Abstract
The endocannabinoid (eCB) system regulates mood, emotion, and stress coping, and dysregulation of the eCB system is critically involved in pathophysiology of depression. The eCB ligand 2-arachidonoylglycerol (2-AG) is inactivated by monoacylglycerol lipase (MAGL). Using chronic unpredictable mild stress (CUS) as a mouse model of depression, we examined how 2-AG signaling in the hippocampus was altered in depressive-like states and how this alteration contributed to depressive-like behavior. We report that CUS led to impairment of depolarization-induced suppression of inhibition (DSI) in mouse hippocampal CA1 pyramidal neurons, and this deficiency in 2-AG-mediated retrograde synaptic depression was rescued by MAGL inhibitor JZL184. CUS induced depressive-like behaviors and decreased mammalian target of rapamycin (mTOR) activation in the hippocampus, and these biochemical and behavioral abnormalities were ameliorated by chronic JZL184 treatments. The effects of JZL184 were mediated by cannabinoid CB1 receptors. Genetic deletion of mTOR with adeno-associated viral (AAV) vector carrying the Cre recombinase in the hippocampus of mTORf/f mice recapitulated depressive-like behaviors induced by CUS and abrogated the antidepressant-like effects of chronic JZL184 treatments. Our results suggest that CUS decreases eCB-mTOR signaling in the hippocampus, leading to depressive-like behaviors, whereas MAGL inhibitor JZL184 produces antidepressant-like effects through enhancement of eCB-mTOR signaling.
Keywords: endocannabinoid, monoacylglycerol lipase, depression, hippocampus, DSI, mTOR
INTRODUCTION
The endocannabinoid (eCB) system regulates mood, emotion, memory, cognition, and stress responses via activation of the cannabinoid receptor (CB1; Hill et al, 2009; Litvin et al, 2013; Lutz, 2009; Varvel et al, 2007). The CB1 antagonist rimonabant increases the incidence of anxiety and depression in clinical trials for the treatment of obesity (Samat et al, 2008), whereas cannabis improves mood in humans (Denson and Earleywine, 2006) and synthetic CB1 agonists produce anxiolytic- and antidepressant-like effects in animal models (Berrendero and Maldonado, 2002; Jiang et al, 2005; Patel and Hillard, 2006; Valjent et al, 2002). Thus, the eCB system represents a promising target for antidepressant medications (Hill et al, 2009). However, direct CB1 agonists may cause unwanted psychotropic effects owing to their indiscriminant activation of CB1 receptors. Inhibitors of eCB degradation hold great promise as therapeutic agents, because they preserve the normal spatial and temporal pattern of CB1 receptor activation.
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are two known endogenous ligands for CB1 receptors. AEA is hydrolyzed by fatty acid amide hydrolase (FAAH), while 2-AG is hydrolyzed primarily by monoacylglycerol lipase (MAGL) (Blankman et al, 2007; Cravatt et al, 1996). FAAH and AEA transport inhibitors produce antidepressant and anxiolytic effects in rodents (Bortolato et al, 2007; Busquets-Garcia et al, 2011; Gobbi et al, 2005; Patel and Hillard, 2006), providing proof of concept that inhibition of eCB degradation produces antidepressant-like effects. MAGL and FAAH are distributed on different neuronal types and subcellular compartments (presynaptic and postsynaptic, respectively) (Cravatt et al, 2001; Tsou et al, 1998). Dysregulation of 2-AG signaling is specifically implicated in human and animal models of depression (Eisenstein et al, 2010; Hill et al, 2008). Studies of the role of 2-AG in mood disorders have only recently became possible with the development of highly selective and potent MAGL inhibitor JZL184 (Long et al, 2009a). Inhibition of 2-AG degradation with MAGL inhibitor JZL184 induces anxiolytic-like effects in marble burying (Kinsey et al, 2011), novelty-suppressed feeding (Sumislawski et al, 2011), and elevated zero/plus maze assays (Busquets-Garcia et al, 2011; Sciolino et al, 2011). However, the consequences of MAGL inhibition on depressive-like behaviors, to our knowledge, have not been examined in any animal models of depression. Chronic unpredictable mild stress (CUS) (Willner et al, 1987) is an animal model that captures core symptoms of depression (Duman, 2007). We sought to understand (1) whether CUS altered eCB-mediated responses in the hippocampus, a brain structure that is critically involved in the pathophysiology of depression (Warner-Schmidt and Duman, 2006); and (2) whether blocking 2-AG inactivation with MAGL inhibitor JZL184 affected CUS-induced alterations of eCB signaling and depressive-like behaviors.
The activation of mammalian target of rapamycin (mTOR) signaling is required for rapid antidepressant action of NMDA receptor antagonist ketamine (Li et al, 2010) and group II/III mGluR antagonist LY341495 (Dwyer et al, 2012). CB1 receptor agonists activate mTOR in the hippocampus and other brain regions, and this signaling pair has been recently implicated in learning, memory, anxiety, and fragile X syndrome (Busquets-Garcia et al, 2013; Busquets-Garcia et al, 2011; Puighermanal et al, 2009). We hypothesized that CB1 receptor-mediated activation of mTOR mediates the antidepressant action of JZL184. To test this hypothesis, we examined whether JZL184 activated mTOR signaling pathway in the hippocampus and whether genetic deletion of mTOR in the hippocampus affected the behavioral effects of JZL184. The present study provided a potential mechanism that mediates antidepressant-like actions of MAGL inhibitor JZL184.
MATERIALS AND METHODS
CUS Paradigm and Drug Treatments
Male C57BL/6J mice (8–10 weeks of age, Jackson Laboratory) were habituated for 1 week. CUS mice were exposed to various stressors for 5 weeks, and time-matched control mice did not receive any stressors. The stressors included restraint, inversion of day/night light cycle, cold room, tilted cage, cage rotation, rat bedding (odor), wet bedding, no bedding, strobe, food and water deprivation, and overcrowding, following published CUS paradigms (Koo and Duman, 2008; Willner et al, 1987). On average, two stressors were administered per day. The stressors and timeline of CUS have been detailed in Supplementary Information and Supplementary Table S1. Animal maintenance and use were in accordance with protocols approved by the Institutional Animal Care and Use Committee of Medical College of Wisconsin.
Slice Preparation and Electrophysiology
The following day after the last overnight stressor (tilted cage), CUS and time-matched control mice were killed, hippocampal slices were prepared for electrophysiology, and hippocampal tissue samples were collected for measuring 2-AG tissue content and western blotting (see below). Mice were anesthetized by isoflurane inhalation and decapitated. Hippocampal slices (250 μm) and whole-cell recordings were made as described previously (Pan et al, 2009). Slices were stored in artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose at room temperature. All solutions were saturated with 95% O2 and 5% CO2. Evoked inhibitory postsynaptic currents (IPSCs) were recorded from hippocampal CA1 pyramidal neurons, while electrical stimulation was delivered by a bipolar tungsten stimulation electrode (WPI) that was placed in the striatum radiatum of the CA1 region, using square pulses (duration, 100 μs; intensity, ∼50 μA; interval, 4–10 s). Glutamate receptor antagonists CNQX (20 μM) and D-AP-5 (50 μM) were present in the ACSF. The internal solution in patch pipettes contained (in mM): 80 Cs-methanesulfonate, 60 CsCl, 2 QX-314, 10 HEPES, 0.2 EGTA, 2 MgCl2, 4 MgATP, 0.3 Na2GTP, and 10 Na2-phosphocreatine (pH 7.2 with CsOH). To induce DSI (depolarization-induced suppression of inhibition), cells were depolarized from −60 to 0 mV for 5 s, and IPSCs were evoked at 4-s intervals. All recordings were performed at 32±1 °C by using an automatic temperature controller (Warner Instrument, Hamden, CT). Series resistance (15–30 MΩ) was monitored throughout the recordings, and data were discarded if the resistance changed by >20%.
Biochemical Detection of 2-AG
Control and CUS mice were anesthetized by isoflurane inhalation and decapitated. The brain was immediately removed, and the hippocampi were dissected out and rapidly frozen on dry ice. 2-AG was extracted from the hippocampus as previously described (Wang et al, 2010). Samples were weighed and placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 186 pmol [2H8]2-AG. They were homogenized with a round-bottomed rod and sonicated in an ice-cold water bath for 30 min. Samples were incubated overnight at −20 °C to precipitate proteins and subsequently centrifuged at 1500 g for 3 min. The supernatants were transferred to a new glass tube and evaporated to dryness under N2 gas. The samples were re-suspended in 300 ml of methanol to recapture any lipids adhering to the glass tube and dried again under N2 gas. Dried lipid extracts were suspended in 20 ml of methanol and stored at −80 °C until analysis. The content of 2-AG was determined using isotope-dilution liquid chromatography–electrospray ionization tandem mass spectrometry (LC-MS/MS) (Patel et al, 2003).
Western Blotting
Control and CUS mice were anesthetized with isoflurane and rapidly decapitated. The hippocampi were dissected out and then homogenized in 0.2 ml lysis buffer (pH 7.6), and western blots were carried out as described previously (Yu et al, 2013) and in Supplementary Information.
Behavior
Open field test (OPT)
Mice were placed individually in one corner of the open field (50 cm length × 45 cm wide × 30 cm deep box) and allowed to freely explore the arena during a 20-min test session. Time in center is defined as the amount of time that was spent in the central 25 × 22.5 cm2 area of the open field.
Sucrose-preference test (SPT)
Mice were individually housed and were trained two times (at the start and completion of the CUS) to drink from two bottles that contained 1% sucrose solution and tap water, respectively, for 24 h. During the SPT, mice were deprived of food and water for 8 h, and the consumption of sucrose solution and water over the next 16 h was measured. The sucrose preference (%) was calculated as sucrose solution consumed divided by the total amount of solution consumed.
Novelty-suppressed feeding (NSF)
The NSF was carried out similar to a published protocol (Santarelli et al, 2003). Mice were deprived of food for 24 h before being placed in a novel environment (a plastic box 45 cm long × 35 cm wide × 20 cm deep) where five food pellets (regular chow) were placed on a piece of white filter paper (11 cm in diameter) in the center of the box. A mouse was placed in one corner of the box and the latency to feed was measured. Feeding was defined as biting not simply sniffing or touching the food. Immediately after the test, the animal was transferred to the home cage, and the latency to feed in the home cage was measured to serve as controls.
Forced swim test (FST)
Mice were placed individually into glass cylinders (13 cm diameter, 25 cm tall) filled to a depth of 18 cm with water (25±1 °C). The mice were placed in the cylinders for 6 min. The time spent immobile during the last 4 min was scored by an observer blind to treatment conditions. Immobility was defined as the cessation of all movements (eg, climbing, swimming) except those necessary for the mouse to keep its head above water (ie, floating).
Intra-Hippocampus Microinjection of Adeno-Associated Virus (AAV)
ROSA26 mice (Jax stock no.: 003474), mTORf/f mice (Jax stock no.: 011009), and C57BL/6J mice were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed in a stereotaxic device (David Kopf Instruments, Tujunga, CA) for surgery. AAV2-Cre-GFP or AAV2-GFP (Penn Vector Core, Philadelphia, PA) was bilaterally microinjected into the hippocampus (0.5 μl per side) at four injection sites with the following coordinates (Paxinos and Franklin, 2001; site1: AP, −2.0 mm; ML, ±1.8; DV, −1.8; site 2: AP, −3.0, ML, ±1.8; DV, −2.8). The animals were allowed to recover for at least 3 weeks before histological and behavioral experiments.
X-Gal Staining
Three weeks after the AAV microinjection, ROSA26 mice were deeply anesthetized by pentobarbital sodium (100 mg/kg, i.p.) and transcardially perfused with 0.1 M sodium phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 4% sucrose-PBS (pH 7.4). After perfusion, the brain was removed and fixed in the same fixative for 1/2 h at 4 °C and was then dehydrated in increasing concentrations of sucrose (20 and 30%) in 0.1 M PBS at 4 °C and frozen on dry ice. Coronal hippocampal sections were made at 30-μm thickness with a cryostat. X-gal staining was done by overnight incubation at 37 °C in PBS solution containing 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6 3H2O, and 1 mg/ml X-Gal. The sections were visualized by using a Nikon Eclipse 80i microscope.
Immunofluorescence Staining
mTORf/f and control mice were transcardially perfused with the fixative as described above, except that brains were post-fixed for 4 h at 4 °C. Coronal hippocampal sections (20 μm) were incubated with primary antibodies against Neuronal Nuclei (NeuN; 1 : 500, Millipore, Billerica, MA) at 4 °C for 48 h. After washing, sections were incubated with goat anti-rabbit IgG-Texas Red (1 : 200, Santa Cruz, Dallas, TX) for 4 h at room temperature. The sections were analyzed by using a Nikon Eclipse TE-2000U confocal microscope. NeuN-immunoreactive or AAV-expressing neurons in the CA1 region of hippocampus from both hemispheres (approximately between 2.0 and 3.0 mm posterior to bregma) were manually counted in two sections from each animal. The coordinates were determined by comparing the brain structures in hippocampal sections with those in mouse stereotaxic atlas (Paxinos and Franklin, 2001).
Chemicals
JZL184 was synthesized in the laboratory of Benjamin Cravatt (Long et al, 2009a). CNQX-Na2, D-AP-5, and all other common chemicals were purchased from Sigma-Aldrich (St Louis, MO). Rimonabant (SR141716A) was obtained from Sanofi-Aventis (Bridgewater, NJ), and WIN55 212-2 was obtained from Tocris Bioscience (Ellisville, MO).
Data Analysis and Statistics
All results are expressed as mean±SEM. The decay time constant (τ) and magnitude of DSI and the depression (%) of IPSCs by CB1 agonists WIN55 212-2 were measured as we have described (Pan et al, 2009). Results for electrophysiology and biochemical assay were analyzed by Student's t-test. Behavioral test results were analyzed by Student's t-test, one-way, or two-way ANOVA followed by Tukey post hoc analysis. The F values and group and experimental degrees of freedom are included in the Results section. Results were considered to be significant at p<0.05.
RESULTS
CUS Decreased eCB-mTOR Signaling in the Hippocampus
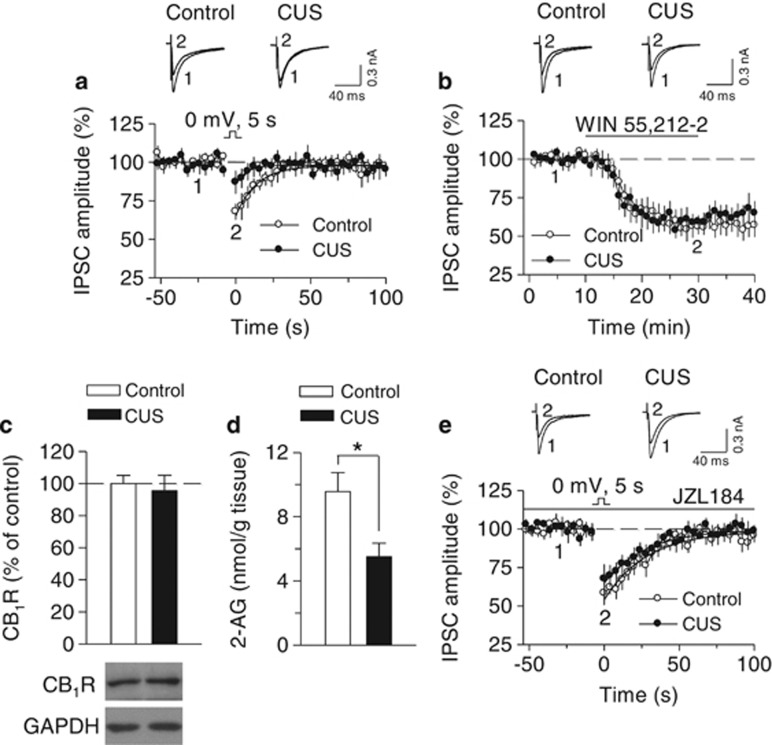
Mice were exposed to CUS for 5 weeks (see Supplementary Table S1). The following day after the last overnight stressor (tilted cage), CUS and time-matched control mice were killed, hippocampal slices were prepared for electrophysiology, and hippocampal tissue samples were collected for measuring 2-AG tissue content and western blotting (see below). DSI is mediated by 2-AG-induced activation of CB1 receptors (Gao et al, 2010; Pan et al, 2009; Tanimura et al, 2010). Measuring DSI provides a good indication of functional status of eCB signaling in the brain. We examined whether DSI in the hippocampus was altered in CUS mice. Whole-cell recordings were made from CA1 pyramidal neurons and DSI was induced by a brief depolarization (5 s) from −60 to 0 mV. We found that the decay time constant (τ) (control, 14.1±1.8 s, n=10; CUS, 7.5±1.4 s, n=11; t19=2.92, p<0.01) and magnitude (control, 27.9±4.0%, n=10; CUS, 9.9±2.1%, n=11; t19=4.09, p<0.001) of DSI were significantly decreased in CUS-exposed mice compared with those in unstressed control mice (Figure 1a).
Figure 1.
Selective MAGL inhibitor JZL184 rescued CUS-induced deficit in DSI in hippocampal CA1 pyramidal neurons. (a) CUS significantly decreased the decay time constant (τ) (n=10–11, p<0.01) and magnitude (p<0.001) of DSI, which was induced by 5-s depolarization from −60 to 0 mV. Sample traces of IPSCs are superimposed on the top. The solid lines are single exponential fitting curves of the decay of DSI. (b) Bath application of the CB1 receptor agonist WIN55212–2 (5 μM) induced similar depression of IPSCs in CA1 pyramidal neurons in hippocampal slices prepared from control and CUS-exposed mice (n=9–9; p>0.05). (c) Representative western blots (bottom) and summarized data (top) showed that CUS did not significantly alter protein levels of CB1 receptor (CB1R) in the hippocampus (p>0.05; n=5 animals each group). Immunoreactivity was normalized to GAPDH and presented as percentage of time-matched control mice. (d) CUS decreased the tissue content of 2-AG in the hippocampus (n=6 mice each group; *p<0.05). (e) Bath application of JZL184 (1 μM) potentiated DSI in control and CUS-exposed mice. DSI in these two groups was not significantly different in the presence of JZL184 (n=7–8, p>0.05).
The CUS-induced impairment in DSI could be explained by a decrease in CB1 receptor responsiveness and/or 2-AG availability. To distinguish between the two possibilities, we first examined the effect of application of a saturating concentration of CB1 agonist WIN55212-2 (5 μM) on evoked IPSCs in CA1 pyramidal neurons in control and CUS-exposed mice. A change of the CB1 receptor agonist-induced depression of IPSCs would suggest a change in CB1 receptor responsiveness. However, we found that WIN55212-2 induced similar depression of IPSCs in control and CUS mice (n=9–9, t16=0.25, p>0.05; Figure 1b). Next, we measured CB1 receptor protein expression using western blotting. There was no significant difference of CB1 receptor protein levels between the control and CUS groups (t8=0.41, p>0.05; Figure 1c). Finally, we used LC-MS/MS to measure tissue contents of 2-AG in the hippocampus dissected from these two groups of mice. 2-AG contents were significantly decreased in CUS-exposed mice compared with those of control mice (t10=2.81, p<0.05; Figure 1d). Together, these results suggest that 2-AG availability, but not CB1 receptor density/sensitivity, was decreased in CUS-exposed mice.
2-AG is hydrolyzed primarily by MAGL (Blankman et al, 2007; Cravatt et al, 1996), while JZL184 is a selective and potent MAGL inhibitor that increases 2-AG levels in the brain (Long et al, 2009a; Pan et al, 2009). We determined whether JZL184 could restore DSI in CUS mice. Bath application of JZL184 (1 μM) prolonged the decay time constant and enhanced the magnitude of DSI in CA1 pyramidal neurons in both control and CUS-exposed mice (Figure 1e), and DSI was not significantly different between these two groups of mice in the presence of JZL184 (τ: control, 28.6±3.7 s, n=7; CUS, 27.8±4.5 s, n=8; t13=0.14, p>0.05; magnitude: control, 39.8±5.1%, n=7; CUS, 30.2±4.2%, n=8; t13=1.47, p>0.05; Figure 1e). Thus, JZL184 rescued CUS-induced deficit in DSI in CA1 pyramidal neurons.
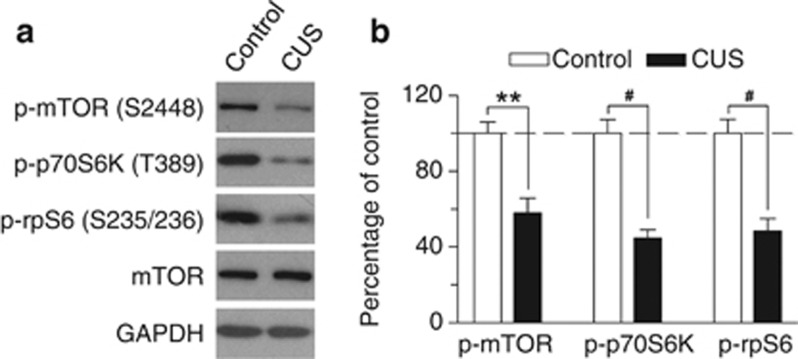
CB1 receptor agonists activate mTOR in the hippocampus (Puighermanal et al, 2009). To determine whether CUS-induced deficiency in 2-AG signaling was associated with alterations of mTOR activation in the hippocampus, we performed western blotting to detect phosphorylated (active) mTOR and its downstream effectors. mTOR phosphorylates its downstream target, the 70-kDa ribosomal protein S6 kinase (p70S6K), at T389 site (Jefferies et al, 1997). The activated p70S6K phosphorylates its target substrate ribosomal protein S6 (rpS6) at S235/236 site, which initiates mRNA translation (Proud, 2007). Western blotting was performed using antibodies against p-mTOR (S2448), p-p70S6K (T389) and p-rpS6 (S235/236). We found that CUS significantly decreased protein levels of p-mTOR (S2448) (t8=4.29, p<0.01), p-p70S6K (T389) (t8=6.254, p<0.001), and p-rpS6 (S235/236) (t8=5.26, p<0.001) compared with those of control group (Figure 2). Thus, the CUS-induced decrease in 2-AG signaling was accompanied by a decrease in mTOR activation in the hippocampus.
Figure 2.
CUS altered mTOR signaling in the hippocampus. (a) Representative western blots and (b) summarized data showed that CUS significantly decreased p-mTOR (S2448), p-p70S6K (T389), and p-rpS6 (S235/236) in the hippocampus (**p<0.01, #p<0.001; n=5 animals each group). Immunoreactivity was normalized to GAPDH and presented as percentage of time-matched control mice.
Chronic JZL184 Treatments Produced Antidepressant-Like Behavioral Effects in CUS Model of Depression
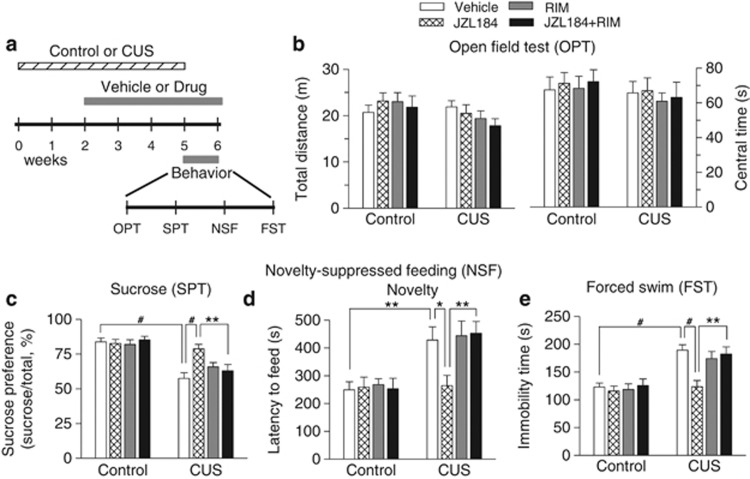
Having shown that CUS decreased 2-AG levels and 2-AG-mediated synaptic depression in the hippocampus, we next examined whether enhancing 2-AG signaling with MAGL inhibitor JZL184 affected depressive-like behavior in a CUS model of depression. Mice were exposed to CUS for a total of 5 weeks. At the beginning of the third week, CUS-exposed mice and time-matched control mice were given i.p. injections of one of the followings every 2 days for 4 weeks: (1) vehicle; (2) JZL184 (8 mg/kg); (3) rimonabant (2 mg/kg); and (4) JZL184+rimonabant. Behavioral tests were carried out in the last week with vehicle or JZL184/rimonabant treatments. The time course of stress exposure, drug treatment, and behavioral tests is shown in Figure 3a. The dose and treatment time of JZL184 were chosen based on previous studies showing that JZL184 irreversibly inhibits MAGL and produces at least two-fold increase in 2-AG levels in the brain at a dose of 8 mg/kg when dissolved in the vehicle used in this study (Kinsey et al, 2013; Long et al, 2009a; Long et al, 2009b; Sumislawski et al, 2011). Repeated administration of JZL184 at this low dose does not induce apparent CB1 receptor desensitization and functional tolerance (Kinsey et al, 2013).
Figure 3.
Chronic JZL184 treatment blocked CUS-induced depressive-like behaviors. (a) Timeline of the CUS exposure, drug treatment, and behavioral tests. (b) Neither CUS nor chronic JZL184 (JZL) treatment affected the total distance traveled (p>0.05) and on time in center (p>0.05) during the first 5-min test session in the OPT. (c) CUS decreased the sucrose preference in the SPT (#p<0.001; Tukey's post hoc test), JZL184 treatment restored CUS-induced reduction of the sucrose preference (#p<0.001), and the effects of JZL184 were blocked by the CB1 antagonist rimonabant (RIM) (**p<0.01). (d) CUS increased the latency to feed in the novel environment in the NSF test (**p<0.01); JZL184 treatment decreased the latency to feed (*p<0.05), which was blocked by rimonabant (**p<0.01). (e) CUS significantly increased the immobility time in the FST (#p<0.001); JZL184 treatment increased the immobility time in CUS-exposed mice (#p<0.001) but not in control mice. The effects of JZL184 was blocked by rimonabant (**p<0.01). n=11–12 animals each group. OPT, open field test; SPT, sucrose-preference test; NSF, novelty-suppressed feeding; FST, forced swim test; Novelty, novel environment.
OPT was used to determine whether CUS induced abnormalities in locomotor activity and anxiety-related behavior. Mice tend to avoid open spaces when exposed to an open field arena. Reduced activity in the center of an open field has been correlated with anxiety- and depression-like behaviors in rodents (El Yacoubi et al, 2003). However, we found that neither CUS nor chronic JZL184/rimonabant treatment altered the total distance traveled (CUS: F1,84=3.30, p>0.05; drug treatment: F3,84=0.46, p>0.05; CUS × drug treatment: F3,84=0.92, p>0.05) and time in center (CUS: F1,84=1.29, p>0.05; drug treatment: F3,84=0.15, p>0.05; CUS × drug treatment: F3,84=0.11, p>0.05) during the first 5-min test session in the OPT (Figure 3b).
Anhedonia is a core symptom of depression (Duman, 2007), which can be assessed with the SPT. Two-way ANOVA showed that CUS (F1,84=50.26, p<0.001) and drug treatment (F3,84=3.18, p<0.05) had significant effects on the sucrose preference, and there was a significant CUS by drug treatment interaction (F3,84=4.26, p<0.01; Figure 3c). Tukey's post hoc tests showed that CUS decreased the sucrose preference (p<0.001), JZL184 treatment restored the sucrose preference in CUS-exposed mice (p<0.001), and the effect of JZL184 was blocked by the CB1 antagonist rimonabant (p<0.01).
The NSF test has been used to measure depression and anxiety (Santarelli et al, 2003). In the NSF test, food pellets are placed in an open field, and a fasting mouse faces the choice between eating and avoiding the novel environment. The increase in the latency to feed indicates reduced interest in novel environment, a common feature of depression. CUS and drug treatment significantly changed the latency to feed in the novel environment in the NSF test (CUS: F1,84=25.56, p<0.001; drug treatment: F3,84=3.35, p<0.05; CUS × drug treatment: F3,84=2.74, p<0.05; Figure 3d). Tukey's post hoc tests showed that chronic JZL184 treatment reversed CUS-induced increase in the latency to feed (p<0.05) but did not affect the latency to feed in control mice (p>0.05), and the effect of JZL184 was blocked by rimonabant (p<0.01). In contrast, neither CUS nor JZL184 treatment affected the latency to feed in the home cage (CUS: F1,84=1.30, p>0.05; drug treatment: F3,84=0.06, p>0.05; CUS × drug treatment: F3,84=1.27, p>0.05; Supplementary Figure S1). Thus, the effects of JZL184 on the latency to feed in the novel environment cannot be explained by possible changes in appetite.
Finally, we used FST to detect depression-like behavior (Porsolt et al, 1977). CUS and drug treatment significantly altered the immobility time in the FST (CUS: F1,84=37.84, p<0.001; drug treatment: F3,84=5.07, p<0.01; CUS × drug treatment: F3,84=3.14, p<0.05; Figure 3e). Tukey's post hoc tests showed that JZL184 reversed CUS-induced increase in the immobility time in the FST (p<0.001), and this effect of JZL184 was blocked by rimonabant (p<0.01).
CUS and Chronic JZL184 Treatments Altered mTOR and Extracellular Signal-Regulated Protein Kinase (ERK) Signaling and Protein Translation Machinery
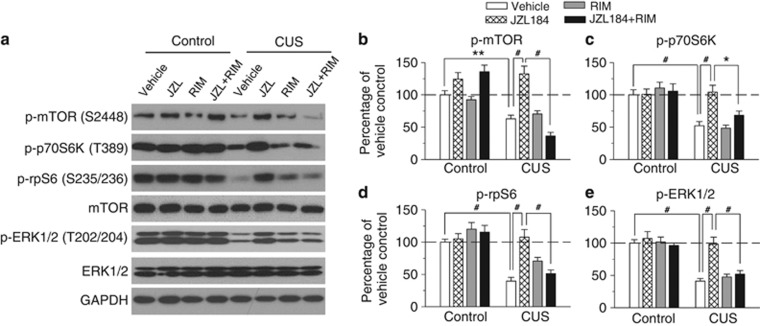
NMDA receptor antagonist ketamine produces rapid antidepressant-like effects via mTOR (Li et al, 2010). We investigated whether CUS and chronic JZL184 treatment altered mTOR signaling in the hippocampus. After the completion of behavioral tests, the mice showed in Figure 3 were euthanized, and the hippocampi were rapidly dissected out. Western blotting was performed using antibodies against p-mTOR (S2448). Two-way ANOVA revealed that CUS and JZL184 treatments had significant effects on p-mTOR (S2448) levels (CUS: F1,40=45.39, p<0.001; drug treatment: F3,40=16.75, p<0.001; CUS × drug treatment: F3,40=16.46, p<0.001). Tukey's post hoc tests showed that CUS decreased p-mTOR (p<0.01) levels in the hippocampus, which were reversed by JZL184 treatments (p<0.001). The effects of JZL184 were blocked by rimonabant (p<0.001; Figure 4a and b).
Figure 4.
CUS and chronic JZL184 treatments altered mTOR and ERK signaling in the hippocampus. (a) Representative western blots for p-mTOR (S2448), p-P70s6k (T389), p-rpS6 (S235/236), total mTOR, p-ERK1/2 (T202/204), and total ERK1/2 from the same groups of mice shown in Figure 3. (b–e) Summarized data showed that CUS significantly decreased p-mTOR (b), p-p70S6K (c), p-rpS6 (d), and p-ERK1/2 (e) in the hippocampus, and these decreases were reversed by JZL184 treatments. CB1 receptor antagonist rimonabant blocked the effects of JZL184 treatments. The p values for Tukey's post hoc test results are shown on the top (*p<0.05, **p<0.01, #p<0.001; n=6 animals each group). Immunoreactivity was normalized to GAPDH and presented as the percentage of the control group with vehicle treatment.
As mentioned earlier, p70S6K and rpS6 are two downstream effectors of mTOR (Jefferies et al, 1997; Proud, 2007). CUS and JZL184 treatments had significant effects on p-p70S6K (T389) (CUS: F1,40=36.56, p<0.001; drug treatment: F3,40=3.94, p<0.05; CUS × drug treatment: F3,40=5.62, p<0.01) and p-rpS6 (S235/236) levels (CUS: F1,40=52.86, p<0.001; drug treatment: F3,40=7.16, p<0.001; CUS × drug treatment: F3,40=6.89, p<0.001). Tukey's post hoc test showed that the levels of p-p70S6K and p-rpS6 were significantly decreased in CUS-exposed mice compared with those of control mice (p<0.001 for both p-p70S6K and p-rpS6), and these decreases were reversed by JZL184 treatments (p<0.001 for both p-p70S6K and p-rpS6). Rimonabant blocked the effects of JZL184 (p<0.05 for p-p70S6K, p<0.001 for p-rpS6; Figure 4a, c and d).
ERK1/2 activation is decreased in the hippocampus of postmortem brain from patients with major depressive disorder (MDD; Duric et al, 2010). We examined whether CUS and JZL184 treatments affected phosphorylated ERK1/2 levels at T202/204 site (p-ERK1/2) in the hippocampus. Two-way ANOVA indicated that CUS and JZL184 treatments had significant effects on p-ERK (T202/204) (CUS: F1,40=68.98, p<0.001; drug treatment: F3,40=8.88, p<0.001), and there was a significant interaction between CUS exposure and JZL184 treatments (F3,40=5.10, p<0.01). Tukey's post hoc test showed that CUS decreased p-ERK (p<0.001) in the hippocampus, which was reversed by JZL184 treatments (p<0.001). The effect of JZL184 treatments on p-ERK was blocked by rimonabant (p<0.001; Figure 4a and e). These results indicate that CUS exposure caused abnormalities in mTOR/ERK signaling in the hippocampus, while JZL184 treatments corrected the CUS-induced deficits.
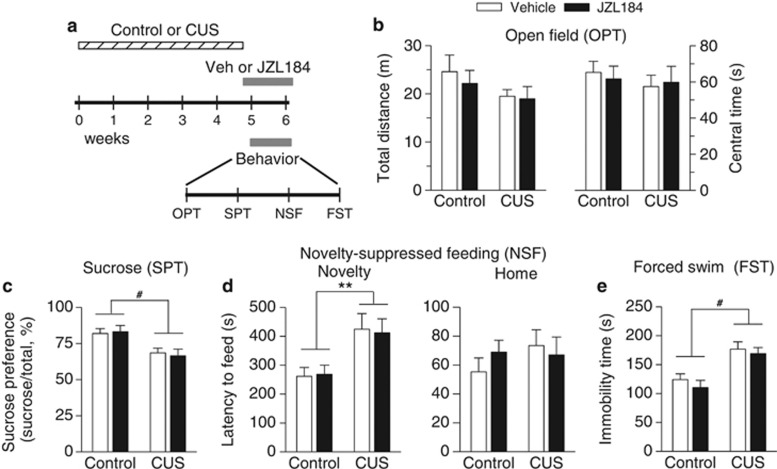
Subchronic JZL184 Treatment did not Affect CUS-Induced Depressive-Like Behavior
We also examined the effects of subchronic JZL184 treatment on CUS-induced depressive-like behavior. Mice were exposed to CUS for a total of 5 weeks. One day before the behavioral tests, CUS-exposed mice and time-matched control mice were given i.p. injections of vehicle or JZL184 (8 mg/kg). The injections were made once every 2 days during the period of behavioral tests (Figure 5a). We found that neither CUS nor subchronic JZL184 treatment altered total distance traveled (CUS: F1,28=2.50, p>0.05; JZL184 treatment: F1,28=0.33, p>0.05) and time in center of the OPT (CUS: F1,28=0.46, p>0.05; drug treatment: F1,28=0.01, p>0.05; Figure 5b). Subchronic JZL184 treatment did not significantly alter CUS-induced anxiety- and depressive-like behaviors, SPT (CUS: F1,28=14.58, p<0.001; JZL184 treatment: F1,28=0.01, p>0.05; Figure 5c), NSF (novelty: CUS: F1,28=13.07, p<0.01; JZL184 treatment: F1,28=0.003, p>0.05; Figure 5d), and FST (CUS: F1,28=23.56, p<0.001; JZL184 treatment: F1,28=0.89, p>0.05; Figure 5e).
Figure 5.
Subchronic JZL184 treatment did not alter anxiety- and depressive-like behaviors induced by CUS. (a) Timeline for CUS exposure, JZL184 treatment, and behavioral tests. (b) Neither CUS nor acute JZL184 treatment affected the total distance traveled (p>0.05) and the time in center (p>0.05) during the first 5-min test session in the OPT. (c–e) CUS significantly changed the sucrose preference (#p<0.001) in the SPT (c), the latency to feed in the novel environment (**p<0.01) in the NSF test (d), and the immobility time (#p<0.001) in the FST (e). However, subchronic JZL184 treatment did not reverse these CUS-induced behavioral alterations. Neither CUS nor JZL184 treatment affected the latency to feed in the home cage in the NSF test (d) (p>0.05, n=8 animals each group).
Hippocampus-Specific Deletion of mTOR Produced Depressive-Like Behaviors and Abrogated the Antidepressant-Like Effects of JZL184 Treatments
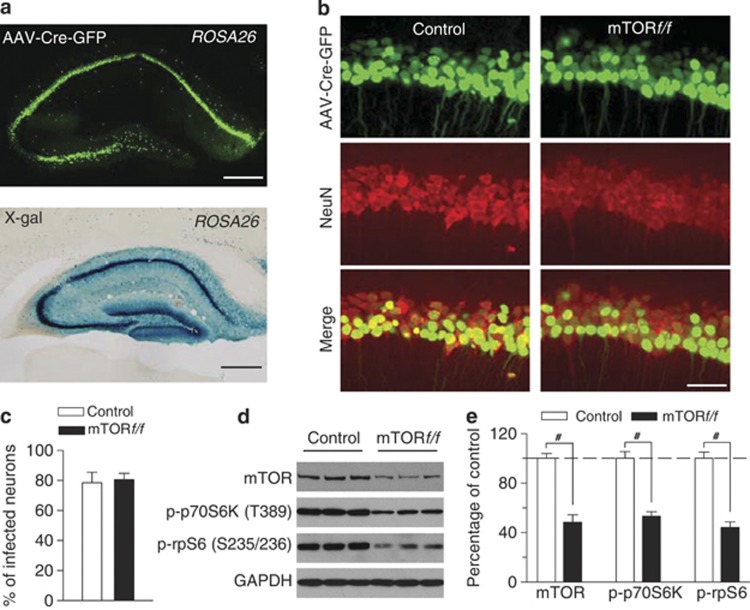
We have shown that CUS decreased mTOR activation in the hippocampus (Figures 2 and 4). To determine whether the impairments of hippocampal mTOR signaling contribute to CUS-induced depressive-like behavior, we used Cre recombinase-expressing AAV type 2 (AAV2-Cre-GFP) to selectively delete mTOR in the hippocampus and examine its effects on depressive-like behavior. To evaluate the effectiveness of the Cre recombinase in the AAVs, we first injected AAV2-Cre-GFP bilaterally into the hippocampi of LacZ reporter mice carrying the reporter cassette in the ROSA 26 locus. Each mouse received four injections that targeted rostral and caudal hippocampus bilaterally (see Materials and Methods). After recovery for 3 weeks, immunofluorescence staining and X-gal staining of brain sections revealed the presence of β-galactosidase in entire hippocampi, including CA1, CA3, and dentate gyrus (Figure 6a), indicating that the AAVs are expressed in all subfields of the hippocampus and Cre recombinase in the viral vector is very effective. We then injected AAV2-Cre-GFP into the hippocampus bilaterally in homozygous mTOR-floxed mice (mTORf/f) and control mice (C57BL/6J), using the same injection procedure. Immunofluorescence staining for NeuN (neuronal marker) indicated that ∼80% of hippocampal CA1 pyramidal neurons expressed AAV2-Cre-GFP in both mTORf/f and control mice 3 weeks after the AAV injection (Figure 6b and c). Western blotting analysis of hippocampal tissues showed that injection of AAV2-Cre-GFP significantly decreased protein level of mTOR in mTORf/f mice (p<0.001; Figure 6d and e). In addition, p-p70S6K (T389) and p-rpS6 (S235/236) levels were significantly decreased in mTORf/f mice (p<0.001; Figure 6d and e). These results further confirmed the effectiveness of AAV2-Cre-GFP in deleting mTOR in the hippocampus.
Figure 6.
AAV2-Cre-GFP-mediated deletion of mTOR in the hippocampus. (a) X-gal staining of hippocampus following intra-hippocampus microinjections of AAV2-Cre-GFP. AAV2-mediated Cre recombinase expression was labeled by LacZ (blue) when injected into Rosa26 reporter mice in the hippocampus. Scale bar: 0.5 mm. (b, c) Immunofluorescence staining for Neuronal Nuclei (NeuN; neuronal marker) and AAV2-Cre-GFP (green) in the hippocampus. Representative images (b) and summarized data (c) showed that AAV2-Cre-GFP infected ∼80% of all hippocampal CA1 pyramidal neurons in both control and mTORf/f mice (n=3 animals each group). Scale bar: 50 μm. (d and e) Representative (d) and summarized data (e) of western blots showed that intra-hippocampal microinjection of AAV2-Cre-GFP significantly decreased protein levels of mTOR, p-p70S6K (T389), and p-rpS6 (S235/236) in the hippocampus (#p<0.001; n=6 animals each group). Immunoreactivity was normalized to GAPDH and presented as the percentage of that of the control mice.
To examine whether mTOR deletion in the hippocampus affects eCB signaling, we compared DSI in CA1 pyramidal neurons in hippocampal slices prepared from control and mTORf/f mice that received intra-hippocampus injection of AAV2-Cre-GFP. We found that DSI in AAV2-Cre-GFP-expressing neurons was not significantly different between the control and mTORf/f groups (Supplementary Figure S2). These results suggest that mTOR deletion does not affect DSI in the hippocampus.
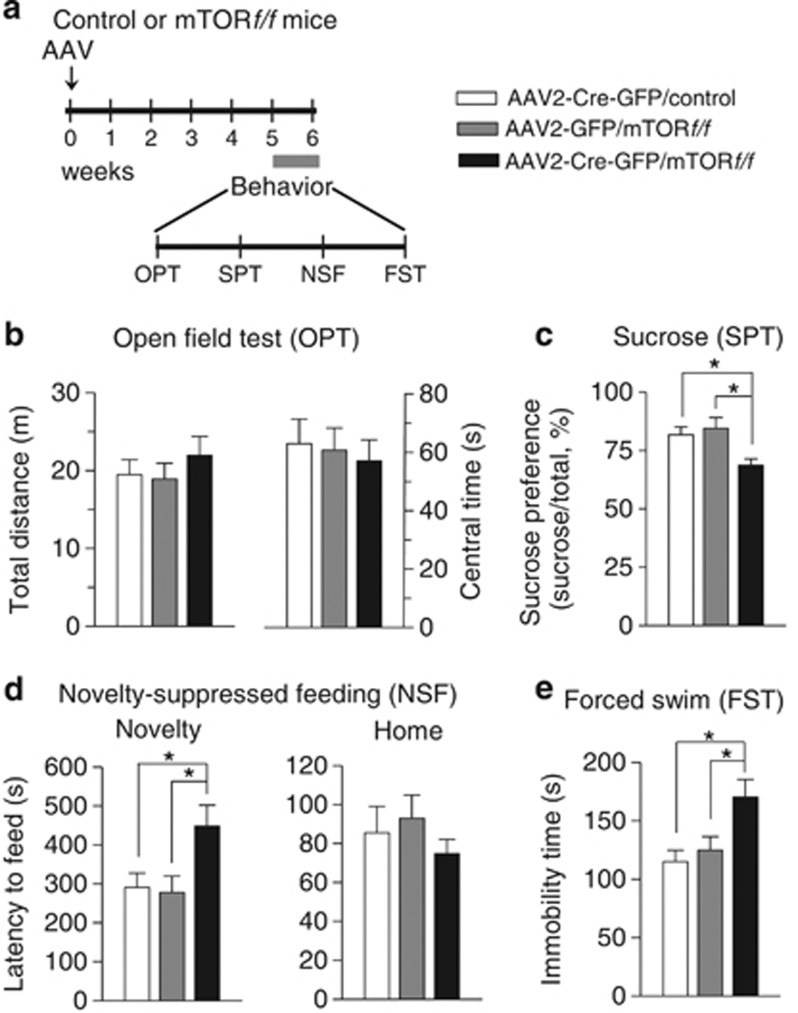
We examined the effect of hippocampus-specific deletion of mTOR on depression-related behavior. AAV2-Cre-GFP was bilaterally injected into the hippocampi of control and mTORf/f mice as described above. In an additional control experiment, AAV2-GFP was bilaterally injected into the hippocampi of mTORf/f mice. The time course of the AAV injection and behavioral tests is shown in Figure 7a. The mice in which mTOR was deleted in the hippocampus appeared grossly normal. A one-way ANOVA revealed that hippocampus-specific deletion of mTOR did not significantly affect locomotor activity (F2,27=0.58, p>0.05) and time in center in the OPT (F2,27=0.16, p>0.05; Figure 7b) but caused a significant decrease in the sucrose preference (F2,27=5.82, p<0.01; Figure 7c) and significant increases in the latency to feed in the novel environment in the NSF test (F2,27=4.54, p<0.05; Figure 7d) and the immobility time in the FST (F2,27=5.36, p<0.05; Figure 7e). However, mTOR deletion did not affect the latency to feed in the home cage in the NSF test (F2,27=0.70, p>0.05; Figure 7d). These results suggest that mTOR deletion induced depressive-like behavior and recapitulated CUS-induced behavioral changes.
Figure 7.
Hippocampus-specific deletion of mTOR produced anxiety- and depressive-like behaviors. (a) Timeline for the AAV mciroinjection and behavioral tests. (b) Hippocampus-specific mTOR deletion did not affect the total distance traveled (p>0.05) and the time in center (p>0.05) in the OPT. (c–e) Hippocampus-specific mTOR deletion significantly decreased the sucrose preference in SPT test (*p<0.05) (c), increased the latency to feed in the novel environment in NSF test (*p<0.05) (d), and the immobility time in FST test (*p<0.05) (e) but did not alter the latency to feed in the home cage in NSF test (p>0.05) (d). n=9–11 animals each group. AAV, AAV2-Cre-GFP or AAV2-GFP.
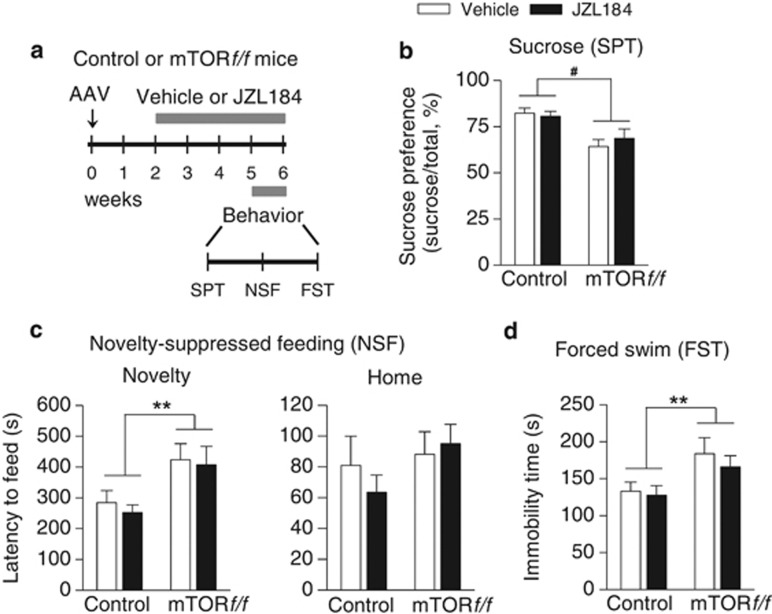
We next determined whether the antidepressant-like action of JZL184 was altered by hippocampus-specific deletion of mTOR. A new group of control or mTORf/f mice received bilateral intra-hippocampal AAV2-Cre-GFP injections. Two weeks after the AAV injections, mice received i.p. injections of vehicle or JZL184 (8 mg/kg) every 2 days for 4 weeks. The time course of the AAV injection, drug treatment, and behavioral tests is shown in Figure 8a. We found that chronic JZL184 treatments did not significantly affect mTOR deletion-induced anxiety- and depressive-like behaviors as shown by SPT (mTOR deletion: F1,33=16.64, p<0.001; JZL184 treatment: F1,33=0.13, p>0.05; Figure 8b), NSF (novelty: mTOR deletion: F1,33=10.65, p<0.01; JZL184 treatment: F1,33=0.29, p>0.05; Figure 8c), and FST (mTOR deletion: F1,33=7.93, p<0.01; JZL184 treatment: F1,33=0.54, p>0.05; Figure 8d). These results suggest that hippocampus-specific deletion of mTOR abrogated the antidepressant-like effects of chronic JZL184 treatments.
Figure 8.
Chronic JZL184 treatments did not affect anxiety- and depressive-like behaviors induced by mTOR deletion in the hippocampus. (a) Timeline for the AAV microinjection, drug treatment, and behavioral tests. (b–d) Hippocampus-specific deletion of mTOR significantly altered the sucrose preference (#p<0.001) in the SPT (b), the latency to feed in the novel environment (**p<0.01) in the NSF test (c), and the immobility time (**p<0.01) in the FST (d). However, chronic JZL184 treatments did not reverse these mTOR deletion-induced behavioral alterations (p>0.05). Neither mTOR deletion nor JZL184 treatment affected the latency to feed in the home cage in the NSF test (c) (p>0.05). n=9–10 mice per group. AAV, AAV2-Cre-GFP.
DISCUSSION
CUS exhibits high predictive, face, and construct validity as an animal model for depression (Willner, 2005). We showed that CUS impaired eCB-mediated retrograde synaptic depression in the hippocampus, and this deficit was rescued by MAGL inhibitor JZL184. Furthermore, CUS induced depressive-like behavior and decreased the activation of mTOR signaling in the hippocampus, whereas chronic JZL184 treatments reversed CUS-induced biochemical and behavioral abnormalities. These results suggest that the impairment of eCB-mTOR signaling contributes to the pathophysiology of depression, while chronic JZL184 treatment produces antidepressant-like effects via activation of eCB-mTOR signaling.
DSI is a form of synaptic plasticity mediated by 2-AG-induced activation of CB1 receptors (Gao et al, 2010; Pan et al, 2009; Tanimura et al, 2010). Consistent with previous finding that chronic restraint stress decreases DSI in the hippocampus (Hu et al, 2011), the present study showed that CUS decreased the magnitude and decay time constant of DSI in hippocampal CA1 pyramidal neurons. In contrast, acute restraint stress enhances DSI and other eCB responses in the hippocampus via glucocorticoid-mediated recruiting of the eCB system (Wang et al, 2012). However, repeated recruiting of eCBs by chronic stress likely overwhelms the eCB system and impairs eCB signaling in the brain, which might explain why DSI was impaired in the hippocampus in mice exposed to chronic stress. CUS decreased 2-AG tissue content in rat hippocampus (Hill et al, 2005). Similarly, we found that the tissue contents of 2-AG were decreased in the hippocampus after CUS exposure, whereas CB1 agonist WIN52212-2-induced depression of IPSCs was not altered by CUS. These results suggest that CUS decreases 2-AG availability without significantly altering the responses of CB1 receptors to CB1 ligands. We have shown that CUS also altered eCB and CB1 receptor-mediated responses in the nucleus accumbens (Wang et al, 2010). The effects of CUS are quite global (Willner, 2005) and many brain regions are likely impacted, though they may be distinctly affected.
The present study showed that CB1 receptor protein expression in the hippocampus was not altered in CUS mice. In contrast, CUS decreased CB1 receptor protein expression in the hippocampus in male rats (Hill et al, 2005; Reich et al, 2009) but increased CB1 receptor protein expression in the hippocampus in female rats (Reich et al, 2009). Thus, species and gender differences may explain the different changes in CB1 receptor protein expression in the hippocampus induced by CUS.
The present study showed that chronic JZL184 treatment reversed CUS-induced depressive-like behaviors in a battery of behavioral tests, and the effects of JZL184 were mediated by the CB1 receptor. Chronic JZL184 treatment did not affect depression-related behaviors in unstressed control mice. Our results are consistent with recent studies showing that acute JZL184 had little effect on anxiety-related behaviors in NSF and elevated plus maze tests, while chronic JZL184 treatment did not significantly affect the latency to feed in unstressed control mice in the NSF test, but decreased the latency to feed in mice that received chronic restraint stress (Sumislawski et al, 2011). The eCBs could act as a stress buffer that dampens the hormonal and behavioral responses to stress and restores emotional homeostasis (Hill et al, 2009). The eCB system remains intact in control mice but is compromised in CUS mice, which might explain why enhancing eCB signaling with JZL184 has relatively little impact on depression-related behavior in unstressed control mice but produces antidepressant-like effects in chronically stressed mice.
Pharmacological blockade or genetic knockout of FAAH produces anxiolytic- and antidepressant-like effects primarily via CB1 receptors (Bambico et al, 2009; Bambico et al, 2007; Bortolato et al, 2007; Gobbi et al, 2005; Kathuria et al, 2003; Patel and Hillard, 2006). Interestingly, a recent study has shown that acute treatments with JZL184 and FAAH inhibitor URB597 induce anxiolytic-like effects in elevated zero and plus maze assays through CB2 and CB1 receptors, respectively (Busquets-Garcia et al, 2011). Thus, both FAAH and MAGL inhibition produce antidepressant- and anxiolytic-like effects. Despite this common behavioral phenotype, FAAH and MAGL inhibitors may exhibit subtle differences in their antidepressant-like action. While 2-AG is a full CB1 agonist, AEA acts as a partial CB1 agonist (Hill et al, 2009). Brain levels of 2-AG are much higher than AEA (Stella et al, 1997). MAGL and CB1 receptors are expressed on presynaptic axonal terminals and are located in close proximity, whereas FAAH is located inside postsynaptic neurons (Cravatt et al, 2001; Gulyas et al, 2004; Tsou et al, 1998). MAGL inhibition may produce greater activation of CB1 receptors than FAAH inhibition. However, whether MAGL inhibition results in more effective antidepressant action remains to be determined.
As mentioned above, acute treatment with JZL184 induces anxiolytic-like effects in elevated zero and plus maze assays, and these effects are mediated by CB2 receptors (Busquets-Garcia et al, 2011). In contrast, we found that subchronic JZL184 treatments did not affect anxiety- and depressive-like behaviors as assessed by OPT, SPT, NSF test, and FST, while chronic JZL184 treatments produced antidepressant-like effects via activation of CB1 receptors. The reason for these discrepancies is not yet clear. We suspect that different behavioral assays and treatment paradigms may be responsible for the different effects observed.
We found that acute JZL184 application normalized CUS-induced deficit in DSI in the hippocampus, while chronic JZL184 treatment is required to produce antidepressant-like effects. These results suggest that normalization of 2-AG signaling is necessary, but not sufficient, to reverse behavioral deficits induced by CUS. Clinically antidepressants have delayed onset in their antidepressant actions, suggesting that slow neurochemical and other changes are required for their clinical effects (Wong and Licinio, 2001). We have shown that chronic JZL184 treatment, but not subchronic JZL184 treatment, produced antidepressant-like effects in CUS model of depression. It is likely that subsequent neuroadaptations following CB1 receptor activation are also required for the manifestation of the antidepressant-like effects of JZL184.
As mentioned earlier, the CB1 antagonist rimonabant increases the incidence of anxiety and depression in clinical trials for the treatment of obesity (Samat et al, 2008). Overall, rimonabant caused anxiety or depression in 1–3% of patients, while placebo caused anxiety or depression in <1% of patients (Moreira and Crippa, 2009). The difference is highly significant in humans. It is surprising that chronic treatment with CB1 antagonist rimonabant (i.p., 2 mg/kg) alone did not affect depression-related behavior in the present study. In contrast, chronic treatment with rimonabant (i.p.,10 mg/kg) increased the immobility time in the FST and decreased sucrose preference in rats, while chronic rimonabant at a lower dose (i.p., 3 mg/kg) had no significant effects on these two behaviors (Beyer et al, 2010). Rats are more vulnerable to CUS than mice (Willner, 2005). Species and dose differences may explain the different behavioral responses to chronic rimonabant treatment. It is worth noting that rimonabant (i.p., 2 mg/kg) is sufficient to block behavioral effects of JZL184. It is unclear whether rimonabant at the high dose (i.p.,10 mg/kg) may affect targets other than CB1 receptors.
A concern of cannabinoid-mimic drugs is their psychoactive effects and abuse potential. However, dual FAAH/MAGL blockade, but not disruption of either FAAH or MAGL alone, produced THC-like drug discrimination responses (Long et al, 2009c). It is thus likely that FAAH or MAGL inhibitors do not share the psychoactive or adverse effects of THC. The following observations suggest that partial blockade of FAAH or MAGL may produce better antidepressant action while minimizing adverse effects. First, low doses of CB1 agonists produce anxiolytic and antidepressant effects (Berrendero and Maldonado, 2002; Jiang et al, 2005; Patel and Hillard, 2006; Valjent et al, 2002), while moderate-to-high doses are generally anxiogenic (Mangieri and Piomelli, 2007; Moreira et al, 2009; Patel and Hillard, 2006). Second, high doses of FAAH inhibitors can increase anxiety levels (Rubino et al, 2008; Scherma et al, 2008). Third, chronic JZL184 at high dose (40 mg/kg), but not low lose (8 mg/kg), causes behavioral tolerance and CB1 receptor desensitization (Busquets-Garcia et al, 2011; Long et al, 2009a). We chose to use low dose of JZL184 (8 mg/kg), which is estimated to produce half-maximal increase in 2-AG levels in the brain (Kinsey et al, 2013; Long et al, 2009b; Sumislawski et al, 2011). Thus, low doses of MAGL or FAAH inhibitors are required for their antidepressant-like behavioral effects. [35S]GTPγS and CB1 receptor binding studies indicate that CB1 receptor expression and function are maintained following repeated administration of low-dose JZL184 (⩽8 mg/kg) (Kinsey et al, 2013). Chronic JZL184 at this low-dose produces antinociceptive and anti-inflammatory effects without inducing apparent CB1 receptor desensitization and functional tolerance (Kinsey et al, 2013).
mTOR and ERK1/2 are serine/threonine protein kinases that are involved in cellular survival, growth, and differentiation (Hoeffer and Klann, 2010). Postmortem studies have showed deficits in mTOR signaling in the prefrontal cortex and ERK1/2 signaling in the hippocampus of subjects diagnosed with MDD (Duric et al, 2010; Jernigan et al, 2011). CB1 receptor agonists activate mTOR (Busquets-Garcia et al, 2013; Busquets-Garcia et al, 2011; Puighermanal et al, 2009) and ERK in the hippocampus and other brain regions (Derkinderen et al, 2003; Pan et al, 2011). mTOR deletion did not significantly affect DSI in hippocampal CA1 pyramidal neurons (Supplementary Figure S2), suggesting that CB1 receptors are upstream of mTOR activation in the hippocampus. CUS decreased DSI and 2-AG tissue contents in the hippocampus (Figure 1). The CUS-induced deficiency in 2-AG signaling and CB1 receptor activation may lead to decreases in mTOR and ERK activation in the hippocampus. In support of this idea, we found that CUS led to decreases in the activation of mTOR and ERK1/2, their downstream effectors p70S6K, rpS6 (Jefferies et al, 1997; Proud, 2007), while these decreases were reversed by chronic JZL184 treatment. Moreover, we showed that genetic deletion of mTOR in the hippocampus with AAV2-Cre-GFP recapitulated the depressive-like behaviors induced by CUS and abrogated the antidepressant-like action of chronic JZL184. The mice in which mTOR was deleted in the hippocampus appeared grossly normal and displayed normal locomotor activity in an OPT. Further, there was no apparent change in the number of hippocampal neurons as shown by NeuN staining. Thus, the depressive-like behaviors in these mice cannot be attributed to deterioration of health conditions. Taken together, these data appear to support a model in which CUS-induced deficits in eCB signaling lead to a decrease in CB1 receptor-mediated mTOR and ERK activation and depressive-like behavior, whereas activation of mTOR and ERK1/2 signaling may constitute a mechanism for the antidepressant-like effects of chronic JZL184 treatments. Nevertheless, our studies do not rule out other mechanisms that may contribute to antidepressant-like effects of JZL84. For example, proinflammatory cytokines such as IL-1beta have been implicated in stress and depression (Koo and Duman, 2008). Chronic JZL184 exhibits neuroprotective effects against Alzheimer's disease and Parkinson's disease via inhibition of prostaglandin-induced neuroinflammation (Chen et al, 2012; Nomura et al, 2011; Piro et al, 2012). The anti-neuroinflammation effects of JZL184 may also contribute to its antidepressant action.
The activation of mTOR and associated increase in the synthesis of synaptic proteins contribute to rapid antidepressant action of NMDA receptor antagonist ketamine (Li et al, 2010) and group II/III mGluR antagonist LY341495 (Dwyer et al, 2012). We show here the CB1-dependent antidepressant-like effects induced by JZL184 treatments are also dependent on mTOR signaling. Thus, activation of mTOR appears to be a final common pathway that multiple signaling molecules can converge to produce antidepressant-like effects. In summary, our studies indicate that chronic JZL184 treatment produces antidepressant-like effects in a CUS model of depression, and these effects are likely mediated by reversing CUS-induced downregulation of mTOR and ERK signaling. Our data suggest that MAGL inhibition represents a useful strategy for the development of antidepressant medications.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by NIH Grants R21 MH095921 and R01 DA024741 and Extendicare Foundation. It was also partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and the National Institutes of Health Grant UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Sciences. We thank Dr Cecilia J Hillard for her critical comments on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Author contributions
All authors designed the experiments. PZ, WW, BP, XL, and ZZ performed the experiments, collected, and analyzed the data. JZL and BFC contributed reagents. PZ and QSL drafted the manuscript.
Supplementary Material
References
- Bambico FR, Duranti A, Tontini A, Tarzia G, Gobbi G. Endocannabinoids in the treatment of mood disorders: evidence from animal models. Curr Pharm Des. 2009;15:1623–1646. doi: 10.2174/138161209788168029. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, et al. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep. 2012;2:1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Denson TF, Earleywine M. Decreased depression in marijuana users. Addict Behav. 2006;31:738–742. doi: 10.1016/j.addbeh.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, et al. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. A silver bullet for the treatment of depression. Neuron. 2007;55:679–681. doi: 10.1016/j.neuron.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Clapper JR, Holmes PV, Piomelli D, Hohmann AG. A role for 2-arachidonoylglycerol and endocannabinoid signaling in the locomotor response to novelty induced by olfactory bulbectomy. Pharmacol Res. 2010;61:419–429. doi: 10.1016/j.phrs.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci. 2009;30:484–493. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Zhang W, Flugge G. Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res Bull. 2011;85:374–379. doi: 10.1016/j.brainresbull.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, O'Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, et al. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, Phan A, Hill MN, Pfaff DW, McEwen BS. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav. 2013;12:479–489. doi: 10.1111/gbb.12045. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mangieri RA, Piomelli D. Enhancement of endocannabinoid signaling and the pharmacotherapy of depression. Pharmacol Res. 2007;56:360–366. doi: 10.1016/j.phrs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, et al. Antiaversive effects of cannabinoids: is the periaqueductal gray involved. Neural Plast. 2009;2009:625469. doi: 10.1155/2009/625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Crippa JA. The psychiatric side-effects of rimonabant. Rev Bras Psiquiatr. 2009;31:145–153. doi: 10.1590/s1516-44462009000200012. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.2001The Mouse Brain in Stereotaxic Coordinates2nd edn.Academic Press: San Diego, San Francisco, New York, Boston, USA; London, UK; Sydney, Australia; Tokyo, Japan [Google Scholar]
- Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, et al. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer's disease. Cell Rep. 2012;1:617–623. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Samat A, Tomlinson B, Taheri S, Thomas GN. Rimonabant for the treatment of obesity. Recent Pat Cardiovasc Drug Discov. 2008;3:187–193. doi: 10.2174/157489008786264014. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology. 2011;36:2750–2761. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, et al. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26:56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, et al. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010;35:2249–2261. doi: 10.1038/npp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhong P, Liu X, Sun D, Gao HQ, Liu QS. Metabotropic glutamate receptor I (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology. 2013;38:1308–1321. doi: 10.1038/npp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.