Abstract
Background
The purpose of this study is to investigate functional outcomes and cost impacts of immediate functional reconstruction performed in conjunction with limb-sparing resection of upper extremity soft tissue sarcomas.
Methods
Patients undergoing simultaneous limb-sparing upper extremity soft tissue sarcoma resection and functional reconstruction between December 1998 and March 2004 were retrospectively identified, their medical records reviewed, and costs of surgery analyzed. Functional outcomes and patient satisfaction were assessed via patient surveys and the Toronto Extremity Salvage Score (TESS).
Results
Thirteen patients met the inclusion criteria. Average follow-up was 43.3 months. Reconstructions included rotational innervated muscle flaps (n = 6), free innervated myocutaneous flaps (n = 1), and tendon transfers or grafts (n = 6). Overall survival was 85 % (n = 11) and disease-free survival was 77 % (n = 10). Average total cost of surgery was $26,655. Patients undergoing reconstruction for hand and forearm sarcomas had significantly higher total costs of surgery than those undergoing reconstruction for elbow and upper arm sarcomas. Survey response rate was 91 % (n = 10). Average TESS score was 76. Of the patients who worked preoperatively, 88 % returned to work postoperatively, and all patients who returned to work currently use their affected limb at work.
Conclusions
Patients undergoing immediate functional reconstruction for upper extremity soft tissue sarcoma resection achieved very good to excellent functional outcomes with quick recovery times and a high return-to-work rate following immediate functional reconstruction, thereby minimizing surgical cost impacts. Immediate functional reconstruction in the same surgical setting is thus a viable strategy following upper extremity soft tissue sarcoma resection.
Electronic supplementary material
The online version of this article (doi:10.1007/s11552-013-9567-9) contains supplementary material, which is available to authorized users.
Keywords: Cost analysis, Functional reconstruction, Sarcoma, Upper extremity
Introduction
It is estimated that there were 10,980 new cases of soft tissue sarcoma diagnosed in the USA in 2011, with an estimated 3,920 deaths [30]. Approximately 35 and 15 % of soft tissue sarcomas occur in the lower and upper extremities, respectively [36]. Historically, treatment of soft tissue sarcomas with simple excision resulted in very poor outcomes [3] with local recurrence rates estimated at 75–90 % [2, 33].
Treatment of soft tissue sarcomas with limb amputation significantly improved outcomes due to markedly reduced local recurrence rates [29, 32], resulting in a 50 % increase in 5-year survival [9]. Limb amputation, therefore, became the standard of care for extremity soft tissue sarcoma despite the morbidity associated with limb loss [14].
In the mid-1970s, wide-margin surgical resection of extremity soft tissue sarcomas with the addition of adjuvant therapy (radiation and/or chemotherapy) achieved recurrence rates, metastasis, and mortality equivalent to that of limb amputation [15, 24, 31, 35]. These advancements in surgical technique, radiation, and adjuvant chemotherapy increased resectability and opportunities for limb reconstruction [25, 27].
Limb-sparing surgery has become the preferred method of treatment for extremity soft tissue sarcomas, with discussion now focusing on the timing, choice, and outcomes of various reconstructive techniques [17, 26, 28]. In cases where adequate tumor resection completely eliminates or compromises crucial limb function, functional reconstructive surgery using innervated muscle or tendon transfers is preferable over non-functional soft tissue reconstruction [1, 8, 11, 12, 17, 22, 28]
We have previously shown that functional reconstructive surgery is costly yet effective in restoring limb utility and improving quality of life parameters following limb-sparing sarcoma resection [23]. However, patient perceptions of functional outcome and surgical cost impacts for immediate upper extremity functional reconstruction have not been directly addressed in prior studies, and comparisons of these parameters between proximal and distal upper extremity functional restoration procedures have not been made.
The purpose of this article is to report functional outcomes and cost comparisons for immediate functional reconstructive surgery of the upper limb following soft tissue sarcoma resection at a high-volume referral center.
Materials and Methods
Patients between December 1998 and March 2004 who underwent functional reconstruction in the same surgical setting as their upper extremity soft tissue sarcoma resection were retrospectively identified in the senior surgeon's database, and their charts were reviewed. The study was approved by our institutional review board. All patients underwent wide tumor resection by the orthopedic oncological surgery team, followed by immediate functional reconstruction by the senior author in the same surgical setting.
Patients were divided into two groups: those undergoing reconstruction for defects proximal to the elbow and those undergoing defects distal to the elbow. The two patient groups were then analyzed along several dimensions: hospital length of stay, intensive care unit days, surgical time, total charges, routine charges, pharmacy charges, radiology charges, laboratory charges, medical supply charges, physical therapy charges, and miscellaneous charges. These data were obtained from internal institutional databases. All monetary charges were adjusted to 2012 US dollars using the United States government's annual Consumer Price Index for All Urban Consumers (CPI-U).
Functional outcomes and patient satisfaction with reconstruction were assessed via patient surveys and the Toronto Extremity Salvage Score (TESS). This scoring system is a validated longitudinal tool for measuring functional outcomes following resection for soft tissue sarcomas [5–7, 23] and has been shown to be superior for monitoring clinical outcomes as compared to the Musculoskeletal Tumor Society Rating Scales [10] and the Short Form 36 questionnaires [5]. TESS surveys consisted of 30 questions presented as 5-point Likert scales. Questions related to the patient's level of difficulty performing everyday activities. The patient-reported response for each question was summed and then scaled to 100. Lower scores indicate greater difficulty with activities of daily living.
For continuous variables, statistical analysis was performed using unpaired two-tailed t tests. Two-tailed Fisher's exact test was used to compare non-continuous variables. Results were considered significant at an alpha level less than or equal to 0.05.
Results
Thirteen of 49 patients undergoing reconstruction for upper extremity soft tissue sarcoma resection met the inclusion criteria. Average age was 55 years (range 30–87). There were four men and nine women. Average follow-up was 43.3 months (range 7–85). Overall survival was 85 % (n = 11) and disease-free survival was 77 % (n = 10). 46 % of patients (n = 6) had undergone prior sarcoma resection. 85 % of patients (n = 11) achieved local disease control, and 23 % of patients (n = 3) developed metastatic disease. Of these, 66 % (n = 2) succumbed to metastatic disease. Excised tumor pathology is presented in Table 1. Average maximum tumor diameter was 5.9 cm (range 1.8–15).
Table 1.
Tumor types in the study population
| Tumor type | No. of patients (%) |
|---|---|
| Malignant fibrous histiocytoma | 5 (38) |
| Synovial cell sarcoma | 2 (15) |
| Invasive dedifferentiated osteosarcoma | 1 (8) |
| Clear cell carcinoma | 1 (8) |
| Epithelioid sarcoma | 1 (8) |
| Inflammatory myofibroblastic sarcoma | 1 (8) |
| Other | 2 (15) |
Reconstructive methods and patient parameters are presented in Tables 2 and 3. Seven patients underwent reconstruction proximal to the elbow (Figs. 1, 2, and 3; Video, Supplemental Digital Content 1), and six patients underwent reconstruction distal to the elbow (Figs. 4 and 5; Video, Supplemental Digital Content 2). Of the patients, 23 % of patients (n = 3) underwent brachytherapy: all had reconstruction for hand and forearm sarcomas; 85 % (n = 11) underwent radiotherapy; 61 % (n = 8) and 53 % (n = 7) received preoperative or postoperative radiotherapy, respectively; and four patients (31 %) received both preoperative and postoperative radiotherapy. Average operative time was 643 min (range 471–925), and average cost of surgery was $26,655 per patient (range $20,924—$40,892).
Table 2.
Reconstructive methods used for immediate functional restoration following upper extremity soft tissue sarcoma excision
| Reconstructive method | No. (%) |
|---|---|
| Pedicled latissimus dorsi | 6 (46) |
| Tendon allograft | 4 (31) |
| Tendon transfer | 2 (15) |
| Free gracilis | 1 (8) |
Table 3.
Patient information
| Patient | Sex | Age | Tumor location | Histology | Tumor grade | Brachytherapy | Radiation | Number of prior excisions | Reconstructive procedure | Complications | Recurrence | Secondary revisions/procedures | Current disease status | TESS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 26 | Right forearm extensor component | Epithelioid sarcoma | 3 | No | Preop, postop | 2 | Non-innervated latissimus free flap; allograft tendons to forearm extensor compartment | None | Yes | Wide local excision of local recurrence; ulnar reconstruction with free fibula flap | Metastatic disease, died of disease | NA |
| 2 | F | 78 | Right biceps | Malignant fibrous histiocytoma | 3 | No | Postop | 5 | Innervated rotational latissimus dorsi pedicle flap for biceps function | Intraoperative brachial artery thrombosis | Yes | Radical resection of local recurrence | Metastatic disease, died of disease | NA |
| 3 | F | 84 | Right forearm flexor pronator group | Malignant fibrous histiocytoma | 3 | No | Preop, postop | 0 | Tendon transfer of the index, long, and ring finger flexor digitorum superficialis to the flexor carpi radialis | Wrist flexor bulge; wrist hyperextension deformity | No | Tenolysis of flexor tendons | Disease free | 61.3 |
| 4 | F | 33 | Left humerus and biceps | Dedifferentiated osteosarcoma | 3 | No | None | 2 | Innervated gracilis myocutaneous free flap for biceps function | Inferior flap epidermolysis | No | Flap debulking | Disease free | 35.3 |
| 5 | M | 29 | Right forearm deep flexor compartment | Synovial cell sarcoma | 3 | Yes | Preop, postop | 0 | Non-innervated latissimus free flap and tendon transfer (palmaris longus to FPL) | None | No | Radio-ulnar joint arthrodesis with iliac bone graft | Disease free | 92.6 |
| 6 | F | 30 | Left biceps | Synovial cell sarcoma | 3 | No | Preop | 1 | Innervated rotational latissimus dorsi pedicle flap for biceps function | Ulnar nerve paresthesias; chronic donor site pain | No | Ulnar nerve decompression and transposition; flap debulking; forearm flexor muscle lengthening; resection of donor site neuroma | Disease free | 98 |
| 7 | F | 75 | Left triceps | Malignant fibrous histiocytoma | 3 | No | Preop | 0 | Innervated rotational latissimus dorsi pedicle flap for triceps function | None | No | None | Disease free | 100 |
| 8 | M | 70 | Left biceps and brachioradialis | Undifferentiated sarcoma with myxoid change | 3 | No | Preop, postop | 0 | Innervated rotational latissimus dorsi pedicle flap for biceps function | Donor site seroma; incisional cellulitis | No | Resection of benign ring finger and antecubital fossa lesions | Disease free | 100 |
| 9 | F | 55 | Left biceps | Malignant fibrous histiocytoma | 3 | No | Preop | 0 | Innervated rotational latissimus dorsi pedicle flap for biceps function | None | No | None | Disease free | NA |
| 10 | F | 38 | Left antecubital fossa and biceps | Dedifferentiated sarcoma | 3 | No | Preop | 0 | Innervated rotational latissimus dorsi pedicle flap for biceps function | None | No | None | Disease free | 98 |
| 11 | F | 67 | Dorsum of left hand | Malignant fibrous histiocytoma | 3 | Yes | Postop | 1 | Rotational radial artery forearm flap; delayed allograft tendon to finger extensors | None | No | None | Disease free | 56.7 |
| 12 | M | 54 | Right wrist | Clear cell sarcoma | 3 | Yes | Postop | 1 | Rotational radial artery forearm flap; allograft tendons to extensor compartment | None | No | Extensor tendon tenolysis | Disease free | 92 |
| 13 | F | 76 | Right radial palm at flexor crease | Inflammatory myofibroblastic sarcoma | 2 | No | None | 0 | Non-innervated lateral arm free flap and allograft tendon for index finger flexion | Tendon rupture | Yes | None | Metastatic disease, alive | 46.7 |
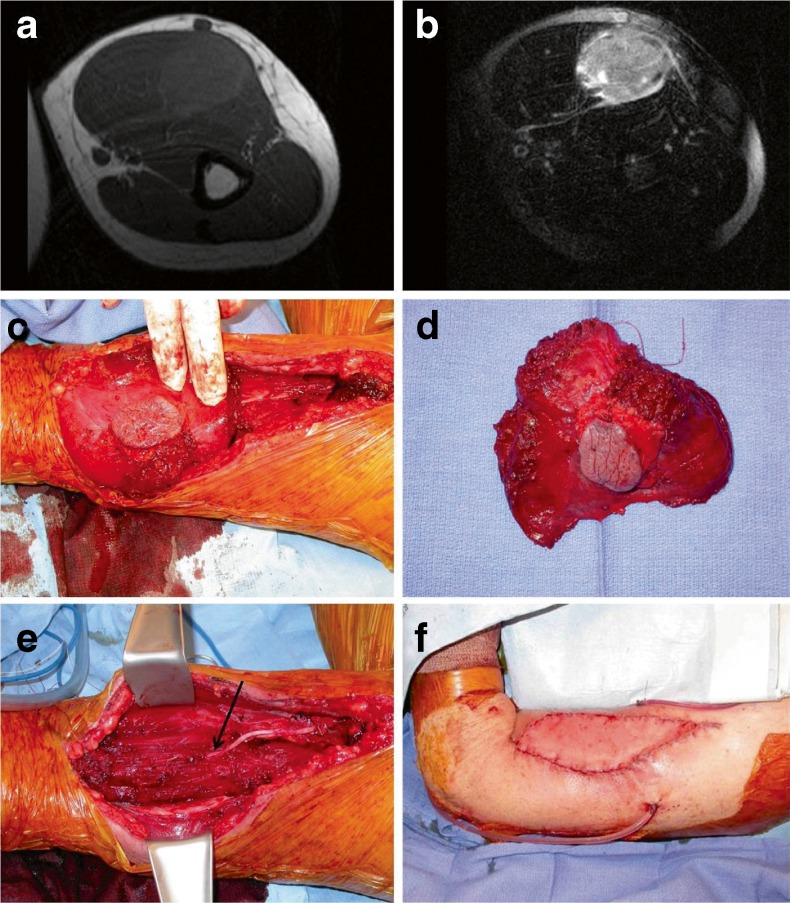
Fig. 1.
Proximal upper extremity reconstruction (patient 8). a Preoperative T1 and b T2-weighted MRI imaging of an undifferentiated sarcoma involving the biceps. c Intraoperative view during sarcoma resection (elbow is to the left). d Resected specimen. e View of postresection defect. Sacrificed musculocutaneous nerve (arrow). f On-table result following tunneled innervated latissimus flap for restoration of biceps function
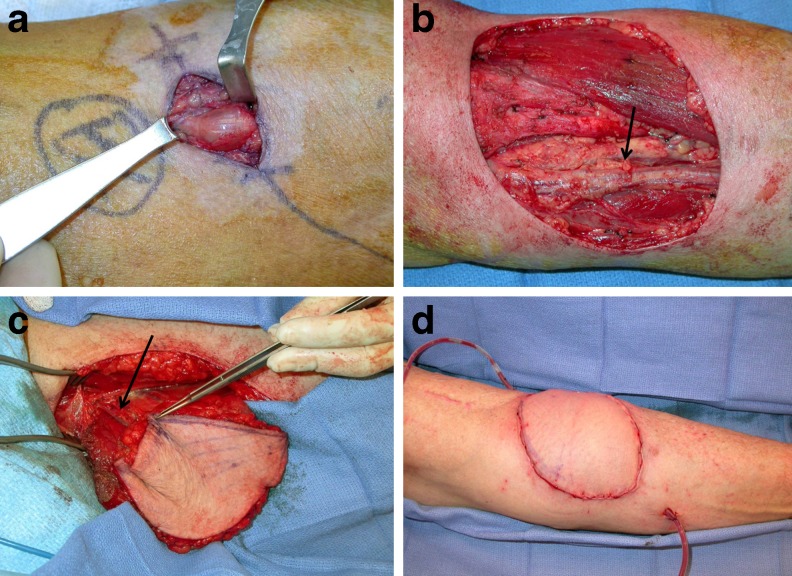
Fig. 2.
Patient 8 subsequently developed lesions suspicious for recurrence in the antecubital fossa and ring finger. These proved to be benign. a View of antecubital lesion (hand is to the right). b Defect following wide local excision. Ulnar artery (arrow). c A left free gracilis flap was harvested for soft tissue coverage. Flap pedicle (arrow). d Image of inset flap following end-to-end anastomosis to the ulnar artery
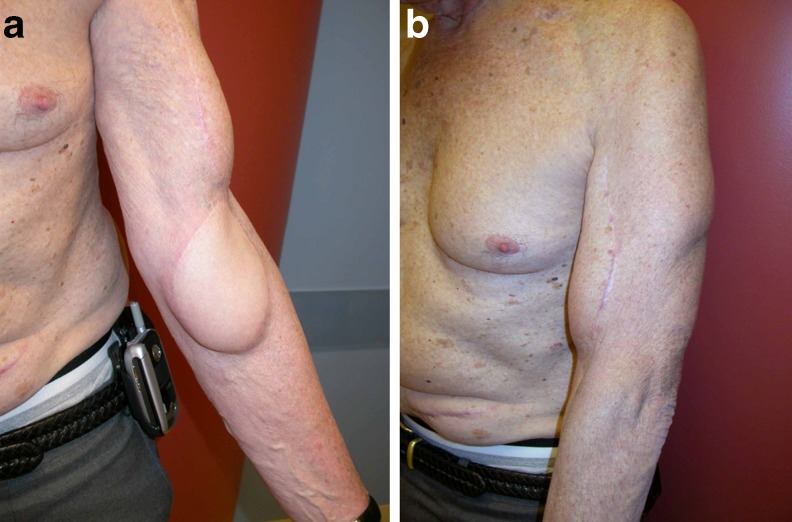
Fig. 3.
Patient 8 result. a Volar view. b Lateral view. Despite the slight bulkiness of the gracilis fasciocutaneous island, the patient was not bothered by this and declined flap debulking
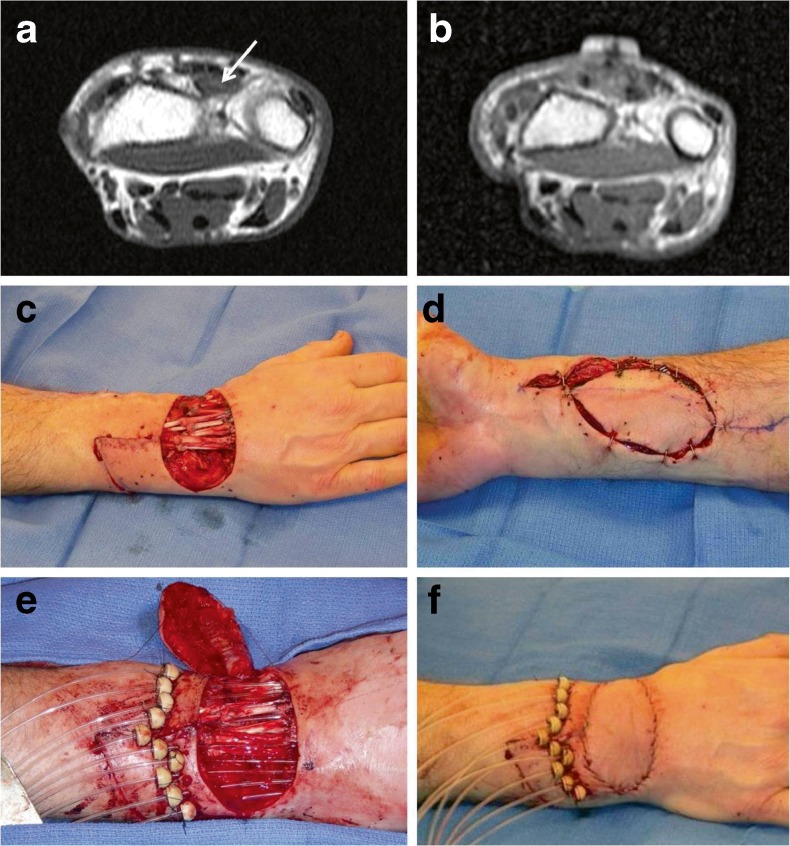
Fig. 4.
Distal upper extremity reconstruction (patient 12). a Preoperative T1-weighted MRI imaging of a clear cell sarcoma (arrow) immediately deep to the hand extensor tendons. b T1-weighted MRI 4 months after surgery demonstrating complete sarcoma resection without recurrence. c Intraoperative view following clear cell sarcoma resection and reconstruction of extensor tendons with allograft tendons. d A pedicled radial artery flap was utilized for soft tissue coverage. e Brachytherapy catheters positioned prior to closure. f On-table result
Fig. 5.
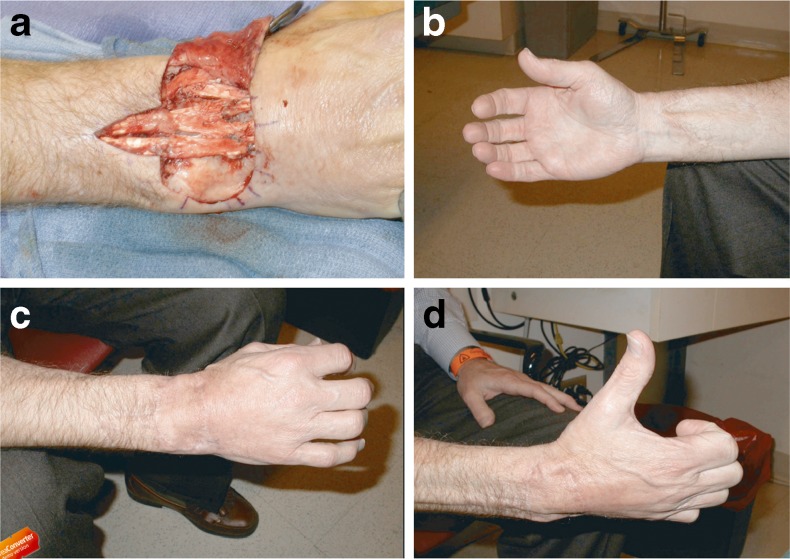
a Patient 12 developed difficulty with wrist extension 9 months postoperatively and underwent tenolysis of allograft tendons. b Volar result, c dorsal result, and d hand with thumb in full abduction 13 months after tenolysis
Complications occurred in six patients (46 %; Table 4). Serious complications, including brachial artery thrombosis, chronic donor site pain, tendon graft rupture, and ulnar nerve paresthesias, occurred in 31 % of patients (n = 6; Table 3).
Table 4.
Complications
| Complication | Incidence (% of total population n = 13) |
|---|---|
| Incisional cellulitis | 2 (15) |
| Donor site seroma | 1 (8) |
| Chronic donor site pain | 1 (8) |
| Ulnar nerve paresthesias | 1 (8) |
| Partial flap epidermolysis | 1 (8) |
| Intraoperative brachial artery thrombosis | 1 (8) |
| Tendon graft rupture | 1 (8) |
Ten of 11 surviving patients returned the survey in the follow-up period, for an average survey response rate of 91 %. Mean TESS score was 76 (range 36.3 to 100). Average consideration of personal disability was 4.1 out of 5 (1 = completely disabled, 2 = severely disabled, 3 = moderately disabled, 4 = mildly disabled, 5 = not at all disabled). 88 % of patients (7 of 8) returned to work postoperatively, and all patients who returned to work currently use their affected limb at work. Average time from discharge to return to work was 2.6 months (range 1–4). No patient stated that they would have preferred amputation to limb-sparing surgery with functional reconstruction.
Patients undergoing functional reconstruction for tumor excisions below the elbow had significantly longer hospital stays yet spent significantly less time in the intensive care unit than patients undergoing functional reconstruction for tumor excisions below the elbow (8.6 days versus 5.2 days, p < 0.006; 0.2 versus 0.7, p < 0.05; see Table 5 for full results). Additionally, they had significantly higher total costs of surgery ($31,929 versus $22,764, p < 0.03). Furthermore, they received more postoperative radiotherapy (83 % versus 29 %), underwent more surgical revisions (1.0 versus 0.28), and had a higher rate of major complications (50 % versus 20 %) than patients in the proximal reconstruction group, though these differences were not statistically significant (p values 0.07, 0.25, and 0.6, respectively).
Table 5.
Comparison of study parameters by site of resection/reconstruction
| Proximal upper extremity (SD) | Distal upper extremity (SD) | Difference | p value | |
|---|---|---|---|---|
| No. of patients | 7 | 6 | ||
| Average age | 54.1 (20.6) | 56 (24.2) | −1.9 | >0.1 |
| Average number of prior excisions | 1.1 (1.9) | 0.7 (0.8) | 0.4 | >0.1 |
| Average tumor grade | 3 (0) | 2.8 (0.4) | 0.2 | >0.1 |
| Average maximum tumor diameter | 7.6 (6.4) | 4.4 (3.3) | 3.2 | >0.1 |
| Brachytherapy (%) | 0 (0) | 50 (55) | −50 | >0.1 |
| Radiotherapy (%) | 86 (38) | 83 (41) | 3 | >0.1 |
| Preoperative radiotherapy (%) | 71 (49) | 50 (55) | 21 | >0.1 |
| Postoperative radiotherapy (%) | 29 (49) | 83 (41) | −54 | >0.1 |
| Length of stay | ||||
| Duration (days) | 5.2 (1.6) | 8.6 (1.9) | −3.4 | 0.006 |
| Intensive care unit (days) | 0.7 (0.5) | 0.2 (0.4) | 0.5 | 0.05 |
| Surgical time (min) | 639 (199) | 648 (176) | −9 | >0.1 |
| Charges (2012 US $) | ||||
| Routine | 9,638 (2,294) | 13,577(4,315) | −3,939 | 0.08 |
| Operating room | 5,834 (1,483) | 6,318 (1,029) | −484 | >0.1 |
| Pharmacy | 1,059 (367) | 1,310 (1,896) | −251 | >0.1 |
| Radiology | 82 (275) | 4,124 (7,353) | −4,042 | >0.1 |
| Laboratory | 1,030 (729) | 1,069 (464) | −39 | >0.1 |
| Supply | 2,495 (700) | 2,468 (1,411) | 27 | >0.1 |
| Therapy | 1,194 (872) | 1,294 (974) | −100 | >0.1 |
| Miscellaneous | 1,432 (1,087) | 1,769 (558) | −337 | >0.1 |
| Total | 22,764 (4,188) | 31,929 (7,528) | −9,167 | 0.03 |
| Average number of revisional procedures | 0.28 (1.2) | 1.0 (1.1) | 0.72 | >0.1 |
| Complication rate (%) | 60 (55) | 33 (51) | 27 | >0.1 |
| Major complication rate (%) | 20 (45) | 50 (54) | −30 | >0.1 |
| Percentage completing survey | 71 | 83 | −12 | >0.1 |
| Average length of follow-up (months) | 57.4 (8.6) | 40.1 (31.5) | −17.3 | >0.1 |
| Average TESS score | 86.3 (28.5) | 69.9 (21.2) | 16.4 | >0.1 |
| Consideration of own disability | 4.6 (0.9) | 3.4 (1.7) | 1.2 | >0.1 |
| Return to work (%) | 75 (5) | 100 (0) | −25 | >0.1 |
| Time to return to work (months) | 2.7 (1.5) | 2.5 (1.3) | 0.2 | >0.1 |
Significant p values in italics
Patients undergoing reconstructions proximal to the elbow had higher average TESS scores (86.3 versus 69.9) and a more favorable global consideration of their disability (4.6 versus 3.9), though these comparisons also did not reach statistical significance (p > 0.10 in both cases).
Discussion
Although limb-sparing surgery with adjuvant radiation and chemotherapy has become the preferred treatment for upper extremity sarcomas, adequate tumor resection can compromise crucial limb function. Functional reconstructive surgery, with its intuitive benefits in restoring limb utility, should be offered to patients as one of many reconstructive options following sarcoma resection [21].
Despite positive outcomes with functional reconstruction, the adoption of this strategy has not been widespread [26]. Functional muscle is not a prerequisite for limb salvage, and functional reconstruction is more technically demanding, time consuming, and expensive than soft tissue-only reconstruction [13, 16, 19, 23]. Controversy also exists regarding the timing of functional reconstruction, with lack of clarity surrounding the definition of immediate reconstruction [20].
The paucity of data regarding complications, outcomes, and monetary cost of immediate functional reconstruction for upper extremity soft tissue defects following sarcoma resection may explain why this technique is not more widespread. Due to their low incidence, investigators have generally combined upper extremity functional reconstruction with lower extremity functional reconstruction in case series [11, 20, 22, 28]. Conversely, articles addressing upper extremity reconstruction following soft tissue sarcoma resection have had few patients with functional reconstruction [1, 34].
In order to clarify the role of this technique in appropriate patients, our study sought to provide single-surgeon data regarding oncologic, surgical, functional, and monetary cost outcomes of concomitant upper extremity soft tissue sarcoma resection and functional reconstruction in the same surgical setting. As we report a single senior surgeon's experience, the study population is relatively small, limiting its power. This is especially true in light of occasional large standard deviations associated with these data. Patients were not randomized with respect to either functional reconstruction or soft tissue coverage, and consequently, no cost parameter comparisons could be made between these groups. However, single-surgeon studies may control for variability better than larger, multi-institutional studies with respect to technique, thereby providing more consistent outcome data with an associated cost of reduced generalizability. Despite these limitations, patient survey response rate was high, and average follow-up time was adequate.
Patients included in the study had oncologic outcomes consistent with both delayed functional and soft tissue-only reconstructions [1, 20, 22, 28]. Although patient perception of functionality following immediate reconstruction was high, patients undergoing reconstruction for sarcomas distal to the elbow had poorer functional outcomes and considered themselves slightly more disabled than patients undergoing reconstruction for sarcomas proximal to the elbow. They also underwent more surgical revisions and had a higher rate of major complications. While none of these differences were statistically significant, they are perhaps unsurprising as the distal upper extremity is a much more functionally complex unit than the proximal upper extremity.
The positive outcomes observed in our study were achieved at an average surgical cost of $26,655, surgical time of 643 min, hospital duration of 6.8 days, minor complication rate of 46 %, and major complication rate of 31 %. The significantly increased costs of surgery and lengths of stay in the distal upper extremity group are explained by the higher rates of brachytherapy and postoperative radiotherapy in this group.
Although return-to-work rates and use of limb upon return to work were investigated, only direct inpatient costs were analyzed. No calculations were made regarding the economic impact of patients being out of work or for the costs of revision procedures following functional reconstruction. No adjustments were made for population differences between the proximal and distal reconstruction groups. As tumor excision and functional reconstruction were performed as single-stage procedures, it was not possible to calculate surgical costs and operative times for only the reconstructive portion of each case.
Overall, this study provides more accurate information to address patient concerns regarding immediate functional reconstructive surgery following limb-sparing upper extremity soft tissue sarcoma excision [4]. Additionally, it highlights the need for further cost and outcome investigations for reconstructive procedures in general and for immediate upper extremity functional reconstructive procedures following limb-sparing sarcoma resection in particular.
In summary, following immediate functional reconstruction, patients achieved very good to excellent functional outcomes with quick recovery times and a high return-to-work rate, thus minimizing surgical cost impacts. Functional recovery may be more difficult for patients undergoing resection and immediate reconstruction of sarcomas of the hand/forearm when compared to the elbow/upper arm. Larger studies are needed to evaluate utility differences between various immediate functional reconstructive techniques.
This study supports the notion that concomitant functional restoration should be offered as a reconstructive option in the same surgical setting when oncologic resection will compromise critical upper limb functionality. In this regard, surgeons who possess both detailed knowledge of hand and upper extremity anatomy and can perform functional limb reconstruction through multiple methods appropriate for the patient's functional and tissue coverage needs are a critical component of modern soft tissue sarcoma therapy [34]. Additionally, the presence of such surgeons at the time of tumor resection is crucial to ultimate patient outcomes through the preservation of small distal extremity structures with critical functionality [21]. This is of increasing importance as evidence mounts that smaller tumor-free resection margins, less than 1 cm in recent studies, can achieve long-term disease-free survival in excess of 65 % [18].
Electronic supplementary material
Patient 8 degree of elbow flexion and flap contour after functional reconstruction (TESS score = 100). (MPG 2646 kb)
Patient 12 functional result (TESS score = 92). (MPG 2138 kb)
Acknowledgment
The authors would like to acknowledge John D. Hundt, M.H.S. for providing cost data.
Conflict of Interest
The authors declare no conflict of interest.
Statement of Human and Animal Rights
All procedures performed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
References
- 1.Barner-Rasmussen I, Popov P, Bohling T, Blomqvist C, Tukiainen E. Microvascular reconstructions after extensive soft tissue sarcoma resections in the upper limb. Eur J Surg Oncol. 2010;36(1):78–83. doi: 10.1016/j.ejso.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Cadman J, Soule FH, Kelly PJ. Synovial sarcoma: an analysis of 134 tumors. Cancer. 1965;18:613–627. doi: 10.1002/1097-0142(196505)18:5<613::AID-CNCR2820180510>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Clark RL, Jr, Martin RG, White EC. A critical review of the management of soft-tissue sarcomas. J Lancet. 1959;79(7):327–331. [PubMed] [Google Scholar]
- 4.Davidge K, Bell R, Ferguson P, Turcotte R, Wunder J, Davis AM. Patient expectations for surgical outcome in extremity soft tissue sarcoma. J Surg Oncol. 2009;100(5):375–381. doi: 10.1002/jso.21301. [DOI] [PubMed] [Google Scholar]
- 5.Davis AM, Bell RS, Badley EM, Yoshida K, Williams JI. Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res. 1999;358:90–100. doi: 10.1097/00003086-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Davis AM, Sennik S, Griffin AM, Wunder JS, O'Sullivan B, Catton CN, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol. 2000;73(4):206–211. doi: 10.1002/(SICI)1096-9098(200004)73:4<206::AID-JSO4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 8.Doi K, Sakai K, Ihara K, Abe Y, Kawai S, Kurafuji Y. Reinnervated free muscle transplantation for extremity reconstruction. Plast Reconstr Surg. 1993;91(5):872–883. doi: 10.1097/00006534-199304001-00021. [DOI] [PubMed] [Google Scholar]
- 9.Emrich LJ, Ruka W, Driscoll DL, Karakousis CP. The effect of local recurrence on survival time in adult high-grade soft tissue sarcomas. J Clin Epidemiol. 1989;42(2):105–110. doi: 10.1016/0895-4356(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 11.Hoy E, Granick M, Benevenia J, Patterson F, Datiashvili R, Bille B. Reconstruction of musculoskeletal defects following oncologic resection in 76 patients. Ann Plast Surg. 2006;57(2):190–194. doi: 10.1097/01.sap.0000216255.18106.e1. [DOI] [PubMed] [Google Scholar]
- 12.Ihara K, Shigetomi M, Kawai S, Doi K, Yamamoto M. Functioning muscle transplantation after wide excision of sarcomas in the extremity. Clin Orthop Relat Res. 1999;358:140–148. doi: 10.1097/00003086-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Kane JM, 3rd, Gibbs JF, McGrath BE, Loree TR, Kraybill WG. Large, deep high-grade extremity sarcomas: when is a myocutaneous flap reconstruction necessary? Surg Oncol. 1999;8(4):205–210. doi: 10.1016/S0960-7404(99)00046-8. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, 3rd, Kraybill WG. Radical operations for soft tissue sarcomas. Surg Oncol Clin N Am. 2005;14(3):633–648. doi: 10.1016/j.soc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Keus RB, Rutgers EJ, Ho GH, Gortzak E, Albus-Lutter CE, Hart AA. Limb-sparing therapy of extremity soft tissue sarcomas: treatment outcome and long-term functional results. Eur J Cancer. 1994;30A(10):1459–1463. doi: 10.1016/0959-8049(94)00302-L. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Youssef A, Subramanian V, Rogers BA, Pollock RE, Robb GL, et al. Upper extremity reconstruction following resection of soft tissue sarcomas: a functional outcomes analysis. Ann Surg Oncol. 2004;11(10):921–927. doi: 10.1245/ASO.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Lee HY, Cordeiro PG, Mehrara BJ, Singer S, Alektiar KM, Hu QY, et al. Reconstruction after soft tissue sarcoma resection in the setting of brachytherapy: a 10-year experience. Ann Plast Surg. 2004;52(5):486–491. doi: 10.1097/01.sap.0000122649.64350.e3. [DOI] [PubMed] [Google Scholar]
- 18.Lehnhardt M, Hirche C, Daigeler A, Goertz O, Ring A, Hirsch T, et al. Soft tissue sarcoma of the upper extremities. analysis of factors relevant for prognosis in 160 patients. Chirurg. 2012;83(2):143–152. doi: 10.1007/s00104-011-2124-6. [DOI] [PubMed] [Google Scholar]
- 19.Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer. 2002;94(8):2256–2264. doi: 10.1002/cncr.10419. [DOI] [PubMed] [Google Scholar]
- 20.Marre D, Buendia J, Hontanilla B. Complications following reconstruction of soft-tissue sarcoma: importance of early participation of the plastic surgeon. Ann Plast Surg. 2012;69(1):73–78. doi: 10.1097/SAP.0b013e31821ee497. [DOI] [PubMed] [Google Scholar]
- 21.Megerle K, Sauerbier M. Reconstructive treatment of soft tissue sarcoma of the upper extremity. J Hand Surg [Am] 2011;36(7):1241–1247. doi: 10.1016/j.jhsa.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu K, Ihara K, Taguchi T. Selection of myocutaneous flaps for reconstruction following oncologic resection of sarcoma. Ann Plast Surg. 2010;64(3):307–310. doi: 10.1097/SAP.0b013e3181b0260e. [DOI] [PubMed] [Google Scholar]
- 23.Nelson AA, Frassica FJ, Gordon TA, Deune EG. Cost analysis of functional restoration surgery for extremity soft-tissue sarcoma. Plast Reconstr Surg. 2006;117(1):277–283. doi: 10.1097/01.prs.0000187140.83705.cf. [DOI] [PubMed] [Google Scholar]
- 24.Pollock RE. Limb-sparing therapy for soft tissue sarcomas. Curr Opin Gen Surg. 1994:224–9. [PubMed]
- 25.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saint-Cyr M, Langstein HN. Reconstruction of the hand and upper extremity after tumor resection. J Surg Oncol. 2006;94(6):490–503. doi: 10.1002/jso.20486. [DOI] [PubMed] [Google Scholar]
- 27.Sarcoma Meta-analysis Collaboration (SMAC). Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev. 2000;(4):CD001419. doi:10.1002/14651858.CD001419. [DOI] [PubMed]
- 28.Serletti JM, Carras AJ, O'Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg. 1998;102(5):1576–1583. doi: 10.1097/00006534-199810000-00036. [DOI] [PubMed] [Google Scholar]
- 29.Shieber W, Graham P. An experience with sarcomas of the soft tissues in adults. Surgery. 1962;52:295–298. [PubMed] [Google Scholar]
- 30.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 31.Simon MA, Enneking WF. The management of soft-tissue sarcomas of the extremities. J Bone Joint Surg Am. 1976;58(3):317–327. [PubMed] [Google Scholar]
- 32.Simon MA, Spanier SS, Enneking WF. Management of adult soft-tissue sarcomas of the extremities. Surg Annu. 1979;11:363–402. [PubMed] [Google Scholar]
- 33.Suit HD, Russell WO, Martin RG. Management of patients with sarcoma of soft tissue in an extremity. Cancer. 1973;31(5):1247–1255. doi: 10.1002/1097-0142(197305)31:5<1247::AID-CNCR2820310533>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Vetter M, Germann G, Bickert B, Sauerbier M. Current strategies for sarcoma reconstruction at the forearm and hand. J Reconstr Microsurg. 2010;26(7):455–460. doi: 10.1055/s-0030-1254229. [DOI] [PubMed] [Google Scholar]
- 35.Williard WC, Hajdu SI, Casper ES, Brennan MF. Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann Surg. 1992;215(3):269–275. doi: 10.1097/00000658-199203000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasko A, Patel R, Pollack A, Pollack RE, editors. Sarcomas of soft tissue and bone. Atlanta: American Cancer Society; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient 8 degree of elbow flexion and flap contour after functional reconstruction (TESS score = 100). (MPG 2646 kb)
Patient 12 functional result (TESS score = 92). (MPG 2138 kb)