Abstract
Cocaine dependence impacts drug-related, dopamine-dependent reward processing, yet its influence on non-drug reward processing is unclear. Here, we investigated cocaine-mediated effects on reward learning using a natural food reinforcer. Cocaine-dependent subjects (N=14) and healthy controls (N=14) learned to associate a visual cue with a juice reward. In subsequent functional imaging sessions they were exposed to trials where juice was received as learned, withheld (negative temporal difference error (NTDE)), or received unexpectedly (positive temporal difference error (PTDE)). Subjects were scanned twice in sessions that were identical, except that cocaine-dependent participants received cocaine or saline 10 min before task onset. In the insula, precentral and postcentral gyri NTDE signals were greater, and PTDE-related function was reduced in cocaine-dependent subjects. Compared with healthy controls, in the cocaine-dependent group PTDE signals were also reduced in medial frontal gyrus and reward-related function, irrespective of predictability, was reduced in the putamen. Group differences in error-related activity were predicted by the time as last self-administered cocaine use, but TDE function was not influenced by acute cocaine. Thus, cocaine dependence seems to engender increased responsiveness to unexpected negative outcomes and reduced sensitivity to positive events in dopaminergic reward regions. Although it remains to be established if these effects are a consequence of or antecedent to cocaine dependence, they likely have implications for the high-cocaine use recidivism rates by contributing to the drive to consume cocaine, perhaps via influence on dopamine-related reward computations. The fact that these effects do not acquiesce to acute cocaine administration might factor in binge-related escalated consumption.
Keywords: cocaine dependence, temporal difference error prediction, reward, motivation, fMRI
INTRODUCTION
Addiction to cocaine and other abused substances is characterized by a cycle involving the compulsion to consume the drug (craving), difficulty-limiting intake (intoxication, bingeing), and negative emotional states (withdrawal, anhedonia)(Goldstein and Volkow, 2011; Koob and LeMoal, 1997). Changes in motivational processing are an essential component of these addiction phenomena (Koob and Volkow, 2010). Indeed, drug dependence engenders functional alterations in dopamine (DA) pathway regions that constitute the brain's reward system (eg the substantia nigra (SN) and ventral tegmental area (VTA) in the midbrain and the basal ganglia structures (ie nucleus accumbens, putamen caudate), and the prefrontal regions (eg, medial prefrontal (mPFC) and orbitofrontal cortices) they principally project to (Diekhof et al, 2008; Haber and Knutson, 2010). Clinical and preclinical investigations confirm the role of these regions in diverse motivational processes, such as incentive salience (Knutson et al, 2000; Knutson et al, 2001; Knutson et al, 2003), the hedonic experience of reward (O'Doherty et al, 2001), and reward learning (Asaad and Eskandar, 2011; Berns et al, 2001; McClure et al, 2003; O'Doherty et al, 2003; Schultz, et al, 1997; Schultz, 1998; Schultz and Dickinson, 2000). Consequently, drug dependence is often considered to be a disease of abnormal reward processing (Volkow et al, 2010).
During reward learning, unpredictability arises from a variety of events, including temporal shifts in reward delivery and the unexpected occurrence of reward-predictive stimuli—so-called ‘temporal difference errors' (TDEs). Transient decreases in dopaminergic function follow omission of temporally anticipated (ie, predicted) rewards, ie negative TDE (NTDE), while positive TDE (PTDE) processing following unanticipated rewards requires phasic increases in DA signaling (McClure et al, 2003; Montague et al, 1996; Montague et al, 2004; Schultz et al, 1997; Schultz, 2000; Schultz, 2002; Schultz, 2007; Wise, 2009). Although TDE signals are typically thought to arise in the midbrain, other brain regions may also be important for error signaling (Roesch et al 2012). For example, gain prediction error computations for monetary rewards can be mediated by mPFC function (Knutson and Wimmer, 2007), whereas orbitofrontal activity correlates with error signals for appetitive rewards (O'Doherty et al, 2003). Abused drugs produce transient increases in DA signaling (Koob et al, 1998; Koob and Volkow, 2010) that become conditioned (Bolieau et al, 2007), leading to positive error signals that increase drug value and reinforce drug-seeking behavior. Thus, drug dependency may be driven in part by changes in TDE reward learning (Redish, 2004).
Neuroimaging studies of cocaine-dependent (CD) adults suggest functional alterations in brain regions that support reward processing, including reward learning. For example, acute cocaine engages the same dopaminergic pathways in CD individuals that mediate acute drug response in preclinical addiction models (Breiter et al, 1997; Kufahl et al, 2005; Risinger et al, 2005). Moreover, large-scale brain networks (eg default mode, salience, and executive control networks; Sutherland et al, 2012) involving mesocorticolimbic DA areas, like the striatum, are dysfunctional in those who abuse cocaine (Gu et al, 2010; Tomasi et al, 2010). Similarly, DA release in the striatum following methlyphenidate or amphetamine challenge is blunted in CD individuals (Martinez et al, 2007; Volkow et al, 1997). Interestingly, this blunting is predictive of choosing cocaine vs alternative rewards (Volkow et al, 1997). Impaired non-drug reward valuation in cocaine dependence likely arises from dysfunction in prefrontal reward regions (Goldstein et al, 2007a; Goldstein et al, 2007b). Furthermore, incentive processing for monetary rewards in motivation-related corticolimbic regions is altered during reward anticipation and receipt in abstinent CD adults, with these differences predicting treatment outcome (Jia et al, 2011).
Dysfunction in reward processing regions in CD individuals may contribute to the incentive to consume cocaine, continued cocaine abuse, and high recidivism rates. Moreover, given the ubiquitous nature of processing for a range of reinforcing stimuli, CD abnormalities in reward processing likely extend beyond drug rewards to non-drug reinforcers. As the reinforcing nature of non-drug stimuli may be critical to maintaining long-term abstinence, understanding the impact of cocaine-mediated changes for non-drug reward is crucial for intervention strategies. However, despite evidence of compromise in reward pathways in CD individuals, the impact of acute cocaine on the mechanisms of non-drug reward processing, including reward learning, is not well delineated in either preclinical or clinical models.
The allostasis hypothesis of drug abuse (eg, Koob and LeMoal, 2007) postulates that chronic exposure to stimulants, like cocaine, engenders a gradual decline in dopaminergic function, which is associated with a preference for the abused substance over other reinforcing stimuli. This hypothesis is complimentary to the computational account of addiction noted above (ie Redish, 2004), whereby acute drug exposure is believed to overstimulate compromised DA reward systems; resulting in aberrant learning signals that also bias toward the drug. As TDE processing has been shown to rely on the integrity of DA systems, TDE learning paradigms have the potential to act as excellent probes of the response of dopaminergic reward pathways to both chronic and acute cocaine exposure.
This study considered the impact of cocaine dependence on reward learning for a non-drug, primary reward. We investigated the neurobiology of TDE processing in CD individuals using a classical conditioning paradigm and considered the relative impact of the trait of cocaine dependence and an acute cocaine administration state on these processes. It was hypothesized that: (1) during cocaine abstinence and compared with controls, CD individuals would show reduced TDE/reward-related activity, due to impaired function in DA pathway regions, and (2) acute cocaine administration would ‘normalize' cocaine-dependent alterations in TDE-related signaling.
MATERIALS AND METHODS
Participants
Healthy controls (HC; N=26) and non-treatment seeking CD (N=22) individuals were recruited from the general population. Participants were right-handed, aged 18–45 years and had no scientific, medical, or ethical contraindications for magnetic resonance imaging (MRI). Participants had no current or past DSM-IV-TR Axis I or II diagnoses, except nicotine dependence in all participants (N=14 HC and 22 CD current/past smokers) and current cocaine dependence in CD participants only. Throughout the study, CD subjects were offered access to treatment services as an alternative to participation. Those expressing a preference for treatment were automatically excluded.
Eight CD subjects were disqualified due to excessive head motion (ie, more than 3 mm/° in any direction between consecutive TRs), resulting in an analysis cohort of N=14. A subsample (N=14) was selected from HC that passed data quality control (N=21) to best match the included CD group (see Table 1 for demographics and Supplementary Table S1 for summary of cocaine use). The subsequent description of methods and results refers only to those included in imaging analyses.
Table 1. Participant Demographics.
| CD (N=14) | HC (N=14) | |
|---|---|---|
| Age at time of testinga (years; mean (SD)) | 42.93 (2.13) | 41.57 (2.14) |
| Years of educationa (mean (SD)) | 13.14 (1.79) | 13.71 (2.49) |
| WAIS full-scale IQa (mean (SD) | 98.86 (8.93) | 105.71 (11.74) |
| WTAR estimated IQa (mean (SD) | 96.50 (13.33) | 100.92 (13.14) |
| Gendera (male: female) | 11: 3 | 12: 2 |
| Ethnicity ratioa (AA: C) | 12: 2 | 11: 3 |
| Smoker past or present (Yes: No) | 14: 0 | 8: 6 |
| Age at first cocaine use (years; mean (SD) and [range]) | 22 (5.80) [14–36] | n/a |
| Years of regular cocaine use (mean (SD) and [range]) | 16.36 (4.99) [8–25] | n/a |
| Number of days used cocaine in week pre-study (mean (SD) and [range]) | 1.79 (1.67) [0–6] | n/a |
| Time since last cocaine use (days; mean (SD) and [range]) | 3.00 (2.28) [0–7] | n/a |
Abbreviations: CD, cocaine dependent; HC, healthy control.
Non-significant (p>0.05) between-group comparisons (independent sample t or χ2).
Procedure
The NIDA-IRP IRB approved this study and subjects provided written, informed consent prior to participation. Participation consisted of task training in a mock scanner and two MRI sessions. CD subjects also completed a ‘drug toleration' session prior to scanning, which included monitoring of blood plasma concentrations of cocaine and metabolites (see Supplementary Methods for details). MRI sessions were identical for HC and CD subjects, except for drug administration. Using a within-subjects, single-blind, randomly counter-balanced design, CD subjects received intravenous cocaine or saline during scanning (N=7 cocaine first). HC were scanned twice to control for the potential timing effects arising from repetition of the experimental paradigms. CD participants were tested on consecutive days and stayed overnight between sessions (Supplementary Figure S1). HC completed experimental sessions on separate days, scheduled as closely as possible.
TDE/Juice Paradigm
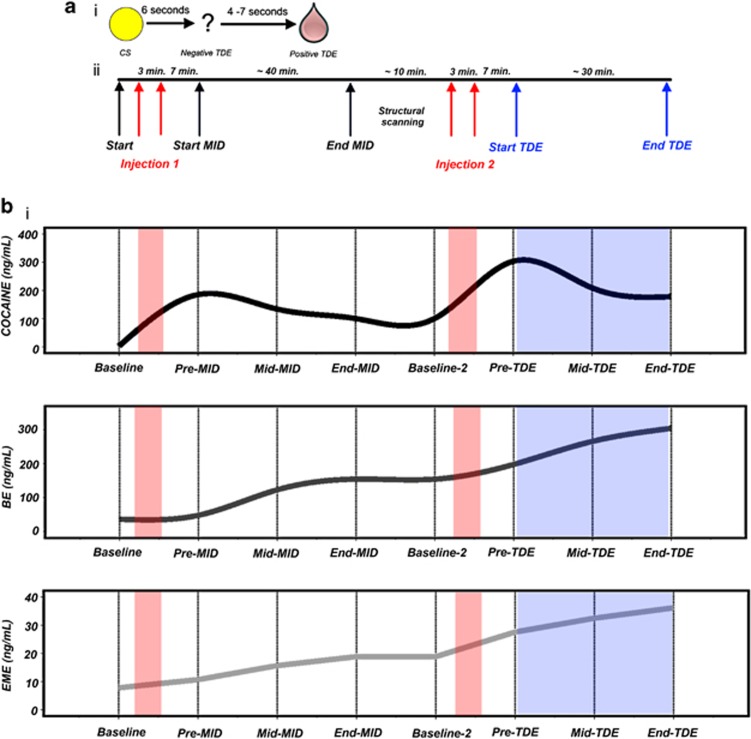
The TDE paradigm is described elsewhere (Rose et al, 2012). In brief, before scanning, participants learned to associate a visual cue and the subsequent receipt of a primary reward (ie, 0.6 ml juice/6 s delay). During scanning the paradigm consisted of trials mimicking learning trials interspersed with trials in which juice was not received as predicted but instead received 4–7 s later, ie ‘catch' trials (Figure 1a(i)). It was intended that omission of the predicted reward would engender NTDE signals, whereas its unanticipated receipt after a pseudo-randomly selected delay would result in a PTDE signal (Hollerman and Schultz, 1998). Normal (N=58) and catch (N=20) trials were divided across three 9-min task ‘blocks'. At the end of each block, participants rated how much they liked the juice on a visual analog scale (range 0–800). CD subjects also rated how high and satisfied they felt, and how much they were craving cocaine (Supplementary Tables S3 and S4). As the TDE measure was passive, requiring no response, participant vigilance was confirmed verbally at the start of each block and visually by inspection of data time series captured in real-time during scanning.
Figure 1.
Experimental timeline and relative plasma concentration of cocaine and metabolites. (a) (i) TDE ‘catch' event (ii) Scanning timeline including timing of cocaine injections relative to imaging tasks. (b) Estimated mean plasma concentrations (ng/ml) of (i) cocaine, (ii) Benzolecgonine (BE), and (iii) Ecgonine methyl ester (EME) across the scanning session. Notes; 1 Metabolite estimates were derived from blood samples obtained during the drug toleration session, which mimicked the scanning session with regards to relative timing of injections and functional measures; Supplementary Table S6 includes a summary of these data. Spline interpolation was used for these graphs. 3: Red shading corresponds to timing of bolus injections of cocaine; blue shading corresponds to TDE task duration. 4: ‘Baseline-2' refers to those samples obtained prior to the commencement of the second cocaine injection.
Timing Paradigm
Accurate temporal difference prediction is critical for the generation of NTDE and PTDE signals. To determine whether general timing processes were potentially compromised in CD individuals, participants completed a test of timing function after their final session. This task is described elsewhere (Rose et al, 2012).
Functional Imaging
Whole-brain echo planar images were acquired on a 3T Siemens Allegra scanner (Erlangen, Germany). Thirty-nine 4-mm slices were acquired in an oblique axial plane (30° to AC–PC) with the following imaging parameters: TR=2000 ms, TE=27 ms, FOV=220 × 220 mm at 64 × 64, and flip angle=78°. T1-weighted MPRAGE structural imaging series were also acquired (voxel=1 mm3).
Using a within-subjects design, participants completed two identical scans. They performed two reward measures per session (Figure 1a(ii)). The TDE task was performed second, ∼70 min after beginning the session, and is the only measure reported here. CD subjects received two 3 min/10 ml bolus injections of 30 mg cocaine hydrochloride/70 kg bodyweight or saline, about 1 h apart. Each bolus was administered ∼10 min prior to each tasks. Drug administration procedures and physiological monitoring for scanning were as described for the toleration session. For the purposes of matching experimental conditions, physiological measures were also obtained for HC.
Characterization
Characterization measures completed upon study entry included indices of psychiatric history, personality, exposure to stressful life events, and cognitive function (see Supplementary Methods). CD subjects provided a detailed history of cocaine use and completed measures of craving and withdrawal.
Data Analysis
Functional imaging data were analyzed using AFNI (Cox, 1996). Data preprocessing and quality control procedures were as previously described (Rose et al, 2012). Data time series were analyzed using voxel-wise, multiple regression in which regressors were expressed as a series of delta functions time-locked to event onset and convolved with a hemodynamic response function and its temporal derivative. Regressors of interest were: visual cue/conditioned stimulus (CS), normal events/unconditioned stimulus (UCS; juice expected/juice delivered), NTDE (juice expected/juice not delivered), and PTDE (juice not expected/juice delivered). In addition, six motion parameters were included as regressors of no interest. Voxel-wise average response amplitude in units of percentage signal change from baseline was calculated for each event type, participant, and session. Resultant activation maps for each of the individual regressors were registered to a higher resolution (1 μl) standard space (Talairach and Tournoux, 1988) and spatially blurred using a 4.2 mm FWHM Gaussian isotropic kernel.
Random effects analyses considered experimental GROUP (HC vs CD), drug CONDITION (cocaine vs saline), and EVENT TYPE (CS, UCS, PTDE & NTDE). SESSION (1 vs 2) was also considered for HC only. Comparisons between HC and CD subjects included regressors averaged across sessions. To account for TDE signals in traditional reward pathways and other brain areas, a priori small volume correction (SVC) analyses in hypothesized DA pathway regions and whole-brain (WB) analyses were carried out. SVC analyses considered bilateral volumes in the SN, striatum (nucleus accumbens, caudate, and putamen), and medial prefrontal cortex (BA10 and BA32) and a midline volume encompassing the VTA, which were defined using a Talairach template. Using the AlphaSim program in AFNI, voxel-wise thresholds corrected for multiple comparisons were calculated using Monte Carlo simulations. Thresholds were calculated separately for WB and SVC analyses and determined as meeting or exceeding minimum cluster extent (KE) criteria at Pcorrected⩽0.05 (ie WB KE=290 voxels; SVC KE=26 voxels). For SVC analyses, this correction accounted for the total volume. The directionality and nature of significant main effects and interactions were confirmed with contrasts that were defined a priori in analysis of variance models and corrected for multiple comparisons. This included contrasts between HC and CD and between individual event types.
Exploratory post-hoc linear regressions were utilized to examine the relationship between cocaine use factors (chronic and acute) and CD changes in reward-related brain activity. As TDE processes are impacted by chronic nicotine exposure (Rose et al, 2012), the relationship between nicotine use and TDE-related function across groups was also explored. Finally, post-hoc regressions were conducted to determine the impact of characteristics that differed between groups on TDE-related brain activity. These latter analyses revealed no significant impact of nicotine or characterization measures on group-related differences in TDE function (see Supplementary Results).
RESULTS
Behavioral Measures
For behavioral measures with multiple indices correction for multiple comparisons (ie, Bonferroni) was considered within each measure (Note: uncorrected values are noted in Supplementary Table S2).
Personality
Compared with HC, CD individuals had higher novelty seeking scores (t(22)=3.34, p<0.007; Cloninger et al, 1994). There were no other group differences in personality.
Affect
CD subjects did not differ from HC on any measure of affect (p>0.05) (Bagby et al, 1994; Beck, 1993; Beck, 1996; Chapman et al, 1976; Kessler et al, 2002; Mroczek and Kolarz, 1998).
Stress
There were no between-group differences in exposure to stressful life events (Brugha et al, 1997), history of childhood abuse, or emotional neglect (Bernstein et al, 1994) (p>0.008).
Cognition
There were no between-group differences on any measure of cognition (Randolph et al, 1998; Wechsler, 2001; Wechsler, 2007), including the timing task (p>0.05), and acute cocaine did not alter timing performance in CD participants (p>0.05).
fMRI
Full details of significant imaging results can be found in Supplementary Table S7.
Acute Cocaine
Acute cocaine did not impact TDE-associated brain activity in WB or SVC analysis (pcorrected>0.05). As HC-only analyses were indicative of no session (1 vs 2) effects, between-group analysis (HC vs CD) focused on the contrast of TDE-related activity averaged across sessions.
Main Effect of Event Type
In general, TDE activation patterns were consistent with previous implementations of this task (McClure et al, 2003). In SVC analyses, there was an effect of EVENT TYPE in the bilateral putamen (Supplementary Figure S3A). Planned contrasts indicated that in the putamen CS and NTDE-related function were equivalent, and that activity for both was lower compared with UCS and PTDE events. WB analysis confirmed EVENT TYPE-related activation in the putamen and further suggested a main effect of the EVENT TYPE in bilateral postcentral gyri and declive, and in the right posterior cingulate (PCC), left BA19, and left cuneus (Supplementary Figure S3B). Activity in the postcentral gyri, PCC, putamen, and left declive was lowest for the CS condition, whereas activity for UCS either exceeded that for all other stimuli or was equivalent only to the unexpected reward/PTDE. In contrast, in the right declive, left cuneus, and left BA19 CS-related function exceeded activity for all other events.
Main Effect of Group
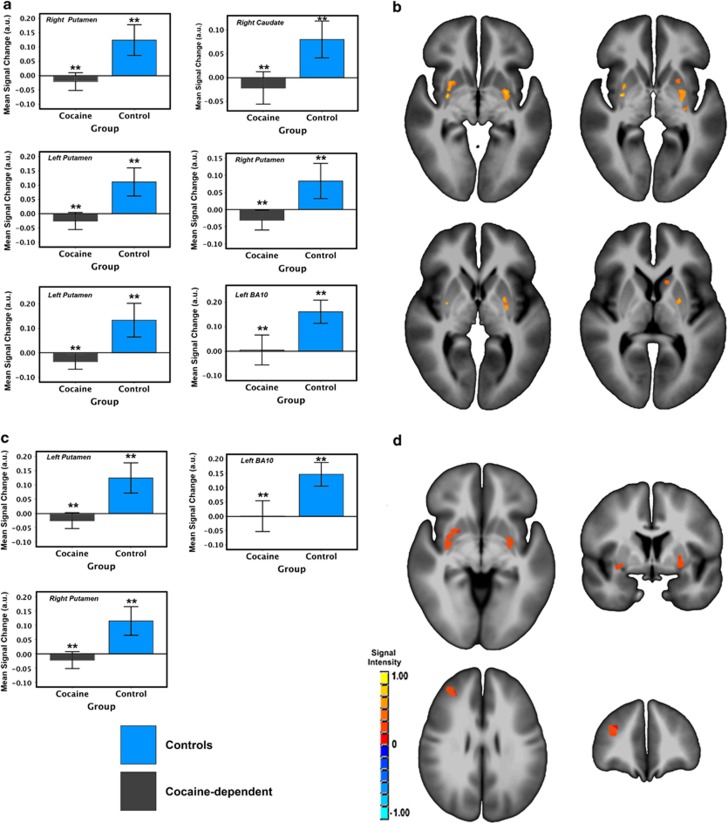
SVC (Figure 2a and b) and WB (Figure 2c and d) analyses revealed GROUP effects in the bilateral putamen and the left middle frontal gyrus (MFG), where the CD group exhibited less neuronal activity than the HC across event types. CD reductions in TDE-related function were also seen in the right caudate in the SVC analysis.
Figure 2.
The main effect of group (HC vs CD) on TDE-related activity in SVC (a and b) and WB (c and d) analyses. Notes: Error bars show ±2: SE; brain images are rendered on the ICBM452 T1 template from AFNI; left=left. **p<0.001.
Group × Event Type Interactions
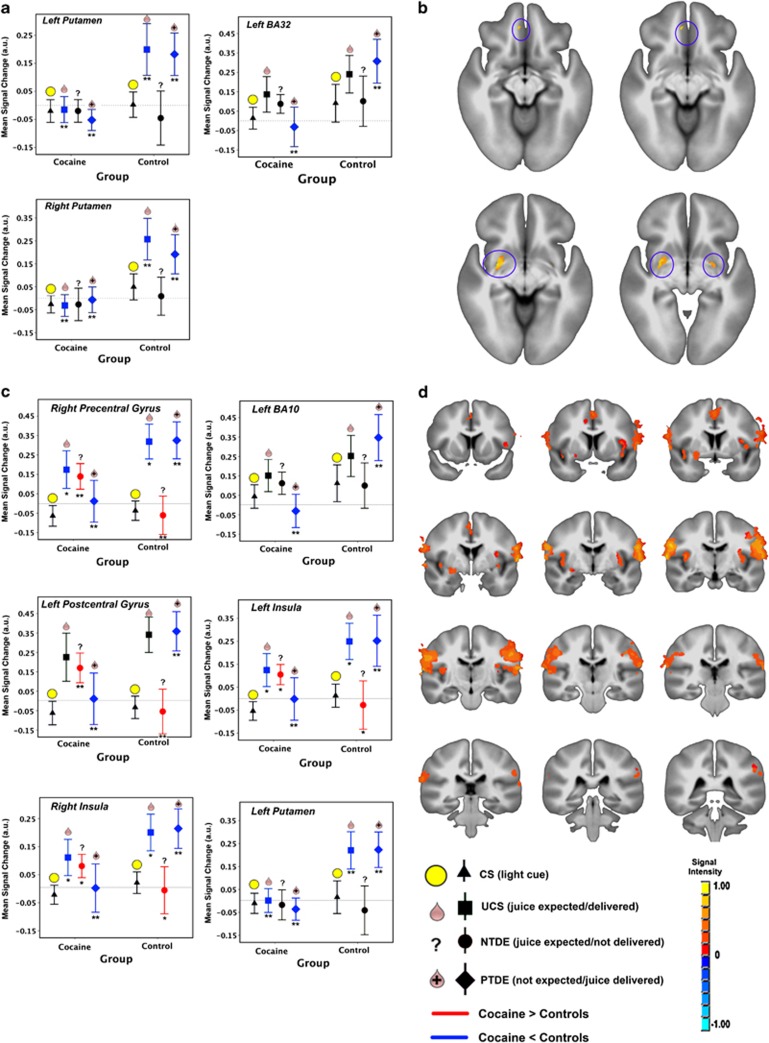
GROUP-by-EVENT TYPE interactions were observed in the bilateral putamen and left BA32 in SVC analyses. Activity in the putamen was lower in CD vs HC subjects for UCS and PTDE events (Figure 3a and b) and did not vary as a function of event type in CD individuals. In left BA32, PTDE-related activity was also lower in CD individuals.
Figure 3.
Group (HC vs CD) by event-type interactions on TDE-related activity in SVC (a and b) and WB (c and d) analyses. Notes: Error bars show SEM; brain images are rendered on the ICBM452 T1 template from AFNI; left=left. **p<0.001, *p<0.05.
WB analyses suggested GROUP-by-EVENT TYPE interactions in the bilateral insula, right precentral gyrus, left postcentral gyrus, left medial frontal gyrus/BA32, and left putamen (Figure 3c and d). In these clusters, PTDE-related activity was lower in CD vs HC subjects. UCS-related activity in the left putamen was also lower in the CD vs HC group. In contrast, NTDE-related activity in CD subjects was greater than in HC in the right precentral gyrus, left postcentral gyrus, and left insula.
Cocaine Use History
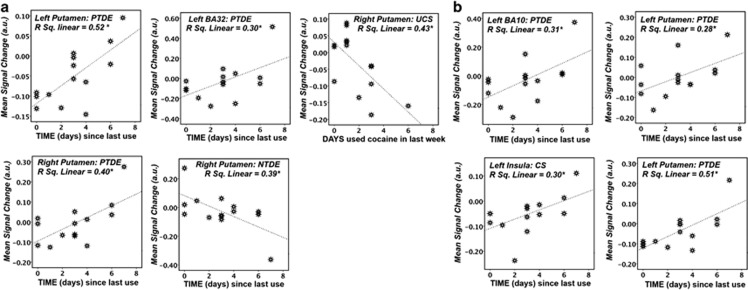
In those regions where GROUP-dependent functional differences (main effects and interactions) were noted, we considered the impact of cocaine-use history on brain activity via linear regression analyses. We included chronic (ie, AGE at first use and YEARS of use) and acute (ie, how many DAYS the subject had used cocaine, the number of ROCKS of cocaine used, total SPENT on cocaine in the week preceding the study, and TIME (days) between last cocaine binge and study entry) factors.
Although chronic-use factors did not predict GROUP-dependent variability in TDE function, TIME and DAYS were associated with GROUP-by-event type interactions. Time was positively correlated with PTDE-dependent activity in bilateral putamen and left BA32 (Figure 4a), CS-related function in the left insula, and activity for UCS in the left putamen (Figure 4b). Conversely, TIME negatively correlated with NTDE events in the right putamen. DAYS was negatively associated with UCS-dependent activity in the right putamen (Figure 4a).
Figure 4.
Significant associations between cocaine-use history and group-dependent differences in TDE-related activity in (a) SVC and (b) WB analysis. *p<0.05.
DISCUSSION
Functional representations of TDE signals were considered in CD individuals in the presence and absence of acute cocaine. Compared with matched HC, reward processing in CD subjects was characterized by greater activity for unpredicted negative outcomes (ie, omission of expected rewards) and less activity for predicted and unpredicted natural rewards in a distributed network of brain regions including major components of the mesocorticolimbic reward system. Moreover, CD individuals exhibited a relative reduction in activity in dopaminergic pathway regions in the basal ganglia and prefrontal cortex, independent of reward outcome event type. Functional alterations in the CD group were predicted by the time since last cocaine use/binge, but not by acute cocaine.
Cocaine Dependence and TDE
Cocaine dependence predicted a general blunting of reward-related function in mesocorticolimbic DA terminal areas (eg putamen, BA10). For CD individuals, activity in the putamen did not vary as a function of event type, suggesting a failure to differentiate between events or simply a failure to respond to reinforcing stimuli. In line with a lack of striatal response to acute drug challenge (Martinez et al, 2007; Volkow et al, 1997) and reduced D2/3 receptor availability in cocaine dependence (Martinez et al, 2004), trait-related decreases in activity in DA pathway regions may be a consequence of reduced presynaptic DA function. Furthermore, this general lowering in activity lends support to the notion that response sensitization in DA-mediated processing is not readily apparent in human cocaine users, even when individuals are not substantially abstinent (Note: even during saline scanning sessions, the maximum duration since last cocaine use would have been approximately 1 day due to drug toleration or cocaine scanning session the previous day). Although this contrasts with observed, persistent decreases in [C11]raclopride binding following repeated stimulant challenge in healthy individuals (Boileau et al, 2006), our participants were drug-dependent and had far greater accumulative exposure to stimulants. Thus, sensitization mechanisms may be critically and differentially impacted by chronic and sustained cocaine consumption.
Reduced activity in reward network regions for non-drug rewards in cocaine dependence may impact incentive salience processing and likely has critical implications for CD individuals. Indeed, decisions to abstain from cocaine and maintain abstinence may rely on satisfaction derived from alternative/non-drug stimuli (eg, food, money, social interactions). As with prior habit formation, failure to respond optimally to non-drug reinforcers likely contributes to continued cocaine consumption (Balleine and O'Doherty, 2010). Intriguingly, here computational activity in reward pathways was not influenced by acute cocaine-administered immediately preceding task performance, thus one potentially ‘desirable' (and hypothesized) effect of drug intake (ie enhanced response to reinforcing stimuli) was not achieved by cocaine administration. This may be a critical factor in escalated cocaine use and the persistent cycle of addiction.
It is interesting that CD deficits in reward learning were noted in the dorsal striatum (DS). Such CD decreases in function are in contrast to preclinical and clinical evidence suggesting increased DS activity accompanying cocaine craving (Everitt and Robbins, 2013; Volkow et al 2006). We saw general reductions in DS activity for non-drug reinforcers, which implies that increased function in DS in CD individuals may be cocaine-specific, occurring only during drug craving and perhaps at the cost of processing for normally reinforcing non-drug stimuli. From a processing perspective, it is postulated that the ventral striatum (VS) mediates error prediction learning on passive measures, whereas active learning requires VS and DS (O'Doherty et al, 2004). Thus, CD deficits in learning-related signals in the putamen may reflect a failure of chronic cocaine users to engage in active learning following errors in reward prediction.
More specific CD changes included lower reward-related activity coupled with increased activity for unpredicted negative outcomes. In the insula, mPFC and pre- and post-central gyri the CD group exhibited a blunting of the response to positive outcomes, irrespective of predictability, and enhanced activity following unpredicted negative events. The lack of GROUP-related discrepancies in the perceived pleasantness of the juice suggests that these data do not likely reflect hedonic differences but rather functional distinctions in the salience of reinforcing outcomes. For example, CD individuals may attribute greater salience to unpredicted negative events than their non-CD counterparts, while simultaneously placing less value on positive/rewarding outcomes. Misattributed salience for negative outcomes may compound the lack of risk-averse behavior in cocaine dependence and increase negative emotional sequelae of addiction, thus driving continued cocaine use.
Intriguingly, regions where saliency-related changes were observed, ie mPFC and insula, are those that have an established role in drug craving and seeking behaviors (Naqvi and Bechara, 2010; Van den Oever et al, 2010). The mPFC and insula form part of a network that mediates interoceptive signals and their affective appraisal, and which communicates this information with the striatum and extended amygdala during reward processing. Aberrant processing in this system is suggested to be a critical factor in drug addiction (Verdejo-Garcia et al, 2012). With regards to the current observations, it is probable that CD functional alterations in these regions disrupt reward and decision-making computations in a manner consistent with poor behavioral regulation and misattribution of salience for positive and negative reinforcers in the environment, perhaps due to misinterpretation of interoceptive signals computed in the insula or their emotional evaluation in mPFC.
Although lower reward-related activity in CD individuals appears contradictory to previous evidence of heightened sensitivity to rewards in cocaine dependence (Jia et al, 2011), in contrast to prior investigations, our participants were not undergoing treatment nor were they seeking treatment. It is probable that the motivational value of cocaine varies between those who do and do not wish to stop using, which in turn may reflect dissociation in the state of brain networks mediating reward processes. Furthermore, while we used a primary reward, Jia et al (2011) found CD increases in reward-related function using money as a reward. Money may have very specific value to CD individuals due to its intrinsic association with the ability to obtain cocaine. Thus, the value of money as a reinforcer in CD populations cannot be wholly extricated from the subjective worth of cocaine itself. As a primary reward, juice lacks this inherent connection to cocaine use. The distinction between cocaine dependence's impact on processing for primary and non-primary rewards is supported by work demonstrating blunted activation in CD individuals while viewing erotic material compared with salient drug cues (Garavan et al, 2000).
It is particularly notable that as time since last pre-study use increased, there was a relative increase in reward-related activity and a decrease in activity related to the NTDE event; ie the more reward processes were effectively ‘normalized'. These differences were not simply due to the absolute time since last cocaine administration (ie∼24 h/time since last session (toleration or scanning)), and thus do not appear related to the bioavailability of cocaine or its metabolites. Alternatively, this effect may be attributed to long-term plastic changes at the level of receptor and/or neurotransmitter function that occurs in the days following an extended cocaine binge. However, this is speculative and requires exploration in both human and preclinical models of cocaine dependence.
Limitations
Despite the novelty and strengths of this study, limitations exist. The number of participants was relatively small for an imaging study, thus replication in larger samples would be advantageous, particularly in determining how periods of use and/or abstinence impact upon group differences. Furthermore, despite having reasonably well-matched groups, we are unable to determine whether or not pre-existing differences (eg, genetics, environmental factors) contributed to functional alterations. Also, the dose and timing of cocaine administered did not replicate that seen naturalistically, thus central and peripheral responses (eg, heart rate/blood pressure) that may act as internal cues (Wise et al, 2008; Wise and Kiyatkin, 2011) were likely quite different in the research setting.
Another limitation is the absence of reward learning effects in regions where group differences were observed, which leaves us unable to determine conclusively whether CD and HC subjects differ in reward learning specifically or in other aspects of reward processing eg outcome/receipt. It should also be noted that PTDE events always occurred after the omitted expected reward (ie, >6 s post-cue), which may have impacted the ‘expectedness' of the juice reward on catch trials, and thus the reliability of the brain response to this reward as a true TDE signal. Although it may be possible to avoid this confounding effect by including catch trials in which the unexpected juice reward is given before the completion of the 6-s interval, this may have an adverse effect on the strength of the learning signal and thus the ability to generate NTDE signals. Alternative modeling of the data (eg, TDE equation modeling) may contribute to a more complete pattern of results.
As the TDE paradigm was always performed ∼70 min after the start of scanning and after performing another reward measure, it is possible that participants may have been fatigued, which could have impacted the results. To combat potential fatigue the reward paradigms were interspersed with rest periods (eg, structural scans, resting state fluctuations). Nonetheless, in recognition of the fact that participants may have still experienced some fatigue at the time of the TDE paradigm, the timing of the task relative to the start of testing was consistent across participants in order to somewhat standardize such effects.
CONCLUSION
Our data provide evidence of altered TDE signaling in cocaine dependence in DA pathway regions known to mediate reward processing. Specifically, cocaine dependence was associated with increased sensitivity for unpredicted negative outcomes, coupled with decreased sensitivity for predicted and unpredicted rewarding outcomes. These group-related differences were not influenced by acute cocaine administration. Abnormalities in reward processing in cocaine dependence may be driven by differences in incentive salience processing for positive and negative reinforcers. These acquired effects of cocaine addiction likely contribute to continued and escalating cocaine consumption and high recidivism rates; effects that may be exacerbated by a failure of acute cocaine administration to ameliorate dependence-related deficits.
FUNDING AND DISCLOSURE
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health, USA. The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge Kimberley Slater for her invaluable support and assistance in running this study. We thank Loretta Spurgeon, Eliscia Smith, NIDA-IRP nursing and recruitment staff, and the individuals who volunteered their time to participate.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Asaad WF, Eskandar EN. Encoding of both positive and negative reward prediction errors by neurons of the primate lateral prefrontal cortex and caudate nucleus. J Neurosci. 2011;31:17772–17787. doi: 10.1523/JNEUROSCI.3793-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale—I: item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The Beck Anxiety Inventory. The Psychological Corporation: London, UK; 1993. [Google Scholar]
- Beck AT. The Beck Depression Inventory—II. The Psychological Corporation: London, UK; 1996. [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, et al. Conditioned dopamine release in humans: a positron emission tomography [11c]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Bebbington PE, Stretch DD, MacCarthy B, Wykes T. Predicting the short-term outcome of first episodes and recurrences of clinical depression: a prospective study of life events, difficulties, and social support networks. J Clin Psychiatry. 1997;58:298–306. doi: 10.4088/jcp.v58n0703. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A Guide to its Development and Use. Washington University: Center for Psychobiology of Personality: St Louis, Missouri; 1994. [Google Scholar]
- Cox RW. AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction. Am J Psychiatry. 2007a;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007b;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event- related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Homme D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE. Splitting the difference: how does the brain code reward episodes. Ann NY Acad Sci. 2007;1104:54–69. doi: 10.1196/annals.1390.020. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2007;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Esber GR, Li J, Daw ND, Schoenbaum G. Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain. Eur J Neurosci. 2012;35:1190–1200. doi: 10.1111/j.1460-9568.2011.07986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis M, et al. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71:206–213. doi: 10.1016/j.biopsych.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Ann Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme: New York, NY; 1988. [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechlser Test of Adult Reading (WTAR): Test Manual. Pearson: San Antonio, TX; 2001. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Pearson: San Antonio, TX; 2007. [Google Scholar]
- Wise RA. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Kiyatkin EA. Differentiating the rapid actions of cocaine. Nat Rev Neurosci. 2011;12:479–484. doi: 10.1038/nrn3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.