Abstract
The molecular profile of epithelial–myoepithelial carcinomas (EMCa) has not been well studied, though a recent association with Harvey rat sarcoma viral oncogene homolog (HRAS) mutations has been noted. To confirm and validate this, we surveyed fifteen EMCa for HRAS codon 61 mutations and correlated HRAS status with clinicopathologic parameters. There were 11 females and 4 males and mean patient age was 64 (range 49–90). Parotid gland was most commonly involved (n = 10) and the most common histologic appearance was that of a ‘classic’ EMCa (7/15). Four of fifteen (26.7 %) cases demonstrated local recurrence, while 2/15 (13.3 %) demonstrated distant metastases. Other variant morphologies included EMCa arising from pleomorphic adenoma (3/15), and high grade EMCa (2/15). HRAS exon 3, codon 61 mutations, p.Q61R (n = 3) and p.Q61 K (n = 1) were identified in 4 of 15 successfully tested EMCAs (14 patients). Two cases were classic type, while the other cases consisted of one oncocytic variant, and one tumor with myoepithelial overgrowth, the latter of which showed the same mutation in both the primary and recurrence. Of note, the high grade EMCa and EMCa ex pleomorphic adenoma were negative for mutations. Given the small number of cases, there were no significant differences between mutation positive and mutation negative cases in terms of age, gender and outcome.
Keywords: Epithelial–myoepithelial carcinoma, HRAS, Mutation, Oncocytic variant
Introduction
RAS proteins are encoded by three proto-oncogenes: HRAS, KRAS, and NRAS for which activating mutations were implicated in many human tumor types. The relative frequency of the three mutant isoforms as well as the distribution of codons involved varies considerably by organ site and tumor type. For instance, while KRAS mutations predominate in lung and colon adenocarcinomas, HRAS (Harvey rat sarcoma viral oncogene homolog) mutations are overrepresented in salivary gland tumors as per the COSMIC dataset [1] and present in about 15 % of salivary cancers as a whole [1]. HRAS has been implicated in salivary tumorigenesis as early as the 1990s: transgenic mice expressing a HRAS p.G12 V mutation developed “aden squamous” carcinomas of submandibular glands [2]. Since then a variety of common tumor types including pleomorphic adenoma (PA), carcinoma ex pleomorphic adenoma (CAxPA), mucoepidermoid carcinoma, and adenocarcinoma not otherwise specified have been reported to have HRAS mutations or protein (p21) overexpression in as many as 1 out of 3 tumors [3–7]. Given the relative frequency of HRAS mutations in common tumor types, particularly pleomorphic adenoma and its transformed malignant counterpart, applicability of HRAS mutational analysis in less common tumor types may provide insights into their molecular pathogenesis.
Among these rarer tumor types is epithelial–myoepithelial carcinoma (EMCa), a low grade biphasic salivary gland malignancy initially described by Donath et al. [8]. This tumor often has a deceptively bland appearance and in many ways resembles PA, though it is rather rare comprising ~2 % of salivary gland malignancies. In some cases EMCa is the actual carcinoma type arising from PA in CAxPA. To date, the tumorigenesis for this rare carcinoma is unclear and there is very little in the literature regarding relevant genetic alterations. However, recently, up to 33 % rate of HRAS codon 61 (exon 3) mutations in EMCa was suggested in a preliminary report by Cros et al. [4]. Thus, in order to confirm/validate these findings, we herein survey fifteen EMCa for HRAS codon 61 mutations and correlate HRAS status with clinicopathologic parameters.
Methods
Case Selection
This study was approved by our Institutional Review Board (IRB# PRO07050360). Sixteen EMCa in fifteen patients (1980–2013) had sufficient and available formalin fixed paraffin embedded tissue for mutational analysis, and were included in the study. Clinicopathologic parameters were reviewed. The parameters for cases 1–10 were previously reported [9, 10], but follow-up was subsequently updated for this study. Tumors were categorized as follows: classic, ex pleomorphic adenoma, high grade, with myoepithelial overgrowth, oncocytic and apocrine variants. Classic tumors fulfilled the WHO 2005 requirement for having a clear cell myoepithelial component [11]. EMCa ex pleomorphic adenoma, EMCa with myoepithelial overgrowth, oncocytic and apocrine variants fulfilled criteria previously described [9, 10]. High grade transformation of either myoepithelial or epithelial components qualified as high grade for this study [12]. Tumors were staged according to 7th edition AJCC rules.
HRAS Mutation Analysis
Tissue cores from tumor targets were obtained as previously described [13]. DNA was isolated from tissue cores using the DN easy tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. For the detection of mutations, DNA was amplified with primers flanking exon 3 of the HRAS gene (forward primer 5′-GTC CTC CTG CAG GAT TCC TA-3′ and reverse primer 5′-CGG GGT TCA CCT GTA CT-3′). PCR products were sequenced in both sense and antisense directions using the BigDye Terminator v3.1 cycle sequencing kit on ABI 3730 (Applied Biosystems, Inc., Foster City, CA) according to the manufacturer’s instructions. The sequences were analyzed using Mutation Surveyor software (SoftGenetics, LLC., State College, PA).
Results
Clinicopathologic parameters and mutational status are summarized in Table 1. This set of tumors had a female predilection (11:4), and mean patient age was 64 (range 49–90). Site distribution was as follows: Parotid—10, Submandibular—2, Maxillary—2, Lung—1. The mean tumor size was 3 cm (range 0.8–6.5 cm). The most common histologic appearance was that of a ‘classic’ EMCa (7/15). Other variant morphologies included EMCa arising from pleomorphic adenoma (3/15), high grade EMCa (2/15), oncocytic EMCa (1/15), apocrine EMCa (1/15), and EMCa with myoepithelial overgrowth (1/15). All cases were node negative. Four of fifteen (26.7 %) cases demonstrated local recurrence, while 2/15 (13.3 %) demonstrated distant metastases. Overall, four patients had died, but only one patient specifically died of disease, within a month of diagnosis. Mean follow up on patients without recurrence or death from disease was 4.6 years.
Table 1.
Clinicopathologic parameters and mutation status
| Case | Gender | Age | Case type | Site | Size (cm) | Histologic subtype | Nodal status | HRAS mutation status | HRAS mutation type | TNM stage | Recurrence | Distant metastasis | Site of metastasis | DFS (years) | OS (years) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 61 | Primary | Parotid | 0.8 | Classic | Negative | T1Nx | N | N | 6.7 | 6.7 | DOC | |||
| 2 | F | 55 | Primary | Submandibular | 2.8 | High grade | Negative | T3NxM1 | N | Y | Pelvic | 0.1 | 0.1 | DOD | ||
| 3 | F | 66 | Primary | Parotid | 2 | Classic | Positive | p.Q61R | T1N0 | 9.5 | DOC | |||||
| 4 | M | 90 | Primary | Parotid | 2.4 | Classic | Negative | Positive | p.Q61R | T2N0 | N | N | 5.4 | 5.4 | DOC | |
| 5 | F | 62 | Recurrence | Parotid | 6.5 | Classic | Negative | T3Nx | Y | LTF | ||||||
| 6 | F | 71 | Recurrence | Maxillary | Classic | Negative | TxNx | Y | N | 1.0 | 7.3 | Alive | ||||
| 7 | M | 78 | Primary | Parotid | 2.2 | Classic | Negative | Failed | T2N0 | N | N | 7.4 | 7.4 | Alive | ||
| 8 | F | 51 | Primary | Parotid | 4.7 | Ex pleomorphic adenoma | Negative | T3Nx | N | N | 1.1 | 1.1 | Alive | |||
| 9 | F | 82 | Primary | Parotid | 4.3 | Oncocytic | Positive | p.Q61 K | T3Nx | N | N | 8.5 | 8.5 | Alive | ||
| 10 | M | 49 | Primary | Parotid | 3 | Apocrine | Negative | T2Nx | N | N | 6.0 | 6.0 | Alive | |||
| 11 | M | 61 | Recurrence | Lung | 3.5 | High grade | Negative | Negative | T2N0M1 | Y | Y | Brain | 2.7 | 4.6 | Alive | |
| 12 | F | 59 | Primary | Parotid | 1.2 | Ex pleomorphic Adenoma | Negative | T1Nx | N | N | 0.3 | 0.3 | Alive | |||
| 13 | F | 68 | Primary and recurrence | Submandibular | 2.7 | Myoepithelial overgrowth | Negative | Positive | p.Q61R | T2N0 | Y | N | 1.2 | 1.2 | Alive | |
| 14 | F | 58 | Primary | Maxillary | 4.1 | Classic | Negative | Negative | T3Nx | N | N | 1.2 | 1.2 | Alive | ||
| 15 | F | 55 | Primary | Parotid | 1.5 | Ex pleomorphic adenoma | Negative | Negative | T1Nx | N | N | 0.1 | 0.1 | Alive |
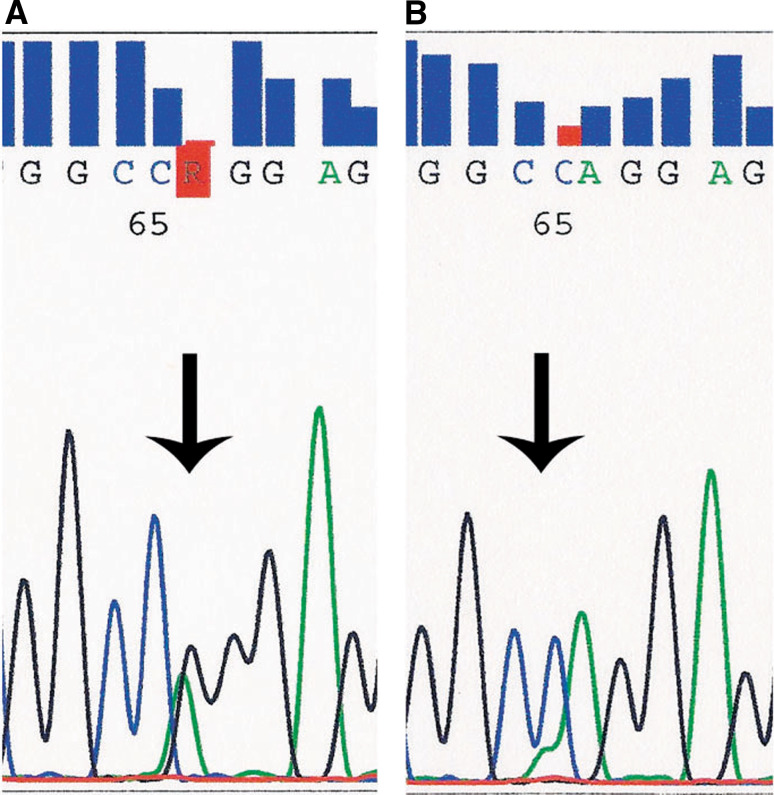
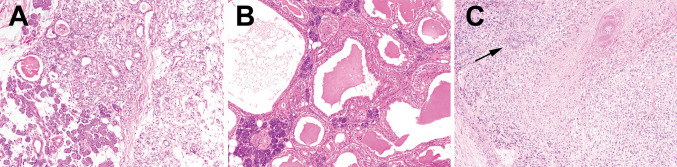
Of the tumors tested, twelve were primary, while four were recurrences. One recurrence was paired to a primary. One case failed, and HRAS exon 3, codon 61—p.Q61R c.182A>G and p.Q61 K, c.183C>A mutations were identified in 4 of 15 successfully tested EMCAs (thus in 4/14 patients) (Fig. 1). Two cases were classic type (Fig. 2a), while the other cases consisted of one oncocytic variant (Fig. 2b), and one tumor with myoepithelial overgrowth (Fig. 2c) the latter of which shows the same mutation in both the primary and recurrence. Of note, the high grade EMCa and EMCa ex pleomorphic adenoma were negative for mutations. Given the small number of cases, there were no significant differences between mutation positive and mutation negative cases in terms of age, gender and outcome.
Fig. 1.
Segments of exon 3 HRAS sequencing electropherogram, forward. a Arrow indicates c.182A>G substitution, resulting in p.Q61R missense heterozygous mutation. b Arrow indicates c.181C>A substitution, resulting in p.Q61 K missense heterozygous mutation
Fig. 2.
Morphologic subtypes of EMCa with HRAS mutations. a This classic EMCa from the parotid (100×) harbored p.Q61R mutation. b This oncocytic EMCa (100×) of parotid on the other hand showed a p.Q61 K mutation. c This submandibular tumor with a p.Q61 K mutation is an aggressive tumor showing myoepithelial overgrowth and necrosis (100×). Arrow indicates the rare compressed ductal component
Discussion
Our findings validate the prior abstract report [4] on the frequency of HRAS mutations in EMCa and provide further clinicopathologic correlates. Almost 1/3 of our cases are positive for the mutation thus adding to the list of salivary gland tumors reported to show HRAS mutations. However, the significance of this finding is not particularly clear in the context of EMCa given the small number of cases. As the initial findings by Cros et al. [4] were reported in abstract form, detailed features of the EMCa in that study are not available for comment. Aside from the small size of this study, another limitation is the restriction of our evaluation of exon 3 codon 61 of HRAS. This limit was based on testing availability, but it is plausible that other EMCa may harbor HRAS mutations in exon 2, codons 12 or 13.
In some salivary carcinoma types, HRAS mutations may be linked to tumor progression. For instance, the HRAS experience in mucoepidermoid carcinomas (MEC) [14, 15] suggests that, in MEC, the frequency of HRAS mutations was greatest in high grade tumors, implying a role in progression. Parenthetically, this draws parallels to the transgenic HRAS p.G12 V murine models showing ‘aden squamous’ carcinomas. Similarly, Halteren et al. [16] reported 3 cases of adenocarcinomas of the parotid gland, not otherwise specified, with HRAS p.Q61R (of 17 adenocarcinomas and 2 pleomorphic adenomas). Of note, adenocarcinomas with HRAS mutation were moderately-to-poorly differentiated, with regional lymph node metastases and perineural invasion.
However, our findings do not seem to show a link to aggressiveness in EMCa. Though only four cases showed mutation, we have found HRAS mutations in classic, indolent and aggressive variants of EMCa, and have found no correlation with outcome measures. Additionally, both the primary and paired recurrence in one case harbored a mutation, suggesting the mutation to be an early event. Furthermore, one lethal EMCa showed no mutation. It appears that these alterations do not necessarily portend a more aggressive course.
Interestingly, this is somewhat similar to the cumulative findings in pleomorphic adenomas and carcinoma ex pleomorphic adenoma. For instance, while HRAS protein was shown to be overexpressed (admittedly not entirely analogous) in carcinomas arising in pleomorphic adenomas [5, 6], Milasin et al. [3] reported that 35 % (6/17) of pleomorphic adenomas of salivary glands harbored exon 2 codon 12 HRAS mutation suggesting that HRAS mutations or overexpression are not necessarily required for malignant transformation. Of note, we had 3 EMCa ex PA, and none showed transformation.
Thus in summary, we confirm that a subset of EMCa demonstrate HRAS codon 61 mutations. These mutations seem to be dispersed across classic and variant morphologies with no correlation with outcome measures based on this small series, suggesting that it does not necessarily denote an aggressive phenotype.
Acknowledgments
Conflict of interest
None.
References
- 1.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen LL, Discafani CM, Gurnani M, Tyler RD. Histopathology of salivary and mammary gland tumors in transgenic mice expressing a human Ha-Ras oncogene. Cancer Res. 1991;51(14):3762–3767. [PubMed] [Google Scholar]
- 3.Milasin J, Pujic N, Dedovic N, Gavric M, Vranic V, Petrovic V, et al. H-Ras gene mutations in salivary gland pleomorphic adenomas. Int J Oral Maxillofac Surg. 1993;22(6):359–361. doi: 10.1016/S0901-5027(05)80668-X. [DOI] [PubMed] [Google Scholar]
- 4.Cros JF, Blons H, Sbidian E, Tartour E, Hans S, Brasnu D, et al. Expression and prognostic significance of directed therapy targets and mutational analysis of the EGFR pathway in malignant salivary gland tumors. J Clin Oncol. 2012;30(Suppl):5554. [Google Scholar]
- 5.Deguchi H, Hamano H, Hayashi Y. c-myc, ras p21 and p53 expression in pleomorphic adenoma and its malignant form of the human salivary glands. Acta Pathol Jpn. 1993;43(7–8):413–422. doi: 10.1111/j.1440-1827.1993.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Dong F, Wang X. Quantitative studies of oncogene ras P21 and P53 gene protein expression in the benign and malignant pleomorphic adenomas of salivary gland]. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi. Chin J Stomatol. 1997;32(4):208–211. [PubMed] [Google Scholar]
- 7.Augello C, Gregorio V, Bazan V, Cammareri P, Agnese V, Cascio S, et al. TP53 and p16INK4A, but not H-KI-Ras, are involved in tumorigenesis and progression of pleomorphic adenomas. J Cell Physiol. 2006;207(3):654–659. doi: 10.1002/jcp.20601. [DOI] [PubMed] [Google Scholar]
- 8.Donath K, Seifert G, Schmitz R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts. Epithelial–myoepithelial carcinoma of the intercalated ducts. Virchows Arch A Pathol Pathol Anat. 1972;356(1):16–31. doi: 10.1007/BF00543554. [DOI] [PubMed] [Google Scholar]
- 9.Seethala RR, Richmond JA, Hoschar AP, Barnes EL. New variants of epithelial–myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med. 2009;133(6):950–959. doi: 10.5858/133.6.950. [DOI] [PubMed] [Google Scholar]
- 10.Seethala RR, Barnes EL, Hunt JL. Epithelial–myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aero digestive tract. Am J Surg Pathol. 2007;31(1):44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca I, Soares J. Epithelial–myoepithelial carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours: Pathology and Genetics Head and Neck Tumours. Lyon: IARCPress; 2005. pp. 225–226. [Google Scholar]
- 12.Roy P, Bullock MJ, Perez-Ordonez B, Dardick I, Weinreb I. Epithelial–myoepithelial carcinoma with high grade transformation. Am J Surg Pathol. 2010;34(9):1258–1265. doi: 10.1097/PAS.0b013e3181e366d2. [DOI] [PubMed] [Google Scholar]
- 13.Prince ME, Ubell ML, Castro J, Ogawa H, Ogawa T, Narayan A, et al. Tissue-preserving approach to extracting DNA from paraffin-embedded specimens using tissue microarray technology. Head Neck. 2007;29(5):465–471. doi: 10.1002/hed.20547. [DOI] [PubMed] [Google Scholar]
- 14.Yoo J, Robinson RA. Ras gene mutations in salivary gland tumors. Arch Pathol Lab Med. 2000;124(6):836–839. doi: 10.5858/2000-124-0836-RGMISG. [DOI] [PubMed] [Google Scholar]
- 15.Yoo J, Robinson RA. H-Ras gene mutations in salivary gland mucoepidermoid carcinomas. Cancer. 2000;88(3):518–523. doi: 10.1002/(SICI)1097-0142(20000201)88:3<518::AID-CNCR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.van Halteren HK, Top B, Mooi WJ, Balm AJ, Rodenhuis S. Association of H-ras mutations with adenocarcinomas of the parotid gland. Int J Cancer. 1994;57(3):362–364. doi: 10.1002/ijc.2910570312. [DOI] [PubMed] [Google Scholar]