Abstract
Being the most vascular tissue of the eye, importance of the choroid has been very well established in various retinal and chorio-retinal diseases. Understanding of the choroidal structures has improved significantly since the evolution of enhanced depth imaging. Quantitative assessment of choroidal measurements has been found to be reproducible using different devices. This review article describes factors affecting choroidal thickness and choroidal changes in several diseases and reports its clinical importance. Evaluation of choroid would provide insight into the pathogenesis, treatment planning and follow up in chorioretinal diseases.
Keywords: Choroid, Choroidal imaging, EDI, Enhanced depth imaging, VKH, Retinitis pigmentosa, Swept source OCT
Introduction
Choroid being the most vascular tissue in the eye, plays an important role in the pathophysiology of various ocular diseases. It provides nutrition to the outer retinal structures. Its role is established in various chorioretinal diseases such as central serous chorioretinopathy,1 Vogt–Koyanagi–Harada disease,2 high myopia-related chorioretinal atrophies,3 age related macular degeneration,4 and polypoidal choroidal vasculopathy.5 Quantitative assessment of choroid has been very challenging with traditional imaging modalities such as indocyanine green angiography and ultrasonography due to limited resolution and repeatability.6,7
Recent advances in optical coherence tomography including enhanced depth imaging have significantly improved understanding of the choroid. The outer limit of the choroid and the sclera cannot usually be reliably identified using conventional spectral domain optical coherence tomography (SD-OCT) due to scattering of light from pigmented retinal pigment epithelium (RPE) layer as well as decreasing sensitivity and resolution with increasing displacement from zero-delay. In SD-OCT, depth information is encoded as different frequencies of the interference spectrum. With increasing depth into tissue, echoes occur further from the point of detection, which is known to be the “zero delay line.” For a retinal OCT, zero delay line is positioned at posterior vitreous to provide clear image of retinal structures. By moving the joystick closer to the eye, zero delay line is focused at the retinal structures to provide better resolution choroidal images. Image averaging, eye tracking, high-speed scanning and low speckle noise produce high-quality choroid images with EDI-OCT.8
Swept source OCT (SS-OCT) is another device that uses a frequency swept laser with a narrowband light source that is rapidly tuned over a broad optical bandwidth that enables the measurement of interference at different optical frequencies or wavelengths sequentially over time.7 No spectrometer or line camera is needed for the Fourier transformation. This increases the imaging speed up to 300,000 axial scans per second and allows a deeper penetration of the sampling beam. SS-OCT offers several potential advantages over SD-OCT, including increased sensitivity through the full imaging depth, decreased fringe washout, better axial resolution over a broad imaging range, and higher detection efficiencies. Being a longer wavelength, it has the potential to image choroid much better than conventional SD-OCT.9
Choroidal imaging using different instruments
Choroidal imaging and thickness measurements have been reported with several commercially available OCT systems including the Cirrus (Carl Zeiss Meditec Inc, Dublin, CA, USA), Topcon 3DOCT 2000 (Topcon Corporation, Tokyo, Japan), Optovue RTVue (Optovue Inc., Fremont, CA, USA), Bioptigen (Bioptigen Inc., Research Triangle Park, NC, USA) and the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany). Spectralis OCT has eye-tracking ability, low speckle noise and averaging (up to 100 B-scans). Cirrus HD-OCT (Carl Zeiss Meditec) lacks eye-tracking ability and can only perform 20 B-scans at a time for each measurement.10
Yamashita et al. performed subfoveal choroidal thickness measurements using three different SD-OCTs: Heidelberg Spectralis-OCT (Spectralis), Cirrus HD-OCT (Cirrus), and Topcon 3D OCT-1000 Mark II (Topcon) and reported a high intraclass correlation coefficients (up to 0.98) as well as high interrater correlation coefficients (up to 0.95) with Spectralis, Cirrus, and Topcon.11 The intermachine correlation coefficient was also significantly high among the machines (P < 0.001, Spearman), 0.97 (Spectralis-Cirrus), 0.96 (Cirrus-Topcon), and 0.98 (Topcon-Cirrus). Similarly, Branchini et al. also reported a high reproducibility in choroidal thickness measurements among Zeiss Cirrus HD-OCT (Carl Zeiss Meditec Inc., Dublin, CA), Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany), and Optovue RTVue (Optovue Inc., Fremont, CA).10
While comparing choroidal thickness measurements between SD-OCT and SS-OCT, Matsuo et al. reported that the choroid measured with SS-OCT was thicker than that measured with SD-OCT instruments, and, thus, the choroidal thickness should not be compared between the SD-OCT and SS-OCT instruments.12
Choroidal thickness measurements
The choroidal thickness so far has been measured manually perpendicularly from the outer edge of the hyperreflective retinal pigment epithelium (RPE) to the inner sclera (choroid–sclera junction) at 500 microns interval from the fovea using the SD-OCT software. Choroidal thickness measurements in normal subjects appear to be highly reproducible.13,14 Shao et al. reported very high reproducibility with a mean difference of 3.14 ± 13.1 μm between the observers.15 Rahman et al. reported that a change >32 mm in subfoveal choroidal thickness probably exceeds interobserver variability.14 Similarly, Chhablani et al. reported highly reproducible manual segmentation of choroid for choroidal volume measurements using the built-in automated retinal segmentation software on Spectralis SD-OCT.16
Choroidal imaging in healthy subjects
Subfoveal choroidal thickness was reported in normal range from 191 ± 74.2 to 354 ± 111 microns.13,14,17–20 This variation could be due to the effect of ethnic differences also. The choroid is thickest subfoveally and thins nasally more than temporally. Inferior macular choroid has been measured thinner than the superior macular choroid.21
Barteselli et al. reported that the mean choroidal volume was 0.228 ± 0.077 mm3 for the center ring and 7.374 ± 2.181 mm3 for the total (Early Treatment Diabetic Retinopathy Study) ETDRS grid.22 The nasal quadrant showed the lowest choroidal volume, and the superior quadrant showed the highest choroidal volume. The temporal and inferior quadrants did not show different choroidal volume values. Ouyang et al. reported that the thickest choroid was found in the outer superior subfield, whereas the thinnest choroid was located in the outer nasal subfield. They reported that the optic nerve head could be a better center to study the regional differences in choroidal thickness compared to foveal thickness.21
Factors affecting choroidal thickness
Age related choroidal thinning in healthy eyes have been reported by numerous studies.13,18,20 Margolis et al.18 reported 15.6 microns decrease in choroidal thickness every decade, similarly 14 microns decrease every decade was reported by Ikuno et al.19 Ding et al. reported that this age-related thinning occurs only in age older than 60 years.20
Bidaut-Garnier et al. reported mean subfoveal choroidal thickness of 341.96 ± 74.7 μm in children.23 Choroidal thickness correlated with age (R2 = 0.056, P = 0.0017), height (R2 = 0.0292, P = 0.028), and weight (R2 = 0.0274, P = 0.033) but not with gender (P = 0.25). It was also inversely correlated to the axial length (R2 = 0.065, P = 0.0008). The nasal choroid appeared thinner than in the temporal area (P < 0.0001). Read and Park associates reported similar results.24,25 Read et al. reported choroidal thinning in myopic children compared to non-myopic children. They reported that the thinning of the choroid was greater than what would be predicted by a simple passive choroidal thinning with axial elongation.26
Wei et al. reported that the subfoveal thickness decreases by 15 microns for every increase in myopic refractive error of 1 D, or by 32 microns for every increase in axial length of 1 mm.17 Fujiwara et al. reported that choroidal thickness decreases by 12.7 μm for each decade of life and by 8.7 μm for each diopter of increasing myopia.27
Gender might play a role in choroidal thickness. Li et al. reported that women have a thinner choroid than men.28 In contrary, adult men have been reported to have thicker choroid than adult females.22 However, in children, Mapelli et al.29 reported a thicker choroid in females with slight significance (P = 0.056), similar to results from the Copenhagen Child Cohort 2000 Eye Study.30 The reason proposed for this difference is that the puberty promotes choroidal thickening in girls, an effect that may be mediated by the pubertal growth spurt. Chen et al. reported no interocular difference in choroidal thickness with 95% limits of agreement of −80 to +83 microns.31
In regard to diurnal variation, Tan et al. reported a significant variation in choroidal thickness in subjects with thicker choroid in the morning compared with those with thin choroids.32 The change in choroidal thickness also correlated with change in systolic blood pressure. Comparing choroidal thickness on two different days, a similar diurnal pattern was observed, with no significant difference between corresponding measurements at the same time point.32 Water drinking test has been reported to cause increase in choroidal thickness.33 Vural et al. reported decrease in choroidal thickness 4 h after coffee drinking.34
Choroidal imaging in various retinal diseases
High myopia
Due to increase in axial length, high myopic eyes have thin choroid (Fig. 1). Flores-Moreno et al. reported decrease in choroidal thickness by 25.9 ± 2.1 μm for each additional millimeter in high myopia.3,35 The choroid was found to be the thinnest at the nasal end. Thickness increased in a graded fashion toward the temporal side and reached maximum at the temporal end. This is in contrast to that in normal subjects, where thickness was highest under the fovea. Thinning of the choroid could be a predictive factor for visual acuity in highly myopic patients because the choroid is responsible for the oxygen and nutrient supply of the outer retina.
Figure 1.
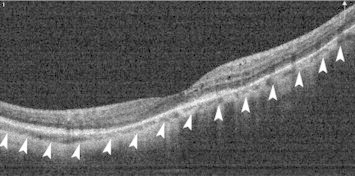
Decrease in choroidal thickness in an eye with high myopia. Outer margin of the choroid is shown as arrow-heads.
Retinitis pigmentosa
It has been hypothesized that there is a primary vascular dysfunction including reduced choroidal as well as retinal blood flow which leads to photoreceptor damage.36 Measurements of choroidal thickness in retinitis pigmentosa patients could be very useful for future therapies, such as suprachoroidal electrode arrays.
Previous studies have shown that the eyes with retinitis pigmentosa tend to have thin choroid, including focal and diffuse thinning.37,38 Ayton et al. reported that the patients with retinitis pigmentosa who have poorer visual acuity or longer duration of symptoms tend to have thinner choroids.39 In contrast, Dhoot and colleagues38 found that there was no correlation between visual acuity and choroidal thickness. However, implication of choroidal thickness in the pathophysiology of retinitis pigmentosa is not clear.
Other inherited disorders
Yeoh et al.37 performed a retrospective observational case series consisting of 20 eyes with a variety of inherited retinal diseases such as Best disease, Stargardt, choroideremia, peripherin retinal degeneration slow (RDS) mutations, and Bietti crystalline retinal dystrophy and reported variable choroidal thinning in these inherited diseases. They reported no association between choroidal thinning and visual acuity or extent of retinal dysfunction on electrophysiology.
Coscas et al. demonstrated choroidal thickening in adult (AOFVD) in contrast with the choroidal thinning observed in advanced AMD.40 Choroidal thickness measurement could help differentiate the challenging diagnosis between exudative AMD and the AOFVD complicated with choroidal neovascularization.
Central serous chorioretinopathy (CSCR)
Indocyanine green angiography shows increased choroidal permeability in CSCR, which may be the cause for increased choroidal thickness (Fig. 2).41 Increased choroidal thickness has been demonstrated in patients with acute CSCR (range, 439–573 μm), which was 214 μm (85%) greater than the mean choroidal thickness of age-matched normal eyes (P ⩽ 0.001). Additionally, studies have reported increased choroidal thickness in both eyes in patients with unilateral CSCR.1,42–44
Figure 2.
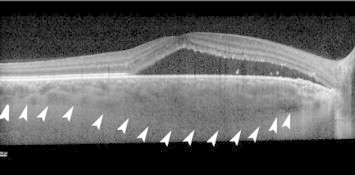
Increased choroidal thickness in an eye with central serous chorioretinopathy. Outer margin of the choroid is shown as arrow-heads.
Maruko et al. measured the subfoveal choroidal thickness before and after the treatment in eyes with chronic CSCR, using SD-OCT. Patients treated with photodynamic therapy (PDT) showed a decrease in the subfoveal choroidal thickness after the treatment, however, patients treated with laser photocoagulation did not demonstrate a reduction in the choroidal thickness.45 Jirarattanasopa et al. showed global choroidal thickening on choroidal macular maps.46 The choroidal thickness may be used as an additional parameter to assist in the differentiation of CSCR from other causes of serous retinal detachment and may indicate the activity of the disease on the follow-up after treatment with PDT.
Age-related macular degeneration (AMD)
SD-OCT has improved the understanding and management of AMD. Being a multifactorial disease, change in choroidal circulation may also contribute to the development of AMD. Therefore, evaluation of choroidal structural changes is important in AMD.
Choroidal thickness seems to be least affected in the early stage of the disease, however, the changes in late stages could be variable.47 Manjunath et al. reported that eyes with AMD on an average had a thinner choroid than that of normal controls (Fig. 3). Furthermore, eyes with exudative AMD had thinner choroids than eyes with nonexudative AMD.48 In a population based study, Jonas et al. reported no significant change in any form of AMD.49 In contrary, Lee et al. reported that the subfoveal choroidal thickness is closely related to the severity of nonexudative AMD, as well as the rate of GA progression.50 Regarding the effect of frequent anti-VEGF injections on choroidal thickness in eyes with neovascular AMD, there are many conflicting reports. Previous reports have shown significant decrease in choroidal thickness in eyes with wet AMD following intravitreal ranibizumab,51 as well as photodynamic therapy (PDT).52 On the contrary, other groups have not confirmed such a change in thickness following anti-VEGF treatment.53
Figure 3.
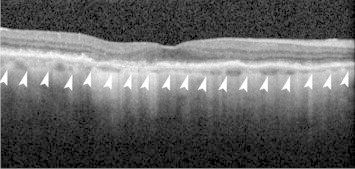
Decreased choroidal thickness in an eye with dry age-related macular degeneration. Outer margin of the choroid is shown as arrow-heads.
With the improved visualization of choroid, Spaide described a distinct entity, termed age-related choroidal atrophy, which has some overlap with dry AMD. He reported decrease in choroidal thickness was associated with loss of visible vessels, implying that age-related choroidal atrophy is a manifestation of small-vessel disease affecting the choroid.54
Koizumi et al. reported that eyes with polypoidal choroidal vasculopathy (PCV) have a thicker subfoveal choroid (293 ± 72.3 μm) when compared with eyes featuring typical neovascular AMD (245 ± 73.1 μm). The thicker choroid could be partially attributed to the dilation of middle and large choroidal vessels or an increase in the choroidal vascular permeability that is observed by ICG.55
Measuring the choroidal thickness may help differentiate between exudative AMD and PCV as well as from CSCR. Both PCV and CSC eyes have thicker choroids than those in normal individuals. Conversely, eyes with exudative AMD have thinner choroids.4,55
Spaide reported the presence of hyperreflective tissue in PEDs, which were found to be serous on conventional SD-OCT. These findings can help explain the pathogenesis of PEDs, retinal vascular anastomosis with choroidal neovascularization, and RPE tears.56
Vogt–Koyanagi–Harada (VKH) disease
Choroidal vessel hyperpermeability has shown to be correlated with increase in choroidal thickness in acute stage.2 Decrease in choroidal thickness with treatment and increase with recurrence have also been reported.57 The choroid was significantly thinner in eyes with VKH, both acute and convalescent compared to normal controls.58 Fong et al. reported loss of focal hyperreflectivity in the inner choroid in both acute and convalescent phases suggestive of permanent structural change to small choroidal vessels.59 Choroidal thickness may be monitored to understand the disease activity and further management.2,57
Diabetic retinopathy
Histopathological studies have shown various choroidal abnormalities, including obstruction of the choriocapillaris, vascular degeneration, choroidal aneurysms, and choroidal neovascularization in diabetic retinopathy.60,61 A large population-based study from China reported choroidal thickening in diabetic patients, however, diabetic retinopathy did not appear to associate with increased choroidal thickness.62 A recent retrospective study from Korea, demonstrated increasing choroidal thickness with increasing severity of retinopathy.63 A recent article from Italy, however, reported a significant thinning of subfoveal choroid in patients with diabetes as compared to controls.64 There are other reports that suggest choroidal thinning in diabetics65–67 and increasing thinning with progressive retinopathy.65 These conflicting reports may reflect dynamic nature of natural history of diabetes and its effect on the eye. Choroidal EDI-OCT imaging might be a useful method to study the contribution of the choroidal circulation to the overall visual dysfunction seen in diabetic patients.
En-face choroidal imaging
Motaghiannezam et al. reported choroidal vascular pattern using en-face images processed from 3D images obtained with SS-OCT prototype.68 The retinal layers, choriocapillaris (CC), Sattler’s layer (SL), Haller’s layer (HL), and the lamina suprachoroid layer (LSL) could be delineated in 2D sagittal tomograms. Long and short posterior ciliary artery branches could also be imaged including their entry sites. Further understanding of these structures in normal individuals and comparison with cadaveric eyes would improve the histological correlation with OCT findings.
Zhang et al. introduced an automated algorithm for segmentation of choroidal vasculature and reported average choroidal vasculature thickness of 172.1 micron and average choriocapillaris-equivalent thickness of 23.1 micron in normal subjects.69
En-face choroidal imaging in CSR has shown focally enlarged choroidal vessels at all the layers of the choroid. Ellabban et al. reported focal choroidal excavations in 7.8% of eyes with CSC. They proposed that these focal choroidal excavations may have been formed from RPE retraction caused by focal scarring of choroidal connective tissue.70 Using en-face imaging, Coscas et al. showed the entire branching neovascular network of CNV within fibrovascular PED (FV-PED) without dye injection.71
In conclusion, choroidal imaging has improved understanding of pathogenesis and diagnostic information of the disease. It also helps in monitoring treatment response in various chorio-retinal diseases. Various factors such as age, axial length, gender, and diurnal variation affect choroidal thickness. Automated segmentation of the choroid and its individual layers would improve the quantitative assessment of choroidal layers. Clinical application of knowledge from choroidal images further needs to be evaluated.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Ross A., Ross A.H., Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22:166–173. doi: 10.1097/ICU.0b013e3283459826. [DOI] [PubMed] [Google Scholar]
- 2.Read R.W., Rao N.A., Cunningham E.T. Vogt–Koyanagi–Harada disease. Curr Opin Ophthalmol. 2000;11:437–442. doi: 10.1097/00055735-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara T., Imamura Y., Margolis R. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Chung S.E., Kang S.W., Lee J.H., Kim Y.T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–845. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi L.A., Sorenson J., Spaide R.F., Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 6.Coleman D.J., Lizzi F.L. In vivo choroidal thickness measurement. Am J Ophthalmol. 1979;88:369–375. doi: 10.1016/0002-9394(79)90635-4. [DOI] [PubMed] [Google Scholar]
- 7.Guyer D.R., Puliafito C.A., Mones J.M. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992;99:287–291. doi: 10.1016/s0161-6420(92)31981-5. [DOI] [PubMed] [Google Scholar]
- 8.Spaide R.F., Koizumi H., Pozzoni M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Adhi M., Duker J.S. Optical coherence tomography–current and future applications. Curr Opin Ophthalmol. 2013;24:213–221. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branchini L., Regatieri C.V., Flores-Moreno I. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–123. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita T., Shirasawa M., Arimura N. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53:1102–1107. doi: 10.1167/iovs.11-8836. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo Y., Sakamoto T., Yamashita T. Comparisons of choroidal thickness of normal eyes obtained by two different spectral-domain OCT instruments and one swept-source OCT instrument. Invest Ophthalmol Vis Sci. 2013;54:7630–7636. doi: 10.1167/iovs.13-13135. [DOI] [PubMed] [Google Scholar]
- 13.Manjunath V., Taha M., Fujimoto J.G., Duker J.S. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(325–329):e321. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman W., Chen F.K., Yeoh J. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 15.Shao L., Xu L., Chen C.X. Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:230–233. doi: 10.1167/iovs.12-10351. [DOI] [PubMed] [Google Scholar]
- 16.Chhablani J., Barteselli G., Wang H. Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:2274–2280. doi: 10.1167/iovs.12-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei W.B., Xu L., Jonas J.B. Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 18.Margolis R., Spaide R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Ikuno Y., Kawaguchi K., Nouchi T., Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 20.Ding X., Li J., Zeng J. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52:9555–9560. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang Y., Heussen F.M., Mokwa N. Spatial distribution of posterior pole choroidal thickness by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:7019–7026. doi: 10.1167/iovs.11-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barteselli G., Chhablani J., El-Emam S. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012;119:2572–2578. doi: 10.1016/j.ophtha.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidaut-Garnier M., Schwartz C., Puyraveau M. Choroidal thickness measurement in children using optical coherence tomography. Retina. 2013 doi: 10.1097/IAE.0b013e3182a487a4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Park K.A., Oh S.Y. Choroidal thickness in healthy children. Retina. 2013;33:1971–1976. doi: 10.1097/IAE.0b013e3182923477. [DOI] [PubMed] [Google Scholar]
- 25.Read S.A., Collins M.J., Vincent S.J., Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci. 2013;54:3586–3593. doi: 10.1167/iovs.13-11732. [DOI] [PubMed] [Google Scholar]
- 26.Read S.A., Collins M.J., Vincent S.J., Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:7578–7586. doi: 10.1167/iovs.13-12772. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara A., Shiragami C., Shirakata Y. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol. 2012;56:230–235. doi: 10.1007/s10384-012-0128-5. [DOI] [PubMed] [Google Scholar]
- 28.Li X.Q., Larsen M., Munch I.C. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci. 2011;52:8438–8441. doi: 10.1167/iovs.11-8108. [DOI] [PubMed] [Google Scholar]
- 29.Mapelli C., Dell’arti L., Barteselli G. Choroidal volume variations during childhood. Invest Ophthalmol Vis Sci. 2013;54:6841–6845. doi: 10.1167/iovs.13-12761. [DOI] [PubMed] [Google Scholar]
- 30.Li X.Q., Jeppesen P., Larsen M., Munch I.C. Subfoveal choroidal thickness in 1323 children aged 11–12 years and association with puberty: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci. 2014;55(1):550–555. doi: 10.1167/iovs.13-13476. [DOI] [PubMed] [Google Scholar]
- 31.Chen F.K., Yeoh J., Rahman W. Topographic variation and interocular symmetry of macular choroidal thickness using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:975–985. doi: 10.1167/iovs.11-8771. [DOI] [PubMed] [Google Scholar]
- 32.Tan C.S., Ouyang Y., Ruiz H., Sadda S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 33.Mansouri K., Medeiros F.A., Marchase N. Assessment of choroidal thickness and volume during the water drinking test by swept-source optical coherence tomography. Ophthalmology. 2013;120:2508–2516. doi: 10.1016/j.ophtha.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vural A.D., Kara N., Sayin N. Choroidal thickness changes after a single administration of coffee in healthy subjects. Retina. 2013 doi: 10.1097/IAE.0000000000000043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Flores-Moreno I., Lugo F., Duker J.S., Ruiz-Moreno J.M. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(314–319):e311. doi: 10.1016/j.ajo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Cellini M., Strobbe E., Gizzi C., Campos E.C. ET-1 plasma levels and ocular blood flow in retinitis pigmentosa. Can J Physiol Pharmacol. 2010;88:630–635. doi: 10.1139/Y10-036. [DOI] [PubMed] [Google Scholar]
- 37.Yeoh J., Rahman W., Chen F. Choroidal imaging in inherited retinal disease using the technique of enhanced depth imaging optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1719–1728. doi: 10.1007/s00417-010-1437-3. [DOI] [PubMed] [Google Scholar]
- 38.Dhoot D.S., Huo S., Yuan A. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013;97:66–69. doi: 10.1136/bjophthalmol-2012-301917. [DOI] [PubMed] [Google Scholar]
- 39.Ayton L.N., Guymer R.H., Luu C.D. Choroidal thickness profiles in retinitis pigmentosa. Clin Exper Ophthalmol. 2013;41:396–403. doi: 10.1111/j.1442-9071.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 40.Coscas F., Puche N., Coscas G. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:64–69. doi: 10.1167/iovs.13-12931. [DOI] [PubMed] [Google Scholar]
- 41.Stanga P.E., Lim J.I., Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110:15–21. doi: 10.1016/s0161-6420(02)01563-4. [quiz 22–13] [DOI] [PubMed] [Google Scholar]
- 42.Brandl C., Helbig H., Gamulescu M.A. Choroidal thickness measurements during central serous chorioretinopathy treatment. Int Ophthalmol. 2014;34(1):7–13. doi: 10.1007/s10792-013-9774-y. [DOI] [PubMed] [Google Scholar]
- 43.Imamura Y., Fujiwara T., Margolis R., Spaide R.F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 44.Kuroda S., Ikuno Y., Yasuno Y. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33:302–308. doi: 10.1097/IAE.0b013e318263d11f. [DOI] [PubMed] [Google Scholar]
- 45.Maruko I., Iida T., Sugano Y. One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina. 2011;31:1921–1927. doi: 10.1097/IAE.0b013e31822bf6b1. [DOI] [PubMed] [Google Scholar]
- 46.Jirarattanasopa P., Ooto S., Tsujikawa A. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119:1666–1678. doi: 10.1016/j.ophtha.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Wood A., Binns A., Margrain T. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152(1030–1038):e1032. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Manjunath V., Goren J., Fujimoto J.G., Duker J.S. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152:663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonas J.B., Forster T.M., Steinmetz P. Choroidal thickness in age-related macular degeneration. Retina. 2013 doi: 10.1097/IAE.0000000000000035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Lee J.Y., Lee D.H., Yoon Y.H. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7812–7818. doi: 10.1167/iovs.13-12284. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki T., Koizumi H., Yamagishi T., Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119:1621–1627. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Maruko I., Iida T., Sugano Y. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151(594–603):e591. doi: 10.1016/j.ajo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Ellabban A.A., Tsujikawa A., Ogino K. Choroidal thickness after intravitreal ranibizumab injections for choroidal neovascularization. Clin Ophthalmol. 2012;6:837–844. doi: 10.2147/OPTH.S30907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spaide R.F. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–810. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Koizumi H., Yamagishi T., Yamazaki T. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:1123–1128. doi: 10.1007/s00417-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 56.Spaide R.F. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644–652. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Maruko I., Iida T., Sugano Y. Subfoveal choroidal thickness after treatment of Vogt–Koyanagi–Harada disease. Retina. 2011;31:510–517. doi: 10.1097/IAE.0b013e3181eef053. [DOI] [PubMed] [Google Scholar]
- 58.Nakai K., Gomi F., Ikuno Y. Choroidal observations in Vogt–Koyanagi–Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250:1089–1095. doi: 10.1007/s00417-011-1910-7. [DOI] [PubMed] [Google Scholar]
- 59.Fong A.H., Li K.K., Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt–Koyanagi–Harada disease. Retina. 2011;31:502–509. doi: 10.1097/IAE.0b013e3182083beb. [DOI] [PubMed] [Google Scholar]
- 60.Fryczkowski A.W. Diabetic choroidal involvement: scanning electron microscopy study. Klin Oczna. 1988;90:145–149. [PubMed] [Google Scholar]
- 61.Fryczkowski A.W., Sato S.E., Hodes B.L. Changes in the diabetic choroidal vasculature: scanning electron microscopy findings. Ann Ophthalmol. 1988;20:299–305. [PubMed] [Google Scholar]
- 62.Xu J., Xu L., Du K.F. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology. 2013;120:2023–2028. doi: 10.1016/j.ophtha.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Kim J.T., Lee D.H., Joe S.G. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–3384. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 64.Querques G., Lattanzio R., Querques L. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017–6024. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 65.Vujosevic S., Martini F., Cavarzeran F. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781–1790. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 66.Esmaeelpour M., Povazay B., Hermann B. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 67.Esmaeelpour M., Brunner S., Ansari-Shahrezaei S. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:6803–6809. doi: 10.1167/iovs.12-10314. [DOI] [PubMed] [Google Scholar]
- 68.Motaghiannezam R., Schwartz D.M., Fraser S.E. In vivo human choroidal vascular pattern visualization using high-speed swept-source optical coherence tomography at 1060 nm. Invest Ophthalmol Vis Sci. 2012;53:2337–2348. doi: 10.1167/iovs.11-7823. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L., Lee K., Niemeijer M. Automated segmentation of the choroid from clinical SD-OCT. Invest Ophthalmol Vis Sci. 2012;53:7510–7519. doi: 10.1167/iovs.12-10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellabban A.A., Tsujikawa A., Ooto S. Focal choroidal excavation in eyes with central serous chorioretinopathy. Am J Ophthalmol. 2013;156:673–683. doi: 10.1016/j.ajo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Coscas F., Coscas G., Querques G. En face enhanced depth imaging optical coherence tomography of fibrovascular pigment epithelium detachment. Invest Ophthalmol Vis Sci. 2012;53:4147–4151. doi: 10.1167/iovs.12-9878. [DOI] [PubMed] [Google Scholar]



