Abstract
Background
The traditional outcome measured following treatment of Dupuytren’s Disease (DD) has been digital range of motion; specifically the gain in digital extension. The outcomes research movement in the last three decades however has been advocating the measurement of outcomes from the patient’s perspective using Health-Related Quality of Life questionnaires (HRQOL). Although several generic and region-specific HRQOL questionnaires exist, there is no guidance as to which one is the most appropriate for this population. The objective of this study is to evaluate the psychometric properties of three self-reported HRQOL outcome measures in patients with DD.
Methods
Patients with DD were enrolled from the practices of three plastic surgeons. Test-retest reliability, concurrent validity and responsiveness of three HRQOL questionnaires were compared in a prospective study design. The HRQOL measures included Health Utilities Index Mark 3 (HUI3), Short Form-36 (SF-36), and the Michigan Hand Questionnaire (MHQ).
Results
All three measures demonstrated good test-retest reliability (ICC = 0.77–0.85). Concurrent validity was found between the HUI3 pain and dexterity attributes and SF-36 physical summary score. The sensitivity of the MHQ to detect changes in the status of the patient was found to be high (effect size = 1.14) whereas that of the SF-36 was trivial.
Conclusions
The HUI3 and the MHQ seem to be reliable and valid tools to assess the HRQOL in patients with Dupuytren’s Disease.
Keywords: Quality of life, Dupuytren’s Disease, Outcomes, Palmar fasciectomy
Background
Dupuytren’s Disease (DD) is a common fibro-proliferative disorder, primarily affecting the palmar fascia of the hand [6]. The epidemiology and pathophysiology of DD has been extensively studied. Historically, DD has been traced to Caucasians of North European descent; however, in the New World, it has been reported in people of any ethnicity and background. A recent study in the USA found that it affects 1 to 7.3 % of the population [13].
Several factors have been associated with DD, such as male gender, positive family history, smoking, alcohol consumption, diabetes, epilepsy, and hypercholesterolemia [13, 28]. None of these factors have been reported to be causative, though they have served as important links to understanding the exact pathophysiological mechanism of DD.
DD was found to be transmitted through autosomal dominant gene with variable penetrance [30, 38]. More recently, certain immunological abnormalities, which cause auto-antibodies to be produced against collagen types 1–4 have also been proposed [31, 38]. However, no strong evidence exists that confirms that these mechanisms in fact result in DD.
Historically, the management of DD has been fasciectomy. Recently, needle aponeurotomy and collagenase injections [23] have been introduced as less invasive management strategies compared to the traditional fasciectomy [2, 6]. Literature has shown that regardless of the management strategy, recurrence is common and the amount of diseased palmar fascia removed is directly proportional to the increased rate of complications [11, 12]. Several outcome measures have been used in DD literature. These outcome measures are physiological (more objective) or patient-reported (more subjective) in nature. The reporting of physiological measures, e.g., Range of Motion (ROM) has been historically favored by hand surgeons. The outcomes research movement in the last three decades however, has been encouraging clinical investigators to use patient-reported outcome measures; specifically those that deal with Health-Related Quality of life (HRQOL) [1, 7, 26].
Quality of Life (QoL) is defined as “An individual’s perception of their position in life, in the context of the culture and values in which they live and in relation to their goals, expectations, standards, and concerns” [21]. HRQOL is a sub-component of QoL, comprises all areas specific to health, i.e., physical, emotional, psychological, social, cognitive, role functioning as well as abilities, relationships, perceptions, life satisfaction and well being [34], and refers to patients’ appraisals of their current level of functioning and satisfaction with it, compared to what they perceived to be ideal [5, 18].
A small number of HRQOL questionnaires have been used in DD to isolate the impact of health on various aspects of functioning providing a holistic picture of patient’s level of functioning [3]. Ball et al. performed a systematic review to assess the outcomes in various surgical treatments used in DD and found significant heterogeneity on both the choice of outcome measures and their reporting [3]. The most commonly used patient-reported outcome measures included Disability of Arm, Shoulder and Hand (DASH) questionnaire, Michigan Hand Questionnaire (MHQ) and Patient Evaluation Measure (PEM). This systematic review did not give us any insight on which is the best measure to use. Hence, it is important to understand the quality and depth of information provided by the outcome measure to guide the assessment of the patient. This can be achieved by examining the psychometric properties of the outcome measures [20].
The aim of this study was to examine the psychometric properties of three commonly used HRQOL instruments in patients with DD: Health Utilities Index Mark 3 (HUI3) [15], Short Form 36 (SF-36) [40, 41] and MHQ [8, 9] in a cohort of patients who underwent palmar fasciectomy. We chose to use a scale from each one of the categories of generic (SF-36), condition specific (MHQ) and a Utility scale (HUI3) as recommended by Guyatt et al. [19]. We believe that the results of the study will assist clinicians and future investigators in the measurement of their DD interventions and economic evaluations comparing various approaches for treating this condition.
Material and Methods
Study Design and Population
Patients with DD, 18 years and older were enrolled prospectively from the practice of three experienced plastic surgeons (AT, CL, and SM) in Hamilton, Ontario, Canada from May 2007 to April 2010. All patients with a diagnosis of Dupuytren’s Disease and bothersome contractures were managed by palmar or digital fasciectomy. The exclusion criteria were the presence of other hand conditions such as carpal tunnel syndrome, rheumatoid arthritis, connective tissue disorder, tenosynovitis, or previous DD surgery on the same hand which, in the opinion of the investigator, would have confounded the assessment of HRQOL. Patients were excluded if they were unable to communicate in English or had undergone previous surgery on the same hand. This study was conducted as a part of a larger study to evaluate the HRQOL in DD which was registered at the outset with http://clinicaltrials.gov/as NCT00468949.
The patients were evaluated using three patient-reported HRQOL questionnaires at 1 week before surgery and 1 day before surgery, 3, 6, and 12 months after surgery. The questionnaires completed by patients included: (1) HUI3 [15], (2) SF-36 [40, 41], and (3) Michigan Hand Outcomes Questionnaire (MHQ) [8, 9] (Table 1). When this study commenced, no validated HRQOL specific to Dupuytren’s Disease existed in literature. Since then, Beaudreuil et al. developed and validated a DD-specific scale—a Unite Rhumatologique des affections de la Main (URAM) scale in 2011 [4]. This DD scale, however, has not been validated by other investigators.
Table 1.
Summary of health-related quality of life measures
| Instrument | Dimensions | No. of items/levels | Scoring and interpretation | Additional comments |
|---|---|---|---|---|
| Health Utility Index Mark 3 (HUI3) [15, 16, 22] | 8; vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain | 45; 5–6 items per attribute | • The responses on the questionnaires (both HUI2 and HUI3) are converted into levels bases on a standardized algorithm • The utility scale being defined for interval −0.36 to 1.00. Negative scores represent states that are considered worst that death by the participants • Self-complete and interviewer administered format |
• Valid, reliable and responsive measure in several populations and can be administered to any individual who is 5 years and older. Combines morbidity and mortality into a single index score • Takes approximately 8 min to complete • Explains 972,000 unique health states that are obtained from the factorials defined for each dimension • The resulting utility score from HUI3 can be used to calculate Quality Adjusted Life Years (QALYs). QALYS an important component of cost-utility analysis (CUA), which is an important type of economic evaluation. CUAs can facilitate comparison of various interventions in Dupuytren’s disease and inform decisions • Permission to use HUI3 should be obtained from Health Utilities Inc. prior to use. The license to use HUI3 is granted one study at a time. Subsequently, the licensing fee is also structured on a one-study-at-a-time model. This is the only disadvantage of using HUI3 in a research study |
| Short Form-36 (SF-36) [39–41] | 8; physical function, role physical, bodily pain, general health, vitality, social functioning, and role emotional and mental health | 36 | • The final score on SF-36 can be interpreted as an 8-scale profile of scores or as a summary measure, i.e., physical component or mental component. The scoring for SF-36 is simple and constitutes an algebraic sum of all the responses for the items in that scale. Each scale is then converted into a 0–100 scale using a transformation formula. • The scale of 0 represents lowest or worst possible level of functioning and 100 represents highest or best possible level of functioning. |
• Widely used, generic multipurpose survey of general health status [14, 39, 40]. Takes 5–10 min for completion. • Easy to use and constitutes an algebraic sum of all the responses for the items in that scale • Licensing fee has been waived for academic use, available from SF-36.org |
| Michigan Hand Questionnaire (MHQ) [8, 9] | (1) Overall hand function, (2) activities of daily living (ADLs), (3) pain, (4) work performance, (5) aesthetics, and (6) patient satisfaction with hand function | 37 | • The subjects respond to each question on every item on a Likert-like scale ranging from 1 to 5. These responses are then added to give a domain score for each of six scales. Each respondent must answer 50 % or more of the items within the scale for responses to be considered sufficient. The scores from each scale are then converted to 0–100 based on algorithm [8, 9] • Higher scores represent better performance for all health domains but pain |
• Hand-specific outcomes instrument, takes approximately 15 min to complete. • The algorithm for scoring is easy to use, and scores can be calculated using SAS or Excel • License fee has been waived for academic and non-academic purposes; available at no cost from University of Michigan, Department of Surgery website |
In addition to the quality of life instruments and physical tests, baseline demographic information was collected for all the enrolled patients. The demographic information constituted age, gender, height, weight, and employment history. Joint ROM was assessed with a goniometer and mean grip strength was calculated by recording the average of three readings on a JAMAR dynamometer. The results from performance-based measures were used to evaluate the HRQOL in patients with DD and will not be discussed further in this article.
Written signed informed consent was obtained from all the study participants. Approval was obtained for this study from the Research Ethics Boards of McMaster University and St. Joseph’s Healthcare Hamilton.
Statistical Analyses
The psychometric properties of the three HRQOL questionnaires were analyzed using the SPSS version 20 (IBM SPSS Inc., Chicago, IL, USA). Descriptive statistics were computed for all instruments for all assessment points. The level of significance for all statistical tests was set a priori at 0.01.
Test-retest reliability of HUI3, SF-36, and MHQ was assessed using Intraclass Correlation Coefficient (ICC) by administering the questionnaires to the surgical patients 1 week and 1 day preoperatively. ICC signifies the agreement between the HRQOL measures at two different time points between which the patient’s level of functioning or quality of life is not expected to change. According to the guidelines described by Landis et al. when the ICC is below 0.7, the level of clinical significance is unacceptable; between 0.70 and 0.79 is fair; between 0.80 and 0.89 is good and when it is above 0.9, it is excellent [27]. The concurrent validity of the three HRQOL measures [33] was investigated by examining the relationship between the scores at baseline and 12 months postoperatively. Pearson correlation coefficients describing the strength of association between the three HRQOL attributes, and between three HRQOL instruments and ROM and grip strength were used to examine the construct validity of the HRQOL questionnaires. We hypothesized that the changed scores will be positively correlated to each other because they are all scored in the same direction, that is measuring improvement.
The extent to which the change over time corresponds with other indicators of change is known as longitudinal construct validity or internal responsiveness [24]. Internal responsiveness was assessed with effect sizes (i.e., the difference (Δ) between the mean follow-up score at 12 months post operatively and the mean baseline score at 1 week divided by the standard deviation of the baseline score). Cohen’s (1997) guidelines were used to facilitate the interpretation of the effect sizes computed as follows: an effect size less than 0.2 was considered trivial, 0.2–0.5 was small, 0.5–0.8 was moderate effect, and greater than 0.8 was considered large effect [10].
Results
Subjects
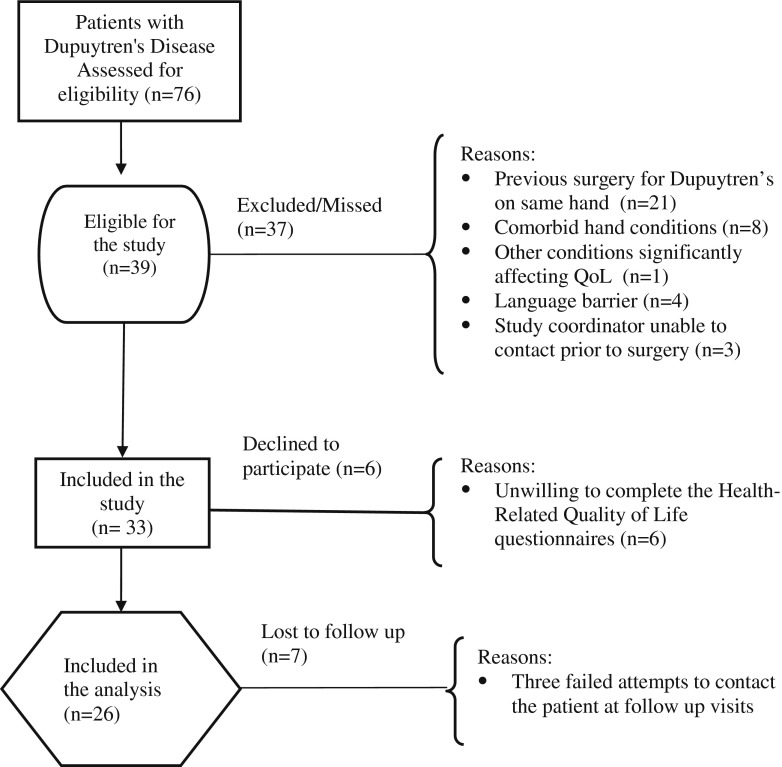
Seventy-six patients were identified to have DD and were eligible for surgery. When the inclusion and exclusion criteria were applied, 39 were found to be eligible to participate in the study (See Fig. 1). Six patients were unwilling to complete the HRQOL questionnaires for the duration of the study and were excluded. Of the remaining 33, 7 patients (21.21 %) were lost to follow-up during the course of the study leaving 26 patients for final analyses. The mean age of the patients was 64.2 ± 7.3 years. At the time of enrolment in the study, 29 % of the patients were employed. The baseline characteristics of the patients are detailed in Table 2. The distribution of the 41 digits in the surgical hand affected by DD in the present study is displayed in Table 3.
Fig. 1.
Patient flow diagram
Table 2.
Baseline characteristics of included patients
| Surgical patients | |
|---|---|
| n = 33 | |
| Mean (standard deviation) | |
| Age (years) | 64.2 (7.3) |
| Gender | |
| Male/female | 28:5 |
| Male (%) | 85 % |
| BMI (kg/m2) | 30.8 (6.0) |
| Employment status | |
| Percentage working | 29 % |
Table 3.
Distribution of involved digits
| Digit involved | N = 33 |
|---|---|
| Index finger | 1 |
| Middle finger | 5 |
| Ring finger | 13 |
| Little finger | 22 |
| Total digits | 41 |
Psychometric Analyses
The test-retest reliability for the SF-36 PCS score in patients with DD was found to be excellent (ICC = 0.948; 95%CI, 0.875–0.979), while that of the HUI3 multiattribute score was good (ICC = 0.853; 95 % CI, 0.688–0.934). The SF-36 MCS score (ICC = 0.77; 95%CI, 0.512–0.901) and the MHQ Total Score for the Affected hand (ICC = 0.793, 95%CI, 0.570–0.908) demonstrated adequate test-retest reliability while that of the HUI3 dexterity attribute was found to be poor (ICC = 0.248; 95%CI, −0.167–0.591) (Table 4).
Table 4.
Intraclass Correlation Coefficients (ICC) and 95 % Confidence Intervals (CIs) of HRQOL scores at 1-week and 1-day preoperatively
| Scale | ICC | 95 % CI | Interpretation of ICC |
|---|---|---|---|
| HUI3 multiattribute score (n = 23) | 0.853 | 0.688–0.934 | Good |
| HUI3 dexterity score (n = 23) | 0.248 | −0.167–0.591 | Poor |
| HUI3 pain score (n = 23) | 0.900 | 0.782–0.956 | Excellent |
| SF-36 physical summary score (n = 20) | 0.948 | 0.875–0.979 | Excellent |
| SF-36 mental summary score (n = 20) | 0.770 | 0.512–0.901 | Adequate |
| MHQ—total score for affected hand (n = 22) | 0.793 | 0.570–0.908 | Adequate |
HUI3 Health Utilities Index Mark 3, SF-36 Short Form-36, MHQ Michigan Hand Questionnaire, ICC intraclass correlation coefficient, CI confidence interval
When evaluating concurrent validity, significant correlations were noted between the HUI3 multiattribute and the HUI3 Dexterity (r = 0.703, p < 0.001) and Pain attributes (r = 0.843, p < 0.001). Furthermore, the HUI3 dexterity attribute correlated significantly with HUI3 Pain attribute (r = 0.782, p < 0.001) (construct validity) and the SF-36 PCS score (r = 0.568, p < 0.001) (construct validity). The HUI3 pain attribute was associated with the SF-36 PCS (r = 0.616, p < 0.001) (construct validity) (Table 5).
Table 5.
Concurrent validity measured by using correlations between changes in HRQOL scales
| Scale | HUI3 dexterity attribute | HUI3 pain attribute | SF-36 physical component summary score | SF-36 mental component summary score | MHQ |
|---|---|---|---|---|---|
| HUI3 multiattribute | |||||
| Pearson correlation | 0.703 | 0.843 | 0.507 | 0.104 | 0.013 |
| p value (two-tailed) | <0.001** | <0.001** | 0.010 | 0.620 | 0.948 |
| n | 26 | 26 | 25 | 25 | 26 |
| HUI3 dexterity attribute | |||||
| Pearson correlation | 0.782 | 0.568 | −0.210 | 0.369 | |
| p value (two-tailed) | <0.001** | 0.003** | 0.313 | 0.064 | |
| n | 26 | 25 | 25 | 26 | |
| HUI3 pain attribute | |||||
| Pearson correlation | 0.616 | −0.065 | 0.154 | ||
| p value (two-tailed) | 0.001** | 0.757 | 0.452 | ||
| n | 25 | 25 | 26 | ||
| SF-36 physical component summary score | |||||
| Pearson correlation | −0.162 | 0.142 | |||
| p value (two-tailed) | 0.438 | 0.498 | |||
| n | 25 | 25 | |||
| SF-36 mental component summary score | |||||
| Pearson correlation | −0.85 | ||||
| p value (two-tailed) | 0.058 | ||||
| n | 25 | ||||
HUI3 Health Utilities Index Mark 3; SF-36 Short Form-36; MHQ Michigan Hand Questionnaire
Our analyses of the association between the HRQOL measures and the ROM and grip strength, at baseline and 12 months postsurgery are presented in the “Appendix”. From the results, the general pattern of associations observed for the HRQOL measures with measures of grip strength and ROM was that the magnitudes of the correlations were below 0.3 except for the MHQ score with measures of ROM at baseline, i.e., with Meta-carpophalangeal joint (MCPJ) (r = 0.358) and Total Passive Extension Deficit (TPED) (r = 0.356) and the SF-36 MCS score with MCPJ (r = 0.441). At 12 months postsurgery, grip strength of the affected limb at 12 months postsurgery with HUI multiattribute score (r = 0.395) and the SF-36 PCS (r = 0.339) as well as TPED with the SF-36 MCS score (r = 0.353).
The responsiveness of each instrument is shown in Table 6. When the effect sizes (internal responsiveness) were assessed, MHQ was found to have a large effect size (1.14 ± 1.4) as compared to HUI3 dexterity which had moderate effect (0.71 ± 0.14). The effect size of HUI3 multiattribute score was found to be low (0.2 ± 0.2) whereas the effect size for the SF-36 PCS score, the SF-36 MCS score, and the HUI3 Pain Score were trivial.
Table 6.
Responsiveness of HRQOL instruments used to measure change in HRQOL of patients undergoing palmar fasciectomy for Dupuytren’s contracture between 1-week preoperatively to 12 months postoperatively
| Scale | Difference | Standard deviationa | Effect size | Magnitude of effect size |
|---|---|---|---|---|
| HUI3 multiattribute score | 0.04 | 0.2 | 0.2 | Small |
| HUI3 dexterity score | 0.1 | 0.14 | 0.71 | Moderate |
| HUI3 pain score | 0.02 | 0.23 | 0.09 | Trivial |
| SF-36 physical component summary score | 0.8 | 9.1 | 0.09 | Trivial |
| SF-36 mental component summary score | −0.4 | 5.7 | −0.07 | Trivial |
| MHQ total for affected hand | 16 | 14 | 1.14 | Large |
HUI3 Health Utilities Index Mark 3, SF-36 Short Form-36, MHQ Michigan Hand Questionnaire
aStandard deviation of sample at baseline
Discussion
In this prospective study, we evaluated the measurement properties of three HRQOL instruments in patients with DD. This is the first study to examine the psychometric properties in patients with DD and hence, adds important information concerning the use of SF-36 in future studies of patients undergoing palmar fasciectomy to the literature. The results of this study indicate that two of the three HRQOL scales used, i.e., the HUI3 and the MHQ can be used to measure HRQOL in patients with Dupuytren’s Disease. Some strengths of our study are a methodologically sound prospective study design with adequate follow-up. We incorporated the time points necessary to evaluate the measurement properties of the outcome measures in DD. This methodology could be used in future surgical trials when evaluating other hand conditions.
Our study was conceived in 2007 and spanned the duration of 4 years until its completion. At the time of conception, no QoL outcome measure that was specific to Dupuytren’s Disease existed. However, in 2011, a DD-specific QoL scale, URAM scale was developed by Beaudreuil et al. [4]. It was not appropriate to introduce this new scale midway in the trial. We recommend however that the URAM scale be used in future trials to assess its validity by other investigators.
All things considered, we believe our choice of outcome measures was aligned with our objectives. We limited ourselves to testing MHQ, hand-specific outcome measure, which is commonly used by the surgeons and allied health professionals in their everyday practice. SF-36 is another well-known and widely used generic health instrument. Hence, it was about time that we reflected on these measures in patients with DD and test them for their measurement properties to assess if they measure what they intend to (or claim to) measure. We decided to refrain from the short-version of these questionnaires, i.e., SF-12 or brief-MHQ because we did not think that we were adding a lot of patient burden. Further, we dismissed DASH questionnaire and related-short versions as DASH is a regional outcome measure, hence not specific to the hand conditions. To identify the self-reported pain and disability issues highly restricted to the hand such as DD, MHQ was a better choice. The outcome measures (including self-report and physical) took approximately 20 min to complete and all the research visits were aligned with the regular clinic visits.
Measurement Properties of Three HRQOL Measures
Test-Retest Reliability
In patients with Dupuytren’s Disease, the HUI3 demonstrated good test-retest reliability (ICC = 0.853, 95%CI, 0.688–0.934). When the test-retest reliability of individual attributes of the HUI3 were closely examined, we found that with the exception of HUI3 dexterity attribute, all other attributes demonstrated adequate to excellent reliability. The test-retest reliability of the HUI3 dexterity attribute was found to be poor. Dexterity in HUI3 is measured on a six-point scale and to determine what may have caused such a result is difficult. Upon reviewing the wording of the questions relating to dexterity in the HUI3 questionnaire, we believe that there may be some subjectivity in regards to how the question could be answered. The combination of the subjectivity of the wording along with the effect of a small sample size may have produced some inaccurate results with respect to test-retest reliability of this attribute.
Test-retest reliability of PCS score of the SF-36 was found to be excellent and that of MCS score was adequate. The low reproducibility of the MCS score of the SF-36 might be due to the fact that the questionnaire was administered for reliability one day before the surgery. Hence, on one hand, though the patients believed that their physical ability did not change much (based on the high ICC for the PCS score), they were definitely more anxious 1 day before surgery (based on the adequate ICC for the MCS score). We anticipated that the MHQ, being a hand-specific questionnaire will demonstrate good to excellent test-retest reliability, although we found the reliability to be adequate. The test-retest reliability of the MHQ has been found to be in fact adequate to good in patients with arthritis [29] and hand/wrist disorders [32]
Concurrent Validity
Concurrent validity tests showed that the HUI3 multiattribute, the HUI3 dexterity and the HUI3 pain scores were all correlated with each other. This is not surprising considering that two of the attributes are subsets of the multiattribute score. Interestingly, the HUI3 pain and the HUI3 dexterity attributes were also correlated with each other despite the fact that there was a statistically significant change from baseline to 12 months postoperative in the HUI3 dexterity attribute while very little change was observed in the HUI3 pain scores.
Finally, the SF-36 PCS score was correlated with the two affected physical attributes of the HUI3 questionnaire: the HUI3 dexterity and the HUI3 pain attributes. Significant correlation was observed between the HUI3 Pain attribute score and the SF-36 PCS score.
When the construct validity of the MHQ was examined by correlating its scores with the HUI3 dexterity attribute, the HUI3 pain attribute, and the SF-36, no significant correlation was found.
Responsiveness
The effect size of the multiattribute HUI3 was 0.2 which is considered a small effect size. Considering however that the dexterity domain of the HUI3 had an effect size of 0.71(moderate effect size), this makes the HUI3 a valid QoL measure for DD.
The effect size of the MHQ (1.14) was found to be large, which implies that the MHQ is a very sensitive scale to capture the change that occurs with surgery in DD. Besides, as every participant in this study improved from pre- to post-surgery using our criteria of moderate to large effect sizes signifying a clinically important and meaningful change, from Table 6, the MHQ (p = 0.001) and the HUI3 dexterity score (p < 0.001) are responsive to the change or improvement observed in the participants.
In terms of the suitability of the chosen HRQOL questionnaires, all questionnaires except the HUI3 pain attribute scores and the SF-36 PCS and MCS scores demonstrated adequate effect size. As expected, as a region-specific questionnaire, the MHQ, demonstrated a large effect size, i.e., 1.14 ± 14, while the HUI3 multiattribute and HUI3 dexterity effect sizes were small and moderate, respectively. The SF-36 Physical Component Summary score, the SF-36 Mental Component Summary score and the HUI3 pain attribute all demonstrated trivial effect sizes, making them unsuitable for measuring change in Dupuytren’s contracture in future studies. Unfortunately the effect size of the SF-36 was only 0.09 which is again a trivial effect. This implies that the SF-36 is not a sensitive measure to capture the change in HRQOL that took place postpalmar fasciectomy in our patient population. Although this finding should be validated in future trials with large sample size, the use of the SF-36 in the assessment of a patient with DD should be questioned.
One of the limitations of our study might be the high loss to follow-up (21.21 %). Obtaining a high degree of compliance is a well-known challenge to the surgical trials [35]. A possible explanation could be that the patients improved significantly from their baseline and hence did not follow-up or possibly the patients did not want to lose time from work to go for the follow-up visit and lose income. Some of the strategies that could be used to overcome these issues are to give compensation (parking and fuel costs) to the patients, and making the follow-up appointment at weekends or evenings so that the patients do not lose time off work. Further, our study evaluated psychometric soundness of the HUI3, MHQ, and SF-36 in patients undergoing palmar fasciectomy only. Considering the variety of non-surgical and surgical treatments options available to patients with DD, we believe the logical next step is to probe the measurement properties of HUI3 and MHQ (alongside URAM) in future studies with alternate treatment approaches such as needle aponeurotomy or collagenase injections.
In conclusion, using questionnaires that are relevant to the objectives of the assessment reduce the patient and clinician time burden and hence improve the continued participation of the patient. Also, summarizing the extra unnecessary information is time-consuming and might hamper the clinical decision-making process. Both, the HUI3 and the MHQ are patient-reported, easy to administer, and feasible to implement in clinical practice. The MHQ has been validated in several hand/wrists disorders. Similarly, the HUI3 has been validated in five general population surveys in Canadian population and has been reported in patients with hip fracture, rheumatic diseases, and stroke [17, 25]. One benefit of including health utility outcome measures such as the HUI3 in future DD studies is that from the utility scores we can calculate Quality Adjusted Life Years (QALY), an important component of cost-effectiveness analysis which none of the other two measures can do [36, 37].
Acknowledgments
We would like to thank Angela Barbas and Kathy Strauss for their assistance in patient recruitment.
Conflict of interest
Achilleas Thoma declares no conflict of interest.
Manraj Nirmal Kaur declares no conflict of interest.
Teegan Aili Ignacy declares no conflict of interest.
Carolyn Levis declares no conflict of interest.
Stuart Martin declares no conflict of interest.
Eric Duku declares no conflict of interest.
Ted Haines declares no conflict of interest.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Approval was obtained for this study from the Research Ethics Boards of McMaster University and St. Joseph’s Healthcare Hamilton, Ontario, Canada.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Funding Received
McMaster Surgical Associates, Department of Surgery, McMaster University and MANUS Canada.
Appendix
Table 7.
Association between the HRQOL measures and the grip strength and Range of Motion at baseline (preoperative) and 12 months postoperatively
| Preoperative | 12 months postoperative | ||||||
|---|---|---|---|---|---|---|---|
| Grip strength for affected limb | MCPJ extension | TPED | Grip strength for affected limb | MCPJ extension | TPED | ||
| HUI3 multiattribute score | Pearson correlation | 0.177 | 0.234 | 0.156 | 0.395 | 0.115 | 0.113 |
| Sig. (2-tailed) | 0.420 | 0.251 | 0.438 | 0.069 | 0.620 | 0.627 | |
| N | 23 | 26 | 27 | 22 | 21 | 21 | |
| HUI3 dexterity score | Pearson correlation | 0.144 | 0.079 | −0.065 | 0.109 | 0.144 | −0.018 |
| Sig. (2-tailed) | 0.512 | 0.701 | 0.746 | 0.630 | 0.534 | 0.939 | |
| N | 23 | 26 | 27 | 22 | 21 | 21 | |
| HUI3 pain score | Pearson correlation | 0.248 | 0.285 | 0.246 | 0.219 | 0.048 | 0.184 |
| Sig. (2-tailed) | 0.253 | 0.159 | 0.216 | 0.327 | 0.835 | 0.425 | |
| N | 23 | 26 | 27 | 22 | 21 | 21 | |
| SF-36 physical summary score (PCS) | Pearson correlation | 0.264 | −0.124 | 0.095 | 0.339 | 0.011 | 0.211 |
| Sig. (2-tailed) | 0.236 | 0.554 | 0.645 | 0.123 | 0.961 | 0.358 | |
| N | 22 | 25 | 26 | 22 | 21 | 21 | |
| SF-36 mental summary score (MCS) | Pearson correlation | −0.295 | 0.441 | 0.073 | 0.171 | −0.273 | 0.353 |
| Sig. (2-tailed) | 0.183 | 0.027 | 0.725 | 0.446 | 0.231 | 0.117 | |
| N | 22 | 25 | 26 | 22 | 21 | 21 | |
| MHQ | Pearson correlation | −0.193 | 0.358 | 0.356 | 0.103 | −0.024 | −0.115 |
| Sig. (2-tailed) | 0.366 | 0.067 | 0.063 | 0.649 | 0.918 | 0.620 | |
| N | 24 | 27 | 28 | 22 | 21 | 21 | |
HUI3 Health Utilities Index Mark 3, SF-36 Short Form-36, MHQ Michigan Hand questionnaire, MCPJ Meta-carpophalangeal Joint, TPED Total Passive Extension Deficit
References
- 1.Alderman AK, Chung KC. Measuring outcomes in hand surgery. Clin Plast Surg. 2008;35(2):239–50. doi: 10.1016/j.cps.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren's contracture. J Hand Surg. 2007;32(6):767–74. doi: 10.1016/j.jhsa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Ball C, Pratt AL, Nanchahal J. Optimal functional outcome measures for assessing treatment for Dupuytren’s disease: a systematic review and recommendations for future practice. BMC Musculoskelet Disord. 2013;14(1):131. doi: 10.1186/1471-2474-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudreuil J, Allard A, Zerkak D, Gerber RA, Cappelleri JC, Quintero N. Unité Rhumatologique des Affections de la Main (URAM) scale: development and validation of a tool to assess Dupuytren’s disease-specific disability. Arthritis Care Res. 2011;63(10):1448–55. doi: 10.1002/acr.20564. [DOI] [PubMed] [Google Scholar]
- 5.Cella DF, Tulsky DS. Measuring quality of life today: methodological aspects. Oncology. 1990;5:29–38. [PubMed] [Google Scholar]
- 6.Chen N, Srinivasan R, Shauver M, Chung K. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6(3):250–5. doi: 10.1007/s11552-011-9326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KC, Burns PB, Davis Sears E. Outcomes research in hand surgery: where have we been and where should we go? J Hand Surg. 2006;31(8):1373–9. doi: 10.1016/j.jhsa.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Chung KC, Hamill JB, Walters MR, Hayward RA. The Michigan Hand Outcomes Questionnaire (MHQ): assessment of responsiveness to clinical change. Ann Plast Surg. 1999;42(6):619–22. doi: 10.1097/00000637-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan hand outcomes questionnaire. J Hand Surg. 1998;23(4):575–87. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical power analysis for the social sciences. 1988.
- 11.Davis T, Becker GW, Rodrigues JN, Ball C, Giele H, Hobby J. Surgery for Dupuytren’s contractures of the fingers. The Cochrane Library. 2012. [DOI] [PMC free article] [PubMed]
- 12.Desai SS, Hentz VR. The treatment of Dupuytren disease. J Hand Surg. 2011;36(5):936–42. doi: 10.1016/j.jhsa.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 13.DiBenedetti D, Nguyen D, Zografos L, Ziemiecki R, Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6(2):149–58. doi: 10.1007/s11552-010-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowrick AS, Gabbe BJ, Williamson OD, Cameron PA. Outcome instruments for the assessment of the upper extremity following trauma: a review. Injury. 2005;36(4):468–76. doi: 10.1016/j.injury.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Feeny D, Furlong W, Boyle M, Torrance G. Multi-attribute health status classification systems. Health utilities index. Pharmacoeconomics. 1995;7(6):490–502. doi: 10.2165/00019053-199507060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The health utilities index (HUI®) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375–84. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 17.Grootendorst P, Feeny D, Furlong W. Health Utilities Index Mark 3: evidence of construct validity for stroke and arthritis in a population health survey. Med Care. 2000;38(3):290–9. doi: 10.1097/00005650-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life: basic sciences review. Ann Intern Med. 1993;70:225–30. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Naylor CD, Juniper E, Heyland DK, Jaeschke R, Cook DJ. Users’ guides to the medical literature XII. How to use articles about health-related quality of life. JAMA. 1997;277:1232–7. doi: 10.1001/jama.1997.03540390062037. [DOI] [PubMed] [Google Scholar]
- 20.Hays R, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res. 1993;2(6):441–9. doi: 10.1007/BF00422218. [DOI] [PubMed] [Google Scholar]
- 21.Herrman H, Metelko Z, Szabo S, Rajkumar S, Kumar S, Vanheck G, et al. Study protocol for the World-Health-Organization project to develop a quality-of-life assessment instrument (WHOQoL) Qual Life Res. 1993;2:153–9. doi: 10.1007/BF00435734. [DOI] [PubMed] [Google Scholar]
- 22.Horsman J, Furlong W, Feeny D, Torrance G. The health utilities index (HUI): Concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FTD, Meals RA. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med. 2009;361(10):968–79. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 24.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness. J Clin Epidemiol. 2000;53(5):459–68. doi: 10.1016/S0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 25.Jones CA, Feeny DH. Agreement between patient and proxy responses of health‐related quality of life after hip fracture. J Am Geriatr Soc. 2005;53(7):1227–33. doi: 10.1111/j.1532-5415.2005.53374.x. [DOI] [PubMed] [Google Scholar]
- 26.Koot H. Quality of life in child and adolescent illness: concepts, methods and findings. 2002
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 28.Lo S, Pickford M. Current concepts in Dupuytren’s disease. Curr Rev Musculoskelet Med. 2013;6(1):26–34. doi: 10.1007/s12178-012-9148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massy-Westropp N, Krishnan J, Ahern M. Comparing the AUSCAN Osteoarthritis Hand Index, Michigan Hand Outcomes Questionnaire, and Sequential Occupational Dexterity Assessment for patients with rheumatoid arthritis. J Rheumatol. 2004;31(10):1996–2001. [PubMed] [Google Scholar]
- 30.Michou L, Lermusiaux JL, Teyssedou JP, Bardin T, Beaudreuil J, Petit-Teixeira E. Genetics of Dupuytren's disease. Joint Bone Spine. 2012;79(1):7–12. doi: 10.1016/j.jbspin.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Picardo NE, Khan WS. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon. 2012;10(3):151–8. doi: 10.1016/j.surge.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Schoneveld K, MsC PT. Clinimetric evaluation of measurement tools used in hand therapy to assess activity and participation. Practice. 2009;1:2. doi: 10.1016/j.jht.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. USA: Oxford University Press; 2008. [Google Scholar]
- 34.Thoma A, Cornacchi S, Lovrics P, Goldsmith C. User’s guide to the surgical literature: how to assess an article on health-related quality of life. Can J Surg. 2008;51(3):215–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Thoma A, Farrokhyar F, McKnight L, Bhandari M. Practical tips for surgical research: how to optimize patient recruitment. Can J Surg. 2010;53(3):205–10. [PMC free article] [PubMed] [Google Scholar]
- 36.Thoma A, McKnight LL. Quality-adjusted life-year as a surgical outcome measure: a primer for plastic surgeons. Plast Reconstr Surg. 2010;125(4):1279. doi: 10.1097/PRS.0b013e3181d0ae58. [DOI] [PubMed] [Google Scholar]
- 37.Thoma A, McKnight L, Knight C. The use of economic evaluation in hand surgery. Hand Clin. 2009;25(1):113–23. doi: 10.1016/j.hcl.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren's contracture unfolded. BMJ Clin Res Ed. 2006;332(7538):397–400. doi: 10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware JEJ. SF-36 health survey update. Spine. 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 40.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care. 1995;33(4):AS264–79. [PubMed] [Google Scholar]
- 41.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]