Abstract
The sinonasal tract may give rise to a broad range of neoplasms that share a “small round blue cell” tumor (SBRCT) appearance on routine histology, but treatment strategies depend on precise tumor classification. Immunohistochemistry for p63 is often employed in the sinonasal SRBCT differential diagnosis because it is highly sensitive for squamous cell carcinoma (SCC). However, p63 staining may be observed in other tumor types, a potential diagnostic pitfall. P40 is a more squamous-specific isoform of p63, and it may be more useful in distinguishing poorly differentiated SCC from its mimickers in the sinonasal tract. Immunohistochemistry for p40 and p63 was performed on 171 sinonasal neoplasms with SRBCT morphology: 73 SCCs (67 poorly differentiated, non-keratinizing, or basaloid types and 6 nasopharyngeal carcinomas), 46 esthesioneuroblastomas, 11 sinonasal undifferentiated carcinomas (SNUCs), 11 lymphomas, 9 melanomas, 7 alveolar rhabdomyosarcomas, 4 solid adenoid cystic carcinomas, 4 NUT midline carcinomas, 4 primitive neuroectodermal tumors (PNETs), and 2 small cell carcinomas. P40 was positive in 72 of 73 SCCs, and showed a diffuse distribution in all but one positive case. P40 immunoexpression was also observed in 13 of 46 (28 %) esthesioneuroblastomas, 6 of 11 (55 %) SNUCs, 2 of 4 (50 %) adenoid cystic carcinomas, 3 of 4 (75 %) NUT midline carcinomas, 1 of 2 (50 %) small cell carcinomas, and 1 of 4 (25 %) PNETs; in the non-SCC tumors, p40 staining was focal in most cases. P63 was positive in every p40-positive tumor. In addition, a p63+/p40− phenotype was seen 5 of 11 (45 %) lymphomas, 4 of 7 (57 %) alveolar rhabdomyosarcomas, 1 of 4 (25 %) PNETs, and 3 of 46 (7 %) esthesioneuroblastomas. All sinonasal melanomas were negative for both markers. In the sinonasal SRBCT differential diagnosis, both p40 and p63 are highly sensitive for SCC, but p40 is more specific. Notably, p40 is consistently negative in lymphomas and alveolar rhabdomyosarcomas, two tumors that are frequently p63-positive. It must be remembered, however, that even diffuse p40 immunostaining is not entirely specific for the squamous phenotype, and therefore it should be utilized as part of an immunohistochemical panel.
Keywords: p40, p63, Squamous cell carcinoma, Esthesioneuroblastoma, Small round blue cell, Tumor
Introduction
The sinonasal tract is a complex anatomic area that may give rise to a very diverse array of neoplasms ranging from epithelial (e.g., squamous cell carcinoma, salivary gland carcinomas) and neuroectodermal (e.g., esthesioneuroblastoma and mucosal melanoma) to mesenchymal (e.g., rhabdomyosarcoma) and hematopoietic (e.g., lymphoma). Many of these sinonasal malignancies have a very similar appearance on routine hematoxylin and eosin-stained sections: that of a “small round blue cell tumor” (SRBCT) characterized by a monotonous proliferation of cells with scant cytoplasm and relatively small nuclear size [1–6]. The difficulty in distinguishing these tumors on routine histology is compounded by the fact that biopsies from the sinonasal region are frequently very small and commonly exhibit obscuring crush artifact [7]. Nevertheless, precise tumor classification is required for establishing prognosis and appropriate treatment strategies.
Immunohistochemistry is often very helpful for narrowing the differential diagnosis of a sinonasal SRBCT. P63, a transcription factor consistently expressed in normal squamous epithelium and squamous cell carcinoma, has been shown to be useful as part of a panel of immunohistochemical markers in this differential diagnosis, primarily due to its very high sensitivity for even poorly differentiated squamous cell carcinoma, particularly when the pattern of staining is strong and diffuse [2, 5, 6]. It is also clear, however, that p63 immunostaining is not limited to sinonasal squamous cell carcinomas, as esthesioneuroblastomas, neuroendocrine carcinomas, sinonasal undifferentiated carcinomas (SNUCs), salivary gland tumors, and even lymphomas may exhibit varying degrees of p63 immunostaining [2, 5, 6, 8–11]. While the conventionally used p63 antibody 4A4 recognizes both the ΔNp63 and TAp63 isoforms of the p63 molecule, p40 is an antibody that recognizes only ΔNp63, the far more squamous-specific form [12]. Recent studies have shown that in certain scenarios, tumors that unexpectedly stain for p63 (e.g., lymphomas, pulmonary adenocarcinomas) are negative for p40 [8, 13–17]. As a result, it is possible that p40 may be more useful than p63 in the evaluation of a sinonasal SRBCT.
Materials and Methods
Formalin fixed and paraffin embedded tissue blocks from 171 sinonasal neoplasms with a poorly differentiated or “small round blue cell” appearance were retrieved from the Surgical Pathology archives of The Johns Hopkins Hospital. The cases included 67 squamous cell carcinomas (only poorly differentiated, basaloid, and/or non-keratinizing types), 46 esthesioneuroblastomas, 11 SNUCs, 11 lymphomas (7 diffuse large B cell and 4 NK/T cell types), 9 mucosal melanomas, 7 alveolar rhabdomyosarcomas, 4 solid adenoid cystic carcinomas, 4 NUT midline carcinomas, 4 primitive neuroectodermal tumors (PNETs), and 2 small cell carcinomas. Six non-keratinizing nasopharyngeal carcinomas were also included because secondary extension of a nasopharyngeal carcinoma into the sinonasal tract can enter into the SRBCT differential diagnosis. Thirty-eight of the cases were tested on whole slides, while the remaining 133 tumors were present on previously constructed tissue microarrays [18–20]. Immunohistochemistry for p40 (Ab-1; Oncogene Research Products, Cambridge, MA; 1:2,000 dilution) was performed on five-micron sections utilizing standard protocols on a Ventana Benchmark XT autostainer (Ventana Medical Systems, Inc. Tucson, AZ). Nuclear staining in <50 % of tumor cells was regarded as “focal” while positivity in >50 % was regarded as “diffuse.”
Results
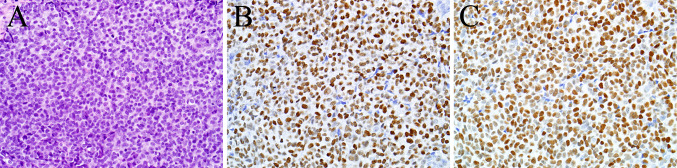
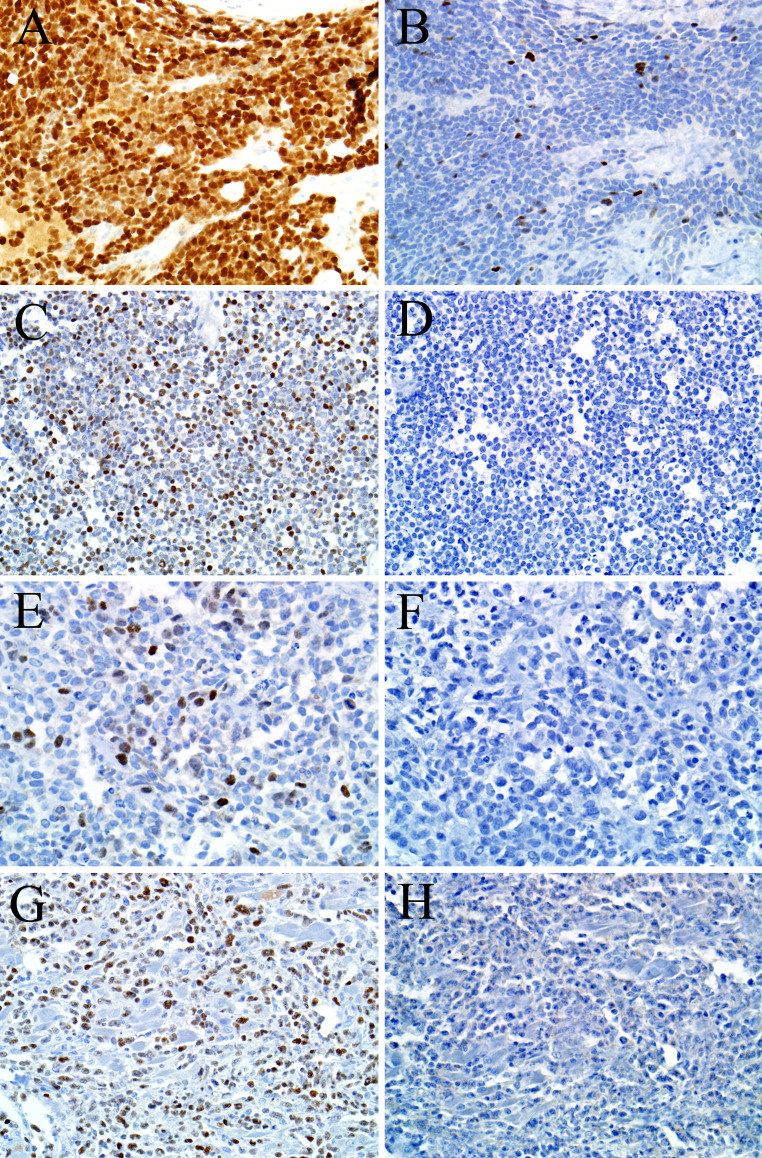
The results are summarized in Table 1. Both p40 and p63 were positive in 66 of 67 (99 %) sinonasal tract squamous cell carcinomas, and for each antibody staining was diffuse in 65 of 66 (99 %) positive cases (Fig. 1). In addition, 6 of 6 (100 %) nasopharyngeal carcinomas were diffusely positive for both immunostains. As expected, both p40 and p63 were positive in a peripheral (i.e., myoepithelial cell) pattern in 2 of 4 (50 %) adenoid cystic carcinomas. Also not surprisingly, 3 of 4 (75 %) of NUT midline carcinomas—a tumor known to exhibit varying degrees of squamous differentiation [20–22]—were positive for both p63 and p40. However, the pattern of staining in NUT midline carcinomas differed. In the p63-positive NUT midline carcinomas, staining was present in almost all tumor cells. On the other hand, for the p40-positive NUT midline carcinomas staining was diffuse in the overtly squamoid areas but only focal in the predominant undifferentiated cell component (Fig. 2a, b).
Table 1.
p63 and p40 immunohistochemisry in sinonasal small round blue cell tumors
| Diagnosis | p63 | p40 | ||||
|---|---|---|---|---|---|---|
| Focal | Diffuse | Any positivity | Focal | Diffuse | Any positivity | |
| Epithelial | ||||||
| Squamous cell carcinoma | 1/67 (1) | 65/67 (97) | 66/67 (99) | 1/67 (1) | 65/67 (97) | 66/67 (99) |
| Sinonasal undifferentiated carcinoma | 2/11 (18) | 4/11 (36) | 6/11 (55) | 2/11 (18) | 4/11 (36) | 6/11 (55) |
| Nasopharyngeal carcinoma | 0/6 (0) | 6/6 (100) | 6/6 (100) | 0/6 (0) | 6/6 (100) | 6/6 (100) |
| Solid adenoid cystic carcinoma | 2/4 (50) | 0/4 (0) | 2/4 (50) | 2/4 (50) | 0/4 (0) | 2/4 (50) |
| NUT midline carcinoma | 0/4 (0) | 3/4 (75) | 3/4 (75) | 3/4 (75) | 0/4 (0) | 3/4 (75) |
| Neuroectodermal | ||||||
| Esthesioneuroblastoma | 15/46 (33) | 1/46 (2) | 16/46 (35) | 12/46 (26) | 1/46 (2) | 13/46 (28) |
| Ewing sarcoma/PNET | 0/4 (0) | 2/4 (50) | 2/4 (50) | 0/4 (0) | 1/4 (24) | 1/4 (25) |
| Small cell carcinoma | 1/2 (50) | 0/2 (0) | 1/2 (50) | 1/2 (50) | 0/2 (0) | 1/2 (50) |
| Melanoma | 0/9 (0) | 0/9 (0) | 0/9 (0) | 0/9 (0) | 0/9 (0) | 0/9 (0) |
| Hematolymphoid | ||||||
| NK/T cell | 2/4 (50) | 0/4 (0) | 2/4 (50) | 0/4 (0) | 0/4 (0) | 0/4 (0) |
| Diffuse large B cell | 0/7 (0) | 3/7 (43) | 3/7 (43) | 0/7 (0) | 0/7 (0) | 0/7 (0) |
| Mesenchymal | ||||||
| Alveolar rhabdomyosarcoma | 4/7 (57) | 0/7 (0) | 4/7 (57) | 0/7 (0) | 0/7 (0) | 0/7 (0) |
Fig. 1.
This example of non-keratinizing squamous cell carcinoma is difficult to distinguish from other types of sinonasal neoplasms in this field (a). Its diagnosis is supported by diffuse immunostaining for p63 (b) and p40 (c)
Fig. 2.
Three of four NUT midline carcinomas exhibited strong p63 immunostaining (a) but only focal p40 immunostaining (b). This case of primitive neuroectodermal tumor was diffusely positive for p63 (c) but negative for p40 (d). Four alveolar rhabdomyosarcomas were focally p63-positive (e), but all cases were negative for p40 (f). Similarly, while five sinonasal lymphomas exhibited p63 immunostaining (g), all cases were p40-negative (h)
Unexpected p40 immunostaining was also encountered. For example, 13 of 46 (28 %) esthesioneuroblastomas were positive for p40 compared to 16 of 46 (35 %) with p63, although only one esthesioneuroblastoma was diffusely positive for both markers. Six of eleven (55 %) SNUCs exhibited both p40 and p63 staining, with 4 of them showing diffuse positivity. One of the two (50 %) small cell carcinomas was focally positive for both p40 and p63. Finally, one of 4 (25 %) PNETs was diffusely positive for p40, compared to two of four (50 %) for p63 (Fig. 2c, d).
P40 immunostaining was absent in hematolymphoid and mesenchymal neoplasms, with 0 of 18 staining. In contrast, p63 was positive in 4 of 7 alveolar rhabdomyosarcomas (all focal) (Fig. 2e, f), 2 of 4 NK/T cell lymhpomas (both focal), and 3 of 7 diffuse large B cell lymphomas (all diffuse) (Fig. 2g, h). Both p40 and p63 were negative in all cases of sinonasal mucosal melanoma.
Overall, both p40 and p63 were 99 % sensitive but p40 was 73 % specific for the diagnosis of squamous cell carcinoma (or nasopharyngeal carcinoma) compared with 60 % for p63. If considering only diffuse staining as positive, the sensitivity of p40 and p63 were both 97 %, but p40 was 94 % specific compared to 87 % for p63.
Discussion
Diagnostic pathology of the sinonasal area is very challenging due to the anatomic complexity and tumor diversity of the region, as well as the propensity for many tumors to share a non-specific small round blue cell morphology. Although diagnostic clues may be gleaned from routine hematoxylin and eosin-stained sections, very often ancillary tools—especially immunohistochemistry—are needed to arrive at the correct diagnosis. P63 is a relatively recent addition to the head and neck pathologist’s armamentarium, and it is often employed in the sinonasal SRBCT differential diagnosis due to its high sensitivity for squamous and myoepithelial cells [2, 5, 6]. Increasing experience with p63, however, has shown that staining may also be unexpectedly encountered in certain tumors that may share a SRBCT appearance. P40 is an antibody specific for the more squamous-specific isoform of p63. In thoracic pathology, for example, p40 has been employed because unlike p63, it only rarely stains pulmonary adenocarcinomas [8, 23–25]. We sought to determine whether p40 offers a similar improvement in distinguishing sinonasal squamous cell carcinoma from its potential mimickers.
Examining 171 sinonasal/nasopharyngeal tumors with a SRBCT appearance, we confirmed that p63 has a very high sensitivity for squamous cell carcinoma in this differential diagnosis, as previously reported [2, 5, 6, 26]. We also showed that p40 demonstrates identically high (99 % each) sensitivity along with superior specificity (73 vs. 60 %) compared to p63. We confirmed that the specificity of p63 for sinonasal squamous cell carcinoma increased dramatically (to 87 %) when only diffuse staining was regarded as positive [2, 5, 6, 26], and we found that this was also true for p40 (to 94 %).
While every p40 positive tumor was also p63 positive, a p63+/p40− immunophenotype was seen in 5 lymphomas, 4 alveolar rhabdomyosarcomas, 3 esthesioneuroblastomas, and 1 PNET. In these tumors, it is presumed that the p63 antibody is detecting the TAp63 isoform that is known not to be squamous-specific [12]. However, even p40 immunoexpression was not limited to squamous cell carcinomas. For adenoid cystic carcinoma and NUT midline carcinoma, p40 immunostaining was expected, but unexpected p40 positivity—usually focal—was observed in some cases of esthesioneuroblastoma, SNUC, small cell carcinoma, and PNET. For SNUC and small cell carcinoma, the p40 immunolabeling may indicate some residual squamous cell carcinoma component, but p40 positivity is difficult to explain in esthesioneuroblastoma and PNET, where squamous differentiation is not known to occur. This aberrant staining shows that even though it is more specific than p63, p40 should still be used in the sinonasal SRBCT differential diagnosis only as part of a panel that should also include cytokeratins, CD45 (common leukocyte antigen), neuroendocrine markers (synaptophysin and chromogranin), melanocytic markers (S100, HMB45, MelanA), desmin, and CD99.
In summary, p40 is useful in the analysis of a sinonasal SRBCT because it is highly specific for squamous cell carcinoma, especially if its pattern of staining is diffuse. In addition, it is more specific than p63 in this setting, with a complete absence of staining in alveolar rhabdomyosarcoma and, most importantly, lymphoma where p63 staining can be diffuse. In contrast, there is no clear advantage to using p63 in place of p40 in the differential diagnosis of a sinonasal SRBCT. At the same time, focal and rarely diffuse p40 staining can be unexpectedly encountered, and therefore it should be interpreted in the context of any histologic clues and other immunostains.
References
- 1.Iezzoni JC, Mills SE. “Undifferentiated” small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Clin Pathol. 2005;124(Suppl):S110–S121. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 2.Chapman-Fredricks J, Jorda M, Gomez-Fernandez C. A limited immunohistochemical panel helps differentiate small cell epithelial malignancies of the sinonasal cavity and nasopharynx. Appl Immunohistochem Mol Morphol. 2009;17(3):207–210. doi: 10.1097/PAI.0b013e31818fc85c. [DOI] [PubMed] [Google Scholar]
- 3.Bridge JA, Bowen JM, Smith RB. The small round blue cell tumors of the sinonasal area. Head Neck Pathol. 2010;4(1):84–93. doi: 10.1007/s12105-009-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills SE, Fechner RE. “Undifferentiated” neoplasms of the sinonasal region: differential diagnosis based on clinical, light microscopic, immunohistochemical, and ultrastructural features. Semin Diagn Pathol. 1989;6(4):316–328. [PubMed] [Google Scholar]
- 5.Wooff JC, Weinreb I, Perez-Ordonez B, Magee JF, Bullock MJ. Calretinin staining facilitates differentiation of olfactory neuroblastoma from other small round blue cell tumors in the sinonasal tract. Am J Surg Pathol. 2011;35(12):1786–1793. doi: 10.1097/PAS.0b013e3182363b78. [DOI] [PubMed] [Google Scholar]
- 6.Bourne TD, Bellizzi AM, Stelow EB, Loy AH, Levine PA, Wick MR, et al. p63 Expression in olfactory neuroblastoma and other small cell tumors of the sinonasal tract. Am J Clin Pathol. 2008;130(2):213–218. doi: 10.1309/TEDD2FCWH8W0H4HA. [DOI] [PubMed] [Google Scholar]
- 7.Stelow EB, Mills SE. Neural, neuroectodermal, and neuroendocrine neoplasms. Biopsy interpretation of the upper aerodigestive tract and ear. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 149.
- 8.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25(3):405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima N, Satoh T, Sueoka N, Sato A, Ide M, Hisatomi T, et al. Clinico-pathological characteristics of p63 expression in B-cell lymphoma. Cancer Sci. 2006;97(10):1050–1055. doi: 10.1111/j.1349-7006.2006.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18(5):645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- 11.Park CK, Oh YH. Expression of p63 in reactive hyperplasias and malignant lymphomas. J Korean Med Sci. 2005;20(5):752–758. doi: 10.3346/jkms.2005.20.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crum CP, McKeon FD. P63 in epithelial survival, germ cell surveillance, and neoplasia. Ann Rev Pathol. 2010;5(Journal Article):349–71. doi: 10.1146/annurev-pathol-121808-102117. [DOI] [PubMed] [Google Scholar]
- 13.Lin Z, Liu M, Li Z, Kim C, Lee E, Kim I. DeltaNp63 protein expression in uterine cervical and endometrial cancers. J Cancer Res Clin Oncol. 2006;132(12):811–816. doi: 10.1007/s00432-006-0130-8. [DOI] [PubMed] [Google Scholar]
- 14.Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad USA. 2000;97(10):5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelosi G, Sonzogni A, Papotti M, Righi L, Rossi G, Viale G. Different prevalence of transactivating (TA) p63 and non-TAp63 isoforms in pulmonary adenocarcinomas: a useful diagnostic tool. Mod Pathol. 2010;23(1s):411A–2A. [Google Scholar]
- 16.Righi L, Graziano P, Fornari A, Rossi G, Barbareschi M, Cavazza A, et al. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology: a retrospective study of 103 cases with surgical correlation. Cancer. 2011;117(15):3416–3423. doi: 10.1002/cncr.25830. [DOI] [PubMed] [Google Scholar]
- 17.Del Vescovo V, Cantaloni C, Cucino A, Girlando S, Silvestri M, Bragantini E, et al. miR-205 Expression levels in nonsmall cell lung cancer do not always distinguish adenocarcinomas from squamous cell carcinomas. Am J Surg Pathol. 2011;35(2):268–275. doi: 10.1097/PAS.0b013e3182068171. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilson MP, Gallia GL, Bishop JA. Among sinonasal tumors, CDX-2 immunoexpression is not restricted to intestinal-type adenocarcinomas. Head Neck Pathol. 2013. In Press. doi:10.1007/s12105-013-0475-7. [DOI] [PMC free article] [PubMed]
- 20.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36(8):1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203(1):16–20. doi: 10.1016/j.cancergencyto.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5(1):31–35. doi: 10.1007/s12105-010-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelosi G, Fabbri A, Rossi G, Maisonneuve P, Sonzogni A, Bresaola E, et al. A two-hit minimalist diagnostic algorithm based on p40 (deltaNp63) and TTF-1 immunostaining upon small biopsy/cellblock samples for differentiating main subtypes of non-small cell lung cancer and sparing material. J Thorac Oncol. 2011;6(6):S335–S336. [Google Scholar]
- 24.Uramoto H, Yamada S, Hanagiri T. Immunohistochemical staining with deltaNp63 is useful for distinguishing the squamous cell component of adenosquamous cell carcinoma of the lung. Anticancer Res. 2010;30(11):4717–4720. [PubMed] [Google Scholar]
- 25.Iacono ML, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, et al. p63 and p73 Isoform Expression in Non-small Cell Lung Cancer and Corresponding Morphological Normal Lung Tissue. J Thorac Oncol. 2011;6(3):473–481. doi: 10.1097/JTO.0b013e31820b86b0. [DOI] [PubMed] [Google Scholar]
- 26.Serrano MF, El-Mofty SK, Gnepp DR, Lewis JS., Jr Utility of high molecular weight cytokeratins, but not p63, in the differential diagnosis of neuroendocrine and basaloid carcinomas of the head and neck. Hum Pathol. 2008;39(4):591–598. doi: 10.1016/j.humpath.2007.08.019. [DOI] [PubMed] [Google Scholar]