Abstract
Salivary duct carcinoma is a highly aggressive salivary gland malignancy that may be misdiagnosed as high-grade mucoepidermoid carcinoma. We utilized tissue microarrays with 78 examples of mucoepidermoid carcinoma and 47 salivary duct carcinomas to evaluate the utility of an immunohistochemical panel consisting of androgen receptor, Her2/neu, p63, and cytokeratin 5/6 in distinguishing these entities. Among all cases in the cohorts, androgen receptor was highly specific for salivary duct carcinoma, while cytokeratin 5/6 and p63 were specific for mucoepidermoid carcinoma. While the rate of unequivocal Her2/neu overexpression among the salivary duct carcinomas was low (8.9 %), discrimination of salivary duct carcinoma was enhanced when this marker was used in combination with androgen receptor due to profound sensitivity. The immunohistochemical panel was particularly efficacious at distinguishing the problematic subset of high-grade mucoepidermoid carcinomas from salivary duct carcinoma. Utilization of this set of immunohistochemical markers allows reliable differentiation of salivary duct and mucoepidermoid carcinoma, a distinction with important prognostic and therapeutic implications.
Keywords: Salivary duct carcinoma, Mucoepidermoid carcinoma, Immunohistochemistry
Introduction
The World Health Organization defines salivary duct carcinoma (SDC) as an aggressive adenocarcinoma whose histopathologic features mimic those of breast ductal carcinoma [1]. Most patients are diagnosed in the sixth or seventh decade of life, and many but not all series demonstrate a significant male predominance [2–22]. The tumor occurs almost exclusively in major salivary glands with the parotid gland being the most common site and some cases arising in a preexisting pleomorphic adenoma (PA) [2–22]. Analogous to breast carcinoma is the frequent overexpression of the growth factor receptor Her2/neu (c-erbB-2); studies examining this marker in SDC have demonstrated expression rates up to 100 % [10, 12–25]. SDC is also notable for its frequent expression of androgen receptor (AR), with most series demonstrating positivity in at least two-thirds of cases [6, 7, 9, 12, 13, 21, 25]. Outcomes are typically poor among patients with SDC; local recurrence, regional metastasis, and distant metastasis are frequent, and many patients die of the disease, although exceptions do rarely occur [2–20, 26]. Given these poor outcomes, therapy for SDC is typically aggressive, consisting of complete surgical excision with lymph node dissection and postoperative radiation with or without adjuvant chemotherapy [21]. Additionally, there is early evidence that trastuzumab and anti-androgen therapy may be efficacious in managing either primary and/or metastatic disease [19, 27–31]. More recent studies have demonstrated abnormalities in the phosphoinositide 3-kinase pathway in some SDCs, creating another parallel with breast carcinoma and additional targets for therapy [32].
Mucoepidermoid carcinoma (MEC) is the most common malignant primary salivary gland tumor and is composed of a mixture of mucous, intermediate, and epidermoid (squamoid) cells [33]. In contrast to SDC, MEC is observed over a wider age range, has a female or less striking male predominance, and occurs in major and minor salivary glands with approximately equal frequency [34–38]. MEC can be graded on the basis of five microscopic features [34], and outcome is significantly correlated with tumor grade [34–38]. The chromosomal translocation t(11;19), resulting in a fusion protein that appears to disrupt the Notch signaling pathway, is observed in a subset of MEC that includes all grades of tumor [39–41].
Recently, Chenevert et al. [41] highlighted the frequency with which high-grade MEC is misdiagnosed, and we have noticed in our own consultation practice that SDC is often mistakenly categorized as high-grade MEC. Given the emerging evidence that biologic therapies such as trastuzumab and anti-androgen agents may have a role in management of SDC, we sought to evaluate the efficacy of a panel of widely available, well-characterized immunohistochemical stains consisting of androgen receptor, Her2/neu, p63, cytokeratin 5/6, and cytokeratin k903 in differentiating SDC from MEC. Specifically, we hypothesized that SDC would show a significantly higher rate of expression of androgen receptor and Her2/neu, while the epidermoid features of MEC would correlate with a greater degree of expression of p63, cytokeratin 5/6, and cytokeratin k903.
Methods
A search of the surgical pathology and teaching files of the Department of Pathology at the University of Michigan identified 47 cases of SDC, including three cases of noninvasive carcinoma arising within a PA and six cases originally misdiagnosed as high-grade MEC, for which slides and/or formalin-fixed, paraffin-embedded blocks were available. The University of Michigan Institutional Review Board provided a waiver of informed consent to obtain these samples. After pathological review to confirm the diagnosis of SDC, a tissue microarray (TMA) was constructed from representative areas of tumor, either primary or metastatic, using the methodology of Nocito, et al. [42]. A similarly constructed, preexisting TMA consisting of 78 cases of confirmed MEC was also utilized in this study. Each case was represented by either two 1-mm diameter cores (SDC TMA) or three 0.7-mm diameter cores (MEC TMA). Criteria for inclusion of cases in the respective TMAs were as follows. A tumor was classified as SDC when it exhibited an infiltrating component characterized by irregular tubules, cribriform nests, and/or single cells. Cytologically, the malignant cells had apocrine features, including abundant pink cytoplasm, marked nuclear pleomorphism, and vesicular chromatin with prominent nucleoli. The presence of an intraductal component was not required for inclusion within the SDC TMA, but when present intraductal growth patterns included cribriform architecture and prominent comedonecrosis. Tumors without these defining features, which were better classified as adenocarcinoma not otherwise specified were excluded, as was carcinoma metastatic to the salivary glands on the basis of patient history. Lastly, all neoplasms included in the SDC array were high-grade; specifically, low-grade cribriform cystadenocarcinoma (so-called low-grade SDC) was excluded. Inclusion in the MEC cohort required the presence of a mixture of mucus, intermediate, and epidermoid (squamoid) cells as well as the lack of the typical intraductal and infiltrating growth patterns as described for SDC. Each of the MECs was graded using standard criteria as a low-, intermediate-, or high-grade tumor [34]; high-grade MECs were characterized by the presence of predominantly solid growth, rare mucous cells, significant cytologic atypia with frequent mitoses, necrosis, and/or perineural invasion. The number of low-, intermediate-, and high-grade MECs included in study were 49, 22, and seven, respectively.
Immunohistochemistry was performed on sections of the TMAs using antibodies directed against androgen receptor (AR), Her2/neu, cytokeratin (CK) 5/6, high molecular weight cytokeratin k903 (34βE12), and p63. Immunohistochemical staining was performed on a DAKO Autostainer (DAKO, Carpinteria, CA) using DAKO LSAB+ and diaminobenzadine (DAB) as the chromogen with the exception of AR and Her2/neu, which used a polymerized detection system (Envision+, DAKO). Serial sections of de-paraffinized TMA sections were labeled with p63 (mouse monoclonal antibody, clone 4A4, 1:400, Thermo Fischer Scientific, Kalamazoo, MI, MS1081), CK 5/6 (mouse monoclonal antibody, clone D5/16 B4, 1:100, Millipore, Billerica, MA, MAB1620), CK k903 (mouse monoclonal antibody, clone 34βE12, 1:50, DAKO, M0630), AR (mouse monoclonal antibody, clone AR441, 1:50, DAKO, M3562) or Her2/neu (rabbit polyclonal antibody, 1:100, DAKO, A0485). Microwave citric acid epitope retrieval was used for all antibodies with the exception of Her2/neu. Appropriate negative (no primary antibody) and positive controls were stained in parallel with each set of tumors studied.
The results of immunohistochemical staining were independently scored by two of the authors (RTB and JBM). For AR, p3, CK 5/6, and CK k903, scores were assigned based on percentage of stained tumor cells, while Her2/neu results were evaluated with an established system used in diagnosis of breast carcinoma [43] (Table 1). In those examples of SDC that arose from a preexisting PA, immunohistochemical staining was evaluated only in the carcinomatous component. For statistical computations, cases were classified as negative (score 0 staining) or positive (score 1+ or more) for AR, p63, CK 5/6, and CK k903 and as negative (score ≤2+) or positive (score 3+) for Her2/neu. In cases in which there was disagreement among the two reviewers that impacted categorization of a case as having positive or negative staining, the case was reviewed jointly until consensus was achieved. For a subset of cases, one or more immunostains was performed on a whole section of tumor and scored as outlined above if its cores were missing and/or lacked neoplastic cells on the corresponding TMA slide.
Table 1.
Criteria for scoring Immunohistochemical results
| Percentage of positive tumor cells | Score |
|---|---|
| Androgen receptor, p63, cytokeratin 5/6, cytokeratin k903 | |
| 0 | 0 |
| 1–25 % | 1+ |
| 26–50 % | 2+ |
| 51–75 % | 3+ |
| 76–100 % | 4+ |
| Staining pattern | Score |
|---|---|
| Her2/neu | |
| None | 0 |
| Weak, incomplete membrane staining | 1+ |
| Weak, circumferential membranous and/or positivity in <30 % of tumor cells | 2+ |
| Intense, circumferential membranous staining in >30 % of tumor cells | 3+ |
Statistical analysis using SPSS software (version 20, IBM Corporation, Armonk, NY) was conducted to determine the sensitivity, specificity, positive predictive value, and negative predictive value of the immunohistochemical staining results with the morphologic classification of a case as an SDC or MEC considered the gold standard diagnosis. Positive predictive values and negative predictive values were calculated even though the overall prevalence of these tumors is low to determine the likelihood of the test being positive when considering a differential diagnosis of SDC and MEC.
Results
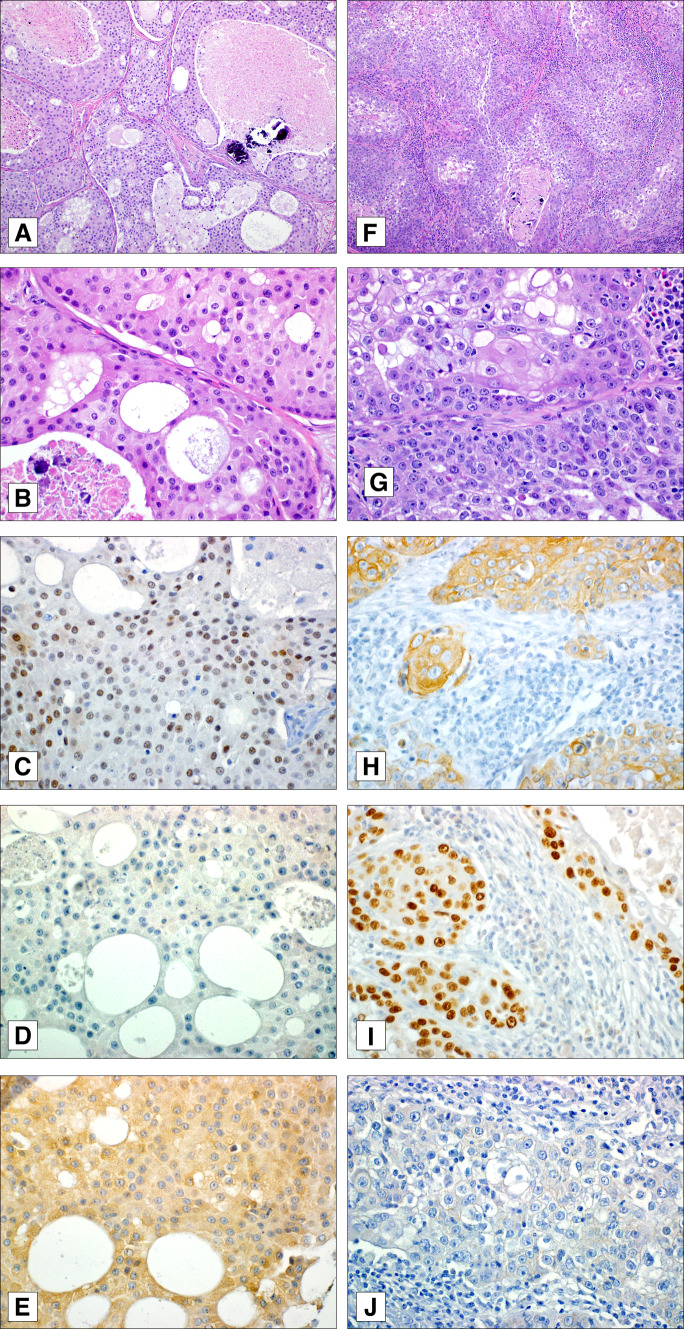
Figure 1 illustrates examples of diagnostically challenging SDC and high-grade MEC and representative results of immunostaining. Table 2 summarizes demographic and basic clinicopathologic features of the patients whose tumors were included in the MEC and SDC TMAs. The proportion of men was greater in the SDC group compared with the MEC group, and the mean and median age was younger in the MEC group compared with the SDC group. Both groups had a preponderance of parotid malignancies compared to other sites.
Fig. 1.
Example of salivary duct carcinoma exhibiting prominent lobular growth with comedonecrosis, microcalcifications, cystic spaces, and large eosinophilic cells with vesicular nuclei (a, b). This case was diffusely, albeit weakly, positive for androgen receptor (c) and lacked cytokeratin 5/6 (d) and nuclear p63 (e) staining. Her2/neu was not overexpressed in this tumor (not shown). A high-grade mucoepidermoid carcinoma with predominantly solid growth with areas of necrosis (f), conspicuous mitoses, vesicular nuclei, and only rare mucous cells (g). This neoplasm was diffusely positive for both cytokeratin 5/6 (h) and p63 (i) and negative for Her2/neu (j) and androgen receptor (not shown)
Table 2.
Demographic and clinicopathologic features of mucoepidermoid carcinoma and salivary duct carcinoma cohorts
| Mucoepidermoid carcinoma (MEC) | Salivary duct carincoma (SDC) | |
|---|---|---|
| Number of patients | 78 | 47 |
| Men (%) | 41 (52.6 %) | 32 (68.1 %) |
| Women (%) | 37 (47.4 %) | 15 (31.9 %) |
| Mean/median age (years) | 49.7/50.7 | 63.3/64.8 |
| Sites | ||
| Parotid gland | 30 | 35 |
| Submandibular gland | 4 | 4 |
| Sublingual gland | 2 | 1 |
| Palate | 24 | 1 |
| Other intraorala | 10 | 0 |
| Parapharyngeal | 1 | 3 |
| Maxilla | 1 | 1 |
| Lacrimal gland | 1 | 2 |
| Lung | 5 | 0 |
| Grade | ||
| Low | 49 | N/A |
| Intermediate | 22 | N/A |
| High | 7 | N/A |
aBase of tongue (4), buccal mucosa (3), mandible (2), retromolar trigone (1)
Cytokeratin k903 did not demonstrate discriminatory power between MEC and SDC, tending to exhibit diffuse staining in both types of tumor, and is thus not discussed further (data not shown). The distribution of immunohistochemical staining for the four remaining immunostains under study is shown in Table 3. Note that the total number of evaluable tumors varied for each stain due to cores that were missing and/or consisted only of non-neoplastic cells on the respective TMA slides. Tables 4 and 5 present the classification of tumors as positive (score ≥1+) or negative for each of the four immunostains when considered individually. The sensitivity, specificity, positive predictive value, and negative predictive value for AR to detect SDC was 73.3, 100, 100, and 83.0 %, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value for Her2/neu to detect SDC was 8.9, 100, 100, and 63.4 %, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value for p63 to detect MEC was 95.4, 87.2, 91.2, and 93.2 %, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value for CK 5/6 to detect MEC was 93.8, 63.8, 77.9, and 88.2 %, respectively. Table 6 shows the classification of tumors as having positive or negative staining results using the combination of stains AR and Her2/neu and p63 and CK 5/6. The results of immunostaining for the high-grade MECs and those SDCs originally misdiagnosed as MEC are displayed in Tables 7 and 8, respectively.
Table 3.
Distribution of Immunohistochemical results
| Marker | Immunohistochemical Score | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | ||
| AR | ||||||
| SDC | 12 (26.7 %) | 8 (17.8 %) | 3 (6.7 %) | 6 (13.3 %) | 16 (35.6 %) | 45 |
| MEC | 59 (100 %) | 0 | 0 | 0 | 0 | 59 |
| Low | 40 (100 %) | 0 | 0 | 0 | 0 | 40 |
| Int. | 14 (100 %) | 0 | 0 | 0 | 0 | 14 |
| High | 5 (100 %) | 0 | 0 | 0 | 0 | 5 |
| Her2/neu | ||||||
| SDC | 29 (64.4 %) | 6 (13.3 %) | 6 (13.3 %) | 4 (8.9 %) | N/A | 45 |
| MEC | 45 (63.4 %) | 26 (36.6 %) | 0 | 0 | N/A | 71 |
| Low | 30 (65.2 %) | 16 (34.8) | 0 | 0 | N/A | 46 |
| Int. | 9 (50 %) | 9 (50 %) | 0 | 0 | N/A | 18 |
| High | 6 (85.7 %) | 1 (14.3 %) | 0 | 0 | N/A | 7 |
| p63 | ||||||
| SDC | 41 (87.2 %) | 5 (10.6 %) | 1 (2.2) | 0 | 0 | 47 |
| MEC | 3 (4.6 %) | 5 (7.7 %) | 10 (15.4 %) | 18 (27.7 %) | 29 (44.6 %) | 65 |
| Low | 3 (7.0 %) | 4 (9.3 %) | 7 (16.3 %) | 14 (32.6 %) | 15 (34.9 %) | 43 |
| Int. | 0 | 1 (6.3 %) | 2 (12.5 %) | 3 (18.8 %) | 10 (62.5 %) | 16 |
| High | 0 | 0 | 1 (16.7 %) | 1 (16.7 %) | 4 (66.7 %) | 6 |
| CK 5/6 | ||||||
| SDC | 30 (63.8 %) | 14 (29.8 %) | 3 (6.4 %) | 0 | 0 | 47 |
| MEC | 4 (6.3 %) | 8 (12.5 %) | 16 (25.0 %) | 14 (21.9 %) | 22 (34.4 %) | 64 |
| Low | 3 (7.3 %) | 6 (14.6 %) | 13 (31.7 %) | 8 (19.5 %) | 11 (26.8 %) | 41 |
| Int. | 0 | 2 (12.5 %) | 2 (12.5 %) | 5 (31.3 %) | 7 (43.8 %) | 16 |
| High | 1 (14.3 %) | 0 | 1 (14.3 %) | 1 (14.3 %) | 4 (57.1 %) | 7 |
AR androgen receptor, CK 5/6 cytokeratin 5/6, SDC salivary duct carcinoma, MEC mucoepidermoid carcinoma, Low low-grade, Int. intermediate-grade, High high-grade
Table 4.
Classification of tumors as positive or negative with AR and Her2/neu
| SDC | MEC | |
|---|---|---|
| AR+ | 33 | 0 |
| AR− | 12 | 59 |
| Her2/neu+ | 4 | 0 |
| Her2neu− | 41 | 71 |
AR androgen receptor, SDC salivary duct carcinoma, MEC mucoepidermoid carcinoma
Table 5.
Classification of tumors as positive or negative with p63 and CK 5/6
| SDC | MEC | |
|---|---|---|
| p63+ | 6 | 62 |
| p63− | 41 | 3 |
| CK 5/6+ | 17 | 60 |
| CK 5/6− | 30 | 4 |
CK 5/6 cytokeratin 5/6, SDC salivary duct carcinoma, MEC mucoepidermoid carcinoma
Table 6.
Classification of tumors as positive or negative with immunostain combinations
| SDC | MEC | |
|---|---|---|
| AR or Her2/neu+ | 35 | 0 |
| AR and Her2/neu− | 10 | 55 |
| p63 or CK 5/6+ | 19 | 63 |
| p63 and CK 5/6− | 28 | 2 |
AR androgen receptor, CK 5/6 cytokeratin 5/6, SDC salivary duct carcinoma, MEC mucoepidermoid carcinoma
Table 7.
Immunohistochemical results for high-grade mucoepidermoid carcinomas
| Patient | AR | Her2/neu | p63 | CK 5/6 |
|---|---|---|---|---|
| 1 | 0 | 0 | 4+ | 0 |
| 2 | 0 | 0 | 3+ | 2+ |
| 3 | 0 | 0 | 4+ | 4+ |
| 4 | 0 | 1+ | 4+ | 4+ |
| 5 | 0 | 0 | 3+ | 4+ |
| 6 | 0 | 0 | 4+ | 3+ |
| 7 | 0 | 0 | 2+ | 4+ |
AR androgen receptor, CK 5/6 cytokeratin 5/6
Table 8.
Immunohistochemical results for salivary duct carcinomas originally diagnosed as mucoepidermoid carcinoma
| Patient | AR | Her2/neu | p63 | CK 5/6 |
|---|---|---|---|---|
| 1 | 3+ | 2+ | 0 | 0 |
| 2 | 4+ | 0 | 0 | 0 |
| 3 | 0 | 3+ | 0 | 0 |
| 4 | 0 | 3+ | 0 | 0 |
| 5 | 0 | 3+ | 0 | 1+ |
| 6 | 0 | 2+ | 1+ | 0 |
AR androgen receptor, CK 5/6 cytokeratin 5/6
Discussion
The cohorts of patients with MEC and SDC included in this study were similar to those in previous reports [2–22, 31–35]. Specifically, the patients diagnosed with SDC were older, showed a stronger male predominance, and had a higher percentage of tumors that arose in major salivary glands. Among the seven SDC patients whose tumor arose outside a major salivary gland, five occurred in conjunction with a PA (two cases of noninvasive disease within a parapharyngeal PA and three cases of carcinoma ex PA in the parapharyngeal space, hard palate, and maxilla).
A previous study demonstrated the frequency with which other entities are mistakenly diagnosed as MEC [38]. Indeed, our SDC cohort included six patients whose tumors were originally diagnosed as MEC. A variety of factors contribute to the difficulty in distinguishing SDC and MEC on morphologic grounds. Firstly, high-grade MECs are designated as such due to the presence of histologic features commonly seen in SDC: predominance of solid over cystic growth, neural invasion, necrosis, and a relatively brisk mitotic rate [31]. Adding to confusion in this differential diagnosis is the occurrence of extracellular mucin and vacuolated cells, some containing mucin, in SDC [8, 9, 16, 25, 38]. A mucin-rich variant of SDC has also been described, in which areas consisting of lakes of mucin with nests and individual cells are admixed with a more SDC typical component, the latter also containing vacuolated cells [13]. Finally, SDC may have a squamoid appearance, and frank focal keratinization has been observed in SDC [4, 5].
The immunohistochemical panel evaluated in this study was able to distinguish SDC and MEC with good sensitivity and specificity. While AR is not perfectly sensitive for SDC, being negative in over a quarter of our cases, it is exquisitely specific in that none of the MECs exhibited any staining with this marker (Table 3). Similarly, diffuse staining (score 3+ or 4+) with either p63 or CK 5/6 is highly specific for MEC as this level of expression was not observed for either marker in any of the SDCs. It is interesting to note that there was variation in the level of expression of both p63 and CK 5/6 according to tumor grade for MEC. In accordance with their more prominent epidermoid component, a greater proportion of intermediate- and high-grade MECs had 3+ or 4+ expression of these markers than did low-grade neoplasms (Table 3). As discussed below, furthermore, the stronger tendency of intermediate- and high-grade MECs to express p63 and CK 5/6 endows the immunohistochemical panel with utility in separating those cases most likely to mimic SDC. Her2/neu offered poor discriminatory power, largely because the majority of both the SDCs and MECs lacked overexpression of this protein. Nonetheless, overexpression (3+ staining) and equivocal results with this marker (2+) were seen only in SDC. Interestingly, the detection of MEC lost power when combining p63 and CK 5/6 due to the decreased specificity of CK 5/6 when compared to p63. This finding may suggest that p63 alone would be a better marker for MEC than CK 5/6 or the combination.
The immunohistochemical panel assessed here appears to offer particular utility in those situations most likely to create diagnostic difficulty, i.e. in separating SDC from high-grade MEC. Specifically, for the seven high-grade tumors included in our MEC cohort, none exhibited expression of AR or overexpression of Her2/neu, while all seven tumors were diffusely positive (score 3+ or 4+) for p63 and/or CK 5/6 (Table 7). Thus the sensitivity and specificity of p63 and CK 5/6 to identify high-grade MEC is 100 %. Similarly, for the subset of SDCs that were incorrectly misdiagnosed as MEC, none of these tumors had more than scattered positivity with both p63 and CK 5/6, and all demonstrated diffuse AR staining and/or 2+ or 3+ Her2/neu staining (Table 8).
There were 14 tumors in the MEC cohort that had a staining score of ≤2+ with both p63 and CK 5/6. Among these cases, 12 were low-grade neoplasms, and two were of intermediate grade. Thus, those MECs that were not diffusely positive for either p63 or CK 5/6 are of the type unlikely to enter the differential diagnosis with SDC. We also noted that three of the MECs with p63 and CK 5/6 staining scores of ≤2+ were represented on the TMA by tissue obtained from blocks that had been decalcified, which may have contributed to falsely weak staining.
Expression of AR is characteristic of SDC. Among our cases, 45 had evaluable material, of which 33 (73.3 %) exhibited some degree of AR expression. Given the limited amount of tumor evaluated in the TMA and our observation that AR positivity was often focal, this rate of AR expression may be lower than would have been observed in whole tissue sections. Nonetheless, the frequency of AR expression reported here is in accordance with other studies, which have documented rates of 67–100 % [6, 7, 9, 12, 13, 25]. The largest previous study to our knowledge to have examined AR expression in SDC found positivity in 56 of 84 cases (67 %) with positive cases constituting a range of those with focal expression to others with diffuse expression [12].
Studies of Her2/neu overexpression in SDC have varied in methodology, with some studies reporting rates of expression and others utilizing criteria analogous to those used in breast carcinoma to examine the rates of unequivocal overexpression [10, 12–20, 22–25]. As discussed above, few of our SDCs exhibited overexpression of Her2/neu, with only 8.9 % of cases having unequivocal overexpression (score 3+). While this rate is lower than that reported by other studies utilizing similar scoring systems, we considered cases as demonstrating overexpression only in the presence of intense, circumferential staining of at least 30 % of neoplastic cells, as is recommended for breast carcinoma [40]; this criterion is more stringent than the 10 % threshold used in the other studies that used a similar scoring modality [12, 20, 22, 23].
It is noteworthy that a recent study by Di Palma, et al. has highlighted the immunophenotypic heterogeneity of SDC and that SDCs may be classified analogously to breast carcinoma. Specifically, expression of AR, Her2/neu, estrogen receptor, progesterone receptor, and epidermal growth factor receptor (EGFR) may be used to categorize SDCs as of Her2, luminal, luminal androgen, or basal phenotypes, although the current prognostic and/or therapeutic implications of such classification are unknown [44]. Among our cases, there were 12 that lacked expression of AR. Two of these tumors would be classified as Her2 phenotype in the schema of Di Palma, et al. on the basis of unequivocal overexpression of Her2/neu, while a third had equivocal Her2/neu positivity (2+) and may also have proven to be of the Her2 phenotype had evaluation for gene amplification been undertaken. Of the 12 AR-negative SDCs, four had 1+ or 2+ expression of CK 5/6, which would warrant classification as the basal subtype. The remaining five AR-negative SDCs also lacked expression of Her2/neu and CK 5/6 but may also represent basal phenotype tumors (rather than indeterminate) if shown to express EGFR. Our results, therefore, also demonstrate variability of immunophenotype in SDC, including the occurrence of the basal phenotype.
While the immunohistochemical panel we studied appears quite efficacious in the differential diagnosis of SDC and MEC, it should be mentioned that detection of the t(11;19) (MECT1-MAML2 fusion) may be an alternative means of differentiating SDC from MEC. While this translocation appears specific for MEC and has been observed in all tumor grades [36–38], it is not seen in all cases. Furthermore, in situ hybridization assays for the t(11;19) translocation are not widely available. The immunohistochemical panel described in this study, in contrast, consists of common markers offered in most clinical pathology laboratories.
Distinguishing SDC and MEC on morphologic grounds can be difficult but is crucial for planning therapy and evaluating patient prognosis. Our results demonstrate that an immunohistochemical panel consisting of AR, Her2/neu, p63, and CK 5/6 can greatly aid in the reliable differentiation of these tumors. These immunohistochemical markers are particularly useful in distinguishing SDC from high-grade MEC.
Acknowledgments
The authors thank Shirley Andrews for her assistance in preparing the manuscript and Mark Deming and Elizabeth Walker for their guidance in presenting the photomicrographs.
References
- 1.Brandwein-Gensler MS, Skálová A, Nagao T. Salivary duct carcinoma. In: Eveson JW, Reichart P, Sidransky D, Barnes L, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 236–237. [Google Scholar]
- 2.Hosal AS, Fan C, Barnes L, Myers EN. Salivary duct carcinoma. Otolaryngol Head Neck. 2003;129(6):720–725. doi: 10.1016/S0194-5998(03)01386-X. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Rao U, Krause J, Contis L, Schwartz A, Scalamogna P. Salivary duct carcinoma: part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78(1):64–73. doi: 10.1016/0030-4220(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 4.Brandwein MS, Jagirdar J, Patil J, Biller H, Kaneko M. Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts): a clinicopathologic and immunohistochemical study of 12 cases. Cancer. 1990;65(10):2307–2314. doi: 10.1002/1097-0142(19900515)65:10<2307::AID-CNCR2820651024>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JE, McKinney BC, Weiland LH, Ferreiro JA, Olsen KD. Salivary duct carcinoma: clinicopathologic and immunohistochemical review of 26 cases. Cancer. 1996;77(2):223–230. doi: 10.1002/(SICI)1097-0142(19960115)77:2<223::AID-CNCR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Fan C, Melhem MF, Hosal AS, Grandis JR, Barnes EL. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor α in salivary duct carcinoma. Arch Otolaryngol. 2001;127(9):1075–1079. doi: 10.1001/archotol.127.9.1075. [DOI] [PubMed] [Google Scholar]
- 7.Fan C, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24(4):579–586. doi: 10.1097/00000478-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Simpson RHW, Clarke TJ, Sarsfield PTL, Babajews AV. Salivary duct adenocarcinoma. Histopathology. 1991;18(3):229–235. doi: 10.1111/j.1365-2559.1991.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 9.Moriki T, Ueta S, Takahashi T, Mitani M, Ichien M. Salivary duct carcinoma: cytologic characteristics and application of androgen receptor immunostaining for diagnosis. Cancer Cytopathol. 2001;93(5):344–350. doi: 10.1002/cncr.9050. [DOI] [PubMed] [Google Scholar]
- 10.Hellquist HB, Karlsson MG, Nilsson C. Salivary duct carcinoma—a highly aggressive salivary gland tumour with overexpression of c-erbB-2. J Pathol. 1994;172(1):35–44. doi: 10.1002/path.1711720108. [DOI] [PubMed] [Google Scholar]
- 11.Delgado R, Vuitch F, Albores-Saavedra J. Salivary duct carcinoma. Cancer. 1993;72(5):1503–1512. doi: 10.1002/1097-0142(19930901)72:5<1503::AID-CNCR2820720503>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Williams MD, Roberts D, Blumenschein GR, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31(11):1645–1652. doi: 10.1097/PAS.0b013e3180caa099. [DOI] [PubMed] [Google Scholar]
- 13.Simpson RHW, Prasad AR, Lewis JE, Skálová A, David L. Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2003;27(8):1070–1079. doi: 10.1097/00000478-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Barnes L, Rao U, Contis L, Krause J, Schwartz A, Scalamogna P. Salivary duct carcinoma: part II. Immunohistochemical evaluation of 13 cases for estrogen and progesterone receptors, cathepsin D, and c-erbB-2 protein. Oral Surg Oral Med Oral Pathol. 1994;78(1):74–80. doi: 10.1016/0030-4220(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 15.Felix A, El-Naggar AK, Press MF, et al. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum Pathol. 1996;27(6):561–566. doi: 10.1016/S0046-8177(96)90162-8. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Barba E, Cortes-Guardiola JA, Minguela-Puras A, Torroba-Caron A, Mendez-Trujillo S, Bermejo-Lopez J. Salivary duct carcinoma: clinicopathological and immunohistochemical studies. J Cranio-Maxillofac Surg. 1997;25(6):328–334. doi: 10.1016/S1010-5182(97)80035-2. [DOI] [PubMed] [Google Scholar]
- 17.Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Löning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103(12):2526–2533. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 18.Etges A, Pinto DS, Jr, Kowalski LP, Soares FA, Araújo VC. Salivary duct carcinoma: immunohistochemical profile of an aggressive salivary gland tumour. J Clin Pathol. 2003;56(12):914–918. doi: 10.1136/jcp.56.12.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 20.Cornolti G, Ungari M, Morassi ML, et al. Amplification and overexpression of HER2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol. 2007;133(10):1031–1036. doi: 10.1001/archotol.133.10.1031. [DOI] [PubMed] [Google Scholar]
- 21.McHugh JB, Visscher DW, Barnes EL. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. 2009;133(11):1763–1774. doi: 10.5858/133.11.1763. [DOI] [PubMed] [Google Scholar]
- 22.Skálová A, Stárek I, Vanecek T, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2003;42(4):348–356. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 23.Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16(8):2266–2274. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugano S, Mukai K, Tsuda H, et al. Immunohistochemical study of c-erbB-2 oncoprotein overexpression in human major salivary gland carcinoma: an indicator of aggressiveness. Laryngoscope. 1992;102(8):923–927. doi: 10.1288/00005537-199208000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Leecy T, Buckland ME, Turner J, Earls P. The use of immunohistochemistry in the diagnosis of primary salivary duct carcinoma: three case reports. Pathology. 2008;40(4):434–437. doi: 10.1080/00313020802040659. [DOI] [PubMed] [Google Scholar]
- 26.Jamal AM, Zhi-Jun S, Xin-Ming C, Yi-Fang Z. Salivary duct carcinoma of the parotid gland: case report and review of the literature. J Oral Maxillofac Surg. 2008;66(8):1708–1713. doi: 10.1016/j.joms.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Prat A, Parera M, Reyes V, et al. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30(5):680–683. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 28.Nashed M, Casasola RJ. Biological therapy of salivary duct carcinoma. J Laryngol Otol. 2009;123(2):250–252. doi: 10.1017/S0022215108002314. [DOI] [PubMed] [Google Scholar]
- 29.Jaspers HCJ, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473–e476. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy J, Krishnamurty DM, Baker JJ, Zhen W, Lydiatt D, Ganti AK. Salivary duct carcinoma responding to trastuzumab-based therapy: case report and review of the literature. Head Neck. 2013 doi: 10.1002/hed.23307. [DOI] [PubMed] [Google Scholar]
- 31.Soper MS, Thompson LDR, Iganej S. Definitive treatment of androgen receptor-positive salivary duct carcinoma with androgen deprivation therapy and external beam radiotherapy. Head Neck. 2013 doi: 10.1002/hed.23383. [DOI] [PubMed] [Google Scholar]
- 32.Griffith CC, Seethala RR, Luvison A, Miller M, Chiosea SI. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37(8):1201–1207. doi: 10.1097/PAS.0b013e3182880d5a. [DOI] [PubMed] [Google Scholar]
- 33.Goode RK, El-Naggar AK. Mucoepidermoid carcinoma. In: Eveson JW, Reichart P, Sidransky D, Barnes L, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 219–220. [Google Scholar]
- 34.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217–1224. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1217::AID-CNCR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Rapidis AD, Givalos N, Gakiopoulou H, et al. Mucoepidermoid carcinoma of the salivary glands: review of the literature and clinicopathological analysis of 18 patients. Oral Oncol. 2007;43(2):130–136. doi: 10.1016/j.oraloncology.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9(7):688–695. doi: 10.1007/BF02574486. [DOI] [PubMed] [Google Scholar]
- 37.Kokemueller H, Brueggemann N, Swennen G, Eckardt A. Mucoepidermoid carcinoma of the salivary glands—clinical review of 42 cases. Oral Oncol. 2005;41(1):3–10. doi: 10.1016/j.oraloncology.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 40.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas—prognostic significance of the MECT1-MAML2 fusion oncogene. Gene Chromosome Cancer. 2006;45(5):470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 41.Chenevert J, Barnes LE, Chiosea SI. Mucoepidermoid carcinoma: a five-decade journey. Virchows Arch. 2011;458(2):133–140. doi: 10.1007/s00428-011-1040-y. [DOI] [PubMed] [Google Scholar]
- 42.Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94(1):1–5. doi: 10.1002/ijc.1385. [DOI] [PubMed] [Google Scholar]
- 43.Hicks DG, Schiffhauer L. Standardized assessment of the HER2 status in breast cancer by immunohistochemisty. Lab Med. 2011;42(8):459–467. doi: 10.1309/LMGZZ58CTS0DBGTW. [DOI] [Google Scholar]
- 44.Di Palma S, Simpson RHW, Marchiò C, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology. 2012;61(4):629–643. doi: 10.1111/j.1365-2559.2012.04252.x. [DOI] [PubMed] [Google Scholar]