Abstract
NUT midline carcinoma (NMC) is a rare, genetically defined, highly lethal undifferentiated carcinoma occurring in the midline location of the neck, head or mediastinum. We present the case of a 23 year-old otherwise healthy Chinese male immigrant who presented with complaints of sore throat and right sided neck mass. The initial treatment was for likely EBV infection with streptococcal superinfection. Although continued investigation was pursued shortly after initial presentation, the mass had enlarged and become necrotic with significant nodal involvement. The mass was diagnosed as an NMC tumor with a novel three-way translocation t(9;15;19; q34;q13;p13.1). Despite aggressive treatment, the patient’s condition progressed rapidly and he died within 3 months of initial diagnosis. Standard therapeutic interventions have been ineffective in the treatment of NMC. Earlier diagnosis could allow characterization of the natural progression of this entity, and allow more time for intervention or development of novel therapies, potentially related to molecular targets. This continues to require a high index of suspicion and early imaging with cytogenetic and immunohistochemical confirmation.
Keywords: NUT midline carcinoma, Undifferentiated carcinoma, Hypopharynx
Introduction
NUT midline carcinoma (NMC) is a lethal undifferentiated carcinoma that can arise in different organs and tissue types but characteristically locates to the midline of head, neck and mediastinum. It is characterized by rearrangement of the NUT gene on chromosome 15 with members of the BRD gene family. The incidence is currently unknown and it is assumed to be rare [1]. However, an early study investigating the presence of NUT rearrangements in pathology specimens of the upper aerodigestive tract known to be undifferentiated carcinoma and not associated with EBV infection reported that approximately 18 % of the tested specimens were consistent with the diagnosis of NUT carcinoma [2]. While originally described as a tumor unique to younger patients, an increased awareness of this tumor type has revealed a broad age distribution extending from birth [3] to the eighth decade [4–6].
Classically the diagnosis has been made by karyotype analysis and demonstration of a characteristic translocation involving chromosomes 15 and 19, t(15;19), in two-thirds of cases, with juxtaposition of the BRD4 (19p13.1) and NUT (15q14) genes resulting in a BRD4-NUT fusion product [6, 7]. In the remaining one-third of cases the NUT gene is coupled with a gene other than BRD4, resulting in a variant translocation. Alternatively, the diagnosis can be rendered by demonstration of the expression of a NUT-fusion protein by immunohistochemistry and confirmation by fluorescence in situ hybridization (FISH).
It has been proposed that the normal function of the NUT protein is to bind and activate the histone acetyltransferase (HAT) of p300 [8], leading to an increase in histone acetylation [9, 10]. The fusion protein created by the translocation, BRD-NUT, may suppress a variety of genes, including those associated with squamous differentiation [11].
In this report we discuss the imaging findings associated with the development of an invasive hypopharyngeal NMC tumor in a 23 year-old male who presented with the chief complaint of sore throat and prominent cervical lymphadenopathy. This tumor displayed a three-way translocation involving chromosomes 9, 15, and 19. While other case reports have been published regarding the various presentations of NMC [10–12], to our knowledge there have been no case reports discussing this particular molecular alteration in the context of radiographic progression [13].
Case Report
The patient was a 23 year-old non-smoking Chinese male immigrant who presented for evaluation after a 3-week history of right sided neck mass with sore throat. He was initially treated by his primary care physician for a presumed pharyngeal infection. Continued enlargement of the adenopathy was associated with tongue swelling and dysarthria despite antibiotic treatment and he was seen by the otolaryngology service for additional evaluation.
At the time of evaluation, the patient reported recent onset of persistent hoarseness but was able to tolerate liquids and soft solids by mouth and a 20-pound weight loss but had no complaints of dyspnea or referred otalgia. He had no significant history of tobacco use or alcohol consumption and no history of prior head and neck surgery or cancer diagnosis. Physical exam was significant for marked enlarged non-tender right level II and III lymphadenopathy and atrophy with rightward deviation of the tongue. Flexible laryngoscopy demonstrated fullness within the hypopharynx and decreased adduction and abduction of the left true vocal cord with speech.
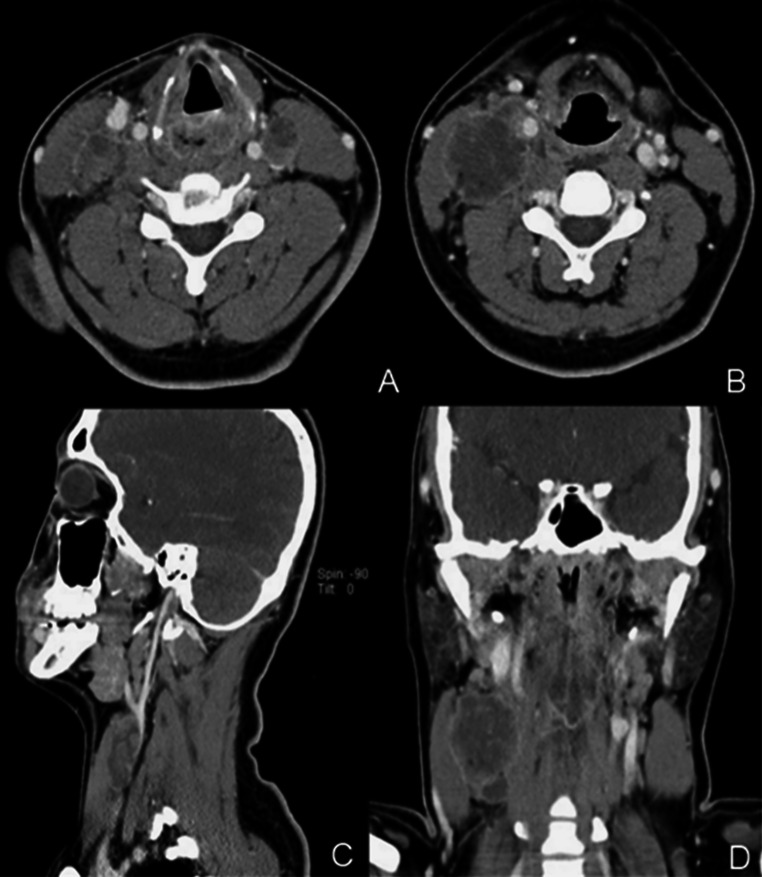
A chest radiograph was negative for significant cardiovascular or pulmonary abnormality. Contrast enhanced multi-detector computed tomography (MDCT) imaging of the soft tissues of the neck revealed a low attenuating irregularly margined mass involving the hypopharynx on the left with extension into the post-cricoid region and aryepiglottic folds bilaterally (Fig. 1). Multiple peripheral enhancing necrotic lymph nodes involving level II, III, and IV cervical nodes were exhibited. The largest IIA node located on the right measuring 3.5 × 3.5 × 3.5 cm exerted mass effect on the right internal jugular vein. Asymmetric thickening was noted in right palatine tonsil, vallecula, and right lingual tonsils.
Fig. 1.
Contrast enhanced MDCT images of this 23-year-old male with NUT midline carcinoma of the neck. a Initial axial CT of the neck shows a low attenuation mass in the left hypopharynx. b Axial CT image shows a large necrotic and peripherally enhancing right cervical node. c Sagittal CT image shows external compression of the right internal jugular vein by an enlarged cervical node. d Coronal CT image shows a low attenuation mass and multiple bulky necrotic nodes
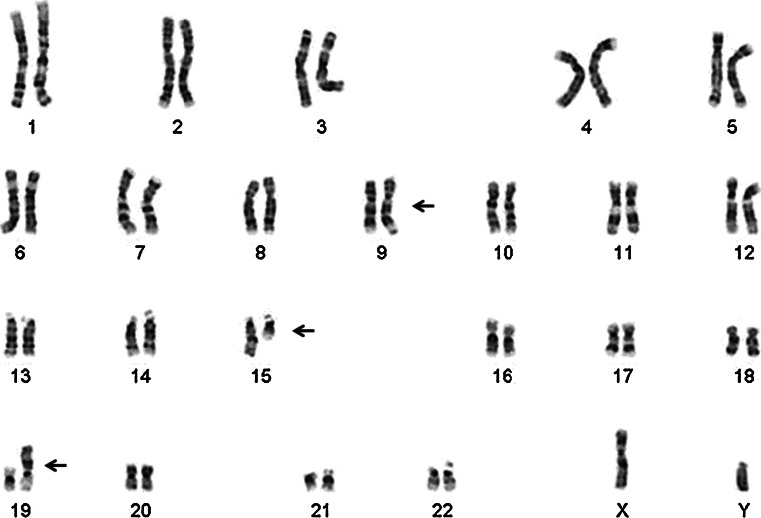
Operative evaluation with microlaryngoscopy and biopsy demonstrated an infiltrative friable mucosal lesion in the hypopharynx extending into the cervical esophagus. The multiple endoscopic tumor biopsies showed only necrotic tumor with bacterial colonization. The fine needle aspirate of a right-sided lymph node yielded limited diagnostic tissue. Analysis of tissue sample from a subsequent excisional biopsy of the lymph node showed a poorly differentiated neoplasm composed of medium-sized, relatively discohesive cells with an epithelioid phenotype (Fig. 2). Immunohistochemical staining revealed tumor cells focally positive for pankeratin and diffusely variably positive for CAM 5.2 (Fig. 3) with some reactivityfor CD30. The tumor cells were negative for CD45, S-100, TTF-1, chromogranin, c-kit, OCT-4, PAX8, CD56, ALK, CD138, and CK7. In addition, in situ hybridization for EBV (EBER) was negative. Karyotype analysis of cells from the lymph node identified a three way translocation, 46XY, t(9;15;19; q34;q13;p13.1), in all cells (Fig. 4), which was diagnostic of a NUT midline carcinoma. Subsequent immunohistochemistry for NUT protein performed at Brigham and Women’s Hospital, Boston, MA confirmed presence of abnormal fusion protein in a speckled, nuclear pattern in all of the cells (Fig. 2).
Fig. 2.
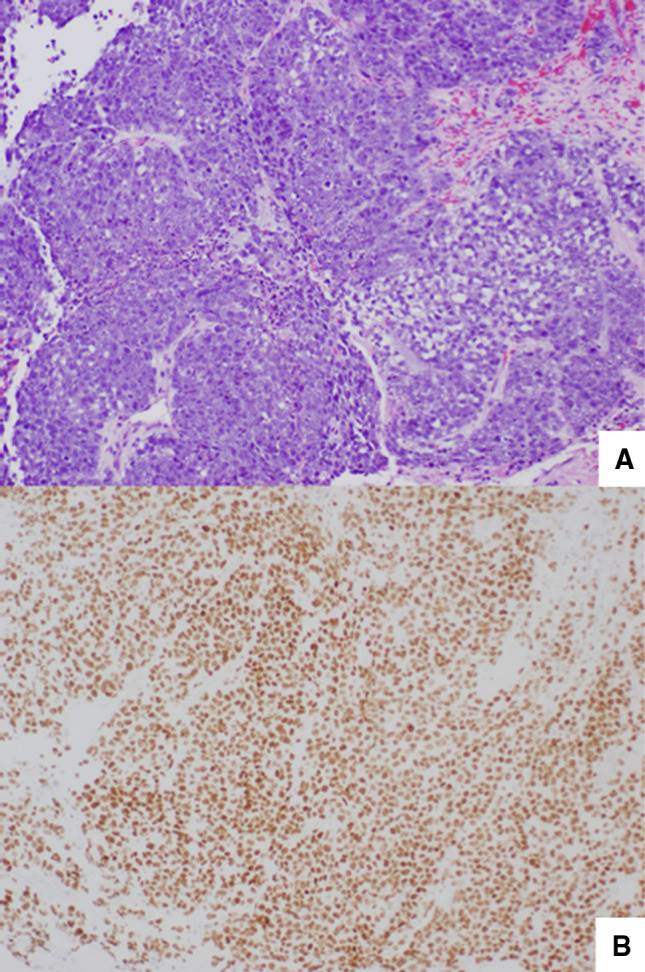
a The tumor demonstrates a solid growth pattern composed of medium-sized, rounded, monotonous cells. H&E stain, original magnification 20×. b Immunohistochemical staining with NUT antibody demonstrates a speckled nuclear pattern within the cells consistent with a diagnosis of NUT midline carcinoma. Immunoperoxidase stain, original magnification 40×
Fig. 3.
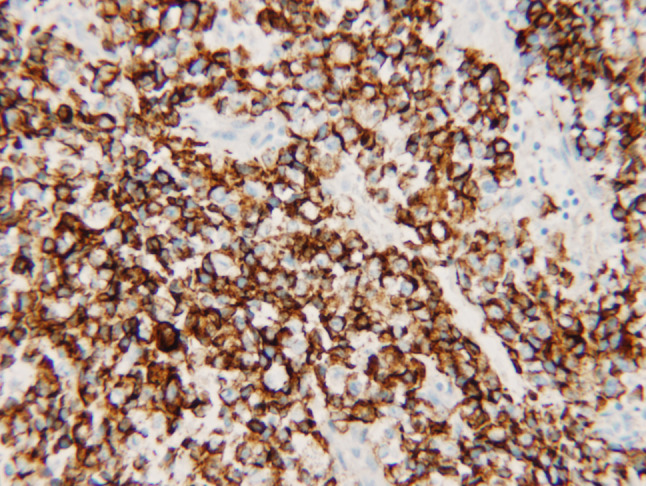
The tumor shows variable weak and patchy staining for CAM 5.2 by immunohistochemistry. Immunoperoxidase stain, original magnification 20×
Fig. 4.
The karyotype identifies the t(9;15;19) translocation, which is diagnostic of NUT midline carcinoma. The translocated chromosome of each pair is indicated by an arrow
Further imaging with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) was notable for interval increase in size of right neck mass, FDG avid left neck lymph nodes, and multiple ground glass densities without FDG uptake in the lungs. The patient was initially treated with chemoradiation with docetaxel, which was changed within 1 month to Romidepsin histone deacetylase (HDAC) inhibitor therapy due to elevated transaminases. Despite these treatments, the patient quickly progressed to the point of palliative care within 3 months of initial diagnosis and expired shortly afterward.
Discussion
Initial characterization of NMC suggested that the tumor is a highly lethal undifferentiated tumor of the midline structures, usually involving the upper aerodigestive tract and containing a characteristic translocation involving chromosome 15 and 19. Subsequent publications have shown this tumor to not only have a broader age distribution that ranges from the first decade to the late eighth decade of life, but also a greater range of anatomic primaries with variations on the classic t(15;19) rearrangement [1, 4]. Infradiafragmatic and non-midline structures have been involved, including medial thigh musculature, pancreas, liver, and bladder [3, 5, 12, 14, 15]. Pathologic evaluation of our patient’s tumor showed a translocation involving three chromosomes resulting in a novel karyotype of 46XY, t(9;15;19; q34;q13;p13.1), and is only the second reported case with a three-way translocation [13].
Histologically, NMC tumors have been typically identified to have an epithelioid component with expression of cytokeratin proteins [7, 14]. In contrast to other poorly differentiated carcinomas, the cells comprising NUT midline carcinoma appear rather uniform with round nuclear contours and are medium-sized [6]. In addition, these tumors may have focal squamous differentiation, as it is an aggressive variant of squamous cell carcinoma [6]. Only one reported case was identified that showed findings suggestive of mesenchymal differentiation. No single tissue type of origin has been successfully identified for these tumors [6].
It has been proposed that the mechanism by which NUT midline carcinoma exerts its lethal effects is through the function of the BRD4-NUT fusion chimeric protein and/or the BRD3-NUT fusion protein [6]. This protein is thought to repress transcription of differentiation-specific genes, particularly genes that are responsible for squamous differentiation [6]. Squamous differentiation is accompanied by arrested cellular proliferation. Therefore, lack of squamous differentiation induced by the chimeric protein is associated with uncontrolled cellular proliferation of a stem-like cell, characteristic of this neoplasm [6]. A recent review of French et al. identified a variety of molecules that may reverse the effect of the fusion protein and could be used therapeutically [6].
In general, the MDCT and MRI signal characteristics of NMC are nonspecific and may mimic a number of pathological entities, including lymphoma, metastatic neuroendocrine tumors and sarcomas. However, a consistent imaging finding on MDCT reported in the literature is a low attenuation mass demonstrating heterogeneous enhancement [10, 12, 13], with tumor and lymph node necrosis often described in the reported cases. Most tumors have reportedly involved midline structures. The MDCT findings in the case we report are concordant with reported cases in the literature with a hypodense mass with associated bulky, necrotic lymph node metastasis (Fig. 1). FDG-PET imaging was also performed in our case for staging. FDG-PET imaging correlated well with the MDCT findings showing hypermetabolic tumor and lymph nodes corresponding to lesions identified on MDCT (Fig. 1). FDG-PET/CT has been used previously to follow the tumor progression and clinical response to treatment [11], and as a result, some have advocated utilizing FDG-PET for the initial staging for NMC after pathologic diagnosis, while others have proposed that interval imaging may be useful to monitor response to treatment [16, 17].
Variable responses to chemotherapy and radiation therapy have been observed in the reported cases, though the treatment outcomes have been extremely poor. There is a single case reported of a patient with an NMC primary located in the iliac bone who responded to treatment with a remission period of over 13 years [18]. The other cases have proven fatal with average survival estimated to be 6.7 months [19]. Recently, progression free survival and overall survival was found to be related to early radiotherapy or more extensive resection of the tumor [19]. Additionally, while a chemoradiation protocol with carboplatin and paclitaxel was advocated as a potential treatment option [20], a recent analysis has thus far failed to show improved outcomes [19].
Conclusions
NMC is an undifferentiated and highly aggressive tumor etiologically related to translocation involving the NUT gene on chromosome 15, and the BRD gene on chromosome 19. Pathologic findings of the discussed case revealed a novel translocation involving a third chromosome, in addition to chromosomes 15 and 19. Consistent with prior reported cases, no definitive tissue or origin was identified. Given the rapid progression and high mortality of the disease after initial presentation, increased awareness of this diagnosis may result in earlier diagnosis of this malignancy and may create an opportunity to initiate aggressive therapy prior to regional or metastatic spread.
Acknowledgments
The authors would like to thank Dr. Christopher French and the Pathology Department at Brigham and Women’s Hospital for providing the immunohistochemistry slide with staining for NUT protein.
References
- 1.French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203:16–20. doi: 10.1016/j.cancergencyto.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 3.Shehata BM, Steelman CK, Abranowsky CR, et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2010;13:481–485. doi: 10.2350/09-10-0727-CR.1. [DOI] [PubMed] [Google Scholar]
- 4.Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5:31–35. doi: 10.1007/s12105-010-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 6.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol Mech Dis. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 7.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 8.Reynoird N, Schwartz BE, Delvecchio M, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–2952. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson BA, Lee EY, French CA, Bauer DE, Vargas SO. BRD4-NUT carcinoma of the mediastinum in a pediatric patient: multidetector computed tomography imaging findings. J Thorac Imaging. 2010;25:93–96. doi: 10.1097/RTI.0b013e3181b5d84d. [DOI] [PubMed] [Google Scholar]
- 11.Niederkohr RD, Cameron MJ, French CA. FDG PET/CT imaging of NUT midline carcinoma. Clin Nucl Med. 2011;36:124–126. doi: 10.1097/RLU.0b013e31821c9a23. [DOI] [PubMed] [Google Scholar]
- 12.Polsani A, Braithwaite KA, Alazraki AL, Abramowsky C, Shehata BM. NUT midline carcinoma: an imaging case series and review of literature. Pediatr Radiol. 2012;42:205–210. doi: 10.1007/s00247-011-2272-3. [DOI] [PubMed] [Google Scholar]
- 13.Toretsky JA, Jenson J, Sun CC, et al. Translocation (11;15;19): a highly specific chromosome rearrangement associated with poorly differentiated thymic carcinoma in young patients. Am J Clin Oncol. 2003;26:300–306. doi: 10.1097/01.COC.0000020960.98562.84. [DOI] [PubMed] [Google Scholar]
- 14.den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 15.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorly-differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teo M, Crotty P, O’Sullivan M, French CA, Walshe JM. NUT midline carcinoma in a young woman. J Clin Oncol. 2011;29:336–339. doi: 10.1200/JCO.2010.32.7486. [DOI] [PubMed] [Google Scholar]
- 17.Rutt AL, Poulik J, Siddiqui AH, et al. NUT midline carcinoma mimicking tonsillitis in an eight-year-old girl. Ann Otol Rhinol Laryngol. 2011;120:546–549. doi: 10.1177/000348941112000810. [DOI] [PubMed] [Google Scholar]
- 18.Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49:1015–1017. doi: 10.1002/pbc.20755. [DOI] [PubMed] [Google Scholar]
- 19.Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18(20):5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santis G, Landau D, Harrison-Phipps K, Neat M, Moonim MT. Successful radical treatment of midline carcinoma with t(15;19) diagnosed by endobronchial ultrasound- derived transbronchial needle aspiration. J Clin Oncol. 2011;29:327–329. doi: 10.1200/JCO.2010.32.7973. [DOI] [PubMed] [Google Scholar]