism that we propose interferes with its biological activity due to its location (D). Formation of the cystine knot permits the rearrangement of the tail by its proximity to the LH α-subunit loop 2 (E). This occurs by migration of the tail from a position where it interferes with formation of the seatbelt latch to a position proximal to the hydrophobic residues in α-subunit loop 2. This removes the inhibition of the seatbelt latch and enables it to form. F, a model of the completely folded hLH heterodimer including the oligosaccharides that are expected to be present in the endoplasmic reticulum. These are processed as the heterodimer is moved through the Golgi. Additionally, the tail, seen in blue, would also be removed because its position in the heterodimer would interfere with signal transduction. The steps in removal of the tail element are unknown.
In this model, the difference in the ability to form the cystine knot is a primary reason for the failure of hLH to fold in the absence of the α-subunit. The cystine knot forms rapidly in hCG in the absence of the α-subunit (5). As a consequence, the introduction of additional cysteines in the β-subunit does not alter the ability of hCG to form a latched seatbelt (Table 4, rows 3, 5, and 7). When the normal seatbelt latch is disrupted (C26A) and the seatbelt is forced to become latched to the α-subunit, the presence of cysteines in the β-subunit disrupts hCG folding. The reason for this is that it is less efficient for the hCG seatbelt latch to cross-link the α-subunit than to cross-link the normal latch site, if it is present. This shows that the hCG β-subunit folds rapidly before it interacts with the α-subunit, confirming data we had observed previously (8–10).
In contrast, not only does the hLH β-subunit fail to fold in the absence of the α-subunit, but in the presence of the α-subunit, its cystine knot is folded slowly. As a consequence, the presence of additional cysteines in the β-subunit reduces the assembly of the heterodimer (Table 4, rows 11, 13, and 15). The reason for this is that these extra cysteines compete with the seatbelt latch for binding to the seatbelt latch site. When these extra cysteines are not present, there is no interference with the ability of the seatbelt latch to interact with its normal latch site (cysteine 26). When this seatbelt latch site was deleted (C26A), the seatbelt latch was able to bind to the cysteine added to the α-subunit (S43C). This observation showed that the seatbelt latch had a preference for binding to its normal seatbelt latch site better than the latch site present in the α-subunit. When cysteine 26 was replaced by alanine, only the F64C analog maintained its level of expression (Table 4, row 14) and had a preference for the cysteine in the α-subunit. This showed that the location of the added cysteines made a difference in their ability to inhibit binding of the latch disulfide to either cysteine 26 or α-subunit S43C, revealing that the location of α-subunit loop 2, adjacent to the β-subunit, is most likely to be in the position occupied in Fig. 3 (D–F) as opposed to being disordered as observed during NMR analysis of hCG α-subunit loop 2 (28). The reason for this is that one β-subunit cysteine substitution (F64C) had a roughly equal ability to latch its seatbelt to β-subunit cysteine 26. When that was eliminated, it was able to latch it to α-subunit S43C. The two other cysteine substitutions added to the β-subunit are farther from the cysteine at position 26 and had much lower abilities to bind this cysteine. Therefore, we propose that during the folding of hLH, the α-subunit acquires the position that is seen in the structures outlined in Fig. 3.
Therefore, although the hLH β-subunit required the presence of the α-subunit to fold, it is clear that formation of the cystine knot occurs more slowly than the formation of the hCG cystine knot and seatbelt latch. We expect that this difference in assembly is caused by residues in the N-terminal region of the hLH β-subunit, most likely residue 8, which is a tryptophan, as opposed to the arginine present at this position in hCG. In most other mammalian lutropins, this residue is a leucine residue. Residue 10, which is a histidine in human LH, is an arginine in hCG and many other mammalian lutropins (29). Thus, we conclude that the presence of residue 8 is most likely responsible for the reduced ability of many mammalian lutropins to fold in the absence of the α-subunit due to the proximity of this residue to the first cysteine (residue 9) of the cystine knot. At this site, both horse and marmoset LH β-subunit contain a leucine residue. Thus, we postulate that the LH of these animals is likely to be folded only in the presence of the α-subunit similarly to what we have observed here for hLH, although they contain an “hCG-like” tail.
The LC Iβ analog was not the only hCG analog containing LH substitutions that affected its folding mechanism, however. Seven different LHβ substitutions in LC IIIβ, also had a similar effect (Table 1, compare rows 10 and 14). Analysis of the locations of these substituted residues showed that three of them (positions 89, 91, and 92) are adjacent to two additional cystine knot cysteines (positions 88 and 90). We conclude that the hLHβ-specific residues influence assembly of the hLH β-subunit due to their potential to impede formation of the cystine knot. As noted earlier, our previous studies with the hCG β-subunit showed that the cystine knot formed early and was followed sequentially by the formation of the tensor disulfide and the latch disulfide (8, 10, 17). This reinforced our thinking that the cystine knot is a critical first step in monomer assembly and is required prior to further disulfide formation.
Because the overall structures of hLH and hCG are similar except for the presence of the long tail on hCG, the assembly of LH must also include latching of the seatbelt tail to the same cysteine in hLH as in hCG. The data in Table 2, rows 1 and 7, show that removal of the hydrophobic tail unique to hLH facilitated its assembly into a heterodimer. Because this process requires the presence of the α-subunit to form the hLH cystine knot, the final assembly of hLH occurs by a wraparound pathway due to the fact that the α-subunit is already present during the initial steps of hLH folding. To explain the role of the hLH β-subunit hydrophobic tail in seatbelt latching, we built a model structure of hLH based on the structure of hCG (2, 6). This is difficult because the tail of the hCG β-subunit is disordered in the crystal structure, and we assumed that the 7 residues of the hLH β-subunit tail are in approximately the same region of the molecule (Fig. 3F). Based on the observation that there are several hydrophobic residues in the hLH β-subunit proximal to this region, including hLH β-subunit residues Pro-24, Val-25, Cys-26, Ile-27, Thr-28, Val-29, Ile-67, Gly-69, Val-70, Gly-71, Gly-72, and Pro-73, and α-subunit residues in loop 2, including Pro-40, Leu-41, Ser-43, Thr-46, Met-47, Leu-48, Val-49, and Pro-50, we propose that the presence of the α-subunit facilitates movement of the hLH β-subunit hydrophobic tail into a position that permits the seatbelt to become latched (Fig. 3, D–F). Based on the data in Table 4, it is clear that the α-subunit is already attached to the β-subunit in a position where both the α- and β-subunit cysteine knots are proximal to one another. This would permit α-subunit loop 2, which has been shown to be disordered in the free subunit (28), to be in the vicinity of these β-subunit residues as well as the β-subunit tail. In our model, the position of the α-subunit hydrophobic residues in loop 2 would allow for the migration of the hydrophobic β-subunit tail away from the seatbelt, enabling it to become latched to the cysteine at position 26. This explains how the hydrophobic tail on the β-subunit prevents formation of the seatbelt latch disulfide and thereby interferes with assembly of the heterodimer in the absence of the α-subunit. Because the location of the tail is proximal to parts of the heterodimer shown to be essential for lutropin activity (30) and is also proximal to the oligosaccharide on α-subunit loop 2 critical for activity (31), the presence of the hydrophobic tail would be expected to interfere with signal transduction. This would explain why the hLH β-subunit tail is removed in mature hLH to complete assembly. In animals such as the horse and marmoset, the hydrophobic tail is absent. It is likely that this change in the LH β-subunit would facilitate the activity of LH in these species when this molecule is expressed during pregnancy. In the case of the marmoset, this change is required to permit activation of the LH receptor, which is missing 27 residues in a key portion of its signaling specificity domain (22).
Thus, the structure of LH in primates and other vertebrates appears to be optimized for its function in reproduction. Its assembly has a built-in mechanism for insuring that the α and β-subunits have combined prior to formation of the heterodimer and that in some species, including humans, there is a component that retards assembly and may also retard activity until it is removed. Both of these components have the ability to restrict assembly of LH and thereby contribute an additional control of its regulation.
Acknowledgments
We thank Donghui Cao for help in the laboratory as well as Darina Dinov and June Thai for help doing research in the preparation of the manuscript. We also thank April De Stefano, who was instrumental in the preparation of the tables.
Footnotes
- CG
- choriogonadotropin
- hCG
- human CG
- FSH
- follitropin
- hFSH
- human FSH
- TSH
- thyrotropin
- hTSH
- human thyrotropin
- LH
- lutropin
- hLH
- human lutropin.
REFERENCES
- 1. Pierce J. G., Parsons T. F. (1981) Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50, 465–495 [DOI] [PubMed] [Google Scholar]
- 2. Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. (1994) Crystal structure of human chorionic gonadotropin. Nature 369, 455–461 [DOI] [PubMed] [Google Scholar]
- 3. Ruddon R. W., Sherman S. A., Bedows E. (1996) Protein folding in the endoplasmic reticulum: lessons from the human chorionic gonadotropin β subunit. Protein Sci. 5, 1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing Y., Lin W., Jiang M., Myers R. V., Cao D., Bernard M. P., Moyle W. R. (2001) Alternatively folded choriogonadotropin analogs. Implications for hormone folding and biological activity. J. Biol. Chem. 276, 46953–46960 [DOI] [PubMed] [Google Scholar]
- 5. Xing Y., Williams C., Campbell R. K., Cook S., Knoppers M., Addona T., Altarocca V., Moyle W. R. (2001) Threading of a glycosylated protein loop through a protein hole: implications for combination of human chorionic gonadotropin subunits. Protein Sci. 10, 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu H., Lustbader J. W., Liu Y., Canfield R. E., Hendrickson W. A. (1994) Structure of human chorionic gonadotropin at 2.6 Å resolution from MAD analysis of the selenomethionyl protein. Structure 2, 545–558 [DOI] [PubMed] [Google Scholar]
- 7. Fox K. M., Dias J. A., Van Roey P. (2001) Three-dimensional structure of human follicle-stimulating hormone. Mol. Endocrinol. 15, 378–389 [DOI] [PubMed] [Google Scholar]
- 8. Xing Y., Myers R. V., Cao D., Lin W., Jiang M., Bernard M. P., Moyle W. R. (2004) Glycoprotein hormone assembly in the endoplasmic reticulum: IV. Probable mechanism of subunit docking and completion of assembly. J. Biol. Chem. 279, 35458–35468 [DOI] [PubMed] [Google Scholar]
- 9. Xing Y., Myers R. V., Cao D., Lin W., Jiang M., Bernard M. P., Moyle W. R. (2004) Glycoprotein hormone assembly in the endoplasmic reticulum: III. The seatbelt and its latch site determine the assembly pathway. J. Biol. Chem. 279, 35449–35457 [DOI] [PubMed] [Google Scholar]
- 10. Xing Y., Myers R. V., Cao D., Lin W., Jiang M., Bernard M. P., Moyle W. R. (2004) Glycoprotein hormone assembly in the endoplasmic reticulum: I. The glycosylated end of human α-subunit loop 2 is threaded through a β-subunit hole. J. Biol. Chem. 279, 35426–35436 [DOI] [PubMed] [Google Scholar]
- 11. Cole L. A., Hartle R. J., Laferla J. J., Ruddon R. W. (1983) Detection of the free β subunit of human chorionic gonadotropin (HCG) in cultures of normal and malignant trophoblast cells, pregnancy sera, and sera of patients with choriocarcinoma. Endocrinology 113, 1176–1178 [DOI] [PubMed] [Google Scholar]
- 12. Campbell R. K., Dean-Emig D. M., Moyle W. R. (1991) Conversion of human choriogonadotropin into a follitropin by protein engineering. Proc. Natl. Acad. Sci. U.S.A. 88, 760–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okayama H., Chen C. (1991) Calcium phosphate mediated gene transfer into established cell lines. Methods Mol. Biol. 7, 15–21 [DOI] [PubMed] [Google Scholar]
- 14. Moyle W. R., Ehrlich P. H., Canfield R. E. (1982) Use of monoclonal antibodies to subunits of human chorionic gonadotropin to examine the orientation of the hormone in its complex with receptor. Proc. Natl. Acad. Sci. U.S.A. 79, 2245–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moyle W. R., Pressey A., Dean-Emig D., Anderson D. M., Demeter M., Lustbader J., Ehrlich P. (1987) Detection of conformational changes in human chorionic gonadotropin upon binding to rat gonadal receptors. J. Biol. Chem. 262, 16920–16926 [PubMed] [Google Scholar]
- 16. Fiddes J. C., Goodman H. M. (1980) The cDNA for the β-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3′-untranslated region. Nature 286, 684–687 [DOI] [PubMed] [Google Scholar]
- 17. Xing Y., Myers R. V., Cao D., Lin W., Jiang M., Bernard M. P., Moyle W. R. (2004) Glycoprotein hormone assembly in the endoplasmic reticulum: II. Multiple roles of a redox sensitive β-subunit disulfide switch. J. Biol. Chem. 279, 35437–35448 [DOI] [PubMed] [Google Scholar]
- 18. Muyan M., Furuhashi M., Sugahara T., Boime I. (1996) The carboxy-terminal region of the β-subunits of luteinizing hormone and chorionic gonadotropin differentially influence secretion and assembly of the heterodimers. Mol. Endocrinol. 10, 1678–1687 [DOI] [PubMed] [Google Scholar]
- 19. Jablonka-Shariff A., Boime I. (2011) A dileucine determinant in the carboxyl terminal sequence of the LHβ subunit is implicated in the regulated secretion of lutropin from transfected GH3 cells. Mol. Cell. Endocrinol. 339, 7–13 [DOI] [PubMed] [Google Scholar]
- 20. Dos Santos S., Mazan S., Venkatesh B., Cohen-Tannoudji J., Quérat B. (2011) Emergence and evolution of the glycoprotein hormone and neurotrophin gene families in vertebrates. BMC Evol. Biol. 11, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakabayashi K., Matsumi H., Bhalla A., Bae J., Mosselman S., Hsu S. Y., Hsueh A. J. (2002) Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J. Clin. Invest. 109, 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller T., Simoni M., Pekel E., Luetjens C. M., Chandolia R., Amato F., Norman R. J., Gromoll J. (2004) Chorionic gonadotrophin β subunit mRNA but not luteinising hormone β subunit mRNA is expressed in the pituitary of the common marmoset (Callithrix jacchus). J. Mol. Endocrinol. 32, 115–128 [DOI] [PubMed] [Google Scholar]
- 23. Bousfield G. R., Liu W. K., Sugino H., Ward D. N. (1987) Structural studies on equine glycoprotein hormones. Amino acid sequence of equine lutropin β-subunit. J. Biol. Chem. 262, 8610–8620 [PubMed] [Google Scholar]
- 24. Jablonka-Shariff A., Boime I. (2013) A novel carboxyl-terminal heptapeptide initiates the regulated secretion of LH from unique sub-domains of the ER. PLoS One 8, e65002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meunier L., Usherwood Y. K., Chung K. T., Hendershot L. M. (2002) A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell 13, 4456–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiss J., Duca K. A., Crowley W. F., Jr. (1990) Gonadotropin-releasing hormone-induced stimulation and desensitization of free α-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology 127, 2364–2371 [DOI] [PubMed] [Google Scholar]
- 27. Reeve J. R., Cheng K. W., Pierce J. G. (1975) Partial reduction of disulfide bonds in the hormone-specific subunits of TSH and LH. Biochem. Biophys. Res. Commun. 67, 149–155 [DOI] [PubMed] [Google Scholar]
- 28. De Beer T., Van Zuylen C. W., Leeflang B. R., Hård K., Boelens R., Kaptein R., Kamerling J. P., Vliegenthart J. F. (1996) NMR studies of the free α subunit of human chorionic gonadotropin. Structural influences of N-glycosylation and the β subunit on the conformation of the α subunit. Eur. J. Biochem. 241, 229–242 [DOI] [PubMed] [Google Scholar]
- 29. Moyle W. R., Campbell Robert K. (1996) in Reproductive Endocrinology, Surgery and Technology (Adashi E. Y., Rock J. A., Rosenwaks Z., eds), pp. 683–724, Lippincott-Raven Publishers, Philadelphia [Google Scholar]
- 30. Moyle W. R., Campbell R. K., Myers R. V., Bernard M. P., Han Y., Wang X. (1994) Co-evolution of ligand-receptor pairs. Nature 368, 251–255 [DOI] [PubMed] [Google Scholar]
- 31. Matzuk M. M., Boime I. (1988) The role of the asparagine-linked oligosaccharides of the α subunit in the secretion and assembly of human chorionic gonadotrophin. J. Cell Biol. 106, 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]