Abstract
Objective:
In this study, we aimed to evaluate and compare the dentinal walls of root-end cavities for the presence of cracks after cavity preparation using US retrotips and Er: YAG laser.
Materials and Methods:
Fifty single-rooted teeth were prepared by Protaper NiTi rotary system and obturated by lateral condensation. Three milimeters of root-end was resected. Twenty teeth were prepared with US retrotip (Group 1), 20 teeth with Er: YAG laser (Group 2), and 10 teeth without retropreparation (control group). The root-end surfaces were examined under a scanning electron microscope (SEM). Then the cracks of the resected root surfaces were evaluated on microphotographs.
Results:
No statistically significant difference was detected between US Group and Laser Group for complete, incomplete, intradentinal, and total number of cracks (P = 0.47, P = 0.80, P = 0.69, P = 0.869, respectively).
Conclusion:
Statistical analysis revealed no significant effect of retropreparation technique on the development of apical cracks (P > 0.05).
Keywords: Er: YAG laser, retropreparation, root-end cavity, ultrasonic retrotips
INTRODUCTION
Despite the fact that the success rate of conventional endodontic therapy has recently been as high as 85-95%, failed cases that cannot be treated conservatively exist.[1] In these cases, endodontic surgery is required. The aim of placing retrograde filling material after apicoectomy and root-end cavity preparation is to prevent the passage of microbial products from the root canal into the periapical tissues by sealing and closing the path from the root canal. The root tip is resected perpendicular to the long axis of the root in 3 mm. Root-end cavity preparation depth is suggested to be 2.5-3 mm, with parallel walls. Ideally, the root-end cavity is compatible with the long axis of the root and anatomically parallel to the root outline. Further, it must provide sufficient retention form. A large number of techniques and devices have been recommended for this procedure.[2] Traditionally, the root-end cavity has been prepared with small, round, and inverted conical burs in high-speed micro handpieces. This technique can cause several problems such as nonparallel cavity walls, difficulty reaching the root tip, and lingual perforation of the root.[3]
Improvement in sonic and ultrasonic (US) retrotips have been of great benefit to root-end treatment. US retrotips have many advantages over traditional apical surgery with high-speed handpieces and burs in that smaller, better-centered, and better-shaped root-end cavities can be prepared. Thus, the risk of perforation is largely reduced. Also, these devices can follow the long axis of the tooth, and apical cavities can be prepared easily and safely. In addition, the cutting bevel on the resected root end can be made perpendicular to the long axis of the root canal, decreasing the number of exposed dentinal tubules and consequent apical leakage.[4,5] However, several studies have demonstrated that, after retrograde cavity preparation, cracks have occured on the surface of the resected root ends.[6,7] On the other hand, other studies have not found any increase in the rate of microcrack formation.[8,9]
With the progress and widespread use of laser technology, a large number of researchers have shown that specific wavelengths of laser can remove dental hard tissues.[10,11] Hibst and Keller[10] and Keller and Hibst[12] reported that, under sufficient water cooling, cavities in enamel and dentin can be opened by means of Er: YAG laser without causing thermal damage to surrounding tissues. Many studies have used this wavelength in apicoectomies.[11,13,14,15]
In this study, we aimed to evaluate and compare root-end surfaces for the presence of cracks after root-end cavity preparation using zirconium nitride-coated US retrotips and Er: YAG laser.
MATERIALS AND METHODS
Fifty extracted, single-rooted maxillary incisor human teeth were used in our study. All teeth were stored in distilled water. The crowns of the teeth were resected at the cementoenamel junction. The root canals were prepared with Protaper (Dentsply/Maillefer, Ballaigues, Switzerland) instruments. Irrigation was copious throughout with a 2.5% sodium hypochlorite solution and EDTA (MD-ChelCream, META BIOMED, Chungbuk, Korea) was used for chelation. The root canals were dried with paper points and (Precise Dental, Zapopan, Mexico) obturated with lateral condensation technique with gutta-percha (Diadent, Choongchong Buk Do, Korea) and AH Plus (Dentsply, DeTrey, Konstanz, Germany) resin based root canal sealer (VDW, Munich, Germany). The teeth were kept at 37°C and 100% humidity for 1 week to ensure setting of the root canal filling material. The apical 3 mm of the root apices was resected perpendicular to the long axis of the tooth by means of a 240-mj, 25-Hz Er: YAG laser (Hoyaconbio Versawe Dental Laser, Fremont, CA, USA) with irrigation. After resection, ten teeth were used as a control. Forty teeth were divided into two groups. The root-end preparations of Group 1 were performed by zirconium nitride-coated US retrotips (ProUltra Tip No. SURG 1, Dentsply/Maillefer Instruments, Ballaigues, Switzerland) with a US device (Mectron, Carasco, Italy) at medium power with water cooling. The retropreparations of Group 2 were performed with 160-mJ, 30-Hz Er: YAG laser fitted with a 1 mm tip (Hoyaconbio Versawe Dental Laser). One tip was used for every ten teeth for all groups. All preparations were made according to the manufacturers’ instructions. The root-end preparations were examined under a scanning electron microscope (SEM) for the presence of cracks. For SEM analysis the specimens were dehydrated in ascending ethanol: water series (30, 50, 70, 90, 100%) dried in open air. After drying, the specimens were sputter-coated with gold using Polaron SC7620 sputter coater and observed by Jeol JSM 5600 SEM at 15.0 kV accelerated voltage and ×25 magnification. The cracks were classified as complete, incomplete, and intradentinal, similar to those of Beling, et al. [Figure 1].[8]
Figure 1.
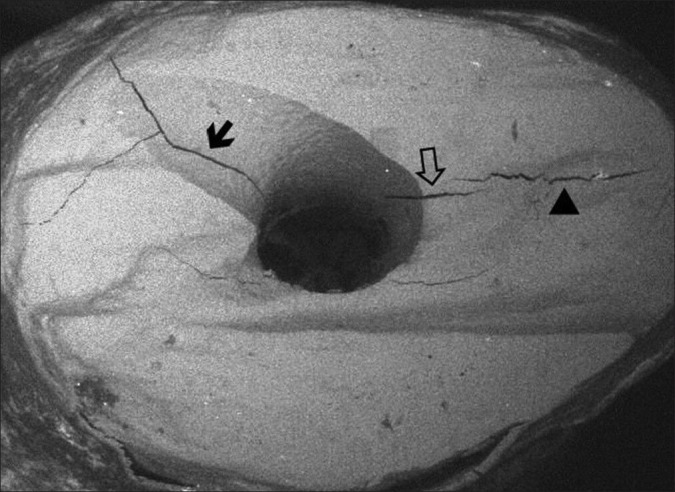
The types of cracks on the resected root surface (SEM, ×50 magnification)
Complete crack
Extending from the canal space to the external root surface.
Incomplete crack
Extending from the canal space to a variable distance into the dentin but ending short of the external root surface.
Intradentinal crack
Confined to dentin and appearing to run in a facial-lingual direction either mesial or distal to the canal.
Data were analyzed using the SPSS 11.5 statistic program (SPSS Inc, Chicago, IL, USA). All the groups were compared using a Kruskal − Wallis Test and Mann − Whitney U Test. A P value equal to or less than 0.05 was accepted as a statistically significant.
RESULTS
According to crack types, mean number of cracks for the groups are shown in Table 1.
Table 1.
Mean number of cracks for the groups according to the crack types
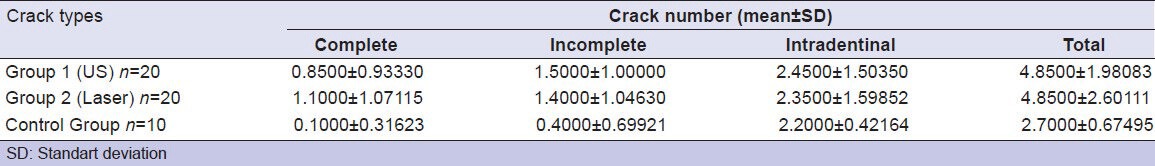
There was significant difference detected among all groups for the complete, incomplete, and total cracks (P = 0.022, P = 0.013, P = 0.018, respectively). For the intradentinal cracks no significant difference was found (P = 0.810).
There was significant difference between US (Group 1) and control groups for the number of complete, incomplete, and total cracks (P = 0.017, P = 0.004, P = 0.003, respectively). For the intradentinal cracks no significant difference was detected between two groups (P = 0.460).
There was significant difference between laser (Group 2) and control groups were found for the number of complete, incomplete, and total cracks (P = 0.008, P = 0.012, P = 0.03, respectively) while no significant difference was found between two groups for intradentinal cracks (P = 0.96).
No statistically significant difference was detected between the US and laser groups for complete, incomplete, intradentinal, and total number of cracks (P = 0.47, P = 0.80, P = 0.69, P = 0.869, respectively).
DISCUSSION
The success rate of endodontic surgery has been raised with the introduction of the operating microscope, US's, and improved root-end filling materials.[16] Results from our in vitro study seem to agree closely with most of the studies on crack formation using US retrotips.[8,17] Layton, et al.,[17] found that significantly more cracks after US root-end preparations than root-end resection only. Frank, et al.,[18] explained that some infractions occured by US root-end preparation. In contrast to this, Morgan, et al.,[19] found that only 1 of 25 roots demonstrated evidence of cracks after US root-end preparation. Results from this in vivo study are similar to those of the studies by Calzonetti, et al.[20] and Gray, et al.,[21] who found no cracks in cadaver teeth after US retropreparations. The clinical situation cannot be duplicated with extracted teeth because for one thing, the periodontal ligament may act as an absorbing factor when root-end cavities are prepared.[22]
Navarre and Steiman,[23] used zirconium nitride-coated and stainless steel US retrotips to examine the relation of retrotip type with crack formation in vitro, reporting no crack formation. Ishikawa, et al.,[24] found no statistically significant difference among zirconium nitride-coated, diamond-coated, and stainless steel US retrotip groups in the number of microcracks in their in vitro study. Taschieri, et al.,[25] used stainless steel and zirconium nitrate retrotips to evaluate the effect of using the US retrotips in root-end preparation. They reported that the type of retrotip did not cause a statistically significant difference in the success of treatment. Based on outcomes in the literature, we prefer to use the zirconium nitride-coated retrotip.
Layton, et al.[17] and De Bruyne and De Moor,[26] reported that more crack formation occurred at the high-frequency setting compared with low-frequency for root-end cavity preparation in vitro. However, Waplington, et al.,[27] reported that US root-end preparations at different power levels did not affect the number of cracks. Taschieri, et al.,[7] adjusted the power of the US device at two levels-half and full-in their in vitro study. A statistically significant difference was found between groups: the number of cracks occurring at full power exceeded those at half power. Bernardes, et al.,[28] did not detect any crack formation at the medium power level in their in vitro study. Taschieri, et al.,[25] performed root-end cavity preparations with stainless steel and zirconium nitrate retrotips without exceeding medium power level to evaluate the success of treatment in vivo. At the end of the year, clinical and radiographic evaluation identified the success rate at 91.3%. Based on outcomes in the literature, the power of US device was set at medium in our study.
Many researchers have used prepared root canals to provide in vivo conditions;[7,24,29,30,31,32] however, others suggest using unprepared teeth.[13,19,33,34] Beling, et al.,[8] reported that there was no significant difference between teeth obturated with gutta-percha and unprepared teeth in terms of type and number of cracks after root resection and retropreparation. On the other hand, Onnink, et al.,[35] found a statistically significant difference between prepared and unprepared teeth in terms of crack formation. We prepared the root canals and obturated them with gutta-percha, using lateral condensation to provide clinical conditions.
Hibst and Keller[10] and Keller and Hibst,[12] reported that under sufficient water cooling, cavities in enamel, and dentin could be opened by means of Er: YAG laser without causing thermal damage to the surrounding tissues. These SEM studies demonstrated that the resected surfaces presented an irregular, clean surface, without a smear layer, and with exposed dentinal tubules.[10,12]
Wallace,[36] evaluated the tendency for crack formation using ErCr: YSGG laser during preparation of retrograde cavities in vitro. Stereomicroscopic views were taken of 36 extracted teeth after retropreparations were performed; only 1 canal-related crack was found. He used unprepared teeth in his study, but we prefer to use prepared and obturated teeth to provide clinical conditions, perhaps explaining the difference between the two studies in the number of cracks. Rahimi, et al.,[37] compared the number of cracks after retropreparation with ErCr: YSGG laser versus using US for the obturated teeth. They found only one crack in the US group, while no crack was observed in the laser group. The results of our study showed no statistically significant difference between the laser and US groups for type and number of cracks.
Komori, et al.[15] used Er: YAG laser for apicoectomy on 13 teeth of 8 patients. Although they used a comparatively slow cutting speed, they reported such advantages as the absence of pain and vibration, low risk of trauma to the surrounding tissues, and lack of risk of contaminating the surgical field. Seven-month clinical and radiological follow-ups showed that treatment was successful. We also used Er: YAG laser for apicoectomy and retropreparation procedures. However, certain clinical limitations such as size of handpiece and angulation and diameter of the fiber still need to be overcome.
The most critical part of SEM analysis is the preparation of a sample. Dehydration and drying procedures may create artifacts in hard tissues. Two different approaches may be used for SEM analysis. Janda,[38] compared “direct” and “indirect” SEM analysis. Direct approach consists of the dehydration and drying of the original sample however indirect approach is carried out by taking impressions with appropriate materials. Even if the indirect SEM analysis may prevent creation of artifacts, this technique can not provide the detailed information of the tooth structure.[38] We have known that the risk of artifact cracks may always exist related technical problems. Our study results showed that there was significant difference between control group and experimental groups for the number of complete and incomplete cracks while for the number of intradentinal cracks no significant difference was found. We thought that most of these intradentinal cracks were occured because of direct SEM analysis.
We preferred to use one rooted teeth with one canal maxillary incisors to eliminate the problems arising from the complexity of root canal anatomy. All preparations were performed by one operator to eliminate the problem of operator differences. Two operators examined and evaluated the SEM views together to reach a consensus.
CONCLUSION
Under the limitations of this in vitro study, the laser irradiation of Er: YAG produces cracks when used for the root-end resection. Also, there is some controversy for using both of zirconium nitride-coated US retrotips and Er: YAG laser in root-end cavity preparations.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Sjogren U, Hagglund B, Sandqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498–504. doi: 10.1016/S0099-2399(07)80180-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim S. Endodontic microsurgery. In: Cohen S, Burns R, editors. Pathways of the pulp. 8th ed. St. Lois: Mosby Corp; 2002. pp. 683–725. [Google Scholar]
- 3.Carr G. Advanced techniques and visual enhancement for endodontic surgery. Endod Rep. 1992;7:6–9. [PubMed] [Google Scholar]
- 4.Gutmann JL, Saunders WP, Nguyen L, Guo IY. Ultrasonic root-end preparation Part I. SEM analysis. Int Endod J. 1994;27:318–24. doi: 10.1111/j.1365-2591.1994.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 5.Gormann MC, Steiman HR, Gartner AH. Scanning electron microscopic evaluation of root-end preparations. J Endod. 1995;21:213–7. doi: 10.1016/s0099-2399(06)80434-6. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd A, Jaunberzins A, Dummer PM, Bryant S. Root end cavity preparation using the micromega sonic retroprep tip. SEM analysis. Int Endod J. 1996;29:295–301. doi: 10.1111/j.1365-2591.1996.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Taschieri S, Testori T, Francetti L, Del Fabro M. Effects of ultrasonic root-end preparation on resected root surfaces: SEM evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:611–8. doi: 10.1016/j.tripleo.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Beiling KL, Marshal JG, Baumgartner JC. Evaluation of cracks associated ultrasonic root-end preparation of gutta-percha filled canals. J Endod. 1997;23:323–6. doi: 10.1016/s0099-2399(97)80415-3. [DOI] [PubMed] [Google Scholar]
- 9.Engel TK, Steiman HR. Preliminary investigation of ultrasonic root end preparation. J Endod. 1995;21:443–5. doi: 10.1016/S0099-2399(06)81524-4. [DOI] [PubMed] [Google Scholar]
- 10.Hibst R, Keller U. Experimental studies of the application of the Er: YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg Med. 1989;9:338–44. doi: 10.1002/lsm.1900090405. [DOI] [PubMed] [Google Scholar]
- 11.Paghdiwala AF. Root resection of endodontically treated teeth by erbium: YAG laser radiation. J Endod. 1993;19:91–4. doi: 10.1016/S0099-2399(06)81203-3. [DOI] [PubMed] [Google Scholar]
- 12.Keller U, Hibst R. Experimental studies of the application of the Er: YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg Med. 1989;9:345–51. doi: 10.1002/lsm.1900090406. [DOI] [PubMed] [Google Scholar]
- 13.Gouw-Soares S, Tanji E, Haypek P, Cardoso W, Eduardo CP. The use of Er: YAG, Nd: YAG and Ga-Al-As lasers in periapical surgery: A 3-year clinical study. J Clin Laser Med Surg. 2001;19:193–8. doi: 10.1089/104454701316918961. [DOI] [PubMed] [Google Scholar]
- 14.Komori T, Yokoyama K, Matsumoto Y, Matsumoto K. Erbium: YAG and holmium: YAG, laser root resection of extracted human teeth. J Clin Laser Med Surg. 1997;15:9–13. doi: 10.1089/clm.1997.15.9. [DOI] [PubMed] [Google Scholar]
- 15.Komori T, Tokoyama K, Takato T, Matsumoto K. Clinical application of the Erbium YAG laser for apicoectomy. J Endod. 1997;23:748–50. doi: 10.1016/S0099-2399(97)80348-2. [DOI] [PubMed] [Google Scholar]
- 16.Creasy JE, Pete M, Sweet M. Surgical trends among endodontists: The results of a web based survey. J Endod. 2009;35:30–4. doi: 10.1016/j.joen.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Layton CA, Marshall JG, Morgan LA, Baumgartner JC. Evaluation of cracks associated with ultrasonic root-end preparation. J Endod. 1996;22:157–60. doi: 10.1016/S0099-2399(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 18.Frank RJ, Antrim DD, Bakland LK. Effect of retrograde cavity preparations on root apexes. Endod Dent Traumatol. 1996;12:100–3. doi: 10.1111/j.1600-9657.1996.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan LA, Marshall JG. The topography of root ends resected with fissure burs and refined with two types of finishing burs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:585–91. doi: 10.1016/s1079-2104(98)90296-7. [DOI] [PubMed] [Google Scholar]
- 20.Calzonetti KJ, Iwanowski T, Komorowski R, Friedman S. Ultrasonic root end cavity preparation assessed by an in situ impression technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:210–5. doi: 10.1016/s1079-2104(98)90428-0. [DOI] [PubMed] [Google Scholar]
- 21.Gray GJ, Hatton JF, Holtzmann DJ, Jenkins DB, Nielsen CJ. Quality of root-end preparations using ultrasonic and rotary instrumentation in cadavers. J Endod. 2000;26:281–3. doi: 10.1097/00004770-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Van Arx T, Walker WA. Microsurgical instruments for root-end cavity preparation following apicoectomy: A literature review. Endod Dent Traumatol. 2000;16:47–62. doi: 10.1034/j.1600-9657.2000.016002047.x. [DOI] [PubMed] [Google Scholar]
- 23.Navarre SW, Steiman R. Root-End fracture during retropreparation: A comparison between zirconium nitride coated and stainless steel microsurgical instruments. J Endod. 2002;28:330–2. doi: 10.1097/00004770-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Sawada N, Kobayashi C, Suda H. Evaluation of root-end cavity preparation using ultrasonic retrotips. Int Endod J. 2003;36:586–90. doi: 10.1046/j.1365-2591.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 25.Taschieri S, Del Fabbro M, Testori T, Francetti L, Weinstein R. Endodontic surgery with ultrasonic retrotips: One-year follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:380–7. doi: 10.1016/j.tripleo.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 26.De Bruyne MA, De Moor RJ. SEM analysis of the integrity of resected root apices of cadaver and extracted teeth after ultrasonic root-end preparation at different intensities. Int Endod J. 2005;38:310–9. doi: 10.1111/j.1365-2591.2005.00949.x. [DOI] [PubMed] [Google Scholar]
- 27.Waplington M, Lumley PJ, Walmsley AD. Incidence of root face alteration after ultrasonic retrograde cavity preparation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:387–92. doi: 10.1016/s1079-2104(97)90247-x. [DOI] [PubMed] [Google Scholar]
- 28.Bernardes RA, de Moraes IG, Garcia RB, Bernardineli N, Baldi JV, Victorino FR, et al. Evaluation of apical cavity preparation with a new type of ultrasonic diamond tip. J Endod. 2007;33:484–7. doi: 10.1016/j.joen.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Rainwater A, Jeansonne B, Sarkar N. Effects of ultrasonic root end preparation on microcrack formation and leakage. J Endod. 2000;26:72–5. doi: 10.1097/00004770-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Khabbaz MG, Kerezoudis NP, Aroni E, Tsatsas V. Evaluation of different methods for the root-end cavity preparation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:237–42. doi: 10.1016/j.tripleo.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 31.Peters CI, Peters OA, Barbakow F. An in vitro study comparing root-end cavities prepared by diamond-coated and stainless steel ultrasonic retrotips. Int Endod J. 2001;34:142–8. doi: 10.1046/j.1365-2591.2001.00367.x. [DOI] [PubMed] [Google Scholar]
- 32.Weston GD, Moule AJ, Bartold PM. A scanning electron microscopic evaluation of root surfaces and the gutta-percha interface following root-end resection in vitro. Int Endod J. 1999;32:450–8. doi: 10.1046/j.1365-2591.1999.00242.x. [DOI] [PubMed] [Google Scholar]
- 33.Gondim E, Jr, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Effect of sonic and ultrasonic retrograde cavity preparation on the integrity of root apices of freshly extracted human teeth: Scanning electron microscopy analysis. J Endod. 2002;28:646–50. doi: 10.1097/00004770-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Tagger M, Tamse A, Katz A, Korzen BH. Evaluation of the apical seal produced by a hybrid root canal filling method, combining lateral condensation and thermatic compaction. J Endod. 1984;10:299–303. doi: 10.1016/S0099-2399(84)80183-1. [DOI] [PubMed] [Google Scholar]
- 35.Onnink PA, Davis RD, Wayman BE. An in vitro comparison of incomplete root fractures associated with three obturation techniques. J Endod. 1994;20:32–7. doi: 10.1016/s0099-2399(06)80024-5. [DOI] [PubMed] [Google Scholar]
- 36.Wallace JA. Effect of Waterlase laser retrograde root-end cavity preparation on the integrity of root apices of extracted teeth as demonstrated by light microscopy. Aust Endod J. 2006;32:35–9. doi: 10.1111/j.1747-4477.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 37.Rahimi S, Yavari HR, Shahi S, Zand V, Shakoui S, Reyhani MF, et al. Comparison of the effect of Er, Cr-YSGG laser and ultrasonic retrograde root-end cavity preparation on the integrity of root apices. J Oral Sci. 2010;52:77–81. doi: 10.2334/josnusd.52.77. [DOI] [PubMed] [Google Scholar]
- 38.Janda R. Preparation of extracted natural human teeth for SEM investigations. Biomaterials. 1995;16:209–17. doi: 10.1016/0142-9612(95)92119-q. [DOI] [PubMed] [Google Scholar]


