Abstract
Extraskeletal Ewing’s sarcoma (EES), or primitive neuroectodermal tumor, is an uncommon neoplasm with low incidence in the head and neck. Occurrences in the larynx are even more exceptional with only two previous reported cases of EES arising from the larynx. We report the case of a 45-year-old woman with a laryngeal Ewing’s sarcoma treated with chemotherapy with radiotherapy to follow. Here we describe the histology, molecular diagnosis and treatment of this unusual tumor.
Keywords: Ewing’s sarcoma, pNET, Extraskeletal, Head and neck, Larynx
Introduction
The Ewing’s sarcoma family of tumors (EFT) includes classic Ewing’s sarcoma of bone, extraskeletal Ewing’s sarcoma (EES) and malignant peripheral primitive neuroectodermal tumor (pNET) of bone and soft tissue [1, 2]. Although all are relatively rare, Ewing’s sarcoma of bone is the second most common bone tumor in children and adolescents. This tumor has a predilection for the long bones or pelvis but has been reported in the bones of the head and neck in about 4 % of cases [3]. EES is even more uncommon than its osseous counterpart and tends to occur in slightly older populations and presents with less pain. The most common sites for EES are the trunk, extremities and retroperitoneum with occurrences in the head and neck in about 18 % of cases [4–6]. When primary to the head and neck region, EES is usually identified in the nasal or oral cavities, sinuses or soft tissues of the neck [2, 4]. Primary development from the larynx is even more unusual and as per the authors’ knowledge only two cases of laryngeal EES have been reported in the literature, one in a nine-month-old male and a second in a 74-year-old male [7, 8]. In this article we report a case of laryngeal EES in a 45-year-old woman. This is the first report of an EES in a female in this very unusual location.
Case Report
A 45-year-old female with a past medical history significant for stage II mammary carcinoma, treated 3 years earlier with modified radical mastectomy, adjuvant cyclophosphamide/daunorubicin, paclitaxel and tamoxifen presented to her oncologist with concerns of a rapidly growing lump in the right side of her neck associated with hoarseness. She had not received radiation therapy as part of her treatment for mammary carcinoma. Ultrasound of the neck showed a 2.9 cm mass in or adjacent to the upper pole of the right thyroid lobe and a separate 1.9 cm extra thyroidal mass anterior to the primary mass. No enlarged lymph nodes were palpated. Fine needle aspiration of the larger mass was performed and was concerning for lymphoma; however definitive diagnosis could not be made.
A positron emission tomography (PET) scan was subsequently obtained that showed fluorodeoxiglucose-avidity (FDG) within the two neck masses without any other lesions identified (Fig. 1). It was still unclear whether the largest mass was intra- or extra thyroidal. The patient was consequently scheduled for an open biopsy. The largest mass was noted to be separate from but infiltrating the thyroid gland, friable and necrotic. Frozen sections of a representative section of the tumor were again suspicious for lymphoma. Flow cytometry was negative. Permanent sections showed a diffuse mass of small round blue cells with scant cytoplasm and inconspicuous nucleoli (Fig. 2). Extensive necrosis was present. Immunohistochemistry demonstrated diffuse membranous staining of tumor cells for CD99 (Fig. 3) and strong nuclear staining for FLI-1. Focal positivity of tumor cells for vimentin, synaptophysin and cytokeratin AE1/AE3 was present. The tumor cells were negative for CD45 (leukocyte common antigen), CD20, CD3, thyroid, muscle, neural, melanoma and vascular markers. Tissue submitted for fluorescence in situ hybridization (FISH) studies showed EWSR1 rearrangement t(11;22) in 95.5 % of cells. The immunohistochemical and molecular panel was diagnostic of ESS. No thyroid tissue was seen in the incisional biopsy.
Fig. 1.
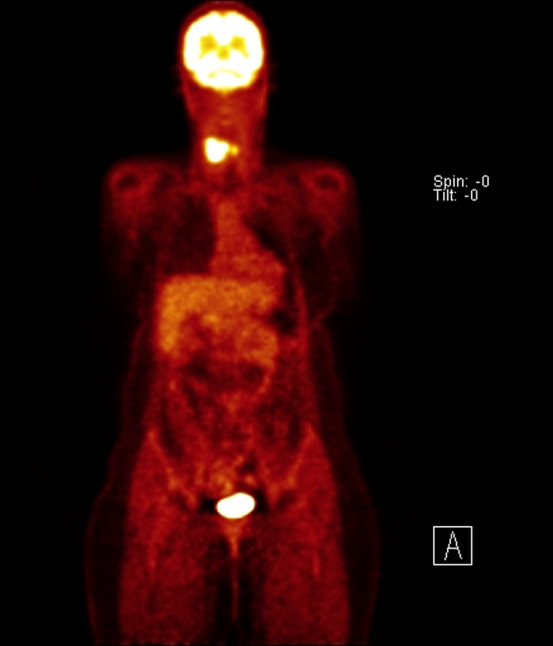
PET scan identified strong FDG-avidity within two neck masses
Fig. 2.
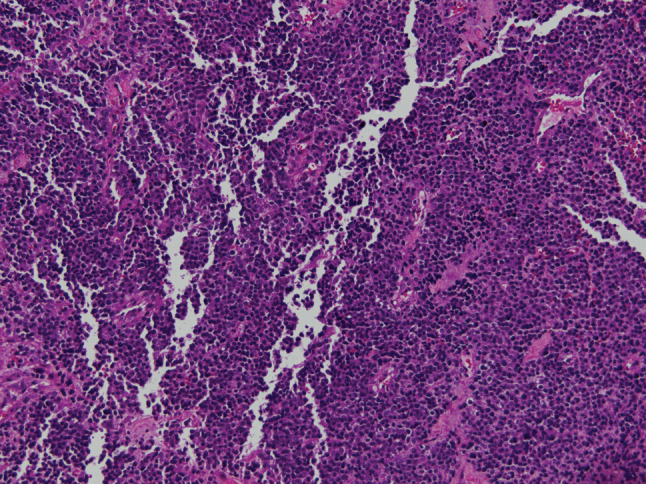
The mass consisted of diffuse sheets of small round blue cells with scant cytoplasm and inconspicuous nucleoli (hematoxylin-eosin, original magnification ×200)
Fig. 3.
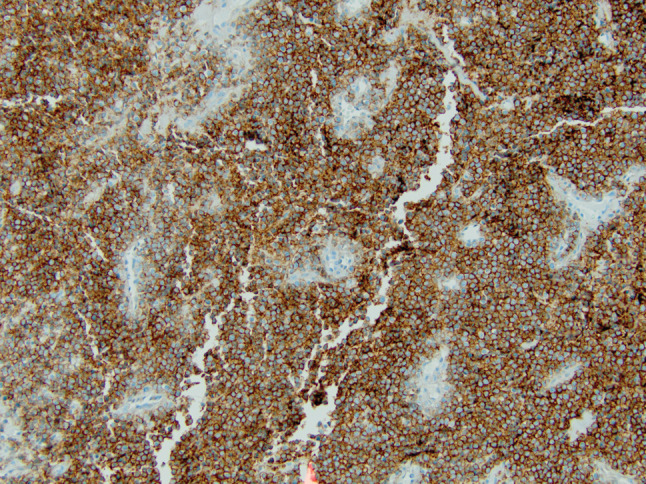
Immunohistochemistry for CD99 demonstrated diffuse, membranous staining of tumor cells. The tumor cells also showed nuclear staining for FLI-1 (not shown)
As the mass continued to grow rapidly, care was transferred, and a direct laryngoscopy with biopsies was performed. The mass was seen emanating from the larynx with fixation of the right cord without airway compromise. Histology was similar to the prior incisional biopsy. The patient began chemotherapy with ifosfamide, etoposide (IE) and vincristine, doxorubicin, cyclophosphamide (VDC) × 4 cycles each. There has been complete response to therapy with no evidence of recurrence on imaging at the time of this report. Radiation therapy to the area will follow.
Discussion
The EFT includes pNET of soft tissue and bone (occasionally referred to as peripheral neuroepithelioma) in addition to classic Ewing’s sarcoma of bone and EES [1, 2]. The tumors in the EFT are thought to represent a continuum of disease according to the degree of neuroectodermal differentiation with ES representing the least differentiated to pNET being most differentiated [1].
Histologically ESS consists of a monotonous population of small, round, hyperchromatic cells with scant cytoplasm. The malignant cells appear undifferentiated and frequently lack any clear morphologic features. Necrosis, Homer-Wright rosettes and a background with neurofibrillary material are variably present [1, 2, 7, 8]. EES identified in the head and neck must be differentiated from a variety of tumors including lymphoma, rhabdomyosarcoma, poorly differentiated salivary gland tumors, olfactory neuroblastoma, undifferentiated nasopharyngeal carcinoma and melanoma [2, 4].
Immunohistochemical staining of ESE is usually distinct and shows diffuse CD99 (MIC2) [1], FLI-1 [9] and vimentin positivity [1]. Variable positive staining for cytokeratin, in about 20 % of cases [2, 10], and neural markers including neuron specific enolase (NSE) [1, 2], synaptophysin [1] and S-100 [2] can be seen. Typically tumors with more differentiation towards pNET exhibit increased staining for neural markers [10]. Perhaps most helpful is negative staining for CD45 (leukocyte common antigen), myogenin/MyoD1 [11], epithelial membrane antigen (EMA) [12, 13], chromogranin [1, 14] and HMB-45 which help to preclude important histologic mimickers from the differential including lymphoma, rhabdomyosarcoma, salivary gland tumors and nasopharyngeal carcinoma, olfactory neuroblastoma and melanoma respectively.
Confirmation of histologic findings can be achieved through molecular and cytogenetic studies. The characteristic chromosomal translocation t(11;22)(q24;12) is found in about 90 % of ES, ESS and pNET [1]. This translocation results in the fusion of the EWSR1 and FLI-1 genes, the end result being a hybrid EWSR1-FLI-1 protein that functions as an aberrant transcriptional regulator [15]. The second most common chromosomal translocation seen in ES is t(22;21)(q22;q12) resulting in EWS-ERG fusion protein in about 10 % of cases [16]. Other specific translocations for ES include EWS-ETV1 t(7;22), EWS-ETV4 t(17;22) and EWS-FEV t(2;22) [16]. FISH or real time-polymerase chain reaction (RT-PCR) can both be used in the molecular diagnosis of ES, ESS and pNET [15, 16].
Aggressive treatment of ESS is suggested and generally includes a combination of multi-agent chemotherapy followed by either wide local excision with post-operative radiotherapy or radiotherapy alone [10, 17]. In this case, although feasible, surgery would have caused significant deficits in vocalization and swallowing function. It was decided that similar or better results could be attained by chemotherapy and radiotherapy alone. Chemotherapy usually includes cyclic combinations, incorporating vincristine, doxorubicin, cyclophosphamide, etoposide, ifosfamide and occasionally actinomycin D [10]. ESS is relatively sensitive to radiotherapy and despite the peculiar location in this case, conventional regimens for ES can be administered in the head and neck. However despite optimal therapy, the overall prognosis is still quite poor with approximately 50 % disease-free 3-year survival [17]. Poor prognostic factors include tumor volume and presence of metastasis at time of diagnosis [2]. ESS arising in the head and neck carries an intermediate prognosis compared to tumors arising in paraspinal and scapular areas (favorable outcomes) and tumors arising in the abdomen (poor outcomes) [4, 18]. Because of the paucity of laryngeal ESS cases, it is unknown if their long-term behavior is different from other head and neck ESS.
Our case highlights an unusual case of ESS arising in the larynx. Only two other primary laryngeal ESS have been described previously in the literature, one in a nine-month-old male and a second in a 74-year-old male [7, 8]. Symptoms may included neck swelling and hoarseness, as seen in our case, or respiratory distress with airway compromise, as described in the 74-year-old male [8]. Our case is the first report of laryngeal ESS in a female or a middle-aged person. The findings in the case suggest that ESS can occur across a broad age range and likely has no sex predilection. We conclude that ESS should be included in the differential of small, round, blue cell tumors of the larynx. Rapid diagnosis should allow for optimal therapy to proceed.
References
- 1.Nikitakis NG, Salama AR, O’Malley BW, Jr, et al. Malignant peripheral primitive neuroectodermal tumor-peripheral neuroepithelioma of the head and neck: a clinicopathologic study of five cases and review of the literature. Head Neck. 2003;25:488–498. doi: 10.1002/hed.10260. [DOI] [PubMed] [Google Scholar]
- 2.Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: incidence, diagnosis, and management. Ann Otol Rhinol Laryngol. 2004;113:533–543. doi: 10.1177/000348940411300705. [DOI] [PubMed] [Google Scholar]
- 3.Siegal GP, Oliver WR, Reinus WR, et al. Primary Ewing’s sarcoma involving the bones of the of the head and neck. Cancer. 1987;60:2829–2840. doi: 10.1002/1097-0142(19871201)60:11<2829::AID-CNCR2820601139>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Hafezi S, Seethala RR, Stelow EB, et al. Ewing’s family of tumors of the sinonasal tract and maxillary bone. Head Neck Pathol. 2011;5:8–16. doi: 10.1007/s12105-010-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada H, Newton WA, Jr, Soule EH, et al. Pathologic features of extraosseous Ewing’s sarcoma: a report from the Intergroup Rhabdomyosarcoma Study. Hum Pathol. 1988;19:442–453. doi: 10.1016/S0046-8177(88)80495-7. [DOI] [PubMed] [Google Scholar]
- 6.Stuart-Harris R, Wills EJ, Phillips J, et al. Extraskeletal Ewing’s sarcoma: a clinical, morphological and ultra-structural analysis of five cases with a review of the literature. Eur J Cancer Clin Oncol. 1986;22:393–400. doi: 10.1016/0277-5379(86)90104-5. [DOI] [PubMed] [Google Scholar]
- 7.Jones JE, McGill T. Peripheral primitive neuroectodermal tumors of the head and neck. Arch Otolaryngol Head Neck Surg. 1995;121:1392–1395. doi: 10.1001/archotol.1995.01890120050009. [DOI] [PubMed] [Google Scholar]
- 8.Yang YS, Hong KH. Extraskeletal Ewing’s sarcoma of the larynx. J Laryngol Otol. 2004;118:62–64. doi: 10.1258/002221504322731682. [DOI] [PubMed] [Google Scholar]
- 9.Folpe AL, Hill CE, Parham, et al. Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000;24:1657–1662. doi: 10.1097/00000478-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein M, Kovar H, Paulussen M, et al. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–519. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Perlman E, Harris CA, et al. Myogenin is a specific marker for rhabdomyosarcoma: an immunohistochemical study in paraffin-embedded tissues. Mod Pathol. 2000;13:988–993. doi: 10.1038/modpathol.3880179. [DOI] [PubMed] [Google Scholar]
- 12.Nagao T, Sato E, Inoue R, et al. Immunohistochemical analysis of salivary gland tumors: application for surgical pathology practice. Acta Histochem Cytochem. 2012;45:269–282. doi: 10.1267/ahc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusterson BA, Mitchell DP, Warburton MJ, et al. Epithelial markers in the diagnosis of nasopharyngeal carcinoma: an immunocytochemical study. J Clin Pathol. 1983;36:628–631. doi: 10.1136/jcp.36.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argani P, Perez-Ordoñez B, Xiao H, et al. Olfactory neuroblastoma is not related to the Ewing family of tumors: absence of EWS/FLI1 gene fusion and MIC2 expression. Am J Surg Pathol. 1998;22:391–398. doi: 10.1097/00000478-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nagao K, Ito H, Yoshida H, et al. Chromosomal rearrangement t(11;22) in extraskeletal Ewing’s sarcoma and primitive neuroectodermal tumour analysed by fluorescence in situ hybridization using paraffin-embedded tissue. J Pathol. 1997;181:62–66. doi: 10.1002/(SICI)1096-9896(199701)181:1<62::AID-PATH687>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Lewis TB, Coffin CM, Bernard PS. Differentiating Ewing’s sarcoma from other round blue cell tumors using a RT-PCR translocation panel on formalin-fixed paraffin-embedded tissues. Mod Pathol. 2007;20:397–404. doi: 10.1038/modpathol.3800755. [DOI] [PubMed] [Google Scholar]
- 17.Jürgens H, Bier V, Harms D, et al. Malignant peripheral neuroectodermal tumors. A retrospective analysis of 42 patients. Cancer. 1988;61:349–357. doi: 10.1002/1097-0142(19880115)61:2<349::AID-CNCR2820610226>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Kimber C, Michalski A, Spitz L, et al. Primitive neuroectodermal tumours: anatomic location, extent of surgery, and outcome. J Pediatr Surg. 1998;33:39–41. doi: 10.1016/S0022-3468(98)90357-8. [DOI] [PubMed] [Google Scholar]


