Abstract
Recent technological advances have improved the whole slide imaging (WSI) scanner quality and reduced the cost of storage, thereby enabling the deployment of digital pathology for routine diagnostics. In this paper we present the experiences from two Swedish sites having deployed routine large-scale WSI for primary review. At Kalmar County Hospital, the digitization process started in 2006 to reduce the time spent at the microscope in order to improve the ergonomics. Since 2008, more than 500,000 glass slides have been scanned in the routine operations of Kalmar and the neighboring Linköping University Hospital. All glass slides are digitally scanned yet they are also physically delivered to the consulting pathologist who can choose to review the slides on screen, in the microscope, or both. The digital operations include regular remote case reporting by a few hospital pathologists, as well as around 150 cases per week where primary review is outsourced to a private clinic. To investigate how the pathologists choose to use the digital slides, a web-based questionnaire was designed and sent out to the pathologists in Kalmar and Linköping. The responses showed that almost all pathologists think that ergonomics have improved and that image quality was sufficient for most histopathologic diagnostic work. 38 ± 28% of the cases were diagnosed digitally, but the survey also revealed that the pathologists commonly switch back and forth between digital and conventional microscopy within the same case. The fact that two full-scale digital systems have been implemented and that a large portion of the primary reporting is voluntarily performed digitally shows that large-scale digitization is possible today.
Keywords: Clinical routine, digital pathology, digital pathology workflow, remote work, whole slide imaging
INTRODUCTION
Many building blocks for implementing digital pathology are available today. Progress has clearly been made in recent years in terms of resolution, image quality, and throughput of scanners; whereas, storage costs have been continuously declining. Therefore, many pathology labs are now facing the question if digital pathology is mature enough to be considered for large-scale implementation in routine clinical practice. In this paper we present the experiences from two Swedish pathology laboratories having deployed whole slide imaging (WSI) for virtually all histopathology, where more than 550,000 scanned slides have been available for primary review to date.
In the Department of Pathology of the Kalmar County Hospital, Sweden, the process towards full scale routine digital pathology started in 2006 for ergonomic reasons since a pathologist had developed cervical spine problems. By developing a new way to use existing technology, a system where the full production of glass slides could be scanned and diagnosed digitally was created. The Kalmar concept was subsequently employed at Linköping University Hospital in 2011. Many practical lessons have been learned that will be summarized in this article and compared to related work. Additionally, in order to assess the current digital practice, a web-based questionnaire was used to gather information from the pathologists in Kalmar and Linköping.
The paper is organized as follows: First, previous research on digital pathology implementations with bearing on digital routine diagnostics will be reviewed. Then the corresponding efforts in Kalmar and Linköping will be described, first the initial setup and activities in the Kalmar concept, followed by a description of the current system design in Kalmar and Linköping. The questionnaire on digital practices and its results are then presented. Finally, the key points of the digital pathology experiences are discussed and main conclusions are given.
RELATED WORK
Reports from Pittsburgh[1] and Toronto[2] describe the increased use of WSI, a decrease in turnaround time and improved user experience for frozen sections when using WSI.
Regarding clinical routine use, Al-Janabi et al.,[3] provides some hands on experience. In 2010 they scanned around 20% of their cases for primary digital diagnosis. 82.1% of the scanned slides could be signed out without the use of the microscope. Deficient image quality, caused by unsharpness or incomplete scanning, prevented a fully digital work flow in 5.3% of the cases.
In 2007, The Department of Pathology of the University Medical Center Utrecht started to scan their full glass production with the initial intention to optimize the preparation and running of the multidisciplinary team meetings.[4] They have since expanded their use to also include quality assurance and education, and now plan to go fully digital, starting to scan the glass slides before primary diagnosis rather than after, as done today.[5]
Even though any pathology lab could benefit from the experience of doing their own internal validation, more formal validation studies[6,7,8,9,10,11,12,13] give detailed insight about when scanned images fail. An unfocused digital slide or lack of nuclear detail might cause the pathologist to miss unexpected findings. In many cases it has also been harder to identify microorganisms like the Helicobacter pylori or the Candida albicans. On the other hand, the microorganisms were rarely completely missed. All of these studies conclude that scanning at ×20 is sufficient for most diagnostic work; whereas, certain types of cases require scanning at ×40 or even the scanning of multiple focal planes. Al-Janabi et al.,[8] compared detection of nucleated red blood cells using light microscopy, digital slides scanned at ×20 and digital slides scanned at ×40. Even though ×40 slides were an improvement compared to ×20 slides, a concordance rate of only 65% was achieved. Except for issues with focus and resolution, the limited field of view of standard desktop displays might induce errors since the chance of detecting something accidently when searching for something else is reduced. On the other hand, these kinds of validation studies detect issues with light microscopy as well. In a study,[11] a case history was probably mixed up during diagnosis with light microscopy, rendering a misidentification of a graft-versus-host colitis pattern.
To revisit the field of view issue, Randell et al.,[14] estimated that the field of view of a Leica DMRB microscope was equivalent to 7.2 million pixels of a digital slide, which means that most computer displays in use today provide a smaller field of view than a conventional microscope. By using larger displays, a 53 million pixel Powerwall[15] or a 11 million pixel three-display configuration,[14] no difference in time to diagnose was found compared to the conventional microscope.
Another speed-related issue within digital pathology is the input device. The computer mouse seems sufficient for panning and zooming for sporadic use of digital slides; whereas, many articles highlight the need for something better as digital diagnostics becomes used more extensively. Many microscope imitating devices have been proposed like the Nikon Ergo Controller or Bioimagene's iSlide, but very little is still known regarding to ergonomic input devices for digital pathology.
Digital pathology also offers new possibilities not available within the conventional microscope. A promising technique is automatic scoring and other kinds of digital image analysis (DIA). In a review, Riber-Hansen et al.,[16] conclude that current DIA methods are able to produce quantitative assessments of immunohistochemically stained slides with a similar variability as manual assessment. In other studies, quantitative DIA methods have been shown to outperform manual work for certain applications, such as in Ki67 proliferation assessment[17] and prediction of recurrence in prostate cancer.[18] However, Riber-Hansen et al.,[16] highlight the fact that a full-scale investigation of DIA methods considering all aspects of the clinical situation is still lacking.
THE KALMAR CONCEPT
We will now turn to our experiences of digital pathology implementations, starting with the initial effort in Kalmar. In the examples from recent previous work, common reasons for engaging in digitization in pathology operations are better telepathology, more convenient multidisciplinary team meetings, improved pathology education, and simplified quality assurance. In Kalmar, the main reason was improved ergonomics.
In 2006, one of the pathologists at the Kalmar County Hospital began having problems with his cervical spine, probably due to the extensive work at the microscope. This was the starting point for an innovation effort led by another consulting pathologist in Kalmar (the main author of this paper, ST). In order to minimize that type of work, the idea came up to use the same kind of technology used for digital telepathology within the routine diagnostics, a technology that had been used in Kalmar since 1999 for frozen sections. The idea was refined into a concrete approach: To let WSI scanners digitize all of the produced histopathology glass slides to enable diagnostic image review and reporting on a computer display. This way the ergonomics would be improved because more diversified working positions were allowed, the neck position would no longer be as fixed, and the digital slides could be navigated using ergonomic input devices.
A project outline was developed in 2006, which led to three different scanner systems being tested during 2007. The choice fell on two Scanscope XT scanners (Aperio, USA) over scanners from Zeiss and Hamamatsu. The Zeiss scanner tested suffered from technical problems that caused glass slides to get stuck in the mechanical transport system. Sometimes glass slides even broke, which was considered unacceptable. The Hamamatsu scanner offered good robustness and image quality, but the provider did not offer a solution for image management or integration with the laboratory information system (LIS).
Together with local partner companies, the Aperio scanners were integrated with the existing LIS, SymPathy (Tieto, Sweden) and image database system, Picsara (Mawell, Sweden). The scanner system was fully integrated and ready for internal validation in March 2008. During a period of 7 months, 14,326 glass slides were scanned to test the mechanical transportation in the scanner. The slides were scanned at ×20 (0.49 microns/pixel) and compressed using JPEG set at a quality level of 70, the highest possible level that could provide stable operation of the scanner system.
A random selection of 10% of the scanned cases, in total 606 cases, was diagnosed by three consultants and one resident pathologist each using both a computer workstation and a microscope. Each case was first reviewed using the digital slides and a description and diagnosis were formulated. Immediately thereafter the same pathologist reviewed the same case using the glass slides and entered the result in an elaborate spreadsheet, noting all discrepancies. The study was designed to limit the disturbance of the normal laboratory throughput. This means that the comparison between microscope and workstation has limitations since no washout period in-between tests or counterbalancing of the alternatives was employed. Any bias was, however, not likely to cause hazardous overestimation of the workstation capacity, but rather to disregard possible limitations of the microscope review. Therefore, for the intended purpose of detecting diagnostic quality flaws in the digital image with microscope review as gold standard, the study design was deemed sufficient.
The comparison only revealed two mismatched diagnoses, a gastric Helicobacter pylori case and a missed fungus in an esophagus biopsy. The former could be explained by the lack of the possibility to review the digital slides in a higher magnification than × 20 and the latter was visible in the digital slide, but was probably missed due to lack of time.
Given the positive validation results, in October 2008 the decision was made to start scanning all histopathology glass slides in Kalmar, except large sections (due to the lack of a large section scanner), and to make WSI available for routine diagnostics on a voluntary basis.
SYSTEM DESIGN
Since 2008 the system has been continuously developed in Kalmar and in the fall of 2010 the system design was copied to the neighboring Department of Pathology at Linköping University Hospital, and an effort to further develop the concept was initiated (again led by ST, having moved to Linköping). The following section will describe the current system design in further detail and why different design decisions were made. Unless otherwise noted, the system implementation is the same in both hospitals. An overview of the corresponding workflow is given in Figure 1.
Figure 1.
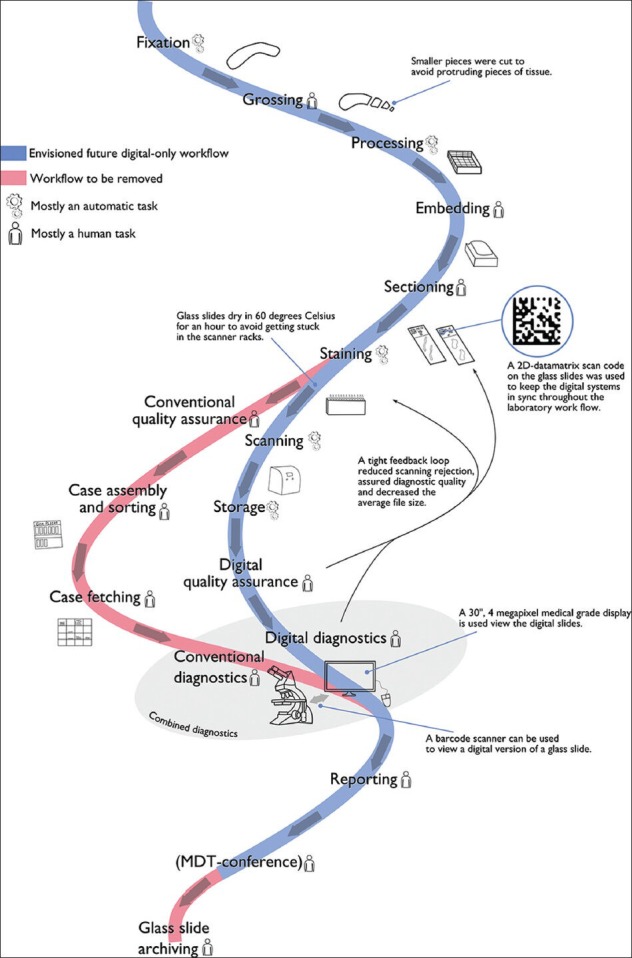
Combined work flow when adding scanning and digital diagnostics. Currently, a conventional and a digital work flow are maintained simultaneously. The red meander-like track belongs to the conventional work flow; whereas, the blue track represents the work flow envisioned with full digitization. Each activity is categorized as having either high or low human involvement, in order to highlight potential benefits from automation
Tissue Preparation
To make glass slides ready for scanning several additional steps have been added to the lab workflow. The refusal rate of the scanners, that is, the percentage of slides with failed scans from a clinical perspective, was initially measured to be 4.7% during the validation in Kalmar. This rate was considered a serious problem due to the additional manual work required to rectify the errors. One essential improvement was to establish a tight feedback loop between pathologists and lab technicians, similar to Pantanowitz et al.[1] The guideline for maximum cut size has been reduced to avoid protruding pieces of tissue causing scanning rejection. Furthermore, to prevent the glass slides from sticking to the scanner racks, the slides are now dried in 60°C for an hour before being scanned. These changes have reduced the rejection rate to well below 1%. Another improvement to the laboratory procedures is increased caution to keep the glass slides clean. This prevents dirt on the glass being considered as valid tissue by the scanners and therefore using precious storage space without aiding the diagnosis. Moreover, glue on the coverslip can cause unfocused digital slides, as can air bubbles between the slide and the coverslip. The average file size per slide was reduced from 0.6 to 0.4 GB after trimming the lab procedures.
To automatically register the scanned slides with the corresponding case in the LIS, the format of the labels attached to the slides has been updated. A two dimensional (2D)-datamatrix scan code is now present on the slide label, containing encoded information about the case ID, which staining that has been used and from what block the tissue section has been sliced. The scanner is then able to recognize the scan code and signals to the LIS once every new image has been scanned. The scanned slides are automatically connected to the corresponding case in the LIS and made available for diagnosis.
Scanning
Both Kalmar and Linköping today use two Aperio ScanScope AT Turbo scanners, with a batch size of 400 glass slides each, to scan most of the slide production.
In Linköping, there are two additional Hamamatsu scanners, a NanoZoomer XR allowing fast scanning at ×40 magnification and a NanoZoomer 2.0-RS dedicated for scanning large sections. In a nearby hospital (Norrköping), a modified Aperio Scanscope CS scanner is used for scanning large sections and frozen sections for telepathology.
During daytime, smaller batches of glass slides are scanned to enable a more flexible work flow including urgent cases. Before the end of the day, the scanners are fully loaded with glass slides to be scanned overnight. A primary quality control procedure is performed by a lab technician or a secretary to check for slides refused by the scanners resulting in a manual rescan of those slides. In Linköping a second quality control step for the scanning has been added, where a lab technician or a secretary verifies that all tissue on the glass slide has been included within the scan area. This way, the risk that pathologists need to order rescans is greatly reduced.
As digital slides have become more important to daily diagnostic work, interruptions in the scanner system have become less acceptable. All scanners are therefore connected to an uninterruptible power supply (UPS), and based on the experiences from Kalmar, the initial halogen light bulbs have been replaced by light-emiting diode (LED) light sources to avoid the need for regular calibration.
The computer operating the scanner is rebooted early in the morning every day to decrease the risk of out of memory issues and the supplier is responsible for the operation of the system through a service level agreement.
Even with careful precautions, the digital systems suffer from occasional malfunctions. The most common problem is that the image viewer crashes sporadically, especially when using the continuous zoom feature. Most issues have been dealt with by restarting the program or the system. More serious problems occur rarely. Once, both scanners broke down at the same time and all cases had to be diagnosed conventionally until the scanners had been repaired.
Storage
Images are stored on a storage area network (SAN) where the scanner sends the image file after each scan. They are handled by the Aperio Spectrum software which keeps a database of the stored images together with scanned metadata from the 2D-datamatrix barcodes. A middleware software, Mawell Picsara, regularly polls the database for new images to make them available to the LIS (SymPathy).
In Kalmar, there is enough storage to keep 6 months of digital slides. Older slides are automatically deleted. This is sufficient to enable primary diagnostics, eventual consultations, and multidisciplinary conferences. In Linköping, no digital slides have been deleted so far. This currently requires a capacity of 130 TB to store 330,000 digital slides, yielding an average size of 0.4 GB per slide.
Workstations
The pathologists work in separate offices. The WSI-enabled offices are equipped with a microscope and a computer workstation placed on a large desk. Each workstation has two displays, a smaller 24” display and a larger 30”, 4 megapixel medical grade display. Three kinds of software are used, the LIS, the middleware software (Picsara) to connect scanned images with cases in the LIS and Aperio ImageScope for viewing the digital slides. Normally, the small display is used for the LIS, the middleware uses a narrow side panel of the large display to list digital slides connected to a case and the remainder of the large display is used by the image viewer. This software combination provides all the information available to diagnose and report a case, including the request, the patient history, possible notes, and scanned sketches from the laboratory. However, it only provides rudimentary means to prioritize cases into electronic work lists. Paper is not used at all to exchange information within the laboratory work flow.
Each computer is equipped with a barcode scanner that is able to scan the 2D-datamatrix barcode on the glass slides. When a barcode is scanned, the corresponding case appears in the LIS. This means that pathologists have the possibility to mix digital and conventional microscopy. It is for example possible to organize the cases in the real world but review images digitally, or to diagnose conventionally but measure margins digitally.
To control the computer and view the digital slides, the pathologists are able to choose an input device that suits them. Even though most pathologists use a computer keyboard and a mouse, a liked addition to navigate digital slides are trackballs with scroll wheels. The trackballs are capable of locking the left mouse button in a down position using a switch button, which makes it less strenuous to pan using the trackball and zoom with the scrolling wheel without having to constantly press down the left mouse button.
In the viewing software (ImageScope) the navigation of the digital slides can be controlled from within an overlaid overview image of the slide. The location of the main viewing area is switched by a click and is marked with a rectangle in the overview. The overview is commonly combined with the aforementioned left mouse button lock technique to navigate around, minimizing the need for large hand movements.
Another navigation possibility is to selectively magnify a specific location using a virtual magnifying glass. That way the context is preserved, while the user is able to look at a feature in full magnification.
Some algorithmic help is available for predictive analyses in breast cancer. Food and Drug Administration (FDA)-approved image analysis algorithms are installed for immunohistochemical nuclear and cell membrane staining. The pathologist can choose a region of interest to be analyzed; the result is then visualized to allow the pathologist to verify the result.
During the primary diagnostic process, the pathologist can also make annotations in the digital slide. It is possible to use circles, squares, or free form figures to surround interesting findings or to write a free text comment. The interface also provides a calibrated ruler to measure for example infiltration depth, margins, and tumor size. The annotations and the measurements are saved to be able to quickly recall interesting findings when revisiting a case or during multidisciplinary team meetings.
Multidisciplinary Team Meetings
In Linköping, the multidisciplinary team meetings for breast cancer, including surgeons, oncologists, pathologists, mammographers, and breast care nurses, use only digital images today. Cases that will be discussed in the meeting are prepared beforehand by the pathologist, marking interesting findings in the digital slides using the built in annotation tool of the viewer unless already done at primary diagnostics. There is no digital worklist system where the presentation can be defined; instead the cases are retrieved manually by using a written list of request IDs that are typed into the digital system. All in all, the physicians judge that the meeting runs significantly faster with digital images than with microscopy use; they describe the effect as now conducting a typical 15-case meeting in 60 min rather than in 90 min as before. This has led to the extension of the multidisciplinary breast conferences to include 25 cases/week in typically 90 min.
Remote Work
It is possible to remotely access the LIS and the digital slide viewer using a remote desktop application over VPN (or Citrix). This kind of access is used by a few pathologists working part-time from home. It is also used to manage the excessive workload through outsourcing of diagnostics of selected case-types to a private clinic. So far over 8,000 cases have been outsourced since mid-2012, with a current rate of around 150 cases per week.
A new digital worklist suitable for remote work is currently being implemented in the LIS, which, together with scanning at × 40 using the Hamamatsu XR scanner, will allow, for example, the consulting pathologists to work full-time performing breast pathology diagnostics for Linköping from a remote location.
Training
The pathologists diagnosing digitally have not received any formal training. Initially, technical workshops were held in order to go through the new software interfaces, how to open slides, how to work with annotations, and where and how to use different features. In addition, the pathologists who participated in the initial validation learned a great deal about digital diagnostics, which has then been conveyed to colleagues through word of mouth. A pathologist who start diagnosing digitally today, learn that through self-validation, reviewing cases with digital slides by WSI, and then as glass slides until they feel comfortable with the new technique. However, this process has not been formalized.
Questionnaire on the Use of Digital Tools in Clinical Routine
The digital workstation is a significant change for the pathologists and much is yet to learn about its impact on the diagnostic review process. In order to increase the understanding of how pathologists with access to digital tools choose to use them in their routine diagnostic work, a web-based questionnaire was designed. The questionnaire was sent out to all the pathologists who were equipped with a digital workstation in Linköping and Kalmar. In Linköping, that means 11 out of 20 pathologists; whereas, all seven pathologists in Kalmar have a digital workstation. In total, the questionnaire reached 18 pathologists and 10 responses were received. One of the responding pathologists reported not using the digital workstation at all and that response was therefore excluded from further analysis.
The questionnaire investigated three topics: To what extent the digital workstation was used in their daily work, to what extent they used the different tools within the workstation, and aspects of how they experienced the digital diagnostic work. In addition, an empty text field was provided for respondents to provide their own comments.
RESULTS
The nine pathologists who responded, used their digital workstation to diagnose 38 ± 28% of their cases, the distribution is depicted in Figure 2. Two of the pathologists who used the digital workstation least, commented that they usually diagnosed their cases with the microscope, but switched to the digital workstation to check digital slides to perform one or several tasks. Such tasks could be checking images from previous biopsies or measuring margins. Those who diagnosed digitally to a greater extent commented that when reviewing digitally they sometimes fell back to using the microscope to perform diagnostic activities such as mitosis counting.
Figure 2.
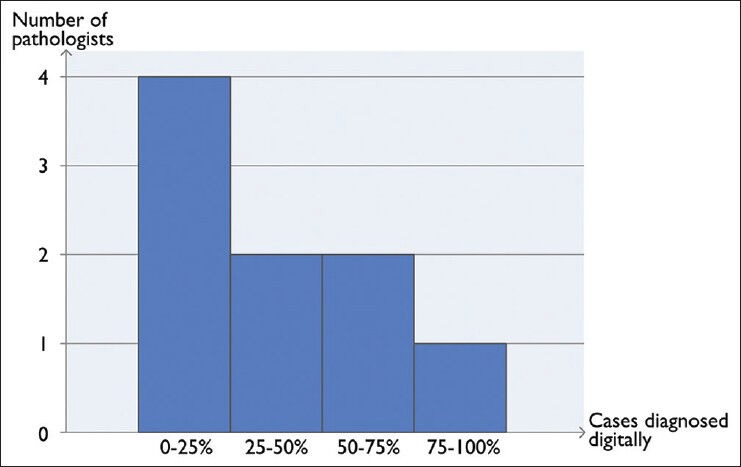
Distribution of histopathological cases diagnosed digitally according to the responses in the survey asking pathologists with access to a digital workstation in Linköping and Kalmar
Most pathologists do not do diagnostic work remotely from home at all; whereas, three out of nine pathologists review cases remotely every week. One pathologist commented that it is complicated to get started and that it requires good workstation hardware, a fast broadband connection, and remote IT support beyond the available level. On the other hand, another pathologist reported having no problems with remote work.
The different tools within the workstation were used to varying degrees. Navigating using the thumbnail overview and to measure using the calibrated ruler was used by everyone; whereas, digital annotations and automatic algorithms was moderately used. The magnifying glass was only used often by a single pathologist. The full statistics are given in Figure 3. Many comments were received requesting more efficient navigation and tool selection. One pathologist suggested to enable the control of more functions from the overview thumbnail in order to minimize hand movements. Another similar suggestion was to implement a mouse-like unit with more hotkeys for zooming, screening mode, annotations, and next case to speed up the interaction.
Figure 3.
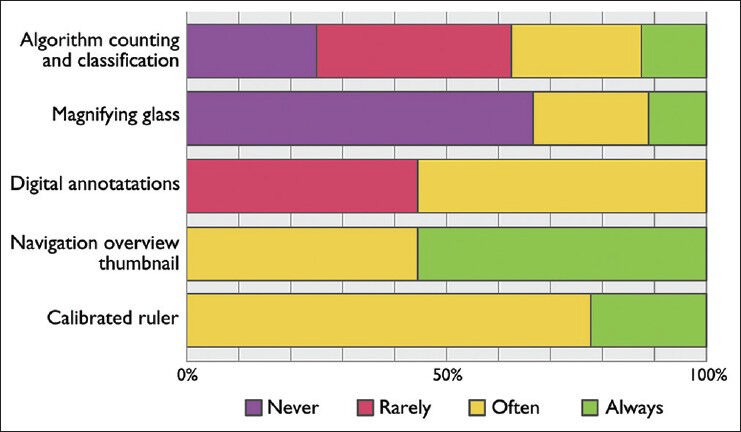
Responses asking pathologists in Kalmar and Linköping with access to a digital workstation: “How often do you use the following digital tools?”
The image quality in the digital environment was generally considered sufficient by the pathologists and the ergonomics of diagnosing digitally was also improved compared to diagnosing using the microscope. However, regarding the speed of the diagnostics and the convenience of reporting, no difference was reported. The full responses are available in Figure 4.
Figure 4.
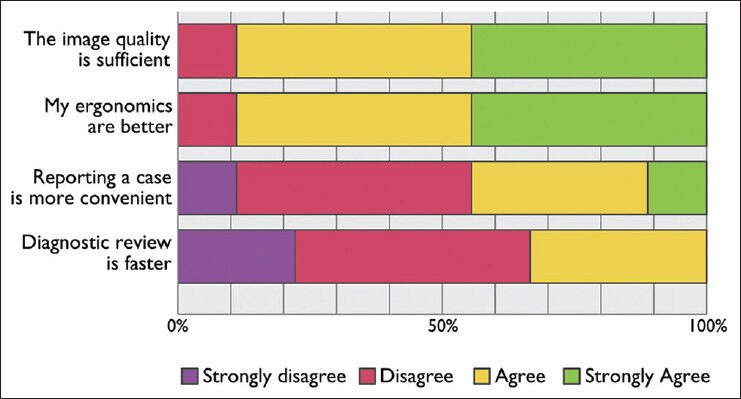
Responses asking pathologists in Kalmar and Linköping with access to a digital workstation: “When diagnosing digitally to what extent do you agree with the following statements?”
Other recurring comments from the free-text part of the questionnaire indicated several important areas of improvement: A fully digital workflow requires a good and functional worklist, the current image viewer software suffers from some lagging, and some cases are not possible to review digitally unless scanning in × 40 is used.
DISCUSSION
From the beginning a major driver behind the transition to digital pathology in Kalmar was the ergonomics. Therefore, it is reassuring, but perhaps not surprising, that most pathologists experience an improvement in that aspect. It is interesting that the digital work situation is described as an improvement despite that several points were made about the limitations of the current digital system: Negative comments about the input devices of the computer, the time to diagnose a case had not been decreased and the convenience to report a case had not been improved. Suboptimal usability of the digital system is one obvious cause for negative assessments, but lack of experience in digital work may also contribute even though we have not found any explicit evidence of this.
Since the case reporting was computerized before the computers were used for reviewing digital slides, a natural expectation would be that bringing both parts into the same environment would be even more convenient. This was not the case. One potential explanation is that the integration between the slide viewer software and the LIS does not provide a unified user experience. The LIS in question has not undergone any significant adaptation to the WSI scenario. Another possible reason would be that the context switching between the microscope and the computer is not so burdensome to begin with.
In Kalmar and Linköping, most pathologists have the possibility to diagnose all their cases digitally. Among those having the choice to diagnose digitally, not everyone has adopted it. One reason could be that certain specialties require high magnification (×40) images more often, which in the current × 20 setup reduces the convenience of using the computer since the pathologist often needs to switch to the microscope. There has been no observation that the feasibility of digital image review depends on the subspecialty area, but certain diagnostic subtasks have been identified as requiring high magnification, such as the search for Helicobacter within gastrointestinal pathology or to count mitoses.
Another reason for the reported low use of the digital workstation for mitosis count specifically within breast pathology could be explained by the fact that the formal protocol for breast cancer requires × 40; whereas, the practice of scanning at the time of the questionnaire was × 20. The routine has since been changed in Linköping to scan all breast histopathology at × 40. Even though it is not directly implied by the survey, a tendency has been observed that, in general, the digital review is more favorable for low magnification tasks rather than for high magnification work. In that case, experienced pathologists would have an easier transition to digital work since they are generally able to diagnose cases at a lower magnification level. The spread in the usage of the available digital tools could also be explained by individuality in technology adoption. Some pathologists will not embrace novelty unless they perceive that a great burden could be relieved; whereas, others find the new technology exciting and attractive per se.
An interesting question is whether the case size (number of slides) affects the preference of digital work. In our experience, the digital system provides a quicker and more convenient startup phase of image review since the request is digital and the digital slides are available one click away. For larger cases, this advantage is less important and the opinion that keeping track of a large set of slides is easier using the glass slides has been voiced. Therefore, we argue that future WSI viewers should aim to improve the slide organization aspect for large cases, to relieve the pathologist of this cognitive burden.
The adoption approach taken in Kalmar and Linköping is, and has been, to offer the digital possibility without making it mandatory to leave the traditional way of working. The adoption level achieved shows that this strategy is a feasible solution. We would argue that the voluntary approach has been necessary in order to maintain high quality diagnostics, while determining the best practices of the new technology. Even though some routines might be transferable to other laboratories, they certainly need to be adapted to the local culture and practice. Whether this approach is the best option for future implementations elsewhere is, therefore, not possible to deduce.
The response rate of the questionnaire was rather low, around 50%. One possibility is that the people who did not use the digital workstation avoided responding which could cause a bias in the result. An example to the contrary is that one pathologist did not diagnose with the workstation at all and responded anyway. The average digital diagnostics rate of 38% was in the order of magnitude of what was expected and increased traceability of the amount of cases diagnosed completely digitally is currently being implemented in order to measure this parameter more precisely. It should also be noted that even when primary review is not done using WSI, the digital slides are still useful for many purposes: For quick overviews, measurements, multidisciplinary team meetings, area annotation for additional stainings on large sections, area annotation for gene analysis, and convenient access to prior slides. Such benefits are acknowledged also in the Utrecht experiences.[5] Inevitably some glass slides will be scanned but never viewed and therefore can be considered as digital waste. This amount is, however, expected to be low, which means that sorting them out beforehand would be inconvenient.
Validation studies have shown that when scanning in × 20 magnification around 80% of the cases can be diagnosed digitally. We believe that trust is a factor that explains part of the gap between the validated level of 80% and the voluntary adoption rate reported here. Until a pathologist has gained full confidence in the digital system, the pathologist will feel obliged to check the original glass slide with the microscope if there is the slightest uncertainty. This creates a tendency for difficult cases to be diagnosed conventionally even though the reason they are difficult may be completely unrelated to better resolution or appearance in the microscope.
The workflow is still driven by passing around cardboard trays with glass slides, no digital worklist is used. With an overall digital usage of 38%, this seems reasonable. Having a digital worklist that drives the workflow is dependent on having a high percentage of the cases diagnosed completely digitally. Disturbances such as a missing cardboard tray when selecting a case from a digital worklist will quickly become very irritating. A complete and well integrated digital solution is preferable, but it has to be accompanied with a high percentage of cases being diagnosed digitally.
One of the main work practice possibilities arising from digitization is remote work. The feasibility of working remotely is underlined by the pathologists in the survey who do this today. It is, however, also clear that such a setup generates more requirements for the information technology (IT) solution compared to purely onsite systems, for example, additional sufficient workstations and IT support, and requirements on higher stringency in the quality control by the lab. Another possibility is to delegate cases to other hospitals or private labs, which was shown to be possible at a significant scale here. As there currently is a great understaffing of pathologists in Sweden, remote work is an important opportunity to solve workload and subspecialty issues.
CONCLUSIONS
The key conclusion of this paper is that implementation of large-scale digitization in clinical routine diagnostics is possible today. The Kalmar/Linköping example with over 500,000 slides that have been scanned for digital primary review, and an overall adoption rate of 38%, shows that major technical and operational hurdles may be overcome.
An improvement in digital diagnostic work, which has clearly been identified in the survey, is the ergonomics. This result confirms the validity of the initial motivation to make the transition to digital. From the reported usage of digital tools we can also conclude that possibilities for an overview image, measurements and annotations are much appreciated parts of the digital environment.
Another key conclusion is that there is still much room for improvement. Several limitations that are preventing further adoption have been identified including the lack of an efficient unified user experience across the LIS and the image viewer, suboptimal input devices as well as speed of digital image navigation.
There are ongoing and planned efforts in Kalmar and Linköping that aim to tackle the remaining limitations and reach an implementation realizing much more of the digital pathology potential for clinical benefits. These efforts include both development of clinical work practices and research initiatives for technology innovations.
ACKNOWLEDGMENTS
This work was supported by VINNOVA, grant 2012-01121, and the Swedish Scientific Council, grant 2011-4138. We would also like to thank the laboratory staff in Kalmar and Linköping.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2014/5/1/14/129452
REFERENCES
- 1.Pantanowitz L, Wiley CA, Demetris A, Lesniak A, Ahmed I, Cable W, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi: 10.4103/2153-3539.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University health network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Al-Janabi S, Huisman A, Nap M, Clarijs R, van Diest PJ. Whole slide images as a platform for initial diagnostics in histopathology in a medium-sized routine laboratory. J Clin Pathol. 2012;65:1107–11. doi: 10.1136/jclinpath-2012-200878. [DOI] [PubMed] [Google Scholar]
- 4.Huisman A, Looijen A, van den Brink SM, van Diest PJ. Creation of a fully digital pathology slide archive by high-volume tissue slide scanning. Hum Pathol. 2010;41:751–7. doi: 10.1016/j.humpath.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Stathonikos N, Veta M, Huisman A, van Diest PJ. Going fully digital: Perspective of a Dutch academic pathology lab. J Pathol Inform. 2013;4:15. doi: 10.4103/2153-3539.114206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Janabi S, Huisman A, Vink A, Leguit RJ, Offerhaus GJ, ten Kate FJ, et al. Whole slide images for primary diagnostics of gastrointestinal tract pathology: A feasibility study. Hum Pathol. 2012;43:702–7. doi: 10.1016/j.humpath.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Al-Janabi S, Huisman A, Vink A, Leguit RJ, Offerhaus GJ, ten Kate FJ, et al. Whole slide images for primary diagnostics in dermatopathology: A feasibility study. J Clin Pathol. 2012;65:152–8. doi: 10.1136/jclinpath-2011-200277. [DOI] [PubMed] [Google Scholar]
- 8.Al-Janabi S, Huisman A, Nikkels PG, ten Kate FJ, van Diest PJ. Whole slide images for primary diagnostics of paediatric pathology specimens: A feasibility study. J Clin Pathol. 2013;66:218–23. doi: 10.1136/jclinpath-2012-201104. [DOI] [PubMed] [Google Scholar]
- 9.Al-Janabi S, Huisman A, Willems SM, Van Diest PJ. Digital slide images for primary diagnostics in breast pathology: A feasibility study. Hum Pathol. 2012;43:2318–25. doi: 10.1016/j.humpath.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: A validation study. BMC Clin Pathol. 2006;6:4. doi: 10.1186/1472-6890-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jukiæ DM, Drogowski LM, Martina J, Parwani AV. Clinical examination and validation of primary diagnosis in anatomic pathology using whole slide digital images. Arch Pathol Lab Med. 2011;135:372–8. doi: 10.5858/2009-0678-OA.1. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: An evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41:1770–6. doi: 10.1016/j.humpath.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Wilbur DC, Madi K, Colvin RB, Duncan LM, Faquin WC, Ferry JA, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: A pilot study using paired subspecialist correlations. Arch Pathol Lab Med. 2009;133:1949–53. doi: 10.1043/1543-2165-133.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randell R, Ruddle RA, Mello-Thoms C, Thomas RG, Quirke P, Treanor D. Virtual reality microscope versus conventional microscope regarding time to diagnosis: An experimental study. Histopathology. 2013;62:351–8. doi: 10.1111/j.1365-2559.2012.04323.x. [DOI] [PubMed] [Google Scholar]
- 15.Treanor D, Jordan-Owers N, Hodrien J, Wood J, Quirke P, Ruddle RA. Virtual reality powerwall versus conventional microscope for viewing pathology slides: An experimental comparison. Histopathology. 2009;55:294–300. doi: 10.1111/j.1365-2559.2009.03389.x. [DOI] [PubMed] [Google Scholar]
- 16.Riber-Hansen R, Vainer B, Steiniche T. Digital image analysis: A review of reproducibility, stability and basic requirements for optimal results. APMIS. 2012;120:276–89. doi: 10.1111/j.1600-0463.2011.02854.x. [DOI] [PubMed] [Google Scholar]
- 17.Gudlaugsson E, Skaland I, Janssen EA, Smaaland R, Shao Z, Malpica A, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology. 2012;61:1134–44. doi: 10.1111/j.1365-2559.2012.04329.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee G, Ali S, Veltri R, Epstein JI, Christudass C, Madabhushi A. Cell orientation entropy (COrE): Predicting biochemical recurrence from prostate cancer tissue microarrays. Med Image Comput Comput Assist Interv. 2013;16:396–403. doi: 10.1007/978-3-642-40760-4_50. [DOI] [PubMed] [Google Scholar]


