Abstract
Background:
Diffuse idiopathic skeletal hyperostosis (DISH) is a systemic noninflammatory disease characterized by ossification of the entheses.
Methods:
This paper reviews the etiopathogenesis, epidemiology, clinical features, differential diagnosis, and treatment of DISH, based on current available literature.
Results:
Exact prevalence and incidence of DISH remains undetermined. Many external and genetic factors have been reported as being contributors to the pathogenesis of DISH. Current theories focus on the pathologic calcification of the anterior longitudinal ligament of the spine as the main physiopathological mechanism of disease. Clinical features are variable from monoarticular sinovitis to airway obstruction, and can be associated to systemic conditions. Comorbidities include obesity, hypertension, diabetes mellitus, hyperinsulinemia, dyslipidemia, and hyperuricemia according to a number of reports.
Conclusions:
DISH is a disease which involves the calcification of the anterior longitudinal ligament of the spine and can be associated with numerous clinical presentations and comorbidities.
Keywords: Anterior Longitudinal Ligament, diffuse idiopathic skeletal hyperostosis, Forestier's disease
INTRODUCTION
Forestier's disease was first described by Jacques Forestier and his student Jaume Rotes-Querol in 1950 under the name “senile ankylosing vertebral hyperostosis”.[8] However, it is now known that this disease is neither limited to the spine nor to older subjects. In 1976, Resnick and Niwayama coined the term “diffuse idiopathic skeletal hyperostosis” (DISH), which is currently widely utilized. Independently of how this condition is named, it consists in a systemic noninflammatory disease characterized by ossification of the entheses – the bony attachment of tendons, ligaments, and joint capsules.[17]
ETIOPATHOGENESIS
While the cause of DISH remains unclear, mechanical factors (such as the location of the aorta contributing to the development of bony bridging on specific sites), genetic factors (HLA genes), environmental exposures (fluoride, vitamin A/retinol), drugs (isotretinoin, etretinate, acitretin and other vitamin A derivatives), and metabolic conditions have been hypothesized to be relevant [Table 3].[4,5,7,15,19,21]
Table 3.
Etiopathogenesis of DISH

Although many external and genetic factors have been reported as being contributors of the pathogenesis of DISH, most of the current theories focus on the pathologic calcification of the anterior longitudinal ligament of the spine. The majority of these theories postulate that this process is due to the abnormal growth and function of the osteoblasts in the osteoligamentary binding.[2] However, it is important to clarify that not all authors accept the association between pathologic calcification and increased bone mineral density.[6]
EPIDEMIOLOGY
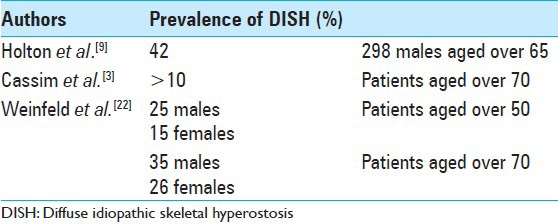
In terms of epidemiology of DISH, it varies in numerous reports – the absence of a consensus about the exact definition of the disease certainly contributes to not being able to determine its exact epidemiology. However, there are a few well-designed studies that try to estimate its prevalence. Holton et al. assessed 298 men aged older than 65 years from the general population. It was found that the prevalence of this disease (using Resnick's definition) in this age group was 42%.[9] Another study from the 1990s postulates that its prevalence is higher than 10% in patients over 70 years.[3] Furthermore, Weinfeld analyzed the data from two large American Midwest metropolitan hospital populations. It was concluded that the prevalence of DISH in this Anglo-Saxon population over 50 years of age is 25% in males and 15% in females. At 70 years of age, there is a 35% and 26% increase in prevalence, respectively.[22] Although the exact prevalence and incidence remains undetermined, it is well known that DISH is more frequent in men, and the incidence increases with age [Table 2] – mainly affecting patients over the age of 40 years.
Table 2.
Epidemiology of DISH-it increases in older males: Variable factors
CLINICAL FEATURES
Various signs and symptoms have been described in patients suffering from DISH, such as polyarticular pain, neck/thoracic/lumbar/extremity pain, acute monoarticular sinovitis, limited range of spinal motion, dysphagia, increased susceptibility to unstable spinal fractures, and different degrees of airway obstruction [Table 5].[23] The frequency and quality of complaints among these subjects varies by the site of the pathologic ossification. In contrast, many individuals who are diagnosed with DISH (visible ossifications on imaging studies acquired for other medical reasons) may be completely asymptomatic. Although DISH affects selectively the spine (with predilection to its thoracic portion – the hallmark of the disease is considered to be the ossification of the anterolateral aspect of the thoracic spine) [Figures 1–3], it is important to emphasize that this condition is not limited to the spine and has often been reported to involve multiple peripheral locations as well (extraspinal entheseal ossfications). These include periarticular hyperostosis of the hands, pelvis, knees, elbows, etc., The enthesophytes seen in DISH must be differentiated from osteophytes due to osteoarthritis and from syndesmophytes due to spondyloarthritis [Table 4]. Some of the features that help differentiate DISH from other pathologic structures are: Developing from joints that are rarely affected by osteoarthritis, such as shoulder, elbow, and metacarpophalangeal joints; developing from bone that is distant from the bone–cartilage junction; affecting distinctive entheses such as the calcaneal spur, quadriceps tendon, among others.[12,13]
Table 5.
Clinical features of DISH

Figure 1.
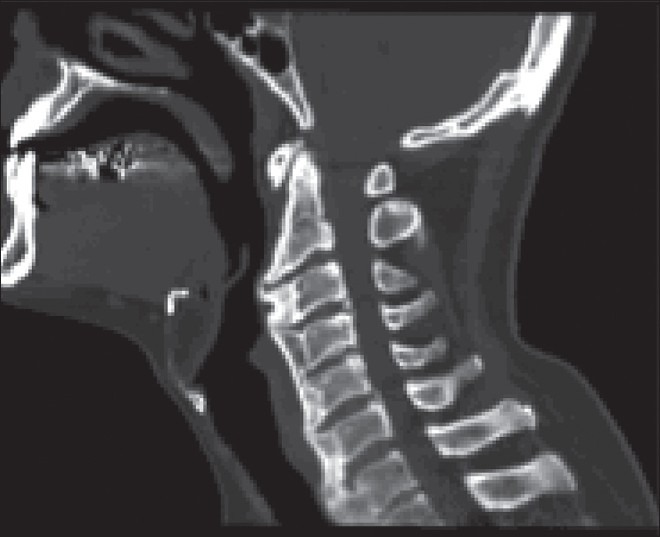
Sagittal cervical CT scan – Bone window
Figure 3.
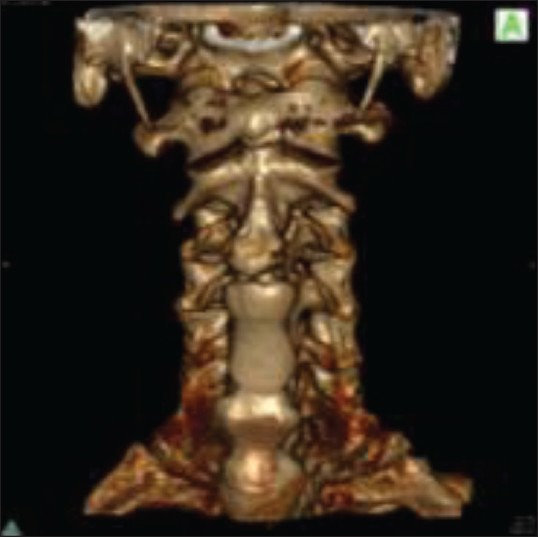
Same CT scan in 3D reconstruction
Table 4.
Pathologic calcification of DISH

Figure 2.
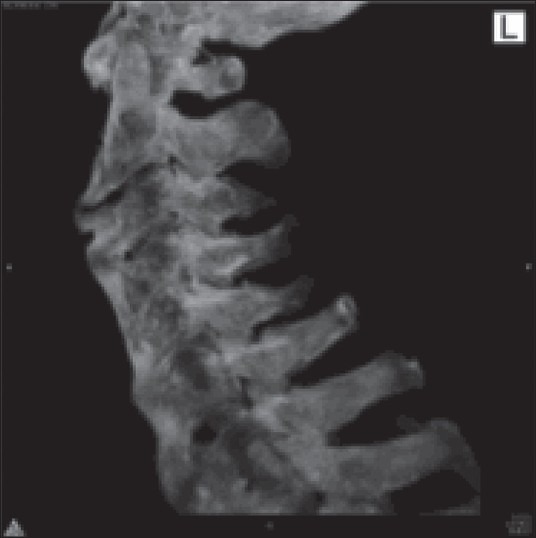
CT scan from the same patient demonstrating hyperostosis and anterior cervical fusion
The most commonly used classification criteria were defined by Resnick and Niwayama and required following anterolateral ossifications of at least four contiguous thoracic vertebral segments, preservation of the intervertebral disk spaces, and absence of apophyseal joint degeneration or sacroiliac inflammatory changes [Table 1].[16]
Table 1.
Criteria to diagnose diffuse idiopathic skeletal hyperostosis according to Resnick and Niwayama

Despite the fact that the presence of constitutional and metabolic abnormalities is not mandatory for making a formal diagnosis of DISH, it is known that systemic conditions are associated with DISH in varying degrees. These comorbidities include obesity, hypertension, diabetes mellitus, hyperinsulinemia, dyslipidemia, and hyperuricemia, according to a number of reports.[10,12,18] Additionally, two recent studies showed that these patients have a higher incidence of risk factors for stroke, higher prevalence of metabolic syndrome, and a higher risk for future coronary events.[11,14]
DIFFERENTIAL DIAGNOSIS
The most common conditions that may also present with bony excrescences, similar to those related to DISH, are Spondylosis Deformans and Ankylosing Spondylitis. The former disease is by far the most common of the disorders to be considered in the differential diagnosis of DISH. Spondylosis Deformans, however, does not affect the anterior longitudinal ligament in the thoracic spine, and that is how one can differentiate these two conditions. The latter disease shares some features seen with DISH, such as a preponderance in males and an association with ligamentous ossification and syndesmophytes. One may distinct these two conditions by noting that in Ankylosing Spondylitis, the bony bridges are slender, vertical, and involve the outer margin of the annulus fibrosus and do not involve the anterior longitudinal ligament. In addition, erosions and bony ankylosis of the sacroiliac and apophyseal joints are not seen in DISH.
TREATMENT
Therapy for DISH is based on symptomatic and empiric treatment. There have been no well-designed studies evaluating the effectiveness of any therapy in this disease. In general, physical therapy, analgesics, sedation, antiinflammatory drugs, and muscle relaxants, associated with appropriate diet, have all been successful in managing the majority of patients with DISH.[1,20]
Even though few articles until now have focused on indications for surgery, it is generally accepted that surgery is indicated for patients with severe symptoms (such as airway obstruction and/or dysphagia) in whom conservative approach has failed.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/4/122/130675
Contributor Information
Fábio A. Nascimento, Email: nascimento.fabio.a@gmail.com.
Luana Antunes Maranha Gatto, Email: luanamaranha@yahoo.com.br.
Roberto Oliver Lages, Email: oliverdrum@hotmail.com.
Heraldo Mello Neto, Email: heraldomello@gmail.com.
Zeferino Demartini, Junior, Email: demartiniz@gmail.com.
Gelson Luis Koppe, Email: koppe@bighost.com.br.
REFERENCES
- 1.Al-Herz A, Snip JP, Clark B, Esdaile JM. Exercise therapy for patients with diffuse idiopathic skeletal hyperostosis. Clin Rheumatol. 2008;27:207–10. doi: 10.1007/s10067-007-0693-z. [DOI] [PubMed] [Google Scholar]
- 2.Atzeni F, Sarzi-Puttini P, Bevilacqua M. Calcium deposition and associated chronic diseases (Atherosclerosis, Diffuse Idiopathic Skeletal Hyperostosis, and Others) Rheum Dis Clin North Am. 2006;32:413–26. doi: 10.1016/j.rdc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Cassim B, Mody GM, Rubin DL. The prevalence of diffuse idiopathic skeletal hyperostosis in African blacks. Br J Rheumatol. 1990;29:131–2. doi: 10.1093/rheumatology/29.2.131. [DOI] [PubMed] [Google Scholar]
- 4.DiGiovanna JJ, Helfgott RK, Gerber LH, Peck GL. Extraspinal tendon and ligament calcification associated with long-term therapy with etretinate. N Engl J Med. 1986;315:1177–82. doi: 10.1056/NEJM198611063151901. [DOI] [PubMed] [Google Scholar]
- 5.DiGiovanna JJ. Isotretinoin effects on bone. J Am Acad Dermatol. 2001;45:S176–82. doi: 10.1067/mjd.2001.113721. [DOI] [PubMed] [Google Scholar]
- 6.Eser P, Bonel H, Seitz M, Villiger PM, Aeberli D. Patients with diffuse idiopathic skeletal hyperostosis do not have increased peripheral bone mineral density and geometry. Rheum. 2010;49:977–81. doi: 10.1093/rheumatology/keq014. [DOI] [PubMed] [Google Scholar]
- 7.Forestier J, Lagier R. Ankylosing hyperostosis of the spine. Clin Orthop Relat Res. 1971;74:65–81. [PubMed] [Google Scholar]
- 8.Forestier J, Rotes-Querol J. Hyperostosis of the spine. Ann Rheum Dis. 1950;9:321–30. doi: 10.1136/ard.9.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holton KF, Denard PJ, Yoo JU, Kado DM, Barrett-Connor E, Marshall LM Osteoporotic Fractures in Men (MrOS) Study Group. Diffuse idiopathic skeletal hyperostosis and its relation to back pain among older men: The MrOS study. Semin Arthritis Rheum. 2011;41:131–8. doi: 10.1016/j.semarthrit.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiss C, Szilagyi M, Paksy A, Poor G. Risk factors for diffuse idiopathic skeletal hyperostosis: A case-control study. Rheum. 2002;41:27–30. doi: 10.1093/rheumatology/41.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Mader R, Novofestovski I, Adawi M, Lavi I. Metabolic syndrome and cardiovascular risk in patients with DISH. Semin Arthritis Rheum. 2009;38:361–5. doi: 10.1016/j.semarthrit.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Mader R, Sarzi-Puttini P, Atzeni F, Olivieri I, Pappone N, Verlaan JJ, et al. Extraspinal manifestations of diffuse idiopathic skeletal hyperostosis. Rheum. 2009;48:1478–81. doi: 10.1093/rheumatology/kep308. [DOI] [PubMed] [Google Scholar]
- 13.Mazieres B. Diffuse idiopathic hyperostosis (Forestier-Rotes-Querol disease): What's new? Joint Bone Spine. 2013;80:466–70. doi: 10.1016/j.jbspin.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Miyazama N, Akiyama I. Diffuse idiopathic skeletal hyperostosis associated with risk factors for stroke. Spine (Phila Pa 1976) 2006;31:E225–9. doi: 10.1097/01.brs.0000214949.75361.f4. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz RW, Boja B, Denko CW. The role of growth factors in degenerative joint disorders. J Rheumatol Suppl. 1991;27:147–8. [PubMed] [Google Scholar]
- 16.Resnick D, Niwayama G. 2nd ed. Philadelphia: WB Saunders; 1988. Diagnosis of bone and joint disorders; pp. 1563–615. [Google Scholar]
- 17.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119:559–68. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 18.Sarzi-Puttini P, Atzeni F. New developments in our understanding of DISH. Curr Opin Rheumatol. 2004;16:287–92. doi: 10.1097/00002281-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Troillet N, Gerster JC. Forestier disease and metabolism disorders. A prospective controlled study of 25 cases. Rev Rheum Ed Fr. 1993;60:274–9. [PubMed] [Google Scholar]
- 20.Umerah BC, Mukherjee BK, Ibekwe O. Cervical spondylosis and dysphagia. J Laryngol Otol. 1981;95:1179–84. doi: 10.1017/s0022215100092008. [DOI] [PubMed] [Google Scholar]
- 21.Utsinger PD, Resnick D, Shapiro R. Diffuse skeletal abnormalities in Forestier disease. Arch Intern Med. 1976;136:763–8. [PubMed] [Google Scholar]
- 22.Weinfeld RM, Olson PN, Maki DD, Griffiths HJ. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 1997;26:222–5. doi: 10.1007/s002560050225. [DOI] [PubMed] [Google Scholar]
- 23.Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18:145–56. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


