Abstract
Trachoma, caused by the obligate intracellular organism Chlamydia trachomatis, is the world’s leading cause of preventable blindness for which a vaccine is needed. We have previously shown that a plasmid-deficient live-attenuated trachoma vaccine delivered ocularly to macaques elicited either solid or partial protective immunity against a virulent ocular challenge. Solidly protected macaques shared the same MHC class II alleles implicating CD4+ T cells in superior protective immunity. Understandably, we sought to define T cell immune correlates in these animals to potentially improve vaccine efficacy. Here, following a two year resting period, these macaques were boosted intramuscularly with the live-attenuated trachoma vaccine and their peripheral T cell anamnestic responses studied. Both solidly and partially protected macaques exhibited a CD4+ and CD8+ T cell anamnestic response following booster immunization. CD8+ but not CD4+ T cells from solidly protected macaques proliferated against soluble chlamydial antigen. We observed a more rapid T cell inflammatory cytokine response in tears of solidly protected animals following ocular re-challenge. Most notably, depletion of CD8+ T cells in solidly protected macaques completely abrogated protective immunity. Collectively, our findings support the conclusion that CD8+ T cells play an important but unexpected role in live-attenuated trachoma vaccine mediated protective immunity.
Introduction
Chlamydia trachomatis is an obligate intracellular bacterial pathogen and the etiologic agent of blinding trachoma, the world’s leading cause of infectious blindness. Trachoma afflicts hundreds of millions of people in sub-Saharan Africa and Asia and is recognized as one of the major neglected tropical infectious diseases of the developing world (1). Current trachoma control strategies of the World Health Organization are focused on mass antibiotic treatment of infected children in hyperendemic areas in attempts to reduce infection rates and burdens in children, interrupting the cycle of transmission and re-infection in adults who are at the greatest risk of developing blinding disease. Despite its benefits, there is debate on its overall effectiveness and sustainability (2). An alternative strategy for trachoma control is vaccination (3). In the face of decades of efforts toward this end, there has been little meaningful progress in the development of a trachoma vaccine using subunit immunogens (4–6).
Recently, we described a plasmid-deficient live-attenuated trachoma vaccine (LATV) that was safe, immunogenic, and protective in macaques (7). We reported that macaques immunized with the LATV were either solidly protected (SP) or partially protected (PP) following challenge with virulent trachoma organisms. SP macaques exhibited transient ocular infections that cleared spontaneously without detectable ocular pathology. SP macaques shared the same MHC class II alleles implicating CD4+ T cells in superior vaccine mediated immunity; a finding consistent with the paradoxical but unambiguous role of CD4+, not CD8+ T cells, in chlamydial murine models of infection (8–12). Regardless, because of the exceptional level of protective immunity generated by the LATV in a relevant nonhuman primate animal model we sought to better define the role of T cells in vaccine mediated immunity. We deemed this to be an important goal since it could lead to knowledge for improving LATV efficacy in humans and the future development of a more conventional subunit trachoma vaccine.
In this study, previously LATV vaccinated macaques were rested for a period of two years and then administered simultaneous intramuscular and ocular vaccine booster immunizations to facilitate the study of chlamydial-specific T cell anamnestic responses in their PBL. Unexpectedly, we report CD8+ T cells play a critical role in LATV mediated solid protective immunity.
Materials and Methods
Nonhuman primates, vaccination, and chlamydial challenge
Six cynomolgus macaques (Macaca fascicularis) described in our original LATV vaccine study were used (7). The animals were housed separately for all experimental studies in the nonhuman primate section at the Rocky Mountain Laboratories veterinary branch. All experimental procedures were performed in accordance with the Guidelines of the Institutional Animal Care and Use Committee. After a two year resting period, macaques were administered with a series of three simultaneous intramuscular (2 x108 inclusion forming unit (IFU)) and ocular (2 x106 IFU/eye) immunizations using the C. trachomatis plasmid-deficient LATV strain (A2497P−). Eight weeks following the last immunization macaques were ocularly challenged with virulent C. trachomatis A2497P+ organisms (2 x 105 IFU/eye).
Infection and disease evaluation
Clinical evaluation and specimen collection for culturing chlamydiae were performed weekly. Chlamydial infection of the macaque conjunctival surface results in inflammation of sub-conjunctival tissues clinically scored as hyperemia and follicle formation. Hyperemia and follicle formation on the upper and lower conjunctivae of both eyes were scored by a veterinary pathologist. Hyperemia was scored as follows: 0, no hyperemia; 1, mild hyperemia; and 2, severe hyperemia. Follicles were scored as follows: 0, no follicles; 1, 1–3 follicles; 2, 4–10 follicles; 3, >10 follicles; and 4, follicles too numerous to count. The clinical disease score for a given animal was the aggregate scores of both hyperemia and follicle formation. The maximum clinical disease score was 24. After clinical pathological scoring, the surfaces of the upper and lower conjunctivae of both eyes were swabbed using a calgiswab (Puritan, Guilford, ME). Ocular swabs were used to monitor chlamydial shedding by culturing organisms in monolayers of cycloheximide treated HeLa 229 cells as previously described (13).
Peripheral blood lymphocyte immunophenotyping
Fluorochrome-conjugated antibodies were incubated with 100 μl of EDTA anticoagulated whole blood for 30 minutes at room temperature. Antibodies used were anti-CD3-Alexa 700 (SP34-2), anti-CD4-FITC (L200), anti-CD20-APC (2H7) all from BD Biosciences San Jose, CA and anti-CD8-PE (DK25, Dako Inc., Carpinteria, CA). Erythrocytes were lysed with multi-species RBC lysis buffer (eBioscience Inc., San Diego, CA) following manufacturer instructions. Lysed specimens were washed once with 3 ml of flow cytometry buffer and centrifuged for 5 minutes at 1200 rpm. Samples were analyzed for four color immunofluorescence and lymphocytes gated based on forward- and side-scatter parameters using a LSRII flow cytometer (BD Biosciences, San Jose, CA) and FlowJo software version 8.8.6 (Tree Star, Inc, Ashland, OR). Total blood counts were calculated using a 950 FS Hematology Analyzer (Drew Scientific Inc., Dallas TX).
Chlamydial soluble antigen
Buffalo Green Monkey Kidney (BGMK) cells were infected with C. trachomatis A2497P+ using a multiplicity of infection (MOI) of 1. Infected BGMK monolayers were fed with Dulbecco’s minimal essential medium (Cellgro, Manassas, VA) supplemented with 10% cynomolgus serum (Innovative Research, Novi, MI) and 10 mg/ml gentamicin. Infected cells were incubated for 42 hrs. at 37° C. The monolayers were washed with Hanks balanced salt solution, removed by scraping, and disrupted by sonication. Host cell debris was removed by centrifugation at 1500 rpm for 15 min at 4°C. The supernatant was collected and centrifuged at 13,500 rpm for 30 min at 4°C to pellet chlamydial organisms. The clarified supernatant was then centrifuged at 100,000 x g for 1 hour at 4°C. The supernatant was collected and concentrated ten fold using an Amicon Ultracel-10K (Millipore, Billerica, MA). The protein concentration was adjusted to 10 mg/ml and aliquots stored at −80° C.
Analysis of chlamydial-specific T cell immune response
Chlamydial-specific T cell expansion and cytokine production from PBMC was done as described (14). Briefly, CFSE (carboxy fluorescein succimidyl ester, Molecular Probes) labeled PBMCs were incubated at 2 x106 cell/ml in deep well tissue culture plates (96 well, Eppendorf) in 1 ml of AIM V medium (Life Technologies) only or pulsed with C. trachomatis A2497P+ elementary bodies (EB) (MOI= 20) or chlamydial soluble antigen (SA) (100 μg/ml) and incubated for five days at 37°C and 5% CO2. Cells were then re-stimulated for six additional hours with medium only or chlamydial antigens in the presence of mAb co-stimulatory signals. The mAb used were 0.5 μg/ml of anti-CD28 (clone CD28.2, Nonhuman Primate Reagent Resource, University of Massachusetts Medical School, Boston, MA), Mab CD49d (clone 9F10, BD Biosciences) and 1X brefeldin A solution (BD Biosciences). The cells were washed and analyzed by flow cytometry. A Live/Dead fixable violet dead cell stain kit (Molecular Probes) was used to discriminate live and dead cells. Dilution of CFSE stain was used to monitor proliferation of CD3+CD4+ and CD3+CD8+ T cells. The percent of cells proliferating was normalized against their respective negative controls which were cells incubated with sucrose-phosphate glutamate buffer (SPG) or SA prepared from mock-infected BGMK monolayers.
Collection of tears and inflammatory cytokine analyses
A polyvinyl acetal ophthalmologic mini sponge (Merocel, Beaver Visitec, Waltham, MA) was placed on the outer third of the lower eyelid margin of both eyes. After five minutes of tear collection, the sponge was recovered and placed in a 2 ml screw cap tube containing 500 μl of PBS. The sponges were incubated for 30 min at 4°C and the liquid sample was eluted by centrifugation and stored at −80°C until use. Cytokines and chemokines were detected using a multiplexed microsphere-based suspension array (Luminex xMAP). Tear samples were analyzed in duplicate using the nonhuman Primate Cytokine Magnetic Bead Panel: PCYTMG-40K-PX23 (EMD Millipore, Billerica, MA).
In vivo depletion of CD8+ T cells and chlamydial challenge
Macaques received by subcutaneous injection 50 mg per kg of the anti-CD8 rhesus recombinant antibody MT807R-IgG1 (CDR-g), NHPRR, University of Massachusetts Medical School, Boston, MA. Antibody was administered two times on days 0 and 21. Blood samples were withdrawn weekly to immunophenotype and quantify CD8+ and CD4+ T cells. Following the second antibody injection animals were ocularly challenged with 2 x104 IFU/eye of virulent C. trachomatis A2497P+. Chlamydial ocular shedding and clinical disease scores were monitored weekly as described above.
Statistical analyses
Differences were compared by an unpaired t-test (two-tailed, unequal variance).
Results
A systemic T cell anamnestic response occurs in vaccinated rested macaques following a combined intramuscular and ocular booster immunization
Our first goal was to boost those ocularly vaccinated macaques used in our original LATV report (7) to generate T cell anamnestic responses in their PBL sufficiently to study LATV mediated T cell immunity. LATV vaccinated macaques that were either SP or PP were rested for a two year period and then given a combined intramuscular and ocular booster immunization with the LATV (Fig. S1). Prior to and after booster immunization we analyzed by flow cytometry the frequency and total numbers of CD4+ and CD8+ T cells in the PBL of these animals (Fig. 1). Total T cell numbers for individual animals in each group and the respective means of the groups showed a significant increase in both CD4+ (p=0.049) and CD8+ T cells (p= 0.009) in the PBL of SP and PP animals. Thus, the LATV booster immunization re-called a systemic T cell anamnestic response.
FIGURE 1.
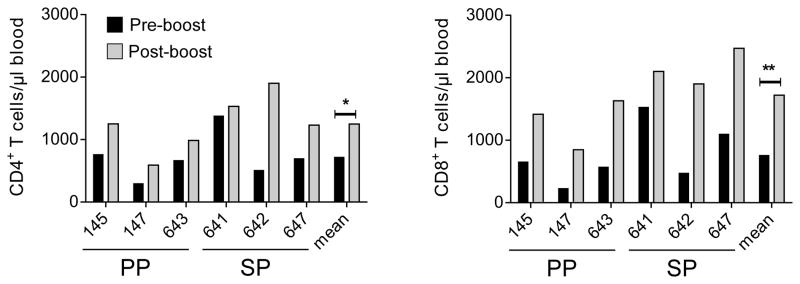
T cell immunophenotypes of PBL in macaques following booster immunization with LATV. Total CD4+ and CD8+ T cell numbers pre- (black bars) and post-booster (grey bars) immunization of individual macaques. The arithmetic means and P values of the PP and SP groups are also shown. The LATV booster vaccination produced a significant increase in both CD4+ (*p= 0.049) and CD8+ (**p= 0.009) T cells.
Protective immunity in rested LATV boosted macaques
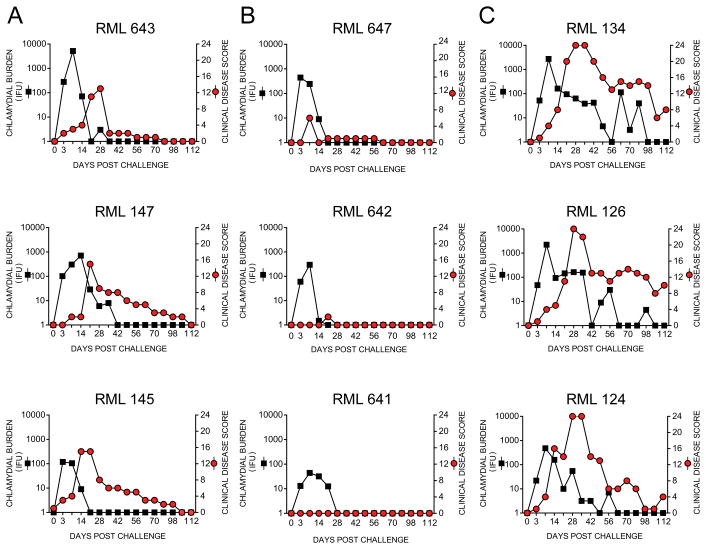
We next challenged the LATV boosted macaques ocularly with virulent plasmid-bearing A2497P+ trachoma organisms to confirm their protective immune status (Fig. 2). PP and SP monkeys were inoculated ocularly with 2 x105 IFU onto the conjunctival surface of both eyes and their infection and ocular clinical pathology monitored (Fig. 2A and B). This challenge dose was ten-fold higher than that used in our initial vaccine efficacy study. A historical naïve control group of similarly A2497P+ infected macaques (13) was used (RML 124, 126, 134) to compare the level of protective immunity conferred by the vaccine (Fig. 2C). The challenged animals retained their original immune status being either PP (RML 145, 147, and 643) or SP (RML 641, 642, and 647) despite the ten fold increase in challenge inoculum. SP animals, although colonized, presented with transient infections of low burden that completely resolved by day 21 post-infection. We observed no clinical pathology in two of the SP macaques (642 and 641) and animal 647 exhibited minimal hyperemia without detectable follicle formation. Compared to SP animals, PP macaques shed greater numbers of organisms for longer periods that were accompanied by moderate clinical pathology scores. Nevertheless, both infection and disease in PP animals was patently less severe compared to infected naïve controls.
FIGURE 2.
Rested LATV boosted macaques are partially or solidly protected following a high dose ocular challenge with virulent trachoma organisms. The six macaques from our original vaccine study (7), which were described as being PP or SP, were boosted with a simultaneous intramuscular/ocular dose of the LATV (Fig. S1). Eight weeks after the booster immunization macaques were challenged ocularly with 2 x 105 IFU; a tenfold higher dose of virulent C. trachomatis A2497P+ than that used in our original vaccine study. (A) Infection and ocular pathology in PP macaques. (B) Infection and pathology in SP macaques. (C) Infection and pathology in historic control naïve macaques. The information in Fig. 2C is reprinted with permission from the Journal of Infectious Diseases (13). The protective immune status of monkeys remained similar to that observed following primary vaccination and challenge preformed 2 years previously despite the increase in challenge dose. Compared to historic naïve controls (RML 134, 126, and 124) (C), boosted macaques were either PP (A) or SP (B). PP animals exhibited infections that resolved between 21 and 42 days that produced mild to moderate pathology. Infections of SP animals were shorter in duration (14–21 days), yielded lower recoverable IFU, and presented with minimal to no ocular pathology. In contrast infection of naïve controls persisted for 60–100 days post-challenge with severe to moderately severe ocular pathology over the entire culture positive period.
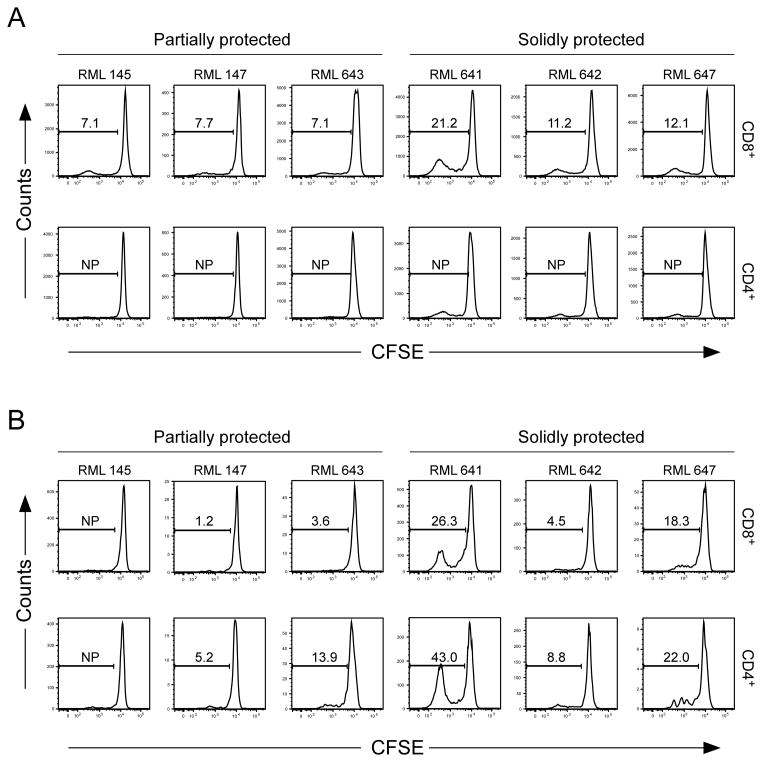
CD8+ T cells from SP macaques proliferate in response to chlamydial soluble antigen
To determine if the T cell anamnestic response of PP and SP boosted macaques was antigen-specific, CFSE-labeled PBMC were stimulated in vitro with C. trachomatis A2497P+ elementary bodies (EB) or a soluble antigen extract (SA) prepared from A2497P+ infected Buffalo Green Monkey Kidney cells (BGMK) (Fig. 3). The SA contains the two well-characterized cytosolically secreted chlamydial virulence factors: chlamydial protease activity factor (CPAF) (15) and plasmid-encoded Pgp3 protein (Pgp3) (16) but lacks chlamydial MOMP and heat shock protein 60 (Fig. S2). CD8+ T cells from both PP and SP macaques proliferated in response to SA, however the proliferative response of CD8+ T cells from SP macaques was noticeably greater (Fig. 3A). In contrast, CD4+ T cells from PP or SP macaques failed to proliferate in response to SA (Fig. 3A). The CD4+ and CD8+ T cell responses against chlamydial EB were variable and inconsistent in magnitude both within and between the groups (Fig. 3B). Intuitively, the preferential stimulation of CD8+ T cells by chlamydial SA is puzzling. A possible explanation for these findings could be that cytosolic CPAF protease in the SA targets chlamydial proteins for proteolytic degradation and these processed proteins are exogenously loaded onto MHC class I molecules of APC (17, 18).
FIGURE 3.
Chlamydial-specific T cell responses in boosted challenged macaques. CFSE labeled PBMC from boosted ocularly challenged macaques were incubated for five days with C. trachomatis strain A2497P+ EBs, A2497P+ SA prepared from infected BGMK cells, or with their respective negative controls; SPG or control SA. Cells were re-stimulated for six additional hours in the presence of anti-CD28 and CD49d and brefeldin A. Chlamydial antigen-specific T cell expansion was monitored by dilution of the CFSE proliferation marker using flow cytometry analysis. The flow analysis was performed on gated live CD3+ CD4+ and CD3+ CD8+ T cells. (A) CD8+ and CD4+ proliferation following incubation with A2497P+ SA. (B) CD8+ and CD4+ proliferation following incubation with A2497P+ EB. The numbers represent the percent of proliferation detected in PBMCs incubated with either chlamydial SA or EB normalized against respective negative control antigens. Note that CD8+ T cells of animals in the SP group (641, 642, and 647) proliferate more intensely and consistently in comparison to PP animals following stimulation with chlamydial SA.
We next asked whether SA stimulated CD8+ T cells from PP and SP macaques exhibited different surface markers that correlated with protective immunity. Flow cytometry analysis of SA stimulated CD8+ T cells revealed expression of interferon-γ (IFN-γ), α4β7 integrin, and granzyme B but we observed no differences in the staining intensity of these molecules between CD8+ T cells of PP and SP animals (Fig. S3). Nonetheless, this finding is consistent with the conclusion that SA stimulated CD8+ T cells possess functional characteristics capable of secreting IFN-γ, homing to mucosal tissues, and potentially functioning as cytolytic T cells against chlamydial infected conjunctival epithelial targets.
A more rapid recall of T cell cytokines occurs in the tears of solidly protected macaques following infectious challenge
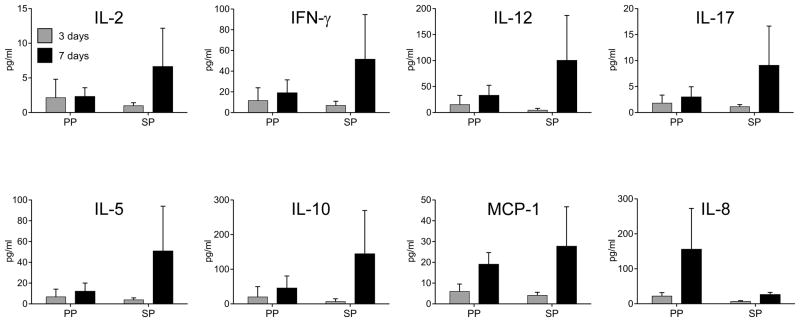
Schenkel et al., (19) described a role for circulating and resident memory CD8+ T cells in the elimination of intracellular pathogens in front line mucosal tissues through two contact-independent effector mechanisms: (i) cytolysis and (ii) secretion of anti-viral cytokines. These authors suggested that vaccines should establish both populations to augment rapid recall of protective immunity. We were not able to directly address a role for cytolytic CD8+ T cells in the present study. However, we did investigate whether PP and SP had a measurable difference in the temporal appearance of T cell anti-inflammatory cytokines in their tears following chlamydial ocular challenge (Fig. 4). Tears were collected from PP and SP animals on days three and seven post-challenge with virulent A2497P+and subjected to immunoassay for the detection of IL-2, IFN-γ, IL-12, IL-17, IL-5, IL-10 and MCP-1. Similar low levels of cytokines were found in the tears of both groups of animals 3 days following infectious challenge. Notably however, there was an increase in tear cytokines in all 3 SP animals on day 7 post-challenge, a time when their infections were resolving. A similar increase in cytokines post-challenge was not observed in the tears of PP macaques. Interestingly, and consistent with the differences in inflammatory pathology that existed between the groups, the inflammatory cytokine IL-8 was only found in the tears of PP animals at day seven (Fig. 2A). These results support the conclusion that SP macaques generated a more rapid recall of T cells to sub-mucosal conjunctival tissue capable of secreting protective anti-chlamydial cytokines, like IFN-γ, that may function in infection resolution. Thus, a rapid recall of T cell secreted cytokines is at least in part a plausible explanation for the superior level of protective immunity observed in SP macaques. We next sought to determine if the source of the T cell cytokine response in SP macaques was the result of CD4+ or CD8+ T cells.
FIGURE 4.
A rapid appearance of inflammatory cytokines in the tears of LATV challenged macaques correlates with protective immune status. Ocular secretions (tears) were collected at three and seven days post chlamydial challenge and were analyzed with a Luminex bead-based multiplex assay to measure local cytokines and chemokines. The graphs show cytokines representing the Th1-Th17 profile (IL-2, IFN-γ, IL-12, IL-17), Th2 (IL-5, IL-10), and the chemoattractant and pro-inflammatory cytokine MCP-1 and IL-8, respectively. Each bar represents the average of cytokine levels detected in PP and SP monkeys ± standard deviation. The SP macaques produced a more intense and rapid (day seven) production of Th1/Th2 cytokines and the MCP-1 chemokine. Interestingly, the pro-inflammatory cytokine IL-8 was not detected in the tears of solidly protected macaques but was found in partially protected animals; findings that are consistent with the ocular pathology exhibited by these animals.
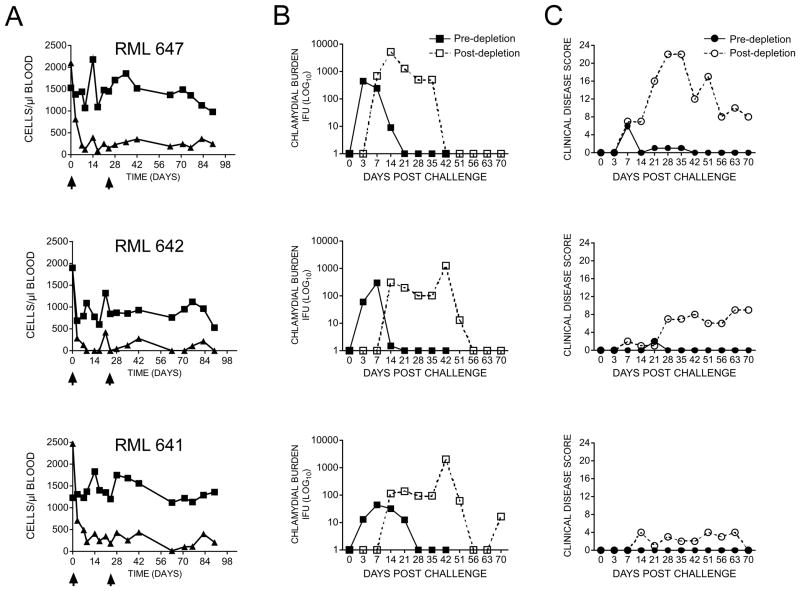
In vivo depletion of CD8+ T cells in SP macaques abrogates protective immunity
Collectively our findings implicated T cells, specifically CD8+ T cells, as an important protective phenotype in SP macaques. To directly test this hypothesis we challenged SP animals prior to and following in vivo depletion of their CD8+ T cells (Fig. 5). Animals were administered two doses of anti-CD8+ mAb and the total numbers of CD8+ T and CD4+ T cells in peripheral blood determined by flow cytometry prior to and following a secondary chlamydial challenge. Treatment with anti-CD8+ antibody significantly reduced and sustained reduced numbers of CD8+ T cells in all animals without affecting the numbers of circulating CD4+ T cells (Fig. 5A). Remarkably, protective immunity against ocular infection was abrogated in all three SP animals (RML 647, 642, and 641) depleted of their CD8+ T cells (Fig. 5B). Infections resulted in an increased burden of ten fold in two animals (RML 647 and 641) and there was dramatic extension in shedding duration in all animals that persisted for 42–56 days post infection. Surprisingly, these exacerbated infections produce a more variable outcome in ocular pathology (Fig. 5C). A single animal (RML 647) presented with severe clinical disease score over the entire infection period; in contrast, only moderate to minimal ocular pathology was observed in animals RML 642 and 641 despite their high infectious burdens. This discordance between infection and ocular disease is not understood.
FIGURE 5.
Depletion of CD8+ T cells in solidly protected macaques abrogates LATV mediated protective immunity. (A) The number of peripheral CD4+ and CD8+ T cells in SP macaques (RML 647, 642, and 641) after subcutaneous administration of two doses of anti-CD8 antibody. A marked decrease (greater or equal to 88%) of CD3+CD8+ (triangles), but not CD3+CD4+ (squares) T cells, was detected following administration of the first dose of antibody. Following the second dose, specific depletion of CD8+ was sustained over the entire experimental period (90 days). Macaques were challenged with 2 x 104 IFU virulent C. trachomatis A2497P+ EBs prior to and after depletion of CD8+ T cells. (B) Infection pre- and post-deletion of CD8+ T cells. (C) Ocular pathology pre- and post-deletion of CD8+ T cells. Depletion of CD8+ T cells completely abrogated the ability of SP macaques to resist challenge infection. Infections in CD8+ depleted animals resulted in greater chlamydial conjunctival burdens that persisted for up to 56 days post-challenge. In contrast, the same LATV immunized macaques infected prior to depletion of CD8+ T cells shed less organisms from their conjunctivae and spontaneously resolved infection between 21–28 days post-challenge.
Discussion
In summary, we present evidence that solid protective immunity elicited by the LATV is mediated by CD8+ T cells. It is unclear how this immunity is generated by the attenuated vaccine. Circulating CD8+ T cells are separated into two functional subsets termed central memory T (Tcm) and effector memory T (Tem) cells (20). Tcm are long-lived with a greater proliferative capacity upon re-exposure to pathogens that have the capacity to permanently reside in peripheral tissues including the mucosae (18, 21); termed tissue- resident memory (Trm) cells. Thus, for reasons not understood at this time, SP vaccinated macaques may have selectively generated Tcm and Trm cells capable of trafficking rapidly to and being retained at the ocular mucosae that were then highly efficient in controlling chlamydial infection. The rapid appearance of T cell inflammatory cytokines in the tears of SP macaques following infection challenge, consisting of IFN-γ, a potent in vitro anti-chlamydial inhibitory cytokine (22), supports this hypothesis. A role for CD4+ T cells in SP LATV protective immunity, either independently or collectively with CD8+ T cells, cannot be ignored however as we previously found a correlation between MHC class II alleles and SP animals, while no correlation was found with any MHC class I or, class IB alleles (7). Perhaps CD4+ T cells play a regulatory role in the differentiation or retention of protective Trm T cells in the conjunctival mucosae.
The principle questions that emerge from our past (7) and current study are; (i) what is the molecular basis for the dramatic infection attenuation of the trachoma plasmid-deficient vaccine strain, and (ii) how does this strong attenuation phenotype play a role in the enhanced ability of the host to generate a protective CD8+ T cell immune response? Indisputably, in models of murine urogenital tract infection with virulent plasmid bearing chlamydial organisms CD8+ T cells are not an important protective phenotype despite the obligate intracellular life-style of the pathogen (11, 23). In contrast, infection of mice generates a protective immune response that is dominated by CD4+ T cells (8–12). Although CD4+ are capable of conferring protective immunity they may be inferior to CD8+ T-cell mediated immunity elicited by the LATV. A logical conclusion from these contrasting immune outcomes is that virulent plasmid-bearing chlamydiae avoid CD8+ T cell immunity by an unknown plasmid function(s). Perhaps in the absence of plasmid-encoded virulence factors infected epithelial cells become unconventional but superior targets for antigen presentation by dendritic cells that are very proficient in the generation of protective memory CD8+ T cells (18). Interestingly, a recent report (24) found that the immunomodulatory ligand PD-L1 contributes to this defective CD8+ T cell response in mice as inhibition of PD-L1 restores protective anti-chlamydial CD8+ T cell immunity. It is not clear how these observations might be related to our findings but it is tempting to speculate that they are associated with plasmid genes or plasmid regulated genes (25–27) that up-regulate PD-L1 in chlamydial infected target cells.
Collectively, defining a role for the chlamydial plasmid in modulating CD8+ T cell immunity is a challenging goal towards understanding chlamydial pathogenesis as it relates to avoidance of adaptive T cell immunity. Studies aimed at molecularly defining the plasmids role in host cell interactions that impact immunity might come from performing comparative transcriptional and proteomic studies on plasmid-bearing and plasmid deficient infected epithelial cells, as well as employing similarly infected epithelial cells as targets to assay CD8+ T cell cytotoxic function.
Supplementary Material
Acknowledgments
We thank Dr. Keith Reimann for assistance in T cell depletion experiments; the Rocky Mountain Veterinary Branch of RML for assistance with nonhuman primates; Aaron Carmody for flow cytometry assistance; Drs. Guangming Zhong and Lihua Song for CPAF and Pgp3 monoclonal antibodies, respectively; Heather Murphy and Anita Mora for graphical art assistance and Kelly Matteson for administrative assistance in preparation of this manuscript.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this article
- LATV
live-attenuated trachoma vaccine
- SP
solidly protected
- PP
partially protected
- IFU
inclusion forming unit
- SA
chlamydial soluble antigen
- EB
elementary body
References
- 1.WHO. Global elimination of blinding trachoma. Resolution adopted by the World Health Assembly. 1998 May 16 [Google Scholar]
- 2.Lakew T, House J, Hong KC, Yi E, Alemayehu W, Melese M, Zhou Z, Ray K, Chin S, Romero E, Keenan J, Whitcher JP, Gaynor BD, Lietman TM. Reduction and return of infectious trachoma in severely affected communities in Ethiopia. PLoS Negl Trop Dis. 2009;3:1–7. doi: 10.1371/journal.pntd.0000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 4.Taylor HR, Prendergast RA. Attempted oral immunization with chlamydial lipopolysaccharide subunit vaccine. Invest Ophthalmol Vis Sci. 1987;28:1722–1726. [PubMed] [Google Scholar]
- 5.Taylor HR, Whittum-Hudson J, Schachter J, Caldwell HD, Prendergast RA. Oral immunization with chlamydial major outer membrane protein (MOMP) Invest Ophthalmol Vis Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 6.Campos M, Pal S, O’Brien TP, Taylor HR, Prendergast RA, Whittum-Hudson JA. A chlamydial major outer membrane protein extract as a trachoma vaccine candidate. Invest Ophthalmol Vis Sci. 1995;36:1477–1491. [PubMed] [Google Scholar]
- 7.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, Sturdevant GL, Lu C, Bakios LE, Randall LB, Parnell MJ, Zhong G, Caldwell HD. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. 2011;208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol. 2012;189:2441–2449. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kari L, Whitmire WM, Carlson JH, Crane DD, Reveneau N, Nelson DE, Mabey DC, Bailey RL, Holland MJ, McClarty G, Caldwell HD. Pathogenic diversity among Chlamydia trachomatis ocular strains in nonhuman primates is affected by subtle genomic variations. J Infect Dis. 2008;197:449–456. doi: 10.1086/525285. [DOI] [PubMed] [Google Scholar]
- 14.Arrode-Bruses G, Sheffer D, Hegde R, Dhillon S, Liu Z, Villinger F, Narayan O, Chebloune Y. Characterization of T-cell responses in macaques immunized with a single dose of HIV DNA vaccine. J Virol. 2010;84:1243–1253. doi: 10.1128/JVI.01846-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193:935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol. 2010;192:6017–6024. doi: 10.1128/JB.00847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosken NA, Bevan MJ, Carbone FR. Class I-restricted presentation occurs without internalization or processing of exogenous antigenic peptides. J Immunol. 1989;142:1079–1083. [PubMed] [Google Scholar]
- 18.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 21.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapoza PA, Tahija SG, Carlin JP, Miller SL, Padilla ML, Byrne GI. Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Invest Ophthalmol Vis Sci. 1991;32:2919–2923. [PubMed] [Google Scholar]
- 23.Perry LL, Feilzer K, Hughes S, Caldwell HD. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infect Immun. 1999;67:1379–1385. doi: 10.1128/iai.67.3.1379-1385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fankhauser SC, Starnbach MN. PD-L1 Limits the Mucosal CD8+ T Cell Response to Chlamydia trachomatis. J Immunol. 2013 doi: 10.4049/jimmunol.1301657. published online 18 December 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson JH, Whitmire WM, Crane DD, Wicke L, Virtaneva K, Sturdevant DE, Kupko JJ, 3rd, Porcella SF, Martinez-Orengo N, Heinzen RA, Kari L, Caldwell HD. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, Carlson JH, Whitmire WM, Kari L, Virtaneva K, Sturdevant DE, Watkins H, Zhou B, Sturdevant GL, Porcella SF, McClarty G, Caldwell HD. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect Immun. 2013;81:636–644. doi: 10.1128/IAI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. Transformation of Chlamydia muridarum reveals a role of Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol. 2013 doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.