Abstract
The introduction of skin substitutes in the last decade has dramatically changed how we think about the concept of “non-healing” wounds. Their use has improved prognosis and reduced morbidity in the treatment of open wounds. This article aims to summarize the development of tissue-engineered skin substitutes, discuss their use, and highlight some specific applications in different clinical settings.
Keywords: Skin substitutes, Wound coverage, Skin grafts, Review
Introduction
Considered the largest organ, the skin provides sensation, thermal, and blood pressure regulations; acts as a barrier against chemical, mechanical, and infectious insults; and prevents dehydration from evaporative water loss [9]. Designing skin substitutes that posses all of these properties has taken a great deal of research and development. The detailed understanding of acute wound healing has allowed for engineered skin substitutes to be tailored toward this goal and therefore significant progress has been made in the development and clinical use of these products. The defining moment in culturing skin was in 1975 when Rheinwald and Green successfully grew human keratinocytes [52, 53]. This breakthrough enabled O'Connor [47] and coworkers to resurface the first burn defect with cultured autologous epithelium in 1981.
When dealing with open or chronic wounds, the choice of coverage is dictated by the nature of the defect. In general, only in severe burns is there a shortage of only autologous skin. In other defects resulting from trauma, chronic ulcers, post-oncological resection, and other causes, the problem is usually a deficiency of the deeper structures and the skin. As an alternative source of coverage, various skin substitutes have been developed to aid in these cases. All tissue-engineered skin substitutes need to comply with three major requirements. They must be safe for the patient, be clinically effective, and be convenient in handling and application [38]. In addition, an optimal skin substitute should have the following features: ability to resist infection, ability to prevent water loss, ability to withstand the shear forces, cost effective, widely available, long shelf life, lack of antigenicity, flexible in thickness, durable with long-term wound stability, conformability to irregular wound surfaces, and ease of application [57].
Development of skin substitutes has decreased complications associated with skin grafts such as donor site availability, immune rejection of allogenic skin grafts, pain, scarring, slow healing, and infection [35]. In addition, the use of skin substitutes has revolutionized the care of burn patients and may be lifesaving in severe situations. In general, skin substitutes have a potentially important role in the treatment of a wide variety of wounds in different clinical scenarios as a temporary or permanent means. Materials used for wound coverage (e.g., Biobrane®, Dermagraft®, and Apligraf®) are primarily indicated for superficial burns, where they provide a barrier against infection, control water loss, and create an environment suitable for epidermal regeneration. Materials intended for definitive wound closure (eg. Alloderm®, Integra®, and Epicel®) restore the epidermal barrier and become incorporated into the healing wound. Anatomically, skin substitutes may be composed of dermis layer, epidermis layer, or combination of both. Histologically, skin substitutes stimulate the host to produce a variety of cytokines and growth factors that promote wound healing [17].
The last few decades have seen a multitude of products released into the market to help with all facets of managing soft tissue injuries. These products range from purely synthetic compounds to both cellular and acellular materials derived from human and animal sources. They may be temporary or permanent, and they may function alone or work by altering the body's own healing capabilities. With so many choices, it can be challenging to select the “proper tool for the job.” Here, we present several of the products on the market that have been employed in our practice.
Indications/Contraindications
Synthetic Products
Biobrane® (UDL Laboratories Inc., Rockford, IL) is a synthetic wound dressing consisting of a silicone film bonded to a knitted nylon mesh coated with porcine collagen/polypeptides. The fabric is a three-dimensional structure that allows blood clot into the matrix and firmly adhere the dressing into the wound until epithelialization occurs. The silicone acts as a semipermeable epidermis that allows for drainage. It is transparent, allowing for direct wound visualization, and has elastic characteristics that allow it to promote motion. A Biobrane® glove can be utilized to help cover hand wounds and burns. It can be applied to any wound that has been appropriately prepared and debrided of nonviable tissue. It can be used to temporarily cover superficial and mid-dermal wounds or as a protective covering over autografts [51]. As the wound heals, the Biobrane® should be trimmed away [22].
Multiple studies have evaluated the efficacy of Biobrane® [5, 12, 18, 32, 33, 60]. Barret et al. prospectively compared the use of Biobrane® with 1 % Silver Sulfadiaxine in the management of partial thickness pediatric burn patients [5]. Ten patients were included in each group and outcome measures included pain, requirement of pain medications, length of hospitalization, rates of healing, and complications. Pain, length of hospitalization, and rate of healing were all superior in the Biobrane® group. No cases of infection were noted in either group. Gerding et al. also examined outcomes between Biobrane® (N = 30) with Silver Sulfadiaxine (N = 26) prospectively [18]. The Biobrane® group fared better in pain, time to healing, and compliance with follow-up care. The average time to healing was 10 days in the Biobrane® group versus 15 days in the Silver Sulfadiaxine group. In addition, a cost analysis revealed the average cost to be $434 for the Biobrane® group versus $504 in patients treated with Silver Sulfadiaxine.
Cassidy et al. examined treatments between Biobrane® and Duoderm® [12]. The authors prospectively randomized pediatric patients with intermediate thickness burns treated with Biobrane® (N = 35) and Duoderm® (N = 37) with an average follow-up of 17 days. Time to healing and pain scores were not significantly different between groups. The Biobrane® was more expensive and the authors concluded Duoderm® should be considered a first-line treatment for intermediate thickness burns in children. Ahuja and Datiashvili described the use of Biobrane® for coverage of wounds with exposed critical structures such as exposed anastomoses of vessels, nerves, and tendons [2]. The authors described its use as an intermediate coverage in five wounds: four replant wounds and one microsurgical reconstruction. All went on to heal without complications.
We have had success using Biobrane® in our upper extremity practice to help create an underlying granulation bed suitable for skin grafting. Biobrane® should only be applied to freshly debrided, clean wounds free of wrinkles with the fabric side lying directly on the wound. The product is sutured, stapled, or taped into place, covered with a compressive dressing and immobilized using a splint for a total of 7–14 days. The wound should be checked every 2–3 days to ensure adherence of the product without complication. When the wound is completely covered with fine granulation tissue, the Biobrane® is removed carefully and the subsequent wound covered with a skin graft.
Transcyte® (Advanced Tissue Sciences, Inc. La Jolla, CA) is a synthetic temporary wound coverage product that is composed of newborn human fibroblast cells cultured on a porcine-coated collagen nylon. It also has a semipermeable silicone membrane. It is indicated for coverage of excised burns prior to autografting or partial thickness burns that do not require grafting [51]. It is typically applied with surgical wrap or adhesive and peels away as the burn wound heals. Noordenbos et al. compared results of Transcyte® and Silver Sulfadiazine for partial thickness burns [46]. The authors performed a prospective analysis on 14 patients and found that the Transcyte®-treated wounds healed more rapidly (11 vs. 18 days). In addition, when measured by the Vancouver Scar Scale, the patients treated with Transcyte® had less hypertrophic scarring compared to those treated with Silver Sulfadiazine. Kumar et al. compared Transcyte®, Biobrane®, and Silver Sulfadiazine in a prospective randomized fashion [32]. Fifty-eight wounds in 33 patients were treated and 20 were treated with Transcyte®, 17 with Biobrane®, and 21 with Silver Sulfadiazine. The investigators found that the average time to re-epithelialization was 7.5 days for Transcyte®, 9.5 for Biobrane®, and 11 days for Silver Sulfadiazine. In addition, Transcyte®-treated wound required the least number of autografts. The authors concluded that Tanscyte® was superior to the others.
Acellular Products
Acellular skin graft substitutes are readily available and include Integra® (Integra LifeSciences, Plainsboro, NJ), Permacol® (Tissue Science Laboratories, Inc., Andover, MD), Matriderm® (Dr. Suwelack Skin and Health Care AG, Germany), EZDerm® (Brennan Medical, St. Paul, MN), and OASIS® (Healthpoint Ltd., Fort Worth, TX).
Integra® is a bilayer membrane that is made of primarily collagen derived from bovine tendons and a small percentage of chondroitin-6-sulfate (from shark cartilage). It is a three-dimensional matrix that allows for the patient's own cells and tissues (fibroblasts, lymphocytes, macrophages, and capillaries) to migrate into it and incorporate over a 2–3-week period. It can be packaged with a silicone protective sheet or alone. In addition, there is a meshed form that is now available that precludes the need for “pie-crusting.” It is designed to cover full thickness wounds and can be placed directly over critical structures such as nerves, arteries, small areas of bone, and tendons. Autografting with split thickness skin can be applied onto the Integra® following incorporation, which is typically 2–3 weeks post-application. Topical negative pressure wound therapy (NPWT) has been shown to accelerate neogenesis and decrease the “take” time for the Integra® [41]. Clinical evaluation of the skin 2 years following application has shown excellent patient satisfaction with respect to softness, mobility, and appearance. Histological analysis has shown an absence of adnexal structures, whereas collagen and elastin fibers were universally present [40].
Integra® has been used extensively in burn patients where significant wound coverage is often necessary [26]. Because of the intricate anatomy and delicate balance of structures in the hand, Integra® has shown promise in treating patients with upper extremity skin defects. Unlike split thickness skin grafts, the neo-dermis that forms with Integra minimizes migrating fibroblasts and results in less wound contracture and adhesions to the underlying structures and thus allows for better tendon gliding [16]. In addition, a non-meshed skin graft can be applied to the Integra® to ultimately provide a more cosmetically appealing result.
Weigert et al. retrospectively reported outcomes with the use of Integra® for traumatic hand wounds in a series of 15 cases [63]. All of the wounds were associated with bone, joint, and/or tendon exposure and the follow-up intervals ranged from 10 to 37 months. Skin grafting was performed at an average of 26 days following application of the Integra. Thirteen of 15 resulted in a successful cosmetic and functional outcome. Multiple case reports of its use in hand surgery have also been published [11, 27, 29, 42, 44, 64]. Carothers et al. described stacking of Integra® to cover a palm wound following tumor resection [11]. It has been successfully utilized in coverage for Apert's syndactyly reconstruction [29] and it can be used to cover donor site defects for major flaps or tissue reconstructions [21]. Moreover, Azzena et al. described a case where Integra® was used to help provide coverage for a degloving injury of the hand [3].
Complications with Integra® are rare, but the most common is loss or partial loss of the dermal substrate. This is most often due to excessive motion between the graft and wound bed or infection. Heimbach et al. noted an overall infection rate of 3.1 % in their series and observed that it was the most common reason for reduced take [25]. Care should be taken to ensure that the wound bed is free of contamination prior to placement of Integra®.
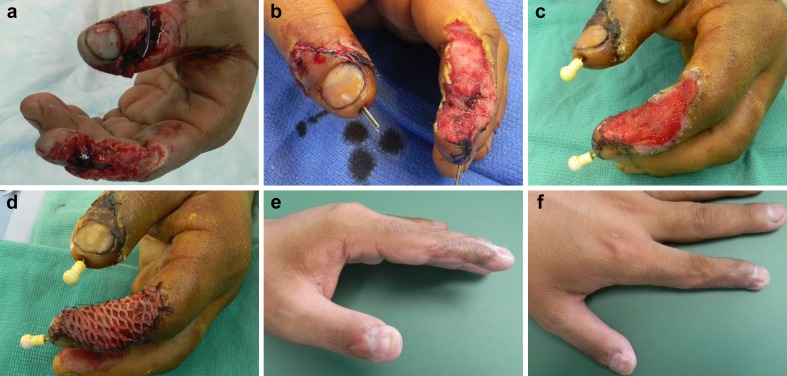
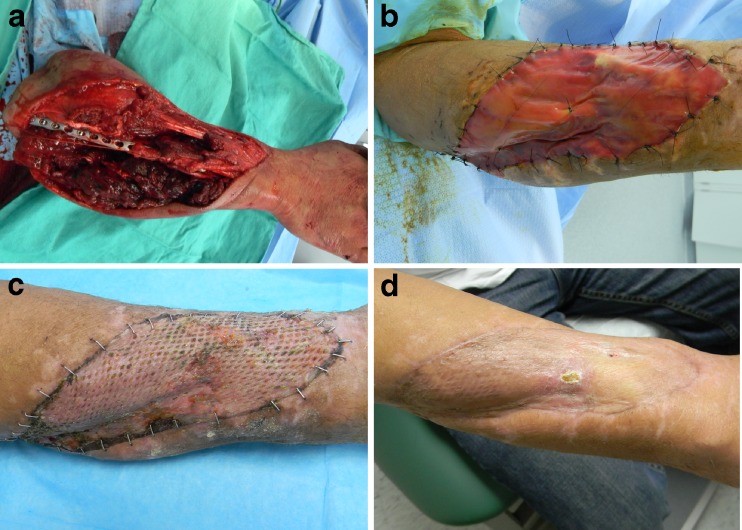
We have significant experience using Integra® in our upper extremity practices and it has proven to be a valuable tool that is straightforward to use. Important to the use of Integra® is preparing the wound for coverage as it must be free of debris and infection and have a suitable bed to receive the product. Achieving this usually included serial debridements and the use of negative pressure dressings. For most cases, we have utilized the Integra® Bilayer wound matrix. It requires 1–2 min of product preparation by soaking it in saline. Surgical gloves should be changed prior to product handling and application. The matrix is then cut to size so that no more than 1–2 mm of matrix overlaps healthy tissue and is then affixed to the wound with sutures or staples insuring there are no gaps between the graft and the underlying wound. The silicone layer is placed superficial to the wound. If a large area of coverage with associated large matrix product is used, the graft may be pie-crusted to allow drainage. Alternatively, a pre-meshed graft may be used for large defects. A compressive dressing or NPWT dressing should be applied over the matrix along with splint immobilization of the recipient site. The need for NPWT dressing is based on the size and depth of the wound and also the type of Integra matrix used. Larger wounds, with deeper, more concave morphologies, and those that require multiple layers of matrix graft benefit from NPWT treatment. The matrix requires 2–3 weeks to mature prior to skin graft coverage. Figures 1 and 2 demonstrate two cases in which Integra® was successfully used in complex wounds of the upper extremity.
Fig. 1.
A 26- year- old male sustained an injury using a saw circular saw. a) Injuries included a distal phalanx fracture of the thumb and index finger. In addition, there was a 2- cm nerve gap associated with the index finger injury. b) After irrigation and debridement, nerve graft conduit, percutaneous pinning. Integra® was applied and covered with a compressive dressing. c) Two weeks following Integra® application. d) Split-thickness, meshed skin graft application harvested from proximal forearm. e, and f) Two months following skin graft application
Fig. 2.
A 48- year- old male sustained this injury while cutting a tree at work. a) The sharp end of the branch impaled the ulnar aspect of his arm. The patient sustained segmental loss of the median nerve measuring 8 cm, severe contusion of his ulnar nerve, a comminuted ulna fracture, and extensive muscle and tendon damage. This patient underwent multiple irrigations and debridements and was subsequently covered with a negative pressure dressing. b) Integra® application. Two weeks following Integra® application, a split-thickness skin graft harvested from the ipsilateral thigh was applied. c) Two weeks following skin graft application. d) Eight weeks following skin graft application showing good healing of the skin graft
Matriderm® is composed of bovine dermis coated with elastin. It is 1 mm thick and composed of types I, III, and V elastin. While it is not available in the USA, it has readily been used in Europe. It is designed to cover wounds in a single stage procedure, usually combined with a thin split-thickness skin graft. Haslik et al. described their experience with the use of Matriderm® for coverage of 17 patients with hand and wrist wounds [23]. All wounds were associated with exposure of critical structures. The overall take rate was 96 % and patient-related outcomes were excellent with no limitations concern hand function. Heckmann et al. reported a series of ten patients who underwent treatment of bone or tendon-exposed wounds with Matriderm® [24]. Nine of ten healed without the need for further reconstructive intervention. However, the authors concluded that functionality is not superior to traditional flaps for skin coverage. Cevelli et al. compared the use of Matriderm® with skin grafting versus skin grafting alone and noted more rapid re-epithelialization in the Matriderm® plus skin graft group at 2 weeks following application [14]. In addition, the quality of the scar was better in the Matridem®-treated group. These results were reiterated in a study examining the use of Matriderm® with skin grafting for burn wounds over the dorsum of the hand [55]. Schneider et al. compared Integra® with Matriderm® in a rat model [56]. Both groups were treated in a two-stage procedure and speed and quality of vascularization was assessed. The found no major differences in rate and quality of vascularization and epithelialization.
Permacol® is a bovine-derived isocyanate collagen cross-linked skin substitute. It is 0.75 mm thick. Macleod et al. examined the use of Permacol® in a rat study and compared it to small intestine submucosa and glycerol-treated ethylene oxide sterilized porcine dermis [37]. The grafts were graded based on degree of acute and chronic inflammation, fibrosis, and stromal response. In addition, vascularity and degree of collagen ingrowth were assessed at 20 weeks post-implantation. The authors concluded that Permacol® was well tolerated in terms of inflammatory response, but the degree of vascular ingrowth was limited. In addition, they concluded that Permacol® had promise, but may benefit from modification to promote a more rapid degree of vascularization. Permacol® has also been utilized as an interposition graft following trapeziectomy in the treatment of basal thumb arthritis. Belcher and Zic performed a comparison study between trapeziectomy alone versus Permacol® interposition [6]. Regrettably, the study had to be aborted due to adverse patient reactions in 6 of 13 cases treated with Permacol®. Three implants had to be removed and histology revealed significant foreign body reactions. The group treated with Permacol® interposition had more pain and less satisfaction compared to trapeziectomy alone. The ideal use of Permacol appears to be for deep structural replacement such as in hernia repair.
EZ-Derm® (Brennen Medical, St Paul, MN) is a processed dermal porcine graft. It can be stored at room temperature and has a shelf life of about 18 months [36]. While it does not become vascularized, it can promote epithelialization by acting as an epidermal barrier. Still et al. reported that its use resulted in decreased hospital stays in the treatment of burn patients [59]. However, over time, the pigskin dries out and can get hard. As a result, it may deleteriously affect function and range-of-motion during the healing process.
OASIS® (Healthpoint Ltd, Fort Worth, TX) is derived from porcine small intestine (jejunum) submucosa (SIS). The serosa, smooth muscle, and mucosa are removed during processing, and the tissue is rendered acellular leaving a scaffold of collagens types I, III, and V, glycosaminoglycans, fibronectin, proteoglycans, and growth factors including TGF-β and FGF-2). It is available in a lyophilized dry formulation or a moist (hydrated) formulation and has the advantage of having a long shelf life [7]. One randomized controlled multicenter series investigated use of OASIS® with compressive therapy versus compressive therapy alone for wound healing of chronic leg ulcers [6]. After 3 months, 55 % of ulcers healed with OASIS® treatment versus 34 % of those treated with compression alone [43]. It is suggested that the biochemical and structural nature of this material promotes tissue specific remodeling. One mechanism for this may be the retention of bioactive growth factors in the matrix from the donor porcine submucosa [39]; another appears to be absorption and incorporation of growth factors from the host wound and serum into the matrix, creating a more favorable environment for wound healing [45]. According to the manufacturer, OASIS® wound matrix is indicated for chronic wounds (diabetic, pressure, venous stasis), burns, trauma, surgical wounds, and full or partial thickness wounds. It is contraindicated in those with a history of reactivity to porcine products or third-degree burns [7].
This wound matrix is available in two formulations: as a single layer of SIS (OASIS®) and as a triple SIS layer formulation (OASIS® Ultra Tri-Layer Matrix) for more challenging wounds. Application instructions per the manufacturer start with wound bed preparation that must be debrided of exudates or devitalized tissues. Bleeding is minimized. OASIS® is applied to the wound such that the entire wound is covered with a slight extension just beyond the wound margins. The matrix is anchored to the adjacent skin with tapes, sutures, staples, or tissue sealant, and subsequently rehydrated with application of sterile saline. A non-adherent dressing is applied over the wound and changed at day 7. After 7 days, the wound is reassessed and if needed, subsequent applications of OASIS® may be entertained. An exudate gel typically forms and appears as a caramel colored or off-white gel; this should be retained at serial wound inspections, as it is part of the healing response.
MatriStem® (ACell®, Lafayette, IN) is a biologic composed of porcine urinary bladder tissue. The product is sterilized with electron beam radiation and processed into a non-crosslinked acellular matrix scaffold. The structure has an intact basement membrane surface and a lamina propria surface. It is indicated for partial and full thickness wounds, pressure ulcers, diabetic ulcers, vascular and venous ulcers, surgical wounds, and traumatic wounds. It is contraindicated in those with sensitivity or allergies to porcine tissue and those with third-degree burns. It is provided in a lyophilized and dehydrated sheet form, and is rehydrated in saline. The wound is prepared by removing necrotic tissue or exudates and freshened about the edges. The wound is then covered with the sheet material and then by a non-adherent dressing followed by an absorptive dressing such as calcium alginate. A hydrogel dressing is then applied to maintain moisture at the wound site. The wound is then inspected every 7 days and a new sheet of MatriStem® applied if needed. The material will turn a caramel color as it is incorporated into the host wound. An alternative formulation is the MatriStem® MicroMatrix, which is a powder formulation which may be sprinkled on the wound. Application of these materials to wounds is believed to promote angiogenesis and cellular recruitment and differentiation to the injury site [4, 19, 20]. To date, published human series are lacking.
Alloderm® (AlloDerm; LifeCell Inc., Branchburg, NJ) is marketed for wound care while Graftjacket® (Wright Medical Technology, Arlington, TN) is licensed for musculoskeletal applications. This material is harvested sterilely from human cadaveric skin, undergoes proprietary processing to remove the epidermis and cellular components, and then freeze-dried [7]. Because cellular components are extracted, it is believed that immunological response is less likely. Although Alloderm® has little function as a barrier, it is intended to serve as a template for native cellular ingrowth for dermal regeneration. It may be used in a one-stage process in which the Alloderm® is applied followed by application of a thin split-thickness skin graft; results suggest that the wound contracture of such constructs is similar to that seen in a thicker split thickness autologous skin graft. Alternatively, it may be used without skin grafting [28, 34, 58, 61]. It became the first available human-derived dermis product in 1994 and was originally indicated for burns; today its use has expanded into wound coverage, head and neck reconstruction, breast reconstruction, and abdominal wall and organ reconstructive purposes [15].
Alloderm® Regenerative Tissue Matrix was previously thought to require refrigeration; however, the manufacturer now states that no adverse effects upon graft performance were noted after exposure to −30 °C for 10 days and 60 °C for 45 days. After opening the package, the product requires rehydration in warm saline until the material becomes supple. Following rehydration, the material must be used within 4 h. A related product which has become recently available is Alloderm® Regenerative Tissue Matrix (RTM) Ready to use; this product is terminally sterilized (unlike the prior product) and does not require rehydration nor refrigeration. It is available in a variety of thicknesses and sizes (0.23–3.30 mm thick, and 4 × 12 to 15 × 20 cm) [15].
The product has an up-down orientation with a dermal side, which is smooth and does not absorb blood and a basement membrane side, which is rough and dull in appearance and absorbent [1]. The manufacturer recommends orienting the basement membrane side towards the integument cavity with the dermal side out if primary closure is to be employed. If primary closure is not used or for coverage of an avascular bed, the dermal side should be placed towards the more vascularized surface with the basement membrane side exposed. The matrix can be then sutured in place under some tension. Contraindications for use include sensitivities to several antibiotics listed on the packaging or Polysorbate 20, as these are used during processing or buffering of the product [15]. As this is human-derived tissue that is harvested sterilely and not terminally sterilized, risks include disease transmission or infection. The tissue is handled in compliance with FDA and state mandates, as well as according to the standards of the American Association of Tissue Banks. Serological screening is performed to assess for HIV, hepatitis, and syphilis. In addition, the processing that removes cellular components is believed to decrease risk of disease transmission. In the breast literature, it has been suggested that use of Alloderm® in association with breast reconstruction results in a higher complication rate (seromas) although no higher loss of tissue expanders and is accompanied by an improved cosmetic result [10, 31, 49, 50]. Likewise, satisfactory results have been seen in applications in which AlloDerm® is used instead of split- or full-thickness skin grafting [30, 54, 58, 62].
Cellular Skin Substitutes
Cellular skin substitutes contain living cells, often fibroblasts and keratinocytes embedded in a collagen or polygalactic scaffold to recreate epidermal and dermal skin layers. The realm of cellular epidermal products consists of culturing autologous skin cells to create sheets of epidermis that may be applied as a skin graft. These products such as Epicel® (Genzyme Corporation, Cambridge, MA) and EpiDex® (Euroderm, Baden, Switzerland), and Apligraf® (Organogenesis Inc., Canton, MA) are useful in cases where healthy donor skin may be limited as in the case of severe burns.
Epicel® is the only cultured epidermal autograft that meets FDA guidelines for safety, quality, and manufacturing. Epicel® is manufactured using donor autologous dermal cells and at some point during the process is combined with residual amounts of murine cells. Hence, the FDA considers Epicel® a xenotransplantation product. It is marketed as a humanitarian use device. It is indicated for severe burn patients who have deep or full thickness burns covering ≥30 % of the total body area. To date, the effectiveness of Epicel® has not been proven in clinical studies. Therefore, long-term safety data is not available.
EpiDex® is a product derived from autologous cells obtained from the outer root sheath of the patient's hair follicles. The cells are cultured and cultivated and subsequently delivered to the treating physician in the form of a package containing six discs, each 1 cm diameter. A single package is able to treat a wound of 10 cm2. The EpiDex® Swiss Field Trial [48] demonstrated the efficacy of EpiDex® to treat intractable leg ulcers that did not heal by conventional wound care methods. By the end of the study, EpiDex® successfully and completely healed 74 % of the leg ulcers. Moreover, the EpiDex® treatment was more cost effective than traditional skin grafts. The authors concluded that EpiDex represents a good alternative to inpatient split-skin mesh treatments.
Apligraf® (Organogenesis Inc., Canton, MA) is the only living, bilayered cell-based product that is FDA approved to heal both diabetic foot ulcers [8] and venous leg ulcers [13]. The lower dermal layer combines bovine type I collage and human fibroblasts, which produce additional matrix proteins. The superficial epidermal layer is formed by promoting human keratinocytes to, first multiply, then differentiate to replicate human epidermis. The keratinocytes and fibroblasts are derived from neonatal foreskins.
The aforementioned cellular products are only representative of many products currently available on the market. These cellular options for wound coverage involve cultured epithelial autografts, which have shown to be efficacious when there is a paucity or contraindication to skin graft harvest due to limited donor site availability. These skin substitutes are expensive options that are seem to be relatively unnecessary for isolated upper extremity injuries.
Discussion
In planning wound coverage in the upper extremity, efforts should include use of the safest and least invasive methods with a goal of achieving optimal functional outcome. The recent development of advanced technology in wound healing has triggered the use of skin substitutes to improve wound healing conditions, in addition to the more traditional methods. When considering the reconstructive ladder, skin grafts may not always represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum has been damaged. In addition, despite adequate graft take, hypertrophic scarring with subsequent cosmetic and functional limitations may develop because of inadequate dermal presence. Similarly, tissue flaps may not be prudent, as many trauma patients have significant comorbidities or associated injuries that may preclude flap reconstruction.
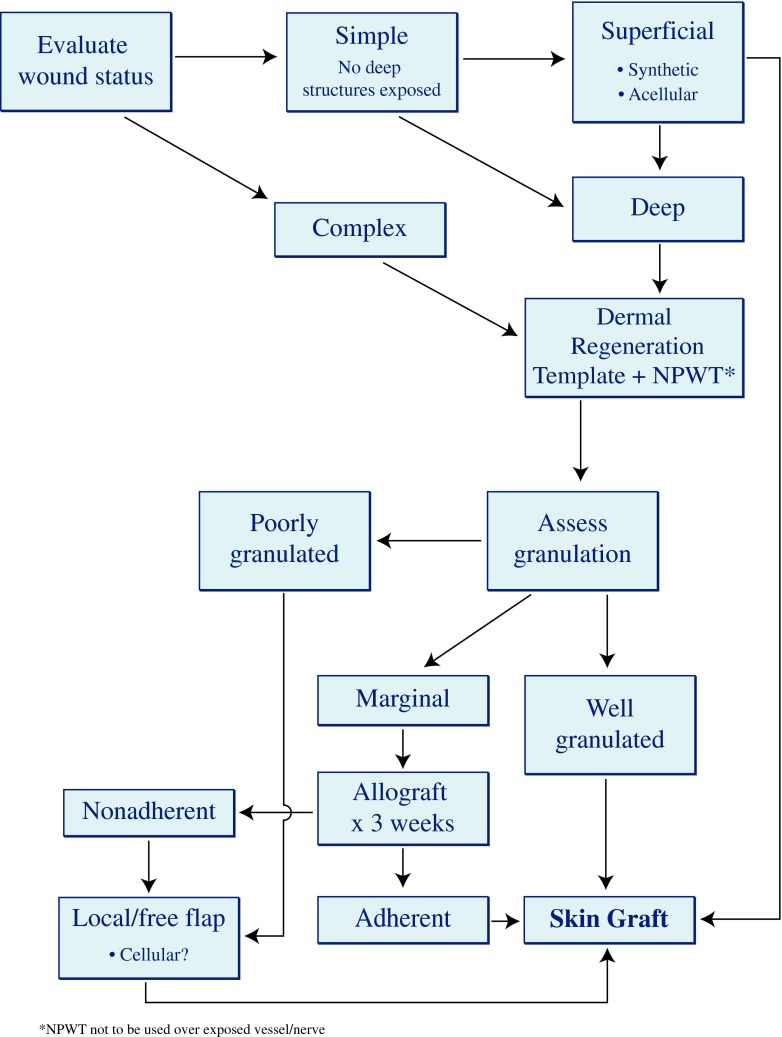
A variety of artificial dermal substitutes have been produced and used in clinical practice to accelerate wound healing and reduce wound contraction. However, it is unclear which product or product family might be better suited for a given clinical scenario. Furthermore, to our knowledge, there have been no reports integrating the relative values of each product into one cohesive algorithm. This review presents an algorithm for upper extremity wound coverage, based on our experience, and focused on the use of skin substitutes (Fig. 3). The algorithm assumes reliable vascular status and an infection-free wound.
Fig. 3.
Wound coverage algorithm for the upper extremity, focused on the use of skin substitutes
The decision making process involved in the management of upper extremity wounds is complex but hinges on certain simple principles. Simple wounds are those that do not contain any exposed vital structure (nerve, artery, tendon, bone), as opposed to complex wounds. If the wound is simple, it needs coverage only to keep it moist in preparation for a skin graft. In this case, any of the acellular products will work. For deeper, complex wounds that require stimulation of granulation tissue prior to grafting, dermal regeneration templates or allograft products combined with negative pressure dressing is our choice. If granulation tissue is of marginal viability, then application of allograft skin for 3–4 weeks can help determine if the wound is suitable for grafting, based on adherence of the allograft. Finally, animal allograft products are less cellular hence result in more fibrosis, while human products maintain more cellular components. For hand coverage, maintaining cellular structures and minimizing fibrosis are key, making human products a more favorable option.
Conclusions
Upper extremity injuries can often be complex requiring soft tissue coverage. This manuscript highlights many of the synthetic, acellular, and cellular options available to the upper extremity surgeon to address such injuries. Many products including Integra®, Biobrane®, and Matriderm® are welcomed additions to the armamentarium of the upper extremity surgeon and can serve as adjuncts or substitutions for complex free tissue transfers or skin grafts. Much research is needed to fully elucidate the potential of these new technologies. However, they have clearly and effectively decreased the morbidity associated with soft tissue coverage in the upper extremity.
Acknowledgments
Conflict of Interest
John Capo is on the Speakers Bureau for Integra Life Sciences and is a consultant for Wright Medical Technology.
Kyle P. Kokko declares no conflict of interest.
Marco Rizzo declares no conflict of interest.
Julie E. Adams declares no conflict of interest.
Ben Shamian declares no conflict of interest.
Brenon Abernathie declares no conflict of interest.
Eitan Melamed declares no conflict of interest.
There were no sources of financial support/funding in the preparation of this manuscript.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Statement of Informed Consent
No patient identifiers are used or visualized in this manuscript.
Contributor Information
John T. Capo, Phone: +1-201-3092427, FAX: +1-212-5987654, Email: njhanddoc@yahoo.com
Kyle P. Kokko, Phone: +1-843-5681769, FAX: +1-212-5987654, Email: Kyle.kokko@nyumc.org
Marco Rizzo, Email: rizzo.marco@mayo.edu.
Eitan Melamed, Phone: +1-201-3092427, FAX: +1-212-5987654, Email: Eitan.melamed@nyumc.org.
References
- 1.Adams JE, Zobitz ME, Reach JS, Jr, et al. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700–9. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja NK, Datiashvili RO. Biobrane in the management of critical microsurgical wounds of the upper extremity. Microsurgery. 2012;32(3):196–200. doi: 10.1002/micr.20966. [DOI] [PubMed] [Google Scholar]
- 3.Azzena B, Amabile A, Tiengo C. Use of acellular dermal regeneration template in a complete finger degloving injury: case report. J Hand Surg [Am] 2010;35(12):2057–60. doi: 10.1016/j.jhsa.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barret JP, Dziewulski P, Ramzy PI, et al. Biobrane versus 1 % silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105(1):62–5. doi: 10.1097/00006534-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Belcher HJ, Zic R. Adverse effect of porcine collagen interposition after trapeziectomy: a comparative study. J Hand Surg (Br) 2001;26(2):159–64. doi: 10.1054/jhsb.2001.0554. [DOI] [PubMed] [Google Scholar]
- 7.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Brem H, Young J, Tomic-Canic M, et al. Clinical efficacy and mechanism of bilayered living human skin equivalent (HSE) in treatment of diabetic foot ulcers. Surg Technol Int. 2003;11:23–31. [PubMed] [Google Scholar]
- 9.Brown EZ Jr., Pederson CW. Nonmicrosurgical coverage of the upper extremity. In: Wolfe SW, editor. Operative hand surgery.6th ed. New York: Churchill-Livingstone; 2011. p. 1645–1720.
- 10.Burke JF, Yannas IV, Quinby WC, Jr, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194(4):413–28. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carothers JT, Brigman BE, Lawson RD, et al. Stacking of a dermal regeneration template for reconstruction of a soft-tissue defect after tumor excision from the palm of the hand: a case report. J Hand Surg [Am] 2005;30(6):1322–6. doi: 10.1016/j.jhsa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy C, St Peter SD, Lacey S, et al. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns. 2005;31(7):890–3. doi: 10.1016/j.burns.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Cavorsi J, Vicari F, Wirthlin DJ, et al. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf) in the treatment of lower-extremity ulcers. Wound Repair Regen. 2006;14(2):102–9. doi: 10.1111/j.1743-6109.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 14.Cervelli V, Brinci L, Spallone D, et al. The use of MatriDerm(R) and skin grafting in post-traumatic wounds. Int Wound J. 2011;8(4):400–5. doi: 10.1111/j.1742-481X.2011.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng A, Saint-Cyr M. Comparison of different ADM materials in breast surgery. Clin Plast Surg. 2012;39(2):167–75. doi: 10.1016/j.cps.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Dantzer E, Queruel P, Salinier L, et al. Dermal regeneration template for deep hand burns: clinical utility for both early grafting and reconstructive surgery. Br J Plast Surg. 2003;56(8):764–74. doi: 10.1016/S0007-1226(03)00366-7. [DOI] [PubMed] [Google Scholar]
- 17.Falanga V, Isaacs C, Paquette D, et al. Wounding of bioengineered skin: cellular and molecular aspects after injury. J Invest Dermatol. 2002;119(3):653–60. doi: 10.1046/j.1523-1747.2002.01865.x. [DOI] [PubMed] [Google Scholar]
- 18.Gerding RL, Emerman CL, Effron D, et al. Outpatient management of partial-thickness burns: Biobrane versus 1 % silver sulfadiazine. Ann Emerg Med. 1990;19(2):121–4. doi: 10.1016/S0196-0644(05)81793-7. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert TW, Nieponice A, Spievack AR, et al. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147(1):61–7. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert TW, Stolz DB, Biancaniello F, et al. Production and characterization of ECM powder: implications for tissue engineering applications. Biomaterials. 2005;26(12):1431–5. doi: 10.1016/j.biomaterials.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Gravvanis AI, Tsoutsos DA, Iconomou T, et al. The use of integra artificial dermis to minimize donor-site morbidity after suprafascial dissection of the radial forearm flap. Microsurgery. 2007;27(7):583–7. doi: 10.1002/micr.20406. [DOI] [PubMed] [Google Scholar]
- 22.Hansen SL, Voigt DW, Wiebelhaus P, et al. Using skin replacement products to treat burns and wounds. Adv Skin Wound Care. 2001;14(1):37–44. doi: 10.1097/00129334-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Haslik W, Kamolz LP, Manna F, et al. Management of full-thickness skin defects in the hand and wrist region: first long-term experiences with the dermal matrix Matriderm. J Plast Reconstr Aesthet Surg. 2010;63(2):360–4. doi: 10.1016/j.bjps.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Heckmann A, Radtke C, Rennekampff HO, et al. One-stage defect closure of deperiosted bone and exposed tendons with MATRIDERM(R) and skin transplantation: possibilities and limitations. Unfallchirurg. 2012;115(12):1092–1098. doi: 10.1007/s00113-011-2003-0. [DOI] [PubMed] [Google Scholar]
- 25.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988;208(3):313–20. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heitland A, Piatkowski A, Noah EM, et al. Update on the use of collagen/glycosaminoglycate skin substitute—6 years of experiences with artificial skin in 15 German burn centers. Burns. 2004;30(5):471–5. doi: 10.1016/j.burns.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Herlin C, Louhaem D, Bigorre M, et al. Use of Integra in a paediatric upper extremity degloving injury. J Hand Surg Eur Vol. 2007;32(2):179–84. doi: 10.1016/j.jhsb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002;55(3):185–93. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- 29.Jung JJ, Woo AS, Borschel GH. The use of Integra(R) bilaminar dermal regeneration template in apert syndactyly reconstruction: a novel alternative to simplify care and improve outcomes. J Plast Reconstr Aesthet Surg. 2012;65(1):118–21. doi: 10.1016/j.bjps.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Jung SN, Chung JW, Yim YM, et al. One-stage skin grafting of the exposed skull with acellular human dermis (AlloDerm) J Craniofac Surg. 2008;19(6):1660–2. doi: 10.1097/SCS.0b013e31818ac258. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129(1):28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 32.Kumar RJ, Kimble RM, Boots R, et al. Treatment of partial-thickness burns: a prospective, randomized trial using Transcyte. ANZ J Surg. 2004;74(8):622–6. doi: 10.1111/j.1445-1433.2004.03106.x. [DOI] [PubMed] [Google Scholar]
- 33.Lal S, Barrow RE, Wolf SE, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock. 2000;14(3):314–8. doi: 10.1097/00024382-200014030-00013. [DOI] [PubMed] [Google Scholar]
- 34.Lattari V, Jones LM, Varcelotti JR, et al. The use of a permanent dermal allograft in full-thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil. 1997;18(2):147–55. doi: 10.1097/00004630-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Lee KH. Tissue-engineered human living skin substitutes: development and clinical application. Yonsei Med J. 2000;41(6):774–9. doi: 10.3349/ymj.2000.41.6.774. [DOI] [PubMed] [Google Scholar]
- 36.Lou RB, Hickerson WL. The use of skin substitutes in hand burns. Hand Clin. 2009;25(4):497–509. doi: 10.1016/j.hcl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Macleod TM, Williams G, Sanders R, et al. Histological evaluation of Permacol as a subcutaneous implant over a 20-week period in the rat model. Br J Plast Surg. 2005;58(4):518–32. doi: 10.1016/j.bjps.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 38.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–80. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 39.McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67(2):637–40. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 40.Moiemen N, Yarrow J, Hodgson E, et al. Long-term clinical and histological analysis of Integra dermal regeneration template. Plast Reconstr Surg. 2011;127(3):1149–54. doi: 10.1097/PRS.0b013e31820436e3. [DOI] [PubMed] [Google Scholar]
- 41.Moiemen NS, Yarrow J, Kamel D, et al. Topical negative pressure therapy: does it accelerate neovascularisation within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns. 2010;36(6):764–8. doi: 10.1016/j.burns.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Moore C, Lee S, Hart A, et al. Use of Integra to resurface a latissimus dorsi free flap. Br J Plast Surg. 2003;56(1):66–9. doi: 10.1016/S0007-1226(02)00413-7. [DOI] [PubMed] [Google Scholar]
- 43.Mostow EN, Haraway GD, Dalsing M, et al. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41(5):837–43. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 44.Murray RC, Gordin EA, Saigal K, et al. Reconstruction of the radial forearm free flap donor site using integra artificial dermis. Microsurgery. 2011;31(2):104–8. doi: 10.1002/micr.20833. [DOI] [PubMed] [Google Scholar]
- 45.Nihsen ES, Zopf DA, Ernst DM, et al. Absorption of bioactive molecules into OASIS wound matrix. Adv Skin Wound Care. 2007;20(10):541–8. doi: 10.1097/01.ASW.0000294756.97425.c9. [DOI] [PubMed] [Google Scholar]
- 46.Noordenbos J, Dore C, Hansbrough JF. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil. 1999;20(4):275–81. doi: 10.1097/00004630-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor NE, Mulliken JB, Banks-Schlegel S, et al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75–8. doi: 10.1016/S0140-6736(81)90006-4. [DOI] [PubMed] [Google Scholar]
- 48.Ortega-Zilic N, Hunziker T, Lauchli S, et al. EpiDex(R) Swiss field trial 2004–2008. Dermatology. 2010;221(4):365–72. doi: 10.1159/000321333. [DOI] [PubMed] [Google Scholar]
- 49.Parks JR, Hammond SE, Walsh WW, et al. Human acellular dermis (ACD) vs. no-ACD in tissue expansion breast reconstruction. Plast Reconstr Surg, 2012. doi:10.1097/PRS.0b013e318262f06e [DOI] [PubMed]
- 50.Parks JW, Hammond SE, Walsh WA, et al. Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg. 2012;130(4):739–46. doi: 10.1097/PRS.0b013e318262f06e. [DOI] [PubMed] [Google Scholar]
- 51.Pham C, Greenwood J, Cleland H, et al. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33(8):946–57. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6(3):317–30. doi: 10.1016/0092-8674(75)90183-X. [DOI] [PubMed] [Google Scholar]
- 53.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43. doi: 10.1016/S0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 54.Rowe NM, Morris L, Delacure MD. Acellular dermal composite allografts for reconstruction of the radial forearm donor site. Ann Plast Surg. 2006;57(3):305–11. doi: 10.1097/01.sap.0000221622.41450.60. [DOI] [PubMed] [Google Scholar]
- 55.Ryssel H, Germann G, Kloeters O, et al. Dermal substitution with Matriderm(®) in burns on the dorsum of the hand. Burns. 2010;36(8):1248–53. doi: 10.1016/j.burns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Schneider J, Biedermann T, Widmer D, et al. Matriderm versus Integra: a comparative experimental study. Burns. 2009;35(1):51–7. doi: 10.1016/j.burns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 58.Sinha UK, Shih C, Chang K, et al. Use of AlloDerm for coverage of radial forearm free flap donor site. Laryngoscope. 2002;112(2):230–4. doi: 10.1097/00005537-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Still J, Donker K, Law E, et al. A program to decrease hospital stay in acute burn patients. Burns. 1997;23(6):498–500. doi: 10.1016/S0305-4179(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 60.Still J, Glat P, Silverstein P, et al. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29(8):837–41. doi: 10.1016/S0305-4179(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 61.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17(2):124–36. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Wax MK, Winslow CP, Andersen PE. Use of allogenic dermis for radial forearm free flap donor site coverage. J Otolaryngol. 2002;31(6):341–5. doi: 10.2310/7070.2002.34423. [DOI] [PubMed] [Google Scholar]
- 63.Weigert R, Choughri H, Casoli V. Management of severe hand wounds with Integra® dermal regeneration template. J Hand Surg Eur Vol. 2011;36(3):185–93. doi: 10.1177/1753193410387329. [DOI] [PubMed] [Google Scholar]
- 64.Wolter TP, Noah EM, Pallua N. The use of Integra in an upper extremity avulsion injury. Br J Plast Surg. 2005;58(3):416–8. doi: 10.1016/j.bjps.2004.04.008. [DOI] [PubMed] [Google Scholar]