Abstract
Accumulating evidence suggests that selective M4 muscarinic acetylcholine receptor (mAChR) activators may offer a novel strategy for the treatment of psychosis. However, previous efforts to develop selective M4 activators were unsuccessful because of the lack of M4 mAChR subtype specificity and off-target muscarinic adverse effects. We recently developed VU0152100, a highly selective M4 positive allosteric modulator (PAM) that exerts central effects after systemic administration. We now report that VU0152100 dose-dependently reverses amphetamine-induced hyperlocomotion in rats and wild-type mice, but not in M4 KO mice. VU0152100 also blocks amphetamine-induced disruption of the acquisition of contextual fear conditioning and prepulse inhibition of the acoustic startle reflex. These effects were observed at doses that do not produce catalepsy or peripheral adverse effects associated with non-selective mAChR agonists. To further understand the effects of selective potentiation of M4 on region-specific brain activation, VU0152100 alone and in combination with amphetamine were evaluated using pharmacologic magnetic resonance imaging (phMRI). Key neural substrates of M4-mediated modulation of the amphetamine response included the nucleus accumbens (NAS), caudate-putamen (CP), hippocampus, and medial thalamus. Functional connectivity analysis of phMRI data, specifically assessing correlations in activation between regions, revealed several brain networks involved in the M4 modulation of amphetamine-induced brain activation, including the NAS and retrosplenial cortex with motor cortex, hippocampus, and medial thalamus. Using in vivo microdialysis, we found that VU0152100 reversed amphetamine-induced increases in extracellular dopamine levels in NAS and CP. The present data are consistent with an antipsychotic drug-like profile of activity for VU0152100. Taken together, these data support the development of selective M4 PAMs as a new approach to the treatment of psychosis and cognitive impairments associated with psychiatric disorders such as schizophrenia.
Keywords: M4, muscarinic, antipsychotic, allosteric, phMRI, VU0152100
INTRODUCTION
The psychotic symptoms and cognitive impairments observed in neuropsychiatric disorders, including schizophrenia, result in debilitating disruptions to the normal daily activities of patients as well as to their families and caregivers (American Psychiatric Association, 2000; Nuechterlein et al, 2004). Unfortunately, clinically available treatments, including typical and atypical antipsychotic drugs (APDs), provide only modest relief for some of the psychotic symptoms observed in these patient populations (Swartz et al, 2008). Treatment outcomes are further constrained by partial responsiveness, treatment resistance, and dose-limiting adverse effects, including extrapyramidal motor side effects and metabolic syndrome associated with many antipsychotics (Lieberman et al, 2003; Parsons et al, 2009). Thus, there remains a tremendous unmet need to develop novel treatment strategies to address the complex psychotic symptoms and cognitive deficits associated with various neuropsychiatric disorders.
Recent studies suggest that selective activators of specific subtypes of muscarinic acetylcholine receptors (mAChRs) may provide an alternative approach for the treatment of the psychotic symptoms and cognitive impairments in patients with schizophrenia, as well as the behavioral disturbances observed in neurologic disorders like Alzheimer's disease (AD). mAChRs are members of the family A G protein-coupled receptors (GPCRs) and consist of five subtypes termed M1–M5 (Bonner et al, 1987; Bonner et al, 1988). These different mAChR subtypes modulate multiple functions within the central nervous system (CNS), including motor control, sleep–wake architecture, cognition, and affective responses (Jones et al, 2012). Previous clinical studies demonstrated that the M1/M4-preferring mAChR agonist xanomeline significantly reduces the behavioral disturbances, including delusions, hallucinations, vocal outbursts and agitation, in AD patients and the psychotic symptoms and some cognitive deficits in individuals with schizophrenia (Bodick et al, 1997; Shekhar et al, 2008). Although encouraging, there have been only two clinical studies with xanomeline published to date indicating that these initial findings should be viewed with caution until further clinical validation is reported. Xanomeline also showed robust efficacy in animal models predictive of APD-like activity comparable to the effects observed with the atypical antipsychotic clozapine, including reversal of psychostimulant-induced hyperlocomotion and disruption of prepulse inhibition (PPI) of the acoustic startle reflex (Andersen et al, 2003; Jones et al, 2005; Perry et al, 2001; Shannon et al, 2000; Stanhope et al, 2001). Unfortunately, xanomeline like other muscarinic agonists ultimately failed in clinical development due to a lack of complete receptor subtype selectivity resulting in adverse side effects associated with non-selective activation of peripheral M2 and M3 mAChRs (Bodick et al, 1997; Shekhar et al, 2008).
The lack of selective ligands for M1 and M4 mAChRs has made it difficult to develop a clear understanding of the relative contributions of these two mAChR subtypes to the clinical and preclinical effects of xanomeline. Development of allosteric ligands provides a novel approach to address these issues (see Jones et al, 2012). Our group and others have identified subtype-selective mAChR ligands that activate a specific receptor subtype at sites that are less highly conserved than the orthosteric binding site of acetylcholine (ACh) and more topographically distinct, termed allosteric sites. These efforts have led to the discovery of highly selective M4 positive allosteric modulators (PAMs) that do not activate M4 directly, but markedly potentiate the response of M4 to ACh (Shirey et al, 2008; Chan et al, 2008; Brady et al, 2008). M4 PAMs bind to an allosteric site and can increase both the affinity of M4 for ACh and the efficiency of coupling of M4 to G proteins (Shirey et al, 2008). Consistent with their effects in cell lines, M4 PAMs potentiate M4-mediated depression of excitatory synaptic transmission at hippocampal CA1 synapses (Shirey et al, 2008). Chemical optimization of early M4 PAMs resulted in the discovery of VU0152100 as a centrally penetrant highly selective M4 PAM (Brady et al, 2008). Interestingly, VU0152100 produced robust efficacy when administered at a single top dose in a preclinical model of APD-like activity (Brady et al, 2008). These studies provided important data supporting the potential for the development of M4 PAMs for the treatment of psychosis. Conversely, LY2033298, another M4 PAM, had no effect alone in preclinical models, but did potentiate the effects of a sub-threshold dose of the non-selective mAChR agonist oxotremorine (Chan et al, 2008; Leach et al, 2010, Suratman et al, 2011). The lack of efficacy observed with LY2033298alone when used in vivo was likely due to the lower potency of this M4 PAM at the rat M4 mAChR and its low cooperativity with the endogenous agonist ACh at the rodent receptor (Suratman et al, 2011). However, these studies also raise a potential concern that there may not be sufficient endogenous cholinergic tone to observe robust activity in key limbic and cortical regions when an M4 PAM is administered alone.
We now report a series of behavioral, neurochemical, and functional imaging studies in which we show that the M4 PAM VU0152100 has robust efficacy in several rodent models predictive of APD-like activity and reversal of cognitive impairments. In addition, we demonstrate corresponding effects of VU0152100 on specific brain regions and circuits that are thought to have an important role in the underlying psychotic symptoms, behavioral, and cognitive disturbances observed in various psychiatric and neurologic disorders.
MATERIALS AND METHODS
Subjects
Adult male Sprague–Dawley rats, weighing 250–275 g, were purchased from Harlan Laboratories (Indianapolis, IN). C57BL/6NTac wild-type mice and M4 knockout (KO) mice were obtained from Taconic Farms (Hudson, NY) through collaboration with Dr Jürgen Wess (National Institute of Diabetes and Digestive and Kidney Disorders, Bethesda, MD). Animals were group housed in large colony rooms under a 12-h light–dark cycle with food and water available ad libitum. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health regulations of animal care covered in Principles of Laboratory Animal Care.
Drugs
D-amphetamine hemisulfate, clozapine, haloperidol, and oxotremorine sesquifumarate were obtained from Sigma-Aldrich (St Louis, MO). Xanomeline L-tartrate was synthesized in-house according to the method of Sauerberg et al (1992) or from commercially available free base xanomeline. VU0152100 was synthesized according to our previously described method (Brady et al, 2008). Amphetamine, xanomeline, and oxotremorine were dissolved in double deionized water; clozapine and haloperidol were dissolved in acidified double deionized water; and VU0152100 was dissolved in 10% Tween 80 plus double deionized water. All solutions were adjusted to a pH of approximately 6–7 using 1 N sodium hydroxide and administered in a volume of 1 ml/kg, with the exception of VU0152100, which was administered in a volume of 2 ml/kg.
Cell Culture, Transfection, Dopamine Transporter Uptake and Binding Assays
HEK 293T cells were cultured, maintained, and transiently transfected with pcDNA 3-rDAT (rat DAT) as described previously (Mazei-Robison and Blakely, 2005). In short, HEK 293T cells were cultured in media (DMEM (Invitrogen), 10% FBS (Hyclone, Carlsbad, CA), 0.1 U/ml penicillin–0.11 gm/ml streptomycin (pen/strep) (Invitrogen), and 2 mM L-glutamine (Invitrogen)) in a humidified incubator at 37 °C and 5% CO2. Cells were plated in 24-well plates at a density of 20 000 cells per well. Trans-IT LT-1 (Mirus, Madison, WI) in serum-free media was used for transfection of cells approximately 24 h after plating using a 1 : 3 DNA:lipid ratio. Assays were conducted approximately 36 h after transfection. [3H] DA transport assays were performed in triplicate in 24-well plates as described previously (Sakrikar et al, 2012). Briefly, cells were washed with Krebs'–Ringer's–HEPES (KRH; 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM HEPES, pH 7.4) buffer and incubated in the uptake assay buffer (KRH, 10 mM glucose, 100 μM pargyline, ascorbic acid, and 10 μM tropolone) before incubation with [3H]DA (3,4-[7-3H]-dihydroxyphenylethylamine, ∼45 Ci/mmol, NEN-Perkin Elmer, Boston, MA) for 15 min. To determine inhibition, parallel wells were incubated with 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine hydrochloride (GBR 12909) (Sigma-Aldrich) or VU0152100 10 min before addition of [3H]DA. For each inhibitor—GBR 12909 and VU 0152100—seven concentrations (0.03, 0.1, 0.3, 1, 3, 10, and 30 μM) were tested to determine IC50 values. MicroScint 20 (Perkin-Elmer, Boston, MA) scintillation mixture was added to the wells at the end of the assay and DA uptake was measured using a TopCount Scintillation Counter. Values reported from transport studies are derived from three replicate experiments. GraphPad Prism was used to fit the inhibition curves and to determine the IC50 values.
In vitro assays on the effects of VU0152100 on human DAT were performed by Eurofins Panlabs (Taipei, Taiwan). Briefly, CHO-K1 cells expressing recombinant human DAT were used (1) to assess if VU0152100 displaces binding of the DAT inhibitor [125I]iometopane ([125I]RTI-55) and (2) to determine if VU0152100 interferes with [3H]dopamine uptake. Detailed assay procedures are available at: https://www.eurofinspanlabs.com/Catalog/Products/ProductDetails.aspx?prodId=wCWTzNIg1Cc%3d (DAT uptake) and https://www.eurofinspanlabs.com/Catalog/Products/ProductDetails.aspx?prodId=umFCt3XtSMg%3d (DAT binding). The IC50 values were estimated by a non-linear, least squares regression analysis using MathIQTM (IDBusiness Solutions, UK) and Ki values were calculated using the equation of Cheng and Prusoff (1973).
Amphetamine-Induced Hyperlocomotion
Locomotor activity was assessed in open field chambers as previously described (see Jones et al, 2008). Briefly, rats were habituated to the chambers for 30 min, then pre-treated with either vehicle or VU0152100 (3–56.6 mg/kg, interperitoneal (i.p.)). Thirty minutes later, rats were administered either vehicle or amphetamine (1 mg/kg, subcutaneous, s.c.) and monitored for another 60 min.
Locomotor activity in mice was examined using open filed boxes (Med Associates, St Albans, VT). Wild-type and M4 KO mice were habituated in the chambers for 90 min before receiving i.p. injections of vehicle (10% Tween 80) or VU0152100 (30 mg/kg). Amphetamine (1.8 mg/kg, i.p.) was injected 30 minutes later and locomotor activity was monitored for another 120 min.
Amphetamine-Induced Disruption of PPI of the Acoustic Startle Reflex
Studies were conducted using startle chambers with a Plexiglas cylinder mounted on a piezoelectric accelerometer for detecting motion as previously described (see Jones et al, 2005). Following a 20-min pre-treatment with vehicle or VU0152100 (3–30 mg/kg, i.p.), rats were injected with amphetamine (3 mg/kg, s.c.), and after an additional 10 min, placed into individual startle chambers for the following paradigm. After a 5-min acclimation period, the rats were presented with five startle stimuli alone, followed by seven randomized presentations of the following trial types: no stimulus, startle pulse alone (120 dB, 40-ms broadband noise burst), prepulse noise alone (82 dB, 40-ms broadband noise burst), and three prepulse (72, 77, or 82 dB; 20 ms) plus startle pulse combinations. The intertrial interval varied pseudorandomly between 15 and 45 s and the interstimulus interval was 100 ms. Background noise of 67 dB was presented continuously. Percent PPI was calculated as 100 × ((mean ASRstartle pulse trials−mean ASRprepulse plus startle pulse trials)/mean ASRstartle pulse trials).
Amphetamine-Induced Disruption of Contextual Fear Conditioning
Studies were conducted using conditioning chambers within sound attenuating cubicles as previously described (see Lebois et al, 2010). All rats were handled and injected with saline for 2 days before conditioning. On the conditioning day, rats were pre-treated with vehicle or VU0152100 (10–56.6 mg/kg, i.p.) for 15 min, followed by administration of either vehicle or amphetamine (4.8 mg/kg, s.c.), and after an additional 15 min, placed in chambers for conditioning with the following paradigm: 2-min habituation, then four presentations of an unconditioned stimulus (0.5 mA 1-s footshock; 79-s intertrial interval) followed by a 45-s interval without stimuli (7-min total). Approximately 24 h after compound treatment and conditioning, rats were re-exposed to the same conditioning context and freezing behavior, defined as motionless posture, excluding respiratory movements, was measured in the absence of any shock stimuli for 7 min. The potential effect of VU0152100 on nociceptive response to the footshook stimulus was also separately measured. Rats were pre-treated with vehicle or VU0152100 (56.6 mg/kg, i.p.) for 30 min, then placed in the conditioning chambers and behavioral changes in footshock threshold were assessed using increasing current from 0 to 0.5 mA in increments of 0.05 mA as described by van der Staay et al (2011).
Fos Immunochemistry
After a 2-h pre-treatment with either vehicle, VU00152100 (30–100 mg/kg, i.p.), clozapine (30 mg/kg, s.c.), or haloperidol (1 mg/kg, s.c.), rats were perfused with 4% paraformaldehyde. Frozen sections were stained to reveal Fos-like immunoreactivity (Fos-LI) using a goat anti-Fos antibody (see Bubser et al, 2005); hypothalamic sections were double-labeled for Fos-LI and orexin A using a mouse anti-orexin A antibody (1 : 1000; R&D Systems, Minneapolis, MN). The density of Fos-like immunoreactive (Fos-li) neurons (number of Fos-li neurons/mm2) was determined as previously described (Bubser et al, 2005).
Pharmacologic Magnetic Resonance Imaging (phMRI)
Isoflurane-anesthetized rats, with preimplanted jugular catheters, underwent endotracheal intubation (14 G catheter), insertion of i.p. catheters (size P50, Braintree Scientific, Braintree, MA), and mechanical ventilation (Kent Scientific, Litchfield, CT; O2:N2O 1 : 2; 2% isoflurane). For scanning, isoflurane was set to 0.88% and pancuronium bromide administered (1 mg/kg, i.p.; BoundTree Medical, Dublin, OH). Heart rate, respiration, and rectal temperature were continuously measured and temperature maintained through a thermocoupled heating unit (SAM-PC; SA Instruments, Encinitas, CA). End-tidal CO2 was continuously monitored (Invivo Research, Orlando, FL). PhMRI data were acquired with a Doty Litz 38-mm transmit-receive radio frequency coil using a 9.4 T Varian magnet controlled by a Varian Inova console (Agilent, Palo Alto, CA). High-resolution fast spin-echo (fse) structural images were collected (repetition time (TR) 2550 ms; effective echo time (TEeff) 40 ms; number of excitations (NEX) 2; 128 × 128 matrix; 35 × 35 mm2 field of view; 11 contiguous slices, 1.5 mm thick). Pre-contrast reference images and post-contrast functional images were acquired (fse: TR 2600 ms; TEeff 36 ms; NEX 2; 64 × 64 matrix). To measure cerebral blood volume (CBV), Molday iron oxide nanoparticles (30 nm; 20 mg/kg, i.v.; BioPAL, Worcester, MA) were injected. For amphetamine interaction experiments, rats were pre-treated for 15 min with vehicle or 56.6 mg/kg VU0152100 (i.p.), then a 15-min baseline was collected, after which rats were administered with vehicle or a 1 mg/kg amphetamine (i.p.) followed by 45 min of continuous acquisition. In a separate study to assess the effects of VU0152100 alone on fractional CBV changes, an initial 15-min baseline was collected, followed by administration of vehicle or VU0152100 (56.6 mg/kg, i.p.), and 45 min of continuous image acquisition.
phMRI data were processed using in-house MATLAB code (MathWorks, Natick, MA) and Analysis of Functional NeuroImages (AFNI; afni.nimh.nih.gov). All masked, motion-corrected (AFNI 2dreg) images were coregistered to the template anatomical images in AFNI. Fractional CBV changes were calculated on a voxel-wise basis for each subject using the equation: ΔCBV(t)=(ln S(t)−ln So)/(ln So−ln Spre), where S(t) is the measured signal at time t, So is the post-contrast baseline signal, and Spre is the pre-contrast baseline (Mandeville et al, 1998). Regions of interest (ROIs), pre-defined on the template based on a rat brain atlas (Paxinos and Watson, 2007), were applied to all subjects. Mean CBV values (left and right hemispheres averaged) were calculated for each subject at the 15- to 25-min interval after the vehicle or amphetamine challenge or at the 15- to 25-min interval after vehicle or VU0152100 alone. Group-averaged maps were colorized according to the voxel CBV value.
Functional Connectivity Analysis
The strength of functional connections between brain regions was quantified by computing the Pearson linear correlation coefficient between the integral of the CBV response to amphetamine for each ROI pair for the vehicle/amphetamine and VU0152100/amphetamine groups (Schwarz et al, 2007b). Correlation coefficients, r, were converted to z-scores using Fisher's transformation (z=ln[(1+r)/(1−r)]/[2*(1/(N−3))0.5]) and thresholded at |Z|>2 (in-house MATLAB code). Permutation analysis (Holmes et al, 1996) was used to identify the correlations that were significantly different (Δz, p<0.05) between vehicle/amphetamine and VU0152100/amphetamine groups (in-house MATLAB code).
Cardiovascular Function
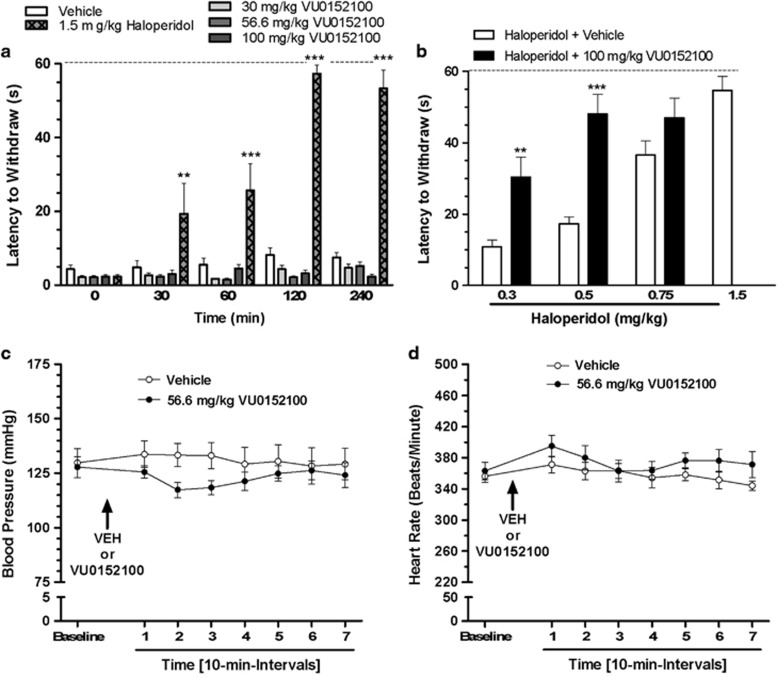
Potential effects of VU0152100 on blood pressure (mm Hg) and heart rate (beats/min) were measured in awake, freely moving rats via a transducer (Biopac Systems, Goleta, CA) connected to a line pre-implanted into the carotid artery (Parasuraman and Raveendran, 2012). VU0152100 (56.6 mg/kg, i.p.) or vehicle were administered after a 30-min baseline recording, and data were collected for 70 additional minutes using Biopac Student Lab Pro Software (Goleta, CA).
Neurochemistry Studies
Rats were pre-treated for 30 min with either vehicle, VU0152100 (10–100 mg/kg, i.p.), or xanomeline (30 mg/kg, i.p.), then killed under isoflurane anesthesia and the medial prefrontal cortex (PFC), nucleus accumbens (NAS), and caudate-putamen (CP) rapidly dissected, frozen on dry ice, and stored at −80 °C until dopamine and serotonin levels were analyzed by HPLC-ECD as described by Hackler et al (2006). Using the same methods, we assessed whether VU0152100 would potentiate the effects of the non-selective mAChR agonist oxotremorine on monoamine metabolite/parent monoamine ratios, an index of monoamine utilization in vivo. Rats were pre-treated for 30 min with vehicle or VU0152100 (56.6 mg/kg) followed by a sub-threshold dose of oxotremorine (0.01 mg/kg, s.c.) or vehicle and killed after another 15 min.
In Vivo Microdialysis and Locomotor Activity
Guide cannulae (BioAnalytical Systems, West Lafayette, IN) were implanted into the NAS (AP +1.5 mm bregma, ML −1.5 mm, DV −7.8 mm) or CP (AP 1.0 mm, ML −2.5 mm, DV −5.5 mm). After recovery for 4–7 days, microdialysis probes (2 mm membrane length) were inserted the night before the experiment using methods previously reported by Perry et al (2001). The following day, rats were placed into open field chambers as used for the amphetamine-induced hyperlocomotion studies. Probes were perfused with artificial cerebrospinal fluid (150 mM NaCl, 3 mM KCl, 1.7 mM CaCl2, and 0.9 mM MgCl2) and dialysate samples were collected in 15-min fractions onto a 96-well plate using a refrigerated fraction collector (EFC-82, Eicom). Following a 2-h equilibration, four baseline samples were collected, followed by a 30-min pre-treatment with either vehicle or VU0152100 (56.6 mg/kg, i.p.). Then all rats were injected with either vehicle or amphetamine (1 mg/kg, s.c.) and dialysate samples were collected for another 120 min. At the end of the study, probes were perfused with methyl blue to verify placement. Dialysates were analyzed using an Eicom ECD-700 HPLC system. Dopamine and its two major metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were separated at a flow rate of 400 μl/min using a mobile phase consisting of 0.1 M citrate-acetate buffer/methanol (80 : 20), 220 mg/l sodium octanesulfonic acid, and 5 mg/l EDTA and quantitated by electrochemical detection. Chromatograms were analyzed using PowerChrom (eDAQ, Denistone East, NSW, Australia). Only animals with accurate probe placement that showed three consecutive stable baseline values (within ⩽20%) were included in the statistical analysis.
Catalepsy
Rats were administered either vehicle, VU0152100 (30–100 mg/kg), or haloperidol (1.5 mg/kg), and catalepsy was assessed at 30, 60, 120, and 240-min post-treatment as previously described (Jones et al, 2008). In a second study, rats were pre-treated for 60 min with a dose of haloperidol (0.3–1.5 mg/kg, i.p.), followed by administration of a 100 mg/kg i.p. dose of VU0152100 or vehicle; then catalepsy was measured after an additional 30 min.
Modified Irwin Test Battery
Effects of vehicle, VU0152100 (56.6 mg/kg, i.p.), or oxotremorine sesquifumarate (1 mg/kg, s.c.) on autonomic and somatosensory system function were assessed at 5, 15, 60, 120, and 240-min post-injection using the Modified Irwin Neurological Test Battery (Irwin, 1968), as previously described (see Jones et al, 2008).
Statistical Analysis
Data were analyzed by one- or two-way ANOVA followed by Dunnett's or Bonferroni's test, or in the case of unequal variance by Kruskal–Wallis test followed by Dunn's test using GraphPad Prism V5.04 (GraphPad Software, La Jolla, CA).
RESULTS
Characterization of VU0152100
Before in vivo testing, we verified that the newly synthesized batch of VU0152100 used in the present studies yielded comparable exposure to our previously reported brain and systemic plasma pharmacokinetic profile when dosed at 56.6 mg/kg (i.p.); specifically, brain AUC0∞ (36.2 μM h) and Cmax values (7.6 μM) observed in our present study were similar to the previously reported AUC0-∞ (19.2 μM h) and Cmax (8.8 μM) values (Brady et al, 2008). Finally, these exposure values represented Cmax free brain concentrations of ∼340 and ∼400 nM, respectively, for the dose of 56.6 mg/kg when the actual amount of unbound or free VU0152100 available in rat brain (rat fu brain=0.045) was taken into consideration.
Based on an initial report that VU0152100 displaced binding of a ligand to the dopamine transporter with a 46% inhibition at 10 μM (Brady et al, 2008), we also estimated the in vitro IC50 and Ki binding values for VU0152100 at the human DAT to be 5.98 and 4.75 μM, respectively, using a recombinant CHO-K1 cells expressing human DAT (Eurofins, Lancaster, PA; see Supplementary Figure S1). In addition, we evaluated the effects of VU0152100 at the rat DAT using HEK cells transiently transfected with the rat DAT and found that the IC50 value was greater than 10 μM (see Supplementary Figure S1).
VU0152100 Reverses Amphetamine-Induced Hyperlocomotion
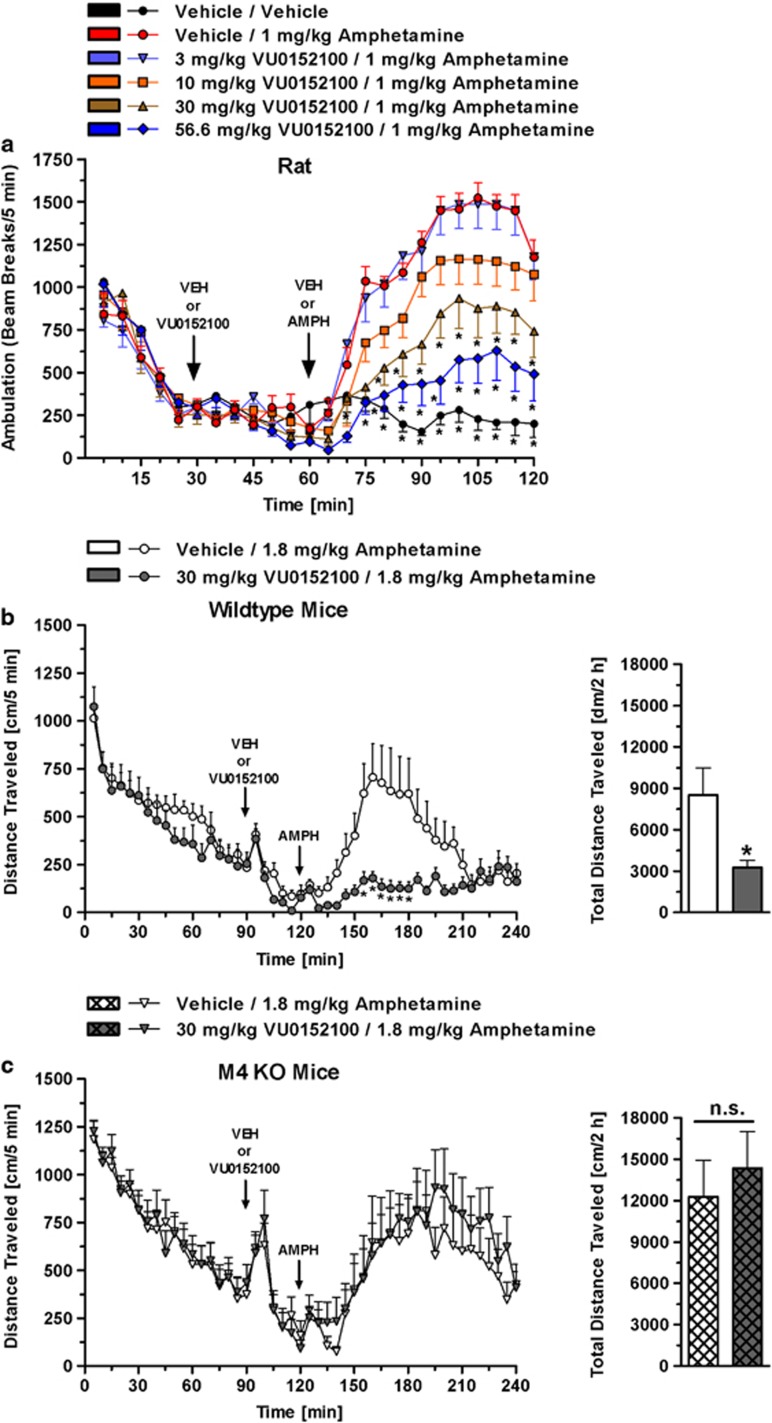
The effects of VU0152100 on amphetamine-induced hyperlocomotion were evaluated in rats. When administered alone, amphetamine (1 mg/kg, s.c.) induced a substantial increase in locomotor activity when compared with the vehicle/vehicle group (Figure 1a). VU0152100 pre-treatment produced a robust dose-dependent reversal of amphetamine-induced hyperlocomotion with significant reversal observed at doses of 30 and 56.6 mg/kg (Figure 1a: F(treatment)5, 1008=11.7, p<0.001, F(time)23, 1008=40.14, p<0.001, and F(interaction)5, 115=20.12, p<0.001).
Figure 1.
VU0152100 reverses amphetamine-induced hyperlocomotion in rats and wild-type mice, but not in M4 KO mice. (a) Amphetamine-induced (1 mg/kg) hyperlocomotion in rats is dose-dependently reversed by VU0152100 (*p<0.05 vs vehicle/amphetamine (Dunnett's test)); (b) VU0152100 reverses amphetamine-induced hyperlocomotion in wild-type mice. (c) In M4 KO mice, amphetamine-induced hyperlocomotion is not blocked by VU0152100 (*p<0.05, vehicle/amphetamine (Bonferroni's test)). Data are mean±SEM of 8 rats or 10–11 mice per group.
VU0152100-Mediated Reversal of Amphetamine-Induced Hyperlocomotion is Absent in M4 KO Mice
Amphetamine elicited a marked locomotor response in wild-type mice that was blocked significantly by pre-treatment with 30 mg/kg VU0152100 (F(treatment)1, 912=65.29, p<0.001, F(time)47, 912=12.02, p<0.001, and F(interaction)47, 912=2.10, p<0.001; see Figure 1b). In M4 KO mice, amphetamine-induced hyperlocomotion was not reversed at any time point by VU0152100 pre-treatment (F(treatment)1, 912=3.13, NS, F(time)47, 912=7.39, p<0.001, and F(interaction)47, 912=0.30, NS; see Figure 1c). Total locomotor activity induced by amphetamine was significantly reduced by VU0152100 in wild-type animals (t19=2.44, p<0.05); pre-treatment with VU0152100 did not alter amphetamine-induced total locomotor activity in M4 KO mice (t19=0.55, NS).
VU0152100 Reverses Amphetamine-Induced Disruption of PPI
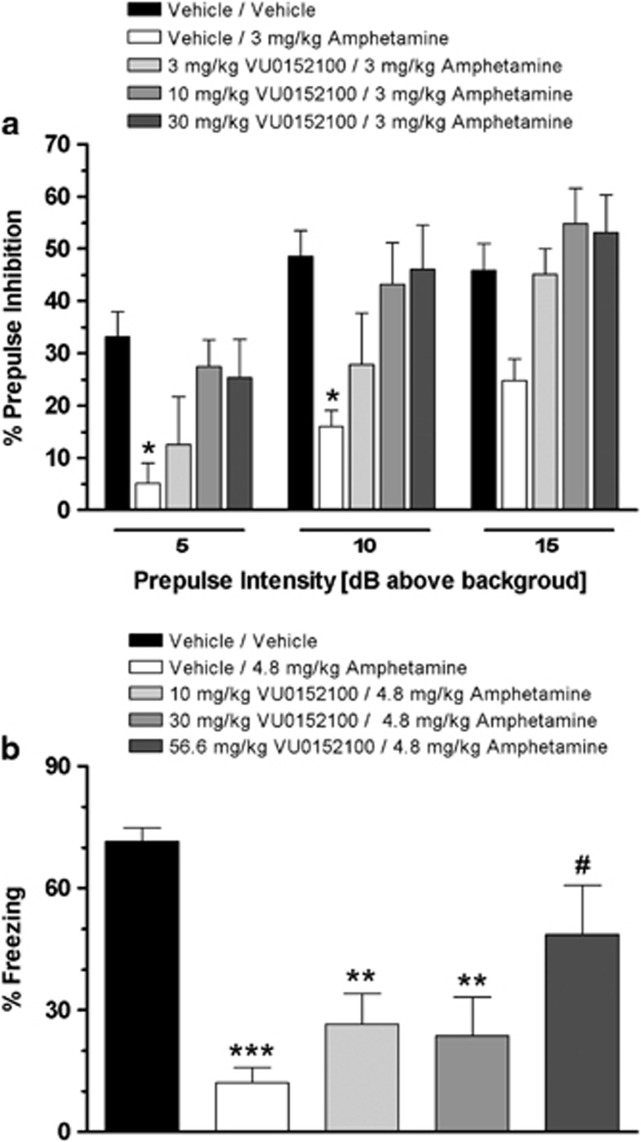
We next evaluated the effects of increasing dose of VU0152100 on amphetamine-induced disruption of PPI of the acoustic startle reflex in rats. As shown in Figure 2a, amphetamine when administered alone produced a robust disruption of PPI as compared with the vehicle/vehicle group across all of the prepulse intensities. However, the disruptive effects of amphetamine were significantly attenuated in the presence of a 30 mg/kg i.p. dose of VU0152100 when compared with the vehicle/amphetamine group at prepulse intensities of 5 and 10 dB with a trend toward significance at 15 dB (F(treatment)4, 136=18.2, p<0.001 and F(prepulse intensity)2, 136=16.0, p<0.001). As a dose-related reversal of amphetamine-induced disruption of PPI was achieved with VU0152100, significant at the 5 and 10 dB prepulse intensities with a dose of 30 mg/kg, higher doses of VU0152100 were not evaluated in this assay. When administered alone, VU0152100 had no effect on startle amplitude or PPI across the dose range tested (data not shown).
Figure 2.
VU0152100 reverses sensorimotor gating and contextual fear conditioning deficits elicited by amphetamine. (a) VU0152100 blocks amphetamine-induced (3 mg/kg) disruption of prepulse inhibition (*p<0.05 vs vehicle/vehicle (Dunnett's test)); (b) The disruptive effects of amphetamine (4.8 mg/kg, s.c.) on the acquisition of a context-dependent fear response are dose-dependently reversed by pre-treatment with VU0152100 (**p<0.01, ***p<0.001 vs vehicle/vehicle, #p<0.05 vs vehicle/amphetamine (Dunnett's test)). Data are mean±SEM of 7–11 rats per group.
VU0152100 Reverses Amphetamine-Induced Disruption of Contextual Fear Conditioning
Contextual fear conditioning is a form of Pavlovian conditioning that is mediated by the hippocampus (Phillips and LeDoux, 1992). As shown in Figure 2b, pre-treatment with a 4.8 mg/kg dose of amphetamine disrupted acquisition of contextual fear conditioning as reflected by a significant decrease in percent freezing behavior relative to the vehicle/vehicle-treated rats. VU0152100 produced a dose-related blockade of the amphetamine-induced disruption of the acquisition of contextual fear conditioning; significant at the 56.6 mg/kg dose (F4, 43=7.66, p=0.001). In contrast, when VU0152100 was administered alone, it had no effect on the acquisition of contextual fear conditioning across the dose range tested (F3, 23=0.47, NS; data not shown). In a separate experiment, pre-treatment with VU0152100 (56.6 mg/kg, i.p.) alone had no effect on the threshold of sensitivity to a footshock stimulus as compared with vehicle-treated rats (data not shown).
VU0152100 does not Induce Fos-LI in Forebrain and Hypothalamus
We next assessed the ability of VU0152100 to induce changes in the expression of Fos-LI in specific cortical and limbic brain regions. In contrast to the increase in Fos-LI expression observed with the typical and atypical antipsychotics, haloperidol and clozapine, respectively, VU0152100 (30 and 100 mg/kg, i.p.) had no effect on Fos-LI expression in the PFC, NAScore, NASshell, or CP (Supplementary Table S1: PFC (H=19.0, p<0.001), NASshell (H=31.9, p<0.0001), NAScore (H=27.3, p<0.0001), and dorsolateral CP (H=39.6, p<0.0001). As weight gain liability of certain antipsychotics correlates with their ability to elicit Fos-LI expression in hypothalamic orexin neurons (Fadel et al, 2002), we also examined the potential of VU0152100 to induce Fos-LI expression in this brain region (Supplementary Table S2). In the medial and lateral part (with respect to the fornix) of the lateral hypothalamus only haloperidol and clozapine, but not VU0152100, increased the number of Fos-li cells/mm2 (medial LH (H=15.3, p<0.01); lateral LH (H=14.7, p<0.01)) and the percentage of orexin cells expressing Fos-LI (medial LH (H=17.8, p<0.01); lateral LH (H=26.4, p<0.0001)) (Supplementary Table S1).
phMRI Quantification of VU0152100-Mediated Suppression of Amphetamine-Induced Regional Brain Activation and Functional Connectivity Pattern
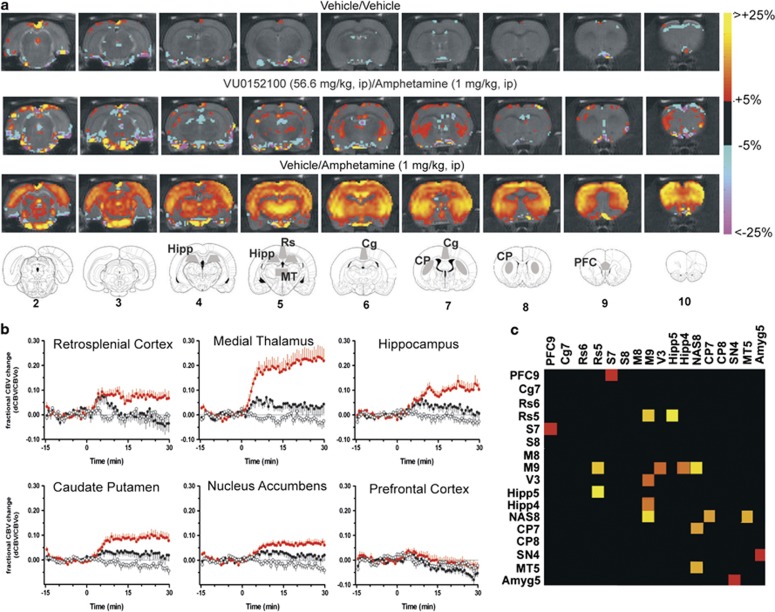
In order to more comprehensively assess the specific brain regions underlying the effects of VU0152100 in reversing the amphetamine-induced changes in behavior, we used phMRI to examine the effects of a behaviorally relevant dose of VU0152100 (56.6 mg/kg, i.p.) on amphetamine-evoked (1 mg/kg, i.p.) alterations in CBV. As shown in the group-averaged CBV maps (Figure 3a) and time courses (Figure 3b), amphetamine robustly increased CBV in the NAS and CP, areas of high dopamine transporter expression, as well as in extrastriatal areas, including the thalamus, hippocampus, and retrosplenial cortex. Figure 3b also shows that a 30-min pre-treatment with VU0152100 (56.6 mg/kg, i.p.) significantly blunted amphetamine-evoked increases in CBV in the medial thalamus (F2, 31=15.7, p<0.0001), hippocampus (F2, 31=11.7, p<0.001), CP (F2, 31=10.3, p<0.001), NAS (F2, 31=9.31, p<0.001), and retrosplenial cortex (F2, 31=3.8, p<0.05). As shown in Figure 3b, in the PFC there were no significant CBV changes in response to amphetamine alone or in combination with VU0152100 (F2, 31=0.94, NS). However, the lack of observed amphetamine effects may have been due in part to the anesthetized preparation and/or the exclusion of the two most anterior brain slices (1.5 mm in thickness) in the analysis to avoid partial volume effects as these slices also include the frontal pole and olfactory cortex, respectively. There were also no significant CBV responses detected in groups treated with vehicle/vehicle (Figure 3b) or VU0152100 alone (data not shown).
Figure 3.
VU0152100 modulates amphetamine-induced cerebral blood volume (CBV) responses. (a) Amphetamine (1 mg/kg, i.p.) elicited CBV increases, reflecting neural activity, while pre-treatment with VU0152100 (56.6 mg/g, i.p.) suppressed this effect in multiple brain areas. (b) Regional CBV time courses for amphetamine and reversal by VU0152100 are shown for the retrosplenial cortex, medial thalamus, hippocampus, NAS, CP, and PFC; note that amphetamine by itself and in combination with VU0152100 did not alter CBV in the PFC. Data are means±SEM of 10–11 animals per group. Functional connectivity analysis of the phMRI data revealed fewer inter-regional correlations in the VU0152100/amphetamine group and the significant ROI–ROI correlation differences between the vehicle/amphetamine group and the VU0152100/amphetamine group (thresholded at p<0.05) are depicted in panel (c). Cells are colored according to the corresponding z-statistic. Abbreviations of regions of interest (number indicates slice): Amyg, amygdala; CP, caudate-putamen; Cg, cingulate; Hipp, hippocampus; MT, medial thalamus; M, motor cortex; NAS, nucleus accumbens; PFC, prefrontal cortex; Pir, piriform cortex; Rs, retrosplenial cortex; S, sensory cortex; SN, substantia nigra; V, visual cortex.
Functional connectivity analysis of the above phMRI data revealed multiple inter-regional correlations in neural activation elicited by amphetamine in rats, while pre-treatment with VU0152100 (56.6 mg/kg, i.p.) modified the amphetamine-induced correlation pattern; fewer significant inter-regional correlations were found in the group pre-treated with VU0152100 before amphetamine (data not shown). As shown in Figure 3c, permutation analysis identified significant differences between the two treatment groups (the statistical threshold was set at p<0.05 and reported as z-scores), including retrosplenium-hippocampus (Δz=3.4499), NAS-motor cortex (Δz=3.3348), retrosplenium-motor cortex (Δz=3.2268), and NAS-medial thalamus (Δz=3.1553). Other ROI pairs with significant differences included the NAS-CP (Δz=3.0555), motor cortex with hippocampus (Δz=2.8753), and visual cortex (Δz=2.7884); PFC-sensory cortex (Δz=2.3990); and substantia nigra-amygdala (Δz=2.3409).
We assessed whether VU0152100 produced alterations in peripheral cardiovascular parameters, including blood pressure and heart rate, which might confound hemodynamic readouts of brain function (Ferrari et al, 2012). Compared with vehicle treatment, VU0152100 (56.6 mg/kg, i.p.) did not change arterial blood pressure (F(treatment)1, 72=9.28, p<0.01, F(time)7, 72=1.44, NS, and F(interaction)7, 72=3.83, NS) or heart rate (F(treatment)1, 72=7.58, p<0.05, F(time)7, 72=7.41, NS, and F(interaction)7, 72=2.47, NS) in awake, freely moving animals (Figures 5c and d).
VU0152100 Potentiates the Effects of a Sub-Threshold Dose of Oxotremorine on Dopamine Utilization in the Forebrain
Previous studies demonstrated that xanomeline produced dose-related increases in the dopamine metabolite DOPAC in the rodent striatum (Bymaster et al, 1994). Here we assessed whether VU0152100, in comparison with xanomeline, alters the ratio of monoamine metabolites/parent monoamines, an index of monoamine utilization, in post mortem brain tissue. In contrast to xanomeline (30 mg/kg), VU0152100 (10–100 mg/kg) alone did not increase dopamine or serotonin utilization in the PFC, NAS, or CP (Table 1: (PFC) DOPAC/DA (F4, 37=5.79, p<0.01), 5-HIAA/5-HT (F4, 39=0.84, NS); (NAS) DOPAC/DA (F4, 38=8.08, p<0.001), 5-HIAA/5-HT (F4, 39=0.57, NS); (CP): DOPAC/DA (F4, 38=0.63, NS), 5-HIAA/5-HT (F4, 38=0.62, NS)). However, when VU0152100 (56.6 mg/kg) was administered in combination with an ineffective dose of the non-selective mAChR agonist oxotremorine (0.01 mg/kg), we observed a marked increase in dopamine, but not serotonin, utilization in the PFC (DOPAC/DA (F3, 30=4.01, p<0.05), HVA/DA (F3, 28=5.63, p<0.01), and 5-HIAA/5-HT (F3, 30=0.95, NS)) and NAS (DOPAC/DA (F3, 30=10.88, p<0.0001), HVA/DA (F3, 30=10.08, p<0.0001), and 5-HIAA/5-HT (F3,30=0.92, NS)) as shown in Table 2. In the CP, the combination of VU0152100 (56.6 mg/kg) with oxotremorine (0.01 mg/kg) reduced dopamine and serotonin utilization (DOPAC/DA (F3, 31=11.04, p<0.0001), HVA/DA (F3, 31=7.27, p<0.001), and 5-HIAA/5-HT (F3, 31=5.09, p<0.01)).
Table 1. Effects of VU0152100 (10–100 mg/kg, i.p.) and Xanomeline (30 mg/kg, i.p.) on Dopamine and Serotonin Utilization in the Rat Forebrain.
| Dose (mg/kg) | N | DOPAC/DA | 5-HIAA/5-HT | |
|---|---|---|---|---|
| Prefrontal cortex | ||||
| Vehicle | 10 | 0.42±0.04 | 0.44±0.02 | |
| VU0152100 | 10.0 | 6 | 0.52±0.03 | 0.49±0.03 |
| VU0152100 | 56.6 | 11 | 0.50±0.02 | 0.47±0.04 |
| VU0152100 | 100.0 | 5 | 0.46±0.04 | 0.54±0.06 |
| Xanomeline | 30.0 | 6 | 0.68±0.08a | 0.50±0.05 |
| Nucleus accumbens | ||||
| Vehicle | 11 | 0.21±0.02 | 0.66±0.07 | |
| VU0152100 | 10.0 | 6 | 0.24±0.01 | 0.66±0.03 |
| VU0152100 | 56.6 | 11 | 0.27±0.02 | 0.68±0.04 |
| VU0152100 | 100.0 | 6 | 0.26±0.02 | 0.62±0.05 |
| Xanomeline | 30.0 | 5 | 0.37±0.01a | 0.75±0.03 |
| Dorsal striatum | ||||
| Vehicle | 10 | 0.13±0.01 | 1.13±0.06 | |
| VU0152100 | 10.0 | 6 | 0.14±0.01 | 1.20±0.07 |
| VU0152100 | 56.6 | 11 | 0.13±0.01 | 1.22±0.07 |
| VU0152100 | 100.0 | 6 | 0.14±0.01 | 1.21±0.02 |
| Xanomeline | 30.0 | 6 | 0.14±0.01 | 1.26±0.06 |
The effects of VU0152100 (10–100 mg/kg, i.p.) or vehicle on monoamine turnover in the medial prefrontal cortex, nucleus accumbens, and dorsolateral caudate-putamen were compared with xanomeline (30 mg/kg, i.p.). Data represent means±SEM; monoamine and metabolite concentrations in vehicle-treated animals were: (PFC) DOPAC 0.30±0.08, DA 0.84±0.15 5-HIAA 4.67±0.65, and 5-HT 10.3±1.21; (NAS) DOPAC 15.38±2.34, DA 71.27±8.29, 5-HIAA 7.77±0.53, and 5-HT 11.24±1.55; (CP) DOPAC 19.77±3.61, DA 135.30±10.86, 5-HIAA 7.56±0.36, and 5-HT 6.88±0.75.
p<0.001 vs vehicle (Bonferroni's test).
Table 2. VU0152100 Potentiates Effect of a Sub-Threshold Dose of Oxotremorine on Monoamine Turnover in the Forebrain.
| Compound 1 | Dose | Compound 2 | Dose | DOPAC/DA | HVA/DA | 5-HIAA/5-HT |
|---|---|---|---|---|---|---|
| Prefrontal cortex | ||||||
| Vehicle | Vehicle | 0.41±0.03 | 0.65±0.07 | 0.37±0.01 | ||
| VU0152100 | 56.6 | Vehicle | 0.44±0.03 | 0.78±0.07 | 0.39±0.02 | |
| Vehicle | Oxotremorine | 0.01 | 0.35±0.02 | 0.56±0.05# | 0.41±0.03 | |
| VU0152100 | 56.6 | Oxotremorine | 0.01 | 0.47±0.01& | 0.86±0.05&& | 0.39±0.02 |
| Nucleus accumbens | ||||||
| Vehicle | Vehicle | 0.22±0.01 | 0.07±0.01 | 0.439±0.060 | ||
| VU0152100 | 56.6 | Vehicle | 0.26±0.01 | 0.10±0.01 | 0.428±0.031 | |
| Vehicle | Oxotremorine | 0.01 | 0.27±0.01 | 0.08±0.01 | 0.503±0.048 | |
| VU0152100 | 56.6 | Oxotremorine | 0.01 | 0.34±0.02***,##,& | 0.13±0.01***,&& | 0.527±0.060 |
| Dorsal striatum | ||||||
| Vehicle | Vehicle | 0.15±0.01 | 0.10±0.01 | 0.88±0.03 | ||
| VU0152100 | 56.6 | Vehicle | 0.16±0.01 | 0.11±0.01 | 0.94±0.04 | |
| Vehicle | Oxotremorine | 0.01 | 0.16±0.01 | 0.09±0.01 | 1.00±0.05 | |
| VU0152100 | 56.6 | Oxotremorine | 0.01 | 0.12±0.01***,###,&&& | 0.08±0.01*,### | 0.81±0.02& |
Following a 15-min pre-treatment with VU0152100 (56.6 mg/kg, i.p.) or vehicle, rats were injected with oxotremorine sesquifumarate (0.01 mg/kg, s.c.) or vehicle (water) and killed 30 min later for the assessment of tissue monoamines and metabolites. Data represent means±SEM of 6–8 animals per group; monoamine and metabolite concentrations (ng/mg protein) in vehicle-treated animals were as follows: (PFC) DOPAC 0.0.48±0.06, DA 1.22±0.15, HVA 0.78±0.07, 5-HIAA 2.13±0.18, and 5-HT 5.86±0.49; (NAS) DOPAC 14.97±0.61, DA 69.49±2.36, HVA 4.62±0.38, 5-HIAA 2.64±0.11, and 5-HT ), 6.82±1.06; (CP) DOPAC 15.65±0.67, DA 102.5±2.22, HVA 9.76±0.42, 5-HIAA 3.08±0.12, and 5-HT 3.51±0.17.
*p<0.05; ***p<0.001 vs vehicle/vehicle; #p<0.05; ##p<0.01; ###p<0.001 vs VU0152100/vehicle; &p<0.05; &&p<0.01; &&&p<0.001 vs vehicle/oxotremorine (Bonferroni's test).
VU0152100 Reverses Amphetamine-Induced Increases in Dopamine Release in the NAS and CP
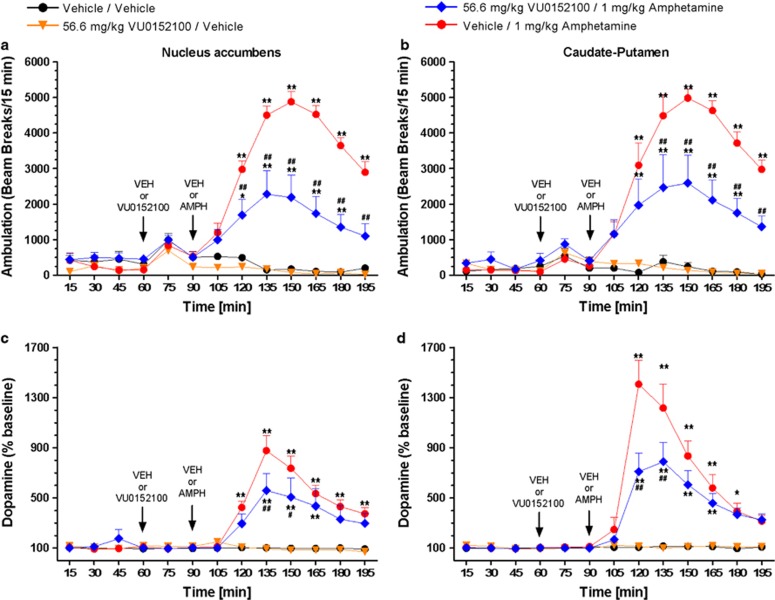
The finding that VU0152100 reverses effects of amphetamine in multiple behavioral models and on CBV in multiple brain regions, raises the possibility that this M4 PAM may act in part by reducing effects of amphetamine on extracellular dopamine levels. The effects of VU0152100 in reversing amphetamine-induced increases in extracellular dopamine levels in the NAS and CP were assessed using in vivo microdialysis in rats at a dose of 56.6 mg/kg (i.p.), which produced maximum efficacy in blocking amphetamine-induced hyperlocomotion and disruption of PPI. As shown in Figures 4a and b, the reversal of amphetamine-induced hyperlocomotion produced by the 56.6 mg/kg dose of VU0152100 was comparable in magnitude to the effects observed in our initial dose response study (Figure 4a: F(treatment)3, 312=30.6, p<0.001, F(time)12, 312=17.5, p<0.0001, and F(interaction)36, 312=34.8, p<0.001; Figure 4b: F(treatment)3, 351=27.1, p<0.001, F(time)12, 351=20.0, p<0.0001, and F(interaction)36, 351=30.7, p<0.001). When administered alone, VU0152100 had no effect on basal dopamine release in either the NAS or CP (Figures 4c and d). However, when given in combination with amphetamine, VU0152100 at the 56.6 mg/kg i.p. dose produced a significant reduction of amphetamine-elicited dopamine release in both the NAS and CP (Figure 4c for NAS: F(treatment)3, 297=19.9, p<0.001, F(time)12, 297=23.3, p<0.001, and F(interaction)36, 297=30.2, p<0.001; Figure 4d for the CP: (F(treatment)3, 351=18.8, p<0.001, F(time)12, 351=27.3, p<0.001, and F(interaction)36, 351=32.4, p<0.001). As shown in Supplementary Figure S2 in the NAS, VU0152100 alone increased extracellular levels of the dopamine metabolites DOPAC (Supplementary Figure S2A: F((treatment)3, 296=16.3, p<0.001, F(time)12, 296=3.87, NS, and F(interaction)36, 296=21.2, p<0.001) and HVA (Supplementary Figure S2B: F(treatment)3, 296=17.9, p<0.001, F(time)12, 296=2.86, NS, and F(interaction)36, 296=17.3, p<0.001). In the CP, amphetamine alone or in combination with VU0152100 reduced DOPAC (Supplementary Figure S2C: F(treatment)3, 334=16.4, p<0.001, F(time)12, 334=11.8, p<0.001, and F(interaction)36, 334=17.7, p<0.001) and HVA (Supplementary Figure S2D: F(treatment)3, 331=13.9, p<0.001, F(time)12, 331=9.98, p<0.001, and F(interaction)36, 331=24.0, p<0.001).
Figure 4.
Pre-treatment with VU0152100 (56.6 mg/kg, i.p.) reverses amphetamine-induced (1 mg/kg, s.c.) hyperlocomotion (a, b) and reduces the amphetamine-elicited increase in extracellular dopamine levels in the nucleus accumbens and caudate-putamen (c, d). Data are mean±SEM of 6–8 rats per group; *p<0.05, **p<0.01 vs vehicle/vehicle; #p<0.05, ##p<0.01 vs vehicle/amphetamine (Dunnett's test).
VU0152100 does not Induce Catalepsy but Exacerbates Haloperidol-Induced Catalepsy
We assessed the time course for the potential induction of catalepsy, a preclinical model of motor impairments and extrapyramidal motor side effects, by VU0152100 when administered alone in comparison with haloperidol as a positive control in rats. As shown in Figure 5a, haloperidol (1.5 mg/kg, s.c.) produced a robust time-dependent induction of cataleptic behavior, significant at 30, 60, 120, and 240-min post-haloperidol administration, while in contrast VU0152100 (30–100 mg/kg, i.p.) was without effect across the dose range and time course tested (F(treatment)4, 125=9.29, p<0.0001, F(time)1, 125=9.98, p<0.0001, and F(interaction)16, 125=26.2, p<0.0001; Figure 5a). However, when given in combination with submaximal doses of haloperidol (0.3 or 0.5 mg/kg), VU0152100 (100 mg/kg, i.p.) significantly enhanced cataleptic behavior compared with rats treated with haloperidol (0.3 or 0.5 mg/kg) alone (F(treatment)1,50=28.6, p<0.0001, F(dose)2, 50=20.53, p<0.0001, and F(interaction)2, 50=4.876, NS; Figure 5b).
Figure 5.
VU0152100 does not cause motor and cardiovascular side effects when given alone, but exacerbates haloperidol-induced catalepsy. (a) VU0152100 (30–100 mg/kg, i.p.) does not induce catalepsy, whereas haloperidol (1.5 mg/kg, s.c.) causes a time-dependent increase in catalepsy (**p<0.01, ***p<0.001 vs vehicle (Bonferroni's test)). (b) VU0152100 (100 mg/kg, i.p.) increases the cataleptogenic effects of submaximal (0.3–0.5 mg/kg, i.p.) doses of haloperidol (**p<0.01, ***p<0.001 vs vehicle/haloperidol (Bonferroni's test)). (c, d) Lack of cardiovascular effects of VU0152100. Rats were injected (arrow) after collecting 30-min baseline data. Following a 15-min post-injection period (acclimation), blood pressure and heart rate data were collected in 10-min intervals. (c) VU0152100 (56.6 mg/kg, i.p.) does not alter blood pressure and (d) heart rate in awake animals. Although two-way ANOVA revealed a significant treatment effect on blood pressure and heart rate there were we no significant differences between vehicle- and VU0152100-treated rats at any time point (Bonferroni test).
VU0152100 does not Produce Side Effects Associated with Activation of Peripheral M2 and M3 mAChRs
Although, VU0152100 is highly selective for M4 relative to the other muscarinic receptor subtypes in vitro (Brady et al, 2008), it was critical to assess the degree of selectivity of the actions of VU0152100 in vivo using the Modified Irwin Neurological Test battery as compared with the non-selective mAChR agonist oxotremorine (Jones et al, 2008). As depicted in Supplementary Table S2, oxotremorine (1.0 mg/kg, s.c.) produced a significant time-related induction of salivation (F(treatment)2, 48=96.0, p<0.01, F(time)4, 48=37.7, p<0.0001, and F(interaction)8, 48=37.7, p<0.0001), lacrimation (F(treatment)2, 48=96.0, p<0.0001, F(time)4, 48=37.7, p<0.0001, and F(interaction)8, 48=37.7, p<0.0001), piloerection (F(treatment)2, 48=164.6, p<0.0001, F(time)4, 48=27.7, p<0.0001, and F(interaction)8, 48=27.7, p<0.0001), and decreased respiratory rate (F(treatment)2, 48=12.5, p<0.01, F(time)4, 48=6.00, p<0.001, and F(interaction)8, 48=6.00, p<0.0001) and core body temperature (F(treatment)2, 48=16.1, p<0.001, F(time)4, 48=2.19, NS, and F(interaction)8, 48=3.41, p<0.01); whereas VU0152100 (56.6 mg/kg, i.p.) produced no changes to any of the measurements in the Modified Irwin Neurological Test battery.
DISCUSSION
In recent years, selective activators of mAChRs have emerged as a new approach for the treatment of psychotic symptoms and cognitive disturbances associated with various neuropsychiatric disorders. In this study, the selective M4 PAM VU0152100 displayed dose-dependent efficacy across multiple behavioral tasks that predict APD-like activity. In contrast to orthosteric mAChR agonists, VU0152100 did not produce adverse effects associated with non-selective activation of peripheral mAChR subtypes or impairments in locomotor activity. phMRI studies also indicated that VU0152100 reduces amphetamine-induced activation of the NAS and CP, as well as certain cortical, thalamic, and hippocampal areas. Functional connectivity analyses of the phMRI data revealed that VU0152100 modulated the activity of neural circuits that included the NAS, retrosplenial and motor cortices, hippocampus and thalamus. Finally, the behavioral effects of VU0152100 were accompanied by reversal of amphetamine-induced increases in extracellular DA levels in the NAS and CP, suggesting that the APD-like activity is mediated in part through modulation of mesolimbic and mesostriatal dopamine function.
The present findings suggest that VU0152100 reverses amphetamine-induced behavioral and neurochemical changes through potentiation of M4 mAChRs. Although the effects of the M4 PAM could be due to a drug–drug interaction between VU0152100 and amphetamine, this seems unlikely because only drug-naive rats were used in acute dosing paradigms that do not allow sufficient time for induction of amphetamine metabolism by VU0152100 (Fahmi and Ripp, 2010; FDA, 2012). Moreover, we previously reported that VU0152100 does not interact with a wide variety of GPCRs, ion channels, transporters, and enzymes that are present in the CNS and could be relevant for the in vivo responses measured (Brady et al, 2008). In this initial study, VU0152100 did displace binding of a ligand to the dopamine transporter with a 46% inhibition at 10 μM. As a follow-up, we have now estimated the in vitro IC50 values for VU0152100 at both the human and rat DAT to be 5.98 μM and >10 μM, respectively. However, at the highest dose of 56.6 mg/kg used in the previous and current studies, VU0152100 achieved only a Cmax free brain concentration of ∼340 nM. This unbound brain concentration of VU0152100 is closely aligned with the concentrations required to potentiate M4 signaling, but well below the concentrations that would be required to impact binding and function of the dopamine transporter. We also performed further studies to definitively confirm a critical role for M4 in the actions of VU0152100. Specifically, we evaluated the effects of VU0152100 in M4 KO mice and demonstrated that the effects of VU0152100 in reversing amphetamine-induced hyperlocomotion are absent in the M4 KO mice. It is, therefore, unlikely that the observed APD-like activity of VU0152100 is because of an off-target-related mechanism. Consistent with this, VU0152100 also reduces cocaine-induced hyperlocomotion, self-administration, and striatal dopamine release, and these effects are abolished in mice with targeted deletion of M4 mAChRs in striatal neurons expressing DA D1 receptors (D1-M4 KO mice; Dencker et al, 2012). These studies, taken together with a growing body of research discussed below suggest that M4 PAMs reduce dopaminergic signaling and have a range of APD-like effects in rodent models.
The present findings support and extend evidence that M4 mAChRs are the likely target involved in the APD-like activity reported with the M1/M4-preferring muscarinic agonist xanomeline (see Jones et al, 2012). M4 mAChRs are expressed in multiple cortical and limbic regions thought to be disrupted in neuropsychiatric disorder like schizophrenia, where they function as both pre- and postsynaptic modulatory receptors (Bubser et al, 2012; Tzavara et al, 2004). M4 KO mice display a hyperdopaminergic phenotype with increased basal and psychostimulant-induced locomotor activity coupled with increased basal and psychostimulant-induced extracellular dopamine levels in the NAS (Gomeza et al, 1999; Schmidt et al, 2011; Tzavara et al, 2004; Koshimizu et al, 2012). Interestingly, muscarinic agonist-induced reductions in accumbal and striatal DA release are absent in M4 KO mice, suggesting that M4 negatively regulates dopaminergic tone in these regions (Threlfell et al, 2010). M4 KO mice also exhibit deficits in PPI and social interaction and enhanced dopamine D1 receptor-mediated effects on locomotor activity, which are recapitulated in D1-M4 KO mice (Gomeza et al, 1999; Jeon et al, 2010; Koshimizu et al, 2012; Thomsen et al, 2010). Importantly, the APD-like effects of xanomeline are also abolished in both M4 KO and D1-M4 KO mice (Woolley et al, 2009; Dencker et al, 2011).
Our data with VU0152100 also validate previously reported effects using the structurally distinct M4 PAM LY2033298 when given in combination with a sub-threshold dose of the non-selective mAChR agonist oxotremorine (Chan et al, 2008; Leach et al, 2010; Suratman et al, 2011). However, LY2033298 does not provide an optimal tool compound for rodent studies in that it has relatively low potency at the rat M4 mAChR (Chan et al, 2008; Leach et al, 2010) and displays only weak cooperativity with ACh, the endogenous agonist of M4 (Suratman et al, 2011), but has high cooperativity with the synthetic mAChR agonist oxotremorine. As LY2033298 induces robust potentiation of oxotremorine, but not ACh effects on M4, it has no behavioral effects when administered alone unless co-administration with oxotremorine (Suratman et al, 2011). Our finding that VU0152100 has robust efficacy in potentiating ACh responses in vitro and in multiple in vivo models when administered alone supports this interpretation. These findings suggest that M4 PAMs that display high cooperativity with ACh at the rodent M4 receptor can potentiate responses to endogenous cholinergic tone to induce robust APD-like activity.
Our finding that VU0152100 attenuates amphetamine-induced increases in extracellular DA in the NAS and CP is consistent with previous studies discussed above and suggests that the in vivo APD-like effects of VU0152100 are mediated in part by reducing DA release. Currently, the mechanism by which M4 activation regulates mesolimbic and mesostriatal dopaminergic transmission is unknown. M4 has been postulated to act as an inhibitory autoreceptor on terminals of cholinergic neurons originating in the pedunculopontine and laterodorsal tegmental nuclei that excite dopaminergic neurons in the ventral tegmental area (Holmstrand and Sesack, 2011; Yeomans, 2012) and activation of M4 autoreceptors in the VTA could reduce cholinergic excitation of dopamine neurons (Tzavara et al, 2004). In addition, activation of cholinergic interneurons in the NAS elicits dopamine release (Cachope et al, 2012). If M4 acts as an inhibitory autoreceptor in NAS and CP, potentiating M4 in each of these regions could also reduce cholinergic-mediated dopamine release. However, it is important to note that while M4-mediated decreases in DA release may contribute to the in vivo effects of VU0152100, other mechanisms are also likely contribute to the APD-like actions of M4 PAMs. Previous studies reported that xanomeline reverses the behavioral effects of both amphetamine and the direct dopamine receptor agonist apomorphine and that these effects are eliminated with genetic deletion of M4 (Jones et al, 2005; Stanhope et al, 2001; Dencker et al, 2011). The ability of M4 activation to inhibit responses to a direct acting dopamine receptor agonist suggests that M4 activation must also act at a level downstream of DA release to induce its overall in vivo effects. M4 receptors are expressed at multiple levels in basal ganglia and forebrain regions and VU0152100 provides an excellent tool for future mechanistic studies aimed at elucidating other actions of M4 PAMs that may contribute to these overall in vivo effects.
Consistent with the behavioral and microdialysis results, VU0152100 suppressed amphetamine-induced increases in CBV in the NAS and CP as well as the medial thalamus, hippocampus, and retrosplenial cortex. Changes in central hemodynamic responses can reflect alterations in neural activity (Kim and Ogawa, 2012) and these alterations can be used to understand spatio-temporal effects of psychoactive drugs (Hackler et al, 2010; Chen et al, 1997; Schwarz et al, 2004). The effects of VU0152100 on brain activity in multiple regions suggest that actions in areas outside of the basal ganglia may also contribute to the in vivo effects of VU0152100. These findings are consistent with previous evidence that cortical projections modulate DA function in the striatum (Desole et al 1992): specifically, the motor and retrosplenial cortices innervate the lateral and medial striatum, respectively, whereas the PFC innervates the NAS (Ebrahimi et al 1992; Sesack et al 1989; van Groen and Wyss 1992, 2003). Using functional connectivity analyses (Schwarz et al, 2007a, 2007b), we characterized the regional correlation patterns in response amplitude to VU0152100. Interestingly, pre-treatment with VU0152100 attenuated several strong connectivity patterns of the NAS and retrosplenial cortex with other brain areas after amphetamine challenge, including correlated activity between NAS and hippocampus, motor cortex, and CP and the retrosplenial cortex with motor cortex and hippocampus. Importantly, the low isoflurane (0.88%) necessary for proper maintenance anesthesia to prevent movement-related artifacts did not result in negative CBV changes to amphetamine. Also, the observed changes in our phMRI studies were not attributable to changes in blood pressure or heart rate. Although additional studies are needed to further investigate the underlying mechanisms of the observed functional changes by VU0152100, these data suggest a role of M4 in direct and indirect modulation of dopaminergic signaling in multiple brain regions.
Although previous studies indicate that activation of M1 mAChRs is critical for learning, memory, and executive functions, the role of M4 mAChRs in modulating cognitive function remains unclear (Bubser et al, 2012; Shirey et al, 2009; Digby et al, 2012; Ma et al, 2009). We found that VU0152100 alone had no effect on PPI or the acquisition of contextual fear conditioning, suggesting that M4 may not be required for normal hippocampal learning and memory processes in young adult rats. These data are in agreement with recent reports that M4 KO mice display normal spatial reference, working, and episodic-like hippocampal memory functions in the Morris water maze test (Koshimizu et al, 2012). Interestingly, we did observe that VU0152100 reversed amphetamine-induced deficits in PPI and contextual fear conditioning. However, as the higher doses of amphetamine used in the cognitive studies can induce increases in both dopamine and norepinephrine (McKittrick and Abercrombie 2007), it will be important in future studies to confirm that these effects of VU0152100 are mediated predominately through reversal of dopaminergic signaling. Finally, VU0152100 provides an important M4-selective tool compound that used along with recently reported M1-selective PAMs (Ma et al, 2009; Shirey et al, 2009) or allosteric agonists (Lebois et al, 2010; Digby et al, 2012) will allow a more complete understanding of the relative roles of these two mAChR subtypes in regulating multiple aspects of cognitive function.
Both M4 KO and D1-M4 KO mice display attenuated antipsychotic-induced catalepsy (Jeon et al, 2010; Fink-Jensen et al, 2011). Our data showed that VU0152100 did not induce catalepsy over the dose range that produced APD-like activity, consistent with the lack of induction in Fos-li expression in the CP. These data suggest that M4 PAMs may not induce the extrapyramidal side effects observed with many APDs. However, VU0152100 did potentiate the induction of catalepsy when given in combination with sub-threshold doses of haloperidol. These findings indicate that the clinical use of VU0152100 may need to be as a stand-alone therapy or restricted to augmentation of atypical APDs.
In summary, these data are consistent with the interpretation that selective modulation of M4 mAChRs has effects in CNS circuits subserving the actions of APDs. Our results support the development of selective M4 PAMs as a novel treatment approach for the psychotic symptoms and cognitive impairments observed in psychiatric and neurologic disorders, including schizophrenia.
FUNDING AND DISCLOSURE
Dr Avison receives funding from NovoNordisk and received funding through NIH. Dr Conn received research support from Bristol Myers Squibb, Johnson & Johnson, and AstraZeneca, the Michael J Fox Foundation, the Thome Foundation and NIH and is an inventor on filed patent applications protecting M4 PAMs for the treatment of schizophrenia. Dr Deutch is supported in part by the US National Institutes of Health (MH077298) and by the Michael J Fox Foundation. He has served as a consultant for Eli Lilly, for which he has received honoraria and travel support. In addition, he has received compensation for editorial board responsibilities on Neuropsychopharmacology (ACNP). He has received travel support to attend meetings of Medical and Scientific Board of the National Parkinson Foundation. Dr Gore received funding from Astra Zeneca and the NIH. Dr Jones received research support from Bristol Myers Squibb, Johnson & Johnson, and AstraZeneca. Dr Jones also received funding through the Michael J Fox Foundation, the Barrus Foundation, and NIH. Dr Lindsley received compensation as a consultant for AbbVie Pharmaceuticals and research support from AstraZeneca, Johnson & Johnson, and Bristol Myers Squibb. Dr Lindsley also received funding through the Michael J Fox Foundation and NIH and is an inventor on filed patent applications protecting M4 PAMs for the treatment of schizophrenia. The remaining authors declare no conflict of interest.
Acknowledgments
We thank Dr Ditte Thorbek for conducting the studies in M4 KO mice and Zou Yue and Tiffany Farmer for their surgical expertise. Blood pressure studies were conducted with Tiffany Farmer at the Vanderbilt Diabetes Resource and Training Center's Metabolic Physiology Shared Resource Core. We thank Amanda Huang for her assistance in analyzing blood pressure and heart rate data and Dr Randy Blakely for help with the rat dopamine uptake assays. This work was funded by grants from NIBIB (T32EB001628) and NIMH (1R01MH099649) to Dr NE Byun and Dr John C Gore, and by a grant from NIMH (R01MH086601) to Dr CK Jones.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- American Psychiatric Association 2000Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Publishing: Washington, DC [Google Scholar]
- Andersen MB, Fink-Jensen A, Peacock L, Gerlach J, Bymaster F, Lundbaek JA, et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in the Cebus apella monkey. Neuropsychopharmacology. 2003;28:1168–1175. doi: 10.1038/sj.npp.1300151. [DOI] [PubMed] [Google Scholar]
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Byun N, Wood MR, Jones CK. Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb Exp Pharmacol. 2012;208:121–166. doi: 10.1007/978-3-642-23274-9_7. [DOI] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Wong DT, Mitch CH, Ward JS, Calligaro DO, Schoepp DD, et al. Neurochemical effects of the M1 muscarinic agonist xanomeline (LY246708/NNC11-0232) J Pharmacol Exp Ther. 1994;269:282–289. [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;26:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–998. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dencker D, Wörtwein G, Weikop P, Jeon J, Thomsen M, Sager TN, et al. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J Neurosci. 2011;31:5905–5908. doi: 10.1523/JNEUROSCI.0370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Weikop P, Sorensen G, Woldbye DP, Wortwein G, Wess J, et al. An allosteric enhancer of M(4) muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacol. 2012;224:277–287. doi: 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desole MS, Miele M, Enrico P, Fresu L, Esposito G, De Natale G, et al. The effects of cortical ablation on d-amphetamine-induced changes in striatal dopamine turnover and ascorbic acid catabolism in the rat. Neurosci Lett. 1992;139:29–33. doi: 10.1016/0304-3940(92)90850-7. [DOI] [PubMed] [Google Scholar]
- Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi A, Pochet R, Roger M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci Res. 1992;14:60. doi: 10.1016/s0168-0102(05)80005-7. [DOI] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmi OA, Ripp SL. Evaluation of models for predicting drug-drug interactions due to induction. Expert Opin Drug Metab Toxicol. 2010;6:1399–1416. doi: 10.1517/17425255.2010.516251. [DOI] [PubMed] [Google Scholar]
- FDA 2012. Guidance for industry: drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations. Guidance for industry: drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf .
- Ferrari L, Turrini G, Crestan V, Bertani S, Cristofori P, Bifone A, et al. A robust experimental protocol for pharmacological fMRI in rats and mice. J Neurosci Methods. 2012;15:9–18. doi: 10.1016/j.jneumeth.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Schmidt LS, Dencker D, Schülein C, Wess J, Wörtwein G, et al. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur J Pharmacol. 2011;656:39–44. doi: 10.1016/j.ejphar.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, et al. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Byun NE, Jones CK, Williams JM, Baheza R, Sengupta S, et al. Selective potentiation of the metabotropic glutamate receptor subtype 2 blocks phencyclidine-induced hyperlocomotion and brain activation. Neuroscience. 2010;168:209–218. doi: 10.1016/j.neuroscience.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Holmstrand EC, Sesack SR. Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons. Brain Struct Funct. 2011;216:331–345. doi: 10.1007/s00429-011-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DP, Cui Y, Davis AA, et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE. Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J Pharmacol Exp Ther. 2005;312:1055–1063. doi: 10.1124/jpet.104.075887. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2012;32:1188–1206. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Leiter LM, Miyakawa T. M4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol Brain. 2012;5:10. doi: 10.1186/1756-6606-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, et al. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois EP, Bridges TM, Lewis LM, Dawson ES, Kane AS, Xiang Z, et al. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M(1) receptor function in the central nervous system. ACS Chem Neurosci. 2010;1:104–121. doi: 10.1021/cn900003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci USA. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Blakely RD. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology. 2005;49:737–749. doi: 10.1016/j.neuropharm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Abercrombie ED. Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem. 2007;100:1247–1256. doi: 10.1111/j.1471-4159.2006.04300.x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Parasuraman S, Raveendran R. Measurement of invasive blood pressure in rats. J Pharmacol Pharmacother. 2012;3:172–177. doi: 10.4103/0976-500X.95521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophrenia Res. 2009;110:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2007The Rat Brain in Stereotoxic Coordinates6th edElsevier: Amsterdam, The Netherlands [Google Scholar]
- Perry KW, Nisenbaum LK, George CA, Shannon HE, Felder CC, Bymaster FP. The muscarinic agonist xanomeline increases monoamine release and immediate gene expression in the rat prefrontal cortex. Biol Psychiat. 2001;49:716–725. doi: 10.1016/s0006-3223(00)01017-9. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, et al. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerberg P, Olesen PH, Nielsen S, Treppendahl S, Sheardown MJ, Honore T, et al. Novel functional M1 selective muscarinic agonists. Synthesis and structure-activity relationships of 3-(1,2,5-thiadiazolyl)-1,2,5,6-tetrahydro-1-methylpyridines. J Med Chem. 1992;35:2274–2283. doi: 10.1021/jm00090a019. [DOI] [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DP, et al. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 2011;216:367–378. doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J, et al. Selective dopamine D(3) receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse. 2004;54:1–10. doi: 10.1002/syn.20055. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Reese T, Bifone A. In vivo mapping of functional connectivity in neurotransmitter systems using pharmacological MRI. NeuroImage. 2007a;34:1627–1636. doi: 10.1016/j.neuroimage.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A. Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to d-amphetamine modified by selective dopamine D3 receptor antagonist SB277011A. Magnetic Resonance Imaging. 2007b;25:811–820. doi: 10.1016/j.mri.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Rasmussen K, Bymaster FP, Hart JC, Peters SC, Swedberg MD, et al. Xanomeline, an M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophrenia Res. 2000;42:249–259. doi: 10.1016/s0920-9964(99)00138-3. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiat. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol. 2008;4:42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- Suratman S, Leach K, Sexton P, Felder C, Loiacono R, Christopoulos A. Impact of species variability and 'probe-dependence' on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br J Pharmacol. 2011;162:1659–1670. doi: 10.1111/j.1476-5381.2010.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, et al. The muscarinic receptor agonist xanomeline has antipsychotic-like activity in the rat. J Pharmacol Exp Ther. 2001;299:782–792. [PubMed] [Google Scholar]
- Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RS, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59:500–506. doi: 10.1176/ps.2008.59.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, et al. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, et al. Attenuation of cocaine's reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–969. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- van Groen A, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA. Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur J Pharmacol. 2009;603:147–149. doi: 10.1016/j.ejphar.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. Muscarinic receptors in brain stem and mesopontine cholinergic arousal functions. Handb Exp Pharmacol. 2012;208:243–259. doi: 10.1007/978-3-642-23274-9_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.