Background: Primed immune responses contribute to vertebrate host defense.
Results: Silkworms acquire resistance to a pathogen by a preinjection of its heat-killed cells or its cell surface peptidoglycans. The amount of antimicrobial peptides is increased at the second round of infection.
Conclusion: Invertebrates acquire infection resistance by peptidoglycan recognition and antimicrobial peptide increase.
Significance: Molecular mechanisms of invertebrate primed immunity were revealed.
Keywords: Antimicrobial Peptides, Immunology, Insect, Invertebrates, Jun N-terminal Kinase (JNK), Peptidoglycan, Silkworm, Tolerance
Abstract
A heightened immune response, in which immune responses are primed by repeated exposure to a pathogen, is an important characteristic of vertebrate adaptive immunity. In the present study, we examined whether invertebrate animals also exhibit a primed immune response. The LD50 of Gram-negative enterohemorrhagic Escherichia coli O157:H7 Sakai in silkworms was increased 100-fold by pre-injection of heat-killed Sakai cells. Silkworms pre-injected with heat-killed cells of a Gram-positive bacterium, Staphylococcus aureus, did not have resistance to Sakai. Silkworms preinjected with enterohemorrhagic E. coli peptidoglycans, cell surface components of bacteria, were resistant to Sakai infection. Silkworms preinjected with S. aureus peptidoglycans, however, were not resistant to Sakai. Silkworms preinjected with heat-killed Sakai cells showed persistent resistance to Sakai infection even after pupation. Repeated injection of heat-killed Sakai cells into the silkworms induced earlier and greater production of antimicrobial peptides than a single injection of heat-killed Sakai cells. These findings suggest that silkworm recognition of Gram-negative peptidoglycans leads to a primed immune reaction and increased resistance to a second round of bacterial infection.
Introduction
All animal species are exposed to pathogenic microorganisms and face the risk of death due to possible infection. The immune system by which animals defend themselves against those pathogens, therefore, plays a highly important role in determining animal fatality. There are two types of immune systems, innate and adaptive. The innate immune system continuously responds to pathogen invasion regardless of the host's infection experience, whereas the adaptive immune system has an enhanced response to pathogens that have previously infected the host. The adaptive immune system and innate immune system work together to confer host defense in vertebrate animals (1).
In the adaptive immune system of vertebrate animals, B-lymphocytes produce immunoglobulins that specifically bind pathogen-derived molecules (2). After vertebrate animals experience an antigen challenge, their immunoglobulin production increases when they next encounter the same antigen (3). Moreover, the cellular immunity provided by T-lymphocytes is also enhanced (4). These enhanced immune functions are called the “booster effect” (5). This effect can work even if the interval between the first and the second immune challenge is long. Adaptive immune systems are characterized by the following features: antigen specificity, enhanced secondary immune responses, and persistence (memory of the antigen) (6, 7).
In contrast, because invertebrate animals, including insects, have no immunoglobulins (8), it has been speculated that only an innate immune system exists for defense against pathogenic microorganisms. Some reports, however, have proposed that invertebrate animals do have immune systems with adaptive characteristics. A hymenopteran insect, the bumble bee, exhibits prolonged survival after infection with a Gram-negative bacterium, Pseudomonas, or a Gram-positive bacterium, Paenibacillus, when first challenged by a sublethal dose of these bacteria (9). Drosophila, a dipteran insect, has an immune pathway that specifically reacts to Streptococcus pneumoniae, a Gram-positive bacterium (10). In the mosquito, malaria infection increases the number of host immune cells, which contributes to host defense during a second malaria infection (11). These reports suggest that invertebrate animals possess an immune system that can be primed by the invasion of microorganisms and defend against the microorganism. The target microbial molecule and the underlying mechanism of the primed immune response in invertebrates, however, remain unclear.
In previous studies, we established an infection model using silkworms (12, 13). This animal has a limited ability to move around due to a long domestication period of more than 4000 years to produce silk. Its body size is sufficient to conduct quantitative injection experiments. These characteristics make the animal highly useful for evaluating pathogen or toxin lethality with quantitative parameters, such as the LD50 (14, 15). Silkworms are susceptible to human pathogens, such as Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, and enterohemorrhagic Escherichia coli (EHEC)2 (12, 16). Genes in S. aureus, Streptococcus pyogenes, or EHEC that are necessary to kill silkworms are also necessary to kill mice (16, 17). Silkworm body fluids contain a factor that inhibits S. aureus virulence, and such inhibitory activity is also observed in mammals (18). Therefore, the interactions between silkworms and pathogens share many common features with those between mammals and pathogens. In the present study, we utilized the silkworm infection model to examine whether the invertebrate immune system shows pathogen selectivity, persistence, and enhanced secondary immune responses.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
E. coli strains, EHEC O-157:H7 Sakai and experimental strain W3110, were aerobically cultured in Luria-Bertani medium at 37 °C. S. aureus NCTC8325-4 strain was aerobically cultured in tryptic soy broth at 37 °C. Serratia marcescens 2170 strain was aerobically cultured in brain heart infusion medium at 30 °C. Each strain was cultured in 10 ml of medium in 50-ml disposable tubes or in 50 ml of medium in 225-ml disposable tubes.
Silkworms
We purchased silkworm eggs (Fu/Yo × Tsukuba/Ne) from Ehime-Sanshu (Ehime, Japan). The hatched larvae were fed with Silkmate (Nihon Nosan) at 27 °C. Fifth instar larvae were fed an antibiotic-free diet (Katakura Co., Tokyo, Japan).
Infections Using Silkworm Larvae or Pupae
Infection experiments were conducted according to the method of Kaito et al. (17). Fifth instar larvae were fed an antibiotic-free diet (Katakura Co., Tokyo, Japan) for 1 day and then injected with bacterial solution using a 1-ml syringe equipped with a 27-gauge needle. The number of injected bacteria was 1 × 107 cfu/larva. After injection, silkworms were incubated at 37 °C without food. Pupal infections were induced in the same manner as larval infections in general, but bacterial solutions were injected into the dorsal side of pupal abdomens, and injected pupae were incubated at 27 °C.
Immunization of Silkworms
Bacteria were cultured in the appropriate liquid medium and centrifuged at 3350 × g for 8 min at room temperature. The resulting pellets were resuspended in 1 volume of saline and then autoclaved at 121 °C for 15 min. The heat-killed cells were diluted in saline, and 25 μl of each aliquot was injected into each silkworm for immunization using 1-ml syringes and 27-gauge sterile needles. After injection, the silkworms were incubated at 27 °C with an antibiotic-free diet.
Preparation of Hemolymph Samples
Hemolymph samples were collected from silkworms to determine their antimicrobial activity. The samples were collected in 1.5-ml Eppendorf tubes by cutting the abdominal prolegs of the silkworm, and phenylthiourea, a melanization inhibitor, was added (final concentration, 100 μm). Collected samples were then centrifuged at 21,500 × g for 5 min at room temperature, and the supernatants were used as the plasma fractions.
Reagents
The glass reagent bottles for saline were depyrogenated by dry heat sterilization at 300 °C for 2 h. To prepare saline, 0.9% (w/v) NaCl was dissolved in milliQ water and autoclaved at 121 °C for 20 min. The saline solution used in this research was stored with the greatest care to prevent contamination by microbial components or other immunogens. Lipopolysaccharides (LPSs) purified from E. coli O-111 were purchased from Wako Pure Chemical Industries (catalog no. 125-05181). Peptidoglycan from E. coli O-111 or S. aureus was purchased from InvivoGen (catalog no. tlrl-pgnec) or Sigma-Aldrich (catalog no. 77140), respectively. Mutanolysin from Streptomyces globisporus ATCC21553 was purchased from Sigma-Aldrich (catalog no. M9901-1KU).
Identification of Antimicrobial Molecule against Sakai
Cation Exchange Column Chromatography
Heat-killed Sakai cells grown to confluence were diluted 100-fold in saline and injected into fifth instar silkworms. The silkworms were fed an antibiotic-free diet and maintained for 12 h. The silkworms were killed to collect a total of 45 ml of hemolymph on ice in the presence of phenylthiourea. The sample was then centrifuged at 3350 × g for 5 min at 4 °C, and the supernatant was diluted with 225 ml of 0.1 m ammonium acetate (pH 7). The diluted sample was applied to a carboxymethyl (CM)-TOYOPEARL (Tosoh, CM-650M) column (bed volume = 80 ml, 2.5 × 16 cm). The column was washed with 5 column volumes of 0.3 m ammonium acetate (pH 7) and then eluted with 300 ml of a linear gradient of 0.3–1.0 m ammonium acetate (pH 7). The fraction volume was 10 ml. The anti-Sakai activity of the fractions was measured. Fractions with anti-Sakai activity were pooled, lyophilized, and dissolved in 1 ml of 0.1 m ammonium acetate (pH 7).
Gel Filtration Chromatography
The fraction with anti-Sakai activity from step A was applied to a gel filtration column for fast protein liquid chromatography (GE Healthcare, Superose 12 10/300 GL). The column was equilibrated with 0.1 m ammonium acetate (pH 7) before application. Fractions of 0.5 ml were collected by eluting with 1.5 column volumes of 0.1 m ammonium acetate (pH 7) at a flow rate of 0.3 ml/min. The anti-Sakai activity and protein concentration of the fractions were measured. The fractions with anti-Sakai activity were analyzed by 15% SDS-polyacrylamide gel electrophoresis.
Determination of Antibacterial Activity
An aliquot of 100 μl of Mueller Hinton broth containing live Sakai cells (106-diluted overnight culture) was poured into microtiter plates. An aliquot of 100 μl of 2-fold serial dilutions of protein samples was added to each well. The plates were incubated overnight at 37 °C. The minimum inhibitory concentrations were determined as the lowest concentration that inhibited Sakai growth. The unit for anti-Sakai activity was determined as follows. An n unit of anti-Sakai activity represents the n-fold diluted sample that inhibited Sakai growth.
Peptide Mass Fingerprinting
The protein band in 15.0% SDS-polyacrylamide gel was excised, digested with trypsin, and subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy analysis (Microflex LRF 20, Bruker Daltonics) as described by Fernandez et al. (19). The peak list was generated using Flex Analysis version 3.0. The five major peaks (63.4% of total signal) were matched with estimated peaks of cecropin.
Analyses of Gene Expression in Silkworm
To analyze the protein expression pattern in the silkworm fat body, we dissected silkworms using sterilized scissors and picked up fat body tissues using tweezers. Tissue samples from at least two silkworms were collected in a 1.5-ml tube. The fat body tissues were homogenized in 700 μl of PBS. To analyze cecropin expression in silkworm hemolymph, hemolymph samples were collected in 1.5-ml tubes on ice and centrifuged to obtain plasma fractions. The protein concentrations were determined using the Bradford assay. The proteins were mixed with Laemmli sample buffer and boiled for 2 min and then electrophoresed in 15.0% SDS-polyacrylamide gel and blotted onto a PVDF membrane (Immobilon-P, Millipore). The membranes were treated with antibodies against phosphorylated c-Jun N-terminal kinase (p-JNK) (catalog no. V793A, Promega) or silkworm cecropin B (catalog no. ab27571, Abcam), followed by treatment with a second antibody against rabbit IgG conjugated with HRP. The membrane was reacted with an HRP substrate (Western Lightning Plus ECL, PerkinElmer Life Sciences) and subsequently exposed to film (Hyperfilm ECL, GE Healthcare). Band intensities were measured by ImageJ software (National Institutes of Health). For mRNA quantification, total RNA was extracted from the fat body using TRIzol reagent (Invitrogen, catalog no. 15596-018) and reverse transcribed to cDNAs by using MultiScribeTM reverse transcriptase (Invitrogen) and random hexamer. Quantitative real-time PCR was performed using cDNA as a template and primers for target mRNAs. The signals were detected using a StepOnePlus real-time PCR system (Applied Biosystems). The primers for cecropin mRNA were 5′-TTGAGCTTCGTCTTCGCGTT-3′ and 5′-TTGCGTCCCACTTTCTCAATT-3′, and the primers for EF-2 mRNA were 5′-GTGCGAGAGCCGGAGAGAC-3′ and 5′-CGAAGAACATAGAGATGGCCG-3′. The data were normalized to EF-2 mRNA.
RESULTS
Preinjection of EHEC O157:H7 Heat-killed Sakai Cells into Silkworms Induces Tolerance to Sakai Infection
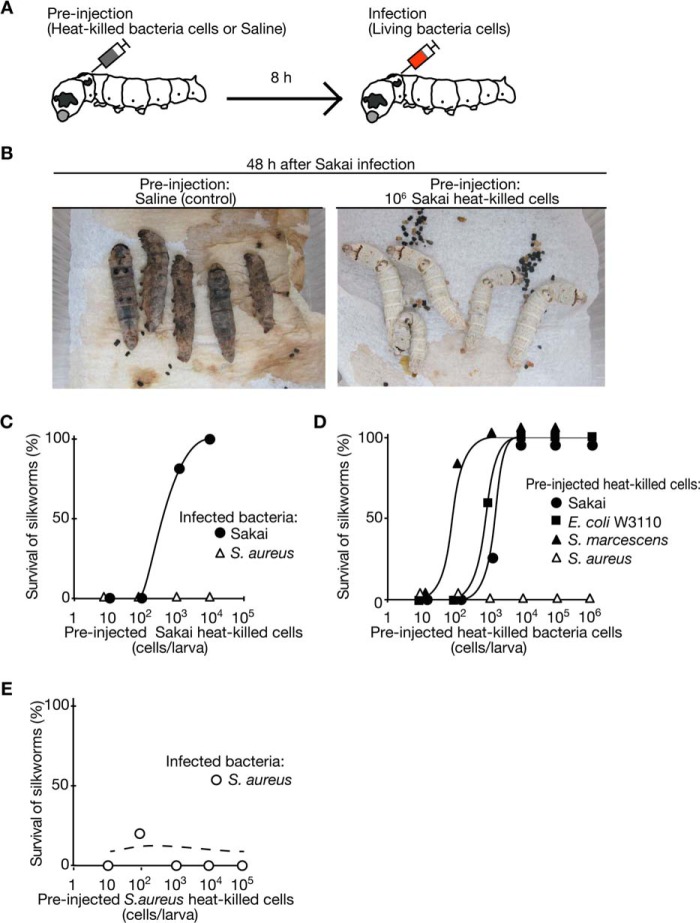
We investigated whether pre-exposure to a particular pathogen inhibits a second round of infection in the silkworm-EHEC infection model. We injected heat-killed Sakai cells (autoclaved at 121 °C for 15 min) or saline (Fig. 1A). At 8 h after this injection, we injected 107 cfu/larva of Sakai live cells into each silkworm. All of the silkworms preinjected with saline were killed by the injection of Sakai live cells, whereas none of the silkworms preinjected with heat-killed Sakai cells died after the injection of Sakai live cells (Fig. 1B). This finding indicates that Sakai infection in silkworms was suppressed by pre-exposure to Sakai cells. To quantify the suppression effect, we determined the dose of Sakai that killed 50% of the silkworms (LD50)at 6, 24, and 56 h after preinjection of heat-killed Sakai cells or saline. The LD50 values of Sakai against silkworms preinjected with heat-killed Sakai cells were more than 100 times higher than those against silkworms preinjected with saline at all time periods (Table 1). These findings indicate that silkworms have an immune system that senses the exposure to heat-killed Sakai cells and suppresses subsequent infection with live Sakai cells, which we considered to be a primed immune response.
FIGURE 1.
Silkworms preinjected with heat-killed Sakai cells show tolerance to Sakai infection. A, the scheme of the immunization experiment in silkworms is presented. We first injected heat-killed bacteria cells into the silkworm hemolymph and then maintained the silkworms. We then injected live Sakai or S. aureus cells into the silkworm hemolymph to evaluate their tolerance to bacterial infection. B, left, a silkworm preinjected with saline and subsequently infected with live Sakai cells (1 × 107 cfu). Silkworms killed by Sakai infection exhibited blackening of the body by melanization. Right, a silkworm preinjected with heat-killed Sakai cells and subsequently infected with live Sakai cells (1 × 107 cfu). C, the effect of preinjection of heat-killed Sakai cells on Sakai or S. aureus infections was examined. We injected 1 × 107 cfu of live Sakai (closed circle) or S. aureus (open triangle) cells into silkworms (n = 10) preinjected with heat-killed Sakai cells. The vertical axis shows the silkworm viability after infection, and the horizontal axis shows the preinjection dose of heat-killed Sakai cells. Data are representative of at least three independent experiments. D, the effects of preinjection of heat-killed cells of different bacterial species on Sakai infection were examined. Heat-killed cells of Sakai, E. coli W3110, S. marcescens 2170, or S. aureus NCTC8325-4 were preinjected into silkworms (n = 10), and the silkworms were infected with 1 × 107 cfu of live Sakai cells. Data are representative of at least three independent experiments. E, the effect of preinjection of heat-killed S. aureus cells on S. aureus infection was examined. Heat-killed S. aureus cells were preinjected into silkworms (n = 10), and the silkworms were infected with 1 × 107 cfu of live S. aureus cells. Data are representative from at least three independent experiments.
TABLE 1.
Inhibition of Sakai infection induced by heat-killed Sakai cells
We evaluated the infection tolerance of silkworms to Sakai by measuring the LD50 values. We injected 2-fold serial dilutions of live Sakai cells into silkworms at 6, 24, or 56 h after preinjection of 106 heat-killed Sakai cells or saline. LD50 values were determined by logistic regression analysis of survival curves.
| Preinjected samples | LD50 values |
||
|---|---|---|---|
| 6 h | 24 h | 56 h | |
| cfu/larva | |||
| Heat-killed Sakai cells | 5.3 × 108 | 3.2 × 108 | 6.3 × 107 |
| Saline | 6.7 × 105 | 5.6 × 105 | 5.0 × 105 |
| -Fold change (heat-killed Sakai cells/saline) | 7.9 × 102 | 5.7 × 102 | 1.3 × 102 |
The Silkworm Immune System Recognizes Gram-negative Bacteria Peptidoglycans
We then examined whether the silkworm primed immune response functions against a specific pathogen species. We injected live S. aureus or Sakai cells into silkworms that were preinjected with heat-killed Sakai cells or saline. The silkworms acquired tolerance to Sakai infection, but not to S. aureus infection, by preinjection of heat-killed Sakai cells in a dose-dependent manner (Fig. 1C). Thus, preinjection of heat-killed Sakai cells did not induce tolerance to S. aureus infections in silkworms.
To determine whether the suppression effect against Sakai infection was specifically induced by pre-exposure to the Sakai strain, we tested the suppression effect against Sakai infection using heat-killed bacterial cells of another E. coli strain, W3110; an S. marcescens strain, 2170; or an S. aureus strain, NCTC8235-4. Preinjection of heat-killed E. coli W3110 or S. marcescens cells induced tolerance to Sakai infection in silkworms to the same degree as preinjection of heat-killed Sakai cells (Fig. 1D). In contrast, preinjection with heat-killed S. aureus cells did not induce tolerance to Sakai infection (Fig. 1D). Therefore, exposure to Gram-negative bacteria, such as E. coli or S. marcescens, conferred silkworm tolerance to Sakai infections, whereas exposure to Gram-positive bacteria, such as S. aureus, did not. These findings suggest that silkworm primed immunity has immunogen selectivity to distinguish between Gram-negative and Gram-positive bacteria. In addition, preinjection of heat-killed S. aureus cells did not induce tolerance to S. aureus infection in silkworms (Fig. 1E), suggesting that silkworm primed immunity does not develop against Gram-positive bacteria.
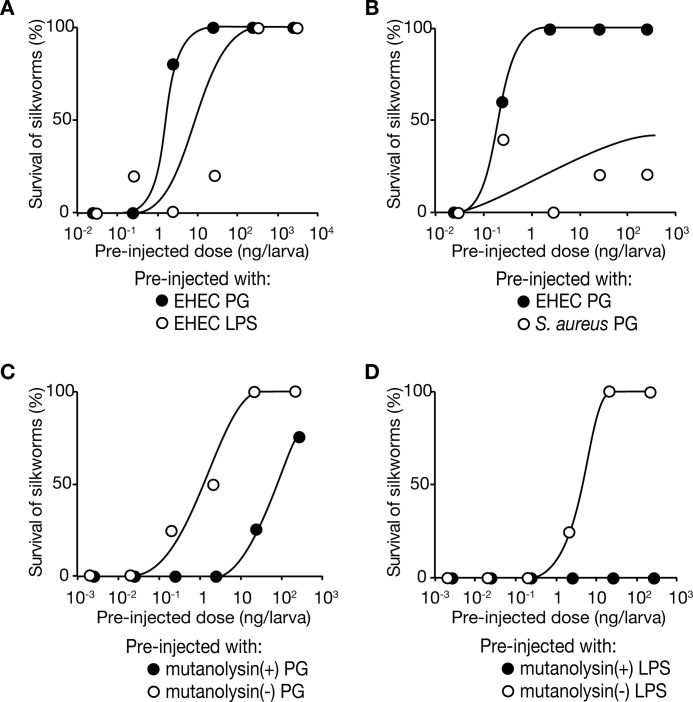
Peptidoglycans are cell surface components whose structures differ between Gram-negative and Gram-positive bacteria. We examined whether preinjection of Gram-negative peptidoglycans induces tolerance to Sakai infections in silkworms. We preinjected silkworms with peptidoglycans purified from EHEC O-111 and maintained the silkworms for 1 day at 27 °C. We then injected 107 cfu/larva of Sakai live cells into the silkworms. Silkworm viability after Sakai infection was increased by preinjection of EHEC peptidoglycans in a dose-dependent manner (Fig. 2A). On the other hand, silkworm viability after Sakai infection was not increased by preinjection of peptidoglycans purified from Gram-positive S. aureus (Fig. 2B). The ED50 value of EHEC peptidoglycans was 0.8 ng/larva, and activity was decreased 100-fold by treatment with mutanolysin, which digests peptidoglycans (Fig. 2C). These findings indicate that the silkworm primed immune response is triggered by the recognition of Gram-negative peptidoglycans and induces tolerance to Sakai infection.
FIGURE 2.
Preinjection of peptidoglycans induced silkworm tolerance to Sakai infection. A, effects of preinjection of peptidoglycans (PG) or LPSs from EHEC O-111 were examined. Peptidoglycan (closed circles) or LPS (open circles) samples were preinjected into silkworms (n = 5), and the silkworms were infected with 1 × 107 cfu live Sakai cells. Data are representative of at least three independent experiments. B, effects of preinjection of peptidoglycans from EHEC O-111 (closed circles) or S. aureus (open circles) were examined. Purified peptidoglycans from EHEC O-111 or S. aureus were preinjected into silkworms (n = 5), and the silkworms were infected with 1 × 107 cfu of live Sakai cells. Data are representative from at least two independent experiments. C, effect of mutanolysin-treated peptidoglycans was examined. Mutanolysin-treated (closed circle) or untreated (open circle) peptidoglycans from EHEC O-111 were preinjected into silkworms (n = 5), and the silkworms were infected with 1 × 107 cfu of live Sakai cells. Data are representative of at least two independent experiments. D, effect of mutanolysin treatment on LPS fraction was examined. Mutanolysin-treated (closed circle) or untreated (open circle) LPS fractions from EHEC O-111 were preinjected into silkworms (n = 5), and the silkworms were infected with 1 × 107 cfu of live Sakai cells. Data are representative of at least two independent experiments.
LPSs represent another type of cell surface molecule that differs between Gram-negative and Gram-positive bacteria. LPSs are present on Gram-negative bacterial surfaces but not Gram-positive bacterial surfaces. Purified LPS fractions are contaminated with peptidoglycans, which activate an immune reaction in Drosophila melanogaster (20, 21). We examined whether an LPS fraction purified from EHEC O-111 conferred silkworm resistance against Sakai infection. At 24 h after preinjection with the LPS fraction, we injected live Sakai cells into the silkworms. The silkworm survival rate was increased by preinjection of the LPS fraction (Fig. 2A). The activity of the LPS fraction of conferring silkworm resistance against Sakai infection was diminished by treatment with mutanolysin, which digests peptidoglycans (Fig. 2D). Thus, the activity of the LPS fraction was attributed to the contaminating peptidoglycans.
Persistence of Infection Tolerance by Silkworm Primed Immunity
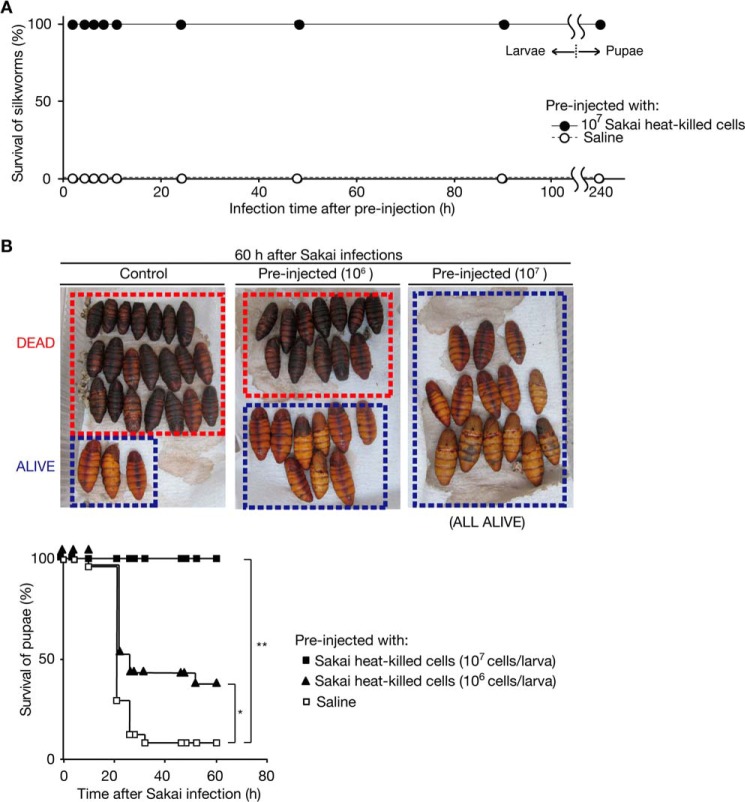
Persistence of infection tolerance is an important characteristic of adaptive immunity in mammals. We evaluated whether the infection tolerance of silkworms triggered by exposure to heat-killed Sakai cells is persistent. We kept silkworms that were preinjected with heat-killed Sakai cells or saline for a long duration and then infected them with live Sakai cells. The silkworms were tolerant to Sakai infection even 90 h after the preinjection (Fig. 3A). Furthermore, tolerance was observed even after the silkworms pupated, 240 h after the preinjection (Fig. 3, A and B). These results suggest that the tolerance of silkworms to Sakai infection triggered by exposure to heat-killed Sakai cells was persistently maintained, even after metamorphosis.
FIGURE 3.
Persistent tolerance to Sakai infection by preinjection of heat-killed Sakai cells in silkworms. A, the persistent immunization effect of heat-killed Sakai cells was examined. The vertical axis shows silkworm viability after 1 × 107 cfu of Sakai infection. The horizontal axis shows the interval between preinjection of heat-killed Sakai cells and injection of live Sakai cells. The preinjection of heat-killed Sakai cells was conducted on the second day of the fifth instar larval stage. Ten silkworms were used for each time point. The point at 240 h on the horizontal axis shows the viability of the pupae after Sakai infection. Data are representative from three independent experiments. B, lethal Sakai infection in silkworm pupae and their acquisition of infection tolerance by preinjection of heat-killed Sakai cells were examined. We preinjected saline (left) or heat-killed Sakai cells (middle and right) into silkworms on the second day of their fifth instar larval stage and maintained those silkworms until they pupated. The pupae (n = 14–24) were then injected with 1 × 107 cfu of live Sakai cells. The pupae killed by Sakai infection were blackened by melanization. The survival curve is presented in the bottom graph. The vertical and horizontal axes show the silkworm pupae viability after Sakai infection and time after Sakai infection, respectively. Asterisks indicate log-rank test p values less than 0.05. (*, p = 0.0338; **, p = 0.0094).
Booster Reaction in the Silkworm Primed Immune Response
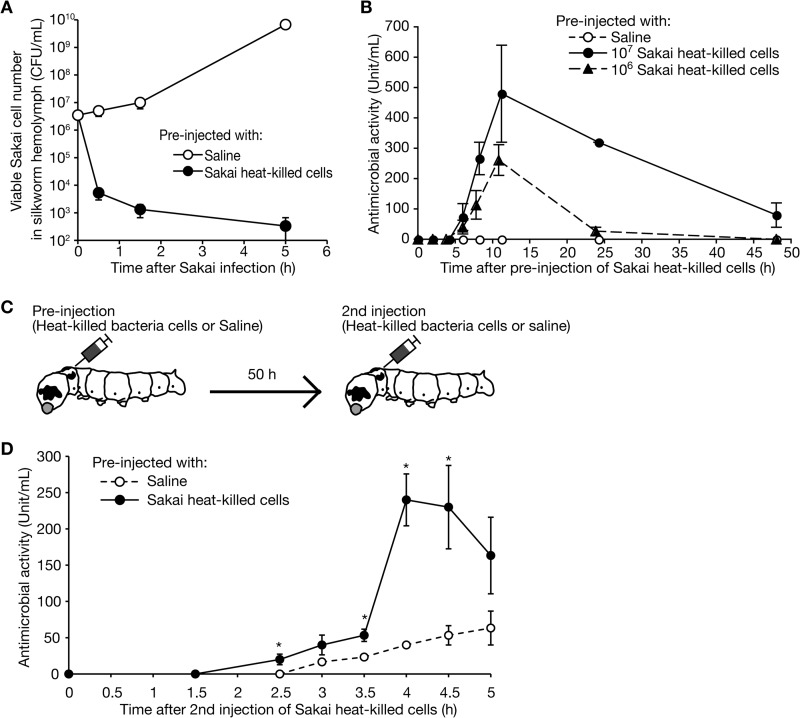
To investigate the molecular mechanisms of the acquired tolerance of the silkworms against Sakai infection, we hypothesized that the clearance of Sakai cells is accelerated in silkworms with acquired tolerance to Sakai infection. We preinjected silkworms with heat-killed Sakai cells or saline and then injected the silkworms with live Sakai cells and measured the numbers of live Sakai cells in the silkworm hemolymph. The number of Sakai cells increased after infection in silkworms preinjected with saline, whereas the number decreased after infection in silkworms preinjected with heat-killed Sakai cells (Fig. 4A). These results suggest that immune reactions leading to the elimination of Sakai cells are activated in silkworms preinjected with heat-killed Sakai cells.
FIGURE 4.
Booster effect of antimicrobial activity after the second injection of heat-killed Sakai cells. A, the viability of Sakai in the silkworm hemolymph was examined. We injected live Sakai cells into silkworms preinjected with heat-killed Sakai cells (closed circles) or saline (open circle). We then collected the hemolymph from the silkworms (n = 3) at different time points after Sakai infection and measured the cfu of Sakai of the hemolymph samples. The vertical axis shows the bacterial cell number in the silkworm hemolymph, and the horizontal axis shows the time after injection of live Sakai cells. Values are shown as mean ± S.E. B, the induction of antimicrobial activity to Sakai in the silkworm hemolymph after preinjection of heat-killed Sakai cells was examined. We collected silkworm hemolymph (n = 4) at different time points after preinjection of heat-killed Sakai cells and then measured the antimicrobial activity against Sakai. The vertical axis shows the amount of antimicrobial activity in the silkworm hemolymph. The definition of units is presented under “Experimental Procedures.” The horizontal axis shows the time after injection of heat-killed Sakai cells. Data are representative from three independent experiments. C, experimental scheme for investigating the booster effect. D, we injected heat-killed Sakai cells at 50 h after the preinjection of heat-killed Sakai cells (closed circles) or saline (open circles). The vertical axis shows the amount of antimicrobial activity to live Sakai cells in silkworm hemolymph samples (n = 12). The horizontal axis shows the time after the second injection of heat-killed Sakai cells. Values are presented as mean ± S.E. (error bars). A representative result of three independent trials is shown. *, Student's t test p values < 0.05.
In the adaptive immune systems of vertebrate animals, a booster reaction contributes to enhance the immune response when the same pathogen invades repeatedly. We hypothesized that a booster reaction contributes to the improved clearance of Sakai cells in silkworms that were preinjected with heat-killed Sakai cells. To investigate this possibility, we conducted a second round of injections of heat-killed Sakai cells into silkworms that had been preinjected with heat-killed Sakai cells and analyzed the production patterns of antimicrobial activity in the hemolymph. Anti-Sakai activity was observed 4–6 h after the preinjection of heat-killed Sakai cells, peaked at 12 h, and returned to baseline at 48 h after the preinjection (Fig. 4B). We then injected heat-killed Sakai cells into the silkworms again 50 h after the first injection of heat-killed Sakai cells or saline (Fig. 4C). The induction of anti-Sakai activity in the silkworms that were preinjected with heat-killed Sakai cells occurred earlier and to a greater degree than that in silkworms that were preinjected with saline (Fig. 4D). The findings suggest that exposure to heat-killed Sakai cells enhanced the induction of antimicrobial activity at the second round of exposure to Sakai cells in silkworms. Thus, a booster reaction works in silkworm immunity.
Contribution of Cecropin to the Silkworm Booster Reaction
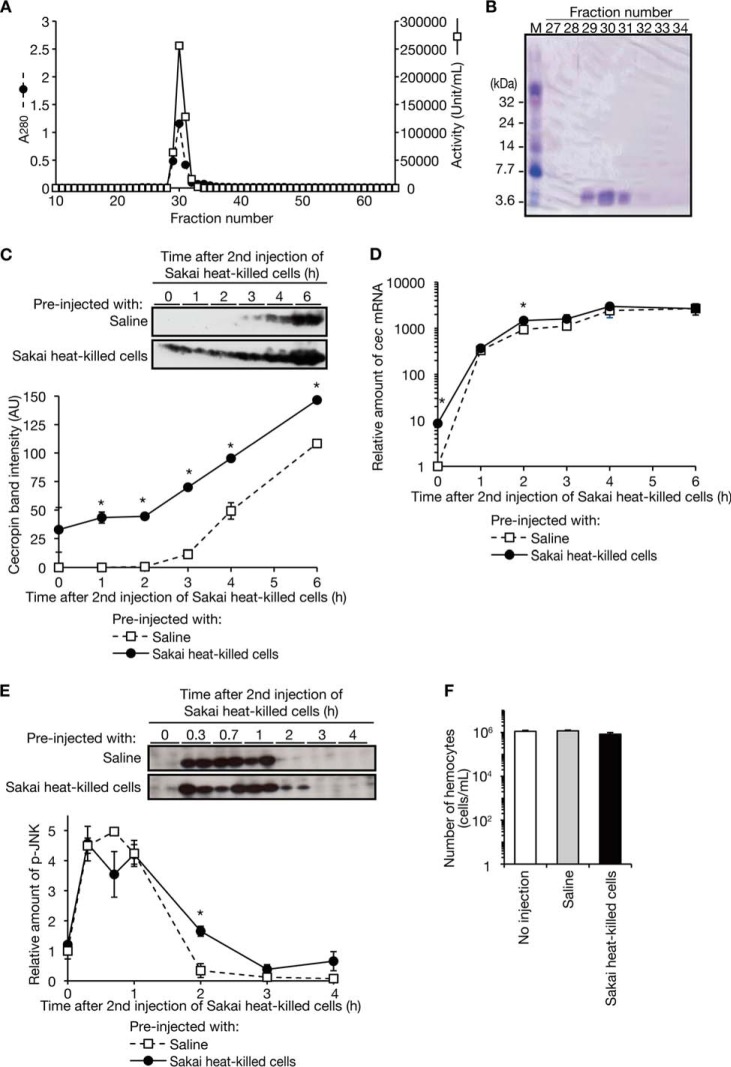
To investigate the molecular mechanism underlying the silkworm booster reaction against Sakai, we purified the antimicrobial molecule against Sakai from the silkworm hemolymph (Table 2). Following cation exchange column chromatography and gel filtration column chromatography, we obtained fractions (fraction numbers 29–31) containing proteins that co-migrated with the antimicrobial activity against Sakai (Fig. 5A). SDS-polyacrylamide gel electrophoresis analysis revealed the presence of a single 4-kDa protein band in the fractions (Fig. 5B). Peptide mass fingerprinting analysis identified the protein as cecropin. We then examined whether the booster reaction in silkworms is attributable to a corresponding increase of the amount of cecropin in the silkworm hemolymph. Within 2 h after the second injection, the amounts of cecropin were constant in silkworms preinjected with heat-killed Sakai cells or saline, whereas the hemolymph from silkworms preinjected with heat-killed Sakai cells contained higher amounts of cecropin at 1 and 2 h after the second injection than silkworms preinjected with saline (Fig. 5C). This finding indicates that at the time of the second injection of heat-killed Sakai cells, cecropin had accumulated in the silkworms preinjected with heat-killed Sakai cells. The level of accumulated cecropin at the second injection is likely to be inadequate to exhibit antimicrobial activity in vitro, which we measured in Fig. 4D. From 3 to 6 h after the second injection of heat-killed Sakai cells, the amount of cecropin increased in both silkworms preinjected with heat-killed Sakai cells and those preinjected with saline (Fig. 5C). Up to 6 h after the second injection of heat-killed Sakai cells, the amount of cecropin was greater in the silkworms preinjected with heat-killed Sakai cells than in those preinjected with saline (Fig. 5C). These findings suggest that the increased amount of cecropin in the hemolymph contributes to the booster reaction by increasing antimicrobial activity.
TABLE 2.
Purification of antimicrobial activity in silkworm hemolymph induced by the injection of heat-killed Sakai cells
The minimum inhibitory concentration of the fraction III against Sakai was 0.47 μg/ml.
| Fraction | Protein | Activity | Specific activity | Purification | Yield |
|---|---|---|---|---|---|
| mg | units | units/mg | -fold | % | |
| I. Hemolymph plasma | 450 | 10,800 | 24 | 1 | 100 |
| II. CM-TOYOPEARL | 13 | 280,000 | 21,000 | 870 | 2600 |
| III. Superose 12 | 0.58 | 128,000 | 221,000 | 9230 | 1100 |
FIGURE 5.
Molecular mechanism of the booster effect of antimicrobial activity. A, the antimicrobial activity induced by an injection of heat-killed Sakai cells was purified. We performed carboxymethyl-TOYOPEARL column chromatography and applied the active fractions to a Superose 12 10/300 GL column, a gel filtration column. We measured the optical absorbance at 280 nm and the antimicrobial activity against live Sakai cells. B, SDS-PAGE analysis of fractions 27–34 of the gel filtration column chromatography was performed. A 15% SDS-polyacrylamide gel was used and stained with Coomassie Brilliant Blue. C, the amount of cecropin peptide in the silkworm hemolymph was measured after the second injection of Sakai heat-killed cells. We injected heat-killed Sakai cells or saline into silkworms (n = 4) and maintained them for 50 h. We then injected heat-killed Sakai cells into all of the silkworms and prepared hemolymph plasma samples at different time points (samples from at least two silkworms were pooled in a tube). Samples containing 1.7 μl of hemolymph plasma fractions were electrophoresed in 15% SDS-polyacrylamide gels, and the gels were subjected to Western blot analysis using anti-cecropin antibody. Band intensities at each time point are shown in the bottom graph. Data represent means ± S.E. (error bars) from two samples that each contained hemolymph plasma from at least two silkworms. *, Student's t test p value less than 0.05. D, the amount of cec (cecropin) mRNA was measured after a second injection of Sakai heat-killed cells. We prepared total RNA from silkworm fat body (n = 4) at different time points and measured the amount of cec mRNA by quantitative RT-PCR using elongation factor-2 mRNA as an internal control. Data represent means ± S.E. from two samples that each contained cDNAs from at least two silkworms. *, Student's t test p value less than 0.05. E, the amount of p-JNK after the second injection of Sakai heat-killed cells was measured. We prepared cell lysates from the fat body (n = 4) at different time points and electrophoresed 10 μg of protein in 12.5% SDS-polyacrylamide gels. Western blot analysis against p-JNK using anti-p-JNK monoclonal antibody was performed. Relative band intensities at each time point against that of saline-preinjected silkworms at 0 h are shown in the bottom graph. *, Student's t test p value less than 0.05. Data are representative from two independent experiments. F, the number of hemocytes in silkworm hemolymph was measured. We collected hemolymph from silkworms (n = 3) at 10 h after the injection of heat-killed Sakai cells or saline or from non-injected silkworms. We counted hemocytes using a hemocytometer. Data represent means ± S.E. of the number of hemocytes in hemolymph samples. Data are representative of two independent experiments.
We then measured the amount of cec mRNA, which encodes cecropin, after the second injection of Sakai heat-killed cells in the fat body, an organ producing antimicrobial peptides. At the time of the second injection, the amount of cec mRNA was greater in the silkworms preinjected with heat-killed Sakai cells than in those preinjected with saline (Fig. 5D). From 0 to 2 h after the second injection of heat-killed Sakai cells, the amount of cec mRNA was increased in both silkworms preinjected with heat-killed Sakai cells and saline. At 2 h, the amount of cec mRNA was slightly higher in silkworms preinjected with heat-killed Sakai cells than in those preinjected with saline, but at 6 h, the amount of cecropin was not different between silkworms preinjected with heat-killed Sakai cells and those preinjected with saline (Fig. 5D). These findings suggest that de novo transcription of cec is not markedly stimulated in silkworms preinjected with heat-killed Sakai cells. To further evaluate the transcription of cec after the second injection of heat-killed Sakai cells, we measured the amount of p-JNK, which promotes the transcription of cec. The amount of p-JNK did not significantly differ between silkworms preinjected with heat-killed Sakai cells and those preinjected with saline, except at 2 h after the second injection, when a slightly larger amount of p-JNK was observed in the silkworms preinjected with heat-killed Sakai cells than in those preinjected with saline (Fig. 5E). These findings suggest that repeated injection of heat-killed Sakai cells did not markedly increase the transcription of cec by p-JNK.
To determine whether the booster reaction was related to the increased number of hemocytes in the hemolymph, we measured the number of hemocytes in silkworms injected with heat-killed Sakai cells or saline. The injection of heat-killed Sakai cells did not significantly change the number of hemocytes in the silkworm hemolymph (Fig. 5F).
DISCUSSION
The findings of the present study revealed that the silkworm, an invertebrate animal without antibodies, has an immune system that can recognize bacterial peptidoglycans to enhance the humoral immune reaction upon subsequent exposure to a pathogen. We demonstrated that the primed immune response of silkworms has pathogen selectivity (Gram-negative versus Gram-positive) and persistence and is enhanced in response to subsequent infection, features similar to those of vertebrate adaptive immune systems. Recent findings in invertebrate animals, such as fruit flies, bumblebees, or copepods, indicate that these animals recognize specific pathogens, and such exposure experience results in persistent resistance against a second round of infection (9, 10, 22). Importantly, we identified that a bacterial molecule, Gram-negative peptidoglycan, is involved in priming the silkworm immune system. The present findings also demonstrated that exposure to a pathogen boosts the humoral immune response in the second round of infection.
The silkworm immune system distinguishes between Gram-negative and Gram-positive bacteria but not between species of Gram-negative bacteria. Thus, silkworm immunity has less specificity than the adaptive immune response in mammals. This is consistent with the fact that the insect immune system recognizes pathogen-associated molecular patterns (23). In Drosophila, peptidoglycan recognition proteins recognize bacterial peptidoglycans (23, 24). We speculate that peptidoglycan recognition proteins recognizing Gram-negative peptidoglycans containing diaminopimelic acid contribute to the primed immune response in silkworms. In addition, we showed that immune tolerance to Sakai, which was acquired at the larval stage, remained after the silkworms pupated. Considering that most larval tissues are digested and reconstructed during pupation (25), our results suggest that a particular compartment is preserved at pupation to provide tolerance against a second round of infection.
We demonstrated the occurrence of a booster reaction in the silkworm humoral immune pathways, which depended on previous exposure to Sakai cells. The booster reaction of silkworm antimicrobial activity was not coupled with the phosphorylation of JNK, by which transcription of the cec gene is activated (Fig. 5E). Further, the amounts of cecropin and cec mRNA remained higher in silkworms preinjected with heat-killed Sakai cells at the time of the second injection (Fig. 5, C and D). Increase rates of cecropin and cec mRNA, however, were comparable between silkworms preinjected with heat-killed Sakai cells and those preinjected with saline (Fig. 5, C and D). These findings suggest that the booster reaction of antimicrobial activity depends on the additive effect of accumulated cecropin and de novo expressed cecropin. Therefore, we consider that the accumulation of cec mRNA and cecropin itself plays a key role in the primed humoral immune response of silkworms. Based on previous reports that the half-lives of particular mRNAs are affected by LPS stimulation in mouse dendritic cells (26), the accumulation of cec mRNA and cecropin can be attributed to a mechanism that modulates the degradation of a specific mRNA in the invertebrate primed immune response.
In mammals, immune cells play an important role in adaptive immune responses. In the first exposure to an antigen, antigen presenting cells present the antigen to immature T-cells, and antigen-specific mature T-cells and B-cells begin to proliferate. At the second exposure to the antigen, the antigen-specific memory cells induce the booster reaction in vertebrate immunity (3). Recent reports also showed in a vertebrate the existence of an immune memory that does not depend on T-cells or B-cells but depends on macrophages (27). Although there was no change in the number of silkworm hemocytes present after preinjection of heat-killed Sakai cells (Fig. 5F), the possible involvement of immune cells in the invertebrate primed immune response cannot be ruled out. Functional alterations of silkworm immune cells induced by exposure to Gram-negative peptidoglycans and their involvement in the development of tolerance to a second round of bacterial infection require further investigation.
This work was supported by Grants-in-aid for Scientific Research 23249009, 24590519, 25117507 and Japan Society for the Promotion of Science research fellowships for young scientists Grant 25-8664 (to A. M.). This study was also supported in part by the Mochida Memorial Foundation and the Genome Pharmaceuticals Institute.
- EHEC
- enterohemorrhagic E. coli
- p-JNK
- phospho-JNK.
REFERENCES
- 1. Weaver C. T., Hatton R. D. (2009) Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9, 883–889 [DOI] [PubMed] [Google Scholar]
- 2. Manis J. P., Tian M., Alt F. W. (2002) Mechanism and control of class-switch recombination. Trends Immunol. 23, 31–39 [DOI] [PubMed] [Google Scholar]
- 3. Woodland D. L. (2004) Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 25, 98–104 [DOI] [PubMed] [Google Scholar]
- 4. Lund F. E., Randall T. D. (2010) Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 10, 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harty J. T., Badovinac V. P. (2008) Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8, 107–119 [DOI] [PubMed] [Google Scholar]
- 6. Litman G. W., Rast J. P., Fugmann S. D. (2010) The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 10, 543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medzhitov R. (2001) Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 8. Babayan S. A., Schneider D. S. (2012) Immunity in society: diverse solutions to common problems. PLoS Biol. 10, e1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadd B. M., Schmid-Hempel P. (2006) Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 16, 1206–1210 [DOI] [PubMed] [Google Scholar]
- 10. Pham L. N., Dionne M. S., Shirasu-Hiza M., Schneider D. S. (2007) A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodrigues J., Brayner F. A., Alves L. C., Dixit R., Barillas-Mury C. (2010) Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaito C., Akimitsu N., Watanabe H., Sekimizu K. (2002) Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32, 183–190 [DOI] [PubMed] [Google Scholar]
- 13. Kaito C., Sekimizu K. (2007) A silkworm model of pathogenic bacterial infection. Drug Discov. Ther. 1, 89–93 [PubMed] [Google Scholar]
- 14. Miyazaki S., Matsumoto Y., Sekimizu K., Kaito C. (2012) Evaluation of Staphylococcus aureus virulence factors using a silkworm model. FEMS Microbiol. Lett. 326, 116–124 [DOI] [PubMed] [Google Scholar]
- 15. Hossain M. S., Hamamoto H., Matsumoto Y., Razanajatovo I. M., Larranaga J., Kaito C., Kasuga H., Sekimizu K. (2006) Use of silkworm larvae to study pathogenic bacterial toxins. J. Biochem. 140, 439–444 [DOI] [PubMed] [Google Scholar]
- 16. Miyashita A., Iyoda S., Ishii K., Hamamoto H., Sekimizu K., Kaito C. (2012) Lipopolysaccharide O-antigen of enterohemorrhagic Escherichia coli O157:H7 is required for killing both insects and mammals. FEMS Microbiol. Lett. 333, 59–68 [DOI] [PubMed] [Google Scholar]
- 17. Kaito C., Kurokawa K., Matsumoto Y., Terao Y., Kawabata S., Hamada S., Sekimizu K. (2005) Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol. Microbiol. 56, 934–944 [DOI] [PubMed] [Google Scholar]
- 18. Hanada Y., Sekimizu K., Kaito C. (2011) Silkworm apolipophorin protein inhibits Staphylococcus aureus virulence. J. Biol. Chem. 286, 39360–39369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez J., Gharahdaghi F., Mische S. M. (1998) Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS). Electrophoresis 19, 1036–1045 [DOI] [PubMed] [Google Scholar]
- 20. Kaneko T., Goldman W. E., Mellroth P., Steiner H., Fukase K., Kusumoto S., Harley W., Fox A., Golenbock D., Silverman N. (2004) Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 20, 637–649 [DOI] [PubMed] [Google Scholar]
- 21. Leulier F., Parquet C., Pili-Floury S., Ryu J. H., Caroff M., Lee W. J., Mengin-Lecreulx D., Lemaitre B. (2003) The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 4, 478–484 [DOI] [PubMed] [Google Scholar]
- 22. Kurtz J., Franz K. (2003) Innate defence: evidence for memory in invertebrate immunity. Nature 425, 37–38 [DOI] [PubMed] [Google Scholar]
- 23. Kurata S. (2010) Extracellular and intracellular pathogen recognition by Drosophila PGRP-LE and PGRP-LC. Int. Immunol. 22, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takehana A., Katsuyama T., Yano T., Oshima Y., Takada H., Aigaki T., Kurata S. (2002) Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl. Acad. Sci. U.S.A. 99, 13705–13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parthasarathy R., Palli S. R. (2007) Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect, Heliothis virescens. Mech. Dev 124, 23–34 [DOI] [PubMed] [Google Scholar]
- 26. Rabani M., Levin J. Z., Fan L., Adiconis X., Raychowdhury R., Garber M., Gnirke A., Nusbaum C., Hacohen N., Friedman N., Amit I., Regev A. (2011) Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 29, 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quintin J., Saeed S., Martens J. H., Giamarellos-Bourboulis E. J., Ifrim D. C., Logie C., Jacobs L., Jansen T., Kullberg B. J., Wijmenga C., Joosten L. A., Xavier R. J., van der Meer J. W., Stunnenberg H. G., Netea M. G. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]