Abstract
Importance
The symptomatic benefits of spinal augmentation (vertebroplasty or kyphoplasty) for the treatment of osteoporotic vertebral compression fractures are controversial. Recent population-based studies using medical billing claims have reported significant reductions in mortality with spinal augmentation compared to conservative therapy, but in non-randomized settings such as these, there is the potential for selection bias to influence results.
Objective
To compare major medical outcomes following treatment of osteoporotic vertebral fractures with spinal augmentation or conservative therapy. Additionally, we will evaluate the role of selection bias using pre-procedure outcomes and propensity score analysis.
Design, Setting, and Participants
Retrospective cohort analysis of Medicare claims for 2002–2006. We compared 30-day and 1-year outcomes in patients with newly-diagnosed vertebral fractures treated with spinal augmentation (augmented; n=10 541) or conservative therapy (control; n=115 851). Outcomes were compared using traditional multivariate analyses adjusted for patient demographics and comorbid conditions. We also used propensity score matching to select 9017 pairs from the initial groups to compare the same outcomes.
Main Outcomes and Measures
Mortality, major complications, and healthcare utilization.
Results
Using traditional covariate adjustments, mortality was significantly lower in the augmented group compared to controls (5.2% vs 6.7% at one year; hazard ratio, 0.83; 95% CI, 0.75–0.92). However, patients in the augmented group who had not yet undergone augmentation (pre-procedure subgroup) had lower rates of medical complications 30 days post-fracture compared to controls (6.5% vs 9.5%; odds ratio, 0.66; 95% CI, 0.57–0.78), suggesting that the augmented group was less medically ill. After propensity score matching to better account for selection bias, one-year mortality was not significantly different between groups. Furthermore, one-year major medical complications were also similar between groups, and the augmented group had higher rates of healthcare utilization, including hospital and intensive care unit admissions and discharges to skilled nursing facilities.
Conclusions and Relevance
After accounting for selection bias, spinal augmentation did not improve mortality or major medical outcomes and was associated with greater healthcare utilization compared to conservative therapy. Our results also highlight how analyses of claims-based data that do not adequately account for unrecognized confounding can arrive at misleading conclusions.
INTRODUCTION
Vertebroplasty and kyphoplasty, together termed spinal augmentation, have until recently received widespread acceptance as effective, minimally invasive treatments for rapid symptomatic relief following osteoporotic vertebral compression fractures. These procedures involve percutaneous injection of bone cement into the collapsed vertebral body.
Numerous observational, non-randomized, and randomized controlled trials (RCT) have supported the use of spinal augmentation for the treatment of vertebral compression fractures1–3. The two largest RCTs comparing vertebroplasty and kyphoplasty to conservative, non-surgical therapies found significant reductions in pain, analgesic usage, and disability with spinal augmentation at 1 month and 1 year.2,3 However, results from two double-blinded RCTs failed to demonstrate improvements in pain or disability with vertebroplasty.4–6 These results suggest that the benefits of spinal augmentation might largely reflect a placebo effect.
The importance of effectively treating osteoporotic compression fractures, however, goes beyond the acute symptoms. Mortality rates are doubled following a fracture, perhaps due to the acute symptoms superimposed on significant underlying comorbid conditions.7–12 Early intervention with spinal augmentation, therefore, has the potential to lessen the risk of death by ameliorating the acute symptoms. Several recent, population-based studies have suggested spinal augmentation is associated with marked reductions in mortality,13–15 supporting this hypothesis. However, a small, retrospective cohort study found similar mortality following vertebroplasty compared to historical controls.16
To clarify these conflicting results, we studied the Medicare population to compare mortality, rates of major medical complications, and several measures of healthcare utilization among patients with osteoporotic vertebral fractures who were treated with either spinal augmentation or conservative therapy. We also investigated the potential role of selection bias by examining pre-procedure outcomes in the augmented group compared to controls and using propensity score analysis.
METHODS
The institutional review board of the University of Washington approved this project.
DATA SOURCE
We used a 20% random sample of the Centers for Medicare and Medicaid Services (CMS) outpatient (physician/supplier; Part B) billing claims and corresponding sample of the inpatient Medicare Provider Analysis and Review (MedPAR; Part A) claims from 2002 through 2006. The Medicare enrollment file (Denominator) was used to determine dates of death. Unique patient identifiers allow linkage of patients among databases as well as the ability to follow them over time.
We excluded beneficiaries receiving Social Security Disability income, those with end-stage renal disease, and those enrolled in a health maintenance organization. These special groups are often excluded from this type of analysis because they may be receiving additional medical coverage from an outside provider that is not included in the Medicare database.17–19 Patients aged less than 66 years were also excluded in order to allow one full year of Medicare eligibility prior to the index event.
PATIENTS AND INDEX FRACTURE
We identified patients using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (eMethods) for thoracic or lumbar vertebral fractures and “pathologic fracture of vertebrae,” which includes osteoporotic fractures. To limit our sample to newly-diagnosed, osteoporotic vertebral fractures, we excluded patients with other diagnoses that could result in fracture (eMethods). In total, we identified 127 238 beneficiaries.
TREATMENTS
We defined treatment groups based on the presence (“augmented group”) or absence (“control group”) of Current Procedural Terminology (CPT) and ICD-9-CM procedural codes for vertebroplasty or kyphoplasty in the year following the index fracture. We excluded patients who underwent spinal augmentation six months or more after their fracture (n=846), allowing at least six months of post-procedure follow-up. Additionally, we excluded patients in the control group with a procedural code for “unlisted procedure, spine” (n=2814) because this code may include spinal augmentation. After these exclusions, 115 851 patients were in the control group and 10 541 patients were in the augmented group.
DEMOGRAPHICS AND MEASURES OF COMORBIDITY
We collected available demographics for all patients. We categorized race as white or non-white. Race was not specified in 0.2% of patients in the control and augmented groups. We determined rural-urban commuting areas (RUCA)20 from patient zip codes. A zip code was not available for less than 0.5% of patients in both groups. We used state subsidization of Medicare premiums and deductibles (buy-in status) as a measure of socioeconomic status.
We accounted for baseline patient comorbid conditions using the modified Quan comorbidity index and individual conditions included in the index.21,22 We also recorded the number of hospitalizations in the prior year as an additional measure of overall disease burden.17
COMPLICATIONS
We evaluated mortality and the occurrence of a major medical complication within one year from the time of index vertebral. Major medical complications included diagnosis codes for cardiorespiratory arrest, acute myocardial infarction, respiratory failure, pulmonary embolism, pneumonia, and stroke as well as relevant procedural codes (eMethods). We chose these complications because they have a major effect on health and are more consistently coded compared to minor complications.17,23
HEALTHCARE UTILIZATION
We recorded general hospital admissions, intensive care unit (ICU) admissions, and discharges to a skilled nursing facility (SNF) during the year following vertebral fracture. We did not include hospital admissions during which spinal augmentation was performed.
STATISTICAL ANALYSIS
We initially compared 30-day and one-year mortality and rates of major medical complications between augmented and control groups using a multivariate Cox proportional hazards model and Kaplan Meier survival curves. We compared hospitalizations, ICU admissions, and discharges to SNFs within one year from the index fracture between groups using multivariate logistic regression. Time points were pre-specified. We adjusted all regression models for year, patient demographics and comorbid conditions, fracture level, hospital admission at the time of fracture, and the use and timing of advanced imaging following fracture.
To examine the potential for unmeasured selection bias between augmented and control groups, we compared rates of major medical complications within 30 days of the index fracture between patients in the augmented group who had not yet undergone spinal augmentation (pre-procedure subgroup) and the control group using multivariate logistic regression, as above.
In addition to standard covariate adjustment, we used propensity score matching methods to better account for selection bias inherent in observational studies in which patients are selected for a given treatment in a non-random manner and compared with untreated patients.24–28 We matched augmented patients with control patients one-to-one using the psmatch2 function29 with the adjustment variables listed above. This technique identified 9085 pairs of adequately matched augmented and control patients. Sixty-eight pairs were excluded because the control patient died before the augmented patient underwent the procedure and, therefore, differences in survival could not be due to spinal augmentation. Propensity scores were equal between treatment groups after matching (mean, 0.18 for both; P=.99, t test). We then repeated outcome analyses described above using matched-sample tests and compared results to the standard covariate analysis.
All analyses were performed using Stata 12 statistical software (StataCorp, College Station, TX).
RESULTS
TRADITIONAL MULTIVARIATE ANALYSIS
Differences in baseline characteristics between the augmented and control groups were small (Table 1). In part due to the absence of a distinct CPT code for kyphoplasty until 2005, the majority (71.0%) of the augmented group in our sample was coded as having only vertebroplasties.
Table 1.
Baseline Characteristics of Patients and Procedures
| No. (%) | |||
|---|---|---|---|
| Characteristic | Control (n = 115 851) |
Augmented (n = 10 541) |
P Value |
| Age, mean (SD) | 80.2 (7.2) | 80.0 (6.7) | .006 |
| Female sex | 89 815 (77.5) | 8244 (78.2) | .11 |
| White race | 109 240 (94.3) | 10 206 (96.8) | <.001 |
| State buy-in | 16 985 (14.7) | 1069 (10.1) | <.001 |
| Quan comorbidity score | .004 | ||
| 0 | 29 267 (25.3) | 2570 (24.4) | |
| 1 | 27 900 (24.1) | 2518 (23.9) | |
| 2 | 21 356 (18.4) | 1905 (18.1) | |
| 3+ | 37 328 (32.2) | 3548 (33.7) | |
| Prior inpatient admissions | <.001 | ||
| 0 | 76 565 (66.1) | 6721 (63.8) | |
| 1 | 18 331 (15.8) | 1783 (16.9) | |
| 2 | 10 584 (9.1) | 1014 (9.6) | |
| 3+ | 10 371 (9.0) | 1023 (9.7) | |
| Chronic pulmonary disease | 35 864 (31.0) | 3476 (33.0) | <.001 |
| Admitted at diagnosis | 26 720 (23.1) | 2894 (27.5) | <.001 |
| Fracture level | <.001 | ||
| Thoracic | 21 504 (18.6) | 2058 (19.5) | |
| Lumbar | 31 453 (27.1) | 3243 (30.8) | |
| Both | 1909 (1.6) | 237 (2.2) | |
| Unspecified | 60 985 (52.6) | 5003 (47.5) | |
| Advanced imaging | 46 512 (40.1) | 9116 (86.5) | <.001 |
| Procedure | |||
| Type | |||
| Vertebroplasty | 7488 (71.0) | ||
| Kyphoplasty | 2797 (26.5) | ||
| Both | 256 (2.4) | ||
| Multiple levels at once | 1776 (17.3) | ||
| Inpatient | 5740 (54.5) | ||
Abbreviations: SD, standard deviation.
MORTALITY
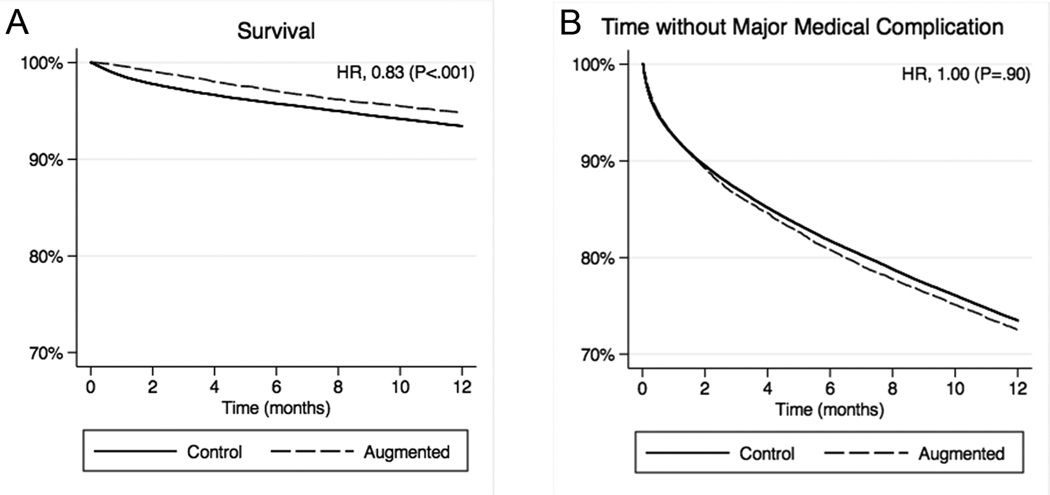
Thirty-day mortality following the index vertebral fracture was markedly lower in the augmented group compared to controls (0.4% vs 1.5%) with an odds ratio (OR) of 0.29 (95% confidence interval [CI], 0.20–0.41; Table 2). At one year, mortality remained significantly lower in the augmented group compared to controls, but the relative difference was attenuated (5.2% vs 6.7%) with a hazard ratio (HR) of 0.83 (95% CI, 0.75–0.92). Figure 1A displays one-year Kaplan-Meier curves for survival.
Table 2.
Mortality, Major Medical Complications, and Resource Utilization.
| No. (%) | ||||
|---|---|---|---|---|
| Control (n = 115 851) |
Augmented (n = 10 541) |
HR or OR (95% CI) |
P Value |
|
| Death | ||||
| 30 days | 1754 (1.5) | 39 (0.4) | 0.29 (0.20–0.41) | <.001 |
| 1 year | 7768 (6.7) | 546 (5.2) | 0.83 (0.75–0.92) | <.001 |
| Major medical complication | ||||
| 30 days | 12 057 (10.4) | 975 (9.3) | 0.85 (0.79–0.92) | <.001 |
| 1 year | 33 497 (28.9) | 3051 (28.9) | 1.00 (0.95–1.04) | .90 |
| Healthcare utilization (1 year) | ||||
| Hospitalizationa | 55 164 (47.6) | 6324 (60.0) | 1.37 (1.30–1.44) | <.001 |
| ICU admission | 13 247 (11.4) | 1470 (13.9) | 1.14 (1.06–1.21) | <.001 |
| SNF discharge | 19 819 (17.1) | 2284 (21.7) | 1.16 (1.10–1.23) | <.001 |
| Wrist fracture | 2387 (2.1) | 194 (1.8) | 0.90 (0.76–1.07) | .23 |
| Hip fracture | 7664 (6.6) | 677 (6.4) | 0.96 (0.87–1.05) | .37 |
| No Procedure (30 days) |
Control (n = 115 851) |
Pre-Procedureb (n = 3023) |
||
| Major medical complications | 12 057 (10.4) | 199 (6.6) | 0.66 (0.57–0.78) | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ICU, Intensive care unit; OR, Odds ratio; SNF, skilled nursing facility.
Hospitalizations after vertebral fracture diagnosis and excluding hospitalization at the time of spinal augmentation procedure.
Pre-procedure subgroup includes patients from the augmented group who had not yet undergone spinal augmentation at 30 days.
Figure 1.
Major medical outcomes following vertebral compression fracture in the traditional multivariate analysis. A, Survival. B, Time without a major medical complication. Hazard ratios (HR) were calculated with Cox proportional hazards model adjusted for year of fracture diagnosis, patient demographics and comorbidities, fracture level, and use of advanced imaging.
MAJOR MEDICAL COMPLICATIONS
The augmented group also had significantly fewer major medical complications compared to controls during the 30 days following the index fracture (9.3% vs 10.4%) with an OR of 0.85 (95% CI, 0.79–0.92; Table 2). At one year, however, rates of complications were equal between groups (28.9% for both; HR, 1.00; 95% CI, 0.95–1.04). Figure 1B displays one-year Kaplan-Meier curves for time without a major medical complication.
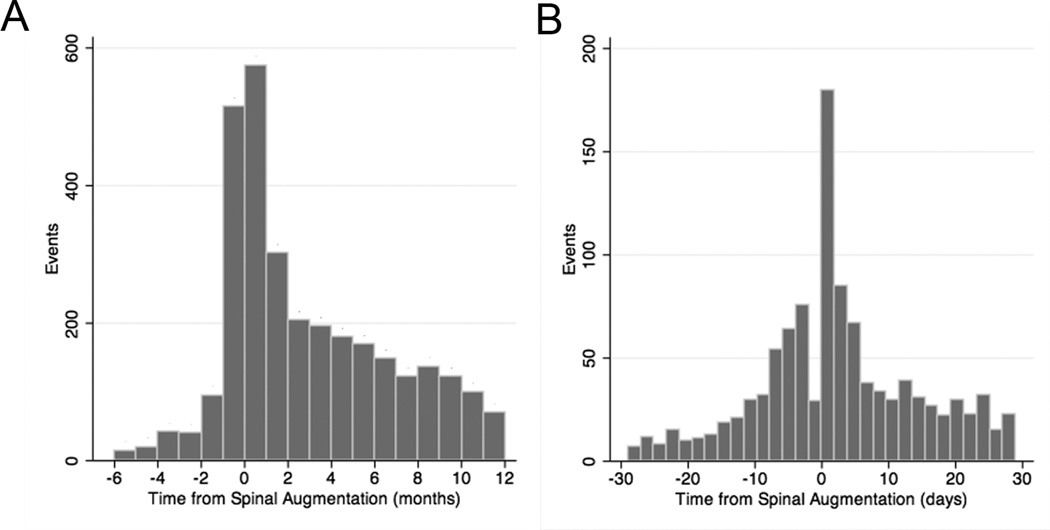
In the augmented group, the first major medical complication occurred after the spinal augmentation in 79.9%, with the greatest peak immediately after the procedure (Figures 2A and B). The risk of death was significantly higher among patients who experienced a major medical complication (OR, 5.59; 95% CI, 5.13–6.09).
Figure 2.
Major medical complications as a function of time from spinal augmentation. A, Overall major medical complications (event) relative to the time of spinal augmentation (day 0). B, Major medical complications (event) within one month of the procedure. Pre-procedure events are indicated as negative time.
HEALTHCARE UTILIZATION
We also tracked additional measures of healthcare utilization during the year following the index vertebral fracture. Compared to controls, the augmented group had significantly higher rates of hospitalizations (excluding those for the spinal augmentation), ICU admissions, and discharges to SNFs (Table 2). In the augmented group, the first of these events occurred after the procedure in a majority of the patients: 4472 of the hospitalizations (70.7%), 1278 of the ICU admissions (86.9%), and 1746 of the SNF discharges (76.4%). Rates of wrist and hip fractures during the year following the index vertebral fracture were similar between groups, suggesting roughly similar severity of osteoporosis.
PRE-PROCEDURE MAJOR MEDICAL COMPLICATIONS
Seventy-one percent of patients in the augmented group underwent spinal augmentation during the first 30 days after the index vertebral fracture. The remaining 3023 (29.0%) patients, who had not yet undergone augmentation at 30 days (pre-procedure subgroup), therefore, offered a unique internal control of overall health between treatment groups. Any differences in thirty-day outcomes could not be due to a procedure that had not yet occurred.
Patients in this pre-procedure subgroup experienced significantly lower rates of major medical complications during the first 30 days than controls (6.6% vs 10.4%) with an OR of 0.66 (95% CI, 0.57–0.78; Table 2).
Mortality cannot be compared in this manner because any patient who died prior to spinal augmentation would automatically be included in the control group (having had no procedure).
PROPENSITY SCORE MATCHED ANALYSIS
We performed propensity score matching to better control for unmeasured factors between groups. Baseline characteristics of the matched-sample groups were similar to those in the traditional analysis reported above but better matched between groups (Table 3).
Table 3.
Baseline Characteristics of Patients and Procedures for Matched Samples
| No. (%) | |||
|---|---|---|---|
| Characteristic | Control (n = 9017) |
Augmented (n = 9017) |
P Value |
| Age, mean (SD) | 80.3 (7.0) | 80.0 (6.7) | .01 |
| Female sex | 7120 (79.0) | 7077 (78.5) | .43 |
| White race | 8776 (97.3) | 8765 (97.2) | .62 |
| State buy-in | 914 (10.1) | 902 (10.0) | .77 |
| Quan comorbidity score | .83 | ||
| 0 | 2195 (24.3) | 2194 (24.3) | |
| 1 | 2134 (23.7) | 2138 (23.7) | |
| 2 | 1660 (18.4) | 1628 (18.1) | |
| 3+ | 3028 (33.6) | 3057 (33.9) | |
| Prior inpatient admissions | .006 | ||
| 0 | 5921 (65.7) | 5707 (63.3) | |
| 1 | 1388 (15.4) | 1562 (17.3) | |
| 2 | 852 (9.5) | 860 (9.5) | |
| 3+ | 856 (9.5) | 888 (9.9) | |
| Chronic pulmonary disease | 2937 (32.6) | 3005 (33.3) | .28 |
| Admitted at diagnosis | 2496 (27.7) | 2536 (28.1) | .51 |
| Fracture level | .05 | ||
| Thoracic | 1611 (17.9) | 1754 (19.5) | |
| Lumbar | 2832 (31.4) | 2786 (30.9) | |
| Both | 208 (2.3) | 211 (2.3) | |
| Unspecified | 4366 (48.4) | 4266 (47.3) | |
| Advanced imaging | 9017 (100) | 9017 (100) | - |
| Procedure | |||
| Type | |||
| Vertebroplasty | 6404 (71.0) | ||
| Kyphoplasty | 2393 (26.5) | ||
| Both | 220 (2.4) | ||
| Multiple levels at once | 1562 (17.8) | ||
| Inpatient | 4944 (56.2) | ||
Abbreviations: SD, standard deviation.
MATCHED-SAMPLE MORTALITY
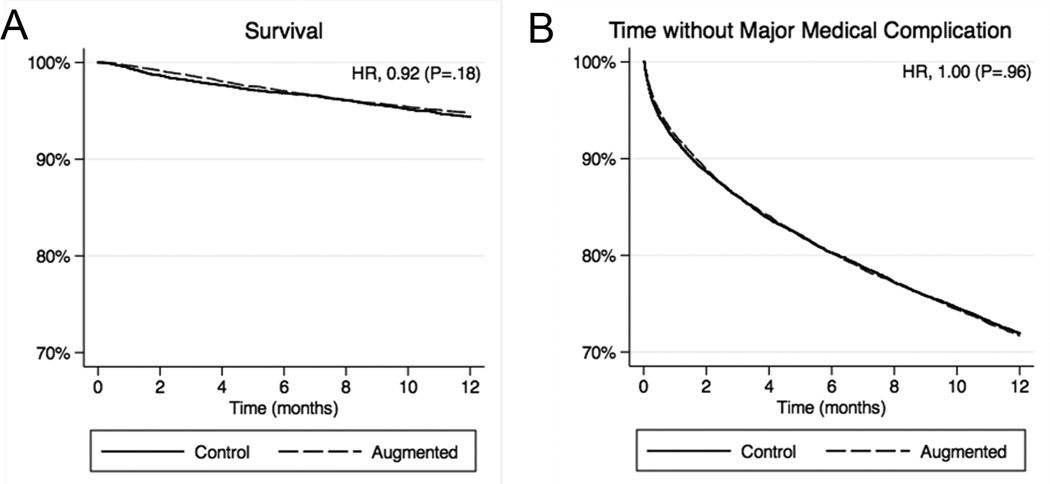
Thirty-day mortality was significantly lower in the augmented group compared to controls in the propensity matched-sample comparison (0.3% vs 0.6%) with an OR of 0.61 (95% CI, 0.39–0.95; Table 4), but this difference was attenuated compared to that in the traditional multivariate analysis. At one year, the difference was no longer statistically significant (5.2% vs 5.6%) with an HR of 0.92 (95% CI, 0.81–1.04). Figure 3A displays one-year Kaplan-Meier curves for survival for the propensity score matched-sample.
Table 4.
Mortality, Major Medical Complications, and Resource Utilization in Propensity Score Matched Groups
| No. (%) | ||||
|---|---|---|---|---|
| Control (n = 9017) |
Augmented (n = 9017) |
HR or OR (95% CI) |
P Value |
|
| Death | ||||
| 30 days | 51 (0.6) | 31 (0.3) | 0.61 (0.39–0.95) | .03 |
| 1 year | 505 (5.6) | 469 (5.2) | 0.92 (0.81–1.04) | .18 |
| Major medical complications | ||||
| 30 days | 947 (10.5) | 860 (9.5) | 0.90 (0.81–0.99) | .03 |
| 1 year | 2709 (30.0) | 2691 (29.8) | 1.00 (0.94–1.06) | .96 |
| Healthcare utilization (1 year) | ||||
| Hospitalizationa | 5023 (55.7) | 5585 (61.9) | 1.31 (1.23–1.39) | <.001 |
| ICU admission | 1187 (13.2) | 1300 (14.4) | 1.11 (1.02–1.21) | .02 |
| SNF discharge | 1901 (21.1) | 2051 (22.8) | 1.10 (1.03–1.19) | .006 |
| Wrist fracture | 191 (2.1) | 169 (1.9) | 0.88 (0.72–1.09) | .24 |
| Hip fracture | 601 (6.7) | 582 (6.5) | 0.97 (0.86–1.09) | .57 |
| No Procedure (30 days) |
Control (n = 2773) |
Pre-Procedureb (n = 2773) |
||
| Major medical complications | 264 (9.5) | 180 (6.5) | 0.66 (0.54–0.80) | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ICU, Intensive care unit; OR, Odds ratio; SNF, skilled nursing facility.
Hospitalizations after vertebral fracture diagnosis and excluding hospitalization at the time of spinal augmentation procedure.
Pre-procedure subgroup includes patients from the augmented group who had not yet undergone spinal augmentation at 30 days.
Figure 3.
Major medical outcomes following vertebral compression fracture in the propensity score matched analysis. A, Survival. B, Time without a major medical complication. Hazard ratios (HR) were calculated with matched-sample Cox proportional hazards model.
MATCHED-SAMPLE MAJOR MEDICAL COMPLICATIONS
Thirty-day rates of major medical complications were borderline lower in the augmented group compared to controls (9.5% vs 10.5%) with an OR of 0.90 (95% CI, 0.81–0.99; Table 4). At one year, the risk of a major medical complication was equivalent between groups (HR, 1.00; 95% CI, 0.94–1.06), as in the traditional multivariate analysis. Figure 3B displays one-year Kaplan-Meier curves for time without a major medical complication in the propensity score matched-sample.
In the augmented group, the initial major medical complication occurred after spinal augmentation was performed in 2123 (78.9%) patients. The risk of death remained significantly higher in patients who experienced a major medical complication compared to those who had not (OR, 6.05; 95% CI, 5.19–7.04).
MATCHED-SAMPLE HEALTHCARE UTILIZATION
As in the complete sample, the augmented group was more likely than controls to require hospitalization, ICU admission, or discharge to a SNF (Table 4). Rates of wrist and hip fractures during the year following fracture diagnosis were similar between groups.
MATCHED-SAMPLE PRE-PROCEDURE COMPLICATIONS
The augmented group in the propensity score matched-sample included 2773 (31.0%) patients who had not yet undergone spinal augmentation at 30 days (pre-procedure subgroup). As in the traditional analysis, major medical complications were significantly less likely in the pre-procedure subgroup compared to controls (OR, 0.66; 95% CI, 0.54–0.80; Table 4), suggesting residual unmeasured confounders are resulting in healthier patients in the augmented group.
COMMENT
The results from this study suggest that treatment of osteoporotic vertebral fractures with spinal augmentation does not improve long-term mortality or major medical complications and increases several measures of healthcare utilization compared to conservative therapy. Our analysis also illustrates how selection bias hidden within billing claims data can significantly alter the results of population-based outcomes research.
Two recent studies using billing claims data reported markedly better survival following spinal augmentation compared to conservative therapy.13,15 Edidin et al. reported spinal augmentation was associated with a 37% lower adjusted risk of death at four years using Medicare claims from 2005–2008 and Zampini et al. showed a 48% lower adjusted risk of inpatient mortality in patients treated with kyphoplasty using the 2005 National Inpatient Sample.13,15
Our initial analysis paralleled these studies. Using standard covariate adjustments, we observed significantly lower mortality in the augmented group compared to controls. However, patients in the augmented group who had not yet undergone augmentation (pre-procedure subgroup) had better 30-day outcomes than controls. Since a procedure that has not yet been performed cannot improve outcomes, we concluded that this difference was due to selection bias: providers performing spinal augmentation were excluding patients at higher risk for complications. We would also expect the ability of the provider to predict a complication would be best in the short term, explaining the markedly lower risk of death at 30 days in the augmented group compared to controls.
To better control for selection bias, we performed propensity score matching and repeated the analyses. Comparing matched samples, improvements in 30-day outcomes in the augmented group were attenuated compared to the traditional analysis and the mortality advantage at one year was lost. Hospitalizations, ICU admissions, and SNF discharges remained significantly higher in the augmented group. The risk of a major medical complication at 30 days remained lower in the pre-procedure subgroup, suggesting residual selection bias.
This study has several strengths. The large sample size provides adequate power to detect small differences in relatively rare events such as death and major medical complications. We also used data from routine clinical practice rather than from selected populations in clinical trials or academic medical centers. However, there are also limitations. Medicare data cannot be used to evaluate symptomatic outcomes such as pain and disability. Diagnoses and procedures may have been miscoded even though they were used for billing and subject to audit. We tracked major complications, which are more reliably coded than minor complications,23 to mitigate this shortcoming. Additionally, there is no ICD code specific to osteoporotic vertebral fractures; we were forced to exclude patients with other comorbid conditions that may have resulted in the fracture.
Our results further challenge the value of spinal augmentation for the treatment of osteoporotic vertebral compression fractures. Spinal augmentation is intended to limit acute pain and disability, and by extension, improve medical outcomes. However, two double-blinded RCTs failed to demonstrate benefits in pain or disability with vertebroplasty,4,5 and the results of this study suggest that long-term mortality and major medical complications are not improved and healthcare utilization is increased with spinal augmentation compared to conservative therapy. Furthermore, our analysis highlights how unmeasured selection bias in billing claims data can lead to overestimation of the benefits of a procedure. This has implications for patients diagnosed with an osteoporotic vertebral fracture, but also for outcomes research in general that uses medical billing claims.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: This research is supported in part by the Radiological Society of North America Research Resident grant RR1139, University of Washington Department of Radiology research grant, Agency for Healthcare Research and Quality grant 1R01 HS019222, National Institute of Arthritis, Musculoskeletal, and Skin Diseases grant 1R01 AR054912, and National Center for Research Resources/National Center for the Advancement of Translational Science grant 1UL1 RR024140.
Role of the Sponsor: The funding agencies had no role in design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Dr Deyo receives honoraria as a board member of the non-profit Informed Medical Decisions Foundation. In the recent past, he has received honoraria as member of an Advisory Committee for the non-profit Robert Wood Johnson Foundation. He receives honoraria from UpToDate for authoring topics on low back pain. His research receives support from grants from the National Institutes of Health and the Agency for Healthcare Research and Quality. His position at Oregon Health and Science University is supported in part by an endowment from Kaiser Permanente. Dr Jarvik served on the Comparative Effectiveness Research Advisory Board for General Electric Healthcare. He is a consultant for HealthHelp (a radiology benefits management company). He is also a co-founder, stockholder, and co-patent holder for PhysioSonics (a high intensity focused ultrasound diagnostics company).
Footnotes
Previous Presentation: The data were presented at the Radiological Society of North America annual meeting on November 27, 2012 in Chicago, Illinois.
Author Contributions: Dr McCullough had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: McCullough, Jarvik.
Collection, management, analysis, and interpretation of the data: McCullough, Comstock, Deyo, Kreuter, Jarvik.
Preparation, review, or approval of the manuscript: McCullough, Comstock, Deyo, Kreuter, Jarvik.
Conflict of Interest Disclosures: The remaining authors have no potential conflicts of interest to report.
REFERENCES
- 1.Muijs SP, van Erkel AR, Dijkstra PD. Treatment of painful osteoporotic vertebral compression fractures: a brief review of the evidence for percutaneous vertebroplasty. J Bone Joint Surg Br. 2011 Sep;93(9):1149–1153. doi: 10.1302/0301-620X.93B9.26152. [DOI] [PubMed] [Google Scholar]
- 2.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010 Sep 25;376(9746):1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009 Mar 21;373(9668):1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 4.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009 Aug 6;361(6):569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009 Aug 6;361(6):557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 6.Staples MP, Kallmes DF, Comstock BA, et al. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ. 2011;343:d3952. doi: 10.1136/bmj.d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008 Jul;90(7):1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 8.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009 Feb 4;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 9.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999 Mar 13;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 10.Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004 Jan;15(1):38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 11.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993 May 1;137(9):1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int. 2004 Feb;15(2):108–112. doi: 10.1007/s00198-003-1516-y. [DOI] [PubMed] [Google Scholar]
- 13.Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011 Jul;26(7):1617–1626. doi: 10.1002/jbmr.353. [DOI] [PubMed] [Google Scholar]
- 14.Edidin AA, Ong KL, Lau E, Kurtz SM. Life expectancy following diagnosis of a vertebral compression fracture. Osteoporos Int. 2012 Mar 16; doi: 10.1007/s00198-012-1965-2. [DOI] [PubMed] [Google Scholar]
- 15.Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clin Orthop Relat Res. 2010 Jul;468(7):1773–1780. doi: 10.1007/s11999-010-1279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald RJ, Achenbach SJ, Atkinson EJ, et al. Mortality in the vertebroplasty population. AJNR Am J Neuroradiol. 2011 Nov-Dec;32(10):1818–1823. doi: 10.3174/ajnr.A2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010 Apr 7;303(13):1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009 Apr 2;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 19.Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008 Jan 31;358(5):464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 20.Morrill R, Cromartie J, Hart G. Metropolitan, urban, and rural commuting areas: Toward a better depiction of the United States settlement system. Urban Geogr. 1999;20(8):727–748. [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23.Lawthers AG, McCarthy EP, Davis RB, Peterson LE, Palmer RH, Iezzoni LI. Identification of in-hospital complications from claims data. Is it valid? Med Care. 2000 Aug;38(8):785–795. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pearl J. Causality: Models, Reasoning and Inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 25.Rosenbaum P, Rubin D. The bias due to incomplete matching. Biometrics. 1985;41(1):103–116. [PubMed] [Google Scholar]
- 26.Rosenbaum P, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 27.Rosenbaum PR. Design of observational studies. 1st ed. New York: Springer; 2009. [Google Scholar]
- 28.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. The Review of Economics and Statistics. 2002;84(1):151–161. [Google Scholar]
- 29.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. 2003 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.