Abstract
Background:
The impact of early mobilization on perioperative comorbidities and length of stay (LOS) has shown benefits in other medical/surgical subspecialties. However, few spinal series have specifically focused on the “pros” of early mobilization for spinal surgery, other than in acute spinal cord injury. Here we reviewed how early mobilization and other adjunctive measures reduced morbidity and LOS in both medical and/or surgical series, and focused on how their treatment strategies could be applied to spinal patients.
Methods:
We reviewed studies citing protocols for early mobilization of hospitalized patients (day of surgery, first postoperative day/other) in various subspecialties, and correlated these with patients’ perioperative morbidity and LOS. As anticipated, multiple comorbid factors (e.g. hypertension, high cholesterol, diabetes, hypothyroidism, obesity/elevated body mass index hypothyroidism, osteoporosis, chronic obstructive pulmonary disease, coronary artery disease and other factors) contribute to the risks and complications of immobilization for any medical/surgical patient, including those undergoing spinal procedures. Some studies additionally offered useful suggestions specific for spinal patients, including prehabilitation (e.g. rehabilitation that starts prior to surgery), preoperative and postoperative high protein supplements/drinks, better preoperative pain control, and early tracheostomy, while others cited more generalized recommendations.
Results:
In many studies, early mobilization protocols reduced the rate of complications/morbidity (e.g. respiratory decompensation/pneumonias, deep venous thrombosis/pulmonary embolism, urinary tract infections, sepsis or infection), along with the average LOS.
Conclusions:
A review of multiple medical/surgical protocols promoting early mobilization of hospitalized patients including those undergoing spinal surgery reduced morbidity and LOS.
Keywords: Decreased cost, early mobilization, length of stay, prehabilitation, reduced morbidity, rehabilitation, spinal surgery
INTRODUCTION
A review of multiple studies’ early mobilization protocols, involving hospitalized medical/surgical patients, indicates getting out of bed “early” (e.g. out of bed the day of surgery [OOBDS] or the first postoperative day or soon thereafter (OOBFD)) reduces perioperative morbidities and length of stay (LOS) [Table 1]. Risks of perioperative complications (morbidity/mortality) increase with the number of attendant comorbid factors including; hypertension, diabetes, obesity/elevated body mass index (BMI), hypothyroidism, osteoporosis, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD) and other factors. If early mobilization protocols “work”, then the incidence of various perioperative/postoperative complications should be reduced (e.g. deep venous thrombosis (DVT)/pulmonary embolism (PE), pneumonia (PN)/atelectasis (AT), urinary tract infections (UTIs), sepsis, myocardial infarction (MI), stroke(S), and others). The reduction of the LOS also decreases attendant hospital costs, while freeing up beds for other patients (increasing hospital revenue).
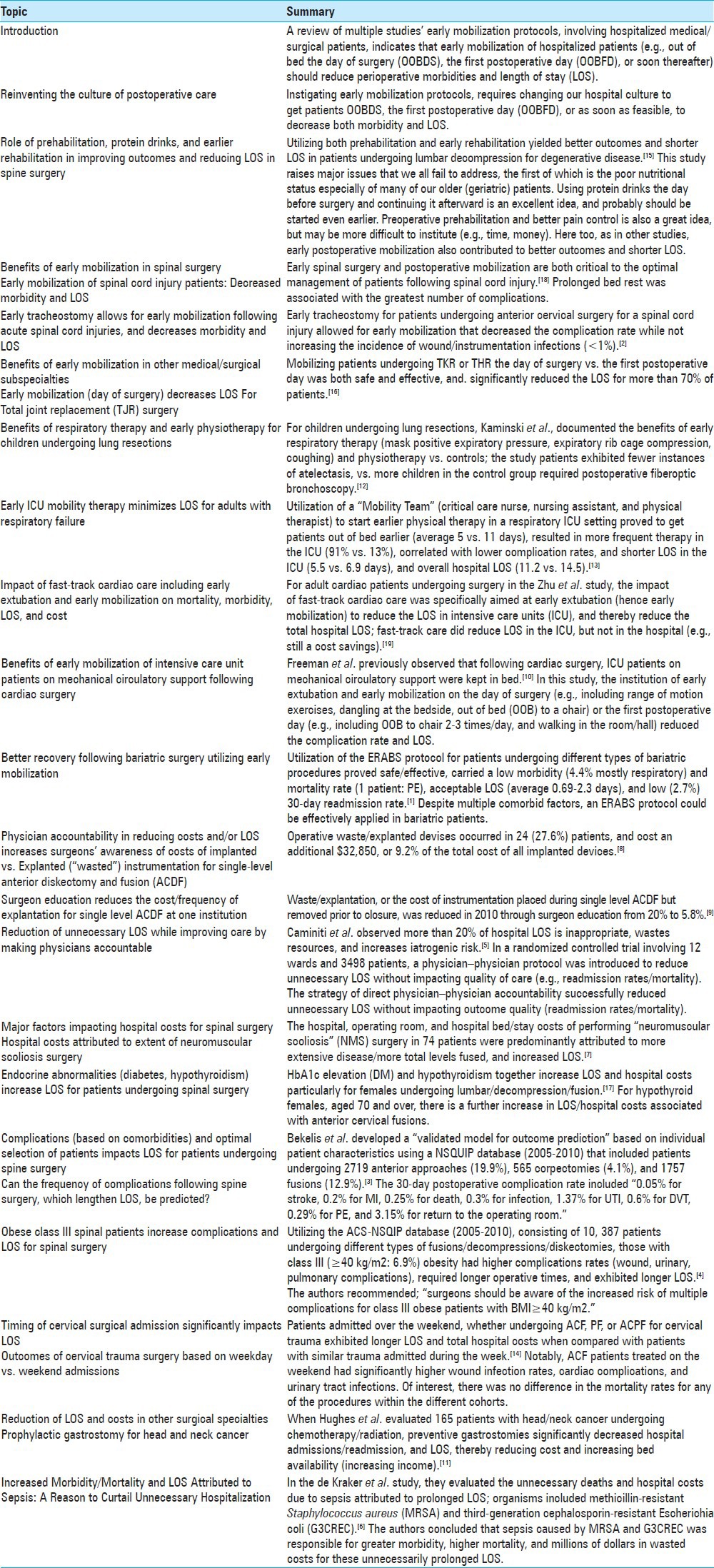
Table 1.
Reviewing benefits of early mobilization
Reinventing the culture of postoperative care
Here we reassess the “culture” surrounding early mobilization of medical/surgical intensive care unit (ICU) patients and/or those who have recently undergone spinal surgery. To accomplish this goal, we reviewed multiple early mobilization protocols along with other factors within these protocols that contributed to a reduction in both morbidity and LOS. Early mobilization was variously defined, but optimally included getting patients OOBDS, the first postoperative day (OOBFD), or very soon thereafter. We paid particular attention to the “pros” of proactive maneuvers associated with early mobilization, and realized that we must educate our patients, our families, our nurses, and ourselves, to improve patients’ health and reduce LOS.
Role of prehabilitation, protein drinks, and earlier rehabilitation in improving outcomes and reducing LOS in spine surgery
Nielsen et al. assessed whether prehabilitation in addition to early rehabilitation would improve outcomes following spinal surgery.[15] Utilizing a randomized design, 60 patients undergoing elective spinal procedures for degenerative disease were treated with both preoperative “prehabiliation” and early postoperative rehabilitation. Of these, 28 had both (e.g. prehabiliation/rehabilitation), while 32 had only standard care. Prehabilitation started 2 months prior to surgery, and involved a stringent exercise regimen, medications for adequate pain control, and protein drinks the day prior to surgery. Early postoperative rehabilitation involved; adequate pain management (with self-administered epidural analgesia), redoubled/intensified mobilization (2-fold more often than routine), and postoperative protein supplements. Postoperative findings included; those in the treatment group exhibited better outcomes versus controls (e.g. mobilization 1-6 days vs. 3-13,), and had shorter LOS (average 5 (range 3-9) vs. average 7 (range 5-15) days). Although no changes were noted regarding postoperative complications, adverse events, and low back radiating pain, patients in the study enjoyed greater postoperative satisfaction.
Benefits of early mobilization in spinal surgery
Early mobilization of spinal cord injury patients decreased morbidity and LOS
In Wang et al. retrospective analysis of patients who sustained acute spinal cord injuries, the interval between surgery and patient mobilization was correlated with postoperative complications and LOS.[18] Medical records of 102 patients with acute spinal injuries were evaluated, and the dates following injury to the dates of admission, operation, and mobilization were recorded. Prolonged bed rest was associated with the greatest number of complications.
Early tracheostomy allows for early mobilization following acute spinal cord injuries, and decreases morbidity and LOS
Babu et al. observed that after spinal cord injury, patients often require not only anterior cervical spine fixation (ACSF), but also tracheostomies.[2] However, the tracheostomies are often delayed by the concern for infecting the ACSF site and instrumentation. This then prolongs immobilization and thereby increases the risk of further deterioration (e.g. especially pulmonary complications). In this study, the beneficial impact of early tracheostomy and, hence, early mobilization was confirmed. Of 1184 patients undergoing ACSF, 20 (1.7%) had postoperative tracheostomies performed an average of 6.9 days (STDEV 4.2) postoperatively (range 0-17 days); half were performed within 6 days, and only 1 resulted in a wound infection. Furthermore, for both groups, there was only a 1% cross-infection rate, with none involving contamination of implants. However, late tracheostomy significantly increased the complication rate (e.g. 9 of 10 PNs occurred prior to delayed tracheostomy).
Benefits of early mobilization in other medical/surgical subspecialties
Early mobilization (day of surgery) decreases LOS for total joint replacement surgery
Tayrose et al. noted that utilizing a protocol that required early mobilization following total joint replacement (TJR) enhanced postoperatively recovery, while reducing costs and LOS.[16] Their protocol required physical therapy (PT) on the day of surgery for either total hip replacement (THR) or total knee replacement (TKR). The study included 900 patients divided into two groups; Group 1 underwent rapid PT started in the recovery room (RR), while Group 2 patients had the standard PT initiated the day after surgery. They observed a significant reduction in total LOS (3.9 days) for Group 1 versus a longer LOS (4.4 days) for the Group 2 patients. Cost savings were attributed to the decreased LOS and, therefore, lesser utilization of in-hospital resources when patients underwent PT in the RR the day of THR or TKR replacement surgery.
Benefits of respiratory therapy and early physiotherapy for children undergoing lung resections
Kaminski et al. evaluated the benefits of early respiratory therapy (e.g., including mask positive expiratory pressure, expiratory rib cage compression, and coughing) and physiotherapy (e.g. arm lifting and walking in <4 postoperative hours/and continued for 3 times/day) in 52 children undergoing lung resections versus 71 controls (who did not receive these early therapies).[12] The treatment group exhibited fewer instances of AT, while more children in the control group required postoperative fiberoptic bronchoscopy. Of interest, no differences were encountered in the average time of drainage/chest tube utilization or LOS.
Early ICU mobility therapy minimizes LOS for adults with respiratory failure
Utilizing a prospective cohort, Morris et al. instituted an active early mobility/PT (“Mobility Team”) protocol versus the “usual care” (control group) to treat patients with acute respiratory decompensation who required mechanical ventilation at the time of admission to a medical ICU.[13] The “Mobility Team”, consisting of the critical care nurse, nursing assistant, and physical therapist, were supposed to initiate the protocol within 48 h of mechanical ventilation. The main outcome measure for those receiving PT was survival until discharge from the hospital. Although clinical variables were similar for both patient groups, the “More PT Protocol” patients were out of bed earlier (5 vs. 11 days on average), had more frequent therapy in the ICU (91% vs. 13% on average), exhibited lower complication rates, had shorter LOS in the ICU (5.5 vs. 6.9 days on average), and shorter overall hospital LOS (11.2 vs. 14.5 on average). Nevertheless, although there were no significant complications attributed to early mobilization, no cost difference (survivors + nonsurvivors) between the “Mobility Team” vs. “Usual” groups could be confirmed.
Impact of fast-track cardiac care including early extubation and early mobilization on mortality, morbidity, LOS, and cost
For adult cardiac patients undergoing surgery in the Zhu et al. study, the impact of fast-track cardiac care was specifically aimed at early extubation, and hence early mobilization, to reduce the LOS in ICUs, and total hospital LOS.[19] To compare the safety/efficacy of fast track versus not fast-track care in adult cardiac surgical patients, the study utilized multiple major databases (e.g. Cochrane, MEDLINE, EMBASE, CINAHL) to identify randomized controlled trials of adults undergoing cardiac bypass, or aortic/mitral valve replacements. In addition to early extubation (and hence early mobilization) to get patients out of surgical ICUs, fast-track interventions also included the use of low-dose opioid-based general anesthesia. Although both groups demonstrated comparable risks of mortality (first postoperative year), and similar postoperative morbidities/complications (e.g. the same rates of MI, reintubation (<24 h), acute renal failure, major bleeding, major sepsis, and wound infections), fast-track care did reduce LOS in the ICU, but not in the hospital (e.g. still a cost savings). Of interest, only one high quality randomized controlled trial showed that early extubation was cost-effective.
Benefits of early mobilization of intensive care unit patients on mechanical circulatory support following cardiac surgery
Freeman et al. previously observed that following cardiac surgery, if ICU patients on mechanical circulatory support were kept in bed, it increased their risk of venous thromboembolism (DVT)/pulmonary emboli (PE), poorer/reduced pulmonary function that increased risk of PN, longer LOS, further deconditioning, and a greater need for postoperative rehabilitation.[10] To counteract the multiple risks of prolonged bed rest, the authors introduced a protocol including early extubation and early mobilization. The protocol on the day of surgery included range of motion exercises, dangling at the bedside, and being out of bed (OOBDS) to a chair. The protocol of getting out of bed the first postoperative day included OOBFD to a chair 2-3 times/day, and walking in the room/hall. Both protocols of early extubation/mobilization successfully reduced the number of the complications and LOS for this patient population.
Better recovery following bariatric surgery utilizing early mobilization
Awad et al. noted few protocols like the “Enhanced Recovery After Bariatric Surgery (ERABS)” were utilized in patients undergoing bariatric surgery.[1] In their series of 226 patients with median BMI of 44.9, patients underwent laparoscopic gastric bypasses (66%), sleeve gastrectomies (21%), and gastric bands (13%). The protocol tracked comorbidities (hypertension (40%), diabetes mellitus (34%), sleep apnea (24%) limited mobility (9%)), morbidity, mortality, readmission rates, and effectiveness of early mobilization. Postoperatively, no anastomoses leaked, 4.4% of patients developed mostly respiratory complications, and one died of a massive pulmonary embolus despite preoperative-inferior vena cava filter (IVC) filter placement. Respective mean LOS for bypasses was 1.88 days, for sleeves 2.30 days, and for bands 0.69 days; six patients (2.7%) required readmission within 30 days.
Physician accountability in reducing costs and/or LOS
Increasing surgeons’ awareness of costs of implanted vs. explanted (“wasted”) instrumentation for single-level anterior diskectomy and fusion
Epstein, Schwall, and Hood evaluated the costs of devices (plates, screws, spacers) implanted versus explanted (wasted or removed prior to closure) during 87 single-level anterior cervical diskectomy and fusion surgical procedures (ACDF) performed over one year at a single institution.[8] Costs to the hospital (without overhead) for implants included: screws (~103,572: 84 patients); plates (~120,694: 85 patients); allograft spacers (~92,776: 64 patients); cages (~38,821: 9 patients); and autografts (no charge; 14 patients); the total was ~355,863. Wasted dollars (~32,850) spent on wasted/explanted devices included: 37 screws (~11,014: 17 patients); 7 plates (~12,743: 5 patients); and 8 allograft spacers (~9093: 7 patients). The authors concluded that operative waste/explanted devises were encountered in 24 (27.6%) patients, costing an additional ~32,850, or adding 9.2%, to the cost of the implanted devices.
Surgeon education reduces the cost/frequency of explantation for single level ACDF at one institution
Epstein, Schwall, and Hood then demonstrated how making surgeons aware of waste/explantation (the cost of implanting devices but removing them prior to closure) occurring during single-level ACDF would substantially reduce costs.[9] The authors prospectively assessed the costs/frequency of wasting/explanting devices utilized to perform single-level ACDF over one year at one institution, before and after surgeon education. Explantation costs/frequencies for the first 4 months of 2010 versus the last 8 months were compared before and after surgeon education. Prior to education, instrumentation was explanted in 45.5% of cases versus after education, which occurred in only 16% of the cases. The explantation rate ((explanted devices/implanted devices) ×100) was lower following education for: screws (12.5-7.7%), plates (9.4-0%), and allograft spacers (7.1-2.9%). Finally, the overall cost of explanted devices was lowered after surgeon education from 20% to 5.8%.
Reduction of unnecessary LOS while improving care by making physicians accountable
Caminiti et al. observed that more than 20% of hospital LOS is inappropriate, wastes resources, and increases iatrogenic risk.[5] In a randomized controlled trial involving 12 wards and 3498 patients, a physician–physician protocol was introduced to reduce unnecessary LOS without impacting quality of care (e.g. measured by readmission rates/mortality). Physicians were informed if their discharges were compatible with the “Delay Tool”, and received their own LOS data (e.g. as audited by ward physicians). Over 12 months, more than 50% of LOS were considered appropriate, and most delayed discharges were attributed to nonmedical problems. Overall, the LOS was significantly reduced by 16% for the “intervention vs. control group”. Furthermore, outcomes, as measured by readmission, or mortality rates within 30 days of discharge, showed no significant differences between the two groups. The authors concluded that utilizing a strategy of direct physician–physician accountability successfully reduced unnecessary LOS without impacting outcome quality (readmission rates/mortality).
Major factors impacting hospital costs for spinal surgery
Hospital costs attributed to extent of neuromuscular scoliosis surgery.
Diefenbach et al. evaluated whether the hospital, operating room, and hospital bed/stay costs of performing “neuromuscular scoliosis” (NMS) surgery in 74 patients could be reduced.[7] Surgical costs reflected the degree/extent of surgical correction (e.g. posterior approaches (76%) with pedicle screws (75%)), LOS (averaged 8 days (range 3-47 days)), and extent of postoperative care. The total surgical cost was ~50,096± ~23,998 while other average costs included implant costs (~13,916), room/ICU costs (~12,483), and bone grafts costs (~6398). Increased total costs were predominantly attributed to more extensive disease/more total levels fused, and increased LOS.
Endocrine abnormalities (Diabetes, Hypothyroidism) Increase LOS for Patients Undergoing Spinal Surgery
Walid and Zaytseva noted that prior studies correlated increased LOS for spine surgery with diabetes, but that these studies did not include assessment of attendant hypothyroidism.[17] Here, the authors reviewed charts (2005 and 2008) of patients who were diabetic and/or hypothyroid undergoing spinal surgery, and evaluated how outcomes and surgical costs correlated with diabetes alone, hypothyroidism alone, or with both risk factors. Surgery included lumbar microdiskectomy (N = 237), anterior cervical decompression and fusion (N = 339), and lumbar decompression and fusion (N = 211). Patients average age was 54.5 years, and for 653 patients glycosylated hemoglobin (HbA1c) levels measured; 32.5% had an HbA1c level ≥ 6.1% and 4.3% had high HbA1c levels and hypothyroidism (this combination increased with age). Those undergoing lumbar decompressions/fusions with both comorbid factors demonstrated an increased LOS and hospital costs. Alternatively, for HbA1c elevation or hypothyroidism, the average LOS was 5 days, but both comorbidities increased the average LOS to 8 days. The average hospital cost without these comorbidities was ~52,449; diabetes increased the cost to ~56,176; both comorbidities (DM/hypothyroidism) further increased the average hospital cost to ~71,352. The authors concluded that cost and LOS increased with age, female gender, and lumbar decompression/fusion, but further increased LOS and cost were observed for hypothyroid females aged ≥ 70 years undergoing anterior cervical decompression/fusion.
Complications (based on comorbidities) and optimal selection of patients impacts LOS for patients undergoing spine surgery
Can the frequency of complications following spine surgery, which lengthen LOS, be predicted?
Bekelis et al. modeled the frequency of complications following spinal surgery using the National Surgical Quality Improvement Program (NSQIP) between 2005 and 2010.[3] This retrospective cohort study of 13,660 patients (NSQIP database) included 2719 anterior approaches (19.9%), 565 corpectomies (4.1%), and 1757 fusions (12.9%). Thirty-day postoperative complications included; “0.05% for S, 0.2% for MI, 0.25% for death, 0.3% for infection, 1.37% for UTI, 0.6% for DVT, 0.29% for PE, and 3.15% for return to the operating room.” Patients who had greater than a 3-day postoperative stay were at increased risk due to the following variables/comorbidities: advanced age, larger operations (fusion, corpectomy), poorer preoperative medical condition (“weight loss, dialysis, peripheral vascular disease, CAD, COPD, diabetes”), higher BMI, more severe neurological deficits inhibiting mobility, and bleeding disorders. The authors developed a “validated model for outcome prediction” based on individual patient characteristics.
Obese class III spinal patients increase complications and LOS for spinal surgery
Buerba et al. identified 10,387 patients undergoing spinal surgery from the American College of Surgeons (ACS)-NSQIP database (2005-2010) who were considered “class III obese” ((≥40 kg/m2: 6.9%).[4] Their aim was to correlate the impact of class III obesity on perioperative spinal complications, and LOS for patients undergoing anterior lumbar fusions (ALF 4.5%), posterior fusions (PF 17.9%), transforaminal or posterior lumbar interbody fusions (TLIF/PLIF 6.3%), discectomies (40.7%), and decompressions (30.5%). Complications and outcomes at 30 postoperative days included PE, DVT, death, system-specific complications (wound, pulmonary, urinary, central nervous system, cardiac), sepsis, and whether patients had ≥ 1 complications. Other variables evaluated were the operating room time, number of blood transfusions, LOS, and reoperation rates. Patients were split into four BMI groups: nonobese (18.5-29.9 kg/m2), obese I (30-34.9 kg/m2: 25.6%), obese II (35-39.9 kg/m2; 11.5%), and obese III (≥40 kg/m2: 6.9%). Patients who were obese in categories I and III had more urinary complications, obese II and III patients had more wound infections/complications, but only obese III patients, had longer operative times and LOS, with greater pulmonary complications.
Timing of cervical surgical admission significantly impacts LOS
Outcomes of cervical trauma surgery based on weekday vs. weekend admissions
In Nandyala et al. retrospective study based on a 34,122 Nationwide Inpatient Sample (2002-2011), the outcomes of cervical trauma surgery (anterior cervical fusions (ACF 11.5%,), posterior cervical fusion (PCF 19.9%), or combined fusions (anterior posterior cervical fusion (APCF) 17.2%)) were analyzed for patients admitted/operated on during the week versus over a weekend.[14] Variables evaluated included clinical data/status, comorbid factors, LOS, costs, mortality, and outcomes. In general, those admitted over the weekend were younger, and included more males with fewer comorbidities versus those admitted during the week. For patients undergoing ACF, the weekend admission LOS was 4.4 days longer and cost more (additional ~10,045) versus patients admitted during the week; they also demonstrated higher infection rates, more cardiac complications, and urinary infections versus those ACF patients admitted on a weekday. Patients undergoing PCF admitted over the weekend also had longer LOS (average 2.6 days longer) and cost more as well (~10,227 additional vs. weekday patients). Similarly, APCF patients admitted over the weekend had longer LOS (average 4.2 days longer), and also cost more (increased ~11,301) versus those admitted during the week. Of interest, there was no difference in the mortality rates for any of the procedures within the different cohorts.
Reduction of LOS and costs in other surgical specialties
Prophylactic gastrostomy for Head and Neck cancer
When Hughes et al. evaluated patients with head/neck cancer undergoing chemotherapy/radiation, they considered the benefits of prophylactic gastrostomy to maintain nutritional status, and potentially improve patient outcomes.[11]
Prophylactic gastrostomies were performed in 165 patients undergoing radical chemoradiation for head/neck cancers; this resulted in fewer complications, fewer admissions/readmissions, and shorter LOS versus those not receiving gastrostomies. The authors concluded that preventive placement of gastrostomy tubes for this patient population significantly decreased hospital admissions/readmission rates and LOS, thereby reducing hospital costs, and increasing bed availability (increasing income).
Increased morbidity/mortality and LOS attributed to sepsis: a reason to curtail unnecessary hospitalization
The de Kraker et al. study involving 31 countries participating in the European Antimicrobial Resistance Surveillance System (EARSS), assessed the unnecessary deaths and hospital costs due to sepsis attributed to prolonged LOS.[6] Sepsis was attributed to methicillin-resistant Staphylococcus aureus (MRSA) and third-generation cephalosporin-resistant Escherichia coli (G3CREC). The excess 30-day mortality and LOS due to MRSA or G3CREC (2007) were as follows: 27,711 MRSA sepsis led to 5503 excess deaths, 255,683 excess days (LOS), and cost 44.0 Euros (~63.1 million dollars), while the 15,183 G3CREC sepsis led to 2712 excess deaths, 120,065 excess days (LOS), and cost 18.1 Euros (~29.7 million dollars). The authors concluded that sepsis caused by MRSA and G3CREC was responsible for greater morbidity, higher mortality, and millions of dollars in wasted costs for these unnecessarily prolonged LOS.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/4/66/130674
REFERENCES
- 1.Awad S, Carter S, Purkayastha S, Hakky S, Moorthy K, Cousins J, et al. Enhanced recovery after bariatric surgery (ERABS): Clinical outcomes from a tertiary referral bariatric centre. Obes Surg. 2013 doi: 10.1007/s11695-013-1151-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu R, Owens TR, Thomas S, Karikari IO, Grunch BH, Moreno JR, et al. Timing of tracheostomy after anterior cervical spine fixation. J Trauma Acute Care Surg. 2013;74:961–6. doi: 10.1097/TA.0b013e3182826ea4. [DOI] [PubMed] [Google Scholar]
- 3.Bekelis K, Desai A, Bakhoum SF, Missios S. A predictive model of complications after spine surgery: The National Surgical Quality Improvement Program (NSQIP) 2005-2010. Spine J. 2013 doi: 10.1016/j.spinee.2013.08.009. In press. [DOI] [PubMed] [Google Scholar]
- 4.Buerba RA, Fu MC, Gruskay JA, Long WD, 3rd, Grauer JN. Obese class III patients at significantly greater risk of multiple complications after lumbar surgery: An analysis of 10,387 patients in the ACS-NSQIP database. Spine J. 2013 doi: 10.1016/j.spinee.2013.11.047. In press. [DOI] [PubMed] [Google Scholar]
- 5.Caminiti C, Meschi T, Braglia L, Diodati F, Iezzi E, Marcomini B, et al. Reducing unnecessary hospital days to improve quality of care through physician accountability: A cluster randomized trial. BMC Health Serv Res. 2013;13:14. doi: 10.1186/1472-6963-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Kraker ME, Davey PG, Grundmann H BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diefenbach C, Ialenti MN, Lonner BS, Kamerlink JR, Verma K, Errico TJ. Hospital cost analysis of neuromuscular scoliosis surgery. Bull Hosp Jt Dis (2013) 2013;71:272–7. [PubMed] [Google Scholar]
- 8.Epstein NE, Schwall GS, Hood DC. The incidence and cost of devices explanted during single-level anterior diskectomy/fusions. Surg Neurol Int. 2011;2:23. doi: 10.4103/2152-7806.77033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein NE, Schwall GS, Hood DC. Reducing the cost and frequency of explantations associated with single-level anterior diskectomy and fusion at a single institution through education. Spine (Phila Pa 1976) 2012;37:414–7. doi: 10.1097/BRS.0b013e3182451540. [DOI] [PubMed] [Google Scholar]
- 10.Freeman R, Maley K. Mobilization of intensive care cardiac surgery patients on mechanical circulatory support. Crit Care Nurs Q. 2013;36:73–88. doi: 10.1097/CNQ.0b013e31827532c3. [DOI] [PubMed] [Google Scholar]
- 11.Hughes BG, Jain VK, Brown T, Spurgin AL, Hartnett G, Keller J, et al. Decreased hospital stay and significant cost savings after routine use of prophylactic gastrostomy for high-risk patients with head and neck cancer receiving chemoradiotherapy at a tertiary cancer institution. Head Neck. 2013;35:436–42. doi: 10.1002/hed.22992. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski PN, Forgiarini LA, Andrade CF. Early respiratory therapy reduces postoperative atelectasis in children undergoing lung resection. Respir Care. 2013;58:805–9. doi: 10.4187/respcare.01870. [DOI] [PubMed] [Google Scholar]
- 13.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 14.Nandyala SV, Marquez-Lara A, Fineberg SJ, Schmitt DR, Singh K. Comparison of perioperative outcomes and cost of spinal fusion for cervical trauma: Weekday versus weekend admissions. Spine (Phila Pa 1976) 2013;38:2178–83. doi: 10.1097/BRS.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: Randomized clinical trial. Clin Rehabil. 2010;24:137–48. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 16.Tayrose G, Newman D, Slover J, Jaffe F, Hunter T, Bosco J., 3rd Rapid mobilization decreases length-of-stay in joint replacement patients. Bull Hosp Jt Dis (2013) 2013;71:222–6. [PubMed] [Google Scholar]
- 17.Walid MS, Zaytseva N. How does chronic endocrine disease affect cost in spine surgery? World Neurosurg. 2010;73:578–81. doi: 10.1016/j.wneu.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Teddy PJ, Henderson NJ, Shine BS, Gardner BP. Mobilization of patients after spinal surgery for acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:2278–82. doi: 10.1097/00007632-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 19.Zhu F, Lee A, Chee YE. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. 2012;10:CD003587. doi: 10.1002/14651858.CD003587.pub2. [DOI] [PubMed] [Google Scholar]


