Abstract
An instance of isolated unilateral temporalis muscle hypertrophy (reactive masticatory muscle hypertrophy with fiber type 1 predominance) confirmed by muscle biopsy with histochemical fiber typing and image analysis in a 62 year-old man is reported. The patient presented with bruxism and a painful swelling of the temple. Absence of asymmetry or other abnormalities of the craniofacial skeleton was confirmed by magnetic resonance imaging and cephalometric analyses. The patient achieved symptomatic improvement only after undergoing botulinum toxin injections. Muscle biopsy is key in the diagnosis of reactive masticatory muscle hypertrophy and its distinction from masticatory muscle myopathy (hypertrophic branchial myopathy) and other non-reactive causes of painful asymmetric temporalis muscle enlargement.
Keywords: Pain, temple; Temporalis muscle hypertrophy; Reactive masticatory muscle hypertrophy; Fiber type 1 predominance; Image analysis; Otsu thresholding
Introduction
The management of a painful unilateral temporalis muscle enlargement mandates a rigorous diagnostic evaluation of potential underlying causes, which may include inflammatory, neoplastic, vascular malformative, and myopathic processes, as well as reactive hypertrophy of the temporalis muscle [1–5]. Isolated unilateral temporalis muscle hypertrophy [4, 5], also known as reactive masticatory muscle hypertrophy (RMMH) [3], is a rare cause of a painful soft tissue swelling of the temple. Τo our knowledge, this is the second report of RMMH with histochemical documentation of muscle hypertrophy with fiber type 1 predominance. The objective of this case report is to enhance awareness of this obscure nosologic entity among practicing pathologists and clinicians across disciplines dealing with the diagnosis and management of head and neck lesions.
Case Report
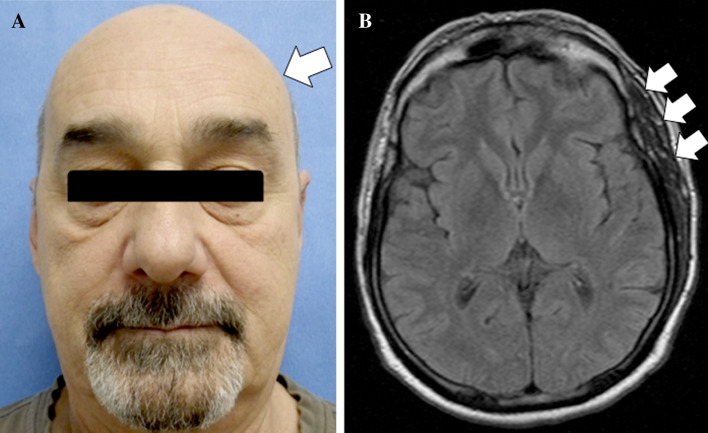
The case of a 62 year-old-male with an eight-year history of painful left temporal swelling of insidious onset is reported. The pain was constant, not paroxysmal, moderately severe and not aggravated by jaw movement. It was unresponsive to analgesics, anticonvulsants and muscle relaxants. Until recently, the patient carried the diagnosis of trigeminal neuralgia that was refractory to treatment. Past medical history includes bruxism, sufficiently severe to warrant a mouth guard, and an ill-defined old left cervical (neck) injury sustained during his military service. Laboratory investigations had been consistently unremarkable (normal range erythrocyte sedimentation rate, C-reactive protein and serum creatine kinase). On physical examination, aside from the conspicuous left temporal hypertrophy which was painful on palpation, there was a slight asymmetry of the angle of the jaw and pterygoids (Fig. 1a). No significant facial changes were discernible involving the soft tissues above the maxilla and the nasolabial fold. Moreover, there was no evidence of perioral, periorbital, and/or neck asymmetry. There was also an absence of alopecia, hyperpigmentation, or vitiligo in the overlying skin (Fig. 1a). Neither retinal nor optic nerve findings were identified.
Fig. 1.
a Patient’s face frontal view demonstrating swelling in the left temporalis muscle region (arrow). b Axial fluid attenuated inversion recovery (FLAIR) MR image showing enlarged left temporalis muscle with normal muscle signal intensity (arrow)
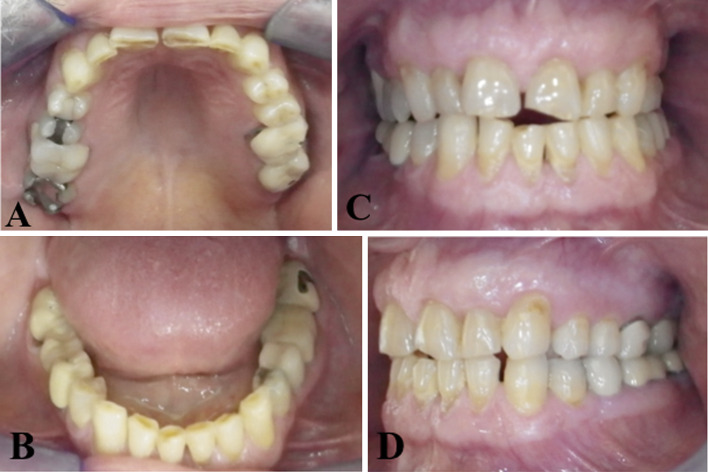
The oral examination was consistent with a history of bruxism. Fractured and abraded porcelain fused to metal crowns with a flat plane occlusion and wear facets, especially in the molar region, were observed (Figs. 2, 3). There was no evidence of ipsilateral lingual, palatal, or gingival atrophy (Fig. 3).
Fig. 2.
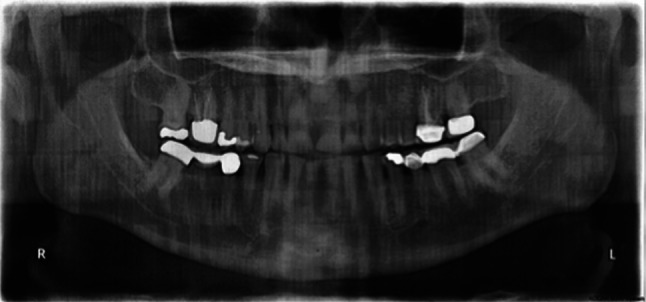
Panoramic film reveals no gross dental or skeletal pathology of the maxilla or mandible. Temporo-mandibular joint positioned well in fossa. Patient’s occlusal plane especially flat on left secondary to bruxism
Fig. 3.
a, b Maxillary and mandibular arches exhibiting occlusal wear of the porcelain fused to metal crowns, exposing the metal of the crowns on the left posterior teeth. c, d Patient’s class III occlusion demonstrates classic features of bruxism including flattening of the occlusal plane, chipped dentition and generalized attrition, all of which are more severe on the left side secondary to excessive occlusal forces
MRI and Cephalometric Analysis
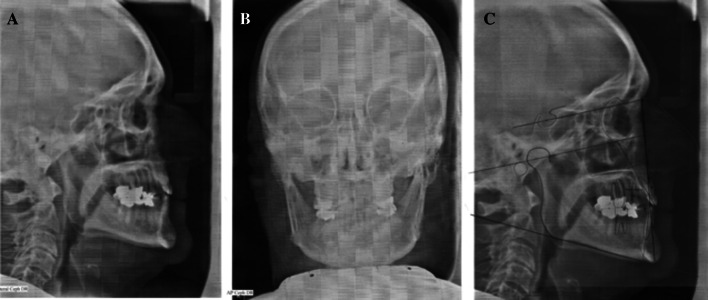
Magnetic resonance imaging (MRI) revealed an enlarged left temporalis muscle with normal muscle signal intensity (Fig. 1b). No contrast enhancement was present (not shown). The CNS was essentially unremarkable. No cranial bone abnormalities were identified. Evaluation of the craniofacial skeleton with MRI, skull A-P plain film and lateral cephalometric analyses demonstrated no evidence of facial skeletal asymmetry (Fig. 4a–c).
Fig. 4.
a Lateral cephalometric film. b Skull A-P plain film demonstrating symmetry of mandibular ramus. c Cephalometric analysis reveals no gross skeletal discrepancies
Muscle Biopsy
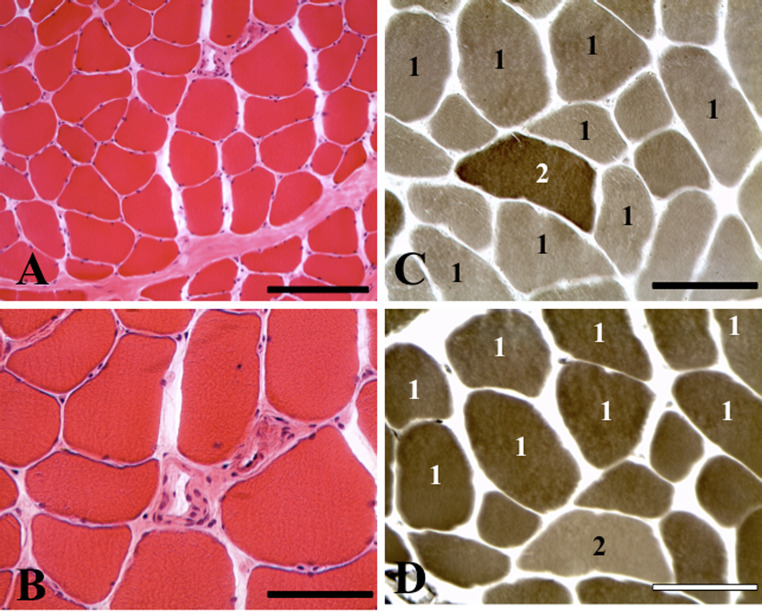
A muscle biopsy of the left temporalis muscle revealed no evidence of an overt inflammatory process, either in the context of focal myositis or fasciitis. A moderately increased number of hypertrophic myofibers was detected, with an absence of myopathic changes or perifascicular atrophy (Fig. 5a, b). There was no evidence of tumor or cavernous hemangioma.
Fig. 5.
Images of representative fields from cryosections of the temporalis muscle biopsy. a, b Hematoxylin and eosin staining shows a moderately increased number of hypertrophic fibers without myopathic or inflammatory changes. c, d Myofibrillar ATPase histochemical preparations preincubated at pH 9.4 (a) and pH 4.3 (b), depicting fiber type 1 predominance. Scale bars (a) 200 μm; (b, c, d) 100 μm
On histochemical preparations stained for myofibrillar ATPase, there was predominance of hypertrophic type 1 fibers (Fig. 5c, d). Rare, randomly dispersed, mildly to moderately atrophic, predominantly type 1 and to a lesser degree, type 2 fibers were also detected. Immunohistochemical preparations for inflammatory markers using antibodies to CD3, CD4, CD8, CD20, and CD68 were essentially negative.
Image Analysis
The Aperio Scanscope XT was used to generate high-resolution (0.25 μm/pixel) whole-slide images of skeletal muscle tissue. Images were converted to hue-saturation-value space, and Otsu thresholding [6] was applied to the saturation channel to segment individual fibers. Image co-registration was performed manually; fibers that were not clearly present in both images, or failed to properly segment in either image, were discarded from analysis. In total, 426 fibers were analyzed. The diameter of each fiber was estimated as the length of the minor axis of a best-fit ellipse.
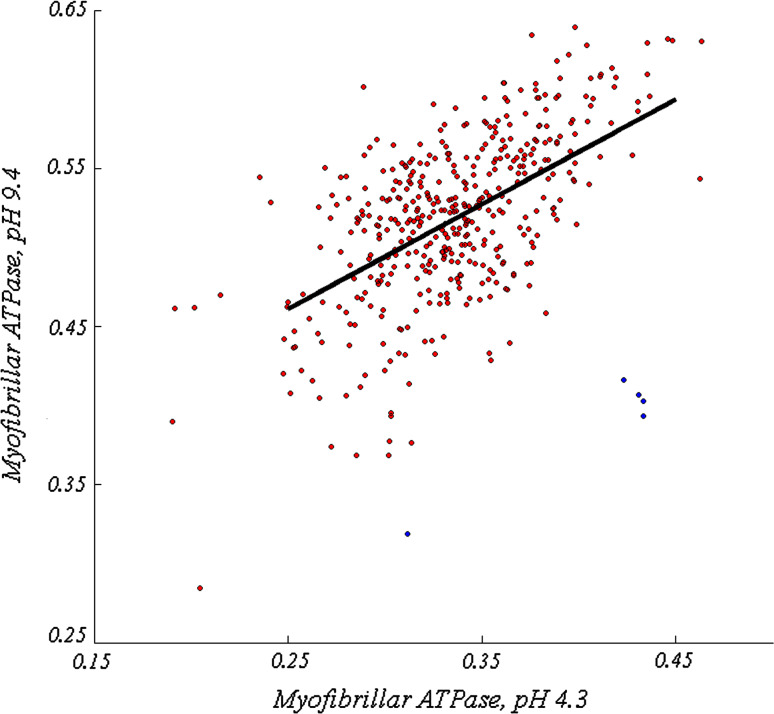
Fiber type was determined by comparing mean pixel intensity within corresponding fibers from myofibrillar ATPase preparations preincubated at pH 4.3 and pH 9.4 respectively. Generally, staining intensity was positively correlated between the two images (Fig. 6, R2 = 0.294). Type 2 fibers typically appear lighter in the pH 4.3 preparation (Fig. 5d) and darker in the pH 9.4 preparation (Fig. 5c). Therefore, type 2 fibers were defined as those that exhibited a substantial departure from the observed correlated behavior. The fibers (Fig. 6, blue) that were greater than 3.5 standard deviations away from the model generated by linear regression (Fig. 6, dark line) were selected. Five putative type 2 fibers were identified using this criterion, and are indicated in Fig. 7 by red arrows.
Fig. 6.
Scatter plot of mean pixel intensities for each of the 426 selected fibers after preincubation at pH 4.3 (abscissa) and pH 9.4 (ordinate). Red points correspond to putative type 1 fibers and blue points correspond to putative type 2 fibers. The linear regression line of all data points is shown in black. Putative type 2 fibers were selected on the basis of their distance from the regression line, consistent with the observation that type 2 fibers are considerably lighter at pH 4.3 and darker at pH 9.4
Fig. 7.
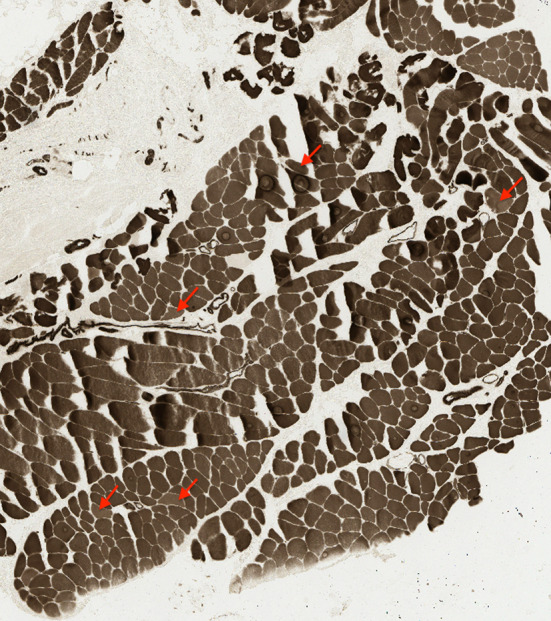
Image of myofibrillar ATPase preincubated at pH 4.3. Red arrows correspond to the points classified as putative type 2 fibers in Fig. 6
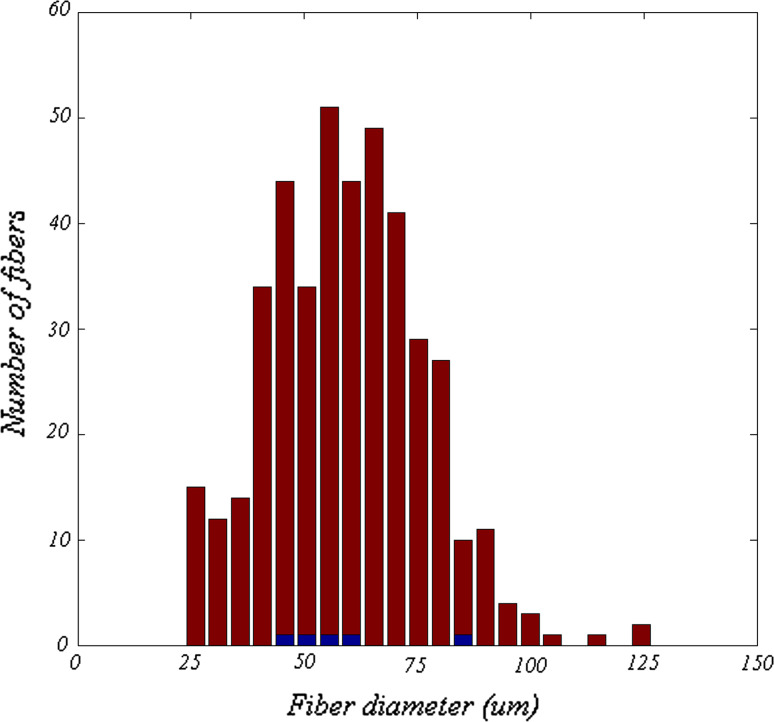
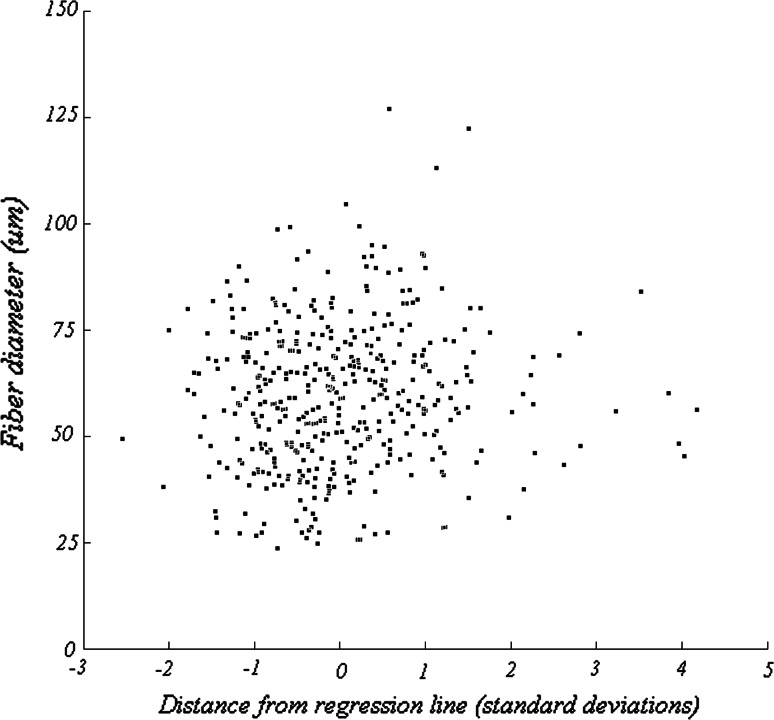
The diameters of putative type 1 and type 2 fibers are shown in Fig. 8. The median diameter of type 1 fibers was larger than that of type 2 fibers (59.0 vs. 56.3 μm), but this difference was not statistically significant (Wilcoxon sign rank test, p = 0.85), although perhaps due only to the small number of type 2 fibers. To demonstrate that this was not a result of our categorization criteria, Fig. 9 shows that a correlation between fiber diameter and distance from the regression line did not exist. These results imply that fiber diameter was independent of differential staining intensity.
Fig. 8.
Distribution of the diameters of the 426 selected fibers. Red bars represent diameters of putative type 1 fibers and blue bars represent diameters of putative type 2 fibers
Fig. 9.
Fiber diameter as a function of the distance from the regression line, shown in Fig. 6, expressed in units of standard deviation. This distance metric is used to assess the relative staining intensities produced as a result of preincubation at pH 4.3 and pH 9.4. Positive values correspond to fibers that are more lightly stained in pH 4.3 preparations and more darkly stained in pH 9.4 preparations. The most positive values likely correspond to type 2 fibers. These data reveal that fiber diameter is independent of the relative staining intensities observed in the population
Discussion
In the absence of myopathic [3, 7], vascular malformative [8], inflammatory [9, 10], or neoplastic [11–13] processes, the diagnosis of RMMH was made on the basis of histologic detection of myofiber hypertrophy and type 1 fiber predominance [3]. Predominance of’ hypertrophied type 1 fibers has also been described in masticatory muscle myopathy (hypertrophic branchial myopathy) [3, 14, 15]. However, no overt myopathic changes were detected in the case presented. The patient was treated with botulinum toxin (Botox) injections and experienced symptomatic relief for the first time.
The term reactive masticatory muscle hypertrophy (RMMH) was coined by Harriman in 1996 who reported that endurance exercise has a greater effect on the muscles of mastication, as compared to appendicular muscles, whereby the former react structurally by hypertrophy and progressive type 1 fiber predominance [3]. In the present study muscle fiber hypertrophy and fiber type 1 predominance were corroborated by image analysis. The median diameter of type 1 fibers was found to be larger than that of type 2 fibers (59.0 vs. 56.3 μm) although this difference was not statistically significant. However, this may be attributed, in part, to the small number of type 2 fibers. Our results confirm and augment previous observations by Harriman [3] with regard to the alteration in fiber type distribution with total dominance of type 1 fibers. At variance with the findings by Harriman, according to which the mean fiber diameter of type 1 fibers in their index case of RMMH was 39 μm [3], the median diameter of type 1 fibers in the present case was 59.0 μm. Interestingly, the latter is similar to the median diameter of 57 μm in type 1 fibers, described by Lambert and colleagues in masticatory muscle myopathy (hypertrophic branchial myopathy) [14].
Masticatory muscles in humans have a somewhat different fiber type composition as compared to trunk or limb muscles [16, 17]. In the latter, myofibrillar ATPase histochemical reaction yields two main fiber types of almost equal size: Type 1 (slow-twitch/oxidative) and type 2 (fast-twitch/glycolytic). Masticatory muscles have small type 2 fibers and a fiber type of intermediate staining intensity between type 1 and type 2 fibers (type 1 M fibers). Given that biopsies of temporalis muscle are exceptionally rare, normal reference values regarding fiber diameter and fiber type distribution in age matched controls are based mainly on postmortem data reported in a small number of publications [3, 16, 17]. Using myofibrillar ATPase histochemistry, Harriman reported preservation of the checkerboard pattern of fiber type composition, similar with that encountered in trunk and limb muscles, in a single temporalis muscle biopsy-derived normal control case from a 31-old-female [3]. In this case, the distribution of type 1 and type 2 fibers was 38 and 36 % respectively; however, transitional 2C fibers at 26 % were significantly increased [3]. In the same study, group mean values of percentage of fiber type composition in control autopsy specimens of central temporalis muscle were reported to be 48 % for type 1 fibers and 52 % for type 2 fibers [3]. In two of seven postmortem specimens of temporalis muscle, fibers exhibited a normal mosaic pattern while in the remaining five specimens they contained variously atrophic type 2 fibers intermingling with normal appearing type 1 fibers [3]. This pattern was also confirmed by myofibrillar ATPase histochemistry in autopsy-derived temporalis muscle samples from 5 women and 5 men aged 71–91 years [17]. Myofiber size of normal human temporalis muscle varies with age [3]. Group mean values of fiber type size in control autopsy specimens of central temporalis muscle are 34 μm for type 1 fibers and 24 μm for type 2 fibers [3]. However, reference values for normal fiber type composition and fiber size remain unclear as they are based on a very small number of postmortem control tissues from disparate ages [3]. Also, these may be confounded by regional heterogeneity of fiber type distribution in human temporalis muscle [16]. Hence, in the most anterior portion of the muscle, type 1 fibers constitute 46 % of all fiber types as compared to the intermediate and the posterior portions where they comprise 57 and 24 % respectively [16].
Collectively, fiber size and fiber type composition in the muscle biopsy of the present case represent a major departure from previously reported findings in normal control temporalis muscle.
Clinicopathologic Correlations
RMMH is typically isolated and unilateral, although unilateral temporalis muscle hypertrophy with contralateral masseter muscle hypertrophy has also been reported [18]. Potential causes, local factors, and triggers in this regard include bruxism, malocclusion, prognathism, dental caries, loss of teeth, temporomandibular joint disease, and the bony deformities produced by craniofacial trauma [3]. In the absence of identifiable triggers, RMMH may be ascribed to psychogenic factors [3].
Distinction of RMMH from non-reactive MMH is mandatory and requires performance of muscle biopsy. The differential diagnosis of unilateral temporalis muscle hypertrophy is summarized in Table 1.
Table 1.
Differential diagnosis of isolated unilateral temporalis muscle swelling
| I. Reactive masticatory muscle hypertrophy (MMH) (progressive fiber type 1 predominance) [3] |
| II. Non-reactive MMH (modified after Harriman [3]) |
| A. Genetic (or possibly genetic) MMH (fiber type 2 hypertrophy) [3] |
| B. Congenital MMH [3] |
| C. Masticatory muscle myopathy (hypertrophic branchial myopathy) [3, 7, 14, 15] |
| D. Vascular malformation—intramuscular cavernous hemangioma [3, 8] |
| E. Inflammatory processes |
| Focal myositis [1] |
| Eosinophilic fasciitis [9] |
| Ascending (necrotizing) fasciitis secondary to odontogenic infections [10] |
| Idiopathic inflammatory myopathy [3] |
| Infective causes—submasseteric abscess (severe pain and trismus) [3] |
| F. Neoplastic processes |
| Benign |
| Lipoma [4, 5] |
| Malignant |
| Intramuscular lymphoma [2] |
| Leukemic infiltration/granulocytic sarcoma [11] |
| Primary soft tissue sarcomas (liposarcoma and rhabdomyosarcoma) [4, 5] |
| Metastatic tumors (carcinoma [12], melanoma [13], and sarcoma) |
The etiology of the left RMMH in the present case is conjectural. The patient’s history of bruxism, confirmed on oral examination by a significant unilateral tendency of abraded occlusion, is considered to be the proximate cause and most likely exacerbating factor. The question of right-sided hemifacial atrophy (involving the side of the face opposite to the left temporal RMMH) in the context of Parry-Romberg syndrome was entertained but effectively, excluded on clinical grounds and on the basis of normal MR imaging, dental analysis, panoramic radiographs, A-P plain film and lateral cephalometric analyses. Hence, the explanation that the RMMH on the left developed on a compensatory basis owing to right-sided hemifacial atrophy is not tenable. Moreover, the facial pain in Parry-Romberg syndrome occurs on the side of hemifacial atrophy while in the case under consideration the pain is localized solely in the vicinity of the temporalis muscle swelling.
It is noteworthy that the only symptomatic pain relief in this patient was achieved after treatment with injections of botulinum A toxin, as previously reported in this clinical setting [3, 5, 19]. One of the drawbacks of this treatment is the need for periodic injections [5]. The reported problem of tolerance to botulinum A toxin, owing to antibody production, may be circumvented by switching to botulinum type F, keeping in mind that the duration of action of botulinum F is shorter than botulinum A [19]. Another treatment option is muscle reduction surgery, which carries potential side effects such as trismus, fibrosis, and decreased range of motion [5]. A symptomatic response to acetominaphen has been reported [20], but analgesic treatment was ineffective in the present case.
This case illustrates an instance of a painful RMMH in a 62 year-old man with bruxism, underscoring the importance of a comprehensive interdisciplinary team approach to diagnosis and treatment planning. The patient attained symptomatic improvement only following botulinum toxin injections. Muscle biopsy is key in the diagnosis of a painful asymmetric temporalis muscle swelling.
Acknowledgments
We thank Dr. Sheldon Winkler, DDS, for his constructive comments.
Conflict of interest
None.
Abbreviations
- A-P
Antero-posterior
- ATP
Adenosine triphosphate
- CNS
Central nervous system
- FLAIR
Fluid attenuated inversion recovery
- MRI
Magnetic resonance imaging
- RMMH
Reactive masticatory muscle hypertrophy
References
- 1.Naumann M, Toyka KV, Goebel HH, et al. Focal myositis of the temporal muscle. Muscle Nerve. 1993;16:1374–1376. doi: 10.1002/mus.880161216. [DOI] [PubMed] [Google Scholar]
- 2.Benson-Mitchell R, Warwick-Brown N, Chappell ME. Non-Hodgkin’s lymphoma presenting as an isolated temporal soft tissue swelling. J Laryngol Otol. 1996;110:161–162. doi: 10.1017/s0022215100133043. [DOI] [PubMed] [Google Scholar]
- 3.Harriman DG. The histochemistry of reactive masticatory muscle hypertrophy. Muscle Nerve. 1996;19:1447–1456. doi: 10.1002/(SICI)1097-4598(199611)19:11<1447::AID-MUS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Lowry TR, Helling E. Unilateral temporal muscle hypertrophy: a rare clinical entity. Ear Nose Throat J. 2003;82:198–199. [PubMed] [Google Scholar]
- 5.Wang BH, Moon SJ, Wang H, et al. Isolated unilateral temporalis muscle hypertrophy. J Neurosurg Pediatr. 2013;11:451–453. doi: 10.3171/2013.1.PEDS12534. [DOI] [PubMed] [Google Scholar]
- 6.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cyber. 1979;9:62–66. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- 7.Mancall EL, Patel AN, Hirschhorn AM. Hypertrophic branchial myopathy: idiopathic enlargement of the masticatory muscles as a neglected myopathic disorder. Neurology. 1974;24:1166–1170. doi: 10.1212/WNL.24.12.1166. [DOI] [PubMed] [Google Scholar]
- 8.Bucci T, De Giulio F, Romano A, et al. Cavernous haemangioma of the temporalis muscle: case report and review of the literature. Acta Otorhinolaryngol Ital. 2008;28:83–86. [PMC free article] [PubMed] [Google Scholar]
- 9.McGuigan C, O’Riordan S, Farrell M, et al. Case report: recurrent temporalis muscle swelling and headache. Neurology. 2003;60:724–725. doi: 10.1212/01.WNL.0000048664.01003.E2. [DOI] [PubMed] [Google Scholar]
- 10.Park E, Hirsch EM, Steinberg JP, et al. Ascending necrotizing fasciitis of the face following odontogenic infection. J Craniofac Surg. 2012;23:e211–e214. doi: 10.1097/SCS.0b013e31824de3e7. [DOI] [PubMed] [Google Scholar]
- 11.Bassichis B, McClay J, Wiatrak B. Chloroma of the masseteric muscle. Int J Pediatr Otorhinolaryngol. 2000;53:57–61. doi: 10.1016/S0165-5876(00)00301-3. [DOI] [PubMed] [Google Scholar]
- 12.Uygur S, Aral M, Kaya B, et al. Giant temporalis muscle metastasis of esophageal carcinoma. J Craniofac Surg. 2011;22:736–737. doi: 10.1097/SCS.0b013e318208bae9. [DOI] [PubMed] [Google Scholar]
- 13.Oittinen HA, O’Shaughnessy M, Cullinane AB, et al. Malignant melanoma of the ciliary body presenting as extraocular metastasis in the temporalis muscle. J Clin Pathol. 2007;60:834–835. doi: 10.1136/jcp.2005.033613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert CD, Young JR. Hypertrophy of the branchial muscles. A case with unusual features. J Neurol Neurosurg Psychiatry. 1976;39:810–816. doi: 10.1136/jnnp.39.8.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagawa Y, Hashimoto K, Kuriyama M. Hypertrophic branchial myopathy with uniform predominance of type 1 fibres. Case report. Scand J Plast Reconstr Surg Hand Surg. 2000;34:391–396. doi: 10.1080/028443100750059200. [DOI] [PubMed] [Google Scholar]
- 16.Korfage JA, Van Eijden TM. Regional differences in fibre type composition in the human temporalis muscle. J Anat. 1999;194:355–362. doi: 10.1046/j.1469-7580.1999.19430355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkeby S, Garbarsch C. Histochemical studies of the masseter, the temporal and small zygomaticomandibular, and the temporomandibular masticatory muscles from aged male and female humans. Fiber types and myosin isoforms. Cranio. 2001;19:174–182. doi: 10.1080/08869634.2001.11746167. [DOI] [PubMed] [Google Scholar]
- 18.Ozturk E, Mutlu H, Sonmez G, et al. Unilateral temporalis muscle hypertrophy with contralateral masseteric hypertrophy. Dentomaxillofac Radiol. 2007;36:296–297. doi: 10.1259/dmfr/12003200. [DOI] [PubMed] [Google Scholar]
- 19.Rokadiya S, Malden NJ. Variable presentation of temporalis hypertrophy—a case report with literature review. Br Dent J. 2006;201:153–155. doi: 10.1038/sj.bdj.4813850. [DOI] [PubMed] [Google Scholar]
- 20.Vordenbäumen S, Groiss SJ, Dihné M. Isolated unilateral temporal muscle hypertrophy: a rare cause of hemicranial headache. Headache. 2009;49:779–782. doi: 10.1111/j.1526-4610.2009.01393.x. [DOI] [PubMed] [Google Scholar]