Abstract
Background
Platelet-rich plasma (PRP) has shown promise in the treatment of tendinopathy, including rotator cuff and lateral epicondylitis. Here, we evaluate the effect of PRP on healing in a rabbit zone II flexor tendon model.
Methods
Thirty New Zealand white rabbits underwent transection and repair of the second and fourth flexor digitorum profundus. Half of the rabbits received autologous PRP intraoperatively, while the other half underwent standard four-strand tendon repair. Tendons were examined at 2, 4, and 8 weeks postoperatively. Range of motion and ultimate tensile strength were assessed on the fourth toes, while second toes underwent histologic analysis with hematoxylin and eosin, Masson Trichrome, and Picrosirius Red, for assessment of cell count, collagen content, and collagen maturity.
Results
There were no significant differences in ultimate tensile strength between treatments at 2, 4, or 8 weeks. There was a trend towards lower tensile strength in the PRP group at 2 weeks. There was no statistically significant difference in excursion or range of motion between PRP and control tendons. Cell counts at 4 weeks were statistically significantly reduced in the PRP tendons as compared to controls. No difference in collagen content or maturity was detected.
Conclusions
In contrast to previous studies, PRP did not significantly improve ultimate tensile strength. PRP-treated tendons exhibited trends towards reduced healing, including a significant reduction in cell counts as well as a smaller increase in collagen deposition over time as compared to controls. Further study is needed to determine the precise effect of PRP on intrasynovial flexor tendon repairs.
Keywords: Platelet-rich plasma, ACP, Tendon, Hand, Zone II
Introduction
Zone II flexor tendon repair remains a surgical challenge due to the complex anatomy in the region. In this zone, both the flexor digitorum profundus and flexor digitorum superficialis must glide within a synovial sheath through a series of fibro-osseous pulleys. Any disruption in gliding, including scar tissue or excess surgical knots can negatively impact a patient’s postoperative function. Early active and passive motion has been shown to improve healing and reduce adhesions [6, 10, 19]. However, overly aggressive early mobility puts the repair at risk for failure, with many failures occurring within the first 3 weeks [42]. Earlier work has focused on optimizing mechanical properties of repair techniques and rehabilitation. Previous work on gap formation at the repair site demonstrated that gapping prevents the accrual of strength and stiffness [15]. As a result, stronger suture constructs with more strands across the repair site have been shown to improve the strength of repairs [13]. Increasing the number of strands also causes additional damage to the tendon, adds bulk, increases technical difficulty, and may cause nutritional compromise thereby impairing healing [38]. Continued improvement in flexor tendon repair may therefore depend on biologic adjuvants to tendon healing [37].
A number of growth factors have been investigated with the goal of stimulating intrinsic tendon healing and enhanced tensile strength while preserving tendon gliding properties and preventing adhesion formation. Platelet derived growth factor (PDGF) acts to stimulate production of hyaluronic acid and fibronectin, two factors that appear to be important in promoting the gliding of flexor tendons [20, 39]. PDGF has also been shown to stimulate collagen deposition in tendons, which in turn might increase tensile strength [43]. Vascular endothelial growth factor (VEGF) is found in synovial fibroblasts and its expression has been shown to increase after flexor tendon injury [5, 44]. IGF-1 has also been found to stimulate type I collagen production, and to be a mitogen for a wide variety of cell types, including but not limited to fibroblasts, chondrocytes, and osteoblasts, and is also expressed at high levels during flexor tendon healing [9, 20, 22]. Basic fibroblast growth factor (bFGF) is bound to cell surfaces and the extracellular matrix, and is released when heparin proteoglycans are degraded [20]. It causes proliferation and migration of keratinocytes, stimulates fibroblasts to produce collagenase, and stimulates proliferation of capillary endothelial cells. Transforming growth factor (TGF-β) has been linked to increased collagen deposition [24]. Inhibition of TGF-β can reduce scarring and fibrosis [4, 8].
Platelet-rich plasma (PRP) contains many of the growth factors thought to be important in tendon healing, including PDGF, IGF-1, TGF-β, VEGF, bFGF, and EGF [2, 20]. PRP is appealing for clinical application as an inexpensive source of growth factors. Because they are autologous, there is no concern for immunologic response. PRP is already widely used in orthopedics for treatment of musculoskeletal injuries [2]. Clinical applications for PRP include rotator cuff repair, Achilles tendon repair, Achilles tendinopathy, and lateral epicondylitis; however, the efficacy of PRP is controversial [3, 7, 11, 12, 17, 26, 30, 31, 33, 35]. We sought to assess the potential benefit of PRP in zone II flexor tendon repair. We hypothesized that PRP would increase tensile strength while minimizing scar tissue and inflammation.
Materials and Methods
All rabbit experiments were performed with procedures approved by our Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines for animal research. All institutional and national guidelines for the care and use of laboratory animals were followed. One fellowship-trained hand surgeon performed all tendon repairs.
Animal Model
We performed a controlled trial of intraoperative application of autologous PRP prepared with a commercially available kit (Arthrex, Naples, FL). Rabbits were randomly assigned to treatment and control conditions, and two toes per rabbit were incised and repaired. Quantitative mechanical testing was performed on the fourth toe to assess outcomes of tendon tensile strength and range of motion. Quantitative histological analysis was performed on the second toe to measure cellularity, collagen deposition, and collagen maturity.
A total of 37 New Zealand White rabbits weighing 3.0–3.5 kg were used in the study.
Preparation of PRP
In both groups, the auricular artery was cannulated intraoperatively and 5 mL of blood was drawn into a double syringe system. In control rabbits, blood was discarded. The syringe was centrifuged at 1,300 rmp for 5 min. The supernatant was then drawn into the inner syringe, which constituted our PRP. Prior to skin closure, 0.25 cc PRP was dripped directly onto the tendon. After skin closure, another 0.25 cc PRP was injected subcutaneously into the repair site. All PRP was used within 30 min of preparation.
Surgical Technique
All rabbits were anesthetized with ketamine 30–35 mg/kg and xylazine 5 mg/kg. Anesthesia was maintained with isofluorane 1–5 % as needed. The left front paw was shaved, prepped, and draped. A digital block was performed by infiltrating 0.25 % bupivacaine into the radial and ulnar web spaces of the second and fourth digits. Fourth toes were incised and repaired first, followed by the second toe. A longitudinal incision was made on the plantar aspect of the left forepaw over the A1 pulley. The flexor tendon sheath was identified and incised between the A1 and A2 pulleys, and the deep flexor tendon identified, isolated, and sharply transected. Tendons were immediately repaired using a four-strand technique with 6–0 prolene suture, with a modified Kessler suture technique augmented with a figure of eight suture. In the treatment group, 0.5 mL of autologous PRP was applied to the repair site. Blood was also drawn from the control group rabbits and discarded. Wounds were closed using buried interrupted absorbable sutures, and sterile dressings were applied.
Postoperatively, a 25-μg-per-hour fentanyl patch was applied to the ear and buprenorphine 0.02–0.05 mg/kg was administered. Forelimbs were immobilized with a bulky dressing and volar splint for a total of 2 weeks postoperatively and rabbits were allowed to roam freely in their cages. Rabbits appearing to be in pain postoperatively were given meloxicam 0.1–0.2 mg/kg as needed. Rabbits were sacrificed at 2, 4, or 8 weeks postoperatively. Repairs found to be ruptured at the time of analysis were replaced in order to ensure five animals per time point. The left forepaws were harvested at 2, 4, and 8 weeks after surgery. Five contralateral paws from control group rabbits were arbitrarily chosen and harvested. The FDP of the fourth digit was incised and repaired on the day of testing for a day 0 control group.
Biomechanical Analysis
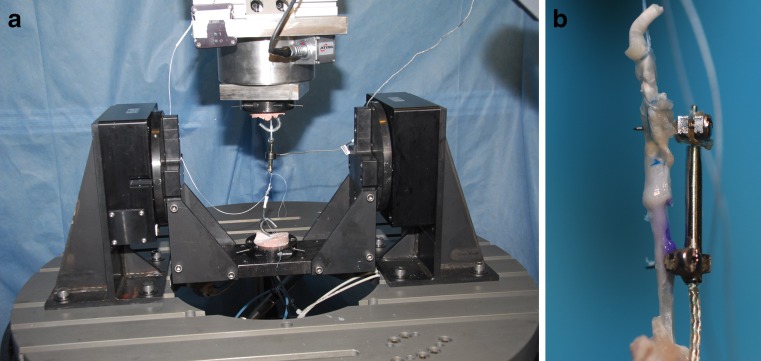
All mechanical testing was performed during the day of sacrifice. Tissues were stored at room temperature after harvest. Forelimbs were amputated at the mid-forearm. The fourth digits were left intact while the remaining digits were carefully removed from the paw. Toes were mounted onto a custom testing jig as previously described using k-wires through the metacarpals and clamps (Fig. 1) [41]. The metacarpophalangeal (MP) and interphalangeal joints were freely mobile. A 0.45-N weight was sutured to the distal end of the toe to achieve full extension. A 4.4-N weight was then sutured to the proximal end of the flexor digitorum profundus tendon and allowed to hang to gravity to produce flexion.
Fig. 1.
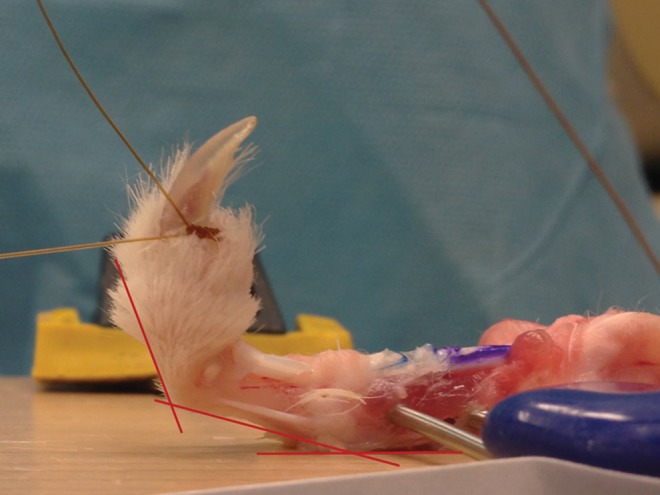
Toes were mounted to a testing jig with k-wires and clamps. Tendon excursion from the A1 pulley was measured in millimeters, and a digital photograph was taken to measure metacarpophalangeal and proximal interphalangeal joint range of motion in degrees
Weights were applied only once so as not to loosen scar tissue. Tendon glide was assessed with measurements of tendon excursion from the A1 pulley using a microcaliper. Digital photographs were taken of each toe in flexion. Angular range of motion (ROM) over the MP and proximal interphalangeal (PIP) joints was measured on the photographs by two of the coauthors, in an independent, blinded fashion.
Tendons were then dissected free from the sheath and surrounding peritendinous scar. The ultimate tensile strengths were determined using a custom materials testing system. An R2000 hexapod robot (Mikrolar, Boston, MA) was used to distract the tendon at a constant velocity of 0.2 mm/s (Fig. 2). The force was recorded with an in-line LCFD-10 (Omegadyne, Sunbury, OH) load cell, with a reported accuracy of ±0.067 N.
Fig. 2.
Tendon breaking strength was tested using a Hexapod R2000 robot (left). A close-up of a tendon mounted to the testing jig (right)
Histological Analysis
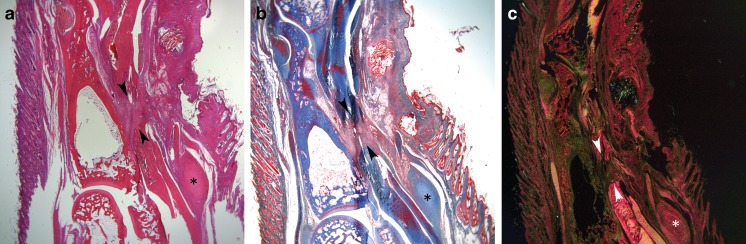
Histological analysis was carried out for 2- and 4-week tendons only. Sections of intact second toes were prepared by our institution’s histology core in a standard fashion. Sagittal sections at a thickness of 5 μm were made. Sections from each specimen were stained with hematoxylin and eosin (H&E), Masson trichrome, and Picrosirius Red (PSR) (Fig. 3). Sections were analyzed with a Leica DM2500 microscope (Wetzlar, Germany) and digitized with a SPOT Insight 4 digital camera using SPOT Advanced software (SPOT Imaging, Sterling Heights, MI). A polarizing filter was used to enhance collagen birefringence of PSR-stained slides.
Fig. 3.
Representative sections from one specimen stained with H&E (a), Masson Trichrome (b), and Picrosirius Red (c). Images are sagittal sections centered on the proximal phalanx with the distal end of the digit at the top of the screen. The asterisk symbol marks the A1 pulley on each specimen, while the scar formed between the proximal and distal ends of the flexor digitorum profundus is marked with arrow heads
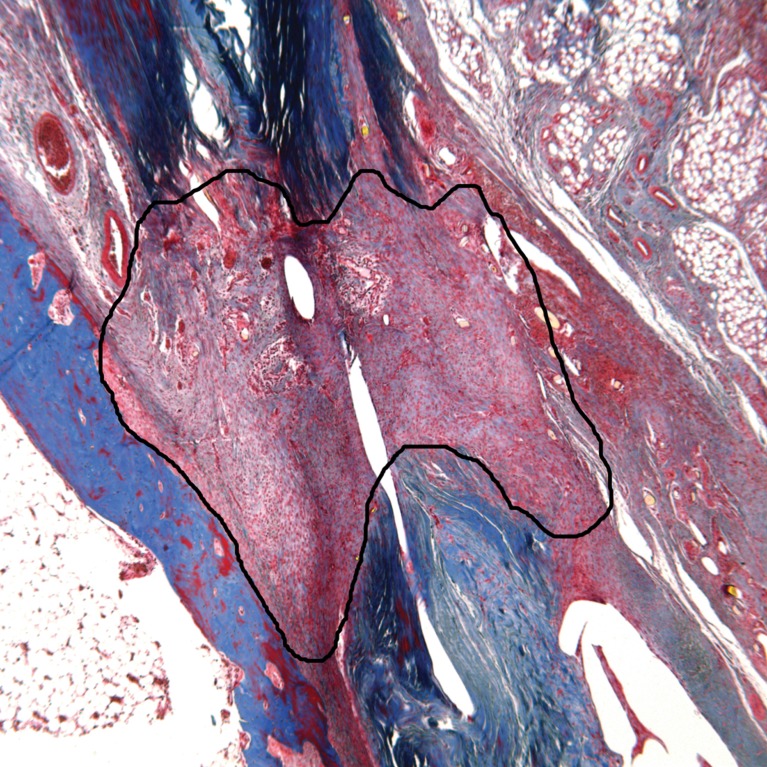
Quantitative analysis was carried out using public domain ImageJ software [21]. Cell counts were performed on H&E-stained specimens at ×630 magnification for five high-powered fields per specimen, distributed throughout the tendon scar. Collagen quantification was carried out at ×50 magnification. One trichrome-stained section per specimen was deconvoluted and thresholded. The scar tissue region of interest was selected and collagen content reported as a percentage of total area (Fig. 4). Collagen maturity was assessed using PSR-stained sections at ×50 magnification viewed under crossed polarized light. Thick, mature fibers appear as yellow, orange, or red while thin, immature fibers appear green or blue [32]. Images were split into red, green, and blue stacks and scar tissue was selected. Red fibers were thresholded and quantified, and reported as a percentage of the area.
Fig. 4.
Collagen analysis was carried out on trichrome-stained sections with color deconvolution and thresholding (not shown). The scar tissue area of interest was selected (above) and collagen was reported as a percentage of the area
Statistics
Significance was set at alpha = 0.05. Excursion was calculated as a mean distance in millimeters and standard deviation. Range of motion was calculated as a mean absolute degrees and standard deviation, while ultimate tensile strength was calculated as a mean force in newtons and standard deviation. Means for biomechanical data points were compared with t tests.
Means for cell counts were compared using a Student’s t test, while collagen content and maturity were compared with the nonparametric Wilcoxon rank sum test.
Results
There were seven repairs found to be ruptured at the time of analysis: two from each of the 2 week control, 2 week PRP, and 8 week PRP groups, and one from the 4 week control group. These seven were replaced so that five rabbits per group in each time point remained for analysis. All rabbits survived to their planned end-point. There were no infections or complications from anesthesia.
Biomechanical Analysis
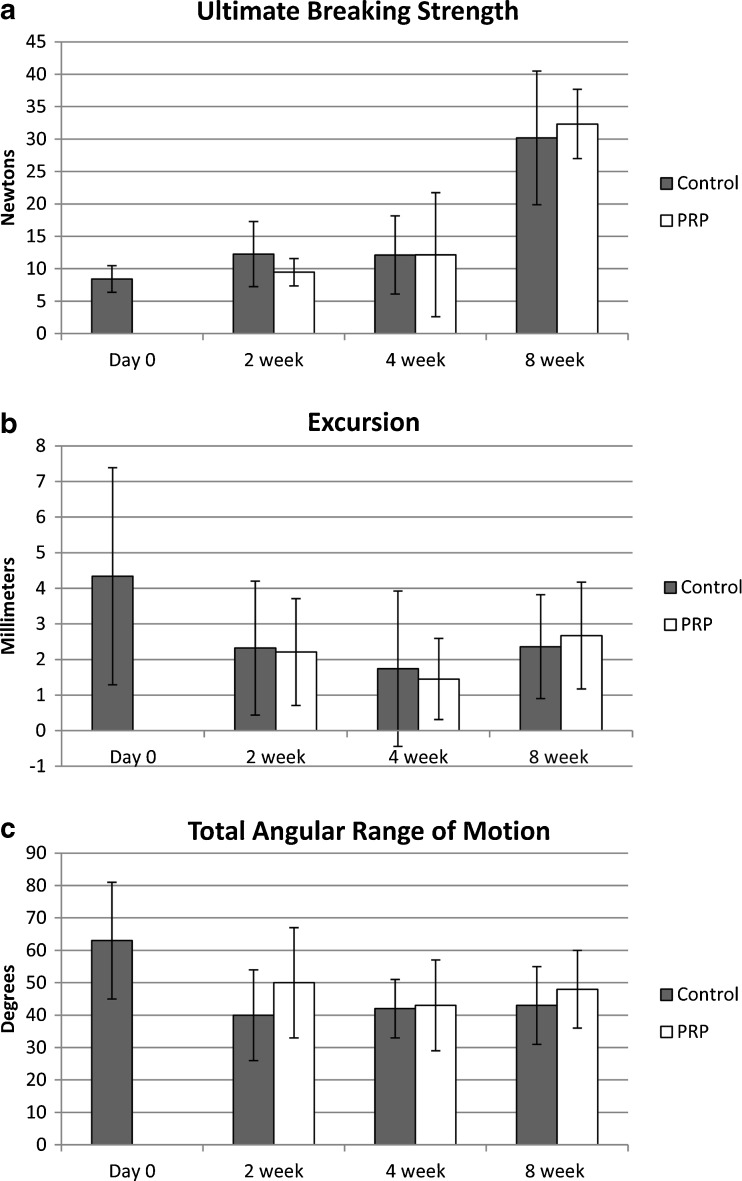
Biomechanical results are summarized in Fig. 5. The ultimate tensile strength was significantly increased in both PRP and control groups at 8 weeks as compared to the other time points (p < 0.0005 for controls and <0.0001 for PRP). There was no significant difference in ultimate tensile strength between the control and PRP groups at 2 weeks (p = 0.30), 4 weeks (p = 0.99), or 8 weeks (p = 0.69). There was a trend towards lower tensile strength in the PRP group at 2 weeks but this was not statistically significant.
Fig. 5.
Results of biomechanical analysis demonstrate no difference between PRP and control tendons for ultimate breaking strength (a), tendon excursion from the A1 pulley (b) or total angular range of motion measured as the sum of the arc of motion at the metacarpophalangeal joint and proximal interphalangeal joints (c)
There was excellent interobserver reliability for MP range of motion measurements, with a correlation coefficient (R squared) of 0.85. There was moderate reliability for PIP ROM, with a correlation coefficient of 0.66. There was no significant difference between time points or treatment groups for excursion, MP range of motion, PIP range of motion, or total range of motion. Total ROM trended higher at 2 and 8 weeks in the PRP group, but did not reach significance (p = 0.29 and p = 0.58 respectively).
Histological Analysis
Cellularity
Cell count per high powered field at 4 weeks was significantly lower in the PRP tendons than for the control tendons (213 vs. 269, p = 0.02) (Table 1). There was no significant difference between PRP and control tendons at 2 weeks (p = 0.85). Over time, both groups exhibited a significant reduction in cell counts, dropping from 311 to 269 in the control group (p = 0.03) and from 314 to 213 in the PRP group (p = 0.0005).
Table 1.
Results of histological analysis for cell counts, collagen content, and collagen maturity
| Cell count mean (95 % CI) |
Collagen content % area (95 % CI) |
Mature collagen Percent (95 % CI) |
|
|---|---|---|---|
| 2-week control | 311 (281–339) | 42 % (33–51) | 19 % (15–23) |
| 2-week PRP | 314 (277–353) | 49 % (42–57) | 19 % (17–21) |
| 4-week control | 269 (244–294) | 62 % (47–76) | 28 % (19–36) |
| 4-week PRP | 213 (171–254) | 57 % (40–73) | 25 % (9–41) |
CI confidence interval, PRP platelet-rich plasma
Collagen Content
There was no statistically significant difference in collagen content between PRP and control tendons at 2 weeks (p = 0.11) or 4 weeks (p = 0.37) (Table 1). There was also no difference between treatment groups in collagen maturity at these time points (p = 0.66 for both 2 and 4 weeks). In control tendons, the increase in collagen content between 2 and 4 weeks approached significance (p = 0.06) and the increase in amount of mature collagen content was significant (p = 0.03). In contrast, collagen content and maturity did not increase in the PRP-treated tendons between 2 and 4 weeks (p = 0.21 and p = 0.11, respectively).
Discussion
Platelet-rich plasma and the ability to deliver supra-physiologic doses of growth factors and cytokines to a tendon repair have theoretical benefits in tissue regeneration. Platelet-rich plasma has been demonstrated to benefit tendon healing in rotator cuff repairs and animal tendon repair models [3, 23, 28, 31, 34]. A recent study by Sato et al. of PRP for flexor tendon repair in a rabbit model demonstrated statistically significant increase in tendon tensile strength from 10.0 to 14.7 N, with addition of PRP-impregnated fibrin matrix at 2 weeks postoperatively as compared to controls repaired without PRP [34]. In the current study, PRP did not improve ultimate tensile strength of zone II flexor tendon repairs as compared to controls at 2, 4, or 8 weeks. In contrast to the findings of the Sato study, at 2 weeks, PRP-treated tendons trended towards decreased tensile strength with an average breaking strength of 9.48 N as compared to 12.26 N for controls.
One possible explanation for the different results is the variation in the PRP preparations. In the study by Sato et al., the PRP was embedded in a fibrin matrix, which allowed for a slow release of growth factors over time [34]. Another popular method of preparation involves a two-step centrifugation process [3, 23, 31, 35]. In a recent study evaluating the effects of different PRP separation methods, Mazzocca et al demonstrated that varying spin processes can affect platelet and white blood cell concentrations [29]. Our PRP was produced in a single, short centrifugation step. While single-step preparation systems (including the one used in the current study) were shown to produce an adequate concentration of platelets for clinical use, it was also shown that the preparations varied considerably in content of white blood cells and growth factors between repeated blood draws on the same individual and between individuals [29]. Another study demonstrated that increasing platelet concentration was associated with increased anabolic signaling, while increased leukocyte concentration is associated with increased catabolic cytokines [40]. The variation in PRP preparation methods, content, and dosing schedule is often cited as a confounding factor when comparing data across studies [1, 7, 18, 25, 36]. We chose the current PRP system for its ease in preparation with a commercial double syringe and single short centrifuge cycle, and its lower current cost for clinical use.
Another goal of the study was to examine the effect of PRP on scar formation and tendon gliding, as measured by range of motion and tendon excursion. Previous work on adhesion formation has identified TGF-β as a key regulator of fibrosis. Suppression of TGF-β both with mannose-6-phosphate or anti-TGF-β antibodies has been shown to suppress adhesion formation and increase flexor tendon range of motion; however, the effect on tensile strength was not evaluated [4, 8]. PDGF-BB has similarly been demonstrated to improve postoperative range of motion and tendon excursion at 3 weeks, without adverse effects on tendon tensile strength [16]. PRP is known to be a source of both TGF-β and PDGF, and PRP has been shown to increase the expression of TGF-β in the first 2 weeks of healing as compared to physiologic levels [27]. We did not observe any significant effects of PRP on range of motion or excursion at any time point, though PRP-treated tendons trended toward greater range of motion at 2 weeks and 8 weeks. These results highlight one difficulty presented by the mixed content of PRP, in that effects may not be easily isolated to individual growth factors. Some of the growth factors may also have opposing effects.
PRP-treated tendons trended towards lower cellularity overall, and this trend was significant at 4 weeks. No distinction was made between cell types, however, and it is unknown whether this reduced cellularity may be attributed to fewer tenocytes, inflammatory cells, or both. Collagen content did not increase significantly between 2 and 4 weeks with PRP, and in that context, the reduced cellularity associated with PRP may be attributable in part to reduced tenocyte or fibroblast content. A recent study by Fernandez-Sarmiento et al. using PRP in the sheep Achilles tendon observed reduced fibroblast densities at both time points as compared to controls [14]. In contrast to the current study, Fernandez-Sarmiento et al. also observed more densely packed collagen and better collagen organization with PRP, and they attributed the reduced fibroblast content and increased collagen content of the PRP-treated sheep tendons to accelerated healing [14]. While our PRP-treated tendons did not demonstrate statistically significant differences in collagen content or collagen maturity as compared to control tendons, they trended towards reduced healing over time. These trends suggest that PRP does not accelerate and may have even impeded tendon healing.
There are a few limitations in our study. We looked at the effect of PRP on tendon healing and adhesions at 2, 4, and 8 weeks. It is possible that the effects of PRP are more pronounced earlier in the healing process, with a protective effect in the early postoperative period. In addition, we tested one PRP preparation and administered a single injection rather than multiple injections or sustained release. Variations in PRP preparation as well as delivery have been demonstrated to differences in concentration of leukocytes, platelets, and growth factors. Lastly, the heterogeneity of PRP preparations limits the generalizability of our results.
PRP may still be a viable source of inexpensive growth factors; however, a better understanding of the effects of each growth factor and its interactions with other signaling molecules is required. Only then will it be possible to manipulate the overall anabolic and catabolic activity of the PRP in order to produce a desired outcome. Improvements in PRP preparations and administration methods may lead to more precise concentration of each of these cytokines and potential future clinical applications in flexor tendon repair in the hand.
Acknowledgments
Statement of Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed
Conflict of Interest
No outside funds were received in support of this study. This study was supported in-kind by Arthrex, Inc. A centrifuge for the production of PRP was loaned to the authors. Arthrex, Inc also provided syringes for the production of PRP and fiberwire suture for use in the study. Author Katie Kollitz declares no other conflict of interest. Author Erin Parsons declares no other conflict of interest. Author Matthew Weaver declares no other conflict of interest. Author Jerry Huang declares the following: Consultant for Arthrex, Inc for education and product development; grants from Arthrex, Inc, for Fellowship Education; honoraria from AO North America as course faculty; payment for service on speakers’ bureau for Auxilium; travel and accommodation expense from Arthrex and Auxilium.
Statement of Informed Consent
This article does not contain human subjects or patient identifying details.
References
- 1.Abate M, Di Gregorio P, Schiavone C, Salini V, Tosi U, Muttini A. Platelet rich plasma in tendinopathies: how to explain the failure. Int J Immunopathol Pharmacol. 2012;25(2):325–34. doi: 10.1177/039463201202500202. [DOI] [PubMed] [Google Scholar]
- 2.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 3.Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029–35. doi: 10.1016/j.arthro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Bates SJ, Morrow E, Zhang AY, Pham H, Longaker MT, Chang J. Mannose-6-phosphate, an inhibitor of transforming growth factor-beta, improves range of motion after flexor tendon repair. J Bone Joint Surg Am. 2006;88(11):2465–72. doi: 10.2106/JBJS.E.00143. [DOI] [PubMed] [Google Scholar]
- 5.Bidder M, Towler DA, Gelberman RH, Boyer MI. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. J Orthop Res. 2000;18(2):247–52. doi: 10.1002/jor.1100180212. [DOI] [PubMed] [Google Scholar]
- 6.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83-A(6):891–9. [PubMed] [Google Scholar]
- 7.Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718–27. doi: 10.1016/j.arthro.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–55. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg [Am] 2008;33(10):1834–42. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Chesney A, Chauhan A, Kattan A, Farrokhyar F, Thoma A. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast Reconstr Surg. 2011;127(4):1583–92. doi: 10.1097/PRS.0b013e318208d28e. [DOI] [PubMed] [Google Scholar]
- 11.de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387–92. doi: 10.1136/bjsm.2010.076398. [DOI] [PubMed] [Google Scholar]
- 12.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. Jama. 2010;303(2):144–9. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 13.Dinopoulos HT, Boyer MI, Burns ME, Gelberman RH, Silva MJ. The resistance of a four- and eight-strand suture technique to gap formation during tensile testing: an experimental study of repaired canine flexor tendons after 10 days of in vivo healing. J Hand Surg [Am] 2000;25(3):489–98. doi: 10.1053/jhsu.2000.6456. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Sarmiento JA, Dominguez JM, Granados MM, et al. Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am. 2013;95(3):246–55. doi: 10.2106/JBJS.K.01659. [DOI] [PubMed] [Google Scholar]
- 15.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81(7):975–82. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32(3):373–9. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200–8. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 18.Gross CE, Hsu AR, Chahal J, Holmes GB., Jr Injectable treatments for noninsertional achilles tendinosis: a systematic review. Foot Ankle Int. 2013;34(5):619–28. doi: 10.1177/1071100713475353. [DOI] [PubMed] [Google Scholar]
- 19.Hatanaka H, Zhang J, Manske PR. An in vivo study of locking and grasping techniques using a passive mobilization protocol in experimental animals. J Hand Surg [Am] 2000;25(2):260–9. doi: 10.1053/jhsu.2000.jhsu25a0260. [DOI] [PubMed] [Google Scholar]
- 20.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg [Am] 2004;29(4):551–63. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Image J, U.S. National Institutes of Health [computer program]. Bethesda, MD, USA http://imagej.nih.gov/ij/1997-2012.
- 22.Jenniskens YM, Koevoet W, de Bart AC, et al. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthr Cartil. 2006;14(11):1136–46. doi: 10.1016/j.joca.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kaux JF, Drion PV, Colige A, et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound Repair Regen. 2012;20(5):748–56. doi: 10.1111/j.1524-475X.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg [Am] 2002;27(4):615–20. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 25.Krogh TP, Bartels EM, Ellingsen T, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med. 2013;41(6):1435–46. doi: 10.1177/0363546512458237. [DOI] [PubMed] [Google Scholar]
- 26.Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–35. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 27.Lyras DN, Kazakos K, Verettas D, et al. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30(11):1101–6. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 28.Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37(11):2117–25. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 29.Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308–16. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 30.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–62. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 31.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Should Elbow Surg. 2011;20(4):518–28. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Rich L, Whittaker P. Collagen and Picrosirius Red staining: a polarized light assessment of fibrillar hue and spacial distribution. Braz J Morphol Sci. 2005;22(2):97–104. [Google Scholar]
- 33.Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245–51. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 34.Sato D, Takahara M, Narita A, et al. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J Hand Surg [Am] 2012;37(7):1356–63. doi: 10.1016/j.jhsa.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38–47. doi: 10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- 36.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 37.Silva MJ, Boyer MI, Gelberman RH. Recent progress in flexor tendon healing. J Orthop Sci. 2002;7(4):508–14. doi: 10.1007/s007760200090. [DOI] [PubMed] [Google Scholar]
- 38.Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg [Am] 2000;25(2):214–35. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 39.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22(5):984–9. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–40. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 41.Tan V, Nourbakhsh A, Capo J, Cottrell JA, Meyenhofer M, O’Connor JP. Effects of nonsteroidal anti-inflammatory drugs on flexor tendon adhesion. J Hand Surg [Am] 2010;35(6):941–7. doi: 10.1016/j.jhsa.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010;92(13):2285–93. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 2001;72(3):287–92. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Liu H, Stile F, et al. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112(6):1613–9. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]