Abstract
Nerve conduits and acellular nerve allograft offer efficient and convenient tools for overcoming unexpected gaps during nerve repair. Both techniques offer guidance for migrating Schwann cells and axonal regeneration though utilizing very different scaffolds. The substantially greater amount of animal and clinical data published on nerve conduits is marked by wide discrepancies in results that may be partly explained by a still poorly defined critical repair gap and diameter size. The available information on acellular allografts appears more consistently positive though this tool is also hampered by a longer but also limited critical length. This article reviews the current relative literature and examines pertinent parameters for application of both acellular allograft and nerve conduits in overcoming short nerve gaps.
Keywords: Acellular nerve allograft, Nerve conduits, Nerve repair
Introduction
Peripheral nerve injuries occur in as many as 3 % of all trauma patients and, also, as the result of surgery, either in oncologic resection or inadvertently [41]. Despite four decades of research on nerve regeneration, clinical outcomes following peripheral nerve repair are unpredictable and often unsatisfactory [57].
When possible, end-to-end repair of severed nerves remains the standard of care; however, regenerating axons are unable to traverse scar tissue or disorganized intraneural architecture within the zone of injury [11, 39, 57]. In order to allow normal regeneration, the resection of damaged nerve segments is often necessary, but debridement may result in gaps which cannot be closed without tension. High tension closures are now well understood to inhibit Schwann cell activation and axon regeneration [58]. Indeed, nerve stretch greater than 15 % compromises tissue perfusion and results in ischemic damage with further scar generation [10, 57]. It is often the case, then, that the surgeon must choose between an inadequate debridement and creating a gap that must be bridged.
Failure to recognize the zone of injury and reticence to sacrifice a donor nerve are likely culprits in under-resection and high-tension closures [59]. However, in recent years, the availability of more convenient repair tools in the form of “off-the-shelf” nerve conduits and acellular nerve allograft allows efficient management of unexpected gaps. For these new tools to be used effectively, their limitations and appropriate application must be appreciated.
Nerve Conduits
The concept of using a tube to connect two severed nerve stumps is inherently simple and appealing: nerve stumps are pulled into the ends of a tube—or tube like structure—which restricts the direction of regenerating axonal processes towards the distal nerve segment while at the same time protecting the regenerating axons from intervening scar tissue or trauma. The protected microenvironment created by the conduit contains an enriched milieu of concentrated neurotrophic factors [13, 29]. A fibrin clot forming between the nerve stumps acts as the scaffold to support the essential migration of Schwann cells and, subsequently, axons [43, 56].
The potential benefits of an “off-the-shelf” conduit include no donor deficit, avoidance of two suture lines, convenience, and proven effectiveness in the right situations. Though there is support for using blood vessels to bridge short gaps [4, 8, 53], harvesting the graft may still require additional time and a second surgical dissection. One study failed to demonstrate any cost savings in harvesting a vein graft compared with using a commercially available conduit [40]. Suture lines (between a formal nerve graft and nerve stumps) are opportune to technical errors resulting in scarring or poor fascicular alignment. With a conduit, axons still have to transition from neural architecture, to fibrin clot scaffolding, and back to neural architecture. Though serial transitions are theoretically opportunities for axon escape or loss, Evans et al. demonstrated that this arrangement may actually encourage end-organ specificity. In a rodent model, purposely malaligned sciatic nerves recovered better if fixed with a conduit than if directly sutured [18]. Schwann cells are essential to guide axons between these transition sites.
Current FDA approved commercially available conduits in the USA are made of either polyglycolic acid (PGA), collagen, or polycaprolactone (PCL). These materials share the common characteristics that they are semi-rigid, to resist collapse and kinking; semi-permeable, to allow the diffusion of oxygen and nutrients to support nerve regeneration; and absorbable. Historically, nonabsorbable conduits, such as silicone, while effective in supporting nerve regeneration, eventually produced an inflammatory reaction and scarring around the nerve. The resultant irritation often required a second surgery for tube removal [31, 32].
In general, there is supportive data for all three conduit materials. Efficacy of collagen and PGA tubes has been demonstrated in primate models [1, 15], though a recent rodent study indicated that polycaprolactone conduits outperformed the other two materials [45]. In this study, the PGA group performed the worst with inferior axon regeneration and several tubes collapsing, but this may have been related to poor sizing of this conduit for the rat sciatic nerve being repaired (the company did not make a conduit small enough diameter for this nerve so the authors used a tube that was too large). Polycaprolactone (PCL) does have a longer degradation life and its crystalline structure remains impermeable to fluid longer than the other two available synthetics [46]. This potential biological advantage, however, must be weighed against challenging handling characteristics. Polycaprolactone tubes are so rigid that passing a microsuture through the wall can be difficult, and concerns about extrusion, fistula formation, and foreign body reaction, especially in areas of thin overlying tissue or around joints, have been raised [7, 14].
Conduit Application
With no convincing clinical data that one commercially available conduit is better than another, the important parameters to keep in mind when considering conduit repair are nerve diameter and gap size. The working length seems to be the most hotly debated topic with most published recommendations indicate a high level of effectiveness for gaps up to 3 cm though repair of even longer gaps has been reported. For small gaps, supportive evidence for conduit effectiveness is quite strong. Median and ulnar nerve repairs approximated with silicone conduits over a 5-mm gap recovered equivalently to primary repairs [31]. Rinker and Liau reported 36 digital nerve repairs using conduits to overcome an average gap of 9 mm and Taras et al. reported on 22 digital nerve repairs (mean gap of 12 mm) with consistent, good results [40, 51]. Haug et al. utilized a battery of tests including functional assessment, sensory testing, ultrasound, and electrodiagnostic testing to conclude that “acceptable” results were obtained in 60 % of 45 digital nerve repairs with average gap of 12 mm and range up to 26 mm [21] (though his scale of “acceptable” was less stringent than others). However, when the focus is shifted to the results of conduit repair across longer gaps, successful regeneration can occur, but the outcome is much less predictable. Weber et al., in a prospective human study comparing autograft to conduit repair of digital nerve injuries, concluded that conduits were superior for defects of up to 3 cm. Deeper analysis of this study, however, reveals that the longest defect in the study was only 25 mm and the average gap size only 7 mm (±5.6 mm). The quality of recovery decreased with longer gaps and one third of repairs for gaps between 5 and 25 mm had poor outcomes [54]. Lohmeyer et al. reported 75 % success in all conduit assisted digital nerve repairs but complete failure in all repairs with a gap greater than 15 mm [28]. Other reports have been even more pessimistic regardless of gap size. Chiriac et al. looked at 27 nerve repairs throughout the upper extremity utilizing PCL conduits for gap sizes averaging 11 mm (up to 25 mm; and diameters ranging from 1.5 to 4 mm). Only six patients, five of which had digital nerve repairs, had satisfactory outcomes [7].
Nerve diameter may also be an issue with conduit efficacy. Though conduits have been shown to support major peripheral nerve regeneration in a primate model, the macaque monkeys used in these studies are comparable in size to a large house cat. Additionally, support of major peripheral nerve repairs has been definitively demonstrated in humans but for very short 3–5-mm gaps [31]. More recently, Dienstknecht et al. reported on nine median nerve repairs using conduits for defects of 1 to 2 cm. Six patients obtained M4 intrinsic muscle recovery and seven patients had static two-point discrimination of 10 mm or less [16]. Interestingly, all patients suffered sharp lacerations (glass, knife, etc.) and all were fixed in less than 18 h raising some question regarding whether a true gap existed. Conduit assisted repair of major peripheral nerve repairs with more legitimate gaps is less optimistic. Chiriac et al. only reported acceptable recovery in 1 out of 12 conduit assisted major peripheral nerve repairs [7]. Moore et al. reported on four out of four major peripheral nerve repair failures and recommend against their use in that particular clinical scenario [34]. Donoghoe et al. cable grafted four separate 2.3-mm diameter conduits to reconstruct two separate 3-cm median nerve defects. Though two-point discrimination and abductor pollicis function reportedly recovered in both patients, patient #2 had no recordable signals during follow-up electrodiagnostic testing [17] leaving some room for skepticism.
Fibrin clot instability seems to be the primary factor leading to these conduit size limitations (Fig. 1). This theory has been validated in the lab by Lundborg three decades ago who demonstrated loss of fibrin stability with an increasing distance between nerve ends within a silicone nerve tube [30]. Failure to predictably form a fibrin clot means that Schwann cells and axons would not have a scaffold to guide and support their migration across the conduit.
Fig. 1.
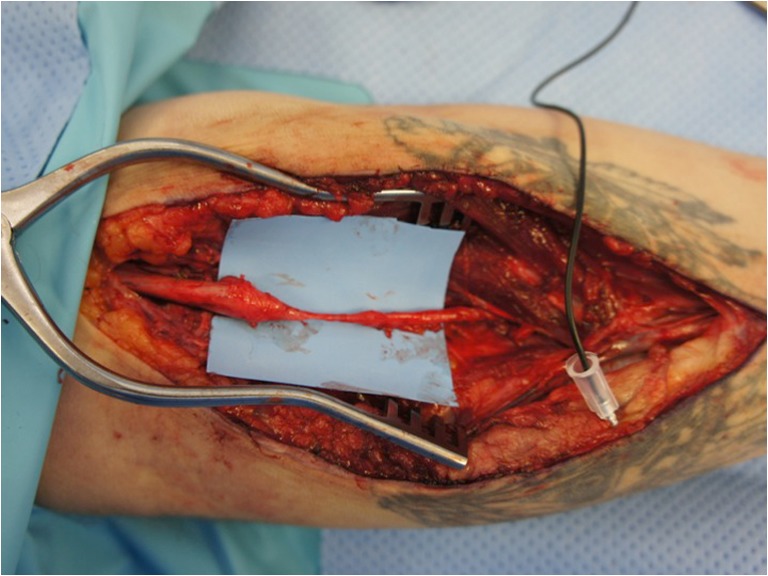
Median nerve in proximal forearm during exploration after failed reconstruction of a 3-cm gap with a nerve conduit. Note the thin and inadequate regeneration
Regardless of the size of the nerve being repaired, the selected conduit diameter should closely fit the stumps. The use of an undersized conduit is not only conceptually poor given the likely resultant constriction of the regenerating nerve, but also technically difficult (to squeeze the stump into a tight conduit) [12, 33]. Asymmetrical fibrosis of the two nerve stumps may create a challenge in choosing an appropriate diameter conduit, though most often, “size-mismatches” are a result of erroneous estimation and judgment by the surgeon. If an oversized tube were mistakenly chosen, however, the cost of replacing the tube with a smaller size would be a substantial obstacle and in many cases the larger tube would be accepted. Despite the theoretical risk of this decision—loss of the protected microenvironment—there is almost no data on the potential consequences. The only paper identified on this topic was from 1968, and studied silastic tube repairs of canine and primate nerves. The authors suggested that the conduit should be two and one half to three times the diameter of the nerve though in a somewhat contradictory and minimally helpful statement, the authors recommended achieving a “watertight seal [45].” We looked at this in a rodent sciatic nerve (1.5 mm diameter) repair model in which a 10-mm gap was bridged with either autograft, or a 1.5-mm, 2-mm or 3-mm collagen nerve tube. Similar to Shin et al.’s observations, the oversized (3 mm) tube suffered from collapse and failed to adequately support axons. The 1.5-mm fitted conduits did function best suggesting that this previously underappreciated parameter is important and might explain some of the unpredictability and inconsistencies associated with conduit use.
Allograft
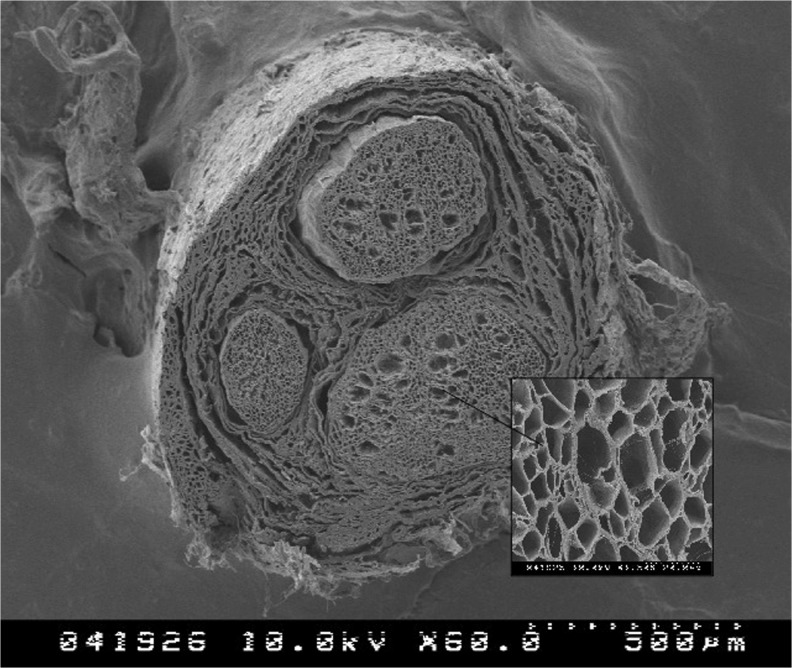
Based on the data available, properly sized conduits are effective bridging tools for 1 to 2-cm gaps in small diameter nerves. However, as many injuries involve larger diameter nerves or larger defects, other options would be beneficial. Since the primary weakness of conduits is the reliance on fibrin clot formation and stability, a significant advantage in an alternative bridging tool would be to provide internal architecture that could provide the crucial scaffolding to support Schwann cell and axon migration [3]. This architecture can be found in nerve allograft (Fig. 2).
Fig. 2.
Electron microscopy image of AxoGen processed acellular nerve allograft demonstrating internal microstructure (courtesy of AxoGen, Inc.)
The idea of using nerve allograft has obvious appeal since it could offer many of the benefits of conduits (off-the-shelf convenience, avoidance of a donor defect, “unlimited” supply) but might offer superior axon support and potentially even some neurotrophic factors or guidance cues. Unfortunately, substantial immunogenicity in human nerve tissue necessitated the concomitant administration of immunosuppressive agents [38]. Though there is data to support this approach as potentially effective, the toxicity of these agents hindered routine use of donated human allograft and its use was relegated to extreme clinical situations or investigational research [35].
Advances in tissue processing, however, overcame this obstacle with the development of detergents and protocols capable of decellularizing and removing immunogenic components from nerve allograft. The removal of Schwann cells is unfortunately critical to creating an immunotolerant product, and, probably, also represents the most significant deficit in this product. However, nerve architecture and guidance cues in the form of laminin are preserved. Though without cells, Wallerian degeneration and the clearing of endoneural tubes will not occur, axon remnants and other inhibitory proteoglycans are removed during processing [27, 37]. Schwann cells are still crucial to axon regeneration and must still migrate from in situ nerve stumps into the nerve allografts to support this activity.
Compared with nerve conduits, there is only a fraction of animal and clinical data available on acellular nerve allograft. Further complicating the interpretation of the available literature is that many labs do not investigate the same material being used clinically. While the only FDA approved acellular allograft available to surgeons (in the USA) is produced from a single company (AxoGen, Inc., Alachua, Florida), labs are studying acellular allograft prepared by a variety of methods including cold preservation, freeze–thaw cycling, irradiation, lyophilization, and detergent preparations which may result in variable graft performance [22, 35, 36, 47]. AxoGen prepares their product by combining proprietary detergent processing and gamma irradiation of human nerve allograft designed to minimize microstructural damage while removing cellular remnants. Further enzymatic removal of chondroitin sulfate proteoglycan from the endoneural tube system is felt to further enhance axon regeneration [35, 37]. There are a few clinically relevant rodent studies that compare acellular allograft (AxoGen, Inc.), with commercially available conduits, and autograft (or isograft). Whitlock et al. found similar functional recovery for allograft and isograft repairs across a 14-mm defect though isograft was superior at 28 mms. The conduit was inferior at 14 mm and demonstrated no recovery when used to bridge a 28-mm gap [55]. Mid graft (conduit) histologic analysis showed equivalent axonal densities and distributions between allograft and isograft at both lengths, but diminished regeneration, especially in the 28 mm group, for conduit repairs [25]. Guisti et al. performed a similar evaluation though with a 1-cm gap and evaluation time points of 3 and 4 months. Equivalent muscle recovery and histology were reported for allograft and autograft repairs at 3 months and, although the histologic similarities were maintained at 4 months, muscle recovery was slightly better for the autograft group at this later time point. The conduit group again consistently underperformed at both time points [20].
There is limited human data available at this time as well (Fig. 3). A study conducted at the Mayo Clinic looked at ten sensory nerve repairs in the upper extremity with gap sizes ranging from 0.5 to 3 cm and noted consistently good results with all patients recovering two-point discrimination of 6 mm or better [26]. The majority of the available outcome data comes from a data acquisition study sponsored by AxoGen, Inc., but involving 25 surgeons across 12 different sites and including data on 76 nerve repairs broken down into 49 sensory, 18 mixed, and 9 motor nerves. Graft lengths ranged from 5 to 50 mm and averaged 22 mm. Though (as often occurs with nonprospective multicenter studies), a variety of assessment measurements were used across the different centers, 87 % of patients achieved M3 or S3 (Medical Research Council grading scale) or better. When broken down further, 9 out of 19 patients obtained M4 or M5 recovery—the true goal of surgical treatment of nerve injuries. These outcomes are comparable to results associated with most upper extremity major nerve repairs [19, 42]. Results were consistent across gap sizes, timing of repair, mechanism of injury, and age of patient. There were no complications related to this relatively new technology and, according to the responsible surgeon, the four revisions were due to inadequate resection of the “zone of injury” of the nerve [5]. Most recently, Taras et al. published their experience in 18 digital nerve repairs utilizing acellular allograft for defects averaging 11 mm. Two patients with repairs of 28 mm and 30 mm were among the 15 out of 18 good or excellent results [50].
Fig. 3.
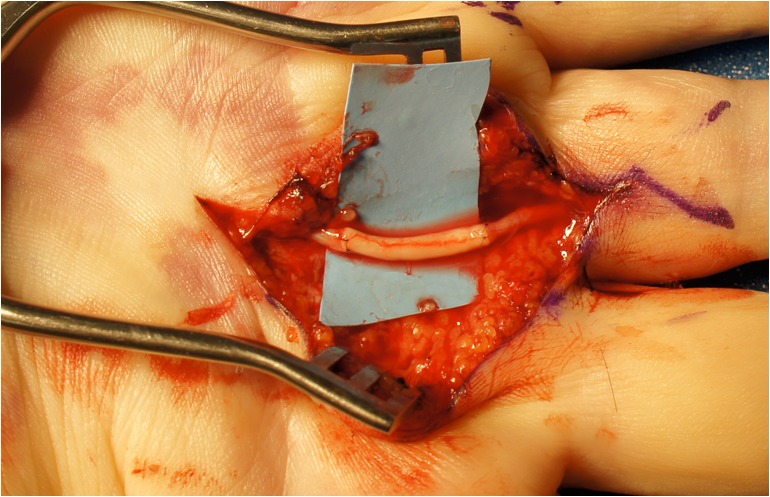
Digital nerve repaired with acellular allograft
The primary drawback of acellular allograft is the dependence on in situ Schwann cell migration into the graft [35, 48]. This migration process is restricted by a gradual quiescence of the Schwann cells which may limit the ultimate effective length of the allograft. Saheb-Al-Zamani et al. demonstrated this utilizing a transgenic rat model (in which axons appeared fluorescent green under a fluorescence-enabled microscope) and comparing axon regeneration over various lengths of acellular allograft and isograft sutured together (for up to 60 mm). In addition to decreasing axon regeneration with increasing allograft length, immunostained cellular senescence markers appeared upregulated at the portion of the allograft where axon regeneration ended [44]. Other disadvantages compared with conduits include the need to keep acellular allograft frozen until implantation and the need to suture them end to end (just like with autograft). These storage requirements limit the potential “off-the-shelf” availability and, at the very least, create additional overhead costs. Though the allograft material with its slightly “denser” feel compared with autograft has favorable handling characteristics (compared with the flimsy sural nerve autograft), end to end coaptations seem more prone to technical errors (such as poor alignment or overtightening so that fascicles are pooched to the side) which certainly inhibit axon regeneration. Using a nerve conduit as a connector (without a true gap) between the acellular allograft and nerve stump may alleviate this disadvantage [24] (Fig. 4a–b). The same technical challenges apply to repairs with autograft as well.
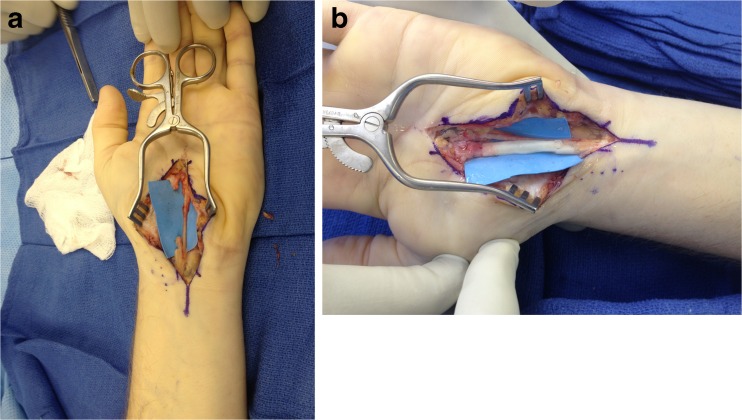
Fig. 4.
Median nerve partially transected during endoscopic carpal tunnel release. a Appearance of nerve following debridement. b Allograft sutured in place with overlying nerve connectors
Though acellular allograft length seems to be limited by the Schwann cell migration problem, this critical gap size is not known. A subgroup of twenty patients with allograft repairs between 30 and 50 mm extrapolated from the multicenter data acquisition study had a 90 % rate of meaningful recovery (S3-4 or M3-5) [9]. Though a 7-cm allograft is available, there is no published outcome information on this size at this time.
The optimal allograft caliber is also not known. The central necrosis and resulting intraneural scarring that occurs in nerve autograft whose thickness exceeds oxygen and nutrient penetration depths should not affect acellular allograft which does not contain living tissue. However, Schwann cells and axons do have metabolic demands that must be met by either diffusion or neovascularization. This process, however, has not been well delineated and it is not clear whether repair of a large diameter nerve is best accomplished with multiple thin allografts (stacked into a “cable graft” such as the current recommendation for autograft) or one thick allograft. Tang et al. tried to answer this question in a rodent model in which they compared sural nerve cable grafts with a single thicker acellular allograft and noted that the thicker graft was better [49]. However, this study is limited by the technical difficulty of creating a multistrand cable graft with rodent sural nerves and the fact that even a “thick” single graft of rodent nerve is far below the threshold of the diffusion hurdle.
Efforts to populate acellular allograft with pluripotent or neurosupportive cells are taking place in many peripheral nerve research facilities with the hope that this will improve axon regeneration and extend the workable length of this repair tool. Many challenges must be overcome to achieve this goal including identifying the correct cell type, isolating and proliferating this cell, and seeding the cell into the allograft material. Current thinking has focused on autologous Schwann cells [2], mesenchymal stem cells [23], bone marrow stromal cells [6], and skin derived precursor cells [52]. Regardless, a substantial amount of work must be accomplished before the potential of this idea can be maximized.
A direct comparison of the clinical results obtainable with acellular allograft repairs of nerve injuries versus those achievable with conduits is not possible based on the current literature. Though currently, only positive results on the clinical use of acellular nerve allograft have been published compared with mixed results for conduits, the disproportionate number of conduit studies may skew impressions. Animal data does seem to favor the allograft which seems to be effective at longer gap sizes than the conduits.
Conclusions and Recommendations
Both conduits and acellular allografts are useful tools for dealing with short nerve gaps. The convenience of either should facilitate adequate nerve debridement and the avoidance of over tensioned repairs. Though with time and mounting experience, the recommended maximum repair length of conduits seems to be decreasing while that of the acellular allograft seems to be increasing, the critical gap sizes for either tool are not known. Patients should be counseled that at least in the research lab, autograft is still the gold standard but in the right situations, either conduits or acellular allograft can achieve equivalent or at least similar results making them excellent options for nonessential nerve repairs and something that should be at least considered for more important nerves. Though autograft donor deficits or complications are typically minimal or rare, significant problems can occur. This risk must be weighed against perceived effectiveness of conduits (for 1–2-cm defects in small diameter nerves or less in larger diameter nerves) and allografts (“off-the-shelf” option up to 5 cm). The exact roles of both tools in the nerve repair algorithm continue to be defined.
Acknowledgments
Conflict of Interest
Jonathan Isaacs has received speaker and consultant fees from Axogen, Inc. and partial research support from Integra LifeSciences Corporation. Timothy Browne declares he has no conflicts of interest.
Statement of Human and Animal Rights
This article does not present original research and, therefore, does not include a statement of human or animal rights.
Statement of Informed Consent
No patient information is included in this manuscript.
Contributor Information
Jonathan Isaacs, Email: jisaacs@mcvh-vcu.edu.
Timothy Browne, Email: timothylbrowne@gmail.com.
References
- 1.Archibald SJ, Shefner J, Krarup C, et al. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 1995;15:4109–23. doi: 10.1523/JNEUROSCI.15-05-04109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aszmann OC, Korak KJ, Luegmair M, et al. Bridging critical nerve defects through an acellular homograft seeded with autologous Schwann cells obtained from a regeneration neuroma of the proximal stump. J Reconstr Microsurg. 2008;24:151–8. doi: 10.1055/s-2008-1076091. [DOI] [PubMed] [Google Scholar]
- 3.Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–8. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Benito-Ruiz J, Navarro-Monzonis A, Piqueras A, et al. Invaginated vein graft as nerve conduit: an experimental study. Microsurgery. 1994;15:105–15. doi: 10.1002/micr.1920150205. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. doi: 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Ou YC, Liao SL, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Chiriac S, Facca S, Diaconu M, et al. Experience of using the bioresorbable copolyester poly(dl-lactide-epsilon-caprolactone) nerve conduit guide Neurolac for nerve repair in peripheral nerve defects: report on a series of 28 lesions. J Hand Surg Eur Vol. 2012;37:342–9. doi: 10.1177/1753193411422685. [DOI] [PubMed] [Google Scholar]
- 8.Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86:928–34. doi: 10.1097/00006534-199011000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg [Am] 2012;37:2340–9. doi: 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Clark WL, Trumble TE, Swiontkowski MF, et al. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg [Am] 1992;17:677–87. doi: 10.1016/0363-5023(92)90316-H. [DOI] [PubMed] [Google Scholar]
- 11.Dahlin LB. Techniques of peripheral nerve repair. Scand J Surg. 2008;97:310–6. doi: 10.1177/145749690809700407. [DOI] [PubMed] [Google Scholar]
- 12.Dahlin LB, Lundborg G. Use of tubes in peripheral nerve repair. Neurosurg Clin N Am. 2001;12:341–52. [PubMed] [Google Scholar]
- 13.Danielsen N, Varon S. Characterization of neurotrophic activity in the silicone-chamber model for nerve regeneration. J Reconstr Microsurg. 1995;11:231–5. doi: 10.1055/s-2007-1006537. [DOI] [PubMed] [Google Scholar]
- 14.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63–8. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- 15.Dellon AL, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988;82:849–56. doi: 10.1097/00006534-198811000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Dienstknecht T, Klein S, Vykoukal J, et al. Type I collagen nerve conduits for median nerve repairs in the forearm. J Hand Surg [Am] 2013;38:1119–24. doi: 10.1016/j.jhsa.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Donoghoe N, Rosson GD, Dellon AL. Reconstruction of the human median nerve in the forearm with the neurotube. Microsurgery. 2007;27:595–600. doi: 10.1002/micr.20408. [DOI] [PubMed] [Google Scholar]
- 18.Evans PJ, Bain JR, Mackinnon SE, et al. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559:315–21. doi: 10.1016/0006-8993(91)90018-Q. [DOI] [PubMed] [Google Scholar]
- 19.Frykman G, Gramyk K. Results of nerve grafting. In: Gelberman R, editor. Operative nerve repair and reconstruction. Philadelphia: JB Lippincott; 1991. pp. 553–68. [Google Scholar]
- 20.Giusti G, Willems WF, Kremer T, et al. Return of motor function after segmental nerve loss in a rat model: comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen) J Bone Joint Surg Am. 2012;94:410–7. doi: 10.2106/JBJS.K.00253. [DOI] [PubMed] [Google Scholar]
- 21.Haug A, Bartels A, Kotas J, et al. Sensory recovery 1 year after bridging digital nerve defects with collagen tubes. J Hand Surg [Am] 2013;38:90–7. doi: 10.1016/j.jhsa.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Hiles RW. Freeze dried irradiated nerve homograft: a preliminary report. Hand. 1972;4:79–84. doi: 10.1016/0072-968X(72)90019-8. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Zhu QT, Liu XL, et al. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658–66. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Isaacs J. Major peripheral nerve injuries. Hand Clin. 2013;29:371–82. doi: 10.1016/j.hcl.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PJ, Newton P, Hunter DA, et al. Nerve endoneurial microstructure facilitates uniform distribution of regenerative fibers: a post hoc comparison of midgraft nerve fiber densities. J Reconstr Microsurg. 2011;27:83–90. doi: 10.1055/s-0030-1267834. [DOI] [PubMed] [Google Scholar]
- 26.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 2009;4:245–9. doi: 10.1007/s11552-009-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krekoski CA, Neubauer D, Zuo J, et al. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–13. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmeyer JA, Sommer B, Siemers F, et al. Nerve injuries of the upper extremity-expected outcome and clinical examination. Plast Surg Nurs. 2009;29:88–93. doi: 10.1097/01.PSN.0000356867.18220.73. [DOI] [PubMed] [Google Scholar]
- 29.Longo FM, Manthorpe M, Skaper SD, et al. Neuronotrophic activities accumulate in vivo within silicone nerve regeneration chambers. Brain Res. 1983;261:109–16. doi: 10.1016/0006-8993(83)91289-1. [DOI] [PubMed] [Google Scholar]
- 30.Lundborg G, Dahlin LB, Danielsen N, et al. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982;76:361–75. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 31.Lundborg G, Rosen B, Dahlin L, et al. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg (Br) 2004;29:100–7. doi: 10.1016/j.jhsb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Merle M, Dellon AL, Campbell JN, et al. Complications from silicon-polymer intubulation of nerves. Microsurgery. 1989;10:130–3. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- 33.Meyer RS, Abrams RA, Botte MJ, et al. Functional recovery following neurorrhaphy of the rat sciatic nerve by epineurial repair compared with tubulization. J Orthop Res. 1997;15:664–9. doi: 10.1002/jor.1100150506. [DOI] [PubMed] [Google Scholar]
- 34.Moore AM, Kasukurthi R, Magill CK, et al. Limitations of conduits in peripheral nerve repairs. Hand (N Y) 2009;4:180–6. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore AM, MacEwan M, Santosa KB, et al. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44:221–34. doi: 10.1002/mus.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myckatyn TM, Mackinnon SE. A review of research endeavors to optimize peripheral nerve reconstruction. Neurol Res. 2004;26:124–38. doi: 10.1179/016164104225013743. [DOI] [PubMed] [Google Scholar]
- 37.Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207:163–70. doi: 10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porayko MK, Textor SC, Krom RA, et al. Nephrotoxic effects of primary immunosuppression with FK-506 and cyclosporine regimens after liver transplantation. Mayo Clin Proc. 1994;69:105–11. doi: 10.1016/S0025-6196(12)61034-9. [DOI] [PubMed] [Google Scholar]
- 39.Prasad AR, Steck JK, Dellon AL. Zone of traction injury of the common peroneal nerve. Ann Plast Surg. 2007;59:302–6. doi: 10.1097/SAP.0b013e3180302668. [DOI] [PubMed] [Google Scholar]
- 40.Rinker B, Liau JY. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg [Am] 2011;36:775–81. doi: 10.1016/j.jhsa.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–73. doi: 10.1002/(SICI)1097-4598(200006)23:6<863::AID-MUS4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Ruijs AC, Jaquet JB, Kalmijn S, et al. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484–94. doi: 10.1097/01.prs.0000172896.86594.07. [DOI] [PubMed] [Google Scholar]
- 43.Rydevik BL, Kwan MK, Myers RR, et al. An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res. 1990;8:694–701. doi: 10.1002/jor.1100080511. [DOI] [PubMed] [Google Scholar]
- 44.Saheb-Al-Zamani M, Yan Y, Farber SJ, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247:165–77. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin RH, Friedrich PF, Crum BA, et al. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91:2194–204. doi: 10.2106/JBJS.H.01301. [DOI] [PubMed] [Google Scholar]
- 46.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30:574–88. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 47.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/S0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 48.Sun XH, Che YQ, Tong XJ, et al. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2009;29:347–53. doi: 10.1007/s10571-008-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang P, Kilic A, Konopka G, et al. Histologic and functional outcomes of nerve defects treated with acellular allograft versus cabled autograft in a rat model. Microsurgery. 2013;33:460–7. doi: 10.1002/micr.22102. [DOI] [PubMed] [Google Scholar]
- 50.Taras JS, Amin N, Patel N, et al. Allograft reconstruction for digital nerve loss. J Hand Surg Am. 2013 doi: 10.1016/j.jhsa.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg [Am] 2011;36:1441–6. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Walsh S, Biernaskie J, Kemp SW, et al. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–107. doi: 10.1016/j.neuroscience.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 53.Wang KK, Costas PD, Bryan DJ, et al. Inside-out vein graft repair compared with nerve grafting for nerve regeneration in rats. Microsurgery. 1995;16:65–70. doi: 10.1002/micr.1920160205. [DOI] [PubMed] [Google Scholar]
- 54.Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–45. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–99. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 56.Williams LR, Longo FM, Powell HC, et al. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218:460–70. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 57.Wood MD, Kemp SW, Weber C, et al. Outcome measures of peripheral nerve regeneration. Ann Anat =Anat Anzeiger: Off Organ Anat Ges. 2011;193:321–33. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Yi C, Dahlin LB. Impaired nerve regeneration and Schwann cell activation after repair with tension. Neuroreport. 2010;21:958–62. doi: 10.1097/WNR.0b013e32833e787f. [DOI] [PubMed] [Google Scholar]
- 59.Zachary LS, Dellon AL. Progression of the zone of injury in experimental nerve injuries. Microsurgery. 1987;8:182–5. doi: 10.1002/micr.1920080403. [DOI] [PubMed] [Google Scholar]