Abstract
Leukemic stem cells (LSC) in acute myeloid leukemia (AML), defined by CD34 and CD38 antigens also express CD33 similar to normal hematopoietic stem cells. Residual LSC are believed to be responsible for relapse in AML after chemotherapy. Leukemic progenitor cell compartments were defined by CD34 and CD38 expression by flow cytometry in 61 new cases of AML. In each of four compartments thus defined, CD34+CD38−, CD34+CD38+, CD34−CD38− and CD34−CD38+, the pattern and intensity of expression of CD33 were studied in comparison to similar progenitor cell compartments in normal bone marrow and peripheral blood stem cell harvests. Post induction bone marrow samples from 10/61 cases were studied for aberrant CD33 expression. The intensity and pattern of expression of CD33 in AML progenitor cells were significantly different compared to normal progenitor cells. In two cases who were in morphological remission post induction, aberrant CD33 expressing progenitor cells were detectable at a frequency of 1.6 and 0.5 % respectively in the bone marrow. Aberrant CD33 expression in bone marrow LSC identified as CD34+CD38− cells in the CD45 dim/low side scatter region on flow cytometry may be useful as minimal residual disease marker after AML therapy. The method involves the use of a limited number of reagents and can be applied to all cases of AML.
Keywords: Acute myeloid leukemia, CD33, Leukemic progenitor cells, Minimal residual disease
Introduction
Leukemic stem cells (LSC) which are believed to be responsible for relapse of acute myeloid leukemia resemble normal hematopoietic stem cells (HSC) in hierarchical development and expression of cell surface antigens CD34 and CD38 [1]. Progenitor cell compartments or subsets can be defined by the expression of CD34 and CD38 [2]. The myeloid antigen CD33 is expressed by HSC as well as LSC in AML in the CD34+CD38− compartment [3]. Asynchronous expression or overexpression of myeloid antigens CD33 and CD13 have not been found in the CD34+CD38− compartment of normal bone marrow but can be seen in the LSC CD34+CD38− cells [5]. LSC frequency in complete remission (CR) in CD34+ AML detected by aberrant marker expression and abnormal light scatter properties of CD34+CD38− cells was shown to be superior in predicting prognosis compared to total blast MRD [6]. LSC detected by CD34+CD38− and ALDH intermediate expression were present in bone marrow samples of AML cases in follow up who later relapsed. The LSC contained the same cytogenetic marker as in blasts. The LSC could be detected 3 months before relapse [7].
In our study, samples from 61 AML patients were studied for the pattern of CD33 expression in the progenitor cell compartment defined by CD34 and CD38, in order to detect differences in expression from similar compartments in normal bone marrow and peripheral blood stem cell harvests. Post-induction bone marrows of 10/61 AML cases were examined for aberrant CD33 expression in stem cell subsets defined by CD34 and CD38. The objective was to formulate a method for minimal residual disease detection which involved a minimum of reagents and was easy to apply at multiple time points after chemotherapy cycles.
The correlation of CD33 expression with karyotype was determined.
Study Design
Patients
Sixty-one new AML patients (35 males, 26 females, median age 32 years range 6–63 years) were enrolled in the study. Karyotypes were available in 19 cases (good risk seven cases, intermediate risk nine cases, and poor risk three cases).Cases of promyelocytic leukemia were excluded. Controls were five cases of idiopathic thrombocytopenic purpura and ten peripheral blood stem cell donors. AML patients received induction chemotherapy, ‘7 + 3’ cytarabine and daunorubicin followed by three cycles of high dose cytarabine as consolidation. Samples were obtained after informed consent according to institutional guidelines.
Flow Cytometry
Peripheral blood and bone marrow samples of AML patients and controls and ten post-induction bone marrows collected in EDTA were processed within 2 h. Samples stained with CD34 PE or PECy7, CD38 FITC, CD45 PerCPCy5.5, CD33 APC at 4 °C were lysed for 10 min in 1 % NH4Cl at room temperature. Data acquisition was performed immediately after lysis on FACS Canto (BD Biosciences) flow cytometer. Data analysis was done with FACS DIVA. Compensation and voltage settings were adjusted with Calibrite beads (BD Biosciences) using in built software for automated adjustment of instrument settings, before each acquisition.
Cells with low side scatter in the dim CD45 region were gated. The regions in the second log decade of both axes were identified as low or dim expression. The subsets CD34+CD38−, CD34+CD38+, CD34−CD38− and CD34−CD38+ were defined in CD34 versus CD38 scatter plots. Each of the subsets was visualized in CD33 versus CD34 contour plots for patterns of antigen expression. The mean fluorescence intensity of CD33 in each subset and the coefficient of variation (cv) of CD33 expression were recorded.
Statistical Analysis
The mean fluorescence intensity and coefficient of variation expression of CD33 in AML cases and controls were compared by the nonparametric Mann–Whitney U test. Spearman’s test was used for the correlation of frequency of subsets and CD33 mean fluorescence intensity (mfi) with total WBC count and peripheral smear blast counts. The Kruskal–Wallis test for non- parametric data was used to test for the significance of association of CD33 mean fluorescence intensity with cytogenetic risk groups (p < 0.05 was considered significant for all analysis). Analysis was performed with SPSS version 11.5 (Chicago, IL, USA).
Results
Comparison of AML Patients and Controls
Patterns of CD33 Expression
(i) CD 33 expression was found at variable levels in all subsets of AML, whether CD34+ or CD34−. CD33 expression was absent in 11/61 cases in the CD34+ CD38- cells, in 1/32 cases in CD34+CD38+ cells, 1/61 in CD34−CD38− and 5/41 in the CD34−CD38+ subset (all the four subsets were not present in some cases). The mean fluorescent intensity (mfi) of CD33 expression in all the subsets was significantly higher in AML compared to controls (p < 0.05) (Table 1).
Table 1.
Mean fluorescence intensity of CD33 expression and frequency of stem cell subsets in AML and Controls
| Cell subsets | CD45 dim/ssc low | CD34+CD38− | CD34+CD38+ | CD34−CD38− |
|---|---|---|---|---|
| Controls (n = 15) | m 1116.1 (50, 2629) | m 1591.3 (116, 5208) | m 2911.3 (498, 12254) | m 953 (50, 3367) |
| f 18.6 (1.2, 55.70) | f 2.7 (0.0, 22.7) | f 0.27 (0.0, 1.70) | f 8.0 (0.3, 53.6) | |
| AML (n = 61) | m 4527.9 (151, 13294) | m 4719 (217, 16379) | m 6613 (411, 20498) | m 4067.5 (135, 13281) |
| f 49.6 (5.5, 92.5) | f 13.9 (0.1, 55.6) | f 9.1 (0.1, 71.8) | f 22.8 (1.20, 88.8) | |
| p value (mfi) | 0.001 | 0.009 | 0.011 | 0.001 |
| p value (f) | 0.001 | 0.001 | 0.001 | 0.002 |
mfi mean fluorescence intensity, m mean, f frequency %, ssc side scatter
CD33 was expressed more uniformly in all the stem cell subsets of AML (lower cv) compared to expression in controls (p 0.024 for CD34+CD38− and p 0.001 for CD34−CD38− cells).
Appearance of Stem Cell Subsets of AML and Control Cases in Contour Plots in the Flow Cytometer
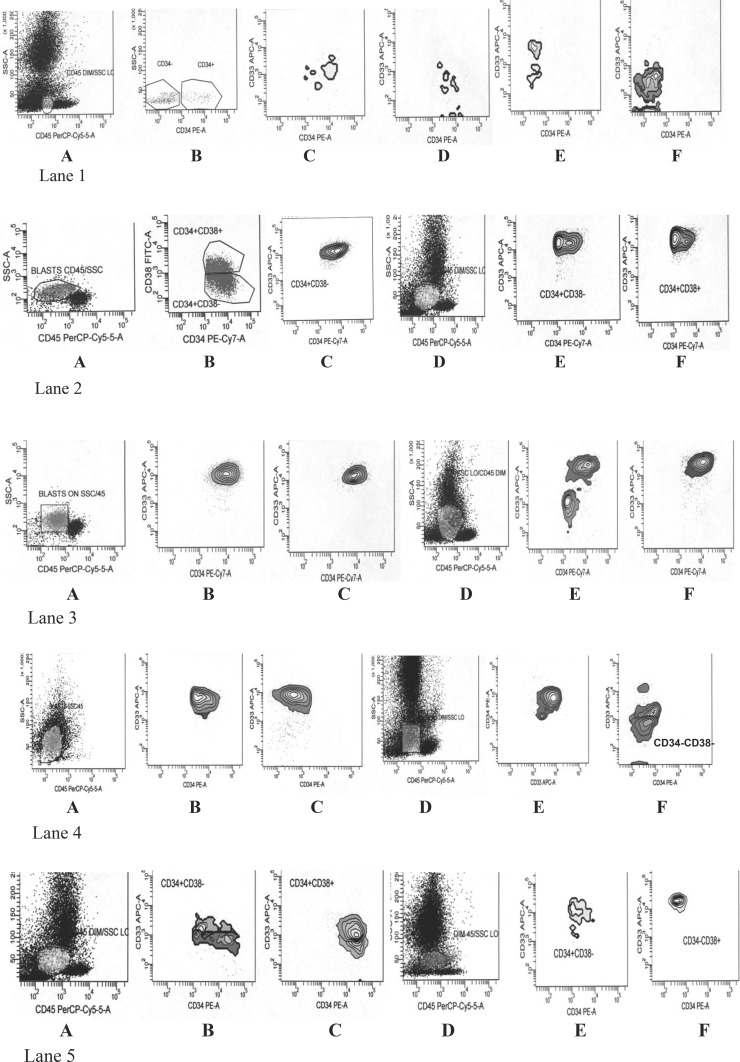
The gated CD34+CD38−, CD34+CD38+, CD34−CD38− and CD34−CD38+ populations defined in the dim CD45/low side scatter region of AML cases and controls were each viewed in contour plots for homogeneity within cell populations with respect to CD33 and CD34 expression. In 55/61 AML at diagnosis, irrespective of the frequency of the subset concerned, at least one of the progenitor cell subsets, most commonly CD34+CD38− followed by CD34–CD38– or the CD33+ fraction of a subset showed a single homogenous, oval or round, cohesive cluster of events with respect to CD33 and CD34 expression (Fig. 1). This was in contrast to similar subsets in all the normal bone marrow samples and PBSC harvests where irregular and multiple discrete clusters were visualized. The lowest frequency of cells at which a subset was clearly visible in the contour plot was 0.1 % of total events.
Fig. 1.
Lane 1 Normal bone marrow. Heterogenous pattern of CD33 expression in CD34+CD38−, CD34+CD38+, CD34−CD38+ and CD34−CD38−, Lane 2 Case 1, A, B, C: AML M4 pre-treatment peripheral blood. Homogenous CD33 expression in CD34+CD38− cells, Case 1, D, E, F: Post-induction bone marrow. Blasts 69 % on morphology. Persistent aberrant CD33 expression in CD34+CD38− and CD34+CD38+cells, Lane 3 Case 2, A, B, C: AML M6 pre-treatment peripheral blood. Single cluster of cells in CD34+CD38− and CD34+CD38+ subsets with homogenous CD33 expression, Case 2, D, E, F: Post-induction bone marrow. Blasts 14 % on morphology. Homogenous CD33 expression in CD34+CD38+ cells subset, Lane 4 Case 3, A, B, C: AML M4 pre-treatment peripheral blood. Single cluster with homogenous CD33 expression in CD34+CD38− and CD34−CD38− cell subset, Case 3, D, E, F: Post-induction bone marrow. Blasts <5 % on morphology. Aberrant CD33 expression in CD34+CD38− cells, Lane 5 Case 4, A, B, C: AML M2 peripheral blood at diagnosis. Heterogenous CD33 expression in CD34+CD38− cells and a more homogenous cluster in CD34+CD38+ cell subset, Case 4, D, E, F: Post-induction bone marrow, blasts <5 % on morphology, showing single homogenous CD33+ cluster in CD34−CD38+ cell subset
Relation to Karyotype
The mean fluorescence intensity of CD33 in the CD34+CD38– subset was significantly related to cytogenetic risk group (Kruskal–Wallis Chi square 7.214, df 2, p 0.027, mean ranks: poor risk 18, intermediate risk 8.39, good risk 8.64). However there was no correlation of CD33 mean fluorescence intensity with the frequency of CD34+CD38− cells, peripheral blood blast counts or subtype of AML.
Comparison of Pre- and Post-induction Samples of Ten AML Patients
Two patients in this group were not in remission by morphological criteria [4]. The other eight patients had less than 5 % blasts in the bone marrow at day 28 post-induction. Two of the latter relapsed at 6 months and 8 months post-induction, respectively.
Mean fluorescent intensity of CD33 increased in all progenitor cell subsets following induction but the increase in CD33 expression was not statistically significant.
Compared to controls however the CD33 mean fluorescence intensity in the progenitor cell subsets of post induction marrow revealed a significantly higher expression of CD33 (p < 0.001 for all subsets) compared to controls, even without data of the two refractory cases.
The two refractory cases showed a pattern of homogenous CD33 expression in contour plots, similar to their pre-treatment samples (Fig. 1). In two patients who were in morphological remission, an aberrant pattern of CD33 antigen expression was seen in CD34+CD38− (Fig. 1, lane 4) and CD34−CD38+ cells (Fig 1, lane 5), at 1.6 and 0.5 % of total events respectively. They continue to be in remission at 1 year, after consolidation chemotherapy. In the two cases who relapsed, an aberrant pattern of CD33 expression was not seen in the post-induction bone marrow samples. Bone marrow samples obtained at later time points were not available in these two cases.
To conclude, AML cells with progenitor cell characteristics of low side scatter and dim CD45 expression were categorized on the basis of CD34 and CD38 expression into four compartments described above. Expression of CD33 in these subsets in AML was different from normal in our study. Examination of post-induction bone marrow in ten AML cases revealed aberrant CD33 expression in two cases which were otherwise in morphological remission. Our method using a four antibody combination is therefore a simple way of detecting minimal residual disease in the form of aberrant CD33 expression in progenitor cell subsets in the bone marrow of AML cases post- chemotherapy. This method however needs validation in a larger number of cases, by comparison with traditional methods of MRD detection by leukemia associated phenotypes (LAIPS) using a panel of antibodies or real time PCR based MRD detection.
Acknowledgments
We thank Dr. Purnima Sharma for help with statistical analysis and Dr. Hema Arora for technical help.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;7:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland HJ, Blair A, Zapf RW. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87:4754–4761. [PubMed] [Google Scholar]
- 3.Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106(13):4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 5.van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AWM, et al. Aberrant marker expression patterns on the CD34+CD38− stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21:1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 6.Terwijn M, Kelder A, Rutten AP, Snel AN, Scholten W, Zweegman S, et al. LSC frequency in CR in CD34+ AML detected by aberrant marker expression and light scatter properties was shown to be superior in predicting prognosis compared to total blast MRD. Blood. 2009;114(22):399a. [Google Scholar]
- 7.Gerber JM, Smith BD, Ngwang B, Vala MS, Morsberger L, Galkin S, et al. A clinically relevant population of leukemic CD34+CD38− cells in acute myeloid leukemia. Blood. 2012;119(15):3571–3577. doi: 10.1182/blood-2011-06-364182. [DOI] [PMC free article] [PubMed] [Google Scholar]